Abstract
Exposure to air pollution poses significant risks to human health, including detrimental effects on the reproductive system, affecting both men and women. Our prospective clinical study aimed to assess the impact of prolonged air pollution exposure on sperm quality in male patients attending a fertility clinic. The current study was conducted at Sri Narayani Hospital and Research Centre in Vellore, Tamil Nadu, India, and the study examined sperm samples obtained from individuals with extended exposure to air pollution. Microscopic analysis, including scanning electron microscopy (SEM), was conducted to evaluate sperm morphology. At the same time, atomic absorption spectroscopy (AAS) determined the presence of heavy metals, including Zinc (Zn), Magnesium (Mg), Lead (Pb) and Cadmium (Cd), known to affect sperm production. Our findings revealed that long-term exposure to air pollution adversely affects sperm quality, manifesting in alterations during the spermatogenesis cycle, morphological abnormalities observed through SEM, and impaired sperm motility. Additionally, epidemiological evidence suggests that elevated levels of cadmium and lead in the environment induce oxidative stress, leading to sperm DNA damage and reduced sperm concentrations. These results underscore the urgent need for environmental interventions to mitigate air pollution and protect reproductive health.
Keywords: Air pollution, Heavy metals, Spermatogenesis, Infertility, Atomic absorption spectroscopy (AAS)
Graphical Abstract
Highlights
-
•
The study revealed that prolonged exposure to air pollution significantly affects sperm quality.
-
•
Morphological abnormalities in sperm cells were observed by SEM analysis.
-
•
Heavy metals in the sperm cells were quantified by AAS analysis.
-
•
Cd and Pb damage the DNA of the sperm cell.
1. Introduction
Air pollution and heavy metal exposure have become global health concerns due to their detrimental impacts on human health. Although their effects on the cardiovascular and respiratory systems have been well investigated, their implications on male fertility and reproductive health have received more attention recently [1]. Exposure to heavy metals and air pollution has a deleterious consequence on sperm quality, which is a vital component of male fertility [2]. Understanding the mechanisms through which these pollutants affect sperm quality is crucial to establishing strategies for reducing their negative impacts.
Prior research has shown that air pollution, particularly fine particulate matter (PM2.5), has been correlated to impaired male reproductive function [3], [4]. Research has demonstrated that PM2.5 can enter the bloodstream and deeply penetrate the respiratory system, with the potential to reach the testes and impact spermatogenesis [5]. Numerous epidemiological research has found links between exposure to PM2.5 and lowered markers of sperm quality in men [6], [7], [8], [9]. An investigation carried out in China, for instance, discovered a correlation between increased ambient PM2.5 levels and reduced sperm motility and morphology in men residing in high-pollution urban regions [10].
Apart from air pollution, exposure to heavy metals has been recognized as a possible risk factor for low-quality sperm. It has been discovered that some heavy metals, including zinc (Zn), lead (Pb), cadmium (Cd), and mercury (Hg), negatively impact sperm parameters [1]. Increased sperm DNA fragmentation and lower sperm motility and count have both been linked to lead exposure [11]. Exposure to Cd has been associated with reduced sperm concentration, motility, count, and morphology, as well as increased fragmentation of sperm DNA [12], [42]. Cadmium causes structural damage to the seminiferous tubules, Sertoli cells, blood testis barrier, and the vascular system of the testis, resulting in the loss of sperm. In addition, it inhibits Leydig cell formation, inhibits its function, and causes DNA damage [40]. There's been evidence linking Hg exposure to reduced sperm motility and morphology [13]. In a study, it is mentioned how oxidative stress driven by heavy metals, damages DNA in spermatozoa [41]. Additionally, Sun et al. [14] observed that a Zn-deficient diet resulted in lower semen quality, faulty autophagy, an imbalance in zinc homeostasis, and abnormalities in testicular structure. Zinc and pentoxifylline significantly increased sperm motility, concentration, inflammatory variables, and metabolic indicators, according to Dadgar et al. [15]. Zinc controls phospholipids and increases ATP for sperm-free motility. Toxic metals such as Pb and Cd that act as endocrine disruptors have been shown to affect hormones responsible for sperm production [16]. Furthermore, Cd can take the role of essential metals like Cu2+, Fe2+, and particularly Zn2+, which is a cofactor for a number of the body's enzymes [17]. The presence of Pb in the human body causes a reduction in both reproductive hormone and its receptors, as well as direct enzymatic action on the alpha estrogenic receptor [18]. This leads to a decrease in antioxidant activities such as catalase and alkaline phosphatase, an increase in reactive oxygen species (ROS) inhibition, and disruption of spermatogenesis [19].
A few clinical studies have directly assessed the effect of air pollution and heavy metal exposure on sperm quality in a controlled clinical setting, and the majority of this evidence comes from laboratory or epidemiological investigations. Thus, the purpose of this prospective research project is to examine the relationship, in a clinical context, between heavy metal exposure, air pollution (PM2.5 and NO2), and sperm quality. We intend to clarify the possible pathways connecting environmental exposures to male infertility by enlisting male participants from a reproductive clinic and performing thorough evaluations of their exposure levels to heavy metals, air pollutants, and sperm quality criteria.
2. Materials and methods
2.1. Institute ethical approval number
This study is part of major research work, and the ethical clearance was approved by the Institutional Human Ethical Committee, VIT, Vellore. Ref No: VIT/IECH/XI/2022/06 & Sri Narayani Hospital and Research Center Ethical Committee, Ref IEC No: 36/04/02/23.
