Summary.
The Sunnybrook Facial Grading System (SFGS) is a widely recognized tool for assessing facial palsy with a total composite score between 0% and 100%.
The current standard of the SFGS Total score is 100% and may not deal with the natural variations of face symmetry observed in healthy individuals.
In this study, SFGS Total score in healthy participants ranged between 65% and 100%.
It is essential to consider that normal facial function does not necessarily correspond to perfect facial symmetry.
By embracing a more realistic approach that acknowledges asymmetry in facial movements, we can enhance patient care and promote a more holistic understanding of facial rehabilitation outcomes.
1. Introduction
The Sunnybrook Facial Grading System (SFGS) is a widely recognized tool for assessing facial palsy and its subsequent recovery, offering a standardized approach for quantifying facial movements [1]. This scale compares the paralyzed hemiface to the healthy hemiface of patients using a total composite score between 0% and 100%, where 100% corresponds to full recovery of facial function. The SFGS evaluate five facial movements, symmetry at rest and the presence of synkinesis. The total composite score is calculated by subtracting the symmetry and synkinesis scores from the dynamic movement score.
This scale and total composite score have proven to be a gold standard in clinical practice and research [2]. Even if it has been shown to be more sensitive in detecting changes following therapeutic intervention, the SFGS remains largely subjective [3]. Moreover, the widespread assumption of achieving a perfect 100% may not deal with the natural variations of face symmetry observed in healthy individuals and in patients with facial palsy [4]. Indeed, there is valuable evidence of asymmetry in the production of facial expressions in the general population, even in the absence of facial palsy [5].
This study aims to provide a more comprehensive understanding of the natural range of SFGS scores among healthy participants, thereby redefining the normative standards and advocating for a more patient‐centred and holistic approach to facial rehabilitation.
1.1. Objectives
The main objective of this study was to describe the distribution of the SFGS scores in the general population. The secondary objective was to analyze any effects of age, gender or side of the face on Sunnybrook Grading System scores.
2. Methods
2.1. Participants
We conducted a cohort study with 111 healthy participants included (57 women and 54 men), aged 18 to 79 years. The inclusion criteria were to be over 18 years old, to have a good level of understanding of spoken French and to have signed the consent form to take part in the research. Participants were recruited on a voluntary basis from our clinical and academic ENT department. Participants had no history of facial palsy or facial plastic surgery, botulinum toxin or hyaluronic acid injection in the face, or central neurological disorders.
2.2. Ethical Considerations
This study adhered to the Declaration of Helsinki and was registered with the CNIL declaration (no 2 217747). Each participant was recruited voluntarily. They signed an informed consent form to take part in the research and were given an anonymity number.
2.3. Design
Each participant underwent two assessments, one with the right hemiface as a reference and the other with the left hemiface as a reference. Two speech therapists specialized in the assessment of facial motor skills evaluated the participants. The first evaluator completed the SFGS in person with the patient. This evaluation was also recorded so that the second evaluator could complete the assessment later using the videos. As highlighted by Cabrol et al. [6], the inter‐rater reliability of the Sunnybrook system in video format is excellent.
2.4. Main Outcomes Measures
Descriptive analyses of the scores were carried out in our population of 111 participants using data from the assessment conducted by the first evaluator in person SFGS composite scores (SFGS‐Total) and sub‐scores at rest (SFGS‐Rest) and in movement (SFGS‐Movement) were studied for both hemifaces as reference, according to three age categories (18–39 years) (40–59 years) and (60–79 years) and gender. Inter‐rater reliability was collected between the two evaluators with Cronbach's alpha. The resultant data provided insights into the expected SFGS scores among healthy individuals and emphasized the natural variability of facial expressions.
As the SFGS data and the age of the patients did not follow a normal distribution (verified beforehand using a Shapiro–Wilk test p < 0.001), we conducted non‐parametric analyses.
To meet our secondary objectives, a Wilcoxon sign‐rank test was used to estimate significant differences between the SFGS‐Total scores according to the reference hemiface.
