Abstract

To discover novel antiviral agents, based on the high antiviral activity of (4-oxo-4H-quinolin-1-yl)-acetic acid hydrazide (C), a series of 4-oxo-4H-quinoline acylhydrazone derivatives were designed, synthesized, and first evaluated for their antiviral and fungicidal activities. Most acylhydrazone derivatives exhibited moderate to good antiviral activities in vivo. The inactive, curative, and protective activities of compounds 4 (51.2, 47.6, and 46.3%), 11 (49.6, 43.0, and 45.2% at 500 mg/L), and 17 (47.1, 49.2, and 44.1%) were higher than those of ribavirin (39.2, 38.0, and 40.8%) at 500 mg/L. Molecular docking showed that compound 4 exhibited a stronger affinity to TMV coat protein (TMV-CP) than ribavirin, with a binding energy (−6.89 kcal/mol) slightly lower than that of ribavirin (−6.08 kcal/mol). Microscale thermophoresis showed that compound 4 (Kd = 0.142 ± 0.060 μM) exhibited a strong binding ability to TMV-CP, superior to that of ribavirin (Kd = 0.512 ± 0.257 μM). The results of transmission electron microscopy showed that compound 4 hindered the self-assembly and growth of TMV. The antifungal activities of most compounds were moderate at 50 mg/L, among which compounds 12 and 21 exhibited a 72.1 and 76.5% inhibitory rate against Physalospora piricola, respectively. Meanwhile, compound 16 exhibited a 60% inhibitory rate against Cercospora arachidicola Hori at 50 mg/L.
Introduction
Plant virus diseases cause approximately 5 billion euros in economic losses to world agriculture every year.1 As a widely distributed virus worldwide, tobacco mosaic virus (TMV) can infect various Solanaceae plants, and its infection is difficult to control in the field. The actual inhibitory effect of commercially available TMV inhibitor Ningnanmycin is lower than 60% in the field.2 Meanwhile, its use is limited by water stickiness and photosensitivity.3 Based on this, it is necessary to develop efficient TMV inhibitors.
Due to their low toxicities, easy decompositions, and unique action mechanism, more and more natural-product-based antiviral agents have been developed and their antiviral mechanism has been investigated in recent years,4 including α-amino phosphonates derivatives,5,6 chalcone derivatives,7,8 dithioacetal derivatives,9−11 myricetin derivatives,12,13 ferulic acid derivatives,14−16 indole derivatives,17,18 limonin derivatives,19 and so forth. However, few antiviral drugs are used in the field due to cost, synthetic complexity, and stability.20,21 Thus, it is still necessary to develop highly stable, easy to synthesize, and efficient plant-based virus inhibitors.
Quinoline alkaloids are widely distributed in nature and drugs, which exhibited broad insecticidal, antifungal, antiviral, antimalarial, anticancer, and anti-inflammatory activities.22,23 Dictamnine (I) extracted from D. dasycarpus exhibited feeding deterrent activity against adults and larvae of T. castaneum as well as S. zeamais adults with EC50 values of 57.6, 47.9, and 91.7 ppm, respectively.24 Liu et al. synthesized quinoline alkaloids II (EC50 = 0.41 μg/mL) and III (EC50 = 0.55 μg/mL) which displayed superior in vitro fungicidal activities against Sclerotinia sclerotiorum.25 Quinoline tricyclic derivative IV exhibited anti-HCV (Hepacivirus) (EC50 = 3.1 μM) and anti-BVDV (Pestivirus) (EC50 = 1.2 μM) activities.26 Wolf et al. synthesized quinoline derivative V which exhibited submicromolar antimalarial activity versus HB3 (chloroquine sensitive) (IC50 = 17.5 nM) and Dd2 (chloroquine-resistant strains of Plasmodium falciparum) (IC50 = 22.7 nM) parasites.27 Compound VI displayed promising cytotoxicity against PC-3 (IC50 = 3.12 μM), DU-145, NCIH460, and 4 T1 cell lines.28 Compound VII could inhibit TNF-α formation with an IC50 value of 2.3 μM.29
Acylhydrazone structure is commonly used in drug design. In 2013, Kaplancikli et al. found that compound VIII exhibited larvicidal activity with LD50 values of 57.4 and 4.35 ppm and LD90 values of 297.8 and 19.1 ppm, respectively, at 24 and 48 h post treatment.30 In 2019, Yang et al. synthesized compound IX which exhibited higher curative, protective, and inactive activities against TMV and CMV than those of ningnanmycin.31 In 2024, Wu et al. found that arylhydrazone derivative X exhibited anti-TMV curative activity (EC50 = 139 μg/mL), which was similar to that of ningnanmycin32 (Figure 1).
Figure 1.
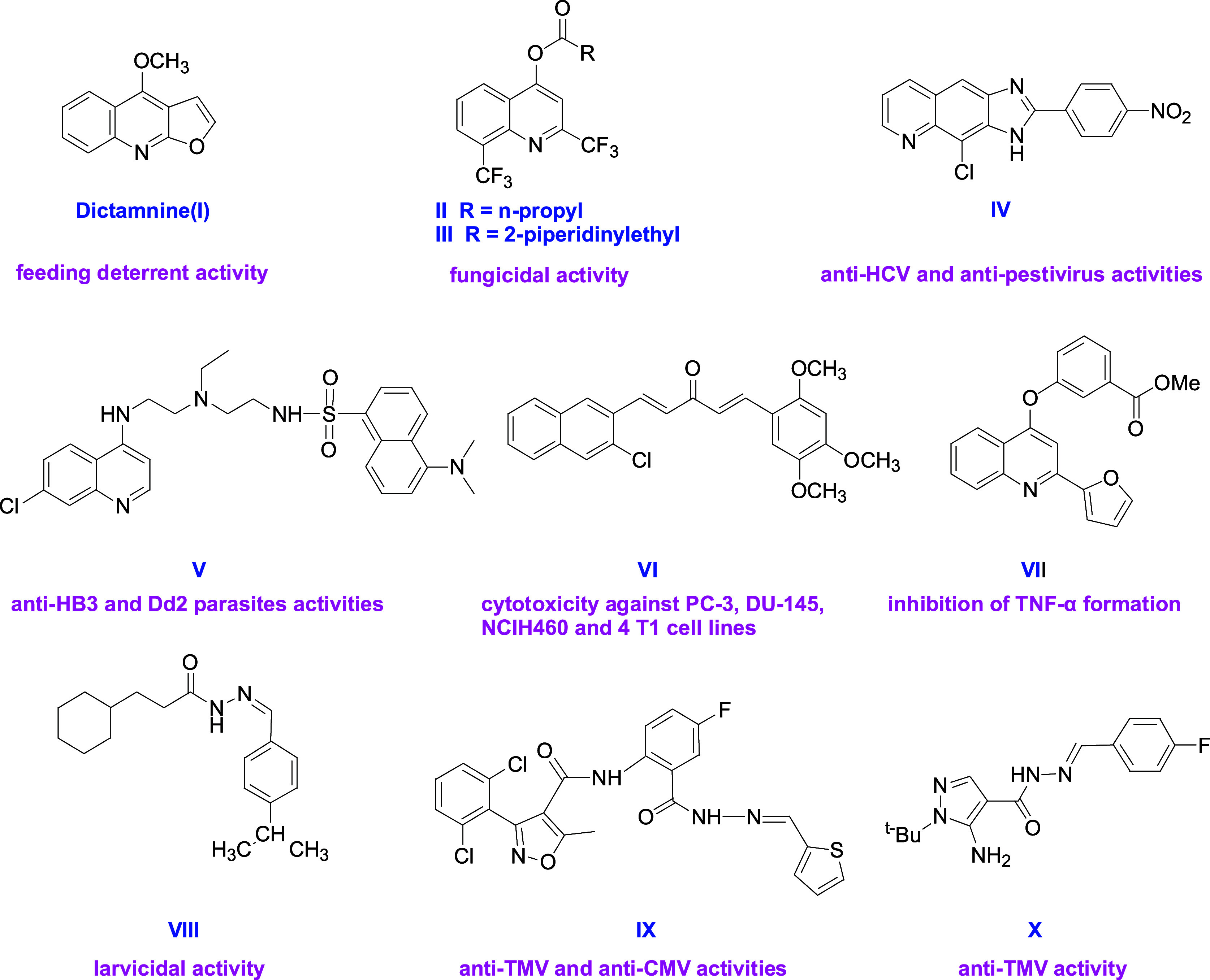
Natural products and drugs containing a quinoline or acylhydrazone structure.
