Abstract
Objective
This study aims to enhance understanding of necrotizing pneumonia and toxic shock syndrome by analyzing an adult case of community-acquired necrotizing pneumonia caused by co-infection of Influenza A (H1N1) and Staphylococcus aureus with LukS-PV and LukF-PV virulence factor genes.
Method
The clinical data of one patient admitted to the intensive care unit (ICU) with co-infection of Influenza A (H1N1) and Staphylococcus aureus was retrospectively analyzed.
Results
The patient exhibited typical clinical manifestations of viral and Staphylococcus aureus co-infection, including necrotizing pneumonia and toxic shock syndrome. The presence of LukS-PV and LukF-PV virulence factor genes of Staphylococcus aureus was detected in the patient's bronchoalveolar lavage fluid. Unfortunately,although antiviral agents (oseltamivir) and antibiotics (linezolid, imipenem-cilastatin) were timely administrated, as well as corticosteroids for anti-inflammatory purposes, the patient's condition was progressively deteriorated and eventually led to death.
Conclusion
Clinical practitioners should be vigilant about the co-infection of Influenza virus and Staphylococcus aureus, particularly when the latter carries virulence factors. The presence of virulence factor genes of Staphylococcus aureus can lead to necrotizing pneumonia with a poor prognosis. This is a particular concern because both infections can be life threatening in young adults.
Keywords: Panton–Valentine leukocidin (PVL)-producing Staphylococcus aureus, Necrotizing pneumonia, Pneumonia, Influenza A virus (H1N1), Co-infection, LukF and LukS genes, Toxic shock syndrome
Introduction
Necrotizing pneumonia, a rare yet severe complication of community-acquired pneumonia (CAP), is characterized by the liquefaction and formation of cavities in lung tissue due to infectious pathogens. Despite accounts for approximately 4 % of CAP cases, its impact is profound [1]. The hallmarks of necrotizing pneumonia include sudden onset, rapid symptom deterioration, airway bleeding, severe respiratory failure, and a high mortality rate. While predominantly afflicting children, fewer cases are reported in adults [2].
The primary pathogens associated with necrotizing pneumonia include Streptococcus pneumoniae, Staphylococcus aureus, and Klebsiella pneumoniae [1]. Notably, strains of Staphylococcus aureus that produce the Panton-Valentine leukocidin (PVL) toxin can lead to rapidly progressive and highly fatal necrotizing pneumonia [3]. In instances where viral infections coexist, their synergistic interaction with bacterial agents further accelerates disease progression [4].
In this report, we present a case of a previously healthy 39-year-old female who succumbed to necrotizing pneumonia despite early and accurate diagnosis and appropriate treatment. The tragic outcome underscores the critical need for vigilance and timely intervention in managing this formidable condition.
Case presentation
In this clinical vignette, we introduce a 39-year-old female with an unremarkable medical history who presented with a constellation of symptoms. She reported experiencing fatigue,headaches, myalgia, and fever, which manifested shortly after her return from a trip to Thailand. Despite self-administering "Paracetamol and Caffeine Tablets" and "Honeysuckle Granules", her symptoms persisted. Subsequently she developed a sore throat, hyperpyrexia cough, white sputum streaked with blood,progressively worsening shortness of breath and palpitations.
Due to the exacerbation of her symptoms, he was admitted to the intensive care unit (ICU) for immediate treatments.Upon admission of the patient, her vital signs were recorded as follows: body temperature of 35.5 °C, heart rate 170 beats per minute with no murmurs detected, respiratory rate of 50 breaths per minute with wet rales in both lower lungs, blood pressure of 143/111 mmHg, and a Glasgow Coma Scale (GCS) score of 13. The capillary refill time was 5 s,and a Symmetrical red rash was observed on the trunk.
