Abstract
Cystic fibrosis (CF) is a genetic disease caused by mutations in the CFTR (cystic fibrosis transmembrane conductance regulator) gene. Although CF is a multiorgan disease, the leading causes of morbidity and mortality are related to progressive lung disease. Current understanding of the effects of the broad spectrum of CFTR mutations on CFTR function has allowed for the development of CFTR modulator therapies. Despite the remarkable impact that these therapies have had, there remains a significant proportion of people with CF (estimated at 10–15% of the global CF population) who are genetically ineligible for, or intolerant of, current CFTR-targeting therapies and whose therapeutic needs remain unmet. Inhaled genetic therapies offer the prospect of addressing the unmet pulmonary treatment need in people with CF, with several approaches, including gene addition therapy (the focus of this review), RNA-based therapies, antisense oligonucleotides, and gene editing, being explored. Various nonviral and viral vectors have been investigated for CF gene addition therapy for mutation-agnostic restoration of CFTR function in the lungs. Lentiviral vectors offer the prospect of highly efficient and long-lasting gene expression, and the potential to be safely and, in contrast to other commonly used viral vectors, effectively redosed. A third-generation lentiviral vector pseudotyped with Sendai virus F and HN envelope proteins (rSIV.F/HN) has been developed for the treatment of CF. Promising preclinical results support the progression of this vector carrying a full-length CFTR transgene (BI 3720931) into a first-in-human clinical trial expected to begin in 2024.
Keywords: CFTR, integrating vectors, lentivirus, genetic therapy, mutation-agnostic treatment
Introduction to Cystic Fibrosis
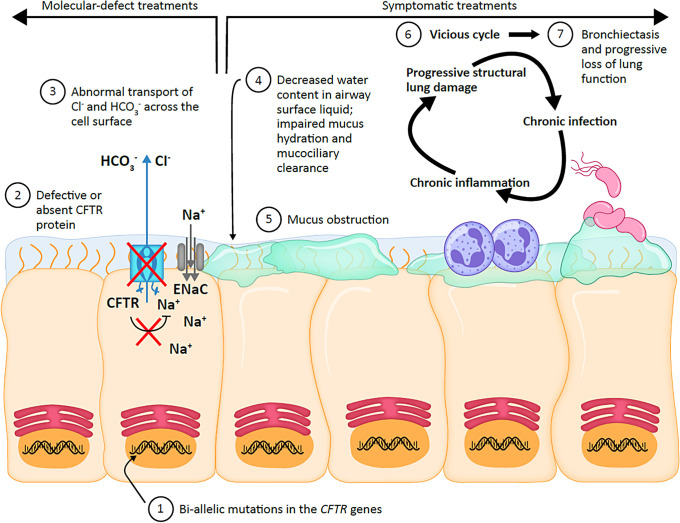
Cystic fibrosis (CF) is an autosomal recessive genetic disorder that is estimated to affect approximately 160,000 individuals across 94 countries (1–3). CF is caused by pathogenic mutations in the CFTR (cystic fibrosis transmembrane conductance regulator) gene (1, 2, 4). The CFTR gene encodes an anion channel that transports chloride and bicarbonate across the apical surface of multiple epithelial cell types in the lungs and other organs (1, 2, 4) (Figure 1). The CFTR channel also regulates sodium transport via the epithelial sodium channel, ENaC (5, 6). Reduced or absent functioning of the CFTR channel in the airways of people with CF results in a cycle of chronic pulmonary infection, inflammation, and progressive structural lung damage (1, 2, 4). Although yet to be fully elucidated, factors implicated in this include impaired mucus hydration and subsequent mucociliary clearance, as well as reduced pH that potentially leads to impaired bacterial killing (1, 2, 4, 7) (Figure 1). Extrapulmonary clinical manifestations can include chronic sinusitis and nasal polyps; exocrine pancreatic insufficiency leading to malnutrition, pancreatitis, and CF-related diabetes; liver and intestinal disease; bone disease; and reduced fertility in both men and women (1, 2, 4, 8).
Figure 1.
Mechanism of disease. CFTR = cystic fibrosis transmembrane conductance regulator; Cl− = chloride ion; ENaC = epithelial sodium channel; HCO3− = bicarbonate ion; Na+ = sodium ion.
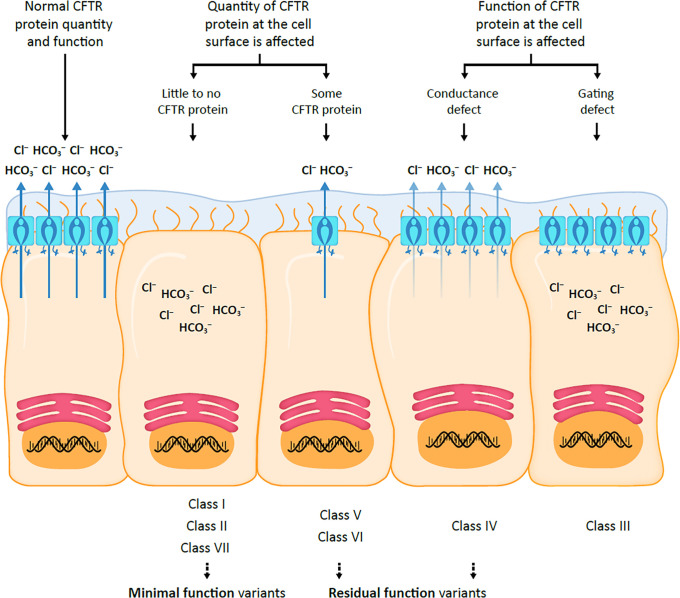
Since the identification of the CFTR gene in 1989, more than 2,000 different variants have been discovered, of which approximately 700 have been determined to be disease-causing mutations (2, 4, 9–11). Seven classes of CFTR mutations have been identified, impacting either the quantity or function of CFTR protein at the cell surface (Figure 2) (7, 12, 13).
Figure 2.
CFTR mutation classes. CFTR = cystic fibrosis transmembrane conductance regulator; Cl− = chloride ion; HCO3− = bicarbonate ion.
An understanding of the effects of mutations on CFTR function has allowed for the development of small molecules that restore defective CFTR function caused by a subset of mutation classes; these treatments are known as CFTR modulators (2, 12).
Current Therapies for CF: CFTR Modulator Therapies
CFTR modulators are systemic therapies, administered orally, that have transformed the landscape of clinical care for people with CF through unprecedented improvements in lung function, pulmonary exacerbations, weight and nutritional status, quality of life, and other clinical outcomes, all of which likely translate into substantially increased life expectancy (11, 14, 15). There are two types of CFTR modulators currently in use: potentiators that enhance channel function and correctors that increase the proportion of mature CFTR protein that is successfully trafficked to the cell surface (7, 14).
To date, four CFTR modulator therapies have been approved for the treatment of CF (11): ivacaftor, a CFTR potentiator; two corrector-potentiator combinations, lumacaftor-ivacaftor and tezacaftor-ivacaftor; and triple combination therapy, elexacaftor-tezacaftor-ivacaftor (ETI). ETI has resulted in robust improvements in lung function (change in percent predicted FEV1 [ppFEV1] of up to 13.8%) and other clinical outcomes, such as reduced frequency of pulmonary exacerbations, increased body mass index, and improved patient-reported quality of life (Cystic Fibrosis Questionnaire– Revised, respiratory domain) (3, 7, 11, 12, 16, 17). Recent results from an observational study showed that ETI restores CFTR function in the airway and intestinal epithelia to ∼40–50% of that of healthy people (15).