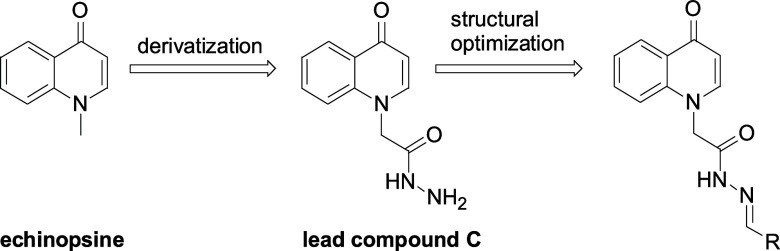
In our previous work, we found that the inactive, curative, and protective activities of echinopsine against TMV were 49.5, 46.1, and 42.6% at 500 mg/L, respectively.33 Further studies indicated that the inactive, curative, and protective activities of compound C (45.9, 31.5, and 47.8%) were also higher than those of commercial ribavirin (39.2, 38.0, and 40.8%) at 500 mg/L. Based on the broad spectrum bioactivities of quinoline and acylhydrazone structures, using compound C as the leading compound, a series of 4-oxo-4H-quinolin-1-yl acylhydrazone derivatives were designed and synthesized (Figure 2). Their anti-TMV and fungicidal activities were evaluated for the first time.
Figure 2.
Design of target compounds.
Materials and Methods
Instruments
1H NMR spectra were recorded at 400 MHz using a Bruker AV400 spectrometer in a CDCl3 or DMSO-d6 solution with tetramethylsilane as the internal standard. High-resolution mass spectrometry (HRMS) data were obtained on a Thermo Q Exactive Focus-MS instrument. The melting points were determined on an SWG X-4 binocular microscope melting point apparatus without correction.
General Synthesis
Ribavirin (Topscience Co., Ltd.), chlorothalonil (Bailing Agrochemical Co., Ltd.), carbendazim (Bailing Agrochemical Co., Ltd.), and other reagents were purchased from commercial sources and used as received. Echinopsine was synthesized according to literature.34 All anhydrous solvents were dried and purified according to the standard techniques.
Synthesis of (4-Oxo-4H-quinolin-1-yl)-acetic Acid Ethyl Ester (B)
To a round bottomed flask was added N,N-dimethylformamide (100 mL), compound A (4.35 g, 30 mmol), potassium carbonate (6.22 g, 45 mmol), and ethyl bromoacetate (8.02 g, 48 mmol). The reaction mixture was stirred for 12 h at room temperature. Water was added and the reaction mixture was extracted with dichloromethane three times. The organic phase was combined, washed with brine, dried over anhydrous Na2SO4, and evaporated under reduced pressure. The residue was subjected to column chromatography eluted with dichloromethane/methanol (v/v, 20/1) to give compound B as a white solid (5.36 g, 77.4%); mp 157–158 °C. 1H NMR (400 Hz, CDCl3): δ 8.45 (d, J = 8.0 Hz, 1H), 7.66–7.62 (m, 1H), 7.47 (d, J = 7.6 Hz, 1H), 7.40–7.37 (m, 1H), 7.19 (d, J = 8.4 Hz, 1H), 6.29 (d, J = 7.6 Hz, 1H), 4.77 (s, 2H), 4.24 (q, J = 7.2 Hz, 2H), 1.25 (t, J = 7.2 Hz, 3H). 13C NMR (100 Hz, CDCl3): δ: 178.4, 167.2, 143.7, 140.2, 132.5, 127.3, 127.1, 124.0, 114.6, 110.8, 62.4, 54.0, 14.1. ESI-HRMS (m/z): calcd for C13H14NO3 [M + H]+, 232.0974; found, 232.0965.
Synthesis of (4-Oxo-4H-quinolin-1-yl)-acetic Acid Hydrazide (C)
Compound B (2.31 g, 10 mmol) and hydrazine hydrate (5 g, 80%, 100 mmol) were dissolved in methanol (300 mL). The reaction mixture was refluxed until compound B was completely consumed. The reaction mixture was cooled to room temperature, and methanol was evaporated under reduced pressure until a large amount of white solid precipitated. The residue was filtered under reduced pressure to afford compound C as a white solid (1.78 g, 81.8%). mp 240–241 °C. 1H NMR (400 Hz, DMSO-d6): δ 9.53 (s, 1H), 8.17 (d, J = 7.2 Hz, 1H), 7.92 (d, J = 7.6 Hz, 1H), 7.72–7.68 (m, 1H), 7.44 (d, J = 8.8 Hz, 1H), 7.39–7.35 (m, 1H), 6.07 (d, J = 7.6 Hz, 1H), 4.86 (s, 2H), 4.36 (s, 2H). 13C NMR (100 Hz, DMSO-d6): δ 176.5, 166.0, 145.7, 140.3, 132.0, 126.5, 125.7, 123.3, 116.0, 108.7, 52.8. ESI-HRMS (m/z): calcd for C11H12N3O2 [M + H]+, 218.0930; found, 218.0924.
General Procedure for the Preparation of Compounds 1–25
The mixture of methanol (50.0 mL), compound C (1 mmol), and aldehydes D1–D25 (1 mmol) was refluxed for 12 h. Then, the reaction mixture was cooled to room temperature, and methanol was evaporated under reduced pressure until a large amount of solid precipitated. The residue was filtered under reduced pressure and the precipitate was washed with methanol to afford compounds 1–25. The data of compounds 1–25 are presented in the Supporting Information.
Biological Assay
The anti-TMV and fungicidal activities of compounds were tested according to the methods reported in the literature,35,36 which can also be found in the Supporting Information.
Morphological Observation via Transmission Electron Microscopy
The samples were treated according to the method reported in the literature.37 The support membrane was dipped in the compound solution for 10 s, then redyed using phosphotungstic acid, dried, and then observed under a transmission electron microscope. The details can be found in the Supporting Information.
Molecular Docking
Molecular docking was performed on the MOE software. The structure preparation function in the MOE was used to prepare the protein structure. The preparation process included assessing the quality of the protein-structure data using defined temperature factors, protein geometry checks, and electron-density checks; adding hydrogen atoms and optimizing their positions; and performing the final energy minimization of the structure. Next, these ligands were minimized by the Amber 10 force field.
Following this, molecular docking was performed with the dock application of the MOE, with two rounds of calculation. A collection of poses were generated from the pool of ligand conformations using the Triangle Matcher method and were further refined using the rigid receptor method in MOE. Finally, the generalized-Born/volume-integral implicit-solvent model developed by Labute, GBVI/WSA dG, was used for scoring each of the generated poses. For each compound, the pose with the lowest score was retained. The compounds were ranked lowest to highest on the basis of their scores.
Microscale Thermophoresis
TMV coat protein (TMV-CP) was purified according to the procedure reported in a previous study.38 The required concentration of compound 4 was mixed with 10–2 mL of the same volume of labeled protein (TMV-CP) and incubated for 5 min. Samples were loaded into special glass capillaries, and the dissociation constant (Kd) values of compounds were measured at 25 °C via microscale thermophoresis using the Monolith NT.115 software (Nano Temper Technologies).39,40 Additional details can be found in the Supporting Information.
Results and Discussion
Synthesis of Target Compounds
The synthetic route of 4-oxo-4H-quinolin-1-yl acylhydrazone is given in Figure 3. The nucleophilic substitution of compound A and ethyl bromoacetate afforded compound B in 77.4% yield, which reacted with hydrazine hydrate to afford compound C in 81.8% yield. The condensation of compound C and aldehydes D1–D25 afford compounds 1–25 in 42.8–95.5% yields, respectively. Compounds 1–25 precipitated from methanol, which made the purification of these compounds easy and suitable for large-scale production.
Figure 3.