A Spearman correlation analysis was performed to understand a potential relationship between age and SFGS‐Total scores. Post hoc comparisons with Kruskall‐Wallis test was used to compare SFGS scores for the different age groups. A Mann–Whitney‐Wilcoxon test was also conducted to determine any significant differences between SFGS‐Total scores according to participant gender.
3. Results
Upon analyzing the SFGS scores, it was observed that the range differed depending on whether the right or left hemiface was considered the reference. Taking the right hemiface as a reference, SFGS‐Total scores ranged from 65% to 100% (median = 96, IQR [91–100]). When the left hemiface was considered as the reference, scores ranged from 78% to 100% (median = 95; IQR [90–100]). No participants showed any synkinesis. No significant differences between the SFGS‐Total scores according to the reference hemiface were found on the whole sample (p = 0.517). The data are reported by age and gender in Table 1. Data distribution using violin plots is reported in Figure 1.
TABLE 1.
Sunnybrook Facial Grading Scores according to age group and gender for the different reference hemifaces.
| Right side as reference | Left side as reference | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFGS‐total | SFGS‐rest | SFGS‐movement | SFGS‐total | SFGS‐rest | SFGS‐movement | ||||||||||||||
| Gender | N | Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max |
| Women | 19 | 96 | 82.00 | 100.00 | 0 | 0.00 | 10.00 | 100 | 92.00 | 100.00 | 96 | 90.00 | 100.00 | 0 | 0.00 | 10.00 | 100 | 92.00 | 100.00 |
| Men | 18 | 98 | 91.00 | 100.00 | 0 | 0.00 | 5.00 | 100 | 92.00 | 100.00 | 96 | 86.00 | 100.00 | 0 | 0.00 | 10.00 | 96 | 88.00 | 100.00 |
| Women | 19 | 100 | 79.00 | 100.00 | 0 | 0.00 | 10.00 | 100 | 84.00 | 100.00 | 95 | 87.00 | 100.00 | 5 | 0.00 | 10.00 | 100 | 88.00 | 100.00 |
| Men | 19 | 96 | 87.00 | 100.00 | 0 | 0.00 | 5.00 | 100 | 88.00 | 100.00 | 92 | 78.00 | 100.00 | 5 | 0.00 | 15.00 | 100 | 84.00 | 100.00 |
| Women | 19 | 95 | 75.00 | 100.00 | 5 | 0.00 | 10.00 | 100 | 80.00 | 100.00 | 95 | 79.00 | 100.00 | 0 | 0.00 | 10.00 | 100 | 84.00 | 100.00 |
| Men | 17 | 91 | 65.00 | 100.00 | 5 | 0.00 | 15.00 | 96 | 80.00 | 100.00 | 92 | 80.00 | 100.00 | 5 | 0.00 | 10.00 | 96 | 80.00 | 100.00 |
FIGURE 1.

Sunnybrook Facial Grading System Total Score (SFGS‐Total score) distribution using violin plots. (A) SFGS‐Total score according to hemifaces reference. (B) SFGS‐Total score according to gender. (C) SFGS‐Total score according to age groups.
Consistency between evaluators were high for right and left SFGS‐Total scores (respectively α = 0.953 and α = 0.926). Right and left SFGS‐Rest scores showed also high inter‐rater reliability (respectively α = 0.860 and α = 0.886) as well as right and left SFGS‐Movement scores (respectively α = 0.957 and α = 0.816).
There was a slight negative correlation between age and SFGS‐Total scores. Indeed, the older the participants, the lower their scores (right side as reference: ρ (109) = −0.37, p < 0.0001, 95% CI [−0.52, −0.20]; left side as reference: ρ (109) = −0.25; p = 0.009; 95% CI [−0.42, −0.06], Figure 2). Post hoc comparisons using Kruskall–Wallis test indicated that there was a significant difference in the SFGS‐Total scores (right side as reference) across age categories (χ2 [2, n = 111] = 13.89, p = 0.001). The median scores were 96/100 for participants aged (18–39 years), 96/100 for participants aged (40–59 years) and 93/100 for participants aged (60–79 years).
FIGURE 2.
