Abstract
The EFSA Panel on Plant Health performed a group pest categorisation for the EU territory of non‐EU Scolytinae (Coleoptera: Curculionidae) on non‐coniferous hosts, which total 6495 known species. Most species attack apparently healthy, weakened or dead trees, either feeding on the phloem (‘bark beetles’ subgroup) or on fungi inoculated into the sapwood (‘ambrosia beetles’ subgroup). Smaller subgroups feed and reproduce in seeds and fruits, or in herbaceous plants. Some species are polygynous, the males initiate a gallery or a chamber on or in a new host and attract females. Others are monogamous, and the females initiate the new galleries. Many species respond to primary volatile attractants emitted by the hosts, and some produce aggregation pheromones that attract conspecifics of both sexes. The species attacking living hosts are often associated with fungi that contribute to weakening the host defences and provide nutrients to the insects. Some are inbreeding; the males in the offspring mate with their sisters and rarely leave their natal tree. The larvae of all species develop and pupate within their hosts. Based on catalogues and other published data, a database was constructed providing information on hosts, feeding and reproductive habits, geographic distribution and the Köppen–Geiger climate types in countries where species occur. The Scolytinae were screened to exclude species in the following categories: (i) 708 species attacking conifers; (ii) 127 species present in at least four EU Member States and (iii) 440 species occurring in areas with climatic conditions not occurring in the EU. Among the remaining 5220 species, 88 species known for their mobility, occupying at least two landmasses separated by geographical barriers and some of which had impact levels documented in literature, were extracted. They were grouped into four subcategories: (i) 12 species with high impact on plant health; (ii) 16 species with low or doubtful impact; (iii) 48 species with no impact; (iv) 12 species with no impact and which had never been recorded as ‘introduced’ in the consulted catalogues but occurring on at least two landmasses. All 88 species could enter the EU with wood or wood products, or with plants for planting, and could establish because host plants are available, and climate is suitable in parts of the EU. Control measures to inhibit introduction are available. There is considerable uncertainty regarding the potential impact of many species. Methods for the reliable identification of many species are lacking. For some species of non‐EU Scolytinae on non‐coniferous hosts, all criteria assessed by EFSA for consideration as potential quarantine pest are met. Nevertheless, the Panel was not able to develop a method to discriminate confidently between species that clearly meet the criteria for potential quarantine pest status and those that do not.
Keywords: ambrosia beetles, bark beetles, broadleaf, pest risk, plant health, plant pest, quarantine
1. INTRODUCTION
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annex 1A, 1B, 1D and 1E (for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of High Risk Plants.
1.2. Interpretation of the Terms of Reference
Non‐EU Scolytinae on non‐coniferous hosts are listed as a group in Annex 1B of the Terms of Reference (ToR) to be subject to pest categorisation to determine whether they fulfil the criteria of potential Union quarantine pests for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform EU decision making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/ 2072. If a pest fulfils the criteria to be potentially listed as a Union quarantine pest, risk reduction options will be identified.
For this group pest categorisation, the terms of reference requests all members of the wider group to be considered. Although the term ‘Scolytinae (non‐European)’ is used In Annex II of Commission Implementing Regulation 2019/2072, the phrase ‘non‐European’ is understood to mean ‘non‐EU’ and refers to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
In this opinion, non‐EU species are interpreted as being species that are not known to be established in the EU, or if present in the EU, they are not widespread or are under official control (i.e. currently treated as Union quarantine pests). Not widespread is understood, in this scientific opinion, to mean occurring in up to three EU Member States.
Species that occur in four or more EU Member States are assumed, in this scientific opinion, to be widely distributed in the EU; such species are excluded from detailed consideration in this categorisation and will not be regarded as non‐EU Scolytinae. Nevertheless, the Panel will begin by compiling a comprehensive global list of species of Scolytinae and filter out species using relevant factors to narrow down and focus the categorisation on the species with the highest potential to satisfy the criteria, which are within the remit of the European Food Safety Authority (EFSA) to assess, necessary to allow a species to be regarded as a potential Union quarantine pest.
This opinion focuses only on species that are using non‐coniferous plant species as their hosts. The non‐EU Scolytinae on coniferous host plants have been addressed in two previous scientific opinions (EFSA PLH Panel, 2020a, 2020b, 2020c).
2. DATA AND METHODOLOGIES
2.1. Data
2.1.1. Literature search
The searches for relevant information on the natural history (i.e. feeding and reproductive habits) and distribution of Scolytinae species, as well as pest‐specific information, were primarily performed in Google Scholar (GS) and in both relevant international and grey literature. Specifically, we looked for known hostplants, feeding and reproductive habits, and geographic distribution of each species.
The beetle‐plant associations were retrieved from different bibliographic resources (Browne, 1961; Schedl, 1962; Smith et al., 2020), but most of them come from authoritative world catalogues and checklists (Atkinson, 2023; Bright, 2014, 2019; Bright & Skidmore, 1997, 2002; Wood, 2007; Wood & Bright, 1992). Additionally, this data was integrated with multiple records coming from a metasearch carried out in GS. Quotation marks (‘’) were used to search for specific keywords, such as the combination of genus and species names. These searches allowed to check online literature in multiple languages, although most of them in English. In order not to get iterative results during each search, the GS option ‘include citations’ was kept unchecked. As a final step, all GS results linked to accessible digital resources (e.g. pdf, html, text files, etc.) were checked one‐by‐one for relevant information, and specifically bark beetle‐broadleaves associations and new distributional records. Records referring to trapping experiments by means of luring substances (e.g. semiochemicals) were excluded because they don't demonstrate the beetle‐plant association clearly, but rather the attractiveness for bark beetles of specific ‘baits’. Therefore, only direct observations of beetles feeding or breeding on plant(s) were considered sufficiently reliable to be included in the dataset.
When available, information on the feeding and reproductive habits of the Scolytinae was obtained directly from relevant literature at species level (e.g. Kirkendall et al., 2015; Wood, 1986), or in some cases at genus level.
The geographic distribution of the Scolytinae species (i.e. every country where the species is considered established) was obtained from the catalogues of Bright (2021) and of Alonso‐Zarazaga et al. (2017, 2023) for the distribution of Palearctic Scolytinae. The geographical units are individual countries or, for large countries (Australia, Brazil, Canada, China, India, Russia and United States), sub‐units such as states or provinces. Geographic distribution was continuously kept updated according to new records published in national and international taxonomic and ecological papers.
2.1.2. Scolytinae database development methodology
Based on the information collected on the biology, host plants and distribution of Scolytinae species, a database was created by the University of Padova, DAFNAE department, under an EFSA art. 36 Tasking Grant (Specific Agreement n. 2 of SA2‐GP/EFSA/ALPHA/2019/01/Lot 3). The database is supported by the following three excel files:
The first file (called ‘Full‐List‐General’) contains the information on the species regarding:
Taxonomy (i.e. species name including author and year of description and tribe to which it belongs);
Biology (i.e. type of reproduction – monogamous, bigamous, polygamous and inbreeding polygynous – and feeding habits – herbiphagous, mycophagous, myelomycetophagous, myelophagous, phloeophagous, spermatophagous, xylomycetophagous and xylophagous);
Impact, reflected by the number of citations in Google Scholar.
For the type of reproduction and feeding habits, the most relevant bibliographic sources are given in two separate columns. When this information was missing, ‘Unknown’ is reported.
A glossary, specific to this opinion, explaining terms used to describe feeding habits and reproductive behaviour of Scolytinae is provided at the end of this opinion.
-
2
The second file (called ‘Full‐List‐Hosts’) refers to the host plants of the Scolytinae species. The information in the database is reported in rows, where each row corresponds to a host plant associated with a specific scolytine species, with information regarding host plant family, genus and species (in three different columns), as well as the relevant bibliographic source. Thus, based on the number of host species, the degree of polyphagy of a species can be quantified. If there are no host plants known for a scolytine species, ‘Unknown’ is reported in the three columns for host plant family, genus and species. When the hosts are conifers, the word ‘Conifers’ is reported in the same three columns.
-
3
The third file (called ‘Full‐List‐Geo’) contains information on the worldwide distribution of species. Each row in the database corresponds to a country in which a species occurs, with a hierarchical classification including biogeographic realm (i.e. Africa, Atlantic, Australasia, Indian Ocean, Nearctic, Neotropical, Oriental, Pacific and Palearctic) and Area (federations of states or provinces such as USA, China, Brazil or macro‐areas such as Asia, Central America, Europe). Then, the status of the species in that country (i.e. ‘Native’or ‘Introduced’) and the bibliographic source is given. Finally, in 31 columns for each species, all the climatic areas according to the Köppen–Geiger classification (Kottek et al., 2006) are given, with the percentage that these climatic areas occupy in each country where the scolytine species occurs. Appendix J shows a simplified map of biogeographic realms.
2.1.3. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organisation (EPPO) Global Database (EPPO, online), the CABI Compendium (online), Atkinson (2023) and relevant scientific literature (Bright, 2014; Bright, 2019; Bright & Skidmore, 1997, 2002; Wood, 2007; Wood & Bright, 1992).
Data about the import of commodity types (non‐coniferous wood products) that could potentially provide a pathway for the pest to enter the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for genus‐ and pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020. TRACES separates pest interceptions and records of non‐compliance and was searched for each; records began in June 2020.
2.2. Methodologies
The Panel performed the pest categorisation for non‐EU Scolytinae on non‐coniferous hosts, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018a, 2018b), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union quarantine pest (QP) is given in Regulation (EU) 2016/2031 Article 3 and Annex I, Section 1 of the Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee et al., 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
TABLE 1.
Pest categorisation criteria under evaluation, as derived from Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column).
| Criterion of pest categorisation | Criterion in regulation (EU) 2016/2031 regarding union quarantine pest (article 3) |
|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in and spread within, the EU territory? If yes, briefly list the pathways for entry and spread |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests' introduction have an economic or environmental impact on the EU territory? |
| Available measures (Section 3.6 ) | Are there measures available to prevent pest entry, establishment, spread or impacts? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met |
The Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs and make a judgement about potential likely impacts in the EU. Whilst the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018a, 2018b). Article 3(d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
For this pest categorisation, a stepwise dichotomous decision tree was developed and used to narrow down the number of species to be considered more fully in the pest categorisation process (Figure 1). A similar approach was used by EFSA PLH Panel (2020a, 2020b, 2020c) for the pest categorisation of non‐EU Tephritidae.
FIGURE 1.

Decision tree to support the categorisation of Scolytinae (numbers in brackets indicate the number of species).
The first step in the decision tree was to exclude all the Scolytinae species that develop in conifers only (Appendix A , 708 spp.). Then, from the remaining 5787 species, all those already known to be present in the EU were excluded, either because they are native, or because they are alien but already widespread in the EU (Appendix B ; 127 spp.). A previous pest categorisation of non‐EU Scolytinae on coniferous hosts determined that occurrence in at least four EU MS was an appropriate threshold to consider a species as widespread in the EU (EFSA PLH Panel, 2020a, 2020b, 2020c). For consistency, the Panel used the same threshold for this opinion. All remaining species occurring in countries having climatic conditions not fitting with those occurring in the EU were also excluded (Appendix C ; 440 spp.). Finally, all species that meet the criteria of having broadleaf hosts, not occurring in the EU but in countries with EU type climates or not occurring widely in the EU, were grouped for further consideration in Appendix D (5220 spp.).
For the species listed in Appendix D, a ‘degree of polyphagy’ was also introduced. A species that feeds on plant species belonging to a single genus was classified as ‘monophagous’; a species that feeds on different plant species belonging to a single family was classified as ‘oligophagous’; a species that feeds on plant species belonging to at least two families was classified as ‘polyphagous’.
The list of 163 species with high mobility (species that have colonised at least two landmasses, i.e. pieces of land surrounded by ocean or sea) listed by Grégoire et al. (2023) was used to identify the species within Appendix D, potentially associated with a higher risk of introduction to the EU (excluding those species belonging to Appendices A, B and C). Then, endemic species within the EU but with very restricted distribution (i.e. Aphanarthrum and Liparthrum species) and species with peculiar hosts mostly absent from the EU, i.e. Hypothenemus hampei (attacking coffee berries) and Coccotrypes rhizophorae (attacking Rhizophora spp.) were removed. Finally, two species absent from the list (Pseudopityophthorus minutissimus and P. pruinosus) were added because they are included in the EPPO A1 list of quarantine species. Thus, from the initial 5220 species present in Appendix D (see Figure 1), 88 species were extracted (Table 2). These 88 species were finally organised in four lists (Appendix E1):
List 1–12 species with high impact on plant health according to the literature (reviewed in Grégoire et al., 2023);
List 2–16 species with low or doubtful impact on plant health;
List 3–48 species with no impact on plant health and recorded as ‘introduced’ or ‘alien’ in at least one country in the catalogues cited in Section 2.1.1;
List 4–12 species with no impact on plant health and not recorded as alien in the main taxonomic catalogues but regarded as mobile by Grégoire et al. (2023).
TABLE 2.
List of highly mobile Scolytinae species: See Appendix E for details regarding the four list subcategories within the table.
| List 1 (species with high impact) |
| Euwallacea fornicatus (Eichhoff, 1868) |
| Euwallacea interjectus (Blandford, 1894) |
| Euwallacea kuroshio Gomez & Hulcr, 2018 |
| Euwallacea perbrevis (Schedl, 1951) |
| Euwallacea validus (Eichhoff, 1875) |
| Hypothenemus crudiae (Panzer, 1791) |
| Pityophthorus juglandis Blackman, 1928 |
| Pseudopityophthorus minutissimus (Zimmermann, 1868) |
| Pseudopityophthorus pruinosus (Eichhoff, 1878) |
| Scolytus schevyrewi Semenov, 1902 |
| Xyleborus ferrugineus (Fabricius, 1801) |
| Xyleborus glabratus Eichhoff, 1877 |
| List 2 (species with low or doubtful impact) |
| Amasa parviseta Knizek & Smith, 2024 |
| Ambrosiodmus rubricollis (Eichhoff, 1875) |
| Ambrosiophilus compressus (Lea, 1894) |
| Coccotrypes carpophagus (Hornung, 1842) |
| Cryphalus dilutus Eichhoff, 1878 |
| Cryphalus discretus Eichhoff, 1878 |
| Cryphalus mangiferae Stebbing, 1914 |
| Euwallacea piceus (Motschulsky, 1863 |
| Euwallacea similis (Ferrari, 1867) |
| Hypothenemus areccae (Hornung, 1842) |
| Hypothenemus birmanus (Eichhoff, 1878) |
| Hypothenemus obscurus (Fabricius, 1801) |
| Hypothenemus seriatus (Eichhoff, 1872) |
| Phloeotribus liminaris (Harris, 1852) |
| Xyleborus affinis Eichhoff, 1868 |
| Xyleborus perforans (Wollaston, 1857) |
| List 3 (species with no impact but introduced or alien) |
| Ambrosiodmus lewisi (Blandford, 1894) |
| Ambrosiodmus minor (Stebbing, 1909) |
| Ambrosiophilus atratus (Eichhoff, 1875) |
| Ambrosiophilus osumiensis (Murayama, 1934) |
| Anisandrus maiche (Kurentzov, 1941) |
| Cnestus mutilatus (Blandford, 1894) |
| Cnestus pseudosolidus (Schedl, 1936) |
| Coccotrypes aciculatus Schedl, 1952 |
| Coccotrypes advena Blandford, 1894 |
| Coccotrypes cyperi (Beeson, 1929) |
| Coccotrypes robustus Eichhoff, 1878 |
| Coccotrypes rutschuruensis Eggers, 1940 |
| Coccotrypes vulgaris (Eggers, 1923) |
| Coptoborus ricini (Eggers, 1932) |
| Cryphalus wapleri Eichhoff, 1872 |
| Cryptocarenus heveae (Hagedorn, 1912) |
| Cyclorhipidion distinguendum (Eggers, 1930) |
| Cyclorhipidion pelliculosum (Eichhoff, 1878) |
| Dryocoetoides cristatus (Fabricius, 1801) |
| Dryoxylon onoharaense (Murayama, 1934) |
| Eccoptopterus spinosus (Olivier, 1795) |
| Eidophelus jalappae (Letzner, 1849) |
| Heteroborips seriatus (Blandford, 1894) |
| Hypothenemus africanus (Hopkins, 1915) |
| Hypothenemus brunneus (Hopkins, 1915) |
| Hypothenemus californicus Hopkins, 1915 |
| Hypothenemus columbi Hopkins, 1915 |
| Hypothenemus elephas (Eichhoff, 1872) |
| Hypothenemus erectus LeConte, 1876 |
| Hypothenemus javanus (Eggers, 1908) |
| Hypothenemus pubescens Hopkins, 1915 |
| Hypothenemus setosus (Eichhoff, 1868) |
| Liparthrum mandibulare Wollaston, 1854 |
| Microborus boops Blandford, 1897 |
| Monarthrum mali (Fitch, 1855) |
| Pagiocerus frontalis (Fabricius, 1801) |
| Premnobius ambitiosus (Schaufuss, 1897) |
| Premnobius cavipennis Eichhoff, 1878 |
| Xyleborinus andrewesi (Blandford, 1896) |
| Xyleborinus artestriatus (Eichhoff, 1878) |
| Xyleborinus exiguus (Walker, 1859) |
| Xyleborinus gracilis (Eichhoff, 1868) |
| Xyleborinus octiesdentatus (Murayama, 1931) |
| Xyleborus bispinatus Eichhoff, 1868 |
| Xyleborus spinulosus Blandford, 1898 |
| Xyleborus volvulus (Fabricius, 1775) |
| Xylosandrus amputatus (Blandford, 1894) |
| Xyloterinus politus (Say, 1826) |
| List 4 (species with no impact and not recorded as alien) |
| Ambrosiodmus obliquus (LeConte, 1878) |
| Coccotrypes distinctus (Motschulsky, 1866) |
| Cryphalus pallidus Eichhoff, 1872 |
| Hypothenemus plumeriae (Nordlinger, 1856) |
| Microperus eucalypticus (Schedl, 1938) |
| Microperus quercicola (Eggers, 1926) |
| Planiculus bicolor (Blandford, 1894) |
| Scolytoplatypus tycon Blandford, 1893 |
| Scolytus dimidiatus Chapuis, 1869 |
| Truncaudum agnatum (Eggers, 1923) |
| Xyleborus africanus Eggers, 1927 |
| Xylosandrus mancus (Blandford, 1898) |
The species in List 1 are most likely to satisfy the criteria to qualify as potential EU quarantine pests.
Appendix E1 presents these four lists in more details, including the following fields:
Present in the EU (Y/N);
Species name;
Impact on plant health according to literature: 0 – no reported impact; 1 – occasional, limited impact; 2 – high impact (Grégoire et al., 2023);
Google Scholar (GS) citations: number of citations addressing the particular species;
Risk Score: score of 0–9, based on the sum of the scores of three traits (reproduction, feeding habits and degree of polyphagy) scoring 0–3 each (see more details below);
Spread score: 0 – species has not spread/invaded elsewhere; 1 – species has spread/invaded at least one area other than the native area (based on information in the different catalogues listed in Section 2.1.3);
Identification methods: A – Readily identifiable with both morphological and molecular methods; B – Identifiable with morphological methods but not by DNA barcoding; C – Identifiable with molecular methods but not by a reliable morphological method; D – No reliable or applicable morphological or molecular tools available (for more information see Appendix F).
Within Appendix D, three criteria identified in previous studies were considered (i.e. type of reproduction, type of feeding habits and degree of polyphagy) (EPPO, 2020; Grousset et al., 2020; Lantschner et al., 2020; Grégoire et al., 2023). These criteria were analysed in all the species recorded as ‘introduced’ in at least one country in the database (not only the species belonging to Appendix D). All the traits of these three criteria were ranked in three risk groups with different points (PT) assigned (based on the percentage of species with an invasion history sharing that type of characteristic) from ‘low risk’ (1 PT – if a given trait is present in less than 10% of the species), to ‘medium risk’ (2 PT – if a given trait is present in between 10% and 40% of the species), up to ‘high risk’ (3 PT – if a given trait is present in more than 40% of the species). Concerning reproduction, 50% of alien species in the world are inbreeding polygynous, so this characteristic was assigned the highest score (3 PT); the same for feeding habits where phloeophagy (50%) or xylomycetophagy (42%) (3 PT) characterise most of the alien species. Finally, a high degree of polyphagy (i.e. polyphagous species) is shared among most alien species (67%) (3 PT). Species with unknown traits, both at genus and tribe levels, scored zero points for those traits. Table 3 presents the scores assigned to each type of the three categories.
TABLE 3.
