Abstract
Background and Aims
Long-term safety and efficacy of mavacamten in patients with obstructive hypertrophic cardiomyopathy (HCM) are unknown. MAVA-LTE (NCT03723655) is an ongoing, 5-year, open-label extension study designed to evaluate the long-term effects of mavacamten.
Methods
Participants from EXPLORER-HCM (NCT03470545) could enrol in MAVA-LTE upon study completion.
Results
At the latest data cut-off, 211 (91.3%) of the 231 patients originally enrolled in MAVA-LTE still received mavacamten. Median (range) time on study was 166.1 (6.0–228.1) weeks; 185 (80.1%) and 99 (42.9%) patients had completed the Week 156 and 180 visits, respectively. Sustained reductions from baseline to Week 180 occurred in left ventricular outflow tract gradients [mean (standard deviation): resting, −40.3 (32.7) mmHg; Valsalva, −55.3 (33.7) mmHg], N-terminal pro B-type natriuretic peptide [median (interquartile range): −562 (−1162.5, −209) ng/L], and EQ-5D-5L score [mean (standard deviation): 0.09 (0.17)]. Mean left ventricular ejection fraction (LVEF) decreased from 73.9% (baseline) to 66.6% (Week 24) and 63.9% (Week 180). At Week 180, 74 (77.9%) of the 95 patients improved by at least one New York Heart Association class from baseline. Over 739 patient-years exposure, 20 patients (8.7%; exposure-adjusted incidence: 2.77/100 patient-years) experienced 22 transient reductions in LVEF to <50% resulting in temporary treatment interruption (all recovered LVEF of ≥50%). Five (2.2%) patients died (all considered unrelated to mavacamten).
Conclusions
Long-term mavacamten treatment resulted in sustained improvements in cardiac function and symptoms in patients with obstructive HCM, with no new safety concerns identified. Transient, reversible reductions in LVEF were observed in a small proportion of patients during long-term follow-up.
Keywords: Obstructive hypertrophic cardiomyopathy, Mavacamten, MAVA-LTE (EXPLORER cohort), Safety, Efficacy, Long-term outcomes
Structured Graphical Abstract
Structured Graphical Abstract.
See the editorial comment for this article ‘Advances in the treatment of hypertrophic cardiomyopathy’, by E. Braunwald, https://doi.org/10.1093/eurheartj/ehae710.
Introduction
Hypertrophic cardiomyopathy (HCM) is a predominantly inherited myocardial disorder defined by left ventricular (LV) hypertrophy that cannot be explained by abnormal loading conditions.1–4 Obstructive HCM affects approximately two-thirds of patients with HCM and is characterized by a dynamic narrowing of the LV outflow tract (LVOT) due to septal hypertrophy combined with systolic anterior motion (SAM) and septal contact of the mitral valve.4–6 Common signs and symptoms of HCM include chest pain, dyspnoea, fatigue, and syncope.2,7 HCM often results in significant impairment of a patient’s functional capacity and quality of life.8
Mavacamten is the first and only cardiac myosin inhibitor approved in five continents for the treatment of adults with symptomatic New York Heart Association (NYHA) Class II–III obstructive HCM.9–14 The 2023 European Society of Cardiology and 2024 American Heart Association/American College of Cardiology guidelines recommend treatment with cardiac myosin inhibitors (such as mavacamten) for adults with obstructive HCM if they have persistent symptoms despite receiving beta-blocker (BB) or calcium channel blocker (CCB) therapy.2,4 In the pivotal randomized, phase 3 EXPLORER-HCM trial (NCT03470545), mavacamten was shown to be superior to placebo in achieving improvements in clinical measures, symptoms, and patient-reported outcomes in patients with obstructive HCM over 30 weeks. The safety and tolerability profiles of mavacamten in EXPLORER-HCM were considered similar to placebo.15
Despite the encouraging results observed in EXPLORER-HCM, the long-term safety and efficacy profiles of mavacamten remain unknown for this first-in-class, cardiac myosin inhibitor that has already entered real-world clinical practice in multiple countries. MAVA-Long-Term Extension (LTE) is an ongoing, 5-year, open-label, active-treatment, extension study (NCT03723655) that was designed to assess the long-term safety and efficacy of mavacamten in the EXPLORER cohort and has already provided short- and medium-term data on extended exposure to mavacamten.16
This publication presents the longest period of exposure to date of a cohort of patients with obstructive HCM treated with mavacamten, reporting updated cumulative results and additional analyses from patients who participated in EXPLORER-HCM and rolled over to MAVA-LTE. A particular focus of the publication is the implications of dosing stability over time; another focus is the sustained effects of long-term mavacamten treatment on outflow gradients and symptom improvement.
Methods
Study design, patients, and dosing
Details of the MAVA-LTE study design have been published previously.16 In brief, patients who completed the EXPLORER-HCM study, including the 8-week post-treatment washout period, had the option to enrol in MAVA-LTE (EXPLORER cohort), irrespective of the treatment received in the parent study (see Supplementary data online, Figure S1). Patients were screened for eligibility using either the results of assessments from the EXPLORER-HCM end-of-study visit (if the patient consented to MAVA-LTE within 28 days of EXPLORER-HCM study completion) or by a full screening visit before enrolment. Background HCM standard-of-care monotherapy with either BBs or CCBs (verapamil or diltiazem) was allowed, provided that the patient had been receiving a stable dose for at least 14 days before screening. Background therapy could be adjusted or discontinued after 24 weeks of mavacamten treatment at the investigator’s discretion. EXPLORER-HCM participants enrolled in MAVA-LTE received mavacamten once daily for a maximum of 252 weeks, irrespective of treatment in the parent study.
Full inclusion and exclusion criteria for patients enrolled in the EXPLORER-HCM trial have been published previously.15,17 Key inclusion criteria for participation in MAVA-LTE (EXPLORER cohort) included a documented resting LV ejection fraction (LVEF) reading of ≥50% by central laboratory-read echocardiography, safety laboratory parameters within normal limits at screening, and no pregnancy or lactation. Inclusion criteria from EXPLORER-HCM related to LVOT gradients and NYHA class did not apply in MAVA-LTE. Intracavity LVOT gradients did not form part of the entry criteria assessment for EXPLORER-HCM or MAVA-LTE. Key exclusion criteria included the following: persistent or permanent atrial fibrillation in patients not receiving anticoagulation for at least 4 weeks beforehand and/or not adequately rate-controlled; a history of syncope or sustained ventricular tachyarrhythmia with exercise between the parent study end-of-study visit and screening; treatment with disopyramide or ranolazine; and background combination therapy of BBs and verapamil or diltiazem.
Assessments of genotype to determine HCM gene pathogenicity and pharmacogenetic screening to determine the cytochrome P450 2C19 (CYP2C19) metabolizer phenotype of patients was performed in EXPLORER-HCM. CYP2C19 genotyping did not inform the dose titration strategy used in MAVA-LTE.
All patients received mavacamten 5 mg once daily as the starting dosage. Scheduled dose titrations occurred at Weeks 4, 8, and 12 based on site-read transthoracic echocardiogram measurements of Valsalva LVOT gradient and LVEF. At Week 4, mavacamten dose was decreased to 2.5 mg if a patient had a Valsalva LVOT gradient of ≤30 mmHg. At Weeks 8 and 12, mavacamten dose was increased by one level (i.e. 2.5 to 5 mg, 5 to 10 mg, and 10 to 15 mg) if Valsalva LVOT gradient was >30 mmHg and LVEF was ≥50%. Dose increases were possible at Week 24 if site-read post-exercise LVOT gradient was ≥50 mmHg or were possible at any time after Week 24 if site-read Valsalva LVOT gradient was >30 mmHg and LVEF was ≥50%, per investigator decision in conjunction with the sponsor medical monitor. After each dose increase, a follow-up visit was scheduled 28 (±7) days later to confirm that LVEF remained within normal range. Following the dose-titration period, patients had scheduled assessments every 12 weeks between the Week 24 and Week 156 visits, and every 24 weeks between the Week 156 and Week 252 visits.
