Abstract
Background and Aims
In patients with atrial fibrillation (AF), recurrent AF and sinus rhythm during follow-up are determined by interactions between cardiovascular disease processes and rhythm control therapy. Predictors of attaining sinus rhythm at follow-up are not well known.
Methods
To quantify the interaction between cardiovascular disease processes and rhythm outcomes, 14 biomarkers reflecting AF-related cardiovascular disease processes in 1586 patients in the EAST-AFNET 4 biomolecule study (71 years old, 45% women) were quantified at baseline. Mixed logistic regression models including clinical features were constructed for each biomarker. Biomarkers were interrogated for interaction with early rhythm control. Outcome was sinus rhythm at 12 months. Results were validated at 24 months and in external datasets.
Results
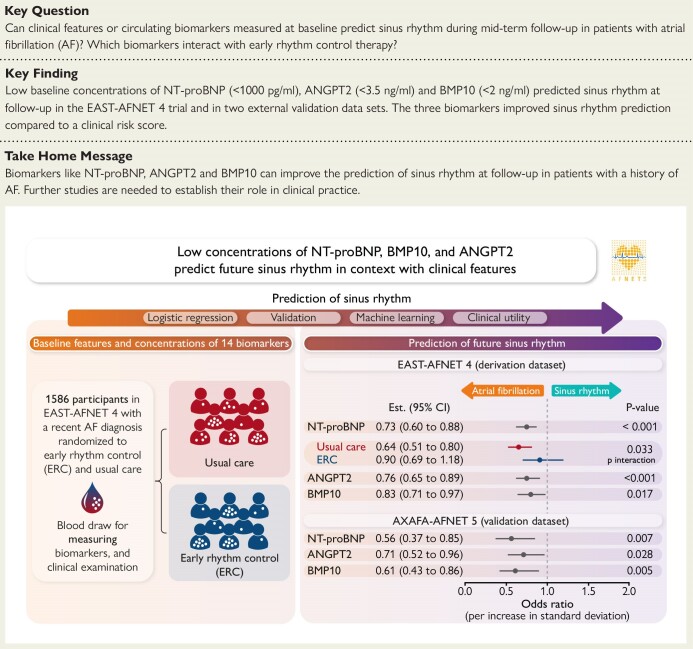
Higher baseline concentrations of three biomarkers were independently associated with a lower chance of sinus rhythm at 12 months: angiopoietin 2 (ANGPT2) (odds ratio [OR] .76 [95% confidence interval .65–.89], P < .001), bone morphogenetic protein 10 (BMP10) (OR .83 [.71–.97], P = .017), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) (OR .73 [.60–.88], P < .001). Analysis of rhythm at 24 months confirmed the results. Early rhythm control interacted with the predictive potential of NT-proBNP (Pinteraction = .033). The predictive effect of NT-proBNP was reduced in patients randomized to early rhythm control (usual care: OR .64 [.51–.80], P < .001; early rhythm control: OR .90 [.69–1.18], P = .453). External validation confirmed that low concentrations of ANGPT2, BMP10, and NT-proBNP predict sinus rhythm during follow-up.
Conclusions
Low concentrations of ANGPT2, BMP10, and NT-proBNP identify patients with AF who are likely to attain sinus rhythm during follow-up. The predictive ability of NT-proBNP is attenuated in patients receiving rhythm control.
Keywords: Atrial fibrillation, Blood biomarker, Sinus rhythm, Rhythm control, Natriuretic peptides, Bone morphogenetic protein 10, Angiopoietin 2, Risk prediction, Risk score
Structured Graphical Abstract
Structured Graphical Abstract.
In patients diagnosed with atrial fibrillation, low concentrations of NT-proBNP, BMP10, and ANGPT2 at baseline predict sinus rhythm at 12-month follow-up in context with clinical features. This was validated in additional datasets, of which AXAFA-AFNET 5 is depicted here. A treatment interaction shows that NT-proBNP’s predictive value is impacted by early rhythm control treatment. AF, atrial fibrillation; ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
See the editorial comment for this article ‘Personalized clinical management of patients with atrial fibrillation: is a biomarker-based strategy for prediction of sinus rhythm persistence ready for prime time?’, by G. Boriani et al., https://doi.org/10.1093/eurheartj/ehae720.
Introduction
In addition to improving atrial fibrillation (AF)-related symptoms,1 rhythm control therapy2 can prevent AF-related cardiovascular events such as stroke, heart failure hospitalizations, and cardiovascular death.3 The cardiovascular complication-reducing effect of early rhythm control therapy shown in the EAST-AFNET 4 study is mainly mediated by attaining sinus rhythm at 12-month follow-up.4 This potentially reflects a reduced AF burden5 and lack of progression to non-paroxysmal patterns of AF.6,7 Predicting sinus rhythm at 12 months could therefore help to identify patients requiring intensive rhythm control, e.g. with AF ablation.3,8 Knowledge of treatable processes contributing to AF at 12-month follow-up can help to develop adjunct therapies aimed at maintaining sinus rhythm and preventing AF progression. Several chronic, interdependent disease processes9,10 contribute to AF. Such processes can be aggravated by presence of AF, attenuated by rhythm control, or exist independent of AF.1,11 Circulating biomarkers provide quantitative proxies for cardiomyocyte death or injury [troponin (TnT)]; atrial metabolic dysfunction and stress [bone morphogenetic protein 10 (BMP10), fatty acid binding protein 3 (FABP3), and insulin-like growth factor binding protein 7 (IGFBP7)]12,13; thrombo-inflammation [D-dimer, C-reactive protein (CRP), interleukin-6 (IL-6)]14,15; vascular and endothelial dysfunction [angiopoietin 2 (ANGPT2), endothelial specific molecule 1 (ESM1)]14,15; frailty [growth differentiation factor 15 (GDF-15)]; and cardiac load [natriuretic peptides like N-terminal pro-B-type natriuretic peptide (NT-proBNP)].16 Quantification of biomarkers selected to reflect these disease processes in a single blood draw identifies patient clusters with different risk of cardiovascular events.17 Whether the disease processes reflected by these biomarkers modify future rhythm in patients with AF has not been investigated.
This analysis of the EAST-AFNET 4 biomolecule study embedded into the Early treatment of Atrial fibrillation for STroke prevention (EAST-AFNET 4) trial2 quantified 14 biomarkers reflecting different disease processes in AF that were defined a priori.9 The ability of each biomarker to predict sinus rhythm at 12-month follow-up in patients with and without early rhythm control therapy was evaluated (Structured Graphical Abstract).
Validation was performed internally at 24 months, by comparing biomarker-based clusters at baseline by association with sinus rhythm at 12- and 24-month follow-up and by machine learning integrating biomarkers and clinical parameters. Clinical utility was assessed by defining and testing threshold values and by comparison with a clinical score. External validation was performed in two independent datasets of patients with AF.
Methods
Details of the prespecified analysis plan of the EAST-AFNET 4 biomolecule study can be found in a separate Supplementary material file (Supplementary file Statistical analysis plan SAP). Post hoc exploratory analyses were added to gain more insight into the main findings.
Derivation dataset (EAST-AFNET 4)
EAST-AFNET 4 randomized patients with recently diagnosed AF and stroke risk factors to systematic early rhythm control or usual care including symptom-based rhythm control.2 All patients were followed up for a median of 5.1 years. The EAST-AFNET 4 biomolecule study collected a baseline blood sample in 1586 patients enrolled in the EAST-AFNET 4 trial.17,18 In brief, all consenting patients provided a blood sample at baseline. Samples were shipped to the core biostorage facility at UKE Hamburg, spun, shock-frozen, and stored at −80°C. EAST-AFNET 4 and its biomolecule study were approved at all participating study sites. Written informed consent was obtained from all patients.
Validation datasets
AXAFA-AFNET 5
The Anticoagulation using the direct factor Xa inhibitor apixaban during Atrial Fibrillation catheter Ablation: Comparison to vitamin K antagonist therapy (AXAFA-AFNET 519) trial was a randomized, investigator-initiated trial comparing continuous vitamin K antagonist therapy to apixaban in 633 patients undergoing a first AF ablation in 49 European and US American study sites. The same 14 biomarkers quantified in the derivation dataset were quantified in the AXAFA-AFNET 5 blood samples using the same assays.20 The outcome of interest was rhythm at the final follow-up visit, 120 days after enrolment.19 All patients provided written informed consent.
BBC-AF atrial fibrillation snapshot
Details of the BBC-AF cohort have been described before.21 In brief, consecutive patients eligible for recruitment had ECG-diagnosed AF or presented with at least two cardiovascular conditions (congestive heart failure, hypertension, diabetes, prior stroke, or vascular disease) to a large teaching hospital (Sandwell and West Birmingham NHS Trust). Patients who did not have a diagnosis of AF underwent 7-day ambulatory ECG monitoring to rule out undiagnosed ECG-documented AF. For this analysis, only patients with ECG-documented AF were included. Follow-up data were collected by assessing local hospital records corroborated against Hospital Episode Statistics data, general practitioner records, and mortality data from NHS Digital, up to 2.5 years after the final patient was recruited.22 This study complied with the Declaration of Helsinki, was approved by the National Research Ethics Service Committee (IRAS ID 97753), and was sponsored by the University of Birmingham. All patients provided written informed consent.
TRUST snapshot
A snapshot of patients enrolled in the Long-term Outcome and Predictors for Recurrence after Medical and Interventional Treatment of Arrhythmias study (TRUST; NCT05521451), with biomarker concentrations and 12-month rhythm status was created. All patients provided written informed consent. A snapshot of all patients with biomarker concentrations and ECG follow-up at 12–18 months was obtained in June 2024 for validation.
Selection of biomarkers and their quantification
Circulating biomarkers were selected by scientists from the EU-funded CATCH-ME consortium based on relevant disease processes and available high-precision high-throughput assays.9 Biomarkers were selected in four steps: (i) members of the consortium identified candidate biomarkers reflecting disease processes known to contribute to AF and its complications, (ii) deep literature and patent searches for candidate biomarkers and additional novel biomarkers were performed, (iii) expert discussion and Delphi-like votes by the consortium defined most promising candidates, and (iv) availability and feasibility checks to perform measurements of thousands of samples with high precision.
Fourteen biomarkers were selected (Table 1 following clinical characteristics); ANGPT2, BMP10, cancer antigen 125 (CA125), CRP, D-dimer, ESM1, FABP3, fibroblast growth factor 23 (FGF23), GDF15, IGFBP7, IL-6, NT-proBNP, TnT, and serum creatinine (sCr).
Table 1.
