Abstract
Introduction
Endoscopic ultrasound (EUS)-guided lumen-apposing metal stents (LAMS) represent a novel tool in therapeutic endoscopy. However, the presence of LAMS may dissuade surgeons from operations with curative-intent. We report three clinical scenarios with deployment of LAMS in patients that subsequently underwent pancreaticoduodenectomy (PD).
Methods
Six patients identified from our IRB-approved pancreas cancer database had EUS-LAMS placement prior to PD. Patient, tumor, treatment-related variables, and outcomes are herein reported.
Results
Two patients underwent a LAMS gastrojejunostomy (GJ) for duodenal obstruction. Another patient underwent LAMS choledochoduodenostomy (CDS) for malignant biliary obstruction. In three patients, a LAMS gastrogastrostomy or jejunogastrostomy was deployed post Roux-en-Y gastric bypass (RYGB) for a EUS-directed transgastric ERCP (EDGE) procedure. The hospital length of stay after LAMS placement was 0–3 days without morbidity. Patients subsequently proceeded to either classic PD (n = 5) or PPPD (n = 1). Interval from LAMS insertion to surgery ranged from 28 to 194 days. Mean PD operative time and EBL were 513 minutes and 560 mL, respectively. Post-PD hospital length of stay was 4–17 days. Clavien-Dindo IIIb morbidity required percutaneous drainage of intra-abdominal collections in two patients. In cases involving LAMS-GJ and CDS, the LAMS directly impacted the surgeon's preference not to perform pylorus preservation.
Conclusions
In this case series, PD following EUS-LAMS was feasible with acceptable morbidity. Additional studies with larger patient populations are needed to evaluate LAMS as a bridge to PD with curative-intent.
Keywords: Advanced endoscopy, Lumen-apposing metal stent, Pancreatic cancer, Pancreaticoduodenectomy
1. Introduction
Endoscopic ultrasound (EUS)-guided lumen-apposing metal stents (LAMS) have become an increasingly useful tool in the armamentarium of advanced endoscopists [1,2]. Therapeutic benefits to patients are apparent due to the minimally invasive nature of the procedure and as evidenced by the evolving indications for its use [1]. LAMS was originally designed for internal drainage of pancreatic fluid collections and is now used, in part, to bypass intestinal obstructions, internally drain gallbladders with complicated cholecystitis, decompress the biliary system, and as a method to gain access to the excluded stomach after a Roux-en-Y gastric bypass [1,[3], [4], [5]]. For patients with advanced cancer, LAMS has demonstrated increasing utility for palliation when definitive surgical resection is deemed futile.
The extension of LAMS as a potential ‘bridge-to-surgery’ with curative-intent has gone largely unrecognized among treatment algorithms for patients with cancer. Due to the novelty and general unfamiliarity with LAMS technology, placement of a LAMS may create a perception of greater disease severity. This combined with concerns over increased operative technical complexity due to the LAMS likely contributes to the continued disuse of LAMS in the curative oncologic domain. Notably, LAMS has since been employed in non-surgical candidates with periampullary cancers resulting from duodenal and malignant distal biliary obstruction (MDBO). The most commonly reported use of LAMS for MDBO involves creation of a palliative choledochoduodenostomy (CDS) to decompress the biliary tree after failed ERCP [6,7]. Several reports now describe the rare occurrence of a pancreaticoduodenectomy (PD) performed after placement of a LAMS CDS [[8], [9], [10]].
Similarly, the world literature is limited to two case reports of a PD conducted after a LAMS gastrojejunostomy (GJ) [11,12]. PD after a LAMS gastrogastrostomy (GG) or jejunogastrostomy (JG) deployed in patients with periampullary tumors with a previous Roux-en-Y gastric bypass (RYGB) for EUS-Directed transGastric ERCP (EDGE) remains undescribed. In this case series, the early surgical experience and short-term outcomes at a high-volume North American (Eastern Atlantic) tertiary referral center are highlighted. Three separate clinical case scenarios are presented in patients that underwent a LAMS procedure prior to a PD with the intent to contribute to a small but growing body of evidence supporting the potential feasibility of LAMS in the setting of surgery with curative-intent.
1.1. Case series presentation
Six patients were identified from our institutional review board (IRB)-approved pancreas cancer database (IRB approval number: 22E.136) who underwent LAMS placement prior to undergoing a PD or pylorus-preserving PD (PPPD) from 2020 to 2023. A retrospective chart review of this cohort was completed, and data recorded including patient demographics, medical and surgical history, oncologic evaluation, radiologic assessment/response evaluation criteria in solid tumors (RECIST), endoscopic LAMS procedures, surgical course, pathologic results, and short-term treatment outcomes. A descriptive analysis of the data was completed. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed during the conduct of this study. A STROBE checklist was used in conjunction for data reporting [13].
