Summary
Cardiovascular diseases (CVDs) and metabolic disorders (MDs) have surfaced as formidable challenges to global health, significantly imperiling human well-being. Recently, microneedles (MNs) have garnered substantial interest within the realms of CVD and MD research. Offering a departure from conventional diagnostic and therapeutic methodologies, MNs present a non-invasive, safe, and user-friendly modality for both monitoring and treatment, thereby marking substantial strides and attaining pivotal achievements in this avant-garde domain, while also unfurling promising avenues for future inquiry. This thorough review encapsulates the latest developments in employing MNs for both the surveillance and management of CVDs and MDs. Initially, it succinctly outlines the foundational principles and approaches of MNs in disease surveillance and therapy. Subsequently, it delves into the pioneering utilizations of MNs in the surveillance and management of CVDs and MDs. Ultimately, this discourse synthesizes and concludes the primary findings of this investigation, additionally prognosticating on the trajectory of MN technology.
Subject areas: Health sciences, Natural sciences, Applied sciences
Graphical abstract
Health sciences; Natural sciences; Applied sciences
Introduction
In recent years, the prevalence and mortality of cardiovascular diseases (CVDs) and metabolic disorders (MDs) have increased, posing huge burdens and challenges to society and healthcare systems. Therefore, monitoring and treating these two types of diseases has become a critical public health imperative. Table 1 summarizes the symptoms and hazards of standard CVDs and MDs, along with available treatments and their limitations and morbidity/mortality details. CVDs broadly refer to diseases related to blood vessels and the heart, such as cardiomyopathy, coronary heart disease, valvular heart disease, coronary atherosclerosis, and intimal hyperplasia, also known as circulatory diseases. A combination of other diseases, unhealthy lifestyles, and environmental and genetic factors typically causes these diseases. If severe, they may pose a threat to the patient’s life.1,2,3,4,5,6 Endocrine MDs refer to diseases associated with hormone imbalances originating from endocrine organs, primarily diabetes, gout, osteoporosis, and obesity. These diseases are often associated with a variety of factors, such as genetic defects, unhealthy lifestyles, environmental factors, and improper medical treatment. MDs interact with each other and can co-exist, which may increase the risk of disability and death.7,8,9,10,11 CVDs and MDs exhibit numerous shared risk factors and interconnections; because of their association, treating both types of diseases requires an integrated approach.
Table 1.
Symptoms and hazards of typical CVDs and MDs available treatments and their shortcomings and morbidity/mortality
| Categorization | Symptoms and hazards | Available treatments | Shortcomings of available treatments | Morbidity/Mortality |
|---|---|---|---|---|
| Myocardial infarction | Most often there is severe and persistent sternal pain complicating cardiac arrhythmia shock or heart failure which can even be life-threatening.12,13 | Thrombolytic therapy: the use of thrombolytic drugs to dissolve the embolism in the blood vessels and eliminate them from the body restoring blood flow14; interventional surgery: the surgical installation of stents to the plug blood vessels to hold the blood vessels open and allow blood flow.15 | Thrombolytic therapy is associated with serious complications such as intracranial hemorrhage cerebral edema and systemic hemorrhage.16 Interventional surgical treatment of heart attack is not easy to implement and is demanding in terms of hardware and software.17 | Among all CVDs acute myocardial infarction ranks first in global mortality a ranking that will remain until 2030.18 |
| Coronary atherosclerosis | Numbness of limbs muscle atrophy lack of blood supply dizziness and headache dyspnea and palpitations.19,20 | Medication: "Anti-Myocardial drugs such as enteric-coated aspirim tablets clopidogrel bisulfate tablets pravastatin sodium etc.21; Surgical treatment: subcutaneous stent implantation balloon dilatation etc.22 | Statins may cause side effects such as liver function damage muscle damage and elevated blood glucose. Surgical treatment of myocardial damage large surgical incisions slow recovery and long hospitalization time.23,24 | Coronary atherosclerosis accounts for 17.9 million deaths worldwide or 31% of all deaths.25 |
| Diabetes | Prolonged hyperglycemia attacks various organs and tissues of the body such as the heart kidneys brain and eyes.26 | Type 1 diabetes: insulin injection therapy; type 2 diabetes: oral antidiabetic drugs agents are required and insulin injection therapy is appropriate.27 | Lack of knowledge of medication by patients leads to wrong timing of medication stopping medication on their own and missing doses.28 Insulin injection with poor patient compliance.29 | The prevalence of diabetes is about 9.3% (463 million people) globally and is expected to increase to 10.9% (700 million people) in 2045.30 |
| Gout | Joint pain hot and swollen and itch processing in the skin if left untreated can disable the joints and large amounts of uric acid deposited in the kidneys can severely damage the kidneys.31 | Medication: febuxostat tablets benzbromarone tablets etc. for uric acid-lowering treatment32; Surgery: removal of gout tophus or correction of joint deformities.33 | Medication may cause side effects such as gastrointestinal upset and kidney damage. Surgical treatment is risky and may cause damage to the joints.34 | Gout has a global prevalence of about 1–4% and an incidence of about 0.1–0.3%.35 |
| Osteoporosis | Severe leg pain may cause difficulty in mobility and fractures are highly likely to occur when patients are subjected to factors such as minor external forces as well as simple movements.36 | Pharmacologic therapy: drugs that inhibit osteoclasts such as alendronate and zoledronic acid which are bisphosphonates and drugs that inhibit bone resorption such as proclcitonin and salmon calcitonin.37,38 | It requires long-term adherence takes a while to see significant results and medication often has side effects such as nausea vomiting diarrhea and headache.39 | The global prevalence of osteoporosis is about 18.3%.40 |
| Obesity | It makes the human body’s behavior very sluggish and is prone to comorbidities such as hypertension hyperglycemia and high cholesterol causing a series of CVDs and cerebrovascular diseases.41 | Pharmacologic treatment: intestinal lipase inhibitor drugs42; surgical treatment: liposuction sleeve gastrectomy.43 | Medication may trigger adverse effects such as flatulence back pain lower extremity pain headache and upper respiratory tract infection.44 Surgical treatment is risky and harmful. | The 2023 edition of the World Obesity Map predicts that by 2035 more than 4 billion people worldwide will be obese or overweight representing 51% of the global population.45 |
As shown in Table 1, many limitations exist in current treatments. The medical profession is constantly exploring new monitoring and treatment methods to reduce the prevalence and mortality rates of CVDs and MDs. As an emerging diagnostic and therapeutic technology, the application of microneedles (MNs) has gradually become a research hotspot in medicine. MNs are composed of a series of micrometer-sized needles attached to the substrate, which can be applied to the skin like a bandage.46 Drugs can penetrate the stratum corneum of the skin through the MNs and directly enter the body fluid circulation. At the same time, non-invasive extraction of body fluid through micrometer-sized needles can also monitor blood glucose, uric acid, and other indicators.47 Due to various factors such as drug characteristics, delivery kinetics, and dosage requirements that affect the therapeutic effect, MN structures with various advantageous features have been developed, including coated MNs, soluble MNs, phase change MNs, and hollow MNs.48 Table S1 provides a detailed introduction to the drug delivery mechanisms and advantages and disadvantages of different types of MNs. In monitoring CVDs and MDs, MNs can track patients’ disease progression and changes by detecting biomarkers and offering timely warnings to prevent related diseases. MNs’ monitoring technology, with its advantages of being wearable, minimally invasive, convenient, and intuitive, has shown significant application prospects in various settings such as community healthcare, home health monitoring, and outdoor medical rescue. Compared to traditional monitoring, it simplifies the operational process, shortens waiting times, alleviates psychological stress on patients, and enhances user experience.49,50,51 In addition, in treating CVDs and MDs, MNs significantly enhances the transdermal absorption capacity of tissues for small and large molecular drugs by creating micro-channels on biological tissues. The advantage of this emerging treatment technology lies in its ability to achieve precise dosage control, effectively preventing potential toxicity risks caused by improper dosages. It not only abandons the inconvenience of traditional injection methods but also ensures that the drug acts directly and effectively on the target area, achieving in situ treatment, which greatly improves the bioavailability of the drug.52
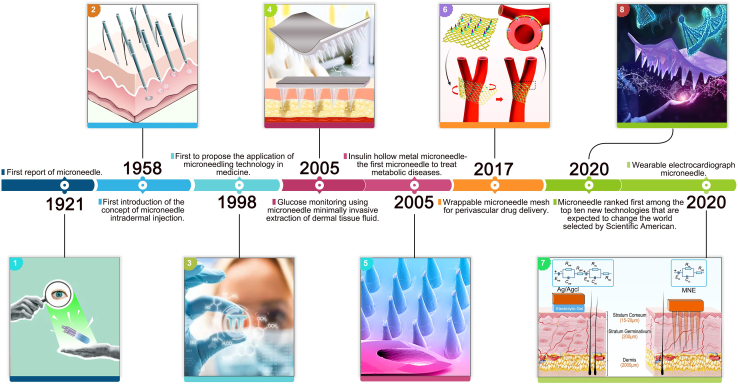
In recent years, MNs have rapidly gained popularity mainly due to the following advantages: Firstly, their design is highly flexible and can be customized in terms of length, shape, and material to meet the monitoring and treatment needs of different scenarios. Secondly, the manufacturing process of MNs is diverse and straightforward, which makes it easy to integrate them with other components, greatly expanding the range and field of MNs applications. Lastly, MNs are cost-effective and support large-scale production, providing a foundation for the commercialization and widespread application of related products. The use of MNs in CVDs and MDs dates back to the first report of MNs in 1921.53 Subsequently, the concept of intradermal injection of MNs was first proposed in 1958,54 and it was first applied for the transdermal delivery of drugs in 199855; the first MN that could be used to extract tissue fluid for glucose monitoring56 and insulin, a hollow metal MN, was introduced in 2005,57 which initiated the application of MNs in the monitoring and treatment of MDs. After over a decade of development, MN technology has matured and transitioned into monitoring and treating CVDs. The first MN for encapsulated vascular drug delivery emerged in 2017.58 The first wearable electrocardiogram MN for monitoring electrocardiogram signals appeared in 2020.59 Scientific Americans rated it the first of the top 10 emerging technologies expected to change the world in the same year.50 In the following years, MNs have continuously improved, and various MNs for CVDs and MDs applications have emerged, providing new options for disease monitoring and treatment (Figure 1).
Figure 1.
Timeline of the development of MN for the monitoring and treatment of CVDs and MDs
Although many high-quality research results have emerged in the field of MN in CVDs and MDs, systematic reviews summarizing this research area are still lacking. Therefore, this study aimed to fill this gap and provide a comprehensive overview of the current status of MN applications in CVDs and MDs. We provide more comprehensive, accurate, and practical reference information for physicians, clinical researchers, and scientific decision-makers and further promote the research and application of MNs in this field.
