Abstract
Introduction
Pancreatic adenocarcinoma (PDAC) is becoming a public health issue with a 5-years survival rate around 10%. Patients with PDAC are often sarcopenic, which impacts postoperative outcome. At the same time, overweight population is increasing and adipose tissue promotes tumor related-inflammation. With several studies supporting independently these data, we aimed to assess if they held an impact on survival when combined.
Methods
We included 232 patients from two university hospitals (CHU de Lille, Institut Paoli Calmette), from January 2011 to December 2018, who underwent Pancreaticoduodenectomy (PD) for resectable PDAC. Preoperative CT scan was used to measure sarcopenia and visceral fat according to international cut-offs. Neutrophil to lymphocyte (NLR) and platelet to lymphocyte ratios (PLR) were used to measure inflammation. For univariate and multivariate analyses, the Cox proportional-hazard model was used. P-values below 0.05 were considered significant.
Results
Sarcopenic patients with visceral obesity were less likely to survive than the others in multivariate analysis (OS, HR 1.65, p= 0.043). Cutaneous obesity did not influence survival. We also observed an influence on survival when we studied sarcopenia with visceral obesity (OS, p= 0.056; PFS, p = 0.014), sarcopenia with cutaneous obesity (PFS, p= 0.005) and sarcopenia with PLR (PFS, p= 0.043). This poor prognosis was also found in sarcopenic obese patients with high PLR (OS, p= 0.05; PFS, p= 0.01).
Conclusion
Sarcopenic obesity was associated with poor prognosis after PD for PDAC, especially in patients with systemic inflammation. Pre operative management of these factors should be addressed in pancreatic cancer patients.
Keywords: Pancreatic adenocarcinoma, Sarcopenia, Systemic inflammatory response, Obesity
Introduction
Pancreatic adenocarcinoma (PDAC) is the third leading cause of cancer death, with a five-year survival rate of 12 % [1]. Despite advances in chemotherapy and radiotherapy, it is projected to become the second leading cause of cancer- related mortality by 2030 [2]. The underlying cause of PDAC remains unknown, but several risk factors such as genetic predispositions, smoking, alcohol use, chronic pancreatitis, diet, and obesity have been identified [3, 4]. To this day, surgical resection accompanied with perioperative chemotherapy remains the only curative option to treat PDAC [5]. However, this approach is linked to high morbidity and mortality rates [6, 7].
When it comes to surgical treatment of various cancer types, anthropometric factors have been shown to influence patient postoperative survival [8–10]. Notably, some studies have shown that obesity and sarcopenia in PDAC are associated with lower survival rates [11, 12]. According to the European working group on sarcopenia in older people (EWGSOP), sarcopenia is defined by low muscle mass and low muscle strength and is assessed using different cut-offs [13]. Obesity, defined by a body mass index (BMI) superior to 30 kg/m2 is associated with poor health outcomes such as cancer [14]. Sarcopenic obesity is prevalent in today’s population and appears to be increasing with age [12, 15] and measurable with CT-Scan [16].
There has been a recent focus on sarcopenia and inflammation with regards to several malignancies. Previous reports have shown that these factors are interrelated and that they are predictors of poor patient outcomes [17, 18]. Recently, platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR) markers have been effectively used to identify systemic inflammatory response (SIR) and to predict post-operative survival in PDAC patients [19].
The aim of this study is to demonstrate the impact of sarcopenia, visceral obesity, sarcopenic obesity, and inflammation status on postoperative oncological outcomes after pancreatoduodenectomy (PD) for PDAC.
Patients and methods
Study population
Participants were identified from the department of digestive and transplant surgery at Lille University Hospital and from the department of surgical oncology at Institut Paoli-Calmette in Marseille. We selected patients who underwent PD for PDAC from the 1st of January 2011 to the 31st of December 2018. Only patients with PDAC comprising of surgically resectable tumors were included in our study. Underage patients, patients treated with neoadjuvant chemotherapy and/or radiotherapy, patients who underwent palliative procedures, and those with low quality CT scans were excluded from our analysis. We believe that being so selective about our patients would be more accurate to study the correlation between sarcopenia, visceral obesity and prognosis. The study complied with French National Health guidelines on research involving human subjects. IRB approval was not needed for this study.
