Abstract
Background
The antiviral efficacy of Evusheld (AZD7442) in patients hospitalized for SARS-CoV-2 is unknown.
Methods
We analysed the evolution of both the nasopharyngeal viral load and the serum neutralization activity against the variant of infection in 199 hospitalized patients (109 treated with Evusheld, 90 treated with placebo) infected with the SARS-CoV-2 virus and included in the randomized, double-blind, trial DisCoVeRy (NCT04315948). Using a mechanistic mathematical model, we reconstructed the trajectories of viral kinetics and how they are modulated by the increase in serum neutralization activity during Evusheld treatment.
Results
Our model identified that the neutralization activity was associated with viral kinetics. Reflecting the variant-dependent neutralization activity of Evusheld, the antiviral activity of Evusheld was larger in patients infected with pre-Omicron or Omicron BA.2 variants than in patients infected with Omicron BA.1 variant. More specifically, the model predicted that Evusheld reduced the median time to viral clearance compared with placebo-treated patients by more than 5 days in patients infected by pre-Omicron (median: 5.9; 80% PI: 2.1–13.6) or Omicron BA.2 (median: 5.4; 80% PI: 2.0–12.4), respectively. The effect was more modest in patients infected by the Omicron BA.1 variant, reducing the median time to viral clearance by 2 days (median: 2.2; 80% PI: 0.4–8.9).
Conclusions
Hospitalized patients treated with Evusheld had a shorter median time to SARS-CoV-2 viral clearance. As Evusheld antiviral activity is mediated by the level of neutralization activity, its impact on viral clearance varies largely according to the variant of infection.
Background
The development of effective vaccines has led to a large reduction in the risk of hospitalization after infection with SARS-CoV-2 virus.1 However, the risk of severe disease still exists, in particular in unvaccinated or fragile populations, such as aged or immunocompromised individuals.1 In those individuals, the use of antiviral treatments, such as small molecules (Paxlovid) or monoclonal antibodies (mAbs), can reduce the risk of severe disease by 50%–90% when administered to outpatients within the first week after symptom onset.2,3 However, the benefit of antiviral treatments at later stages of the disease, i.e. when patients are hospitalized, is lower and their use remains controversial.4,5
Evusheld (AZD7442) is a cocktail of two human mAbs, tixagevimab and cilgavimab, that reduces the risk of hospitalization or death by 50% if administered within seven days of symptom onset.6 Because of its modified Fc effector function, Evusheld has an extended half-life of 90 days,7 which makes it also an attractive candidate for pre-exposure prophylaxis in fragile populations.8,9 However, the emergence of Omicron variants in 2022 has been associated with a marked decrease in the efficacy of Evusheld as measured by serum neutralization activity,10,11 leading the FDA and other regulatory agencies to progressively suspend its use.12
The clinical efficacy of Evusheld has been evaluated in 2021–2022 in the randomized, placebo-controlled, phase III, DisCoVeRy clinical trial.13 Patients were included throughout the Alpha, Delta and Omicron variant waves. Evusheld administration led to a large and rapid increase in the serum neutralization activity but no significant difference in clinical or virological outcomes was found.13 However, the pre-specified statistical analysis was underpowered due to a premature interruption of inclusions.
In order to further address the benefit of Evusheld in hospitalized patients, we aimed to use mathematical modelling techniques to evaluate more precisely the impact of Evusheld on viral kinetics, which is likely a prerequisite to clinical benefit.14,15 In 2022, we demonstrated, using a similar approach, that remdesivir, a small-molecule antiviral drug, accelerated viral clearance in patients from the DisCoVeRy clinical trial.4 While the antiviral activity was modest, it was the first demonstration that remdesivir had an effect on nasopharyngeal viral load, a result that was later confirmed in more detailed virological studies.16 Here we extend this approach to assess the antiviral activity of Evusheld on different SARS-CoV-2 strains in patients from the DisCoVeRy clinical trial.
Methods
Data collection
DisCoVeRy is a randomized, phase III platform clinical trial (NCT 04315948), evaluating antiviral drugs in hospitalized patients with SARS-CoV-2 infection.13 Patients in the present analysis were randomized into two treatment arms between April 2021 and June 2022 across 63 sites in Europe (mostly France, but also Belgium, Norway, Luxembourg, Greece and Portugal). They received either a placebo (saline solution) or Evusheld, which was administered with a single intravenous infusion of 600 mg (300 mg of tixagevimab + 300 mg of cilgavimab) within 24 h after randomization. All patients or their legal representatives provided fully informed written consent.
Neutralization and viral load measurements
Blood-neutralizing antibody titers were measured against Delta, Omicron BA.1 and Omicron BA.2 variants (lineages B.1.617.2, B.1.1.529 and BA.2 respectively) at day 0 (baseline pre-treatment), day 2 and day 7 (both ± 1 day), but only the neutralization against the variant of infection was reported. Of note, we assumed that the neutralization activity was similar against all pre-Omicron variants,17 and was similar between Omicron BA.2 and Omicron BA.5 as supported by in vitro studies.11 When the virus genotype was not available, the variant of infection was assumed to be the dominant variant in the population, i.e. representing more than 95% of circulating variants at the time of symptom onset.18,19 If several variants were circulating with a prevalence >5%, no imputation was made and the patient was not included in the analysis. Neutralization activity was quantified as 50% effective dilution , measuring the number of dilutions required to halve viral level, with a limit of detection (LoD) of 30.