‘Risk scores’ expressed as point (PT) for the traits of the three categories: Reproduction, Feeding Habits and Degree of polyphagy.
| Biological feature | Low risk (1 PT) | Medium risk (2 PT) | High risk (3 PT) |
|---|---|---|---|
| Reproduction | Bigamous [2%] |
Monogamous [35%] Polygamous [16%] |
Inbreeding polygynous [50%] |
| Feeding Habits |
Myelophagous [8%] Herbiphagous [2%] Xylophagous [2%] Mycophagous [1%] |
Spermatophagous [11%] |
Phloeophagous [50%] Xylomycetophagous [42%] |
| Degree Of Polyphagy | Monophagous [8%] | Oligophagous [21%] | Polyphagous [67%] |
| Known To Be Invasive | – | – | +1 PT |
Notes: Square brackets show the percentage of species that share each trait. The sum of the percentages within a feature may be greater than 100% because, for species with unknown traits, all features known at the genus or tribe level were included.
Thus, a ‘risk score’ based on three traits was calculated for each species in Appendix D, ranging from a minimum of 0, where details of traits were unknown for each category, to a maximum of 9 (high risk for each category). Lastly, an additional point was assigned to those species that have already spread into new territories, i.e. considered mobile according to Grégoire et al. (2023), raising the maximum total score to 10 points (i.e. species with ‘high risk’ traits score for each of the three categories and that are known to have invaded at least one state/country).
Consultation with the European Union Reference Laboratory 1
EFSA has consulted the European Union Reference Laboratory (EURL) for Insects and Mites on the identification of Scolytinae species. The EU reference laboratory considered whether national reference laboratories of Member States would realistically be able to identify Scolytinae to species level. More specifically, they analysed whether individual species could be reliably distinguished from other Scolytinae. To inform such judgement, the EU reference laboratory considered whether there were reliable morphological or molecular methods available to identify the 88 species listed in Appendix E1 and E2, i.e. the Scolytinae with broadleaf hosts that are found in separate landmasses and in countries with an EU type climate and are either not present or have limited distribution in the EU. A reliable morphological identification of adults was judged feasible if it was supported by published methods which were deemed sufficiently discriminative and robust. In addition, also the experience by the EURL in applying the method was taken into account. Furthermore, the EURL had to consider whether the method could be used by generalist entomological diagnosticians working in a national reference laboratory i.e. diagnosticians not specialised in the identification of species of Scolytinae (see excerpt from report of EURL insects and mites in Appendix F of this opinion, where a synoptic table is presented of the EURL evaluation of the 88 species listed in Appendix E, with reference to available morphological and molecular diagnostic methods).
3. PEST CATEGORISATION
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes, the identity of the pest is well established, and the non‐EU Scolytinae species are described in worldwide, regional or national catalogues and faunae.
The subfamily Scolytinae is a hyper‐diverse insect group of coleopterans belonging to the large weevil family Curculionidae, currently including 6495 known species worldwide. Traditionally, Scolytinae are separated into two major ecological subgroups: (i) bark beetles that feed on inner bark of woody plants; and (ii) ambrosia beetles that farm fungi inside the xylem of the host trees and feed on them. In addition, another subgroup of species feed and reproduce also in seeds and fruits, or in herbaceous plants, especially in tropical and sub‐tropical areas. Scolytines were fully listed in the world catalogue of Bright (2021) and in other publications (i.e. Bright, 2014; Bright & Skidmore, 1997, 2002; Wood & Bright, 1992), as well as in the online catalogue of Atkinson (2023) for species native to, or introduced in, the Americas. Keys and documentation have been published, e.g. by Wood (1982, 2007), Gomez et al. (2018) and Atkinson (2023) for the Nearctic and Neotropic regions; and by Schedl (1981) and Pfeffer (1994) for the Western Palearctic region. The taxonomy is continuously evolving, e.g. with the reassignment of species to different genera, new synonymies or the description of new species.
The EPPO code 2 (EPPO, 2019; Griessinger & Roy, 2015) for the Scolytinae is 1SCOLS (EPPO, online).
3.1.2. Biology of the pest
Scolytinae are associated with a remarkable variety of plants (Marchioro et al., 2024; Ruzzier et al., 2023). Both adults and larvae feed and develop either in the sapwood or phloem of recently dead or dying trees, or in different parts of live plants (i.e. phloem or sapwood, seeds, fruits, shoots, roots) according to species. Also, many species rely on symbiotic and mutualistic relationships with yeasts and other fungi (often phytopathogenic), and bacteria carried on the body surface or stored inside specific exoskeletal cavities (a.k.a., mycangia). Some of these microorganisms invade the plant tissues and contribute to overcome the constitutive and induced defences of living hosts, causing strong dieback and wood degradation (e.g. Beaver et al., 1989; Hulcr & Stelinski, 2017). Some species are thus economically important pests, weakening and killing healthy trees and, in many cases, damaging the wood because of the staining caused by their associated fungi and the structural weakening caused by their galleries (Hofstetter et al., 2015; Raffa et al., 2015).
A general introduction to the biology of bark and ambrosia beetles is provided by Raffa et al. (2015) and Kirkendall et al. (2015). Many scolytine species live in the inner bark of trees (phloem and cambium) and often also slightly engraving the outer sapwood. Other species are instead xylophagous (i.e. feed directly in the wood) or xylomycetophagous (i.e. feed on symbiotic fungi that they farm in the galleries, or the chambers excavated in the wood). Other species are pith‐feeding (myelophagous) or seed/fruit‐feeding (spermatophagous).
Among the majority of Scolytinae, males remain with females in their tunnel systems until most or all eggs have been laid. Species may be polygamous (harem polygyny) or monogamous. In the polygamous bark beetles, the males leave their natal system, create a new gallery in a new host and attract several females, which will excavate their own egg tunnel. Instead, in the monogamous species, each female initiates the colonisation of a new host by boring a new gallery and attracts one male. In the ambrosia beetles, polygyny is much more common than among bark beetles, and mating occurs among siblings (inbreeding) in the natal system before emergence of the females, which initiate alone a new colony. Currently, 1627 species are known to have adopted an inbreeding strategy; all the remaining species are outbreeders (Kirkendall et al., 2015).
The galleries or the brood chambers vary in shape and size between the different species, often creating specific patterns (Faccoli, 2015). Each female excavates an egg gallery or an egg chamber. The eggs are either laid individually in niches along the gallery or in batches in a chamber. The larvae develop either in individual galleries at the end of which they pupate or gregariously in a common chamber. In some species, the young adults must proceed to maturation feeding, before or after emergence from the natal tree. In the latter case, they may feed on fresh bark tissues or on young twigs of healthy trees, in some case vectoring plant disease. Dispersal occurs by flight, except for the males of many inbreeding species, which do not fly but die within the natal galleries after mating with siblings.
The chemical ecology of Scolytinae is very complex (Raffa et al., 2015). Most species respond to primary volatile attractants released from their hosts, such as alpha‐pinene and other monoterpenes, or ethanol when the hosts are dying or dead, and their tissues start to ferment. In addition to primary attractants, during the process of host colonisation, many species produce aggregation pheromones that attract conspecifics of both sexes. This results in the mass attack and quick colonisation of the new hosts, overcoming their defences.
3.1.3. Host range/species affected
3387 plant species in 207 families and 1437 genera are recorded as hosts in the database for the 6495 Scolytinae species investigated. For 2652 species of Scolytinae the hosts are however unknown (most of them originated from Neotropical and Oriental regions), and 152 species develop on both broadleaves and conifers. The most attacked families are Fabaceae (attacked by 11% of the Scolytinae species), Fagaceae (7%), Moraceae (6%), Lauraceae (6%) and Malvaceae (5%). The most attacked genera are Quercus (5%), Ficus (4%), Shorea (3%), Theobroma (2%) and Dipterocarpus (2%). In Table 4, the 10 most attacked families and genera are reported with the corresponding number of Scolytinae species infesting them.
TABLE 4.
The most attacked host plant families and genera with the number of Scolytinae species recorded on them.
| Family | No of Scolytinae species | Genus | No of Scolytinae species |
|---|---|---|---|
| Fabaceae | 736 | Quercus | 311 |
| Fagaceae | 424 | Ficus | 286 |
| Moraceae | 408 | Shorea | 201 |
| Lauraceae | 368 | Theobroma | 133 |
| Malvaceae | 348 | Dipterocarpus | 116 |
| Euphorbiaceae | 329 | Prunus | 112 |
| Dipterocarpaceae | 291 | Inga | 112 |
| Anacardiaceae | 255 | Acacia | 106 |
| Burseraceae | 237 | Albizia | 106 |
| Sapindaceae | 229 | Artocarpus | 106 |
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, visual detection and trapping allow Scolytinae to be detected, although detection accuracy depends on the target species. Morphological and molecular identification methods are available.
Detection
Scolytinae can be detected visually by the symptoms caused by their own activity and by the fungi they often vector:
White wood sawdust from entry holes produced by wood‐inhabiting species (‘ambrosia beetles’);
Brown sawdust from entry holes produced by phloem‐inhabiting species (‘bark beetles’);
Galleries excavated in the phloem by bark beetle parent adults and their larvae. These galleries often show distinct patterns characterising particular species;
Circular or oval entry or exit holes and galleries and chambers excavated in the sapwood by ambrosia beetle parent adults (a single female in inbreeding species) and their larvae;
Blue‐black discolouration of the attacked wood due to symbiotic fungi inoculated by the insects;
On living trees, sap bleeding/oozing from entry and exit holes, gum or sugar exudates at entry holes, wilting, leaf dieback, loss of bark, epicormic growth and, ultimately, dying of the tree.
Scolytinae can also be monitored by trapping. Most species respond to semiochemicals, either produced by conspecifics (pheromones), or by the living or dying hosts (e.g. ethanol in non‐conifer species). A comprehensive list of these semiochemicals is provided by the Pherobase (El‐Sayed, 2024). Different trap models and colours are commercially available: vane‐traps, flight barriers, funnel‐traps. Recent comparisons of trap models and colours have been published by Allison and Redak (2017) and Cavaletto et al. (2020), respectively. Ideally, traps should be at the same time very sensitive and very cheap, to allow for maximal chances to detect low density populations (Liebhold & Tobin, 2008). Host volatiles attract a very large number of species and are used for monitoring entries near entry points (Rabaglia et al., 2008, 2019; Rassati et al., 2014; Rassati, Faccoli, Marini, et al., 2015; Rassati, Faccoli, Petrucco Toffolo, et al., 2015). As host volatiles are not specific, an enormous work of sorting and analysis in necessary for identifying the catches. However, specific pheromones can be used for detecting previously identified high risk species (Rabaglia et al., 2008, 2019).
For clearly recognisable species, or symptoms, or for specific host‐trees, a citizen science approach can be developed. Based on iNaturalist records and host‐plant distribution maps, Potgieter et al. (2024) developed a citizen monitoring program for the polyphagous shot hole borer, Euwallacea fornicatus in South Africa.
Identification
Based on the consultation with the EURL Insects and Mites, it is important to recognise that the identification of Scolytinae is based primarily on adult morphological characters and that there is a lack of information to distinguish between species if only immature stages are found (see Appendix F, excerpt from EURL report). Nevertheless, Hulcr et al. (2015) provide a comprehensive synthesis of the taxonomy and phylogenetics of the Scolytinae including an overview of the identification keys, picture databases, catalogues and methods currently available. They point out, however, that no more than 25% of the Scolytine species are listed in identification keys. After the fundamental contributions of Wood (1982), Wood & Bright (1992) and Bright (2021), considerable additions and corrections are still made annually to the taxonomy of the Scolytinae, with hundreds of new species added or moved to different genera and hundreds of synonymies detected since 1992. A recent global catalogue updating previous work (Wood, 1982; Wood & Bright, 1992) has been published by Bright (2021). Wood (1986) also published a world reclassification of the genera of the Scolytinae.
Dichotomic keys are available for North and Central America (Wood, 1982), South America (Wood, 2007), Europe (e.g. Balachowsky, 1949; Freude et al., 1981; Pfeffer, 1994), and various other areas in the world, or for particular groups in a limited area (Rabaglia et al., 2006). Hulcr et al. (2015) also provide a list of public databases on Scolytinae taxonomy and images.
DNA barcoding is increasingly used for species identification, with some accuracy (for example, Jordal and Kambestad (2014) report one misidentification out of 70 species analysed). However, Cognato et al. (2020) created COI and CAD DNA barcode databases including 165 species of Xyleborini (out of 316 species in the whole Southeast Asian fauna), and matched their analyses to morphological identifications by taxonomic experts. They concluded that barcoding alone is often uncertain without the support of morphological expertise. In addition to adults or immature stages, indirect identification can also be achieved: three Xylosandrus species and Xyleborinus saxesenii were also identified from DNA extracted from their frass or from gallery sections (Rizzo et al., 2021, 2024).
Available methods for identification: A summary of the feedback by the EURL indicating how many of the 88 species in Appendix E have reliable morphological and/or molecular methods available for their identification is shown in Table 5. For more details on EURL feedback on the individual species, see Appendix F.
TABLE 5.
The number of Scolytinae species from Appendix E in four categories (A–D) indicating whether reliable morphological or molecular methods are available for their identification (Only adults can be identified using morphological methods).
| Category | Number of species | Reliable morphological methods available | Reliable molecular methods available |
|---|---|---|---|
| A | 20 | Yes | Yes |
| B | 47 | Yes | No |
| C | 1 | No | Yes |
| D | 20 | No | No |
| 88 |
Key to categories:
A. Readily identifiable with current tools: both reliable morphological and molecular identification is possible.
B. Morphologically‐based identification possible: reliable morphological methods possible but cannot be reliably confirmed by DNA barcoding.
C. Molecularly‐based identification possible but cannot be confirmed by a reliable morphological identification.
D. Identification currently problematic: there are no reliable or applicable morphological or molecular tools.
Of the 88 taxa categorised in Appendix E, 20 cannot reliably be identified at present. If only immature specimens are available, only 21 of the 88 species can be reliably identified (see Appendix F, excerpt from EURL report).
The E. fornicatus species complex contains harmful invasive species with traits that score highly. There are, however, challenges in identifying species within the complex. Defining the species in the complex is evolving, and accurate identification requires specialist expert experience.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
Most of the non‐EU Scolytinae on non‐coniferous hosts, with known hosts, occur in the Americas (3230 species, among which 2078 species occur in North America, Central America and West Indies, and 1536 species occur in South America), and Asia (1916 species) (Figure 2). Moreover, 1704 and 1281 species, respectively are distributed only in North America and in Asia (Figure 3). 915 species are spread in more than one continent, and 13 species have a pan‐global distribution covering six continents (Figure 4).
FIGURE 2.

Global distribution of non‐EU Scolytinae on non‐coniferous hosts (Source: Literature; for details see Appendix B).
FIGURE 3.

Number of non‐EU Scolytinae species on non‐coniferous hosts reported exclusively from one continent.
FIGURE 4.

Number of non‐EU Scolytinae species on non‐coniferous hosts distributed in world continents.
Among the 88 species at high risk of introduction (Appendix E), most occur in the Americas (72 species, in particular 71 species in North and Central America, and 45 species in South America), and in Asia (59 species). Eight species (Ambrosiophilus compressus, Cnestus pseudosolidus, Cryphalus pallidus, Microperus quercicola, P. minutissimus, Pseudopityophthorus pruinosus, Scolytoplatypus tycoon and Xyleborus africanus) occur in only one continent, whereas three species (E. fornicatus, Hypothenemus seriatus and Xyleborus affinis) occur in all the six inhabited continents.
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed.
By definition, non‐EU Scolytinae are not present in the EU, or if present are not widely distributed (here considered to mean that they occur in up to three EU Member States) and are under official control.
19 non‐native species are present in three or less EU Member States (Table 6).
TABLE 6.
Non‐native non‐EU Scolytinae species present in three or less EU Member States, and their impact at world level.
| Species | EU MSs | Impact* | |
|---|---|---|---|
| Score | Reference | ||
| Amasa parviseta Knížek & Smith (until recently described as Amasa sp. near truncata) | ES; FR; PT | 1 | Flechtmann and Cognato (2011) |
| Ambrosiodmus rubricollis (Eichhoff, 1875) | IT; PT; SL | 1 | Kirkendall and Faccoli (2010) |
| Ambrosiophilus atratus (Eichhoff, 1875) | IT; SL | 0 | Rabaglia et al. (2006) |
| Anisandrus maiche (Kurentzov, 1941) | IT; SL | 0 | Terekhova and Skrylnik (2012) |
| Cnestus mutilatus (Blandford, 1894) | IT | 0 | CABI (2009) |
| Coleobothrus luridus (Wollaston, 1860) | HR | 0 | Jordal (2006) |
| Cryphalus dilutus Eichhoff, 1878 | FR; IT; MT | 1 | Gugliuzzo et al. (2023) |
| Cyclorhipidion distinguendum (Eggers, 1930) | FR | 0 | Barnouin et al. (2020) |
| Cyclorhipidion pelliculosum (Eichhoff, 1878) | DE | 0 | No data on damage |
| Dryoxylon onoharaense (Murayama, 1934) | IT | 0 | No data on damage |
| Euwallacea fornicatus sensu lato (Eichhoff, 1868) | (DE)** | 2 | Smith et al. (2019) |
| Euwallacea validus (Eichhoff, 1875) | FR | 2 | Cognato et al. (2015) |
| Liparthrum mandibulare Wollaston, 1854 | FR; IT; ES | 0 | No data on damage |
| Monarthrum mali (Fitch, 1855) | IT | 0 | Kirkendall et al. (2008) |
| Phloeotribus liminaris (Harris, 1852) | BE; IT | 1 | Pennacchio et al. (2004) |
| Pityophthorus juglandis Blackman, 1928 | FR; IT | 2 | EPPO (2020) |
| Pityophthorus juglandis Blackman, 1928 | FR; IT | 2 | Rabaglia et al. (2006) |
| Xyleborus bispinatus Eichhoff, 1868 | FR; IT; ES | 0 | Faccoli et al. (2016) |
| Xyloterinus politus (Say, 1826) | FR | 0 | No data on damage |
Impact (from the literature, in Grégoire et al., 2023) – 0: no recorded impact in the reference provided or no data on damage available; 1: moderate or doubtful impact; 2: high impact. Impact scores are based on literature around the world and not specific to the EU. Impact scores relate both to the insect and their associated fungi.
In brackets: transient, under eradication.
3.3. Regulatory status
3.3.1. Commission Implementing Regulation 2019/2072
Non‐EU Scolytinae species are listed in Commission Implementing Regulation 2019/2072 as Scolytinae spp. (non‐European). Details are presented in Table 7.
TABLE 7.
Non‐EU Scolytinae spp. in Commission Implementing Regulation 2019/2072.
| Annex II | List of Union quarantine pests and their respective codes assigned by EPPO |
| Part A | Pests not known to occur in the Union territory |
| 3. Insects and mites | |
| 72. | Scolytinae spp. (non‐European) [1SCOLF] |
As described in Section 1.2, the phrase ‘non‐European’ is understood to mean ‘non‐EU’ and refers to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
3.3.2. Hosts or species affected that are prohibited from entering the Union from third countries
The list of hosts affected by non‐EU Scolytinae and prohibited from entering the EU is shown in Table 8.
TABLE 8.
