Abstract
Background:
Alopecic sarcoidosis is an uncommon cutaneous manifestation of sarcoidosis. Scarring and nonscarring alopecic sarcoidosis have been reported; however, information on the epidemiology, systemic disease associations, and treatment efficacy is limited.
Objective:
To address these gaps, we conducted a retrospective chart review and systematic literature review of alopecic sarcoidosis cases.
Methods:
Full-text English publications from PubMed, Scopus, and Google Scholar from inception to August 2023 were analyzed. Treatment evidence quality was assessed using the modified Oxford Centre for Evidence-Based Medicine rating scale. Three patients with biopsy-proven alopecic sarcoidosis were included as a case series, all demonstrating systemic sarcoidosis and 2 requiring multiple therapies. Among 1778 search results, 60 articles representing 77 cases of alopecic and scalp sarcoidosis were included. Patients were categorized into 4 distinct alopecic subgroups. Black patients constituted the majority of all subgroups.
Results:
Extracutaneous sarcoidosis burden was high across all alopecic subgroups, with ocular disease appearing overrepresented. Topical and oral corticosteroids were the main treatments. Though scarring alopecia patients had poor outcomes despite receiving immunomodulators/cx, limited data suggest potential efficacy of tumor necrosis factor-alpha inhibitors.
Limitations:
This study has a small sample size.
Conclusion:
Our findings underscore the importance of evidence-based strategies for improving alopecic sarcoidosis management. Prompt diagnosis and systemic evaluation, especially for scarring alopecia, are essential for timely intervention to optimize patient outcomes.
Keywords: alopecia, cutaneous sarcoidosis, extracutaneous sarcoidosis, nonscarring, sarcoidosis, scarring, skin of color, treatment
What is known about this subject in regard to women and their families?
Sarcoidosis, characterized by noncaseating granulomatous inflammation, demonstrates variable incidence and prevalence and may disproportionally impact women. While alopecia secondary to sarcoidosis is rare, it often manifests as scarring hair loss, particularly impacting Black women.
The adverse psychosocial effects and impacts on quality of life in women caused by alopecia, especially when leading to scarring, may be profound. In addition to the burden of systemic sarcoidosis, women with alopecic sarcoidosis often experience increased levels of anxiety, depression, and social stigma, which can affect relationships and overall well-being.
Despite its significant impact, there is limited literature on the epidemiology, systemic disease associations, and treatment efficacy of alopecic sarcoidosis.
What is new from this article as messages for women and their families?
Alopecic sarcoidosis frequently presents with systemic involvement, requiring thorough evaluation and monitoring.
Given the potential for scarring hair loss, early recognition and aggressive therapeutic approaches are essential to potentially prevent or halt scarring alopecia.
Additional research is warranted to advance our understanding of sarcoid alopecia and optimize therapy, with a particular focus on investigating the potential benefits of tumor necrosis factor-alpha inhibitors.
Introduction
Sarcoidosis is a multisystem disease characterized by noncaseating granulomatous inflammation with an unknown etiology and variable disease course.1–3 Sarcoidosis can impact any organ, potentially leading to chronic inflammation, fibrosis, organ dysfunction, or death.1–3 The pathogenesis and prognosis of sarcoidosis are multifactorial, influenced by antigenic triggers, genetics, gender, access to care, race, and income.1–3 Approximately 30% of patients with systemic sarcoidosis in the United States develop skin manifestations.1,2 Cutaneous sarcoidosis can serve as an initial indicator of systemic disease and may profoundly impact the quality of life.1–4 Specific skin lesions, classified by the presence of granulomas on histology, may provide prognostic insights into systemic disease.1 Certain specific skin lesions are chronic and occur more frequently among patients with skin of color, who also face a higher likelihood of developing severe extracutaneous disease.1,2,5,6
Alopecic sarcoidosis is an uncommonly reported cutaneous manifestation of sarcoidosis with limited literature suggesting a higher prevalence among Black patients.1,2,7,8 Both scarring (SA) and nonscarring alopecic (NSA) sarcoidosis have been reported; however, data guiding evaluation and treatment of alopecic sarcoidosis are limited.7,8 Considering the impact of SA on quality of life, and the potential prognostic implications of alopecic sarcoidosis on extracutaneous disease, it is important that dermatologists approach scalp disease in sarcoidosis comprehensively. Herein, we present a three-patient case series and systematic review of alopecic sarcoidosis to identify clinicopathologic features of SA and NSA, epidemiologic data, associations with cutaneous and extracutaneous disease, and response to therapy.
Patients and methods
Case series
This case series included 3 patients with biopsy-proven alopecic sarcoidosis at NYU Langone Health between 2014 and 2024 and was deemed institutional review board exempt given fewer than 4 cases presented. Baseline characteristics are demonstrated in Table 1.
Table 1.
Summary of sarcoidosis alopecia case series
| Case no. | Age at biopsy (y) | Sex | Race | Clinical description | Histopathological description | Other skin manifestations | Systemic features | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40s | Female | White | Cicatricial alopecia, patches, scaling, and erythema | Granulomatous dermatitis comprising well-circumscribed aggregates of epithelioid histiocytes with a sparse admixed infiltrate | Indurated plaques on the face and lower extremities | Hilar and paratracheal adenopathy, pulmonary | Fluocinonide 0.05 % ointment BID, IL TAC, HCQ 200 mg BID | At 7 months: stabilization of hair loss, no new lesions |
| 2 | 70s | Female | Black | Cicatricial alopecia, ulcers, scaling, pink dyspigmentation, and raised borders | Granulomatous dermatitis comprising nodular and loose aggregates of epithelioid histiocytes with admixed lymphocytes and neutrophils | Papulonodules on the face raised plaque on the arm | Ocular | Clobetasol 0.05% solution daily, Mupirocin 2% ointment BID, Doxycycline 40 mg daily | Lost to follow-up |
| 3 | 40s | Female | Black | Cicatricial alopecia, patches, annular plaques, atrophy, pink dyspigmentation, hyperpigmented borders, and scaling | Granulomatous dermatitis comprising epithelioid histiocytes with a few multinucleated forms and a scant accompanying lymphocytic infiltrate | Erythematous to hyperpigmented, indurated, scaly plaques on face, neck, trunk, and extremities | Pulmonary | Clobetasol 0.05% solution daily, Tacrolimus 0.1% ointment BID, Doxycycline 100 mg BID, HCQ 200 mg BID | At 9 months: significant improvement, with partial hair regrowth, thinning plaques |
BID, twice a day IL; HCQ, hydroxychloroquine; TAC, intralesional triamcinolone.