However, despite the remarkable impact that CFTR modulator therapies have had on CF clinical care, there is still a significant proportion of people with CF who are ineligible for, or intolerant of, CFTR modulator therapies and for whom there remains a high unmet therapeutic need (10–15% of the global CF population, based on current estimates) (11, 18–21).
Unmet Need for New Therapeutic Approaches for CF
Available CFTR modulator therapies are only beneficial when there is sufficient CFTR protein with therapeutic binding sites for them to act on (22). Various CF-causing mutations, including frameshift, nonsense, and splicing mutations, result in no CFTR protein or very low amounts of CFTR protein that is truncated and dysfunctional. As these mutations are largely insensitive to CFTR modulator therapies, people possessing them are ineligible for such therapies (22, 23). The most recent estimates indicate that approximately 5% of people with CF in the United States aged 12 years and older have mutations that are ineligible for CFTR modulator therapy, and these mutations are more common in ethnically and racially minoritized people with CF (24). Thus, there is a higher proportion of minoritized people with CF who are genetically ineligible for CFTR modulator therapy than in the overall CF population, resulting in a CF health disparity (25, 26). In addition, not all people with CF who are genetically eligible for modulator therapies are able to tolerate them because of adverse effects (12, 27). In sum, there is an unmet need for new therapeutic approaches for people with CF who still rely on traditional CF care (symptom-directed treatments), and alternative approaches are needed to restore CFTR function (11, 12). This will help to close the widening health outcome gap between modulator-treated and non–modulator-treated people with CF (11).
In addition, for those who are eligible for and able to tolerate CFTR modulators, lifelong daily oral administration is required, and significant drug–drug interactions have been reported (18, 19). For example, triazoles (first-line antifungal treatment in people with CF) have been associated with significant, bidirectional drug–drug interactions with CFTR modulators through interference with the cytochrome P450 system (28). Therefore, longer-lasting therapeutic approaches that reduce the need for concomitant therapies could reduce the treatment burden for people with CF, although if administered by inhalation, the systemic benefit of CFTR modulator therapy would be lost. Clearly, even in the CFTR modulator era, there is an ongoing need for new treatment strategies targeting CFTR.
Genetic Therapies in Development for CF
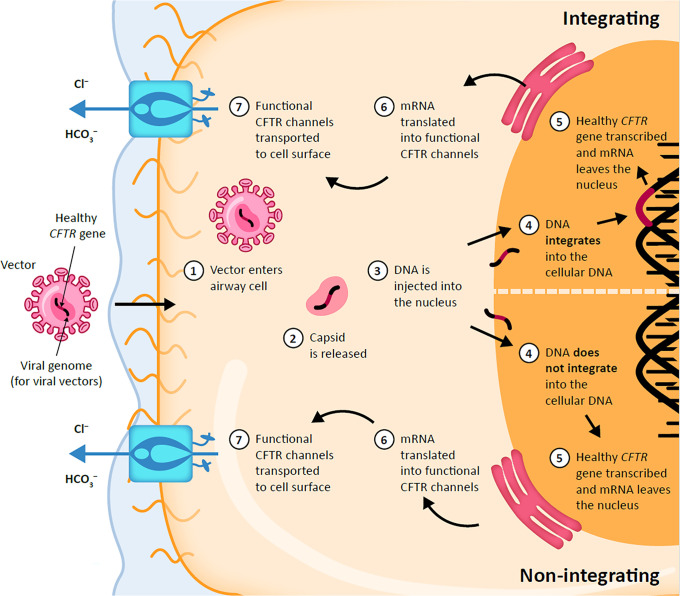
Inhaled genetic therapies offer the prospect of addressing the unmet pulmonary treatment need in people with CF (29, 30). There are several approaches being investigated for CF, including gene addition therapy (the focus of this review) (Figure 3), RNA-based therapies such as mRNAs and transfer RNAs, antisense oligonucleotides (ASOs), and gene editing. Currently, these therapeutic approaches are in clinical use for several indications but are still in preclinical or early clinical trial stages for CF (29).
Figure 3.
Viral gene therapy for cystic fibrosis. CFTR = cystic fibrosis transmembrane conductance regulator; Cl− = chloride ion; HCO3− = bicarbonate ion.
There are several RNA molecules being assessed for their therapeutic potential in CF (29). A promising mutation-agnostic therapeutic approach for CF is full-length CFTR mRNA addition (30). Three early-phase clinical trials of inhaled mRNA therapy for CF (CFTR mRNA encapsulated in a lipid nanoparticle) are in progress (30–32): RCT2100 in healthy volunteers (33), ARCT-032 in healthy volunteers and adults with CF (34), and VX-522 in adults with CF who are ineligible for CFTR modulator therapy (35).
ASOs are short, synthetic, single-stranded nucleic acids that bind to mutant mRNA transcripts; in doing so, they modulate gene expression either through mRNA repair permitting transcript read-through or by correcting splicing mutations (36, 37). There are several ASOs targeting nonsense and splicing mutations under preclinical and clinical development for treatment of CF (22). In 2023, a phase I study of SPL84, an ASO for the treatment of people with CF with at least one 3849 + 10kbC→T variant, was completed (38). SPL84 was shown to be safe and well tolerated in healthy volunteers who received a single dose (38). A phase II efficacy study is planned for 2024 (38).
Gene editing to correct CFTR mutations is still in the early preclinical development stage, with both in vivo clustered regularly interspaced short palindromic repeats (CRISPR)-based and non–CRISPR-based gene editing approaches assessed in animal models (39).
Gene Addition Therapy
Gene addition therapy is a mutation-agnostic approach that refers to the treatment of a genetic disease through the introduction of the normal gene into cells via a vector (Figure 3). Many gene therapy trials for CF have been performed since the identification of the CFTR gene (36 trials involving approximately 600 people with CF); however, for various reasons relating to efficacy and safety, no gene therapy for CF has reached the market to date (18). There are two types of vectors that have been investigated for CF gene therapy, namely nonviral (e.g., liposomes) and viral vectors (e.g., based on adenoviruses [Ads], adeno-associated viruses [AAVs], herpes simplex viruses [HSVs], and lentiviruses). The potential benefits and limitations of these vectors are shown in Table 1.
Table 1.
Benefits and Limitations of Nonviral and Viral Vectors
| Nonviral Vectors | Viral Vectors |
||||
|---|---|---|---|---|---|
| Ad Vectors | AAV Vectors | HSV Vectors | Lentiviral Vectors | ||
| Large packaging capacity | ✓ | ✓ | ✓ | ✓ | |
| Almost no risk of insertional oncogenesis | ✓ | ✓ | ✓ | ✓ | |
| Noninflammatory | ✓ | ✓ | ✓ | ✓ | |
| Transduction of terminally differentiated epithelial cells | ✓ | ✓ | ✓ | ✓ | ✓ |
| Integrating vector (gene expression for the lifetime of the transduced cell)* | ✓ | ||||
| Successful readministration to the lung without loss of efficacy | ✓† | ✓ | |||
Definition of abbreviations: AAV = adeno-associated virus; Ad = adenovirus; HSV = herpes simplex virus.