SARS-CoV-2 viral load was measured at baseline and days 2, 7, 14 and 28 (all ±1 day) after randomization in nasopharyngeal swabs if the patient was still hospitalized. We followed the same methodology of normalized viral load developed previously to allow the comparison of respiratory samples of different cell richness.4 Results were provided in RNA copies/104 cells, with a LoD of 10 copies/104 cells.
Only patients with at least one viral load and one neutralization data were included in the modelling. All observations (neutralization activity and viral load) below the LoD were considered as censored.
Modelling the serum neutralization activity against the variant of infection
We modelled the progressive increase in the neutralization activity, , from the time of infection (t = 0) using an adapted sigmoid Gompertz model:20
| (1) |
In this model, , τ and g capture serum neutralization activity kinetics in the absence of treatment, and represent the maximal level of neutralization (in the absence of treatment), the time of detectable neutralization activity and the steepness of increase in neutralization kinetics, respectively. To represent the impact of Evusheld on the neutralization activity, we introduced the parameter , which represents the neutralization activity conferred by Evusheld after its administration at time t = tx. Of note the model assumes no pharmacological delay between Evusheld injection and its effect on neutralization activity, consistent with the rapid pharmacokinetics of Evusheld after IV infusion with less than 1 h to reach maximum drug concentration.7
Modelling the association between neutralization activity and viral load dynamics
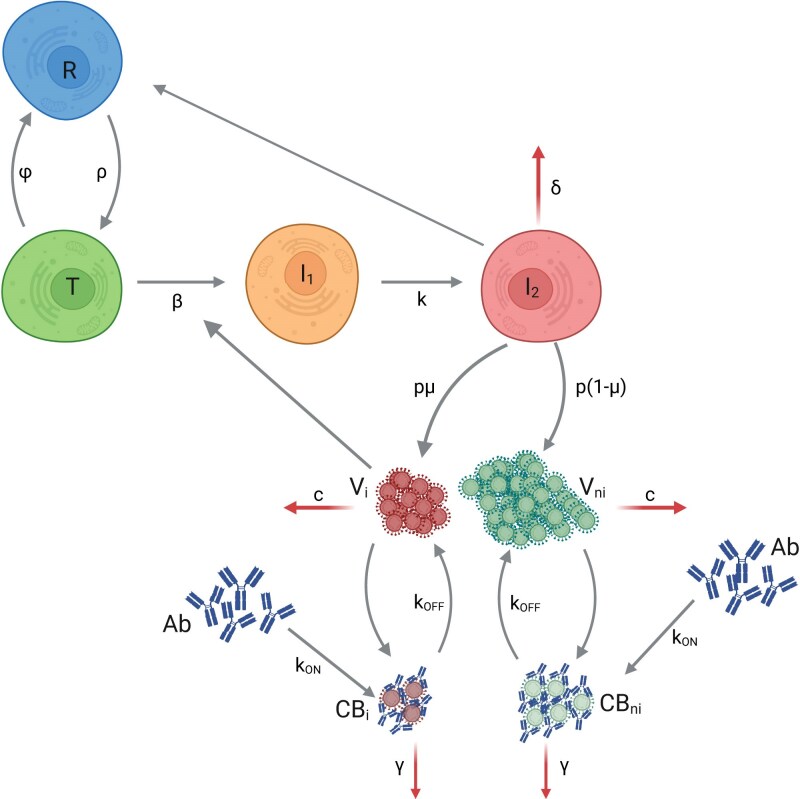
We used a viral dynamic model with an eclipse phase and a refractory compartment to describe the viral load dynamics from the time of infection (t = 0) to viral clearance21 (Figure 1).
Figure 1.
Schema of the mechanistic model of viral dynamics, integrating the dynamics of neutralization activity. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
We simultaneously modelled viral dynamics and neutralization activity. The model assumes that neutralizing antibodies bind to viral particles, leading to a complex virus-antibodies, noted CB, which prevents cell infection. This complex is then eliminated at rate γ and dissociate at rate . The measured viral load, V, is the sum of free and bound viruses.
The model is defined by the following system of ordinary differential equations:
| (2) |
Infection starts at t = 0 with only one productively infected cell , i.e. , , , , , , with the initial number of susceptible cells. is the basic reproductive number and can be written as . We assumed that we observe only a proportion f of the number of viral particles V present in the nasopharyngeal tract, , with .4 We fixed the eclipse phase to 4 d−1,22 the clearance rate c to 10 d−1,22 the proportion of infectious viruses produced to 10−4,23 the rate of complex dissociation, , to 2.16 d−1,21 and the elimination rate of bound viruses γ to = 30 d−1.21
To reconstruct the time of infection, the incubation time was fixed at 5 days for all individuals.24 Thus, we estimated the following parameters: and and their potential associated random effects.
In supplementary analyses, we also tested other mechanistic models as well as a shorter incubation period of 3 days [see Appendix (available as Supplementary data at JAC Online)].
Evaluating the impact of neutralization activity on viral load dynamics
The impact of Evusheld on neutralization evolution and viral load dynamics was evaluated through simulations of 5000 in silico Evusheld-treated profiles using the estimated parameters of the final model (Table S1) and the same distribution of baseline characteristics as in the original population (Table 1).
Table 1.