2.2. Study population and number
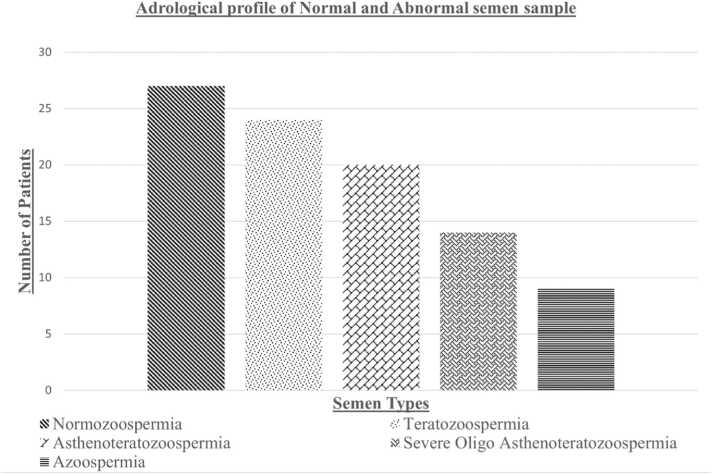
The study was conducted among the patients (aged 23–47) attending the Department of Reproductive Medicine unit at Sri Narayani Hospital and Research Centre, Vellore, Tamil Nadu, India. We included fertile and infertile men who are exposed to air pollution based on their occupation to evaluate and know the differences. The semen samples were collected from Sri Narayani Hospital and Research Centre, Vellore, Tamil Nadu, India. The number of samples collected was N = 94 (both fertile and infertile). The andrological profile of the Study subjects are mentioned in Fig. 1, Teratozoospermia (n = 24), Asthenoteratozoospermia (n = 20), Severe Oligo Asthenoteratozoospermia (n = 14), Azoospermia (n = 9), and Normozoospermia (n = 27).
Fig. 1.
Andrological profile showing semen type and the number of patients.
2.3. Sample collection and analysis
Plastic sterile containers were issued to the patients to collect the semen samples. Collected samples were taken to the laboratory immediately for further examination. As per Agarwal et al. [20] and WHO guidelines 5th laboratory manual for the examination of human semen tests is done. The macroscopic analysis of sperm comprises the following parameters: Appearance (colour of semen sample), liquification, Volume, and pH. The Makler chamber (Fig. 2) is used for counting sperm cells. Labomed microscope was used to observe the morphology, presence of agglutination, and white blood cells of the sperm cells. A drop of fresh semen sample was placed on the clean sterile glass slide followed by covering with a coverslip and it was observed under the 20 ×, 40 ×, and 100 × magnifications (usually oil drop) to observe the difference in the sperm cells.
Fig. 2.
Makler counting chamber.
We included patients who have been exposed to air pollution for a long duration, such as traffic police, courier delivery personnel, construction field workers, roadside shopkeepers, and local bus drivers. Patients who were currently using supplemental or other medications for infertility were excluded from this research. Subjects with varicocele, a high white blood cell (WBC) count in the semen, sexually transmitted diseases, alcohol consumption, or a smoking history were excluded from the study due to increased seminal ROS levels, which reduce antioxidant activity and cause abnormal sperm cell activity, DNA damage, and morphological defects.
2.4. SEM analysis of morphological differences in sperm
The preparation of sperm samples for scanning electron microscopy (SEM) analysis followed the protocol outlined in the World Health Organization (WHO) Laboratory Manual for the Examination and Processing of Human Semen [21]. Fresh semen samples were collected by masturbation, diluted with an equal volume of phosphate-buffered saline (PBS) to remove excess seminal plasma, and centrifuged at 500–1000 g for 5–10 min to pellet the sperm cells. The pellet was resuspended in 2.5 % glutaraldehyde in PBS and fixed for 2–4 h at room temperature or overnight at 4 °C. The fixed sample was washed three times with PBS for 10 min each, followed by post-fixation in 1–2 % osmium tetroxide in PBS for 1–2 h at room temperature to enhance membrane contrast and stability. The sample was then dehydrated through a graded ethanol series (30 %, 50 %, 70 %, 90 %, and 100 %), each for 10–15 min, with a final dehydration step in 100 % ethanol for at least 30 min. For drying, hexamethyldisilazane (HMDS) was used as a transitional fluid, replacing the final ethanol step with two changes of HMDS for 10 min each, followed by air drying, or a critical point dryer was used following the manufacturer's instructions. The dried sperm cells were mounted onto SEM stubs using double-sided carbon tape or conductive adhesive, ensuring even distribution. The mounted samples were then coated with a thin layer of gold or platinum (5–20 nm) using a sputter coater. Finally, the coated samples were placed into the SEM chamber, and the instrument settings were adjusted for optimal imaging to capture detailed sperm morphology, including the head, midpiece, and tail.
2.5. Sample preparation for estimation of Zn, Mg, Pb, and Cd
A 1 mL of semen sample was centrifuged at 8000 g for 20 min and the supernatant was collected and transferred to a new Eppendorf tube without disturbing the pellet. The collected supernatant was used in Atomic absorption spectroscopy (AAS) analysis to identify the presence of heavy metals. Standards for the heavy metals were prepared using High-performance liquid chromatography (HPLC) grade water and nitric acid with appropriate concentration as given below. Compound preparation for 1000 PPM is done by dividing the Molecular weight of the element by its atomic weight. Then it must be weighed as per the value in weighing balance and diluted in 1 L HPLC grade water, then prepare the standards by doing serial dilution method. Similarly, for preparation of 100 PPM, we must take 10 mL of Stack solution and dilute it with 90 mL of HPLC grade water.
3. Results
3.1. Evaluation of sperm quality
Each sample was handled carefully while doing the semen analysis to find the normal and abnormal sperm cells. A total motile count including progressive, non-progressive, and immotile sperm cells are the main factor in predicting male infertility. Sperm concentrations in normal males vary from 15 million to more than 200 million per mL of sperm. If the person has fewer than 15 million sperm per mL or fewer than 39 million sperm overall per ejaculate, it is considered a low sperm count [22].