Because of the turnover of transduced airway epithelial cells, a single dose may not be sufficient to achieve lifelong therapeutic benefit in humans. Therefore, the ability to safely and effectively readminister may also be a prerequisite for a successful gene therapy.
Multiple repeat dosing without loss of expression has been demonstrated with the nonviral CFTR (cystic fibrosis transmembrane conductance regulator) gene liposome complex pGM169/GL67A (42). However, it remains unclear whether multiple repeat dosing is possible without loss of efficacy for all nonviral vectors, as there is currently insufficient clinical experience with newer vectors.
Nonviral vectors
Nonviral vectors offer many benefits, including an almost unlimited packaging capacity, negligible risk of insertional oncogenesis (as they do not integrate into the DNA of transduced cells), and increased biocompatibility (low cytotoxicity and immunogenicity) compared with viral vectors (36, 41). However, efficient delivery of nonviral vectors to the lung remains a challenge (41). In a phase IIb trial, monthly repeated administration of the nonviral CFTR gene liposome complex pGM169/GL67A over a 1-year period was shown to be safe, well tolerated and produced significant lung function benefit compared with placebo (36, 42). In addition, multiple repeat dosing without loss of expression was demonstrated with this vector (42). However, the magnitude of efficacy was modest and insufficient to warrant further development, highlighting the need for more potent vectors (19, 42).
In addition to liposomes, there are several other nonviral vectors in development for the treatment of CF, including lipid nanoparticles, extracellular vesicles, and polymeric nanoparticles (43). Although several nonviral vectors are in clinical (RCT2100, ARCT-032, and VX-522 using lipid nanoparticles) (33–35) and preclinical (Nanite using polymeric nanoparticles) (44) development to deliver CFTR mRNA to people with CF, there are currently no ongoing clinical trials using nonviral vectors for CFTR gene addition. There are, however, several nonviral vectors for gene addition therapy in early development (extracellular vesicles [45] and polymeric nanoparticles [46]).
Viral vectors
Viral vectors may offer the advantage of improved efficiency of gene delivery (Figure 3). Ad vectors were one of the first viral vectors investigated for use in gene therapy because of their natural lung tropism and large carrying capacity (40). However, traditional Ad vectors have been considered unsuitable for gene therapy for CF because of their short duration of expression requiring frequent readministration and vector-induced immune responses preventing efficient gene expression after readministration (30, 40). As such, helper-dependent Ad (HD-Ad) vectors have since been developed based on Ad vectors through the removal of viral genes; this makes HD-Ad vectors less immunogenic after repeat administration (47). No clinical trials using HD-Ad vectors for CFTR gene addition are currently underway.
Although there is evidence of some degree of integration emerging, AAVs have typically been considered a type of vector that does not integrate into the DNA of transduced cells and therefore offer a very low risk of insertional oncogenesis (31, 36, 48). At low doses, AAVs only elicit modest inflammatory responses, with evidence from clinical trials suggesting that immunogenicity is dose dependent (49, 50). They also have a small packaging capacity and thus cannot carry the full-length CFTR coding sequence coupled with typical transcriptional control sequences (30, 31, 36). Furthermore, efficacy of AAVs can be impacted by preexisting immunity as well as adaptive immune responses after vector administration (51, 52). Importantly, efficacious readministration has not been demonstrated with AAVs (30). 4D-710 is an AAV vector–based gene therapy that is currently being assessed in a phase I/II single-dose trial in adults with CF who are ineligible for, or unable to tolerate, CFTR modulator therapy (NCT05248230) (31, 36, 53). To overcome the packaging limitation, the vectors carry CFTR minigenes, in which a portion of the coding sequence has been removed (54); alternatively, novel AAV/bocavirus hybrid vectors have been created that sufficiently extend the packaging capacity to allow full-length CFTR cDNA to be used (55, 56).
HSV-based vectors are nonintegrating and comparatively transient expression vectors that have a large packaging capacity and are considered noninflammatory (36). Although they are better known for their use in the nervous system and for skin diseases, HSV-1 vectors are also being developed for use in the lung (36). Although efficacy after repeated administration with HSV vectors has been shown to be maintained after cutaneous administration (36), at the time of writing, the authors are not aware of data showing that efficacy is maintained in the lungs. KB407 is a replication-defective HSV-1 vector encoding two copies of the full-length human CFTR gene for the treatment of CF (30, 36). Duration of expression is short, and safety of weekly administration is currently being assessed in a phase I/IIa clinical trial in adults with CF, regardless of their CFTR genotype (NCT05504837) (36, 57, 58).
Lentiviral vectors integrate into the DNA of transduced cells, allowing for long-term gene expression throughout the lifetime of the cell after a single dose (18, 31, 36, 40). They have a large packaging capacity and can therefore carry the full-length CFTR coding sequence (30, 36). Lentiviral vectors are recognized for their very weak inflammatory properties (59). Moreover, in contrast to Ad, AAV, and HSV, lentiviral vectors have been successfully readministered to the respiratory epithelium without loss of efficacy (30, 60). Lentiviral vectors can be modified with different viral envelope proteins (known as pseudotyping) to improve their tropism for different primary cell types (61).
A highly successful gene therapy for CF would require efficient and long-lasting gene expression. The ability to safely and effectively redose therapy may also be a prerequisite given the turnover of transduced airway epithelial cells. Lentiviral vectors have the potential to meet these needs and are the focus of the remainder of this review.
Gene Therapy with Lentiviral Vectors
There are several lentiviral vector–based gene therapies that have been investigated for the pulmonary treatment of CF. These include lentiviral vectors pseudotyped with VSV-G (62) and GP64 (63) envelope proteins, both of which are in preclinical development. The CFTR transgene DNA was detected in five out of six nonhuman primates treated with a lentiviral vector pseudotyped with VSV-G envelope proteins (62). Evidence of functional CFTR was reported in newborn CF pigs that received a lentiviral vector pseudotyped with GP64 envelope proteins (63). In addition, a lentiviral vector pseudotyped with Sendai virus envelope is expected to enter phase I/II testing in 2024 and will be discussed in further detail later in this review.
Expression beyond the lifetime of the surface epithelial cells, which would thereby remove the need for repeated dosing, would require progenitor and/or stem cell transduction. A lentiviral vector has been shown to transduce human airway progenitor cells (primary basal cells) in vitro without altering the differentiation potential (64). In vivo, such basal cells in the pseudostratified proximal airways are difficult to target; however, progenitor and/or stem cells of the distal lower airways may be more readily transduced related to the simpler, single-cell layer anatomy of this region.
Safety concerns associated with viral vector–based gene therapy, in particular integrating vectors such as lentiviral vectors, include acute inflammation and genotoxic events such as insertional oncogenesis caused by activation of protooncogenes or disruption of tumor suppressor genes (65, 66). Although not new, considerable advances have been made recently to improve the safety of lentiviral vectors.