List of plants, plant products and other objects that can be hosts for non‐EU Scolytinae, whose introduction into the Union from certain third countries is prohibited (Source: Commission Implementing Regulation (EU) 2019/2072, Annex VI).
| List of plants, plant products and other objects whose introduction into the union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN code | Third country, group of third countries or specific area of third country | |
| 2. |
Plants of Castanea Mill. and Quercus L., with leaves, other than fruit and seeds |
ex 0602 10 90 ex 0602 20 20 ex 0602 20 80 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 99 ex 0604 20 90 ex 1404 90 00 |
Third countries other than Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐ Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Turkey, Ukraine and the UnitedKingdom |
| 3. |
Plants of Populus L., with leaves, other than fruit and seeds |
ex 0602 10 90 ex 0602 20 20 ex 0602 20 80 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 99 ex 0604 20 90 ex 1404 90 00 |
Canada, Mexico, United States |
| 4. | Isolated bark of Castanea Mill. |
ex 1404 90 00 ex 4401 40 90 |
All third countries |
| 5. | Isolated bark of Quercus L., other than Quercus suber L. |
ex 1404 90 00 ex 4401 40 90 |
Canada, Mexico, United States |
| 6. |
Isolated bark of Acer saccharum Marsh. |
ex 1404 90 00 ex 4401 40 90 |
Canada, Mexico, United States |
| 7. | Isolated bark of Populus L. |
ex 1404 90 00 ex 4401 40 90 |
The Americas |
| 8 | Plants for planting of Chaenomeles Ldl., Crateagus L., Cydonia Mill., Malus Mill., Prunus L., Pyrus L. and Rosa L., other than dormant plants free from leaves, flowers and fruits |
ex 0602 10 90 ex 0602 20 20 ex 0602 20 80 ex 0602 40 00 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
Third countries other than Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐ Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Turkey, Ukraine and the United Kingdom |
| 9. | Plants for planting of Cydonia Mill., Malus Mill., Prunus L. and Pyrus L. and their hybrids, and Fragaria L., other than seeds |
ex 0602 10 90 ex 0602 20 20 ex 0602 90 30 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
Third countries other than Albania, Algeria, Andorra, Armenia, Australia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canada, Canary Islands, Egypt, Faeroe Islands, Georgia, Iceland, Israel, Jordan, Lebanon, Libya, Liechtenstein, Moldova, Monaco, Montenegro, Morocco, New Zealand, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (SeveroZapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐ Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Syria, Tunisia, Turkey, Ukraine, the United Kingdom and United States other than Hawaii |
| 10. | Plants of Vitis L., other than fruits |
0602 10 10 0602 20 10 ex 0604 20 90 ex 1404 90 00 |
Third countries other than Switzerland |
| 11. |
Plants of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids, other than fruits and seeds |
ex 0602 10 90 ex 0602 20 20 0602 20 30 ex 0602 20 80 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 ex 0604 20 90 ex 1404 90 00 |
All third countries |
| 12. |
Plants for planting of Photinia Ldl., other than dormant plants free from leaves, flowers and Fruits |
ex 0602 10 90 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
China, Democratic People's Republic of Korea, Japan, Republic of Korea and United States |
| 13. | Plants of Phoenix spp. other than fruit and seeds |
ex 0602 20 20 ex 0602 20 80 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 50 ex 0602 90 70 ex 0602 90 99 ex 0604 20 90 ex 1404 90 00 |
Algeria, Morocco |
| 18. | Plants for planting of Solanaceae other than seeds and the plantscovered by entries 15, 16 or 17 |
ex 0602 90 30 ex 0602 90 45 ex 0602 90 46 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
Third countries other than: Albania, Algeria, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Egypt, Faeroe Islands, Georgia, Iceland, Israel, Jordan, Lebanon, Libya, Liechtenstein, Moldova, Monaco, Montenegro, Morocco, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (SeveroKavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Syria, Tunisia, Turkey, Ukraine and the United Kingdom |
3.3.3. Legislation addressing the organisms vectored by Scolytinae (Commission Implementing Regulation 2019/2072)
Many symbiotic organisms (fungi, bacteria, yeasts, nematodes) are vectored by Scolytinae (Hofstetter et al., 2015; Kirisits, 2004; Raffa et al., 2015). Several common genera of fungi associated with bark and ambrosia beetles, Ophiostoma, Ceratocystiopsis, Grosmannia, Ceratocystis, Leptographium include recognised phytopathogenic species. In the 20th century, the world spread of the Dutch elm disease was caused by Ophiostoma ulmi and O. novo‐ulmi vectored by Scolytus species (Brasier & Buck, 2001; Smith & Hulcr, 2015). The recently emerged pest, Pityophthorus juglandis kills walnuts because of its association with the ascomycete Geosmithia morbida (EPPO, 2020). The North American P. minutissimus and P. pruinosus qualify as potential quarantine species in the EU because they vector the oak pathogen Bretziella (Ceratocystis) fagacearum (EFSA PLH Panel, 2019). It is the combined action of the scolytine Xyleborus glabratus and of its symbiont Harringtonia (Raffaelea) lauricola that caused the death of millions of Persea borbonia after the pair had arrived in North America (Smith & Hulcr, 2015). Four phytopathogenic species are regulated in the EU (Table 9). See also Appendix G.
TABLE 9.
Organisms vectored by non‐EU Scolytinae spp. in Commission Implementing Regulation 2019/2072 (9 October 2023).
| Annex II | Union quarantine pests Part A (not known to occur in the EU) |
|---|---|
| B. Fungi and oomycetes | |
| 5. | Bretziella fagacearum m (Bretz) Z.W de Beer, T.A. Duong & M.J. Wingfield, comb. nov. [CERAFA] |
| 20. | Neocosmospora ambrosia (Gadd & Loos) L. Lombard & Crous [FUSAAM] |
| 21. | Neocosmospora euwallaceae (S. Freeman, Z. Mendel, T. Aoki & O'Donnell) Sandoval‐Denis, L. Lombard & Crous [FUSAEW] |
| Union quarantine pests Part B (known to occur in the EU) | |
| B. Fungi and oomycetes | |
| 1. | Geosmithia morbida Kolarík, Freeland, Utley & Tisserat [GEOHMO] |
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Yes, non‐EU Scolytinae can enter the EU. Interceptions are reported in Europhyt and Traces.
Comment on plants for planting as a pathway.
Plants for planting (excluding pollen) provide a pathway for entry (9 out of 216 interceptions were on plants). Most interceptions are on wood and wood packaging material.
Potential pathways for non‐EU Scolytinae into the EU are summarised in Table 10.
TABLE 10.
Potential pathways for non‐EU Scolytinae into the EU.
| Pathways (e.g. host/intended use/source) | Life stage | Relevant mitigations within Implementing Regulation 2019/2072 |
|---|---|---|
| Host plants for planting | All stages | Annex VII, point 32 |
| Cut branches | All stages | Annex VII, point 32 |
| Dunnage (solid wood packaging material) | All stages | ISPM 15 |
| Wood with bark | All stages | Annex VII, points 79 and 81 |
Annex VII of EU 2019/2072 details the special requirements necessary for specified plants, plant products and other objects originating from third countries which could provide a pathway for entry of non‐EU Scolytinae. Details are at points 32, 79 and 81 of Annex VII.
Analysis of import data
Import data for 13 different HS codes of selected non‐coniferous wood and wood products (Appendix H) were extracted from Eurostat for the 6‐year period 2017–2022. The sum of annual imports from the world's biogeographic realms are shown in Figure 5. A global map indicating the realms is shown in Appendix J. Details of imports by HS code from each realm are provided in Appendix H. Most of the non‐coniferous wood is sourced from the Palearctic, Asia and Oriental region. Strictly, non‐EU European countries (and EU countries) would be considered as within the Palearctic region but have been shown separately for the purposes of this of opinion.
FIGURE 5.
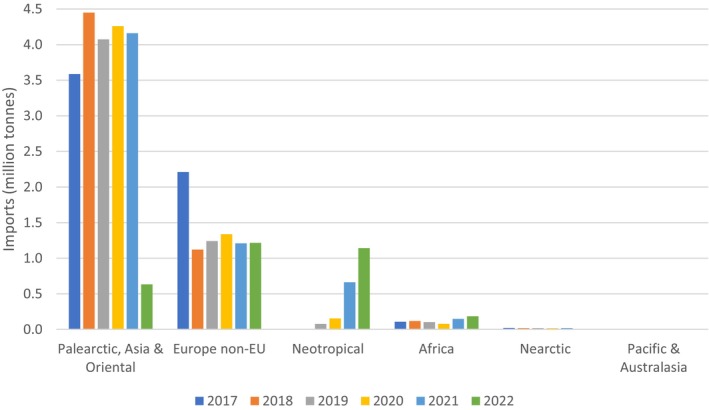
EU annual imports on non‐coniferous wood (selected HS codes) from the world's biogeographic realms (in million tonnes).
Approximately 48% of non‐native Scolytinae established in the EU originate from the Palearctic, Asia and Oriental region. Table 11 summarises EU imports by HS code from all realms in descending order of total imports in the period 2017 to 2022.
TABLE 11.
Summary of EU annual imports of selected non‐coniferous woods, 2017–2022 (in tonnes). Detailed descriptions of HS codes are provided in Appendix H. (Source Eurostat, https://trade.ec.europa.eu/access‐to‐markets/en/statistics).
|
HS code Short description |
2017 | 2018 | 2019 | 2020 | 2021 | 2022 | Mean |
|---|---|---|---|---|---|---|---|
|
4403 95 Betula > 15 cm |
2,742,345 | 3,352,120 | 3,027,133 | 3,360,609 | 3,370,329 | 415,457 | 2,711,332 |
|
4415 Boxes, crates |
813,936 | 977,812 | 974,836 | 945,571 | 1,085,232 | 1,147,349 | 990,789 |
|
4403 96 Betula < 15 cm |
1,130,244 | 849,722 | 938,010 | 846,014 | 719,047 | 201,870 | 780,818 |
|
4403 98 Eucalyptus |
1068 | 1636 | 70,996 | 147,066 | 695,660 | 1,194,634 | 351,843 |
|
4403 97 Populus |
568,484 | 315,954 | 300,040 | 392,856 | 158,079 | 33,911 | 294,887 |
|
4403 49 Tropical wood |
104,455 | 107,887 | 94,407 | 66,670 | 93,551 | 106,723 | 95,616 |
|
4403 12 Non‐coniferous rough wood |
478,085 | 139 | 680 | 3382 | 469 | 1664 | 80,737 |
|
4403 93 Fagus > 15 cm |
24,245 | 42,718 | 45,988 | 31,707 | 35,807 | 64,584 | 40,842 |
|
4403 91 Quercus |
31,381 | 23,740 | 24,547 | 19,227 | 23,895 | 10,057 | 22,141 |
|
4403 94 Fagus < 15 cm |
26,500 | 29,147 | 24,960 | 20,931 | 17,030 | 8509 | 21,180 |
|
4406 12 Railway sleepers |
18,992 | 20,473 | 19,408 | 19,889 | 12,936 | 14,137 | 17,639 |
|
4403 42 Teak wood |
0 | 0 | 0 | 0 | 0 | 3186 | 531 |
|
4403 41 Maranti wood |
13 | 1 | 20 | 0 | 1513 | 0 | 258 |
| Sum | 5,939,748 | 5,721,349 | 5,521,025 | 5,853,922 | 6,213,548 | 3,202,081 | 5,408,612 |
Interceptions
In assembling information for this group categorisation, we found that 165 Scolytinae species had already invaded areas outside of their native range, including into the EU. Interception data in the Traces and Europhyt databases indicate imported wood and wood packaging material provide the most interceptions. This aligns with the Scolytinae literature which reports that the primary pathways facilitating international spread are traded wood and wood packaging material (Brockerhoff et al., 2003; Lantschner et al., 2020; Ward et al., 2023).
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. A search of the Europhyt and Traces databases revealed 216 unique records where ‘Scolytidae’ were recorded as the harmful organism intercepted. Previously Scolytidae was regarded as a family of Coleoptera but nowadays the members are classified as Scolytinae, a subfamily of weevils (Curculionidae). Because all non‐EU Scolytinae are regarded as quarantine pests (EU 2019/2072, Annex II, Part A point 65), it was not necessary for interceptions to be identified further (Appendix I). Almost 95% of EU interceptions of ‘Scolytidae’ were associated with traded wood or wood packaging material. The majority of interceptions (67.6%, 146 of 216) were intercepted with wood and bark commodities such as CN 4407 (Sawn wood of a thickness exceeding 6 mm). 27.3% (59 of 216) of the interceptions were associated with wooden packaging material such as crates, dunnage and pallets; 4.2% (9 of 216) were associated with plants and two records (0.9%) did not indicate what the Scolytidae were found on or in. Table 12 shows the sources of interceptions of Scolytidae on wood and bark.
TABLE 12.
Scolytidae interceptions associated with coniferous and non‐coniferous wood and bark.
| Year | 2002 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country of origin | ||||||||||||||||
| Cameroon | 8 | 5 | 13 | 14 | 3 | 12 | 2 | 1 | 1 | 59 | ||||||
| Congo | 5 | 6 | 6 | 3 | 5 | 8 | 3 | 1 | 1 | 1 | 39 | |||||
| United States | 1 | 1 | 2 | 3 | 1 | 2 | 2 | 1 | 13 | |||||||
| Russian Federation | 1 | 2 | 4 | 7 | ||||||||||||
| Central African Republic | 2 | 1 | 2 | 1 | 6 | |||||||||||
| Equatorial Guinea | 1 | 1 | 1 | 1 | 4 | |||||||||||
| Gabon | 2 | 2 | 4 | |||||||||||||
| Bulgaria* | 2 | 1 | 3 | |||||||||||||
| Dem. Rep. Congo | 3 | 3 | ||||||||||||||
| Ukraine | 3 | 3 | ||||||||||||||
| Romania* | 2 | 2 | ||||||||||||||
| Switzerland | 2 | 2 | ||||||||||||||
| Canada | 1 | 1 | ||||||||||||||
| Sum | 1 | 3 | 2 | 3 | 8 | 16 | 14 | 22 | 23 | 13 | 23 | 9 | 5 | 2 | 2 | 146 |
Bulgaria and Romania joined the EU on 1st January 2007.
Note that the interceptions above include interceptions with coniferous and non‐coniferous wood.
Table 13 shows the sources of interceptions of Scolytidae with wood packaging material.
TABLE 13.
Scolytidae interceptions with coniferous and non‐coniferous wood packaging material.
| Year | 1999 | 2000 | 2001 | 2005 | 2006 | 2007 | 2008 | 2009 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country of origin | ||||||||||||||||
| China | 3 | 5 | 1 | 1 | 2 | 1 | 4 | 7 | 4 | 8 | 1 | 2 | 39 | |||
| India | 2 | 3 | 1 | 1 | 7 | |||||||||||
| Brazil | 1 | 1 | 1 | 3 | ||||||||||||
| Portugal | 1 | 1 | 2 | |||||||||||||
| Russian Federation | 1 | 1 | 2 | |||||||||||||
| Canada | 1 | 1 | ||||||||||||||
| Honduras | 1 | 1 | ||||||||||||||
| Pakistan | 1 | 1 | ||||||||||||||
| Romania* | 1 | 1 | ||||||||||||||
| Turkey | 1 | 1 | ||||||||||||||
| Unknown | 1 | 1 | ||||||||||||||
| Sum | 3 | 5 | 3 | 3 | 3 | 4 | 3 | 4 | 3 | 5 | 8 | 4 | 8 | 1 | 2 | 59 |
Bulgaria and Romania joined the EU on 1st January 2007.
Some EU interceptions of Scolytinae have been identified to genus and/or species level. These are shown as separate records to interceptions of ‘Scolytidae’ in Europhyt and Traces. Individual searches of each of the 39 genera known to have spread and become invasive were searched in Europhyt and Traces. Six of the 39 genera have been intercepted in the EU (Table 14). Xyleborus represent over 35% of interceptions where a scolytine genus is named. The majority of interceptions of named genera are from Asia (82.9%, 150 of 181 interceptions) (See also Appendix I).
TABLE 14.
Country of origin and year of interception for named genera of Scolytinae reported in Europhyt or Traces.
| Genus | From | 2011 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Sum | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xyleborus | China | 38 | 13 | 51 | ||||||||
| Vietnam | 6 | 6 | ||||||||||
| Cameroon | 2 | 1 | 3 | |||||||||
| India | 2 | 2 | ||||||||||
| USA | 2 | 2 | ||||||||||
| Costa Rica | 1 | 1 | ||||||||||
| Sub‐total | 65 | |||||||||||
| Xylosandrus | China | 5 | 28 | 8 | 41 | |||||||
| USA | 6 | 2 | 7 | 15 | ||||||||
| Sub‐total | 56 | |||||||||||
| Xyleborinus | China | 11 | 15 | 4 | 10 | 5 | 45 | |||||
| USA | 3 | 3 | ||||||||||
| Sub‐total | 48 | |||||||||||
| Scolytus | USA | 3 | 4 | 7 | ||||||||
| Sub‐total | 7 | |||||||||||
| Cryphalus | China | 1 | 1 | |||||||||
| Malaysia | 2 | 2 | ||||||||||
| Sub‐total | 3 | |||||||||||
| Coccotrypes | China | 2 | 2 | |||||||||
| Sub‐total | 2 | |||||||||||
| Sum | 181 | |||||||||||
The following species are recorded in Europhyt or TRACES: Coccotrypes cyperi, Scolytus multistriatus, Xyleborinus artestriatus, X. saxesenii, X. affinis, X. volvulus, Xylosandrus compactus and Xylosandrus crassiusculus.
3.4.2. Establishment
Is the pest able to become established in the EU territory?
Yes, some of the Scolytinae species are able to establish in the EU territory because a) their host plants are present, or they are sufficiently polyphagous to attack new hosts; and b) some are found in countries with climates matching those in the EU.
Climatic mapping is the principal method for identifying areas that could provide suitable conditions for the establishment of a pest, taking key abiotic factors into account (Baker, 2002). The availability of hosts is considered in Section 3.4.2.1. Climatic factors are considered in Section 3.4.2.2.
3.4.2.1. EU distribution of main host plants
Among the 65 species within Appendix D that scored 10/10, all showing a very high degree of polyphagy, 52 are xylomycetophagous and 13 show different feeding habits. In this respect, xylomycetophagous species find suitable hosts more easily, as the only limitation for their establishment is the survival of the symbiont fungus on which the insect feeds, and these fungi can generally grow in many different plants. Thus, the xylomycetophagous species may infest large numbers of host plants (a mean of 20 host families with a range of 2–90, and 69 host plants with a range of 3–703 per species), many of which are even still unknown because never recorded so far. For these reasons, the risk of establishment in the EU Member States of xylomycetophagous species is less dependent upon the availability of known hosts. Moreover, the 13 non‐xylomycetophagous species in the list also have many recorded hosts (mean of 22 host families with a range of 3–53, and 50 host plants with a range of 3–156 per species).
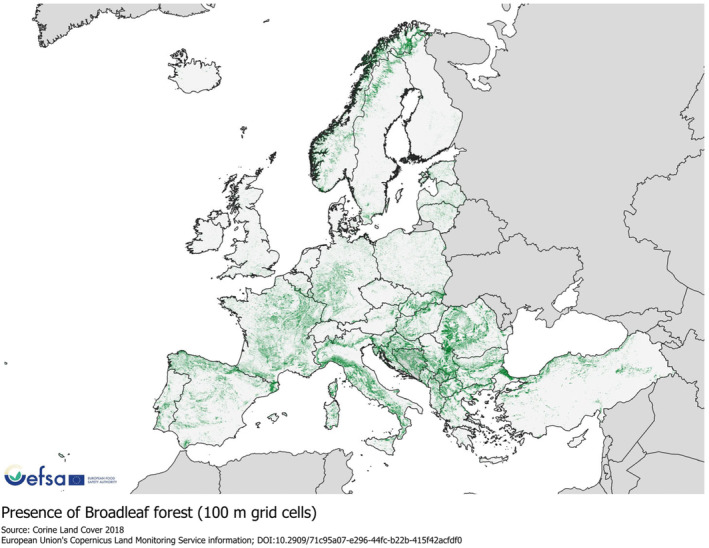
A range of broadleaf trees grow widely within the EU. In European deciduous forests, common broadleaved species include Quercus spp. (oaks), Fagus sylvatica (European beech), Carpinus betulus (hornbeam), Fraxinus spp. (ash) and Castanea spp. (chestnut); at higher altitudes Fagus spp. are the dominant broadleaf deciduous species (Dreiss & Volin, 2014). The distribution of broadleaf forests in Europe is shown in Figure 6 (CORINE Land Cover, 2018).
FIGURE 6.
The distribution of European broadleaf forests (data source: CORINE Land Cover, 2018).
The distribution of specific broadleaf tree species is shown in Figure 7.
FIGURE 7.