Data collection
Clinical data including demographics, ASA score, Charlson comorbidity score, preoperative body weight, height, BMI, laboratory data, tumor characteristics were retrieved from patients’ medical records. Recorded data comprised of: intraoperative (operative time, blood loss, type of pancreatic anastomosis, vascular reconstruction), histological (histologic classification, degree of differentiation, and resection margin status), postoperative (length of stay, post-operative complications as defined by the Clavien-Dindo classification, and pancreatic surgery complications as defined by guidelines of International Study Group of pancreatic surgery) [20], and follow-up data (adjuvant therapy rate, recurrence rate, overall survival, and disease-free survival).
Muscle mass, subcutaneous and visceral adipose tissue measurements
Preoperative CT scans were performed at least one month prior to the surgery and analyzed using the MYRIAN software. Total abdominal muscle area (TAMA) comprising of transversus abdominis, external and internal obliques, rectus abdominis, psoas muscles and para spinal muscles, was measured on one image slice at vertebral level L3. Subcutaneous fat area (SFA) and visceral fat area (VFA) were also measured using this method. We calculated skeletal muscle index (SMI) as follow: TAMA measured at L3 (cm2) divided by height (m2). Since there is no gold standard cut off for sarcopenia, we used Prado’s cut-offs, based on BMI and SMI. Indeed, their study held a large variability of body composition in cancer patients with statistics to support their number choices [13] : obese (BMI>30 kg/m2) women with SMI<38.5 cm2/m2, non-obese (BMI<30 kg/m2) women with SMI<32 cm2/m2, obese men with SMI<52.4 cm2/m2, and non-obese men with SMI<42 cm2/m2. Visceral obesity was defined as visceral fat area VFA>130 cm2 measured at L3 level. Subcutaneous obesity was classified as high for patients with subcutaneous fat area SFA>177 cm2. In line with other studies, median measures were used as gold-standard cut-offs have yet to be defined [21, 22].
Inflammatory markers
Biological data were collected retrospectively from six-month old laboratory data. As reported in previous studies, NLR>3 was defined as high, and the PLR cut-off was 150 [23–26].
Statistical analysis
Computation was performed using the R software, with significant P-values being lower than 0.05. Continuous variables were compared using student t-test and categorical variables were analyzed using Fisher’s test. The Kaplan-Meier method was used to calculate survival curves that were then compared with the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional-hazard model.
Results
A total of 364 patients underwent PD during the study period. Among them, 88 patients were excluded due to unavailable preoperative CT scans. Of the 276 remaining patients, 20 were excluded as they were previously treated with neoadjuvant therapy and 24 were excluded as their treatment comprised of a procedure different from PD. Of the 232 patients included in our study, 49% were sarcopenic and 10% suffered from sarcopenic obesity. Sarcopenic patients were more likely to have low BMI, SMI, and cutaneous adipose tissue (Table 1).
Table 1.
Characteristics of the study population
| Caracteristics | All patients n = 196 | Sarcopenia | |||||
|---|---|---|---|---|---|---|---|
| N | No ; n = 98 | Yes ; n = 94 | |||||
| Age$ | 196 | 67,47 | 9,82 | 67,73 | 8,69 | 67,63 (+/10,36) | 10,36 |
| Sex (n male %)** | 195 | 108 | 55,4 | 37 | 37,8 | 68 | 72,3 |
| ASA Score$ | 196 | 2,11 | 0,64 | 2,08 | 0,65 | 2,15 | 0,62 |