Temporary treatment interruption and permanent treatment discontinuation criteria have also been published previously.16 Following a protocol amendment on 2 February 2022, the interval between the time of temporary treatment interruption due to an LVEF of <50% and the follow-up visit was extended from 2–4 to 4–6 weeks. The criteria for temporary treatment interruption were also revised in the amendment by removing interruption criteria related to mavacamten pharmacokinetics and QT interval corrected using Fridericia’s formula (QTcF) prolongation (except in Germany).
This study complies with the principles outlined in the Declaration of Helsinki. The research protocol was approved by the respective locally appointed ethics committees, and written informed consent was obtained from all patients in the study.
Study assessments
Site-read and central-read echocardiograms were performed at each visit to assess resting and Valsalva LVOT gradients and LVEF. Assessments at baseline included echocardiograms read only by the central laboratory. Left atrial volume index (LAVI), the ratio of early mitral inflow velocity and mitral annular early diastolic velocity (E/e′), posterior wall thickness, interventricular septum thickness, LV end-diastolic and end-systolic volume, LV stroke volume, and the presence or absence of SAM were assessed only by the central laboratory. Other efficacy assessments included serum N-terminal pro B-type natriuretic peptide (NT-proBNP) concentrations, the severity of heart failure assessed using the NYHA functional classification, and the Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness of Breath (HCMSQ SoB) domain score. The HCMSQ SoB domain score ranges from 0 to 18 and assesses patient-reported shortness of breath in a 7-day recall period (see Supplementary data online, Appendix S1).18 Lower scores indicate less symptoms whereas a higher score indicates a greater symptom burden. Participants’ reports of general health status were assessed using the EQ-5D-5L index score, ranging from 0 to 1 with a lower score indicating worse health status.
Safety endpoints included the frequency and severity of treatment-emergent adverse events (TEAEs), serious TEAEs, and deaths. Cardiovascular serious TEAEs of clinical interest were also reported and were defined as serious TEAEs related to major adverse cardiovascular events, atrial fibrillation, ventricular arrhythmias, syncope/pre-syncope, cardiac failure, hypotension, and QTcF prolongation.
The data cut-off date for the interim analysis reported here was 31 August 2023. When relevant, differences in efficacy and safety results between this analysis and a previous interim analysis (data cut-off: 31 August 2021; median time on study: 62.3 weeks) are presented.
Statistical analyses
All efficacy analyses were performed on the intention-to-treat population (all patients who were enrolled), and all safety analyses were performed on the safety population (all patients who received at least one dose of mavacamten). Descriptive statistics for efficacy parameters were provided by time point for the number of patients who had reached that time point at the data cut-off date and for mean change and median change from baseline with associated standard deviation (SD) and interquartile range (IQR), respectively. Descriptive statistics were also provided for safety parameters by counts and percentages. No formal hypothesis testing was performed for efficacy or safety analyses.
Results
Baseline characteristics
In total, 231 (94.7%) of the 244 patients who completed the EXPLORER-HCM study were enrolled in MAVA-LTE and constituted the intention-to-treat and safety populations for the study. Of the 231 patients enrolled in MAVA-LTE, 185 (80.1%) and 99 (42.9%) had completed Week 156 and Week 180 visits, respectively, by the 31 August 2023 interim analysis data cut-off date. At this date, 19 (8.2%) patients had permanently discontinued both treatment and study; reasons for treatment discontinuation were as follows: adverse events of ejection fraction decreased (n = 3), cardiac failure (n = 1), fatigue (n = 1), muscle weakness (n = 1), and systemic lupus erythematosus (n = 1); death (n = 5); lost to follow-up (n = 1); meeting stopping criteria (n = 3, of which n = 2 were due to LVEF-related results and n = 1 was due to QTcF-related results); and patient withdrawal (n = 3). One additional patient had permanently discontinued treatment but not study owing to an adverse event of acute myocardial infarction (see Supplementary data online, Figure S2). Thus, 211 (91.3%) of the 231 patients remained on treatment at the data cut-off date. Overall, the median (IQR) time on study was 166.1 (159.6, 189.3) weeks, resulting in an exposure of 739 patient-years. The majority of patients in the study were either CYP2C19 normal metabolizers [NMs; n = 86 (37.2%)] or intermediate metabolizers [n = 59 (25.5%)]; only three (1.3%) were CYP2C19 poor metabolizers (PMs). Other baseline demographics and disease characteristics of the EXPLORER cohort of MAVA-LTE are summarized in Table 1.
Table 1.
Baseline demographics and disease characteristics
| Parameter | EXPLORER cohort of MAVA-LTE (n = 231) |
|---|---|
| Age, years | 60.0 (11.9) |
| Female, n (%) | 91 (39.4) |
| BMI, kg/m2 | 29.7 (5.2) (n = 229) |
| History of atrial fibrillation, n (%) | 41 (17.7) |
| CYP2C19 metabolizer phenotype,a n (%) | |
| Poor metabolizer | 3 (1.3) |
| Intermediate metabolizer | 59 (25.5) |
| Normal metabolizer | 86 (37.2) |
| Rapid metabolizer | 53 (22.9) |
| Ultrarapid metabolizer | 7 (3.0) |
| Not poor metabolizerb | 4 (1.7) |
| Unknown | 19 (8.2) |
| HCM genetic testing,a n (%) | |
| Pathogenic | 49 (21.2) |
| Variant of uncertain significance | 67 (29.0) |
| Negative | 61 (26.4) |
| Unknown | 54 (23.4) |
| Background HCM therapy, n (%) | |
| Beta-blockersc | 176 (76.2) |
| Calcium channel blockersd | 38 (16.5) |
| NYHA class, n (%) | |
| Class I | 14 (6.1) |
| Class II | 152 (65.8) |
| Class III | 65 (28.1) |
| Received placebo in EXPLORER-HCM, n (%) | 116 (50.2) |
| NT-proBNP, ng/L, median (IQR) | 766.0 (323.0, 1593.0) (n = 230) |
| Resting LVOT gradient, mmHg | 48.3 (31.9) |
| Valsalva LVOT gradient, mmHg | 69.5 (33.3) (n = 228) |
| LVEF, % | 74.0 (5.9) (n = 230) |
| LAVI, mL/m2 | 38.3 (13.0) (n = 227) |
| E/e′ average ratio | 17.5 (6.9) (n = 222) |
| Time on study, weeks, median (range) | 166.1 (6.0–228.1) |
Data presented are mean (SD) unless otherwise stated. Baseline is defined as the last non-missing measurement before the first dose of mavacamten in MAVA-LTE.
BMI, body mass index; CYP2C19, cytochrome P450 2C19; E/e′, ratio between early mitral inflow velocity and mitral annular early diastolic velocity; HCM, hypertrophic cardiomyopathy; IQR, interquartile range; LAVI, left atrial volume index; LTE, Long-Term Extension; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
aData were collected in the parent study.
b‘Not poor metabolizer’ is defined as any patient who was identified as not being a poor metabolizer but for whom a definitive cumulative CYP2C19 phenotype could not be established during genotyping.
cBeta-blocker use at baseline derived from concomitant medication data in MAVA-LTE.
dCalcium channel blocker use was limited to diltiazem and verapamil.