Baseline characteristics and biomarkers in the EAST-AFNET 4 biomolecule study
| Treatment group | Early rhythm control | Usual care | P-value* | |
|---|---|---|---|---|
| n | 800 | 786 | ||
| Sex: female | 355 (44%) | 358 (46%) | .639 | |
| Age (years) | 71 [66, 75] | 71 [66, 76] | .711 | |
| BMI | 28.7 [25.6, 32.1] | 29.0 [25.6, 32.5] | .699 | |
| Blood pressure (systolic, mmHg) | 135 [123, 150] | 135 [125, 148] | .730 | |
| Blood pressure (diastolic, mmHg) | 80 [74, 90] | 80 [74, 90] | .716 | |
| LVEF (%) | 60 [55, 65] | 60 [55, 65] | .873 | |
| AF type (first episode) | 290 (36%) | 270 (34%) | ||
| AF type (paroxysmal) | 302 (38%) | 288 (37%) | .839 | |
| AF type (persistent) | 208 (26%) | 228 (29%) | .202 | |
| Other clinical characteristics | ||||
| Diabetes | 207 (26%) | 189 (24%) | .400 | |
| Hypertension | 494 (62%) | 512 (65%) | .170 | |
| Chronic kidney disease | 98 (12%) | 97 (12%) | .956 | |
| Estimated glomerular filtration rate (mL/min 1.73 m²) | 75 [63–87] | 76 [64–87] | .734 | |
| Previous stroke or transient ischaemic attack | 114 (14%) | 81 (10%) | .017 | |
| Chronic obstructive pulmonary disease | 63 (8%) | 61 (8%) | .991 | |
| Diastolic LA diameter (mm) | 42 [38, 47] | 43 [39, 47] | .730 | |
| NYHA class | ||||
| No heart failure | 523 (65%) | 509 (65%) | ||
| I | 82 (10%) | 88 (11%) | .555 | |
| II | 164 (21%) | 160 (20%) | .985 | |
| III | 31 (4%) | 29 (4%) | .882 | |
| EHRA score | ||||
| I | 232 (29%) | 236 (30%) | ||
| II | 386 (48%) | 374 (48%) | .679 | |
| III | 122 (15%) | 122 (15%) | .914 | |
| IV | 8 (1%) | 9 (1%) | .839 | |
| Missing | 52 (7%) | 45 (6%) | ||
| Biomarker (unit) | Coefficient of variation | |||
| NT-proBNP (pg/mL) | 1.51 | 441 [175–966] | 467 [187–1036] | .537 |
| ANGPT2 (ng/mL) | .70 | 2.53 [1.87–3.65] | 2.53 [1.87–3.75] | .456 |
| BMP10 (ng/mL) | .24 | 2.10 [1.82–2.41] | 2.11 [1.83–2.45] | .507 |
| FGF23 (pg/mL) | 1.27 | 155 [115–218] | 153 [115–211] | .244 |
| ESM1 (ng/mL) | .76 | 2.04 [1.64–2.59] | 2.05 [1.63–2.63] | .818 |
| GDF15 (pg/mL) | .80 | 1333 [990–2000] | 1359 [971–2005] | .078 |
| IGFBP7 (ng/mL) | .26 | 102 [90.7–117] | 102 [90.1–117] | .457 |
| IL-6 (pg/mL) | 6.62 | 2.56 [1.64–4.04] | 2.68 [1.67–4.18] | .479 |
| FABP3 (ng/mL) | .50 | 32.0 [26.3–39.6] | 31.9 [26.4–39.6] | .837 |
| D-dimer (µg/mL) | 1.74 | .17 [.09–.34] | .16 [.08–.36] | .506 |
| TnT (ng/L) | 2.26 | 11.1 [8.02–16.6] | 11.4 [8.21–16.7] | .337 |
| CRP (mg/L) | 3.28 | 2.02 [.96–4.99] | 2.38 [1.04–4.75] | .392 |
| sCr (µmol/L) | .29 | 81.7 [70.7–95.5] | 80.4 [70.0–94.5] | .771 |
| CA125 (U/mL) | 1.51 | 11.5 [8.08–15.9] | 11.1 [7.93–16.1] | .433 |
Estimated glomerular filtration rate (eGFR) was calculated as CKD EPI, Chronic Kidney Disease Epidemiology Collaboration.
AF, atrial fibrillation; SR, sinus rhythm; ERC, early rhythm control; UC, usual care; BMI, body mass index; LVEF, left ventricular ejection fraction; LA, left atrium; NYHA, New York Heart Association Functional Classification of heart failure; EHRA, European Heart Rhythm Association score; ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; CA125, cancer antigen 125; CRP, C-reactive protein; ESM1, endothelial specific molecule 1; FABP3, fatty acid binding protein 3; FGF23, fibroblast growth factor 23; GDF15, growth differentiation factor 15; IGFBP7, insulin-like growth factor binding protein 7; IL-6, interleukin-6; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TnT, cardiac troponin; sCr, serum creatinine.
* P-values were calculated on the unimputed dataset using mixed logistic regression model with site as random effect, for biomarkers additionally adjusted for sex, age, body mass index, diastolic blood pressure, left ventricular ejection fraction, and AF type. Distributions are shown as mean and SD for normally distributed values, as median and IQR for non-normally distributed values and biomarkers, and as frequency (percentage) for nominal features.
Blood samples were collected at all participating sites and shipped to the core lab at University Heart and Vascular Center (UHZ) Hamburg by courier at ambient temperatures (24–48 h transport time). Upon arrival at UHZ, samples were spun, shock-frozen, and stored at −80°C for analysis. Biomarkers were centrally quantified using pre-commercial and commercial high-throughput, high-precision platforms (Roche, Penzberg, Germany) in EDTA plasma. The biomarker quantification was provided as an in-kind contribution of Roche to the CATCH ME consortium. Blood samples were shipped to, and quantifications were conducted at the Roche biomarker research facility in Penzberg, Germany.
Statistical methods
As rhythm is a secondary outcome analysis of the EAST-AFNET 4 trial, all results are exploratory. Biomarker concentrations were 1% winsorized23 from above and logarithmically transformed (log base e) to normalize skewed concentration ranges for all datasets. Concentrations below the detection limit for CA-125 and D-dimer were replaced with the lowest available value. For the initial testing of prespecified hypotheses, all 14 biomarkers were used. Validations were done with predictive biomarkers. This analysis does not take into account the probability of chance findings because of performance of multiple comparisons with 14 biomarkers. As a consequence, results should be interpreted as explorative/hypothesis generating and call for further validation.
Patients in AF at the time of blood sampling showed higher concentrations in most biomarkers (see Supplementary data online, Table S1). Rhythm at time of blood sampling was therefore included as a confounder in all subsequent analyses in addition to the features predicting rhythm at 12 months in the main EAST-AFNET 4 dataset.4
Mixed logistic regression models were used to assess the predictive value of the 14 biomarkers on rhythm at 12 months, with study centre as a random intercept. The lme4 R package24 was used. Each biomarker was assessed in a separate model adjusted for sex, age, rhythm at baseline, body mass index, diastolic blood pressure, AF pattern (first episode, paroxysmal, persistent), left ventricular ejection fraction, rhythm at baseline, and randomized group (usual care or early rhythm control).4 Nested models with additional interaction terms between treatment type and the biomarker of interest were constructed. To obtain P-values for the interaction, each nested model pair was compared by ANOVA for their goodness of fit. Odds ratios (ORs) and P-values for the biomarker effects under different treatment types were calculated by reference cell coding.25 Missing values in heart rhythm and left ventricular ejection fraction were imputed in a 60-times multiple imputed dataset as described earlier,2 following the recommendations of White, Royston, and Wood.26,27 A sensitivity analysis constructed prediction models for recurrent AF at 12- and 24-month follow-up without imputation.
To further explore the effect of rhythm on the biomarkers, mixed regression models were repeated in subgroups split by baseline rhythm (sinus rhythm or AF) and by rhythm control therapy (early rhythm control or usual care) and ORs for the outcome sinus rhythm at 12 months were calculated using the methods described above.
As internal validation, analysis was repeated for sinus rhythm at 24-month follow-up. As sensitivity analysis, the analysis was repeated for recurrent AF up to 24 months.
As additional internal validation, patient clusters formed using all biomarker concentrations agnostic to clinical features17 were tested for prediction of presence of sinus rhythm at 12- and 24-month follow-up. The lowest-risk cluster was used as a reference.
As another means of internal validation, we applied a random forest machine learning model (ML) and made use of a mixed effect random forest (MERF) wrapper to account for the centre as a random effect. The ML model was fitted with the features used for confounding the generalized linear model as well as of all 14 biomarkers at once. To assess the variable importance, we used the models’ inherent Gini-based feature importance as well as the model agnostic SHapley Additive exPlanations (SHAP) values.
Clinical utility
Cut-off values for clinically useful probabilities of sinus rhythm at 12 months (80%) and for AF at 12 months (40%) were determined for all biomarkers that predicted the main outcome. A clinical risk score was developed based on a recent meta-analysis28: three accepted clinical features predicting recurrent AF, namely left atrial size, AF pattern, and age, were dichotomized with a point scored for persistent AF yes, anterior–posterior left atrial diameter > 50 mm, age > 75 years (see Supplementary data online, Table S2). As many patients with one of these three features attain sinus rhythm at 12 months, the score was considered as predictive of high risk of AF at 12 months if at least two of the three factors were present. Each of the biomarkers that were independently associated with sinus rhythm at 12 months was added to this clinical score separately, as well as in combination. If at least one biomarker was above the cut-off value, the patient was regarded as high risk of not attaining sinus rhythm. The confusion matrices for correctly and incorrectly classified patients at high-risk-classified of not attaining sinus rhythm were calculated for the reference clinical score alone and all additional, biomarker-enriched scores.
Biomarkers’ predictive values were tested in the validation datasets using univariate and multivariate models restricted to the features that predicted sinus rhythm at 12 months in the derivation dataset.
Python version 3.8.13 was employed for data pre-processing and visualization, R version 4.2.2 for statistical computations.29 Relevant code will be made publicly available (https://github.com/UCCSHH).
Results
Derivation analysis dataset
The 1586 patients with a recent history of AF and stroke risk factors (age 71 years, 45% women) with clinical features, biomarker concentrations, and cardiovascular outcomes were equally assigned to both randomized treatment groups (Table 1, Supplementary data online, Figure S1).
Association of biomarker concentrations with attaining sinus rhythm at 12 months
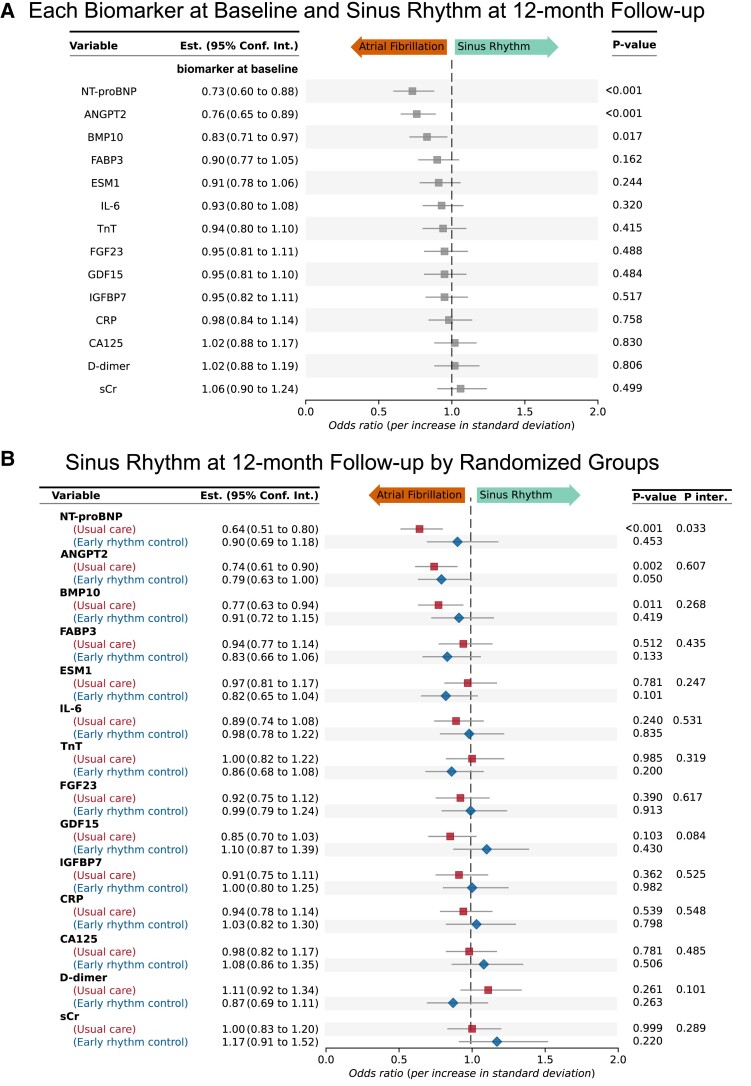
Three biomarkers (ANGPT2, BMP10, and NT-proBNP) showed lower concentrations at baseline in patients who were in sinus rhythm at the 12-month follow-up (Figure 1A). These three biomarkers were independently associated with sinus rhythm at the 12-month follow-up after multiple corrections for clinical features, early rhythm control, and baseline rhythm (Figure 1A). NT-proBNP interacted with early rhythm control therapy at 12-month follow-up (P = .033) and low NT-proBNP concentrations only predicted sinus rhythm at 12 months in patients randomized to usual care (Figure 1B). Early rhythm control impacted on the rhythm-predicting effect of NT-proBNP and dampened its predictive value in this group. There was no significant interaction detected between early rhythm control and any of the other 13 biomarkers in this dataset (Figure 1B).