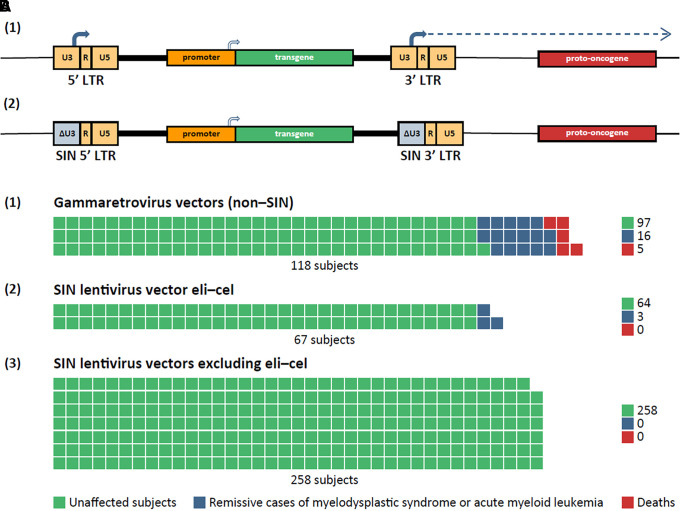
As lentiviral vectors are integrating vectors, insertional oncogenesis is a safety concern (18, 31, 40, 66). Transcriptionally active long terminal repeats (LTRs) with strong promoter and enhancer sequences have been identified as major drivers of genotoxicity in early generations of lentiviral vectors (first-generation vectors included large portions of the lentiviral genome; second-generation vectors excluded the lentiviral accessory genes vif, vpr, vpu, and nef) (66, 67). Subsequently, the safety of lentiviral vectors has been improved through the development of third-generation self-inactivating (SIN) vectors (68, 69), which lack lentiviral tat (67) and the transcriptional control elements in the 3′LTR and are therefore much less capable of activating protooncogenes (36, 40, 66, 70) (Figure 4A). Indeed, in a 2022 systematic literature review and meta-analysis that assessed the incidence of genotoxicity in hematopoietic stem and progenitor cells by vector type, no oncogenic events were reported across 34 studies using lentiviral vectors (71). Subsequent to publication of the review, three oncogenic cases associated with vector insertion were reported in trials of the lentiviral vector elivaldogene autotemcel (eli-cel; Skysona™) designed to treat cerebral adrenoleukodystrophy (72). The vector includes a transgene promoter derived from a retroviral LTR element (MNDU3 promoter), which has been implicated as the responsible genotoxic element (72). Overall, excluding eli-cel–treated subjects, SIN lentiviral vectors have demonstrated a reassuring safety profile (Figure 4B). In contrast, in the 2022 systematic literature review and meta-analysis mentioned above, first-generation (non-SIN) gammaretroviral vectors were responsible for 21 oncogenic events across 20 studies (n = 118 subjects) (71).
Figure 4.
Safety of lentiviral gene therapy. (A) Deletion of long terminal repeat (LTR) promoter/enhancer reduces genotoxic risks. (1) Integrated second-generation retroviral vector with intact LTRs (non–self-inactivating [non-SIN]). The promoter/enhancer element in the U3 region of the 3′ LTR (solid arrow) has the potential to activate (dotted arrow) a cellular protooncogene. (2) Deletion of the U3 element (ΔU3) to ablate the LTR-initiated genotoxic effect is a feature of all modern SIN vectors (third-generation vectors). Host genomic DNA is shown as a thin horizontal black line. (B) Waffle charts of frequency of occurrence of oncogenic events after retroviral gene therapy (each square represents an individual subject). (1) Subjects treated with early-generation gammaretroviral vectors without SIN deletions. (2) Subjects treated with elivaldogene autotemcel (eli-cel), a SIN lentiviral vector (i.e., with ΔU3 deletions in the LTRs) but whose internal transgene promoter is derived from a retroviral LTR. (3) Subjects treated with SIN lentiviral vectors lacking any LTR-derived promoter/enhancers. Eli-cell data and inferences from Jun 9–10, 2022 meeting of the U.S. Food and Drug Administration Cellular, Tissue, and Gene Therapies Advisory Committee (72); other data from Reference 71.
An additional safety feature in the production of third-generation lentiviral vectors involves the splitting of the necessary viral genes onto several different plasmids, thereby reducing the risk of recombination and replication-competent vector development during manufacturing (a hypothetical risk and not seen to date) (73, 74). Lentiviral vectors have been further modified to prevent recombination in vivo, such as minimizing sequence similarities between the vector genome and viral gag/pol transcripts (75).
Several third-generation lentiviral vector–based gene therapies have been approved for various indications (Table 2). In addition, several clinical trials using lentiviral vectors for the treatment of various indications, including metabolic disorders, cancers, immune disorders, and rare genetic diseases, are ongoing (40).
Table 2.
Approved Lentiviral Vector-based Gene Therapies
| Approved Lentiviral Gene Therapy | Indication | Approval Agency (yr) |
|---|---|---|
| Zynteglo | β-thalassemia | EMA (2019)* (83); FDA (2022) (84) |
| Libmeldy or Lenmeldy | Metachromatic leukodystrophy | EMA (2020) (85); FDA (2024) (86) |
| Skysona | Cerebral adrenoleukodystrophy | EMA (2021)* (87); FDA (2022) (88) |
| Lyfgenia | Sickle cell disease | FDA (2023) (89) |
| Kymriah | CAR T therapy for certain cancer types | FDA (2017) (90); EC (2022) (91) |
| Breyanzi | CAR T therapy for certain cancer types | FDA (2021) (92); EC (2022) (93) |
rSIV.F/HN: A Third-Generation Lentiviral Vector
Historically, no viral vector has met the requirements for clinical use in CF. Major shortcomings included poor gene transfer (in part due to the receptors for viral vectors being predominantly located on the basolateral surface of airway epithelium) and limited capacity for repeat administration (36, 76).
A third-generation lentiviral vector pseudotyped with Sendai virus F and HN envelope proteins (rSIV.F/HN) has been developed as a platform for the treatment of CF (76). The receptors for the F/HN pseudotype are located on the apical surface of airway epithelia, thus enabling cell entry upon inhaled delivery (76, 77). The vector has also been designed to mitigate safety risks, as it is SIN and replication incompetent (76). As mentioned above, concerns regarding the safety of integrating viral vectors were raised after the development of leukemia in people with severe combined immunodeficiency after treatment with a first-generation γ retroviral vector–transduced bone marrow (76, 78). In addition to the improved biosafety of third-generation lentiviral vectors, the risk of insertional oncogenesis is also influenced by the target cell population and is likely to be considerably lower in differentiated nondividing epithelial cells compared with rapidly dividing bone marrow stem cells (76, 78). Indeed, a nonexhaustive insertion site analysis in genomic DNA from nasal and lung airway epithelial cells extracted from mice transduced in vivo by instilled rSIV.F/HN revealed no preference for integration near known oncogenic loci; insertion sites were amplified using linear amplification–mediated PCR adapted for SIV LTRs, and sequence analysis revealed that 73% of insertion sites were located in transcription units, a frequency distribution pattern typical of lentiviruses (70). A comprehensive insertion site analysis performed later in primary human bronchial epithelial cells from people with CF (F508del/F508del) transduced ex vivo by rSIV.F/HN also concluded that the insertion site distribution was consistent with the rSIV.F/HN vector being of low genotoxic potential (data not shown).