Cardiac function, symptoms, and patient-reported outcomes
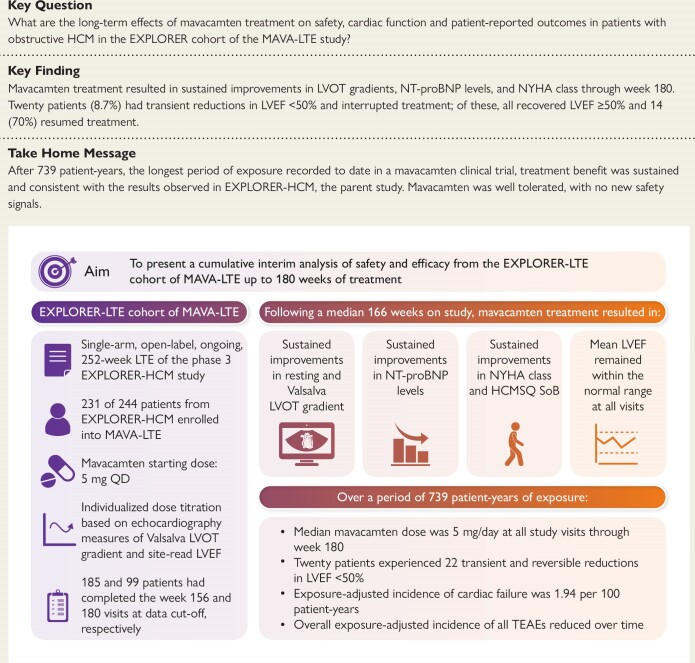
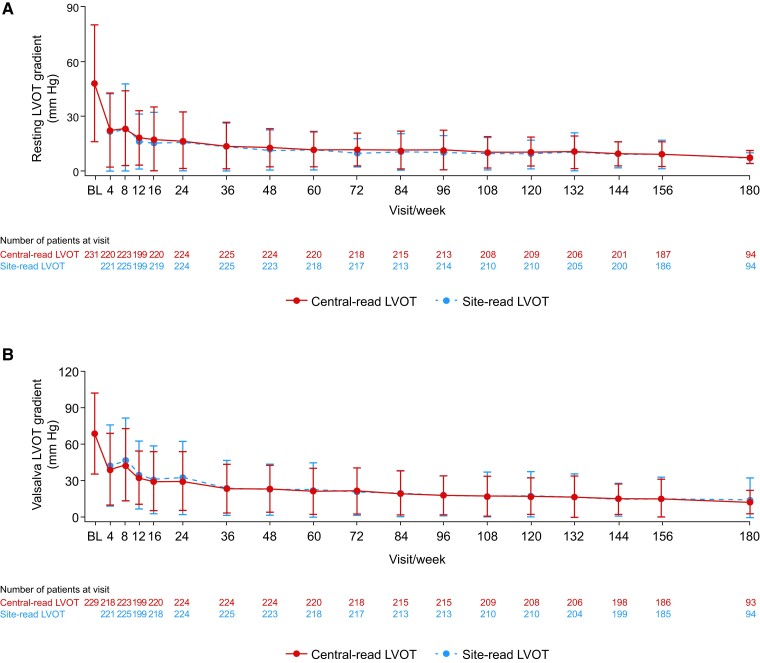
The notable improvements in resting and Valsalva LVOT gradients observed in EXPLORER-HCM were sustained in the current interim analysis of MAVA-LTE through Weeks 156 and 180, as confirmed by site- and central-read echocardiograms (Figure 1). The mean (SD) change in central-read resting LVOT gradient from baseline was −40.2 (32.8) mmHg at Week 156 (n = 187) and −40.3 (32.7) mmHg at Week 180 (n = 94); the mean (SD) changes in central-read Valsalva LVOT gradients from baseline during the same weeks were −55.3 (37.3) mmHg (n = 184) and −55.3 (33.7) mmHg (n = 91), respectively. In total, 191 (82.7%) patients achieved a central-read Valsalva LVOT gradient of ≤30 mmHg and remained at or below the 30 mmHg threshold until the data cut-off (Figure 2). For these patients, the median (range) duration from baseline to achieving a central-read Valsalva LVOT gradient of ≤30 mmHg was 74.1 (2.7–207.3) weeks.
Figure 1.
Mean (standard deviation) resting left ventricular outflow tract (A) and Valsalva left ventricular outflow tract (B) gradients over time. Baseline values represent those from the beginning of MAVA-LTE, not the beginning of EXPLORER-HCM. For re-enrolled patients, the baseline value is considered the first measurement following re-enrolment. The number of patients at each time point reflects the number of evaluable results from those who had reached the stated week by the interim cut-off date. BL, baseline; HCM, hypertrophic cardiomyopathy; LTE, Long-Term Extension; LVOT, left ventricular outflow tract
Figure 2.
Proportion of patients who achieved a Valsalva left ventricular outflow tract gradient of ≤30 mmHg by visit. The values above the bars indicate the n (%) of patients who achieved a Valsalva left ventricular outflow tract gradient of ≤30 mmHg at each time point
Lateral E/e′ ratio decreased from baseline to Week 144 and Week 180 [mean (SD) change in lateral E/e′ ratio: −3.7 (6.4), n = 181, and −5.1 (6.6), n = 88, respectively]. Reductions in LAVI from baseline to Week 144 and Week 180 were also found [mean (SD) change in LAVI: −3.5 (10.4) mL/m2, n = 193, and −5.5 (9.7) mL/m2, n = 62, respectively) (see Supplementary data online, Figure S3). The median (IQR) change in posterior wall thickness from baseline was −0.5 (−1.9, 0.9) mm at Week 144 (n = 192) and 0.4 (−0.9, 1.6) mm at Week 180 (n = 89). The median (IQR) change in interventricular septum thickness from baseline was −1.0 (−2.8, 1.0) mm at Week 144 (n = 198) and 0.5 (−1.0, 2.4) mm at Week 180 (n = 92). Median (IQR) increases from baseline for LV end-diastolic and end-systolic volume were observed at Week 156 [6.5 (−4.3, 25.0) mL, n = 175, and 8.5 (2.6, 18.2) mL, n = 175, respectively] and Week 180 [LV end-diastolic volume: 9.5 (−4.3, 22.0) mL, n = 93, and LV end-systolic volume: 8.6 (3.1, 17.8) mL, n = 93]. LV stroke volume was relatively consistent from baseline to Weeks 156 and 180 [median (IQR) change in LV stroke volume: 0.06 (−9.3, 8.3) mL, n = 175, and −0.8 (−10.2, 6.9) mL, n = 93, respectively]. The proportion of patients with SAM continued to decrease from 77.6% (177/228 patients) at baseline to 14.0% (18/129 patients) at Week 156 and 5.3% (5/94 patients) at Week 180.
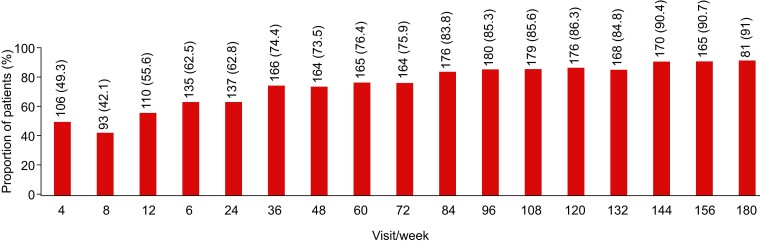
Mavacamten treatment resulted in a reduction in median (IQR) plasma NT-proBNP concentrations from 766 (323, 1593) ng/L at baseline (n = 230) to 118 (58, 304) ng/L at Week 180 (n = 89) (Figure 3). At baseline, 22 (9.6%) of the 230 patients with evaluable data had a NT-proBNP concentration of <124 ng/L (indicative of NT-proBNP levels being in normal range). The proportion of patients with NT-proBNP levels < 124 ng/L increased to 28.4% at Week 4 (62/218 patients), 43.2% at Week 156 (80/185 patients), and 53.8% at Week 180 (50/93 patients). The median (IQR) change in NT-proBNP concentrations from baseline was −504 (−1160, −143) ng/L at Week 156 (n = 179) and −562 (−1162.5, −209) ng/L at Week 180 (n = 88).
Figure 3.