2. Results
Two males and four females with a mean age of 63.5 years (SD ± 8.3 years) and mean Charlson comorbidity index of 5 were identified and found to have a hepatopancreatobiliary or duodenal malignancy that underwent EUS-guided LAMS prior to undergoing PD or PPPD at our institution. The indications for LAMS proceeding to PD are presented herein among three clinical scenarios: Scenario I- duodenal obstruction in two patients, Scenario II- distal biliary obstruction in one patient, and Scenario III - LAMS as part of an EDGE procedure in three patients with periampullary cancers.
2.1. Lumen-apposing metal stent procedural details
The technical aspects behind performing EUS-guided LAMS for creation of a bilioenteric or enteroenteric fistula has been previously described. Briefly, EUS LAMS is a single-staged transluminal stenting procedure between two nonadherent lumens of the digestive tract using a bi-flanged covered metal stent establishing lumen-to-lumen apposition (Fig. 1). [14,15] Technical considerations for each of the clinical scenarios (CS I-III) are summarized below.
Fig. 1.
A: Structural depiction of bi-flanged covered lumen apposing metal stent (LAMS). B: Schematic of electrocautery-enhanced LAMS deployment. (Credit for this figure is given to Boston Scientific – AXIOS™ stent).
In the cases of LAMS GJ (CS I; patients 1 and 2), EUS was performed with LAMS deployment between the stomach and proximal jejunal lumens separated by less than 1 cm and devoid of blood vessels. In each case a 20 mm × 10 mm electrocautery-enhanced LAMS was interpositioned under EUS, fluoroscopic, and endoscopic guidance. The LAMS was subsequently dilated with an 18 mm balloon (Fig. 2A).
Fig. 2.
CT and Endoscopic Images stratified by clinical scenario (CS). A: CS 1: EUS-guided LAMS gastrojejunostomy. B: CS 2: EUS-guided LAMS choledochoduodenostomy. C: CS 3: EUS-guided LAMS gastrogastrostomy* CT: computed tomography; EUS: endoscopic ultrasound; LAMS: lumen apposing metal stent; EDGE procedure: endoscopic ultrasound directed transgastric ERCP *gastrogastrostomy from gastric pouch to remnant stomach.
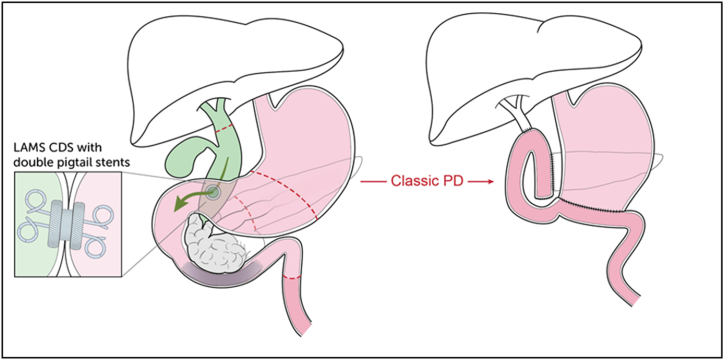
In the case of LAMS CDS (CS II; patient 3), the major papilla was unable to be identified due to mass effect upon the second portion of the duodenum, and biliary cannulation was not attempted. EUS revealed dilation of the common bile duct to 2 cm with a sludge-filled lumen. There were no abnormal lymph nodes and the portal vein appeared uninvolved. A 10 mm × 10 mm lumen apposing metal stent was deployed from the duodenal bulb to the common bile duct. Two 6 French x 4 cm double pigtail stents were placed co-axially across the LAMS to optimize drainage (Fig. 2B).
In CS III (patients 4–6), a LAMS GG or LAMS JG was placed as part of the EDGE procedure. EUS was performed and the excluded stomach visualized. The distance between the gastric pouch or proximal jejunum and excluded stomach was less than 1 cm and an avascular path for the LAMS was confirmed. A 20 mm × 10 mm electrocautery-enhanced LAMS was deployed creating a JG or GG and each was dilated with an 18 mm balloon. The proximal flange of the LAMS was sutured in place (Fig. 2C).
2.2. LAMS outcomes
Overall, the hospital length of stay after LAMS placement ranged from 0 to 3 days. There were no complications related to the LAMS procedure, including no perforation, stent migration or bleeding events. In the two patients (CS I) who had a LAMS GJ for duodenal obstruction, the stomach was decompressed, and each patient was able to progress to an oral diet and achieve preoperative nutritional parameters. The LAMS CDS (CS II) successfully decompressed the biliary tree with normalization of serologic bilirubin levels and hepatic profile. In each of the three cases of LAMS as part of the EDGE procedure (CS III), EUS was able to be performed through the LAMS so that the periampullary tumors could be biopsied. The LAMS also allowed for ERCP and biliary drainage (Table 1).