In mouse models, the rSIV.F/HN vector was shown to transduce the respiratory epithelium with an efficiency of ∼15%, a level that is likely to be relevant for clinical benefit in humans (70). Transduction with the vector led to stable gene expression that persisted for at least 2 years after transduction (78). In addition, monthly repeat administration was possible without significant loss of gene expression; the degree of expression was identical after one and three doses and was >4 log orders higher than in nontransduced mice (78). No evidence of chronic toxicity was observed during the 2-year follow-up period (78). Moreover, preexisting and acquired immune responses appear not to interfere with vector readministration efficacy (70).
Furthermore, in nonhuman primates that received a single aerosolized dose of the rSIV.F/HN vector carrying a reporter gene, no evidence of toxicity was reported (79). Transduction levels in airway epithelial cells ranged from 9% to 12%, and high amounts of vector-specific mRNA were detected (79). The rSIV.F/HN vector has also been shown to transduce human lungs maintained in an ex vivo lung perfusion system (unpublished data) and achieve persistent gene expression in ovine precision-cut lung slices (78).
The rSIV.F/HN vector carrying a reporter gene has been shown to transduce differentiated human airway epithelium ex vivo (78). In addition, persistent gene expression was observed in differentiated human air–liquid interface cultures at amounts far higher than air–liquid interface cultures transduced with the previous nonviral gene transfer agent GL67A (78).
In preparation for a first-in-human clinical trial, the rSIV.F/HN vector carrying a codon-optimized and CpG-depleted CFTR (soCFTR2) cDNA under the control of a CpG-free hybrid promoter (hCEF) (rSIV.F/HN-hCEF-soCFTR2) has been assessed in key translational preclinical studies (70). Codon optimization allows for enhanced translation, thus increasing CFTR transgene expression. CpG motifs within plasmid DNA have been shown to induce acute inflammatory responses when delivered to the lungs of CF animal models and people with CF (80). As such, the use of CpG-depleted cDNA has previously resulted in a reduction in host inflammatory response (80). Preclinical data have shown that codon optimization and CpG depletion of CFTR cDNA does not alter the structure and function of CFTR in cell culture and can lead to persistent CFTR expression in vivo in mice (80). In these translational preclinical studies, rSIV.F/HN-hCEF-soCFTR2 led to expression of functional chloride channels in vitro as assessed by the iodide efflux assay (70). Furthermore, when transduced into the nasal epithelium of CF knockout mice, significant amounts of vector-specific mRNA were detectable after both 1 and 4 weeks after transduction (70). In addition, rSIV.F/HN-hCEF-soCFTR2 transduction resulted in a significant increase in CF intestinal organoid swelling (a specific indicator of CFTR channel activity) compared with nontransduced controls, suggesting partial restoration of channel activity (70). Together, these preclinical results support the progression of the vector into a first-in-human clinical trial.
Introduction to the First-in-Human Trial of BI 3720931 (Lenticlair™ 1)
rSIV.F/HN-hCEF-soCFTR2 is now being developed as BI 3720931 in a first-in-human phase I/II trial (81). This trial will investigate safety, tolerability, and efficacy and is expected to begin in 2024. Adult males and females of nonchildbearing potential with a clinical diagnosis of CF who are genetically ineligible for CFTR modulator therapy will be recruited into the trial.
Phase I is an open-label dose-escalation trial (minimum of n ≥ 9). Participants will receive a single low, medium, or high dose of orally inhaled, nebulized BI 3720931 (1:1:1) plus standard of care, with 24 weeks of follow-up. The primary endpoint is the occurrence of any drug-related treatment-emergent adverse event (AE) within 24 weeks. Secondary endpoints are occurrence of a treatment response, defined as a change from baseline ≥5% ppFEV1 within 8 weeks after dosing, absolute change from baseline in ppFEV1 at Week 24 after dosing, and occurrence of dose-limiting toxicity up to Week 24 after dosing. Interim safety and efficacy results from a minimum of 8 weeks after dosing will inform dose selection for phase II.
Phase II (n = 27) is a randomized, double-blind, placebo-controlled, dose-expansion trial with a 4-week screening period, followed by randomization to a single dose of nebulized BI 3720931 (one of two dose strengths) or placebo plus standard of care (1:1:1) with 24 weeks of follow-up. The primary endpoint is absolute change from baseline in ppFEV1 at Week 8 after dosing. Secondary endpoints include absolute change from baseline in ppFEV1 and occurrence of serious AEs and drug-related treatment-emergent AEs up to Week 24 after dosing.
After trial participation, all participants will be asked to enter a separate extension trial for 15 years of follow-up (in accordance with regulatory guidance from the U.S. Food and Drug Administration) to investigate any potential delayed AEs (Lenticlair™-ON) (82). Participants may enter other investigational trials during this time pending eligibility criteria of these other trials.
Conclusions
Despite the unprecedented improvements in CF clinical care due to the introduction of CFTR modulator therapies, a need for disease-modifying treatments still exists for people with CF who are ineligible for, or intolerant of, these therapies. Various genetic approaches, including gene addition therapy, provide opportunities to address this unmet need. Lentiviral vectors have been successful for several indications and hold promise for CF gene therapy because of their long duration of gene expression, high packaging capacity, noninflammatory nature, and ability to transduce both dividing and nondividing cells. The rSIV.F/HN lentiviral vector has shown favorable preclinical safety and efficacy results, with a first-in-human trial expected to start in 2024. This approach has the potential to address the unmet need in CF clinical care and help to close the widening health outcome gap between modulator-treated and non–modulator-treated people with CF.
Footnotes
Supported by Boehringer Ingelheim International GmbH; the Wellcome Trust Health Innovation Fund, the National Institutes of Health and Care Research through the Imperial Biomedical Research Centre, the Royal Brompton Clinical Research Facility, and Senior Investigator Awards (E.W.F.W.A. and J.C.D.); the Cystic Fibrosis Foundation (D.P.); and German Federal Ministry of Education and Research grant 82DZL009B1 and German Research Foundation grant CRC 1449 – project 431232613 (M.A.M.). The authors did not receive payment related to the development of the manuscript. Laura Cottino, Ph.D., of Nucleus Global, provided writing, editorial support, and formatting assistance, which was contracted and funded by Boehringer Ingelheim. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Author Contributions: All authors contributed to the conceptualization, writing, and review of the manuscript. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors.