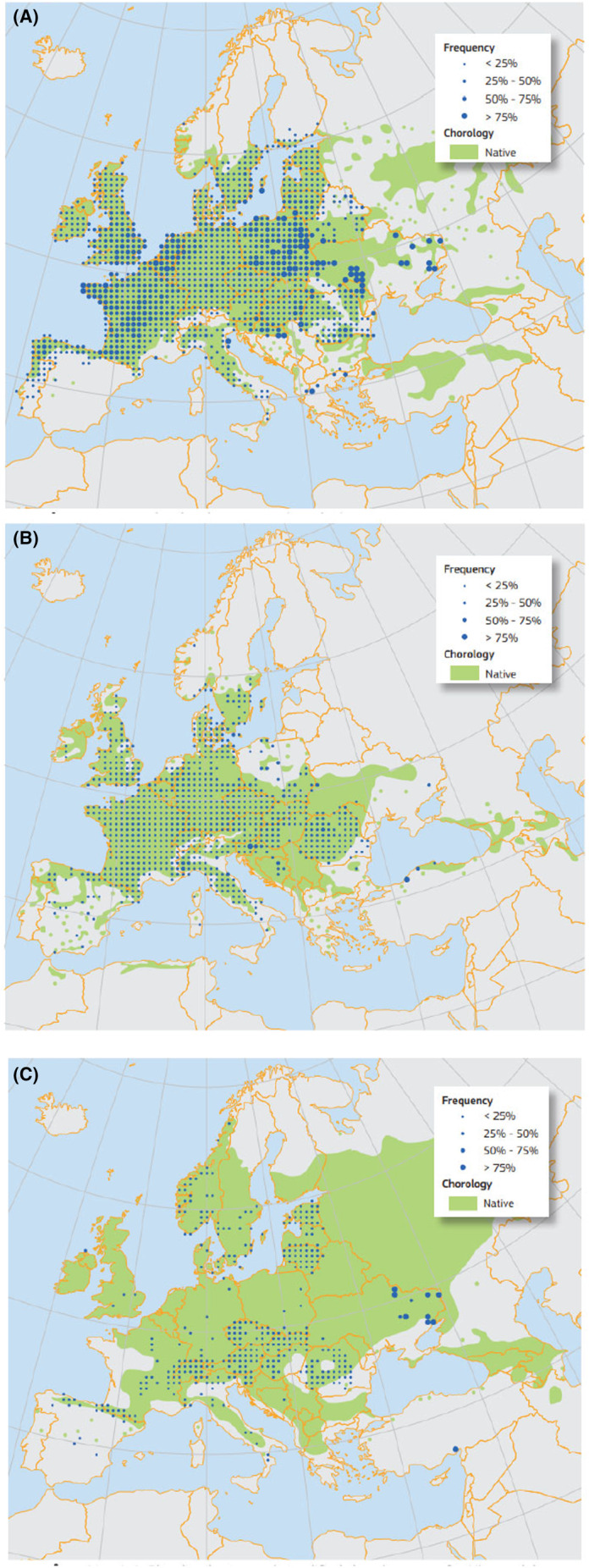
(a) Plot distribution and simplified chorology map for Quercus robur. Frequency of Quercus robur occurrences within the field observations as reported by the National Forest Inventories (Eaton et al., 2016). https://ies‐ows.jrc.ec.europa.eu/efdac/download/Atlas/pdf/Quercus_robur_petraea.pdf. (b) Plot distribution and simplified chorology map for Prunus avium. Frequency of Prunus avium occurrences within the field observations as reported by the National Forest Inventories (Welk et al., 2016). (c) Plot distribution and simplified chorology map for Ulmus glabra. Frequency of Ulmus glabra occurrences within the field observations as reported by the National Forest Inventories (Caudullo & de Rigo, 2016). (a–c) Plot distributions and simplified chorology maps for specific broadleaf tree (non‐coniferous) hosts of non‐EU Scolytinae.
3.4.2.2. Climatic conditions affecting establishment
All the species within Appendix D associated with a higher risk of introduction in the EU are distributed in non‐European countries having at least 50% of climatic compatibility with the EU MSs, and half of these species present a compatibility of 92%. Moreover, 57, 46 and 38 out of 57 species, respectively, show compatibility with ‘Temperate oceanic’, ‘Continental uniform precipitation cold summer’, and ‘Temperate dry hot summer’ climatic areas, the most highly represented climatic types in the EU with a surface of 50%, 14% and 13%, respectively. Considering this, climate compatibility does not seem to be a limitation for the establishment of these species in the EU.
3.4.3. Spread
Describe how the pest would be able to spread within the EU territory following establishment?
The pest would be able to spread within the EU territory by autonomous or semi‐passive flight, or transported in wood, wood products, solid wood packaging material, plants for planting (excluding pollen) and, possibly, by hitchhiking.
Comment on plants for planting as a mechanism of spread.
Living plants can be infested by Scolytinae, especially by xylomycetophagous species, often with little external symptoms except a few small entry holes and some sawdust.
Spread can occur by flight, with transported wood and wood products, or with plants for planting. An example of a probable combination of factors is given by Marchioro and Faccoli (2021), on the spread of P. juglandis in the Veneto region (Italy) during the 2013–2020 period. They measured an average annual spread of 9.4 km, likely to be due to direct flight but also recorded single dispersal events at longer distances (maximum 40 km), possibly due to the transportation of infested material.
Spread by flight. Only a few examples of wind‐tunnel experiments on non‐coniferous Scolytinae are available. Kees et al. (2017) found that P. juglandis could fly at most 3.6 km in 24 h. Seo et al. (2017) recorded shorter distances for X. glabratus and Monarthrum mali (an average 17.6 m in 24 h for X. glabratus; over 100 m for M. mali). More examples relating to species attacking coniferous hosts are provided and will be considered here, as these species do not differ in size or behaviour from the species attacking non‐coniferous hosts. Anecdotal information is available regarding conifer bark beetles found in the tundra as far as 170 km north of the limit of spruce in Finland (Nilssen, 1984). Flight‐mill experiments suggest that a fraction of an Ips sexdentatus population is able to fly more than 45 km (Jactel & Gaillard, 1991). In field experiments in New‐Zealand, Chase et al. (2017) trapped Hylurgus ligniperda and Hylastes ater more than 25 km from the nearest host‐trees. The capacity of Dendroctonus ponderosae to travel long distances by flight has been demonstrated in British Columbia by Jackson et al. (2008), using meteorological radar recordings coupled with aerial samplings with a drogue net pulled by an airplane. Beetles were caught up to 800 m above the forest canopy at a mean density of 4950 beetles/ha and covering 30–110 km/day. Semi‐passive, wind‐directed high‐altitude flight is reported or strongly suspected among many insect species (Reynolds et al., 2017), including plant pests (EFSA PLH Panel, 2018a, 2018b) or vectors of animal or human diseases (Hendrickx et al., 2008). Recent experimental results based on pheromone trap transects from an outbreak hotspot in the French Ardennes to the French and British coasts along the English Channel provide evidence that Ips typographus can fly several hundreds of km (Inward et al., 2024). This could explain the recent establishment of breeding populations in southern England (Blake et al., 2024).
Transportation with wood, wood products and solid wood packaging material. Recent reviews are provided by Meurisse et al. (2019) and Turner et al. (2021). Large numbers of interceptions have been reported from these commodities.
Plants for planting (excluding pollen) are a known pathway, including for species developing in young twigs, such as Xylosandrus morigerus (Kirkendall & Faccoli, 2010), X. crassiusculus (EFSA PLH Panel, 2020a, 2020b, 2020c) or X. compactus (Anses, 2017).
3.5. Impacts
Would the pests' introduction have an economic or environmental impact on the EU territory?
Yes, as illustrated by the large impact of native Scolytinae species and of species introduced in new territories, some non‐EU Scolytinae species would have an economic or environmental impact on hosts in the EU territory.
The history of forest health includes many episodes of damaging outbreaks caused by native or introduced Scolytinae, causing economic, environmental and social damage (Smith & Hulcr, 2015). Some native species are major pests in their range. In Canada, D. ponderosae killed about 730 million m3 of Pinus contorta in British Columbia between 1999 and 2015 (British Columbia Government, 2024). In Europe, more than 25 million m3 of spruce were killed by I. typographus in the Czech Republic alone, between 2018 and 2019 (Hlásny et al., 2021). These well‐known species, however, attack conifers and are not known to have crossed geographic barriers, except D. ponderosae taking advantage of winter warming to cross the Rocky Mountains from British Columbia to Alberta (Robertson et al., 2009), and I. typographus crossing the English Channel by flight (Inward et al., 2024). Most of the Scolytinae attacking non‐coniferous hosts that cause massive outbreaks are known as very mobile, especially when associated with pathogenic fungi that help them to overcome tree resistance. A classic example is the Eurasian species S. multistriatus, vector of the highly virulent pathogens associated with the Dutch elm disease. The disease was caused in a first trans‐ and inter‐continental wave by O. ulmi and, in a second wave, by O. novo‐ulmi and developed devastating pandemics in the twentieth century (Brasier, 1991; Brasier & Buck, 2001). During the spread of O. novo‐ulmi in the 1970s, most mature European elms were killed, as well as hundreds of millions in North America (Brasier & Buck, 2001). More recently, in the early 2000s, the redbay ambrosia beetle, X. glabratus, innocuous in its native range in southeast Asia, was introduced into southeastern US (Smith & Hulcr, 2015), together with its associated fungus Raffaelea lauricola. The pair killed more than 300 million redbay trees (Persea borbonia L. Spreng.) as well as attacking several other Lauraceae, including avocado, Persea americana Mill. (Hughes et al., 2017). Similarly, the walnut twig beetle, P. juglandis and its associated fungus, G. morbida, were living innocuously on weakened branches and twigs of Juglans major in their native range in Arizona and New Mexico. They encountered J. nigra and J. regia at the end of the 20th century or the very beginning of this century and started massively killing these new hosts (EPPO, 2024). They also rapidly spread along the west coast of the USA as well as to several states in the east of the country, crossed the Atlantic and established in Italy (Montecchio & Faccoli, 2014) and France (Saurat et al., 2023). Other examples involving introduced Scolytinae, their associated fungi and their impact in newly invaded areas include E. fornicatus, X. crassiusculus and X. compactus.
Broadleaf trees provide a range of ecosystem services, including provisioning services, (e.g. timber and fruit production), carbon sequestration, clean drinking water, erosion prevention, recreation, tourism, aesthetics, property value security and others (Dreiss & Volin, 2014). In addition to killing trees and damaging their wood (staining due to fungal activity and structural weakening caused by the galleries), Scolytinae exert an important environmental impact in all these respects (Grégoire et al., 2015).
Major uncertainties in predicting impact: (i) the possible future international spread of species so far only known as local pests (review of North American harmful species by Smith & Hulcr, 2015); (ii) the emergence of novel insect‐pathogen‐host associations after establishment in a new territory, with major consequences on new hosts. Examples: Ophiostoma novo‐ulmi (Brasier & Buck, 2001); X. glabratus and R. lauricola (Smith & Hulcr, 2015).
The emergence of unexpected pests (‘unknown unknowns’) is documented by the history of the Google Scholar citations used in this study, as illustrated for three recently emerged pests, X. glabratus; P. juglandis and G. morbida (the bark beetle vector and fungal agent of the Thousand Canker Disease of walnuts); E. fornicatus (Figure 8). These three species were almost unknown and would have been considered harmless before 2005.
FIGURE 8.

Google Scholar citations against time for three scolytine species that recently established and caused damage in new territories.
3.6. Available measures and their limitations
Are there measures available to prevent pest entry, establishment, spread or impacts such that the risk becomes mitigated?
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to some host plants for planting (see 3.3.2).
Additional potential risk reduction options and supporting measures are shown in Sections 3.6.1.1 and 3.6.1.2.
3.6.1.1. Additional potential risk reduction options
Potential additional control measures are listed in Table 15.
TABLE 15.
Selected control measures (a full list is available in EFSA PLH Panel, 2018a, 2018b) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance.
| Control measure/risk reduction option (blue underline = Zenodo doc, blue = WIP) | RRO summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Require pest freedom | Plant or plant product comes from country officially free from pest or from pest free area or (plants for planting) from pest free production sites or places of production | Entry/Spread |
| Growing plants in isolation | Places of production of plants for planting are insect proof, with complete physical isolation | Entry/Spread |
| Managed growing conditions | Plants collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime | Entry/Spread |
| Roguing and pruning | Plants which have shown symptoms giving rise to the suspicion of contamination by the pests have been rogued out at that place and the plants have undergone appropriate treatment to rid them of specified pests | Entry/Spread/Impact |
| Biological control and behavioural manipulation |
Biological control: many natural enemies (predators, parasitoids and pathogens) of scolytine species have been recorded and have a variable and fluctuating impact on natural populations, but none have so far been successfully released against Scolytinae attacking non‐coniferous hosts (Kenis et al., 2004; Wegensteiner et al., 2015) Pheromone mass‐trapping has been unsuccessfully attempted against Scolytus multistriatus (Birch et al., 1982). In spite of previous claims, recent experimental results demonstrate that mass‐trapping is also inefficient against conifer Scolytinae (Kuhn et al., 2022) Mating disruption appears ineffective to date, as illustrated by the lack of effectiveness of pheromone mass‐trapping Push‐pull strategies (repelling the pest from stands under protection while attracting them to non‐host stands) is under investigation (e.g. Rivera et al., 2020), but no practical application has been developed so far Sterile Insect Techniques (SIT) have not been used against Scolytinae. One difficulty would be to mass‐produce the insects |
Spread/Impact |
| Chemical treatments on crops including reproductive material | Contact insecticides can be applied on wood and wood products; contact and systemic insecticides can be used to protect plants for planting | Entry/Spread/Establishment/Impact |
| Chemical treatments on consignments or during processing |
Use of chemical compounds that may be applied to plants or to plant products after harvest, during process or packaging operations and storage. The treatments addressed in this risk mitigation measure are:
Host wood has been treated (e.g. fumigation, chemical pressure impregnation, ionising irradiation) prior to export |
Entry/Spread |
| Physical treatments on consignments or during processing |
The following categories of physical treatments belong to this measure: irradiation /ionisation; mechanical cleaning (brushing, washing); sorting and grading, and; removal of plant parts (e.g. debarking wood) Host wood is bark free and free from holes; Host wood bark and at least 2.5 cm of the outer sapwood are removed; Wood chips processed into pieces of not more than specified thickness and width |
Entry/Spread |
| Cleaning and disinfection of facilities, tools and machinery |
The physical and chemical cleaning and disinfection of facilities, tools, machinery, transport means, facilities and other accessories (e.g. boxes, pots, pallets, palox, supports, hand tools). The treatments addressed in this risk mitigation measure are: washing, sweeping and fumigation Prior to their export, machinery and vehicles which have been operated for agricultural or forestry purposes are cleaned and free from soil and plant debris |
Entry/ Spread |
| Waste management | Treatment of the waste from roguing and pruning (deep burial, composting, incineration, chipping, production of bio‐energy…) in authorised facilities and official restriction on the movement of waste | Establishment/ Spread |
| Heat and cold treatments |
Controlled temperature treatments aimed to kill or inactivate pests without causing any unacceptable prejudice to the treated material itself. The measures addressed here are: autoclaving; steam; hot water; hot air; cold treatment Host wood is heat treated to achieve a minimum temperature of 56°C for a minimum duration of 30 continuous minutes throughout the entire profile of the wood Host wood is kiln dried to below 20% moisture |
Entry/ Spread |
| Conditions of transport | Commodities can be handled and packaged in ways to prevent infestation after leaving the place of production | Entry/ Spread |
| Controlled atmosphere | Treatment of plants by storage in a modified atmosphere (including modified humidity, O2, CO2, temperature, pressure) | Entry/ Spread (via commodity) |
| Post‐entry quarantine and other restrictions of movement in the importing country |
This measure covers post‐entry quarantine (PEQ) of relevant commodities; temporal, spatial and end‐use restrictions in the importing country for import of relevant commodities; Prohibition of import of relevant commodities into the domestic country ‘Relevant commodities’ are plants, plant parts and other materials that may carry pests, either as infection, infestation or contamination |
Establishment/ Spread |
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 16.
TABLE 16.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018a, 2018b) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance.
| Supporting measure (blue underline = Zenodo doc, blue = WIP) | Summary | Risk element targeted (entry/ establishment/ spread/impact) |
|---|---|---|
| Inspection and trapping |
ISPM 5 (FAO, 2023) defines inspection as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques |
Entry/Establishment/ Spread |
| Laboratory testing | Examination, other than visual, to determine if pests are present using official diagnostic protocols. Diagnostic protocols describe the minimum requirements for reliable diagnosis of regulated pests | Entry/Spread |
| Sampling |
According to ISPM 31 (FAO, 2008), it is usually not feasible to inspect entire consignments, so phytosanitary inspection is performed mainly on samples obtained from a consignment. It is noted that the sampling concepts presented in this standard may also apply to other phytosanitary procedures, notably selection of units for testing For inspection, testing and/or surveillance purposes the sample may be taken according to a statistically based or a non‐statistical sampling methodology |
Entry/Establishment/Spread |
| Phytosanitary certificate and plant passport |
According to ISPM 5 (FAO, 2023) a phytosanitary certificate and a plant passport are official paper documents or their official electronic equivalents, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements: (a) export certificate (import) (b) plant passport (EU internal trade) |
Entry/establishment/Spread |
| Certified and approved premises | Mandatory/voluntary certification/approval of premises is a process including a set of procedures and of actions implemented by producers, conditioners and traders contributing to ensure the phytosanitary compliance of consignments. It can be a part of a larger system maintained by the NPPO in order to guarantee the fulfilment of plant health requirements of plants and plant products intended for trade. Key property of certified or approved premises is the traceability of activities and tasks (and their components) inherent the pursued phytosanitary objective. Traceability aims to provide access to all trustful pieces of information that may help to prove the compliance of consignments with phytosanitary requirements of importing countries | Entry/Spread |
| Certification of reproductive material (voluntary/official) | Plants come from within an approved propagation scheme and are certified pest free (level of infestation) following testing; Used to mitigate against pests that are included in a certification scheme | Entry/Spread |
| Delimitation of Buffer zones | ISPM 5 (FAO, 2023) defines a buffer zone as ‘an area surrounding or adjacent to an area officially delimited for phytosanitary purposes in order to minimise the probability of spread of the target pest into or out of the delimited area, and subject to phytosanitary or other control measures, if appropriate’. The objectives for delimiting a buffer zone can be to prevent spread from the outbreak area and to maintain a pest free production place (PFPP), site (PFPS) or area (PFA) | Spread |
| Surveillance | Surveillance to guarantee that plants and produce originate from a pest free area could be an option | Entry/Spread |
3.6.1.3. Biological or technical factors limiting the effectiveness of measures
Identification of species if species names are used in phytosanitary regulation rather than ‘non‐EU Scolytinae’.
Use of chemical or biological treatments in large natural areas (woods, forests), or in urban areas where no pesticides are allowed, or their use is regulated.
Surveillance of large, wooded areas (also surrounding production places and points of introduction) is challenging.
3.7. Uncertainty
At this point in a pest categorisation for a single species, only key uncertainties are normally considered. However, in this group pest categorisation a wider discussion of the uncertainties in determining the species most likely to satisfy quarantine pest status for the EU is warranted. There are uncertainties regarding species identification, entry, establishment and impact.
Species identification: as noted in Section 3.1.4, no more than 25% of the Scolytine species are listed in identification keys, reliable methods for the identification of many species are not currently available, and many that are available rely on adult specimens. It may be difficult for non‐specialised laboratories to identify some species. Dealing with a group consisting of several thousand species does not lend itself to having stable species nomenclature and it is inevitable that changes in taxonomy will occur; consequently, maintaining a correct species identification is challenging and could result in some uncertainties due to recent taxonomic reviews (e.g. Knizek & Smith, 2024) which may change species names causing a mismatch between the names reported in previous morphological identification keys and the new nomenclature. Finally, updated and reliable tools for molecular and/or morphological identification are not available for all species or genera, although protocols for molecular identification are quickly improving.
Entry: Whilst some species absent from the EU are known to have colonised new territories, the vast majority are not known to have displayed any mobility. This, however, largely depends upon the specificities of international trade (origin, destination and nature of the commodities).
Establishment: even though the range of host plants has been described for many species, some of these are very polyphagous or could adapt to new hosts, allowing establishment in areas devoid of the recognised normal hosts.
Impact: Two different scales have been used in this study to assess impact: (a) direct information from the literature, for a limited number of species; (b) a risk score based upon biological features. These scales are not always congruent. The uncertainties associated with the Google Scholar citations have been discussed in Section 3.5.
As a group, some species in Appendix D will likely be able to enter, establish, spread and cause impact in the EU. The challenge is to identify which of the species from Appendix D are most likely to do this. Two approaches were used. One focused on those species that are known to have spread between land masses (Appendix E1); the other focussed on species traits and developed ‘risk scores’ (Appendix D and E2). A summary of the number of species with risk scores from 10 (strong likelihood of satisfying criteria for EU quarantine pest status) to 0 (weak likelihood of satisfying criteria for EU quarantine pest status) is provided in Table 17.
TABLE 17.
