Abstract

The Dothideomycete fungal pathogen Pyrenophora tritici-repentis (Ptr) is the causal agent of the tan spot disease of wheat. The proteinaceous necrotrophic effectors ToxA and ToxB are well characterized. A nonproteinaceous effector called ToxC has also been partially characterized. Ptr produces a number of other small molecular weight compounds, but these remain poorly characterized. In this study, two novel compounds, designated ToxE1 and ToxE2, capable of inducing chlorotic symptoms on wheat leaves in a cultivar-specific manner, were purified from Ptr liquid cultures. There is no evidence that these compounds correspond to ToxC. Most isolates produced ToxE1, ToxE2, or both, and both compounds were detected in infected wheat leaves. The structures of both analogues were elucidated by NMR spectroscopy and comprise a phthalide core structure with an amide moiety. We postulate that these compounds have a general phytotoxic effect and may have an ancillary role in disease development.
Keywords: specialized metabolites, phytotoxin, tan spot, phthalide, ToxE, yellow leaf spot, ToxC
1. Introduction
The Dothideomycete fungal pathogen Pyrenophora tritici-repentis (Ptr) (syn. Drechslera, Helminthosporium), a causal agent of tan spot disease (also called yellow (leaf) spot) in wheat (Triticum aestivum), has been detected in most wheat-growing areas around the world. Ptr is an economically important foliar disease causing major yield loss especially in regions where conservation tillage practices are employed1 by inducing host death to obtain nutrients from necrotic tissue.2 The disease is typified by the presence of dark necrotic lesions surrounded by tan-colored chlorotic haloes.
Research into the tan spot has been dominated by two small proteins, ToxA and ToxB, that are secreted during infection and growth in culture. These proteins interact with the products of specific wheat genes (Tsn1 and Tsc2) to cause necrosis/chlorosis and promote disease in a pathogen isolate- and host genotype-specific manner. These proteins are archetypal necrotrophic effectors (NE),3,4 and ToxA insensitivity has been used to select for disease resistance in wheat germplasm collections.5 The structure of a third NE, ToxC,6 that interacts with the wheat gene Tsc1 has remained elusive. Recently, a conserved hypothetical gene most likely encoding a vacuolar membrane protein that is required but not sufficient for the production of ToxC was identified.7 Ptr populations have been divided into eight races on the basis of whether they express ToxA, ToxB, and ToxC.8,9
The role of secondary (or specialized) metabolites (SMs) in the virulence of Ptr has received scant attention. Just two classes of SMs have been purified from Ptr, the anthraquinones and triticones. Anthraquinones are tricyclic aromatic ketones and have been purified from many fungal, plant, and insect species.10,11 Catenarin, emodin, helminthosporin, and islandicin have been detected in culture filtrates of Ptr, catenarin and emodin in infected kernels, and catenarin in infected leaves.12,13 Catenarin, a red pigmented compound, is thought to cause red smudge symptom on wheat kernels.14
Triticones are characterized by a spirocyclic γ-lactam core structure, and six triticones (triticone A–F) have been purified from Ptr.15 The biosynthetic gene cluster responsible for production of triticones, Ttc, was recently identified in Ptr when deletion of the core biosynthetic gene prevented the production of 38 triticone or triticone-like compounds in culture filtrates.16 Infection assays using ttc mutants showed that while triticones A and B were phytotoxic, they did not contribute to virulence but may act to deter other microbes in the phyllosphere.
Culture filtrates of Ptr contain many as-yet uncharacterized SMs. Mining the Ptr M4 genome revealed 39 biosynthetic gene clusters potentially capable of producing SMs;17 only one, Ttc, which produces the triticones, has been characterized in any detail.16 Here, we report the purification, structural elucidation, and functional characterization of novel chlorosis-inducing specialized metabolites from Ptr that consist of two bioactive phthalides differing by a CHOH group, designated ToxE1 and ToxE2. Their molecular structures were resolved by nuclear magnetic resonance (NMR) spectroscopy supported by density functional theory (DFT) calculations.
2. Materials and Methods
2.1. Microbes and Plant Materials, and Culture Filtrate Production
Ptr isolate V1, collected in 2014 from Victoria, Australia, was grown on V8-PDA agar as described previously.18 To prepare the fungal liquid cultures, V1 was grown in Fries 3 media as described.16 The culture medium was filtered through three layers of milk filter paper (Daviesway, Heidelberg Heights, Victoria, Australia) followed by centrifugation of filtrate at 10,000 rpm for 1 h. The filtrate was then vacuum filtered through a Whatman No. 1 filter paper followed by a 0.2 μm membrane filter. The filamentous fungus Parastagonospora nodorum used in this study was provided by the Department of Primary Industries and Regional Development,19 and Alternaria infectoria was previously isolated from infected wheat leaves.20Saccharomyces cerevisiae and Escherichia coli were supplied by the School of Molecular Life Sciences Curtin University. The plant materials of wheat (Triticum aestivum) and barley (Hordeum vulgare) cultivars were supplied by Grain Research and Development Corporation (GRDC); canola (Brassica napus) cultivars were kindly provided by Mark Derbyshire (Curtin University); thale cress (Arabidopsis thaliana), Brachypodium distachyon, and Nicotiana benthamiana were supplied by School of Molecular Life Sciences Curtin University; and perennial ryegrass (Lolium perenne) cultivars were obtained from Symonds Seed (WA, Australia). The panel of Ptr isolates with different races were previously described.21,23
2.2. Toxin Purification
The culture filtrate (2 L) was extracted using dispersive solid phase extraction (dSPE) using 10 g of Bondesil-C18, 40 μm sorbent (Agilent, Santa Clara, California, USA) per L of culture filtrate. The sorbent was recovered by vacuum filtration and washed with 2 × 50 mL of water, followed by 45% (v/v) methanol. Active compounds were eluted with 70% methanol and further purified by high-performance liquid chromatography (HPLC) coupled to a diode array detector (DAD). Chromatographic separation was performed on an isocratic elution at 60% acetonitrile through an Agilent C18 semipreparative column (PrepHT, 150 mm × 21.2 mm × 5 μm). The active UV absorbing compounds were collected manually by monitoring 220, 254, 280, and 420 nm.
2.3. Liquid Chromatography Mass Spectrometry (LC-MS)
High-resolution mass spectrometry (MS) data were collected using the Q Exactive hybrid quadrupole-Orbitrap mass spectrometer (Thermo Scientific, Waltham, Massachusetts, United States) in positive ion mode with a C18 column (ACQUITY UPLC BEH C18, 100 mm × 2.1 mm, 1.7 μm, Waters, Milford, Massachusetts, United States) column. Chromatographic separation was achieved using 0.1% formic acid in water as mobile phase A and 0.1% formic acid in acetonitrile as mobile phase B at a flow rate of 0.4 mL/min. Chromatographic conditions held the column at 5% B for 1 min and then increased to 30% over 5 min, and B was then further increased to 99% over a further 5 min and held for 2 min. Mobile phase B was dropped back down to 5% over 0.1 min and maintained for a further 4 min (total time of 17 min). The injection volume was 5 μL. The scan range was 80 to 1200 m/z at a resolution of 70,000 full width half-maximum (FWHM). The automatic gain control (AGC) target values were 1 × 106 and 1 × 105, respectively. The heated electrospray ionization (HESI) source parameters were set as follows: sheath gas flow rate 40 units, aux gas flow rate 10 units, spray voltage 3 kV, capillary temperature 350 °C, and aux gas heater temperature 400 °C.
