Abstract
Background
Diagnosing sternal wound infection (SWI) following median sternotomy remains laborious and troublesome, resulting in high mortality rates and great harm to patients. Early intervention and prevention are critical and challenging. This study aimed to develop a simple risk prediction model to identify high-risk populations of SWI and to guide examination programs and intervention strategies.
Methods
A retrospective analysis was conducted on the clinical data obtained from 6715 patients who underwent median sternotomy between January 2016 and December 2020. The least absolute shrink and selection operator (LASSO) regression method selected the optimal subset of predictors, and multivariate logistic regression helped screen the significant factors. The nomogram model was built based on all significant factors. Area under the curve (AUC), calibration curve and decision curve analysis (DCA) were used to assess the model's performance.
Results
LASSO regression analysis selected an optimal subset containing nine predictors that were all statistically significant in multivariate logistic regression analysis. Independent risk factors of SWI included female [odds ratio (OR) = 3.405, 95% confidence interval (CI) = 2.535–4.573], chronic obstructive pulmonary disease (OR = 4.679, 95% CI = 2.916–7.508), drinking (OR = 2.025, 95% CI = 1.437–2.855), smoking (OR = 7.059, 95% CI = 5.034–9.898), re-operation (OR = 3.235, 95% CI = 1.087–9.623), heart failure (OR = 1.555, 95% CI = 1.200–2.016) and repeated endotracheal intubation (OR = 1.975, 95% CI = 1.405–2.774). Protective factors included bone wax (OR = 0.674, 95% CI = 0.538–0.843) and chest physiotherapy (OR = 0.446, 95% CI = 0.248–0.802). The AUC of the nomogram was 0.770 (95% CI = 0.745–0.795) with relatively good sensitivity (0.798) and accuracy (0.620), exhibiting moderately good discernment. The model also showed an excellent fitting degree on the calibration curve. Finally, the DCA presented a remarkable net benefit.
Conclusions
A visual and convenient nomogram-based risk calculator built on disease-associated predictors might help clinicians with the early identification of high-risk patients of SWI and timely intervention.
Keywords: Sternal wound infection, Nomogram, Median sternotomy, Prediction model, Postcardiac surgical complications
Highlights.
A retrospective cohort study was conducted based on a large clinical dataset of 6715 patients who underwent median sternotomy.
The LASSO-logistic regression method was utilized to identify the risk and protective factors associated with sternal wound infection.
A visual and computable risk nomogram model for sternal wound infection was constructed based on all significant predictive factors, providing a convenient and efficient tool for the clinical identification of high-risk patients and offering reference and guidance for early clinical decision-making.
Background
Since its first application in 1957, median sternotomy has become widely recognized as the standard surgical approach for cardiac surgery [1], thereby rendering sternal wound infection (SWI) one of the most prevalent and troublesome complications associated with this surgical incision [1]. Despite gradual advancements in surgical technique, the routine administration of prophylactic antibiotics and the continuous optimization of blood glucose management, the incidence rate of SWI still varies between 0.5 and 10% [2]. Without timely diagnosis and appropriate treatment, the mortality rate could reach as high as 47% [3].
Currently, once a diagnosis of SWI is established, many reconstruction algorithms based on factors such as wound depth, the extent and location of sternal defect and the patient's comorbidities could help yield satisfactory outcomes [4]. Undoubtedly, the process involving recurrent debridement and surgical reconstruction could cause additional mental and physical distress, prolong hospital stays and increase medical costs [5]. To explore the pathogenesis and reduce the incidence of SWI, numerous studies have found many associated risk factors including, but are not limited to, advanced age, obesity, diabetes, chronic obstructive pulmonary disease (COPD), smoking, prolonged surgery duration, use of blood products and bone wax, re-operation, and prolonged mechanical ventilation [1, 5]. However, a global consensus on the critical factors has not yet been reached within the academic community. Compared to the evolving surgical reconstruction strategies and the ongoing improvement of perioperative management, the early identification of high-risk patients remains the foremost task and the major challenge in clinical practice.
Several meticulously designed scoring methodologies and risk assessment tools have been established to predict the likelihood of postoperative infections at the surgical site following cardiac surgery [6–8]. However, there remains a scarcity of predictive models specifically tailored to SWI. The Gatti scoring system has been validated to effectively predict the risk of SWI for patients who underwent bilateral internal mammary artery (BIMA) grafting in coronary artery bypass grafting (CABG) surgery [9]. Furthermore, most of the current prediction models are based on small-sample clinical data, the corresponding risk factors do not cover the entire disease course, and these models often lack visual representation, thereby limiting their clinical applicability. Additionally, studies focusing on SWI risk factors within the Chinese population are relatively scarce.
Thus, based on a large-sample clinical data, this study conducted an analysis of risk and protective factors in patients receiving median sternotomy and developed a nomogram-based risk prediction model. The study aimed to provide a convenient and effective tool for clinical staff to screen out high-risk patients for SWI and to offer reference and guidance for early clinical decision-making.
Methods
Study design
This study was designed as a retrospective cohort study. Between January 2016 and December 2020, a total of 23,604 individuals requiring surgical intervention for an assortment of cardiac conditions were admitted to Xijing Hospital of the Air Force Medical University. We reviewed the historic medical records of these 23,604 cases to identify clinically valuable predictors and to develop a nomogram model for predicting the risk of developing SWI.
Patient selection and diagnostic criteria
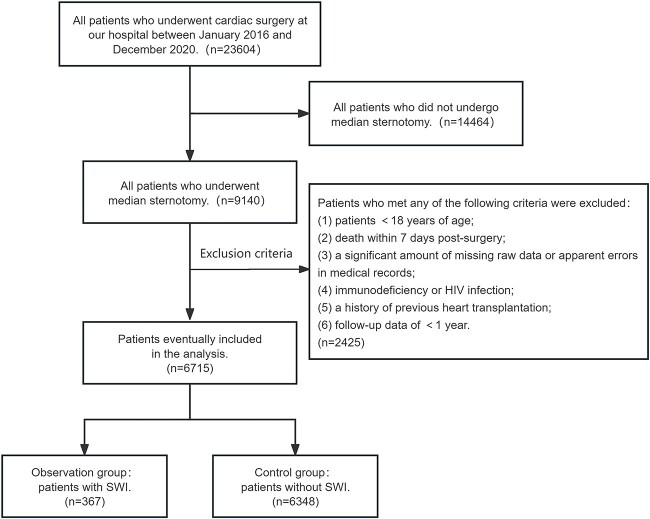
The process of selecting the target population is depicted in Figure 1. All patients who underwent full median sternotomy in the department of cardiovascular surgery at Xijing Hospital during the study period were included. Subsequently, further screening was conducted using the following exclusion criteria: (1) patients <18 years of age; (2) death within 7 days post-surgery; (3) a significant amount of missing raw data or apparent errors in medical records; (4) immunodeficiency or HIV infection; (5) a history of previous heart transplantation; and (6) follow-up data of <1 year. In this study, the diagnostic criteria for SWI employed the version published by the US Centers for Disease Control and Prevention (CDC) [10].