| Score 0-2 (n(%)) | 149 | 76 | 76 | 77,6 | 69 | 73,4 | |
| Score 3-4 (n(%)) | 47 | 24 | 22 | 22,4 | 25 | 26,6 | |
| Charlson$ | 115 | 2,96 | 1,55 | 3,24 | 1,49 | 2,73 | 1,59 |
| Score 0-2 (n(%)) | 45 | 39,13 | 18 | 30,5 | 24 | 46,15 | |
| Score 3-4 (n(%)) | 52 | 45,22 | 28 | 47,5 | 23 | 44,23 | |
| Score >4 (n(%)) | 18 | 15,65 | 13 | 22 | 5 | 9,62 | |
| Medical history (n(%)) | |||||||
| High blood pressure | 133 | 63 | 47,4 | 37 | 54,4 | 23 | 37,7 |
| Ischemic heart disease | 114 | 12 | 10,5 | 8 | 13,8 | 4 | 7,7 |
| Heart failure | 114 | 1 | 0,9 | 0 | 0 | 1 | 1,9 |
| Kidney failure | 114 | 1 | 0,9 | 1 | 1,7 | 0 | 0 |
| COPD* | 115 | 4 | 3,5 | 4 | 6,8 | 0 | 0 |
| Respiratory failure | 114 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chronic pancreatitis | 113 | 6 | 5,3 | 3 | 5,3 | 3 | 5,8 |
| Diabetes | 133 | 31 | 23,3 | 18 | 26,5 | 12 | 19,7 |
| Cirrhosis | 103 | 1 | 1 | 1 | 2 | 0 | 0 |
| Discovery circumstances (n(%)) | |||||||
| Abdominal pain | 112 | 57 | 50,9 | 31 | 54,4 | 25 | 49 |
| Jaundice | 114 | 80 | 70,2 | 41 | 70,7 | 36 | 69,2 |
| Acute cholitis | 110 | 8 | 7,3 | 3 | 5,5 | 4 | 7,8 |
| Worsening diabetes | 111 | 10 | 9 | 7 | 12,5 | 3 | 5,9 |
| Pancreatitis | 113 | 19 | 17,1 | 8 | 14,3 | 11 | 21,6 |
| Malnutrition (n(%)) | 115 | ||||||
| moderate | 51 | 44,35 | 23 | 39 | 26 | 50 | |
| severe | 29 | 25,22 | 15 | 25,4 | 13 | 25 | |
| BMI$** | 192 | 24,54 | 4,13 | 26,10 | 4,34 | 22,82 | 3,19 |
| SMI$**__ | 193 | 46,46 | 13,08 | 52,77 | 12,48 | 39,87 | 10,23 |
| Sarcopenia (n (%)) | 192 | 94 | 49 | 0 | 0 | 1 | 100 |
| Cutaneous fat (cm2/m2)$** | 196 | 197 | 99 | 240,46 | 102,96 | 148,73 | 68,36 |
| Visceral fat (cm2/m2)$* | 193 | 146 | 94 | 159,67 | 95,22 | 129,45 | 88,03 |
| Ca 19-9$ | 81 | 495,79 | 851 | 430,37 | 874,58 | 562,04 | 853,97 |
| Albumin$ | 115 | 37,03 | 6,24 | 37,7 | 5,86 | 36,81 | 6 |
| Pre-albumin$ | 114 | 0,19 | 0,07 | 0,19 | 0,07 | 0,20 | 0,08 |
| CRP$ | 85 | 17,90 | 29,90 | 16,77 | 25,6 | 19,10 | 34,26 |
| NLR$ | 161 | 3,50 | 2,35 | 3,17 | 2,26 | 3,78 | 2,40 |
| PLR$ | 160 | 181,09 | 98,22 | 171,4 | 84,85 | 191,3 | 109,08 |
| Post operative complications : | |||||||
| Clavien Dindo > 3a (n(%)) | 130 | 28 | 21,5 | 17 | 24,6 | 11 | 18,6 |
| Anatomopathology | |||||||
| pT (n(%)) | 192 | ||||||
| pT1-pT2 | 51 | 26,6 | 30 | 31,2 | 21 | 22,6 | |
| pT3-pT4 | 141 | 73,4 | 66 | 68,8 | 72 | 77,4 | |
| Vascular embolus (n(%)) | 154 | 65 | 42,2 | 28 | 36,4 | 36 | 48,6 |
| Lymphatic embolus (n(%)) | 108 | 20 | 18,5 | 9 | 16,7 | 9 | 17,6 |
| Resection (n(%)) | 194 | ||||||
| R0 | 169 | 87,1 | 86 | 87,8 | 80 | 86 | |
| R1 | 24 | 12,4 | 11 | 11,2 | 13 | 14 | |
| R2 | 1 | 0,5 | 1 | 1 | 0 | 0 | |
| N+$ | 189 | 2,47 | 2,64 | 2,37 | 2,75 | 2,54 | 2,5 |
| N+/N$ | 189 | 0,19 | 0,43 | 0,20 | 0,58 | 0,17 | 0,18 |
| Adjuvant chemotherapy (n(%)) | 112 | 83,9 | 46 | 80,7 | 44 | 86,3 | |
| Mortality at 90 days (n(%)) | 196 | 8 | 4,1 | 5 | 5,1 | 3 | 3,2 |
| Dead (n(%)) | 196 | 135 | 69 | 64 | 65,3 | 69 | 73,4 |
| Days before death$ | 196 | 636 | 478 | 656 | 534 | 626 | 427 |
| Survival in days$ | 196 | 1074 | 876 | 1164 | 959 | 969 | 764 |
ASA American society of anesthesiologists, COPD Chronic obstructive pulmonary disease, BMI Body mass index, SMI Squeletal muscle index, NLR Neutrophil to lymphocyte ratio, PLR Platelet to lymphocyte ratio
$= mean (+/- SD)
*= p-value < 0,005
**= p-value < 0,001
We studied the correlation between sarcopenia and inflammatory markers. No correlation was observed between sarcopenia and NLR or PLR. However, sarcopenic patients tended to present with higher NLR and PLR compared to non-sarcopenic patients.