2.4. NMR and Infrared (IR) Spectroscopy
NMR spectra were obtained with Bruker Avance III HD 500 MHz and 600 Hz NMR spectrometers in methanol-d4 and acetone-d6, and data analysis was carried out using TopSpin v4.0.1 and Mestrenova software packages. Data obtained in methanol-d4 were referenced to the residual solvent signals at δ 3.31 ppm for 1H spectra and δ 49.1 ppm for 13C spectra relative to TMS. Data obtained in acetone-d6 were referenced to the residual solvent signal at δ 2.05 ppm for 1H spectra and δ 29.9 ppm for 13C spectra relative to TMS. The infrared spectrum was collected using a Nicolet iS50 FTIR fitted with a dedicated single-bounce diamond attenuated total reflection (ATR) purged with dry nitrogen. The absorbance was ATR corrected. Density functional theory (DFT) analysis was performed as previously described.100 NMR chemical shift calculations for the molecule were also determined using GNN (graph neural network) NMR shift prediction software Chemical Shift Calculator using Deep Learning CASCADE.22
2.5. Detection of ToxE Compounds in Fungal Culture Filtrates
An LC-QMS (single quadrupole) was used in selected ion monitoring mode to quantify ToxE1 and ToxE2 produced in the fungal culture filtrates. Mobile phase B was acetonitrile with 0.1% formic acid, and mobile phase A was water with 0.1% formic acid. Using a Phenomenex C18 column (Kinetix, 10 mm × 2.1 mm ID × 2.6 μm; Torrance, California, USA), chromatographic conditions held the column at 0% B for 0.08 min, and then increased to 100% over 10 min, and B was then held for a further 3.3 min and then decreased to 0% B over 0.3 min and finally maintained for 2.3 min (16 min total). The flow rate was 0.75 mL/min, and the injection volume was 3 μL. All mass spectral data analysis was carried out using AnalyzerPro v5.7.0.176.
2.6. In Planta Detection of ToxE1 and ToxE2 Using LC-MS
Two-week-old wheat cultivar “Eagle Rock” seedlings were inoculated with Ptr isolate M4 as described.18,23 Isolate M4 was used for infection assays due to its relative ease of sporulation in comparison to V1 and comparable production of ToxE1 and ToxE2 in the fungal culture filtrate. Second leaves of infected seedlings were snap frozen and freeze-dried at 1, 3, 5, and 7 days post inoculation. Leaves were ground to a fine powder, and 10 mg was extracted with 2 × 500 μL of methanol and 1 × 500 μL of 50:50 water:methanol. Solvents from the extracts were removed and reconstituted in 100 μL of 95:5 water:acetonitrile to analyze by accurate mass LC-Orbitrap-MS. Four biological samples were prepared for each time point. Samples were collected using parallel reaction monitoring mode (PRM) targeting ToxE1 and ToxE2 masses and a full scan. The scan range was 80 to 1200 m/z at a resolution of 70,000 FWHM in full-scan mode and 35,000 FWHM in PRM. LC parameters are as described in Section 2.3.
2.7. Phytotoxicity Assays
Infiltration of purified ToxE compounds was carried out on wheat (Triticum aestivum), canola (Brassica napus), thale cress (Arabidopsis thaliana), Nicotiana benthamiana, Brachypodium distachyon, perennial ryegrass (Lolium perenne), and barley (Hordeum vulgare). Seeds were sown in grade 2 vermiculite (the Perlite & Vermiculite Factory, Jandakot, WA, Australia) in seed trays, fertilized during sowing with soluble all-purpose Thrive N:P:K 25:5:8.8 (Yates Australia, Padstow NSW) at the concentration of 1 g/L, and grown at 22 °C in a 12 h day/night cycle in a growth chamber. Wheat, B. distachyon, ryegrass, and barley were infiltrated upon full expansion of the second leaf. Canola, A. thaliana, and N. benthamiana were infiltrated upon full expansion of the third leaf. All plant bioassays were done in triplicate, and symptoms were evaluated 7 days post infiltration. For the evaluation of Australian commercial wheat cultivars (Supporting Information Table S1), ToxE-induced chlorosis symptoms were scored on a 0 to 5 scale (Supporting Information Figure S1). For the seedling growth inhibition assay, second leaf of cultivar “Eagle Rock” was infiltrated with ToxE compounds at concentrations of 2, 20, and 200 μg/mL, respectively. Mock infiltration was included as a control. The length of the third leaf was recorded at a weekly interval for the duration of 4 weeks. A minimum of four biological replicates were measured.
2.8. Segregation Analysis of F1 Progeny and Double Haploid Wheat Population
Australian wheat cultivar “Eagle Rock” (sensitive to ToxE2) was reciprocally crossed to wheat line “95ZEE10” (insensitive to ToxE2) that was obtained from the CIMMYT and ICARDA wheat breeding program. Seeds from the F1 generation were planted in seedling trays containing vermiculite as described in Section 2.7. Leaves of 2-week old F1 progenies were subjected to infiltration with purified ToxE2 at the concentration of 200 μg/mL. For the segregation analysis, a double haploid (DH) wheat population consisting of 251 lines derived from the “Eagle Rock” × “95ZEE10” cross was used. The DH population was developed by the Western Australian Agriculture Authority (WAAA) at the Department of Primary Industries and Regional Development, South Perth, Western Australia. The lines were assayed for ToxE2 sensitivity similar to that of the F1 progenies. Three biological replicates were tested for each DH line. The parent lines were assayed along with the DH lines as controls. Sensitive reactions were scored 7 days post infiltration. Goodness of fit to a hypothesized 1:1 Mendelian segregation ratio was tested. Chi-square test (JMP 16.0.0) was used in the segregation analysis of the DH population.
2.9. Antimicrobial Assays
Antimicrobial activities of ToxE1 and ToxE2 were assessed in fungal and bacterial species using a disk diffusion method.24 The antifungal activity for each ToxE compound was tested against Saccharomyces cerevisiae, Alternaria infectoria, and P. nodorum. Briefly, mycelial plugs of filamentous fungi were each placed on V8-PDA plates, and 10 μL of ToxE solution at the concentrations of 2.0, 0.5, and 0.1 mg/mL was applied to 5 mm filter paper disks placed on the inoculated plates (equivalent to 20, 5, and 1 μg per disk, respectively). Plates were grown at room temperature for 36 to 72 h, and plates were observed for any growth inhibition. S. cerevisiae was cultured to an optical density (OD) of approximately 0.6–0.8, and 100 μL of culture was spread on YPD agar plates and grown at 30 °C. Antibacterial activity was similarly tested against Gram-negative bacteria Escherichia coli. Briefly, bacterial suspensions were grown to approximately 0.6–0.8 OD and 100 μL culture was spread on LB agar plates. Filter paper disks placed on the inoculated plates were infused with 10 μL of ToxE solution at the concentrations of 2.0, 0.5, and 0.1 mg/mL. For S. cerevisiae, plates were incubated for 24 h at 30 °C while E. coli at 37 °C. All disk assays were performed with three biological replicates.
3. Results and Discussion
3.1. Discovery and Structural Elucidation of Two Novel Phthalide Phytotoxins from Ptr
The culture filtrate of Ptr is known to contain a variety of proteinaceous and small molecular weight phytoactive compounds.14−16,25−27 In this study, two novel compounds were discovered using dispersive solid phase extraction (dSPE) and HPLC of Ptr isolate V1. A bioassay-guided purification of the culture filtrate was initially performed to identify bioactive fractions (details of the purification are provided in Supporting Information S2). Fractionation of the methanol eluate of the dSPE identified two peaks with phytotoxic activity, which we refer to as ToxE1 and ToxE2.