Figure 1.
Flowchart showing the process of selecting patients for the study. HIV human immunodeficiency virus, SWI sternal wound infection
Data collection
According to previous researches and our clinical observations, historical data was extracted from our institution's digital medical documentation. Basic information included sex, birth, smoking and drinking history, and date of operation. Previous operation history included cardiac interventional surgery and re-operation. Primary admission diagnosis included congenital heart disease, coronary heart disease, rheumatic heart disease, dilated macrovascular disease, cardiomyopathy, infectious endocarditis, pericardial disease, and cardiac and mediastinal tumor. Comorbidities included heart failure, hypertension, diabetes, hyperlipemia, COPD, hypoproteinemia, anemia. Surgical details included bone wax, BIMA grafting, vasotransplantation, valve transplantation, duration of surgery, and emergency surgery. Postoperative medical records included laboratory results, length of intensive care unit (ICU) stay, chest physiotherapy (CPT), number of endotracheal intubation, duration of mechanical ventilation, New York Heart Association (NYHA) class, postoperative pulmonary infection, sternal complications manifestations and length of total hospital stay.
Definitions
Based on previous researches and reports, explicit definitions for certain variables are provided here. Re-operation meant at least one sternotomy or open-chest surgery were performed during hospitalization due to emergent conditions following the initial median sternotomy. Dilated macrovascular disease predominantly encompassed aneurysms and aortic dissections [11]. CPT primarily referred to mechanical-assisted sputum clearance techniques, which can effectively promote sputum drainage [12]. According to the 2015 European Society of Cardiology guidelines for the diagnosis and management of pericardial diseases, pericardial diseases mainly referred to conditions such as pericarditis, pericardial effusion and cardiac tamponade in clinical practice [13]. Prolonged mechanical ventilation met the definition from the Society of Thoracic Surgeons, referring to mechanical ventilation that endures for >24 h after cardiothoracic surgery [14]. Cases with an ICU stay >14 days were defined as ‘prolonged ICU stay’ due to a research finding that >14 days of ICU stay caused a higher risk of mortality [15]. Heart failure was defined as NYHA class III or higher [16]. Repeated endotracheal intubation (Re-intubation) was defined as more than one on-record mechanical ventilation procedure with an interval of >24 h from the first extubation during the entire hospitalization period [17]. Cases exceeding the 75th percentile of the length of total hospital stay were categorized as ‘prolonged hospitalization’. Likewise, all cases with a surgery duration greater than or equal to the 75th percentile were classified as ‘prolonged surgery duration’. Moreover, the definition for hypoproteinemia in this study was widely accepted in clinical practice (serum albumin <35 g/l or total protein <60 g/l) [18]. Patients ≥65 years old were picked out and categorized as ‘advanced age’. According to the World Health Organization, the diagnostic criterion for anemia in adult males is a hemoglobin concentration <130 g/l, while in non-pregnant adult females, it is <120 g/l [19]. Season was determined based on the date of operation. Finally, the study's terminal event was the development or diagnosis of SWI, characterized by non-healing or delayed healing status at the sternal incision during a 1-year follow-up after median sternotomy.
Statistical analysis
Excel (Microsoft) and R software (version 4.3.2) were respectively utilized for data processing and statistical analysis. Variables with a missing proportion >5% were directly excluded. The random forest algorithm was used to fill the remaining missing values in the dataset. Predictor screening and prediction model construction were conducted in reference to methods reported in previous studies [20, 21]. To obtain the optimal subset of predictors, least absolute shrink and selection operator (LASSO) regression analysis employs a constraint on the model parameters to minimize prediction error for a response variable. This constraint causes the regression coefficients for certain variables to shrink toward zero. Consequently, variables with a regression coefficient of zero after the shrinkage process are excluded from the model, while those with non-zero regression coefficients are saved and considered to be most strongly associated with the response variable. When the partial likelihood deviance reached the minimum value, 10-fold cross-validation could further centralize and normalize the included variables and then pick the best lambda value. The subset of predictors derived from ‘Lambda.1se’ could build a predictive model with a strong performance based on the most concise independent variables. Hence, to obtain the optimal subset of predictors, the LASSO method was chosen to analyze the whole dataset. A variance inflation factor (VIF) was calculated among these predictors. No collinearity means no VIF value of each predictor exceeding five. Afterward, a multivariate logistic regression analysis was performed to identify the significant factors by introducing the subset of predictors selected in the LASSO method. A nomogram was created using all statistically significant factors from multivariate logistic regression analysis. The discrimination ability of the nomogram was evaluated by receiver operating characteristic curve and area under the curve (AUC). A high-performance nomogram model requires an AUC of ≥0.7 [22]. The bootstrap method with 1000-times resample was used to generate the calibration curve and the decision curve for internal validation of the nomogram. The calibration curve was used to assess the model's prediction accuracy, and the decision curve analysis (DCA) evaluated the clinical utility of the model by measuring the net benefit under different threshold probabilities.
The sample size for multivariate logistic regression analysis satisfied the rule-of-thumb that a minimum of 10 positive events corresponds to per variable [23]. Since the 10-fold cross-validation method and bootstrap method automatically separated the whole dataset into training and validation sets in R [22], a separate validation set was not utilized when dividing the dataset. The data was analyzed using R packages including ‘missForest’, ‘rms’, ‘glmnet’, ‘rmda’, ‘pROC’. In terms of reproducibility, the seed number was set to 2023. Season was transformed into dummy variables (spring, summer, autumn), with winter defined as the reference level. All variables included in LASSO analysis and multivariate logistic regression analysis were binary variables, the endpoint was SWI. For all tests, a p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
Among the 23,604 patients initially included in our study, a total of 6715 patients met the inclusion and exclusion criteria, and 367 of these 6715 patients eventually developed SWI. The overall incidence rate of SWI was 5.47% (Table S1, see online supplementary material). Table S1 depicts a comprehensive summary of the demographic details, clinical characteristics and postoperative outcomes for these 6715 patients. Among the 6715 patients, the mean age was 51.3 years old, 2243 patients were female, 24.7% had a history of alcohol consumption and 7.8% had a smoking history.