Principles and modalities of MN for disease monitoring and disease treatment
MNs are emerging medical tools that act on the skin in a minimally invasive manner for disease monitoring and treatment. For disease monitoring, MNs focus on sampling modal analysis of the skin to obtain insightful results. MNs can be combined with biosensors or electrodes to improve monitoring accuracy and provide patients with more personalized and effective treatment plans. In terms of disease treatment, MNs can deliver drugs precisely to the localized area that needs to be treated. Compared to other treatment methods, such as oral, injection, and topical application, MNs can increase the drug permeability by several orders of magnitude, thereby significantly improving the therapeutic effect.60,61,62
Principles and modalities of MNs for CVD and MD monitoring
In medicine, biomarkers, such as proteins and glucose in the blood, reflect the health and physiological state of the human body.63 Biosignals are signals that can reflect the state of life activities of an organism, such as cardiac, cerebral, and myoelectric signals.64 For monitoring CVDs and MDs, MNs are mainly used to collect biomarkers/biosignals by subcutaneous pricking, which are detected and analyzed to assess the body’s health status. MNs are used in two ways for monitoring CVDs and MDs. One approach involves incorporating color-rendering reactions and MNs during the preparation process. In the preparation process, the MN is directly added to the matrix material to produce a color-changing response with the monitoring substance, which occurs after the MN is pierced into the skin and comes into contact with the tissue fluid, and the concentration of the biomarker can be roughly determined according to the depth of the color.65 Although this method is simple, convenient, and intuitive, it risks significant error since the concentration range is estimated through visual observation. Another approach involves extracting physiological fluids (such as blood tissue) from patients or obtaining biosignals through specific biosensors or electrodes on the MNs. Analyzing the extracted fluids or received signals enables real-time monitoring and display of relevant information.66 In contrast, the second method provides more accurate detection results and allows the real-time monitoring of biomarkers/biosignals.
Principles and modalities of MNs in the treatment of CVDs and MDs
The skin is divided into three main layers, the stratum corneum, epidermis, and dermis, with thicknesses of 10–20 μm, 50–150 μm, and 1–4 mm, respectively.67 MNs are a novel physically facilitated penetration method with tip lengths ranging from 25 to 2000 μm and tip radii ranging from 1 to 25 μm.68 MN can penetrate the skin barrier from the microchannels in the epidermis and dermis without damaging the nerves or blood vessels, and the small size and tip of the needle can accurately locate the target area to achieve painless targeted treatment.69 As a minimally invasive transdermal device, MNs can deliver controlled and sustained drug release, facilitating the transdermal penetration of a wide range of substances, such as small molecule peptides and nanopharmaceuticals.70 MN penetrates the skin and stratum corneum to deliver drugs directly to the skin. The cuticle can deliver drugs directly to lesion sites, greatly improving drug bioavailability. Compared with oral use and injections, MN transdermal drug delivery technology circumvents the first-pass reaction of the liver to oral drugs and mitigates the local irritation associated with injections; this enhances therapeutic efficacy and promotes increased patient adherence to the treatment.71
Currently, MNs have gained momentum in the therapeutic field of CVDs and MDs, and many studies have shown that MNs can achieve transdermal delivery of various active drugs. Generally, they can be categorized as channel-based MNs systems and non-channel-based MNs.72 In channel-based MNs systems such as solid MNs and hollow MNs, In solid MNs systems, numerous micrometer-sized holes are created by interacting with the skin’s surface. After the solid MNs are removed, drugs such as ointments and gels are applied to the activated MNs sites73; the drug in hollow MNs are preloaded into the hollow structure or needle cavity of the needle body and diffused into the body through the pores of the needle body by pressure.74 This method contrasts with non-channel-based MNs systems, including coated MNs, dissolvable MNs, and phase-change MNs. When coated MNs punctures the skin, the drug film on the surface of the needle body dissolves within the skin75; the needle body of dissolvable MNs contains the drug itself, and after puncturing the skin, drug release is achieved with the dissolution of the needle body76; phase-change MNs after puncturing the skin achieves drug release (Table S1; Figure S1).77
MNs in monitoring and treatment of CVDs
Monitoring of CVDs is an indispensable and essential part of health management and disease prevention; however, the currently used monitoring methods, such as blood pressure monitoring and electrocardiogram signal monitoring, have many problems, such as limited sensitivity and specificity.78,79,80,81 The traditional treatments for CVDs mainly include general, drug, and surgical treatments. Although traditional treatments have some efficacy, they have many drawbacks, such as low efficacy, high risk, and low patient compliance.82
In recent years, the emergence of MN has brought new hope for personalized medicine to improve therapeutic efficacy and reduce side effects. Numerous research outcomes have surfaced from the application of MNs in monitoring and treating CVDs. MNs enable more precise, comprehensive, and efficient tracking in the monitoring phase. In treating CVDs, MNs can adjust drug release rates as needed and tailor their structure and shape to the therapeutic site’s characteristics for personalized treatment.
MN in monitoring CVDs
Currently, research on the use of MNs for CVD monitoring remains relatively limited, owing to the underutilization and limited promotion of MN technology, coupled with the inherent challenges associated with CVD monitoring. To address the requirement for prolonged electrocardiography (ECG) signal monitoring in patients with heart disease, Satti et al.59 devised a rigid poly-perylene-coated MN electrode array. This innovation enables long-term ECG signal monitoring with low contact impedance and a more stable bio-potential, surpassing the performance of traditional silver-silver chloride electrodes. Physicians can diagnose arrhythmia promptly and accurately by receiving output signals from the MN electrodes. In addition, this MN electrode is suitable for long-term monitoring without affecting the ECG signal quality.
As the development and application of MN gradually spread and the demand for CVD monitoring increases, research on MN in CVD monitoring will increase. MNs still have room for improvement in this field, which can be combined with other medical technologies to achieve more accurate, comprehensive, and efficient CVD monitoring goals through various technical means and methods.
MN in the treatment of CVDs
Considering the significant differences in how MNs are used to treat myocardial and vascular diseases, we provide an overview of these two areas in this section. The intramyocardial injection of therapeutic agents is a common method for treating myocardial diseases.
MN in the treatment of myocardial diseases
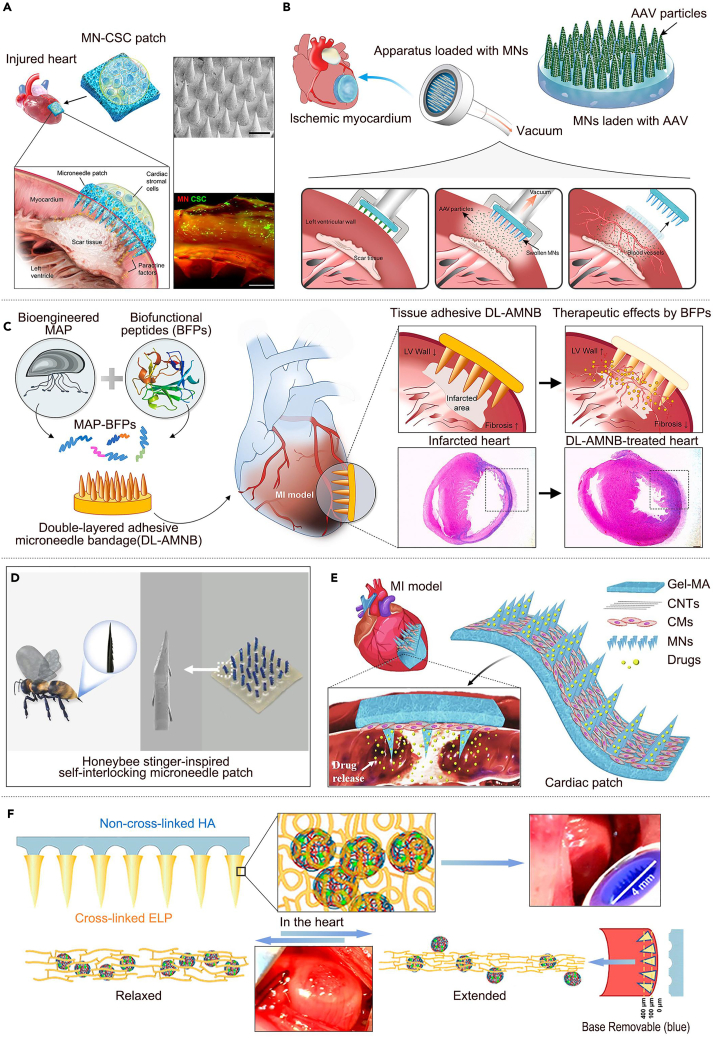
Currently, most therapeutic agents are extruded from the myocardium because of continuous dynamic muscle contraction at the site, resulting in very low retention of therapeutic agents after injection, with almost only 5–15% of the therapeutic agents reported to be retained in the myocardium.83 To address this problem, Tang et al.84 developed an MN patch that integrates cardiac stromal cells (CSCs) to treat cardiac tissue regeneration after acute myocardial infarction. Compared with the traditional cellular delivery method, utilizing the polymeric MN system to create a “channel” between the host myocardium and the therapeutic CSCs effectively promoted cardiac repair, protected cardiac functions, and angiomyogenesis (Figure 2A). Similarly, Shi et al.85 developed a phase-change MN coated with an adeno-associated virus, allowing the drug to be uniformly distributed inside the myocardium. MNs significantly improved cardiac function by enhancing the expression of vascular endothelial growth factor, which led to functional angiogenesis and activation of the Akt signaling pathway, demonstrating the superiority of MN over direct injection (Figure 2B). Their study demonstrated the feasibility and advantages of MN therapy for myocardial diseases.
Figure 2.
MNs are used in the treatment of myocardial infarction
(A) An MN patch integrating CSCs for therapeutic heart regeneration after acute myocardial infarction. Adapted with permission.84 Copyright 2018, AAAS.
(B) An adeno-associated virus adeno-associated virus gene therapy MN patch for treating ischemic myocardial disease. Adapted with permission.85 Copyright 2020, AAAS.
(C) A double-layered adhesive MN patch based on biofunctionalized adhesive protein for cardiac tissue regeneration. Adapted with permission.86 Copyright 2021, Elsevier.
(D) A self-interlocking MN patch inspired by honeybee stings for myocardial infarction treatment. Adapted with permission.87 Copyright 2022, Elsevier.
(E) A conductive MN patch integrating induced cardiomyocytes for myocardial infarction therapy. Adapted with permission.88 Copyright 2021, Elsevier.
(F) A detachable MN patch loaded with MSCF-loaded poly NP for myocardial regeneration. Adapted with permission.89 Copyright 2022, American Chemical Society.