Patient characteristics
| Patient characteristics | Evusheld (n = 109) | Placebo (n = 90) |
|---|---|---|
| Male gender | 69 (63%) | 65 (72%) |
| Age ≥ 65 years | 55 (50%) | 52 (58%) |
| Fully or partially vaccinated | 49 (45%) | 44 (49%) |
| Clinical status (seven-point ordinal scale) | ||
| 3 or 4 | 86 (79%) | 78 (87%) |
| 5 | 23 (21%) | 12 (13%) |
| Variant of infection | ||
| Pre-Omicron | 66 (61%) | 59 (66%) |
| Omicron BA.1 | 32 (29%) | 20 (22%) |
| Omicron BA.2/5 | 11 (10%) | 11 (12%)a |
| Positive serology test at randomization | 69 (63%) | 59 (66%) |
| Viral load at randomization | ||
| Patients with missing viral load | 7 (6%) | 3 (3%) |
| Viral load under LOD | 6 (6%) | 5 (6%) |
| Normalized viral load (log10copies/104cells) | 4.25 (3.02–5.23) | 4.25 (3.45–5.40) |
| Neutralization activity against the variant of infection at randomization | ||
| Missing data | 2 (2%) | 2 (2%) |
| Data below the limit of detection | 56 (52%) | 48 (55%) |
| Neutralization level (log10ED50)b | 1.48 (1.48–2.33) | 1.48 (1.48–2.55) |
| Time from symptom onset to hospitalization (days) | 7.00 (6.00–9.00) | 7.00 (5.00–9.00) |
| Duration of hospital stay (days) | 8 (5–12) | 8 (5–14) |
| Deaths at D90 | 12 (11%) | 16 (18%) |
Data are n (%) or median (IQR).
aIncluding one patient infected with Omicron BA.5.
bData below LoD are censored to the LoD estimate.
Then, for each virtual patient, we calculated the viral kinetics with and without treatment (i.e. assuming D = 0 in Eq. 1), and we calculated for each individual the gain in viral load decline as well as in the time to reach undetectable viral load. Finally, to evaluate the association between the level of neutralization and viral dynamics, we used the same virtual patient population to explore the effects of different levels of neutralization activity, fixing D at 10–106 in Eq. 1.
We also used the individual patient’s predictions (i.e. Empirical Bayes Estimates, EBEs) to evaluate the association between the neutralization activity at day 5 and the time to viral clearance.
Parameter estimation and simulation
The association between patient characteristics (sex, vaccination status, age > 65 years, variant of infection and clinical status at inclusion, Table 1) and the estimated parameters of the mathematical model were tested using the COSSAC algorithm.25
We estimated the parameters of the model by computing the maximum-likelihood estimator using the stochastic approximation expectation-maximization algorithm implemented in Monolix Software 2018R2.26 The simulations were performed with Simulix Software 2018R2. All figures were performed using R.4.2.0.
Results
Baseline characteristics
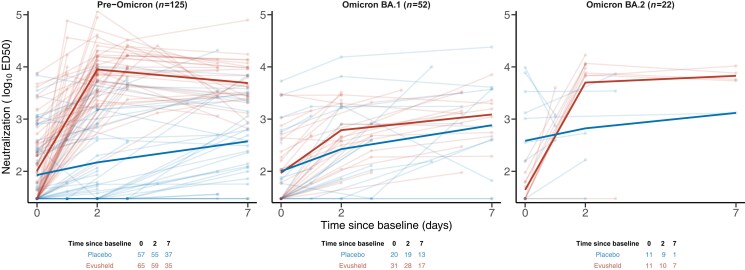
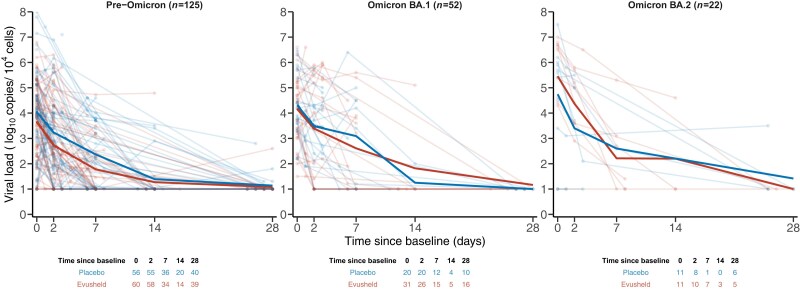
Between April 2021 and June 2022, 226 hospitalized patients were randomized in the two treatment arms with 218 individuals with at least one analysable viral load and one neutralization activity. After the imputation of the missing variant of infection (see Methods), 199 patients were included in the analysis (placebo, n = 90; Evusheld, n = 109, Figure S1). Most patients were male (n = 134, 67%) and older than 65 years (n = 107, 54%), and about half of them were vaccinated (n = 93, 47%) (Table 1). Most patients were infected by pre-Omicron variants (n = 125, 63%), while Omicron BA.1 accounted for about a quarter of infections (n = 52, 26%), and only a few patients were infected by Omicron BA.2 (n = 21, 10%) or BA.5 (n = 1, 1%). Patients arrived late at the hospital, time since symptom onset was 7 days (median: 7; IQR: 6–9). Neutralization activity increased from baseline in both arms, with a marked rise before D2 in patients infected with pre-Omicron and Omicron BA.2 variants in the Evusheld arm (Figure 2). Most patients were included in the study after the peak of viral load, during the clearance phase of viral load (Figure 3). Virological trajectories and levels of neutralization were highly heterogeneous within the population (Figures 2 and 3). At baseline, only a few patients had missing viral load or neutralization measurements (n = 10, 5% and n = 4, 2% respectively); only very few individuals had a viral load below the LoD (n = 11, 6%) conversely most patients had an undetectable level of neutralization (n = 104, 52%, Table 1).