Screening for predictive factors
A total of 36 binary independent variables from Table S1, excluding winter, were introduced into the LASSO regression analysis to obtain the optimal subset of predictors that were strongly associated with SWI. Figure S1 was a regression coefficient profile plot against the log(lambda) sequence. As the parameter log(lambda) increased, the regression coefficient (i.e. the vertical coordinate value) converged and eventually reached 0. The order in which regression coefficients of the variables converged to 0 represented the order of the variable's importance in predicting SWI. After cross-validation, nine variables with non-zero coefficients were finally saved under the penalty with lambda.1se criteria (Figure 2) and considered as the optimal subset of predictors for SWI, including female, drinking, smoking, COPD, re-operation, bone wax, CPT, heart failure and re-intubation. The VIF of these nine predictors ranged from 1.004 to 2.306, indicating no collinearity (Table S2, see online supplementary material). Meanwhile, multivariate logistic regression analysis revealed that these nine predictors were all statistically significant (Table 1). According to the odds ratio (OR) values, female [OR = 3.405, 95% confidence interval (CI) = 2.535–4.573], COPD (OR = 4.679, 95% CI = 2.916–7.508), drinking (OR = 2.025, 95% CI = 1.437–2.855), smoking (OR = 7.059, 95% CI = 5.034–9.898), re-operation (OR = 3.235, 95% CI = 1.087–9.623), heart failure (OR = 1.555, 95% CI = 1.200–2.016) and re-intubation (OR = 1.975, 95% CI = 1.405–2.774) were identified as the independent risk factors. At the same time, bone wax (OR = 0.674, 95% CI = 0.538–0.843) and CPT (OR = 0.446, 95% CI = 0.248–0.802) were independent protective factors.
Figure 2.
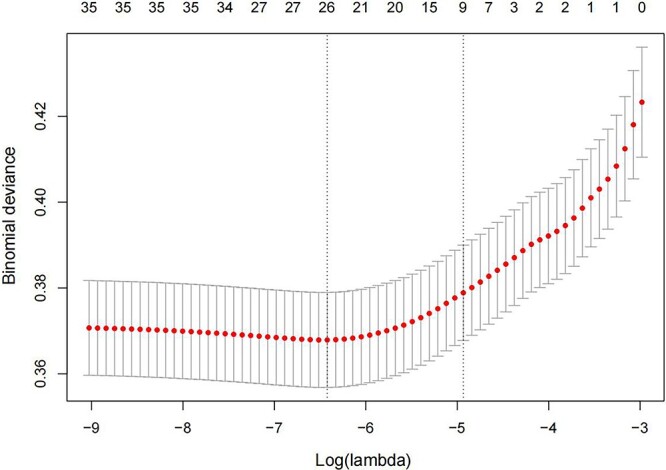
Discriminative features selection by the least absolute shrink and selection operator (LASSO) model. By identifying the optimal penalization coefficient (lambda) in the LASSO model with 10-fold cross-validation, the partial likelihood deviance (binomial deviance) curve was plotted against log(lambda). Dotted vertical lines were drawn at the value with the minimum criteria (Left) and 1 standard error of the minimum criteria (Right)
Table 1.
Multivariable logistic regression analysis
| βa | OR (95% CI) | P value | |
|---|---|---|---|
| Intercept | –3.333 | 0.036 (0.019–0.068) | <0.001 |
| Femaleb | 1.225 | 3.405 (2.535–4.573) | <0.001 |
| COPD | 1.543 | 4.679 (2.916–7.508) | <0.001 |
| Drinking | 0.706 | 2.025 (1.437–2.855) | <0.001 |
| Smoking | 1.954 | 7.059 (5.034–9.898) | <0.001 |
| Re-operation | 1.174 | 3.235 (1.087–9.623) | 0.035 |
| Bone wax | –0.395 | 0.674 (0.538–0.843) | <0.001 |
| CPT | –0.808 | 0.446 (0.248–0.802) | 0.007 |
| Heart failure | 0.442 | 1.555 (1.200–2.016) | <0.001 |
| Re-intubation | 0.680 | 1.975 (1.405–2.774) | <0.001 |
OR odds ratio, CI confidence interval, COPD chronic obstructive pulmonary disease, CPT chest physiotherapy, Re-intubation repeated endotracheal intubation. aβ is the regression coefficient. bThe reference level was male
Prediction model development
After multivariate logistic regression analysis, the above nine significant predictors were integrated into the nomogram (Figure 3). The nomogram model should be applied as follows. If a predictor has a value of 1, this implies that the patient carries that predictor. Then, the value of each predictor should be mapped onto the scores on the first horizontal line (axis 1). The points of nine predictors determined on the scale are added to obtain the total points (axis 11). Drawing a vertical line from the total points scale to the last axis could ascertain the probability of SWI (axis 12). A higher total point indicates a higher risk of developing SWI for a patient. For instance, if a female patient had been diagnosed with COPD and heart failure, had a history of alcohol consumption and smoking, and also had a history of re-operation and re-intubation, accompanied by a situation that bone wax was not used intraoperatively and CPT was not applied postoperatively, her probability of developing SWI would increase by 80% after median sternotomy.
Figure 3.
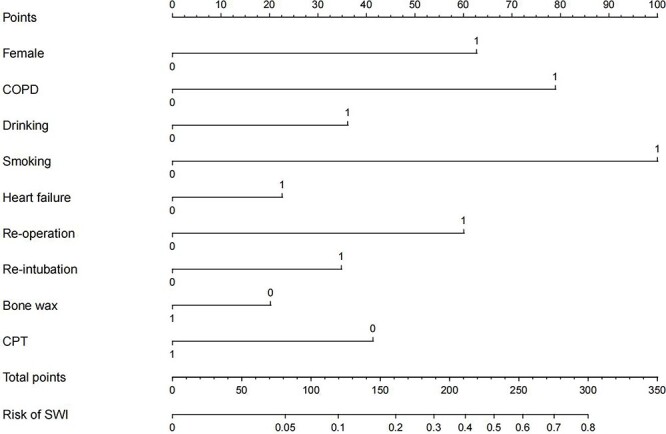
Nomogram to predict the risk of sternal wound infection (SWI) after median sternotomy. The nomogram offered a visual and quantifiable scoring system baesd on a combination of clinical characteristics to estimate the probability of developing SWI. COPD chronic obstructive pulmonary disease, CPT chest physiotherapy
Predictive performance and model validation
As shown in Figure 4 and Figure S2 (see online supplementary material), the nomogram model exhibited moderately good discrimination, with an AUC of 0.770 (95% CI, 0.745–0.795), a sensitivity of 0.798 and an accuracy of 0.620. The calibration curve also showed an excellent goodness-of-fit, indicating that the observed probability was nearly consistent with the predicted probability (Figure 5). Within the risk threshold range of 0–22%, the DCA demonstrated that the nomogram model could bring benefits to patients in predicting the risk of developing SWI (Figure 6).