ToxE1 was obtained as an off-white, amorphous powder (melting point 90–93 °C). The molecular formula of ToxE1 was established as C21H27NO7 (calculated exact mass: 405.1782) using high-resolution-mass spectrometry by identification of the M+H adduct (experimental m/z 406.1863, calculated m/z 406.1860, <1 ppm error) and M+Na adduct (experimental m/z 428.1679, calculated m/z 428.1680, <1 ppm error), implying 9 degrees of unsaturation (Figure 1 and Figure S3A). The UV/vis spectrum showed absorption maxima at 218 and 308 nm (Figure S3B). The IR spectrum suggested the presence of a broad O–H or N–H stretch occurring at >3200 cm–1 and alkyl C–H stretches in the 2900 cm–1 region. The IR spectrum also has a carbonyl stretch at 1733 cm–1 and an additional band potentially arising from an alcohol or carboxylic acid (1086 cm–1) (Figure S3C). The presence of these absorptions and the lack of any other strong absorptions correlates with the functional groups present on ToxE1. The 13C NMR spectra (Table S2 and Figures S4 and S5) showed a total of 21 carbons, corroborating the molecular formula derived from high-resolution mass spectrometry. A Distortionless Enhancement by Polarization Transfer (DEPT-135) experiment (Figure S6) in conjunction with 1H NMR analysis (Table S2 and Figure S7 and S8) assigned eight quaternary, eight methine (=CH– or >CH−), and five methyl (−CH3) carbons. No methylene (−CH2−) carbons were detected. A total of 23 nonexchangeable protons were represented in the 1H NMR spectrum in methanol-d4 (Figure S7), consigning four protons as exchangeable. Three of the quaternary carbons, C-7, C-13, and C-19, were downfield shifted to the carbonyl region (δ 170.81, 169.40, and 175.34, respectively). Analysis of compound 1 in acetone-d6 showed the presence of an amide proton at δ 8.31 (Figure S8) accounting for one carbonyl signal. The lack of an aldehyde proton and no carbonyl signal downfield suitable for a ketone indicate the two remaining carbonyls are carboxylic acids or carboxylic esters. Two doublets at δ 8.23 and δ 7.22, representing a single proton each, occur in the aromatic region and show ortho- coupled protons of a tetra-substituted aromatic ring (J = 8.0 Hz). Formation of a second ring completes the 9 degrees of unsaturation and forms the basis of a phthalide backbone. Five methyl groups were identified, of which two equivalent methyl groups, H-11 and H-12, are coupled to a proton on a chiral carbon (C-9) based on the septet of doublets observed for H-10 (Figures S7 and S8). Deshielding of two methyl groups, H-18 and H-21, suggests attachment to an olefinic carbon (Figures S6 and S7). The heteronuclear single quantum coherence spectroscopy (HSQC) spectrum (Figure S9) unambiguously assigned all protons to their respective carbons. The long-distance allylic coupling constants seen for H-17, H-18, and H-21 (J = 1.3 Hz) in the 1H NMR are corroborated by cross peaks in the correlation spectroscopy (COSY) spectrum (Figure S10) for H-17/H-21 and H-18/H-21 (Figure 2). The E configuration of the alkene bond was confirmed by the NOESY correlations occurring between H-17 and H-15 and H-17 and H-20 (Figure 2 and Figure S12) and by direct correlation between H-18 and H-21 in the ROESY NMR spectrum (Figure S13). Detailed analysis of the remaining 2D NMR correlations established the structure of ToxE1 as shown in Figure 2 (Figures S11 and S12). Supplementary data for the support of the proposed structure via DFT calculations, including DP4+ analysis28 and CASCADE prediction,22 are provided in Figure S14.
Figure 1.
Structures of the ToxE analogues identified from Pyrenophora tritici-repentis.
Figure 2.
Key 2D NMR correlations for ToxE1.
The second chlorosis-inducing compound, ToxE2, was identified as a C-8 isopropyl analogue (Figure 1). Similar to ToxE1, this compound was obtained as an off-white amorphous powder (melting point 88–91 °C) however at a lower quantity of approximately 20% of ToxE1 in the filtrate. The low quantities of ToxE2 limited structural data to UV, MS, and 1H NMR. High-resolution-mass spectrometry revealed an M+H adduct of m/z 376.1762 (calculated m/z 376.1755, 2.0 ppm error) and M+Na adduct of m/z 398.1578 (calculated m/z 398.1574, 1.8 ppm error), indicative of a C20H25NO6 molecular formula for ToxE2 (calculated exact mass: 375.1676) (Figure S15A). ToxE2 displayed a similar UV/vis spectrum to ToxE1 (Figure S15B). The difference in molecular formula between ToxE1 and ToxE2 correlates with a difference of CH2O (30 Da). Comparison of their MS/MS spectra showed five mass fragments (including precursor ion) offset by 30 Da and three fragments of equal mass (Figure S16). Comparison of the proton NMR revealed the loss of the H-9 signal and the retention of all other signals (Figure S17). The splitting pattern and chemical shift for the majority of the signals remained the same with notable exceptions including the upfield shift of H-4 by 0.2 ppm and a change to the coupling constant of H-10; lastly, a single methyl doublet was shifted upfield by approximately 0.3 ppm.
Phthalide derivative compounds have been identified from a number of Dothideomycete plant pathogens Alternaria kikuchiara,29A. solani,30A. tagetica,31A. porri,32 and Mycosphaerella fijiensis;33 however, all the identified metabolites lack the amide moiety of ToxE compounds. Only about 300 phthalides have been isolated.34 Phthalides extracted from plants and human fungal pathogens are well-studied in clinical research due to their antimicrobial, antioxidant, and cytotoxic properties.34−38 Phthalides are also found in lichens, liverworts, and bacteria;34,39 however, little is known about phthalides in phytopathogenic fungi. This is the first discovery of a fungal phytotoxin in the phthalide class that contains an amide moiety.
3.2. Both ToxE1 and ToxE2 Elicit Chlorosis in a Cultivar-Specific Manner
To assess the phytotoxicity levels of ToxE, leaves of cultivar “Eagle Rock” were infiltrated with the purified compounds at the concentrations of 20, 50, 100, 150, 200, and 300 μg/mL using a 1 mL needleless syringe and then incubated in a growth chamber with a photoperiod of 12 h for 7 days. Chlorosis was observed at 100 μg/mL and was maximal at 200 μg/mL for ToxE1 (Figure 3). ToxE2 elicited weak chlorotic symptoms at 50 μg/mL with a maximum intensity observed at 100 μg/mL and greater. Subsequent plant infiltrations used 200 μg/mL for both compounds.
Figure 3.

Response of wheat leaves (cultivar “Eagle Rock”) to purified ToxE1 and ToxE2. Concentrations of ToxE compounds infiltrated are displayed on the left. Photographs of representative leaves were taken 7 days post infiltration.