The number of species in each risk score category and mean number of google scholar citations (Source: Appendix D).
| ‘Risk score’ | Number of species | % of species | Mean number of Google scholar citations per species | Standard deviation of mean citations |
|---|---|---|---|---|
| 10 | 57 | 1.1 | 117.0 | 222.3 |
| 9 | 454 | 8.7 | 9.0* | 24.4 |
| 8 | 547 | 10.5 | 10.7 | 35.0 |
| 7 | 490 | 9.4 | 3.7 | 10.7 |
| 6 | 1656 | 31.7 | 2.7 | 16.6 |
| 5 | 1506 | 28.9 | 1.6 | 2.5 |
| 4 | 218 | 4.2 | 2.2 | 2.9 |
| 3 | 172 | 3.3 | 1.5 | 2.0 |
| 2 | 26 | 0.5 | 1.7 | 2.2 |
| 1 | 34 | 0.7 | 1.0 | 1.2 |
| 0 | 60 | 1.1 | 1.0 | 1.0 |
| 5220 | 100.0 |
Mean and standard deviation of Google Scholar citations for species with risk score 9 excludes coffee berry borer which has 11,500 citations (outlier).
Of 88 non‐EU Scolytinae on non‐coniferous hosts occurring on distinct land masses (i.e. indicating mobility as per Grégoire et al. (2023)), 12 species can be regarded as most likely to satisfy the criteria for EU potential quarantine pest status (Table 2: List 1 and Appendix E1, List 1). The cases for other species in Table 2, Lists 2–4 and Appendix E1, Lists 2–4) become progressively weaker.
When categorising species from a group as either quarantine or non‐quarantine organisms there could be two possible types of error. One error type is to consider a species a quarantine pest erroneously because in truth it fails to satisfy the necessary criteria for quarantine pest status. This type of error can impact trade and gives the exporters and inspectors more work but its effects on the plants in the EU are minimal or non‐existent because no harm would result if the organism was introduced into the risk assessment area. The other error is that an organism that does satisfy the criteria to be a quarantine pest is judged not to be a quarantine pest. The consequences resulting from this type of error could be much larger to its hosts in the risk assessment area. Considering the lack of information for very many of the 4384 species of Scolytinae identified as having non‐coniferous hosts, the resulting uncertainty makes it very likely that both types of errors are possible.
Using ‘highly mobile’ in the sense of Grégoire et al. (2023) and only listing the 12 species in Table 2, List 1, as EU quarantine pests is likely to result in some harmful non‐EU Scolytinae not being categorised as quarantine pests. However, categorising the 44 species with risk scores of 10 in Appendix E2 as EU quarantine pests is likely to result in some species being categorised as quarantine species when in reality their introduction into the EU will not have significant impact.
4. CONCLUSIONS
Some species among the non‐EU Scolytinae attacking non‐coniferous hosts more strongly satisfy the criteria that are within the remit of EFSA to assess for them to be regarded as a potential Union quarantine pest (e.g. the 12 species in Table 2, List 1 or the 44 species with risk scores of 10 in Appendix E2) than others. Uncertainties exist over the magnitude of potential impacts for many species in the entire group.
Table 18 provides a summary of the PLH Panel conclusions.
TABLE 18.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column).
| Criterion of pest categorisation | Panel's conclusions against criterion in regulation (EU) 2016/2031 regarding union quarantine pest | General uncertainties |
|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest is well established, and the non‐EU Scolytinae species are described in worldwide, regional or national catalogues and faunae | Although the identity is well established, species identification may prove difficult |
| Absence/presence of the pest in the EU (Section 3.2 ) |
By definition, non‐EU Scolytinae are not present in the EU, or if present are not widely distributed (here considered to mean that they occur in up to three EU Member States) and are under official control 5201 species are absent from the EU; an additional 19 spp. are present in three EU MSs or less |
None presently, but when dealing with such a large number of species, the distribution of an individual species could change rapidly |
| Pest potential for entry, establishment and spread in the EU (Section 3.4 ) | Yes, many species are able to enter into, become established in, and spread within, the EU territory. Their main pathways are wood, solid wood packaging material and plants for planting (excluding pollen) | None |
| Potential for consequences in the EU (Section 3.5 ) | Some species in the group have demonstrated their capacity to have a high economic and environmental impact | There is a high uncertainty regarding the potential impact of species that have not (yet) expanded beyond their native range |
| Available measures (Section 3.6 ) | Yes, measures are available | There is a high uncertainty regarding the possibility to detect and hence prevent entry of many of the species of the group which have immature stages hidden deep in the sapwood |
| Conclusion (Section 4 ) |
For some species of non‐EU Scolytinae on non‐coniferous hosts, all criteria assessed by EFSA above for consideration as a potential quarantine pest are met (the 12 species in Table 2, List 1 or the 44 species with risk scores of 10 in Appendix E2). However, other species with lower scores still present a plant health risk to the EU The Panel was not able to develop a method to discriminate confidently between species that clearly meet the criteria for potential quarantine pest status and those that do not |
|
| Aspects of assessment to focus on/scenarios to address in future if appropriate | Research on the following issues would be beneficial:
|
|
GLOSSARY
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2023).
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2023).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2023).
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2023).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2023).
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment.
- Hitchhiker
An organism sheltering or transported accidentally via inanimate pathways including with machinery, shipping containers and vehicles; such organisms are also known as contaminating pests or stowaways (Toy & Newfield, 2010).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2023).
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2023).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2023).
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2023).
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2023).
ABBREVIATIONS
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
GLOSSARY SPECIFIC TO THIS OPINION AND THE BIOLOGY OF SCOLYTINAE
Feeding habit
Herbiphagous eats herbaceous (non‐woody) plants
Mycophagous eats fungi (not growing in wood)
Myelophagous eats pith in twigs and branches
Phloeophagous feeds on phloem
Spermatophagous eats seeds and fruits
Xylomycetophagous eats fungi growing in wood
Xylophagous eats woods
Reproduction
Bigamous a male mates with only two females
Inbreeding polygynous a male mates with multiple female siblings
Monogamous a male mates with one female
Polygamous a male mates with multiple females
Number of hosts
Monophagous hosts are species in one genus
Oligophagous more than one genus but all in the same plant family
Polyphagous hosts in more than one plant family
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2021‐00710
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A. Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L. Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen, Lucia Zappalà
MAP DISCLAIMER
The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Supporting information
Scolytinae of conifers
Scolytinae with broadleaf hosts, native or widespread in the EU
Scolytinae with broadleaf hosts, not present in the EU, occurring in countries with non‐EU climate types
Scolytinae with broadleaf hosts, not present in the EU or present with limited distribution occurring in countries which have an EU climate type
Appendices E1 and E2 are available under the Supporting Information section on the online version of the scientific output.
ACKNOWLEDGEMENTS
EFSA wishes to acknowledge: the contribution by the University of Padova, DAFNAE department (Massimo Faccoli, Matteo Marchioro, Isabel Martinez, Giacomo Ortis, Davide Rassati and Enrico Ruzzier) in developing the global database of Scolytinae species, within the EFSA art. 36 Tasking Grant with UNIPD Specific Agreement n. 2 of SA2‐GP/EFSA/ALPHA/2019/01/Lot 3; the contribution of the European Reference Laboratory (EURL) for Insects & Mites to this opinion, namely Helga Reisenzein, Richard Gottsberger, Elena Bacher, Martina Moritz (AGES) and Philippe Reynaud, Raphaelle Mouttet, Pascal Rousse (ANSES).
APPENDIX A. Scolytinae of conifers
A.1.
These 708 species have coniferous hosts and are therefore outside the scope of this pest categorisation.
Appendix A is available under the Supporting Information section on the online version of the scientific output.
APPENDIX B. Scolytinae with broadleaf hosts, native or widespread in the EU
B.1.
These 127 species of Scolytinae have broadleaf hosts and are either native to the EU or occur in four or more EU Member States. They do not satisfy criteria for further consideration as potential EU quarantine pests.
Appendix B is available under the Supporting Information section on the online version of the scientific output.
APPENDIX C. Scolytinae with broadleaf hosts, not present in the EU, occurring in countries with non‐EU climate types
C.1.
These 440 species of Scolytinae have broadleaf hosts and are not known to occur in the EU; they occur in countries with non‐EU climate types. It is not clear that they satisfy all criteria to be potential EU quarantine pests.
Appendix C is available under the Supporting Information section on the online version of the scientific output.
APPENDIX D. Scolytinae with broadleaf hosts, not present in the EU or present with limited distribution occurring in countries which have an EU climate type
D.1.
Scolytinae that have broadleaf hosts, are not known to occur in the EU or are present with limited distribution (i.e. occur in 3 or less EU Member States) and the countries in which they occur share a climate type with the EU are listed in the Excel sheet (5220 species).
Appendix D is available under the Supporting Information section on the online version of the scientific output.
APPENDIX E. Scolytinae from Appendix D with high mobility across landmasses
E.1.
From the list of 5220 species in Appendix D, 88 species that show high mobility across landmasses (Gregoire et al., 2023) most likely satisfy the required criteria for potential EU quarantine pest status. They have been further subdivided into 4 lists:
List 1–12 species with high impact on plant health according to the literature (reviewed in Grégoire et al., 2023) (most likely to satisfy EU potential quarantine pest status);
List 2–16 species with low or doubtful impact on plant health (could be potential quarantine pests but no current strong evidence for likely significant impact in the EU if introduced. However, recall the examples of Xyleborus glabratus, Pityophthorus juglandis and Euwallacea fornicatus in Section 3.5 as organisms that would have been considered harmless before 2005, but are now causing significant impact where introduced).
List 3–48 species with no impact on plant health and recorded as ‘introduced’ or ‘alien’ in at least one country in the catalogues cited in Section 2.1.1 (could be potential quarantine pests but as with list 2, there is a lack of evidence for impact).
List 4–12 species with no impact on plant health and never recorded as alien (could be potential quarantine pests but as with list 2, there is a lack of evidence for impacts) (see Appendix E1).
The same 88 species are also shown in Appendix E2 with ‘risk scores’ shown.
Appendices E1 and E2 are available under the Supporting Information section on the online version of the scientific output.
APPENDIX F. Synoptic table of EURL evaluation of the 88 species in Appendix E, with reference to available morphological and molecular diagnostic methods
F.1.
| Species | Morphology | Molecular | Synthesis | |||
|---|---|---|---|---|---|---|
| Nb of methods | References a | Sequences available? | Barcoding possible? b | Category c | EURL comments | |
| Euwallacea fornicatus | 8 | 1, 2, 3, 4, 6, 17, #18, #42 | x | Q | D | Euwallacea fornicatus species complex (lacking E. fornicatior); complex identifiable with morphological and molecular tools but specific resolution barely feasible and/or not reliable; RT‐PCR usable as alternative to barcoding |
| Euwallacea kuroshio | 6 | 1, 4, 6, 17, #18, #42 | x | Q | D | |
| Euwallacea perbrevis | 6 | 1, 4, 6, 17, #18, #42 | x | Q | D | |
| Euwallacea interjectus | 7 | 2, 3, 5, 6, 17, #18, #42 | x | Q | B | ITP sequence available; DNA barcoding gap |
| Euwallacea validus | 6 | 2, 3, 6, 17, #18, #42 | x | Y | A | ITP sequence and sequence records sufficient |
| Hypothenemus crudiae | 5 | 7, #18, 24, 25, 28 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Pityophthorus juglandis | 4 | 7, 8, 9, #18 | x | Y | A | Q‐Bank sequence; sequence records sufficient |
| Pseudopityophthorus minutissimus | 2 | 7, #18 | x | Q | B | DNA barcoding gap |
| Pseudopityophthorus pruinosus | 2 | 7, #18 | x | Q | B | DNA barcoding gap; taxonomic data uncertainties |
| Scolytus schevyrewi | 2 | 10, #18 | x | Q | A | Q‐Bank sequence available; DNA barcoding gap |
| Xyleborus ferrugineus | 10 | 2, 3, 6, 7, 17, #18, 24, 28, 35, #42 | x | Y | A | ITP sequence and sequence records sufficient |
| Xyleborus glabratus | 6 | 2, 3, 6, 17, #18, #42 | x | Y | A | ITP and Q‐Bank sequences; sequence records sufficient |
| Amasa truncata | 2 | #19, 22 | N | B | Not actually an invasive species (11, 22)? (cf. 3.1) | |
| Ambrosiodmus rubricollis | 5 | 2, 3, 7, #18, #42 | x | Y | A | ITP sequence and sequence records sufficient |
| Ambrosiophilus compressus | 2 | 17, #19 | N | B | Taxonomy to be updated (13) (cf. 3.1) | |
| Coccotrypes carpophagus | 5 | 7, 14, #18, 24, 28 | x | Q | B | DNA barcoding gap |
| Cryphalus dilutus | 3 | 15, #18, #42 | x | Q | B | Taxonomic data uncertainties; DNA barcoding gap |
| Cryphalus discretus | 3 | 37, 38, #42 | N | B | Not an invasive species (15) (cf. 3.1) | |
| Cryphalus mangiferae | 5 | 14, #18, 28, 37, 38 | x | Y | A | Sequence records sufficient; taxonomic data uncertainties; DNA barcoding gap |
| Euwallacea piceus | 3 | 6, 12, 17 | x | Q | B | Taxonomy to be updated (16) (cf. 3.1); ITP sequence available; DNA barcoding gap |
| Euwallacea similis | 6 | 2, 3, 6, 14, 17, #42 | x | Q | B | ITP sequence available; DNA barcoding gap |
| Hypothenemus areccae | 6 | 7, 14, #18, #19, 24, 28 | x | Q | D | Morphological identification of Hypothenemus species difficult (cf 3.2); DNA barcoding gap |
| Hypothenemus birmanus | 3 | 7, 14, #18 | x | Q | D | Morphological identification of Hypothenemus species difficult (cf 3.2); DNA barcoding gap |
| Hypothenemus obscurus | 5 | 7, 14, #18, 24, 28 | x | Y | C | Morphological identification of Hypothenemus species difficult (cf 3.2); Q‐Bank sequence and sequence records sufficient |
| Hypothenemus seriatus | 4 | 7, 14, #18, 24 | x | Q | D | Sometimes considered as a synonym of H. obscurus; morphological identification of Hypothenemus species difficult (cf 3.2); DNA barcoding gap |
| Phloeotribus liminaris | 3 | 7, #18, 20 | x | Q | D | DNA barcoding gap |
| Xyleborus affinis | 11 | 2, 3, 6, 7, 14, 17, #18, #19, 24, 28, #42 | x | Y | A | ITP sequence and sequence records sufficient |
| Xyleborus perforans | 4 | 6, 17, #19, 21 | x | Y | A | ITP sequence and sequence records sufficient |
| Ambrosiodmus lewisi | 6 | 2, 3, 6, 17, #18, #42 | x | Q | B | ITP sequence available; DNA barcoding gap |
| Ambrosiodmus minor | 5 | 3, 6, 17, #18, #42 | x | Q | B | ITP sequence available; DNA barcoding gap |
| Ambrosiophilus atratus | 7 | 2, 3, 6, 17, #18, 36, #42 | x | Q | B | ITP sequence available; DNA barcoding gap |
| Ambrosiophilus nodulosus | 4 | 3, 6, 17, #42 | N | B | Taxonomy to be updated (6) (cf. 3.1) | |
| Anisandrus maiche | 5 | 3, 6, 17, #18, #42 | x | Y | A | ITP sequence and sequence records sufficient |
| Cnestus mutilatus | 7 | 2, 3, 6, 14, 17, #18, #42 | x | Y | A | ITP sequence and sequence records sufficient |
| Cnestus pseudosolidus | 1 | 23 | N | B | Barely invasive species (cf. 3.3) | |
| Coccotrypes aciculatus | 3 | 7, 14, #18 | x | Q | B | DNA barcoding gap |
| Coccotrypes advena | 5 | 7, 14, #18, 24, 28 | x | Q | B | DNA barcoding gap |
| Coccotrypes cyperi | 5 | 7, 14, #18, 24, 28 | x | Q | B | DNA barcoding gap |
| Coccotrypes robustus | 5 | 7, 14, #18, 24, 28 | N | B | ||
| Coccotrypes rutschuruensis | 2 | 14, #18 | N | B | ||
| Coccotrypes vulgaris | 2 | #18,40 | N | B | ||
| Coptoborus ricini | 4 | 2, 3, 14, #18 | N | B | ||
| Cryphalus wapleri | 1 | 41 | N | B | Barely invasive species (cf. 3.3) | |
| Cryptocarenus heveae | 5 | 7, 14, #18, 24, 28 | N | B | Taxonomic data uncertainties | |
| Cyclorhipidion distinguendum | 3 | 17, #18, #42 | x | Y | A | ITP sequence and sequence records sufficient; taxonomic data uncertainties |
| Cyclorhipidion pelliculosum | 6 | 2, 3, 14, 17, #18, #42 | x | Y | A | ITP sequence and sequence records sufficient; taxonomic data uncertainties |
| Dryocoetoides cristatus | 4 | 14, #18, 24, 28 | x | Q | B | DNA barcoding gap |
| Dryoxylon onoharaense | 4 | 3, 17, #18, #42 | x | Q | B | ITP sequence available; DNA barcoding gap |
| Eccoptopterus spinosus | 2 | 17, #18 | x | Q | B | ITP sequence available; DNA barcoding gap |
| Eidophelus jalappae | 3 | 7, 14, #18 | N | B | ||
| Heteroborips seriatus | 3 | 3, 17, #18 | x | Q | B | ITP sequence available; DNA barcoding gap |
| Hypothenemus africanus | 5 | 7, 14, #18, 24, 28 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Hypothenemus brunneus | 5 | 7, 14, #18, 24, 28 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Hypothenemus californicus | 5 | 7, 14, #18, 24, 28 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Hypothenemus columbi | 5 | 7, 14, #18, 24, 28 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Hypothenemus elephas | 2 | #18, 25 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2); not an invasive species (25) (cf. 3.1) | |
| Hypothenemus erectus | 3 | 7, 14, #18 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Hypothenemus javanus | 3 | #7, 14, 18 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Hypothenemus pubescens | 5 | 7, 14, #18, 24, 28 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Hypothenemus setosus | 5 | 7, 14, #18, 24, 28 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Liparthrum mandibulare | 2 | 26, 27 | x | Q | B | Actually present in Europe (26, 27) (cf. 3.3); DNA barcoding gap. |
| Microborus boops | 4 | 7, 14, #18, 28 | x | Q | B | DNA barcoding gap |
| Monarthrum mali | 4 | 7, #18, 24, 28 | x | Y | A | Sequence records sufficient |
| Pagiocerus frontalis | 4 | 7, 14, #18, 28 | x | Y | A | Sequence records sufficient |
| Premnobius ambitiosus | 3 | 7, 14, #18 | x | Y | A | Sequence records sufficient |
| Premnobius cavipennis | 6 | 2, 7, 14, #18, 24, 28 | x | Q | B | DNA barcoding gap |
| Xyleborinus andrewesi | 6 | 3, 6, 17, #18, 28, #42 | x | Q | B | ITP sequence available; DNA barcoding gap; taxonomic data uncertainties |
| Xyleborinus artestriatus | 5 | 3, 6, 17, #18, #42 | x | Y | A | ITP sequence and sequence records sufficient |
| Xyleborinus exiguus | 3 | 6, 17, #18 | x | Q | B | ITP sequence available; DNA barcoding gap; taxonomic data uncertainties |
| Xyleborinus gracilis | 7 | 2, 3, 14, #18, 24, 28, #42 | x | Q | B | DNA barcoding gap |
| Xyleborinus octiesdentatus | 5 | 3, 6, 17, #18, #42 | x | Q | B | ITP sequence available but DNA barcoding gap |
| Xyleborus bispinatus | 7 | 3, 7, 14, #18, 28, 35, #42 | x | Q | B | DNA barcoding gap |
| Xyleborus spinulosus | 7 | 3, 7, 14, #18, 24, 28, #42 | x | Q | B | DNA barcoding gap; taxonomic data uncertainties |
| Xyleborus volvulus | 9 | 2, 3, 7, 14, 17, #18, 24, 28, #42 | x | Q | B | DNA barcoding gap; taxonomic data uncertainties |
| Xylosandrus amputatus | 6 | 3, 6, 17, #18, 39, #42 | x | Y | A | DNA barcoding gap |
| Xyloterinus politus | 3 | 7, 14, #18 | x | Y | A | Sequence records sufficient |
| Ambrosiodmus obliquus | 5 | 2, 3, #18, 28, #42 | x | Q | B | DNA barcoding gap |
| Coccotrypes distinctus | 2 | 14, #18 | x | Q | B | DNA barcoding gap |
| Cryphalus pallidus | 1 | 33 | N | D | Only one description of poor quality available for morphological identification | |
| Hypothenemus plumeriae | 2 | #18, 28 | N | D | Morphological identification of Hypothenemus species difficult (cf 3.2) | |
| Microperus eucalypticus | 1 | 32 | N | B | Only one description available for morphological identification | |
| Microperus quercicola | 3 | 17, 29, 30 | x | Q | B | DNA barcoding gap |
| Planiculus bicolor | 2 | 6, 17 | x | Q | B | ITP sequence available; DNA barcoding gap |
| Scolytoplatypus tycon | 1 | 31 | x | Q | B | DNA barcoding gap |
| Scolytus dimidiatus | 3 | 17, #18, 28 | x | Q | B | DNA barcoding gap |
| Truncaudum agnatum | 2 | 6, 17 | x | Q | B | ITP sequence available; DNA barcoding gap; taxonomic data uncertainties |
| Xyleborus africanus | 1 | 34 | N | D | Only one illustrated description available for morphological identification | |
| Xylosandrus mancus | 3 | 6, 17, 39 | x | Y | A | ITP sequence and sequence records sufficient |
Note: # indicates a picture resource without discriminating key.
