Abstract
With the size of the aging population increasing worldwide, the effective diagnosis and treatment of neurodegenerative diseases (NDDs) has become more important. Two-dimensional (2D) materials offer specific advantages for the diagnosis and treatment of NDDs due to their high sensitivity, selectivity, stability, and biocompatibility, as well as their excellent physical and chemical characteristics. As such, 2D materials offer a promising avenue for the development of highly sensitive, selective, and biocompatible theragnostics. This review provides an interdisciplinary overview of advanced 2D materials and their use in biosensors, drug delivery, and tissue engineering/regenerative medicine for the diagnosis and/or treatment of NDDs. The development of 2D material-based biosensors has enabled the early detection and monitoring of NDDs via the precise detection of biomarkers or biological changes, while 2D material-based drug delivery systems offer the targeted and controlled release of therapeutics to the brain, crossing the blood–brain barrier and enhancing treatment effectiveness. In addition, when used in tissue engineering and regenerative medicine, 2D materials facilitate cell growth, differentiation, and tissue regeneration to restore neuronal functions and repair damaged neural networks. Overall, 2D materials show great promise for use in the advanced treatment of NDDs, thus improving the quality of life for patients in an aging population.
Keywords: Two-dimensional (2D) material, Neurodegenerative disease, Early diagnosis, Drug delivery, Regenerative medicine
Introduction
Neurodegenerative diseases (NDDs) are a significant global public health issue that affects millions of individuals and their families [1]. NDDs are characterized by the progressive degeneration of the structure and function of the nervous system, leading to a range of debilitating symptoms and, ultimately, a decline in cognitive and motor abilities [2]. NDDs have a strongly negative impact on the quality of life of individuals and represent a considerable socioeconomic burden due to the cost of treatment and long-term care and the loss of productivity associated with caregiving [3]. In addition, as the global population ages, the prevalence of NDDs is likely to increase, placing greater strain on healthcare systems and resources [4]. It has thus become vital to develop effective means to successfully diagnose and treat NDDs as part of a cohesive strategy to ameliorate the socioeconomic effect of these diseases. For this reason, a range of research initiatives have been pursued, including the investigation of the genetic and environmental factors contributing to NDDs, the design and testing of novel therapeutic approaches, and the fabrication of more effective diagnostic tools.
Two-dimensional (2D) materials with a single-atom thickness, such as graphene and other nanosheets (NSs), have become increasingly important in biomedical research due to their exceptional mechanical strength, electrical conductivity, and biocompatibility, facilitating their use in a range of potential applications [5–7]. For example, the high electrical conductivity of 2D materials have been exploited in biosensing applications, with functionalized NSs utilized to detect specific biomolecules associated with target diseases [8]. The high surface-to-volume ratio of 2D materials enhances their biomolecular interactions, contributing to the development of ultrasensitive biosensors. In addition, 2D materials can serve as effective biocompatible carriers for pharmaceutical compounds because their large surface area allows for high drug loadings [9–11]. Their tunable surface properties also support targeted drug delivery, enhancing their therapeutic efficacy and reducing side effects.
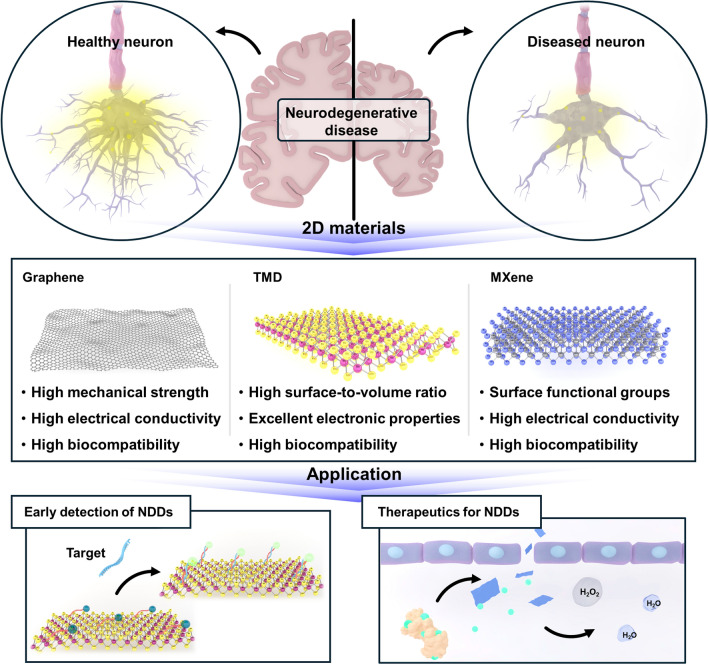
Among the various materials explored for the effective management of NDDs, 2D materials have shown exceptional suitability for biomedical applications. Wherein, this discovery has led to numerous studies investigating their potential and application. In this review, we provide a brief overview of 2D materials that have been employed for the diagnosis and/or treatment of NDDs (Fig. 1). Specifically, we summarize studies that have employed 2D materials in biosensors for the early diagnosis of NDDs, in drug delivery systems for effective NDD treatment, and in efficient neural tissue regeneration applications. As such, this review highlights promising advancements in the use of 2D materials for biomedical applications associated with NDDs.
Fig. 1.
Schematic illustration of application of 2D nanomaterial for neurodegenerative disease
Properties of 2D materials for biomedical applications
Due to their advantageous physicochemical properties, 2D materials have emerged as versatile components of numerous biomedical applications. In addition to the widely studied graphene, transition metal dichalcogenides (TMDs), MXenes, other 2D materials such as black phosphorus, silicene, borophene and tunable crystalline vanadate groups to 2D forms such as cerium vanadate and iron vanadate offer distinct advantages for specific needs, including drug delivery, biosensing, and tissue engineering [12–19]. Continued research into their synthesis, functionalization, and biocompatibility will broaden their impact on healthcare technologies for NDDs.
Graphene
Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, has been employed in numerous biomedical applications because of its unique combination of a high mechanical strength, electrical conductivity, biocompatibility, and surface area [12, 20]. For example, graphene-based drug delivery systems have been designed for the targeted and controlled release of therapeutic agents [12]. The large surface area of graphene allows for high drug loading, and its functionalization can be tailored to enhance biocompatibility and allow specific targeting. Graphene oxide (GO) has also been extensively studied as a carrier for anticancer agents, antibiotics, and anti-inflammatory drugs, leading to the development of drug delivery systems with greater drug solubility, stability, and bioavailability and fewer side effects due to targeted delivery to affected tissues [21]. Furthermore, the high electrical conductivity and surface area of graphene make it an ideal candidate for use in highly sensitive biosensors [22]. Functionalized graphene can interact selectively with biomolecules, enabling the detection of specific markers associated with target NDDs. For example, graphene-based biosensors have been developed for the detection of amyloid-beta (Aβ) peptides as biomarkers of Alzheimer’s disease (AD) [23]. The sensitivity and specificity of graphene-based biosensors allow for early and accurate diagnosis, facilitating timely intervention and treatment. In addition, the structural similarity between graphene and the extracellular matrix of tissue and its excellent mechanical properties make it suitable for use in tissue engineering applications [24]. Graphene-based scaffolds provide a supportive framework for cell adhesion, proliferation, and differentiation. In neural tissue engineering, graphene has been employed to enhance the growth and maturation of neurons, which provides a promising means for the repair of neural tissues damaged by neurodegenerative conditions [25–27]. Its biocompatibility and ability to promote cell–material interactions also make graphene a valuable component in the design of biomimetic structures for tissue regeneration.
Transition metal dichalcogenides (TMDs)
TMDs, a class of 2D materials, have gained significant attention in biomedical research due to their advantageous electronic, optical, and mechanical properties. TMDs such as molybdenum disulfide (MoS2) and tungsten disulfide (WS2) represent a versatile platform for various biomedical applications, including drug delivery, therapeutic interventions, and tissue engineering [28, 29]. As the understanding of these materials becomes stronger and their fabrication techniques improve, the potential for TMDs to address NDD diagnosis and treatment challenges continues to grow, offering innovative solutions for improved patient outcomes. For example, MoS2 and WS2 have shown potential in drug delivery applications, with their layered structure allowing for the efficient loading and controlled release of therapeutic agents [30–32]. The large surface area of TMDs can accommodate a high drug payload, and their surface can be easily functionalized to improve their biocompatibility and targeting. Thus, TMD-based drug delivery systems offer greater treatment effectiveness while minimizing side effects. In addition, the high surface-to-volume ratio, and excellent electronic properties of TMDs make them suitable for biosensing applications [33–35]. Functionalized TMDs can selectively bind to biomolecules, enabling the development of highly sensitive biosensors for the detection of specific disease markers related to NDDs such as AD or Parkinson's disease (PD) [36]. Moreover, TMDs have shown promise in tissue engineering due to their biocompatibility and ability to support cell growth [37]. MoS2 has been investigated for its potential in promoting osteogenic differentiation in bone tissue engineering. The layered structure of TMDs provides a suitable environment for cellular adhesion and proliferation, making them particularly suitable for use in scaffolds for tissue regeneration.
MXenes
MXenes, a family of 2D transition metal carbides or nitrides, have recently emerged as promising materials for biomedical applications, including drug delivery, biosensing, imaging, and tissue engineering. Due to their unique combination of electrical, mechanical, and surface properties, MXenes are a versatile group of materials for the diagnosis, treatment, and understanding of various medical conditions, including NDDs. In therapeutic applications, MXenes, particularly titanium carbide (Ti3C2Tx), have shown potential as drug delivery agents [38]. Their layered structure allows for efficient drug loading, and the surface functional groups can be modified to enhance their biocompatibility and facilitate controlled release. MXene-based drug delivery systems offer a platform for targeted and sustained drug delivery, improving therapeutic efficacy while minimizing side effects. In addition, the excellent electrical conductivity of MXenes makes them ideal for developing highly sensitive biosensors [39]. Functionalized MXene nanosheets can be tailored to selectively bind to specific biomolecules, enabling the detection of disease-related markers. MXene-based biosensors can thus play a crucial role in early diagnosis by identifying biomarkers associated with AD and PD, providing a rapid and sensitive diagnostic tool [40, 41]. Additionally, MXenes have been used in tissue engineering applications due to their biocompatibility and ability to support cell adhesion and proliferation [42]. MXene-based scaffolds can serve as platforms for tissue regeneration, promoting the growth and differentiation of cells. Their unique properties, including electrical conductivity, can be harnessed to modulate cellular behavior, making them valuable components in the development of biomimetic structures for tissue engineering.
Application of 2D materials in biosensors for the early detection of NDDs
To date, 2D materials have been widely used in the development of highly sensitive and specific biosensing applications for NDDs [43]. Their outstanding optical, electrical and electrochemical properties had made 2D materials as a strong contender as probes and platforms for developing a robust biosensor especially for the detection of biologically active molecules. Phase engineering, crystallinity, metallicity, stability, sheet’s size, surface functionalization and degree of exfoliation are the important aspects in regulating the 2D material’s properties [7]. As an instance, MXene and TMDs such as MoS2 and WS2 offer an ideal platform for biosensors due to their large surface area and tunable electronic properties. With its exceptional electrical conductivity, graphene also provides a foundation for biosensors that can rapidly and accurately identify biomolecular signatures linked to neurodegenerative conditions.