Figure 4.
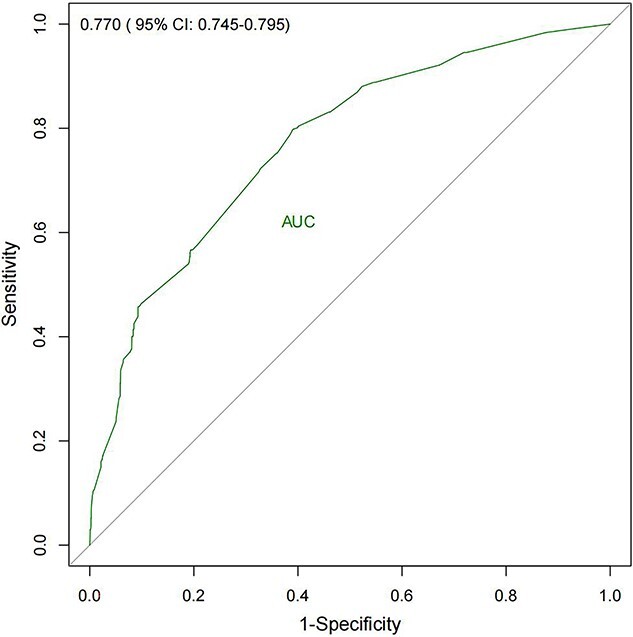
Receiver operator characteristic curve of the nomogram model. CI confidence interval, AUC area under the curve
Figure 5.
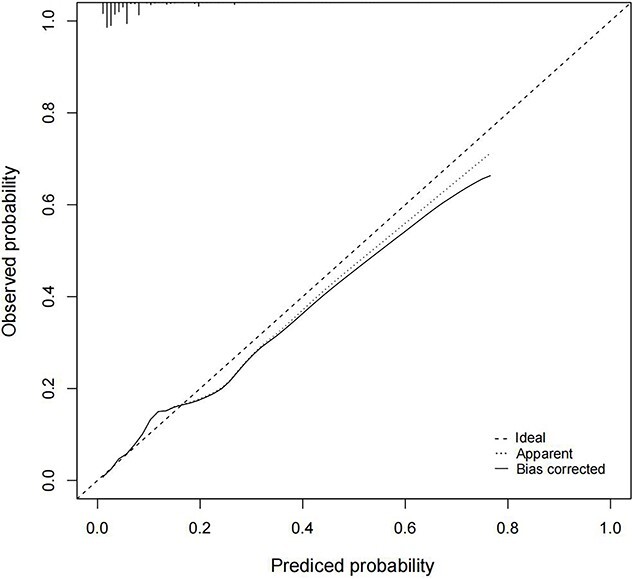
Internal calibration curves for the nomogram model. The predicted probability of sternal wound infection (SWI) was plotted on the x-axis and the actual observed probability of SWI was plotted on the y-axis. The diagonal line (45° line) meant a perfect prediction by an ideal model. The apparent calibration curve (dotted line) represented the calibration of the nomogram model. The bias-corrected curve (solid line) represented the actual predictive performance after correcting the optimism with 1000-times bootstrap resampling. A closer fit to the diagonal line represented a better prediction
Figure 6.
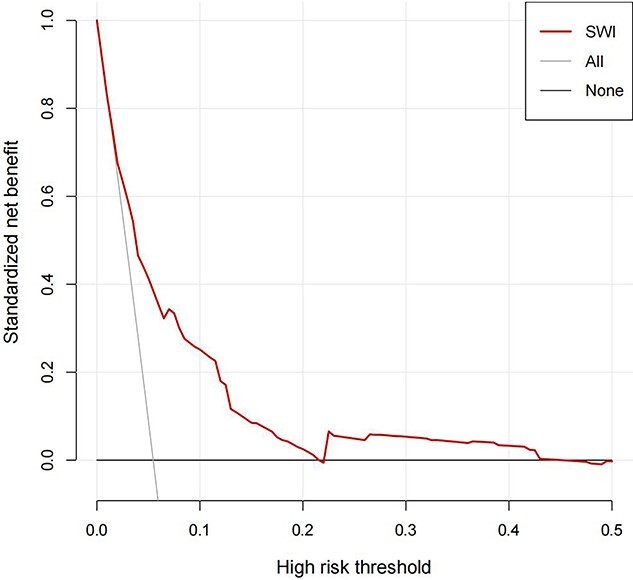
Internal decision curve analysis for the nomogram model. The vertical axis (y-axis) represented the net benefit. The black solid horizontal line represented the assumption that no patient had sternal wound infection (SWI). The grey solid line represented the assumption that all patients had SWI. This decision curve analysis could provide a potential net benefit within the defined range (0–22%)
Discussion
In this study, we conducted a retrospective study on patients who underwent median sternotomy in cardiac surgery over a 5-year period. Although the diagnostic criteria and classification definitions for SWI are updated from time to time, we adhered to the most widely accepted diagnostic criteria defined by the US CDC to ensure the continuity and acceptability of our results [10]. Our sample size was comparable to the largest single-center cohort previously reported in China by Pan et al. [24], but the incidence rate was slightly different. They reported an overall incidence rate of SWI at 1.33% in 7944 patients who underwent median sternotomy, whereas our rate was 5.47%. Two reasons may account for the variation in incidence rates across studies. Firstly, some patients did not return to the original medical institution for postoperative follow-up. Secondly, the diagnosis of SWI was made beyond the predefined study period. Consequently, the incidence rate of SWI may have been underestimated in some studies for the aforementioned reasons.
SWI can be categorized into superficial SWI and deep SWI based on the depth of infection infiltration. Superficial SWI invades the skin, subcutaneous tissue and deep fascia, while deep SWI usually presents with mediastinitis and sternal osteomyelitis [1]. To avoid misdiagnosis and ensure a sufficient number of positive cases, the observation group included all subtypes of SWI. The exclusion of cases resulting in death within 7 days was implemented to mitigate the competing risk of other life-threatening outcomes during disease progression. In order to enhance readability and interpretability of the results, all independent variables were included as binary independent variables throughout each step of data analysis.