Table 1.
Distribution of Patient demographics and outcomes after LAMS interventions.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Age (years) | 62 | 51 | 60 | 61 | 78 | 69 |
| Sex | Male | Female | Male | Female | Female | Female |
| Tumor Location | HOP | Duodenum-D3 | Peri-ampullary | Duodenum-D2-D3 | HOP | HOP |
| Tumor Size | 2.6 × 3.2cm | 4.5 × 2.6cm | 2.4 × 2.3cm | 9.0 × 5.5cm* | 1.3 × 1.1cm | 1.8 × 2.3cm |
| Endoscopic Interventions (LAMS excluded) | FNA | CBD and duodenal stents, FNA | duodenal stent, FNA | CBD stent, FNA | CBD and PD stents, FNA | CBD stent, FNA |
| Indication for LAMS | Duodenal Obstruction | Duodenal Obstruction | MDBO | RYGB | RYGB | RYGB |
| LAMS Intervention | G-J | G-J | CDS | J-G | G-G | G-G |
| LOS after LAMS | 0 days | 1 day | 3 days | 2 days | 1 day | 2 days |
| Interval from LAMS to Surgery | 181 days | 98 days | 49 days | 28 days | 28 days | 194 days |
| Neoadjuvant Therapy | None | FOLFOX | None | None | None | FOLFIRINOX |
HOP: Head of Pancreas; FNA: Fine-needle Aspiration; CBD: Common Bile Duct; PD: Pancreatic Duct; EUS: Endoscopic Ultrasound; MDBO: Malignant Distal Biliary Obstruction; RYGB: Roux-en-Y Gastric Bypass; G-J: Gastrojejunostomy; J-G: Jejunogastrostomy CDS: Choledochoduodenostomy; G-G: Gastrogastrostomy; LAMS: Lumen opposing metal stent; LOS: Length of stay; FOLFOX: folinic acid, fluorouracil, oxaliplatin; FOLFIRINOX: folinic acid, fluorouracil, irinotecan, oxaliplatin *Circumferential wall thickening of the second and third portion of the duodenum (measurement from gross pathology).
Two patients received and completed neoadjuvant chemotherapy after LAMS placement. In all cases, patients proceeded with PD (five cases) or PPPD (one case). The time from LAMS insertion to surgery ranged from 28 to 194 days (Table 1).
2.3. Pancreaticoduodenectomy operative details
In each case, an open exploratory laparotomy was performed, and the abdominopelvic cavity evaluated to verify absence of visible metastatic disease prior to proceeding. PD was then conducted, preserving the pylorus in one of the six cases [16]. Operative reconstruction entailed either performance of an end-to-side two-layered invagination or transpancreatic U-suture technique with a duct-to-mucosa pancreaticojejuniostomy (PJ) anastomosis [17,18], end-to-side one-layer hepaticojejunostomy (HJ), and lastly an end-to-side two-layered GJ or duodenojejunostomy (DJ) [19]. In all three PDs performed after RYGB, the gastric remnant (GR) was spared, performing either a GR-PD or GR-PPPD with the jejuno-gastric remnant anastomosed for drainage in-series with the PJ and HJ to a single jejunal limb raised from the old common channel. Restoration of gastrointestinal continuity thereafter occurred between the new limb with respective biliopancreatic and GR drainage anastomosed to the distal portion of the old alimentary limb [20]. Prior to abdominal closure, two drains were positioned in place, one near the HJ and one near the PJ. A standard post-Whipple accelerated recovery pathway (WARP) at our institution directed the management of patient recovery [21].
2.4. Operative conduct as relates to in situ LAMS
In case scenario I, a LAMS GJ was employed for patients 1 and 2. For each patient, the LAMS was positioned between the corpus of the stomach and proximal jejunum. In both cases the LAMS traversed the transverse mesocolon creating significant inflammation and fibrosis necessitating a time-intensive (≥ 1 hour) lysis of adhesions to adequately assess the LAMS-associated inflammation and potential implications toward resection and reconstruction.