Moreover, we assessed the impact of sarcopenia, NLR and PLR on oncological outcomes after PD for PDAC. Sarcopenic patients were shown to have a lower progression-free survival (PFS) compared to non-sarcopenic patients (p=0.09). However, overall survival (OS) was similar for sarcopenic patients and non-sarcopenic patients (p=0.2). Regarding inflammatory status, no significant differences were observed in PFS and OS between patients with high NLR and those with low NLR. Similar results were observed regarding PLR status. With regards to obesity status, OS and OFS were not impacted by VFA and SFA after PD for PDAC.
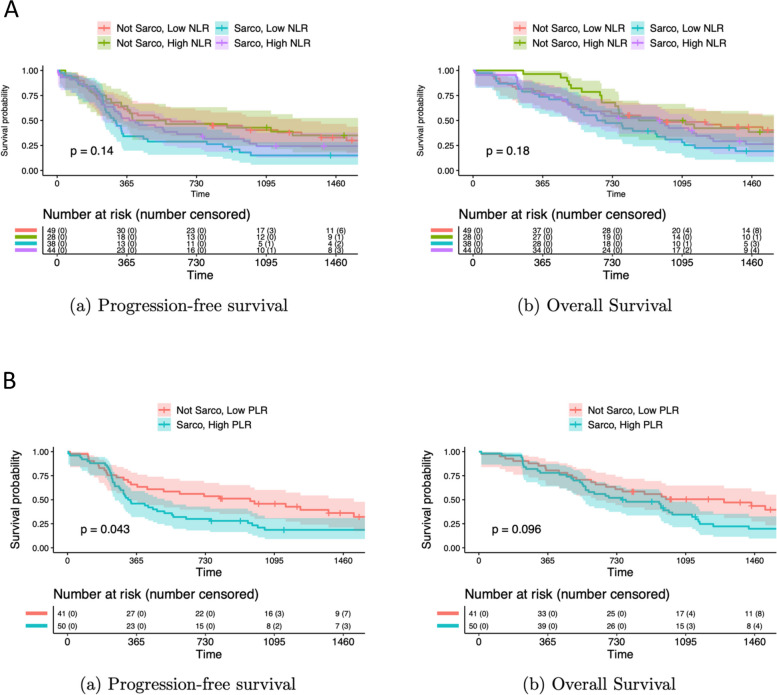
We then investigated patients’ survival outcomes based on their sarcopenia and inflammatory status. We found that sarcopenic patients with high NLR tended to have worse prognosis than non-sarcopenic patients with high NLR, but that the difference was not significant (Fig. 1A). However, PFS was significantly lower for sarcopenic patients with high PLR compared to non-sarcopenic patients with low PLR (p=0.043). Moreover, OS appeared to be lower for sarcopenic patients with high PLR compared to non-sarcopenic patients with low PLR (p=0.096) (Fig. 1B).
Fig. 1.
Survival outcome based on sarcopenia and inflammatory status, A Survival in sarcopenic and non-sarcopenic patients with high or low NLR, B Survival in sarcopenic and non- sarcopenic patients with high or low PLR
Furthermore, we quantified the impact of sarcopenia and obesity on survival. Patients who were classified as sarcopenic and viscerally obese demonstrated lower survival rates compared to non-sarcopenic and non-obese patients (OS, p= 0.014; PFS, p=0.05) (Fig. 2A). Furthermore, sarcopenic patients with cutaneous obesity had a lower PFS (p = 0.0059) compared to non-sarcopenic and non-cutaneous obese patients (Fig. 2B).
Fig. 2.