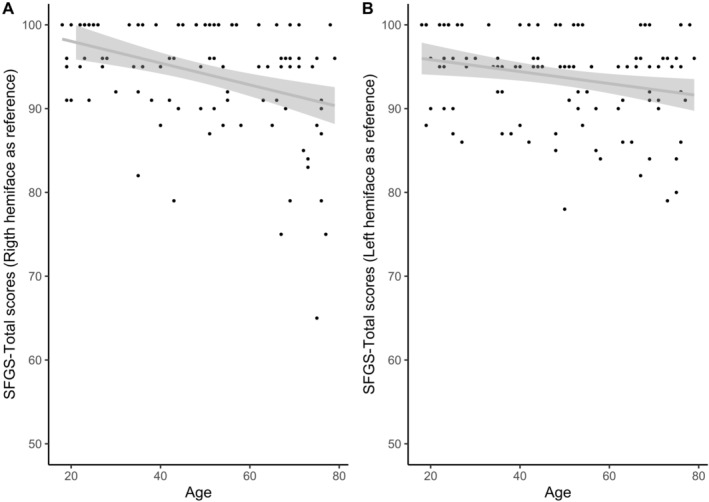
Correlation between SFGS‐Total scores and age of the participants. A. Right hemiface as reference. B. Left hemiface as reference.
Although women appear to have better scores than men when the left hemiface is the reference (women's median SFGS‐Total scores = 95, IQR [91–100], men's median SFGS‐Total scores = 95, IQR [88–100]), no significant difference was found between SFGS scores in women versus men (p = 0.091). When the right hemiface is the reference, no significant difference was found (p = 0.631).
4. Discussion
The results of this study show the different total scores of the SFGS according to the hemiface taken as reference. Right and left SFGS Total scores showed high inter‐rater reliability consistent with findings by Cabrol et al. [6]. Facial movements on the right and left sides are not significantly different, whereas the literature reports a greater expressiveness of the left hemiface in right‐handers [5].
Picard et al. [7], who standardized an oro‐facial motor assessment scale (the MBLF protocol), found an age‐ and gender‐related effect on normal facial function in their validation study. An age effect was also found in our study: older participants demonstrated lower SFGS‐Total scores. This phenomenon can be attributed to the reduced tonicity of the cheeks observed in elderly adults, resulting from the aging of soft tissues or alterations in the stomatognathic system [8]. There was no significant gender effect in our study, although women's facial expressivity appeared to be greater than men's when the reference hemiface was on the left side.
The current emphasis on achieving perfect facial symmetry as the ultimate goal may then create unrealistic expectations for patients. Being aware of the natural variations in face symmetry is crucial to patient‐centered care. The patient and the practitioner should be aware that the normal SFGS score ranges from 65% to 100% (median = 96, IQR [91–100]). The results of our study are consistent with those of Neely et al. [9], where patients with Grade 1 according to the House‐Brackmann classification, indicating normal facial function, had scores ranging from 95% to 100%. This range constitutes a more realistic and achievable target. This will reassure the patient who is dissatisfied with his recovery. The main limitation of the study lies in the subjectivity of the scale. The development of automated systems would take better account of the degree of asymmetry in normal participants and patients with facial palsy [10].
It is essential, especially for young practitioners clinical guidance, to consider that normal facial function does not necessarily correspond to perfect facial symmetry. This awareness contributes to a more realistic clinical practice tailored to patients experiencing facial palsy. Patients who have made significant progress in their recovery journey may still have minor residual asymmetries, which should not overshadow their overall functional improvements. By solely focusing on achieving perfect symmetry, we risk overlooking the broader aspects of facial rehabilitation, such as restoring functional movements, improving emotional expression and enhancing quality of life.
We are also aware that the SFGS score can be lowered by the simple presence of synkinesis complicating a previous facial palsy. Thus, it is obvious that a patient with this type of sequelae will be much more disabled than a healthy participant with the same SFGS score, less than 100%, but without any history of facial palsy.
5. Conclusion
By embracing a more realistic approach that acknowledges natural variations and asymmetry in facial movements, we can enhance patient care, foster a sense of achievement and promote a more holistic understanding of facial rehabilitation outcomes.