Systematic literature review
We performed a systematic literature review of alopecic sarcoidosis and scalp sarcoidosis without alopecia to describe clinicopathologic features of SA and NSA, epidemiologic data, co-existing cutaneous and extracutaneous disease, treatment modalities, and efficacy.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines. Since this study used publicly available data from published studies, it was deemed institutional review board exempt.
Information sources and search
Two authors (C.O. and M.S.) conducted independent searches of PubMed, Scopus, and Google Scholar from their inception through August 2023. Search terms used in various combinations included: sarcoid, sarcoidosis, alopecia, hair, scalp, cicatricle, scarring, and non-scarring. The full search strategy is available in the Supplementary Methods, http://links.lww.com/IJWD/A57.
Inclusion criteria
Publications that described at least 1 case of sarcoidosis alopecia or scalp sarcoidosis were included. Publications without full-text versions in English were excluded.
Study selection, data extraction, and quality assessment
Two authors (C.O. and M.S.) independently screened the title and abstract of each retrieved record and reviewed 15 articles independently to test the inclusion criteria, all decisions were unanimous. Both authors independently reviewed full-text publications that met inclusion criteria and extracted descriptive and quantitative data into a standardized Excel (Microsoft) spreadsheet. Extracted data included: demographics, presentation, exam features, differential diagnosis, additional cutaneous findings, extracutaneous disease, treatment, and reported efficacy. Quality assessment of reported treatments was performed by both authors using the modified Oxford Centre for Evidence-Based Medicine’s Level of Evidence rating scale.9
Data analysis
Statistical analyses were performed using STATA V.18 (College Station, TX: StataCorp).
Continuous variables are reported as means with standard deviation and categorical variables are displayed as percentages. P values were calculated with Pearson χ2 for categorical variables and Student’s t test or analysis of variance for continuous variables as appropriate. P values <.05 were considered statistically significant.
Results
Case series
Case 1
A White female in her 40s presented with a 20-year history of an eruption initially limited to her legs but progressing over 6 months to involve her scalp. She noted scalp redness, itch, and hair loss accompanied by fatigue and persistent cough. Physical examination of the scalp revealed a scarring alopecic, pink and yellow, scaly patch (Fig. 1A). Indurated plaques on the face and lower extremities were also noted.
Fig. 1.
Alopecic sarcoidosis. (A) Scarring alopecic, pink, and yellow, scaly patchy on the scalp. (B) Atrophic, pink plaques of scarring alopecia with hyperpigmented borders found to be late-stage scarring alopecic sarcoidosis.
Four-millimeter punch biopsies from the scalp and lower extremity demonstrated granulomatous inflammation with epithelioid histiocytes throughout the dermis. Stainings for periodic acid-Schiff and acid-fast bacilli were negative. Polarizing microscopy revealed no foreign material. Evaluation by pulmonology revealed mediastinal and right hilar lymphadenopathy with numerous pulmonary nodules, but normal pulmonary function tests. Cardiac and ophthalmologic workups were negative.
The patient’s hair loss persisted despite topical and intralesional steroids. Subsequently, the patient was started on hydroxychloroquine 400 mg daily. At her 7-month follow-up, she reported stabilization of hair loss. Her follow-up scalp examination demonstrated loss of follicular ostia without erythema or scaling.
Case 2
A Black female in her 70s was referred to dermatology for a 3-year history of progressive hair loss with painful and pruritic scalp lesions. Physical examination revealed SA, ulcers with light pink dyspigmentation, and diffuse scaling. Raised plaques on the left cheek and upper extremities and papulonodules at the medial canthi were observed. A scalp punch biopsy demonstrated a noncaseating granulomatous dermatitis with epithelioid histiocytes in the dermis. Ophthalmologic examination demonstrated bilateral intermediate uveitis consistent with ocular sarcoidosis. While undergoing further workup, the patient was lost to follow-up.
Case 3
A Black female in her 40s presented with a 12-year history of a widespread eruption extending to her scalp, causing progressive hair loss for several years. She reported arthralgia, palpitations, and dyspnea. She reported that prior skin biopsies were consistent with sarcoidosis. Previous treatments included topical steroids, oral steroids, and hydroxychloroquine. Physical exam was notable for atrophic, pink plaques of SA with hyperpigmented borders (Fig. 1B). Flesh-colored to hyperpigmented nodules on the right nasal ala and large indurated plaques on the face, neck, trunk, and extremities were observed. A 4-mm punch biopsy of an alopecic plaque on the scalp demonstrated aggregates of epithelioid histiocytes in the dermis. Stainings for periodic acid-Schiff and acid-fast bacilli were negative.
Pulmonary evaluation revealed mild hilar lymphadenopathy and normal pulmonary function tests. Due to extensive cutaneous involvement, the patient was started on hydroxychloroquine 400 mg daily and doxycycline 200 mg daily. At the 9-month follow-up, she reported significant improvement with thinning plaques, minimal inflammation, and partial hair regrowth (Table 1).
Systematic review
Of 1778 search results, 171 were duplicates and 1529 were excluded by title and abstract screening (Supplemental Material, http://links.lww.com/IJWD/A57). Among 78 full-text articles that were assessed, 60 met inclusion criteria, representing 77 patients.7,8,10–68 About 58 of 60 publications were either case series or case reports. Most studies received poor-quality levels of evidence ratings. (Table 2 and Supplemental Material, http://links.lww.com/IJWD/A57). Cases were categorized as SA, NSA, scalp sarcoidosis without alopecia, or undefined if alopecia type was not specified.
Table 2.