Survival outcome based on sarcopenia and obesity, A Survival in sarcopenic and non-sarcopenic patients with visceral obesity, B Survival in sarcopenia and non-sarcopenic patients with cutaneous obesity
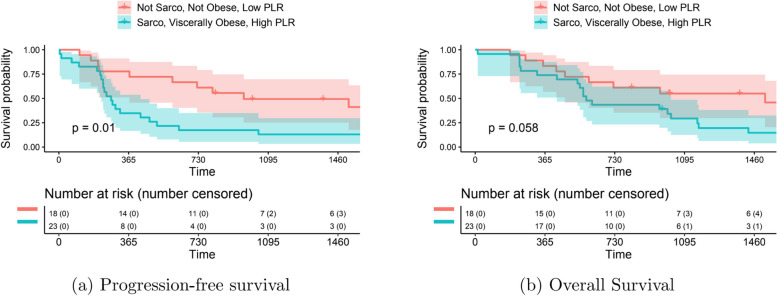
Among our study participants, 20 were considered sarcopenic, viscerally obese, and had a high NLR. Although the difference is not significant, these patients had lower rates of OS and PFS compared to non-sarcopenic, non-obese patients with low NLR. Moreover, sarcopenic obesity in patients with high PLR had a lower PFS (p=0.01) and OS (p=0.05) compared to non-sarcopenic, non-obese patients with low PLR (Fig. 3).
Fig. 3.
Survival outcome based on sarcopenia, visceral obesity and PLR status, A Progression free survival, B Overall survival
Finally, a multivariate analysis with Cox proportional-hazard ratio was performed to identify the risk factors of worse prognosis after PD for PDAC. Based on the multivariate analysis, the sarcopenic visceral obesity variable was independent from poor prognostic factors. The result was significant for OS (HR, 2.17; 95% CI,1.2–4.6, p=0.04) (Fig. 4A) and PFS (HR, 2.34; 95% CI,1.16–4.7, p=0.018). Lastly, lower PFS was associated with high PLR and cutaneous obesity (p= 0.08 and p= 0,064 respectively) (Fig. 4B).
Fig. 4.
Multivariate analysis (COX Proportional Hazard ratio model, p= 0,05), A Overall survival, B Progression free Survival
Discussion
This study is the first to examine the relationship between SIR, sarcopenia, and visceral obesity in PDAC. Our results demonstrate an interdependence between inflammation and sarcopenia and highlight their impact on positive patient outcomes. Interestingly, we found that sarcopenic patients with visceral obesity were more likely to relapse following PDAC and presented reduced post-operative survival. Our data suggests that preoperative evaluation of sarcopenia, inflammation and visceral obesity status could help to determine postoperative patient outcomes and thus assess if a patient is an appropriate candidate for surgery.
Based on our results, sarcopenia alone does not impact oncological outcomes after PD for PDAC (OS, p= 0.2; PFS, P= 0.09). Several papers have identified sarcopenia as a risk factor for poor prognosis in many cancers including PDAC [10]. Previous research suggest that tumor aggressiveness is correlated with sarcopenic status [26]. Leads on decreasing sarcopenia have been established, but the data is lacking for patients undergoing PD. Physical training against resistance and improved nutrition (nutritional supplements by vitamin D, ß-methylbutyrate and creatine) up to three months before surgery have been proposed to enhance post-operative survival [27, 28]. Several randomized controlled studies to identify an adequate preoperative program for sarcopenic patients before highly complex procedures are in progress.
In cancer patients, sarcopenia is frequently associated with inflammation. Indeed, tumors create an inflammatory environment by producing diverse cytokines, altering local balance, and attracting neutrophils [29]. Moreover, chronic inflammation generates neoplastic processes by inducing DNA damage [30]. It has been suggested that PLR is a useful prognostic factor in some cancer types since platelets enhance tumor development by promoting angiogenesis through the cytokine vascular endothelial growth factor (VEGF). Due to the involvement of lymphocytes and neutrophils in PDAC and the tumoral microenvironment, NLR has also been associated with PDAC survival [31, 32]. NLR has been used as a prognostic factor in several cancers [33–35] including PDAC [35]. When NLR is highly elevated, surgery should be delayed, and neoadjuvant treatment should be prioritized. In fact, neoadjuvant chemotherapy has been shown to lower NLR and improve postoperative prognosis in patients with breast cancer [34]. Moreover, the normalization of PLR and NLR after the first cure of FOLFOX in patients with gastric cancer was associated with better OS [36]. As previously reported, these findings indicate that sarcopenia and inflammation reflect aggressive tumor process [16, 37, 38].
