Abstract
Background and aims
In all age, FoShou as a Chinese medicinal herb has been active in various kinds of Traditional Chinese medicine formula to treating diabetes. Hesperidin (HES), the main monomeric component of FoShou, has been extensively investigated for interventions with pathogenic mechanism of diabetes as well as subsequent treatment of associated complications. Islet β-cells have an essential effect on dynamically regulating blood sugar. Functional abnormalities in these cells and their death are strongly associated with the onset of diabetes. Therefore, induction of islet endocrine cell lineage re-editing for damaged βcell replenishment would be a promising therapeutic tool. Previously, it has been found that HES can protect islet β-cells in vivo, But, the regenerative function of HES in islet β cells and its role in promoting differential non-β cells transdifferentiation into β cells and cell fate rewriting associated mechanisms remain unclear.This work focused on investigating whether HES can induce islet α cells transdifferentiation into β cells for achieving damaged β cell regeneration and the causes and possible mechanisms involved in the process.
Materials and methods
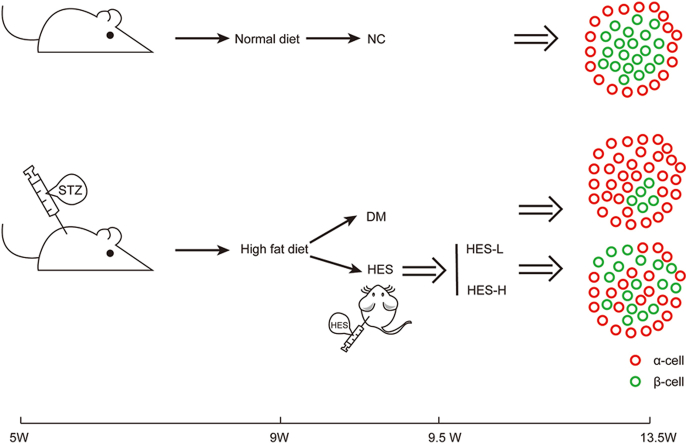
In brief, 60 mg/kg/d streptozotocin (STZ) was administered intraperitoneally in each male C57bL/6J mouse raised by the high-sugar and high-fat diet (HFD) to create a diabetic mouse model with severe β-cell damage. After 28 consecutive days of HES treatment (160 mg/kg; 320 mg/kg; once daily, as appropriate). Tracing the dynamics of α as well as β cell transformation, together with β cells growth and apoptosis levels during treatment by cell lineage tracing. The self-enforcing transcriptional network on which the cell lineage is based is used as a clue to explore the underlying mechanisms. Guangdong Pharmaceutical University's Animal Experiment Ethics Committee (GDPulac2019180) approved all animal experiments.
Results
Localization by cell lineage we find that transdifferentiated newborn β-cells derived from α cells appeared in the islet endocrine cell mass of DM mice under HES'action. Compared to the model group, expressed by Tunel staining and CXCL10 levels the overall apoptosis rate of β-cells of the pancreas were reduced,the inflammatory infiltration feedback from HE staining were lower.Ki-67 positive cells showed enhanced β-cell proliferation. Decreased HbA1c and blood glucose contents, elevated C-Peptide and insulin contents which respond to ability of nascent beta cells. Also upregulated the mRNA levels of MafA, Ngn3, PDX-1, Pax4 and Arx. Moreover, increased the expression of TGR5/cAMP-CREB/GLP-1 in mouse intestinal tissues and GLP-1/GLP-1R and cAMP-CREB/IRS2/PDX-1 in pancreatic tissues.
Conclusions
HES directly affects β-cells, apart from being anti-apoptotic and reducing inflammatory infiltration. HES promotes GLP-1 release by intestinal L cells by activating the TGR5 receptor in DM mouse and regulating its response element CREB signaling. GLP-1 then uses the GLP-1/GLP-1R system to act on IRS2, IRS2 as a port to influence α precursor cells to express PDX-1, with the mobilization of Pax4 strong expression than Arx so that α cell lineage is finally reversed for achieving β cell endogenous proliferation.
Keywords: Hesperidin, β cell regeneration, α cell, Cross-spectrum transdifferentiation, Glucagon-like peptide-1, IRS2
Graphical abstract
1. Introduction
Diabetes mellitus (DM) comprises various metabolic disturbances resulting from a variety of factors (including genetics, immune dysfunction, cellular metabolism, and toxin accumulation factors) that induce pancreatic islet β-cell dysfunction or even apoptosis, which eventually leads to insufficient insulin secretion and a series of metabolic disorders, such as abnormal metabolism of sugar, fat, water, and electrolytes. Recently, DM exhibits a dramatically increasing incidence, and has become a highly prevalent metabolic disease for all human beings. By 2040, the global prevalence of DM may grow to 642 million [1].
Pancreatic β-cell functional deficiency and apoptosis is the central mechanism in DM pathogenesis, causing permanent endogenous insulin deficiency and irreversible hyperglycemia [[2], [3], [4]]. Therefore, more and more articles are conducted to achieve islet β-cell protection and proliferation under diabetic condition, especially searching for the novel source of islet β-cells in this process.
The α and β cells within pancreatic islet endocrine cells have same origin during embryonic development, mature through the same pathway, and play similar roles in regulating blood glucose [5]. In addition, during DM development,the dysfunction and reduced β cell number are accompanying with the reduced α cell number, but α cells function degree essentially remains normal. To date, the function of α cells remains a mystery [6]. The known functions of α cells include the production of the hormone glucagon, which maintains blood glucose through triggering glucose generation in the liver. However, more and more articles also identify other functions of α-cells, like the source for maintaining normal β-cell function or number or even for regeneration of β-cells [[7], [8], [9]].
With regard to extremely damaged pancreatic β-cells, α-cells may be the progenitors of β-cells [10,11]. Whereas the genealogy of cells during cell development needs to be maintained by the expression of self-reinforcing transcriptional networks [12], the nature of these networks is critical during cell development, and the molecular mechanisms underlying the differentiation state of cells can facilitate cell reprogramming [7,13]. Similarly, nuclear factors can be modified to execute a new differentiation program at the expense of another one, which, in turn, helps reverse α-cell lineage for achieving β-cell neogenesis while alleviating persistent pancreatic β-cell apoptosis.
The process can be often characterized by GLP-1 involvement, which is considered an important growth factor to expand pancreatic α precursor cells. As reported in several articles, GLP-1 exerts protection on and even promotes islet β-cell regeneration within diabetic mice, in which β-cells are probably derived from α precursor cell transdifferentiation [14,15].
Flavonoids reportedly exert protection for pancreatic β-cells [16]. For example, both naringenin and quercetin can increase pancreatic β-cell survival while ensuring normal insulin production from β-cells in a high glucose state by inhibiting the oxidative stress pathway [[17], [18], [19]]. It is reported that kaempferol counteracts damage to the β-cells in high glucose conditions by inhibiting caspase-3 protein and upregulating Bcl-2 protein [20].
Hesperidin (HES), another flavonoid, has been widely distributed within several plant species, including the fruits of FoShou and citrus fruits. Previous studies have shown that HES exhibits positive effects in terms of both preventing and treating diabetes [21,22]. We have previously verified that HES modulates IRS1-GLUT2 pathway within HepG2 cells through TLR4 for mitigating insulin resistance [23]. It has also been shown to modulate glycolysis- and gluconeogenesis-related enzyme activities [24] and exert protection for pancreatic β-cells [23]. However, whether HES can promote α cell transdifferentiation in β cells to facilitate endogenous islet β cell proliferation, along with the involvement of GLP-1 and the mechanisms underlying the possible transdifferentiation process, is still not known.