In our study, females were found to have a higher risk of developing SWI compared to males. Our result aligned with a big meta-analysis conducted by Balachandran et al., who discovered that females had a substantially higher incidence rate of SWI than males [25]. Copeland et al. revealed a correlation between increased breast size and an increased risk of SWI and speculated that the weight of unsupported breasts might cause increased inferolateral tension in the lower segment of the sternal incision, potentially resulting in wound dehiscence and secondary infection [26]. Besides, relatively excessive fat tissue in the chest region had also been shown to affect the penetration of antibiotics and the delivery of nutrients [27]. Although the presence of breasts could impact the healing of sternal wound anatomically and physiologically, and made female gender an independent risk factor for SWI in various studies, we believe that further investigation is warranted. Future research should explore more aspects that may contribute to the higher risk of developing SWI in the female population by categorizing the female population based on age, body shape, breastfeeding situation, dressing habits, etc.
According to previous studies, smokers have a higher risk of delayed union or non-union after open or closed fractures [28, 29], and they are more susceptible to surgical incision problems [30]. Firstly, as a potent vasoconstrictor, nicotine reduces peripheral blood flow, causing skin and tissue hypoxia, thereby hindering wound healing [28]. In our nomogram model, we did not find any significant collinearity between smoking and COPD. However, the repeated coughing caused by smoking and COPD was confirmed to exert pressure along the sternum incision line, causing repeated rubbing against the fractured end of the sternum and increasing contact force on the fixed wire [31]. This situation could increase the probability of sternum fracture and wire breakage, creating a conducive environment for bacterial invasion and colonization [31]. Consequently, any impairment in the healing of the surgical incision or an increased risk of infection can significantly compromise the quality of recovery following sternal closure [1].
Drinking was an independent risk factor in our study. A series of published studies have demonstrated the adverse effects of alcohol on bone health, including higher fracture rates [32, 33] and higher infection rates at surgical sites [34]. Excessive alcohol consumption may elevate oxidative stress levels, potentially delaying wound closure and enhancing the risk of infection at surgical sites [35]. Delayed wound healing weakens the protective barrier around the wound, providing fertile ground for pathogen colonization and increasing the probability of opportunistic infections. It is noteworthy that China has a rich cultural and historical background of substance consumption, particularly with regard to alcohol and tobacco [36]. We believe that clinicians have a duty to inform patients about the harmful effects of alcohol and tobacco on wound healing after cardiac surgery. However, smoking and drinking remained risk factors for SWI in our study and we supposed that a history of smoking and alcohol consumption, as well as their poor adherence to suggestions on healthy lifestyle, may impact vascular function and have a driving effect on the development of SWI to some extent.
Undoubtedly, reconstruction is often an unavoidable choice following postoperative complications such as bleeding or sternal wire breakage, representing a re-injury for the patient. Numerous studies have found that the risk of SWI increases with re-operation [5, 37]. Re-exposure of the mediastinum to the external environment increases the risk of contamination and infection [25]. Furthermore, excessive loss of blood and bodily fluids, hypotension and tissue damage caused by surgical dissection can significantly impair the early healing of the sternal incision [38]. In addition, our finding was consistent with some specific researches which identified heart failure as a risk factor for SWI [39, 40]. Heart failure can lead to varying degrees of cardiac dysfunction and hemodynamic disturbances [41]. A reduced cardiac output results in decreased tissue perfusion and hypoxia, potentially compromising the blood supply to the wound and the rate of wound healing, thereby increasing the likelihood of surgical wound infection [41].
Research has reported that bone wax promotes blood coagulation by occluding Haversian canals in cortical bone and medullary cavities in cancellous bone, thereby achieving hemostasis [42]. Although bone wax was a protective factor in our study, debate on its safety is ongoing. Studies suggested that bone wax may contribute to sternal complications after cardiac surgery for many reasons, including foreign body reactions, impaired bone regeneration, infections and thrombus formation [43, 44]. However, a study conducted by Ozdemir and Aykut found no evidence supporting a higher risk of SWI in patients treated with bone wax during the CABG [45]. A prospective study by Prziborowski et al. did not observe an increased risk of SWI in patients using bone wax, which reinforced the safety of its use [46]. In recent years, many emerging materials have been developed with the aim of replacing bone wax to promote sternal hemostasis and healing [42]. Nonetheless, bone wax remains the most commonly used hemostatic material in many clinical departments for the following reasons: new bone hemostatic agents often can not effectively integrate hemostasis, antimicrobial and osteogenesis properties [42]; compared with western developed countries, the high costs associated with the development and application of new bone hemostatic agents hinder their widespread adoption in emerging healthcare markets [47]; and there is still a lack of extensive prospective clinical trials to further validate the properties of these agents [47]. We also hypothesize that differences in study conclusions may relate to variations in methods and locations of application by clinicians. Thus, it seems imprudent to discard a product with >127 years of use until more multifunctional, accessible and cost-effective alternatives are developed. Despite occasional negative reports, based on the aforementioned statements, we still believe that currently the benefits of using bone wax as a hemostatic material outweigh the drawbacks.
Unplanned postoperative re-intubation represents an unlooked-for event that often signifies an unfavorable postoperative progression. It indicates grave adverse outcomes that may be life-threatening following cardiac surgery [48]. Due to unstable cardiopulmonary function after cardiac surgery, patients risk developing various postoperative complications that may necessitate further endotracheal intubation, including bleeding, infection, arrhythmia and respiratory failure [48–50]. Previous studies have rarely addressed the direct relationship between re-intubation and SWI. A study by Beverly et al. validated that patients requiring re-intubation faced a significantly higher risk of failed extubation, pneumonia and composite sepsis compared to those who did not require re-intubation [49]. We speculated that re-intubation probably increased the possibility of disseminated infection. Re-intubation has been shown to prolong the total duration of mechanical ventilation [40], and prolonged mechanical ventilation has been validated as a risk factor for SWI [51]. In patients subjected to prolonged mechanical ventilation, timely sputum clearance is hindered, fostering an environment conducive to bacterial growth [52]. Another study confirmed that the increased intrathoracic positive pressure caused by mechanical ventilation also contributed to sternal instability, affecting the closure of the sternum [53]. Indeed, re-intubation can be further decomposed into several underlying factors, including the number of repeated airway manipulations, the total duration of mechanical ventilation, the intensity of intrathoracic positive pressure, and the airway damage and contamination caused by repeated airway manipulation. Therefore, there is a substantial need for further investigation to ascertain which of these factors plays the predominant role in the development of SWI. Interestingly, our study was the first to substantiate that CPT was a protective factor against SWI, further emphasizing the importance of optimizing airway management and preventing pulmonary infection following cardiac surgery.