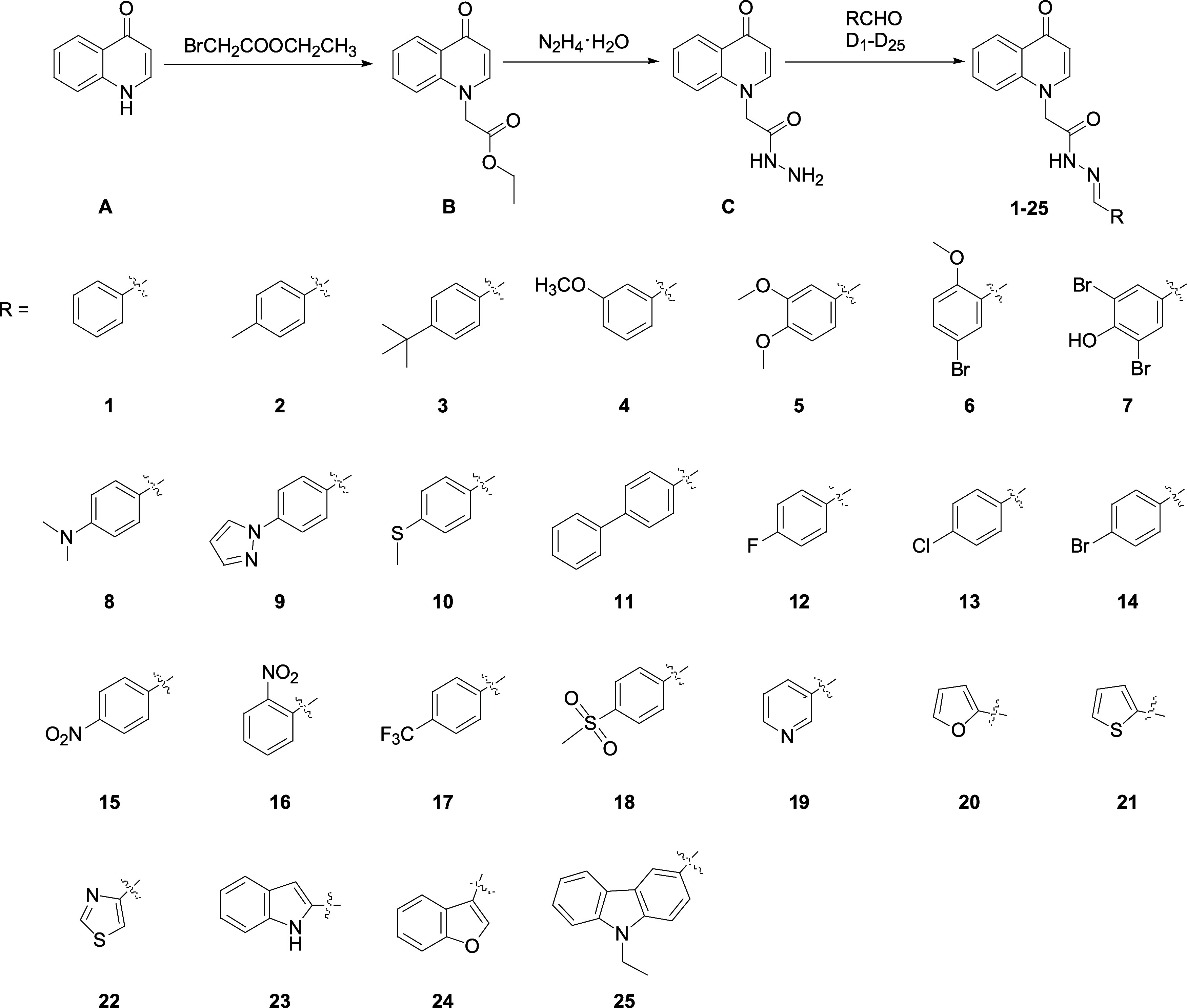
Synthesis of 4-oxo-4H-quinolin-1-yl acylhydrazone.
Configuration of Compounds 1–25
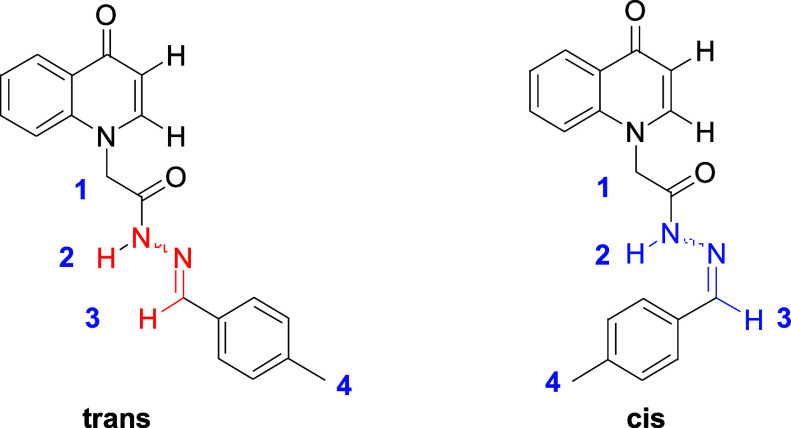
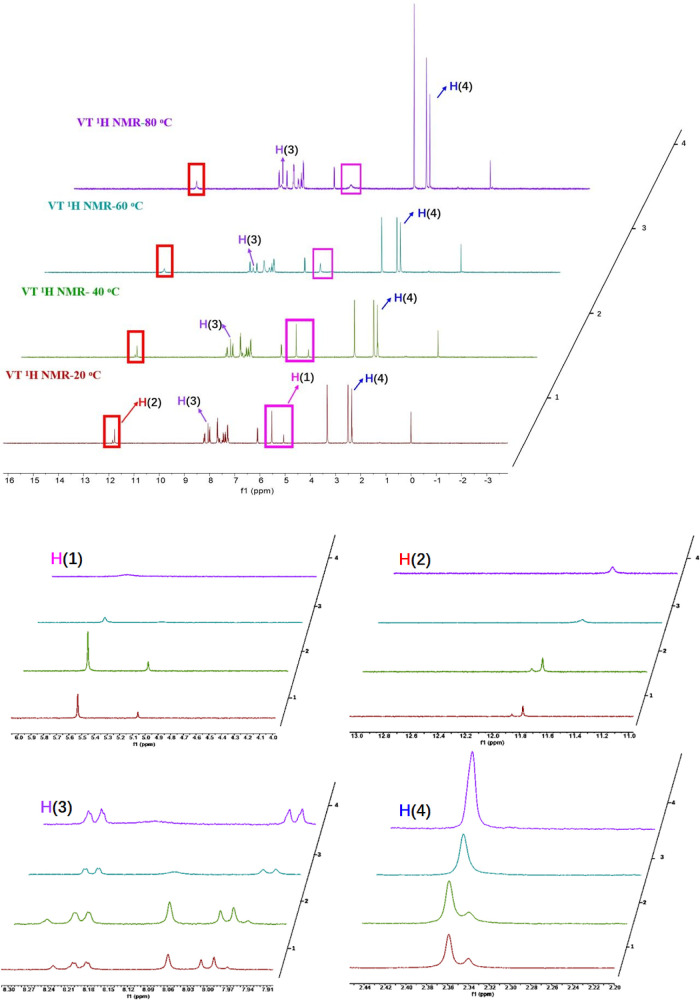
It is interesting that compounds 1–25 exhibited two sets of signals in the 1H NMR spectra at room temperature. Initially, this phenomenon was considered to be caused by the impurities in the compounds. Further purification of these compounds ruled out this possibility. According to previous literature,41,42 the condensation of hydrazide and aldehyde afforded both cis and trans isomers (Figure 4), the mutual transformation of which may be hindered at room temperature, resulting in two sets of peaks on 1H NMR. The ratio of cis and trans isomers can be determined by integral area of peaks in 1H NMR, which can be attributed to the fact that the relative thermodynamical stabilities of cis isomers and trans isomers were different. The hypothesis was confirmed by variable temperature 1H NMR studies of compound 2 in DMSO-d6 (Figure 5). The coalescence of signals was observed in the 1H NMR spectrum with the increase of temperature (20–40–60–80 °C).
Figure 4.
Structure of compound 2.
Figure 5.
Variable-temperature 1H NMR of compound 2 in DMSO-d6.