Figure 1.
Low concentrations of the biomarkers NT-proBNP, angiopoietin 2, and bone morphogenetic protein 10 predict sinus rhythm at 12-month follow-up in the derivation dataset (EAST-AFNET 4). Odds ratios for sinus rhythm at 12-month follow-up (A) and odds ratios by randomized treatment group (B). Forest plot showing odds ratios for each biomarker for the outcome sinus rhythm at 12-month follow-up and 95% confidence intervals. The odds ratio for NT-proBNP shows an interaction between NT-proBNP concentrations and randomized treatment group (early rhythm control or usual care). All odds ratios are corrected for clinical features, age, sex, EAST study centre, rhythm at baseline, atrial fibrillation type, randomized treatment group, body mass index, diastolic blood pressure, and left ventricular ejection fraction. Even after multiple confounding, high biomarker concentrations indicate lower odds of sinus rhythm at 12-month follow-up. Low concentrations of NT-proBNP predict sinus rhythm at 12-month follow-up in patients with usual care (only symptomatic rhythm control). High concentrations of NT-proBNP do not necessarily predict lack of sinus rhythm at 12 months if patients receive early rhythm control. ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; CA125, cancer antigen 125; CRP, C-reactive protein; D-dimer, ESM1, endothelial specific molecule 1; FABP3, fatty acid binding protein 3; FGF23, fibroblast growth factor 23; GDF15, growth differentiation factor 15; IGFBP7, insulin-like growth factor binding protein 7; IL-6, interleukin-6; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TnT, cardiac troponin; sCr, serum creatinine
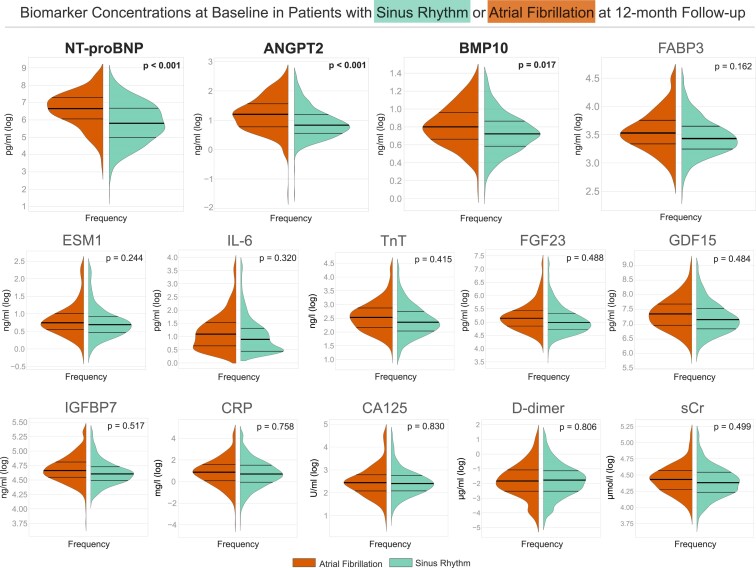
Biomarker concentrations distributions depicted in violin plots after log transformation (Figure 2) show lower concentrations in sinus rhythm vs. AF at 12 months. Numbers of mean biomarker concentrations by rhythm at 12-month follow-up and by randomized treatment group are given in Table 2.
Figure 2.
Biomarker concentration distributions at baseline in patients with sinus rhythm (teal right part of each plot) or atrial fibrillation (orange left part of each plot) at 12-month follow-up. Violin plot of the distribution of log-transformed biomarker concentrations for each of 14 biomarkers at baseline, split by the outcome of rhythm at 12-month follow-up. Log-transformed biomarker concentrations are shown on the y-axis and the kernel estimated frequency on the x-axis. Central thick horizontal lines are the median and the thinner lines represent interquartile range. N-terminal pro-B-type natriuretic peptide, angiopoietin 2, and bone morphogenetic protein 10 show an association with sinus rhythm at 12-month follow-up based on the acceptance of a Type 1 error of 5%. P-values were calculated using mixed logistic regression model with site as random effect, adjusted for age, sex, rhythm at baseline, randomized group (early rhythm control or usual care), body mass index, diastolic blood pressure, and left ventricular ejection fraction, those clinical features that were associated with outcomes including sinus rhythm in the main EAST-AFNET 4 trial. ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; CA125, cancer antigen 125; CRP, C-reactive protein; D-dimer, ESM1, endothelial specific molecule 1; FABP3, fatty acid binding protein 3; FGF23, fibroblast growth factor 23; GDF15, growth differentiation factor 15; IGFBP7, insulin-like growth factor binding protein 7; IL-6, interleukin-6; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TnT, cardiac troponin; sCr, serum creatinine
Table 2.
Baseline biomarker concentrations are shown split by rhythm at 12-month follow-up (sinus rhythm or atrial fibrillation) and by randomized group (early rhythm control or usual care)
| Randomization group | Early rhythm control | Usual care | SR vs. AF 12 months | ||
|---|---|---|---|---|---|
| Rhythm at 12-month follow-up | Sinus rhythm 12-month FU | Atrial fibrillation 12-month FU | Sinus rhythm 12-month FU | Atrial fibrillation 12-month FU | P-value* |
| NT-proBNP (pg/mL) | 377 [164–859] |
750 [376–1351] |
294 [127–700] |
782 [437–1454] |
.001 |
| ANGPT2 (ng/mL) | 2.34 [1.78–3.41] |
3.45 [2.43–5.62] |
2.24 [1.7–3.09] |
3.31 [2.15–4.62] |
.001 |
| BMP10 (ng/mL) | 2.08 [1.8–2.39] |
2.21 [1.96–2.58] |
2.04 [1.79–2.34] |
2.24 [1.94–2.66] |
.010 |
| FGF23 (pg/mL) | 151 [112–209] |
179 [125–238] |
141 [108–197] |
168 [129–226] |
.429 |
| ESM1 (ng/mL) | 2.01 [1.61–2.52] |
2.17 [1.75–2.88] |
1.98 [1.57–2.52] |
2.09 [1.73–2.66] |
.218 |
| GDF15 (pg/mL) | 1304 [958–1934] |
1441 [997–2008] |
1254 [911–1782] |
1589 [1071–2347] |
.461 |
| IGFBP7 (ng/mL) | 100 [89–114] |
108 [93–126] |
98.8 [88.5–110] |
104 [94.7–119] |
.487 |
| IL-6 (pg/mL) | 2.47 [1.57–3.88] |
2.6 [1.76–4.62] |
2.37 [1.56–3.6] |
3.02 [1.98–4.65] |
.417 |
| FABP3 (ng/mL) | 31.4 [25.6–39] |
35.3 [28.3–43.4] |
30.4 [25.7–37.8] |
33.5 [28.0–42.0] |
.151 |
| D-dimer (µg/mL) | .17 [.08–.33] |
.19 [.1–.36] |
.16 [.08–.32] |
.16 [.08–.32] |
.638 |
| TnT (ng/L) | 10.6 [7.81–15.7] |
13.0 [9–17.6] |
10.3 [7.53–15.5] |
12.5 [8.68–17.7] |
.415 |
| CRP (mg/L) | 2 [.95–4.65] |
1.97 [.9–4.63] |
2.07 [.93–4.37] |
2.52 [1.12–4.87] |
.910 |
| sCr (µmol/L) | 81.3 [70–95] |
83 [72.7–94.8] |
79.5 [68.0–91.9] |
84.4 [72–97.2] |
.541 |
| CA125 (U/mL) | 11.4 [8.0–15.8] |
12.3 [8.3–17.1] |
10.8 [7.8–15.7] |
11.4 [7.96–15.9] |
.779 |
AF, atrial fibrillation; SR, sinus rhythm; FU, follow-up; ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; CA125, cancer antigen 125; CRP, C-reactive protein; D-dimer, ESM1, endothelial specific molecule 1; FABP3, fatty acid binding protein 3; FGF23, fibroblast growth factor 23; GDF15, growth differentiation factor 15; IGFBP7, insulin-like growth factor binding protein 7; IL-6, interleukin-6; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TnT, cardiac troponin; sCr, serum creatinine.
* P-values were calculated using mixed logistic regression model with site as random effect, adjusted for sex, age, body mass index, diastolic blood pressure, left ventricular ejection fraction. Values are shown as median [IQR].
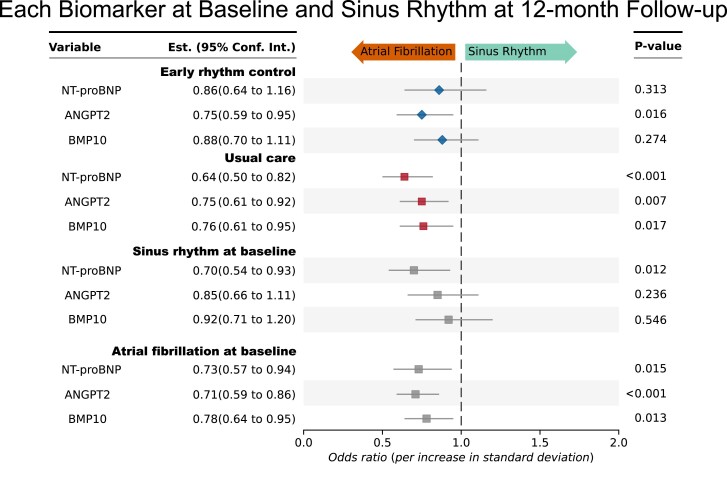
Baseline biomarker concentrations depending on baseline rhythm in the derivation dataset and clinical features are shown in Table 3, extended information shown in Supplementary data online, Table S1. Post hoc subgroup analyses by rhythm at the time of baseline assessment (sinus rhythm or AF) and by randomized group (early rhythm control or usual care) find NT-proBNP mainly associated with sinus rhythm at 12 months in patients under usual care. BMP10 and ANGPT2 retained their predictive ability shown in the joint group of all patients also if only the subgroup patients in AF at the time of blood sampling were analysed (Figure 3).
Table 3.