These 2D material-based biosensors hold promise for the early and precise diagnosis of NDDs, facilitating timely intervention and personalized treatment strategies. Moving forward, as research in nanotechnology advances, the integration of 2D materials into biosensing devices offers a transformative approach to improving NDD diagnosis, thus improving patient outcomes. In brief, biosensor is a detection system that detects biological analyte measured by signals generation allowing for signals quantification. There are various forms of sensing signals that can be measured as an instance optical and electrical. In this section, we aimed to highlight in depth the sensing mechanisms that are harnessed together with 2D materials such as FET, electrochemical, fluorescence and SERS based systems.
Graphene-based biosensors
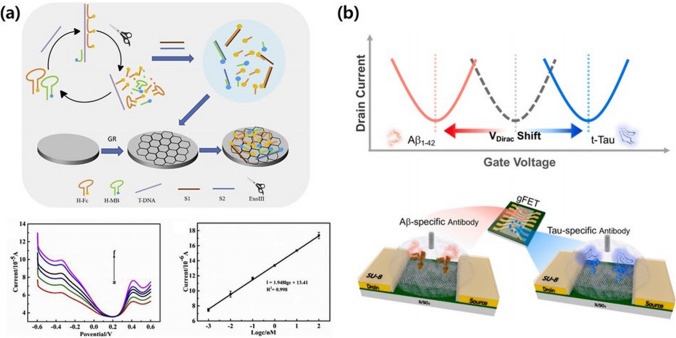
Because graphene exhibits superior electrical properties compared to other materials, research has been conducted on its utility for the diagnosis of NDDs in field-effect transistor (FET) systems that detect biomarkers such as neurotransmitters [44]. For example, Liu et al. developed a novel radiometric electrochemical biosensor for the quantitative detection of trinucleotide repeat sequences associated with NDDs, specifically d(CAG)n (Fig. 2a) [45]. The biosensor utilized Exo III-assisted recycling amplification and a graphene-modified electrode, incorporating hairpin DNA labeled with ferrocene and methylene blue. Following hybridization with the target DNA, Exo III digestion released target and report fragments, which were selectively adsorbed by the graphene-modified electrode, generating twin electrochemical signals. The signal ratio of ferrocene to methylene blue had a linear relationship with the number of repeats, allowing for the accurate determination of the repeat length and exhibiting a low detection limit of 0.22 pM. Xu et al. [46] presented a robust on-chip graphene FET (GFET) biosensing method for the ultrafast and sensitive detection of glial fibrillary acidic protein, a discriminative blood biomarker for neurological diseases such as traumatic brain injury. The GFET biosensor had a low limit of detection (LOD) of 20 fg/mL in a buffer solution and 231 fg/mL in the plasma of a patient, with a rapid sample-to-result time of less than 15 min. A comparison with state-of-the-art single-molecule array (Simoa) technology and enzyme-linked immunosorbent assays revealed a competitive LOD, faster detection times, and user-friendly features, positioning the GFET biosensing platform as a promising tool for point-of-care diagnosis and the monitoring of traumatic brain injuries in various clinical settings, including GP surgeries and patient homes [46]. Salehirozveh et al. reported a cost-efficient and highly sensitive method for the detection of Aβ42 biomarkers, which are crucial for early AD diagnosis. A reduced graphene oxide (rGO) FET with an RNA aptamer allowed for the ultrasensitive, label-free detection of Aβ42 in cerebrospinal fluid and serum samples [47]. The self-assembled rGO channel produced a linear response in the gate voltage shift following the interaction between Aβ42 and the RNA aptamer. Operating within a concentration range of 1 ng/mL to 1 pg/mL at pH 7.4, the rGO FET proved to be a low-cost, specific, and highly sensitive tool for the detection of very low concentrations of Aβ42, paving the way for potential applications in serum-sample measurements and AD diagnostics. Ge et al. [48] introduced a solution-gated graphene transistor (SGGT) biosensor for the detection of GAA trinucleotide repeats, which are associated with Friedreich’s ataxia. This novel biosensor used G-quadruplex DNAzymes and graphene channels to quantify captured target DNA using a robust catalytic current signal that was dependent on peroxidase-like activity. The SGGT biosensor exhibited excellent self-amplifying performance, yielding significantly stronger current signals compared to conventional electrochemistry. With an ultra-low LOD of 32.25 fM, a broad linear range of 100 fM to 100 nM, and exceptional selectivity, this biosensor holds great promise for early NDD diagnosis, particularly for conditions such as Friedreich’s ataxia. Park et al. developed a highly sensitive and multiplexed method for the detection of key AD biomarkers Aβ1-42 and t-Tau in biofluids using an rGO FET (Fig. 2b) [49]. This approach exhibited a wide logarithmically linear detection range and an impressive femtomolar-level LOD in various biofluids, including human plasma and artificial cerebrospinal fluid. Based on the distinct surface charge of the core biomarkers arising from their isoelectric points, the rGO FET biosensor platform produced a unique output signal for each biomarker, enhancing its specificity. This technology has significant potential for early AD diagnosis in clinical settings, offering both sensitivity and accurate analyte analysis based on the surface charge of the target molecules.
Fig. 2.
Graphene-based biosensors for the diagnosis of neurodegenerative diseases (NDDs). a electrochemical biosensor which employs a dual-signal approach alongside Exo III-assisted recycling and the selective adsorption characteristics of graphene for identifying the trinucleotide repeat sequence d(CAG)n. Reproduced with permission and copyright (2019) Elsevier B.V. [45]. b Graphene-based field-effect transistor (gFET) for multiplex detection of Aβ1-42 and t-Tau using specific antibody-modified reduced graphene oxide. Reproduced with permission and copyright (2020) Elsevier B.V. [49]
In addition to FET-based graphene biosensors, other analytical methods have also been developed using the electrochemical and electromagnetic characteristics of graphene. Sethi et al. presented a label-free biosensor consisting of a dual layer of graphene and rGO for the detection of plasma-based Aβ1-42 biomarkers in AD [23]. The platform was modified with 1-pyrenebutyric acid N-hydroxysuccinimide ester to immobilize H31L21 antibodies, and the modifications were characterized using various techniques. Differential pulse voltammetry revealed a low LOD of 2.398 pM over a broad linear range from 11 pM to 55 nM, exhibiting excellent specificity in the presence of interfering species. Successful validation using spiked human and mice plasma samples and immunohistochemistry, and magnetic resonance imaging data obtained from brain analysis demonstrated the biosensor's potential for the accurate electrochemical determination of Aβ1-42 in bio-fluidic samples. Park et al. produced an electrochemical sensor by modifying an electrode with rGO sheet (rGS)–gold nanoparticle (AuNP) complexes for the simultaneous determination of dopamine (DA) and ascorbic acid (AA) in a mixture within a range of 0.1–100 μM [50]. The rGS enhanced the selectivity of the biosensor through interactions with DA, while the AuNPs improved its sensitivity due to their high conductivity and large surface area. The sensor exhibited significantly higher electrocatalytic activity compared to rGS or AuNPs individually, as indicated by scanning electron microscopy (SEM), cyclic voltammetry (CV), and differential pulse voltammetry measurements. The sensor exhibited a linear response in the range of 0.1–100 μM for DA with an LOD of 0.098 M in the presence of 400 M AA, making it a potential candidate for medical biosensors in the diagnosis of NDDs. The same research group also developed a multiplexing biosensor for the quantification of norepinephrine (NE) in the presence of interfering substances such as AA and uric acid, which are commonly present in biological fluid samples tested for NDDs such as AD and PD [51]. The researchers fabricated a multi-modified electrode incorporating rGS and AuNPs to achieve the highly selective and sensitive detection of NE. The developed sensor demonstrated a wide detection range of 0.2–10 µM) and an impressive LOD of 200 nM even in the presence of a concentration of AA.
Besides electrical detection methods, Choi et al. demonstrated a novel neurotransmitter detection method by developing a GO-hybrid nanosurface-enhanced Raman scattering (SERS) array for the selective and sensitive detection of DA [52]. The GO-hybrid nano-SERS array rapidly and reliably measured a wide range of DA concentrations from 10⁻4 to 10⁻⁹ M. The proposed method was used to measure DA from differentiating neural stem cells (NSCs), offering insights into the characterization of neuronal differentiation. This SERS-based detection method, with its potential for in-situ detection at the single-cell level, represents a unique tool for investigating single-cell signaling pathways associated with DA and other neurotransmitters, shedding light on their roles in neurological processes. The graphene-based biosensors for early detection of NDDs are delineated in Table 1.
Table 1.
Graphene-based biosensors for early detection of NDDs
| Graphene-based biosensors | Target | Detection range | LOD | Refs | |
|---|---|---|---|---|---|
| FET-based | Radiometric electrochemical biosensor | d(CAG)n | – | 0.22 pM | [45] |
| On-chip graphene FET | glial fibrillary acidic protein | – |
20 fg/mL 231 fg/mL |
[46] | |
| rGO | Aβ42 | 1 ng/mL–1 pg/mL | – | [47] | |
| SGGT biosensor | GAA | 100 fM–100 nM | 32.25 fM | [48] | |
| rGO-FET |
Aβ1-42 t-Tau |
10–1–10–5 pg/mL |
21.8 fM 222.0 fM |
[49] | |
| Electrochemical, electromagnetic based | Dual layer graphene and rGO | Aβ1-42 | 11 pM–55 nM | 2.398 pM | [23] |
| rGO sheets-AuNP |
DA AA |
0.1–100 μM |
0.098 M 400 M |
[50] | |
| GS-AuNP | NE | 0.2–10 μM | 200 nM | [51] | |
| SERS-based | GO-hybrid nano SERS array | DA | 10⁻4–10⁻⁹ M | – | [52] |
MXene-based biosensors
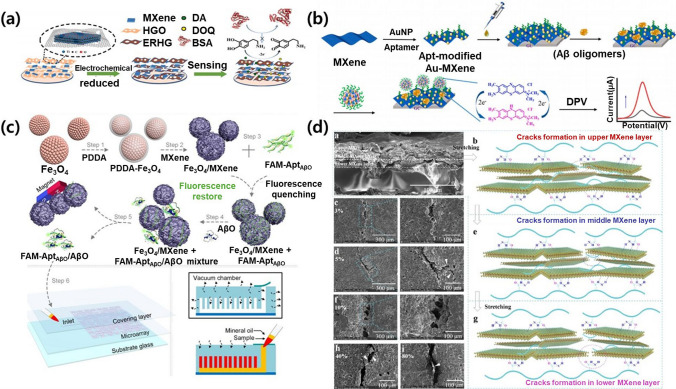
Similar to graphene, MXenes offer electrochemical characteristics that are advantageous for the detection of NDD biomarkers, wherein the MXene-based biosensors are summarized in Table 2. Zhang et al. [53] presented an MXene-based electrochemical sensor with anti-biofouling properties for use in complex biological fluids (Fig. 3a). The sensor, based on a glassy carbon electrode (GCE) modified with the MXene Ti3C2Tx and electrochemically reduced holey graphene (ERHG), exhibited excellent electrochemical performance. ERHG prevented the agglomeration and oxidation of the MXene, increasing its stability, while the hydrophilicity and conductivity of the MXene contributed to a high electron transfer rate. The MXene/ERHG/GCE sensor demonstrated a wide detection range for DA from 0.2 to 125 μM with a low LOD (0.044 μM) in phosphate-buffered saline. Importantly, the hydrophilicity of the MXene/ERHG sensor reduced non-specific protein adsorption, leading to strong anti-biofouling properties and the potential for use in biosensing applications for serum and artificial cerebrospinal fluid. Lv et al. reported a sensitive electrochemical method for the detection of AD biomarkers, specifically Aβ1-42 oligomers, addressing previous deficiencies in signal transduction (Fig. 3b) [54]. Their method employed a double amplification strategy, utilizing a Ti3C2Tx decorated with AuNPs (Au-MXene) as the electrode substrate. This configuration enhanced the electrochemical response due to its unique surface area and charge mobility while providing numerous binding sites for aptamers specific to Aβ1-42 oligomers. Incorporating AuNPs into covalent organic frameworks further enhanced the electron–hole separation efficiency, with toluidine blue serving as an electron mediator. The proposed electrochemical assay was proven to be a highly efficient platform for the detection of Aβ1-42 oligomers, exhibiting a wide linear range and an ultralow LOD, suggesting potential applications in the molecular diagnostics of AD serum samples.