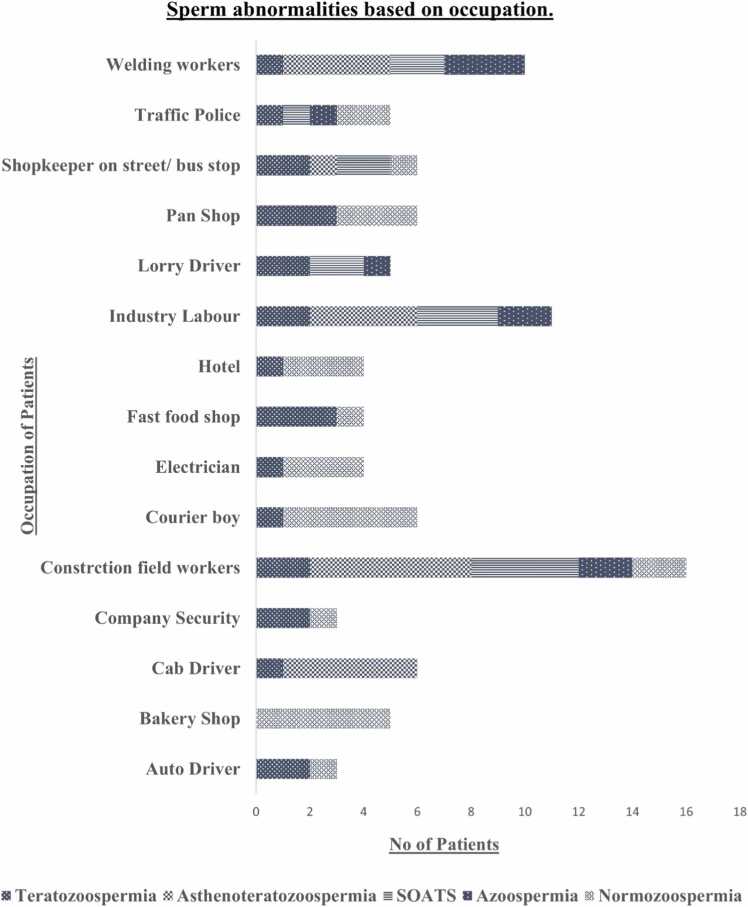
According to Fig. 3, Sperm quality is destructively impacted by those who work in industries, exposed to welding smoke, where chemicals/contaminants are present. Also, in inhaling tobacco smoke, Traffic police/shopkeepers on the street are being exposed to heavy metals, and people being around industrial pollutants. Bus/auto/cab drivers, the person sitting in one place for a long duration, courier boys, and other professions involving extended exposure to high temperatures like those in foundries or requiring particular physical labor facing high scrotal temperatures confirm abnormal progressive motility and morphology. Most of them have sperm cell morphological defects (Teratozoospermia), which might enable them to conceive naturally if they follow their doctor's instructions and stay in good health. Study participants include those with severe oligo Asthenoteratozoospermia (SOAT) and Asthenoteratozoospermia (AST) sperm types. Patients who are heavily exposed to air pollutants at work, irrespective of their age, should follow their doctor's advice to prepare for In vitro fertilization (IVF) and Intrauterine insemination (IUI) therapy.
Fig. 3.
Graph indicating the occupation and number of patients. AST – Asthenoteratozoospermia, AZO – Azoospermia, NOR – Normozoospermia, SOATS – Severe Oligo Asthenoteratozoospermia, TERT – Teratozoospermia.
3.2. Morphology analysis
Basic sperm morphology was identified by comparing the classifications given in the World Health Organisation (WHO) 2021 laboratory manual for the examination and processing of human semen, 6th edition [23]. The mean standard error of the study and the normal and abnormal semen are given in Table 1.
Table 1.
Mean ± standard error of normal and abnormal semen.
| S.no. | Experiment parameters | Normozoospermia | Teratozoospermia | Asthenoteratozoospermia | Severe oligo Asthenoteratozoospermia | Azoospermia |
|---|---|---|---|---|---|---|
| 1 | Volume (mL) | 1.4 ± 1.5 | 1.0 ± 1.2 | 1.0 ± 1.5 | 0.5 ± 1.0 | 0.5 ± 1.5 |
| 2 | pH | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 |
| 3 | Sperm count % | 212.9 ± 17.0 | 124 ± 12.0 | 81 ± 13 | 8.8 ± 1.5 | 0 |
| 4 | Progressive % | 35 ± 1.3 | 9.1 ± 1.3 | 8.25 ± 1.6 | 0 ± 0 | 0 |
| 5 | Non-progressive % | 26.6 ± 1.4 | 28.5 ± 2.4 | 22.2 ± 2.4 | 18.6 ± 6.6 | 0 |
| 6 | Immotile % | 42.0 ± 1.8 | 62.2 ± 3.3 | 65 ± 4.9 | 74.2 ± 8.6 | 0 |
| 7 | Morphology Normal% | 2.1 ± 0.1 | 1.4 ± 0.1 | 1.45 ± 0.2 | 0 | 0 |
| 8 | Head | 87.7 ± 0.1 | 84.8 ± 3.6 | 79.6 ± 6.0 | 0 | 0 |
| 9 | Midpiece | 5.1 ± 0.1 | 4.7 ± 0.2 | 4.5 ± 0.3 | 0 | 0 |
| 10 | Tail | 5 ± 0 | 4.7 ± 0.2 | 4.5 ± 0.3 | 0 | 0 |
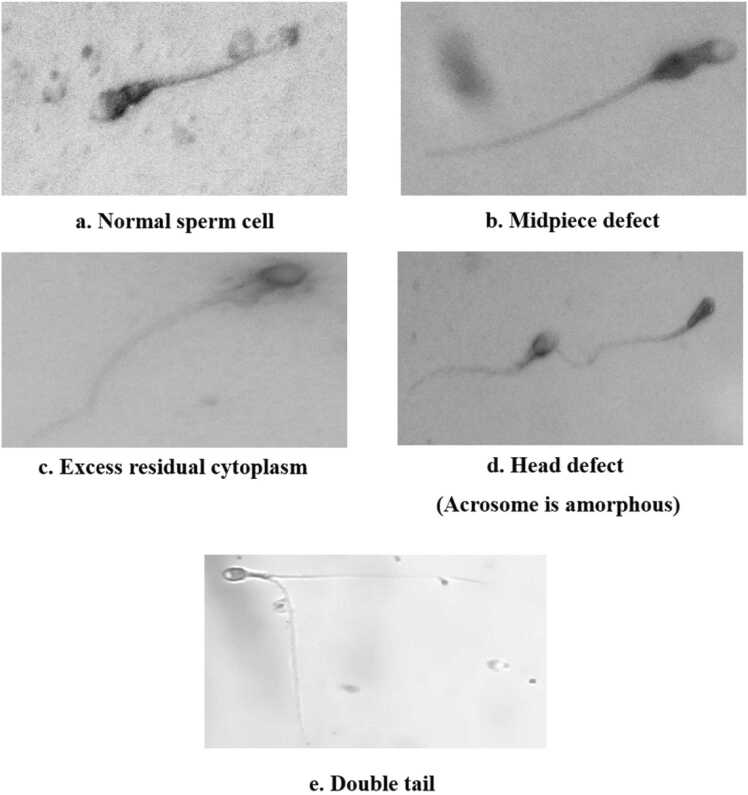
In Fig. 4, we observed the differences in sperm cells. They are Normal sperm cells, Midpiece defect, Excess residual cytoplasm, Head defect (Acrosomes are amorphous), and Double tail.