Author Contributions
Diane Picard: conceptualization, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing – original draft preparation. Remi Hervochon: resources, supervision, validation, writing – review and editing. Elodie Lannadere: conceptualization, project administration, supervision, validation, writing – review and editing. Cloe Cabos: data curation, investigation, writing – review and editing. Loeiza Gourves: data curation, investigation, writing – review and editing. Frederic Tankere: resources, supervision, validation, writing – review and editing. Peggy Gatignol: conceptualization, investigation, methodology, project administration, supervision, validation, writing – review and editing.
Ethics Statement
This study adhered to the Declaration of Helsinki and was registered with the CNIL declaration (no 2 217747). Each participant was recruited voluntarily. They signed an informed consent form to take part in the research and were given an anonymity number.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/coa.14217.
Funding: The authors received no specific funding for this work.
[Correction added on 02 October 2024, after first online publication: The abstract section has been removed in this version.]
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Ross B. G., Fradet G., and Nedzelski J. M., “Development of a Sensitive Clinical Facial Grading System,” Otolaryngology and Head and Neck Surgery 114, no. 3 (1996): 380–386. [DOI] [PubMed] [Google Scholar]
- 2. Fattah A. Y., Gurusinghe A. D. R., Gavilan J., et al., “Facial Nerve Grading Instruments: Systematic Review of the Literature and Suggestion for Uniformity,” Plastic and Reconstructive Surgery 135, no. 2 (2015): 569–579. [DOI] [PubMed] [Google Scholar]
- 3. Mat Lazim N., Ismail H., Abdul Halim S., Nik Othman N. A., and Haron A., “Comparison of 3 Grading Systems (House‐Brackmann, Sunnybrook, Sydney) for the Assessment of Facial Nerve Paralysis and Prediction of Neural Recovery,” Medeniyet Medical Journal 38, no. 2 (2023): 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martineau S., Perrin L., Kerleau H., Rahal A., and Marcotte K., “Comparison of Objective Facial Metrics on Both Sides of the Face Among Patients With Severe Bell's Palsy Treated With Mirror Effect Plus Protocol Rehabilitation Versus Controls,” Facial Plastic Surgery & Aesthetic Medicine 26, no. 2 (2024): 172–179. [DOI] [PubMed] [Google Scholar]
- 5. Sackeim H. A., Gur R. C., and Saucy M. C., “Emotions Are Expressed More Intensely on the Left Side of the Face,” Science 202, no. 4366 (1978): 434–436. [DOI] [PubMed] [Google Scholar]
- 6. Cabrol C., Elarouti L., Montava A. L., et al., “Sunnybrook Facial Grading System: Intra‐Rater and Inter‐Rater Variabilities,” Otology & Neurotology 42, no. 7 (2021): 1089–1094. [DOI] [PubMed] [Google Scholar]
- 7. Picard D., Lannadere E., Robin E., et al., “Oro‐Facial Motor Assessment: Validation of the MBLF Protocol in Facial Palsy,” European Archives of Oto‐Rhino‐Laryngology 278, no. 4 (2021): 1017–1025. [DOI] [PubMed] [Google Scholar]
- 8. Zimbler M. S., Kokoska M. S., and Thomas J. R., “Anatomy and Pathophysiology of Facial Aging,” Facial Plastic Surgery Clinics of North America 9, no. 2 (2001): 179–187. [PubMed] [Google Scholar]
- 9. Neely J. G., Cherian N. G., Dickerson C. B., and Nedzelski J. M., “Sunnybrook Facial Grading System: Reliability and Criteria for Grading,” Laryngoscope 120, no. 5 (2010): 1038–1045, 10.1002/lary.20868. [DOI] [PubMed] [Google Scholar]
- 10. Miller M. Q., Hadlock T. A., Fortier E., and Guarin D. L., “The Auto‐eFACE: Machine Learning‐Enhanced Program Yields Automated Facial Palsy Assessment Tool,” Plastic and Reconstructive Surgery 147, no. 2 (2021): 467–474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