Although fungi are known as prolific secretors of secondary metabolites, there are only a small number of SM that have been identified as virulence factors that facilitate pathogenicity in wheat disease,40 and none are phthalides. Ochracinic acid, a phthalide derivative, was isolated from another dothideomycete plant–pathogen Alternaria kikuchiara, the causal agent of black spot disease on Japanese pears, and later from Mycosphaerella fijiensis, the causal agent of black Sigatoka disease on bananas and plantains.29,33 Ochracinic acid was toxic to both banana and plantain but did not display host cultivar selectivity. To determine whether the ToxE-induced chlorotic response was dependent on host genotype, a selection of Australian wheat cultivars representing varying resistance levels to tan spot disease was assayed for their sensitivity. ToxE1 and ToxE2 induced chlorotic symptoms in some but not all cultivars (Figure 4 and Table 1). Infiltration of 200 μg/mL of ToxE2 produced clear symptoms in around 50% of the cultivars tested. ToxE1 was generally less phytotoxic and induced clear symptoms in only 25% of cultivars. This result demonstrated that the phytotoxic effects of ToxE1 and ToxE2 were dependent on the host genotypes. The selectivity of the host-induced chlorosis did not correlate with the tan spot disease susceptibility of the wheat cultivars tested. While further work on genetic analysis is required, absence of correlation suggests interplay of many virulence factors in tan spot disease development of which ToxE may have an ancillary role. Further analysis of the ToxA, ToxB, and ToxC differential lines that showed mild symptoms and absence of selectivity on the three tan spot wheat differentials indicates that neither molecule corresponds to ToxC (Figure S18).
Figure 4.

ToxE1 and ToxE2 elicited cultivar-specific chlorosis. Leaves of wheat cultivars exhibiting varying degrees of chlorosis upon infiltration with ToxE1 and ToxE2 at the concentration of 200 μg/mL. Cultivar names and their resistance ratings to tan spot disease are indicated (MRMS = moderately resistant to moderately susceptible, MS = moderately susceptible, MSS = moderately susceptible to susceptible, and SVS = susceptible to very susceptible).
Table 1. Toxin Sensitivities of 40 Australian Wheat Cultivars to ToxE1 and ToxE2.
| cultivars | tan spot disease resistance ratinga | ToxE1 (mean ± SE) | ToxE2 (mean ± SE) |
|---|---|---|---|
| Arrino | MS | 0.0 ± 0.0 | 1.0 ± 0.0 |
| Bremer | MSS | 2.3 ± 0.3 | 2.7 ± 0.3 |
| Calingiri | MS | 0.0 ± 0.0 | 1.0 ± 0.0 |
| Carnamah | MSS | 1.7 ± 0.3 | 3.3 ± 0.3 |
| Clearfield WHT STL | S | 2.0 ± 0.9 | 2.3 ± 0.3 |
| Correll | SVS | 0.3 ± 0.3 | 2.3 ± 0.3 |
| Cosmick | MRMS | 0.0 ± 0.0 | 3.3 ± 0.3 |
| Devil | MRMS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| DS Pascal | MS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| EGA Bonnie Rock | MRMS | 0.0 ± 0.0 | 2.7 ± 0.3 |
| EGA Wedgetail | MSS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Espada | MS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Fortune | MRMS | 0.3 ± 0.3 | 2.0 ± 0.5 |
| Grenade CL Plus | S | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Halberd | S | 0.0 ± 0.0 | 0.3 ± 0.3 |
| Harper | MSS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Hydra | MRMS | 0.7 ± 0.3 | 2.0 ± 0.0 |
| Illabo | MS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Justica CL Plus | SVS | 3.3 ± 0.5 | 3.5 ± 0.3 |
| King Rock | MRMS | 3.0 ± 0.5 | 2.7 ± 0.3 |
| Kinsei | MS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Kiora | MSS | 0.0 ± 0.0 | 1.0 ± 0.0 |
| LRPB Havoc | MRMS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| LRPB Kittyhawk | MRMS | 1.0 ± 0.0 | 3.0 ± 0.0 |
| LRPB Scout | SVS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| LRPB Spitfire | MSS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mace | MRMS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Magenta | MRMS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Ninja | MRMS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Sheriff CL Plus | MRMS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Shield | MSS | 0.0 ± 0.0 | 1.0 ± 0.0 |
| Sunlamb | MRMS | 0.0 ± 0.0 | 2.3 ± 0.7 |
| Suntop | MSS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Supreme | MS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Tammarin Rock | SVS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Tungsten | MSS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Vixen | MRMS | 0.0 ± 0.0 | 1.0 ± 0.0 |
| Wyalkatchem | MRMS | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Yitpi | SVS | 1.0 ± 0.0 | 0.7 ± 0.3 |
| Zen | MRMS | 0.0 ± 0.0 | 0.3 ± 0.3 |
Tan spot disease resistance ratings were obtained from the Australian Wheat Variety Crop Sowing Guide (2023–2015), where MRMS = moderately resistant to moderately susceptible, MS = moderately susceptible, MSS = moderately susceptible to susceptible, S = susceptible, and SVS = susceptible to very susceptible.
3.3. Genetics of ToxE2-Induced Chlorosis in Wheat
Since ToxE2 induced a stronger symptom and was more prevalent in the Australian wheat panel, we progressed to investigate the genetic control of host chlorosis induced by ToxE2. To examine the inheritance of ToxE2 sensitivity in wheat, we analyzed the F1 from a cross between “Eagle Rock” and “95ZEE10”, which are sensitive and insensitive to ToxE2, respectively (Figure S19). All the F1 seedlings from the cross “Eagle Rock” × “95ZEE10” developed chlorosis on the infiltrated leaves (Table 2). A similar response was also observed for all the F1 seedlings from the reciprocal cross “95ZEE10” × “Eagle Rock”, suggesting that sensitivity to ToxE2 was dominant. To further investigate the genetics that conditioned ToxE2 sensitivity in wheat, 251 DH lines derived from the F1 cross between “Eagle Rock” × “95ZEE10” were used to test for allele segregation at the receptor loci. The chi-square test showed that the segregation of ToxE2 sensitivity was significantly different from the expected 1:1 ratio (Table 3). Since this result did not fit the one locus segregation model, it is plausible that there is more than one locus controlling the phenotype. Whether multiple genes contribute additively to the phenotype or interact epigenetically cannot be determined at this stage of the analysis, but this observation could partly explain the lack of association between ToxE sensitivity with tan spot susceptibility when assessed on the Australian wheat panel. Overall, these results support the quantitative inheritance of wheat reaction to tan spot disease.4
Table 2. Reaction of Parental Lines, F1 Progenies, and Double Haploid (DH) Wheat Lines to the Ptr ToxE2 Phytotoxina.
| ToxE2 sensitivity |
|||
|---|---|---|---|
| lines/cross | generation | sensitive | insensitive |
| Eagle Rock | parental | + | – |
| 95ZEE10 | parental | – | + |
| Eagle Rock × 95ZEE10 | F1 progeny | 13 | 0 |
| 95ZEE10 × Eagle Rock | F1 progeny | 8 | 0 |
“+”, sensitive; “–”, insensitive.
Table 3. Segregation of Double Haploid (DH) Wheat Population Derived from Cross “Eagle Rock” × “95ZEE10” for Chlorosis Induced by ToxE2.
| ratio
(sensitive: insensitive) |
||
|---|---|---|
| observed | expected | chi-square; Prob > ChiSqa |
| 156:95 | 1:1 | χ2 = 7.52; P = 0.0061 |
A fit to the expected ratio is accepted if P > 0.05 (chi-square test).
3.4. Detection of ToxE1 and ToxE2 in Ptr Culture Filtrate
Necrotrophic fungal pathogens such as Ptr and P. nodorum secrete multiple NEs in cultures.27,41−43 In Ptr, the secreted NE corresponds to the isolate race classification.8,9 To examine if the production of ToxE was limited to certain races of Ptr, a panel of 37 Ptr isolates from races 1, 2, 3, 4, 5, 7, and 8 were quantified for the production of ToxE1 and ToxE2 in culture filtrate using LC-MS (Table 4). ToxE1 and ToxE2 were detected in 32 and 25 isolates respectively and ToxE1 was always more abundant. The presence of both toxins in all races tested including 2, 4, 5, and 7 which are non-ToxC producing races further confirms that neither of these molecules are ToxC.