Further, to make the MN patch adhere better to the myocardial surface and act as a synergistic therapeutic agent, Lim et al.86 developed a biofunctional MN system by fusing four biofunctional peptides with angiogenic potential into double-layer adhesive MN bandages. Long-term retention of the therapeutic peptides and strong underwater adhesion between the MN patch and myocardium were observed. The dual-layer adhesive MN bandage system adhered more firmly to the tissue, promoting the repair and regeneration of the cardiac muscle (Figure 2C). Similarly, Lu et al.87 developed a self-locking MN patch inspired by the honeybee stinger. This innovative design enhances the puncture process, effectively minimizing wall stress and strain in the myocardial infarction zone. As a result, it contributes to preserving cardiac function and morphology. The tip of the MN is similar to the stinger of a honeybee and is capable of natural interlocking in a dynamically beating heart. Simultaneously, microbleeds and spontaneous coagulation induced by MN puncture provided additional adhesions (Figure 2D). Sun et al.88 developed novel induced pluripotent stem cell-derived cardiomyocytes to improve the therapeutic efficacy of cardiomyocyte-carrying MN patches. The cardiac patch consisted of a drug-encapsulated MN bottom layer, a parallel-aligned carbon nano-tube conductive middle layer, and a gelatin-methacrylate hydrogel scaffold top layer that could target the damaged area for drug release. The carbon nano-tube layer helped to maintain synergistic intercellular interactions, resulting in an effective treatment of myocardial infarction (Figure 2E). In addition, to increase the transdermal efficiency of MN-loaded drugs to improve therapeutic efficacy, Hu et al. further89 developed a detachable MN patch containing mesenchymal stromal cell-secreted factors-loaded poly (lactic-co-glycolic acid) nanoparticles. These nanoparticles were able to promote the proliferation of damaged and reduced cardiomyocytes. The MN patches promoted myocardial recovery and reduced cardiomyocyte apoptosis (Figure 2F). Researchers have designed a self-locking structure for MNs and explored adhesive MN substrate materials to enable MNs to better adhere to the surface of the myocardium for continuous treatment. MNs can continuously deliver drugs or myocardial cells into the myocardium through microchannels opened by the needle tip. Compared to a single direct injection of myocardial cells, the retention rate and therapeutic effect of myocardial cells are significantly improved. In summary, MNs have improved drug retention rate and tissue repair effect in the treatment of myocardial diseases, demonstrating their potential in improving drug delivery efficiency, precise targeted therapy, and improving drug release. These revelations provide broad prospects for the application of MNs in the treatment of tumors, diabetes, skin repair, nerve regeneration and other diseases.
MN in the treatment of vascular diseases
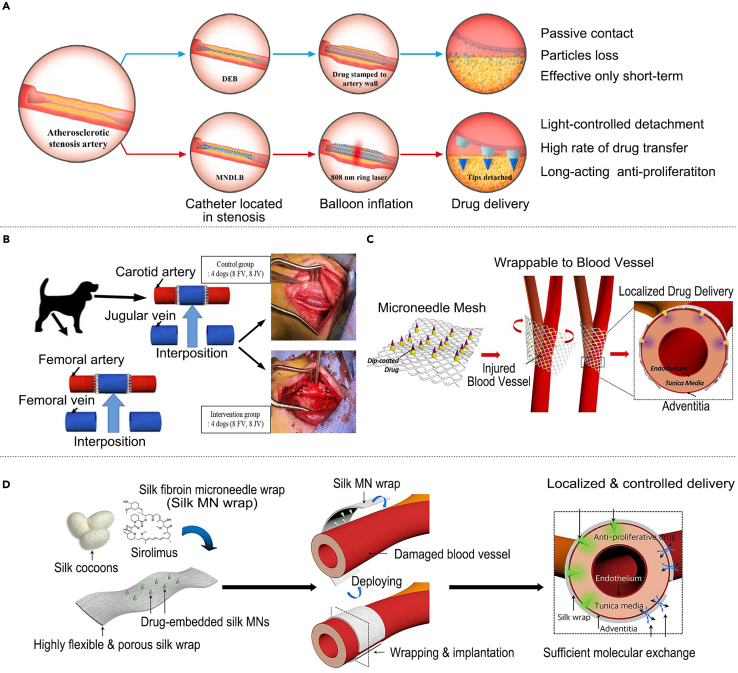
Coronary atherosclerosis is a chronic arterial lesion, and current treatments include drug therapy, interventional therapy, and coronary artery bypass grafting surgery; however, there are problems, such as short-lived therapeutic effects and high relapse rates.16 To realize targeted slow-release drug delivery for treating coronary atherosclerosis, Huang et al.90 developed a novel drug-loaded balloon with a separable MN tip on its surface. This balloon was introduced using a near-infrared ring laser built into the inner shaft of the catheter to activate the MN tip. The drug can be released slowly by embedding it in the vasculature, releasing the antiproliferative paclitaxel, which can achieve a therapeutic effect lasting more than half a year (Figure 3A). Endothelial hyperplasia is currently treated with surgery or drugs; however, there are problems such as high risk and limited effectiveness.91 To address this problem, Kim et al.92 investigated the role of the filipin protein MN embedded with sirolimus in the drug delivery efficacy inhibition of neointimal hyperplasia and vascular remodeling. This MN patch delivered drugs to the intimal layer of the vein grafts, prevented vein graft dilatation, and inhibited neointimal hyperplasia (Figure 3B). To enhance the fit of MN to blood vessels and improve their therapeutic effect, Lee et al.58 developed a biodegradable MN array made of flexible woven surgical mesh by a transfer molding method for treating endothelial hyperplasia. This MN mesh significantly increased drug delivery efficiency and inhibited neointimal hyperplasia while greatly reducing the possible side effects of other perivascular drug delivery devices (Figure 3C). Based on the above study, Lee et al.93 replaced the flexible woven surgical mesh substrate with a porous sericin protein substrate, and this MN device could provide an effective guarantee for drug delivery. Simultaneously, it preserves vascular tissues’ dilation and structural integrity, ensuring that blood vessels can undergo normal substance exchange with the surrounding environment. Additionally, there is no concern about triggering robust inflammatory reactions following the material’s degradation (Figure 3D).
Figure 3.
MN for use in the treatment of coronary atherosclerosis and CVD intimal hyperplasia
(A) A drug-loaded balloon with a built NIR-controlled tip-separable MN for arteriosclerosis treatment. Adapted with permission.90 Copyright 2022, Elsevier.
(B) A sirolimus-embedded silk fibroin MN for neointimal hyperplasia treatment. Adapted with permission.92 Copyright 2023, Mdpi.
(C) An MN mesh can be wrapped around blood vessels for neointimal hyperplasia treatment. Adapted with permission.58 Copyright 2017, Elsevier.
(D) Highly flexible and porous silk fibroin MN for vascular intimal hyperplasia treatment. Adapted with permission.93 Copyright 2021, Elsevier.
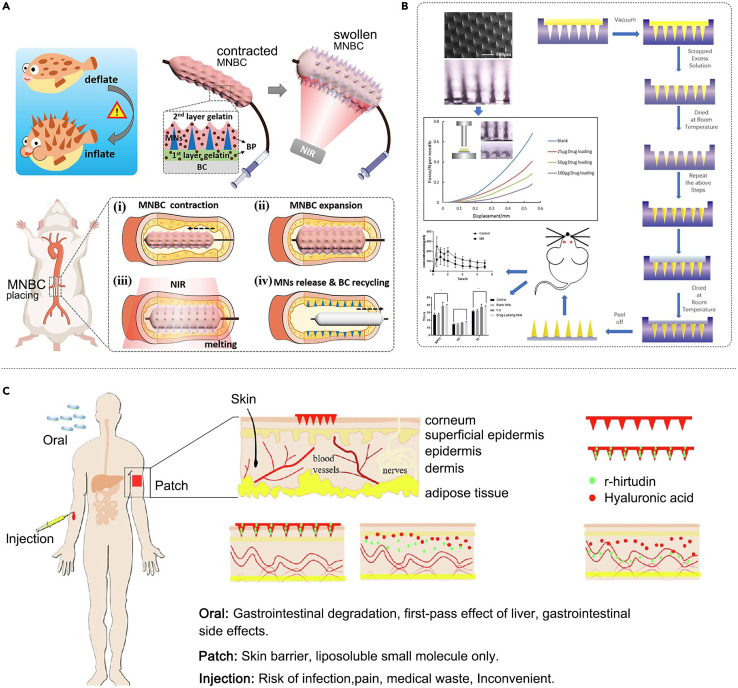
In addition, researchers have explored the application of MN in the treatment of other vascular diseases; balloon catheters are valuable in the treatment of CVDs, but their function still needs to be improved to avoid the problem of restenosis caused by the intervention of intravascular drug delivery. Zhang et al.94 proposed a smart balloon catheter inspired by globefish’s thorn-hiding and deflating–inflating characteristics. It is decorated with an invisible MN for endovascular drug delivery to inhibit post-intervention restenosis. The MN is hidden under the outermost protective gelatin layer during implantation, allowing it to move smoothly within the vessel. Upon arrival at the destination, the embedded black phosphorus converts the near-infrared light into heat, melting the gelatin layer so that the MN can be exposed and penetrate the vessel and then detach from the balloon catheter and remain within the vessel for sustained release of the drug (Figure 4A). Recombinant hirudin is a commonly used drug for the prevention of CVDs; however, its subcutaneous and intravenous administration involves the risk of bleeding. Men et al.95 prepared a recombinant hirudin MN patch using polypeptide anticoagulant drugs as matrix materials for transdermal administration. This MN patch showed a significant potential for preventing thromboembolic diseases without causing bleeding (Figure 4B). Similarly, Wu et al.96 developed a three-dimensional printing method to fabricate a mold for MN patches, enabling MN personalization. Researchers used this mold to prepare soluble r-hirudin-loaded and hyaluronic acid-based MN as matrix materials for thromboembolic disease treatment. The MN patch exhibited significant potential for preventing thromboembolic diseases and was more therapeutically effective than conventional administration (Figure 4C). Overall, MNs can be conveniently attached to blood vessels without causing damage to the blood vessels and can aid slow and continuous drug release according to the dissolution of the polymer, reducing the frequency of treatment and maintaining a stable blood concentration, thus shortening the treatment period and alleviating various side effects that are promising for the treatment of vascular diseases.
Figure 4.
MN is used in the treatment of other vascular diseases
(A) A globefish-inspired balloon catheter with intelligent MN for vascular disease treatment. Adapted with permission.94 Copyright 2022, AAAS.
(B) A recombinant hirudin MN patch for the treatment of thrombotic diseases. Adapted with permission.95 Copyright 2022, Elsevier.
(C) A dissolving MN patch loaded with R-hirudin for thromboembolic disease treatment. Adapted with permission.96 Copyright 2022, Elsevier.
The successful application of MN technology in the treatment of vascular diseases reveals its potential in the treatment of other diseases. MNs can be conveniently fixed on target tissues without causing damage, and slow and sustained drug release can be achieved through polymer dissolution, reducing treatment frequency and maintaining stable drug concentration, shortening treatment cycles, and reducing side effects. These advantages make MNs have broad prospects in the treatment of chronic diseases, skin diseases, tumors, etc., which can significantly improve drug delivery efficiency and treatment effectiveness, while enhancing patient compliance.
MNs in monitoring and treatment of MDs
MDs represent a challenge in contemporary medical practice, encompassing a range of conditions, such as diabetes, gout, osteoporosis, and obesity. Monitoring these diseases involves biomarker testing, a critical yet demanding process that often requires frequent sample collection from patients, with some methods posing potential harm to patient health.7 Traditional treatments for MDs generally include dietary control, exercises, and surgery when necessary.8 Although traditional treatments can improve MDs to a certain extent, they are often limited by their suboptimal efficacy and the risk of significant side effects.
MNs, as an emerging technology, exhibit promising applications in monitoring and treating MDs, supported by an expanding body of research. In disease monitoring of MDs, MNs enable single- and multiple-indicator tracking. The treatment of MDs using MNs enhances therapeutic effectiveness and improves patient compliance and comfort, addressing the key limitations of traditional treatment strategies.