Figure 2.
Neutralization activity of Evusheld against the variant of infection. Light lines and dots are individual observations. Solid lines are the mean of each treatment arm. Observations under LoD are imputed to the LoD estimate. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
Viral load in treated and untreated individuals according to the variant of infection. Light lines and dots are individual observations. Solid lines are the mean of each treatment arm. Observations under LoD are imputed to the LoD estimate. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Estimation of the neutralization and viral load dynamics parameters
The individual models reproduced neutralization activity and virological trajectories, fitting the observations (Figures S2–S5, S13–S14). The model estimated that, in absence of treatment, partially or fully vaccinated patients had a higher level of neutralization than non-vaccinated individuals, with maximum levels after infection, noted , estimated to 6272 and 1501, respectively (P < 10−4, Wald test), irrespective of the variants of infection. Evusheld led to an increase in neutralization activity that was higher in patients infected with pre-Omicron or Omicron BA.2 than in patients infected with Omicron BA.1. Indeed, the neutralization activity conferred by Evusheld, noted D, was equal to 263 for Omicron BA.1 compared with 5956 (P < 10−15, Wald test) and 4325 (P < 10−8, Wald test) for pre-Omicron and Omicron BA.2, respectively.
The model also estimated the impact of this neutralization activity on viral kinetic parameters. We estimated the binding rate of Evusheld to virus particles, noted , estimated at (95% CI: 0.0004–0.0036), with no significant differences across variants. The within-host reproduction number, was estimated at 4.51 (95% CI: 2.69–6.33), the viral production p was estimated at (95% CI: –), and the loss rate of infected cells, δ, was estimated at (95% CI: 0–4.86). No association between patient characteristics and the viral dynamics parameters was found. All parameter estimates are presented in Table S1.
Impact of Evusheld on the neutralization activity and time to viral clearance
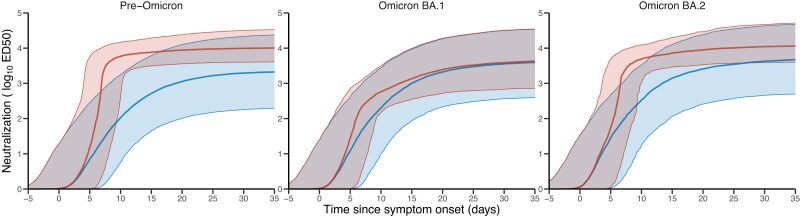
Next, we evaluated in more detail by simulations the impact of both infection and treatment on neutralization activity and viral kinetics (see Methods). Treatment led to a substantial increase in neutralization (Figure 4). At 12 h post-treatment initiation, we estimated that the median level of neutralization activity was equal to 6369 (80% PI: 6369–15 092), 467 (80% PI: 168–2073) and 5143 (80% PI: 1997–12 145) in pre-Omicron-, Omicron BA.1- and Omicron BA.2-treated patients, respectively, as compared with 36 (80% PI: 1–950), 72 (80% PI: 1–1597) and 113 (80% PI: 1–1783) in untreated patients. The differences tended to reduce over time, due to the progressive increase in antibody response in untreated individuals. One month after infection, we estimated that the median level of neutralization activity was equal to 9493 (80% PI: 3888–28 766), 3309 (80% PI: 622–25 781) and 10 142 (80% PI: 3729–41 290) in Evusheld-treated patients infected with pre-Omicron, Omicron BA.1 and Omicron BA.2, respectively, as compared with 1653 (80% PI: 150–17 864), 2978 (80% PI: 304–25 379) and 3471 (80% PI: 372–35 228) in untreated patients.
Figure 4.
Model-based evolution of neutralization activity according to the variant of infection. Red: Median and 10–90 prediction interval of neutralization activity in Evusheld-treated individuals; blue: median and 10–90 prediction interval of neutralization activity in placebo-treated individuals. Parameters and patient characteristics are sampled from the viral dynamic model (see Methods). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
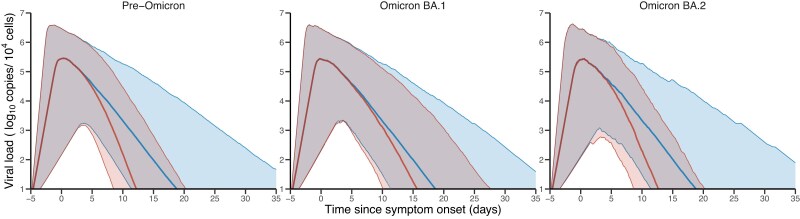
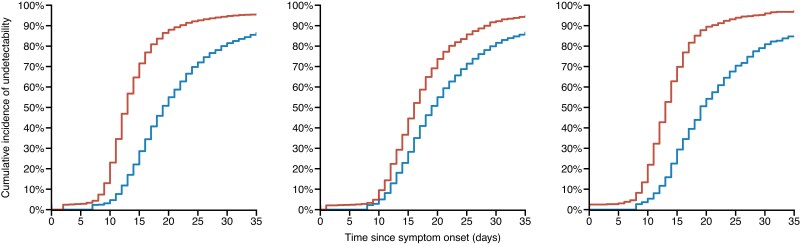
These higher levels of neutralization activity induced by Evusheld led to a more rapid viral decline (Figure 5). Thus, we predict a shorter time to viral undetectability for individuals in the Evusheld arm, for all infection variants (Figure 6). We estimated that Evusheld reduced the time to viral undetectability by more than 5 days in patients infected by pre-Omicron (median: 5.9; 80% PI: 2.1–13.6) or Omicron BA.2 (median: 5.4; 80% PI: 2.0–12.4), respectively (Figure S6). The effect was more modest in patients infected by the Omicron BA.1 variant, reducing the time to viral undetectability by 2 days (median: 2.2; 80% PI: 0.4–8.9).