In the present investigation, we explored the compositional state of pancreatic islet endocrine cells after HES administration by combining STZ and HFD to create a diabetic mouse model with severely damaged pancreatic islet β-cells and used the molecular imaging immunofluorescence technique to profile the tracing of pancreatic islet endocrine cells and to identify the key transcription factors and potential underlying mechanisms associated with pancreatic islet endocrine cells.
2. Materials and Methods
2.1. Chemicals and reagents
HES (CAS: #520–26–3, LOT: N22GS168518), streptozotocin (STZ) (CAS:18883–66–4, LOT: C12195306), and carboxymethyl cellulose sodium (CMCNa) were provided by MACKLIN (CAS: 9085–26–1, LOT: C12962727). Bicinchoninic acid (BCA) protein assay kit as well as super-sensitive enhanced chemiluminescent (ECL) reagent was purchased in Meilunbio (MA0082). Antibodies against PDX-1 (ab34150), Ki-67 (GB111141), and NKX6.1 (ab221549) were provided by Abcam. Anti-MafB (SC376387), anti-Neurogenin3 (SC374442), and anti-insulin (SC8033) antibodies were provided by Santa Cruz (Dallas, USA). Anti-glucagon (15954-1-AP) antibodies were provided by Proteintech (USA).
2.2. Animals and treatment
The 4-6-week-old C57BL/6 mice (body mass: 15–17 g/mice) were provided by Guangdong Zhiyuan Biomedical Technology Co. The animals were housed in the SPF class breeding room at the Guangdong Pharmaceutical University Experimental Animal Center under standard environmental conditions of 20∼26 °C, relative humidity of 40∼70 %, along with light/dark cycle of 12-h/12-h, with free access to water and standard diet. Guangdong Pharmaceutical University's Animal Experiment Ethics Committee (GDPulac2019180) approved all animal experiments.
The diabetic mouse model with extensive pancreatic β-cell damage was constructed as described by Chaudhry Z et al. [25,26]. C57 mice were randomized as four groups: negative control (NC), diabetic (DM), HES low dose (HES-L), and HES high dose (HES-H) (10 mice per group). Except for the NC group, all groups were fed a high-fat diet (including 35 % fat, 18 % protein, together with 47 % carbohydrate) for a 3-weel period, followed by injection of STZ (60 mg/kg/d) for four days, while HFD was continued. HES was given to low- (HES-L; 160 mg/kg/d) and high-dose (HES-H; 320 mg/kg/d) groups for 28 days. The dose conversion method of HES was referred to as the standard dose conversion between adults and mice in the 2015 edition of Chinese Pharmacopoeia. The standard weight adult dose shown in the pharmacopoeia was converted using the equivalent dose conversion method with a conversion factor of Rab = 12.33 to obtain the maximum and minimum dose for standard weight mice. Blood glucose measurement by tail prick in mice at 0 d before modeling, after STZ injection (-4d), 0 d before and 3, 7, 14, 21, and 28 d after hesperidin administration. Blood glucose was determined in mice during oral glucose tolerance test (OGTT) 28 days after hesperidin administration. Fasting blood glucose was measured before OGTT, and then recorded at 30/60/120 min after gavage of glucose in the mice. Mice weight was recorded at 0 d before modeling, after STZ injection (−4 d), 0 d before and 3, 7, 14, 21, and 28 d after hesperidin administration. The amount of food consumed was recorded at 0 d before modeling, after STZ injection (−4 d), and 7, 14, 21, 28 d after hesperidin administration. Next, we collected blood and pancreatic tissue samples. We preserved the extracted samples under −80 °C prior to analyzed.
2.3. Biochemical tests
Blood samples were centrifuged for a 15-min period at 3000 rpm to obtain serum. The insulin (INS), glucagon (GC), glycated hemoglobin (HbA1C), C-peptide, CXCL10, together with glucagon-like peptide-1 (GLP-1) contents in serum were measured with corresponding mouse enzyme-linked immunosorbent assay (ELISA) kits (Cat. no.: MM-0579M1, MM-0661M1, MM-0159M1, MM-058OM1, MM-45188M1, MM-0027M1, respectively). (Meimian Industrial Co.,Ltd., Jiangsu, China). The manufacturer's instructions were followed for all ELISAs.
2.4. Hematoxylin and eosin
Sections were dewaxed, gradient ethanol dewaxed, and hematoxylin stained for 10 s, followed by rapid underwater rinsing for 15 min and eosin staining for 30 s. After dehydration with gradient ethanol, neutral resin was added for sealing.
2.5. Immunofluorescence
The sections were dewaxed, immersed in citric acid-sodium citrate buffer, and heated for 13 min. After cooling every sections in tap water to achieve antigen repair, sections were incubated using buffer consisting of goat serum and 5 % BSA for a 1-h period under ambient temperature. Following primary antibody incubation (antibodies against INS, GC, Ki-67, NKX6.1, and MafB at dilutions 1:300, 1:300, 1:1000, 1:500, and 1:100, respectively) for 12 h under 4 °C, sections were further subjected to another 1-h secondary antibody incubation (AF488 goat anti-mouse, AF488 goat anti-rabbit, and AF594 goat anti-rabbit each; 1:1000) in dark under ambient temperature. Finally, DAPI was added to block sections. For assessing cell apoptosis, we conducted TUNEL assay using in situ cell death kit in line with specific protocols (Fluorescein, Roche Diagnostics, UK). EDTA antibody repair solution was used for antibody repair.
During immunofluorescence staining, the cells were quantified for each pancreas in sections with equal spacing (covering the whole pancreas). Moreover, Image J software (Media Cybernetics, Rockville, MD, USA) was employed to manually determine positive cell number for three mice from each group.
2.6. RT-PCR
We utilized TRIzol (Cat. No.: R401–01; Vazyme Biotech Co. Ltd.) for extracting RNA. The conventional reversal was performed with HiScript II Q-RT SuperMix for qPCR (+gDNA wiper) (Cat. No.: R223–01; Vazyme Biotech Co. Ltd.), while mircoRNA reversal with HiScript II Q Select RT SuperMix for qPCR (+gDNA wiper) (Cat. No.: R223–01; Vazyme Biotech Co. Ltd.); DL2000 Plus DNA Marker (Cat. No.: MD101–02; Vazyme Biotech Co. Ltd.); GelRed Nucleic Acid Gel Stain, 10,000X in water (Cat. No.: 41003; Biotium Scientific Beijing).
Table 1 displays the primer sequences synthesized by the Tsingke Biotechnology Company (Beijing, China). Each experiment was carried out thrice, with GAPDH being the endogenous reference for normalizing gene levels by 2−ΔΔCT approach.
Table 1.
List of primer sequences.
| Gene | Primer | Sequence (5′-3′) | PCR Products |
|---|---|---|---|
| Mus GAPDH | Forward | ATGGGTGTGAACCACGAGA | 229bp |
| Reverse | CAGGGATGATGTTCTGGGCA | ||
| Mus ngn3 | Forward | ACACAACAACCTTTTCCCGG | 226bp |
| Reverse | CCCGATCATTGGCCTTCTTG | ||
| Mus Mafa | Forward | GCACATTCTGGAGAGCGAGA | 225bp |
| Reverse | CACAGAAAGAAGTCGGGTGC | ||
| Mus Pdx-1 | Forward | AAATCCACCAAAGCTCACGC | 241bp |
| Reverse | TTTTCCACTTCATGCGACGG | ||
| Mus Pax4 | Forward | AAAGAGTTTCAGCGTGGGC | 314bp |
| Reverse | CAGTAGGGTTGGAGATAGGC | ||
| Mus Arx | Forward | TTTGGCAGGCTCTTTTCCAC | 214bp |
| Reverse | GGATGTTGAGCTGCGTGAG |
2.7. Western blotting
Protein lysis buffer containing proteinase/phosphatase inhibitors was used to extract the total proteins. After mixing supernatants with loading buffer, the mixed solution was subjected to 10-min boiling, followed by protein separation using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), then transfer to polyvinylidene fluoride (PVDF) membranes. Non-fat milk (5 %) was used as a blocking solution under ambient temperature for a 1.5-h period, membranes were then incubated using primary antibodies under 4 °C overnight, followed by additional 1-h incubation Using horseradish peroxidase (HRP)-labeled secondary antibody under ambient temperature. Bio-Rad chemiluminescence imagers with enhanced chemiluminescence (ECL) substrates were then used to detect membranes. Image Lab software was applied in quantifying band intensities in each lane.