Table 2.
MXene-based biosensors for the early detection of NDDs
| Mxenes-based biosensor | Target | Detection range | LOD | Refw | |
|---|---|---|---|---|---|
| Electrochemical based | GCE modified MXene and ERHG | DA | 0.2–125 μM | 0.044 μM | [53] |
| Au-MXene | Aβ1-42 | 0.01–180 pg/mL | 4.27 fg/mL | [54] | |
| (d-Ti3C2Tx) and (MWCNTs) | Aβ42 | 1.0 fg/mL–100.0 fg/mL | 0.3 fg/mL | [55] | |
| MXene/Lip-Qu@AuNP on GCE | DA | 1–10 μM |
0.35 μM (CV) 0.72 μM (differential pulse voltammetry) |
[56] | |
| MXene@Fe3O4 (M/F) | DA | 1–250 μM | 0.48 μM | [57] | |
| FRET-based | (3D) Fe3O4/MXene nanosphere | Aβ Oligomers (AβO) | 0.10–200 nM | ~ 0.05 nM | [41] |
| Nacre-mimetic nanocomplexes | PD Symptoms | GF = 296.8 | ɛ < 10% | [58] | |
Fig. 3.
MXene-based biosensors for the diagnosis of NDDs. a Preparation of MXene-electrochemically reduced holey graphene (ERHG) on a glass carbon electrode and its antifouling process for dopamine sensing in complex biological fluids. Reproduced with permission and copyright (2022) Elsevier B.V. [53]. b Illustration of electrochemical determination of Aβ1-42 oligomers with the Au-MXene substrate and the aptamer/toluidine blue-Au@covalent organic frameworks (Apt/TB-Au@COFs). Reproduced with permission and copyright (2024) Elsevier B.V. [54]. c Schematic diagram of the AβO recognition strategy based on FRET technology and the microfluidic chip with a sandwich-like structure. Reproduced with permission and copyright (2022) American Chemical Society [41]. d Crack-propagation sensing mechanism using Nacre-inspired PAM-MXene nanocomposites induced by the nacre-mimetic “brick-and-mortar” architecture and the abundant hydrogen bond interfacial interactions. Reproduced with permission and copyright (2023) American Chemical Society [58]
Özcan et al. developed a rapid and straightforward electrochemical biosensor for the sensing of the Aβ42 protein, a key player in AD [55]. The biosensor was based on a composite of delaminated Ti3C2Tx (d-Ti3C2Tx) and multi-walled carbon nanotubes (MWCNTs), incorporating molecularly imprinted polymers (MIPs). XRD, XPS, SEM, UV/Vis spectroscopy, AFM, CV, and EIS analyses confirmed the properties of the d-Ti3C2TX, MWCNTs, and composite. The Aβ42-imprinted biosensor exhibited a linear detection range of 1.0–100.0 fg/mL and an impressive LOD of 0.3 fg/mL. The biosensor demonstrated excellent stability, repeatability, reproducibility, and reusability and was successfully employed to analyze Aβ42 in plasma samples. Lee et al. reported an electrochemical approach for the selective sensing of DA, a potential neurological biomarker for human aging and neurodegenerative disorders such as AD [56]. The sensor was constructed using a titanium-based MXene and quercetin (Qu)-loaded liposomes functionalized with AuNPs (MXene/Lip-Qu@AuNPs). The MXene/Lip-Qu@AuNP composite was coated on a GCE for DA sensing, utilizing the high conductivity of the MXene for electrochemical measurements. The modified GCE exhibited effective DA sensing in the presence of various interferents, with LODs of 0.35 and 0.72 μM in CV and differential pulse voltammetry analysis, respectively, within a linear concentration range of 1–10 μM. The proposed sensor was successfully employed to detect DA in human serum samples, illustrating its convenience, selectivity, and sensitivity in DA detection. Zhang et al. [57] described a comprehensive platform utilizing the magnetic-field-directed self-assembly of a MXene@Fe3O4 (M/F) nanocomposite for the cultivation and in-situ analysis of differentiated dopaminergic neurons from human pluripotent stem cells (hPSCs). The M/F nanocomposite enhanced neurite outgrowth, leading to the development of more intricate ramifications, and the stronger magnetic field intensity also promoted neurite outgrowth and the differentiation of mature neurons from hPSCs. The platform demonstrated exceptional conductivity, enabling the real-time monitoring of the release of the DA neurotransmitter from hPSC-derived dopaminergic neurons. Overall, this integrated platform offers a reliable and efficient means for the in-vitro generation of human neurons with controllable quality, which has significant implications for biological research, disease modeling, and cell replacement therapy.
Due to the photoluminescence of MXenes, Wen et al. [41] designed a biosensor based on Förster resonance energy transfer (FRET) technology (Fig. 3c). MXenes exhibit strong fluorescence quenching, particularly with fluorophore-labeled DNA, making it valuable for use in biosensor applications. However, the common fluorescence background poses a challenge to MXene-based fluorescence biosensors. To address this, Wen et al. synthesized three-dimensional (3D) Fe3O4/MXene nanospheres, with Fe3O4 used to remove the fluorescence background via magnetic quenching. These nanospheres were employed as quenchers in FRET for the detection of Aβ oligomers (AβO). The study utilized a fluorescent reporter group (FAM) attached to an aptamer (FAM-AptAβO) as a probe, with the Fe3O4/MXene nanospheres quenching the fluorescence, and the- presence of AβO leading to fluorescence restoration. Determining the AβO concentration was achieved by monitoring the restored fluorescence intensity. A microfluidic chip based on poly(dimethylsiloxane) was also employed as a detection platform, offering simplicity, high accuracy, and low sample volumes. The developed Fe3O4/MXene nanosphere-based microfluidic chip has the potential for use in the point-of-care testing of chronic diseases such as AD. Du et al. [58] developed nacre-mimetic nanocomposites with a “brick-and-mortar” architecture using polyacrylamide and Ti3C2Tx NSs produced via a layer-by-layer spin-coating technique (Fig. 3d). This nanocomposite-based strain sensor exhibited ultrahigh sensitivity within a low strain range (ε < 10%), which was attributed to its bioinspired hierarchical structure and hydrogen bond-enhanced interfacial interactions. The strain sensor demonstrated high reliability, a broad working sensing range, a short response time, skin-like tensile stress, and excellent durability, making it suitable for accurate diagnosis and the identification of PD symptoms. This work opens new avenues for the design of highly sensitive strain sensors for monitoring subtle signals and aiding in disease diagnosis.
TMD-based biosensors
Materials based on 2D layered TMDs, such as WS2, have received significant interest for their unique properties. For example, WS2 NSs derived from exfoliation or hydrothermal methods have been used in gas sensors and biosensors based on their interaction with DNA and nucleotides [59, 60]. While WS2 has been shown to be able to discriminate between single-stranded and double-stranded DNA, its electrocatalytic oxidation of nucleobases remains unexplored, presenting a potential avenue for future biomedical applications. The TMD-based biosensors are laid out in Table 3.
Table 3.
TMD-based biosensors for the early detection of NDDs
| TMD-based biosensor | Target | Detection range | LOD | Refs | |
|---|---|---|---|---|---|
| Electrochemical based | WS2 NS/Graphite | Adenine guanine | 0.5–20 μM |
5 × 10–8 M 9 × 10–8 M |
[61] |
| Manganese-doped MoS2 | DA |
0–5 μM (10% serum, artificial sweat) |
50 pM (buffer solution) 5 nM (10% serum) 50 nM (artificial sweat) |
[62] | |
| FET-based | 2D MoS2 | GSH | 10 µM–500 mM | 703 nM | [63] |
| Optical based | CM-dex-WS2 | miR-29a | 0–20 nM | – | [64] |
| MoS2 NS2 | AβO | 0.01–20 μM | 3.1 nM | [65] | |
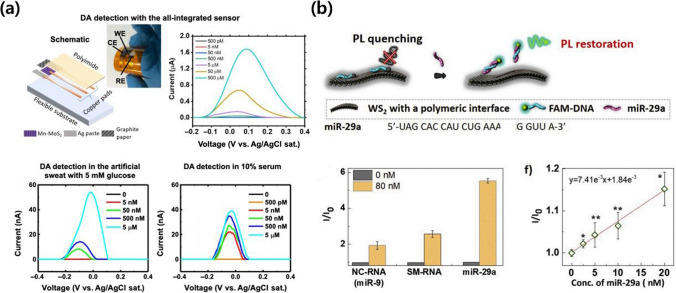
Zhang et al. [61] successfully synthesized a hybrid material consisting of WS2 NSs grown in situ on graphite microfibers. The resulting WS2 NS/graphite microfiber electrode displayed outstanding electrocatalytic oxidation activity towards adenine and guanine. This electrode demonstrated high sensitivity, selectivity, and stability in the detection of these nucleobases. The fibrous morphology and flexibility of the electrode were indicative of its ability to adapt to various environments, such as targeted cells and in-vivo settings. Overall, their study presented a promising platform for sensitive and selective detection. Lei et al. [62] developed an ultrasensitive electrochemical DA sensor utilizing manganese-doped MoS2 synthesized via a scalable two-step approach with approximately 2.15 atomic % Mn (Fig. 4a). The sensor demonstrated selective DA detection and an impressive sensitivity, with an LOD of 50 pM in a buffer solution, 5 nM in 10% serum, and 50 nM in artificial sweat. Density functional theory calculations and scanning transmission electron microscopy identified the presence of Mn defects, including Mn on top of a Mo atom (MntopMo) and Mn substituting for a Mo atom (MnMo). The sensor performance was attributed to the interaction between physisorption at MnMo sites and chemisorption at MntopMo sites at different DA concentrations. These findings highlight the potential of metal-doped layered materials for the construction of tunable and highly sensitive biosensors for the versatile detection of biomolecules.