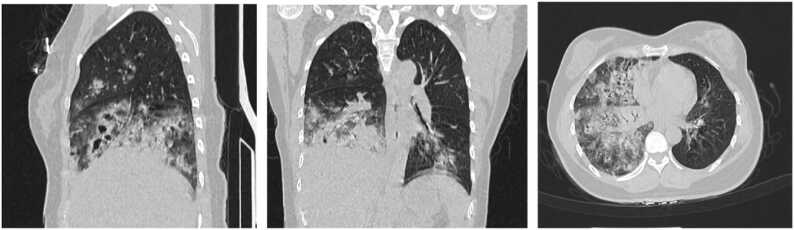
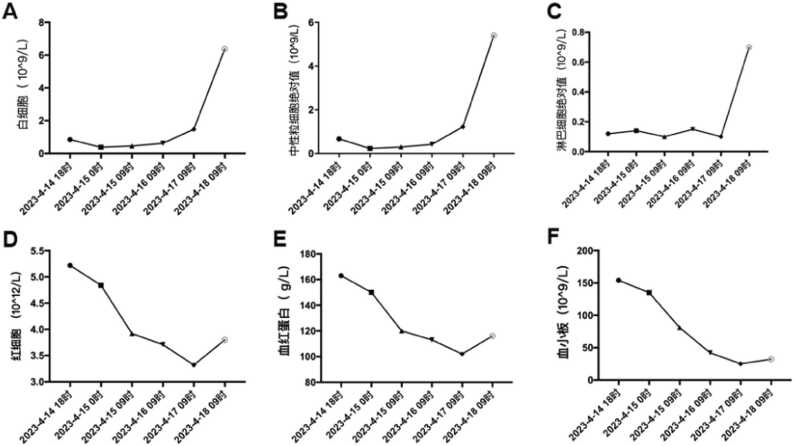
A chest CT scan revealed patchy and nodular opacities, some of which exhibited cavitation, in the right and left lower lobes of the lungs,the detailed performance is shown in Fig. 1. Laboratory findings upon admission are summarized in Table 1 for extreme values and detailed changes are depicted in Fig. 2. Tests for HIV antibodies, hepatitis A, B, and C antibodies, tuberculosis antibodies (PPD), tuberculosis DNA, pneumonia-associated antibodies, and fungal D-glucan tests were all negative. No obvious abnormalities were observed in the head CT scan.
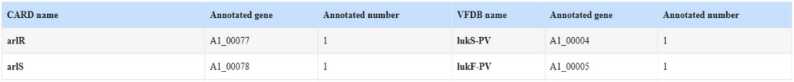
Fig. 3.
Annotation results from the CARD (Comprehensive Antibiotic Resistance Database) and VFDB (Virulence Factor Database) databases.
Fig. 1.
Chest CT shows patchy and nodular opacities with consolidation in the right lung and left lower lobe. Some lesions exhibit cavitations.
Table 1.
Important indicators before patient admission and death.
| The first day of hospitalization | Day of death | Normal range | |
|---|---|---|---|
| Leukocyte(10^9/L) | 0.85 | 6.37 | 3.50 −9.50 |
| Absolute value of lymphocyte(10^9/L) | 0.12 | 0.7 | 0.80 −4.00 |
| Erythrocyte(10^12/L) | 5.22 | 3.8 | 3.80 −5.10 |
| Hemoglobin(g/L) | 163 | 116 | 110 −150 |
| Platelet(10^9/L) | 154 | 32 | 125 −350 |
| C-reactive protein(mg/L) | 353.70 | 554 | ≤ 6.00 |
| IL−6(flow cytometry)(pg/mL) | >10000 | >10000 | 0.373 −0.463 |
| Erythrocyte Sedimentation Rate(ESR)(mm/h) | 91 | 92 | 0 −20 |
| Procalcitonin(PCT)(ng/mL) | >200 | >200 | <0.05 |
| Blood Lactic Acid(mmol/L) | 5.20 | 13.40 | 0.1 −1.0 |
| Prothrombin time(PT)(second) | 14.90 | 14.50 | 11.0 −14.3 |
| Activated partial thromboplastin time(APTT)(second) | 35.90 | 45.50 | 32.00 −43.00 |
| Fibrinogen(g/L) | 7.04 | 6.70 | 2.00 −4.00 |
| Thrombin time(TT)(seconf) | 16.30 | 17.60 | <21.00 |
Fig. 2.
Changes in the absolute values of white blood cells, neutrophils, lymphocytes, red blood cells, hemoglobin, and platelets of the patient.
She underwent a 1-hour bundle therapy for sepsis upon admission, tracheal intubation and mechanical ventilation, analgesia, and sedation. Empirical treatment consisted of antiviral therapy with oseltamivir, antibiotics including imipenem and moxifloxacin, and anti-inflammatory therapy with dexamethasone. Fresh frozen plasma was administered to correct coagulation disorders. The patient's disease is poorly controlled, the infection progressively worsens, progresses to multiple organ dysfunction syndrome(MODS), and persists in exacerbations, necessitating the initiation of continuous renal replacement therapy.
At the same time, Fiberoptic bronchoscopy examination at the bedside revealed congested and swollen mucosa in the right bronchus, with bloody secretions containing necrotic tissue visible within the bronchus. Respiratory tract specimen culture and metagenomic next-generation sequencing (mNGS) revealed positive results for influenza A virus (H1N1) and Staphylococcus aureus with the D+ β-LAC(+) phenotype, indicating coinfection with H1N1 influenza virus and Staphylococcus aureus. he anti-infective regimen was adjusted to imipenem plus linezolid based on susceptibility results.