2.8. Statistical analysis
Data were analyzed with GraphPad PRISM (version 9.2.0), with the data being represented by mean ± SEM. Analyses on significance among multiple groups were performed using one-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons. P < 0.05 stood for statistical significance.
3. Results
3.1. Roles of HES in non-fasting blood glucose levels, OGTT levels, body weight, and food intake
Diabetes is typically characterized by higher blood glucose levels, the increased food consumption, along with weight loss [27]. This work discovered that STZ-mediated diabetic mice significantly higher blood glucose contents in comparison with NC mice (P < 0.001, Fig. 1A). Further, the blood glucose values decreased significantly in diabetic mice after HES treatment (P < 0.05, Fig. 1A). According to OGTT analysis, DM group had evidently increased blood glucose contents 0, 30, 60, and 120 min following 2 g/kg/d glucose administration (P < 0.0001). (Fig. 1B). In comparison with DM group, HES-exposed diabetic mice showed the rapider reduction of blood glucose contents, with significantly contents at every time point (P < 0.05, Fig. 1B). the development of diabetes mellitus will make the pancreatic islet function gradually decline, and then make insulin sensitivity decline and insufficient secretion, so that the body's glucose cannot be fully utilized, so as to consume the body fat and protein into energy to supply the body's needs. Or dehydration due to water electrolyte disorders can also cause weight loss [28]. Unstoppable weight loss is also a red flag with diabetes, and it often indicates poor blood sugar control or an exacerbation of the condition. STZ-induced diabetic mice had evidently increased food intake (P < 0.05, Fig. 1D), along with the remarkably reduced body weight (P < 0.0001, Fig. 1C). HES treatment in diabetic mice significantly reduced the food intake (P < 0.01, Fig. 1D) while suppressing body weight loss (P < 0.05, Fig. 1C).
Fig. 1.
HES improves blood glucose content, body weight, glucose tolerance as well as food consumption of DM mice. (A) blood glucose in mice at 0d before modeling, after STZ injection (-4d), 0d before and 3, 7, 14, 21, and 28d after hesperidin administration. (B) blood glucose contents within mice in OGTT after 28 days of hesperidin administration. (C) body weight in mice at 0d before modeling, after STZ injection (-4d), 0d before and 3, 7, 14, 21, and 28d after hesperidin administration. (D) mice eating at 0d before modeling, after STZ injection (-4d), and 7, 14, 21, and 28d after hesperidin treatment. Results are represented by mean ± SEM (n = 10 in each group). Two groups were compared by one-way ANOVA and Tukey-Kramer multiple comparisons. p < 0.05 stood for statistical significance. *P < 0.05 compared with NC group. (***P < 0.001 vs NC. ****P < 0.0001) vs NC. (#P < 0.05, ##P < 0.01) vs DM group.
3.2. Roles of HES in serum INS, GC, HbA1C, C-peptide, CXCL10, and GLP-1 contents
Compared with the NC group, STZ-mediated diabetic mice exhibited significantly reduced C-peptide and INS contents in serum; however, these levels normalized after HES treatment (P < 0.01, Fig. 2A and D). In contrast, diabetic mice exhibited significantly higher serum levels of HbA1C and GC compared to the normal group; however, these levels also normalized after HES treatment (P < 0.01, Fig. 2C and B). We also examined serum CXCL10 content within mice as it is related to pancreatic β-cell apoptosis [29]. We observed that CXCL10 contents in serum remarkably elevated within the diabetic mice in comparison with the NC group; however, again, these levels normalized after HES treatment (P < 0.05, Fig. 2F). Additional evidence showed that serum GLP-1 levels within diabetic mice remarkably decreased in comparison with NC group, and again, HES treatment effectively normalized these levels (P < 0.01, Fig. 2E). And this indication also provided clues for us to subsequently trace the source of nascent β-cells.
Fig. 2.
HES alleviates functions of pancreatic β-cells and oxidative stress within DM mice (A) INS, (B) GC, (C) HbA1C, (D) C-Peptide, (E) GLP-1, (F) CXCL10 levels in serum measured using ELISA kit. Results are represented by mean ± SEM (n = 10 each). Two groups were compared by one-way ANOVA and Tukey-Kramer multiple comparisons. (**P < 0.01) vs. NC group. (#P < 0.05, ##P < 0.01) vs DM group.
3.3. Role of HES in inflammatory infiltration in mouse islet cells
Microscopic observation of islet endocrine cell clusters at 100X magnification in hematoxylin-eosin-stained mouse pancreatic follicles revealed that relative to NC group, DM group showed fewer islets, which were scattered in a sporadic fashion and were structurally atrophied. These observations in the diabetic mice seemed to ameliorate after HES treatment (Fig. 3).
Fig. 3.
Morphological effects of HES on the pancreas of DM mice.
At 400X magnification, the mice in the NC group exhibited intact islets with round or oval cell clusters and clear borders. In contrast, the islet endocrine cell clusters of DM group mice were disorganized, with epithelial cell vacuolation, edema, interstitial congestion, inflammatory cell infiltration, peripheral omental adipose tissue necrosis, and a faintly visible outline of adipocytes. These observations in the diabetic mice improved after HES treatment (Fig. 3). These findings suggested that HES exerted a protective and anti-inflammatory role on islet endocrine cell mass in diabetic mice.
3.4. Effect of HES on the α- and β-cell numbers within diabetic mice
We evaluated islet morphology as well as α- and β-cell numbers in each group of mice by double staining with insulin+/glucagon+ immunofluorescence. The overall islet area in the DM group showed extreme atrophy (Fig. 4F) (P < 0.01), and the ins+ positive region was barely visible, with less than 20 % of its expression (Fig. 4E) (P < 0.001). The number of β-cells represented decreased dramatically (Fig. 4C),Among them, there were less than 100 signal points in the individual islet endocrine cell clumps (P < 0.001) and less than 20%with respect to the α-cell ratio presentation (Fig. 4D) (P < 0.0001). In contrast, the Gcg+ area tended to expand in comparison with NC group, while alpha cell number represented by the average individual islet mass could exceed 150 (Fig. 4D) (both P < 0.01), exceeding insulin-positive block proportion by nearly 80 % (Fig. 4D) (P < 0.0001); The persistent atrophy of DM islets was alleviated after HES intervention (P < 0.05, P < 0.01) (Fig. 4F), ins+ positive area increased (P < 0.01) (Fig.4D), and β-cell number elevated nearly 2 times relative to model group (Fig. 4C) (P < 0.01); In contrast, Post-HES treatment, the proportion of Gcg+ area was reduced (P < 0.01) (Fig. 4D) α-cell number was reduced (P < 0.05, P < 0.01) (Fig. 4B); Composition structural analysis. In mice of the NC group, the islets had mature β-cells within center whereas α-cells in periphery; thus, showing the typical central-peripheral structure (Fig. 4A). In contrast, among STZ-exposed diabetic mice, α-cells invaded islet center, disrupting the original central-peripheral structure, and very few remaining β-cells were scattered in the glucagon+ area (Fig. 4A). Meanwhile, we found that islet structure in diabetic mice started to recover, and the previously scattered INS+ cells reappeared in the central islets, and the Gcg+ cells moved outward from the central islet location. This indicates that the newborn islet β-cells may exist at the expense of α cells.