Fig. 4.
TMD-based biosensors for the diagnosis of NDDs. a all-integrated electrochemical sensor fabricated on a polyimide (PI) sheet with Mn-MoS2 electrode and the quantification of DA. Reproduced with permission and copyright (2020) AAAS [62]. b Sensing mechanism and quantification data for miR-29a detection on the aptamer-modified WS2 interface using quenching of the fluorescence of FAM-DNA. Reproduced with permission and copyright (2020) Elsevier B.V. [64]
Based for FET system, Rawat et al. introduced a biosensor using two-dimensional MoS2 for the detection and quantification of glutathione (GSH), an important biomarker for various diseases, including cancers [63]. The sensor employed glutathione-S-transferase (GST) immobilized on the MoS2 surface, taking advantage of the electrochemical activity of GSH and 1-chloro-2,4-dinitrobenzene in the presence of GST. The MoS2-based sensor demonstrated high selectivity, with a significantly larger current compared to blank tests. It exhibits a sensitivity of 704 pA/µM, a low LOD of 703 nM, and a broad linear detection range of 10 µM to 500 mM. Additionally, the sensor had excellent repeatability and stability, suggesting that it was a promising approach for cancer detection and quantification.
Similar to MXene-based biosensors, TDM materials can be employed in optical biosensors for the visual detection of NDD biomarkers. An example of this is Kim et al., who developed an engineering interface of ultrathin WS2 NSs for effective fluorescence quenching in nanobiosensors (Fig. 4b) [64]. Four different dextran polymers were employed to modify the WS2 NS interface, influencing the adsorption kinetics and thermodynamics of a DNA probe. Functionalization with carboxyl group-bearing dextran (CM-dex-WS2) and trimethylammonium-modified dextran (TMA-dex-WS2) led to significantly faster adsorption rates for the fluorescein-labeled DNA probe (FAM-DNA) compared to pristine dextran (dex-WS2) or phenoxy groups-bearing dextran (PhO-dex-WS2). Isothermal titration calorimetry measurements revealed that CM-dex-WS2 exhibited a stronger adsorption strength for FAM-DNA, complicating subsequent desorption. Conversely, TMA-dex-WS2 had a more suitable adsorption strength that was favorable for desorption, leading to effective fluorescence restoration after hybridization with a target microRNA (miR-29a) and enabling selective and sensitive recognition in human serum in the presence of non-complementary RNA and single-base-mismatched RNA. Kong et al. presented a novel "off-to-on" fluorescent sensing platform for AβO using dye (FAM)-labeled single-strand DNA (FAM-ssDNA)-conjugated MoS2 NSs [65]. The strong adsorption of ss-DNA to MoS2 NSs quenched the fluorescence of FAM, creating an off state. In the presence of AβO, a hybrid structure formed between AβO and FAM-ssDNA, leading to the dissociation of FAM-ssDNA from MoS2 NSs and the significant recovery of the fluorescence, thus transitioning to an on state. The proposed assay had a linear relationship with AβO concentration, ranging from 0.01 to 20 μM, with an LOD of 3.1 nM. The feasibility of the sensor was verified using hippocampus and cortex tissues from APP/PS1 double-transgenic AD mice. The MoS2 NSs also had a therapeutic effect on AD by inhibiting Aβ aggregations and degrading previously formed Aβ fibrils, indicating their potential for use in future AD-related research.
Application of 2D materials in therapeutics for NDDs
A diverse range of NDD therapeutics based on 2D materials has been developed in past research. Serving as effective drug carriers, materials such as GO and layered double hydroxides enhance targeted drug delivery to the brain, overcoming the blood–brain barrier [66, 67]. Many 2D materials also have inherent neuroprotective and regenerative characteristics, shielding neurons from pathological factors and promoting tissue repair [67, 68]. In addition, anti-aggregation properties interfere with toxic protein aggregates, and anti-inflammatory effects modulate neuroinflammatory responses, potentially slowing the progression of NDDs [69, 70]. Moreover, functionalized 2D materials allow for targeted drug delivery in precise therapeutic interventions [71]. These materials also offer a non-invasive approach for photothermal therapy, and other strategies involving enhanced blood flow, oxygenation, and combination therapies highlight the versatility of 2D materials in addressing the complex challenges associated with NDDs.
Graphene-based therapeutics for NDD treatment
Due to its exceptional conductivity, stability, and biocompatibility, graphene has received attention for use in various biomedical applications, including stem cell research, cell imaging, drug delivery, and photothermal therapy. Its versatile structures, such as 2D lattices, fibers, and 3D graphene (3DG) foams, offer a diverse range of potential platforms. Notably, 3DG has shown promise in enhancing the neuronal differentiation of NSCs and providing a favorable microenvironment for NSC growth, suggesting therapeutic potential for NDDs and neuronal disorders [72–74]. Furthermore, 3DG has demonstrated the ability to enhance NSC differentiation, particularly into astrocytes and neurons. Fang et al. reported metabolic reconfiguration induced by 3DG, establishing correlations between metabolic changes and NSC proliferation rates on different scaffolds. In their study, specific pathways linked to greater amino acid incorporation and improved glucose metabolism were identified. These findings have potential implications for NDDs, particularly PD, providing insights into the fate of NSCs on 3DG and laying a foundation for future research.
Zhang et al. investigated the role of three types of graphene quantum dots (GQDs) in modulating protein liquid–liquid phase separation (LLPS), particularly in stress granule (SG) formation (Fig. 5a) [75]. The findings showed that GQDs were effective in preventing SG formation, promoting SG disassembly, and directly interacting with the SG-containing fused in sarcoma (FUS) protein. Additionally, GQDs effectively inhibited and reversed FUS LLPS, preventing abnormal phase transitions and amyloid aggregation. The study highlights the distinct activity of GQDs with different edge-site structures in binding to FUS monomers and fibrils, providing insights for the rational design of GQDs as effective modulators of protein LLPS for therapeutic applications.
Fig. 5.
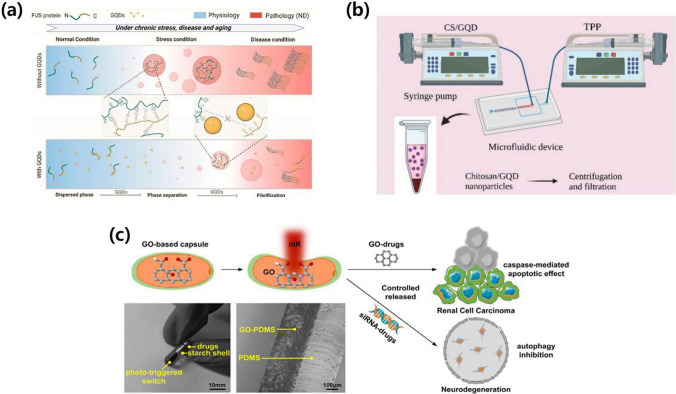
Graphene-based therapeutic strategies for NDDs treatment. a Developed graphene quantum dot (GQD)-assisted prevention of amyloid fibrils accumulation. Fused in sarcoma (FUS) could be phase separation to form liquid-like droplets. Reproduced with permission and copyright (2023) American Chemical Society [75]. b The microfluidic device was designed and fabricated for the synthesis of GQDs, chitosan (CS) nanoparticle, and GQD/CS for drug delivering agents against AD. Reproduced with permission and copyright (2023) Wiley-VCH. [77]. c Graphene oxide (GO)-based nanocapsules with photo-triggered drug released ability for implantable clear cell renal cell carcinoma (ccRCC) or neurodegeneration therapy. Reproduced with permission and copyright (2022) Elsevier B.V. [81]
Based on its positive effect on neural cells, graphene has been utilized as a potential drug delivery system. For AD, Chen et al. introduced GO nanoflakes to intracellular Aβ42 aggregates and tested their resulting cytotoxicity using yeast Saccharomyces cerevisiae as a model organism [76]. The findings reveal that GO nanoflakes effectively penetrate yeast cells, mitigating Aβ42 toxicity. Proteomics data and subsequent experiments demonstrated that GO treatment altered the cellular metabolism, enhancing cellular resistance to misfolded protein stress and oxidative stress, while reducing intracellular Aβ42 oligomers. Notably, GO treatment also reduced HTT103QP toxicity in the Huntington’s disease (HD) yeast model. These results provide a foundation for the rational design of GO nanoflake-based therapies to alleviate the cytotoxicity associated with Aβ42 and other misfolded proteins implicated in neurodegenerative pathology. Mohebichamkhorami et al. demonstrated the potential of ultrasmall nanoparticles (NPs) composed of chitosan (CS) and GQDs as theranostic agents for AD (Fig. 5b) [77]. Synthesized using a microfluidic-based method, these NPs demonstrated transcellular transfer and brain targeting abilities after intranasal delivery. In-vitro experiments revealed their ability to enter the cytoplasm of C6 glioma cells, impacting cell viability in a dose and time-dependent manner. In streptozotocin-induced AD-like models, intranasal administration of the NPs improved memory recovery in the radial arm water maze test. In-vivo bioimaging confirmed the detectability of the NPs in the brain, facilitated by the use of GQDs as diagnostic markers. However, the NPs did not affect Aβ plaque clearance or enhance markers of neural regeneration. The observed memory improvement in treated AD rats may be attributed to neuroprotection via anti-inflammatory effects and regulation of the brain tissue microenvironment, which warrants further investigation.
Kaliyaperumal et al. reported a neuroprotective nanocomposite drug with blue luminescent GQDs as a potential inhibitor of α-synuclein (α-syn) in PD treatment [78]. By chemically synthesizing methyl N-allyl N-benzoylmethionate (MABM) and functionalizing the GQD surface, the nanocomposite targeted brain delivery. Spectroscopic and simulation analyses confirmed defibrillation through interactions between N-terminal amino acids and MABM-GQD NPs, rendering α-syn non-toxic. In-vitro experiments demonstrated the ability of the drug to cross the blood–brain barrier and prevent neuronal loss in neuroblastoma cells. In-vivo cerebral blood flow analysis via magnetic resonance imaging supported the potential therapeutic application of this nanocomposite for the treatment of PD. Xiong et al. developed a lactoferrin (Lf)-GO-puerarin (Pue) nanoplatform as a novel brain drug delivery strategy for the treatment of PD [79]. This nanoplatform demonstrated effective brain targeting in both in vivo and in vitro settings. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice, intravenous administration of the GO-based nanoplatform successfully mitigated PD-related neuron damage, improved neurobehavioral deficits, and exhibited significant antioxidant effects. Importantly, the treatment had no apparent adverse effects or toxicity in major organs or blood samples, highlighting its potential as a safe and efficient therapeutic approach for PD.