Baseline clinical characteristics used as confounders and biomarker concentrations in the derivation dataset (EAST-AFNET 4 biomolecule study) at baseline by randomized group and by baseline rhythm
| Group | Early rhythm control | Usual care | P-value | ||
|---|---|---|---|---|---|
| Baseline rhythm | Sinus rhythm | Atrial fibrillation | Sinus rhythm | Atrial fibrillation | Rhythm* |
| N | 452 | 348 | 438 | 348 | |
| Women | 220 (49%) | 135 (39%) | 221 (51%) | 137 (39%) | <.001 |
| Age, years | 70 [65–75] |
71 [67–76] |
71 [66–75] |
72 [66–76] |
.035 |
| BMI | 28.7 [25.8–31.6] |
28.4 [25.5–32.9] |
28.4 [25.4–31.4] |
29.4 [25.9–33.3] |
.022 |
| Blood pressure (diastolic) (mmHg) | 80 [72, 87] |
80 [76, 90] |
80 [71, 89] |
80 [76, 90] |
<.001 |
| LVEF (%) | 60 [57, 65] |
59 [50, 64] |
60 [59, 65] |
60 [51, 64] |
<.001 |
| AF type: first episode | 172 (38%) | 118 (34%) | 155 (35%) | 115 (33%) | |
| AF type: paroxysmal | 235 (52%) | 67 (19%) | 223 (51%) | 65 (19%) | <.001 |
| AF type: persistent or long-standing persistent | 45 (10%) | 163 (47%) | 60 (14%) | 168 (48%) | <.001 |
| Biomarker concentrations | |||||
| NT-proBNP (pg/mL) | 228 [121–467] |
890 [506–1496] |
253 [124–504] |
934 [529–1603] |
<.001 |
| ANGPT2 (ng/mL) | 2.20 [1.65–2.76] |
3.39 [2.29–5.14] |
2.12 [1.63–3.00] |
3.35 [2.31–4.81] |
<.001 |
| BMP10 (ng/mL) | 2.03 [1.73–2.30] |
2.22 [1.96–2.58] |
2.01 [1.76–2.29] |
2.25 [1.96–2.69] |
<.001 |
| FGF23 (pg/mL) | 139 [106–194] |
178 [128–247] |
140 [110–192] |
170 [130–243] |
.003 |
| ESM1 (ng/mL) | 1.97 [1.58–2.44] |
2.14 [1.74–2.84] |
1.96 [1.57–2.56] |
2.15 [1.74–2.78] |
.002 |
| GDF15 (pg/mL) | 1251 [938–1847] |
1478 [1058–2188] |
1259 [914–1761] |
1585 [1065–2272] |
<.001 |
| IGFBP7 (ng/mL) | 99.0 [89.3–111.2] |
106.8 [93.6–125] |
99.2 [87.9–111] |
105 [93.8–123] |
<.001 |
| IL-6 (pg/mL) | 2.22 [1.50–3.58] |
3.03 [1.99–4.88] |
2.42 [1.58–3.89] |
3.02 [1.95–4.59] |
.041 |
| FABP3 (ng/mL) | 30.2 [25.1–38.1] |
34.2 [28.2–42.1] |
30.9 [25.6–37.9] |
33.3 [27.1–42.6] |
.020 |
| D-dimer (µg/mL) | .17 [.08–.32] |
.18 [.09–.36] |
.15 [.08–.32] |
.18 [.09–.4] |
.267 |
| TnT (ng/L) | 10.1 [7.39–14.5] |
12.7 [9–18.8] |
10.7 [7.6–15.7] |
12.5 [8.73–18.3] |
.436 |
| CRP (mg/L) | 1.76 [.87–4.29] |
2.48 [1.09–5.78] |
2.08 [.93–4.52] |
2.58 [1.26–5.03] |
.130 |
| sCr (µmol/L) | 80.0 [69.0–93.7] |
84.0 [71.0–97.0] |
79.6 [68.1–92.0] |
83.9 [71.0–97.2] |
.296 |
| CA125 (U/mL) | 11.1 [8.01–14.9] |
12.3 [8.4–16.9] |
10.8 [8.02–15.7] |
11.4 [7.84–16.7] |
.052 |
Rhythm at time of blood sampling was included as a fix factor in the analyses of outcome. Distributions are shown as mean and SD for normally distributed values, as median and IQR for non-normal distributed values and biomarkers, and as frequency (percentage) for nominal features. For biomarker concentrations, there were no differences between the randomized groups, but differences between sinus rhythm and AF during the baseline visit.
AF, atrial fibrillation; ERC, early rhythm control; UC, usual care; BMI, body mass index; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; CA125, cancer antigen 125; CRP, C-reactive protein; D-dimer, ESM1, endothelial specific molecule 1; FABP3, fatty acid binding protein 3; FGF23, fibroblast growth factor 23; GDF15, growth differentiation factor 15; IGFBP7, insulin-like growth factor binding protein 7; IL-6, interleukin-6; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TnT, cardiac troponin; sCr, serum creatinine.
* P-values were calculated in the unimputed, pooled dataset (ERC and UC combined) using mixed logistic regression model with site as random effect, for the biomarkers additionally adjusted for sex, age, body mass index, diastolic blood pressure, left ventricular ejection fraction, and AF type, the clinical features that were associated with outcomes including sinus rhythm in the main EAST-AFNET 4 dataset.4
Figure 3.
Biomarkers measured at baseline predicting sinus rhythm at 12-month follow-up in all participants of the biomarker study, separately analysed by rhythm at baseline (atrial fibrillation at baseline or sinus rhythm at baseline) and randomized treatment group (early rhythm control or usual care), respectively, in a post hoc analysis. Of the three biomarkers identified to be predictive of sinus rhythm in the whole cohort, NT-proBNP, ANGPT2, and BMP10, all three biomarkers retained their predictive value in the subgroup of patients randomized to usual care. All three biomarkers also retained their predictive value in the subgroup of patients in atrial fibrillation during blood draw at baseline. ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; NT-proBNP, N-terminal pro-B-type natriuretic peptide
Internal validations
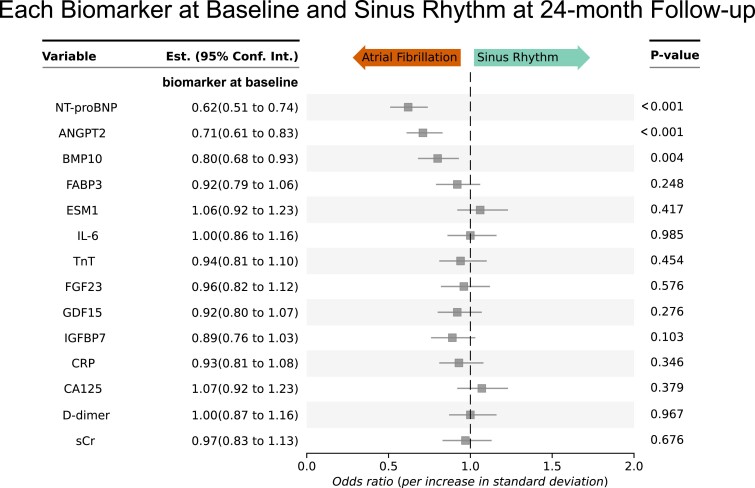
As a first internal validation, the same analysis was performed for the 24-month follow-up. The same biomarkers, ANGPT2, BMP10, and NT-proBNP, were consistently associated with sinus rhythm at 24-month follow-up (Figure 4).
Figure 4.
Internal validation: angiopoietin 2, bone morphogenetic protein 10, and NT-proBNP biomarkers at baseline predict sinus rhythm at 24-month follow-up even after correction for multiple confounders. Odds ratios are shown for sinus rhythm at 24-month follow-up. This analysis provides an internal validation of the biomarkers predicting sinus rhythm at 12-month follow-up (Figure 1). All odds ratios are corrected for clinical age, sex, study site, rhythm at baseline, randomized treatment group (early rhythm control or usual care), body mass index, diastolic blood pressure, and left ventricular ejection fraction, those clinical features that were associated with outcomes including sinus rhythm in the main EAST-AFNET 4 trial.4 Low concentrations of NT-proBNP, ANGPT2, and BMP10 predict sinus rhythm at 24-month follow-up in patients. Accordingly, high concentrations predict lack of sinus rhythm at 24-month follow-up. ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; CA125, cancer antigen 125; CRP, C-reactive protein; D-dimer, ESM1, endothelial specific molecule 1; FABP3, fatty acid binding protein 3; FGF23, fibroblast growth factor 23; GDF15, growth differentiation factor 15; IGFBP7, insulin-like growth factor binding protein 7; IL-6, interleukin-6; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TnT, cardiac troponin; sCr, serum creatinine
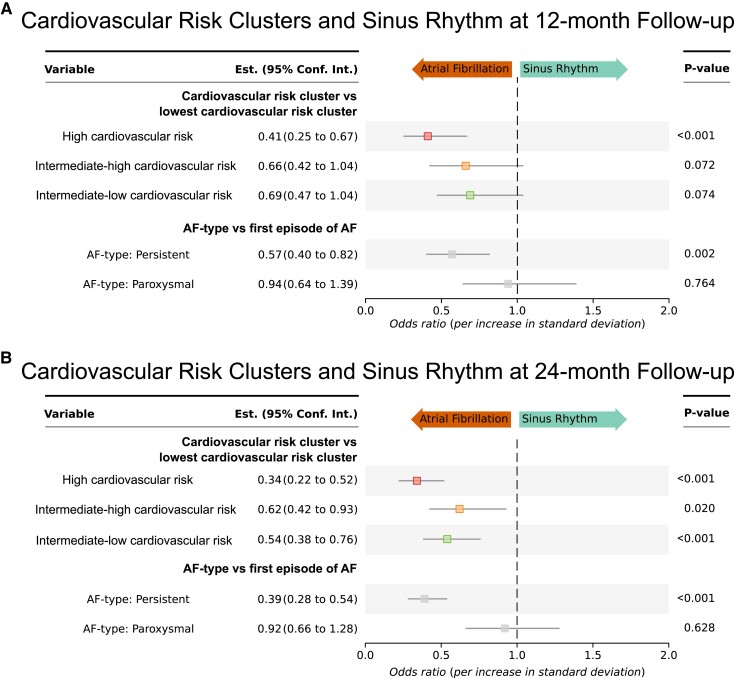
Repeating the analysis for recurrent AF up to 24 months showed similar results (see Supplementary data online, Table S3). As further internal validation analysis, unsupervised biomarker-based clustering of EAST patients previously performed was applied to sinus rhythm at 12-month follow-up. Clusters separated by risk of cardiovascular complications, with patients assigned to the high-risk cardiovascular outcome cluster showing a lower likelihood of sinus rhythm at 12 months, patients in the two intermediate cardiovascular risk biomarker clusters showing an intermediate likelihood of sinus rhythm, all tested against the low cardiovascular risk cluster, with the low-risk outcome patient cluster showing the highest likelihood of sinus rhythm at 12 months (Figure 5A). These findings were consistent for the biomarker-based clusters at 24-month follow-up (Figure 5B).
Figure 5.
Validation applying biomarker-based clusters indicating cardiovascular outcome risk: patients at high risk of cardiovascular complications as estimated by biomarker-based clusters have reduced odds of sinus rhythm at 12-month and 24-month follow-up. Odds ratio for the high cardiovascular outcome risk (red) and intermediate cardiovascular outcome risk biomarker clusters (orange and green) for sinus rhythm at 12-month follow-up (A, above) and at 24-month follow-up (B, bottom) tested against the low cardiovascular risk cluster (not depicted as used as reference). All odds ratios are corrected for age, sex, study centre, rhythm at baseline, atrial fibrillation type (depicted in grey odds ratios below the cluster odds ratios), randomized treatment group (early rhythm control or usual care), as well as body mass index, diastolic blood pressure, and left ventricular ejection fraction, the clinical features that were associated with outcomes including sinus rhythm in the main EAST-FNET 4 trial.4 AF, atrial fibrillation
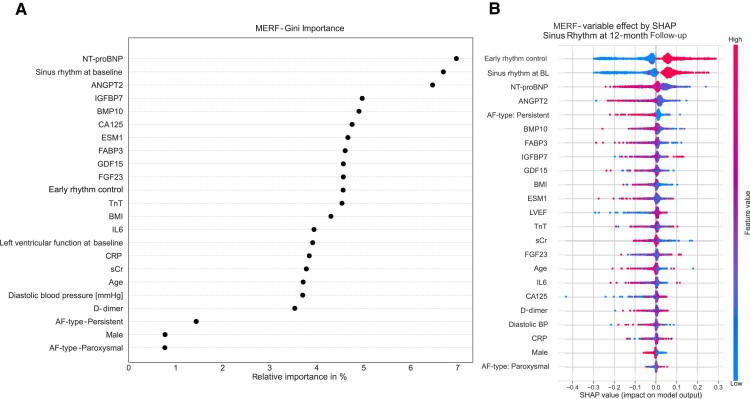
As further internal validation, a random forest classifier was trained on the EAST-AFNET 4 dataset. Its feature performance evaluation confirmed the importance of the three biomarkers alongside AF pattern, rhythm at baseline, and early rhythm control for the outcome of sinus rhythm (Figure 6).