Figure 5.
Model-based kinetics of viral load according to the variant of infection. Red: Median and 10–90 prediction interval of viral load in Evusheld-treated individuals; blue: median and 10–90 prediction interval of viral load in placebo-treated individuals. Parameters and patient characteristics are sampled from the viral dynamic model (see Methods). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 6.
Model-based cumulative incidence of time to viral undetectability according to the variant of infection. Red: Evusheld-treated individuals; blue: placebo-treated individual. Parameters and patient characteristics are sampled from the viral dynamic model (see Methods). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
As a way to verify our model predictions, we also assessed the association between neutralization activity and time to viral clearance using individual predicted parameters in fitted patients (see Methods). While the model unsurprisingly confirmed that treated patients infected by a pre-Omicron variant had higher neutralization activity (Figure S7), our results showed that treated patients had a shorter predicted time to viral clearance, with a reduction of 2.7 days compared with placebo patients (respectively, median: 12.9; IQR: 10.9–15.3 days versus median: 15.6; IQR: 13.2–20.3 days, P < 10−4, Wilcoxon test, Figure S8). Interestingly, and irrespective of the variant of infection, a higher level of neutralization was associated with a faster time to viral clearance (Figure S9).
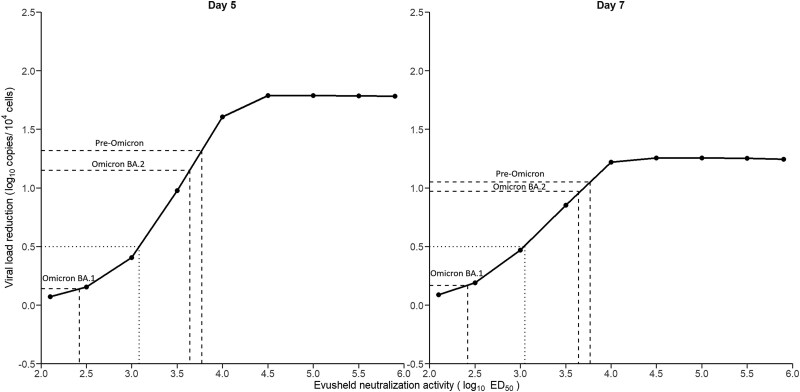
Because larger neutralizing activity levels are associated with more profound viral reduction, we next aimed to estimate more precisely the levels of Evusheld neutralization activity required to achieve a desired decline in viral load. To achieve a 0.5 log difference in viral load levels at day 5 compared with untreated patients, which was associated with a reduction in the risk of severe disease in outpatients,14,15 the neutralization activity level conferred by Evusheld, noted D (see Methods), was required to be greater than 1200 (Figure 7). Because the neutralization activity is the combination of both Evusheld and the treatment-independent endogenous immune response, this corresponds to serum neutralization activity equal to 350 and 1550 in untreated and treated patients, respectively. To achieve a 1 log difference in viral load levels at day 5 compared with untreated patients, the neutralization activity level conferred by Evusheld was required to be greater than 3300, which is close to what was obtained against pre-Omicron or Omicron BA.2 (see Table S1). This corresponds to serum neutralization activity equal to 350 and 3650 in untreated and treated patients, respectively. Interestingly our results identified that the effect saturated for neutralization activity levels greater than 30 000, with maximal effect on viral reduction equal to about 1.8 log at day 5 (Figure 7). The reduction in viral load tended to be less dramatic on day 7 due to the large proportion of individuals who had already achieved viral clearance. Reflective of the critical impact of the timing of initiation, an earlier administration of the treatment, i.e. before symptom onset, could lead to a larger decline in viral load, provided that neutralization activity is greater than 10 000 (Figure S10).
Figure 7.
Association between the neutralization activity of Evusheld and viral reduction. x-axis: neutralization activity of Evusheld; y-axis: median viral load reduction compared with untreated profiles at day 5 (left) and day 7 (right) after treatment initiation. The dotted lines represent, for each variant, the predicted viral reduction following the increase in neutralization activity by Evusheld estimated in our population. Parameters and patient characteristics are sampled from the viral dynamic model (see Methods).
Discussion
Using a mathematical model integrating viral load and neutralization activity, we found that Evusheld reduced the time to SARS-CoV-2 viral clearance in hospitalized patients by 5–6 days in patients infected with pre-Omicron or Omicron BA.2 variants, and by 2 days in patients infected with Omicron BA.1 variant.