In a study by Kang et al. [80], GQDs emerged as a potential therapeutic agent for Niemann-Pick disease type C (NPC). GQDs, known for their negligible long-term toxicity and blood–brain barrier penetration, reduced cholesterol aggregation in lysosomes through physical interactions. Additionally, GQDs promoted autophagy and restored defective autophagic flux, mitigating the atypical accumulation of autophagic vacuoles. The authors claimed that the injection of GQDs inhibited the loss of Purkinje cells in the cerebellum and reduced microglial activation, thus alleviating those functions impaired by NPC. This suggests that GQDs are useful for the treatment of NPC and related disorders. In addition, Jia et al. presented a novel drug delivery approach using GO-based milli-capsules for cancer and NDD treatment to overcome limitations associated with the imprecise control of drug concentrations and treatment-related toxicity (Fig. 5c) [81]. The milli-capsules took advantage of the penetration ability of near-infrared (NIR) light and the NIR-absorption capability of GO, leading to the controlled release of the drug through photomechanical deformation and wireless NIR manipulation. The drug dosage was precisely controlled by varying the radiation level, demonstrating a wide range from 0 lg (0 drops) to 225 lg (five drops). In-vitro evaluation of the cytotoxicity in clear cell renal cell carcinoma (ccRCC) revealed that the milli-capsules inhibited ccRCC cell proliferation in a GO-concentration-dependent manner, allowing for accurate GO-dosing control. This approach, which can be extended to other drugs such as siRNA, represents a promising strategy for effective and targeted drug administration with minimal side effects in cancer and NDD therapy.
Graphene has also been employed in regenerative medicine for NDD treatment. Li et al. demonstrated the potential of GO in clearing Aβ and treating AD by activating autophagy [82]. Their research found that GO inhibited the mTOR signaling pathway by activating AMPK, inducing autophagy in microglia and neurons. GO enhanced microglia-mediated Aβ phagocytosis by improving the autophagy capacity of microglia. In co-culture conditions with microglia and neurons, GO induced autophagy in both cell types, particularly in microglia, leading to enhanced Aβ clearance and neuroprotection. Importantly, GO was non-cytotoxic for the microglia and neurons and reduced Aβ toxicity for neurons through effective clearance, highlighting its potential as a therapeutic candidate for AD. Asaki et al. developed a novel hybrid scaffold, Mo0.25Co1.257W0.25S3 GO (MCWS/GO), which was characterized for its potential in treating spinal cord and sciatic nerve injuries [83]. The composite exhibited low cytotoxicity and a minimal number of necrotic cells. Testing on rats with damage to their spinal cord and sciatic nerve revealed that MCWS/GO actively regenerated nerve tissues, as evidenced by the results for pathology, physiology, and gene expression tests for nerve growth factors. The upregulation of the BDNF, NT3, and NGF genes and the downregulation of p75 NTR indicated the positive impact of the proposed scaffold on nerve regeneration. These findings suggest that MCWS/GO is a promising nanomaterial for neural injury therapy, opening avenues for the further investigation of NDDs. Guo et al. presented 3D bacterial cellulose–graphene foam (3D-BC/G) as a novel scaffold for the culturing of NSCs with promising implications for NDD therapy [84]. Prepared using in-situ bacterial cellulose interfacial polymerization on porous graphene foam, 3D-BC/G not only supported NSC growth and adhesion but also maintained the stemness and enhanced proliferation. Phenotypic analysis revealed that 3D-BC/G induced the selective differentiation of NSCs into neurons, forming a neural network. The scaffold demonstrated good biocompatibility with primary cortical neurons, enhancing neuronal network activity. RNA-Seq analysis of gene expression and signaling pathways provided valuable insights, suggesting that 3D-BC/G is a promising 3D conductive substrate for NSC research and neural tissue engineering. Pradhan et al. fabricated an injectable hydrogel composed of acetylcholine-functionalized GO and poly (acrylic acid) to address the spatial imbalance in the cholinergic system associated with brain damage [85]. The hydrogel was not cytotoxic, promoted neurite outgrowth, stabilized microtubule networks, and enhanced the expression of key neural markers in rat cortical primary neurons. Additionally, the hydrogel exhibited significant potential in neuroregeneration and facilitated the rapid recovery of a sham-injured mouse brain. The observed enhancement of the reactive astrocytes in the hippocampal dentate gyrus region also illustrated its utility for neural repair in a damaged brain. The results collectively suggest that this neuroregenerative hydrogel effectively maintains the cholinergic balance through the local release of acetylcholine, which is crucial for brain repair.
MXene-based therapeutics for NDD treatment
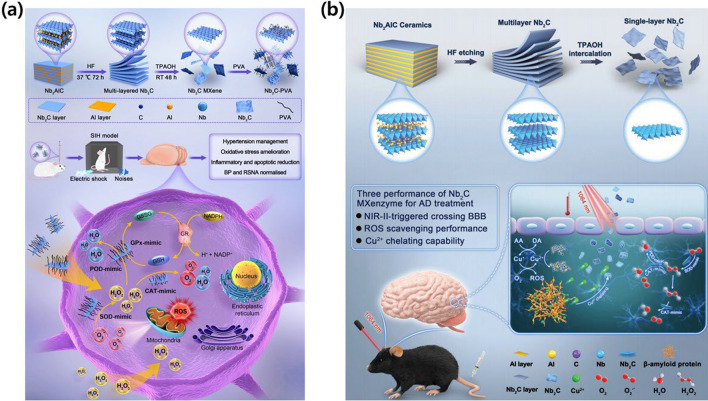
A niobium carbide (Nb2C) MXenzyme, which is an MXene-based nanozyme, has been developed with multiple enzyme-mimicking activities, including superoxide dismutase (SOD)-, catalase (CAT)-, glutathione peroxidase (GPx)-, and peroxidase (POD)-like functions [86]. This nanozyme demonstrates excellent antioxidase-like activity, making it suitable for biomedical applications treating conditions such as inflammation, cerebral stroke, PD, and AD. In both in-vitro and in-vivo experiments, the Nb2C MXenzyme exhibited high biosafety and the effective scavenging of toxic reactive oxygen species (ROS), thus protecting cells from oxidative damage, while also suppressing inflammatory cytokines. Yang et al. reported a novel approach for hypertension treatment utilizing the Nb2C MXenzyme (Fig. 6a) [87]. The biocompatible Nb2C MXenzyme effectively removed ROS and inhibited inflammatory factors, reducing the oxidative stress associated with hypertension. Their study systematically demonstrated the cytoprotective effects of Nb2C MXenzyme in a stress-induced hypertension rat model, illustrating its potential to alleviate inflammatory responses and reduce blood pressure. This advanced strategy not only provides new opportunities for nanozymes in hypertension treatment but also expands the application of 2D MXene nanosystems in biomedicine. Feng et al. presented a 2D vanadium carbide (V2C) MXenzyme designed to mimic the functionality of up to six naturally occurring enzymes, including SOD, CAT, POD, GPx, thiol peroxidase, and haloperoxidase [86]. Based on these enzyme-mimicking properties, the 2D V2C MXenzyme demonstrated high biocompatibility and robust in-vitro cytoprotection against oxidative stress. Critically, this nanoenzyme rebalanced redox homeostasis without disrupting the endogenous antioxidant state and exhibited therapeutic efficacy in vivo, demonstrating a benign effect in both inflammation and neurodegeneration animal models. These findings highlight the potential of MXenzymes as a remedial nanoplatform for diseases associated with ROS-mediated inflammation and neurodegeneration. Du et al. investigated the critical role of transition-metal dyshomeostasis, particularly copper ions (Cu2+), in the pathogenesis of AD and developed an effective therapeutic strategy based on a 2D Nb2C MXene-based nano-chelator for the effective binding of toxic Cu2+ ions (Fig. 6b) [88]. This nano-chelator exhibited MXenzyme properties, effectively scavenging overproduced ROS associated with neuroinflammation and apoptosis in AD. Computational simulation was employed to elucidate the underlying mechanisms. Notably, the Nb2C MXenzyme had a benign photothermal effect, enhancing the blood–brain barrier permeability under NIR laser irradiation, thus overcoming the limitations of conventional anti-AD therapeutic agents. This work not only presents a promising strategy for combating AD using Nb2C-based neuroprotective nano-chelators but also expands the biomedical applications of MXenes in treating central nervous system diseases mediated by transition-metal dyshomeostasis and ROS. Hu et al. exhibited the detrimental effects of excessive ROS-induced inflammatory responses in ischemic stroke, hindering neurological functional recovery [89]. A 2D V2C-based nanozyme is designed with the ability to mimic four natural enzymes, including SOD, POD, CAT, and GPx. The synthesized V2C demonstrated robust antioxidative properties by catalyzing the transformation of harmful ROS such as superoxide anion radicals and hydrogen peroxide into water and oxygen. It also effectively scavenged highly toxic hydroxyl radicals, thus suppressing intracellular ROS levels and mitigating oxidative stress. The nanozyme also significantly reduced cerebral infarct volumes, providing neuroprotection against ischemic stroke by inhibiting cell apoptosis and counteracting inflammation. Additionally, the 2D V2C MXene was employed as a nanoscale contrast agent for T1-weighted magnetic resonance imaging, highlighting its potential as a theranostic nanozyme for ROS-related brain diseases. Seitak et al. also developed a novel drug delivery system using MXene for the controlled release of a therapeutic protein [90]. The study investigated the structural, chemical, and morphological properties of the synthesized MXene using electron microscopy and X-ray diffraction. Testing with murine macrophages (RAW 264.7) revealed no significant toxicity at concentrations below 1 mg/mL. Additionally, the MXene demonstrated the efficient loading and controlled release of FITC catalase, showing promise as a nanocarrier for therapeutic proteins in drug delivery systems.
Fig. 6.
MXene-based therapeutic strategies for NDDs treatment. a 2D niobium carbide MXenzyme with multiple enzyme-mimicking activities to catalyze anti-inflammatory response and ROS scavenging. Reproduced with permission and copyright (2023) American Chemical Society [87]. b 2D niobium carbide MXenzyme under NIR-II irradiation to capture superfluous Cu2+ with high BBB permeability. Reproduced with permission and copyright (2022) Wiley-VCH. [88]
MXenes have also been used in tissue regeneration for NDD treatment. Wei et al. explored the potential of a stable 3D conductive hydrogel for enhancing the differentiation efficiency of transplanted NSCs in the repair of central nervous system injuries [91]. The hydrogel was created by cross-linking Matrigel with the MXene Ti3C2Tx. The resulting composite had a 3D microporous network structure that resembled the extracellular matrix and provided a conducive substrate for NSC growth. The inclusion of Ti3C2Tx enhanced the electrical conductivity and biocompatibility of the hydrogel. The findings demonstrated that the MXene-Matrigel hydrogel effectively promoted the proliferation and differentiation of NSCs, offering valuable insights into the regulatory role of conductive hydrogels in neural tissue engineering. Tang et al. reported the use of ultrathin Nb2C to enhance the performance of retinal progenitor cells (RPCs) for retinal regeneration [92]. RPC-based transplantation holds promise for the restoration of sight, but its efficacy is often compromised by imprecise neurogenic differentiation and oxidative stress in the damaged retinal environment. Their study demonstrated that the moderate photothermal effects of Nb2C significantly improved the neuronal differentiation of RPCs by activating intracellular signaling pathways. Comprehensive biomedical assessments and theoretical calculations also showed that Nb2C effectively protected RPCs by scavenging free radicals. Subretinal transplantation of MXene-assisted RPCs into rd10 mice, a model of retinal degeneration, resulted in dramatically higher neuronal differentiation, contributing to the efficient restoration of retinal architecture and visual function. The dual intrinsic function of MXene synergistically aided in RPC transplantation, thus advancing vision-restoration research and multifunctional nanomedicine.