Figure 6.
Validation by random forest analyses identified highest importance for similar biomarkers, alongside rhythm at baseline and AF pattern, as predictors of sinus rhythm at 12-month follow-up (A—importance, B—SHAP value). AF, atrial fibrillation; ANGPT2, angiopoietin 2; BL, baseline; BMI, body mass index; BMP10, bone morphogenetic protein 10; CA125, cancer antigen 125; CRP, C-reactive protein; D-dimer, ESM1, endothelial specific molecule 1; FABP3, fatty acid binding protein 3; FGF23, fibroblast growth factor 23; GDF15, growth differentiation factor 15; IGFBP7, insulin-like growth factor binding protein 7; IL-6, interleukin-6; MERF, mixed effect random forest; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TnT, cardiac troponin; sCr, serum creatinine; SHAP, SHapley Additive exPlanations
Clinical utility
Thresholds to predict a high probability of attaining sinus rhythm (>80%, low risk of AF) or a high probability of recurrent AF at follow-up (>40%, high risk of AF) were determined for each biomarker (Table 4, Supplementary data online, Figures S2–S4). To compare them to clinical features predicting sinus rhythm, a score combining clinical features predicting recurrent AF was created integrating age, left atrial size, and AF pattern28 (see Supplementary data online, Table S2). Adding biomarkers using these thresholds improved identification of patients at risk of not attaining sinus rhythm at 12-month follow-up (Table 5, Supplementary data online, Table S4).
Table 4.
Threshold concentrations for NT-proBNP, BMP10, and ANGPT2 determined in the derivation dataset (EAST-AFNET 4 biomolecule study)
| Biomarker | Low threshold (>80% sinus rhythm at 12 months) | High threshold (>40% AF at 12 months) |
|---|---|---|
| NT-proBNP (pg/mL) |
<1000 | >1500 |
| BMP10 (ng/mL) |
<2 | >3 |
| ANGPT2 (ng/mL) |
<3.5 | >3.5 |
The lower threshold was defined as the nearest round concentration below which 80% of patients attained sinus rhythm at 12 months. The higher threshold was defined as the nearest rounded concentration above which 40% of patients were in AF at 12 months.
AF, atrial fibrillation; ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Table 5.
Estimated clinical utility of adding NT-proBNP, BMP10, and ANGPT2 alone or in combination to a clinical risk score to predict sinus rhythm at 12 months
| Patients reclassified as high risk of not attaining sinus rhythm at 12 M (N) | Confusion matrix | ||||
|---|---|---|---|---|---|
| Predicted sinus rhythm (actual patients in SR: N = 1081) | Predicted: AF (actual patients in AF: N = 365) | ||||
| Patients in sinus rhythm at 12 M | Patients in AF at 12 M | Patients in AF at 12 M | Patients in sinus rhythm at 12 M | ||
| Clinical model (LA size > 50 mm, persistent AF, age > 75 years) | Reference | 813 (77%) | 245 (23%) | 75 (40%) | 112 (60%) |
| +NT-proBNP | 135 | 743 (79%) | 201 (21%) | 129 (40%) | 191 (60%) |
| +BMP10 | 240 | 670 (79%) | 175 (21%) | 161 (37%) | 279 (63%) |
| +ANGPT2 | 301 | 650 (82%) | 145 (18%) | 198 (40%) | 303 (60%) |
| +NT-proBNP and BMP10 | 298 | 638 (80%) | 158 (20%) | 183 (37%) | 315 (63%) |
| +NT-proBNP and ANGPT2 | 345 | 625 (83%) | 130 (17%) | 215 (39%) | 332 (61%) |
| +ANGPT2 and BMP10 | 410 | 570 (82%) | 125 (18%) | 223 (36%) | 394 (64%) |
| +NT-proBNP and BMP10 and ANGPT2 | 441 | 551 (83%) | 115 (17%) | 234 (36%) | 416 (64%) |
Sinus rhythm at 12 months was initially predicted by a clinical risk score based on three validated clinical features (LA size > 50 mm, persistent AF, age > 75 years) alone. This reference score was then combined with one, a combination of two, or all three binarized predictive biomarkers (biomarker thresholds: NT-proBNP < 1000 pg/mL or >1500 pg/mL, ANGPT2 < 3.5 ng/mL or >3.5 ng/mL, BMP10 < 2 ng/mL or >3 ng/mL, Table 4). If either the clinical risk score is ≥2 or any of the biomarkers added to the model surpasses its threshold, the model predicts failure to attain sinus rhythm at 12-month follow-up and predicts AF instead. All numbers indicate number of patients with percentages of the predicted class in brackets. There were 140 missing values in outcomes and 225 missing values in LA size. The additional use of biomarkers for prediction can lead to differing missing values in predictions made for participants with available outcome data.
AF, atrial fibrillation; ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SR, sinus rhythm; 12 M, 12-month follow-up.
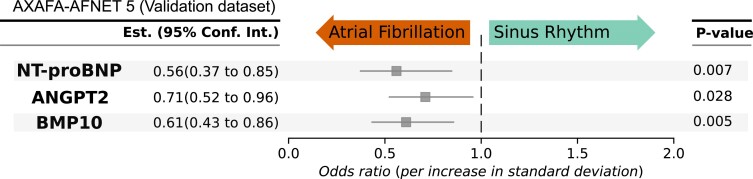
External validation
Several separate validation datasets (AXAFA-AFNET 5 trial, BBC-AF, and TRUST cohort snapshot Supplementary data online, Tables S5–S7) were used. The biomarkers NT-proBNP, BMP10, and ANGPT2 were confirmed as predictive of sinus rhythm in the final follow-up in AXAFA-AFNET 5 (Figure 7, Structured Graphical Abstract). The clinical utility of adding the biomarkers to clinical predictors was validated in both cohorts using the thresholds derived in EAST-AFNET 4 (see Supplementary data online, Tables S8 and S9).
Figure 7.
External validation of the prediction of sinus rhythm at the end of follow-up by baseline biomarkers in AXAFA-AFNET 5. AXAFA-AFNET 5 enrolled 674 patients undergoing a first AF ablation with at least one stroke risk factor. Patients were randomized to apixaban or vitamin K antagonist therapy without affecting rhythm. Individual models with rhythm at baseline, age, and sex were constructed to determine whether each biomarker predicts sinus rhythm at the end of follow-up 120 days after randomization, 549 patients with sinus rhythm, 71 patients with atrial fibrillation, 620 patients with baseline biomarkers completed follow-up. *P-values were calculated using logistic regression, adjusted for sex, age, rhythm at baseline, and treatment group. ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; NT-proBNP, N-terminal pro-B-type natriuretic peptide
Discussion
Main findings
Three out of 14 candidate biomarkers, BMP10, ANGPT2, and NT-proBNP, are associated with sinus rhythm at 12-month and 24-month follow-up after correcting for clinical features. Low NT-proBNP, low ANGPT2, and low BMP10 concentrations independently predict sinus rhythm in patients at follow-up. NT-proBNP is less predictive of rhythm in patients receiving rhythm control therapy. Adding these biomarkers to a clinical score identifying patients with a low probability of sinus rhythm at 12 months (positive with two out of three features: left atrial size > 50 mm, persistent AF, or age > 75 years) refined risk prediction (Structured Graphical Abstract).
Relevance for clinical care and research
In view of the growing choice of medical,2,30 interventional,2,31 and surgical32 treatment options for patients with AF, selecting the best strategy and the patients most benefitting from rhythm control therapy gains importance. Biomarker-based risk estimators have so far mainly been developed to refine anticoagulation decisions in patients with AF.33–35 Actionable biomarkers to guide rhythm control therapy are lacking. Similar to stroke prevention estimators, rhythm estimators face the challenge of random factors determining a binary outcome (AF or sinus rhythm). The present results suggest that NT-proBNP, BMP10, and ANGPT2 can stratify patients at high and low risk of attaining sinus rhythm alone and in combination. These biomarkers reflect and identify diseases processes that promote future AF, pointing to potential therapeutic targets for adjunct therapy supporting rhythm control. While a simple clinical score combining enlarged left atrial size, persistent AF, and older age predicted future sinus rhythm reasonably well, adding biomarkers reclassifies a clinically relevant number of patients at high risk of not attaining sinus rhythm at the price of also classifying more patients in sinus rhythm as high risk.
Effect of baseline rhythm on biomarker concentrations
This study shows that ANGPT2 and BMP10 provide additional information on future sinus rhythm when combined with NT-proBNP, especially in patients who are in AF at the time of blood sampling. Most biomarkers studied were elevated when the blood sample was taken in AF. Furthermore, NT-proBNP lost its ability to predict sinus rhythm in patients on rhythm control therapy.20,36 The effects of baseline rhythm on the concentrations and predictive ability of biomarkers should be further investigated in patients with AF undergoing rhythm control therapy.
Interpretation of NT-proBNP
NT-proBNP is released by atrial cardiomyocytes in response to stretch and strain, thereby acutely regulating fluid balance in the body, resulting in high concentrations during AF.37 In heart failure, NT-proBNP is also released by ventricular cardiomyocytes, further enhancing its concentrations. Atrial stretch has proarrhythmic effects including shortening of the atrial effective refractory period38 and conduction slowing,39 partially explaining its prediction of sinus rhythm in this study. NT-proBNP reflects short- and mid-term cardiac load in patients with AF, probably explaining its interaction with rhythm control. The possibility that elevated NT-proBNP concentrations predict rhythm during follow-up have been reported before.40–47 NT-proBNP is also associated with incident AF48–51 and with cardiovascular events in patients with and without AF and heart failure.22 This analysis demonstrates that the rhythm-predicting ability of NT-proBNP is reduced in patients treated with rhythm control therapy.
The NT-proBNP thresholds associated with a high risk of AF at 12 months in this study (>1500 pg/mL) are comparable to the thresholds associated with cardiovascular events, but higher than currently used thresholds e.g. for AF screening52 or for diagnosing heart failure with AF and heart failure with preserved ejection fraction.53 Based on the present analysis, higher thresholds may have better clinical utility. This warrants further analysis.
Interpretation of BMP10 and ANGPT2
BMP10 and ANGPT2 are tightly regulated circulating biomarkers, illustrating their signalling roles in regulating disease processes contributing to AF.3 Mechanistic studies of their role in AF are needed to define more precise clinical use cases for these biomarkers in patients with AF.
ANGPT2 is a vascular growth factor required for angiogenic remodelling.54 Overexpression of ANGPT2 in murine models promotes perivascular cardiac inflammation and fibrosis.55 Pro-inflammatory molecules such as thrombin increase ANGPT2 expression in vitro56 and inhibition of thrombin in animals with persistent AF improves atrial cardiomyopathy.15 Thus, ANGPT2 mediates the inflammatory communication between endothelial cells and myocardium in AF. Low ANGPT2 might reflect preserved vascular integrity, reducing the inflammatory burden in atrial vascular beds and thereby slowing AF progression.