MN in monitoring MDs
Currently, MNs are widely used to monitor single indicators, such as blood glucose and uric acid (UA), and play an essential role in the surveillance of multiple indicators. Blood glucose monitoring remains the cornerstone of diagnosing and managing diabetes mellitus.97 For instance, Zeng et al.98 developed a glucose-responsive colloidal crystal MN device that leveraged a polymer nucleus for support, with an outer layer comprising glucose-responsive colloidal crystals for monitoring. This innovative design allows the visual detection of glucose concentration changes through color shifts within a 5-min window (Figure 5A). Li et al.99 further advanced the field using a fluorescence-amplified origami MN device, enabling quantitative blood glucose monitoring. The device contains glucose molecules and the enzyme glucose oxidase and utilizes a DNA tubular origami structure. When the glucose oxidase converts a glucose molecule into a proton signal, the DNA tubular structure is driven to unfold so that the fluorescent molecule and neighboring quencher are separated, thus restoring the fluorescent signal. The patient’s blood glucose level was monitored by evaluating the degree of recovery of the DNA origami fluorescence signal in the MN patch (Figure 5B). MN technology can also seamlessly integrate with biosensors to enhance real-time biomarker monitoring. Cheng et al.100 introduced a touch-actuated MN device with a biosensor for refined glucose monitoring employing a transdermal-reverse iontophoresis extraction-electrochemical detection strategy to improve glucose extraction and monitoring precision significantly; the results of this research are expected to be widely used in clinical practice and significantly improve the efficiency of glucose monitoring and treatment of diabetes (Figure 5C). UA monitoring, vital in conditions such as gout, has also seen advancements in MN technology.101 Zhang et al.102 developed a wearable MN colorimetric device with built-in sampling and real-time analysis. The device utilizes polyvinyl alcohol MN embedded in uricase to catalyze the oxidation of UA extracted from tissue fluids and produce hydrogen peroxide. Polypyrrole nanoparticles in the display layer triggered a reaction between hydrogen peroxide and 33′55′-tetramethylbenzidine to produce a color change. Thus, blood glucose levels can be monitored by color changes, while UA levels can be precisely analyzed using a smartphone (Figure 5D).
Figure 5.
Application of MN for MDs monitoring
(A) A GCC MN patch for blood glucose monitoring. Adapted with permission.98 Copyright 2020, Elsevier.
(B) A fluorescence-amplified origami MN device for blood glucose monitoring. Adapted with permission.99 Copyright 2022, Wiley-VCH.
(C) A touch-actuated glucose sensor fully integrated with MN array and reverse iontophoresis for diabetes monitoring. Adapted with permission.100 Copyright 2022, Elsevier.
(D) A wearable colorimetric MN device for uric acid monitoring. Adapted with permission.102 Copyright 2022, Elsevier.
(E) MEAB and MPEA for simultaneously detecting glucose uric acid and cholesterol monitoring. Adapted with permission.103 Copyright 2019, Elsevier.
(F) A colorimetric dermal tattoo biosensor fabricated by MN patch for multiplexed biomarker detection. Adapted with permission.104 Copyright 2021, AAAS.
Recognizing the multifaceted nature of MDs, which often involve numerous biochemical changes, a comprehensive assessment is imperative. To address this issue, Gao et al.103 developed a flexible MN electrode array-based biosensor and a multichannel portable electrochemical analyzer to detect blood glucose, UA, and cholesterol simultaneously. The biosensor employed magnetorheological mapping lithography to prepare the MN electrode arrays and achieved multifunctional detection through biofunctionalization (Figure 5E). He et al.104 introduced a colorimetric skin tattoo biosensor fabricated from a four-region segmented MN patch. This MN device can reflect changes in the concentration of biomarkers by presenting color changes, including monitoring indicators such as pH, glucose, UA, and temperature. At the same time, the device can monitor changes in multiple biomarkers in vitro, ex vivo, and in vivo and enables the monitoring of concentration changes for at least 4 days (Figure 5F).
Currently, MN has made significant progress in the field of MD monitoring, capable of real-time monitoring and multi-indicator detection, thereby improving the accuracy and comprehensiveness of monitoring. In addition, this method of monitoring has also brought new diagnostic, preventive, and management strategies to other disease areas. It is expected to promote the implementation of personalized medicine and early intervention, thereby improving patients' quality of life and reducing long-term medical costs.
MN in the treatment of MDs
Currently, research on the application of MN for the treatment of MDs is primarily focused on managing diabetes. Consequently, this section aims to delineate the utilization of MN in the context of diabetes treatment, followed by their application in managing other MDs.
MN in the treatment of diabetes mellitus
Diabetes mellitus is a chronic disorder that poses a significant threat to human health. Traditional management primarily involves subcutaneous injection of insulin, but there are often side effects such as pain and inflammation.30 To solve this problem, MNs have emerged as an innovative therapeutic approach for diabetes treatment; they can be categorized into two modalities: insulin MN patches for transdermal delivery and intraluminal unfolding MN syringes for oral administration. The insulin MN patches represent a pivotal advancement in drug delivery. These patches adhere to the skin, with the MN penetrating the epidermal layer to facilitate direct insulin transport to body tissues. A notable example is the development by Cao et al.105 of a continuous insulin delivery MN patch. This patch incorporates filipin protein as a drug delivery medium, enabling insulin release regulated by the expansion of the needles, thereby stabilizing blood glucose levels (Figure 6A). In contrast, intraluminal unfolding MN syringes offer an alternative insulin administration route specifically designed for oral delivery. This method involves placing an MN syringe within a gastrointestinal cavity, such as the stomach or intestine. Upon unfolding, the MN syringe efficiently delivered insulin to the intestinal tissues. A pioneering development in this area is the intraluminal unfolding MN syringe designed by Alex et al.106 for oral insulin delivery. This device propels drug-loaded MNs into the intestinal tissue, facilitating effective oral drug administration. Additionally, the versatility of this device extends its potential for loading multiple MN formulations, positioning it as a novel platform for oral drug delivery (Figure 6B).
Figure 6.
MN is used in the treatment of diabetes
(A) A silk fibroin MN patch for insulin delivery. Adapted with permission.105 Copyright 2022, Elsevier.
(B) A luminal unfolding MN injector for oral delivery of macromolecules for insulin delivery. Adapted with permission.106 Copyright 2019, Springer Nature.
(C) A basal-bolus insulin regimen integrated MN patch for intraday postprandial glucose control. Adapted with permission.107 Copyright 2020, AAAS.
(D) An MN patch loaded with nanovesicles for glucose transporter-mediated insulin delivery. Adapted with permission.108 Copyright 2022, American Chemical Society.
(E) A pH-responsive MN patch for controlled insulin delivery. Adapted with permission.109 Copyright 2021, Tsinghua University Press.
(F) A glucose-responsive insulin MN patch for blood glucose control. Adapted with permission.110 Copyright 2020, Nature Publishing Group.
(G) An electro-responsive silk fibroin MN for controlled release of insulin delivery. Adapted with permission.111 Copyright 2023, Elsevier.
However, conventional MNs encounter challenges in achieving an on-demand insulin supply. Researchers have made advancements in MN material structures and drug carriers to address this issue. Chen et al.107 developed an integrated insulin MN patch specifically tailored for intraday postprandial glucose control. This patch facilitates transdermal insulin delivery with varying release kinetics, effectively covering postprandial glucose fluctuations, thereby improving daytime glucose stability and preventing complications (Figure 6C). Chen et al.108 introduced an MN patch containing nanovesicles for glucose transporter-mediated insulin delivery. Under hyperglycemic conditions, glucose competitively interacts with glucosamine-modified insulin within vesicles, leading to its rapid release and in vivo glucose regulation. Incorporating additional (glucosamine-modified-insulin) into vesicles prolongs the effective release time, offering a method for blood glucose control without inducing hypoglycemia (Figure 6D).
As research progresses, a series of smart environmentally responsive MN patches have emerged as MN capable of sensing changes in the surrounding environment, such as blood glucose levels, pH of reactive oxygen species, light temperature, electric field strength, and ion concentration, by coordinating the internal functions of the material and then making corresponding adjustments based on this environmental information. Luo et al.109 developed a pH-responsive MN array patch for insulin release control using pH-sensitive insulin-loaded nanoparticles (SNPI) and pH-insensitive nanoparticles (iSNPG+C). This MN patch allowed transdermal glucose-responsive insulin delivery with rapid insulin release under hyperglycemic conditions, mitigating the risk of skin inflammation (Figure 6E). Similarly, Yu et al.110 developed a glucose-responsive insulin patch using an insulin- and glucose-responsive polymer matrix polymerized via in situ light curing. Under hyperglycemic conditions, phenylboronic acid units in the polymer matrix form glucose-boronate complexes, leading to the swelling of the polymer matrix and the promotion of rapid insulin release (Figure 6F). Qi et al.111 developed an electro-responsive silk fibroin MN device to control insulin release. The disulfide cross-linking sites were reduced by energizing the device, increasing MN solubility, and promoting insulin release. The sulfhydryl groups were oxidized upon power cessation, forming disulfide bonds and reducing the MN solubility, resulting in a slower release rate (Figure 6G). Overall, The application of MN in the treatment of diabetes has made groundbreaking progress. This therapeutic approach not only enhances the bioavailability of medications but also enables on-demand drug delivery, significantly reducing side effects and improving patient compliance. It offers patients a more convenient and comfortable treatment option. Furthermore, the principles and design of MN for diabetes have provided new ideas for the treatment of other diseases, especially in pain management, dermatological treatments, and vaccination. It has paved the way for personalized and precise treatment, promoting the development of transdermal drug delivery toward a more humanized and intelligent direction.
MN for other MDs
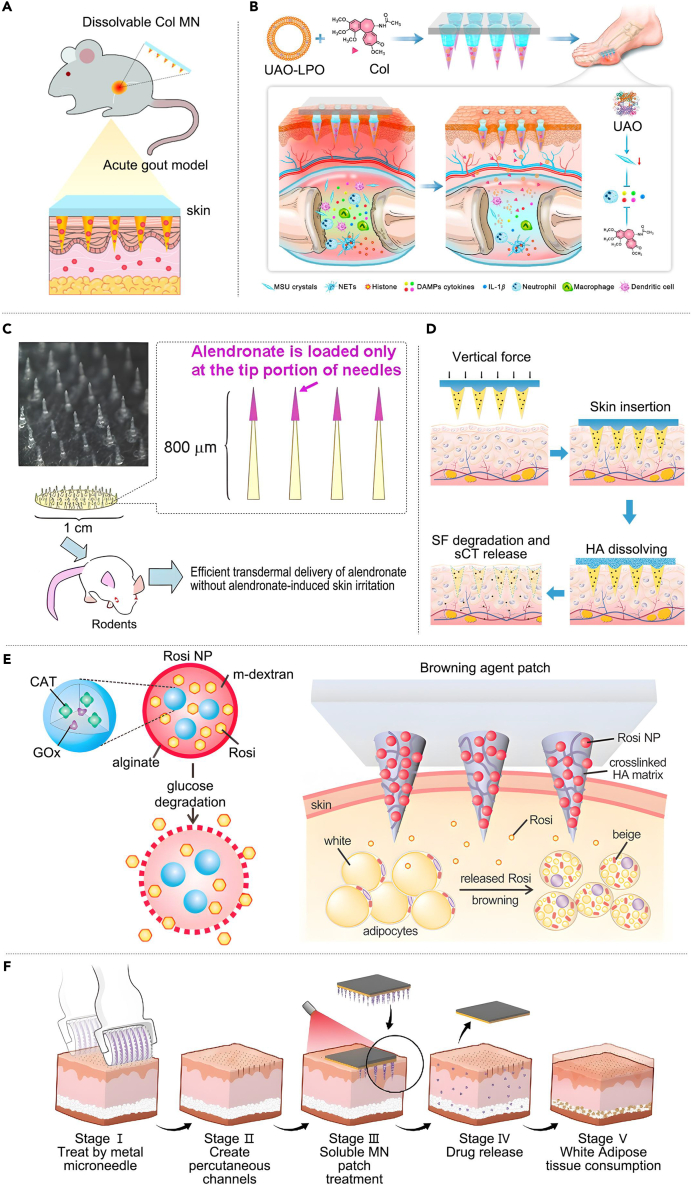
Gout is characterized by intermittent attacks, and patients need to take large amounts of UA-lowering drugs for an extended period to alleviate this condition, leading to substantial side effects.112 To address this problem, Liu et al.113 developed a colchicine-MN single-drug system to treat acute gout in rats. This MN delivery system significantly alleviated inflammation and pain in rat knee joints at a lower dose with fewer side effects (Figure 7A). Compared with the MN single-drug system, the MN dual-drug system is more advantageous in terms of therapeutic effects. Yang et al.114 developed a detachable MN dual-drug system of chitosan carrying both colchicine and uricase-liposomes that achieved up to one week of sustained drug delivery. Compared with traditional delivery methods, this system can reduce doses, minimize side effects, and improve patient compliance (Figure 7B).