Median (interquartile range) N-terminal pro B-type natriuretic peptide over time. Baseline values represent those from the beginning of MAVA-LTE, not the beginning of EXPLORER-HCM. For re-enrolled patients, the baseline value is considered the first measurement following re-enrolment. The number of patients at each time point reflects the number of evaluable results from those who had reached the stated week by the interim cut-off date. BL, baseline; HCM, hypertrophic cardiomyopathy; LTE, Long-Term Extension; NT-proBNP, N-terminal pro B-type natriuretic peptide
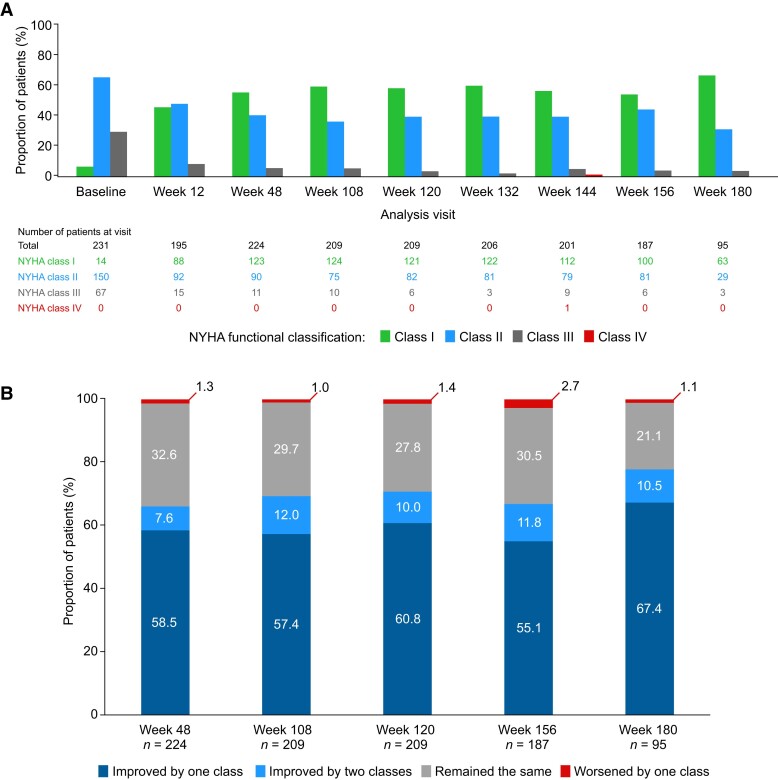
In addition to sustained improvements in LVOT gradients, lateral E/e′, LAVI, and NT-proBNP levels, continued improvements in NYHA class over time were observed (Figure 4). At Week 180, 64/95 (67.4%) patients and 10/95 (10.5%) patients had improved by one and two classes from baseline, respectively. Consequently, most patients were NYHA Class I at Week 180 [63 patients (66.3%)]. Of the 224 patients for whom data were available at Week 48, 123 (54.9%) were NYHA Class I; of these, 60 continued to remain in NYHA Class I until their latest available assessment at the data cut-off date. Overall, 108 (46.8%) patients achieved a complete response—defined as achieving NYHA Class I and a Valsalva LVOT gradient of ≤30 mmHg—during the study and retained a complete response until the data cut-off. At Week 180, three patients (3.2%) were NYHA Class III. Of these three patients, two were NYHA Class III at baseline, and one worsened from NYHA Class II at baseline to NYHA Class III at Week 180. This patient, at the time of NYHA class worsening, had ongoing atrial fibrillation (deemed to be unrelated to the study drug) and had been receiving a stable dose of mavacamten 15 mg for 168 weeks. The patient had reductions in LVOT gradient from baseline to week 180 (resting LVOT gradient decreased from 50.7 to 7.1 mmHg and Valsalva LVOT gradient decreased from 58.1 to 8.3 mmHg) but had a marked increase in NT-proBNP concentrations from baseline (556 ng/L) to Week 180 (2774 ng/L). At Week 144, one patient was NYHA Class IV for a single visit. The patient had been in NYHA Class I from baseline to Week 132, but experienced a serious TEAE of atrial fibrillation with rapid ventricular response and TEAEs of extreme fatigue and shortness of breath which were reported at Week 144. This patient subsequently improved to NYHA Class II at Week 156.
Figure 4.
New York Heart Association class distribution over time (A) and change in New York Heart Association class from baseline (B). The number of patients at each time point refers to the number of patients who had a visit scheduled at each week and for whom data are available. NYHA, New York Heart Association
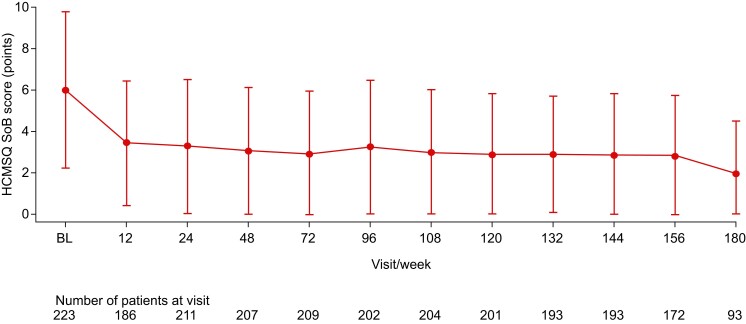
Rapid improvement in the HCMSQ SoB domain score was observed with mavacamten treatment during the first 12 weeks [mean (SD) change from baseline: −2.7 (3.3) points, n = 181] and was sustained through to Week 156 [−3.4 (3.7) points, n = 167] and Week 180 [−3.7 (3.7) points, n = 90] (Figure 5). The EQ-5D-5L index score followed a similar trend to the HCMSQ SoB domain score, showing improvement during the first 12 weeks [mean (SD) change from baseline: 0.08 (0.16) points, n = 188] which was maintained up to Week 180 [0.09 (0.17) points, n = 92] (see Supplementary data online, Figure S4).
Figure 5.
Mean (standard deviation) Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness of Breath domain score over time. The number of patients at each time point refers to the number of patients who had a visit scheduled at each week and for whom data are available. Lower scores indicate less shortness of breath. When the mean value minus the standard deviation value was <0, the lower error bar was set to 0. BL, baseline; HCMSQ SoB, Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness of Breath
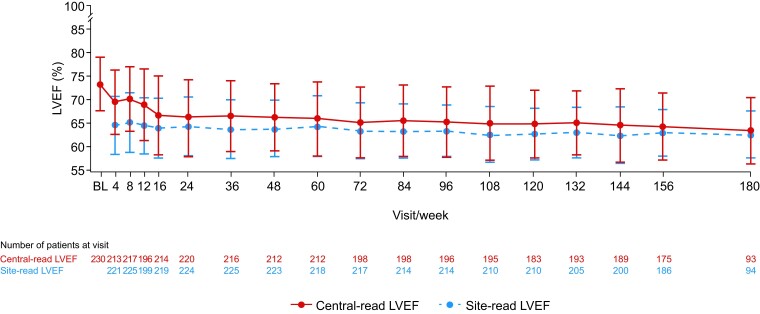
Decreases were observed in central-read LVEF from baseline to Week 156 [mean (SD) change: −9.6% (8.3%), n = 175] and Week 180 [−11.0% (8.9%), n = 93] (Figure 6). At every study visit, the mean site-read LVEF was between 62.9% and 65.7% and the mean central-read LVEF was between 63.9% and 73.9%.
Figure 6.