Our investigation also focused on the impact of VFA and SFA on PDAC prognosis. Our results suggest that visceral and subcutaneous obesity do not impact patient survival. Nonetheless, sarcopenic and viscerally obese patients had poorer OS and PFS, regardless of their BMI. Similarly, Okumura et al. reported that sarcopenic visceral obesity is closely associated with mortality and recurrence [16, 39, 40]. These findings demonstrate that the redistribution of muscle mass and adipose tissue is a key factor to consider when predicting patient prognosis. An emerging topic about fat distribution is myosteatosis and its association with sarcopenia. More studies are reporting the influence of muscle fat on muscle strength and physical activity leading to a worse post operative prognosis in cancer patients regardless of sarcopenia [41, 42].
Our results highlight the prognostic role of sarcopenic visceral obesity associated with inflammation in patients with PDAC. Visceral fat increases proinflammatory cytokine levels, leading to chronic inflammation and consequently increased carcinogenesis [43, 44]. Moreover, adipokines and myokines affect the immune system and promote inflammation [43]. Thus, visceral adiposity, loss of muscle mass, and inflammation are interrelated factors that predict poor patient outcomes [45].
Our study presented some limitations. First, the retrospective study design can be subject to bias, suggesting the need for a prospective study in the future. Second, cut-offs to define sarcopenia, visceral obesity, and inflammation in patients with PDAC were not previously validated due to the lack of a current consensual definition [46]. Third, our analysis only focused on patients undergoing PD, preventing generalization of our results to other treatments or surgical interventions. Finally, sarcopenia was assessed based on preoperative CT scan by measuring the TAMA. In the future, more effective methods could be used to determine sarcopenic state such as dual X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA) [47].
Sarcopenic obesity and SIR are associated with poorer patient outcomes and lower survival rates. Our results suggest that the prognosis of pancreatic cancer patients can be improved with various treatment adjustments. Sarcopenia, visceral obesity, and inflammatory status being predictive of poor prognosis, patients who are assessed for these factors should be considered borderline resectable PDAC and should be treated with neoadjuvant chemotherapy [45]. When sarcopenia is screened for in daily practice, the importance of nutritional care and exercising should be explained to patients. Nutrition can be improved with adequate protein intake of 1.2 g/kg/day. Progressive resistance training (PRT) [48] and supplementation can improve muscle mass, muscle strength and physical performance [47, 49, 50]. Our analysis provides a framework for assessing patient preoperative parameters to determine the most appropriate treatment approach for resectable PDAC. Several protocols can be implemented to improve patient survival and should include exercise,
nutrition modification or neoadjuvant chemotherapy. Further studies should be performed to assess the efficiency of these measures in determining PDAC treatment outcomes.
Authors’ contributions
KB wrote the entire Manuscript. MEA contributed by helping with the structure of the research, the data, the in-site reviewing. YR contributed by reading and correcting the final form of the manuscript. CAB contributed by helping doing the statistics after the data were collected. ST, GP, OT and JG contributed by letting me access the patients' data.
Funding
No fundings were received by authors for this word.
Availability of data and materials
Some data are available.
Data availability
Data that support the findings of this study were collected from the onsite hospital software (in Lille and Marseille).