Originally Published in Press as DOI: 10.1164/rccm.202402-0389CI on September 5, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Shteinberg M, Haq IJ, Polineni D, Davies JC. Cystic fibrosis. Lancet . 2021;397:2195–2211. doi: 10.1016/S0140-6736(20)32542-3. [DOI] [PubMed] [Google Scholar]
- 2.Ong T, Ramsey BW. Cystic fibrosis: a review. JAMA. 2023;329:1859–1871. doi: 10.1001/jama.2023.8120. [DOI] [PubMed] [Google Scholar]
- 3. Guo J, Garratt A, Hill A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J Cyst Fibros . 2022;21:456–462. doi: 10.1016/j.jcf.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 4. Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med . 2020;8:65–124. doi: 10.1016/S2213-2600(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science . 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 6. Mall M, Hipper A, Greger R, Kunzelmann K. Wild type but not deltaF508 CFTR inhibits Na+ conductance when coexpressed in Xenopus oocytes. FEBS Lett . 1996;381:47–52. doi: 10.1016/0014-5793(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 7. Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med . 2020;201:1193–1208. doi: 10.1164/rccm.201910-1943SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shteinberg M, Taylor-Cousar JL, Durieu I, Cohen-Cymberknoh M. Fertility and pregnancy in cystic fibrosis. Chest . 2021;160:2051–2060. doi: 10.1016/j.chest.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Functional Translation of CFTR 2. CFTR2 variant list history. 2023. https://cftr2.org/mutations_history
- 10.Cystic Fibrosis Mutation Database. CFMDB statistics. 2011. http://genet.sickkids.on.ca/StatisticsPage.html
- 11. Taylor-Cousar JL, Robinson PD, Shteinberg M, Downey DG. CFTR modulator therapy: transforming the landscape of clinical care in cystic fibrosis. Lancet . 2023;402:1171–1184. doi: 10.1016/S0140-6736(23)01609-4. [DOI] [PubMed] [Google Scholar]
- 12. Hisert KB, Birket SE, Clancy JP, Downey DG, Engelhardt JF, Fajac I, et al. Understanding and addressing the needs of people with cystic fibrosis in the era of CFTR modulator therapy. Lancet Respir Med . 2023;11:916–931. doi: 10.1016/S2213-2600(23)00324-7. [DOI] [PubMed] [Google Scholar]
- 13. De Boeck K. Cystic fibrosis in the year 2020: a disease with a new face. Acta Paediatr . 2020;109:893–899. doi: 10.1111/apa.15155. [DOI] [PubMed] [Google Scholar]
- 14. Taylor-Cousar JL, Mall MA, Ramsey BW, McKone EF, Tullis E, Marigowda G, et al. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res . 2019;5:00082-2019. doi: 10.1183/23120541.00082-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graeber SY, Vitzthum C, Pallenberg ST, Naehrlich L, Stahl M, Rohrbach A, et al. Effects of elexacaftor/tezacaftor/ivacaftor therapy on CFTR function in patients with cystic fibrosis and one or two F508del alleles. Am J Respir Crit Care Med . 2022;205:540–549. doi: 10.1164/rccm.202110-2249OC. [DOI] [PubMed] [Google Scholar]
- 16. Caley LR, Jarosz-Griffiths HH, Smith L, Gale L, Barrett J, Kinsey L, et al. Body mass index and nutritional intake following elexacaftor/tezacaftor/ivacaftor modulator therapy in adults with cystic fibrosis. J Cyst Fibros . 2023;22:1002–1009. doi: 10.1016/j.jcf.2023.06.010. [DOI] [PubMed] [Google Scholar]
- 17. Gruber W, Welsner M, Blosch C, Dillenhoefer S, Olivier M, Brinkmann F, et al. Long-term follow-up of health-related quality of life and short-term intervention with CFTR modulator therapy in adults with cystic fibrosis: evaluation of changes over several years with or without 33 weeks of CFTR modulator therapy. Healthcare (Basel) . 2023;11:2873. doi: 10.3390/healthcare11212873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sui H, Xu X, Su Y, Gong Z, Yao M, Liu X, et al. Gene therapy for cystic fibrosis: challenges and prospects. Front Pharmacol . 2022;13:1015926. doi: 10.3389/fphar.2022.1015926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alton E, Boyd AC, Davies JC, Gill DR, Griesenbach U, Harman TE, et al. Gene therapy for respiratory diseases: progress and a changing context. Hum Gene Ther . 2020;31:911–916. doi: 10.1089/hum.2020.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graeber SY, Mall MA. The future of cystic fibrosis treatment: from disease mechanisms to novel therapeutic approaches. Lancet . 2023;402:1185–1198. doi: 10.1016/S0140-6736(23)01608-2. [DOI] [PubMed] [Google Scholar]
- 21. Allen L, Allen L, Carr SB, Davies G, Downey D, Egan M, et al. Future therapies for cystic fibrosis. Nat Commun . 2023;14:693. doi: 10.1038/s41467-023-36244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim YJ, Krainer AR. Antisense oligonucleotide therapeutics for cystic fibrosis: recent developments and perspectives. Mol Cells . 2023;46:10–20. doi: 10.14348/molcells.2023.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson JJ, Mao Y, White TR, Jr, Foye C, Oliver KE. Features of CFTR mRNA and implications for therapeutics development. Front Genet . 2023;14:1166529. doi: 10.3389/fgene.2023.1166529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cystic Fibrosis Foundation. Bethesda, MD: 2022. Patient registry annual data report.https://www.cff.org/sites/default/files/2021-11/Patient-Registry-Annual-Data-Report.pdf [Google Scholar]
- 25. McGarry ME, Gibb ER, Oates GR, Schechter MS. Left behind: the potential impact of CFTR modulators on racial and ethnic disparities in cystic fibrosis. Paediatr Respir Rev . 2022;42:35–42. doi: 10.1016/j.prrv.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGarry ME, McColley SA. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol . 2021;56:1496–1503. doi: 10.1002/ppul.25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kramer-Golinkoff E, Camacho A, Kramer L, Taylor-Cousar JL. A survey: understanding the health and perspectives of people with CF not benefiting from CFTR modulators. Pediatr Pulmonol . 2022;57:1253–1261. doi: 10.1002/ppul.25859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jansen AME, Eggermont MN, Wilms EB, Aziz S, Reijers M, Roukema J, et al. Evaluation of the drug-drug interaction between triazole antifungals and cystic fibrosis transmembrane conductance regulator modulators in a real-life cohort. Med Mycol . 2024;62:myae020. doi: 10.1093/mmy/myae020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egan ME. Non-modulator therapies: developing a therapy for every cystic fibrosis patient. Clin Chest Med . 2022;43:717–725. doi: 10.1016/j.ccm.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 30. Allaire NE, Griesenbach U, Kerem B, Lueck JD, Stanleigh N, Oren YS. Gene, RNA, and ASO-based therapeutic approaches in cystic fibrosis. J Cyst Fibros . 2023;22:S39–S44. doi: 10.1016/j.jcf.2022.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor-Cousar JL, Boyd AC, Alton E, Polineni D. Genetic therapies in cystic fibrosis. Curr Opin Pulm Med . 2023;29:615–620. doi: 10.1097/MCP.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 32.ReCode Therapeutics. ReCode therapeutics announces first participants dosed in a phase 1 healthy volunteer clinical study of inhaled mRNA-based genetic medicine, RCT2100, for the treatment of cystic fibrosis. 2024. https://recodetx.com/recode-therapeutics-announces-first-participants-dosed-in-a-phase-1-healthy-volunteer-clinical-study-of-inhaled-mrna-based-genetic-medicine-rct2100-for-the-treatment-of-cystic-fibrosis/