Table 4. Levels of ToxE1 and ToxE2 Produced in the Culture Filtrate of Ptr Isolatesa.
| isolate | race | ToxE1 (mg/L ± SE) | ToxE2 (mg/L ± SE) |
|---|---|---|---|
| Australia | |||
| 134 | race 1 | <DL | <DL |
| 239 | race 1 | ND | ND |
| 5213 | race 1 | ND | ND |
| 11,137 | race 1 | 0.21 ± 0.10 | 0.06 ± 0.04 |
| 16FRG050 | race 1 | 1.75 ± 0.40 | 0.32 ± 0.24 |
| M4 | race 1 | 0.07 ± 0.03 | <DL |
| N3789 | race 1 | <DL | ND |
| N8690 | race 1 | 0.68 ± 0.33 | 0.12 ± 0.10 |
| Ptr16-083 | race 1 | <DL | ND |
| Q4304 | race 1 | <DL | <DL |
| Q5277 | race 1 | ND | ND |
| Q8092 | race 1 | 0.09 ± 0.01 | 0.04 ± 0.01 |
| S2084 | race 1 | <DL | ND |
| S3361 | race 1 | 0.91 ± 0.41 | 0.34 ± 0.27 |
| S6561 | race 1 | <DL | ND |
| V1 | race 1 | 0.13 ± 0.04 | 0.10 ± 0.00 |
| V2042 | race 1 | <DL | ND |
| W0184MC | race 1 | 0.55 ± 0.21 | 0.12 ± 0.09 |
| W0160HY | race 2 | <DL | <DL |
| W0236C | race 1 | 2.24 ± 0.44 | 0.93 ± 0.46 |
| W0238WY2 | race 1 | 0.07 ± 0.02 | ND |
| W0239MG1 | race 2 | 5.09 ± 0.40 | 1.48 ± 0.15 |
| W1087 | race 1 | <DL | <DL |
| WAI1483 | race 1 | <DL | ND |
| WAI376 | race 2 | 0.29 ± 0.12 | 0.09 ± 0.01 |
| USA | |||
| DW5 | race 5 | 0.37 ± 0.15 | 0.07 ± 0.04 |
| DW7 | race 5 | 0.11 ± 0.05 | 0.05 ± 0.01 |
| Germany | |||
| SN001A | race 3 | 16.56 ± 2.02 | 9.95 ± 2.47 |
| Denmark | |||
| EW7m1 | race 3 | 2.09 ± 1.59 | 0.96 ± 0.00 |
| EW306-2-1 | race 1 | 2.22 ± 2.11 | 0.71 ± 0.99 |
| EW4-4 | race 1 | 0.25 ± 0.10 | 0.05 ± 0.00 |
| United Kingdom | |||
| CC143 | race 1 | 0.31 ± 0.12 | 0.08 ± 0.00 |
| Tunisia | |||
| T199 | race 7 | ND | ND |
| T205 | race 4 | <DL | <DL |
| Algeria | |||
| Alg130 | race 5 | <DL | <DL |
| Alg215 | race 8 | 0.31 ± 0.18 | 0.09 ± 0.00 |
| Canada | |||
| 90-2 | race 4 | ND | ND |
ND—not detected. <DL—detected, but below the detection limit.
3.5. Chlorosis Induced by ToxE1 and ToxE2 Is Light-Dependent
Light dependency of ToxE1 and ToxE2 phytotoxic activities was investigated by examining the development of the chlorotic symptom in the presence or absence of light. Leaves of wheat seedlings were infiltrated with either ToxE1 or ToxE2 at the concentration of 200 μg/mL and incubated under the photoperiod of 12 h light/dark settings. When the infiltration zone was kept in darkness for 5 days, chlorosis failed to develop (Figure 5). Chlorosis was fully established in the light-exposed seedlings, providing evidence that the chlorotic activity of ToxE1 and ToxE2 is light dependent.
Figure 5.

ToxE1 and ToxE2 induced light-dependent chlorosis on wheat. The second leaves of cultivar “Eagle Rock” were infiltrated with ToxE compounds at the concentration of 200 μg/mL and incubated under two different light–dark regimes. Top panel shows leaves with chlorosis symptoms induced by ToxE1 and ToxE2 under the 12 h photoperiod. Wheat leaves failed to develop chlorosis when kept in the dark throughout the incubation period (bottom panel).
3.6. ToxE Compounds Detected In Planta during Ptr Infection
To determine if Ptr produces ToxE1 and ToxE2 during infection, metabolite extraction was performed on tan spot-infected wheat leaves, sampled at days 1, 3, 5, and 7 postinfection (dpi), and analyzed by LC-MS. Unlike Ptr triticone A/B,16 ToxE1 was detected at the abundance of 4.9 × 105 in the leaf material at 1 dpi while the earliest detection of ToxE2 was 3 dpi (Figure S20) with the abundance of 2.4 × 105. At the later infection period, both ToxE1 and ToxE2 were detected in the diseased leaves. Active secretion of ToxE1 and ToxE2 by Ptr during infection further supports the role of these compounds in virulence.
3.7. ToxE2 Inhibits Seedling Leaf Development
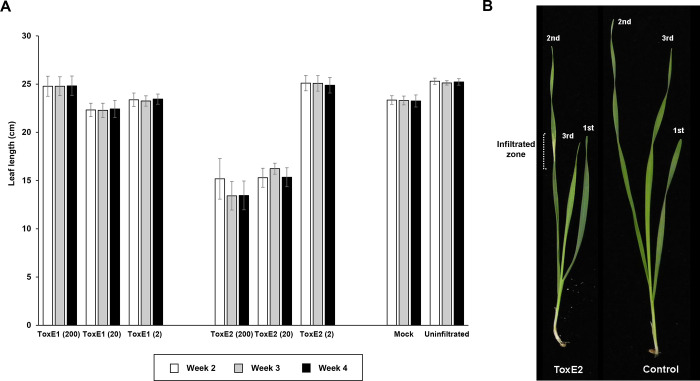
While conducting the plant bioassays, inhibitory activity of ToxE2 on wheat leaves was observed. To further explore the effect of ToxE compounds on plant growth, a time-scale experiment was conducted to measure growth extension of seedling leaves after infiltrating with ToxE compounds. ToxE2 but not ToxE1 inhibited the growth of newly emerged leaves (Figure 6A). When ToxE2 was infiltrated onto the second fully expanded leaf, the third leaf that emerged from the tiller exhibited slower extension growth in comparison to the seedling that was not subjected to ToxE2 infiltration (Figure 6B). Growth reduction was observed at concentrations of 20 and 200 μg/mL, while 2 μg/mL ToxE2 had no effect. Many fungal metabolites possess a wide spectrum of biological activities including inhibitory/stimulatory effect on seed germination and root and shoot elongation.44,45 Despite a long history of research on plant–pathogen interactions, our knowledge of pathogen metabolites with inhibition or promotion effects on plant growth is limited. A small number of pathogenic fungi have been shown to produce SM mimicking phytohormones,46−48 postulated to modulate plant growth during host colonization. Although further investigation is necessary, ToxE2 may play a role in manipulating plant growth to facilitate infection. To the best of our knowledge, this is the first report of a phytopathogenic toxin from phthalide derivatives that inhibits leaf growth.
Figure 6.