Effectiveness of reported treatments for alopecic sarcoidosis with quality of evidence ratings
| Treatment | Quality level of evidencea | Study supports potential effectiveness (No. studies) |
Study does not support effectiveness (No. studies) |
|---|---|---|---|
| Corticosteroids | |||
| Oral | 4 | 215,23 | 38,15,17 |
| 5 | 1411,14,21,34,35,37,40,43,45,54,57–59,61 | 1113,16,20,33,41,48,52,55,56,63,65 | |
| Intralesional | 3 | 118 | 0 |
| 4 | 123 | 37,15,17 | |
| 5 | 431,43,48,64 | 222,56 | |
| Topical | 4 | 123 | 37,8,47 |
| 5 | 714,35,37,49,54,58,68 | 713,20,22,29,33,46,56 | |
| Intravenous | 5 | 0 | 141 |
| Intramuscular | 5 | 168 | 0 |
| Antibiotics | |||
| Tetracycline | 4 | 0 | 27,17 |
| 5 | 231,61 | 113 | |
| Clarithromycin | 4 | 0 | 117 |
| Clindamycin | 4 | 0 | 17 |
| Rifampin | 4 | 0 | 17 |
| Cephalosporin | 4 | 17 | 0 |
| 5 | 0 | 161 | |
| Linezolid | 5 | 161 | 0 |
| Ciprofloxacin | 5 | 161 | 0 |
| Immunomodulators/immunosuppressants | |||
| Hydroxychloroquine | 4 | 0 | 28,17 |
| 5 | 331,37,64 | 213,39 | |
| Chloroquine | 4 | 123 | 215,60 |
| Methotrexate | 4 | 123 | 117 |
| 5 | 235,58 | 213,39 | |
| Mycophenolate Mofetil | 5 | 0 | 113 |
| Infliximab | 4 | 117 | 0 |
| Topical tacrolimus | 4 | 0 | 117 |
| 5 | 156 | 0 | |
| Pentoxifylline | 5 | 0 | 156 |
| Miscellaneous | |||
| Ketoconazole shampoo | 5 | 131 | 0 |
| Allopurinol | 4 | 0 | 117 |
| Topical minoxidil | 5 | 135 | 0 |
| Carpronium | 5 | 0 | 129 |
| Fusidic acid | 5 | 145 | 0 |
| Potassium permanganate compresses | 5 | 145 | 0 |
Quality defined by the JAMA Dermatology Quality Rating Scheme for Studies and Other Evidence (modified Oxford Centre for Evidence-Based Medicine, 2011 criteria): level 1, systematic review with meta-analysis, properly powered and conducted randomized clinical trial (not included in this analysis); 2, well-designed controlled trial without randomization; prospective comparative cohort trial (not included in this analysis); 3, case–control studies; retrospective cohort study; 4, case series with or without intervention, cross-sectional study; and 5, case reports, opinion of respected authorities.
Scarring alopecia
A total of 47 SA cases secondary to sarcoidosis were identified (mean age: 52.8 years, female: 86% [37/43]) (Table 3).7,8,10,11,13–19,23–25,27,28,30,31,33,35,37–39,41,44,46,48,52,53,56–58,60–64,66–68 Black patients were the most affected racial group (75.9% [22/29], P < .001). Common physical exam descriptors were scaly, atrophic, and indurated plaques with raised borders that were shiny, erythematous, yellow, or dyspigmented. Scalp ulceration was less commonly reported.5,11,13,44,57,61,64
Table 3.
Characteristics of cases of sarcoidosis alopecia and scalp sarcoidosis
| All cases | Scarring alopecia | Nonscarring alopecia | Scalp sarcoidosis without alopecia | Undefined alopecia | ||
|---|---|---|---|---|---|---|
| Characteristics, n (%) | N = 77 | n = 47 | n = 18 | n = 6 | n = 6 | P value |
| Demographics | ||||||
| Age (y), mean (SD) | 48.9 (16.2) | 52.8 (15.1) | 40.1 (16.3) | 54.7 (19.3) | 40.8 (8.84) | .02 |
| Sex | <.001 | |||||
| Female | 51 (70.8%) | 37 (86%) | 8 (47.1%) | 1 (16.7%) | 5 (83.3%) | |
| Male | 21 (29.2%) | 6 (14%) | 9 (52.9%) | 5 (83.3%) | 1 (16.7%) | |
| Not stated | 5 | 4 | 1 | 0 | 0 | |
| Race | .39 | |||||
| Black | 32 (71.1%) | 22 (75.9%) | 5 (55.6%) | 1 (50%) | 4 (80%) | |
| White | 9 (20%) | 5 (17.2%) | 3 (33.3%) | 0 | 1 (20%) | |
| Asian | 4 (8.8%) | 2 (6.9%) | 1 (11.10%) | 1 (50%) | 0 | |
| Not stated | 32 | 18 | 9 | 4 | 1 | |
| Sarcoidosis information | ||||||
| Cutaneous sarcoidosis elsewhere | ||||||
| Yes | 42 (54.5%) | 27 (57.4%) | 8 (44.4%) | 3 (50%) | 4 (66.7%) | .73 |
| No/not stated | 35 (45.5%) | 20 (42.6%) | 10 (55.6%) | 3 (50%) | 2 (33.3%) | |
| Systemic sarcoidosis | ||||||
| Yes | 55 (71.4%) | 31 (66%) | 13 (72.2%) | 6 (100%) | 5 (83.3%) | .32 |
| No/not stated | 22 (28.6%) | 16 (34%) | 5 (27.8%) | 0 | 1 (16.7%) | |
| Systemic sarcoidosis organs involved | ||||||
| Pulmonary | 49 (89.1%) | 27 (87.1%) | 12 (92.3%) | 6 (100%) | 4 (80%) | .23 |
| Cardiac | 3 (5.5%) | 2 (6.5%) | 1 (7.7%) | 0 | 0 | .88 |
| Hepatic | 5 (9.1%) | 2 (6.5%) | 2 (15.4%) | 0 | 1 (20%) | .48 |
| Splenic | 3 (5.4%) | 1 (3.2%) | 1 (7.7%) | 0 | 1 (20%) | .33 |
| Bone | 4 (7.3%) | 2 (6.5%) | 0 | 0 | 2 (40%) | .01 |
| Muscular | 1 (1.8%) | 0 | 1 (7.7%) | 0 | 0 | .35 |
| Arthritic/arthropathy/joint | 1 (1.8%) | 0 | 1 (7.7%) | 0 | 0 | .35 |
| Neurologic | 2 (3.6%) | 0 | 2 (15.4%) | 0 | 0 | .08 |
| Ocular/ophthalmic | 11 (20%) | 1 (3.2%) | 5 (38.5%) | 3 (50%) | 2 (40%) | .001 |
| Extrathoracic lymphadenopathy | 1 (1.8%) | 0 | 1 (7.7%) | 0 | 0 | .35 |
| Mucosal | 3 (5.5%) | 1 (3.2%) | 1 (7.7%) | 0 | 1 (20%) | .34 |
| Glandular | 1 (1.8%) | 0 | 0 | 0 | 1 (20%) | .007 |
| Vasculitis (arteritis) | 2 (3.6%) | 2 (6.5%) | 0 | 0 | 0 | .72 |
| Renal | 1 (1.8%) | 1 (3.2%) | 0 | 0 | 0 | .89 |
| Other: hypercalcemia | 3 (5.5%) | 3 (9.7%) | 0 | 0 | 0 | .72 |
Reported P values compare values between alopecia subgroups using Pearson χ2 for categorical variables and analysis of variance for continuous variables as appropriate.