ANGPT2 is associated with recurrent AF in patients after AF ablation20 and with prevalent AF in unselected hospitalized patients.57 ANGPT2 is elevated in patients with kidney disease,58 acute lung injury,59 and sepsis,60 conditions associated with AF. ANGPT2 can also predict heart failure hospitalization in patients with AF,61 similar to NT-proBNP.22 This study is the first to suggest that ANGPT2 can predict sinus rhythm in patients with AF with and without rhythm control therapy. Further research into treatable atrial disease processes regulated by ANGPT2 is warranted.
BMP10 is selectively expressed in and released by atrial cardiomyocytes.16,62 BMP10 is part of the TGFβ growth factor family and regulates vascular smooth muscle cell tone.63 Its function in the atria is not well known. BMP10 concentrations are reduced in hereditary forms of pulmonary arterial hypertension,64 possibly reflecting reduced atrial metabolism. Its inverse correlation and possible repression by PITX2 in atrial cardiomyocytes16,65 may suggest that elevated BMP10 concentrations could identify a reversible atrial metabolic defect13,17 that may be aggravated by the genomic basis of AF on chromosome 4q25.13
High concentrations of BMP10 are associated with recurrent AF,57,66 and with cardiovascular events17,67 and stroke in patients with AF. BMP10 may also be associated with atrial fibrosis.68 Lower BMP10 concentrations in patients in sinus rhythm,20 combined with its prediction of future sinus rhythm (Figure 1) suggest that a possible BMP10-mediated metabolic defect could partially be secondary to the metabolic demands of AF. Taken together, these results suggest that BMP10 is a potentially actionable biomarker indicative of atrial myopathy and atrial metabolic dysfunction. Further research into the atrial effects of BMP10 and its relation to AF burden5 are warranted.
Biomolecule-based clustering of patients agnostic to clinical features previously identified four subgroups of patients with AF with a gradual increase in cardiovascular events.17 The three biomarkers associated with sinus rhythm at 12 months in this study are among the six dominant biomarkers defining these patient clusters.17 The biomarker-based clusters show a risk gradient for sinus rhythm at 12 months (Figure 5). At difference to the prior study that defined patient clusters based on biomarker concentrations agnostic to clinical information, this analysis shows that the three biomarkers NT-proBNP, ANGPT2, and BMP10 predict sinus rhythm in context with clinical parameters. Of note, a simple clinical score (see Supplementary data online, Table S2) was already quite useful in identifying patients who will attain sinus rhythm. This information can help clinicians to select different intensities of rhythm control therapy depending on the likelihood of attaining sinus rhythm. NT-proBNP, ANGPT2, and BMP10 can refine that selection (Table 5). The present result and the biomarker-clustering also identify potentially treatable drivers of recurrent AF and or cardiovascular events in patients with AF. Based on the known atrial effects of BMP10 and ANGPT2, antihypertensive therapy and metabolic interventions such as SGLT2 inhibitor therapy12 could have beneficial effects in patients with elevated BMP10 and ANGPT2 concentrations.16,67,69 The underlying disease processes suggest that the same biomarkers could also be useful to identify patients at risk of AF. The present analysis identifies potentially actionable biomarkers suitable to select the intensity of rhythm control therapy. Further research into the mechanistic links between these biomarkers with baseline and future rhythm, and further evaluations of their clinical utility in different scenarios are warranted.
Strengths and limitations
Central quantification of the biomarkers using high-precision assays combined with the rigorous, near-complete follow-up at 12 and 24 months in a controlled clinical trial is a strength of this analysis. The consistent findings at both time points may suggest that the effects can be extrapolated to even longer follow-up, but this would require validation. Another strength of the analysis is the collection of samples in a broad range of care settings in adequately treated patients with AF, and external validation both in a controlled clinical trial and in cohorts of patients with AF enrolled in routine care settings. Validation of the findings using the same assays in different clinical datasets is a strength, but also limits the findings to the assays used in this study.
The study has important limitations. Although the statistical analysis plan was prespecified and validation was possible in different datasets, all results are explorative. This study is limited to 14 preselected biomarkers. Selected biomarkers intentionally reflect overlapping disease processes, creating redundancy that enables robust definition of disease pathways. Collinearity of biomarkers was more deeply investigated in a previous study defining biomarker-based patient clusters agnostic to clinical features.17
Additional biomarkers in AF may emerge from hypothesis-free quantification of many molecules at once e.g. by RNA-sequencing of cardiac tissue,70 quantification of circulating RNAs, and by proteomics.71,72 Repeat blood samples were not obtained and no information on changes over time is available. Some data on the changes of BMP10 and NT-proBNP over time have been published.20,36
While NT-proBNP can be measured in clinical routine as in vitro diagnostic devices with regulatory approval, the assays for ANGPT2 and BMP10 are not approved for clinical use, restricting them to research settings. Only the consenting portion of the total EAST-AFNET study participants was included in the biomarker study (two-thirds), hence there could be a considerable selection bias. Due to time required to setup the biobank, the first 400 patients were not invited to participate in the biomarker study.
The present study used serum creatinine rather than estimated glomerular filtration rate in the analyses as the formulas used to estimate kidney function rely on clinical parameters that are used in the regression model, including age, sex, and body mass index. Serum creatinine was not a major predictor of sinus rhythm. Whether estimated kidney function is a better predictor of sinus rhythm was not studied.
Validation datasets were smaller than the derivation dataset and therefore did not allow for multiple confounding. Post hoc subgroup analysis by baseline rhythm in EAST-AFNET 4 may have underestimated effects due to smaller group sizes. Almost all patients received guideline-recommended anticoagulation, rate and rhythm control, and often effective treatment of concomitant conditions. Twenty-four hour blood pressure may provide more granular prognostic information than office-based blood pressure, but 24 h blood pressure readings were not available for this analysis.
Left atrial size was used in the clinical score rather than left atrial volume. Indexed left atrial volume can provide more detailed information on left atrial size compared to left atrial diameter, but the predictive value of left atrial volume for recurrent AF is less well established than left atrial size.28 Indexed left atrial volume was not available in a sufficient number of patients to be assessed in this study. The predictive ability of the different biomarker-based models is only valid for the specific AF prevalences in the cohorts studied. Further research into the clinical utility of the biomarkers identified here is warranted.
The blood samples studied here stem from patients with predominantly Caucasian ethnicity, which may limit the generalizability of the findings to other ethnic groups. Validation in other ethnicities is therefore needed.
Testing the relationship between specific blood biomarker levels and a remote outcome observed 12 months later is challenging. In order to limit acute effects of the specific biomarker levels at baseline, we corrected for the acute rhythm at baseline, among other clinical parameters. Prediction of future rhythm by biomarkers depends on several factors, including the underlying biology of each biomarker, spontaneous variations in concentrations, and assay quality. Lack of predictive ability in this study does not rule out relevant biological function of a given molecule. The proposed interventions countering the disease processes associated with biomarkers require further testing.
Conclusion
In conclusion, these findings suggest that NT-proBNP, ANGPT2, and BMP10 can be combined to identify patients with AF at high risk of not attaining sinus rhythm. The disease processes related to ANGPT2 and BMP10 emerge as likely contributors to future rhythm in patients with and without rhythm control therapy. NT-proBNP elevations interact with early rhythm control, potentially suggesting repeat assessment of NT-proBNP to monitor the effectiveness of rhythm control.
Supplementary Material
Acknowledgements
We thank AFNET staff. We would like to thank all participants, study centers and investigators of the studies contributing to the datasets analysed. We would like to thank all members of CATCH ME and MAESTRIA consortia and their scientific advisory board members.
Contributor Information
Larissa Fabritz, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Hamburg, Germany; Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Martinistr. 52, Hamburg 20246, Germany; Atrial Fibrillation NETwork (AFNET), Mendelstr. 11, 48149 Münster, Germany; Institute of Cardiovascular Sciences, Wolfson Drive, University of Birmingham, B15 2TT Birmingham, UK; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France.
Christoph Al-Taie, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Hamburg, Germany; Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Martinistr. 52, Hamburg 20246, Germany; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France.
Katrin Borof, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany.
Günter Breithardt, Atrial Fibrillation NETwork (AFNET), Mendelstr. 11, 48149 Münster, Germany; Department of Cardiology II (Electrophysiology), University Hospital Münster, Germany.
A John Camm, Clinical Sciences, St George’s University, London, UK.
Harry J G M Crijns, Department of Cardiology, University Hospital Maastricht, Maastricht, The Netherlands.
Victor Roth Cardoso, Institute of Cardiovascular Sciences, Wolfson Drive, University of Birmingham, B15 2TT Birmingham, UK; MRC Health Data Research UK (HDR), Midlands Site, Birmingham, UK; Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, UK.
Winnie Chua, Institute of Cardiovascular Sciences, Wolfson Drive, University of Birmingham, B15 2TT Birmingham, UK; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France.
Silke van Elferen, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Hamburg, Germany; Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany; Computational and Systems Biology at Hamburg University, Germany.
Lars Eckardt, Atrial Fibrillation NETwork (AFNET), Mendelstr. 11, 48149 Münster, Germany; Department of Cardiology II (Electrophysiology), University Hospital Münster, Germany.
Georgios Gkoutos, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Hamburg, Germany; Institute of Cardiovascular Sciences, Wolfson Drive, University of Birmingham, B15 2TT Birmingham, UK; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France; MRC Health Data Research UK (HDR), Midlands Site, Birmingham, UK; Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, UK.
Andreas Goette, Atrial Fibrillation NETwork (AFNET), Mendelstr. 11, 48149 Münster, Germany; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France; Department of Cardiology and Intensive Care Medicine, St. Vincenz Hospital, Paderborn, Germany; Medical Faculty, Otto-von-Guericke University, Magdeburg, Germany.
Eduard Guasch, Hospital Clinic de Barcelona, Institute of Biomedical Research August Pi Sunyer (IDIBAPS), Hospital Clinic, CIBERCV, University of Barcelona, Catalonia, Spain.
Stéphane Hatem, MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France; Department of Cardiology, Sorbonne Université, Faculté de médecine UPMC, Assistance Publique-Hôpitaux de Paris, Pitié-Salpêtrière Hospital, Paris, France.
Andreas Metzner, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany.
Lluís Mont, Hospital Clinic de Barcelona, Institute of Biomedical Research August Pi Sunyer (IDIBAPS), Hospital Clinic, CIBERCV, University of Barcelona, Catalonia, Spain.
Vaishnavi Ameya Murukutla, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Hamburg, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Martinistr. 52, Hamburg 20246, Germany; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France.
Julius Obergassel, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Hamburg, Germany; Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France.
Andreas Rillig, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Martinistr. 52, Hamburg 20246, Germany.
Moritz F Sinner, Department of Medicine I, University Hospital Munich, Ludwig Maximilian University of Munich (LMU), Munich, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site Munich Heart Alliance, Munich, Germany.
Renate B Schnabel, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Martinistr. 52, Hamburg 20246, Germany; Atrial Fibrillation NETwork (AFNET), Mendelstr. 11, 48149 Münster, Germany.
Ulrich Schotten, Atrial Fibrillation NETwork (AFNET), Mendelstr. 11, 48149 Münster, Germany; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France; Department of Physiology, Maastricht University, Maastricht, The Netherlands.
Laura C Sommerfeld, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Hamburg, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Martinistr. 52, Hamburg 20246, Germany; Institute of Cardiovascular Sciences, Wolfson Drive, University of Birmingham, B15 2TT Birmingham, UK; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France.
Ursula-Henrike Wienhues-Thelen, Roche Diagnostics, Penzberg, Germany.
Antonia Zapf, German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Martinistr. 52, Hamburg 20246, Germany; Atrial Fibrillation NETwork (AFNET), Mendelstr. 11, 48149 Münster, Germany; Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg Eppendorf, Hamburg, Germany.