Fig. 4.
Morphological changes in sperm cells.
The head of a normal sperm cell (Fig. 4a) contains the nucleus, which carries the genetic material (DNA) normally needed for fertilization. The midpiece is the segment just behind the head and contains mitochondria that provide energy for the sperm's movement. The tail, or flagellum, is a long, whip-like structure that propels the sperm forward. Sperm size abnormalities can show up in a variety of ways and may be linked to problems with conception.
Microcephalic sperm (very little head) may have trouble transporting enough genetic material, which might interfere with conception. Large-head (Macrocephalic) sperm cells have difficulties in entering the egg and carrying out fertilization. Disturbances in the midpiece as shown in Fig. 4(b) might compromise the sperm's ability to supply energy and thus its motility.
The presence of excess residual cytoplasm as shown in Fig. 4(c) impacts sperm morphology and function. During spermatogenesis, sperm cells undergo a process of cytoplasmic reduction, where excess cytoplasm is shed. If this process is incomplete, residual cytoplasmic bodies may remain attached to the sperm which causes a spermatogenesis defect. When the acrosome is not well as shown in Fig. 4(d) may affect the sperm's ability to penetrate and fertilize the egg during the acrosome reaction by reducing sperm motility. It is also considered to be a morphological disorder. A sperm's ability to swim toward the egg may be impaired by structural changes in its tail. It plays a crucial role in sperm motility by housing mitochondria, which provide energy for the sperm's movement. A sperm cell with an elongated double tail as shown in Fig. 4(e) is considered to have an abnormal morphology. The specific causes of this abnormality can vary, and it may be associated with factors such as genetic abnormalities, exposure to toxins, or issues during sperm development. The presence of double tails impacts fertility.
3.3. SEM analysis of sperm morphology
3.3.1. Morphological abnormalities
3.3.1.1. Head abnormalities
Scanning electron microscopy (SEM) is a powerful tool for detecting a variety of head abnormalities, which can significantly impact a sperm's fertilizing capability. As shown in Fig. 5(a), these abnormalities can include unusually large or small heads, as well as heads that are tapered or amorphous. Such morphological defects can hinder the sperm's ability to interact properly with the egg, thereby affecting fertilization success rates.
Fig. 5.
SEM images of sperm cells. A) Sperm cells with abnormal morphology, B) sperm cells with debris and contamination, C) sperm cells with acrosome defects.
3.3.1.2. Midpiece defects
Structural issues in the midpiece, such as bending or swelling, can also be observed using Scanning electron microscopy (SEM). The midpiece contains mitochondria that provide the energy required for sperm motility, and defects in this region can impair the sperm's movement and overall function.
3.3.1.3. Tail defects
The tail is essential for sperm motility, and Scanning electron microscopy (SEM) can identify abnormalities such as coiled, bent, or multiple tails. These defects can severely impact the sperm's ability to swim efficiently towards the egg, thus reducing the likelihood of successful fertilization.
3.3.2. Debris and contaminants
Scanning electron microscopy (SEM) images, as depicted in Fig. 5(b), often reveal the presence of non-sperm cells and debris within semen samples. These extraneous elements can include epithelial cells, leukocytes, and other contaminants, which may indicate infection or contamination. The presence of such debris not only affects the clarity of SEM imaging but also provides insights into the overall health and condition of the semen sample, potentially pointing to underlying medical conditions that need to be addressed.
3.3.3. Acrosome
The acrosome is a cap-like structure covering the anterior part of the sperm head and plays a crucial role in fertilization by enabling the sperm to penetrate the egg. Scanning electron microscopy (SEM) analysis can identify acrosomal abnormalities, such as detachment or irregular shapes, which are critical indicators of sperm dysfunction. Fig. 5(c) illustrates examples of such acrosomal defects. Detecting these abnormalities is essential for understanding issues related to male fertility and potential challenges in the fertilization process.