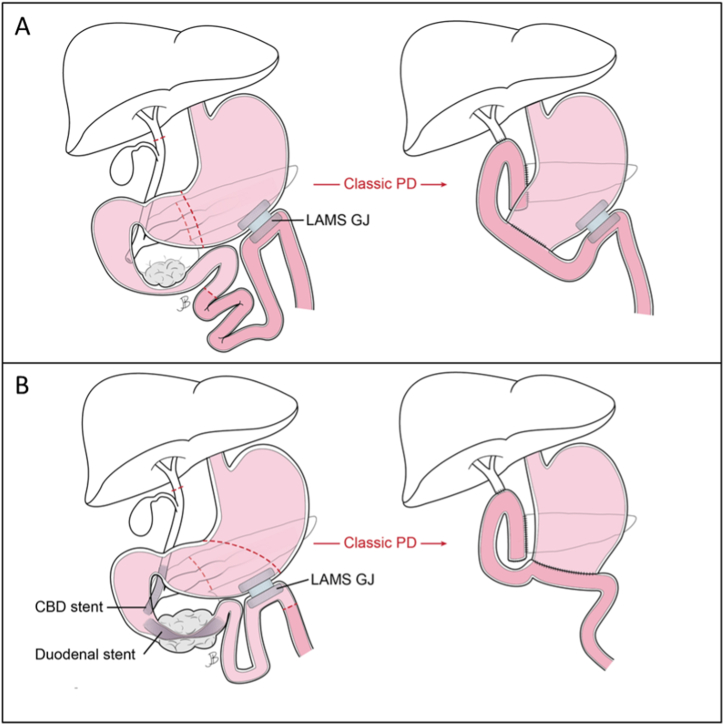
For patient 1, the placement of the LAMS in the more-distal jejunum allowed for the LAMS GJ to remain in situ. Pylorus preservation was not performed due extensive adhesions involving the antrum and duodenum. Sufficient mobilization of the remaining afferent biliopancreatic limb allowed reconstruction of the PJ and HJ anastomoses (Fig. 3A).
Fig. 3.
Clinical Scenario 1: A: EUS-guided LAMS gastrojejunostomy for malignant duodenal obstruction and subsequent classic PD (LAMS left in situ) (Patient 1). B: EUS-guided LAMS gastrojejunostomy for malignant duodenal obstruction with subsequent classic PD (LAMS removed with specimen) (Patient 2). EUS: Endoscopic Ultrasound; LAMS: lumen apposing metal stent; PD: pancreaticoduodenectomy; CBD: common bile duct.
In patient 2, the more proximal positioning of the LAMS in the jejunum, in close proximity to the ligament of Trietz, required the separate extraction of both the gastric and jejunal flanges of the LAMS. The LAMS-created fistulous tract was resected en bloc with the PD specimen, precluding pylorus preservation (Fig. 3B) [12].
In case scenario II (patient 3), the LAMS CDS was removed en bloc with the specimen (Fig. 4). Notably, extensive adhesions were encountered which were believed to be attributable to the LAMS, again necessitating a classic PD.
Fig. 4.
Clinical Scenario 2: EUS-guided LAMS choledochoduodenostomy with double-pigtail stents with subsequent classic PD (LAMS removed with specimen) (Patient 3). EUS: endoscopic ultrasound; LAMS: lumen apposing metal stent; PD: pancreaticoduodenectomy; CDS: choledochoduodenostomy.
Lastly in case scenario-III, for each of the patients with a history of RYGB (patients 4–6), the GG or JG positioning of the LAMS appeared to have no overall impact on the surgeon's decision to perform pylorus preservation. In each case, the LAMS JG or GG was left in situ (Fig. 5).
Fig. 5.
Clinical Scenario 3: Roux-en-Y gastric bypass anatomy. A: EUS-guided LAMS jejunogastrostomy* for completion of EDGE procedure with subsequent gastric remnant PPPD (LAMS left in situ) (Patient 4). B: EUS-guided LAMS gastrogastrostomy for completion of EDGE procedure with subsequent gastric remnant classic PD (LAMS left in situ) (Patients 5–6). EUS: endoscopic ultrasound; LAMS: lumen apposing metal stent; EDGE procedure: endoscopic ultrasound directed transgastric ERCP; PPPD: pylorus-preserving pancreaticoduodenectomy; PD: pancreaticoduodenectomy *jejunogastrostomy from proximal alimentary limb to remnant stomach.
2.5. Pancreaticoduodenectomy operative outcomes
Mean operative time was 513 minutes, and mean EBL was 560 mL. The initial post-PD hospital length of stay ranged from 4 to 17 days. Patients 2 and 5 experienced Clavien-Dindo IIIb [22] morbidity requiring percutaneous drainage of intra-abdominal collections post-operatively. Patient 2 was readmitted to the hospital 5 days after discharge with signs of sepsis. The patient was successfully treated with antibiotics and image-guided drainage of fluid collections in the gallbladder fossa and right retroperitoneum from a Grade B23 postoperative pancreatic fistula. Patient 5 was noted to have a bile leak on the first post-operative day at which time a percutaneous transhepatic biliary drain was placed and later developed a Grade A23 postoperative pancreatic fistula that was controlled with a surgical drain from the index operation. There were no 90-day mortality events (Table 2). At the time of this report, patient 5 was recently deceased, surviving 721 days after surgery, while all other patients remain alive, at a mean of 361 days post-PD.