Figure 7.
MN for use in the treatment of other MDs
(A) Transdermal delivery of Col using dissolvable MN arrays to treat acute gout. Adapted with permission.113 Copyright 2022, Taylor& Francis.
(B) A chitosan detachable MN carrying both Col and UAO-LPO for gout treatment. Adapted with permission.114 Copyright 2023, Elsevier.
(C) A nitrogen-containing bisphosphonate ALN soluble MN for osteoporosis treatment. Adapted with permission.115 Copyright 2017, Mdpi.
(D) A salmon calcitonin separable MN system for osteoporosis treatment. Adapted with permission.116 Copyright 2023, American Chemical Society.
(E) An MN patch for locally induced adipose tissue browning to treat obesity. Adapted with permission.117 Copyright 2017, Springer Berlin Heidelberg.
(F) A black phosphorus-modified Rosi soluble MN patch for treating obesity. Adapted with permission.118 Copyright 2020, Elsevier.
Osteoporosis is a systemic MD that is usually treated with oral infusion or local injection of bisphosphonates, which often yield suboptimal therapeutic outcomes and may induce adverse effects such as pain.119 Addressing this, Katsumi et al.115 developed a self-fusing MN for osteoporosis-related fractures. A micrometer-sized needle, loaded with the nitrogen-containing bisphosphonate alanophosphonate, concentrates alendronate (ALN) at the needle tip. This design facilitates rapid drug delivery, minimizes drug loss, and enhances drug bioavailability (Figure 7C). Li et al.116 developed a salmon calcitonin detachable MN system for osteoporosis treatment. The system, comprising a filipin protein tip and a hyaluronic acid substrate, allows for slow drug release by dissolving the HA substrate after skin penetration, offering a non-injectable strategy for osteoporosis treatment (Figure 7D).
Obesity is one of the most serious public health concerns of the 21st century, and traditional treatments mainly include diet control, physical exercise, and surgery. However, the treatment effect is commonly unsatisfactory and may produce serious side effects.120 Zhang et al.117 addressed this issue by developing an MN patch to induce local fat browning. This innovative patch delivered a browning agent to the subcutaneous fat cells, promoting the ‘browning’ reaction and inhibiting fat cell hypertrophy, thereby improving metabolism and treating obesity (Figure 7E). Peng et al.118 introduced a black phosphorus-modified soluble MN patch for delivery of rosiglitazone. First, a transcutaneous channel was established using metallic MN and subsequently under near-infrared light irradiation; the soluble MN patch efficiently delivered rosiglitazone into deep tissues to reduce adipose tissue (Figure 7F).
Although MN has received less attention in treating other MDs, it has demonstrated favorable therapeutic effects. Nevertheless, it faces various challenges that warrant further in-depth research and exploration; this field holds immense potential and has a broad scope of applications.
Conclusion
Due to the lack of effective conventional products, monitoring and treatment of cardiovascular and MDs is a daunting challenge for clinical doctors. The drug delivery and monitoring system based on MN transdermal delivery is actively being studied. The focus of this method is to overcome skin barriers, deliver effective doses of drugs to the lesion site, or extract tissue fluid for monitoring. All the above studies have demonstrated that MNs have witnessed rapid advancements in the monitoring and treating CVDs and MDs. As an innovative disease monitoring modality, MNs offer two benefits: alleviating patient discomfort and enhancing monitoring comfort while overcoming deficiencies in traditional detection methods, thereby improving the precision and sensitivity of monitoring accuracy. As a novel drug delivery method, MNs enhance drug bioavailability, enable targeted drug delivery, and mitigate the side effects associated with systemic therapy, in contrast to traditional injection and oral intake methods.
Nevertheless, there are still many difficulties to be overcome before the practical application of MN to the clinical treatment of CVDs and MDs. First, it is necessary to overcome the difficulties of improving the drug-carrying capacity of MN patches as well as the transdermal efficiency of drugs in order to improve the therapeutic effect. Second, it is necessary to pay attention to the biocompatibility and safety of MN fabrication materials, to track in real time the inflammatory and immune responses that may occur in vivo after the action of MN patches, and to find timely and effective countermeasures. In addition, before clinical application, the relevant technology must undergo rigorous clinical trials to determine its safety and effectiveness and obtain regulatory approval.
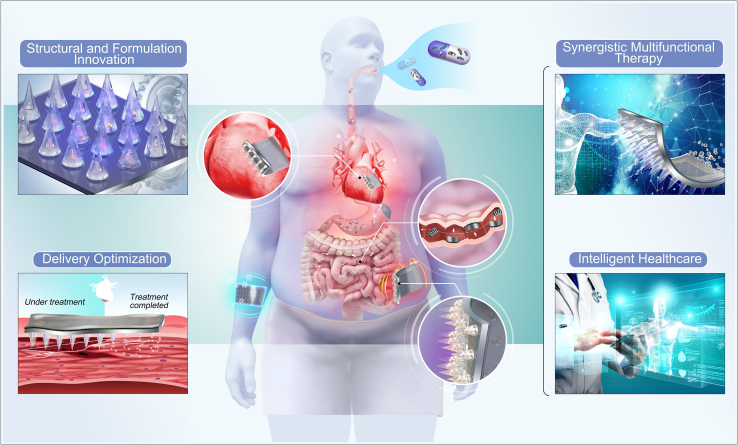
In the future, the theoretical foundation of MNs is anticipated to become more comprehensive, paving the way for broader applications (Figure 8). Structural and Formulation Innovation. Current MN systems have many problems, such as low drug loading, insufficient mechanical strength, insufficient toughness, and easy deformation. These problems are mainly related to the current structure of the MN and matrix materials. In the future, it is expected that the structure of MN bodies will be improved so that their volume remains unchanged but can have more drug loading. Simultaneously, it is also possible to search for new matrix materials and research new formula ratios to make MN have better mechanical strength and toughness of MNs.
Figure 8.
Future directions of MN
Microneedles (MNs) represent a cutting-edge approach in addressing the global health challenges posed by cardiovascular diseases (CVDs) and metabolic Disorders (MDs). This comprehensive overview delves into recent advances, highlighting MNs' role in painless, safe, and convenient monitoring and treatment compared to traditional approaches. The study covers MN principles, modalities, and their application in CVDs and MDs, providing insights into the promising future developments in this innovative field.
Delivery optimization
Although the matrix materials used for making MN can usually be decomposed into harmless byproducts that are easy to metabolize, further verification is needed to determine whether the long-term deposition of matrix materials in tissues, especially in the myocardium and blood vessels, causes unnecessary immune reactions in the body. Given this situation, future researchers are expected to develop a novel delivery mechanism in which MNs quickly miss their target and are eliminated from the body when partial treatment of inflammation is completed or unnecessary immune reactions occur, minimizing damage to the body.
Synergistic multifunctional therapies
A single-drug treatment may not be ideal for most diseases, and combining other treatment methods is important. Future MN systems are expected to integrate electromagnetic light, heat, ultrasound stimulation, acupoint stimulation, and other methods for collaborative treatment.
Intelligent healthcare
Currently, the application of MN is mainly focused on percutaneous drug delivery for disease treatment. Some researchers have begun to explore the monitoring of diseases with MN; however, most of them monitor a single indicator, and the response to diseases is not comprehensive. At the same time, there is a lack of MN systems participating in the entire disease treatment process, such as the monitoring analysis treatment. Intelligence is an inevitable trend for future MN systems that will integrate multiple fields such as drug delivery, biosensing, diagnosis, and electronic control to achieve full process control from disease monitoring and diagnosis to treatment. Furthermore, MN can be combined with digital technology and big data analysis to form cloud data for the unified transmission of results detected by wearable MN devices to hospital databases through the Internet. Professional medical staff can make judgments and provide remote diagnosis and treatment.
In conclusion, MNs hold immense promise for the future and are poised to replace current methodologies for monitoring and treating various diseases. However, it is crucial to note that most current MN studies are based on case series or small-sample randomized trials. Further large-scale randomized controlled clinical trials are imperative for gradually promoting MN applications to bridge the gap between laboratory studies and clinical applications.
Acknowledgments
This work was supported by grants from High-level hospital clinical research fees of Fu Wai Hospital, CAMS (grant 2023-GSP-QN-23) and the National Natural Science Foundation of China (82300345).
Author contributions
X.N.Z. and M.L. collected and interpreted studies and was a major contributor to the writing and editing of the manuscript. Y.H., J.Z., S.R.S., and J.X. provided direction and guidance throughout the preparation of this manuscript. Q.G., X.Y.K., J.Y.S., Y.H., and H.X. reviewed and made significant revisions to the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110615.
Contributor Information
Jing Xu, Email: cardioxujing@163.com.
Songren Shu, Email: shusongren@126.com.
Jian Zhuang, Email: zhuangjian@mail.buct.edu.cn.
Yuan Huang, Email: clevelandhuangyuan@163.com.