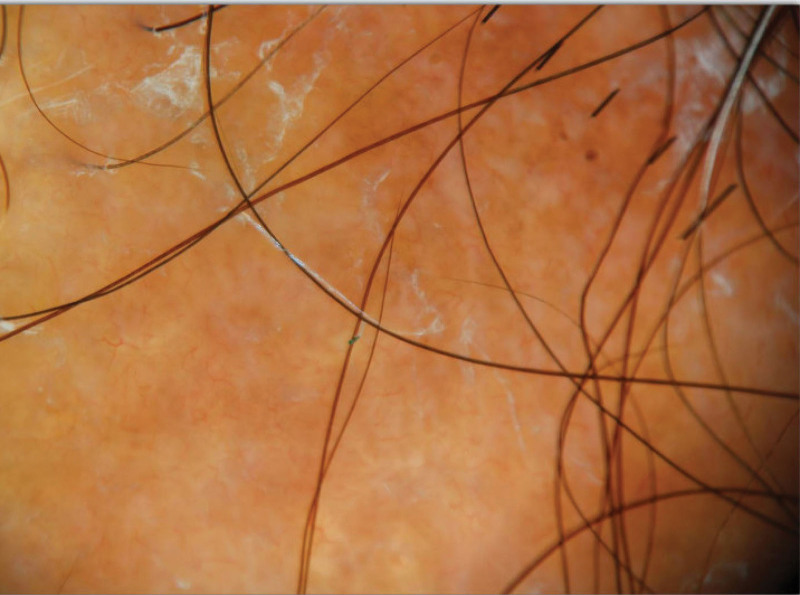
Trichoscopy of scalp sarcoidosis demonstrated perifollicular and follicular yellow-orange, pale spots with perifollicular scaling, telangiectasias, and dystrophic hairs (Fig. 2).48 Before the biopsy, a wide differential diagnosis of cicatricial alopecia was suggested, including discoid lupus erythematosus (DLE), necrobiosis lipoidica, lichen planopilaris, scleroderma, frontal fibrosing alopecia, psoriasis, central centrifugal cicatricial alopecia, and morphea. Most patients underwent scalp punch biopsies (87.2% [41/47]), which demonstrated dermal nodular infiltrates of noncaseating granulomas invading hair follicles with sparse lymphocytic infiltrates (Fig. 3). Complete follicular destruction (7.3% [3/41]), necrobiotic collagen (14.6% [6/41]), and loss of elastic fibers (2.4% [1/41]) were uncommonly reported. Special stains and polarized light examination were reported in 46.3% of biopsies (19/41), with no micro-organisms or foreign bodies found.
Fig. 2.
Trichoscopy of scalp sarcoid demonstrating yellow globules, telangiectasias, and perifollicular scale. Representative image obtained from an NYU Langone Health patient.
Fig. 3.
Dermal nodular infiltrates of noncaseating granulomas surrounding hair follicle with sparse lymphocytic infiltrate (hematoxylin and eosin). Representative image obtained from the Section of Dermatopathology in NYU Langone Health’s Ronald O. Perelman Department of Dermatology.
Most patients (57.4% [27/47]) had additional cutaneous sarcoidosis findings with a statistically significant predominance of facial involvement (59% [16/27], P = .040). Involvement of the extremities (upper: 29.6% [8/27], lower: 33.3% [9/27]) and trunk (18.5% [5/27]) was reported. Plaques (44% [12/27]) and papules (33% [9/27]) were common morphologic findings. Nodules (11.1% [3/27]), ulcers (7.4% [2/27]), and infiltrative scar sarcoidosis (7.4% [2/27]) were uncommon.
Most patients had systemic sarcoidosis (66.6% [31/47], P = .03) with a predominance of lung involvement (87.1% [27/31]). Other organs impacted included the heart (6.5% [2/31]), liver (6.5% [2/31]), vasculature (6.5% [2/31]), bone (6.5% [2/31]), spleen (3.2% [1/31]), kidneys (3.2% [1/31]), eyes (3.2% [1/31]), and mucosa (3.2% [1/31]). Three patients developed severe hypercalcemia.
Nonscarring alopecia
Fewer cases of NSA were identified (n = 18) (Table 3).7,8,27,29,32,34,40,42,43,45,47–51,65 On average, NSA patients were over 10 years younger than SA patients (40.1 years NSA vs 52.8 years SA, P = .02) with a more equal sex distribution (female: 47.1%, P < .001). No significant difference in race was observed between alopecia subgroups (P = .39).
NSA was described as scaly, erythematous plaques, or patches. Most cases were confined to the scalp; however, 3 patients developed nonscalp alopecia involving the lower extremities.32,50,65 One patient presented with total alopecia of the scalp and body, with 2 biopsies demonstrating granulomatous inflammation.34
Differential diagnoses prior to biopsy were alopecia areata, lichen planopilaris, DLE, necrobiosis lipoidica, follicular mucinosis, folliculitis decalvans, or tinea capitis. Scalp punch biopsies were performed in 94.4% (17/18) of cases, revealing dermal noncaseating granulomas adjacent to pilosebaceous units. Loss of hair follicles was described in 3/17 (17.6%) cases. Few cases reported completion of special stains and polarized light microscopy (23.5% [4/17]).
Notably, 44.4% (8/18) of NSA patients had additional cutaneous manifestations. Unlike SA patients, involvement of the extremities (upper: 37.5% [3/8], lower: 62.5% [5/8]) was more common than facial involvement (25% [2/8]), but not statistically significant (upper extremities P = .52, lower extremities P = .22). Plaques and subcutaneous nodules were the most described cutaneous findings.
Most NSA patients had systemic sarcoidosis (72.2% [13/18]) with a predominance of pulmonary sarcoidosis (92.3% [12/13]). Patients also exhibited involvement of the eyes (38.5% [5/13]), liver (15.4% [2/13]), central nervous system (15.4% [2/13]), heart (7.7% [1/13]), spleen (7.7% [1/13]), muscle (7.7% [1/13]), joints (7.7% [1/13]), nose (7.7% (1/13)), larynx (7.7% (1/13)), and extrathoracic lymph nodes (7.7% (1/13)).
Scalp sarcoidosis without alopecia and undefined alopecia
Scalp sarcoidosis without concomitant alopecia was reported in 6 cases (male:83.3% [5/6], mean age: 54.7 years) and described as erythematous, infiltrated scaly plaques (Table 3).20,21,36,54,55,59 A biopsy was obtained in all cases and demonstrated sarcoidal features.
A total of 50% (3/6) of patients had additional cutaneous sarcoidosis findings with papules, plaques, and nodules found on the face, trunk, and extremities. All 6 patients had evidence of systemic sarcoidosis with pulmonary sarcoidosis seen in all cases and ocular involvement was reported in 3 patients.