Declarations
Ethics approval and consent to participate
The study complied with French National Health guidelines on research involving human subjects. IRB approval was not needed for this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/12/2024
For author "Mehdi El Amrani", "El Amrani" should be the Family name and not "Amrani"
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. 10.3322/caac.21763. PMID: 36633525. 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 2.Gordon-Dseagu VL, Devesa SS, Goggins M, Stolzenberg-Solomon R. Pancreatic cancer incidence trends: evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int J Epidemiol. 2018;47(2):427–39. 10.1093/ije/dyx232. PMID: 29149259; PMCID: PMC5913617. 10.1093/ije/dyx232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–705. 10.3748/wjg.v22.i44.9694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker AE. Pancreatic ductal adenocarcinoma: Risk factors, screening, and early detection. WJG. 2014;20(32):11182. 10.3748/wjg.v20.i32.11182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Principe DR, Underwood PW, Korc M, Trevino JG, Munshi HG, Rana A. The current treatment paradigm for pancreatic ductal adenocarcinoma and barriers to therapeutic efficacy. Front Oncol. 2021;11:688377. 10.3389/fonc.2021.688377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Amrani M, Clement G, Lenne X, Farges O, Delpero JR, Theis D, et al. Failure-to-rescue in patients undergoing pancreatectomy: is hospital volume a standard for quality improvement programs? Nationwide analysis of 12,333 patients. Ann Surg. 2018;268(5):799–807. 10.1097/SLA.0000000000002945 [DOI] [PubMed] [Google Scholar]
- 7.El Amrani M, Lenne X, Clément G, Turrini O, Theis D, Pruvot FR, et al. Referring patients to expert centers after pancreatectomy is too late to improve outcome. Inter-hospital transfer analysis in nationwide study of 19,938 patients. Ann Surg. 2020;272(5):723–30. 10.1097/SLA.0000000000004342 [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Zhang W, Huang Y, Chen W, Wu R, Chen X, et al. Sarcopenia is associated with the neutrophil/lymphocyte and platelet/lymphocyte ratios in operable gastric cancer patients: a prospective study. CMAR. 2018;10:4935–44. 10.2147/CMAR.S175421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mungan İ, Dicle ÇB, Bektaş Ş, Sarı S, Yamanyar S, Çavuş M, et al. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer? Military Med Res. 2020;7(1):9. 10.1186/s40779-020-00234-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Amrani M, Vermersch M, Fulbert M, Prodeau M, Lecolle K, Hebbar M, et al. Impact of sarcopenia on outcomes of patients undergoing pancreatectomy: a retrospective analysis of 107 patients. Medicine (Baltimore). 2018;97(39):e12076. 10.1097/MD.0000000000012076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsunaga T, Miyata H, Sugimura K, Motoori M, Asukai K, Yanagimoto Y, et al. Prognostic significance of sarcopenia and systemic inflammatory response in patients with esophageal cancer. Anticancer Res. 2019;39(1):449–58. 10.21873/anticanres.13133 [DOI] [PubMed] [Google Scholar]
- 12.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity - definition, etiology and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35. 10.1016/S1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 14.Salaün H, Thariat J, Vignot M, Merrouche Y, Vignot S. Obésité et cancer. Bull Cancer. 2017;104(1):30–41. 10.1016/j.bulcan.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 15.Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr. 2000;54(3):S92–103. 10.1038/sj.ejcn.1601030 [DOI] [PubMed] [Google Scholar]
- 16.Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Yao S, et al. Visceral adiposity and sarcopenic visceral obesity are associated with poor prognosis after resection of pancreatic cancer. Ann Surg Oncol. 2017;24(12):3732–40. 10.1245/s10434-017-6077-y [DOI] [PubMed] [Google Scholar]
- 17.Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017;3(12):e172319. 10.1001/jamaoncol.2017.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–66. 10.1016/j.cmet.2012.06.011. Epub 2012 Jul 12. 10.1016/j.cmet.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 19.Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21(9):2807–15. 10.3748/wjg.v21.i9.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–91. 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 21.Heus C, Cakir H, Lak A, Doodeman HJ, Houdijk AP. Visceral obesity, muscle mass and outcome in rectal cancer surgery after neo-adjuvant chemo-radiation. Int J Surg. 2016;29:159–64. 10.1016/j.ijsu.2016.03.066. Epub 2016 Apr 6. 10.1016/j.ijsu.2016.03.066 [DOI] [PubMed] [Google Scholar]
- 22.Antoun S, Bayar A, Ileana E, Laplanche A, Fizazi K, di Palma M, et al. High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur J Cancer. 2015;51(17):2570–7. 10.1016/j.ejca.2015.07.042 [DOI] [PubMed] [Google Scholar]