Diabetes has been confirmed as a significant risk factor in the development of SWI [5]. Increased blood glucose levels have detrimental effects on the immune system, impairing wound healing and increasing the risk of infection [5]. Therefore, stringent perioperative blood glucose control is essential for reducing the risk of SWI in patients with diabetes. In our findings, diabetes was not identified as a risk factor. Different conclusions may be attributed to variations in the perioperative blood glucose management strategies and the levels of health education about blood glucose across different medical institutions. In our clinical practice, regardless of the presence of diabetes in patients, we aim to maintain intraoperative blood glucose levels at <10 mmol/l to counteract the potential adverse effects of perioperative hyperglycemia.
It has to be said that there is a delay in monitoring sternal incision and mediastinum following cardiac surgery in some cases. At early stages of SWI, some patients may not immediately exhibit significant sternal symptoms or signs after discharge, and their daily activities remain temporarily unaffected. Unfortunately, the infective condition has already been intractable upon their arrival at the hospital. Additionally, some cases of sternal instability presented insufficient evidence of infection during our clinical observations, which was consistent with a previous report [54]. We supposed that the routine preventive use of antibiotics and the continuously optimized management of perioperative blood glucose also induced atypical symptoms and caused delayed diagnosis. Now, experienced clinicians can refer to the nomogram results for a comprehensive analysis and proactively devise examination and intervention plans, shifting forward the commencement of interventions before necessary hospitalization.
Limitations
Although this study presented statistical analysis results based on a large clinical dataset, several limitations must be acknowledged. Firstly, the study was a single-center retrospective investigation, which may restrict the ability to comprehensively explain the influence of unknown variables and could introduce potential bias into the results. Secondly, the correlation between individual variables and SWI may differ due to population heterogeneity, differences in data collection quality, and variations in clinical feature definitions, which may limit the universal applicability of the results. Thirdly, the impact of the type of open-heart surgery on SWI was not assessed as it is difficult to quantify the surgical proficiency of different physicians. Fourthly, external validation was not conducted due to the scarcity of positive samples and the absence of comparable external datasets. Finally, the lack of deeper cooperation among different clinical disciplines and the restricted access to medical data obstructed a more detailed investigation into certain variables. Given these limitations, there is an urgent need for a multicenter, prospective, randomized controlled trial within the Chinese population.
Conclusions
In this study, we determined the overall incidence of SWI in patients who underwent median sternotomy. SWI was associated with several risk factors, including female, drinking, smoking, COPD, re-operation, heart failure and re-intubation. Bone wax and CPT were identified as protective factors against SWI. Based on these significant predictors, a nomogram model was established for early prediction of SWI, and internal validation confirmed the model's good performance. Clinicians could use this convenient and visual tool to compute the risk of SWI for each patient at an early stage. For patients at higher risk, clinicians can optimize the strategies of examination and treatment, making a better allocation of medical resources.
Supplementary Material
Acknowledgements
Firstly, we truly appreciate the professional suggestions and support from the clinicians in the Department of Cardiovascular Surgery at our center. Secondly, we also thank Professor He for her constructive guidance and assistance in statistical methodology. We acknowledge the efforts of our collaborators in collecting and organizing all clinical data. Especially, we would like to thank our friend Danny, master of computer science from Google, for his valuable assistance in refining the language of our paper. Lastly, we give our deepest gratitude to our supervisor for his selfless dedication, consistent encouragement and meticulous guidance, which have immensely enriched our academic journey.
Contributor Information
Yang Chen, Department of Burns and Cutaneous Surgery, Xijing Hospital of Air Force Medical University, Xi'an, 710032, Shaanxi, People's Republic of China.
Fei He, School of Public Management, Northwest University, Xi'an, 710127, Shaanxi, People's Republic of China.
Fan Wu, Department of Cardiovascular Surgery, Xijing Hospital of Air Force Medical University, Xi'an, 710032, Shaanxi, People's Republic of China.
Xiaolong Hu, Department of Burns and Cutaneous Surgery, Xijing Hospital of Air Force Medical University, Xi'an, 710032, Shaanxi, People's Republic of China.
Wanfu Zhang, Department of Burns and Cutaneous Surgery, Xijing Hospital of Air Force Medical University, Xi'an, 710032, Shaanxi, People's Republic of China.
Shaohui Li, Department of Burns and Cutaneous Surgery, Xijing Hospital of Air Force Medical University, Xi'an, 710032, Shaanxi, People's Republic of China.
Hao Zhang, Department of Burns and Cutaneous Surgery, Xijing Hospital of Air Force Medical University, Xi'an, 710032, Shaanxi, People's Republic of China.
Weixun Duan, Department of Cardiovascular Surgery, Xijing Hospital of Air Force Medical University, Xi'an, 710032, Shaanxi, People's Republic of China.
Hao Guan, Department of Burns and Cutaneous Surgery, Xijing Hospital of Air Force Medical University, Xi'an, 710032, Shaanxi, People's Republic of China.
Abbreviations
AUC: Area under the curve; BIMA: Bilateral internal mammary artery; CABG: Coronary artery bypass grafting; COPD: Chronic obstructive pulmonary disease; CDC: Centers for disease control and prevention; CPT: Chest physiotherapy; DCA: Decision curve analysis; ICU: Intensive care unit; LASSO: Least absolute shrinkage and selection operator; OR: Odds ratio; Re-intubation: Repeated endotracheal intubation; SWI: Sternal wound infection; VIF: Variance inflation factor.
Authors’ contributions
YC designed and carried out the study, collected and analysed all the data and wrote the draft. FH provided full support and supervision in statistical methodology. FW, XH, SL helped with data investigation and collection. WZ and HZ assisted in the data cleaning and manual verification. HG and WD contributed to the conception of the study, provided administrative support and edited the manuscript. All authors reviewed the manuscript and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki. The protocol was reviewed and approved by the Ethics Committee of Xijing Hospital (KY20232189-C-1). We confirm that all data does not contain any identifiable patient information and are securely encrypted. Moreover, as the findings of this study were based on the analysis of historical clinical data without direct patient contact, biological sample collection, and provision of research reports to patients, thus no additional risks were imposed on the patients. Therefore, a waiver of informed consent was approved.
Conflict of interest
None declared.
Funding
This work was supported by Natural Science Foundation of Shannxi Province of China (2023-JC-YB-208).