Tanja Zeller, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Hamburg, Germany; Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Martinistr. 52, Hamburg 20246, Germany.
Paulus Kirchhof, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg Eppendorf, Martinistr. 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Martinistr. 52, Hamburg 20246, Germany; Atrial Fibrillation NETwork (AFNET), Mendelstr. 11, 48149 Münster, Germany; Institute of Cardiovascular Sciences, Wolfson Drive, University of Birmingham, B15 2TT Birmingham, UK; MAESTRIA Consortium, European Union’s Horizon 2020 research and innovation programme, agreement number 965286, Sorbonne Université, Paris, France.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
A.J.C. receives personal funds from Acesion, Incarda, Menarini, Milestone, Sanofi, Bayer, Anthos, Daiichi Sankyo, Pfizer, Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and Johnson and Johnson. H.J.G.M.C. discloses advisory board fees from InCarda Therapeutics, Roche Diagnostics, Daiichi Sankyo, Sanofi, Acesion, and Atricure; speaker fee from Medtronic. U.S. received consultancy fees or honoraria from Università della Svizzera Italiana (USI, Switzerland) and Roche Diagnostics (Switzerland). U.S. was supported by a grant from EP Solutions Inc. (Switzerland) and is co-founder and shareholder of YourRhythmics BV, a spinoff company of the University Maastricht. R.B.S. has received lecture fees and advisory board fees from BMS/Pfizer and Bayer outside this work. L.F. received institutional research grants by EU 633196 [CATCH ME] and EU 965286 [MAESTRIA], British Heart Foundation (AA/18/2/34218), German Center for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK), and several biomedical companies active in the field of research. L.F. is listed as inventor on two issued patents held by the employing institution (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). P.K. received research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Center for Cardiovascular Research, from several drug and device companies active in atrial fibrillation and has received honoraria from several such companies in the past, but not in the last three years. P.K. is listed as inventor on two issued patents held by the employing institution (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). A.G. reports consulting fees from Daiichi Sankyo and payment or honoraria from Daiichi Sankyo, Bayer, Boehringer, Pfizer, Bristol-Meyers Squibb, Boston Scientific, and Medtronic. L.E. received consulting fees from Boston Scientific and lecture fees from various medical companies. G.B. received DSMB or advisory board fees from Charité and Asklepios Hamburg with support from Medtronic. A.M. received consulting fees from Medtronic and Biosense Webster and received lecture honoraria or travel support from Medtronic, LifeTech, Biosense-Webster, Daiichi Sankyo, Boston Scientific, Haemonetics, Bayer, Bristol Meyers Squibb, Atricure, and Pfizer.
Data Availability
Anonymized data may be provided upon reasonable request. Please contact AFNET info:info@kompetenznetz-vorhofflimmern.de. Source code will be made available at GitHub under UCCSHH by the University Center of Cardiovascular Science, Hamburg (https://github.com/UCCSHH).
Funding
The EAST-AFNET 4 trial and its biomolecule study were funded by Bundesministerium für Bildung und Forschung (BMBF), Deutsches Zentrum für Herz-Kreislauf-Forschung (DZHK), Atrial Fibrillation NETwork (AFNET), European Heart Rhythm Association (EHRA), St. Jude Medical–Abbott, Sanofi, and the German Heart Foundation. The biomolecule analysis was funded by EU 633196 [CATCH ME] and EU 965286 [MAESTRIA]. Further support came from EU IMI 116074 [BigData@Heart], British Heart Foundation (PG/20/22/35093; AA/18/2/34218), German Center for Cardiovascular Research supported by the German Ministry of Education and Research [DZHK, grant numbers DZHK (FKZ 81X2800182, 81Z0710116, and 81Z0710110)], German Research Foundation (Ki 509167694), and Leducq Foundation. AXAFA was funded by German Center for Cardiovascular Research (DZHK) and the BMS-Pfizer alliance. L.F. received institutional research grants by EU 633196 [CATCH ME] and EU 965286 [MAESTRIA]. British Heart Foundation (AA/18/2/34218), German Center for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK). U.S. reports grants of the Dutch Heart Foundation (CVON2014–09, RACE V Reappraisal of Atrial Fibrillation: Interaction between hyperCoagulability, Electrical remodeling, and Vascular Destabilisation in the Progression of AF, and grant number 01-002-2022-0118, EmbRACE: Electro-Molecular Basis and the theRapeutic management of Atrial Cardiomyopathy, fibrillation and associated outcomEs), EU 965286 [MAESTRIA]. R.B.S. has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under the grant agreement no. 648131, from the European Union’s Horizon 2020 research and innovation programme under the grant agreement no. 847770 (AFFECT-EU), from the European Union’s Horizon Europe research and innovation programme under the grant agreement ID: 101095480 and German Center for Cardiovascular Research (DZHK e.V.) (81Z1710103 and 81Z0710114); German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031L0239). Wolfgang Seefried project funding German Heart Foundation. A.G. has received research grants by EU 965286 [MAESTRIA]. L.E. received research support by DFG and German Heart Foundation. L.M. received institutional research and educational grants from Abbott Medical, Medtronic, Boston Scientific, and Johnson & Johnson. J.O. received research grants from German Heart Foundation, University of Hamburg and German Federal Ministry of Education and Research. A.Z. received grants to the institution for statistical analysis from EU Horizon 2020, Biotronik, and Adrenomed AG. T.Z. is funded by the German Research Foundation, the EU Horizon 2020 programme, the EU ERANet and ERAPreMed programmes, the German Centre for Cardiovascular Research (DZHK, 81Z0710102), and the German Ministry of Education and Research.
Ethical Approval
Derivation and validation cohorts have ethical approval. EAST-AFNET 4: Ethikkommission der Ärztekammer Westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms-Universität Münster, 09/02/2011, ref: 2010-274-f-A AXAFA-AFNET 5: Ethics Committee at the Medical Faculty of the University of Leipzig (Ethik-Kommission an der Medizinischen Fakultät der Universität Leipzig), 20/01/2015, ref: 341/14-ff BBC-AF: National Research Ethics Service Committee, Integrated Research Application System Identification: (IRAS ID 97753), REC12/WM/0344; 3 Dec 2012 TRUST: Ethikkommission der Ärztekammer Hamburg, 2020-10066-BO-ff, initial vote of 2021-01-25, first patient recruited 2021-03-17, 2nd amendment voted at 2023-5-11.
Pre-registered Clinical Trial Number
EAST-AFNET 4 ISRCTN number, ISRCTN04708680; ClinicalTrials.gov number, NCT01288352; EudraCT number, 2010-021258-20, AXAFA-AFNET 5 ISRCTN87711003, NCT 02227550, EudraCT number: 2014-002442-45.TRUST: NCT05521451.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 3. Linz D, Andrade JG, Arbelo E, Boriani G, Breithardt G, Camm AJ, et al. Longer and better lives for patients with atrial fibrillation: the 9th AFNET/EHRA consensus conference. Europace 2024;26:euae070. 10.1093/europace/euae070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckardt L, Sehner S, Suling A, Borof K, Breithardt G, Crijns H, et al. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST-AFNET 4 trial. Eur Heart J 2022;43:4127–44. 10.1093/eurheartj/ehac471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becher N, Metzner A, Toennis T, Kirchhof P, Schnabel RB. Atrial fibrillation burden: a new outcome predictor and therapeutic target. Eur Heart J 2024;45:2824–38. 10.1093/eurheartj/ehae373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrade JG, Deyell MW, Khairy P, Champagne J, Leong-Sit P, Novak P, et al. Atrial fibrillation progression after cryoablation versus radiofrequency ablation: the CIRCA-DOSE trial. Eur Heart J 2023:44;765–76. 10.1093/eurheartj/ehac692 [DOI] [PubMed] [Google Scholar]
- 7. Kuck KH, Lebedev DS, Mikhaylov EN, Romanov A, Geller L, Kalejs O, et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2021;23:362–9. 10.1093/europace/euaa298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag V, et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med 2022:388;105–16. 10.1056/NEJMoa2212540 [DOI] [PubMed] [Google Scholar]
- 9. Fabritz L, Guasch E, Antoniades C, Bardinet I, Benninger G, Betts TR, et al. Expert consensus document: defining the major health modifiers causing atrial fibrillation: a roadmap to underpin personalized prevention and treatment. Nat Rev Cardiol 2016;13:230–7. 10.1038/nrcardio.2015.194 [DOI] [PubMed] [Google Scholar]
- 10. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet 2018;50:1234–9. 10.1038/s41588-018-0171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154–62. 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Billing AM, Kim YC, Gullaksen S, Schrage B, Raabe J, Hutzfeldt A, et al. Metabolic communication by SGLT2 inhibition. Circulation 2024;149:860–84. 10.1161/CIRCULATIONAHA.123.065517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reyat J, Sommerfeld L, O'Reilly M, Roth Cardoso V, Thiemann E, Khan A, et al. PITX2-deficiency leads to atrial mitochondrial dysfunction. Cardiovasc Res 2024:cvae169. 10.1093/cvr/cvae169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 2015;12:230–43. 10.1038/nrcardio.2015.2 [DOI] [PubMed] [Google Scholar]
- 15. Spronk HM, De Jong AM, Verheule S, De Boer HC, Maass AH, Lau DH, et al. Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation. Eur Heart J 2017;38:38–50. 10.1093/eurheartj/ehw119 [DOI] [PubMed] [Google Scholar]
- 16. Reyat JS, Chua W, Cardoso VR, Witten A, Kastner PM, Kabir SN, et al. Reduced left atrial cardiomyocyte PITX2 and elevated circulating BMP10 predict atrial fibrillation after ablation. JCI Insight 2020;5:e139179. 10.1172/jci.insight.139179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fabritz L, Chua W, Cardoso VR, Al-Taie C, Borof K, Suling A, et al. Blood-based cardiometabolic phenotypes in atrial fibrillation and their associated risk: EAST-AFNET 4 biomolecule study. Cardiovasc Res 2024:120;855–68. 10.1093/cvr/cvae067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kany S, Al-Taie C, Roselli C, Pirruccello JP, Borof K, Reinbold C, et al. Association of genetic risk and outcomes in patients with atrial fibrillation: interactions with early rhythm control in the EAST-AFNET4 trial. Cardiovasc Res 2023;119:1799–810. 10.1093/cvr/cvad027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A, et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–55. 10.1093/eurheartj/ehy176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chua W, Khashaba A, Canagarajah H, Nielsen JC, di Biase L, Haeusler KG, et al. Disturbed atrial metabolism, shear stress, and cardiac load contribute to atrial fibrillation after ablation: AXAFA biomolecule study. Europace 2024;26:euae028. 10.1093/europace/euae028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G, et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur Heart J 2019;40:1268–76. 10.1093/eurheartj/ehy815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brady PF, Chua W, Nehaj F, Connolly DL, Khashaba A, Purmah YJV, et al. Interactions between atrial fibrillation and natriuretic peptide in predicting heart failure hospitalization or cardiovascular death. J Am Heart Assoc 2022;11:e022833. 10.1161/JAHA.121.022833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dixon WJ, Yuen KK. Trimming and winsorization: a review. Statistische Hefte 1974;15:157–70. 10.1007/BF02922904 [DOI] [Google Scholar]
- 24. Bates DMM, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 25. Figueiras A, Domenech-Massons JM, Cadarso C. Regression models: calculating the confidence interval of effects in the presence of interactions. Stat Med 1998;17:2099–105. [DOI] [PubMed] [Google Scholar]