3.4. Heavy metals analysis
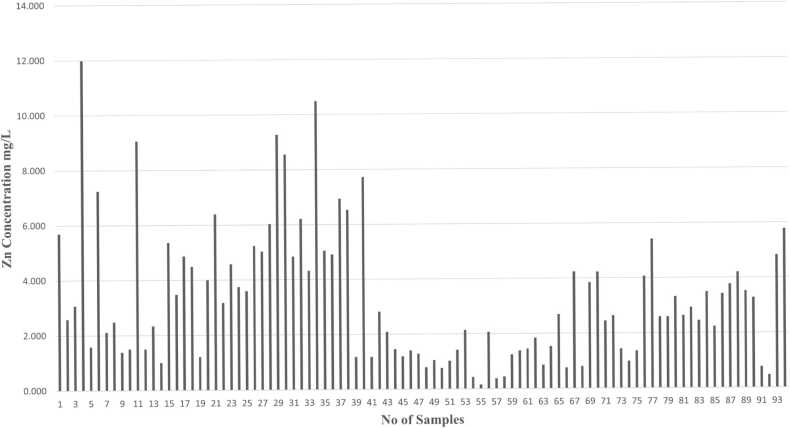
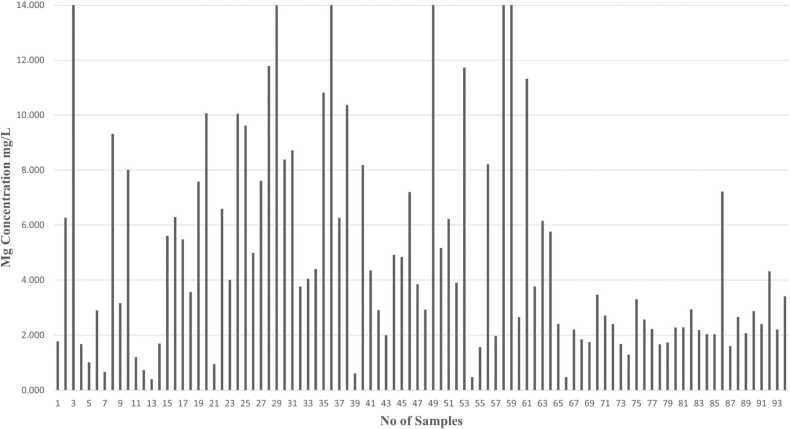
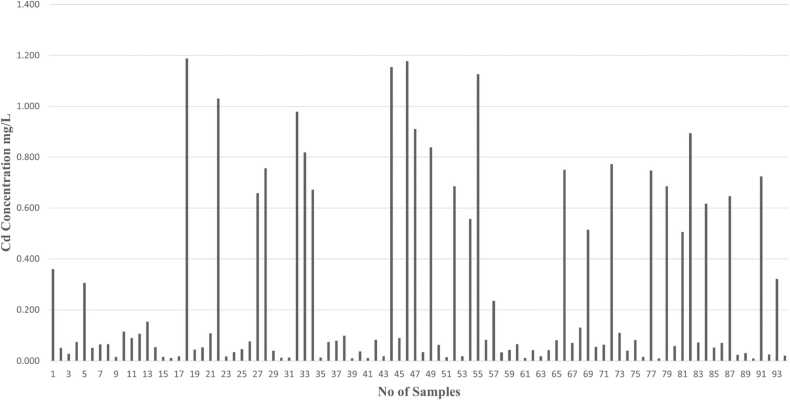
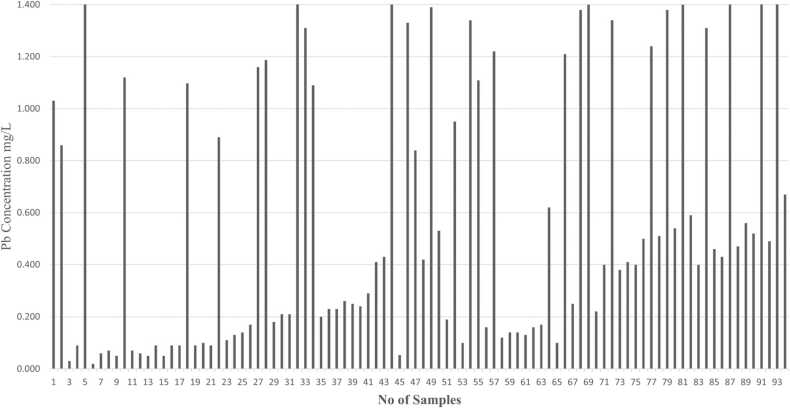
Metals like Zn, Mg, Cd, and Pb concentrations are identified by using Atomic Absorption Spectroscopy (AAS) analysis in human semen samples. Graphs have been plotted based on the number of samples and concentration of metals (mg/L) present in human semen samples.
The normal range of zinc concentration in the human seminal fluid can vary slightly depending on the laboratory and the specific measurement techniques used. However, in general, the normal range for zinc in seminal fluid is commonly reported to be between 0.0024 and 0.0067 mg/L. Excessive zinc levels may interfere with sperm motility, affecting the sperm's ability to swim and navigate the female reproductive tract. Abnormalities in prostatic secretions, potentially affecting the overall semen composition of patients associated with higher Zn concentration as shown in Fig. 6. Elevated oxidative stress can lead to cellular damage and negatively impact sperm quality if the Zn is in high concentration. Excessive zinc may have pro-inflammatory effects, leading to inflammation in the reproductive organs.
Fig. 6.
Graph indicates the concentration of zinc (Zn) in different human semen samples.
Magnesium in seminal fluid is often around 8–12 mmol/L. Magnesium is involved in the regulation of various hormones, including those related to reproduction. Magnesium is involved in sperm motility and function. Adequate magnesium levels as shown in Fig. 7, may support healthy sperm development and enhance sperm motility, which is crucial for fertilization.
Fig. 7.
Graph indicates the concentration of magnesium (Mg) in different human semen samples.
As shown in Fig. 8, the person’s high exposure to cadmium results in an imbalance between the production of reactive oxygen species (ROS) and in turn increased oxidative stress can damage lipids, proteins, and DNA in sperm cells. We can conclude that lead exposure has been associated with a decrease in sperm count, motility, and morphology. In Fig. 9 we observe that patients having high levels of Pb concentration in semen will have poor sperm quality. Elevated levels of Pb induce oxidative stress, which can result in DNA damage in sperm cells. Also, it affects fertility and increases the risk of developmental abnormalities in offspring.
Fig. 8.
Graph indicates the concentration of cadmium (Cd) in different human semen samples.
Fig. 9.
Graph indicates the concentration of lead (Pb) in different human semen samples.