Fig. 4.
HES alters the composition of islet endocrine cells in DM mice. (alpha- and beta-cell localization within pancreas were measured using insulin+ (green), glucagon+ (red) immunoreactivity and quantified with Image J software.) (A) Typical images for islets displaying insulin+,glucagon+ immunoreactivity in every group of mice. Scale bar = 100 μm. (B,C) Quantitative analyses of alpha- and beta-cells on each islet slice. (D) Beta-to-alpha-cell area ratio. (E) Percentage beta cells and αcells of total islet cells conted. (F) Islet area. Data are represented by mean ± SEM of 3 mice. (**P<0.01, ***P<0.001, ****P<0.0001) vs. NC group. (#P<0.05, ##P<0.01) vs. DM group.
3.5. Anti-apoptotic effect of HES on pancreatic islet cells of diabetic mice
Islet cell apoptosis of mice was assessed through TUNEL assay. Compared to the NC group, we observed that close to 80 % of apoptotic signaling sites were expressed in the islet endocrine cell mass of mice in the DM state (P < 0.01) (Fig. 5A and B), suggesting that the apoptotic program is ongoing in islet cells (P < 0.01) (Fig. 5A and B). Further, we observed a reduction in the apoptotic signals in diabetic mice exposed to HES administration (P < 0.05, P < 0.01) (Fig. 5A and B). Apoptosis range can be controlled below 60 % by HES demonstrating that HES significantly inhibited the apoptosis of pancreatic β-cells.
Fig. 5.
HES decrease islet apoptosis in DM mice. (The islets apoptosis was analyzed through TUNEL assay and quantitatively examined by ImageJ software). (A) Typical image for islets exhibiting TUNE (red) Scale bar = 100 μm. (B) Immunoreactivity in every group. Results are mean ± SEM in 3 mice. (**P<0.01) vs. NC group. (#P<0.05, ##P<0.01) vs. DM group.
3.6. Role of HES in damaged pancreatic islet cell growth within diabetic mice
Islet cell proliferation within diabetic mice was assessed using Ki-67+/INS+ immunofluorescence double staining. Our results showed that Ki-67+ cells of NC mice showed major localization within islet periphery, with a larger area and higher number of INS+ cells (Fig. 6A and B). On the other hand, in diabetic mice, Ki-67+ cells were barely visible and had a smaller area, expressed in less than 1 %,Meanwhile with fewer INS+ cells (P < 0.05; Fig. 6A and B). Driven by the role of HES, both Ki-67+ and INS+ cells in diabetic mice started to increase in area and number; and Ki-67+ cells were mainly located in the INS+ region (P < 0.01; Fig. 6A and B). Based on the above results, HES induces pancreatic β-cell proliferation.
Fig. 6.
HES promotes pancreatic β-cell growth of DM mice. (Beta cells and Ki-67 localization was analyzed by insulin+ (green), Ki-67+ (red) immunoreactivity and quantitatively analyzed by Image J software.) (A) Typical images for islets displaying insulin+, Ki-67 + immunoreactivity of every group. Scale bar = 50 μm. (B) Quantification of the area of Ki-67-positive regions. (C) Quantification of the region of overlap between INS+ and Ki-67+ immunoreactivity. Results are mean ± SEM in 3 mice. (*P<0.05, ***P<0.001) vs. NC group. (##P<0.01) vs. DM group.
3.7. Role of HES in endocrine cell lineage in pancreatic islets in diabetic mice
Islet β-cell endogenous proliferation in HES-treated diabetic mice was assessed using insulin islet glucagon immunofluorescence staining as well as Ki-67+/INS+. However, the area of nascent islet β-cells we found tended to be in the positive range of the original α-cells. We, therefore, suspected that the newborn islet β-cells might be obtained based on α-cells. For testing this hypothesis, we traced the α-cell lineage using β-cell-specific marker gene NKX6.1. We were surprised to find that pancreatic islet α-cells in HES-treated diabetic mice released NKX6.1 signals, compared to model set data (P < 0.01; Fig. 7A–D). NKX6.1 signal level was effectively increased in comparison with DM group (Fig. 7B) (P < 0.05, P < 0.01), while the portion of this overlap with the α cell signal reached 30 %–40 % (Fig. 7C) (P < 0.001), and the proportional relationship between α cell and NKX6.1 signal expression showed a completely opposite trend (Fig. 7D) (P < 0.001). It indicates that the α-cells started to converge into the β cells at the time. This finding indicated that α-cells are no longer pure α-cells but are gradually in the process of transformation to β-cells.
Fig. 7.
Localization of the α cells lineage in nascent β cells. (A) Photographs are representative showing immunostaining for NKX6.1 and glucagon. The alpha cells indicated by arrows in the image are magnified in the lower left corner. Typically, α-cells after HES treatment began to show positive signals of NKX6.1, a transcription factor specific to β-cells. (B, C, D) Quantitative analysis on NKX6.1 expression in α-cells. Data are mean ± SEM for 3 mice. (*P<0.05) vs. NC group. (##P<0.01) vs. DM group.
Further, we assessed the α-cell-specific marker gene, MafB, to trace α-cell lineage on newborn pancreatic β-cells. Our results showed that the MafB gene signal, which is only present in α-cells, was also released in the newborn β-cells (P < 0.01; Fig. 8A and B). with the expression ranging from 15 % to 30 % (P < 0.01) (Fig. 8C). This finding demonstrated the cross-spectral transdifferentiation of nascent β-cells derived from α-cells again.
Fig. 8.
β cells lineage localization in αcells. (A) Photographs are representative of immunostaining showing MafB and insulin. The β-cells indicated by arrows are magnified in the lower left corner of the image. It is noteworthy that after HES treatment nascent β-cells started to show positive signals for the α-cell-specific transcription factor MafB. (B, C) Quantification of β-cell expression of MafB. Data are mean ± SEM for 3 mice. (*P<0.05) vs. NC group. (##P<0.01) vs. DM group.
3.8. Effect of HES on critical transcription factors related to α-cell transdifferentiation into β cells
HES remarkably up-regulated Pax4 gene expression in comparison with DM and NC groups (P < 0.01) (Fig. 9). Meanwhile decreased Arx gene level (P < 0.01), and with regard to trends,Pax4 was even highly expressed at the Arx gene level (Fig. 9). The high expression of Pax4 gene in Arx is also an important inflection point that drives α precursor cells towards β-cell differentiation. Islet cell subtypes are determined by cross-suppression of Pax4 and Arx factors,and the ratio of the two is even directly implicated in or determines α-and β-cell lineage selection [30].The maintenance of a high Pax/arx ratio is associated with a shift in favor of the maintenance of the β-cell phenotype,and vice versa in favor of α-lineage programming [7,9,31,32].
Fig. 9.
HES promotes ectopic expression of PDX-1, NGN3, together with MafA transcription factors within pancreatic tissues in diabetic mice, Simultaneous inversion of the expression of the alpha cell fate determinant gene Pax4 with ARX. (A) mRNA expression levels of MafA, Ngn3, and PDX-1, important transcription factors related to pancreatic islet growth and development and regeneration, were detected by RT-PCR. (B) Ratio of grayscale values of the target and internal reference bands. Results are mean ± SEM (n = 3 each). Two groups were compared by one-way ANOVA and Tukey-Kramer multiple comparisons. (**P<0.01) vs. NC group. (#P<0.05, ##P<0.01) vs. DM group.