Supplemental information
References
- 1.Han C., Song Q., Ren Y., Chen X., Jiang X., Hu D. Global prevalence of prediabetes in children and adolescents: A systematic review and meta-analysis. J. Diabetes. 2022;14:434–441. doi: 10.1016/j.ijbiomac.2023.124684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh P., Youden B., Carrier A., Oakes K., Servos M., Jiang R., Lin S., Nguyen T.D., Zhang X. Photoresponsive polymeric microneedles: An innovative way to monitor and treat diseases. J. Contr. Release. 2023;353:1050–1067. doi: 10.1016/j.jconrel.2022.12.036. [DOI] [PubMed] [Google Scholar]
- 3.Mbituyimana B., Ma G., Shi Z., Yang G. Polymer-based microneedle composites for enhanced non-transdermal drug delivery. Appl. Mater. Today. 2022;29 doi: 10.1016/j.apmt.2022.101659. [DOI] [Google Scholar]
- 4.Zhang X.P., He Y.T., Li W.X., Chen B.Z., Zhang C.Y., Cui Y., Guo X.D. An update on biomaterials as microneedle matrixes for biomedical applications. J. Mater. Chem. B. 2022;10:6059–6077. doi: 10.1039/d2tb00905f. [DOI] [PubMed] [Google Scholar]
- 5.Xu N., Xu W., Zhang M., Yu J., Ling G., Zhang P. Microneedle-Based Technology: Toward Minimally Invasive Disease Diagnostics. Adv. Mater. Technol. 2022;7 doi: 10.1021/acs.analchem.3c02242. [DOI] [Google Scholar]
- 6.Xi X., Li H., Chen S., Lv T., Ma T., Jiang R., Zhang P., Wong W.H., Zhang X. Unfolding the genotype-to-phenotype black box of cardiovascular diseases through cross-scale modeling. iScience. 2022;25 doi: 10.1016/j.isci.2022.104790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore L.E., Vucen S., Moore A.C. Trends in drug- and vaccine-based dissolvable microneedle materials and methods of fabrication. Eur. J. Pharm. Biopharm. 2022;173:54–72. doi: 10.1016/j.ejpb.2022.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Bao L., Park J., Bonfante G., Kim B. Recent advances in porous microneedles: materials, fabrication, and transdermal applications. Drug Deliv. Transl. Res. 2022;12:395–414. doi: 10.1007/s13346-021-01045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mdanda S., Ubanako P., Kondiah P.P.D., Kumar P., Choonara Y.E. Recent Advances in Microneedle Platforms for Transdermal Drug Delivery Technologies. Polymers. 2021;13:2405. doi: 10.3390/polym13152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S., Kong N., Feng C., Shajii A., Bejgrowicz C., Tao W., Farokhzad O.C. Drug Delivery Strategies for the Treatment of Metabolic Diseases. Adv. Healthcare Mater. 2019;8 doi: 10.1002/adhm.201801655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y.P., Tian M.Y., Yang Y.D., Li H., Zhao T.T., Zhu J., Mou F.F., Cui G.H., Guo H.D., Shao S.J. Schwann cells-derived exosomal miR-21 participates in high glucose regulation of neurite outgrowth. iScience. 2022;25 doi: 10.1016/j.isci.2022.105141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bras-Rosario L., Joao A., Rocha I., Tiago J., Pinto F., Sequeira A. Arterial Chemoreflex Protective Effect on Left Ventricle Energetics and Changes after Myocardial Infarct. Faseb. J. 2018;32:884. doi: 10.1096/fasebj.2018.32.1_supplement.884.9. [DOI] [Google Scholar]
- 13.Kern K.B., Hanna J.M., Young H.N., Ellingson C.J., White J.J., Heller B., Illindala U., Hsu C.-H., Zuercher M. Importance of Both Early Reperfusion and Therapeutic Hypothermia in Limiting Myocardial Infarct Size Post–Cardiac Arrest in a Porcine Model. JACC Cardiovasc. Interv. 2016;9:2403–2412. doi: 10.1016/j.jcin.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Kamal N., Smith E.E., Jeerakathil T., Hill M.D. Thrombolysis: Improving door-to-needle times for ischemic stroke treatment – A narrative review. Int. J. Stroke. 2018;13:268–276. doi: 10.1177/1747493017743060. [DOI] [PubMed] [Google Scholar]
- 15.Perdana Putra H.B., Syakdiyah N.H., Pintaningrum Y., Debi Mustika Martin M.A. TCTAP A-016 Immediate Versus Deferred Stenting for ST Elevation Myocardial Infarct With High Burden Thrombus: A Meta-Analysis From Different Perspective. J. Am. Coll. Cardiol. 2023;81:S10–S11. doi: 10.1016/j.jacc.2023.03.025. [DOI] [Google Scholar]
- 16.Zheng Z., Lei C., Liu H., Jiang M., Zhou Z., Zhao Y., Yu C.-Y., Wei H. A ROS-Responsive Liposomal Composite Hydrogel Integrating Improved Mitochondrial Function and Pro-Angiogenesis for Efficient Treatment of Myocardial Infarction. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202200990. [DOI] [PubMed] [Google Scholar]
- 17.Ji X., Meng Y., Wang Q., Tong T., Liu Z., Lin J., Li B., Wei Y., You X., Lei Y., et al. Cysteine-Based Redox-Responsive Nanoparticles for Fibroblast-Targeted Drug Delivery in the Treatment of Myocardial Infarction. ACS Nano. 2023;17:5421–5434. doi: 10.1021/acsnano.2c10042. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez Rico C., Konate K., Josse E., Nargeot J., Barrère-Lemaire S., Boisguérin P. Therapeutic Peptides to Treat Myocardial Ischemia-Reperfusion Injury. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.792885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholz M., Horn K., Pott J., Gross A., Kleber M.E., Delgado G.E., Mishra P.P., Kirsten H., Gieger C., Müller-Nurasyid M., et al. Genome-wide meta-analysis of phytosterols reveals five novel loci and a detrimental effect on coronary atherosclerosis. Nat. Commun. 2022;13:143. doi: 10.1038/s41467-021-27706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shreyder K., Chaudhry G.M., Resnic F. When you hear hoofs, it might be zebra. genetically proven arvc in a post cardiac arrest patient with coronary atherosclerosis. J. Am. Coll. Cardiol. 2021;77:2211. doi: 10.1016/s0735-1097(21)03566-x. [DOI] [Google Scholar]
- 21.Dani S.I.D. P1650Three year clinical outcomes after treatment of coronary atherosclerosis with Sirolimus coated balloon. Eur. Heart J. 2018;39 doi: 10.1093/eurheartj/ehy565.p1650. [DOI] [Google Scholar]
- 22.Choi H.Y., Choi S., Ruel I., Genest J. Repurposing the anti-cancer drug docetaxel for the prevention and treatment of atherosclerosis. Atherosclerosis. 2022;355:69. doi: 10.1016/j.atherosclerosis.2022.06.431. [DOI] [Google Scholar]
- 23.Waters D.D. Utility of Biomarkers and Imaging in the Development of Drugs for the Treatment of Coronary Atherosclerosis. Can. J. Cardiol. 2012;28:687–692. doi: 10.1016/j.cjca.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Martín-Reyes R., Calvo-Orbe L., Moreno R. Treatment of coronary atherosclerosis aneurysm with a combination of stent-graft and in-stent drug-eluting stent. J. Invasive Cardiol. 2011;23:E66–E68. [PubMed] [Google Scholar]
- 25.Ziegler T., Kupatt C. Sonodynamic Therapy of Atherosclerotic Plaques: Breaking the Cycle. JACC (J. Am. Coll. Cardiol.): Basic to Translat. Sci. 2020;5:66–68. doi: 10.1016/j.jacbts.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu T., Feng H., He M., Yue R., Wu S. Efficacy of artemisinin and its derivatives in animal models of type 2 diabetes mellitus: A systematic review and meta-analysis. Pharmacol. Res. 2022;175 doi: 10.1016/j.phrs.2021.105994. [DOI] [PubMed] [Google Scholar]
- 27.Sly B., Russell A.W., Sullivan C. Digital Interventions to improve safety and quality of inpatient diabetes management: a systematic review. Int. J. Med. Inf. 2022;157 doi: 10.1016/j.ijmedinf.2021.104596. [DOI] [PubMed] [Google Scholar]
- 28.Xia X., Xiao J. Natural Ingredients from Medicine Food Homology as Chemopreventive Reagents against Type 2 Diabetes Mellitus by Modulating Gut Microbiota Homoeostasis. Molecules. 2021;26:6934. doi: 10.3390/molecules26226934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho K.Y., Nakamura A., Oba-Yamamoto C., Tsuchida K., Yanagiya S., Manda N., Kurihara Y., Aoki S., Atsumi T., Miyoshi H. Switching to Once-Daily Insulin Degludec/Insulin Aspart from Basal Insulin Improves Postprandial Glycemia in Patients with Type 2 Diabetes Mellitus: Randomized Controlled Trial. Diabetes Metab. J. 2020;44:532–541. doi: 10.4093/dmj.2019.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 31.Kasper I.R., Juriga M.D., Giurini J.M., Shmerling R.H. Treatment of tophaceous gout: When medication is not enough. Semin. Arthritis Rheum. 2016;45:669–674. doi: 10.1016/j.semarthrit.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 32.FitzGerald J.D., Dalbeth N., Mikuls T., Brignardello-Petersen R., Guyatt G., Abeles A.M., Gelber A.C., Harrold L.R., Khanna D., King C., et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020;72:744–760. doi: 10.1002/acr.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pisaniello H.L., Fisher M.C., Farquhar H., Vargas-Santos A.B., Hill C.L., Stamp L.K., Gaffo A.L. Efficacy and safety of gout flare prophylaxis and therapy use in people with chronic kidney disease: a Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN)-initiated literature review. Arthritis Res. Ther. 2021;23:130. doi: 10.1186/s13075-021-02416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C., Luo X., Li M., Cui L., Li X., Han L., Wang X., Ren W., He Y., Sun W., et al. Reporting quality of clinical practice guidelines regarding gout and hyperuricemia according to the RIGHT checklist: systematic review. Syst. Rev. 2021;10:99. doi: 10.1186/s13643-021-01645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh J.A., Gaffo A. Gout epidemiology and comorbidities. WB Saunders. 2020;50:S11–S16. doi: 10.1016/j.semarthrit.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 36.He X.-Y., Yu H.-M., Lin S., Li Y.-Z. Advances in the application of mesenchymal stem cells, exosomes, biomimetic materials, and 3D printing in osteoporosis treatment. Cell. Mol. Biol. Lett. 2021;26:47. doi: 10.1186/s11658-021-00291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woźniczka M., Błaszczak-Świątkiewicz K. New Generation of Meso and Antiprogestins (SPRMs) into the Osteoporosis Approach. Molecules. 2021;26:6491. doi: 10.3390/molecules26216491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seely K.D., Kotelko C.A., Douglas H., Bealer B., Brooks A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021;22:9452. doi: 10.3390/ijms22179452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thurner G.C., Haybaeck J., Debbage P. Targeting Drug Delivery in the Elderly: Are Nanoparticles an Option for Treating Osteoporosis? Int. J. Mol. Sci. 2021;22:8932. doi: 10.3390/ijms22168932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salari N., Ghasemi H., Mohammadi L., Behzadi M.H., Rabieenia E., Shohaimi S., Mohammadi M. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021;16:609. doi: 10.1186/s13018-021-02772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rong B., Wu Q., Saeed M., Sun C. Gut Microbiota-A positive contributor in the process of intermittent fasting-mediated obesity control. Anim. Nutr. 2021;7:1283–1295. doi: 10.1016/j.aninu.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muvhulawa N., Dludla P.V., Mthembu S.X., Ziqubu K., Tiano L., Mazibuko-Mbeje S.E. Rutin attenuates tumor necrosis factor-α-induced inflammation and initiates fat browning in 3T3-L1 adipocytes: Potential therapeutic implications for anti-obesity therapy. South Afr. J. Bot. 2023;160:697–704. doi: 10.1016/j.sajb.2023.07.043. [DOI] [Google Scholar]