In 6 cases, alopecia subtype was not defined by authors (female: 83.3% [5/6], mean age: 40.8 years, Black race: 80% [4/5]) (Table 3).8,12,22,26 Biopsies were obtained in 83.3% (5/6) and demonstrated granulomatous inflammation. Most patients had additional cutaneous manifestations of sarcoidosis (66.7% [4/6]) and systemic disease (83.3% [5/6]).
Treatment
Treatment was reported in 48/77 cases (Table 4).7,8,11,13–18,20–23,29,31,33–35,37,39–41,43,45–49,52,54–61,63–65,68 The average number of treatment agents given across all cases was 2.3. Topical and oral corticosteroids were the most reported treatment. SA patients commonly received oral corticosteroids (SA: 66.7% [20/30] vs NSA: 41.7% [5/12], P = .02). SA and NSA patients received similar amounts of topical corticosteroids (SA: 40% [12/30] vs NSA: 41.7% [5/12]; P = .92).
Table 4.
Treatment is given to patients with sarcoidosis alopecia/scalp sarcoidosis, stratified by alopecia subtype
| All patients | Scarring alopecia | Nonscarring alopecia | Scalp sarcoidosis without alopecia | Undefined alopecia | ||
|---|---|---|---|---|---|---|
| N = 77 | n = 47 | n = 18 | n = 6 | n = 6 | P value | |
| Treatment given | ||||||
| Yes | 48 (62.3%) | 30 (63.8%) | 12 (66.7%) | 5 (83.3%) | 1 (16.7%) | .08 |
| No/not stated | 29 (37.7%) | 17 (36.2%) | 6 (33.3%) | 1 (16.7%) | 5 (83.3%) | |
| Total number of treatments given, mean (SD) | 2.3 (1.6) | 2.6 (1.8) | 1.8 (1.4) | 1.4 (0) | 2 | .29 |
| Number of systemic treatments given, mean (SD) | 1.4 (1.4) | 1.8 (1.5) | 0.75 (1.1) | 1 (0) | 0 | .097 |
| Treatment class | ||||||
| Corticosteroids | ||||||
| Oral | 30 (62.5%) | 20 (66.7%) | 5 (41.7%) | 5 (100%) | 0 | .02 |
| Intralesional | 13 (27.1%) | 9 (30%) | 3 (25%) | 0 | 1 (100%) | .75 |
| Topical | 20 (41.7%) | 12 (40%) | 5 (41.7%) | 2 (40%) | 1 (100%) | .92 |
| Intravenous | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Intramuscular | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Antimicrobials | ||||||
| Tetracycline | 6 (12.5%) | 5 (16.7%) | 1 (8.3%) | 0 | 0 | .64 |
| Clarithromycin | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Ketoconazole (topical) | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Clindamycin | 1 (2.1%) | 0 | 1 (8.3%) | 0 | 0 | .35 |
| Rifampin | 1 (2.1%) | 0 | 1 (8.3%) | 0 | 0 | .35 |
| Cephalexin | 1 (2.1%) | 0 | 1 (8.3%) | 0 | 0 | .35 |
| Cefepime | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Linezolid | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Ciprofloxacin | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Immunomodulators/immunosuppressants | ||||||
| Chloroquine | 3 (6.3%) | 3 (10 %) | 0 | 0 | 0 | .57 |
| Hydroxychloroquine | 8 (16.6%) | 8 (26.7%) | 0 | 0 | 0 | .13 |
| Methotrexate | 6 (12.5%) | 6 (20 %) | 0 | 0 | 0 | .25 |
| Mycophenolate mofetil | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Tacrolimus (topical) | 3 (6.3%) | 3 (10%) | 0 | 0 | 0 | .57 |
| Allopurinol | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Pentoxifylline | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Biologics | ||||||
| Infliximab | 2 (4.2%) | 2 (6.7%) | 0 | 0 | 0 | .73 |
| Other | ||||||
| Minoxidil (topical foam) | 1 (2.1%) | 1 (3.3%) | 0 | 0 | 0 | .89 |
| Carpronium | 1 (2.1%) | 0 | 1 (8.3%) | 0 | 0 | .35 |
| Fusidic acid (topical) | 1 (2.1%) | 0 | 1 (8.3%) | 0 | 0 | .35 |
| Potassium permanganate compress | 1 (2.1%) | 0 | 1 (8.3%) | 0 | 0 | .35 |
Reported P values compare values between alopecia subgroups using Pearson χ2 for categorical variables and analysis of variance for continuous variables as appropriate.
SA patients were additionally treated with hydroxychloroquine (26.7% [8/30]), chloroquine (10% [3/30]), methotrexate (20% [6/30]), mycophenolate mofetil (3.3% [1/30]), and tetracycline antibiotics (16.7% [5/30]). Two SA patients were treated with infliximab (6.7% [2/30]).17
SA patients had poor treatment outcomes (Supplemental Material, http://links.lww.com/IJWD/A57). Of patients receiving oral corticosteroids, only 10% (2/20) reported partial or complete hair regrowth while 30% (6/20) had stabilization of hair loss and 40% (8/20) had no improvement. Most SA patients receiving topical (58.3% [7/12]) or intralesional steroids (66.6% [6/9]) similarly reported no improvement of alopecia. SA patients who received hydroxychloroquine (50% [4/8]) or methotrexate (33.3% [2/6]) did not have improvement in hair loss. Both patients treated with infliximab had hair regrowth. Notably, their biopsies demonstrated superficial and deep granulomatous inflammation without comment on scarring or follicular destruction.17
NSA patients generally had better treatment outcomes (Supplemental Material, http://links.lww.com/IJWD/A57). About 60% (3/5) of NSA patients given oral corticosteroids reported a complete response of their hair loss to treatment. And 80% of patients (4/5) who received topical corticosteroids had no improvement, while only 20% (1/5) reported a complete response. Some patients with scalp sarcoidosis without alopecia had complete (20% [1/5]) or partial (40% [2/5]) response with oral corticosteroids.
Discussion
This case series and systematic review highlight important clinical and pathologic features of alopecic sarcoidosis. While there was a wide range of clinical descriptions of scalp disease, both SA and NSA often presented as scaly, atrophic, erythematous, or dyspigmented plaques or patches. The wide range of differential diagnoses considered before biopsy underscores the potential for cutaneous sarcoidosis to mimic several conditions, highlighting the importance of obtaining a biopsy in the evaluation of alopecia, especially when scarring or systemic disease is a consideration.