Data availability
The datasets used and/or analysed in this study are available from the corresponding author on reasonable request.
References
- 1. Song Y, Chu W, Sun J, Liu X, Zhu H, Yu H, et al. Review on risk factors, classification, and treatment of sternal wound infection. J Cardiothorac Surg 2023;18:184. Published 2023 May 19. 10.1186/s13019-023-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lemaignen A, Birgand G, Ghodhbane W, Alkhoder S, Lolom I, Belorgey S, et al. Sternal wound infection after cardiac surgery: incidence and risk factors according to clinical presentation. Clin Microbiol Infect 2015;21:674.e11–8. [DOI] [PubMed] [Google Scholar]
- 3. Singh K, Anderson E, Harper JG. Overview and management of sternal wound infection. Semin Plast Surg 2011;25:025–33. 10.1055/s-0031-1275168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schiraldi L, Jabbour G, Centofanti P, Giordano S, Abdelnour E, Gonzalez M, et al. Deep sternal wound infections: evidence for prevention, treatment, and reconstructive surgery. Arch Plast Surg 2019;46:291–302. 10.5999/aps.2018.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phoon PHY, Hwang NC. Deep sternal wound infection: diagnosis, treatment and prevention. J Cardiothorac Vasc Anesth 2020;34:1602–13. 10.1053/j.jvca.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 6. Bustamante-Munguira J, Herrera-Gómez F, Ruiz-Álvarez M, Figuerola-Tejerina A, Hernández-Aceituno A, et al. A new surgical site infection risk score: infection risk index in cardiac surgery.J Clin Med 2019;8(4):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raja SG, Rochon M, Jarman JWE. Brompton Harefield infection score (BHIS): development and validation of a stratification tool for predicting risk of surgical site infection after coronary artery bypass grafting. Int J Surg 2015;16:69–73. [DOI] [PubMed] [Google Scholar]
- 8. Friedman ND, Bull AL, Russo PL, Leder K, Reid C, Billah B.. An alternative scoring system to predict risk for surgical site infection complicating coronary artery bypass graft surgery. Infect Control Hosp Epidemiol 2007;28:1162–8. [DOI] [PubMed] [Google Scholar]
- 9. Perrotti A, Gatti G, Dorigo E, Sinagra G, Pappalardo A, Chocron S.. Validation of a predictive scoring system for deep sternal wound infection after bilateral internal thoracic artery grafting in a cohort of French patients. Surg Infect 2017;18:181–8. [DOI] [PubMed] [Google Scholar]
- 10. Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017;152:784–91. [DOI] [PubMed] [Google Scholar]
- 11. Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet 2015;385:800–11. 10.1016/S0140-6736(14)61005-9. [DOI] [PubMed] [Google Scholar]
- 12. AARC (American Association for Respiratory Care) clinical practice guideline. Postural drainage therapy. Respir Care 1991;36:1418–26. [PubMed] [Google Scholar]
- 13. Adler Y, Charron P. The 2015 ESC guidelines on the diagnosis and management of pericardial diseases. Eur Heart J 2015;36:2873–4. 10.1093/eurheartj/ehv479. [DOI] [PubMed] [Google Scholar]
- 14. D'Agostino RS, Jacobs JP, Badhwar V, Paone G, Rankin JS, Han JM, et al. The Society of Thoracic Surgeons adult cardiac surgery database: 2016 update on outcomes and quality. Ann Thorac Surg 2016;101:24–32. 10.1016/j.athoracsur.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 15. LaPar DJ, Gillen JR, Crosby IK, Sawyer RG, Lau CL, Kron IL, et al. Predictors of operative mortality in cardiac surgical patients with prolonged intensive care unit duration. J Am Coll Surg 2013;216:1116–23. 10.1016/j.jamcollsurg.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Molenkamp S, Waterbolk TW, Mariani MA, WerkerPM.. Predictors of complications after pectoralis major transposition for sternum dehiscence. Ann Plast Surg 2017;78:208–12. [DOI] [PubMed] [Google Scholar]
- 17. Ingraham NE, Tignanelli CJ, Menk J, Chipman JG.. Pre- and Peri-operative factors associated with chronic critical illness in liver transplant recipients. Surg Infect 2020;21:246–54. 10.1089/sur.2019.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martins F, Freitas F, Martins L, Dartigues JF, Barat M. Spinal cord injuries--epidemiology in Portugal's central region. Spinal Cord 1998;36:574–8. 10.1038/sj.sc.3100657. [DOI] [PubMed] [Google Scholar]
- 19. Wouters HJCM, Klauw MM, Witte T, Stauder R, Swinkels DW, Wolffenbuttel BHR, et al. Association of anemia with health-related quality of life and survival: a large population-based cohort study. Haematologica 2019;104:468–76. 10.3324/haematol.2018.195552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. You P, Zhou X, He P, Zhang J, Mao T, Li X, et al. A nomogram prediction model for sternal incision problems. Int Wound J 2022;19:253–61. 10.1111/iwj.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mo R, Shi R, Hu Y, Hu F.. Nomogram-based prediction of the risk of diabetic retinopathy: a retrospective study. J Diabetes Res 2020;2020:7261047. Published 2020 Jun 7. 10.1155/2020/7261047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yalçın N, Kaşıkcı M, Çelik HT, Allegaert K, Demirkan K, Yiğit Ş, et al. Novel method for early prediction of clinically significant drug-drug interactions with a machine learning algorithm based on risk matrix analysis in the NICU. J Clin Med 2022;11:4715. Published 2022 Aug 12. 10.3390/jcm11164715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR.. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–9. 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 24. Pan L, Mo R, Zhou Q, Wang D.. Deep sternal wound infection after cardiac surgery in the Chinese population: a single-Centre 15-year retrospective study. J Thorac Dis 2017;9:3031–7. 10.21037/jtd.2017.08.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balachandran S, Lee A, Denehy L, Lin KY, Royse A, Royse C.. Risk factors for sternal complications after cardiac operations: a systematic review. Ann Thorac Surg 2016;102:2109–17. [DOI] [PubMed] [Google Scholar]
- 26. Copeland M, Senkowski C, Ulcickas M, Mendelson M, Griepp RB.. Breast size as a risk factor for sternal wound complications following cardiac surgery. Arch Surg 1994;129:757–9. 10.1001/archsurg.1994.01420310089016. [DOI] [PubMed] [Google Scholar]