On the second day in the ICU, the patient's complete blood count revealed a sharp decrease. Bone marrow biopsy results indicated: 1) hyperplasia of the granulocytic system with toxic changes and poor maturation;2) decreased proliferation of erythroid system;and 3) hyperplasia of the megakaryocytic system with poor maturation. Unfortunately she showed no response to blood products transfusion. Tragically, on the fourth day of admission, the patient's condition deteriorated further and did not respond to treatment,the patient succumbed to her illness.
The patient's bronchoalveolar lavage fluid underwent DNA extraction and quality inspection. DNA samples tmeeting quality standards were then utilized for library preparation and high-throughput sequencing (HTS) using High Throughput Sequencing technology. Additionally, conventional gel PCR was employed to detect the lukF and lukS genes, which constitute the two-component toxin known as Panton-Valentine leukocidin (PVL) [5].
Discussion
In this case, we encountered a young woman with no prior history of similar infections or compromised immune system. Within 48 h of admission, both her blood and bronchoalveolar lavage fluid (BALF) samples tested positive for influenza A virus H1N1 and Staphylococcus aureus with the D+ β-LAC(+) phenotype. The pro-inflammatory effect of the influenza virus in the lungs is well-documented. It triggers the release of cytokines and leads to the infiltration of immune cells (including neutrophils, monocytes, and macrophages) into lung tissue [4]. While cytokine response is crucial for virus clearance during influenza pneumonia, excessive and dysregulated cytokine release—commonly referred to as a "cytokine storm"—is closely linked to the incidence and mortality of influenza-related diseases [4], [6].
Specific strains of Staphylococcus aureus can produce an array of exotoxins, including Panton-Valentine leukocidin (PVL), exfoliative toxins, enterotoxins, and hemolysins, rendering them highly virulent [7]. Notably, PVL, composed of the lukF and lukS genes and detectable via conventional gel PCR, activates macrophages, neutrophils, and monocytes, leading to uncontrolled cell death. The dying cells release copious proteases and other active compounds, which spill into surrounding tissues, inducing damage [8]. Neutrophils harbor significant serine protease stores in vesicles, which are uncontrollably released during PVL-induced death of neighboring tissue cells. PVL can cause massive lysis of recruited immune cells, ultimately leading to necrosis of lung tissue [6]. Specifically, in lung tissue, inadequate protease inhibitor activity in the alveolar space can result in substantial lung tissue destruction, culminating in necrotizing pneumonia [9]. In cases where the lungs are repeatedly infected and heavily infiltrated by immune cells, the presence of Staphylococcus aureus strains producing the Panton-Valentine leukocidin (PVL) becomes critical.
During the viral infection, IFN-I produced by T cells disrupted tissue defense against bacteria,inhibited the clearance of alveolar macrophages and impairing the natural killer cell response, thereby compromising the immune system and promoting secondary pulmonary infections [10]. Simultaneously, the expression of pro-apoptotic proteins by the H1N1 influenza virus further heightened the host's susceptibility to Staphylococcus aureus infection [11]. Conversely, viral infection rapidly upregulated the expression of Staphylococcus aureus virulence factors, intensifying the pro-inflammatory and cytotoxic effects of PVL on neutrophils [11], [12].
Thus, the concurrent infection of influenza A virus H1N1 and Staphylococcus aureus and PVLmay be a key factor contributing to the rapid progression of the patient's condition [13]. This complex interplay of viral and bacterial factors underscores the challenges in managing severe respiratory infections and highlights the importance of timely intervention and vigilant monitoring [14]. More importantly, this toxin-producing strain of Staphylococcus aureus is relatively uncommon in mainland China,suggesting a possible link to the patient's travel history to Thailand.