The pathophysiology of alopecic sarcoidosis remains unclear. While a high burden of scarring alopecic sarcoidosis was described in this review, cutaneous sarcoidosis rarely involves scarring or ulceration. This raises the possibility that patients with alopecic sarcoidosis may have a primary concomitant alopecia that initiates inflammation and scarring, to which granulomas hone to in the setting of sarcoidosis. To highlight this hypothesis, one case series demonstrated patients with alopecic sarcoidosis with histologic evidence of co-existing DLE and alopecia areata.27 However, definitive conclusions cannot be drawn and further research is required as to why alopecic sarcoidosis can scar.
Black patients constituted a significant proportion of patients who developed alopecia secondary to sarcoidosis. Though it remains unclear why alopecic sarcoidosis appears to be more common among Black patients, this disproportionate impact may point to underlying environmental exposures or barriers to care and delayed care as contributing factors.1 Statistically significant differences in age and sex were observed between SA and NSA. The greater involvement of SA among older patients may reflect the longevity of the disease process or delayed diagnosis, suggesting the importance of early treatment to prevent scarring hair loss.
Most patients with alopecic sarcoidosis had additional manifestations of cutaneous sarcoidosis; however, the distribution of cutaneous disease varied among subtypes. All forms of alopecic and scalp sarcoidosis were associated with high rates of systemic involvement, aligning with established prevalence patterns of extracutaneous sarcoidosis. Pulmonary sarcoidosis predominated across all groups, as expected, given this is the most impacted organ in sarcoidosis.5,69 Considering reported rates of ocular sarcoidosis, ocular involvement was overrepresented in this systematic review, possibly influenced by the high representation of female and Black patients, who have an established increased burden of ocular sarcoidosis.69 Yet this illustrates for dermatologists that referring patients for eye screening is imperative.
In general, alopecic sarcoidosis had poor treatment outcomes. Most patients received corticosteroids.70,71 NSA patients had moderate treatment responses with topical and oral corticosteroids. In addition to receiving corticosteroids, SA patients typically received corticosteroid-sparing immunomodulators and immunosuppressants. Two cases illustrated the response of hair loss secondary to SA with infliximab.17 This is consistent with prior literature demonstrating infliximab’s benefit for severe, chronic, and cutaneous sarcoidosis,72 highlighting for dermatologists that when treating alopecic sarcoidosis, rapid deployment of tumor necrosis factor inhibitors may be required. Treatment with other immune therapies can also be considered. While azathioprine and leflunomide have been described as treatments for sarcoid, neither were reported as therapies for alopecic sarcoidosis. Moreover, azathioprine has demonstrated low efficacy in treating cutaneous sarcoid.73 The role of Janus Kinase inhibitors for alopecic sarcoidosis is underexplored; however, prior data suggests potential benefits for cutaneous sarcoidosis.74–76
Interpreting this data comes with several limitations including heterogeneity in patient characteristics, variability in treatment and treatment response measures, small sample size, and the absence of blinding, randomization, or control groups in these case reports. Additionally, considering the high likelihood of publication bias with the use of case report data, the rate of systemic associations is likely an overestimation. Despite these limitations, given the importance of early recognition of specific signs of cutaneous sarcoidosis, and as SA adversely affects quality of life,77 dermatologists should understand and recognize alopecic sarcoidosis and consider aggressive therapy to prevent scarring hair loss.
In conclusion, this review highlights the multifaceted nature and significant impact of alopecic sarcoidosis, especially among patients with skin of color, and the associations with cutaneous and extracutaneous disease. The heterogeneity in the presentation, clinical features, and treatment outcomes of SA and NSA in sarcoidosis underscores the need for further research to investigate the underlying factors that drive these differences. Additionally, challenges in treating and diagnosing sarcoidosis highlight the need for clinical trials and standardized treatment approaches to provide evidence-based guidance for clinicians.
Conflicts of interest
None.
Funding
None.
Study approval
N/A
Author contributions
All authors participated in the writing of this manuscript. CO, MS, SI, and ASC participated in the research design and data analysis.
Patient consent
Informed written consent was received from all patients for whom photographs are present in the manuscript.
Data availability
Template data collection forms, extracted data from included studies, and data used for all analyses are available from the corresponding author (A.C.) upon request.
Supplementary data
Supplementary material associated with this article can be found at http://links.lww.com/IJWD/A57.
Supplementary Material
Footnotes
Published online 13 September 2024
C.O. and M.S. contributed equally as cofirst authors.
References
- 1.Ezeh N, Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Dermatol Clin 2023;41:455–70. [DOI] [PubMed] [Google Scholar]
- 2.Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol 2012;66:121.e1–14. [DOI] [PubMed] [Google Scholar]
- 3.Drent M, Crouser ED, Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med 2021;385:1018–32. [DOI] [PubMed] [Google Scholar]
- 4.Saketkoo LA, Russell AM, Jensen K, et al. Health-related quality of life (HRQoL) in sarcoidosis: diagnosis, management, and health outcomes. Diagnostics (Basel) 2021;11:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baughman RP, Teirstein AS, Judson MA, et al. ; Case Control Etiologic Study of Sarcoidosis (ACCESS) Research Group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164:1885–9. [DOI] [PubMed] [Google Scholar]