ToxE2 inhibits leaf growth. (A) Effect of ToxE2 on the leaf length (cm) of wheat seedlings. The length of the third leaf was measured at a weekly time course upon infiltrations of ToxE1 and ToxE2 at the concentrations of 2, 20, and 200 μg/mL. Data points represent the mean length of minimum four biological replicates for each treatment (mean ± SE). B) Growth phenotypes of ToxE2 infiltrated (200 μg/mL) and untreated (control) wheat seedlings.
3.8. ToxE2 Exhibits Nonhost Plant Specificity
Fungal phytotoxins can be classified as host-specific or nonhost specific toxins.49,50 Host-specific toxins are active toward the host plants of the toxin-producing fungi, while nonhost-specific toxins have a broader range of activity causing symptoms not limited to the host of the fungi. To classify the phytotoxic activity of ToxE1 and ToxE2, leaves of nonhost plants were infiltrated with ToxE compounds. Three monocots (Brachypodium, ryegrass, and barley) and three dicots (canola, Arabidopsis, and Nicotiana benthamiana) were infiltrated with 200 μg/mL toxins. All five barley cultivars, four ryegrass varieties, three Brachypodium lines, and the one N. benthamiana line used showed no response to ToxE1 or ToxE2 (Figure 7A,B). In contrast, five canola varieties (“Bonito”, “Cobbler”, “Mako”, “Skipton”, and “Surpass”) and Arabidopsis “Columbia” displayed varying degrees of symptoms when infiltrated with ToxE2 while no symptom was observed for ToxE1 (Figure 7C,D). Although ToxE2 exhibited nonhost specificity, the spectrum of plant species was not as broad as typical nonhost toxins.16
Figure 7.
Response of leaves from nonhost monocots and dicots upon infiltration with ToxE1 and ToxE2. (A, B) Leaves of monocots (barley, Brachypodium, and ryegrass) and Nicotiana benthamiana, displaying absence of symptom, while (C) Arabidopsis “Columbia” and (D) canola varieties exhibited varying degrees of symptoms for ToxE2.
3.9. ToxE Has No Obvious Antimicrobial Activity
Since many phytotoxins also possess antimicrobial activities,16,44,51 the antimicrobial effect of ToxE compounds was also explored. Growth inhibition of fungal and bacterial species by ToxE1 and ToxE2 was tested using a disk diffusion assay. Disks were infused with 20 μg of the respective ToxE compounds and placed onto fungal plates of S. cerevisiae, Alternaria infectoria, and P. nodorum as well as bacterial plates of E. coli. No zone of inhibition was observed on all plates (Figure S21), which suggests that ToxE compounds interact mainly with the host.
In conclusion, we have identified two SM of unique chemical structures from Ptr capable of inducing chlorosis on wheat. The discovery of novel phytotoxic SM compounds from Ptr emphasizes the complex nature of plant–pathogen interactions. The cultivar-specific nature of the chlorosis suggests a specific interaction with particular wheat genes. Genetic linkage mapping analysis of the interaction is underway. Our current hypothesis is that these compounds underpin one of more of the minor quantitative trait loci (QTLs) found in the wheat tan spot interaction.4,52
Acknowledgments
This project was funded by the Grains Research and Development Corporation (GRDC) and Curtin University (project code CUR00023), and the Australian Government Research Training Program (RTP) Scholarship. The authors thank Lise N. Jo̷rgensen (Department of Agroecology, Aarhus University, Slagelse, Denmark), Hamida Benslimane (Département de Botanique, Ecole National Supérieure Agronomique (ENSA), Hassan Badi, El-Harrach, Algiers, Algeria), Stephen E. Strelkov (Department of Agricultural, Food and Nutritional Science, University of Alberta, Canada), Judith Turner (Fera Science Ltd., United Kingdom), Timothy Friesen (US Department of Agriculture, USA), Huyen Phan, Fran Lopez-Ruiz, and Steven Chang (CCDM, Curtin University) for supplying the isolates. We thank M.J. Muria-Gonzalez for the NMR data analysis support. We thank GRDC for the supply of wheat and barley seeds and Mark Derbyshire (CCDM, Curtin University) for the canola seeds, Julie Nicol (University of Sydney) for the wheat seeds; and Catherine Cupitt (CCDM, Curtin University) for the generation of wheat crosses and providing technical assistance with leaf measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.4c02533.
Scoring scale of ToxE-induced chlorosis on wheat (Figure S1); detailed bioassay-guided purification of ToxE1 and ToxE2 (Supporting Information S2); characterization data (UV and IR spectra, 1H NMR, 13C NMR, DEPT-135, HSQC, HMBC, COSY, NOESY, ROESY, HR-MS, and MS/MS spectra, prediction of chemical shifts using DFT and CASCADE (Figures S3–S17); bioassays of tan spot wheat differential lines (Figure S18); phenotype of wheat parent line “Eagle Rock” and “95ZEE10” in response to ToxE2 infiltration (Figure S19); detection of ToxE1 and ToxE2 in infected wheat leaves (Figure S20); growth inhibition assay of ToxE1 and ToxE2 on fungal and bacterial species (Figure S21); Australian wheat commercial cultivars used in this study (Table S1); and 1H and 13C NMR assignments for ToxE1 and ToxE2 (Table S2) (PDF)
Author Contributions
Conceptualization, C.R., R.P.O., and C.S.M.; methodology, C.R., Y.H.C., G.N., and P.T.S.; draft preparation, C.R., R.P.O., and P.T.S.; and review and editing, R.P.O. and P.T.S. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Savary S.; Willocquet L.; Pethybridge S. J.; Esker P.; McRoberts N.; Nelson A. The global burden of pathogens and pests on major food crops. Nature ecology & evolution 2019, 3 (3), 430–439. 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- Oliver R.Achieving durable disease resistance in cereals; Burleigh Dodds Science Publishing: 2021 10.1201/9781003180715. [DOI] [Google Scholar]
- Ciuffetti L. M.; Manning V. A.; Pandelova I.; Betts M. F.; Martinez J. P. Host-selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora tritici-repentis–wheat interaction. New Phytologist 2010, 187 (4), 911–919. 10.1111/j.1469-8137.2010.03362.x. [DOI] [PubMed] [Google Scholar]
- Faris J. D.; Liu Z.; Xu S. S. Genetics of tan spot resistance in wheat. Theor. Appl. Genet. 2013, 126 (9), 2197–2217. 10.1007/s00122-013-2157-y. [DOI] [PubMed] [Google Scholar]
- Vleeshouwers V. G.; Oliver R. P. Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol. Plant-Microbe Interact. 2014, 27 (3), 196–206. 10.1094/MPMI-10-13-0313-IA. [DOI] [PubMed] [Google Scholar]