Other than that,the patient's physical examination revealed cool and cyanotic extremities, along with a symmetrical rash on the trunk. Given the clinical presentation and relevant examination results, toxic shock syndrome (TSS) was considered as a possible diagnosis. Clinical manifestations of TSS encompass fever/chills, hypotension (which may include orthostatic hypotension or fainting), diffuse erythematous rash (peeling of the skin may occur 1–2 weeks later), and involvement of multiple organ systems [15], [16]. Systemic involvement can manifest as gastrointestinal symptoms (abdominal pain, vomiting, and/or watery diarrhea), myalgia, mucosal involvement (vaginal, oral-pharyngeal, or conjunctival), renal involvement, hepatic involvement, thrombocytopenia, and neurological symptoms (headache, somnolence, altered consciousness, irritability, restlessness, and hallucinations) [16]. The symptoms and signs of TSS can rapidly develop within 48 h and often occur in previously healthy individuals. The major causative factors of TSS are the heat-stable exotoxins C and F produced by TSS-related Staphylococcus aureus, now confirmed to be the same substance and collectively referred to as TSST-1 [17]. TSST-1 can cause fever, desquamative rash, and shock, and it increases sensitivity to endotoxins. Infections with toxin-producing strains can lead to multiple organ system dysfunctions in the body, resulting in a high mortality rate [16]. Therefore, we suspect that another reason for the rapid progression of the patient's condition is the production of TSST-1 toxin by the infecting Staphylococcus aureus. TSST-1 acts as a superantigen that stimulates immune cells, bypassing the normal MHC-restricted antigen processing, leading to an "inflammatory cytokine storm". This could explain why the patient experienced such severe inflammation and rapid progression. Unfortunately, the detection of TSST-1 toxin is currently not available to us.
In summary, necrotizing pneumonia with toxic shock syndrome was diagnosed in time for this case. Cure of the infection has been reported particularly when therapy was started early before the patients enter into a lung destructive or septic stage [6]. In severe infections caused by toxin producing bacteria, the treatment aims should be to kill the bacteria, prevent formation of new toxin and inhibit the action of circulating toxin. The primary aim, to kill the bacteria, can be accomplished with a variety of bactericidal drugs including cloxacillin for MSSA and vancomycin for MRSA. If their concentration is above the MIC, these drugs will also inhibit toxin production. Linezolid was chosen for its ability to inhibit protein synthesis by binding to the 50S subunit and preventing the formation of the 70S ribosome complex, thereby inhibiting the initiation stage of bacterial protein synthesis and preventing the production of bacterial superantigens. Its early action involves a unique binding site in the ribosomal assembly stage of protein synthesis, which avoids cross-resistance with other categories of antibacterial drugs. Therefore, linezolid is also an ideal choice for treating TSS. This can be countered by combining cloxacillin with clindamycin or linezolid which inhibit the PVL toxin induced by cloxacillin.This has led certain authorities to suggest combining clindamycin with linezolid and rifampicin in severe PVL-SA infection [18].
During the diagnosis and treatment process of this case, although we could not definitively determine whether the influenza A virus H1N1 and PVL-producing Staphylococcus aureus were sequentially or simultaneously infected, and the detection of TSST-1 toxin was not available, the virulence of Staphylococcus aureus has been increasing worldwide [19], posing a significant threat to severe necrotizing diseases. However, the data on PVL-encoding phage types, lukSF-PV gene variation and chromosomal phage insertion sites for PVL-positive S. aureus are limited, especially in China [20], [21]. Therefore, considering the synergistic pathogenic effects between influenza viruses and respiratory bacteria, clinicians should pay particular attention to patients' travel history to areas with active flu seasons and a high prevalence of PVL-producing Staphylococcus aureus [3]. This case report serves as a reminder for clinicians to promptly recognize and correctly diagnose similar patients.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report.
Funding
This article has no funding.
CRediT authorship contribution statement
Huibin Chen: Writing – review & editing, Writing – original draft. Hui Duan: Supervision, Conceptualization. Jinling Zhao: Data curation. Kang Sun: Data curation. Keji Shan: Supervision, Formal analysis.
Conflicts of interest
I, Huibin Chen declare that there are no conflicts of interest in relation to the manuscript titled“Necrotizing Pneumonia Induced by Influenza A (H1N1) Concurrent with Staphylococcus aureus Infection: a Case Report”submitted to IDCases. IDCasesI confirm that the results and interpretations reported in the manuscript are original and have to been plagiarized. I certify that I have read and understand the IDCases conflict of interest policy, and I understand that failure to disclose a conflict of interest may result in the manuscript being rejected or retracted. I also certify that I have disclosed any financial or non-financial relationships that may be interpreted as constituting a conflict of interest in relation to this manuscript. I understand that this information will be subject to peer review, and I am willing to provide further information or clarification if required. I confirm that I have no known conflicts of interest that would influence the results or interpretation of the data presented in this manuscript, and I understand that failure to disclose a conflict of interest is unethical and may result in sanctions being imposed on me. Huibin Chen.