Table 2.
Distribution of pancreaticoduodenectomy operative and treatment outcomes.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Type of Surgery | Classic PD | Classic PD | Classic PD | PPPD | Classic PD | Classic PD |
| LAMS-related Intraoperative Complications | Dense Adhesions | Dense Adhesions | Dense Adhesions | None | None | None |
| Operative Intervention on LAMS | Left in Place | Removed with Specimen | Removed with Specimen | Left in Place | Left in Place | Left in Place |
| Lymph Nodes Resected | 38 | 16 | 29 | 24 | 12 | 11 |
| Postoperative Complications | None | IAA, POPF (Grade B)a | None | None | Biliary Fistula, POPF (Grade A)a | None |
| Post-PD LOS | 4 days | 5 days | 7 days | 6 days | 17 days | 5 days |
| 30-Day Readmission | No | Yes | No | No | No | No |
| Final Pathology | IPMN with Invasive AdenoCa | Duodenal AdenoCa | IPMN with Invasive AdenoCa | Ampullary AdenoCa | PDAC | PDAC |
| Pathologic Stage | IA – T1cN0M0 | IIIA – T3N1M0 | III – T3N2M0 | IIA – T3aN0M0 | IIA – T3N0M0 | IA – T1aN0M0 |
PD: Pancreaticoduodenectomy; PPPD: Pylorus-preserving pancreaticoduodenectomy; IAA: Intra-abdominal abscess; POPF: Postoperative Pancreatic Fistula; IPMN: Intraductal Papillary Mucinous Neoplasm; AdenoCa: Adenocarcinoma; PDAC: Pancreatic Ductal Adenocarcinoma.
Grading for POPF is based on the International Study Group of Pancreatic Surgery Grading System [23]; LOS: Length of stay.
3. Discussion
This case series highlights an early assessment of potential feasibility of performing a PD after EUS-LAMS in three clinical scenarios including duodenal obstruction (LAMS GJ), distal biliary obstruction (LAMS CDS), and as part of an EDGE procedure (LAMS JG or GG). To date, utilization of EUS-guided LAMS technology has largely been employed for palliation in patients with inoperable malignancies and patients deemed unfit for surgery. Concerns of risk from intraoperative interference of the stents has precluded patient candidacy for surgery with oncologic curative-intent. To our knowledge, the world literature comprises only a composite reporting of 35 patients who have undergone LAMS prior to PD [[8], [9], [10], [11], [12]]. Herein, we report our short-term outcomes among three clinical scenarios, each with varying implications.
In clinical scenario I, a LAMS GJ to bypass duodenal obstructions provided durable decompression while permitting an oral diet. The LAMS GJ served as a bridge-to-surgery in both patients as defined by our preoperative critical pathway [24] with the opportunity to optimize nutritional parameters, maintain a physiologic ECOG performance status ≤2, and the ability to undergo neoadjuvant therapy (FOLFOX) for patient 2. In this case scenario, the surgeons reportedly encountered LAMS-associated adhesions that affected pylorus preservation. At the time of PD, the LAMS was left in situ in patient 1, and removed with the specimen in patient 2. A post-operative intra-abdominal abscess and grade B post-operative pancreatic fistula (POPF) [23] occurred in patient 2, that was successfully managed with percutaneous drainage. This patient subsequently recovered well and completed adjuvant chemotherapy. In patient 1, who has both a surgical GJ and a LAMS GJ, another important consideration is concern over the increased risk of marginal ulceration and bile reflux gastritis. In our early institutional experience, the LAMS can be endoscopically removed, and the epithelialized fistulous tract closed. However, there are currently no reports, to our knowledge, on the optimal management strategy in this scenario.
In a prior single case experience involving LAMS GJ prior to PD, the LAMS GJ was removed intraoperatively. After removal of the LAMS, the gastrotomy was stapled closed, and the involved loop of proximal jejunum was removed as part of the specimen. An uncomplicated surgical GJ was performed as part of the reconstruction and there were no perceived LAMS-related postoperative complications [11].