Preliminary Structure–Activity Relationship Analysis
In Vivo Anti-TMV Activity
The anti-TMV activities in vivo of compounds B, C, and 1–25 are listed in Table 1. In order to make the antiviral activity results more reliable, commercial plant virus inhibitor ribavirin was taken as a control. All compounds exhibited moderate to good anti-TMV activities, especially, compound 4 (51.2, 47.6, and 46.3% at 500 mg/L; 16.8, 14.0, and 15.8% at 100 mg/L) and compound 11 (49.6, 43.0, and 45.2% at 500 mg/L; 19.8, 10.1, and 20.9% at 100 mg/L) exhibited higher inactive, curative, and protective activities against TMV than ribavirin (39.2, 38.0, and 40.8% at 500 mg/L; 12.1, 10.1, and 13.4% at 100 mg/L) both at 500 and 100 mg/L. Compound 17 (47.1, 49.2, and 44.1%) exhibited higher inactive, curative, and protective activities against TMV than ribavirin at 500 mg/L, while its inactive (7.5%) activity was lower than that of ribavirin (12.1%) at 100 mg/L. The inactive, curative, and protective activities of compound 23 (40.1, 32.5, and 43.9%) were equivalent to those of ribavirin at 500 mg/L, while the compound did not exhibit any inactive and protective activities at 100 mg/L. Biphenyl-substituted acylhydrazone derivative 11 (49.6, 43.0, and 45.2% at 500 mg/L) exhibited equivalent antiviral activity to corresponding 3-positon hydrazone derivative of echinopsine (46.2, 45.0, and 41.7% at 500 mg/L).33
Table 1. In Vivo Antiviral Activities of Compounds B, C, and 1–25 against TMVa.
| relative
inhibition rate (%) |
||||
|---|---|---|---|---|
| compd | concentration (mg/L) | inactive effect | curative effect | protective effect |
| 500 | 32.1 ± 1.6 g–j | |||
| 100 | 0 | |||
| C | 500 | 45.9 ± 3.3 b–d | 31.5 ± 2.0 f | 47.8 ± 4.8 b |
| 100 | 17.2 ± 1.1 | 8.2 ± 0.5 | 11.4 ± 2.9 | |
| 1 | 500 | 29.3 ± 1.4 h–k | ||
| 100 | 0 | |||
| 2 | 500 | 26.7 ± 3.0 j–l | ||
| 100 | 0 | |||
| 3 | 500 | 24.1 ± 1.0 k–m | ||
| 100 | 0 | |||
| 4 | 500 | 51.2 ± 1.9 b | 47.6 ± 2.5 b | 46.3 ± 1.8 bc |
| 100 | 16.8 ± 0.3 | 14.0 ± 0.5 | 15.8 ± 2.5 | |
| 5 | 500 | 11.3 ± 3.2 op | ||
| 100 | 0 | |||
| 6 | 500 | 7.4 ± 2.1 p | ||
| 100 | 0 | |||
| 7 | 500 | 23.8 ± 2.5 k–m | ||
| 100 | 0 | |||
| 8 | 500 | 28.1 ± 1.3 ijk | ||
| 100 | 0 | |||
| 9 | 500 | 38.1 ± 3.2 e–g | ||
| 100 | 5.1 ± 0.8 | |||
| 10 | 500 | 15.4 ± 2.0 no | ||
| 100 | 0 | |||
| 11 | 500 | 49.6 ± 3.9 bc | 43.0 ± 2.7 bc | 45.2 ± 1.1 bc |
| 100 | 19.8 ± 2.8 | 10.1 ± 2.0 | 20.9 ± 0.4 | |
| 12 | 500 | 27.4 ± 3.9 i–k | ||
| 100 | 0 | |||
| 13 | 500 | 18.6 ± 0.7 mn | ||
| 100 | 0 | |||
| 14 | 500 | 31.7 ± 2.5 h–j | ||
| 100 | 0 | |||
| 15 | 500 | 16.1 ± 4.8 no | ||
| 100 | 0 | |||
| 16 | 500 | 14.6 ± 4.0 no | ||
| 100 | 0 | |||
| 17 | 500 | 47.1 ± 1.9 bc | 49.2 ± 3.0 b | 44.1 ± 1.4 bc |
| 100 | 7.5 ± 0.2 | 17.7 ± 1.5 | 19.0 ± 1.8 | |
| 18 | 500 | 32.7 ± 3.8 g–j | ||
| 100 | 7.0 ± 1.9 | |||
| 19 | 500 | 20.5 ± 3.6 l–n | ||
| 100 | 0 | |||
| 20 | 500 | 29.7 ± 3.6 h–k | ||
| 100 | 0 | |||
| 21 | 500 | 12.3 ± 1.7 op | ||
| 100 | 0 | |||
| 22 | 500 | 23.9 ± 1.1 k–m | ||
| 100 | 0 | |||
| 23 | 500 | 40.1 ± 2.7 c–e | 32.5 ± 4.1 de | 43.9 ± 1.0 bc |
| 100 | 0 | 6.6 ± 1.9 | 0 | |
| 24 | 500 | 33.5 ± 0.6 g–i | ||
| 100 | 0 | |||
| 25 | 500 | 35.2 ± 4.8 f–h | ||
| 100 | 0 | |||
| Ribavirinb | 500 | 39.2 ± 3.5 d–f | 38.0 ± 2.2 cd | 40.8 ± 1.0 c |
| 100 | 12.1 ± 1.4 | 10.1 ± 1.6 | 13.4 ± 0.7 | |
A one-way analysis of variance followed by Duncan’s test was used for significant differences at p < 0.05, marking with the letters. Different letters indicate significant differences.
Ribavirin was used as the control.
The inactive, curative, and protective activities of hydrazide C (45.9, 31.5, and 47.8% at 500 mg/L) were higher than that of compound B, which indicated that the introduction of a hydrazide functional group increases the antiviral activity. Substituted benzaldehydes and heterocyclic aldehydes were selected to react with hydrazide C to investigate the effect of phenyl rings and heterocycles. Overall, introducing the substituted phenyl rings may increase or decrease antiviral activities, while introducing heterocycles generally makes the antiviral activity lower than compound C. For derivatives containing electron-donating groups substituted phenyl (2–11), the structure–activity relationship shows the following: m-methoxy (4) > p-phenyl (11) > p-1-pyrazolyl (9) > nonsubstituent (1) > p-dimethylamino (8) > p-methyl (2) > p-tert-butyl (3) > methylthio (10). For derivatives containing electron-withdrawing groups substituted phenyl (12–18), the structure–activity relationship shows the following: p-trifluoromethyl (17) > p-methylsulfonyl (18) > p-bromo (14) > nonsubstituent (1) > p-fluoro (12) > p-chloro (13) > p-nitro (15). Thus, there was no obvious linear relationship between anti-TMV activity and electron-donating and electron-withdrawing abilities. The antiviral activities of derivatives containing monosubstituted benzene were higher than those containing polysubstituted benzene. For instance, compared with the disubstituted compound 5 (inactive, 11.3%, 500 mg/L), the monosubstituted compound 4 (inactive, 51.2%, 500 mg/L) exhibited higher activity. For derivatives containing heterocycles, derivatives containing benzoheterocyclic rings exhibited higher antiviral activities than that containing furan, pyridine, thiazole, and thiophene.
TEM Analysis
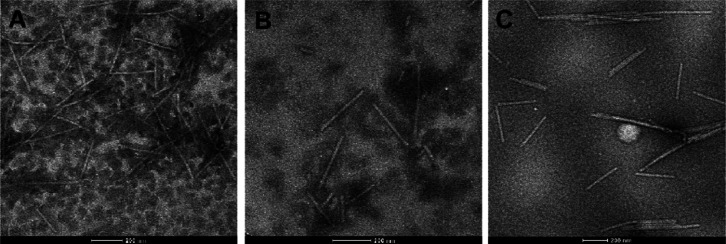
The inhibitory effect of compound 4 and ribavirin (500 mg/L) on the morphology of TMV particles was observed by transmission electron microscopy (TEM) (Figure 6). TMV particles in the blank control exhibited a relatively complete rod shape, while TMV particles treated with a compound 4 solution with a concentration of 500 mg/L fragmented into shorter rods, similar to the effect of the 500 mg/L ribavirin control. It was speculated that compound 4 inhibited the assembly of TMV particles.
Figure 6.
TEM images of TMV particles at 200 nm: (A) blank control, (B) compound 4, and (C) ribavirin.
Molecular Docking
The molecular docking diagrams of ribavirin and compound 4 with TMV-CP are shown in Figure 7. Both compound 4 and ribavirin can be docked into the pocket of TMV-CP. Compound 4 was larger than ribavirin so that it bonded more tightly to the protein pocket than ribavirin. Thus, the docking pocket between compound 4 and TMV-CP was more in line with the lock-key principle than that between ribavirin.
Figure 7.
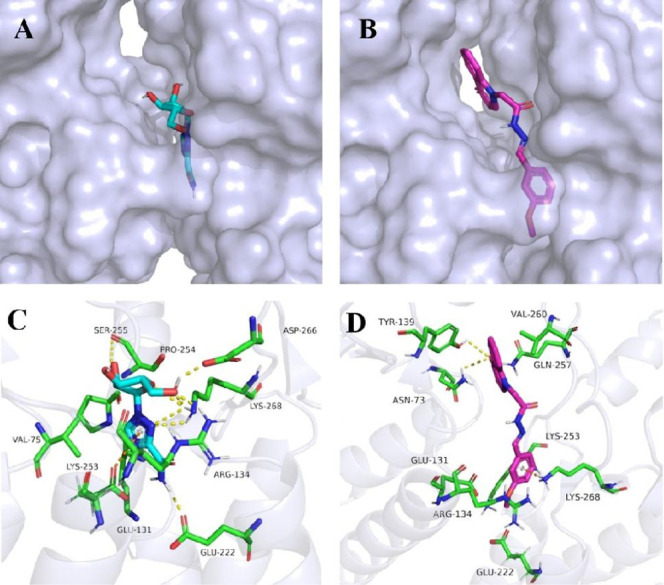
Molecular docking diagrams of ribavirin with TMV-CP (A,C) and compound 4 with TMV-CP (B,D).