Mean (standard deviation) left ventricular ejection fraction over time. Baseline values represent those from the beginning of MAVA-LTE, not the beginning of EXPLORER-HCM. For re-enrolled patients, the baseline value is considered the first measurement following re-enrolment. The number of patients at each time point reflects the number of evaluable quantitative results from those who had reached the stated week by the interim cut-off date. Some central-read LVEF measurements were qualitative and, therefore, did not contribute to the overall mean LVEF value for the study visit. BL, baseline; HCM, hypertrophic cardiomyopathy; LTE, Long-Term Extension; LVEF, left ventricular ejection fraction
Tolerability results
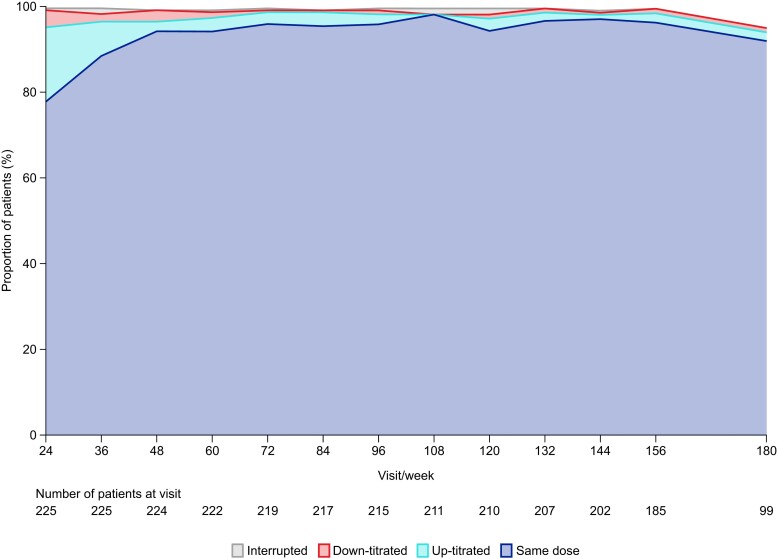
After dose titration, the proportion of patients receiving mavacamten 2.5, 5, 10, and 15 mg doses at Week 24 was 33.5%, 22.8%, 26.3%, and 17.4%, respectively. At Week 180, the proportion of patients receiving mavacamten 2.5, 5, 10, and 15 mg doses was 25.5%, 31.9%, 29.8%, and 12.8%, respectively. Among the 94 patients with available data who reached the Week 180 visit, 65 (69.1%) received the same dose at Week 24 (first visit following initiation of the maintenance phase) and Week 180. At Week 24 and beyond, of the 223 patients for whom data were available, 62 (27.8%) had 1 dose change and 33 (14.8%) had 2 or more dose changes. Of these 33 patients, 8 (24.2%) had their dose down-titrated once owing to having experienced an event of LVEF < 50% at Week 24 or beyond. From Week 48 onwards, at least 90% of patients received the same dose as they received at the previous visit (Figure 7). The mavacamten dose received by the three CYP2C19 PMs included in the study was stable from Week 24 to their latest available assessment at the data cut-off date (5 mg, n = 2; 2.5 mg, n = 1). The median mavacamten dose among patients with available data was 5 mg at all study visits through to Week 180.
Figure 7.
Change in mavacamten dose by study visit. The number of patients at each time point refers to the number of patients who had a study visit scheduled at each week and for whom data are available. Categories relate to the change in mavacamten dose from the previous visit. For Week 24, the previous visit was Week 16
At baseline, 176 patients (76.2%) were receiving BB therapy and 38 (16.5%) CCB therapy, and 17 (7.4%) were not on background obstructive HCM therapy (Table 1). At the current interim analysis data cut-off, seven patients had discontinued BB therapy and six patients had discontinued CCB therapy [n = 13 (5.6% of participants)] and were receiving only mavacamten (median dose at data cut-off: 5 mg).
Safety results
Treatment-emergent adverse events
A summary of TEAEs and serious TEAEs experienced by patients in the study is presented in Table 2. In total, 33 patients (14.3%) experienced atrial fibrillation episodes (15 of whom had a history of atrial fibrillation at time of enrolment) and 14 patients (6.1%) experienced cardiac failure episodes during the study (only one of these patients had a concomitant LVEF of <50%). Since the previous interim analysis data cut-off date (24 months of extended follow-up), 12 additional patients experienced atrial fibrillation and 6 additional patients experienced cardiac failure.
Table 2.
Summary of treatment-emergent adverse events and serious treatment-emergent adverse events up to the 31 August 2023 data cut-off
| Number (%) of patients | Number of events | |
|---|---|---|
| Any TEAE | 228 (98.7) | 1870 |
| Severity | ||
| Mild | 66 (28.6) | 1283 |
| Moderate | 119 (51.5) | 506 |
| Severe | 39 (16.9) | 77 |
| Fatal | 4 (1.7) | 4 |
| Drug-related TEAE | 54 (23.4) | 128 |
| Cardiovascular drug–related TEAE of clinical interest | 28 (12.1) | 40 |
| Serious TEAE | 63 (27.3) | 117 |
| Cardiovascular serious TEAE of clinical interesta | 28 (12.1) | 40 |
| Drug-related serious TEAE | 10 (4.3) | 10b |
| LVEF < 50% | 20 (8.7) | 22c |
| LVEF < 40% | 6 (2.6) | 6c |
| Death | 5 (2.2) | 5d,e |
Treatment-emergent adverse events were recorded and defined based on the discretion of the principal investigator.
LVEF, left ventricular ejection fraction; QTcF, QT interval corrected using Fridericia’s formula; TEAE, treatment-emergent adverse event.
aDefined as serious TEAEs related to major adverse cardiovascular events, atrial fibrillation, ventricular arrhythmias, syncope/pre-syncope, cardiac failure, hypotension, and QTcF prolongation.
bIncludes cardiac failure (n = 3), ejection fraction decreased (n = 5), atrial fibrillation (n = 1), and atrial flutter (n = 1).
cNumber of events that occurred while patients were receiving treatment at the time of the event.
dOwing to bacterial endocarditis (n = 1), cardiac arrest (n = 1), acute myocardial infarction (n = 1; sudden death without an autopsy performed), intracerebral haemorrhage due to arteriovenous malformation (n = 1), and progression of liver metastases with cholangitis and new onset biliary dilatation (n = 1); all unrelated to treatment.
eOf the five patients who died, one did not experience an individual TEAE considered to be of fatal severity.
In total, 10 patients (4.3%) received new implantable cardioverter-defibrillators during the study; all were indicated for primary prevention of sudden cardiac death.
Exposure-adjusted safety
Overall, the exposure-adjusted incidence of all TEAEs from Day 1 to Week 252 of the study was 174.6 per 100 patient-years, which is a decrease from 187.7 per 100 patient-years between Day 1 and Week 60 in the study (Table 3). Analysis of specific TEAEs showed that the exposure-adjusted incidence of atrial fibrillation and cardiac failure, respectively, reduced from 6.57 and 3.55 per 100 patient-years between Day 1 and Week 60 to 4.50 and 1.94 between Day 1 and Week 252.
Table 3.
Incidence and exposure-adjusted incidence of treatment-emergent adverse events
| Number (%) of patients | Exposure-adjusted incidence per 100 PY from Day 1 to Week 60 | Exposure-adjusted incidence per 100 PY from Day 1 to Week 252 | |
|---|---|---|---|
| Patients with ≥1 TEAE | 228 (98.7) | 187.7 | 174.6 |
| Cardiac-related TEAEs | |||
| Hypertension | 36 (15.6) | 7.67 | 5.35 |
| Atrial fibrillation | 33 (14.3) | 6.57 | 4.50 |
| Dyspnoea | 23 (10.0) | 3.59 | 3.11 |
| Palpitations | 16 (6.9) | 3.95 | 2.10 |
| Cardiac failure | 14 (6.1) | 3.55 | 1.94 |
| Ejection fraction decreased | 13 (5.6) | 2.12 | 1.51 |
| Other TEAEs | |||
| COVID-19 infection | 92 (39.8) | 6.80 | 15.06 |
| Dizziness | 41 (17.7) | 8.43 | 6.13 |
| Nasopharyngitis | 36 (15.6) | 7.83 | 5.41 |
| Fatigue | 29 (12.6) | 8.17 | 4.27 |
| Back pain | 24 (10.4) | 5.03 | 3.46 |
| Arthralgia | 24 (10.4) | 4.32 | 3.45 |
| Headache | 23 (10.0) | 7.35 | 3.34 |
Table includes treatment-emergent adverse events of any grade reported in ≥10% of patients, except for cardiac failure, ejection fraction decreased, and palpitations, for which the proportions of patients were <10%. Treatment-emergent adverse events were recorded and defined based on the discretion of the principal investigator.