As pertains to clinical scenario II, a LAMS CDS prior to PD is the most studied scenario to date. A recent cohort analysis published in patients with MDBO compared 28 patients who underwent LAMS CDS to 128 patients who had trans-papillary stenting prior to PD that revealed a lower overall rate of surgical complications in the LAMS CDS group [10]. Notably, a French multicenter study did not report upon the consequent adverse effects of the LAMS CDS on the surgical hepaticojejunostomy. An increased risk of biliary fistula formation secondary to a LAMS CDS has gone unreported [[8], [9], [10]]. Previously noted in our single LAMS CDS patient, adhesions surrounding the LAMS CDS precluded pylorus preservation.
In clinical scenario III (patients 4–6), the patients underwent a LAMS GG (gastric pouch to gastric remnant) or JG (jejunal alimentary limb to gastric remnant) during an EDGE procedure to facilitate performance of an EUS and ERCP to obtain a tissue diagnosis and biliary drainage. In each patient, surgical resection during the PD entailed sparing of the gastric remnant (GR). The LAMS GG or JG was left in place in each patient after resection. In retrospect, the LAMS was inconsequential regarding choice of operative reconstruction in these cases. Moreover, we do acknowledge that performance of classic PD versus PPPD is likely inconsequential given that the altered anatomy in the RYGB patient obviates the inherent physiologic advantages of pylorus preservation, and therefore was not influential in the surgical decision making [25]. Notably, a classic PD was ultimately decided upon in patients 5 and 6 over concerns of antropyloric GR tumor encroachment. In patient four, a GR-PPPD did not preclude R0 resection for a T3a ampullary adenocarcinoma and was preferred by the lead surgeon. Among the case scenario III patients, patient 5 experienced a biliary fistula and grade A POPF [23] that were successfully managed with percutaneous drainage. In patient 4, the JG LAMS was removed three months after surgery.
Overall, there is a small, but growing, body of literature assessing the feasibility of PD after EUS-LAMS [[8], [9], [10], [11], [12]]. A review article by Vanella et al. evaluated the existing data for successful PD after EUS-LAMS proposing strategies for mitigating LAMS-associated surgical complications. Regarding LAMS CDS, it was proposed that LAMS placement from the duodenal bulb to the most distal common bile duct allows for the LAMS to remain within the surgical margins of the intended specimen therefore permitting more space for bile duct transection and surgical HJ reconstruction. For LAMS GJ, placing the LAMS from the stomach to the most proximal jejunum may minimize the amount of jejunum requiring resection prior to reconstruction [26]. To our knowledge, current world literature is devoid of reports describing PD after LAMS GG or JG performed as a part of the EDGE procedure in patients with post RYGB anatomy. In our series of three patients with this clinical scenario, the LAMS GG or JG did not directly impact operative resection or reconstruction considerations as site of the LAMS was apart from the intended surgical specimen otherwise not directly affecting the operative field.
Importantly, the overall morbidity rate after PD approximates 40–50 % [[27], [28], [29]]. Our early experience suggests that inclusion of LAMS in the treatment armamentarium for periampullary cancers prior to PD confers acceptable feasibility and risk. Notably, among the three cases involving EUS LAMS GJ (patients 1–2) and CDS (patient 3), the LAMS did appear to impact the ability to perform pylorus preservation. In the three patients who had a prior RYGB (patients 4–6), the JG or GG LAMS placed during the EDGE procedure did not affect operative resection or reconstruction. Indeed, our sample size is small and in this preliminary assessment the favorable short-term outcomes provide reasonable equipoise to consider further studies involving pancreatic resection after LAMS.
There are clearly limitations of this small case series. These patients were treated at a high-volume pancreatic surgery center with advanced endoscopists who are experienced in placing LAMS. Therefore, the results of this case series may not be generalizable to other institutions or patient populations. This is a retrospective study, and the small patient cohort is subject to selection bias. Finally, there is no control group in this study, limiting the ability to assess the impact of EUS-LAMS prior to PD on short-term treatment outcomes. We present our institution's experience with these clinical scenarios with an aim to assess potential feasibility. More studies are needed with larger sample sizes comparing perioperative and oncologic outcomes, including the individual assessment for each type of LAMS as it becomes increasingly utilized as indications expand.
4. Conclusion
In this case series, we found that PD after EUS-guided LAMS represents a potentially feasible option with acceptable morbidity in select patients. LAMS as a bridge to definitive surgical resection warrants further consideration with additional studies including a larger sample size to draw definitive conclusions.
Funding
No funding was received for this study.
Ethics and informed consent
The patient information included in this series was obtained from our institutional review board-approved pancreatic cancer database at our institution. Consent from the involved patients was obtained for publication.