As shown in Figure 7C,D, the carbonyl group of the quinolone structure of compound 4 can form hydrogen bonding with amino acids Asn73, Tyr139, and Gln257. The benzene ring of compound 4 formed a Pi–cation interaction with Lys268. Compound 4 also had hydrophobic interactions with amino acids Glu131, Arg134, Glu222, and Lys253. Hydrogen bonding interactions, charge interactions, and hydrophobic interactions all played important roles in the binding of compound 4 and TMV-CP. Ribavirin formed hydrogen bonds with amino acids Arg134, Glu222, Ser255, Asp266, and Lys268 and also formed Pi–anion interactions with amino acid Glu131.
Although compound 4 exhibited slightly fewer hydrogen bonding interactions with TMV-CP than ribavirin, the nonpolar interactions (van der Waals forces and hydrophobic interactions) between compound 4 and TMV-CP were superior to ribavirin. Compound 4 was more compatible with the binding pocket of TMV-CP and exhibited antiviral activities that were better than those of ribavirin. According to molecular simulation data in our previous work,43 the TMV-CP and compound 4 complex was stable.
Microscale Thermophoresis Analysis
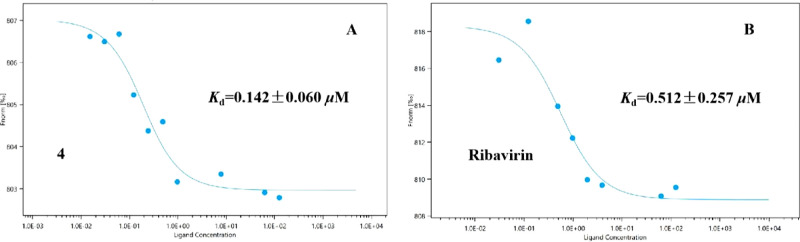
Microscale thermophoresis results of compound 4 and ribavirin with TMV-CP are shown in Figure 8, which indicated that compound 4 (Kd = 0.142 ± 0.060 μM) showed stronger binding affinities toward TMV-CP than ribavirin (Kd = 0.512 ± 0.257 μM). These results were consistent with the molecular docking and anti-TMV inactivation activity results.
Figure 8.
Microscale thermophoresis results for compounds 4 (A) and ribavirin (B) with TMV-CP.
Fungicidal Activity
The fungicidal activities of compounds 1–25 were also evaluated and commercial fungicides carbendazim and chlorothalonil were used as the positive control (Table 2). All compounds exhibited moderate bactericidal activities against 14 kinds of phytopathogenic fungi, which were attributed to their poor solubilities. Compound 4 exhibited more than 40% inhibitory rate against three fungi at 50 mg/L. Compounds 15 and 16 exhibited 72.1 and 76.5% inhibitory rate against Physalospora piricola at 50 mg/L, respectively. Compound 19 exhibited a 60% inhibitory rate against Cercospora arachidicola Hori at 50 mg/L.
Table 2. Fungicidal Activitiesa of Compounds 1–25 against 14 Kinds of Phytopathogens (50 mg/L, Inhibition Rate/%).
| Compd | Asb | Fgb | Mgb | Pcb | Ssb | Bcb | Rsb | Fcb | Chb | Ppb | Rcb | Bmb | Wab | Fmb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30.8 ± 2.3 | 14.3 ± 1.2 | 25.0 ± 1.4 | 21.4 ± 1.1 | 19.5 ± 0.7 | 33.3 ± 1.6 | 14.3 ± 1.3 | 22.4 ± 1.9 | 22.9 ± 2.1 | 16.2 ± 0.5 | 7.3 ± 1.4 | 15.6 ± 1.7 | 16.3 ± 1.3 | 9.1 ± 0.9 |
| 2 | 30.8 ± 1.6 | 14.3 ± 1.3 | 25.0 ± 2.1 | 26.2 ± 1.8 | 12.2 ± 1.2 | 25.0 ± 1.5 | 17.9 ± 1.7 | 28.6 ± 2.3 | 11.4 ± 0.9 | 17.6 ± 1.4 | 4.9 ± 0.7 | 11.1 ± 2.1 | 14.0 ± 1.3 | 6.1 ± 0.7 |
| 3 | 30.8 ± 2.3 | 16.7 ± 1.3 | 12.5 ± 0.9 | 23.8 ± 1.5 | 22.0 ± 1.9 | 29.2 ± 1.7 | 17.9 ± 1.8 | 10.2 ± 1.6 | 20.0 ± 1.4 | 35.3 ± 2.1 | 12.2 ± 1.5 | 8.9 ± 0.7 | 16.3 ± 1.3 | 18.2 ± 1.1 |
| 4 | 61.5 ± 3.2 | 38.1 ± 1.9 | 12.5 ± 1.3 | 38.1 ± 1.7 | 36.6 ± 1.5 | 14.6 ± 0.7 | 57.1 ± 2.3 | 36.7 ± 1.4 | 48.6 ± 1.2 | 38.2 ± 1.7 | 39.0 ± 1.3 | 33.3 ± 1.4 | 39.5 ± 1.1 | 27.3 ± 0.9 |
| 5 | 30.8 ± 2.1 | 14.3 ± 1.5 | 25.0 ± 1.1 | 14.3 ± 0.9 | 24.4 ± 1.1 | 18.8 ± 1.9 | 12.5 ± 1.4 | 16.3 ± 1.3 | 11.4 ± 1.2 | 22.1 ± 1.5 | 15.9 ± 1.1 | 11.1 ± 0.7 | 16.3 ± 1.2 | 21.2 ± 1.9 |
| 6 | 30.8 ± 1.3 | 21.4 ± 1.5 | 25.0 ± 1.1 | 2.4 ± 0.5 | 12.2 ± 1.2 | 14.6 ± 1.7 | 14.3 ± 1.1 | 16.3 ± 1.6 | 17.1 ± 0.9 | 55.9 ± 1.4 | 11.0 ± 1.5 | 13.3 ± 1.3 | 27.9 ± 1.2 | 21.2 ± 1.7 |
| 7 | 30.8 ± 2.3 | 19.0 ± 1.7 | 12.5 ± 1.2 | 23.8 ± 2.1 | 19.5 ± 1.3 | 25.0 ± 1.1 | 25.0 ± 1.6 | 16.3 ± 1.7 | 25.7 ± 1.9 | 25.0 ± 1.3 | 7.3 ± 0.9 | 17.8 ± 1.4 | 20.9 ± 1.5 | 18.2 ± 1.2 |
| 8 | 30.8 ± 2.3 | 23.8 ± 1.9 | 12.5 ± 0.7 | 14.3 ± 1.8 | 12.2 ± 1.3 | 25.0 ± 1.2 | 35.7 ± 2.1 | 16.3 ± 1.6 | 14.3 ± 0.9 | 29.4 ± 2.3 | 11.0 ± 1.1 | 13.3 ± 1.4 | 16.3 ± 1.7 | 9.1 ± 0.5 |