- 43.Wang J., Li G., Ji G., Hu Y., Zhang W., Ji W., Yu J., Han Y., Cui G., Wang H., et al. Habenula volume and functional connectivity changes following laparoscopic sleeve gastrectomy for obesity treatment. Biol. Psychiatr. 2024;95:916–925. doi: 10.1016/j.biopsych.2023.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Tao X., Liu Y., Ding Z., Xie S., Cao W., Li X. Injectable cell-targeting fiber rods to promote lipolysis and regulate inflammation for obesity treatment. Biomater. Sci. 2023;11:5663–5673. doi: 10.1039/d3bm00619k. [DOI] [PubMed] [Google Scholar]
- 45.World Obesity Day Atlases . World Obesity Federation Global Obesity Observatory; 2023. Obesity Atlas 2023.https://data.worldobesity.org/publications/?cat=19 [Google Scholar]
- 46.Arya J., Prausnitz M.R. Microneedle patches for vaccination in developing countries. J. Contr. Release. 2016;240:135–141. doi: 10.1016/j.jconrel.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pham Q.D., Björklund S., Engblom J., Topgaard D., Sparr E. Chemical penetration enhancers in stratum corneum — Relation between molecular effects and barrier function. J. Contr. Release. 2016;232:175–187. doi: 10.1016/j.jconrel.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 48.Xu J., Xu D., Xuan X., He H. Advances of Microneedles in Biomedical Applications. Molecules. 2021;26:5912. doi: 10.3390/molecules26195912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babity S., Roohnikan M., Brambilla D. Advances in the Design of Transdermal Microneedles for Diagnostic and Monitoring Applications. Small. 2018;14 doi: 10.1002/smll.201803186. [DOI] [PubMed] [Google Scholar]
- 50.Wang S., Zhao M., Yan Y., Li P., Huang W. Flexible Monitoring, Diagnosis, and Therapy by Microneedles with Versatile Materials and Devices toward Multifunction Scope. Research. 2023;2023 doi: 10.34133/research.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X., Wang Y., Chi J., Zhao Y. Smart Microneedles for Therapy and Diagnosis. Research. 2020;2020 doi: 10.34133/2020/7462915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun L., Fan L., Bian F., Chen G., Wang Y., Zhao Y. MXene-Integrated Microneedle Patches with Innate Molecule Encapsulation for Wound Healing. Research. 2021;2021 doi: 10.34133/2021/9838490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pires L.R., Gaspar J. Micro and nano-needles as innovative approach in nanomedicine. Nanostr. Biomat. Regen. Med. 2020;1:379–406. doi: 10.1016/b978-0-08-102594-9.00014-0. [DOI] [Google Scholar]
- 54.Creelman B., Frivold C., Jessup S., Saxon G., Jarrahian C. Manufacturing readiness assessment for evaluation of the microneedle array patch industry: an exploration of barriers to full-scale manufacturing. Drug Deliv. Transl. Res. 2022;12:368–375. doi: 10.1007/s13346-021-01076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henry S., McAllister D.V., Allen M.G., Prausnitz M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharmaceut. Sci. 1998;87:922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 56.Wang P.M., Cornwell M., Prausnitz M.R. Minimally Invasive Extraction of Dermal Interstitial Fluid for Glucose Monitoring Using Microneedles. Diabetes Technol. Therapeut. 2005;7:131–141. doi: 10.1089/dia.2005.7.131. [DOI] [PubMed] [Google Scholar]
- 57.Davis S.P., Martanto W., Allen M.G., Prausnitz M.R. Hollow Metal Microneedles for Insulin Delivery to Diabetic Rats. IEEE Trans. Biomed. Eng. 2005;52:909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- 58.Lee J., Kim D.-H., Lee K.J., Seo I.H., Park S.H., Jang E.H., Park Y., Youn Y.-N., Ryu W. Transfer-molded wrappable microneedle meshes for perivascular drug delivery. J. Contr. Release. 2017;268:237–246. doi: 10.1016/j.jconrel.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Satti A.T., Park J., Park J., Kim H., Cho S. Fabrication of Parylene-Coated Microneedle Array Electrode for Wearable ECG Device. Sensors. 2020;20:5183. doi: 10.3390/s20185183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chi J., Sun L., Cai L., Fan L., Shao C., Shang L., Zhao Y. Chinese herb microneedle patch for wound healing. Bioact. Mater. 2021;6:3507–3514. doi: 10.1016/j.bioactmat.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen W., Wainer J., Ryoo S.W., Qi X., Chang R., Li J., Lee S.H., Min S., Wentworth A., Collins J.E., et al. Dynamic omnidirectional adhesive microneedle system for oral macromolecular drug delivery. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abk1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C., Zhao Z., Lv H., Yu J., Zhang P. Microneedles-mediated Drug Delivery System for the Diagnosis and Treatment of Melanoma. Colloids Surf., B. 2022;219 doi: 10.1016/j.colsurfb.2022.112818/. [DOI] [PubMed] [Google Scholar]
- 63.Gumus E., Bingol H., Zor E. Lateral flow assays for detection of disease biomarkers. J. Pharm. Biomed. Anal. 2023;225 doi: 10.1016/j.jpba.2022.115206. [DOI] [PubMed] [Google Scholar]
- 64.Wu Y., Ghoraani B. Biological Signal Processing and Analysis for Healthcare Monitoring. Sensors. 2022;22:5341. doi: 10.3390/s22145341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sang M., Cho M., Lim S., Min I.S., Han Y., Lee C., Shin J., Yoon K., Yeo W.H., Lee T., et al. Fluorescent-based biodegradable microneedle sensor array for tether-free continuous glucose monitoring with smartphone application. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adh1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X., Huang X., Mo J., Wang H., Huang Q., Yang C., Zhang T., Chen H.J., Hang T., Liu F., et al. A Fully Integrated Closed-Loop System Based on Mesoporous Microneedles-Iontophoresis for Diabetes Treatment. Adv. Sci. 2021;8 doi: 10.1002/advs.202100827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed Saeed AL-Japairai K., Mahmood S., Hamed Almurisi S., Reddy Venugopal J., Rebhi Hilles A., Azmana M., Raman S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020;587 doi: 10.1016/j.ijpharm.2020.119673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang T., Sun B., Guo J., Wang M., Cui H., Mao H., Wang B., Yan F. Active pharmaceutical ingredient poly(ionic liquid)-based microneedles for the treatment of skin acne infection. Acta Biomater. 2020;115:136–147. doi: 10.1016/j.actbio.2020.08.023. [DOI] [PubMed] [Google Scholar]
- 69.Heikenfeld J., Jajack A., Feldman B., Granger S.W., Gaitonde S., Begtrup G., Katchman B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019;37:407–419. doi: 10.1038/s41587-019-0040-3. [DOI] [PubMed] [Google Scholar]
- 70.Miller P.R., Narayan R.J., Polsky R. Microneedle-based sensors for medical diagnosis. J. Mater. Chem. B. 2016;4:1379–1383. doi: 10.1039/c5tb02421h. [DOI] [PubMed] [Google Scholar]
- 71.Zanchetti A. Obesity, diabetes, and antidiabetic treatment: blood pressure and vascular effects. J. Hypertens. 2016;34:165–166. doi: 10.1097/HJH.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 72.Chen B.Z., He Y.T., Zhao Z.Q., Feng Y.H., Liang L., Peng J., Yang C.Y., Uyama H., Shahbazi M.-A., Guo X.D. Strategies to develop polymeric microneedles for controlled drug release. Adv. Drug Deliv. Rev. 2023;203 doi: 10.1016/j.addr.2023.115109. [DOI] [Google Scholar]
- 73.Bhadale R.S., Londhe V.Y. Solid microneedle assisted transepidermal delivery of iloperidone loaded film: Characterization and Skin deposition studies. J. Drug Deliv. Sci. Technol. 2023;79 doi: 10.1016/j.jddst.2022.104028. [DOI] [Google Scholar]
- 74.Ghate V., Renjith A., Badnikar K., Pahal S., Jayadevi S.N., Nayak M.M., Vemula P.K., Subramanyam D.N. Single step fabrication of hollow microneedles and an experimental package for controlled drug delivery. Int. J. Pharm. 2023;632 doi: 10.1016/j.ijpharm.2022.122546. [DOI] [PubMed] [Google Scholar]
- 75.Terashima S., Tatsukawa C., Takahashi T., Suzuki M., Aoyagi S. Fabrication of hyaluronic acid hollow microneedle array. Jpn. J. Appl. Phys. 2020;59 doi: 10.35848/1347-4065/ab7312. [DOI] [Google Scholar]
- 76.Liu L., Wang Q., Liao H., Ye J., Huang J., Li S., Peng H., Yu X., Wen H., Wang X. Soluble microneedle patch with photothermal and NO-release properties for painless and precise treatment of ischemic perforator flaps. J. Mater. Chem. B. 2021;9:7725–7733. doi: 10.1039/d1tb00491c. [DOI] [PubMed] [Google Scholar]
- 77.Wei C., You C., Zhou L., Liu H., Zhou S., Wang X., Guo R. Antimicrobial hydrogel microneedle loading verteporfin promotes skin regeneration by blocking mechanotransduction signaling. Chem. Eng. J. 2023;472 doi: 10.1016/j.cej.2023.144866. [DOI] [Google Scholar]
- 78.Nikolaou P.E., Efentakis P., Abu Qourah F., Femminò S., Makridakis M., Kanaki Z., Varela A., Tsoumani M., Davos C.H., Dimitriou C.A., et al. Chronic Empagliflozin Treatment Reduces Myocardial Infarct Size in Nondiabetic Mice Through STAT-3-Mediated Protection on Microvascular Endothelial Cells and Reduction of Oxidative Stress. Antioxidants Redox Signal. 2021;34:551–571. doi: 10.1089/ars.2019.7923. [DOI] [PubMed] [Google Scholar]
- 79.Caforio A.L.P., Malipiero G., Marcolongo R., Iliceto S. Myocarditis: A Clinical Overview. Curr. Cardiol. Rep. 2017;19:63. doi: 10.1007/s11886-017-0870-x. [DOI] [PubMed] [Google Scholar]
- 80.Karthika C.L., Ahalya S., Radhakrishnan N., Kartha C.C., Sumi S. Hemodynamics mediated epigenetic regulators in the pathogenesis of vascular diseases. Mol. Cell. Biochem. 2021;476:125–143. doi: 10.1007/s11010-020-03890-9. [DOI] [PubMed] [Google Scholar]
- 81.Le T., Clark I., Fortunato J., Sharma M., Xu X., Hsiai T.K., Cao H. Interfacing Bioelectronics and Biomedical Sensing. Interf; 2020. Electrocardiogram: Acquisition and Analysis for Biological Investigations and Health Monitoring; pp. 117–142. [DOI] [Google Scholar]