PY, patient-years; TEAE, treatment-emergent adverse event.
Serious treatment-emergent adverse events
In the safety population (n = 231), 117 serious TEAEs occurred in 63 (27.3%) patients, 40 cardiovascular serious TEAEs of clinical interest occurred in 28 (12.1%) patients, and 10 drug-related serious TEAEs occurred in 10 (4.3%) patients. The 10 serious TEAEs considered to be related to study drug were cardiac failure (n = 3), ejection fraction decreased (n = 5), atrial fibrillation (n = 1), and atrial flutter (n = 1). In all five patients who experienced drug-related serious TEAEs of ejection fraction decreased, LVEF recovered to ≥50% after treatment interruption or discontinuation. At the data cut-off date, 6 of the 10 patients who experienced drug-related serious TEAEs remained on treatment. Details of the five additional patients who experienced a drug-related serious TEAE since the previous interim analysis are provided in Supplementary data online, Appendix S2. No CYP2C19 PM experienced a serious TEAE during the study.
Protocol-defined treatment interruptions
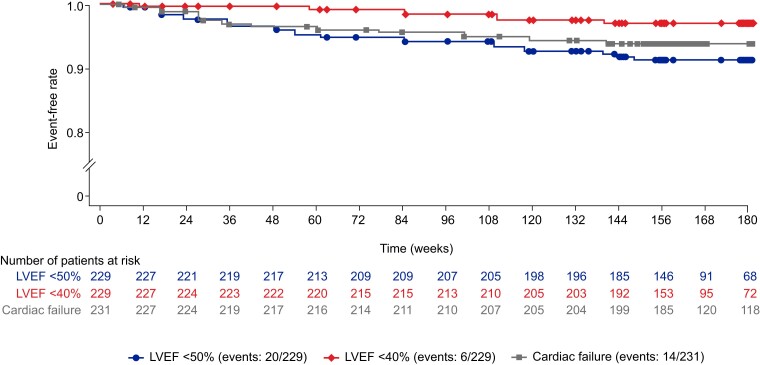
Overall, 20 patients (8.7%) experienced 22 transient reductions in site-read LVEF of <50% (range, 30%–48%) and interrupted treatment—an increase of 8 patients since the previous data cut-off (see Supplementary data online, Table S1). This equates to an exposure-adjusted incidence for LVEF <50% of 2.77 per 100 patient-years. Of these 20 patients, 14 resumed treatment and 6 discontinued treatment (one of whom was re-enrolled at a later date). Six of the 20 patients (2.6% of the overall study cohort) experienced an LVEF of <40%. The median (range) time from treatment initiation to an LVEF of <50% in the 20 patients was 48 (4–144) weeks, whereas the median (range) time from treatment interruption to recovery to an LVEF of ≥50% was 4 (1–12) weeks. One (5%) of the 20 patients experienced a serious TEAE of cardiac failure which was considered a precipitating event of LVEF <50%; this TEAE was considered unrelated to treatment and was resolved 8 days before the transient reduction in LVEF. For further information on these 20 patients, see Supplementary data online, Appendix S3. No additional events of an LVEF of <50% or <40% occurred from Week 156 onwards (Figure 8 and Supplementary data online, Figure S5). No patient experienced an LVEF of <30% during treatment in the study (see Supplementary data online, Figure S6).
Figure 8.
Event-free rate of occurrences of left ventricular ejection fraction <50%, left ventricular ejection fraction <40%, and treatment-emergent adverse events of cardiac failure over time. For patients with <56 days between enrolment periods, events contribute based on initial dosing (left ventricular ejection fraction <50% and left ventricular ejection fraction <40% analyses). LVEF, left ventricular ejection fraction
In total, 10 patients (4.3%) temporarily interrupted treatment owing to a mavacamten plasma concentration >1000 ng/mL before its removal as a protocol-defined treatment interruption criterion on 2 February 2022.
Treatment discontinuations
In total, 13 patients (5.6%) experienced TEAEs that resulted in permanent treatment discontinuation (see Supplementary data online, Table S2). Of these 13 patients, 4 were CYP2C19 NMs, 3 were intermediate metabolizers, 3 were rapid metabolizers, 1 was an ultrarapid metabolizer, none were PMs, and 2 did not have their metabolizer genotype recorded. All five deaths that occurred during the study were considered to be unrelated to mavacamten treatment by investigators.
Two (0.9%) of the 231 patients were recorded as having undergone septal reduction therapy on study Days 54 and 78 after permanent discontinuation of treatment. In each case, the decision to undergo septal reduction therapy was not due to a failure to respond to mavacamten treatment. For further information on these two patients, see Supplementary data online, Appendix S4.
Discussion
In this updated cumulative analysis of the EXPLORER cohort of the ongoing MAVA-LTE study, mavacamten treatment was associated with sustained improvements in echocardiographic parameters, cardiac biomarkers, symptoms, and patient-reported outcomes in patients with obstructive HCM over a long-term treatment period (Structured Graphical Abstract). The present study adds to our understanding of the long-term favourable safety and efficacy profile of mavacamten and demonstrates a decrease in the incidence of TEAEs with prolonged exposure.
Overall, 91.3% of patients who enrolled in the EXPLORER cohort of MAVA-LTE remained on treatment at the latest data cut-off date of 31 August 2023, thus demonstrating the high retention of patients on study over a median period of 166.1 weeks (range: 6.0–228.1 weeks). The present safety analysis indicates that mavacamten treatment is generally well tolerated over a long-term follow-up, with 33 patients with TEAEs of atrial fibrillation, 14 patients with TEAEs of cardiac failure, and 10 patients with drug-related serious TEAEs reported after 739 patient-years of exposure. Of note, the proportion of patients who experienced a TEAE of atrial fibrillation in this study (14%) was lower than the rate of atrial fibrillation observed in a large retrospective study of patients with HCM (18%).19 Furthermore, the proportion of patients without a medical history of atrial fibrillation who experienced a TEAE of atrial fibrillation in MAVA-LTE (7.8%) was similar to that observed in the EMPEROR-Preserved trial comparing empagliflozin with placebo in patients with heart failure with preserved ejection fraction (8.0%; median follow-up of 26.2 months).20,21 Nevertheless, the exposure-adjusted incidence of atrial fibrillation, cardiac failure, and ejection fraction decreased in the present analysis was lower than that observed in the previous interim analysis of MAVA-LTE,16 and the overall exposure-adjusted incidence of all TEAEs reduced over time. Furthermore, the incidence of events of an LVEF of <50% in the study population declined in frequency with time.16 All patients who experienced an LVEF of <50% or <40% subsequently recovered to an LVEF of ≥50% at a later visit following treatment interruption. As of the current interim analysis data cut-off date, 15 (75%) of the 20 patients who experienced an LVEF of <50% remain on treatment. Analysis of the characteristics of the 20 patients who experienced an LVEF of <50% in the EXPLORER cohort of MAVA-LTE revealed that there was no obvious association between transient lowering of LVEF and patients receiving a specific dose or possessing a specific pathogenic gene variant or CYP2C19 metabolizer genotype. Most patients who experienced an LVEF of <50% also experienced TEAEs at the time of the event, with atrial fibrillation or flutter (n = 8) being the most common concurrent illness observed, which likely acted as a trigger for LVEF < 50% in these patients. The proportion of patients who experienced a reduction in LVEF to <40% was low [n = 6 (2.6%)], no patient experienced an LVEF of <30% during treatment, and only one patient with an LVEF of <50% had a precipitating TEAE of cardiac failure. The results indicate that the three participants who were CYP2C19 PMs successfully tolerated long-term mavacamten treatment; all three received stable mavacamten doses of 5 mg (n = 2) or 2.5 mg (n = 1) and were still receiving treatment at the data cut-off date. All five deaths recorded were considered to be unrelated to the study drug. Overall, no new safety concerns were identified. It should be noted that the safety analysis included data from Week 180—the first visit that occurred after the longer-term follow-up interval of 24 weeks—and beyond. Therefore, these results provide further evidence that mavacamten maintains a favourable safety profile (irrespective of CYP2C19 metabolizer phenotype) in a large cohort of patients with obstructive HCM over long-term treatment.