| 9 | 38.5 ± 1.3 | 9.5 ± 0.5 | 18.8 ± 1.5 | 14.3 ± 1.1 | 12.2 ± 1.8 | 25.0 ± 1.5 | 35.7 ± 2.2 | 18.4 ± 0.7 | 25.7 ± 1.4 | 27.9 ± 1.2 | 19.5 ± 1.7 | 15.6 ± 2.1 | 14.0 ± 1.5 | 21.2 ± 1.9 |
| 10 | 30.8 ± 2.1 | 2.4 ± 0.6 | 12.5 ± 1.2 | 23.8 ± 1.6 | 7.3 ± 1.4 | 14.6 ± 1.2 | 3.6 ± 1.1 | 12.2 ± 1.3 | 22.9 ± 1.5 | 58.8 ± 3.2 | 18.3 ± 1.8 | 13.3 ± 0.9 | 14.0 ± 1.7 | 9.1 ± 1.3 |
| 11 | 38.5 ± 2.4 | 9.5 ± 0.7 | 25.0 ± 1.2 | 26.2 ± 1.5 | 22.0 ± 1.4 | 25.0 ± 1.8 | 35.7 ± 2.2 | 22.4 ± 1.3 | 22.9 ± 1.7 | 17.6 ± 1.6 | 6.1 ± 1.1 | 11.1 ± 0.9 | 14.0 ± 1.7 | 9.1 ± 1.2 |
| 12 | 23.1 ± 1.1 | 21.4 ± 1.4 | 18.8 ± 1.7 | 14.3 ± 1.6 | 7.3 ± 1.2 | 25.0 ± 1.4 | 32.1 ± 2.1 | 16.3 ± 1.5 | 20.0 ± 1.3 | 10.3 ± 0.9 | 7.3 ± 1.1 | 15.6 ± 1.7 | 16.3 ± 1.6 | 18.2 ± 1.3 |
| 13 | 30.8 ± 1.3 | 2.4 ± 0.9 | 18.8 ± 1.7 | 14.3 ± 1.3 | 7.3 ± 0.5 | 14.6 ± 1.3 | 12.5 ± 1.4 | 24.5 ± 1.3 | 11.4 ± 1.1 | 17.6 ± 1.5 | 7.3 ± 1.3 | 8.9 ± 0.7 | 27.9 ± 2.3 | 36.4 ± 1.8 |
| 14 | 23.1 ± 1.5 | 2.4 ± 0.6 | 31.3 ± 2.4 | 21.4 ± 1.8 | 2.4 ± 0.4 | 20.8 ± 2.1 | 1.8 ± 0.7 | 22.4 ± 1.3 | 8.6 ± 0.9 | 32.4 ± 2.3 | 14.6 ± 1.1 | 4.4 ± 0.6 | 18.6 ± 1.7 | 15.2 ± 1.3 |
| 15 | 30.8 ± 1.1 | 7.1 ± 1.3 | 12.5 ± 1.2 | 14.3 ± 0.6 | 12.2 ± 1.4 | 27.1 ± 2.4 | 17.9 ± 1.3 | 24.5 ± 1.6 | 40.0 ± 1.5 | 72.1 ± 2.6 | 45.1 ± 1.6 | 40.0 ± 1.3 | 34.9 ± 1.5 | 33.3 ± 1.9 |
| 16 | 30.8 ± 1.4 | 23.8 ± 1.7 | 25.0 ± 2.1 | 14.3 ± 0.7 | 24.4 ± 1.1 | 20.8 ± 1.2 | 35.7 ± 1.5 | 18.4 ± 1.3 | 8.6 ± 0.5 | 26.5 ± 1.1 | 9.8 ± 0.9 | 13.3 ± 1.5 | 23.3 ± 1.6 | 6.1 ± 1.2 |
| 17 | 15.4 ± 1.3 | 19.0 ± 1.2 | 25.0 ± 1.9 | 9.5 ± 0.8 | 22.0 ± 1.3 | 18.8 ± 1.5 | 3.6 ± 0.7 | 26.5 ± 1.7 | 14.3 ± 1.3 | 47.1 ± 1.4 | 7.3 ± 1.1 | 8.9 ± 0.9 | 11.6 ± 1.2 | 27.3 ± 2.2 |
| 18 | 30.8 ± 2.7 | 7.1 ± 1.3 | 12.5 ± 1.5 | 31.0 ± 2.0 | 7.3 ± 1.4 | 18.8 ± 1.1 | 14.3 ± 1.7 | 16.3 ± 1.2 | 14.3 ± 0.7 | 41.2 ± 1.3 | 18.3 ± 1.6 | 8.9 ± 0.9 | 18.6 ± 1.2 | 9.1 ± 0.9 |
| 19 | 46.2 ± 2.2 | 26.2 ± 0.9 | 31.3 ± 1.1 | 14.3 ± 1.2 | 36.6 ± 1.6 | 20.8 ± 0.9 | 26.8 ± 1.4 | 26.5 ± 0.7 | 60.0 ± 2.1 | 44.1 ± 2.8 | 43.9 ± 1.5 | 46.7 ± 1.1 | 46.5 ± 1.3 | 36.4 ± 1.4 |
| 20 | 38.5 ± 0.5 | 14.3 ± 1.4 | 12.5 ± 1.2 | 14.3 ± 1.1 | 36.6 ± 1.7 | 20.8 ± 1.3 | 35.7 ± 1.8 | 16.3 ± 2.0 | 17.1 ± 0.9 | 39.7 ± 1.3 | 8.5 ± 0.7 | 20.0 ± 1.1 | 16.3 ± 1.6 | 21.2 ± 1.5 |
| 21 | 38.5 ± 1.3 | 9.5 ± 1.4 | 12.5 ± 0.9 | 26.2 ± 2.3 | 17.1 ± 1.7 | 31.3 ± 2.1 | 35.7 ± 1.5 | 12.2 ± 1.6 | 17.1 ± 1.1 | 76.5 ± 2.7 | 14.6 ± 0.8 | 17.8 ± 1.2 | 14.0 ± 0.7 | 21.2 ± 1.8 |
| 22 | 7.7 ± 1.2 | 2.4 ± 0.5 | 6.3 ± 0.8 | 26.2 ± 1.5 | 7.3 ± 0.9 | 25.0 ± 2.1 | 26.8 ± 1.1 | 12.2 ± 1.6 | 22.9 ± 1.5 | 32.4 ± 1.3 | 7.3 ± 0.5 | 8.9 ± 0.8 | 14.0 ± 1.7 | 21.2 ± 1.4 |
| 23 | 15.4 ± 1.5 | 14.3 ± 1.1 | 25.0 ± 1.3 | 31.0 ± 2.1 | 12.2 ± 0.9 | 10.4 ± 1.2 | 3.6 ± 0.5 | 6.1 ± 1.3 | 17.1 ± 1.1 | 51.5 ± 2.3 | 25.6 ± 0.7 | 8.9 ± 1.1 | 11.6 ± 0.9 | 18.2 ± 1.3 |
| 24 | 23.1 ± 1.3 | 19.0 ± 1.2 | 12.5 ± 1.7 | 19.0 ± 0.9 | 12.2 ± 0.5 | 20.8 ± 1.5 | 17.9 ± 1.5 | 22.4 ± 1.2 | 20.0 ± 1.3 | 17.6 ± 1.2 | 13.4 ± 1.4 | 11.1 ± 1.8 | 9.3 ± 0.7 | 9.1 ± 1.1 |
| 25 | 46.2 ± 2.7 | 26.2 ± 1.3 | 25.0 ± 1.4 | 26.2 ± 2.1 | 43.9 ± 1.9 | 20.8 ± 1.3 | 21.4 ± 1.6 | 26.5 ± 1.2 | 17.1 ± 1.3 | 32.4 ± 2.3 | 9.8 ± 0.7 | 15.6 ± 1.5 | 16.3 ± 1.2 | 21.2 ± 1.1 |
| carbendazim | 98 ± 1.4 | 29 ± 1.7 | 98 ± 1.2 | 100 ± 0.0 | 86 ± 1.4 | 100 ± 0.0 | 99 ± 0.8 | 96 ± 1.2 | 98 ± 0.7 | 100 ± 0.0 | 75 ± 1.1 | |||
| chlorothalonil | 100 ± 0.0 | 73 ± 0.9 | 100 ± 0.0 | 73 ± 1.3 | <50 | 100 ± 0.0 | <50 | 100 ± 0.0 | 100 ± 0.0 | 91 ± 1.3 | 91 ± 0.7 | 86 ± 1.2 | 100 ± 0.0 | 100 ± 0.0 |
Average of three replicates; all results are expressed as mean ± SD.
As, Alternaria solani; Fg, Fusarium graminearum; Mg, magnaporthe grisea; Pc, Phytophthora capsici; Ss, Sclerotinia sclerotiorum; Bc, Botrytis cinerea; Rs, Rhizoctonia solani; Fc, Fusarium oxysporium f. sp. cucumeris; Ch, Cercospora arachidicola Hori; Pp, Physalospora piricola; Rc, Rhizoctonia cerealis; Bm, Bipolaris maydis; Wa, watermelon anthracnose; and Fm, Fusarium moniliforme.