Li et al. investigated the impact of 2D Ti3C2Tx films on the electrophysiological maturation of NSCs [93]. Using whole-cell patch-clamp recording, the study examined the direct effects of Ti3C2Tx on NSC-derived neurons and its influence on the formation and performance of neural networks. Ti3C2Tx films with a thickness of several hundred nanometers were fabricated and shown to have high biocompatibility. NSCs cultured on Ti3C2Tx films exhibited enhanced differentiation into neurons with higher efficiency and longer neurites, thus promoting NSC maturation. The study also revealed that Ti3C2Tx selectively increased the amplitude of the voltage-gated Ca2+ current, contributing to the longer neurites. Ti3C2Tx thus demonstrates promise as a conductive neural interface or scaffold in stem cell therapy and nerve tissue engineering, influencing the morphology, physiology, and functionality. Driscoll et al. introduced MXtrodes, a class of soft, large-scale bioelectronic interfaces designed for the high-resolution mapping and modulation of excitable networks [94]. MXtrodes utilize the MXene Ti3C2, a two-dimensional transition metal carbide nanomaterial, for scalable and cost-effective solution processing. The electrochemical properties of MXtrodes surpassed those of conventional materials, eliminating the need for conductive gels in epidermal electronics. The versatility of MXtrodes was validated in various applications, including the mapping of large-scale neuromuscular networks in humans, cortical neural recording, and microstimulation in swine and rodent models. Importantly, MXtrodes were compatible with standard clinical neuroimaging modalities, thus representing a promising advancement in soft bioelectronic interfaces for transformative diagnostics, monitoring, and treatment.
TMD-based therapeutics for NDD treatment
To treat NDDs, reducing inflammation is important. For this reason, TMD materials have been actively investigated for their ability to reduce inflammatory responses, particularly for the treatment of AD. Qiu et al. developed a novel theranostic nanoplatform for the real-time assessment of therapeutic outcomes for AD treatment (Fig. 7a) [95]. Using a two-step process with a second NIR-II laser, the platform enables light-controlled NP delivery for effective antioxidant therapy under initial irradiation at 1064 nm. Subsequent irradiation activated a DNA nanomachine, leading to fluorescent signal recovery triggered by the presence of AβO and Ca2+. This innovative approach not only distinguished AD mice from wild-type mice but also offered guidance for AD treatment by monitoring intracellular calcium overload levels in the brain. This study thus established a non-invasive, image-based strategy for early prognosis predictions in AD treatment, with implications for precise medicine planning and prognosis stratification. Ren et al. reported the development of mitochondria-targeted nanozymes, specifically (3-carboxypropyl) triphenyl-phosphonium bromide-conjugated1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N [amino(polyethyleneglycol)-2000]-functionalized MoS2 QDs (TPP-MoS2 QDs) [96]. These nanozymes effectively mitigated Aβ aggregate-mediated neurotoxicity and eliminated Aβ aggregates in AD mice. Importantly, TPP-MoS2 QDs induced a shift in microglial polarization from the proinflammatory M1 phenotype to the anti-inflammatory M2 phenotype. The nanozymes exhibited bifunctional activity, crossed the blood–brain barrier, targeted mitochondria, and regulated proinflammatory and anti-inflammatory substances, thus preventing spontaneous neuroinflammation. In contrast to conventional NP affinity methods, this mitochondria-targeted approach represents a promising pathway to alleviate AD pathology through a combination of nanozymes and the modulation of microglial polarization. Qi et al. reported a novel approach that utilized nanocomposites with both drug loading and photothermal conversion properties to address oxidative stress and Aβ1-42 deposition, two prominent factors associated with AD [97]. MoS2 QDs functionalized with lipids (MoS2 QD/lipid) served as a carrier, enhancing water dispersibility and avoidance of immune clearance. Curcumin-loaded MoS2 QD/lipid (MoS2 QD/lipid-Cur) overcame drug solubility and bioavailability issues, demonstrating sustained release and long-term effectiveness. In both early-stage therapeutic targets, MoS2 QD/lipid-Cur exhibited outstanding performance, significantly reducing Aβ1-42 mediated neurotoxicity, while NIR irradiation enhanced blood–brain barrier permeability. This versatile nanocomposite demonstrated great potential for use in multi-target AD therapy.
Fig. 7.
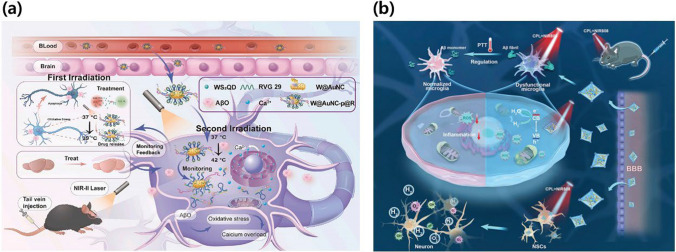
TMD-based therapeutic strategies for NDDs treatment. a PCM/WS2QDs@AuNC for serial activation of therapy and monitoring modules by activating second near-infrared laser. Reproduced with permission and copyright (2023) Elsevier B. V. [95]. b Stimulus-responsive TMD-based nanocomplex, UiO-66-NH2@l-MoS2 QDs@PA-Ni (MSP-U), for the treatment of AD by using decomposition of the formed Aβ plaque. Reproduced with permission and copyright (2023) Wiley-VCH. [100]
Qi et al. reported a breakthrough approach utilizing macrophage membranes (MMs) for the active targeting of inflammation, functionalizing MoS2 QDs to create a nanodrug (MoS2 QD/MM) [98]. This nanodrug could eliminate ROS and inhibit Aβ1-42 deposition, leading to combined multi-target therapy with NIR. MoS2 QD/MM demonstrated targeted therapeutic effects in terms of ROS elimination and Aβ1-42 deposition, effectively reducing Aβ1-42-mediated cytotoxicity. MM modification enhanced brain targeting, and NIR irradiation actively facilitated the crossing of the blood–brain barrier. In-vivo behavioral analysis revealed that the combined treatment group of APP/PS1 mice exhibited a similar exploration desire and learning ability to the wild-type group. MoS2 QD/MM is thus an excellent nanodrug with multiple effects, particularly for NDDs characterized by complex pathogenesis. Wang et al. developed a multifunctional nanocomposite with high NIR absorption properties that combined MoS2 NSs and gold nanorods (AuNRs) [99]. The nanocomposite, synthesized via electrostatic self-assembly, exhibited high stability and excellent biocompatibility. It was employed to modulate the aggregation of Aβ peptides, destabilize mature fibrils under NIR irradiation, and counteract Aβ-induced ROS associated with neurotoxicity. Thioflavin T (ThT) fluorescence assays and transmission electron microscopy (TEM) confirmed the inhibitory and destabilizing effects, while cell viability and ROS assays verified the alleviation of Aβ-induced oxidative stress and cell toxicity. Significantly, both the MoS2 NSs and AuNRs served as NIR photothermal agents, enhancing the disruption of Aβ fibrils and improving cell viability through localized heat generation under low-power NIR irradiation. This study provides valuable insights into the design of multifunctional systems for the treatment of amyloid-related diseases. Tan et al. designed stimulus-responsive chiral UiO-66-NH2@l-MoS2 QDs@PA-Ni (MSP-U) for AD therapy (Fig. 7b) [100]. MSP-U stimulated NSC differentiation, induced in-situ hydrogen (H2) production, and effectively cleared Aβ plaques. l-MoS2 QDs, modified with l-cysteine (l-Cys), enhanced NSC differentiation into neurons under circularly polarized NIR radiation. Doped-phytic acid nickel (PA-Ni) enhanced the scavenging of ROS at the lesion site through photocatalytic H2 production. Loading l-MoS2 QDs with UiO-66 suppressed electron–hole recombination, improved charge separation, and enhanced photogenerated electron transport for efficient H2 production. The photothermal effect of MSP-U also contributed to Aβ plaque clearance. In-vivo assessments revealed that MSP-U enhanced spatial cognition and memory, highlighting its potential as a promising candidate for AD treatment.
TMD materials have also been utilized in tissue regeneration to improve the therapeutic effect for NDDs. For example, Alamri et al. demonstrated the protective capabilities of MoS2 QDs coated with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethyleneglycol)-2000] (DSPE-PEG) linked with (3-carboxypropyl) triphenylphosphonium-bromide (TPP) on the secondary structure of proteins in AD-affected brain tissue [101]. A cohort of 15 male SWR/J mice was divided into three groups: a control group, a second group induced with AD through daily doses of AlCl3 and D-galactose for 49 consecutive days, and a third group receiving the same AD-inducing doses but treated with DSPE-PEG-TPP-MoS2 QDs. After separating the brain tissue from the skull, the molecular structures were analyzed using Fourier-transform infrared (FTIR) spectroscopy and the curve fitting method for the amide I peak. The analysis revealed a significant increase in β-sheet structures and a concurrent reduction in turn and α-helix structures in the AD group compared to the control group, with no statistically significant differences observed between the treated and control mice. Multivariate analysis of the FTIR spectral region for protein amide molecular structures highlighted the strong similarity between the treated and normal mice, suggesting that DSPE-PEG-TPP-MoS2 QDs can protect brain tissue proteins against the pathogenic influences of AD. Feng et al. investigated the use of 2D materials to impede the formation of supercompact polyglutamine (polyQ) structures in the Huntington protein (Htt) exon-1, which is associated with the age of HD onset [102]. The polyQ domain, particularly at lengths greater than 40, has been linked to the development of highly compact aggregate structures leading to HD. Based on the concept of “folding-upon-binding”, in which the protein structure depends on the binding substrate, molecular dynamics simulations were employed to analyze the binding of two polyQ peptides, one with a normal length of 22 (Q22) and another with a typical HD-causing length of 46 (Q46), to graphene and MoS2 NSs. The simulations revealed that Q22 unfolded and elongated on both NSs upon binding, whereas Q46 remained mostly collapsed, representing distinct Q-length-specific behavior. Hydrophobic interactions with graphene/MoS2 promoted the stretching of polyQ, but the internal hydrogen bonds within polyQ created a competitive environment influencing the folding/binding behaviors of different Q-lengths. These findings hold clinical relevance for the understanding of HD and highlight the potential applications of 2D materials in disease-related protein aggregation.