- 27. Teppa R, Sude NS, Karanam VPK, Mallipudi BVP.. Relevance of subcutaneous fat thickness as a risk factor for surgical site infections in abdominal surgeries. Cureus 2022;14:e20946. Published 2022 Jan 4. 10.7759/cureus.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pearson RG, Clement RG, Edwards KL, Scammell BE.. Do smokers have greater risk of delayed and non-union after fracture, osteotomy and arthrodesis? A systematic review with meta-analysis. BMJ Open 2016;6:e010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop 2013;37:883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sørensen LT. Wound healing and infection in surgery: the clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg 2012;147:373–83. [DOI] [PubMed] [Google Scholar]
- 31. McGregor WE, Trumble DR, Magovern JA. Mechanical analysis of midline sternotomy wound closure. J Thorac Cardiovasc Surg 1999;117:1144–50. [DOI] [PubMed] [Google Scholar]
- 32. Zhang X, Yu Z, Yu M, Qu X.. Alcohol consumption and hip fracture risk. Osteoporos Int 2015;26:531–42. [DOI] [PubMed] [Google Scholar]
- 33. Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA Jr, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 2008;121:406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shao J, Zhang H, Yin B, Li J, Zhu Y, Zhang Y.. Risk factors for surgical site infection following operative treatment of ankle fractures: a systematic review and meta-analysis. Int J Surg 2018;56:124–32. 10.1016/j.ijsu.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 35. Rosa DF, Sarandy MM, Novaes RD, Freitas MB, do Carmo Gouveia Pelúzio M, Gonçalves RV.. High-fat diet and alcohol intake promotes inflammation and impairs skin wound healing in Wistar rats. Mediat Inflamm 2018;2018:4658583. Published 2018 Jul 24. 10.1155/2018/4658583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang R, Li B, Jiang Y, Guan Y, Wang G, Zhao G.. Smoking cessation mutually facilitates alcohol drinking cessation among tobacco and alcohol co-users: a cross-sectional study in a rural area of shanghai, China. Tob Induc Dis 2019;17:85.Published 2019 Nov 25. 10.18332/tid/114076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kubota H, Miyata H, Motomura N, Ono M, Takamoto S, Harii K, et al. Deep sternal wound infection after cardiac surgery. J Cardiothorac Surg 2013;8:132. Published 2013 May 20. 10.1186/1749-8090-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ottino G, De Paulis R, Pansini S, Rocca G, Tallone MV, Comoglio C, et al. Major sternal wound infection after open-heart surgery: a multivariate analysis of risk factors in 2,579 consecutive operative procedures. Ann Thorac Surg 1987;44:173–9. [DOI] [PubMed] [Google Scholar]
- 39. Grossi EA, Culliford AT, Krieger KH, Kloth D, Press R, Baumann FG, et al. A survey of 77 major infectious complications of median sternotomy: a review of 7,949 consecutive operative procedures. Ann Thorac Surg 1985;40:214–23. 10.1016/s0003-4975(10)60030-6. [DOI] [PubMed] [Google Scholar]
- 40. Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM.. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg 2003;23:943–9. 10.1016/s1010-7940(03)00137-4. [DOI] [PubMed] [Google Scholar]
- 41. Rhou YJ, Henshaw FR, McGill MJ, Twigg SM.. Congestive heart failure presence predicts delayed healing of foot ulcers in diabetes: an audit from a multidisciplinary high-risk foot clinic. J Diabetes Complicat 2015;29:556–62. 10.1016/j.jdiacomp.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 42. Pradeep A, Rangasamy J, Varma PK. Recent developments in controlling sternal wound infection after cardiac surgery and measures to enhance sternal healing. Med Res Rev 2021;41:709–24. 10.1002/med.21758. [DOI] [PubMed] [Google Scholar]
- 43. Vestergaard RF, Nielsen PH, Terp KA, Søballe K, Andersen G, Hasenkam JM.. Effect of hemostatic material on sternal healing after cardiac surgery. Ann Thorac Surg 2014;97:153–60. [DOI] [PubMed] [Google Scholar]
- 44. Lazar HL, Salm TV, Engelman R, Orgill D, Gordon S.. Prevention and management of sternal wound infections. J Thorac Cardiovasc Surg 2016;152:962–72. [DOI] [PubMed] [Google Scholar]
- 45. Ozdemir AC, Aykut K. Efficacy and safety of bone wax to reduce sternal bleeding following coronary bypass surgery. Ann Thorac Cardiovasc Surg 2014;20:213–6. 10.5761/atcs.oa.12.02186. [DOI] [PubMed] [Google Scholar]
- 46. Prziborowski J, Hartrumpf M, Stock UA, Kuehnel RU, Albes JM. Is bone wax safe and does it help? Ann Thorac Surg 2008;85:1002–6. 10.1016/j.athoracsur.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 47. Zhou H, Ge J, Bai Y, Liang C, Yang L.. Translation of bone wax and its substitutes: history, clinical status and future directions. J Orthop Translat 2019;17:64–72Published 2019 Apr 11. 10.1016/j.jot.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siddiqui KM, Samad K, Jonejo F, Khan MF, Ahsan K.. Factors affecting reintubations after cardiac and thoracic surgeries in cardiac intensive care unit of a tertiary care hospital. Saudi J Anaesth 2018;12:256–60. 10.4103/sja.SJA_631_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beverly A, Brovman EY, Malapero RJ, Lekowski RW, Urman RD.. Unplanned reintubation following cardiac surgery: incidence, timing, risk factors, and outcomes. J Cardiothor Vasc an 2016;30:1523–9. 10.1053/j.jvca.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 50. Brovman EY, Steen TL, Urman RD. Associated risk factors and complications in vascular surgery patients requiring unplanned postoperative reintubation. J Cardiothor Vasc an 2017;31:554–61. 10.1053/j.jvca.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 51. Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg 2001;20:1168–75. 10.1016/s1010-7940(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 52. Modrau IS, Ejlertsen T, Rasmussen BS. Emerging role of Candida in deep sternal wound infection. Ann Thorac Surg 2009;88:1905–9. 10.1016/j.athoracsur.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 53. Yang J, Zhang B, Qu C, Liu L, Song Y.. Analysis of risk factors for sternal wound infection after off-pump coronary artery bypass grafting. Infect Drug Resist 2022;Volume 15:5249–56Published 2022 Sep 6. 10.2147/IDR.S381422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fu RH, Weinstein AL, Chang MM, Argenziano M, Ascherman JA, Rohde CH. Risk factors of infected sternal wounds versus sterile wound dehiscence. J Surg Res 2016;200:400–7. 10.1016/j.jss.2015.07.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed in this study are available from the corresponding author on reasonable request.