- 33.ClinicalTrials.gov. A phase 1 study evaluating safety and tolerability of RCT2100 in healthy participants. 2024. https://classic.clinicaltrials.gov/ct2/show/NCT06237335
- 34.ClinicalTrials.gov. Safety, tolerability, and pharmacokinetics of ARCT-032 in healthy adult subjects. 2023. https://classic.clinicaltrials.gov/ct2/show/NCT05712538
- 35.ClinicalTrials.gov. A phase 1/2 study of VX-522 in participants with cystic fibrosis (CF) 2022. https://classic.clinicaltrials.gov/ct2/show/NCT05668741
- 36. McLachlan G, Alton E, Boyd AC, Clarke NK, Davies JC, Gill DR, et al. Progress in respiratory gene therapy. Hum Gene Ther . 2022;33:893–912. doi: 10.1089/hum.2022.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kreda SM. Oligonucleotide-based therapies for cystic fibrosis. Curr Opin Pharmacol . 2022;66:102271. doi: 10.1016/j.coph.2022.102271. [DOI] [PubMed] [Google Scholar]
- 38.SpliSense. SpliSense successfully completed phase 1 study of SPL84, RNA-based therapy, for the treatment of cystic fibrosis. 2023. https://www.prnewswire.com/news-releases/splisense-successfully-completed-phase-1-study-of-spl84-rna-based-therapy-for-the-treatment-of-cystic-fibrosis-301918897.html?xd_co_f=NjAxNDI1MTAtMzczYy00YWExLTkzZmQtYmI5YjI0OTA2YTU2#:~:text=JERUSALEM%2C%20Sept.%206%2C%202023,a%20first%2Din%2Dhuman%2C
- 39. Wang G. Genome editing for cystic fibrosis. Cells . 2023;12:1555. doi: 10.3390/cells12121555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther . 2021;6:53. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zu H, Gao D. Non-viral vectors in gene therapy: recent development, challenges, and prospects. AAPS J . 2021;23:78. doi: 10.1208/s12248-021-00608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alton E, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, et al. UK Cystic Fibrosis Gene Therapy Consortium Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med . 2015;3:684–691. doi: 10.1016/S2213-2600(15)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Turuvekere Vittala Murthy N, Vlasova K, Renner J, Jozic A, Sahay G. A new era of targeting cystic fibrosis with non-viral delivery of genomic medicines. Adv Drug Deliv Rev . 2024;209:115305. doi: 10.1016/j.addr.2024.115305. [DOI] [PubMed] [Google Scholar]
- 44.Cystic Fibrosis News Today. CF Foundation invests up to $2M in Nanite’s gene therapy approach. 2023. https://cysticfibrosisnewstoday.com/news/cf-foundation-invests-2m-nanites-gene-therapy-delivery-method/
- 45.Carmine Therapeutics. Carmine Therapeutics announces first close of series A to develop non-viral gene therapy. 2022. https://www.carminetherapeutics.com/post/carmine-therapeutics-announces-first-close-of-series-a-to-develop-non-viral-gene-therapy
- 46.ViaNautis. ViaNautis Bio announces $25 million series A financing to drive the next generation of genetic nanomedicines. 2023. https://vianautis.com/vianautis-bio-announces-25-million-series-a-financing-to-drive-the-next-generation-of-genetic-nanomedicines/
- 47. O’Neal WK, Zhou H, Morral N, Langston C, Parks RJ, Graham FL, et al. Toxicity associated with repeated administration of first-generation adenovirus vectors does not occur with a helper-dependent vector. Mol Med . 2000;6:179–195. [PMC free article] [PubMed] [Google Scholar]
- 48. Dalwadi DA, Calabria A, Tiyaboonchai A, Posey J, Naugler WE, Montini E, et al. AAV integration in human hepatocytes. Mol Ther . 2021;29:2898–2909. doi: 10.1016/j.ymthe.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ertl HCJ. Immunogenicity and toxicity of AAV gene therapy. Front Immunol . 2022;13:975803. doi: 10.3389/fimmu.2022.975803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol Ther . 2020;28:723–746. doi: 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muhuri M, Maeda Y, Ma H, Ram S, Fitzgerald KA, Tai PW, et al. Overcoming innate immune barriers that impede AAV gene therapy vectors. J Clin Invest . 2021;131:e143780. doi: 10.1172/JCI143780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood . 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ClinicalTrials.gov. 4D-710 in adult patients with cystic fibrosis (CF) 2022. https://classic.clinicaltrials.gov/ct2/show/NCT05248230
- 54. Ostedgaard LS, Zabner J, Vermeer DW, Rokhlina T, Karp PH, Stecenko AA, et al. CFTR with a partially deleted R domain corrects the cystic fibrosis chloride transport defect in human airway epithelia in vitro and in mouse nasal mucosa in vivo. Proc Natl Acad Sci U S A . 2002;99:3093–3098. doi: 10.1073/pnas.261714599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fakhiri J, Schneider MA, Puschhof J, Stanifer M, Schildgen V, Holderbach S, et al. Novel chimeric gene therapy vectors based on adeno-associated virus and four different mammalian bocaviruses. Mol Ther Methods Clin Dev . 2019;12:202–222. doi: 10.1016/j.omtm.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yan Z, Zou W, Feng Z, Shen W, Park SY, Deng X, et al. Establishment of a high-yield recombinant adeno-associated virus/human bocavirus vector production system independent of bocavirus nonstructural proteins. Hum Gene Ther . 2019;30:556–570. doi: 10.1089/hum.2018.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ClinicalTrials.gov. A study assessing KB407 for the treatment of cystic fibrosis. 2022. https://classic.clinicaltrials.gov/ct2/show/NCT05504837
- 58.Krystal Biotech. Krystal Biotech announces FDA acceptance of KB407 IND application for cystic fibrosis clinical trial. 2022. https://ir.krystalbio.com/news-releases/news-release-details/krystal-biotech-announces-fda-acceptance-kb407-ind-application
- 59. Ku M-W, Charneau P, Majlessi L. Use of lentiviral vectors in vaccination. Expert Rev Vaccines . 2021;20:1571–1586. doi: 10.1080/14760584.2021.1988854. [DOI] [PubMed] [Google Scholar]
- 60. Sinn PL, Arias AC, Brogden KA, McCray PB. Lentivirus vector can be readministered to nasal epithelia without blocking immune responses. J Virol . 2008;82:10684–10692. doi: 10.1128/JVI.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gutierrez-Guerrero A, Cosset FL, Verhoeyen E. Lentiviral vector pseudotypes: precious tools to improve gene modification of hematopoietic cells for research and gene therapy. Viruses . 2020;12:1016. doi: 10.3390/v12091016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Farrow N, Cmielewski P, Delhove J, Rout-Pitt N, Vaughan L, Kuchel T, et al. Towards human translation of lentiviral airway gene delivery for cystic fibrosis: a one-month CFTR and reporter gene study in marmosets. Hum Gene Ther . 2021;32:806–816. doi: 10.1089/hum.2020.267. [DOI] [PubMed] [Google Scholar]