Presentations
The content of this manuscript has been presented as a poster presentation in two local meetings in Philadelphia in 2023: the Metropolitan Philadelphia American College of Surgeons meeting, and the Thomas Jefferson Pancreatic Cancer and Related Diseases Symposium. The content of this manuscript was presented in part as an oral presentation at the Academic Surgical Congress meeting, February 6–8, 2024, Washington, DC.
Data availability statement
The patient data used to complete this study was not deposited into a publicly available repository. The patient information for this study was obtained through an institutional review board approved institutional database. Data may be made available upon request.
CRediT authorship contribution statement
Luke T. Meredith: Writing – review & editing, Writing – original draft, Supervision, Methodology, Data curation, Conceptualization. David Baek: Writing – original draft, Data curation, Conceptualization. Alisha Agarwal: Writing – original draft, Data curation, Conceptualization. Faisal Kamal: Writing – review & editing, Supervision, Conceptualization. Anand R. Kumar: Writing – review & editing, Supervision, Conceptualization. Alexander Schlachterman: Writing – review & editing, Supervision, Conceptualization. Thomas E. Kowalski: Writing – review & editing, Supervision, Conceptualization. Charles J. Yeo: Writing – review & editing, Supervision, Conceptualization. Harish Lavu: Writing – review & editing, Supervision, Conceptualization. Avinoam Nevler: Writing – review & editing, Supervision, Conceptualization. Wilbur B. Bowne: Writing – review & editing, Writing – original draft, Supervision, Methodology, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Thomas Kowalski, MD reports a relationship with Boston Scientific that includes: consulting or advisory. Anand Kumar, MD reports a relationship with Olympus that includes: consulting or advisory. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to express gratitude to Jennifer Brumbaugh, MA, for the illustrations displayed in Fig. 3, Fig. 4, Fig. 5, and to the Thomas Jefferson University Hospital nursing staff and surgical residents, who provided excellent care to the patients reported in this case series.
Footnotes
Departmental Twitter: @JEFFsurgery.
References
- 1.Bang J.Y., Varadarajulu S. Lumen-apposing metal stents for endoscopic ultrasonography-guided interventions. Dig. Endosc. 2019;31(6):619–626. doi: 10.1111/den.13428. [DOI] [PubMed] [Google Scholar]
- 2.Guzman-Calderon E., Chacaltana A., Diaz R., et al. Head-to-head comparison between endoscopic ultrasound guided lumen apposing metal stent and plastic stents for the treatment of pancreatic fluid collections: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2022;29(2):198–211. doi: 10.1002/jhbp.1008. [DOI] [PubMed] [Google Scholar]
- 3.Saumoy M., Yarber C., Kahaleh M. Novel uses of lumen-apposing metal stents. Gastrointest Endosc Clin N Am. 2018;28(2):197–205. doi: 10.1016/j.giec.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Tyberg A., Karia K., Gabr M., et al. Management of pancreatic fluid collections: a comprehensive review of the literature. World J. Gastroenterol. 2016;22(7):2256–2270. doi: 10.3748/wjg.v22.i7.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda I., Takahashi K. Endoscopic management of walled-off pancreatic necrosis. Dig. Endosc. 2021;33(3):335–341. doi: 10.1111/den.13699. [DOI] [PubMed] [Google Scholar]
- 6.On W., Paranandi B., Smith A.M., et al. EUS-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing metal stents in patients with malignant distal biliary obstruction: multicenter collaboration from the United Kingdom and Ireland. Gastrointest. Endosc. 2022;95(3):432–442. doi: 10.1016/j.gie.2021.09.040. [DOI] [PubMed] [Google Scholar]
- 7.Teoh A.Y.B., Napoleon B., Kunda R., et al. EUS-guided choledocho-duodenostomy using lumen apposing stent versus ERCP with covered metallic stents in patients with unresectable malignant distal biliary obstruction: a multicenter randomized controlled trial (DRA-MBO trial) Gastroenterology. 2023;165(2):473–482 e472. doi: 10.1053/j.gastro.2023.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Fabbri C., Fugazza A., Binda C., et al. Beyond palliation: using EUS-guided choledochoduodenostomy with a lumen-apposing metal stent as a bridge to surgery. a case series. J Gastrointestin Liver Dis. 2019;28(1):125–128. doi: 10.15403/jgld.2014.1121.281.eus. [DOI] [PubMed] [Google Scholar]