NGN3, MafA and PDX-1 are critical transcription factors related to β-cell developmental maturation [33]. According to our results, the mRNA expression of all these factors was remarkably upregulated within pancreatic tissues of HES-treated diabetic mice (Fig. 9) (P < 0.05, P < 0.01), which synchronously corresponded to the former Ki-67 expression results.
3.9. Role of HES in GLP-1 secretion in diabetic mice
We conjecture that HES can stimulate GLP-1 secretion by activating the TGR5 pathway in enteroendocrine cells, then GLP-1 further influences α-cell profiles in the pancreas [34]. In the previous serum indicator results. The serum GLP-1 content in each group was analyzed by enzymatic assay, compared with the DM group, it was found that HES could effectively promote GLP-1 levels in DM mice (P < 0.01) (Fig. 2E). which is sufficient to explain HES treatment in diabetic mice significantly reduced the food intake (P < 0.01, Fig. 1D) while suppressing body weight loss (P < 0.05, Fig. 1C).Because GLP-1 contributes to the ileocecal brake, a circuit that regulates the rate of gastric emptying, the rate of nutrients entering the duodenum is balanced with the rate of nutrient absorption in the upper small intestine, restoring homeostasis to the system [35]. Thus, we started by exploring the factors that lead to elevated GLP-1 levels. Feedback from protein immunoblotting results of upstream and downstream targets of TGR5 receptor by ileal tissue of each group of mice, relative to model group, TGR5/cAMP-CREB/GLP-1 signals levels were increased to different degrees in every group after HES intervention (P < 0.01, P < 0.001) (Fig. 10A–E). It is suggested that the factors that enable HES to promote the rise of GLP-1 levels in DM rats are likely to originate from the guidance of TGR5 receptor activation. Which Consistent with the report that TGR5 induces GLP-1 secretion in cultured mouse enteroendocrine cells [34].
Fig. 10.
HES promotes TGR5 expression in DM mouse intestinal tissue and stimulates the release of GLP-1 (A) Representative images showing TGR5, cAMP, CREB, and GLP-1 protein blots. (B,C,D,E) Quantification of TGR5, cAMP, CREB, and GLP-1. Results are mean ± SEM (n = 3 each). Two groups were compared by one-way ANOVA and Tukey-Kramer multiple comparisons. (*P < 0.05, **P<0.01, ***P < 0.001) vs. NC group. (##P<0.01, ###P < 0.001) vs. DM group.
3.10. Role of GLP-1 in α-cell transdifferentiation in β-cells
GLP-1 has been shown to promote β-cell regeneration both in vitro and in vivo [[36], [37], [38]], and has an effect on β-cell differentiation and neogenesis [31,39,40]. This may be attributed to the role of the GLP-1/GLP-1R signaling axis in the expansion and differentiation of pancreatic endocrine cells during their development [41,42]. so the expression of GLP-1 in mouse pancreatic tissues is the key to verify the establishment of the subsequent mechanism of the effect of GLP-1 on the regeneration of β-cells. Through comparing the expression of WB results within mouse pancreatic tissues, we noticed that GLP-1 and its sole receptor GLP-1R would be effectively facilitated by HES (Fig. 11A–C) (P < 0.01, P < 0.001).
Fig. 11.
HES promotes the GLP-1 and GLP-1R protein levels within pancreatic tissues of DM mice. (A) Representative images showing GLP-1and GLP-1R protein blots. (B, C) Quantitative analysis on GLP-1 as well as GLP-1R. Results are mean ± SEM (n = 3 each). Two groups were compared by one-way ANOVA and Tukey-Kramer multiple comparisons. (*P < 0.05, **P<0.01) vs. NC group. (##P<0.01) vs. DM group.
Next, we compared each protein expression within the cAMP-CREB/IRS2/PDX-1 pathway in the pancreatic tissue of each group of mice. It was shown that HES did increase the expression level of each protein under this pathway of cAMP-CREB/IRS2/PDX-1 (P < 0.05, P < 0.01) (Fig. 12A–E). It means that, Before prompting α precursor cells to express the transcription factor PDX-1 regarding the developmental initiation of β-cell maturation, may be triggered by activation of the IRS2 receptor.
Fig. 12.
HES facilitates the protein levels of cAMP, CREB, IRS2 and PDX-1 within pancreatic tissues in DM mice. (A) Representative images showing IRS2, cAMP, CREB, and PDX-1 protein blots. (B,C,D,E) Quantification of IRS2, cAMP, CREB, and PDX-1. Results are mean ± SEM (n = 3 each). Two groups were compared by one-way ANOVA and Tukey-Kramer multiple comparisons. (*P < 0.05, **P<0.01) vs. NC group. (#P < 0.05,##P<0.01) vs. DM group.
4. Discussion
Diminishing pancreatic β-cell function and deficiency exacerbate DM progression. The promotion of endogenous proliferation of β-cells seems to hold a great therapeutic potential by slowing down the progression of DM or even reversing it. In this work, we evaluated neonatal β-cell origin within diabetic mice under HES treatment, including the self-replication of islet β-cells (Fig. 6A–C), α-to-β-cell transdifferentiation (Fig. 4A–F, Fig. 7A–D, Fig. 8A–C). The ability of GLP-1 to promote the α-cell transdifferentiation in β-cells is reported by several previous articles [43]. This data shows that both pathways are dependent on HES-induced GLP-1 expression. Using serum biochemistry and intestinal and pancreatic protein quantification, we detected a rise in GLP-1 to varying degrees in HES-treated mice (Fig. 2E; Fig. 10A and E; Fig. 11A and B).
How are GLP-1 levels promoted in diabetic mice under HES treatment? There is increasing evidence that colonic and distal ileal enteroendocrine L cells produce GLP-1 [44,45]. GLP-1 synthesis involves the activation of TGR5 [46]. Previous studies on gut-restricted pre-glucagon knockout mice also showed that intestine was the major GLP-1 source within the blood [47].
TGR5 is a membrane receptor that combines with G protein-coupled bile acid receptor, Gpbar1. Its activation is achieved through bile acid through ligand internalization, induces the production of cAMP, and subsequently activates transcription factor home cAMP responsive-element binding protein (CREB). As a result, it causes target gene expression [48]. This process also paves the way to later switch the spectrum of α-cells (Fig. 10A–E).
G protein-coupled receptors (GPCRs) are huge receptor families, like TGR5, GPR119, GLP-1R, etc. [49]. They play several roles in a variety of pathways, including those involved in maintaining energy homeostasis and glucose metabolism. All GPCR receptor families contain a “7-transmembrane structural domain” that, after binding extracellular space ligands, activates several effector pathways to convert extracellular signals into downstream cascades [50].
But GPCR pathway will not be restricted on plasma membrane. It is controlled spatiotemporally using endocytic pathways, such as GLP-1 [51,52]. This endocytic trafficking approach also allows for the construction of adaptive systems that maintain intercellular homeostatic relationships as well as receptor-regulated pathway localization together with duration to achieve specific cellular responses [53]. Such an approach opens the door for GLP-1 to be effectively internalized into α precursor cells and further change the direction of its transcriptional gene expression.
We inferred that the activation of TGR5 receptor may be responsible for GLP-1 levels, which were promoted in diabetic mice after HES treatment. The signaling properties based on G protein-coupled receptor family also coincided with a possibility that GLP-1, which promotes proliferation by HES, can efficiently be internalized into the cells and continue to promote the conversion of α precursor cells.