4. Discussion
Sperm are intricate cell machinery that have been shaped by evolution to increase the likelihood of fertilization. Their mobility and hydrodynamics are mostly determined by the sperm head's form and the mechanics of the whip-like sperm tail. Minor alterations to these structures may determine whether sperm fertilization succeeds or fails in polyandrous species, where females mate with many men. Therefore, one of the main factors influencing evolutionary change is the structure of the sperm [24]. our study demonstrates that sperm morphological abnormalities, particularly in the head region, are prevalent in samples with compromised fertility. Amorphous and tapered heads, as well as acrosomal defects, were the most common morphological abnormalities observed. Additionally, midpiece and tail defects further impair sperm functionality. The presence of debris and contaminants indicated potential infections or contamination, further impacting sperm quality. Previous investigations have also found that aberrant sperm morphology is primarily associated with amorphous and tapered heads of the sperm [25], [26].
It is very important to observe that exposure to pollutants and heavy metals has led to many health issues for mankind in recent days. Most of the health problems are very common when compared to reproductive system dysfunction. The Zn may be a marker for sperm abnormalities and male infertility if the content of Zn in the seminal fluid aligns with the parameters of semen quality [27]. According to the literature, Zn is essential for living beings because nearly 350 enzymes require Zn for their regular activity, mainly the oxidant defense system. Kumosani et al. [28] stated that the calcium pump is regulated by Zn in controlling Ca2+ ATPase, which triggers intracellular Ca2+ in animal cells. Endre et al. [29] reported that molecular configurations like DNA, RNA, and protein molecules are maintained by Zn. Parameswari and Sridharan [30], reported that the metal toxicant Cd, which is released by smoking, alters the concentration of ROS in seminal plasma. It is anticipated that this imbalance will affect the morphology of sperm DNA, leading to male infertility. According to the Auld [31] study, concentrations of Zn show differences in infertile and fertile men. Danscher et al. [32] reported that the presence of a high concentration of Zn in seminal plasma leads to an increase in sperm count, concentration, motility, and good morphology. Mg2+ appears to have a small impact on DNA integrity. The lack of distinction among permeabilized and non-permeabilized sperm in the results with both ions supports the participation of an intracellular mechanism. Heavy metals may harm the human male reproductive system by disrupting the hypothalamic-pituitary axis or directly influencing spermatogenesis, which results in poor sperm quality [33]. Some metals, namely Pb and Cd have been identified as reproductive toxins and/or probable endocrine disruptors. Several studies found a substantial adverse relationship between blood and seminal Pb levels and sperm quality in both exposed and unexposed males in the workplace [34], [35], [36], [37], [38], [39]. Cd is thought to be an endocrine disruptor, although the exact mechanisms involved are unknown. Adults often have normal laboratory tests in the range of 1.8–2.6 mg/dL. Children's levels range from 1.7 to 2.1 mg/dL of magnesium in their blood. Higher levels of magnesium in the blood (hypermagnesemia) cause kidney, liver, mortality-related, cardiovascular, and neurological disorders. As the graph represents in Fig. 3, Most of the patients exposed to air pollution were affected by sperm count, morphology, and motility issues. Morphological defects of normal and abnormal sperm cells are given in Fig. 4. The concentration of heavy metals in every patient is shown in Fig. 5, Fig. 6, Fig. 7, Fig. 8. Reducing exposure to cadmium and lead is crucial for maintaining reproductive health. This can include adopting protective measures in occupational settings, avoiding contaminated environments, and making lifestyle choices that minimize exposure. It's important to note that the impact of heavy metal exposure on sperm quality can depend on the duration and level of exposure. Occupational exposure, environmental contamination, or exposure through certain behaviours (such as smoking, as cadmium is present in tobacco smoke) can cause increased levels of these metals in the human body. In the process of ART fertilization, if we try to remove the heavy metals by separating the healthy sperm cells from the seminal plasma, then the chance of fertility will be higher.
5. Conclusion
The study highlights the significant impact of occupational and environmental exposures on male fertility, particularly through the influence of heavy metals such as Zn, Cd, and Pb. SEM analysis of sperm morphology revealed widespread abnormalities in individuals exposed to high levels of these metals, underscoring the critical need for monitoring and mitigating environmental exposures. Occupational settings involving prolonged exposure to high temperatures, chemicals, and pollutants were strongly correlated with reduced sperm quality, emphasizing the importance of protective measures in these environments. The findings advocate for comprehensive semen analysis in diagnosing male infertility and call for targeted interventions to reduce heavy metal exposure and improve reproductive health outcomes. Additional research is essential to understand the mechanisms behind these effects and develop strategies to enhance fertility in affected populations. Also, to determine the biological mechanisms underlying the observed associations.
CRediT authorship contribution statement
Abilash D: Writing – review & editing, Writing – original draft. Sridharan T B: Validation, Supervision, Investigation.
Compliance with Ethical Standards
The study was approved by the Institutional Human Ethical Committee, VIT, Vellore. Ref No: VIT/IECH/XI/2022/06 & Sri Narayani Hospital and Research Centre Ethical Committee, Ref IEC No: 36/04/02/23.
Funding
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are very much thankful to the Management of Vellore Institute of Technology, Vellore as well as to the Management, Research and Development, Department of Reproductive Medicine, Sri Narayani Hospital and Research Centre, Sripuram, Vellore.
Conflict of Interest
The authors declare that there are no competing interests.
Handling Editor: Dr. L.H. Lash
Data availability
Data will be made available on request.