Conclusion and future perspectives
In conclusion, 2D materials have played a vital role in diagnostic and therapeutic applications for NDDs. These materials provide a range of options for the effective diagnosis and treatment of NDDs due to their ultrathin atomic-layer structure and specific surface area. Integrating 2D materials with biomolecules has emerged as a groundbreaking approach for the detection of various sensitive pathological substances. For therapeutic applications, including drug delivery systems (DDS) and regenerative medicine, considerable attention has been directed toward 2D materials, particularly for neural repair and regeneration. These materials exhibit remarkable physicochemical properties and exceptional biological activity, including a large surface-area-to-thickness ratio, high adhesion levels, and high flexibility. Moreover, their biocompatibility can be tailored, and they can act as effective nano-carriers with electrical properties. Eventually, various 2D materials have already been reported for its contribution to the advancements in synaptic modulation, neuroinflammation reduction, stem cell fate regulation, and injured neural cells and tissues repairment.
Although 2D materials have made a notable contribution to the treatment of NDDs, several important manufacturing issues remain. In diagnostics, biosensing systems may be prone to false positives or negatives due to variations in indicator concentration and probe degradation. Assessing whether 2D materials can outperform current clinical guidelines in vivo necessitates comprehensive support from big data analysis. In addition to functional group modification, defect state regulation, single-atom loading, and the construction of heterojunctions, another important question is whether these materials can remain stable in vivo. The inherent hydrophobicity of graphene, weak interactions with other media, and strong van der Waals forces also complicate the dispersion of graphene within NSs. In therapeutic applications, the optimization and screening of effective 2D materials are crucial, given that only a limited number of them are suitable. Surface modification or functionalization based on disease-specific requirements is also essential, and the implementation of an efficient screening system could expedite this process. Moreover, the biological effects of 2D materials on neuronal function, particularly after severe nerve injury, remain contentious.
Looking ahead, future research should focus on optimizing 2D materials by tailoring their design and applications conscientiously to specific NDDs and their intended purposes. In terms of biosafety aspects, standardized and large-scale studies are expected to assure the safety and viability of 2D materials for diagnosis and therapeutic modalities in NDDs. Biocompatibility may be influenced by physical and chemical properties, hence, emphasizing the need to optimize these properties to meet the clinical requirements. Besides, the selection and designing of 2D materials for NDDs diagnosis and treatment is also crucial to boost targeting and efficacy in diagnosis and treatment course. Participation from third party evaluations or assessment by research bodies, especially for its ethical considerations into clinical translation is greatly anticipated. Ultimately, research efforts should also be concentrated to navigate blueprint for regulatory approvals of 2D materials application in the clinical settings.
While the focus of this paper was exclusively on 2D materials, it remains possible for other organic or inorganic substances to be used in a hybrid form to amplify the sensing or therapeutic effects of these materials. Moreover, these materials would be useful in applications that require the simultaneous detection of bio-signals and tissue regeneration. For example, in constructing in-vitro human nervous system models that closely replicate real tissue, 2D materials such as graphene can aid in building models capable of the real-time detection of signals from neural cells while mimicking neural tissue characteristics. In addition, 2D materials can initiate nerve cell differentiation and enhance maturity, enabling the sensitive measurement of various bio-signals. This use of microphysiological models not only advances the study of the pathophysiology of NDDs but also holds promise for drug screening. Overall, 2D materials offer considerable potential across a wide range of NDD research applications, enhancing a deeper understanding of these diseases and paving the way for novel treatment strategies.
Acknowledgements
This work was supported by GRDC Cooperative Hub through the National Research Foundation (NRF) of Korea funded by the Ministry of Science and ICT (Grant Number RS-2023-00259341), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No.2016R1A6A1A03012845), and National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2022R1C1C1008797).
Author contributions
Jin-Ha Choi and Jeong-Woo Choi organized the structure of the manuscript. Jin-Ha Choi and Jeong-Woo Choi wrote the whole manuscript. Jeong-Woo Choi revised the manuscript. Izzati Haizan revised the overall illustrations and figures, added Fig. 1, and included a table. All authors collaboratively wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023;186:693–714. [DOI] [PubMed] [Google Scholar]
- 2.Rekatsina M, Paladini A, Piroli A, Zis P, Pergolizzi JV, Varrassi G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv Ther. 2020;37:113–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maresova P, Hruska J, Klimova B, Barakovic S, Krejcar O. Activities of daily living and associated costs in the most widespread neurodegenerative diseases: a systematic review. Clin Interv Aging. 2020;15:1841–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zahra W, Rai SN, Birla H, Singh SS, Dilnashin H, Rathore AS, Singh SP. The global economic impact of neurodegenerative diseases: opportunities and challenges. Bioeconomy for Sustainable Development; 2020. pp. 333–345.
- 5.Murali A, Lokhande G, Deo KA, Brokesh A, Gaharwar AK. Emerging 2D nanomaterials for biomedical applications. Mater Today. 2021;50:276–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H, Feng W, Chen Y. Two-dimensional biomaterials: material science, biological effect and biomedical engineering applications. Chem Soc Rev. 2021;50:11381–485. [DOI] [PubMed] [Google Scholar]
- 7.Rohaizad N, Mayorga-Martinez CC, Fojtů M, Latiff NM, Pumera M. Two-dimensional materials in biomedical, biosensing and sensing applications. Chem Soc Rev. 2021;50:619–57. [DOI] [PubMed] [Google Scholar]
- 8.Choi HK, Choi J-H, Yoon J. An updated review on electrochemical nanobiosensors for neurotransmitter detection. Biosensors (Basel). 2023;13:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouyang J, Rao S, Liu R, Wang L, Chen W, Tao W, Kong N. 2D materials-based nanomedicine: from discovery to applications. Adv Drug Deliv Rev. 2022;185:114268. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Fan T, Chen W, Li Y, Wang B. Recent advances of two-dimensional materials in smart drug delivery nano-systems. Bioact Mater. 2020;5:1071–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng L, Wang X, Gong F, Liu T, Liu Z. 2D nanomaterials for cancer theranostic applications. Adv Mater. 2020;32:1902333. [DOI] [PubMed] [Google Scholar]
- 12.Song S, Shen H, Wang Y, Chu X, Xie J, Zhou N, Shen J. Biomedical application of graphene: from drug delivery, tumor therapy, to theranostics. Colloids Surf B Biointerfaces. 2020;185:110596. [DOI] [PubMed] [Google Scholar]
- 13.Dutta RR, Devi R, Dutta HS, Gogoi S. Transition metal dichalcogenides for biomedical applications. In: Pramanik S, Sundar D, editors. Two-Dimensional Nanostructures for Biomedical Technology. Elsevier; 2020. p. 211–47. [Google Scholar]
- 14.Lu B, Zhu Z, Ma B, Wang W, Zhu R, Zhang J. 2D MXene nanomaterials for versatile biomedical applications: current trends and future prospects. Small. 2021;17:2100946. [DOI] [PubMed] [Google Scholar]
- 15.Pandey A, Nikam AN, Fernandes G, Kulkarni S, Padya BS, Prassl R, Das S, Joseph A, Deshmukh PK, Patil PO. Black phosphorus as multifaceted advanced material nanoplatforms for potential biomedical applications. Nanomaterials. 2020;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You Y, Yang C, Zhang X, Lin H, Shi J. Emerging two-dimensional silicene nanosheets for biomedical applications. Mater Today Nano. 2021;16:100132. [Google Scholar]
- 17.Ou M, Wang X, Yu L, Liu C, Tao W, Ji X, Mei L. The emergence and evolution of borophene. Adv Sci. 2021;8:2001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farasati Far B, Maleki-baladi R, Fathi-karkan S, Babaei M, Sargazi S. Biomedical applications of cerium vanadate nanoparticles: a review. J Mater Chem B. 2024;12:609–36. [DOI] [PubMed] [Google Scholar]
- 19.Javad Javid-Naderi M, Valizadeh N, Banimohamad-Shotorbani B, Shahgolzari M, Shayegh F, Maleki-baladi R, Sargazi S, Fathi-karkan S. Exploring the biomedical potential of iron vanadate nanoparticles: a comprehensive review. Inorg Chem Commun. 2023;157:111423. [Google Scholar]
- 20.Magne TM, de Oliveira Vieira T, Alencar LMR, Junior FFM, Gemini-Piperni S, Carneiro SV, Fechine LMUD, Freire RM, Golokhvast K, Metrangolo P. Graphene and its derivatives: understanding the main chemical and medicinal chemistry roles for biomedical applications. J Nanostructure Chem. 2022;12:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniyal M, Liu B, Wang W. Comprehensive review on graphene oxide for use in drug delivery system. Curr Med Chem. 2020;27:3665–85. [DOI] [PubMed] [Google Scholar]
- 22.Yildiz G, Bolton-Warberg M, Awaja F. Graphene and graphene oxide for bio-sensing: general properties and the effects of graphene ripples. Acta Biomater. 2021;131:62–79. [DOI] [PubMed] [Google Scholar]
- 23.Sethi J, Van Bulck M, Suhail A, Safarzadeh M, Perez-Castillo A, Pan G. A label-free biosensor based on graphene and reduced graphene oxide dual-layer for electrochemical determination of beta-amyloid biomarkers. Microchim Acta. 2020;187:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maleki M, Zarezadeh R, Nouri M, Sadigh AR, Pouremamali F, Asemi Z, Kafil HS, Alemi F, Yousefi B. Graphene oxide: a promising material for regenerative medicine and tissue engineering. Biomol Concepts. 2020;11:182–200. [DOI] [PubMed] [Google Scholar]