- 6.Rybicki BA, Major M, Popovich J, Jr., Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997;145:234–41. [DOI] [PubMed] [Google Scholar]
- 7.House NS, Welsh JP, English JC, 3rd. Sarcoidosis-induced alopecia. Dermatol Online J 2012;18:4. [PubMed] [Google Scholar]
- 8.Katta R, Nelson B, Chen D, Roenigk H. Sarcoidosis of the scalp: a case series and review of the literature. J Am Acad Dermatol 2000;42:690–2. [PubMed] [Google Scholar]
- 9.Oxford Centre for Evidence-Based Medicine. Levels of Evidence. 2009. [Cited 2023 December 3]. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 [Google Scholar]
- 10.Maurice P, Goolamali S. (63) Sarcoidosis of the scalp presenting as scarring alopecia. Br J Dermatol 1988;119:116–7. [Google Scholar]
- 11.Ronchese F. Annular sarcoiodosis. Arch Dermatol 1951;64:806–7. [Google Scholar]
- 12.Russell B. Atrophic alopecia due to granulomatous infiltration of scalp in systemic sarcoidosis. Proc R Soc Med 1965;58:243–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harman KE, Calonje E, Robson A, Black MM. Case 1. Sarcoidosis presenting as a scarring alopecia resembling necrosis lipoidica. Clin Exp Dermatol 2003;28:565–6. [DOI] [PubMed] [Google Scholar]
- 14.Douri T, Chawaf AZ, Alrefaee BA. Cicatricial alopecia due to sarcoidosis. Dermatol Online J 2003;9:16. [PubMed] [Google Scholar]
- 15.Golitz LE, Shapiro L, Hurwitz E, Stritzler R. Cicatricial alopecia of sarcoidosis. Arch Dermatol 1973;107:758–60. [PubMed] [Google Scholar]
- 16.Yazdani Abyaneh MA, Raghu P, Kircher K, Kutzner H, Kortz A, Carlson JA. Circumscribed cicatricial alopecia due to localized sarcoidal granulomas and single-organ granulomatous arteritis: a case report and systematic review of sarcoidal vasculitis. J Cutan Pathol 2015;42:746–56. [DOI] [PubMed] [Google Scholar]
- 17.Tu J, Chan J. Cutaneous sarcoidosis and infliximab: evidence for efficacy in refractory disease. Australas J Dermatol 2014;55:279–81. [DOI] [PubMed] [Google Scholar]
- 18.Chong WS, Tan HH, Tan SH. Cutaneous sarcoidosis in Asians: a report of 25 patients from Singapore. Clin Exp Dermatol 2005;30:120–4. [DOI] [PubMed] [Google Scholar]
- 19.Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol 1999;38:841–5. [DOI] [PubMed] [Google Scholar]
- 20.Akhdari N, Skalli HD, Lakhdar H. Erythematous lesions on the scalp. Sarcoidosis. Arch Dermatol 2004;140:1003–8. [DOI] [PubMed] [Google Scholar]
- 21.Bhushan P, Thatte SS, Singh A. Key messages from a rare case of annular sarcoidosis of scalp. Indian Dermatol Online J 2016;7:192–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charakida A, Teixeira F, Kubba F, Anton A, Schulman D, Cintra ML. A rare manifestation of scalp sarcoidosis. Int J Dermatol 2023;62:e9–e10. [DOI] [PubMed] [Google Scholar]
- 23.Mosam A, Morar N. Recalcitrant cutaneous sarcoidosis: an evidence-based sequential approach. J Dermatolog Treat 2004;15:353–9. [DOI] [PubMed] [Google Scholar]
- 24.Henderson CL, Lafleur L, Sontheimer RD. Sarcoidal alopecia as a mimic of discoid lupus erythematosus. J Am Acad Dermatol 2008;59:143–5. [DOI] [PubMed] [Google Scholar]
- 25.Thomas CC. Sarcoidosis. Arch Dermatol Syphil 1943;47:58–73. [Google Scholar]
- 26.Bluefarb SM, Szymanski FJ, Rostenberg A, Jr. Sarcoidosis as a cause of patchy alopecia. AMA Arch Derm 1955;71:602–4. [DOI] [PubMed] [Google Scholar]
- 27.Sode T, Ogwumike E, Hosler GA, Khalid I. Sarcoidosis coexisting with distinct forms of alopecia on the scalp: a case series. Am J Dermatopathol 2023;45:478–81. [DOI] [PubMed] [Google Scholar]
- 28.Morrison J. Sarcoidosis in the Bantu: necrotizing and mutilating forms of the disease. Br J Dermatol 1974;90:649–55. [DOI] [PubMed] [Google Scholar]
- 29.Nonomura Y, Otsuka A, Miyachi Y, Kabashima K. Sarcoidosis of the scalp presenting as patchy alopecia; analysis of transforming growth factor-β expression in the affected area by immunostaining. Eur J Dermatol 2013;23:115–6. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Mori M, Muraoka S, et al. Sarcoidosis presenting as a scarring alopecia: report of a rare cutaneous manifestation of systemic sarcoidosis. Dermatology 1996;193:144–6. [DOI] [PubMed] [Google Scholar]
- 31.Ranasinghe GC, Hogan S, Ibrahim O, Piliang MP. Sarcoidosis presenting as frontal fibrosing alopecia: a master mimicker or a coincidental finding? Am J Dermatopathol 2018;40:73–5. [DOI] [PubMed] [Google Scholar]
- 32.Dan L, Relic J. Sarcoidosis presenting as non-scarring non-scalp alopecia. Australas J Dermatol 2016;57:e112–3. [DOI] [PubMed] [Google Scholar]
- 33.Saxe N, Benatar SR, Bok L, Gordon W. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol 1984;120:93–6. [PubMed] [Google Scholar]
- 34.Smith SR, Kendall MJ, Kondratowicz GM. Sarcoidosis--a cause of steroid responsive total alopecia. Postgrad Med J 1986;62:205–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prohaska J, Demaree E, Powers J, Cook C. Scalp sarcoidosis presenting as cicatricial alopecia. J Am Osteopath Assoc 2018;118:824–6. [DOI] [PubMed] [Google Scholar]
- 36.Ito T, Yamamoto T. Scalp sarcoidosis with alopecia areata showing Renbök phenomenon. J Dermatol 2020;47:e454–5. [DOI] [PubMed] [Google Scholar]
- 37.La Placa M, Vincenzi C, Misciali C, Tosti A. Scalp sarcoidosis with systemic involvement. J Am Acad Dermatol 2008;59(5 Suppl):S126–7. [DOI] [PubMed] [Google Scholar]
- 38.Cheraghi N, Robinson A, O’Donnell P, Belazarian L. Scalp sarcoidosis: a sign of systemic sarcoidosis. Dermatol Online J 2014;20:doj_21767. [PubMed] [Google Scholar]
- 39.Long T, Bindernagel R, Austin B, Tarbox M. Severe scalp sarcoidosis in an unlikely patient. JAAD Case Rep 2020;6:1165–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huisman P, Van Royen E. Skin uptake of Gallium 67 in cutaneous sarcoidosis. Acta Derm Venereol 1985;65:243–7. [PubMed] [Google Scholar]
- 41.Frieder J, Kivelevitch D, Menter A. Symptomatic hypercalcemia and scarring alopecia as presenting features of sarcoidosis. Proc (Bayl Univ Med Cent) 2018;31:224–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costabel U, Guzman J, Baughman RP. Systemic evaluation of a potential cutaneous sarcoidosis patient. Clin Dermatol 2007;25:303–11. [DOI] [PubMed] [Google Scholar]
- 43.Cho HR, Shah A, Hadi S. Systemic sarcoidosis presenting with alopecia of the scalp. Int J Dermatol 2004;43:520–2. [DOI] [PubMed] [Google Scholar]