- 63. Cooney AL, Abou Alaiwa MH, Shah VS, Bouzek DC, Stroik MR, Powers LS, et al. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight . 2016;1:e88730. doi: 10.1172/jci.insight.88730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cooney AL, Thurman AL, McCray PB, Jr, Pezzulo AA, Sinn PL. Lentiviral vectors transduce lung stem cells without disrupting plasticity. Mol Ther Nucleic Acids . 2021;25:293–301. doi: 10.1016/j.omtn.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gene therapy needs a long-term approach. Nat Med . 2021;27:563. doi: 10.1038/s41591-021-01333-6. [DOI] [PubMed] [Google Scholar]
- 66. David RM, Doherty AT. Viral vectors: the road to reducing genotoxicity. Toxicol Sci . 2017;155:315–325. doi: 10.1093/toxsci/kfw220. [DOI] [PubMed] [Google Scholar]
- 67. Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia . 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol . 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miyoshi H, Blömer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol . 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alton EW, Beekman JM, Boyd AC, Brand J, Carlon MS, Connolly MM, et al. Preparation for a first-in-man lentivirus trial in patients with cystic fibrosis. Thorax . 2017;72:137–147. doi: 10.1136/thoraxjnl-2016-208406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tucci F, Galimberti S, Naldini L, Valsecchi MG, Aiuti A. A systematic review and meta-analysis of gene therapy with hematopoietic stem and progenitor cells for monogenic disorders. Nat Commun . 2022;13:1315. doi: 10.1038/s41467-022-28762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.U.S. Food & Drug Administration. Cellular, Tissue, and Gene Therapies Advisory Committee June 9-10, 2022 meeting announcement. 2022. https://www.fda.gov/advisory-committees/advisory-committee-calendar/cellular-tissue-and-gene-therapies-advisory-committee-june-9-10-2022-meeting-announcement-06092022
- 73. Schambach A, Zychlinski D, Ehrnstroem B, Baum C. Biosafety features of lentiviral vectors. Hum Gene Ther . 2013;24:132–142. doi: 10.1089/hum.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol Ther Methods Clin Dev . 2016;3:16017. doi: 10.1038/mtm.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tareen SU, Nicolai CJ, Campbell DJ, Flynn PA, Slough MM, Vin CD, et al. A Rev-independent gag/pol eliminates detectable psi-gag recombination in lentiviral vectors. Biores Open Access . 2013;2:421–430. doi: 10.1089/biores.2013.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mitomo K, Griesenbach U, Inoue M, Somerton L, Meng C, Akiba E, et al. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes. Mol Ther . 2010;18:1173–1182. doi: 10.1038/mt.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Munday RJ, Coradin T, Nimmo R, Lad Y, Hyde SC, Mitrophanos K, et al. Sendai F/HN pseudotyped lentiviral vector transduces human ciliated and non-ciliated airway cells using α 2,3 sialylated receptors. Mol Ther Methods Clin Dev . 2022;26:239–252. doi: 10.1016/j.omtm.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Griesenbach U, Inoue M, Meng C, Farley R, Chan M, Newman NK, et al. Assessment of F/HN-pseudotyped lentivirus as a clinically relevant vector for lung gene therapy. Am J Respir Crit Care Med . 2012;186:846–856. doi: 10.1164/rccm.201206-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Griesenbach U, McLachlan G, Sinadinos A, Cheminay C, Ashour J, Meng C, et al. F/HN pseudotyped lentiviral vector-mediated transduction of non-human primates [abstract] J Cyst Fibros . 2023;22:WS17.06. [Google Scholar]
- 80. Varathalingam A, Lawton AE, Munkonge FM, Chan M, Pringle IA, Greisenbach U, et al. Novel CPG-depleted and codon-optimised CFTR CDNAs maintain the structure and function of CFTR protein Pediatr Pulmonol 2005. 40 275 16037925 [Google Scholar]
- 81. Davies JC, Mall MA, Polineni D, Donaldson SH, Fajac I, Jain R, et al. Lenticlair 1: a phase 1/2 trial evaluating the safety, tolerability and efficacy of an inhaled F/HN-pseudotyped lentiviral vector for CF gene therapy in people with CF ineligible for CFTR modulators. J Cyst Fibros . 2024;23:P045. [Google Scholar]
- 82. Davies JC, Mall MA, Polineni D, Donaldson SH, Fajac I, Jain R, et al. Lenticlair-ON: an extension trial examining long-term safety and efficacy outcomes associated with an inhaled F/HN-pseudotyped lentiviral vector for CF gene therapy in people with CF. J Cyst Fibros . 2024;23:P046. [Google Scholar]
- 83.Pharmaceutical Technology. Bluebird turns heads with high pricing of gene therapy Zynteglo in Europe. 2019. https://www.pharmaceutical-technology.com/analyst-comment/zynteglo-gene-therapy-2019/?cf-view
- 84.Genetic Engineering & Biotechnology News. FDA approves Bluebird Bio’s lentiviral gene therapy to treat beta-thalassemia. 2022. https://www.genengnews.com/news/fda-approves-bluebird-bios-lentiviral-gene-therapy-to-treat-beta-thalassemia/
- 85.European Medicines Agency. New gene therapy to treat rare genetic disorder metachromatic leukodystrophy. 2020. https://www.ema.europa.eu/en/news/new-gene-therapy-treat-rare-genetic-disorder-metachromatic-leukodystrophy
- 86.U.S. Food & Drug Administration. FDA approves first gene therapy for children with metachromatic leukodystrophy. 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-children-metachromatic-leukodystrophy
- 87.European Medicines Agency. First gene therapy to treat children with rare inherited neurological disease. 2021. https://www.ema.europa.eu/en/news/first-gene-therapy-treat-children-rare-inherited-neurological-disease
- 88.Kiem HP.FDA approves SKYSONA, second lentiviral vector gene therapy in the US. 2022. https://asgct.org/publications/news/september-2022/eli-cel-second-lentiviral-vector-gene-therapy
- 89.U.S. Food & Drug Administration. FDA approves first gene therapies to treat patients with sickle cell disease. 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease
- 90.U.S. Food & Drug Administration. FDA approval brings first gene therapy to the United States. 2017. https://www.fda.gov/news-events/press-announcements/fda-approval-brings-first-gene-therapy-united-states
- 91.Novartis. Novartis Kymriah receives EC approval as first CAR-T cell therapy for adults with relapsed or refractory follicular lymphoma. 2022. https://www.novartis.com/news/media-releases/novartis-kymriah-receives-ec-approval-first-car-t-cell-therapy-adults-relapsed-or-refractory-follicular-lymphoma
- 92.U.S. Food & Drug Administration. FDA approves new treatment for adults with relapsed or refractory large-B-cell lymphoma. 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-adults-relapsed-or-refractory-large-b-cell-lymphoma
- 93.Bristol Myers Squibb. Bristol Myers Squibb receives European Commission approval for CAR T cell therapy Breyanzi (lisocabtagene maraleucel) for certain forms of relapsed or refractory large B-cell lymphoma. 2022. https://news.bms.com/news/details/2022/Bristol-Myers-Squibb-Receives-European-Commission-Approval-for-CAR-T-Cell-Therapy-Breyanzi-lisocabtagene-maraleucel-for-Certain-Forms-of-Relapsed-or-Refractory-Large-B-cell-Lymphoma/default.aspx
- 94.European Medicines Agency. Zynteglo. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/zynteglo
- 95.European Medicines Agency. Syksona. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/skysona