Conclusions
In summary, a series of 4-oxo-4H-quinolin-1-yl acylhydrazone derivatives were synthesized. Their anti-TMV activities and fungicidal activities were investigated. The bioassays results showed that compounds 4 and 11 exhibited higher inactive, curative, and protective activities than ribavirin both at 500 and 100 mg/L. The inactive, curative, and protective activities of compound 17 against TMV were higher than those of ribavirin at 500 mg/L, while its inactive activities were lower than those of ribavirin at 100 mg/L. Molecular docking, TEM, and microscale thermophoresis experiments showed that compound 4 hindered the self-assembly of TMV-CP and thus prevented TMV from infecting tobacco plants. Compounds 15 and 16 exhibited 72.1 and 76.5% inhibitory rate against P. piricola at 50 mg/L, respectively. Compound 19 exhibited a 60% inhibitory rate against C. arachidicola Hori at 50 mg/L. Further studies on structural optimization are in progress in our laboratory.
Acknowledgments
The authors would like to acknowledge support from the National Natural Science Foundation of China (22001190 and 21702144) and Fundamental Research Program of Shanxi Province (202303021221016 and 20210302123180).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c05046.
1H NMR, 13C NMR, and ESI–HRMS data for compounds 1–25; 1H NMR and 13C NMR spectrum for compounds B, C, and 1–25; detailed bioassay procedures for the anti-TMV activities; and detailed bioassay procedures for the fungicidal activities (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Pallás V.; Sánchez-Navarro J. A.; James D. Recent advances on the multiplex molecular detection of plant viruses and viroids. Front. Microbiol. 2018, 9, 1–11. 10.3389/fmicb.2018.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X. H.; Hu D. Y.; Li P.; Wu J.; Chen X. W.; Xue W.; Song B. A. Design, synthesis, antiviral activity and three-dimensional quantitative structure-activity relationship study of novel 1,4-pentadien-3-one derivatives containing the 1,3,4-oxadiazole moiety: Antiviral activity of novel 1,4-pentadien-3-onederivatives containing the 1,3,4-oxadiazole moiety. Pest Manag. Sci. 2016, 72, 534–543. 10.1002/ps.4018. [DOI] [PubMed] [Google Scholar]
- Chen M. H.; Chen Z.; Song B. A.; Bhadury P. S.; Yang S.; Cai X. J.; Hu D. Y.; Xue W.; Zeng S. Synthesis and Antiviral Activities of Chiral Thiourea Derivatives Containing an α-Aminophosphonate Moiety. J. Agric. Food Chem. 2009, 57, 1383–1388. 10.1021/jf803215t. [DOI] [PubMed] [Google Scholar]
- Chi Y.; He H. W.; Chen C. Y.; Zhao S. Y.; Zhou H.; Xu D.; Liu X. L.; Xu G. Furofuran lignans for plant protection: discovery of sesamolin and its derivatives as novel anti-tobacco mosaic virus and antibacterial agents. J. Agric. Food Chem. 2023, 71, 10798–10808. 10.1021/acs.jafc.3c03257. [DOI] [PubMed] [Google Scholar]
- Lan X. M.; Xie D. D.; Yin L. M.; Wang Z. Z.; Chen J.; Zhang A. W.; Song B. A.; Hu D. Y. Novel á, â-unsaturated amide derivatives bearing á-amino phosphonate moiety as potential antiviral agents. Bioorg. Med. Chem. Lett. 2017, 27, 4270–4273. 10.1016/j.bmcl.2017.08.048. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Ye Y. Q.; Liu S. S.; Shao W. B.; Liu L. W.; Yang S.; Wu Z. B. Design, synthesis and anti-TMV activity of novel á-aminophosphonate derivatives containing a chalcone moiety that induce resistance against plant disease and target the TMV coat protein. Pestic. Biochem. Physiol. 2021, 172, 104749. 10.1016/j.pestbp.2020.104749. [DOI] [PubMed] [Google Scholar]
- Gan X. H.; Hu D. Y.; Chen Z.; Wang Y. J.; Song B. A. Synthesis and antiviral evaluation of novel 1,3,4-oxadiazole/thiadiazole-chalcone conjugates. Bioorg. Med. Chem. Lett. 2017, 27, 4298–4301. 10.1016/j.bmcl.2017.08.038. [DOI] [PubMed] [Google Scholar]
- Zhou D. G.; Xie D. D.; He F. C.; Song B. A.; Hu D. Y. Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Bioorg. Med. Chem. Lett. 2018, 28, 2091–2097. 10.1016/j.bmcl.2018.04.042. [DOI] [PubMed] [Google Scholar]
- Chen J.; Shi J.; Yu L.; Liu D. Y.; Gan X. H.; Song B. A.; Hu D. Y. Design, synthesis, antiviral bioactivity, and defense mechanisms of novel dithioacetal derivatives bearing a strobilurin moiety. J. Agric. Food Chem. 2018, 66, 5335–5345. 10.1021/acs.jafc.8b01297. [DOI] [PubMed] [Google Scholar]
- Zu G. C.; Chen J. X.; Song B. A.; Hu D. Y. Synthesis, Anti-tomato spotted wilt virus activities, and interaction mechanisms of novel dithioacetal derivatives containing a 4(3H)-quinazolinone pyrimidine ring. J. Agric. Food Chem. 2021, 69, 14459–14466. 10.1021/acs.jafc.1c03555. [DOI] [PubMed] [Google Scholar]
- Ran L. L.; Yang H. Y.; Luo L. Z.; Huang M. X.; Hu D. Y. Discovery of potent and novel quinazolinone sulfide inhibitors with anti-ToCV activity. J. Agric. Food Chem. 2020, 68, 5302–5308. 10.1021/acs.jafc.0c00686. [DOI] [PubMed] [Google Scholar]
- Tang X.; Zhang C.; Chen M.; Xue Y. N.; Liu T. T.; Xue W. Synthesis and antiviral activity of novel myricetin derivatives containing ferulic acid amide scaffolds. New J. Chem. 2020, 44, 2374–2379. 10.1039/c9nj05867b. [DOI] [Google Scholar]
- Liu T. T.; Peng F.; Cao X.; Liu F.; Wang Q. F.; Liu L. W.; Xue W. Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety. ACS Omega 2021, 6, 30826–30833. 10.1021/acsomega.1c05256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X. H.; Hu D. Y.; Wang Y. J.; Yu L.; Song B. A. Novel trans-ferulic acid derivatives containing a chalcone moiety as potential activator for plant resistance induction. J. Agric. Food Chem. 2017, 65, 4367–4377. 10.1021/acs.jafc.7b00958. [DOI] [PubMed] [Google Scholar]
- Ren X. L.; Li X. Y.; Yin L. M.; Jiang D. H.; Hu D. Y. Design, synthesis, antiviral bioactivity, and mechanism of the ferulic acid ester containing sulfonamide moiety. ACS Omega 2020, 5, 19721–19726. 10.1021/acsomega.0c02421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F.; Wei P. P.; Yu G.; Guo S. X.; Zheng Z. G.; Chen S. H.; Dai A. L.; Zhang R. F.; Wu Z. X.; Wu J. Synthesis of trans-methyl ferulate bearing an oxadiazole ether as potential activators for controlling plant virus. Bioorg. Chem. 2021, 115, 105248. 10.1016/j.bioorg.2021.105248. [DOI] [PubMed] [Google Scholar]
- Chen L. W.; Hao Y. K.; Song H. J.; Liu Y. X.; Li Y. Q.; Zhang J. J.; Wang Q. M. Design, synthesis, characterization, and biological activities of novel spirooxindole analogues containing hydantoin, thiohydantoin, urea, and thiourea moieties. J. Agric. Food Chem. 2020, 68, 10618–10625. 10.1021/acs.jafc.0c04488. [DOI] [PubMed] [Google Scholar]
- Wang T. N.; Li L.; Zhou Y. N.; Lu A. D.; Li H. Y.; Chen J. X.; Duan Z. Y.; Wang Q. M. Structural simplification of marine natural products: discovery of hamacanthin derivatives containing indole and piperazinone as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2021, 69, 10093–10103. 10.1021/acs.jafc.1c04098. [DOI] [PubMed] [Google Scholar]