- 9.Gaujoux S., Jacques J., Bourdariat R., et al. Pancreaticoduodenectomy following endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents an ACHBT - SFED study. HPB (Oxford) 2021;23(1):154–160. doi: 10.1016/j.hpb.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Janet J., Albouys J., Napoleon B., et al. Pancreatoduodenectomy following preoperative biliary drainage using endoscopic ultrasound-guided choledochoduodenostomy versus a transpapillary stent: a multicenter comparative cohort study of the ACHBT-French-SFED intergroup. Ann. Surg Oncol. 2023;30(8):5036–5046. doi: 10.1245/s10434-023-13466-8. [DOI] [PubMed] [Google Scholar]
- 11.Vanella G., Tamburrino D., Dell'Anna G., et al. Endoscopic ultrasound-guided gastrojejunostomy does not prevent pancreaticoduodenectomy after long-term symptom-free neoadjuvant treatment. Endoscopy. 2022;54(4):E143–E145. doi: 10.1055/a-1408-1180. [DOI] [PubMed] [Google Scholar]
- 12.Yu T.Z., Agnihotri A., Zheng R., et al. Salvage endoscopic ultrasound-guided gastrojejunostomy as a bridge to definitive surgical therapy for duodenal adenocarcinoma presenting with duodenal stent obstruction. Clin J Gastroenterol. 2023;16(3):387–391. doi: 10.1007/s12328-023-01781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Mussetto A., Fugazza A., Fuccio L., et al. Current uses and outcomes of lumen-apposing metal stents. Ann. Gastroenterol. 2018;31(5):535–540. doi: 10.20524/aog.2018.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prepared by ATC. Law R.J., Chandrasekhara V., et al. Lumen-apposing metal stents (with videos) Gastrointest. Endosc. 2021;94(3):457–470. doi: 10.1016/j.gie.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Yeo C.J. Pylorus-preserving pancreaticoduodenectomy. Surg Oncol Clin N Am. 1998;7(1):143–156. [PubMed] [Google Scholar]
- 17.Grobmyer S.R., Kooby D., Blumgart L.H., Hochwald S.N. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J. Am. Coll. Surg. 2010;210(1):54–59. doi: 10.1016/j.jamcollsurg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy E.P., Yeo C.J. Dunking pancreaticojejunostomy versus duct-to-mucosa anastomosis. J Hepatobiliary Pancreat Sci. 2011;18(6):769–774. doi: 10.1007/s00534-011-0429-y. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy E.P., Brumbaugh J., Yeo C.J. Reconstruction following the pylorus preserving Whipple resection: PJ, HJ, and DJ. J. Gastrointest. Surg. 2010;14(2):408–415. doi: 10.1007/s11605-009-1066-5. [DOI] [PubMed] [Google Scholar]
- 20.Morano W.F., Shaikh M.F., Gleeson E.M., et al. Reconstruction options following pancreaticoduodenectomy after Roux-en-Y gastric bypass: a systematic review. World J. Surg. Oncol. 2018;16(1):168. doi: 10.1186/s12957-018-1467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavu H., McCall N.S., Winter J.M., et al. Enhancing patient outcomes while containing costs after complex abdominal operation: a randomized controlled trial of the whipple accelerated recovery pathway. J. Am. Coll. Surg. 2019;228(4):415–424. doi: 10.1016/j.jamcollsurg.2018.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clavien P.A., Barkun J., de Oliveira M.L., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann. Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 23.Bassi C., Dervenis C., Butturini G., et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy E.P., Rosato E.L., Sauter P.K., et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution--the first step in multidisciplinary team building. J. Am. Coll. Surg. 2007;204(5):917–923. doi: 10.1016/j.jamcollsurg.2007.01.057. ; discussion 923-914. [DOI] [PubMed] [Google Scholar]
- 25.Kang C.M., Lee J.H. Pathophysiology after pancreaticoduodenectomy. World J. Gastroenterol. 2015;21(19):5794–5804. doi: 10.3748/wjg.v21.i19.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanella G., Tamburrino D., Capurso G., et al. Feasibility of therapeutic endoscopic ultrasound in the bridge-to-surgery scenario: the example of pancreatic adenocarcinoma. World J. Gastroenterol. 2022;28(10):976–984. doi: 10.3748/wjg.v28.i10.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron J.L., He J. Two thousand consecutive pancreaticoduodenectomies. J. Am. Coll. Surg. 2015;220(4):530–536. doi: 10.1016/j.jamcollsurg.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 28.He J., Ahuja N., Makary M.A., et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford) 2014;16(1):83–90. doi: 10.1111/hpb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruangsin S., Sunpaweravong S., Laohawiriyakamol S. Achievement of benchmark outcomes by a young surgical attendant performing pancreatoduodenectomies. Langenbeck's Arch. Surg. 2023;408(1):404. doi: 10.1007/s00423-023-03132-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The patient data used to complete this study was not deposited into a publicly available repository. The patient information for this study was obtained through an institutional review board approved institutional database. Data may be made available upon request.