- 44.Andersen KE. Systemic sarcoidosis with necrobiosis lipoidica-like scalp lesions. Acta Derm Venereol 1977;57:367–9. [PubMed] [Google Scholar]
- 45.Omar SI, Genedy RM, Zaid SAA. Systemic sarcoidosis with psoriasiform plaques and patchy nonscarring alopecia. Adv Skin Wound Care 2021;34:1–4. [DOI] [PubMed] [Google Scholar]
- 46.Bleehen SS. Systemic sarcoidosis with scalp involvement. Proc R Soc Med 1969;62:348–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishikawa M, Ohtsuka M, Yamamoto T. Three cases of scalp sarcoidosis with alopecia. Actas Dermosifiliogr (Engl Ed) 2018;109:933–4. [DOI] [PubMed] [Google Scholar]
- 48.Torres F, Tosti A, Misciali C, Lorenzi S. Trichoscopy as a clue to the diagnosis of scalp sarcoidosis. Int J Dermatol 2011;50:358–61. [DOI] [PubMed] [Google Scholar]
- 49.Kim JC, Lee ES. Unusual case of scalp sarcoidosis with alopecia: an only manifestation of cutaneous sarcoidosis without systemic involvement. Ann Dermatol 2022;34:154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rapp SE. An unusual cause of hair loss. Arch Dermatol 2002;138:259–64. [DOI] [PubMed] [Google Scholar]
- 51.Greer KE, Harman LE, Jr., Kayne AL. Unusual cutaneous manifestations of sarcoidosis. South Med J 1977;70:666–8. [DOI] [PubMed] [Google Scholar]
- 52.Paolino G, Panetta C, Didona D, Donati M, Donati P. Atrophic and annular scarring alopecia of the scalp as a finding in underlying systemic sarcoidosis. Acta Dermatovenerol Croat 2017;25:298–9. [PubMed] [Google Scholar]
- 53.Abdel Bary A, Eldeeb M, Hassan E. Cicatricial alopecia: do clinical, trichoscopic, and histopathological diagnosis agree? Acta Dermatovenerol Alp Pannonica Adriat 2021;30:129–36. [PubMed] [Google Scholar]
- 54.Tisack A, Jiang A, Veenstra J. Crusted, ulcerated plaques on the scalp and face. Clin Exp Dermatol 2021;46:199–202. [DOI] [PubMed] [Google Scholar]
- 55.Boda D, Cutoiu A, Bejenariu N, Caruntu C. Cutaneous sarcoidosis of the scalp unmasking systemic involvement: a case report. Exp Ther Med 2021;22:1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Enany G, Nada H, Nagui N, et al. Diffuse cicatricial alopecia and multiple telangiectatic indurated leg plaques. Int J Dermatol 2021;60:e222–6. [DOI] [PubMed] [Google Scholar]
- 57.Karrakchou B, Fliti A, Meziane M, Senouci K. Erythroderma with total scarring alopecia. BMJ Case Rep 2023;16:e250782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ford PG, Jorizzo JL, Hitchcock MG. Previously undiagnosed sarcoidosis in a patient presenting with leonine facies and complete heart block. Arch Dermatol 2000;136:712–4. [DOI] [PubMed] [Google Scholar]
- 59.Thaipisuttikul Y, Kateruttanakul P. Sarcoidosis mimics lepromatous leprosy: a case report. J Med Assoc Thai 2007;90:171–4. [PubMed] [Google Scholar]
- 60.Knight L, Ngwanya M. Sarcoidosis of the scalp: the largest single-institutional case series. Int J Dermatol 2019;58:e149–51. [DOI] [PubMed] [Google Scholar]
- 61.Fram G, Kohli S, Jiang A, Kaatz S. Sarcoidosis presenting as facial and scalp ulceration with secondary bacterial infection of the skin. BMJ Case Rep 2019;12:e231769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barker C, Nyers E, Kramish C, Scribner J. Scarring alopecia and scalp pruritus. JAAD Case Rep 2022;30:134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghosh A, Sengupta S, Coondoo A, Gharami RC. Single lesion of sarcoidosis presenting as cicatricial alopecia: a rare report from India. Int J Trichology 2014;6:63–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai CF, Lee HC, Chu CY. Sarcoidal alopecia mimicking discoid lupus erythematosus: report of a case and review of the literature. Dermatologica Sinica 2014;32:43–6. [Google Scholar]
- 65.Felix RH. (15) Alopecia of the shin: a presenting sign of sarcoidosis. Br J Dermatol 1983;109:66–7. [Google Scholar]
- 66.Dash SS, Malhotra AK, Bhatti SS, Karak AK, Gupta S. Discoid lupus erythematosus-like sarcoidosis. Clin Exp Dermatol 2007;32:442–3. [DOI] [PubMed] [Google Scholar]
- 67.Cather JC, Menter MA. Hair loss and plaquelike skin lesions. Proc (Bayl Univ Med Cent) 2001;14:101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Starace M, Brandi N, Baraldi C, Maria B. Scalp sarcoidosis with systemic involvement: a case report and literature review. EMJ 2019;4:63–7. [Google Scholar]
- 69.Haimovic A, Sanchez M, Judson MA, Prystowsky S. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. Extracutaneous disease. J Am Acad Dermatol 2012;66:719.e1–719.e10. [DOI] [PubMed] [Google Scholar]
- 70.Haimovic A, Sanchez M, Judson MA, Prystowsky S. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. Cutaneous disease. J Am Acad Dermatol 2012;66:699.e1–18; quiz 717. [DOI] [PubMed] [Google Scholar]
- 71.Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med 2020;41:689–99. [DOI] [PubMed] [Google Scholar]
- 72.Stagaki E, Mountford WK, Lackland DT, Judson MA. The treatment of lupus pernio: results of 116 treatment courses in 54 patients. Chest 2009;135:468–76. [DOI] [PubMed] [Google Scholar]
- 73.Rosenbach M, Baughman R. Sarcoidosis In: Garg A, Merola J, Fitzpatrick L, editors. Interdisciplinary approaches to overlap disorders in dermatology & rheumatology. Cham: Springer International Publishing AG; 2022. p.199–223. [Google Scholar]
- 74.Alam M, Fang V, Rosenbach M. Treatment of cutaneous sarcoidosis with tofacitinib 2% ointment and extra virgin olive oil. JAAD Case Rep 2021;9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dai C, Shih S, Ansari A, Kwak Y, Sami N. Biologic therapy in the treatment of cutaneous sarcoidosis: a literature review. Am J Clin Dermatol 2019;20:409–22. [DOI] [PubMed] [Google Scholar]
- 76.Chapman S, Kwa M, Gold LS, Lim HW. Janus kinase inhibitors in dermatology: part I. A comprehensive review. J Am Acad Dermatol 2022;86:406–13. [DOI] [PubMed] [Google Scholar]
- 77.Singh R, Wilborn D, Lintzeri DA, Blume-Peytavi U. Health-related quality of life (hrQoL) among patients with primary cicatricial alopecia (PCA): a systematic review. J Eur Acad Dermatol Venereol 2023;37:2462–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Template data collection forms, extracted data from included studies, and data used for all analyses are available from the corresponding author (A.C.) upon request.