The effect of Evusheld on viral kinetics is larger than what we previously estimated for remdesivir, a nucleotide analogue, that reduced the time to viral clearance by 0.7 days on average, and by 2 days in patients with high viral load at inclusion.4 How the larger antiviral effect of Evusheld would translate in terms of clinical benefit is unknown. In the DisCoVeRy trial, there was no significant clinical benefit of Evusheld on the primary endpoint (clinical status at day 15, measured by the WHO seven-point ordinal scale).27 However, the clinical interpretation of the results was hampered by the fact that the study was stopped prematurely due to a lack of recruitment and was thus underpowered. Interestingly, remdesivir, despite a lower antiviral activity, was found to reduce the risk of death or progression to ventilation significantly28 and is to date the only antiviral drug that showed clinical benefit in hospitalized patients. Although the comparison with remdesivir needs to be done with caution, as most results were obtained in the pre-Omicron era and a largely unvaccinated population, the larger antiviral activity found for Evusheld suggests that monoclonal antibodies warrant further investigation in hospitalized patients.
From a methodological point of view, and again reminiscent of our previous work with remdesivir, our results show that mathematical modelling is critical to analysing viral dynamics. Indeed, viral load analysis is made difficult by the variability in both viral load data and the timing of treatment initiation with respect to disease onset. This explains why statistical approaches that do not integrate the biological knowledge of host/pathogen/treatment often fail to identify treatment antiviral activity.15,16 In contrast, mathematical modelling can provide higher statistical power. In fact, our methodology previously identified for the first time an effect of remdesivir on viral clearance,4 and this effect was later confirmed in another study where viral load was frequently measured.16 To illustrate the benefit of viral dynamic modelling, we calculated the statistical power to identify a significant effect of Evusheld on the viral decline between baseline and day 7 (see Appendix). Assuming the same effect of Evusheld that was identified by our model in pre-Omicron infected patients, we found that the statistical power in a 1:1 randomized study with n = 60 patients per arm (as in our study) would be only 42%, and only 63% with n = 100, as in the overall population of our study. This illustrates that both frequent viral load data and modelling analyses are warranted to evaluate antiviral drugs29 and explains why published studies failed to identify an antiviral effect of monoclonal antibodies in hospitalized patients. Further, mathematical modelling can characterize the dose–effect relationship of a drug. Here we identified that serum neutralization ED50 of 1000 is required to achieve 0.5 log viral load reduction at day 5 compared with untreated individuals, a threshold that was identified with a 50% reduction in disease progression (i.e. hospitalization) in outpatients. In order to achieve a virological benefit larger than 1 log, we estimated that serum neutralization levels need to be larger than 3300. Because this result is likely not specific to Evusheld, it could be used beforehand to anticipate the virological benefit of a monoclonal antibody in hospitalized patients.
This study has several limitations, in particular due to data limitations. First only a limited number of individuals were infected by Omicron BA.2 (n = 22). Although our results suggesting that Evusheld regained some activity against Omicron BA.2 is consistent with previous studies,10 they will need to be confirmed in larger populations. Second, while the use of a viral dynamic model increases the statistical power to identify an effect on the kinetics of viral load, the amount of viral load data remains critical, as elegantly demonstrated by the PLATCOV study.16 In addition, as hospitalized patients arrive late in their infection, some parameters related to the early infections had to be fixed to ensure model identifiability. For instance, we assumed a fixed duration of the incubation period equal to 5 days. Although we verified that using a shorter value of 3 days, as proposed for Omicron variants,30 did not modify our results, it is possible that the model underestimates variability in the viral kinetic profiles. Furthermore, the virological measurements scheduled in the trial design were not sufficient to identify intrinsic differences in the viral dynamics across variants of infection. Finally, our model assumed no loss of Evusheld neutralizing activity over time. Although this hypothesis is reasonable given the long half-life of the drug and the short time-frame of an acute infection,7 it will be interesting to evaluate the evolution of Evusheld in the long-term.
Obviously, our results confirm the reduction in antiviral activity against Omicron variants. This has led the FDA to withdraw Evusheld approval in January 2023,12 as is the case for most monoclonal antibodies at the date where this paper is written. Beyond the specific case of AZD7442, our study opens opportunities for future research and exploration of monoclonal antibodies. The development of second-generation monoclonal antibodies, with retained activity against circulating viruses, will need to be evaluated. While there is no question that the earlier the better for antiviral treatment31 our results show that monoclonal antibodies can accelerate viral clearance in the nasopharyngeal compartment. This is likely a prerequisite to clinical benefit which warrants to pursue the evaluation of antiviral drugs in hospitalized patients.
Supplementary Material
Acknowledgements
We thank Alan S. Perelson and Tin Phan (LANL) for valuable discussions on the modelling of the effect of monoclonal antibodies. We thank all participants who consented to enrol in the trial, as well as all study and site staff whose indispensable assistance made the conduct of the DisCoVeRy trial possible.
Contributor Information
Maxime Beaulieu, Université Paris Cité et Université Sorbonne Paris Nord, Inserm, IAME, F-75018 Paris, France.
Alexandre Gaymard, Hospices Civils de Lyon, Laboratoire de Virologie, Institut des Agents Infectieux de Lyon, Centre National de Référence des virus respiratoires France Sud, F-69317 Lyon, France; Université Claude Bernard Lyon 1, Virpath, CIRI, INSERM U1111, CNRS UMR5308, ENS Lyon, F69372 Lyon, France.