But with this, a new question arose, how does GLP-1 promote cross-spectrum transdifferentiation of α-cells? It is yet unclear how GLP-1 transforms the fate of pancreatic islet cells; a bridge between in and out of cells remains missing. In order to investigate this issue, we assessed both GLP-1 together with GLP-1R, both were GLP-1 receptors, within pancreatic tissue in diabetic mice. GLP-1 release in L cells is activated via cAMP elevation. GLP-1 just possesses one recognized receptor, that is, GLP-1R [54]. It is a ligand-specific receptor that acts primarily through the Gs signaling pathway3, and it also belongs to the category of GPCRs [54,55]. GLP-1R mostly locates within pancreas (β/δ-cells) [56]. When GLP-1 is exposed, before dissipation within the specified half-life time, the receptor can gradually accumulate in the body [57], where the long-term relation of G protein α subunits (Gαs) that possess activated receptors facilitates the persistent production of cAMP [58]. Moreover, cAMP has been recognized as a crucial endocytic player in regulating GPCR signaling and trafficking [59,60]. The GLP-1 hormone binds to GLP-1R in a specific manner [54]. GLP1R activation within pancreatic β-cells, which causes insulin secretion, also depends on stimulation of cAMP and elevation of plasma glucose concentration [61]. Our results also demonstrated that cAMP expression (Fig. 10A and C) followed the same trend as GLP-1 (Fig. 10A and E) and GLP-1R (Fig. 11A–C). This finding indicates that GLP-1 expression is associated with elevated cAMP levels during both its release from intestinal L cells and its binding to its receptor GLP-1R.
TGR5 activated by INT-777 and oleanolic acid(OA) in pancreatic β-cells along with gut endocrine cells, separately, can selectively induce Gαs. This, in turn, elevated the intracellular levels of cAMP and Ca2+. On the one hand, further activation of Epac in intestinal endocrine cells increases the hydrolysis of phosphoinositide(PI). and eventually releases GLP-1 as well as peptide YY (PYY) [62]. Besides, insulin is released from the pancreatic β cells. Embryonic stem cells (ESCs) can help pancreatic islet endocrine cells grow and differentiate during development by activating GLP-1/GLP-1R signal shaft [41,42].
The key transcription factor for pancreatic development, PDX-1, is expressed by ESCs that derive from the primitive undivided epithelial cells of the intestinal duct. At this stage, the endocrine precursor transcription factor Ngn3 initiates the delineation of the pancreatic endocrine cell lineage [63]. The relative Arx and Pax4 expression levels govern the α-/β-cell differentiation [7,9,31,32]. Our findings also demonstrated higher mRNA expression levels of Pax4 within pancreas in comparison with model group HES administration group compared to Arx (Fig. 9). GLP-1 can act as an important growth factor for α precursor cell expansion [64,65].
β-cell neogenesis promoted via GLP-1/GLP-1R system is inseparable from IRS2 [66]. GLP-1 activates CREB, which, in turn, increases IRS2 transcription [67]. IRS2 plays an indispensable role in islet β-cell development. It acts by maintaining the function and viability of β-cells. IRS2 can achieve islet quality control, including insulin, IGF1/2, GLP-1, growth hormone, and d-glucose, by regulating downstream growth factors within β-cells [68]. The corresponding cAMP and Ca2+ ions can, in turn, increase IRS2 activity within β-cells [69]. Noteworthily, during this process, insulin also elevates IRS2 levels within pancreatic β-cells. According to our results, IRS2 was up-regulated within pancreas in diabetic mice after HES administration (Fig. 12A–D).
Other transcription factors, including MafA, NGN3, or Pdx1, have essential roles in controlling functions of mature β-cells together with glucose-triggered insulin production from adequate upgrowth-induced nascent islet β-cells [70]. Among these, PDX-1 regulates component binding and enhances insulin gene transcription. It has an important effect on overall early pancreatic development, β-cell differentiation and maturation [71]. This work used immunofluorescence to assess the alteration of α-to-β cell composition ratio after HES administration (Fig. 4A–F). Similarly, the association with increased PDX-1 protein and mRNA expression within pancreatic tissues validates the previous view (Fig. 9, Fig. 12E). It is an essential factor for endocrine and exocrine pancreatic cell maturation. The first node of influence of IRS2 in reversing the fate of the α-cell lineage after being activated also originates from the production of the stimulatory transcription factor PDX-1 [71]. Our data showed that the HES treatment of the diabetic mice led to varying degrees of upregulation of MafA, PDX-1, and NGNG3 mRNAs in pancreatic tissues (Fig. 9). This finding indicated that HES plays a role in promoting the elevation in key transcription factors associated with pancreatic islet growth and regeneration.
When pancreatic progenitor cells express PDX-1, the PDX-1 promoter forcibly overexpresses Ngn3, which, in turn, drives the pancreatic islet progenitor cells population to begin to appear biased toward an α-cell precursor population; Early endocrine lineage cells are α precursor cells, and they are defined by the presence of the endocrine-promoting transcription factor Ngn3 [72,73]. Ngn3 accounts for a sign of initiating the early primitive endocrine lineage [74].Ngn3+ progenitor cells are divided into α-/β-cells based on relative Pax4 and Arx expression [75,76]. Subsequently, the respective specific transcription factors of α- and β-cells were then committed to the delineation of α- and β-cell lineage fates, respectively [77]. Our findings from the islets of HES-treated diabetic mice can also explain why α- and β-cells present themselves with positive immunofluorescence results for specific transcription factors (Fig. 7, Fig. 8A).
5. Conclusion
In conclusion, HES can alter the compositional state of endocrine cells within pancreas in diabetic mice. Co-localization with positive cells with NKX6.1+/Gcg+; MafB+/Ins+ confirmed that the nascent β-cells were possibly obtained based on α-cells. This process involves HES-induced activation of TGR5 receptors in diabetic mice and the subsequent GLP-1 upregulation. GLP-1 then uses GLP-1/GLP-1R system for mediating IRS2 upregulation, thus stimulating the forced expression of Ngn3 via the PDX-1 promoter. The drug causes overexpression of PAX4 mRNA. PAX4 mRNA expression is even higher than Arx, this promotes α precursor cell development in β cells.
Abbreviations Full Name
| HES Hesperidin |
|---|
| DM NC SPF Diabetes mellitus Normal control Specific pathogen free |
| CXCL10 HFD Ins Gcg RT-PCR WB GLP-1 SOD GSH CXCL10 ELISA IF HE TGR5 cAMP CREB PDX-1 IRS2 MafA MafB PYY TUNEL OA PI ESCs Chemokine ligand-10 High-fat diet Insulin Glucagon Reverse transcription PCR Western Blotting Glucagon-like peptide-1 superoxide dismutase l-Glutathione Chemokine 10 Enzyme-linked immunosorbent assay immunofluorescence Hematoxylin and Eosin The G protein-cpupled receptor Cyclic Adenosine Monophosphate cAMP-responsive-element binding Pancreatic and duodenal homeobox 1 Insulin receptor substrate 2 V-Maf Musculoaponeurotic Fibrosarcoma Oncogene Homolog A V-Maf Musculoaponeurotic Fibrosarcoma Oncogene Homolog B peptide YY Terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling Oleanolic acid Phosphoinositide Embryonic stem cells |
Ethics approval and consent for participation
Our animal protocols gained approval from Ethics Committee for Animal Experimentation of Guangdong Pharmaceutical University (Guangdong, China) (protocol code GDPulac2019180, March 15th, 2022).