The considerable reductions in LVOT gradients, E/e′, and LAVI from baseline observed at short- and medium-term persisted through Weeks 156 and 180.15,16 Results from a randomized crossover trial in patients with NYHA Class II–III obstructive HCM previously demonstrated that metoprolol reduced LVOT gradients and improved quality of life compared with placebo, but had no effect on exercise capacity or NT-proBNP levels.22,23 In this study, a reduction of approximately 85% in median NT-proBNP concentrations from baseline to Week 180 indicated that long-term treatment with mavacamten decreased NT-proBNP concentrations to normal levels.23 Overall, improvements in echocardiographic parameters and NT-proBNP levels, in addition to improvements in NYHA functional class and patient-reported outcome scores, demonstrate the sustained efficacy of mavacamten in patients with obstructive HCM over the highest cumulative drug exposure (739 patient-years) recorded in a mavacamten clinical trial to date. Furthermore, the high proportions of patients who improved to NYHA Class I at Week 180 from baseline (67.4%) and who achieved and sustained up to their latest available assessment a Valsalva LVOT gradient of ≤30 mmHg (82.7%) and complete response (46.8%) suggest that long-term mavacamten treatment resulted in most patients experiencing resolution of LVOT obstruction and relief of symptoms.
Routine echocardiographic assessments of patients treated with mavacamten are required to monitor safety and efficacy. Consistent with the known mechanism of action of mavacamten,24 a reduction in mean LVEF from baseline to Weeks 156 and 180 was observed. Mean site-read and central-read LVEF values at these time points were similar to the corresponding values in the previous analysis,16 suggesting that the reduction in LVEF stabilized after the initial 24-week dose-titration period.
Dose distribution in this study revealed that 69% of patients were receiving the same dose at Week 180 as the one after the Week 24 assessment, and ≥90% of patients received the same dose from visit to visit from Week 48 onwards. This analysis provided further evidence that monitoring of site-read measures of efficacy (Valsalva LVOT gradient) and safety (LVEF) for the purposes of dose optimization was sufficient to provide the correct dose in a majority of patients, without consideration of mavacamten plasma concentration or knowledge of CYP2C19 phenotype. The results observed following the first 24-week interval between visits in this study also provide initial evidence of the potential feasibility of reducing the frequency of echocardiographic assessments during long-term mavacamten therapy.
The findings of this interim analysis should be interpreted in the context of some limitations. Although the change from baseline results could be used to establish improvements in efficacy parameters from the beginning of the study, the design of MAVA-LTE as an open-label, single-arm study prevents the comparison with a control arm. However, the results observed in MAVA-LTE up to Weeks 156 and 180 are broadly similar to those observed in the 30-week, randomized, placebo-controlled parent study.15 Although approximately 80% of participants reached Week 156 by the data cut-off date, fewer than half of the patients who currently remain on treatment in the study had reached Week 180 by the data cut-off date. Some of the parameters presented here that were measured by echocardiography (e.g. posterior wall thickness and interventricular septum thickness) are more accurately measured and characterized by cardiac magnetic resonance imaging. A cardiac magnetic resonance study in a subset of patients in MAVA-LTE is currently ongoing to assess the long-term effects of mavacamten on cardiac remodelling.
In conclusion, over a 166-week median follow-up period, long-term treatment with mavacamten resulted in sustained and clinically relevant improvements in LVOT gradients and several other echocardiographic measurements, NT-proBNP levels, NYHA class, and quality of life as measured by patient-reported outcomes in patients with obstructive HCM. Furthermore, long-term treatment with mavacamten was well tolerated, with minimal changes in dose across the study population after the dose-titration period and with an observed reduction in the incidence of TEAEs with extended follow-up. Transient reductions in LVEF were observed in a small proportion of patients during long-term therapy, but all cases were reversible following treatment interruption.
Supplementary Material
Acknowledgements
The authors thank all patients who participated in this study and their families. They also thank the clinical study teams who participated for their contributions. All authors contributed to and approved the manuscript. Medical writing and editorial support was provided by Thomas Crighton, PhD, of Oxford PharmaGenesis, Oxford, UK, funded by Bristol Myers Squibb, Princeton, NJ, USA.
Contributor Information
Pablo Garcia-Pavia, Department of Cardiology, Hospital Universitario Puerta de Hierro Majadahonda, IDIPHISA, CIBERCV, Manuel de Falla 2, 28222, Madrid, Spain; Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain; Universidad Francisco de Vitoria (UFV), Pozuelo de Alarcon, Spain.
Artur Oręziak, Department of Arrhythmia, National Institute of Cardiology, Warsaw, Poland.
Ahmad Masri, Division of Cardiovascular Medicine, School of Medicine, Oregon Health & Science University, Portland, OR, USA.
Roberto Barriales-Villa, Inherited Cardiovascular Diseases Unit, Complexo Hospitalario Universitario A Coruña, INIBIC, CIBERCV (ISCIII), A Coruña, Spain.
Theodore P Abraham, School of Medicine, University of California San Francisco, San Francisco, CA, USA.
Anjali T Owens, Center for Inherited Cardiac Disease, Division of Cardiovascular Medicine, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, USA.
Morten K Jensen, School of Medicine, Aarhus University Hospital, Aarhus, Denmark.
Wojciech Wojakowski, Chief Division of Cardiology and Structural Heart Diseases, Medical University of Silesia, Katowice, Poland.
Tim Seidler, Department of Cardiology and Pulmonology, University of Göttingen, Göttingen, Germany; Cardiology, Kerckhoff Clinic, Bad Nauheim, Germany.
Albert Hagege, AP-HP, Hôpital Européen Georges Pompidou, Paris, France.
Neal K Lakdawala, Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Andrew Wang, Cardiology, Duke University Hospital, Durham, NC, USA.
Matthew T Wheeler, Cardiovascular Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Lubna Choudhury, Cardiovascular Medicine, Northwestern University, Feinberg School of Medicine, Chicago, IL, USA.
Ganesh Balaratnam, Bristol Myers Squibb, Princeton, NJ, USA.
Ashish Shah, Bristol Myers Squibb, Princeton, NJ, USA.
Shawna Fox, Bristol Myers Squibb, Princeton, NJ, USA.