Detailed reference list below.
Yes, No or Questionable.
The categorisation of identification feasibility is based on one of the following scores: (A) Readily identifiable with the current tools: both reliable morphological and molecular identification is possible. (B) Morphologically‐based identification possible: reliable morphological methods possible but can currently not be reliably confirmed by DNA barcoding. (C) Molecularly‐based identification possible but cannot be confirmed by a reliable morphological identification. (D) Identification currently problematic: to date, there are no reliable or applicable morphological or molecular tools.
Detail of references listed for morphological identification
Gomez, D. F., Skelton, J., Steininger, M. S., Stouthamer, R., Rugman‐Jones, P. F., Sittichaya, W., Rabaglia R. J., & Hulcr, J. (2018). Species delineation within the Euwallacea fornicatus (Coleoptera: Curculionidae) complex revealed by morphometric and phylogenetic analyses. Insect Systematics and Diversity, 2, 1–11.
Rabaglia, R. J., Dole, S. A., & Cognato, A. I. (2006). Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occuring north of Mexico, with an illustrated key. Annals of the Entomological Society of America, 99, 1034–1056.
Gomez, D. F., Rabaglia, R. J., Fairbanks, K., & Hulcr, J. (2018a). North American Xyleborini north of Mexico: A review and key to genera and species (Coleoptera, Curculionidae, Scolytinae). ZooKeys, 768, 19–68.
Smith, S., Gómez, D. F., Beaver, R. A., Hulcr, J., & Cognato, A. I. (2019). Reassessment of the species in the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) complex after the rediscovery of the “lost” type specimen. Insects, 10, 261.
Beaver, R. A., Sittichaya, W., & Liu, L. Y. (2014). A synopsis of the Scolytine Ambrosia Beetles of Thailand (Coleoptera: Curculionidae: Scolytinae). Zootaxa, 3875, 1–82.
Smith, S., Beaver, R. A., & Cognato, A. I. (2020). A monograph of the Xyleborini (Coleoptera, Curculionidae, Scolytinae) of the Indochinese Peninsula (except Malaysia) and China. ZooKeys, 983, 1–442.
Wood, S. L. (1982). The bark and Ambrosia beetles of North and Central America (Coleoptera: Scolytidae) a taxonomic Monographi. The great Basin Naturalist, 6, 150–203.
Bright, D. E. (1981). Taxonomic monograph of the genus Pityophthorus Eichhoff in north and central America (Coleoptera: Scolytidae). The Memoirs of the Entomological Society of Canada, 113, 1–378.
Seybold, S., Dallara, P. L., Hishinuma, S. M., & Flint, M. L. (2013). Detecting and identifying walnut twig beetle: Monitoring guidelines for the invasive vector of thousand cankers disease of walnut. In Detecting and identifying walnut twig beetle: monitoring guidelines for the invasive vector of thousand cankers disease of walnut: UC IPM Program, University of California Agriculture and Natural Resources. 13 pp. www.ipm.ucdavis.edu/thousandcankers.
LaBonte, J. R. (2010). The baded Elm Bark Beetle, Scolytus schevyrewi Semenov (Coleoptera, Curculionidae, Scolytinae) in North America: A taxonomic review and modifications to the Wood (1982) key to the species of Scolytus Geoffroy in North and Central America. ZooKeys, 56, 207–218.
Barnouin, T., Soldati, F., Roques, A., Faccoli, M., Kirkendall, L. R., Mouttet, R., Daubree, J.‐B., & Noblecourt, T. (2020). Bark beetles and pinhole borers recently or newly introduced to France (Coleoptera: Curculionidae, Scolytinae and Platypodinae). Zootaxa, 4877, 51–74.
Hulcr, J., & Cognato, A. I. (2013). Xyleborini of New Guinea, a Taxonomic Monograph (Coleoptera: Curculionidae: Scolytinae): Entomological Society of America.
Smith, S., Beaver, R. A., Sittichaya, W., & Cognato, A. I. (2022). One hundred eighteen taxonomic changes among Xyleborine ambrosia beetles (Coleoptera: Curculionidae: Scolytinae). Zootaxa, 5194, 151–175.
Wood, S. L. (2007). Bark and ambrosia beetles of South America (Coleoptera: Scolytidae): Monte L. Bean Life Science Museum.
Justesen, M. J., Hansen, A. K., Knížek, M., Lindelow, A., Solodovnikov, A., & Ravn, H. P. (2023). Taxonomic reappraisal of the European fauna of the bark beetle genus Cryphalus (Coleoptera, Curculionidae, Scolytinae). ZooKeys, 1179, 63–105.
Hulcr, J., & Cognato, A. I. (2010). New genera of Palaeotropical Xyleborini (Coleoptera: Curculionidae: Scolytinae) based on congruence between morphological and molecular characters. Zootaxa, 2717, 1–33.
Smith, S. M., Beaver, R. A., Cognato, A. I., Hulcr, J., & Redford, A. J. (2019). Southeast Asian Ambrosia Beetle ID. In Southeast Asian Ambrosia Beetle ID. USDA APHIS Identification Technology Program (ITP) and Michigan State University. https://idtools.org/sea_ambrosia
Atkinson, T. H. (2024). Bark and ambrosia beetles of the Americas. http://www.barkbeetles.info
CSIRO. (2024). ALA: Atlas of Living Australia. https://www.ala.org.au/
Pennacchio, F., Faggi, M., Gatti, E., Caronni, F., Colombo, M., & Roversi, P. F. (2004). First record of Phloeotribus liminaris (Harris) from Europe (Coleoptera Scolytidae). Redia, 87, 85–89.
CABI. (2022). Invasive species compendium. www.cabi.org/isc. In Invasive Species Compendium. www.cabi.org/isc
Knizek, M., & Smith, S. M. (2024). A new widely distributed invasive alien species of Amasa ambrosia beetles (Coleoptera: Curculionidae: Scolytinae: Xyleborini). Zootaxa, 5403, 385–390.
Dole, S. A., & Beaver, R. A. (2008). A review of the Australian species of Xylosandrus Reitter (Coleoptera: Curculionidae: Scolytinae). The Coleopterists Bulletin, 62, 481–492.
Bright, D. E., & Torres, J. A. (2006). Studies on West Indian Scolytidae (Coleoptera) 4 A review of the Scolytidae of Puerto Rico, U.S.A. with descriptions of one new genus, fourteen new species and notes on new synonymy (Coleoptera: Scolytidae). Koleoterologische Rundschau, 76, 389–428.
Atkinson, T. H., & Flechtmann, C. H. W. (2021). New species, new records and synonymy of Brazilian species of Hypothenemus Westwood, 1834 (Coleoptera: Curculionidae: Scolytinae). Insecta Mundi, 846, 1–33.
Lagarde, M., & Noblecourt, T. (2018). Une nouvelle espèce de Scolyte pour la France: Liparthrum mandibulare (Wollaston, 1854) (Coleoptera Curculionidae Scolytinae). L'Entomologiste, 74, 191–192.
Israelson, G. (1990). A key to the Macaronesian Hypoborini, with description of two new species (Coleoptera, Scolytidae). Bocagiana Museu Municipal do Funchal, 137, 1–11.
Bright, D. E. (2019). A taxonomic monograph of the Bark and Ambrosia Beetles of the West Indies (Coleoptera: Curculionoidea: Scolytidae) Studies on West Indian Scolytidae (Coleoptera) 7. Occasional Papers of the Florida State Collection of Arthropods, 12, 1–491.
Sittichaya, W., Smith, S. M., Beaver, R. A., & Thaochan, T. (2021). Revision of the xyleborine ambrosia beetle genus Microperus Wood, 1980 (Curculionidae, Scolytinae, Xyleborini) of Thailand with four new species and four newly recorded species. ZooKeys, 1074, 191–214.
Lin, C.‐S., & Smith, S. M. (2024). Review of the genus Microperus Wood, 1980 (Coleoptera, Curculionidae, Scolytinae) of Taiwan with the description of two newly recorded species and the first description of males of six species. Taiwania, 69, 151–167.
Beaver, R. A., & Gebhardt, H. (2006). A review of the oriental species of Scolytoplatypus Schaufuss (Coleoptera, Curculionidae, Scolytinae). Deutsche Entomologische Zeitschrift, 53(2), 155–178.
Schedl, K. E. (1938). Scolytidae and Platypodidae. Contribution, 49. New species from Australia and the Fiji Islands with some revisional notes. Transactions of the Royal Society of South Australia, 62(1), 34–52.
Eichhoff, W. J. (1872). Neue exotische Tomiciden‐Arten. Berliner Entomologische Zeitschrift, 15(2–3), 131–136.
Nunberg, M. (1963). Die Gattung Xyleborus Eichhoff (Coleoptera Scolytidae). Ergänzungen, Berichtigungen und Erweiterung der Diagnosen (II. Teil). Annales du Musée Royal du Congo Belge, Ser. 8, Sciences Zoologiques, 115, 127.
Atkinson, T. H., Carrillo, D., Duncan, R. E., & Peña, J. E. (2013). Occurrence of Xyleborus bispinatus (Coleoptera: Curculionidae: Scolytinae) Eichhoff in southern Florida. Zootaxa, 3669(1), 96–100.
Faccoli, M. (2008). First record of Xyleborus atratus Eichhoff from Europe, with an illustrated key to the European Xyleborini (Coleoptera: Curculionidae: Scolytinae). Zootaxa, 1772(1), 55–62.
Johnson, A. J., Hulcr, J., Knížek, M., Atkinson, T. H., Mandelshtam, M. Y., Smith, S. M., & Jordal, B. H. (2020). Revision of the bark beetle genera within the former Cryphalini (Curculionidae: Scolytinae). Insect Systematics and Diversity, 4(3).
Johnson, A. J., Knížek, M., Atkinson, T. H., Jordal, B. H., Ploetz, R. C., & Hulcr, J. (2017). Resolution of a global mango and fig pest identity crisis. Insect Systematics and Diversity, 1(2).
Dole, S. A., & Cognato, A. I. (2010). Phylogenetic revision of Xylosandrus Reitter (Coleoptera: Curculionidae: Scolytinae: Xyleborina). Proceedings of the California Academy of Sciences, 61(7), 451.
Eggers, H. (1923). Neue indomalayaische Borkenkäfer (Ipidae). Zoologische Mededelingen, 7(11), 129–220.
Eichhoff, W. J. (1872). Neue exotische Tomiciden‐Arten. Berliner Entomologische Zeitschrift, 15(2–3), 131–136.
Hulcr, J. (2012). Xyleborini North of Mexico. https://ambrosiasymbiosis.org/northamericanxyleborini
APPENDIX G. EU regulated plant pathogens vectored by Scolytinae
G.1.
Table G.1 shows which species of Scolytinae from List 1 and List 2 of Appendix E are known vectors of EU regulated plant pathogens, together with the name of the pathogens they vector (source: EPPO, online). The Scolytinae species from list 1 are species with high impact on plant health according to the literature reviewed in Grégoire et al. (2023) and most likely satisfy EU potential quarantine pest status. Table G.1 also indicates the names of non‐regulated plant pathogens vectored by the Scolytinae in List 1 or 2 from Appendix E.
TABLE G.1.
Non‐EU Scolytinae vectors of EU regulated and non‐regulated plant pathogens (Source EPPO, online, June 2024).
| Vectored pathogen. (EU regulatory status is shown in brackets) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Appendix E List | Scolytinae species | Bretziella agacearum (Annex II A) | Fusarium euwallaceae a (Annex II A) | Fusarium ambrosium (Annex II A) | Geosmithia morbida (Annex II B) | Harringtonia lauricola EPPO Alert list, 2014 | Neocosmospora akasia | Neocosmospora awan | Neocosmospora rekana | Neocosmospora warna | Neocosmospora meka |
| 1 | Euwallacea fornicatus (Eichhoff, 1868) sensu stricto | ✓ | |||||||||
| Euwallacea fornicatus sensu lato | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 2 | Euwallacea similis (Ferrari, 1867) | ✓ | ✓ | ✓ | ✓ | ||||||
| 1 | Xyleborus glabratus Eichhoff, 1877 | ✓ | |||||||||
| 1 | Euwallacea perbrevis (Schedl, 1951) | ✓ | ✓ | ✓ | ✓ | ||||||
| 1 | Pityophthorus juglandis Blackman, 1928 | ✓ | |||||||||
| 1 | Pseudopityophthorus minutissimus (Zimmermann, 1868) | ✓ | |||||||||
| 1 | Pseudopityophthorus pruinosus (Eichhoff, 1878) | ✓ | |||||||||
As the taxonomic controversy about Fusarium and Neocosmospora has not been resolved, the EPPO Secretariat prefers to use the name Fusarium euwallaceae S. Freeman, Z. Mendel, T. Aoki & O'Donnell rather than Neocosmospora euwallaceae (S. Freeman, Z. Mendel, T. Aoki & O'Donnell) Sandoval‐Denis, L. Lombard & Crous to minimise confusion (EPPO (online), June 2024).
EPPO (online), June 2024) also report Scolytus intricatus, Xyleborus affinis, Xyleborinus gracilis, Xyleborinus saxesenii, Xyleborus bispinatus, Xyleborus volvulus and Xylosandrus crassiusculus as potential vectors of Harringtonia lauricola the pathogen causing laurel wilt and which has been on the EPPO Alert list since 2014.
EPPO (online), June 2024) does not indicate that all Scolytinae species in Appendix E List 1 and List 2 are vectors of plant pathogens (Table G.2). However, information on vectors and their associated pathogens is a relatively new feature of the EPPO online database (vector information in place since April 2023) and additional data will be added over time with a focus on regulated (quarantine) pests (EPPO, online, June 2024).
TABLE G.2.
Non‐EU Scolytinae not reported as vectors of EU regulated or non‐regulated plant pathogens in EPPO online (June 2024).
| Appendix E list | Scolytinae species |
|---|---|
| 1 | Euwallacea kuroshio Gomez & Hulcr, 2018 |
| 1 | Euwallacea validus (Eichhoff, 1875) |
| 1 | Hypothenemus crudiae (Panzer, 1791) |
| 1 | Scolytus schevyrewi Semenov, 1902 |
| 1 | Euwallacea interjectus (Blandford, 1894) |
| 1 | Xyleborus ferrugineus (Fabricius, 1801) |
| 2 | Amasa parviseta Knizek & Smith, 2024 |
| 2 | Ambrosiodmus rubricollis (Eichhoff, 1875) |
| 2 | Ambrosiophilus compressus (Lea, 1894) |
| 2 | Coccotrypes carpophagus (Hornung, 1842) |
| 2 | Cryphalus dilutus Eichhoff, 1878 |
| 2 | Cryphalus discretus Eichhoff, 1878 |
| 2 | Cryphalus mangiferae Stebbing, 1914 |
| 2 | Euwallacea piceus (Motschulsky, 1863) |
| 2 | Hypothenemus areccae (Hornung, 1842) |
| 2 | Hypothenemus birmanus (Eichhoff, 1878) |
| 2 | Hypothenemus obscurus (Fabricius, 1801) |
| 2 | Hypothenemus seriatus (Eichhoff, 1872) |
| 2 | Phloeotribus liminaris (Harris, 1852) |
| 2 | Xyleborus perforans (Wollaston, 1857) |
APPENDIX H. HS import codes and import data for selected non‐coniferous wood
H.1.
HS import codes and their text descriptions of selected non‐coniferous wood are shown below. The quantity of EU imports from third countries for each code were extracted from Eurostat for the 6 year period 2017–2022. Code HS 4415 may contain a mix of coniferous and non‐coniferous wood products.
| HS code | Description |
|---|---|
| 4403 12 | Wood in the rough, treated with paint, stains, creosote or other preservatives, non‐coniferous (excl. rough‐cut wood for walking sticks, umbrellas, tool shafts and the like; wood in the form of railway sleepers; wood cut into boards or beams, etc.) |
| 4403 41 | Dark red meranti a , light red meranti and meranti bakau wood in the rough, whether or not stripped of bark or sapwood, or roughly squared (excl. rough‐cut wood for walking sticks, umbrellas, tool shafts and the like; wood cut into boards or beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4403 42 | Teak wood in the rough, whether or not stripped of bark or sapwood, or roughly squared (excl. rough‐cut wood for walking sticks, umbrellas, tool shafts and the like; wood cut into boards or beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4403 49 | Tropical wood in the rough, whether or not stripped of bark or sapwood, or roughly squared (excl. teak, dark red meranti, light red meranti, meranti bakau, acajou d'Afrique, iroko, sapelli, okoumé and sipo; rough‐cut wood for walking sticks, umbrellas, tool shafts and the like; wood cut into boards or beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4403 91 | Oak ‘Quercus spp.’ in the rough, whether or not stripped of bark or sapwood, or roughly squared (excl. rough‐cut wood for walking sticks, umbrellas, tool shafts and the like; wood in the form of railway sleepers; wood cut into boards or beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4403 93 | Beech ‘Fagus spp.’ in the rough, of which the smallest cross‐sectional dimension is ≥15 cm, whether or not stripped of bark or sapwood, or roughly squared (excl. wood in the form of railway sleepers; wood cut into beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4403 94 | Beech ‘Fagus spp.’ in the rough, of which the smallest cross‐sectional dimension is < 15 cm, whether or not stripped of bark or sapwood, or roughly squared (excl. rough‐cut wood for walking sticks, umbrellas, tool shafts and the like; wood in the form of railway sleepers; wood cut into boards or beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4403 95 | Birch ‘Betula spp.’ in the rough, of which the smallest cross‐sectional dimension is ≥15 cm, whether or not stripped of bark or sapwood, or roughly squared (excl. sawlogs; wood in the form of railway sleepers; wood cut into beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4403 96 | Birch ‘Betula spp.’ in the rough, of which the smallest cross‐sectional dimension is < 15 cm, whether or not stripped of bark or sapwood, or roughly squared (excl. rough‐cut wood for walking sticks, umbrellas, tool shafts and the like; wood in the form of railway sleepers; wood cut into boards or beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4403 97 | Poplar and aspen ‘Populus spp.’ in the rough, whether or not stripped of bark or sapwood, or roughly squared (excl. rough‐cut wood for walking sticks, umbrellas, tool shafts and the like; wood in the form of railway sleepers; wood cut into boards or beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4403 98 | Eucalyptus ‘Eucalyptus spp.’ in the rough, whether or not stripped of bark or sapwood, or roughly squared (excl. rough‐cut wood for walking sticks, umbrellas, tool shafts and the like; wood in the form of railway sleepers; wood cut into boards or beams, etc.; wood treated with paint, stains, creosote or other preservatives) |
| 4406 12 | Railway or tramway sleepers ‘cross‐ties’ of wood, not impregnated, non‐coniferous |
| 4415 | Packing cases, boxes, crates, drums and similar packings, of wood; cable‐drums of wood; pallets, box pallets and other load boards, of wood; pallet collars of wood (excl. containers specially designed and equipped for one or more modes of transport) |
Meranti wood is a hardwood (non‐coniferous) tropical wood from species of Shorea.