- 23.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 24.Yang JJ. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. WJG. 2015;21(9):2807. 10.3748/wjg.v21.i9.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Wei Q, Fan J, Cheng S, Ding W, Hua Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis containing 8252 patients. Clin Chim Acta. 2018;479:181–9. 10.1016/j.cca.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 26.Li B, Zhou P, Liu Y, Wei H, Yang X, Chen T, et al. Platelet-to-lymphocyte ratio in advanced Cancer: review and meta-analysis. Clin Chim Acta. 2018;483:48–56. 10.1016/j.cca.2018.04.023 [DOI] [PubMed] [Google Scholar]
- 27.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NEP, Dhatariya K, et al. Perioperative nutrition: recommendations from the ESPEN expert group. Clin Nutr. 2020;39(11):3211–27. 10.1016/j.clnu.2020.03.038 [DOI] [PubMed] [Google Scholar]
- 29.Baazim H, Antonio-Herrera L, Bergthaler A. The interplay of immunology and cachexia in infection and cancer. Nat Rev Immunol. 2022;22(5):309–21. 10.1038/s41577-021-00624-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kay J, Thadhani E, Samson L, Engelward B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair. 2019;83:102673. 10.1016/j.dnarep.2019.102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pointer DT, Roife D, Powers BD, Murimwa G, Elessawy S, Thompson ZJ, et al. Neutrophil to lymphocyte ratio, not platelet to lymphocyte or lymphocyte to monocyte ratio, is predictive of patient survival after resection of early-stage pancreatic ductal adenocarcinoma. BMC Cancer. 2020;20(1):750. 10.1186/s12885-020-07182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236(4):297–304. 10.1620/tjem.236.297. PMID: 26250537. 10.1620/tjem.236.297 [DOI] [PubMed] [Google Scholar]
- 33.Lorite MJ, Cariuk P, Tisdale MJ. Induction of muscle protein degradation by a tumour factor. Br J Cancer. 1997;76(8):1035–40. 10.1038/bjc.1997.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi H, Noh H, Cho IJ, Lim ST, Han A. Changes in neutrophil to lymphocyte ratio (NLR) during neoadjuvant treatment correlated with patients’ survival. Breast Cancer. 2020;27(5):871–9. 10.1007/s12282-020-01083-2 [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Wei Q, Fan J, Cheng S, Ding W, Hua Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis containing 8252 patients. Clin Chim Acta. 2018;479:181–9. 10.1016/j.cca.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. 10.1186/1471-2407-13-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawada T. Neutrophil-to-lymphocyte ratio as an indicator of prognosis in patients with pancreatic cancer. HPB. 2019;21(12):1791. 10.1016/j.hpb.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 38.Shusterman M, Jou E, Kaubisch A, Chuy JW, Rajdev L, Aparo S, et al. The neutrophil-to-lymphocyte ratio is a prognostic biomarker in an ethnically diverse patient population with advanced pancreatic cancer. J Gastrointest Canc. 2020;51(3):868–76. 10.1007/s12029-019-00316-8 [DOI] [PubMed] [Google Scholar]
- 39.Moon HG, Ju YT, Jeong CY, Jung EJ, Lee YJ, Hong SC, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol juill. 2008;15(7):1918–22. 10.1245/s10434-008-9891-4 [DOI] [PubMed] [Google Scholar]
- 40.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63(1):131–40. 10.1016/j.jhep.2015.02.031 [DOI] [PubMed] [Google Scholar]
- 41.Kim DW, Ahn H, Kim KW, Lee SS, Kim HJ, Ko Y, Park T, Lee J. Prognostic value of sarcopenia and myosteatosis in patients with resectable pancreatic ductal adenocarcinoma. Korean J Radiol. 2022;23(11):1055–66. 10.3348/kjr.2022.0277. Epub 2022 Aug 31. PMID: 36098341; PMCID: PMC9614291. 10.3348/kjr.2022.0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016;35(5):1103–9. 10.1016/j.clnu.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 43.Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 2012;4(8):535–46. 10.18632/aging.100482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White PB, True EM, Ziegler KM, Wang SS, Swartz-Basile DA, Pitt HA, et al. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J Gastrointest Surg. 2010;14(12):1888–94. 10.1007/s11605-010-1349-x [DOI] [PubMed] [Google Scholar]
- 45.Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW, Kishiwada M, Kitagawa H, Michalski CW, Wolfgang CL. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11. 10.1016/j.pan.2017.11.011. Epub 2017 Nov 22 PMID: 29191513. 10.1016/j.pan.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 46.van der Werf A, Langius JAE, de van der Schueren MAE, Nurmohamed SA, van der Pant KAMI, Blauwhoff-Buskermolen S, et al. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr. 2018. [DOI] [PMC free article] [PubMed]
- 47.Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16:170. 10.1186/s12877-016-0349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu C, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;2009(3):CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denison HJ, Cooper C, Sayer AA, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging. 2015;10:859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holeček M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. 2017;8(4):529–41. 10.1002/jcsm.12208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some data are available.
Data that support the findings of this study were collected from the onsite hospital software (in Lille and Marseille).