We declare that our animal experimental protocols gained approval from Animal Ethics Committee of Guangdong Pharmaceutical University, acceptance number GDPulacspf2017668.
We declare that all experimental methods were performed following review form of Animal Ethics Committee of Guangdong Pharmaceutical University. These include necessity of animal experiments, rationality of using animals, the rationality of using the number of animals, whether the surgical protocol meets the ethical requirements, whether the animal care measures meet the requirements, the rationality of the experimental cycle, and the guarantee of harmless disposal of animals after the experiments are strictly enforced by the regulations.
We state that every experiment was performed following ARREW reporting guidelines for unit allocation and animal numbers.
Consent for publication
Not applicable.
Data and material availability
The data utilized in the present work can be obtained from the manuscript.
Funding
The present work was supported by the National key R&D Plan “Research on Modernization of Traditional Chinese Medicine” [grant number 2018YFC1704200]; and the Major Basic and Applied Basic Research Projects of Guangdong Province of China [grant number 2019B030302005].
Data availability statement
Additional data are made available in supplementary tables of this manuscript. The authors will supply the relevant data in response to reasonable requests.
CRediT authorship contribution statement
Wang Zhang: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Lele Wu: Investigation. Ru Qu: Resources. Tianfeng Liu: Resources. Jiliang Wang: Resources. Ying Tong: Resources. Weijian Bei: Writing – review & editing, Data curation. Jiao Guo: Data curation. Xuguang Hu: Funding acquisition.
Declaration of competing interest
Our authors claim no competing interests.
Acknowledgments
Our thanks should go to Guangdong Pharmaceutical University College of Traditional Chinese Medicine for providing advanced research equipment and Xiao Huan for his technical guidance during the experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35424.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ogurtsova K., et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Cornell S. Continual evolution of type 2 diabetes: an update on pathophysiology and emerging treatment options. Therapeut. Clin. Risk Manag. 2015;11:621–632. doi: 10.2147/TCRM.S67387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding L., Gysemans C., Mathieu C. β-Cell differentiation and regeneration in type 1 diabetes. Diabetes Obes Metab. 2013;15(Suppl 3):98–104. doi: 10.1111/dom.12164. [DOI] [PubMed] [Google Scholar]
- 4.Remedi M.S., Emfinger C. Pancreatic β-cell identity in diabetes. Diabetes Obes Metab. 2016;18(Suppl 1):110–116. doi: 10.1111/dom.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gromada J., Franklin I., Wollheim C.B. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr. Rev. 2007;28(1):84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 7.Thorel F., et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho J.M., et al. A novel dipeptidyl peptidase IV inhibitor DA-1229 ameliorates streptozotocin-induced diabetes by increasing β-cell replication and neogenesis. Diabetes Res. Clin. Pract. 2011;91(1):72–79. doi: 10.1016/j.diabres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hasani K., et al. Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev. Cell. 2013;26(1):86–100. doi: 10.1016/j.devcel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Meier J.J., et al. Diminished glucagon suppression after β-cell reduction is due to impaired α-cell function rather than an expansion of α-cell mass. Am. J. Physiol. Endocrinol. Metab. 2011;300(4):E717–E723. doi: 10.1152/ajpendo.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henquin J.C., Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54(7):1720–1725. doi: 10.1007/s00125-011-2118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arda H.E., Benitez C.M., Kim S.K. Gene regulatory networks governing pancreas development. Dev. Cell. 2013;25(1):5–13. doi: 10.1016/j.devcel.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collombat P., Mansouri A. Turning on the beta-cell identity in the pancreas. Cell Cycle. 2009;8(21):3450–3451. doi: 10.4161/cc.8.21.9791. [DOI] [PubMed] [Google Scholar]
- 14.Miao X.Y., et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013;39:71–79. doi: 10.1016/j.peptides.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Bulotta A., et al. Cultured pancreatic ductal cells undergo cell cycle re-distribution and beta-cell-like differentiation in response to glucagon-like peptide-1. J. Mol. Endocrinol. 2002;29(3):347–360. doi: 10.1677/jme.0.0290347. [DOI] [PubMed] [Google Scholar]
- 16.Ghorbani A., Rashidi R., Shafiee-Nick R. Flavonoids for preserving pancreatic beta cell survival and function: a mechanistic review. Biomed. Pharmacother. 2019;111:947–957. doi: 10.1016/j.biopha.2018.12.127. [DOI] [PubMed] [Google Scholar]
- 17.Annadurai T., et al. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012;68(3):307–318. doi: 10.1007/s13105-011-0142-y. [DOI] [PubMed] [Google Scholar]
- 18.Adewole S.O., Caxton-Martins E.A., Ojewole J.A. Protective effect of quercetin on the morphology of pancreatic beta-cells of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2006;4(1):64–74. doi: 10.4314/ajtcam.v4i1.31196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maciel R.M., et al. Antioxidant and anti-inflammatory effects of quercetin in functional and morphological alterations in streptozotocin-induced diabetic rats. Res. Vet. Sci. 2013;95(2):389–397. doi: 10.1016/j.rvsc.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 2011;670(1):325–332. doi: 10.1016/j.ejphar.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoud A.M., et al. In vivo and in vitro antidiabetic effects of citrus flavonoids; a study on the mechanism of action. Int. J. Diabetes Dev. Ctries. 2015;35(3):250–263. [Google Scholar]
- 22.Homayouni F., et al. Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes; a randomized double-blind controlled clinical trial. Phytother Res. 2018;32(6):1073–1079. doi: 10.1002/ptr.6046. [DOI] [PubMed] [Google Scholar]
- 23.Hanchang W., et al. Hesperidin ameliorates pancreatic beta-cell dysfunction and apoptosis in streptozotocin-induced diabetic rat model. Life Sci. 2019;235 doi: 10.1016/j.lfs.2019.116858. [DOI] [PubMed] [Google Scholar]
- 24.Jung U.J., et al. The Hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004;134(10):2499–2503. doi: 10.1093/jn/134.10.2499. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhry Z.Z., et al. Streptozotocin is equally diabetogenic whether administered to fed or fasted mice. Lab Anim. 2013;47(4):257–265. doi: 10.1177/0023677213489548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckhardt B.A., et al. Accelerated osteocyte senescence and skeletal fragility in mice with type 2 diabetes. JCI Insight. 2020;5(9) doi: 10.1172/jci.insight.135236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayramoglu G., et al. Carvacrol partially reverses symptoms of diabetes in STZ-induced diabetic rats. Cytotechnology. 2014;66(2):251–257. doi: 10.1007/s10616-013-9563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krysiak R., Handzlik-Orlik G., Okopień B. [Diabetes insipidus] Przegl. Lek. 2014;71(12):711–719. [PubMed] [Google Scholar]
- 29.Antonelli A., et al. CXCR3, CXCL10 and type 1 diabetes. Cytokine Growth Factor Rev. 2014;25(1):57–65. doi: 10.1016/j.cytogfr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Napolitano T., et al. Pax4 acts as a key player in pancreas development and plasticity. Semin. Cell Dev. Biol. 2015;44:107–114. doi: 10.1016/j.semcdb.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Collombat P., et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138(3):449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unger R.H., Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A. 2010;107(37):16009–16012. doi: 10.1073/pnas.1006639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y., et al. PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Res. Ther. 2017;8(1):1–7. doi: 10.1186/s13287-017-0694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsuma S., Hirasawa A., Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 2005;329(1):386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 35.Gribble F.M., Reimann F. Metabolic Messengers: glucagon-like peptide 1. Nat. Metab. 2021;3(2):142–148. doi: 10.1038/s42255-020-00327-x. [DOI] [PubMed] [Google Scholar]