References
- 1.Darboe S., et al. Prevalence of Panton-valentine leukocidin (PVL) and antimicrobial resistance in community-acquired clinical Staphylococcus aureus in an urban gambian hospital: A 11-year period retrospective pilot study. Front Cell Infect Microbiol. 2019;9:170. doi: 10.3389/fcimb.2019.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krutikov M., Rahman A., Tiberi S. Necrotizing pneumonia (aetiology, clinical features and management) Curr Opin Pulm Med. 2019;25(3):225–232. doi: 10.1097/MCP.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 3.Bubeck Wardenburg J., et al. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13(12):1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 4.Yangyang Li, Peng peng. Progress of research on community-acquired methicillin-resistant Staphylococcus aureus. Chin J Nosocomiology. 2011;21(05):1056–1058. [Google Scholar]
- 5.Niemann S., et al. Combined action of influenza virus and Staphylococcus aureus panton-valentine leukocidin provokes severe lung epithelium damage. J Infect Dis. 2012;206(7):1138–1148. doi: 10.1093/infdis/jis468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillet Y., et al. Pragmatic management of Panton-Valentine leukocidin-associated staphylococcal diseases. Int J Antimicrob Agents. 2011;38(6):457–464. doi: 10.1016/j.ijantimicag.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Small C.L., et al. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol. 2010;184(4):2048–2056. doi: 10.4049/jimmunol.0902772. [DOI] [PubMed] [Google Scholar]
- 8.Wenyu Wang, et al. Necrotizing pneumonia due to influenza A virus and bacteria infection with good prognosis:A report of 1 case. Chin J Pract Pediatr. 2020;35(12):990–992. [Google Scholar]
- 9.Scheiblauer H., et al. Interactions between bacteria and influenza A virus in the development of influenza pneumonia. J Infect Dis. 1992;166(4):783–791. doi: 10.1093/infdis/166.4.783. [DOI] [PubMed] [Google Scholar]
- 10.Kreienbuehl L., Charbonney E., Eggimann P. Community-acquired necrotizing pneumonia due to methicillin-sensitive Staphylococcus aureus secreting Panton-Valentine leukocidin: a review of case reports. Ann Intensive Care. 2011;1(1):52. doi: 10.1186/2110-5820-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S.S., et al. A case of severe pseudomembranous tracheobronchitis complicated by co-infection of Influenza A (H1N1) and Staphylococcus aureus in an immunocompetent patient. Tube Respir Dis (Seoul) 2015;78(4):366–370. doi: 10.4046/trd.2015.78.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loffler B., et al. Pathogenesis of Staphylococcus aureus necrotizing pneumonia: the role of PVL and an influenza coinfection. Expert Rev Anti Infect Ther. 2013;11(10):1041–1051. doi: 10.1586/14787210.2013.827891. [DOI] [PubMed] [Google Scholar]
- 13.Gillet Y., et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359(9308):753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 14.Burnham J.P., Kollef M.H. Understanding toxic shock syndrome. Intensive Care Med. 2015;41(9):1707–1710. doi: 10.1007/s00134-015-3861-7. [DOI] [PubMed] [Google Scholar]
- 15.Minjun Chu, Mingbiao Ma, Tingyi Du. Research progress of Staphylococcus aureus toxic shock toxin-1. Int J Lab Med. 2021;42(12) 1521-1524+1536. [Google Scholar]
- 16.Ma X., et al. Staphylococcal Panton-Valentine leukocidin induces pro-inflammatory cytokine production and nuclear factor-kappa B activation in neutrophils. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen S.A.H., et al. Life-threatening necrotizing pneumonia with Panton-Valentine leukocidin-producing, methicillin-sensitive staphylococcus aureus in a healthy male co-infected with influenza B. Infect Dis Rep. 2021;14(1):12–19. doi: 10.3390/idr14010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakthavatchalam , Y.D. Nabarro , L.E.B. Ralph , R. Veeraraghavan , B.Diagnosis and management of Panton-Valentine leukocidin toxin associated.2017 8(7):10.1080/21505594.2017.1362532. [DOI] [PubMed]
- 19.Ma X., et al. Staphylococcal Panton-Valentine leukocidin induces pro-inflammatory cytokine production and nuclear factor-kappa B activation in neutrophils. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epidemiology and clinical features of Skin and Soft Tissue Infections Caused by PVL-Positive and PVL-Negative Methicillin-Resistant Staphylococcus aureus Isolates in inpatients in China: a single-center retrospective 7-year study." Emerg Microbes Infect 13(1): 2316809. [DOI] [PMC free article] [PubMed]
- 21.Zhao H., et al. Typing of panton-valentine leukocidin-encoding phages and lukSF-PV gene sequence variation in Staphylococcus aureus from China. Front Microbiol. 2016;7:1200. doi: 10.3389/fmicb.2016.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]