EU imports of non‐coniferous wood and wood products from biogeographic realm by HS code for the years 2017–2022 (tonnes) (Note: Europe, non‐EU is not a biogeographic realm but was separated out for this opinion) See Appendix J for a simplified map of realms.
| Realm | HS code | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|---|
| Palearctic, Asia and Oriental | 4403 95 | 2,697,332 | 3,346,032 | 3,026,855 | 3,359,572 | 3,363,217 | 411,659 |
| 4403 96 | 632,528 | 711,209 | 733,445 | 567,947 | 529,585 | 91,604 | |
| 4403 97 | 180,276 | 296,000 | 225,129 | 247,967 | 155,671 | 28,634 | |
| 4415 | 73,895 | 93,798 | 86,004 | 80,428 | 111,697 | 92,021 | |
| 4403 49 | 1438 | 745 | 218 | 921 | 92 | 8386 | |
| 4406 12 | 208 | 496 | 2069 | 1014 | 252 | 0 | |
| 4403 91 | 567 | 70 | 454 | 490 | 186 | 136 | |
| 4403 93 | 696 | 444 | 0 | 359 | 170 | 199 | |
| 4403 12 | 35 | 1 | 305 | 58 | 64 | 372 | |
| 4403 94 | 6 | 0 | 0 | 435 | 36 | 136 | |
| 4403 98 | 0 | 0 | 58 | 0 | 7 | 4 | |
| 4403 41 | 12 | 1 | 20 | 0 | 0 | 0 | |
| 4403 42 | 0 | 0 | 0 | 0 | 0 | 16 | |
| Europe non‐EU 3 | 4415 | 721,189 | 865,796 | 870,819 | 847,242 | 945,265 | 1,012,529 |
| 440396 | 497,716 | 138,513 | 204,564 | 278,068 | 189,462 | 107,761 | |
| 440397 | 388,144 | 19,927 | 74,823 | 144,889 | 2318 | 5109 | |
| 440312 | 478,030 | 91 | 348 | 3306 | 385 | 1238 | |
| 440393 | 23,527 | 42,274 | 45,988 | 31,349 | 35,637 | 64,357 | |
| 440394 | 26,494 | 29,147 | 24,960 | 20,496 | 16,994 | 8371 | |
| 440391 | 20,672 | 14,105 | 12,692 | 7729 | 10,267 | 9287 | |
| 440395 | 45,012 | 6083 | 278 | 1037 | 7112 | 3797 | |
| 440612 | 3276 | 3941 | 4814 | 2994 | 1622 | 820 | |
| 440349 | 6346 | 983 | 1809 | 301 | 36 | 51 | |
| 440398 | 15 | 25 | 35 | 4 | 1 | 1580 | |
| 440341 | 0 | 0 | 0 | 0 | 35 | 0 | |
| 440342 | 0 | 0 | 0 | 0 | 0 | 34 | |
| Neotropical | 440398 | 54 | 234 | 69,598 | 145,892 | 649,724 | 1,115,806 |
| 4415 | 2966 | 3578 | 3437 | 4007 | 7090 | 16,971 | |
| 440349 | 2849 | 2929 | 3788 | 3897 | 3277 | 5303 | |
| 440342 | 0 | 0 | 0 | 0 | 0 | 3017 | |
| 440341 | 0 | 0 | 0 | 0 | 1479 | 0 | |
| 440612 | 0 | 0 | 0 | 0 | 53 | 0 | |
| 440312 | 0 | 47 | 0 | 1 | 0 | 0 | |
| 440391 | 12 | 23 | 0 | 0 | 1 | 0 | |
| 440397 | 0 | 0 | 0 | 0 | 0 | 17 | |
| Africa | 440349 | 93,764 | 103,214 | 88,582 | 61,456 | 89,959 | 92,980 |
| 440398 | 984 | 1376 | 1252 | 1161 | 45,924 | 77,239 | |
| 440612 | 12,620 | 13,752 | 11,881 | 15,666 | 11,008 | 13,317 | |
| 4415 | 116 | 149 | 34 | 46 | 90 | 256 | |
| 440342 | 0 | 0 | 0 | 0 | 0 | 115 | |
| 440312 | 0 | 0 | 27 | 17 | 19 | 28 | |
| 440391 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nearctic | 440391 | 10,104 | 9542 | 11,402 | 10,961 | 13,441 | 627 |
| 4415 | 7829 | 6181 | 6364 | 5500 | 5432 | 8715 | |
| 440612 | 2889 | 2285 | 645 | 208 | 0 | 0 | |
| 440397 | 65 | 27 | 88 | 0 | 90 | 152 | |
| 440349 | 51 | 3 | 2 | 87 | 176 | 0 | |
| 440393 | 22 | 0 | 0 | 0 | 0 | 28 | |
| 440398 | 12 | 0 | 31 | 6 | 0 | 0 | |
| 440312 | 20 | 0 | 0 | 0 | 0 | 25 | |
| 440395 | 0 | 6 | 0 | 0 | 0 | 0 | |
| 440342 | 0 | 0 | 0 | 0 | 0 | 3 | |
| 440394 | 0 | 0 | 0 | 0 | 0 | 2 | |
| 440341 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 440396 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pacific and Australasia | 4415 | 93 | 118 | 71 | 249 | 237 | 679 |
| 440349 | 7 | 12 | 7 | 9 | 10 | 3 | |
| 440398 | 3 | 1 | 22 | 3 | 4 | 5 | |
| 440391 | 0 | 0 | 0 | 0 | 0 | 6 | |
| 440312 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 440612 | 0 | 0 | 0 | 0 | 0 | 0 |
APPENDIX I. Invasive genera of Scolytinae and whether they have been intercepted in the EU
I.1.
Recognising that all non‐EU Scolytinae are regarded as quarantine pests (EU 2019/2072, Annex II, Part A point 65), it is not necessary for interceptions of Scolytinae to be identified to genus or species level. As such, only genera that were identified to genus or species level can be positively regarded as having been intercepted. Six genera of Scolytinae are reported as having been intercepted in the EU:
Coccotrypes
Cryphalus
Scolytus
Xyleborinus
Xyleborus
Xylosandrus
Other genera are known to contain invasive species and searches were conducted in Europhyt and Traces but no positive identifications were found for the 33 genera listed below:
|
|
|
It is possible that some or all of the 33 genera above have been intercepted in the EU but were recorded as ‘Scolytidae’.
APPENDIX J. World biogeographic realms
J.1.

EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , … MacLeod, A. (2024). Pest categorisation of non‐EU Scolytinae on non‐coniferous hosts. EFSA Journal, 22(9), e8889. 10.2903/j.efsa.2024.8889
Adopted: 19 June 2024
Appendix A, B, C… are available under the Supporting Information section .
Notes
The European Council and the Commission have designated EU Reference Laboratories (EURL) with the aim to ensure high‐quality, uniform testing in the EU and support Commission activities on risk management and risk assessment in the area of laboratory analysis. The consortium between ANSES (France) and AGES (Austria) is designated as the European Union Reference Laboratory for the identification of regulated insect and mite species under Commission Implementing Regulation (EU) 2019/530 of 27 March 2019.
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (EPPO, 2019; Griessinger & Roy, 2015).
Europe non‐EU is not a realm but is shown to distinguish imports between non‐EU Europe and other parts of the Palearctic and Oriental realms.
REFERENCES
- Allison, J. D. , & Redak, R. A. (2017). The impact of trap type and design features on survey and detection of bark and woodboring beetles and their associates: A review and meta‐analysis. Annual Review of Entomology, 62, 127–146. [DOI] [PubMed] [Google Scholar]
- Alonso‐Zarazaga, M. A. , Barrios, H. , Borovec, R. , Bouchard, P. , Caldara, R. , Colonnelli, E. , … Yunakov, N. N. (2017). Cooperative catalogue of palaearctic Coleoptera Curculionoidea. Monografías Electrónicas SEA, 8(1), 729. [Google Scholar]
- Alonso‐Zarazaga, M. A. , Barrios, H. , Borovec, R. , Bouchard, P. , Caldara, R. , Colonnelli, E. , … Yunakov, N. N. (2023). Cooperative catalogue of palaearctic Coleoptera Curculionoidea. Monografías Electrónicas SEA, 14, 780. [Google Scholar]
- Anses . (2017). Anses opinion on a request for an express risk assessment (ERA) on Xylosandrus compactus (Eichhoff) identified in metropolitan France. Anses, Maisons‐Alfort, 68. https://www.anses.fr/fr/system/files/SANTVEG2016SA0170Ra.pdf [Google Scholar]
- Atkinson, T. H. (2023). Bark and Ambrosia Beetles of the Americas. https://www.barkbeetles.info/americas_index.php
- Baker, R. H. A. (2002). Predicting the limits to the potential distribution of alien crop pests. In Hallman G. J. & Schwalbe C. P. (Eds.), Invasive arthropods in agriculture: Problems and solutions (pp. 207–241). Science Publishers Inc. [Google Scholar]
- Balachowsky, A. (1949). Faune de France. 50. Coléoptères Scolytides. Librairie de la Faculté des Sciences. [Google Scholar]
- Barnouin, T. , Soldati, F. , Roques, A. , Faccoli, M. , Kirkendall, L. R. , Mouttet, R. , Daubrée, J.‐B. , & Noblecourt, T. (2020). Bark beetles and pinhole borers recently or newly introduced to France (Coleoptera: Curculionidae, Scolytinae and Platypodinae). Zootaxa, 4877(1), 51–74. [DOI] [PubMed] [Google Scholar]
- Beaver, R. A. , Wilding, N. , Collins, N. , Hammond, P. , & Webber, J. (1989). Insect‐fungus relationships in the bark and ambrosia beetles. Insect‐Fungus Interactions, 121, 143. [Google Scholar]
- Birch, M. C. , Miller, J. C. , & Paine, T. D. (1982). Evaluation of two attempts to trap defined populations ofScolytus multistriatus . Journal of Chemical Ecology, 8, 125–136. 10.1007/BF00984010 [DOI] [PubMed] [Google Scholar]
- Blake, M. , Straw, N. , Kendall, T. , Whitham, T. , Manea, I. A. , Inward, D. , Jones, B. , Hazlitt, N. , Ockenden, A. , Deol, A. , Brown, A. , Ransom, E. , Smith, L. , & Facey, S. (2024). Recent outbreaks of the spruce bark beetle Ips typographus in the UK: Discovery, management, and implications. Trees, Forests and People, 100508, 13. 10.1016/j.tfp.2024.100508 [DOI] [Google Scholar]
- Brasier, C. M. (1991). Ophiostoma novo‐ulmi sp. nov., causative agent of current Dutch elm disease pandemics. Mycopathologia, 115, 151–161. [Google Scholar]
- Brasier, C. M. , & Buck, K. W. (2001). Rapid evolutionary changes in a globally invading fungal pathogen (Dutch elm disease). Biological Invasions, 3, 223–233. [Google Scholar]
- Bright, D. E. (2014). A catalog of Scolytidae and Platypodidae (Coleoptera), Supplement 3 (2000–2010), with notes on subfamily and tribal reclassifications. Insecta Mundi, 0356, 1–336. [Google Scholar]
- Bright, D. E. (2019). A taxonomic monograph of the bark and ambrosia beetles of the West Indies (Coleoptera: Curculionoidea: Scolytidae). Studies on West Indian Scolytidae (Coleoptera) 7. Occasional Papers of the Florida State Collection of Arthropods, 12, 1–491. [Google Scholar]
- Bright, D. E. (2021). Catalog of Scolytidae (Coleoptera), supplement 4 (2011–2019) with an annotated checklist of the world fauna (Coleoptera: Curculionoidea: Scolytidae), a doctoral dissertation. Colorado State University Libraries. https://mountainscholar.org/handle/10217/229307 [Google Scholar]
- Bright, D. E. , & Skidmore, R. E. (1997). Catalog of Scolytidae and Platypodidae (Coleoptera), supplement 1 (1990–1994) (p. 368). NRC Research Press. [Google Scholar]
- Bright, D. E. , & Skidmore, R. E. (2002). Catalog of Scolytidae and Platypodidae (Coleoptera), supplement 2 (1995–1999) (p. 523). NRC Research Press. [Google Scholar]
- British Columbia Government . 2024. https://www2.gov.bc.ca/gov/content/industry/forestry/managing‐our‐forest‐resources/forest‐health/forest‐pests/bark‐beetles/mountain‐pine‐beetle/responding‐to‐the‐1999‐2015‐outbreak#Key%20facts
- Brockerhoff, E. G. , Knížek, M. , & Bain, J. (2003). Checklist of indigenous and adventive bark and ambrosia beetles (Curculionidae: Scolytinae and Platypodinae) of New Zealand and interceptions of exotic species (1952–2000). New Zealand Entomologist, 26(1), 29–44. [Google Scholar]
- Browne, F. G. (1961). The biology of Malayan Scolytidae and Platypodidae. Malayan Forest Records, 22, 266. [Google Scholar]
- CABI . (2009). Xylosandrus mutilatus (camphor shoot beetle). 10.1079/cabicompendium.57239 [DOI]
- CABI Compendium . (online). CABI compendium. CAB International. https://www.cabidigitallibrary.org/journal/cabicompendium [Google Scholar]
- Caudullo, G. , & de Rigo, D. (2016). Ulmus ‐ elms in Europe: Distribution, habitat, usage and threats. In San‐Miguel‐Ayanz J., de Rigo D., Caudullo G., Houston Durrant T., & Mauri A. (Eds.), European atlas of Forest tree species (p. e01bd40). Publ. Off EU. [Google Scholar]
- Cavaletto, G. , Faccoli, M. , Marini, L. , Spaethe, J. , Magnani, G. , & Rassati, D. (2020). Effect of trap color on captures of bark‐and wood‐boring beetles (Coleoptera; Buprestidae and Scolytinae) and associated predators. Insects, 11(11), 749. 10.3390/insects11110749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase, K. D. , Kelly, D. , Liebhold, A. M. , Bader, M. K. F. , & Brockerhoff, E. G. (2017). Long‐distance dispersal of non‐native pine bark beetles from host resources. Ecological Entomology, 42(2), 173–183. [Google Scholar]
- Cognato, A. I. , Hoebeke, E. R. , Kajimura, H. , & Smith, S. M. (2015). History of the exotic ambrosia beetles Euwallacea interjectus and Euwallacea validus (Coleoptera: Curculionidae: Xyleborini) in the United States. Journal of Economic Entomology, 108(3), 1129–1135. 10.1093/jee/tov073 [DOI] [PubMed] [Google Scholar]
- Cognato, A. I. , Sari, G. , Smith, S. M. , Beaver, R. A. , Li, Y. , Hulcr, J. , Jordal, B. H. , Kajimura, H. , Ching‐San, L. , Pham, T. , Singh, S. , & Sittichaya, W. (2020). The essential role of taxonomic expertise in the creation of DNA databases for the identification and delimitation of Southeast Asian Ambrosia beetle species (Curculionidae: Scolytinae: Xyleborini). Frontiers in Ecology and Evolution, 8, 17. [Google Scholar]
- CORINE Land Cover . (2018). Coordination of information on the environment. Vector/raster 100 m, Europe, 6‐yearly. https://land.copernicus.eu/en/products/corine‐land‐cover/clc2018
- Dreiss, L. M. , & Volin, J. C. (2014). Forests: Temperate Evergreen and deciduous. In Encyclopedia of natural resources. CRC Press. 10.1081/E-ENRL-120047447214 [DOI] [Google Scholar]
- Eaton, E. , Caudullo, G. , Oliveira, S. , & de Rigo, D. (2016). Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In San‐Miguel‐Ayanz J., de Rigo D., Caudullo G., Houston Durrant T., & Mauri A. (Eds.), European atlas of Forest tree species (e01c6df+). Publ. Off EU. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger, M. , Bragard, C. , Caffier, D. , Candresse, T. , Chatzivassiliou, E. , Dehnen‐Schmutz, K. , Grégoire, J.‐C. , Jaques Miret, J. A. , MacLeod, A. , Navajas Navarro, M. , Niere, B. , Parnell, S. , Potting, R. , Rafoss, T. , Rossi, V. , Urek, G. , Van Bruggen, A. , Van Der Werf, W. , … Gilioli, G. (2018a). Guidance on quantitative pest risk assessment. EFSA Journal, 16(8), 5350. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger, M. , Bragard, C. , Caffier, D. , Candresse, T. , Chatzivassiliou, E. , Dehnen‐Schmutz, K. , Gilioli, G. , Grégoire, J.‐. C. , Jaques Miret, J. A. , Navarro, M. N. , Niere, B. , Parnell, S. , Potting, R. , Rafoss, T. , Rossi, V. , Urek, G. , Van Bruggen, A. , Van der Werf, W. , … MacLeod, A. (2018b). Scientific Opinion on the pest risk assessment of Spodoptera frugiperda for the European Union. EFSA Journal, 16(8), 5351. 10.2903/j.efsa.2018.5351 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Plant Health Panel) , Bragard, C. , Dehnen‐Schmutz, K. , Di Serio, F. , Gonthier, P. , Jacques, M.‐A. , Jaques Miret, J. A. , Fejer Justesen, A. , MacLeod, A. , Magnusson, C. S. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , Zappala, L. , … Milonas, P. (2019). Scientific Opinion on the pest categorisation of Pseudopityophthorus minutissimus and P. Pruinosus . EFSA Journal, 17(1), 5513. 10.2903/j.efsa.2019.5513 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Dehnen‐Schmutz, K. , Di Serio, F. , Gonthier, P. , Jacques, M.‐A. , Jaques Miret, J. A. , Justesen, A. F. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , Zappala, L. , … MacLeod, A. (2020a). Pest categorisation of non‐EU Tephritidae. EFSA Journal, 18(1), 5931. 10.2903/j.efsa.2020.5931 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Dehnen‐Schmutz, K. , Di Serio, F. , Gonthier, P. , Jacques, M.‐A. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Thulke, H.‐H. , van der Werf, W. , Civera, A. V. , Yuen, J. , Zappala, L. , … Milonas, P. (2020b). Scientific Opinion on the list of non‐EU Scolytinae of coniferous hosts. EFSA Journal, 18(1), 5933. 10.2903/j.efsa.2020.5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Dehnen‐Schmutz, K. , Di Serio, F. , Gonthier, P. , Jacques, M.‐A. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Thulke, H.‐H. , Van der Werf, W. , Civera, A. V. , Yuen, J. , Zappala, L. , … Milonas, P. (2020c). Scientific Opinion on the pest categorisation of non‐EU Scolytinae of coniferous hosts. EFSA Journal, 18(1), 5934. 10.2903/j.efsa.2020.5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy, A. , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Benfenati, E. , Chaudhry, Q. M. , Craig, P. , … Younes, M. (2017). Scientific opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal, 15(8), 4971. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Sayed, A. M. (2024). The Pherobase: Database of Pheromones and Semiochemicals. https://www.pherobase.com
- EPPO (European and Mediterranean Plant Protection Organization) . (2019). EPPO codes. https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO (European and Mediterranean Plant Protection Organization) . (2020). EPPO Datasheet: Pityophthorus juglandis . https://gd.eppo.int/taxon/PITOJU/datasheet
- EPPO (European and Mediterranean Plant Protection Organization) . (2024). Pityophthorus juglandis. EPPO datasheets on pests recommended for regulation. https://gd.eppo.int
- EPPO (European and Mediterranean Plant Protection Organization) EPPO A1 list of quarantine species. https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list
- EPPO (European and Mediterranean Plant Protection Organization) . (online). EPPO global database. https://gd.eppo.int
- Faccoli, M. (2015). European bark and ambrosia beetles: Types, characteristics and identification of mating systems. WBA Handbooks 5. ISBN‐13: 9788890332340. [Google Scholar]
- Faccoli, M. , Campo, G. , Perrotta, G. , & Rassati, D. (2016). Two newly introduced tropical bark and ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) damaging figs (Ficus carica) in southern Italy. Zootaxa, 4138(1), 189–194. [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) . (2008). ISPM (international standards for Phytosanitary measures) No 31. Methodologies for sampling of consignments. FAO. https://www.ippc.int/static/media/files/publication/en/2016/11/ISPM_31_2008_Sampling_of_consignments_EN.pdf [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) . (2013). ISPM (international standards for Phytosanitary measures) No 11. Pest risk analysis for quarantine pests. FAO. https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014‐04‐30_201405121523‐494.65%20KB.pdf [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) . (2023). ISPM (international standards for Phytosanitary measures) No 5. Glossary of phytosanitary terms. FAO. https://assets.ippc.int/static/media/files/publication/en/2023/07/ISPM_05_2023_En_Glossary_PostCPM‐17_2023‐07‐12_Fixed.pdf [Google Scholar]
- Flechtmann, C. A. H. , & Cognato, A. I. (2011). First report of Amasa truncata (Erichson) (Coleoptera Curculionidae: Scolytinae) in Brazil. The Coleopterists Bulletin, 65(4), 417–421. [Google Scholar]
- Freude, H. , Harde, K. W. , & Lohse, G. A. (1981). Die Käfer Mitteleuropas, band 10 (Bruchidae, Anthribidae, Scolytidae, Platypodidae, Curculionidae). Goecke and Evers, Krefeld, 1981, 69. [Google Scholar]