- 36.Yang L., et al. Puerarin protects pancreatic β-cells in obese diabetic mice via activation of GLP-1R signaling. Mol. Endocrinol. 2016;30(3):361–371. doi: 10.1210/me.2015-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y.F., et al. Loss of miR-182 affects B-cell extrafollicular antibody response. Immunology. 2016;148(2):140–149. doi: 10.1111/imm.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima M.J., et al. Efficient differentiation of AR42J cells towards insulin-producing cells using pancreatic transcription factors in combination with growth factors. Mol. Cell. Endocrinol. 2012;358(1):69–80. doi: 10.1016/j.mce.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 39.van der Meulen T., Huising M.O. Role of transcription factors in the transdifferentiation of pancreatic islet cells. J. Mol. Endocrinol. 2015;54(2):R103–R117. doi: 10.1530/JME-14-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lund A., et al. Glucagon and type 2 diabetes: the return of the alpha cell. Curr Diab Rep. 2014;14(12):555. doi: 10.1007/s11892-014-0555-4. [DOI] [PubMed] [Google Scholar]
- 41.Bai L., Meredith G., Tuch B.E. Glucagon-like peptide-1 enhances production of insulin in insulin-producing cells derived from mouse embryonic stem cells. J. Endocrinol. 2005;186(2):343–352. doi: 10.1677/joe.1.06078. [DOI] [PubMed] [Google Scholar]
- 42.Sanz C., Blázquez E. New gene targets for glucagon-like peptide-1 during embryonic development and in undifferentiated pluripotent cells. Am. J. Physiol. Endocrinol. Metab. 2011;301(3):E494–E503. doi: 10.1152/ajpendo.00116.2011. [DOI] [PubMed] [Google Scholar]
- 43.Kim W., Egan J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kauth T., Metz J. Immunohistochemical localization of glucagon-like peptide 1. Use of poly- and monoclonal antibodies. Histochemistry. 1987;86(5):509–515. doi: 10.1007/BF00500625. [DOI] [PubMed] [Google Scholar]
- 45.Eissele R., et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Invest. 1992;22(4):283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 46.Katsuma S., et al. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J. Biol. Chem. 2005;280(20):19507–19515. doi: 10.1074/jbc.M412385200. [DOI] [PubMed] [Google Scholar]
- 47.Song Y., et al. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metab. 2019;30(5):976–986.e3. doi: 10.1016/j.cmet.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawamata Y., et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003;278(11):9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 49.Cvijic M.E., et al. GPCR profiling: from hits to leads and from genotype to phenotype. Drug Discov. Today Technol. 2015;18:30–37. doi: 10.1016/j.ddtec.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Rohrer D.K., Kobilka B.K. G protein-coupled receptors: functional and mechanistic insights through altered gene expression. Physiol. Rev. 1998;78(1):35–52. doi: 10.1152/physrev.1998.78.1.35. [DOI] [PubMed] [Google Scholar]
- 51.Calebiro D., Maiellaro I. cAMP signaling microdomains and their observation by optical methods. Front. Cell. Neurosci. 2014;8:350. doi: 10.3389/fncel.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jong Y.I., Harmon S.K., O'Malley K.L. GPCR signalling from within the cell. Br. J. Pharmacol. 2018;175(21):4026–4035. doi: 10.1111/bph.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lobingier B.T., von Zastrow M. When trafficking and signaling mix: how subcellular location shapes G protein-coupled receptor activation of heterotrimeric G proteins. Traffic. 2019;20(2):130–136. doi: 10.1111/tra.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992;89(18):8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee Y.S., Jun H.S. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63(1):9–19. doi: 10.1016/j.metabol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Richards P., et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widmann C., Dolci W., Thorens B. Agonist-induced internalization and recycling of the glucagon-like peptide-1 receptor in transfected fibroblasts and in insulinomas. Biochem. J. 1995;310(Pt 1):203–214. doi: 10.1042/bj3100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Girada S.B., et al. Gαs regulates Glucagon-Like Peptide 1 Receptor-mediated cyclic AMP generation at Rab5 endosomal compartment. Mol Metab. 2017;6(10):1173–1185. doi: 10.1016/j.molmet.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bahouth S.W., Nooh M.M. Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks. Cell. Signal. 2017;36:42–55. doi: 10.1016/j.cellsig.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pavlos N.J., Friedman P.A. GPCR signaling and trafficking: the long and short of it. Trends Endocrinol Metab. 2017;28(3):213–226. doi: 10.1016/j.tem.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gromada J., Holst J.J., Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch. 1998;435(5):583–594. doi: 10.1007/s004240050558. [DOI] [PubMed] [Google Scholar]
- 62.Kumar D.P., et al. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem. Biophys. Res. Commun. 2012;427(3):600–605. doi: 10.1016/j.bbrc.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saleh M., Gittes G.K., Prasadan K. Alpha-to-beta cell trans-differentiation for treatment of diabetes. Biochem. Soc. Trans. 2021;49(6):2539–2548. doi: 10.1042/BST20210244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ellingsgaard H., et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kilimnik G., et al. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of ß-cell regeneration. Islets. 2010;2(3):149–155. doi: 10.4161/isl.2.3.11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park S., et al. Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J. Biol. Chem. 2006;281(2):1159–1168. doi: 10.1074/jbc.M508307200. [DOI] [PubMed] [Google Scholar]
- 67.Jhala N.C., et al. Infiltration of Helicobacter pylori in the gastric mucosa. Am. J. Clin. Pathol. 2003;119(1):101–107. doi: 10.1309/YDTX-KE06-XHTH-FNP2. [DOI] [PubMed] [Google Scholar]
- 68.Li Y., et al. Complete genome sequence of the novel lytic avian pathogenic coliphage NJ01. J. Virol. 2012;86(24):13874–13875. doi: 10.1128/JVI.02727-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amacker-Françoys I., et al. The metabolisable hexoses D-glucose and D-mannose enhance the expression of IRS-2 but not of IRS-1 in pancreatic beta-cells. Exp. Clin. Endocrinol. Diabetes. 2005;113(8):423–429. doi: 10.1055/s-2005-865803. [DOI] [PubMed] [Google Scholar]
- 70.Hang Y., Stein R. MafA and MafB activity in pancreatic β cells. Trends Endocrinol Metab. 2011;22(9):364–373. doi: 10.1016/j.tem.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McKinnon C.M., Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia. 2001;44(10):1203–1214. doi: 10.1007/s001250100628. [DOI] [PubMed] [Google Scholar]
- 72.Schwitzgebel V.M., et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127(16):3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 73.Harb G., et al. Ectopic expression of neurogenin 3 in neonatal pig pancreatic precursor cells induces (trans)differentiation to functional alpha cells. Diabetologia. 2006;49(8):1855–1863. doi: 10.1007/s00125-006-0299-z. [DOI] [PubMed] [Google Scholar]
- 74.Johansson K.A., et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell. 2007;12(3):457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Seymour P.A., Sander M. Historical perspective: beginnings of the beta-cell: current perspectives in beta-cell development. Diabetes. 2011;60(2):364–376. doi: 10.2337/db10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Courtney M., et al. In vivo conversion of adult α-cells into β-like cells: a new research avenue in the context of type 1 diabetes. Diabetes Obes Metab. 2011;13(Suppl 1):47–52. doi: 10.1111/j.1463-1326.2011.01441.x. [DOI] [PubMed] [Google Scholar]
- 77.Habener J.F., Stanojevic V. α-cell role in β-cell generation and regeneration. Islets. 2012;4(3):188–198. doi: 10.4161/isl.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data are made available in supplementary tables of this manuscript. The authors will supply the relevant data in response to reasonable requests.