Clément Massonnaud, Université Paris Cité et Université Sorbonne Paris Nord, Inserm, IAME, F-75018 Paris, France; Département d’Épidémiologie, Biostatistique et Recherche Clinique, AP-HP, Hôpital Bichat, F75018 Paris, France.
Nathan Peiffer-Smadja, Université Paris Cité et Université Sorbonne Paris Nord, Inserm, IAME, F-75018 Paris, France; AP-HP, Hôpital Bichat, Service de Maladies Infectieuses et Tropicales, F-75018 Paris, France; National Institute for Health Research, Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Imperial College London, London, UK.
Maude Bouscambert-Duchamp, Hospices Civils de Lyon, Laboratoire de Virologie, Institut des Agents Infectieux de Lyon, Centre National de Référence des virus respiratoires France Sud, F-69317 Lyon, France; Université Claude Bernard Lyon 1, Virpath, CIRI, INSERM U1111, CNRS UMR5308, ENS Lyon, F69372 Lyon, France.
Guislaine Carcelain, Immunology Department, Robert Debré Hospital, Assistance Publique Hôpitaux de Paris, Paris, France; Université Paris Cité, INSERM U976, Paris, France.
Guillaume Lingas, Université Paris Cité et Université Sorbonne Paris Nord, Inserm, IAME, F-75018 Paris, France.
France Mentré, Université Paris Cité et Université Sorbonne Paris Nord, Inserm, IAME, F-75018 Paris, France; Département d’Épidémiologie, Biostatistique et Recherche Clinique, AP-HP, Hôpital Bichat, F75018 Paris, France.
Florence Ader, Département des Maladies Infectieuses et Tropicales, Hospices Civils de Lyon, Hôpital de la Croix-Rousse, F-69004 Lyon, France; Université Claude Bernard Lyon 1, CIRI, INSERM U1111, CNRS UMR5308, ENS Lyon, F-69372 Lyon, France.
Maya Hites, Clinic of Infectious Diseases, Hôpital Universitaire de Bruxelles (HUB), Université Libre de Bruxelles, Brussels, Belgium.
Pascal Poignard, Groupe de Recherche en Infectiologie Clinique CIC-1406, Inserm—CHUGA—Université Grenoble Alpes, Grenoble, France; Univ. Grenoble Alpes, CEA, CNRS, Institut de Biologie Structurale (IBS), Grenoble, France; Laboratoire de Virologie, Center Hospitalier Universitaire Grenoble-Alpes, Grenoble, France.
Jérémie Guedj, Université Paris Cité et Université Sorbonne Paris Nord, Inserm, IAME, F-75018 Paris, France.
Funding
This work was supported by several sources: the European Commission (EU-Response, Grant 101015736), the DIM One Health Île-de-France (R20117HD) and AstraZeneca.
Transparency declarations
M.H. reports grants from The Belgian Center for Knowledge (KCE), the Fonds Erasme-COVID-Université Libre de Bruxelles and the EU-Horizon program, for the submitted work; and has received support for attending meetings from Pfizer; support for participation on an advisory board for therapeutics on COVID-19; and support for leadership for the Belgian guidelines on therapeutics for COVID-19 and acting as a treasurer for the Belgian Society of Clinical Microbiology and Infectious Diseases. F.M. reports grants and consulting fees from Da Volterra, grants from Sanofi, and consulting fees from Ipsen, outside the submitted work. All other authors declare no competing interests.
F.M., P.P., J.G. and M.B. were involved in the design of the study. M.B.D., A.G., P.P. and M.B. were responsible for the analysis. J.G. and M.B. wrote the original draft, and all authors contributed to the refinement of the manuscript and approved it.
Supplementary data
Figures S1 to S14 and Tables S1 to S4 are available as Supplementary data at JAC Online.
References
- 1. Kirwan PD, Charlett A, Birrell P et al. Trends in COVID-19 hospital outcomes in England before and after vaccine introduction, a cohort study. Nat Commun 2022; 13: 4834. 10.1038/s41467-022-32458-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Najjar-Debbiny R, Gronich N, Weber G et al. Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis 2023; 76: e342–9. 10.1093/cid/ciac443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abani O, Abbas A, Abbas F et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2022; 399: 665–76. 10.1016/S0140-6736(22)00163-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lingas G, Néant N, Gaymard A et al. Effect of remdesivir on viral dynamics in COVID-19 hospitalized patients: a modelling analysis of the randomized, controlled, open-label DisCoVeRy trial. J Antimicrob Chemother 2022; 77: 1404–12. 10.1093/jac/dkac048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO Solidarity Trial Consortium . Repurposed antiviral drugs for COVID-19—interim WHO solidarity trial results. N Engl J Med 2021; 384: 497–511. 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hobbs FDR, Montgomery H, Padilla F et al. Outpatient treatment with AZD7442 (Tixagevimab/Cilgavimab) prevented COVID-19 hospitalizations over 6 months and reduced symptom progression in the TACKLE randomized trial. Infect Dis Ther 2023; 12: 2269–87. 10.1007/s40121-023-00861-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forte-Soto P, Albayaty M, Brooks D et al. Safety, tolerability and pharmacokinetics of half-life extended severe acute respiratory syndrome coronavirus 2 neutralizing monoclonal antibodies AZD7442 (Tixagevimab-Cilgavimab) in healthy adults. J Infect Dis 2023; 227:1153–63. 10.1093/infdis/jiad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levin MJ, Ustianowski A, De Wit S et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for prevention of COVID-19. N Engl J Med 2022; 386: 2188–200. 10.1056/NEJMoa2116620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anon . Update on AZD7442 STORM CHASER trial in post-exposure prevention of symptomatic COVID-19. https://www.astrazeneca.com/media-centre/press-releases/2021/update-on-azd7442-storm-chaser-trial.html.