- 82.Tan X., Liang Y., Rajpura J.R., Yedigarova L., Noone J., Xie L., Inzucchi S., de Havenon A. Once-weekly glucagon-like peptide-1 receptor agonists vs dipeptidyl peptidase-4 inhibitors: cardiovascular effects in people with diabetes and cardiovascular disease. Cardiovasc. Diabetol. 2023;22:319. doi: 10.1186/s12933-023-02051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu R., Sun H., Yu K., Zhong Y., Shi H., Wei Y., Su X., Xu W., Luo Q., Zhang F., et al. Interleukin-37 and Dendritic Cells Treated With Interleukin-37 Plus Troponin I Ameliorate Cardiac Remodeling After Myocardial Infarction. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang J., Wang J., Huang K., Ye Y., Su T., Qiao L., Hensley M.T., Caranasos T.G., Zhang J., Gu Z., Cheng K. Cardiac cell–integrated microneedle patch for treating myocardial infarction. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aat9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi H., Xue T., Yang Y., Jiang C., Huang S., Yang Q., Lei D., You Z., Jin T., Wu F., et al. Microneedle-mediated gene delivery for the treatment of ischemic myocardial disease. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aaz3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim S., Park T.Y., Jeon E.Y., Joo K.I., Cha H.J. Double-layered adhesive microneedle bandage based on biofunctionalized mussel protein for cardiac tissue regeneration. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121171. [DOI] [PubMed] [Google Scholar]
- 87.Lu Y., Ren T., Zhang H., Jin Q., Shen L., Shan M., Zhao X., Chen Q., Dai H., Yao L., et al. A honeybee stinger-inspired self-interlocking microneedle patch and its application in myocardial infarction treatment. Acta Biomater. 2022;153:386–398. doi: 10.1016/j.actbio.2022.09.015. [DOI] [PubMed] [Google Scholar]
- 88.Sun L., Zhu X., Zhang X., Chen G., Bian F., Wang J., Zhou Q., Wang D., Zhao Y. Induced cardiomyocytes-integrated conductive microneedle patch for treating myocardial infarction. Chem. Eng. J. 2021;414 doi: 10.1016/j.cej.2021.128723. [DOI] [Google Scholar]
- 89.Hu S., Zhu D., Li Z., Cheng K. Detachable Microneedle Patches Deliver Mesenchymal Stromal Cell Factor-Loaded Nanoparticles for Cardiac Repair. ACS Nano. 2022;16:15935–15945. doi: 10.1021/acsnano.2c03060. [DOI] [PubMed] [Google Scholar]
- 90.Huang L., Fang H., Zhang T., Hu B., Liu S., Lv F., Zeng Z., Liu H., Zhou W., Wang X. Drug-Loaded Balloon with Built-In NIR Controlled Tip-Separable Microneedles for Long-Effective Arteriosclerosis Treatment. Bioact. Mater. 2023;23:526–538. doi: 10.1016/j.bioactmat.2022.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Melnik T., Jordan O., Corpataux J.-M., Delie F., Saucy F. Pharmacological prevention of intimal hyperplasia: A state-of-the-art review. Pharmacol. Ther. 2022;235 doi: 10.1016/j.pharmthera.2022.108157. [DOI] [PubMed] [Google Scholar]
- 92.Kim J.-H., Jang E.H., Ryu J.-Y., Lee J., Kim J.H., Ryu W., Youn Y.-N. Sirolimus-Embedded Silk Microneedle Wrap to Prevent Neointimal Hyperplasia in vain Graft Model. Int. J. Mol. Sci. 2023;24:3306. doi: 10.3390/ijms24043306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee J., Jang E.H., Kim J.H., Park S., Kang Y., Park S., Lee K., Kim J.H., Youn Y.N., Ryu W. Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery. J. Contr. Release. 2021;340:125–135. doi: 10.1016/j.jconrel.2021.10.024. [DOI] [PubMed] [Google Scholar]
- 94.Zhang X., Cheng Y., Liu R., Zhao Y. Globefish-Inspired Balloon Catheter with Intelligent Microneedle Coating for Endovascular Drug Delivery. Adv. Sci. 2022;9 doi: 10.1002/advs.202204497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Men Z., Lu X., He T., Wu M., Su T., Shen T. Microneedle patch-assisted transdermal administration of recombinant hirudin for the treatment of thrombotic diseases. Int. J. Pharm. 2022;612 doi: 10.1016/j.ijpharm.2021.121332. [DOI] [PubMed] [Google Scholar]
- 96.Wu M., Xia T., Li Y., Wang T., Yang S., Yu J., Liang Q., Shen T., Yu M., Zhao B. Design and fabrication of r-hirudin loaded dissolving microneedle patch for minimally invasive and long-term treatment of thromboembolic disease. Asian J. Pharm. Sci. 2022;17:284–297. doi: 10.1016/j.ajps.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pasquel F.J., Lansang M.C., Dhatariya K., Umpierrez G.E. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021;9:174–188. doi: 10.1016/S2213-8587(20)30381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeng Y., Wang J., Wang Z., Chen G., Yu J., Li S., Li Q., Li H., Wen D., Gu Z., Gu Z. Colloidal crystal microneedle patch for glucose monitoring. Nano Today. 2020;35 doi: 10.1016/j.nantod.2020.100984. [DOI] [Google Scholar]
- 99.Li X., Xu X., Wang K., Chen Y., Zhang Y., Si Q., Pan Z., Jia F., Cui X., Wang X., et al. Fluorescence-Amplified Origami Microneedle (FAOM) Device for Quantitatively Monitoring Blood Glucose. Adv. Mater. 2023;35 doi: 10.1002/adma.202208820. [DOI] [PubMed] [Google Scholar]
- 100.Cheng Y., Gong X., Yang J., Zheng G., Zheng Y., Li Y., Xu Y., Nie G., Xie X., Chen M., et al. A touch-actuated glucose sensor fully integrated with microneedle array and reverse ionto phoresis for diabetes monitoring. Biosens. Bioelectron. 2022;203 doi: 10.1016/j.bios.2022.114026. [DOI] [PubMed] [Google Scholar]
- 101.Schild L., Roch-Ramel F., Diezi J. Renal transport of organic ions and uric acid. Dis. Kidney. 1997:243–264. [Google Scholar]
- 102.Zhang P., Wu X., Xue H., Wang Y., Luo X., Wang L. Wearable transdermal colorimetric microneedle patch for Uric acid monitoring based on peroxidase-like polypyrrole nanoparticles. Anal. Chim. Acta. 2022;1212 doi: 10.1016/j.aca.2022.339911. [DOI] [PubMed] [Google Scholar]
- 103.Gao J., Huang W., Chen Z., Yi C., Jiang L. Simultaneous detection of glucose, uric acid and cholesterol using flexible microneedle electrode array-based biosensor and multi-channel portable electrochemical analyzer. Sens. Actuators, B. 2019;287:102–110. doi: 10.1016/j.aca.2022.339911. [DOI] [Google Scholar]
- 104.He R., Liu H., Fang T., Niu Y., Zhang H., Han F., Gao B., Li F., Xu F. A Colorimetric Dermal Tattoo Biosensor Fabricated by Microneedle Patch for Multiplexed Detection of Health-Related Biomarkers. Adv. Sci. 2021;8 doi: 10.1002/advs.202103030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cao J., Liu Y., Qi Z., Tao X., Kundu S.C., Lu S. Sustained release of insulin from silk microneedles. J. Drug Delivery Sci. 2022;74 doi: 10.1016/j.jddst.2022.103611. [DOI] [Google Scholar]
- 106.Abramson A., Caffarel-Salvador E., Soares V., Minahan D., Tian R.Y., Lu X., Dellal D., Gao Y., Kim S., Wainer J., et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 2019;25:1512–1518. doi: 10.1038/s41591-019-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen B.Z., Zhang L.Q., Xia Y.Y., Zhang X.P., Guo X.D. A basal-bolus insulin regimen integrated microneedle patch for intraday postprandial glucose control. Sci. Adv. 2020;6 doi: 10.1038/s41591-019-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Q., Xiao Z., Wang C., Chen G., Zhang Y., Zhang X., Han X., Wang J., Ye X., Prausnitz M.R., et al. Microneedle Patches Loaded with Nanovesicles for Glucose Transporter-Mediated Insulin Delivery. ACS Nano. 2022;16:18223–18231. doi: 10.1021/acsnano.2c05687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luo F.-Q., Chen G., Xu W., Zhou D., Li J.-X., Huang Y.-C., Lin R., Gu Z., Du J.-Z. Microneedle-array patch with pH-sensitive formulation for glucose-responsive insulin delivery. Nano Res. 2021;14:2689–2696. [Google Scholar]
- 110.Yu J., Wang J., Zhang Y., Chen G., Mao W., Ye Y., Kahkoska A.R., Buse J.B., Langer R., Gu Z. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 2020;4:499–506. doi: 10.1038/s41551-019-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qi Z., Tao X., Tan G., Tian B., Zhang L., Kundu S.C., Lu S. Electro-responsive silk fibroin microneedles for controlled release of insulin. Int. J. Biol. Macromol. 2023;242 doi: 10.1016/j.ijbiomac.2023.124684. [DOI] [PubMed] [Google Scholar]
- 112.Stamp L.K., Farquhar H. Treatment advances in gout. Best Pract. Res. Clin. Rheumatol. 2021;35 doi: 10.1016/j.berh.2021.101719. [DOI] [PubMed] [Google Scholar]
- 113.Liu Y., Zhu X., Ji S., Huang Z., Zang Y., Ding Y., Zhang J., Ding Z. Transdermal delivery of colchicine using dissolvable microneedle arrays for the treatment of acute gout in a rat model. Drug Deliv. 2022;29:2984–2994. doi: 10.1080/10717544.2022.2122632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang Y., Li Z., Huang P., Lin J., Li J., Shi K., Lin J., Hu J., Zhao Z., Yu Y., et al. Rapidly separating dissolving microneedles with sustained-release colchicine and stabilized uricase for simplified long-term gout management. Acta Pharm. Sin. B. 2023;13:3454–3470. doi: 10.1016/j.apsb.2023.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Katsumi H., Tanaka Y., Hitomi K., Liu S., Quan Y.S., Kamiyama F., Sakane T., Yamamoto A. Efficient Transdermal Delivery of Alendronate, a Nitrogen-Containing Bisphosphonate, Using Tip-Loaded Self-Dissolving Microneedle Arrays for the Treatment of Osteoporosis. Pharmaceutics. 2017;9:29. doi: 10.3390/pharmaceutics9030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y., Ju X.-J., Fu H., Zhou C.-H., Gao Y., Wang J., Xie R., Wang W., Liu Z., Chu L.-Y. Composite Separable Microneedles for Transdermal Delivery and Controlled Release of Salmon Calcitonin for Osteoporosis Therapy. ACS Appl. Mater. Interfaces. 2023;15:638–650. doi: 10.1021/acsami.2c19241. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y., Liu Q., Yu J., Yu S., Wang J., Qiang L., Gu Z. Locally Induced Adipose Tissue Browning by Microneedle Patch for Obesity Treatment. ACS Nano. 2017;11:9223–9230. doi: 10.1021/acsnano.7b04348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peng H., Zhang C., Wang M., Zhang W., Xu B., Yan X., Xin H., Wang X. Black phosphorus modified soluble microneedle patch for painless, effective and accurate body slimming. Appl. Mater. Today. 2020;19 doi: 10.1016/j.apmt.2020.100577. [DOI] [Google Scholar]
- 119.Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43–49. doi: 10.1080/13697137.2021.1951697. [DOI] [PubMed] [Google Scholar]
- 120.Dietz W.H. The Obesity (Under) Treatment Conundrum. Obesity. 2019;27:1928–1929. doi: 10.1002/oby.22641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.