- Huang X. B.; Li T. Z.; Shan X. J.; Lu R. F.; Hao M.; Lv M.; Sun Z. Q.; Xu H. High value-added use of citrus industrial wastes in agriculture: semisynthesis and anti-tobacco mosaic virus/insecticidal activities of ester derivatives of limonin modified in the B ring. J. Agric. Food Chem. 2020, 68, 12241–12251. 10.1021/acs.jafc.0c05588. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Botanical pesticides, past, present, and future. ACS Symp. Ser. (Am. Chem. Soc.) 1989, 387, 1–10. 10.1021/bk-1989-0387.ch001. [DOI] [Google Scholar]
- Isman M. B. Botanical insecticides: for richer, for poorer. Pest Manag. Sci. 2008, 64, 8–11. 10.1002/ps.1470. [DOI] [PubMed] [Google Scholar]
- Shang X. F.; Morris-Natschke S. L.; Liu Y. Q.; Li X. H.; Zhang J. Y.; Lee K. H. Biology of quinoline and quinazoline alkaloids. Alkaloids 2022, 88, 1–47. 10.1016/bs.alkal.2021.08.002. [DOI] [PubMed] [Google Scholar]
- Cai Q. F.; Song H. Y.; Zhang Y.; Zhu Z. N.; Zhang J.; Chen J. X. Quinoline derivatives in discovery and development of pesticides. J. Agric. Food Chem. 2024, 72, 12373–12386. 10.1021/acs.jafc.4c01582. [DOI] [PubMed] [Google Scholar]
- Liu Z. L.; Xu Y. J.; Wu J. E.; Goh S. H.; Ho S. H. Feeding deterrents from dictamnus dasycarpus turcz against two stored-product insects. J. Agric. Food Chem. 2002, 50, 1447–1450. 10.1021/jf010838l. [DOI] [PubMed] [Google Scholar]
- Yang G. Z.; Zhu J. K.; Yin X. D.; Yan Y. F.; Wang Y. L.; Shang X. F.; Liu Y. Q.; Zhao Z. M.; Peng J. W.; Liu H. Design, synthesis, and antifungal evaluation of novel quinoline derivatives inspired from natural quinine alkaloids. J. Agric. Food Chem. 2019, 67, 11340–11353. 10.1021/acs.jafc.9b04224. [DOI] [PubMed] [Google Scholar]
- Carta A.; Briguglio I.; Piras S.; Corona P.; Boatto G.; Nieddu M.; Giunchedi P.; Marongiu M. E.; Giliberti G.; Iuliano F.; Blois S.; Ibba C.; Busonera B.; La Colla P. Quinoline tricyclic derivatives. Design, synthesis and evaluation of the antiviral activity of three new classes of RNA-dependent RNA polymerase inhibitors. Bioorg. Med. Chem. 2011, 19, 7070–7084. 10.1016/j.bmc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Ekoue-Kovi K.; Yearick K.; Iwaniuk D. P.; Natarajan J. K.; Alumasa J.; de Dios A. C.; Roepe P. D.; Wolf C. Synthesis and antimalarial activity of new 4-amino-7-chloroquinolyl amides, sulfonamides, ureas and thioureas. Bioorg. Med. Chem. 2009, 17, 270–283. 10.1016/j.bmc.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri Ramya P.; Guntuku L.; Angapelly S.; Karri S.; Digwal C. S.; Babu B. N.; Naidu V. G. M.; Kamal A. Curcumin inspired 2-chloro/phenoxy quinoline analogues: Synthesis and biological evaluation as potential anticancer agents. Bioorg. Med. Chem. Lett. 2018, 28, 892–898. 10.1016/j.bmcl.2018.01.070. [DOI] [PubMed] [Google Scholar]
- Chen Y. L.; Zhao Y. L.; Lu C. M.; Tzeng C. C.; Wang J. P. Synthesis, cytotoxicity, and anti-inflammatory evaluation of 2-(furan-2-yl)-4-(phenoxy) quinoline derivatives. Part 4. Bioorg. Med. Chem. 2006, 14, 4373–4378. 10.1016/j.bmc.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Tabanca N.; Wedge D. E.; Ali A.; Khan I. A.; Kaplancikli Z. A.; Altintop M. D. Antifungal, mosquito deterrent, and larvicidal activity of N-(benzylidene)-3-cyclohexylpropionic acid hydrazide derivatives. Med. Chem. Res. 2013, 22, 2602–2609. 10.1007/s00044-012-0250-4. [DOI] [Google Scholar]
- Yang Z. B.; Li P.; He Y. J. Design, synthesis, and bioactivity evaluation of novel isoxazole-amide derivatives containing an acylhydrazone moiety as new active antiviral agents. Molecules 2019, 24, 3766. 10.3390/molecules24203766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F. L.; Liu Z. X.; Zheng Y.; Song D. D.; Chen K.; Wu Z. B. Repairing host damage caused by tobacco mosaic virus stress: design, synthesis, and mechanism study of novel oxadiazole and arylhydrazone derivatives. J. Agric. Food Chem. 2024, 72, 11351–11359. 10.1021/acs.jafc.3c09463. [DOI] [PubMed] [Google Scholar]
- Cui P. P.; Cai M. J.; Meng Y. N.; Yang Y.; Song H. J.; Liu Y. X.; Wang Q. M. Design, synthesis and biological activities of echinopsine derivatives containing acylhydrazone moiety. Sci. Rep. 2022, 12, 2935. 10.1038/s41598-022-06775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J.; Meszaros Z.; Dutka F.; Komives T.; Marton A. F. Alkylation of quinolines with trialkylphosphates. Tetrahedron Lett. 1977, 18, 4545–4546. 10.1016/S0040-4039(01)83562-0. [DOI] [Google Scholar]
- Wang Z. W.; Wei P.; Xizhi X.; Liu Y. X.; Wang L. Z.; Wang Q. M. Design, synthesis, and antiviral activity evaluation of phenanthrene-based antofine derivatives. J. Agric. Food Chem. 2012, 60, 8544–8551. 10.1021/jf302746m. [DOI] [PubMed] [Google Scholar]
- Liu Y. X.; Zhang P. X.; Li Y. Q.; Song H. B.; Wang Q. M. Design, synthesis, and biological evaluation of 2-benzylpyrroles and 2-benzoylpyrroles based on structures of insecticidal chlorfenapyr and natural pyrrolomycins. Mol. Divers. 2014, 18, 593–598. 10.1007/s11030-014-9515-9. [DOI] [PubMed] [Google Scholar]
- Yang Y. Y.; Zhang J.; Li X. X.; He F. C.; Wu R.; Hu D. Y.; Song B. A. Discovery of dithioacetal derivatives containing sulfonamide moiety of novel antiviral agents by TMV coat protein as a potential target. ACS Omega 2020, 5, 22596–22602. 10.1021/acsomega.0c03306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Y.; Song B. A.; Hu D. Y.; Wang Z. C.; Zeng M. J.; Yu D. D.; Chen Z.; Jin L. H.; Yang S. The development and application of new crystallization method for tobacco mosaic virus coat protein. Virol. J. 2012, 9, 279. 10.1186/1743-422x-9-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Z.; Xie D. D.; Gan X. H.; Zeng S.; Zhang A. W.; Yin L. M.; Song B. A.; Jin L. H.; Hu D. Y. Synthesis, antiviral activity, and molecular docking study of trans-ferulic acid derivatives containing acylhydrazone moiety. Bioorg. Med. Chem. Lett. 2017, 27, 4096–4100. 10.1016/j.bmcl.2017.07.038. [DOI] [PubMed] [Google Scholar]
- Bhyravbhatla B.; Watowich S. J.; Caspar D. L. D. Refined atomic model of the four-layer aggregate of the tobacco mosaic virus coat protein at 2.4-Å resolution. Biophys. J. 1998, 74, 604–615. 10.1016/S0006-3495(98)77819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. T.; Yang R. X.; Liu L. X.; Zhang J. J.; Liu Y. X.; Li Y. Q.; Wang L. Z.; Song H. J.; Wang Q. M. Design, synthesis, and bioactivity of aldisine derivatives containing oxime and hydrazine moieties based on hydrogen bonds. J. Agric. Food Chem. 2023, 71, 11016–11025. 10.1021/acs.jafc.3c02480. [DOI] [PubMed] [Google Scholar]
- Liu Y. X.; Song H. J.; Huang Y. Q.; Li J. R.; Zhao S.; Song Y. C.; Yang P. W.; Xiao Z. X.; Liu Y. X.; Li Y. Q.; Shang H.; Wang Q. M. Design, Synthesis, and Antiviral, Fungicidal, and Insecticidal Activities of Tetrahydro-β-carboline-3-carbohydrazide Derivatives. J. Agric. Food Chem. 2014, 62, 9987–9999. 10.1021/jf503794g. [DOI] [PubMed] [Google Scholar]
- Chen S. H.; Yang Z. K.; Sun W.; Tian K.; Sun P.; Wu J. TMV-CP based rational design and discovery of α-Amide phosphate derivatives as anti plant viral agents. Bioorg. Chem. 2024, 147, 107415. 10.1016/j.bioorg.2024.107415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.