References
- 1.Mitra S., Chakraborty A.J., Tareq A.M., Emran T.Bin, Nainu F., Khusro A., Idris A.M., Khandaker M.U., Osman H., Alhumaydhi F.A., Simal-Gandara J. Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J. King Saud. Univ. Sci. 2022;34 doi: 10.1016/J.JKSUS.2022.101865. [DOI] [Google Scholar]
- 2.Chao H.H., Zhang Y., Dong P.Y., Gurunathan S., Zhang X.F. Comprehensive review on the positive and negative effects of various important regulators on male spermatogenesis and fertility. Front. Nutr. 2022;9 doi: 10.3389/FNUT.2022.1063510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omolaoye T.S., Skosana B.T., Ferguson L.M., Ramsunder Y., Ayad B.M., Du Plessis S.S. Implications of exposure to air pollution on male reproduction: the role of oxidative stress. Antioxidants. 2024;13 doi: 10.3390/antiox13010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Wei J., Zhao S., Zeng Q., Sun S., Cao W. Ambient fine particulate matter constituents and semen quality among adult men in China. J. Hazard. Mater. 2024;465 doi: 10.1016/j.jhazmat.2023.133313. [DOI] [PubMed] [Google Scholar]
- 5.Wang L., Luo D., Liu X., Zhu J., Wang F., Li B., Li L. Effects of PM2.5 exposure on reproductive system and its mechanisms. Chemosphere. 2021;264 doi: 10.1016/J.CHEMOSPHERE.2020.128436. [DOI] [PubMed] [Google Scholar]
- 6.Liu J., Dai Y., Li R., Yuan J., Wang Q., Wang L. Does air pollution exposure affect semen quality? Evidence from a systematic review and meta-analysis of 93,996 Chinese men. Front. Public Health. 2023;11 doi: 10.3389/FPUBH.2023.1219340/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najafi T.F., Roudsari R.L., Namvar F., Ghanbarabadi V.G., Talasaz Z.H., Esmaeli M. Air pollution and quality of sperm: a meta-analysis. Iran. Red. Crescent Med. J. 2015;17:26930. doi: 10.5812/IRCMJ.17(4)2015.26930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L., Xu T., Wang Q., Ni H., Yu X., Song C., Li Y., Li F., Meng T., Sheng H., Cai X., Dai T., Xiao L., Zeng Q., Guo P., Wei J., Zhang X. Exposure to fine particulate matter constituents and human semen quality decline: a multicenter study. Environ. Sci. Technol. 2023;57:13025–13035. doi: 10.1021/ACS.EST.3C03928/ASSET/IMAGES/LARGE/ES3C03928_0004.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y., Zhu J., Tang Q., Wang J., Feng J., Zhou Y., Li J., Pan F., Han X., Lu C., Wang X., Langston M.E., Chung B.I., Wu W., Xia Y. Exposure to particulate matter may affect semen quality via trace metals: evidence from a retrospective cohort study on fertile males. Chemosphere. 2024;346 doi: 10.1016/J.CHEMOSPHERE.2023.140582. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Zhu Q., Lin J., Cai J. Association of exposure to particulate matter air pollution with semen quality among men in China. JAMA Netw. Open. 2022;5 doi: 10.1001/JAMANETWORKOPEN.2021.48684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang T., Ru Y.F., Wu B., Dong H., Chen L., Zheng J., Li J., Wang X., Wang Z., Wang X., Shen X., Wu J., Qian J., Miao M., Gu Y., Shi H. Effects of low lead exposure on sperm quality and sperm DNA methylation in adult men. Cell Biosci. 2021;11:1–11. doi: 10.1186/S13578-021-00665-7/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamkovicova M., Toman R., Martiniakova M., Omelka R., Babosova R., Krajcovicova V., Grosskopf B., Massanyi P. Sperm motility and morphology changes in rats exposed to cadmium and diazinon. Reprod. Biol. Endocrinol. 2016;14:1–7. doi: 10.1186/S12958-016-0177-6/FIGURES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henriques M.C., Loureiro S., Fardilha M., Herdeiro M.T. Exposure to mercury and human reproductive health: a systematic review. Reprod. Toxicol. 2019;85:93–103. doi: 10.1016/J.REPROTOX.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Sun B., Ma J., Te L., Zuo X., Liu J., Li Y., Bi J., Wang S. Zinc-deficient diet causes imbalance in zinc homeostasis and impaired autophagy and impairs semen quality in mice. Biol. Trace Elem. Res. 2023;201:2396–2406. doi: 10.1007/S12011-022-03324-1. [DOI] [PubMed] [Google Scholar]
- 15.Dadgar Z., Shariatzadeh S.M.A., Mehranjani M.S., Kheirolahi A. The therapeutic effect of co-administration of pentoxifylline and zinc in men with idiopathic infertility. Ir. J. Med. Sci. 2023;192:431–439. doi: 10.1007/S11845-022-02931-0. [DOI] [PubMed] [Google Scholar]
- 16.Chinyere Nsonwu-Anyanwu A., Raymond Ekong E., Jeremiah Offor S., Francis Awusha O., Chukwuma Orji O., Idiongo Umoh E., Aleruchim Owhorji J., Rowland Emetonjor F., Adanna Opara Usoro C., Abbas A. Heavy metals, biomarkers of oxidative stress and changes in sperm function: a case-control study. Int. J. Reprod. Biomed. 2019;17:163–174. doi: 10.18502/ijrm.v17i3.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puentes-Díaz N., Chaparro D., Morales-Morales D., Flores-Gaspar A., Alí-Torres J. Role of metal cations of copper, iron, and aluminum and multifunctional ligands in Alzheimer’s disease: experimental and computational insights. ACS Omega. 2023;8:4508–4526. doi: 10.1021/ACSOMEGA.2C06939/ASSET/IMAGES/MEDIUM/AO2C06939_0010.GIF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tariba Lovaković B., Lovaković T., Blanka Cadmium, arsenic, and lead: elements affecting male reproductive health. COTox. 2020;19:7–14. doi: 10.1016/J.COTOX.2019.09.005. [DOI] [Google Scholar]
- 19.Bhardwaj J.K., Paliwal A., Saraf P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021;35 doi: 10.1002/JBT.22823. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal A., Sharma R., Gupta S., Finelli R., Parekh N., Selvam M.K.P., Pompeu C.P., Madani S., Belo A., Darbandi M., Singh N., Darbandi S., Covarrubias S., Sadeghi R., Arafa M., Majzoub A., Caraballo M., Giroski A., McNulty K., Durairajanayagam D., Henkel R. Standardized laboratory procedures, quality control and quality assurance are key requirements for accurate semen analysis in the evaluation of infertile male. World J. Mens. Health. 2022;40:52. doi: 10.5534/WJMH.210022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.F. Luis, G. Moncayo, World Health Organization, WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed., World Health Organization, 2010.