- Effertz R. J.; Meinhardt S. W.; Anderson J. A.; Jordahl J. G.; Francl L. J. Identification of a chlorosis Inducing toxin from Pyrenophora tritici-repentis and the chromosomal location of an insensitivity locus in wheat. Phytopathology 2002, 92 (5), 527–533. 10.1094/PHYTO.2002.92.5.527. [DOI] [PubMed] [Google Scholar]
- Shi G.; Kariyawasam G.; Liu S.; Leng Y.; Zhong S.; Ali S.; Moolhuijzen P.; Moffat C. S.; Rasmussen J. B.; Friesen T. L.; et al. A Conserved Hypothetical Gene Is Required but Not Sufficient for Ptr ToxC Production in Pyrenophora tritici-repentis. Mol. Plant-Microbe Interact. 2022, 35 (4), 336–348. 10.1094/MPMI-12-21-0299-R. [DOI] [PubMed] [Google Scholar]
- Lamari L.; Sayoud R.; Boulif M.; Bernier C. C. Identification of a new race in Pyrenophora tritici-repentis: implications for the current pathotype classification system. Canadian Journal of Plant Pathology 1995, 17 (4), 312–318. 10.1080/07060669509500668. [DOI] [Google Scholar]
- Lamari L.; Strelkov S. E.; Yahyaoui A.; Orabi J.; Smith R. B. The Identification of Two New Races of Pyrenophora tritici-repentis from the Host Center of Diversity Confirms a One-to-One Relationship in Tan Spot of Wheat. Phytopathology 2003, 93 (4), 391–396. 10.1094/PHYTO.2003.93.4.391. [DOI] [PubMed] [Google Scholar]
- Gessler N.; Egorova A.; Belozerskaya T. Fungal anthraquinones. Applied biochemistry and microbiology 2013, 49 (2), 85–99. 10.1134/S000368381302004X. [DOI] [Google Scholar]
- Wouters J. High performance liquid chromatography of anthraquinones: analysis of plant and insect extracts and dyed textiles. Studies in Conservation 1985, 30 (3), 119–128. 10.1179/sic.1985.30.3.119. [DOI] [Google Scholar]
- Bouras N.; Strelkov S. E. The anthraquinone catenarin is phytotoxic and produced in leaves and kernels of wheat infected by Pyrenophora tritici-repentis. Physiological and molecular plant pathology 2008, 72 (1–3), 87–95. 10.1016/j.pmpp.2008.06.001. [DOI] [Google Scholar]
- Kachlicki P.; Wakuliński W. Analysis of anthraquinone pigments of Pyrenophora tritici-repentis (Died.) Drechs. Acta Agrobot. 2013, 53, 97–105. 10.5586/aa.2002.010. [DOI] [Google Scholar]
- Wakuliński W.; Kachlicki P.; Sobiczewski P.; Schollenberger M.; Zamorski C.; Łotocka B.; Sarova J. Catenarin production by isolates of Pyrenophora tritici-repentis (Died.) Drechsler and its antimicrobial activity. Journal of Phytopathology 2003, 151 (2), 74–79. 10.1046/j.1439-0434.2003.00683.x. [DOI] [Google Scholar]
- Hallock Y. F.; Lu H. S.; Clardy J.; Strobel G. A.; Sugawara F.; Samsoedin R.; Yoshida S. Triticones, spirocyclic lactams from the fungal plant pathogen Drechslera tritici-repentis. J. Nat. Prod. 1993, 56 (5), 747–754. 10.1021/np50095a012. [DOI] [Google Scholar]
- Rawlinson C.; See P. T.; Moolhuijzen P.; Li H.; Moffat C. S.; Chooi Y. H.; Oliver R. P. The identification and deletion of the polyketide synthase-nonribosomal peptide synthase gene responsible for the production of the phytotoxic triticone A/B in the wheat fungal pathogen Pyrenophora tritici-repentis. Environ. Microbiol 2019, 21 (12), 4875–4886. 10.1111/1462-2920.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolhuijzen P. M.; Muria-Gonzalez M. J.; Syme R.; Rawlinson C.; See P. T.; Moffat C. S.; Ellwood S. R. Expansion and Conservation of Biosynthetic Gene Clusters in Pathogenic Pyrenophora spp. Toxins 2020, 12 (4), 242. 10.3390/toxins12040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat C. S.; See P. T.; Oliver R. P. Generation of a ToxA knockout strain of the wheat tan spot pathogen P yrenophora tritici-repentis. Molecular plant pathology 2014, 15 (9), 918–926. 10.1111/mpp.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon P. S.; Thomas S. W.; Spanu P.; Oliver R. P. The utilisation of di/tripeptides by Stagonospora nodorum is dispensable for wheat infection. Physiological and Molecular Plant Pathology 2003, 63 (4), 191–199. 10.1016/j.pmpp.2003.12.003. [DOI] [Google Scholar]
- Moffat C. S.; See P. T.; Oliver R. P. Leaf yellowing of the wheat cultivar Mace in the absence of yellow spot disease. Australasian Plant Pathology 2015, 44 (2), 161–166. 10.1007/s13313-014-0335-2. [DOI] [Google Scholar]
- See P. T.; Marathamuthu K.; Cupitt C.; Iagallo E.; Moffat C. A race profile of tan spot in Australia reveals race 2 isolates harbouring ToxC1. Phytopathology 2023, 113 (7), 1202–1209. 10.1094/PHYTO-11-22-0422-R. [DOI] [PubMed] [Google Scholar]
- Moolhuijzen P. M.; See P. T.; Shi G.; Powell H. R.; Cockram J.; Jørgensen L. N.; Benslimane H.; Strelkov S. E.; Turner J.; Liu Z.; Moffat C. S. A global pangenome for the wheat fungal pathogen Pyrenophora tritici-repentis and prediction of effector protein structural homology. Microbial Genomics 2022, 8 (10), 1. 10.1099/mgen.0.000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala S.; James P. J. C.; Nealon G. L.; Fromont J.; Gomez O.; Vuong D.; Lacey E.; Flematti G. R. Dendrillic Acids A and B: Nitrogenous, Rearranged Spongian Nor-Diterpenes from a Dendrilla sp. Marine Sponge. J. Nat. Prod. 2023, 86 (3), 482–489. 10.1021/acs.jnatprod.2c01087. [DOI] [PubMed] [Google Scholar]
- Guan Y.; Sowndarya S. V. S.; Gallegos L. C.; John P. C. S.; Paton R. S. Real-time prediction of 1H and 13C chemical shifts with DFT accuracy using a 3D graph neural network. Chem. Sci. 2021, 12 (36), 12012–12026. 10.1039/D1SC03343C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W.; Kirby W. M. M.; Sherris J. C.; Turck M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45 (4_ts), 493–496. 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- Sugawara F.; Takahashi N.; Strobel G. A.; Strobel S. A.; Lu H. S.; Clardy J. Triticones A and B, novel phytotoxins from the plant pathogenic fungus Drechslera tritici-repentis. J. Am. Chem. Soc. 1988, 110 (12), 4086–4087. 10.1021/ja00220a085. [DOI] [Google Scholar]
- Ballance G. M.; Lamari L.; Bernier C. C. Purification and characterization of a host-selective necrosis toxin from Pyrenophora tritici-repentis. Physiological and Molecular Plant Pathology 1989, 35 (3), 203–213. 10.1016/0885-5765(89)90051-9. [DOI] [Google Scholar]
- Strelkov S. E.; Lamari L.; Ballance G. M. Characterization of a Host-Specific Protein Toxin (Ptr ToxB) from Pyrenophora tritici-repentis. Mol. Plant-Microbe Interact. 1999, 12 (8), 728–732. 10.1094/MPMI.1999.12.8.728. [DOI] [Google Scholar]
- Grimblat N.; Zanardi M. M.; Sarotti A. M. Beyond DP4: an Improved Probability for the Stereochemical Assignment of Isomeric Compounds using Quantum Chemical Calculations of NMR Shifts. Journal of Organic Chemistry 2015, 80 (24), 12526–12534. 10.1021/acs.joc.5b02396. [DOI] [PubMed] [Google Scholar]