- 26. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 27. van Buuren S, Karin G-O. mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 28. Dretzke J, Chuchu N, Agarwal R, Herd C, Chua W, Fabritz L, et al. Predicting recurrent atrial fibrillation after catheter ablation: a systematic review of prognostic models. Europace 2020;22:748–60. 10.1093/europace/euaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Team RC . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2022. http://www.R-project.org/. [Google Scholar]
- 30. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 31. Sohns C, Fox H, Marrouche NF, Crijns H, Costard-Jaeckle A, Bergau L, et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med 2023;389:1380–9. 10.1056/NEJMoa2306037 [DOI] [PubMed] [Google Scholar]
- 32. Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. 10.1056/NEJMoa2101897 [DOI] [PubMed] [Google Scholar]
- 33. Hijazi Z, Lindback J, Alexander JH, Hanna M, Held C, Hylek EM, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37:1582–90. 10.1093/eurheartj/ehw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hijazi Z, Oldgren J, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet 2016;387:2302–11. 10.1016/S0140-6736(16)00741-8 [DOI] [PubMed] [Google Scholar]
- 35. Pol T, Hijazi Z, Lindback J, Oldgren J, Alexander JH, Connolly SJ, et al. Using multimarker screening to identify biomarkers associated with cardiovascular death in patients with atrial fibrillation. Cardiovasc Res 2022;118:2112–23. 10.1093/cvr/cvab262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gkarmiris KI, Lindback J, Alexander JH, Granger CB, Kastner P, Lopes RD, et al. Repeated measurement of the novel atrial biomarker BMP10 (bone morphogenetic protein 10) refines risk stratification in anticoagulated patients with atrial fibrillation: insights from the ARISTOTLE trial. J Am Heart Assoc 2024;13:e033720. 10.1161/JAHA.123.033720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamaji T, Ishibashi M, Nakaoka H, Imataka K, Amano M, Fujii J. Possible role for atrial natriuretic peptide in polyuria associated with paroxysmal atrial arrhythmias. Lancet 1985;1:1211. 10.1016/S0140-6736(85)92883-1 [DOI] [PubMed] [Google Scholar]
- 38. Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation 1997;96:1686–95. 10.1161/01.CIR.96.5.1686 [DOI] [PubMed] [Google Scholar]
- 39. Walters TE, Lee G, Spence S, Larobina M, Atkinson V, Antippa P, et al. Acute atrial stretch results in conduction slowing and complex signals at the pulmonary vein to left atrial junction: insights into the mechanism of pulmonary vein arrhythmogenesis. Circ Arrhythm Electrophysiol 2014;7:1189–97. 10.1161/CIRCEP.114.001894 [DOI] [PubMed] [Google Scholar]
- 40. Wachter R, Lahno R, Haase B, Weber-Kruger M, Seegers J, Edelmann F, et al. Natriuretic peptides for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemia—the Find-AF study. PLoS One 2012;7:e34351. 10.1371/journal.pone.0034351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zografos T, Maniotis C, Katsivas A, Katritsis D. Relationship between brain natriuretic peptides and recurrence of atrial fibrillation after successful direct current cardioversion: a meta-analysis. Pacing Clin Electrophysiol 2014;37:1530–7. 10.1111/pace.12477 [DOI] [PubMed] [Google Scholar]
- 42. Asselbergs FW, van den Berg MP, Bakker SJ, Signorovitch JE, Hillege HL, van Gilst WH, et al. N-terminal pro B-type natriuretic peptide levels predict newly detected atrial fibrillation in a population-based cohort. Neth Heart J 2008;16:73–8. 10.1007/BF03086122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hwang HJ, Son JW, Nam BH, Joung B, Lee B, Kim JB, et al. Incremental predictive value of pre-procedural N-terminal pro-B-type natriuretic peptide for short-term recurrence in atrial fibrillation ablation. Clin Res Cardiol 2009;98:213–8. 10.1007/s00392-009-0744-3 [DOI] [PubMed] [Google Scholar]
- 44. Freestone B, Gustafsson F, Chong AY, Corell P, Kistorp C, Hildebrandt P, et al. Influence of atrial fibrillation on plasma von Willebrand factor, soluble E-selectin, and N-terminal pro B-type natriuretic peptide levels in systolic heart failure. Chest 2008;133:1203–8. 10.1378/chest.07-2557 [DOI] [PubMed] [Google Scholar]
- 45. Xu X, Tang Y. Relationship between brain natriuretic peptide and recurrence of atrial fibrillation after successful electrical cardioversion: an updated meta-analysis. Braz J Cardiovasc Surg 2017;32:530–5. 10.21470/1678-9741-2017-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Darkner S, Goetze JP, Chen X, Henningsen K, Pehrson S, Svendsen JH. Natriuretic propeptides as markers of atrial fibrillation burden and recurrence (from the AMIO-CAT trial). Am J Cardiol 2017;120:1309–15. 10.1016/j.amjcard.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 47. den Uijl DW, Delgado V, Tops LF, Ng AC, Boersma E, Trines SA, et al. Natriuretic peptide levels predict recurrence of atrial fibrillation after radiofrequency catheter ablation. Am Heart J 2011;161:197–203. 10.1016/j.ahj.2010.09.031 [DOI] [PubMed] [Google Scholar]
- 48. Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, et al. N-terminal pro-B-type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke 2014;45:1646–50. 10.1161/STROKEAHA.114.004712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schrage B, Geelhoed B, Niiranen TJ, Gianfagna F, Vishram-Nielsen JKK, Costanzo S, et al. Comparison of cardiovascular risk factors in European population cohorts for predicting atrial fibrillation and heart failure, their subsequent onset, and death. J Am Heart Assoc 2020;9:e015218. 10.1161/JAHA.119.015218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Svennberg E, Henriksson P, Engdahl J, Hijazi Z, Al-Khalili F, Friberg L, et al. N-terminal pro B-type natriuretic peptide in systematic screening for atrial fibrillation. Heart 2017;103:1271–7. 10.1136/heartjnl-2016-310236 [DOI] [PubMed] [Google Scholar]
- 51. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation 2009;120:1768–74. 10.1161/CIRCULATIONAHA.109.873265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Engdahl J, Svennberg E, Friberg L, Al-Khalili F, Frykman V, Kemp Gudmundsdottir K, et al. Stepwise mass screening for atrial fibrillation using N-terminal pro b-type natriuretic peptide: the STROKESTOP II study design. Europace 2017;19:297–302. 10.1093/europace/euw319 [DOI] [PubMed] [Google Scholar]
- 53. Parwani AS, Kaab S, Friede T, Tilz RR, Bauersachs J, Frey N, et al. Catheter-based ablation to improve outcomes in patients with atrial fibrillation and heart failure with preserved ejection fraction: rationale and design of the CABA-HFPEF-DZHK27 trial. Eur J Heart Fail 2024. 10.1002/ejhf.3373. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 54. Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell 2002;3:411–23. 10.1016/S1534-5807(02)00217-4 [DOI] [PubMed] [Google Scholar]
- 55. Chen JX, Zeng H, Reese J, Aschner JL, Meyrick B. Overexpression of angiopoietin-2 impairs myocardial angiogenesis and exacerbates cardiac fibrosis in the diabetic db/db mouse model. Am J Physiol Heart Circ Physiol 2012;302:H1003–12. 10.1152/ajpheart.00866.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang YQ, Li JJ, Hu L, Lee M, Karpatkin S. Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood 2002;99:1646–50. 10.1182/blood.V99.5.1646 [DOI] [PubMed] [Google Scholar]
- 57. Chua W, Cardoso VR, Guasch E, Sinner MF, Al-Taie C, Brady P, et al. An angiopoietin 2, FGF23, and BMP10 biomarker signature differentiates atrial fibrillation from other concomitant cardiovascular conditions. Sci Rep 2023;13:16743. 10.1038/s41598-023-42331-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bontekoe J, Lee J, Bansal V, Syed M, Hoppensteadt D, Maia P, et al. Biomarker profiling in stage 5 chronic kidney disease identifies the relationship between angiopoietin-2 and atrial fibrillation. Clin Appl Thromb Hemost 2018;24:269S–76S. 10.1177/1076029618808909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med 2006;12:1286–93. 10.1038/nm1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kumpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, et al. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 2008;12:R147. 10.1186/cc7130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Benz AP, Hijazi Z, Lindback J, Connolly SJ, Eikelboom JW, Kastner P, et al. Plasma angiopoietin-2 and its association with heart failure in patients with atrial fibrillation. Europace 2023;25:euad200. 10.1093/europace/euad200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kahr PC, Piccini I, Fabritz L, Greber B, Scholer H, Scheld HH, et al. Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. PLoS One 2011;6:e26389. 10.1371/journal.pone.0026389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang L, Rice M, Swist S, Kubin T, Wu F, Wang S, et al. BMP9 and BMP10 act directly on vascular smooth muscle cells for generation and maintenance of the contractile state. Circulation 2021;143:1394–410. 10.1161/CIRCULATIONAHA.120.047375 [DOI] [PubMed] [Google Scholar]
- 64. Hodgson J, Swietlik EM, Salmon RM, Hadinnapola C, Nikolic I, Wharton J, et al. Characterization of GDF2 mutations and levels of BMP9 and BMP10 in pulmonary arterial hypertension. Am J Respir Crit Care Med 2020;201:575–85. 10.1164/rccm.201906-1141OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Steimle JD, Canozo G, Park FJ, Kadow M, Samee ZA, Martin MAH, et al. Decoding the PITX2-controlled genetic network in atrial fibrillation. JCI Insight 2022;7:e158895. 10.1172/jci.insight.158895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hennings E, Aeschbacher S, Coslovsky M, Paladini RE, Meyre PB, Voellmin G, et al. Association of bone morphogenetic protein 10 and recurrent atrial fibrillation after catheter ablation. Europace 2023;25:euad149. 10.1093/europace/euad149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hennings E, Blum S, Aeschbacher S, Coslovsky M, Knecht S, Eken C, et al. Bone morphogenetic protein 10-A novel biomarker to predict adverse outcomes in patients with atrial fibrillation. J Am Heart Assoc 2023;12:e028255. 10.1161/JAHA.122.028255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Winters J, Kawczynski MJ, Gilbers MD, Isaacs A, Zeemering S, Bidar E, et al. Circulating BMP10 levels associate with late postoperative atrial fibrillation and left atrial endomysial fibrosis. JACC Clin Electrophysiol 2024;10:1326–40. 10.1016/j.jacep.2024.03.003 [DOI] [PubMed] [Google Scholar]
- 69. Hijazi Z, Benz AP, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW, et al. Bone morphogenetic protein 10: a novel risk marker of ischaemic stroke in patients with atrial fibrillation. Eur Heart J 2023;44:208–18. 10.1093/eurheartj/ehac632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Winters J, Isaacs A, Zeemering S, Kawczynski M, Maesen B, Maessen J, et al. Heart failure, female sex, and atrial fibrillation are the main drivers of human atrial cardiomyopathy: results from the CATCH ME Consortium. J Am Heart Assoc 2023;12:e031220. 10.1161/JAHA.123.031220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barallobre-Barreiro J, Gupta SK, Zoccarato A, Kitazume-Taneike R, Fava M, Yin X, et al. Glycoproteomics reveals decorin peptides with anti-myostatin activity in human atrial fibrillation. Circulation 2016;134:817–32. 10.1161/CIRCULATIONAHA.115.016423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ko D, Benson MD, Ngo D, Yang Q, Larson MG, Wang TJ, et al. Proteomics profiling and risk of new-onset atrial fibrillation: Framingham Heart Study. J Am Heart Assoc 2019;8:e010976. 10.1161/JAHA.118.010976 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data may be provided upon reasonable request. Please contact AFNET info:info@kompetenznetz-vorhofflimmern.de. Source code will be made available at GitHub under UCCSHH by the University Center of Cardiovascular Science, Hamburg (https://github.com/UCCSHH).