- 22.S.W. Leslie, T.L. Soon-Sutton, M.A. Khan, Male Infertility, StatPearls, 2023. 〈https://www.ncbi.nlm.nih.gov/books/NBK562258/〉 (Accessed 17 March 2024). [PubMed]
- 23.WHO Laboratory Manual for the Examination and Processing of Human Semen, sixth edition, World Health Organization, Geneva, 2021
- 24.Korneev D., Merriner D.J., Gervinskas G., de Marco A., O’Bryan M.K. New insights into sperm ultrastructure through enhanced scanning electron microscopy. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.672592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen H.T.T., Dang H.N.T., Nguyen T.T.T., Nguyen T.Van, Dang T.C., Nguyen Q.H.V., Le M.T. Correlations between abnormalities of morphological details and DNA fragmentation in human sperm. Clin. Exp. Reprod. Med. 2022;49 doi: 10.5653/cerm.2021.04777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iqbal I., Mustafa G., Ma J. Deep learning-based morphological classification of human sperm heads. Diagnostics. 2020;10 doi: 10.3390/diagnostics10050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickram S., Muthugadhalli S., Jayaraman G., Kamini R., Ramesh Pathy M., Sridharan B. Influence of trace elements and their correlation with semen quality in fertile and infertile subjects. Turk. J. Med. Sci. 2013;43:1000–1007. doi: 10.3906/SAG-1211-54. [DOI] [Google Scholar]
- 28.Kumosani T.A., Elshal M.F., Al-Jonaid A.A., Abduljabar H.S. The influence of smoking on semen quality, seminal microelements and Ca2+-ATPase activity among infertile and fertile men. Clin. Biochem. 2008;41:1199–1203. doi: 10.1016/J.CLINBIOCHEM.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Endre L. The role of zinc in human health. J. Trace Elem. Exp. Med. 1990;3:337–375. 〈https://cir.nii.ac.jp/crid/1570291224795895680〉 accessed March 17, 2024. [Google Scholar]
- 30.Parameswari R., Sridharan T.B. Cigarette smoking and its toxicological overview on human male fertility—a prospective review. Toxin Rev. 2021;40:145–161. doi: 10.1080/15569543.2019.1579229. [DOI] [Google Scholar]
- 31.D.S. Auld, The ins and outs of biological zinc sites, n.d. 〈 10.1007/s10534-008-9184-1〉. [DOI] [PubMed]
- 32.G. Dnnschcrl, R. Ham, E. Fjerdingstad’, H. Rcbbez, Zinc content of human ejaculate and the motility of sperm cells, Wiley Online Library G Danscher, R Hammen, E Fjerdingstad, H RebbeInternational Journal of Andrology, 1978•Wiley Online Library, vol. 1, 1978, pp. 576–81. 〈 10.1111/j.1365-2605.1978.tb00628.x〉. [DOI]
- 33.Wyrobek A.J., Schrader S.M., Perreault S.D., Fenster L., Huszar G., Katz D.F., Osorio A.M., Sublet V., Evenson D. Assessment of reproductive disorders and birth defects in communities near hazardous chemical sites. III. Guidelines for field studies of male reproductive disorders. Reprod. Toxicol. 1997;11:243–259. doi: 10.1016/S0890-6238(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 34.Eibensteiner L., Sanz A.D.C., Frumkin H., Gonzales C., Gonzales G.F. Lead exposure and semen quality among traffic police in Arequipa, Peru. Int. J. Occup. Environ. Health. 2005;11:161–166. doi: 10.1179/OEH.2005.11.2.161. [DOI] [PubMed] [Google Scholar]
- 35.Tališman S., Cvitković P., Jurasović J., Pizent A., Gavella M., Ročić B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ. Health Perspect. 2000;108:45. doi: 10.1289/EHP.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurasovi´c J.J., Cvitkovi´ccvitkovi´c P., Pizent A., Božoˇbožočolak B., Telišman S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. BioMetals. 2004;17:735–743. doi: 10.1007/s10534-004-1689-7. [DOI] [PubMed] [Google Scholar]
- 37.De Rosa M., Zarrilli S., Paesano L., Carbone U., Boggia B., Petretta M., Maisto A., Cimmino F., Puca G., Colao A., Lombardi G. Traffic pollutants affect fertility in men. Hum. Reprod. 2003;18:1055–1061. doi: 10.1093/HUMREP/DEG226. [DOI] [PubMed] [Google Scholar]
- 38.Maric T., Fucic A., Aghayanian A. Environmental and occupational exposures associated with male infertility. Arch. Ind. Hyg. Toxicol. 2021;72:101. doi: 10.2478/AIHT-2021-72-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marić T., Fučić A., Aghayanian A. Association of blood and seminal plasma cadmium and lead levels with semen quality in non-occupationally exposed infertile men in Abakaliki, South East Nigeria. J. Fam. Reprod. Health. 2017;11:97. doi: 10.2478/aiht-2021-72-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Q., Li X., Ge R.S. Toxicological effects of cadmium on mammalian testis. Front. Genet. 2020;11:527. doi: 10.3389/fgene.2020.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aitken R.J. Sperm DNA integrity: a special issue exploring the causes, consequences, and treatment of DNA damage in human spermatozoa. Andrology. 2023;11(8):1541–1544. doi: 10.1111/andr.13503. [DOI] [PubMed] [Google Scholar]
- 42.Cannarella R., Gül M., Rambhatla A., Agarwal A. Temporal decline of sperm concentration: role of endocrine disruptors. Endocrine. 2023;79(1):1–16. doi: 10.1007/s12020-022-03136-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.