Sheila M Hegde, Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Iacopo Olivotto, Cardiology Unit, Meyer Children’s Hospital, IRCCS, Florence, Italy.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
P.G.-P. has received consulting fees from BioMarin Pharmaceutical, Bristol Myers Squibb, Cytokinetics, Lexeo Therapeutics, and Rocket Pharmaceuticals and has received speaker fees from Bristol Myers Squibb. A.O. has received personal fees from Bristol Myers Squibb and has received investigator fees from Cytokinetics. A.M. has received research grants from Attralus, Cytokinetics, Ionis Pharmaceuticals/Akcea Therapeutics, Pfizer, Ultromics, and the Wheeler Foundation and has received consulting fees from Alnylam Pharmaceuticals, Attralus, Bristol Myers Squibb, Cytokinetics, Eidos Therapeutics, Haya Therapeutics, Intellia Therapeutics, Ionis Pharmaceuticals, Lexicon Pharmaceuticals, Pfizer, and Tenaya Therapeutics. R.B.-V. has received advisory board and personal fees from Amicus Therapeutics, Bristol Myers Squibb, Cytokinetics, Pfizer, and Sanofi. A.T.O. has received consulting fees from Alexion Pharmaceuticals, BioMarin Pharmaceuticals, Bristol Myers Squibb, Cytokinetics, Edgewise Therapeutics, Imbria, Lexeo Therapeutics, Pfizer, Renovacor, Stealth BioTherapeutics, and Tenaya Therapeutics and has served on the executive committee of the Hypertrophic Cardiomyopathy Medical Society. M.K.J. has received lecture fees from Bristol Myers Squibb. W.W. has received speaker fees from Bristol Myers Squibb. T.S. has received grants, advisory board fees, consulting fees, and/or lecture fees from Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Corvia, Cytokinetics, Edwards Life Sciences, Medtronics, MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb, Novartis, Pfizer, and Teleflex. A.H. has received consultant/advisor fees from Alnylam Pharmaceuticals, Amicus Therapeutics, Bayer, Bristol Myers Squibb, MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb, Pfizer, and Sanofi Genzyme and has received steering committee fees for SEQUOIA-HCM from Cytokinetics. N.K.L. has received consulting fees from Bristol Myers Squibb, Cytokinetics, Pfizer, Sarepta Therapeutics, and Tenaya Therapeutics; has received grants from Pfizer; and has served as the Vice Chair for the American Heart Association Council on Scientific Sessions Planning, Genomics and Precision Medicine Section. A.W. has received advisory board and consulting fees from Bristol Myers Squibb and Cytokinetics; has received grants, personal fees, and fees for presenting from Bristol Myers Squibb; and has served on the executive committee of the Hypertrophic Cardiomyopathy Medical Society. M.T.W. has received personal fees and research support from Bristol Myers Squibb. G.B., A.S., and S.F. are employees of Bristol Myers Squibb and own stock in Bristol Myers Squibb. S.M.H. serves on the faculty of the Cardiovascular Imaging Core Laboratory at Brigham and Women’s Hospital, and her institution has received payments for her core laboratory services from Bristol Myers Squibb and Cytokinetics. I.O. has received grants from Amicus Therapeutics, Boston Scientific, Bristol Myers Squibb, Cytokinetics, Genzyme, and Menarini International and has received consulting fees from Amicus Therapeutics, Cytokinetics, Genzyme, MS Pharma, Rocket Pharmaceuticals, and Tenaya Therapeutics. T.P.A. and L.C. have no relevant competing interests to disclose.
Data Availability
Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosurecommitment.html.
Funding
This study was supported by Bristol Myers Squibb, Princeton, NJ, USA.
Ethical Approval
This study complies with the principles outlined in the Declaration of Helsinki. The research protocol was approved by the respective locally appointed ethics committees, and written informed consent was obtained from all patients in the study.
Pre-registered Clinical Trial Number
The pre-registered clinical trial number is NCT03723655.
References
- 1. Alamo L, Ware JS, Pinto A, Gillilan RE, Seidman JG, Seidman CE, et al. Effects of myosin variants on interacting-heads motif explain distinct hypertrophic and dilated cardiomyopathy phenotypes. eLife 2017;6:e24634. 10.7554/eLife.24634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, et al. 2023 ESC guidelines for the management of cardiomyopathies: developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur Heart J 2023;44:3503–626. 10.1093/eurheartj/ehad194 [DOI] [PubMed] [Google Scholar]
- 3. Garfinkel AC, Seidman JG, Seidman CE. Genetic pathogenesis of hypertrophic and dilated cardiomyopathy. Heart Fail Clin 2018;14:139–46. 10.1016/j.hfc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice guidelines. J Am Coll Cardiol 2024;83:2324–405. 10.1016/j.jacc.2024.02.014 [DOI] [PubMed] [Google Scholar]
- 5. Schultze M, Zema C, Carroll R, Hurst M, Borchert J, Zhong Y, et al. Population estimates of obstructive and non-obstructive hypertrophic cardiomyopathy in the UK and Germany. Eur Heart J 2022;43:ehac544.1747. 10.1093/eurheartj/ehac544.1747 [DOI] [Google Scholar]
- 6. Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006;114:2232–9. 10.1161/CIRCULATIONAHA.106.644682 [DOI] [PubMed] [Google Scholar]
- 7. Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 2017;121:749–70. 10.1161/CIRCRESAHA.117.311059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zaiser E, Sehnert AJ, Duenas A, Saberi S, Brookes E, Reaney M. Patient experiences with hypertrophic cardiomyopathy: a conceptual model of symptoms and impacts on quality of life. J Patient Rep Outcomes 2020;4:102. 10.1186/s41687-020-00269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. CAMZYOS (mavacamten) . US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214998s000lbl.pdf (05 June 2024, date last accessed).
- 10. CAMZYOS (mavacamten) . Australian product information. https://apps.medicines.org.au/files/bqpcamzo.pdf (05 June 2024, date last accessed).
- 11. CAMZYOS (mavacamten) . Canadian product monograph. https://www.bms.com/assets/bms/ca/documents/productmonograph/CAMZYOS_EN_PM.pdf (05 June 2024, date last accessed).
- 12. CAMZYOS (mavacamteno) . Brazilian bula profissional. https://www.bms.com/assets/bms/brazil/documents/Camzyos_Bula_Profissional.pdf (05 June 2024, date last accessed).
- 13. CAMZYOS (mavacamtenum) . Professional information, Swissmedic. https://www.swissmedic.ch/swissmedic/en/home/about-us/publications/public-summary-swiss-par/public-summary-swiss-par-camzyos.html (05 June 2024, date last accessed).
- 14. CAMZYOS (mavacamten) . European summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/camzyos-epar-product-information_en.pdf (05 June 2024, date last accessed).
- 15. Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020;396:759–69. 10.1016/S0140-6736(20)31792-X [DOI] [PubMed] [Google Scholar]
- 16. Rader F, Oręziak A, Choudhury L, Saberi S, Fermin D, Wheeler MT, et al. Mavacamten treatment for symptomatic obstructive hypertrophic cardiomyopathy: interim results from the MAVA-LTE study, EXPLORER-LTE cohort. JACC Heart Fail 2024;12:164–77. 10.1016/j.jchf.2023.09.028 [DOI] [PubMed] [Google Scholar]
- 17. Ho CY, Olivotto I, Jacoby D, Lester SJ, Roe M, Wang A, et al. Study design and rationale of EXPLORER-HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy. Circ Heart Fail 2020;13:e006853. 10.1161/CIRCHEARTFAILURE.120.006853 [DOI] [PubMed] [Google Scholar]
- 18. Reaney M, Allen V, Sehnert AJ, Fang L, Hagège AA, Naidu SS, et al. Development of the hypertrophic cardiomyopathy symptom questionnaire (HCMSQ): a new patient-reported outcome (PRO) instrument. Pharmacoecon Open 2022;6:563–74. 10.1007/s41669-022-00335-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siontis KC, Geske JB, Ong K, Nishimura RA, Ommen SR, Gersh BJ. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc 2014;3:e001002. 10.1161/JAHA.114.001002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 21. Filippatos G, Farmakis D, Butler J, Zannad F, Ferreira JP, Ofstad AP, et al. Empagliflozin in heart failure with preserved ejection fraction with and without atrial fibrillation. Eur J Heart Fail 2023;25:970–7. 10.1002/ejhf.2861 [DOI] [PubMed] [Google Scholar]
- 22. Dybro AM, Rasmussen TB, Nielsen RR, Andersen MJ, Jensen MK, Poulsen SH. Randomized trial of metoprolol in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2021;78:2505–17. 10.1016/j.jacc.2021.07.065 [DOI] [PubMed] [Google Scholar]
- 23. Welsh P, Campbell RT, Mooney L, Kimenai DM, Hayward C, Campbell A, et al. Reference ranges for NT-proBNP (N-terminal pro-B-type natriuretic peptide) and risk factors for higher NT-proBNP concentrations in a large general population cohort. Circ Heart Fail 2022;15:e009427. 10.1161/CIRCHEARTFAILURE.121.009427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grillo MP, Erve JCL, Dick R, Driscoll JP, Haste N, Markova S, et al. In vitro and in vivo pharmacokinetic characterization of mavacamten, a first-in-class small molecule allosteric modulator of beta cardiac myosin. Xenobiotica 2019;49:718–33. 10.1080/00498254.2018.1495856 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosurecommitment.html.