- Gomez, D. F. , Rabaglia, R. J. , Fairbanks, K. E. , & Hulcr, J. (2018). North American Xyleborini north of Mexico: A review and key to genera and species (Coleoptera, Curculionidae, Scolytinae). ZooKeys, 768, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoire, J.‐C. , Jactel, H. , Hulcr, J. , Battisti, A. , Inward, D. , Petter, F. , & Grousset, F. (2023). Cosmopolitan Scolytinae: Strong common drivers, but too many singularities for accurate prediction. In Jactel H., Orazio C., Robinet C., Douma J. C., Santini A., Battisti A., Branco M., Seehausen L., & Kenis M. (Eds.), Conceptual and technical innovations to better manage invasions of alien pests and pathogens in forests. NeoBiota (Vol. 84, pp. 81–105). 10.3897/neobiota.84.89826 [DOI] [Google Scholar]
- Grégoire, J.‐C. , Raffa, K. F. , & Lindgren, B. S. (2015). Economics and politics of bark beetles. In Vega F. E. & Hofstetter R. W. (Eds.), Bark beetles. Biology and ecology of native and invasive species (pp. 585–613). Elsevier/Academic Press. [Google Scholar]
- Griessinger, D. , & Roy, A.‐S. (2015). EPPO codes: a brief description. https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Grousset, F. , Grégoire, J.‐C. , Jactel, H. , Battisti, A. , Benko Beloglavec, A. , Hrašovec, B. , Hulcr, J. , Inward, D. , Orlinski, A. , & Petter, F. (2020). The risk of bark and ambrosia beetles associated with imported non‐coniferous wood and potential horizontal phytosanitary measures. Forests, 11, 342. 10.3390/f11030342 [DOI] [Google Scholar]
- Gugliuzzo, A. , Gusella, G. , Leonardi, G. R. , Costanzo, M. B. , Ricupero, M. , Rassati, D. , Biondi, A. , & Polizzi, G. (2023). From a cause of rapid fig tree dieback to a new threat to mango production: The invasive bark beetle Cryphalus dilutus Eichhoff (Coleoptera: Curculionidae, Scolytinae) and its associated fungi found on mango trees in Europe. EPPO Bulletin, 53(3), 663–670. [Google Scholar]
- Hendrickx, G. , Gilbert, M. , Staubach, C. , Elbers, A. , Mintiens, K. , Gerbier, G. , & Ducheyne, E. (2008). A wind density model to quantify the airborne spread of Culicoides species during north‐western Europe bluetongue epidemic, 2006. Preventive Veterinary Medicine, 87(1–2), 162–181. 10.1016/j.prevetmed.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Hlásny, T. , Zimová, S. , Merganičová, K. , Štěpánek, P. , Modlinger, R. , & Turčáni, M. (2021). Devastating outbreak of bark beetles in The Czech Republic: Drivers, impacts, and management implications. Forest Ecology and Management, 490, 119075. 10.1016/j.foreco.2021.119075 [DOI] [Google Scholar]
- Hofstetter, R. W. , Dinkins‐Bookwalter, J. , Davis, T. S. , & Klepzig, K. D. (2015). Symbiotic associations of bark beetles. In Vega F. E. & Hofstetter R. W. (Eds.), Bark beetles. Biology and ecology of native and invasive species (pp. 209–245). Elsevier/Academic Press. [Google Scholar]
- Hughes, M. A. , Riggins, J. J. , Koch, F. H. , Cognato, A. I. , Anderson, C. , Formby, J. P. , Dreaden, T. , Ploetz, R. , & Smith, J. A. (2017). No rest for the laurels: Symbiotic invaders cause unprecedented damage to southern USA forests. Biological Invasions, 19(7), 2143–2157. 10.1007/s10530-017-1427-z [DOI] [Google Scholar]
- Hulcr, J. , Atkinson, T. H. , Cognato, A. I. , Jordal, B. H. , & McKenna, D. D. (2015). Morphology, taxonomy, and phylogenetics of bark beetles. In Vega F. E. & Hofstetter R. W. (Eds.), Bark Beetles. Biology and ecology of native and invasive species (pp. 41–84). Elsevier/Academic Press. [Google Scholar]
- Hulcr, J. , & Stelinski, L. L. (2017). The ambrosia symbiosis: From evolutionary ecology to practical management. Annual Review of Entomology, 62, 285–303. [DOI] [PubMed] [Google Scholar]
- Inward, D. J. G. , Caiti, E. , Barnard, K. , Hasbroucq, S. , Reed, K. , & Grégoire, J.‐C. (2024). Evidence of cross‐channel dispersal into England of the forest pest Ips typographus . Journal of Pest Science, 1–15. 10.1007/s10340-024-01763-4 [DOI] [Google Scholar]
- Jackson, P. L. , Straussfogel, D. , Lindgren, B. S. , Mitchell, S. , & Murphy, B. (2008). Radar observation and aerial capture of mountain pine beetle, Dendroctonus ponderosae Hopk. (Coleoptera: Scolytidae) in flight above the forest canopy. Canadian Journal of Forest Research, 38(8), 2313–2327. 10.1139/X08-066 [DOI] [Google Scholar]
- Jactel, H. , & Gaillard, J. (1991). A preliminary study of the dispersal potential of Ips sexdentatus (Boern) (Col., Scolytidae) with an automatically recording flight mill. Journal of Applied Entomology, 112, 138–145. 10.1111/j.1439-0418.1991.tb01039.x [DOI] [Google Scholar]
- Jordal, B. H. (2006). Community structure and reproductive biology of bark beetles (Coleoptera: Scolytinae) associated with Macaronesian Euphorbia shrubs. European Journal of Entomology, 103(1), 71. [Google Scholar]
- Jordal, B. H. , & Kambestad, M. (2014). DNA barcoding of bark and ambrosia beetles reveals excessive NUMT s and consistent east‐west divergence across Palearctic forests. Molecular Ecology Resources, 14(1), 7–17. [DOI] [PubMed] [Google Scholar]
- Kees, A. M. , Hefty, A. R. , Venette, R. C. , Seybold, S. J. , & Aukema, B. H. (2017). Flight capacity of the walnut twig beetle (Coleoptera: Scolytidae) on a laboratory flight mill. Environmental Entomology, 46, 633–641. [DOI] [PubMed] [Google Scholar]
- Kenis, M. , Wermelinger, B. , & Grégoire, J.‐C. (2004). Research on parasitoids and predators of Scolytidae in living trees in Europe – A review. In Lieutier F., Day K., Battisti A., Grégoire J. C., & Evans H. (Eds.), Bark and Wood boring insects in living trees in Europe, a synthesis (pp. 237, 583–290). Kluwer. [Google Scholar]
- Kirisits, T. (2004). Fungal associates of European bark beetles with special emphasis on the Ophiostomatoid fungi. In Lieutier F., Day K., Battisti A., Grégoire J. C., & Evans H. (Eds.), Bark and Wood boring insects in living trees in Europe, a synthesis (pp. 181–235). Kluwer. 10.1007/978-1-4020-2241-8_11 [DOI] [Google Scholar]
- Kirkendall, L. R. , Biedermann, P. H. , & Jordal, B. H. (2015). Evolution and diversity of bark and ambrosia beetles. In Vega F. E. & Hofstetter R. W. (Eds.), Bark beetles. Biology and ecology of native and invasive species (pp. 85–156). Elsevier/Academic Press. [Google Scholar]
- Kirkendall, L. R. , Dal Cortivo, M. , & Gatti, E. (2008). First record of the ambrosia beetle, Monarthrum Mali (Curculionidae, Scolytinae) in Europe. Journal of Pest Science, 81(3), 175–178. [Google Scholar]
- Kirkendall, L. R. , & Faccoli, M. (2010). Bark beetles and pinhole borers (Curculionidae, Scolytinae, Platypodinae) alien to Europe. Zookeys, 56, 227–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knizek, M. , & Smith, S. M. (2024). A new widely distributed invasive alien species of Amasa ambrosia beetles (Coleoptera: Curculionidae: Scolytinae: Xyleborini). Zootaxa, 5403(3), 385–390. [DOI] [PubMed] [Google Scholar]
- Kottek, M. , Grieser, J. , Beck, C. , Rudolf, B. , & Rubel, F. (2006). World map of the Köppen_Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. 10.1127/0941-2948/2006/0130 [DOI] [Google Scholar]
- Kuhn, A. , Hautier, L. , & San, M. G. (2022). Do pheromone traps help to reduce new attacks of Ips typographus at the local scale after a sanitary cut? PeerJ, 10, e14093. 10.7717/peerj.14093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantschner, M. V. , Corley, J. C. , & Liebhold, A. M. (2020). Drivers of global Scolytinae invasion patterns. Ecological Applications, 30(5), e02103. [DOI] [PubMed] [Google Scholar]
- Liebhold, A. M. , & Tobin, P. C. (2008). Population ecology of insect invasions and their management. Annual Review of Entomology, 53, 387–408. [DOI] [PubMed] [Google Scholar]
- Marchioro, M. , & Faccoli, M. (2021). Dispersal and colonization risk of the Walnut Twig Beetle, Pityophthorus juglandis, in southern Europe. Journal of Pest Science, 95(1), 303–313. [Google Scholar]
- Marchioro, M. , Vallotto, D. , Ruzzier, E. , Besana, L. , Rossini, M. , Ortis, G. , Faccoli, M. , & Martinez‐Sañudo, I. (2024). The first host plant dataset of Curculionidae Scolytinae of the world: Miscellaneous tribes. Scientific Data, 11(1), 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurisse, N. , Rassati, D. , Hurley, B. P. , Brockerhoff, E. G. , & Haack, R. A. (2019). Common pathways by which non‐native forest insects move internationally and domestically. Journal of Pest Science, 92(1), 13–27. 10.1007/s10340-018-0990-0 [DOI] [Google Scholar]
- Montecchio, L. , & Faccoli, M. (2014). First record of thousand cankers disease Geosmithia morbida and walnut twig beetle Pityophthorus juglandis on Juglans nigra in Europe. Plant Disease, 98(5), 696. 10.1094/PDIS-07-14-0719-PDN [DOI] [PubMed] [Google Scholar]
- Nilssen, A. C. (1984). Long‐range aerial dispersal of bark beetles and bark weevils (Coleoptera, Scolytidae and Curculionidae) in northern Finland. Annales Entomologici Fennici, 50(2), 37–42. [Google Scholar]
- Pennacchio, F. , Faggi, M. , Gatti, E. , Caronni, F. , Colombo, M. , & Roversi, P. F. (2004). First record of Phloeotribus liminaris (Harris) in Europe (Coleoptera Scolytidae). Redia, 87, 85–89. [Google Scholar]
- Pfeffer, A. (1994). Zentral‐ und Westpalaarktische Borken‐ und Kernkafer (Coleoptera: Scolytidae, Platypodidae). Entomologia Basiliensia, 17, 5–310. [Google Scholar]
- Potgieter, L. J. , Cadotte, M. W. , Roets, F. , & Richardson, D. M. (2024). Monitoring urban biological invasions using citizen science: The polyphagous shot hole borer (Euwallacea fornicatus). Journal of Pest Science, 1–13. [Google Scholar]
- Rabaglia, R. , Duerr, D. , Acciavatti, R. , & Ragenovich, I. (2008). Early detection and rapid response for non‐native bark and ambrosia beetles. US Department of Agriculture Forest Service, Forest Health Protection . [Google Scholar]
- Rabaglia, R. J. , Cognato, A. I. , Hoebeke, E. R. , Johnson, C. W. , Labonte, J. R. , Carter, M. E. , & Vlack, J. J. (2019). Early detection and rapid response: A 10‐year summary of the USDA Forest Service program of surveillance for non‐native bark and Ambrosia beetles. American Entomologiste, 65(1), 29–42. 10.1093/ae/tmz015 [DOI] [Google Scholar]
- Rabaglia, R. J. , Dole, S. A. , & Cognato, A. I. (2006). Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occurring north of Mexico, an illustrated key. Annals of the Entomological Society of America, 99, 1034–1056. [Google Scholar]
- Raffa, K. F. , Grégoire, J. C. , & Lindgren, B. S. (2015). Natural history and ecology of bark beetles. In Vega F. E. & Hofstetter R. W. (Eds.), Bark beetles. Biology and ecology of native and invasive species (pp. 1–40). Elsevier/Academic Press. [Google Scholar]
- Rassati, D. , Faccoli, M. , Marini, L. , Haack, R. A. , Battisti, A. , & Petrucco Toffolo, E. (2015). Exploring the role of wood waste landfills in early detection of non‐native wood‐boring beetles. Journal of Pest Science, 88, 563–572. 10.1007/s10340-014-0639-6 [DOI] [Google Scholar]
- Rassati, D. , Faccoli, M. , Petrucco Toffolo, E. , Battisti, A. , & Marini, L. (2015). Improving the early detection of alien wood‐boring beetles in ports and surrounding forests. Journal of Applied Ecology, 52, 50–58. 10.1111/1365-2664.12347 [DOI] [Google Scholar]
- Rassati, D. , Toffolo, E. , Roques, A. , Battisti, A. , & Faccoli, M. (2014). Trapping wood boring beetles in Italian ports: A pilot study. Journal of Pest Science, 87, 61–69. 10.1007/s10340-013-0499-5 [DOI] [Google Scholar]
- Reynolds, D. R. , Chapman, J. W. , & Drake, V. A. (2017). Riders on the wind: The aeroecology of insect migrants. In Chilson P. B., Frick W., Kelly J., & Liechti F. (Eds.), Aeroecology (pp. 145–178). Springer. 10.1007/978-3-319-68576-2_7 [DOI] [Google Scholar]
- Rivera, M. J. , Martini, X. , Conover, D. , Mafra‐Neto, A. , Carrillo, D. , & Stelinski, L. L. (2020). Evaluation of semiochemical based push‐pull strategy for population suppression of ambrosia beetle vectors of laurel wilt disease in avocado. Scientific Reports, 10(1), 2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, D. , Da Lio, D. , Bartolini, L. , Salemi, C. , Del Nista, D. , Aronadio, A. , Pennacchio, F. , Binazzi, F. , Francardi, V. , Garonna, A. P. , & Rossi, E. (2021). TaqMan probe assays on different biological samples for the identification of three ambrosia beetle species, Xylosandrus compactus (Eichoff), X. Crassiusculus (Motschulsky) and X. Germanus (Blandford)(Coleoptera Curculionidae Scolytinae). 3 Biotech, 11(6), 1–15. 10.1007/s13205-021-02786-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, D. , Stabile, I. , Marrucci, A. , Ranaldi, C. , Zubieta, C. G. , d'Agostino, A. , Bartolini, L. , Pennacchio, E. , Rossi, E. , & Garonna, A. P. (2024). Identification of the ambrosia beetle Xyleborinus saxesenii (Ratzeburg, 1837)(Coleoptera: Curculionidae: Scolytinae) from frass and adult DNA by TaqMan probe real‐time‐PCR. EPPO Bulletin, 54 (1), 84–94. 10.1111/epp.12970 [DOI] [Google Scholar]
- Robertson, C. , Nelson, T. A. , Jelinski, D. E. , Wulder, M. A. , & Boots, B. (2009). Spatial–temporal analysis of species range expansion: The case of the mountain pine beetle, Dendroctonus ponderosae . Journal of Biogeography, 36, 1446–1458. 10.1111/j.1365-2699.2009.02100.x [DOI] [Google Scholar]
- Ruzzier, E. , Ortis, G. , Vallotto, D. , Faccoli, M. , Martinez‐Sañudo, I. , & Marchioro, M. (2023). The first full host plant dataset of Curculionidae Scolytinae of the world: Tribe Xyleborini LeConte, 1876. Scientific Data, 10(1), 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurat, C. , Mouttet, R. , Jeandel, C. , Prost, J. , Tellez, D. , & Ioos, R. (2023). First report of thousand cankers disease caused by the fungus Geosmithia morbida and its vector Pityophthorus juglandis on Juglans regia in France. New Disease Reports, 47(1), 4 pp. [Google Scholar]
- Schedl, K. E. (1962). Scolytidae und Platypodidae Afrikas II, Familie Scolytidae. Revista de Entomolia de Moçambique, 5(1), 595–1352. [Google Scholar]
- Schedl, K. E. (1981). Familie: Scolytidae (Borken‐ und Ambrosiakafer). In Die Kafer Mitteleuropas, Band 10 (pp. 34–99, 280–295). Goecke and Evers. [Google Scholar]
- Seo, M. , Martini, X. , Rivera, M. J. , & Stelinski, L. L. (2017). Flight capacities and diurnal flight patterns of the ambrosia beetles, Xyleborus glabratus and Monarthrum Mali (Coleoptera: Curculionidae). Environmental Entomology, 46(3), 729–734. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Beaver, R. A. , & Cognato, A. I. (2020). A monograph of the xyleborini (Coleoptera, Curculionidae, Scolytinae) of the Indochinese peninsula (except Malaysia) and China. ZooKeys, 983, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Gomez, D. F. , Beaver, R. A. , Hulcr, J. , & Cognato, A. I. (2019). Reassessment of the species in the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) complex after the rediscovery of the “lost” type specimen. Insects, 10(9), 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , & Hulcr, J. (2015). Scolytus and other economically important bark and ambrosia beetles. In Vega F. E. & Hofstetter R. W. (Eds.), Bark beetles: Biology and ecology of native and invasive species (pp. 495–532). Elsevier/Academic Press. [Google Scholar]
- Terekhova, V. V. , & Skrylnik, Y. Y. (2012). Biological peculiarities of the alien for Europe Anisandrus maiche stark (Coleoptera: Curculionidae: Scolytinae) bark beetle in Ukraine. Russian Journal of Biological Invasions, 3(2), 139–144. [Google Scholar]
- Toy, S. J. , & Newfield, M. J. (2010). The accidental introduction of invasive animals as hitchhikers through inanimate pathways: A New Zealand perspective. Revue Scientifique et Technique (International Office of Epizootics), 29(1), 123–133. [DOI] [PubMed] [Google Scholar]
- Turner, R. M. , Brockerhoff, E. G. , Bertelsmeier, C. , Blake, R. E. , Caton, B. , James, A. , MacLeod, A. , Nahrung, H. F. , Pawson, S. M. , Plank, M. J. , Pureswaran, D. S. , Seebens, H. , Yamanaka, T. , & Liebhold, A. M. (2021). Worldwide border interceptions provide a window into human‐mediated global insect movement. Ecological Applications, 31(7), e02412. 10.1002/eap.2412 [DOI] [PubMed] [Google Scholar]
- Ward, S. F. , Brockerhoff, E. G. , Turner, R. M. , Yamanaka, T. , Marini, L. , Fei, S. , & Liebhold, A. M. (2023). Prevalence and drivers of a tree‐killing bark beetle, Ips typographus (Coleoptera, Scolytinae), in international invasion pathways into the USA. Journal of Pest Science, 96(2), 845–856. [Google Scholar]
- Wegensteiner, R. , Wermelinger, B. , & Herrmann, M. (2015). Natural enemies of bark beetles: Predators, parasitoids, pathogens, and nematodes. In Vega F. E. & Hofstetter R. W. (Eds.), Bark beetles: Biology and ecology of native and invasive species (pp. 247–304). Elsevier/Academic Press. [Google Scholar]
- Welk, E. , de Rigo, D. , & Caudullo, G. (2016). Prunus avium in Europe: Distribution, habitat, usage and threats. In San‐Miguel‐Ayanz J., de Rigo D., Caudullo G., Houston Durrant T., & Mauri A. (Eds.), European atlas of Forest tree species (p. e01491d+). Publ. Off EU. https://forest.jrc.ec.europa.eu/media/atlas/Prunus_avium.pdf [Google Scholar]
- Wood, S. L. (1982). The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. The Great Basin Naturalist Memoirs, 6, 1–1359. [Google Scholar]
- Wood, S. L. (1986). A reclassification of the genera of Scolytidae (Coleoptera). The Great Basin Naturalist Memoirs, 10, 1–126. [Google Scholar]
- Wood, S. L. (2007). Bark and ambrosia beetles of South America. Brigham Young University. [Google Scholar]
- Wood, S. L. , & Bright, D. E. (1992). A catalog of Scolytidae and Platypodidae (Coleoptera), part 2. Taxonomic index (volumes A,B). The Great Basin Naturalist Memoirs, 13, 1–11553. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scolytinae of conifers
Scolytinae with broadleaf hosts, native or widespread in the EU
Scolytinae with broadleaf hosts, not present in the EU, occurring in countries with non‐EU climate types
Scolytinae with broadleaf hosts, not present in the EU or present with limited distribution occurring in countries which have an EU climate type
Appendices E1 and E2 are available under the Supporting Information section on the online version of the scientific output.