- 10. Bruel T, Hadjadj J, Maes P et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med 2022; 28: 1297–302. 10.1038/s41591-022-01792-5 [DOI] [PubMed] [Google Scholar]
- 11. Bruel T, Stéfic K, Nguyen Y et al. Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies. Cell Rep Med 2022; 3: 100850. 10.1016/j.xcrm.2022.100850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. U.S. Food and Drug Administration. FDA announces Evusheld is not currently authorized for emergency use in the U.S. FDA 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us.
- 13. Hites M, Massonnaud CR, Lapique EL et al. Tixagevimab-cilgavimab (AZD7442) for the treatment of patients hospitalized with COVID-19 (DisCoVeRy): a phase 3, randomized, double-blind, placebo-controlled trial. J Infect 2024;88:106120. 10.1016/j.jinf.2024.106120 [DOI] [PubMed] [Google Scholar]
- 14. Mitjà O, Corbacho-Monné M, Ubals M et al. Hydroxychloroquine for early treatment of adults with mild coronavirus disease 2019: a randomized, controlled trial. Clin Infect Dis 2021; 73: e4073–81. 10.1093/cid/ciaa1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parienti JJ, de Grooth HJ. Clinical relevance of nasopharyngeal SARS-CoV-2 viral load reduction in outpatients with COVID-19. J Antimicrob Chemother 2022; 77: 2038–9. 10.1093/jac/dkac104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jittamala P, Schilling WHK, Watson JA et al. Clinical antiviral efficacy of remdesivir in coronavirus disease 2019: an open-label, randomized controlled adaptive platform trial (PLATCOV). J Infect Dis 2023; 228: 1318–25. 10.1093/infdis/jiad275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao Y, Wang J, Jian F et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022; 602: 657–63. 10.1038/s41586-021-04385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emma B Hodcroft. “CoVariants: SARS-CoV-2 Mutations and Variants of Interest.” https://covariants.org/. 2021.
- 19. Santé publique France. OLD Données de laboratoires pour le dépistage : Indicateurs sur les mutations (SI-DEP)—data.gouv.fr. https://www.data.gouv.fr/fr/datasets/donnees-de-laboratoires-pour-le-depistage-a-compter-du-18-05-2022-si-dep/.
- 20. Tjørve KMC, Tjørve E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: an addition to the unified-Richards family. PLoS One 2017; 12: e0178691. 10.1371/journal.pone.0178691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phan T, Zitzmann C, Chew KW et al. Modeling the emergence of viral resistance for SARS-CoV-2 during treatment with an anti-spike monoclonal antibody. PLoS Pathog 2024; 20: e1011680. 10.1371/journal.ppat.1011680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ke R, Zitzmann C, Ho DD et al. In vivo kinetics of SARS-CoV-2 infection and its relationship with a person’s infectiousness. Proc Natl Acad Sci U S A 2021; 118: e2111477118. 10.1073/pnas.2111477118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonçalves A, Maisonnasse P, Donati F et al. SARS-CoV-2 viral dynamics in non-human primates. PLoS Comput Biol 2021; 17: e1008785. 10.1371/journal.pcbi.1008785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Néant N, Lingas G, Le Hingrat Q et al. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc Natl Acad Sci U S A 2021; 118: e2017962118. 10.1073/pnas.2017962118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ayral G, Si Abdallah JF, Magnard C et al. A novel method based on unbiased correlations tests for covariate selection in nonlinear mixed effects models: the COSSAC approach. CPT Pharmacometrics Syst Pharmacol 2021; 10: 318–29. 10.1002/psp4.12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delyon B, Lavielle M, Moulines E. Convergence of a stochastic approximation version of the EM algorithm. Ann Stat 1999; 27: 94–128. 10.1214/aos/1018031103 [DOI] [Google Scholar]
- 27. World Health Organization. COVID-19 Therapeutic Trial Synopsis. https://www.who.int/publications-detail-redirect/covid-19-therapeutic-trial-synopsis.
- 28. Pan H, Peto R, Henao Restrepo AM et al. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO solidarity randomised trial and updated meta-analyses. Lancet 2022; 399: 1941–53. 10.1016/S0140-6736(22)00519-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watson JA, Kissler SM, Day NPJ et al. Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies. Antimicrob Agents Chemother 2022; 66: e0019222. 10.1128/aac.00192-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galmiche S, Cortier T, Charmet T et al. SARS-CoV-2 incubation period across variants of concern, individual factors, and circumstances of infection in France: a case series analysis from the ComCor study. Lancet Microbe 2023; 4: e409–17. 10.1016/S2666-5247(23)00005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonçalves A, Bertrand J, Ke R, et al. Timing of antiviral treatment initiation is critical to reduce SARS-Cov-2 viral load. CPT Pharmacometrics Syst Pharmacol 2020;9:509–14. 10.1002/psp4.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.