- Kameda K.; Namiki M. A New phthalide from a fungus, Alternaria Kikuchiana. Chem. Lett. 1974, 3 (12), 1491–1492. 10.1246/cl.1974.1491. [DOI] [Google Scholar]
- Gamboa-Angulo M. M.; Alejos-González F.; Peña-Rodríguez L. M. Homozinniol, a New phytotoxic Metabolite from Alternaria solani. J. Agric. Food Chem. 1997, 45 (1), 282–285. 10.1021/jf960134p. [DOI] [Google Scholar]
- Gamboa-Angulo M. M.; Escalante-Erosa F.; García-Sosa K.; Alejos-González F.; Delgado-Lamas G.; Peña-Rodríguez L. M. Natural zinniol derivatives from Alternaria tagetica. Isolation, synthesis, and structure-activity correlation. J. Agric. Food Chem. 2002, 50 (5), 1053–1058. 10.1021/jf010641t. [DOI] [PubMed] [Google Scholar]
- Suemitsu R.; Ohnishi K.; Horiuchi M.; Morikawa Y.; Sakaki Y.; Matsumoto Y. Structure of Porriolide, a New Metabolite from Alternaria porri. Biosci., Biotechnol., Biochem. 1993, 57 (2), 334–335. 10.1271/bbb.57.334. [DOI] [PubMed] [Google Scholar]
- Stierle A. A.; Upadhyay R.; Hershenhorn J.; Strobel G. A.; Molina G. The phytotoxins of Mycosphaerella fijiensis, the causative agent of Black Sigatoka disease of bananas and plantains. Experientia 1991, 47 (8), 853–859. 10.1007/BF01922472. [DOI] [Google Scholar]
- León A.; Del-Ángel M.; Ávila J. L.; Delgado G.. Phthalides: Distribution in Nature, Chemical Reactivity, Synthesis, and Biological Activity. In Progress in the Chemistry of Organic Natural Products; Kinghorn A. D.; Falk H.; Gibbons S.; Kobayashi J. I., Eds.; Springer International Publishing: 2017; pp 127–246. [DOI] [PubMed] [Google Scholar]
- Awasthi A.; Singh M.; Rathee G.; Chandra R. Recent advancements in synthetic methodologies of 3-substituted phthalides and their application in the total synthesis of biologically active natural products. RSC Adv. 2020, 10, 12626–12652. 10.1039/D0RA00701C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullady E. L.; Millett W. P.; Yoo H.-D.; Weiskopf A. S.; Chen J.; DiTullio D.; Knight-Connoni V.; Hughes D. E.; Pierceall W. E. A phthalide with in Vitro Growth Inhibitory Activity from an Oidiodendron Strain. J. Nat. Prod. 2004, 67 (12), 2086–2089. 10.1021/np040123n. [DOI] [PubMed] [Google Scholar]
- Pannek J.; Gach J.; Boratyński F.; Olejniczak T. Antimicrobial activity of extracts and phthalides occurring in Apiaceae plants. Phytother Res. 2018, 32 (8), 1459–1487. 10.1002/ptr.6098. [DOI] [PubMed] [Google Scholar]
- Tianpanich K.; Prachya S.; Wiyakrutta S.; Mahidol C.; Ruchirawat S.; Kittakoop P. Radical Scavenging and Antioxidant Activities of Isocoumarins and a phthalide from the Endophytic Fungus Colletotrichum sp. J. Nat. Prod. 2011, 74 (1), 79–81. 10.1021/np1003752. [DOI] [PubMed] [Google Scholar]
- Puder C.; Zeeck A.; Beil W. New biologically active rubiginones from Streptomyces sp. J. Antibiot. 2000, 53 (4), 329–336. 10.7164/antibiotics.53.329. [DOI] [PubMed] [Google Scholar]
- Stergiopoulos I.; Collemare J.; Mehrabi R.; De Wit P. J. Phytotoxic secondary metabolites and peptides produced by plant pathogenic Dothideomycete fungi. FEMS Microbiol Rev. 2013, 37 (1), 67–93. 10.1111/j.1574-6976.2012.00349.x. [DOI] [PubMed] [Google Scholar]
- Liu Z. H.; Faris J. D.; Meinhardt S. W.; Ali S.; Rasmussen J. B.; Friesen T. L. Genetic and Physical Mapping of a Gene Conditioning Sensitivity in Wheat to a Partially Purified Host-Selective Toxin Produced by Stagonospora nodorum. Phytopathology 2004, 94 (10), 1056–1060. 10.1094/PHYTO.2004.94.10.1056. [DOI] [PubMed] [Google Scholar]
- Friesen T. L.; Zhang Z.; Solomon P. S.; Oliver R. P.; Faris J. D. Characterization of the interaction of a novel Stagonospora nodorum host-selective toxin with a wheat susceptibility gene. Plant Physiol. 2008, 146 (2), 682–693. 10.1104/pp.107.108761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.-F.; Francl L. J.; Jordahl J. G.; Meinhardt S. W. Structural and Physical Properties of a Necrosis-Inducing Toxin from Pyrenophora tritici-repentis. Phytopathology 1997, 87 (2), 154–160. 10.1094/PHYTO.1997.87.2.154. [DOI] [PubMed] [Google Scholar]
- Xu D.; Xue M.; Shen Z.; Jia X.; Hou X.; Lai D.; Zhou L. Phytotoxic Secondary Metabolites from Fungi. Toxins 2021, 13, 261. 10.3390/toxins13040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A.; Sawamura S.; Kawakami Y.; Sakamura S. Dihydrogladiolic Acid, another phytotoxin from Phoma asparagi Sacc. Agric. Biol. Chem. 1985, 49 (6), 1891–1892. 10.1080/00021369.1985.10867001. [DOI] [Google Scholar]
- Nguyen T. A. N.; Higa T.; Shiina A.; Utami Y. D.; Hiruma K. Exploring the roles of fungal-derived secondary metabolites in plant-fungal interactions. Physiological and Molecular Plant Pathology 2023, 125, 102021 10.1016/j.pmpp.2023.102021. [DOI] [Google Scholar]
- Reineke G.; Heinze B.; Schirawski J.; Buettner H.; Kahmann R.; Basse C. W. Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Molecular Plant Pathology 2008, 9 (3), 339–355. 10.1111/j.1364-3703.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemannstöns P.; Voß T.; Hedden P.; Gaskin P.; Tudzynski B. Deletions in the Gibberellin Biosynthesis Gene Cluster of Gibberella fujikuroi by Restriction Enzyme-Mediated Integration and Conventional Transformation-Mediated Mutagenesis. Appl. Environ. Microbiol. 1999, 65 (6), 2558–2564. 10.1128/AEM.65.6.2558-2564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert T. J.; Dunkle L. D.; Ciuffetti L. M. Host-selective toxins and avirulence determinants: what’s in a name?. Annu. Rev. Phytopathol 2002, 40, 251–285. 10.1146/annurev.phyto.40.011402.114210. [DOI] [PubMed] [Google Scholar]
- Berestetskiy A. O. A review of fungal phytotoxins: from basic studies to practical use. Applied Biochemistry and Microbiology 2008, 44 (5), 453–465. 10.1134/S0003683808050013. [DOI] [PubMed] [Google Scholar]
- Yang X.-L.; Zhang S.; Hu Q.-B.; Luo D.-Q.; Zhang Y. Phthalide derivatives with antifungal activities against the plant pathogens isolated from the liquid culture of Pestalotiopsis photiniae. Journal of Antibiotics 2011, 64 (11), 723–727. 10.1038/ja.2011.82. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Salsman E.; Wang R.; Galagedara N.; Zhang Q.; Fiedler J. D.; Liu Z.; Xu S.; Faris J. D.; Li X. Meta-QTL analysis of tan spot resistance in wheat. Theor. Appl. Genet. 2020, 133 (8), 2363–2375. 10.1007/s00122-020-03604-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.