- 25.Girao AF, Sousa J, Dominguez-Bajo A, Gonzalez-Mayorga A, Bdikin I, Pujades-Otero E, Casan-Pastor N, Hortigüela MJ, Otero-Irurueta G, Completo A. 3D reduced graphene oxide scaffolds with a combinatorial fibrous-porous architecture for neural tissue engineering. ACS Appl Mater Interfaces. 2020;12:38962–75. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Rauti R, Scaini D, Antman-Passig M, Meshulam O, Naveh D, Ballerini L, Shefi O. Graphene-based nanomaterials for neuroengineering: recent advances and future prospective. Adv Funct Mater. 2021;31:2104887. [Google Scholar]
- 27.Magaz A, Li X, Gough JE, Blaker JJ. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater Sci Eng C. 2021;119:111632. [DOI] [PubMed] [Google Scholar]
- 28.Lu J, Chen M, Dong L, Cai L, Zhao M, Wang Q, Li J. Molybdenum disulfide nanosheets: from exfoliation preparation to biosensing and cancer therapy applications. Colloids Surf B Biointerfaces. 2020;194:111162. [DOI] [PubMed] [Google Scholar]
- 29.Niknam S, Dehdast SA, Pourdakan O, Shabani M, Koohi MK. Tungsten disulfide nanomaterials (WS2 NM) application in biosensors and nanomedicine: a review. Nanomed Res J. 2022;7:214–26. [Google Scholar]
- 30.Hassani F, Heydarinasab A, Panahi HA, Moniri E. Surface modification of tungsten disulfide nanosheets with PH/thermosensitive polymer and polyethylenimine dendrimer for near-infrared triggered drug delivery of letrozole. J Mol Liq. 2023;371:121058. [Google Scholar]
- 31.Li G, Meng F, Lu T, Wei L, Pan X, Nong Z, Wei M, Liao C, Li X. Functionalised molybdenum disulfide nanosheets for Co-delivery of doxorubicin and SiRNA for combined chemo/gene/photothermal therapy on multidrug-resistant cancer. J Pharm Pharmacol. 2021;73:1128–35. [DOI] [PubMed] [Google Scholar]
- 32.Xie M, Li J, Deng T, Yang N, Yang M. Modification of magnetic molybdenum disulfide by chitosan/carboxymethylcellulose with enhanced dispersibility for targeted photothermal-/chemotherapy of cancer. J Mater Chem B. 2021;9:1833–45. [DOI] [PubMed] [Google Scholar]
- 33.Lee M-H, Thomas JL, Su Z-L, Yeh W-K, Monzel AS, Bolognin S, Schwamborn JC, Yang C-H, Lin H-Y. Transition metal dichalcogenides to optimize the performance of peptide-imprinted conductive polymers as electrochemical sensors. Microchim Acta. 2021;188:203. [DOI] [PubMed] [Google Scholar]
- 34.Tajik S, Dourandish Z, Nejad FG, Beitollahi H, Jahani PM, Di Bartolomeo A. Transition metal dichalcogenides: synthesis and use in the development of electrochemical sensors and biosensors. Biosens Bioelectron. 2022;216:114674. [DOI] [PubMed] [Google Scholar]
- 35.Abhilash S, Sarika S, Ambadi S, Akhila M, Sumi VS, Sreekala CO, Rijith S. Fabrication and performance evaluation of RGO-PANI supported NiP/TMD based biosensors for electrochemical detection of dopamine. Mater Today Commun. 2024;38:107946. [Google Scholar]
- 36.Tufail S, Sherwani MA, Shamim Z, Goh KW, Alomary MN, Ansari MA, Almosa AA, Ming LC, Abdullah ADI, Khan FB. 2D nanostructures: potential in diagnosis and treatment of Alzheimer’s disease. Biomed Pharmacother. 2024;170:116070. [DOI] [PubMed] [Google Scholar]
- 37.Kumar A, Sood A, Han SS. Molybdenum disulfide (MoS2)-based nanostructures for tissue engineering applications: prospects and challenges. J Mater Chem B. 2022;10:2761–80. [DOI] [PubMed] [Google Scholar]
- 38.Liu A, Liu Y, Liu G, Zhang A, Cheng Y, Li Y, Zhang L, Wang L, Zhou H, Liu J. Engineering of surface modified Ti3C2Tx MXene based dually controlled drug release system for synergistic multitherapies of cancer. Chem Eng J. 2022;448:137691. [Google Scholar]
- 39.Wu X, Ma P, Sun Y, Du F, Song D, Xu G. Application of MXene in electrochemical sensors: a review. Electroanalysis. 2021;33:1827–51. [Google Scholar]
- 40.Chavan SG, Rathod PR, Koyappayil A, Go A, Lee M-H. “Two-step” signal amplification for ultrasensitive detection of dopamine in human serum sample using Ti3C2Tx-MXene. Sens Actuators B Chem. 2024;404:135308. [Google Scholar]
- 41.Wen X-H, Zhao X-F, Wang X-H, Wang Y, Guo J-C, Zhou H-G, Zuo C-T, Lu H-L. Fe3O4/MXene nanosphere-based microfluidic chip for the accurate diagnosis of Alzheimer’s disease. ACS Appl Nano Mater. 2022;5:15925–33. [Google Scholar]
- 42.Huang R, Chen X, Dong Y, Zhang X, Wei Y, Yang Z, Li W, Guo Y, Liu J, Yang Z. MXene composite nanofibers for cell culture and tissue engineering. ACS Appl Bio Mater. 2020;3:2125–31. [DOI] [PubMed] [Google Scholar]
- 43.Li S, Ma L, Zhou M, Li Y, Xia Y, Fan X, Cheng C, Luo H. New opportunities for emerging 2D materials in bioelectronics and biosensors. Curr Opin Biomed Eng. 2020;13:32–41. [Google Scholar]
- 44.Krishnan SK, Nataraj N, Meyyappan M, Pal U. Graphene-based field-effect transistors in biosensing and neural interfacing applications: recent advances and prospects. Anal Chem. 2023;95:2590–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Ge Z, Chen M, He H, Zhang X, Wang S. Ratiometric electrochemical biosensor based on Exo III-assisted recycling amplification for the detection of CAG trinucleotide repeats. Biosens Bioelectron. 2019;142:111537. [DOI] [PubMed] [Google Scholar]
- 46.Xu L, Ramadan S, Akingbade OE, Zhang Y, Alodan S, Graham N, Zimmerman KA, Torres E, Heslegrave A, Petrov PK. Detection of glial fibrillary acidic protein in patient plasma using on-chip graphene field-effect biosensors, in comparison with ELISA and single-molecule array. ACS Sens. 2021;7:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salehirozveh M, Dehghani P, Zimmermann M, Roy VAL, Heidari H. Graphene field effect transistor biosensors based on aptamer for amyloid-β detection. IEEE Sens J. 2020;20:12488–94. [Google Scholar]
- 48.Ge Z, Ma M, Chang G, Chen M, He H, Zhang X, Wang S. A novel solution-gated graphene transistor biosensor for ultrasensitive detection of trinucleotide repeats. Analyst. 2020;145:4795–805. [DOI] [PubMed] [Google Scholar]
- 49.Park D, Kim JH, Kim HJ, Lee D, Lee DS, Yoon DS, Hwang KS. Multiplexed femtomolar detection of Alzheimer’s disease biomarkers in biofluids using a reduced graphene oxide field-effect transistor. Biosens Bioelectron. 2020;167:112505. [DOI] [PubMed] [Google Scholar]
- 50.Park D-J, Choi J-H, Lee W-J, Um SH, Oh B-K. Selective electrochemical detection of dopamine using reduced graphene oxide sheets-gold nanoparticles modified electrode. J Nanosci Nanotechnol. 2017;17:8012–8. [Google Scholar]
- 51.Lee EJ, Choi J-H, Um SH, Oh B-K. Electrochemical sensor for selective detection of norepinephrine using graphene sheets-gold nanoparticle complex modified electrode. Korean J Chem Eng. 2017;34:1129–32. [Google Scholar]
- 52.Choi J-H, Kim T-H, El-Said WA, Lee J-H, Yang L, Conley B, Choi J-W, Lee K-B. In situ detection of neurotransmitters from stem cell-derived neural interface at the single-cell level via graphene-hybrid SERS nanobiosensing. Nano Lett. 2020;20:7670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Li C, Yang Y, Han J, Huang W, Zhou J, Zhang Y. Anti-biofouling Ti3C2TX MXene-holey graphene modified electrode for dopamine sensing in complex biological fluids. Talanta. 2022;247:123614. [DOI] [PubMed] [Google Scholar]
- 54.Lv Y, Zhou Y, Dong H, Xu M, Zhang J, Yan M. Ultrasensitive electrochemical detection of amyloid-beta oligomers using double amplification strategy by MXene substrate and covalent organic framework-based probe. Talanta. 2024;266:125134. [DOI] [PubMed] [Google Scholar]
- 55.Özcan N, Medetalibeyoglu H, Akyıldırım O, Atar N, Yola ML. Electrochemical detection of amyloid-β protein by delaminated titanium carbide MXene/multi-walled carbon nanotubes composite with molecularly imprinted polymer. Mater Today Commun. 2020;23:101097. [Google Scholar]
- 56.Lee J, Paul J, Sangubotla R, Kim J. MXene-coated liposome-quercetin-functionalized gold nanoparticles for electrochemical detection of dopamine. Phys Status Solidi (a). 2023;220:2300218. [Google Scholar]
- 57.Zhang Y, Cao F, Xu M, Li X, Tao M, Wu S, Xu W, Liu Y, Zhu W. Integration of magnetic-field-directed self-assembly-based cell culture and biosensing platform for improving HPSCs-derived neurons and quantitative detection of neurotransmitter. ACS Appl Mater Interfaces. 2023;15:58230–40. [DOI] [PubMed] [Google Scholar]
- 58.Du Y, Liu Y, Lu W, Zhang X, Wang A, Kong J. Nacre-inspired MXene nanocomposite-based strain sensor with ultrahigh sensitivity in a small strain range for Parkinson’s disease diagnosis. ACS Appl Mater Interfaces. 2023;15:50413–26. [DOI] [PubMed] [Google Scholar]
- 59.Liu D, Tang Z, Zhang Z. Comparative study on NO2 and H2S sensing mechanisms of gas sensors based on WS2 nanosheets. Sens Actuators B Chem. 2020;303:127114. [Google Scholar]
- 60.Wei X, Liu C, Qin H, Ye Z, Liu X, Zong B, Li Z, Mao S. Fast, Specific, and ultrasensitive antibiotic residue detection by monolayer WS2-based field-effect transistor sensor. J Hazard Mater. 2023;443:130299. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Han D, Wang S, Zhang X, Yang R, Ji Y, Yu X. Electrochemical detection of adenine and guanine using a three-dimensional WS2 nanosheet/graphite microfiber hybrid electrode. Electrochem Commun. 2019;99:75–80. [Google Scholar]
- 62.Lei Y, Butler D, Lucking MC, Zhang F, Xia T, Fujisawa K, Granzier-Nakajima T, Cruz-Silva R, Endo M, Terrones H. Single-atom doping of MoS2 with manganese enables ultrasensitive detection of dopamine: experimental and computational approach. Sci Adv. 2020;6:eabc4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rawat B, Mishra KK, Barman U, Arora L, Pal D, Paily RP. Two-dimensional MoS2-based electrochemical biosensor for highly selective detection of glutathione. IEEE Sens J. 2020;20:6937–44. [Google Scholar]
- 64.Kim H-I, Yim D, Jeon S-J, Kang TW, Hwang I-J, Lee S, Yang J-K, Ju J-M, So Y, Kim J-H. Modulation of oligonucleotide-binding dynamics on WS2 nanosheet interfaces for detection of Alzheimer’s disease biomarkers. Biosens Bioelectron. 2020;165:112401. [DOI] [PubMed] [Google Scholar]
- 65.Kong L, Zhou X, Shi G, Yu Y. Molybdenum disulfide nanosheets-based fluorescent “off-to-on” probe for targeted monitoring and inhibition of β-amyloid oligomers. Analyst. 2020;145:6369–77. [DOI] [PubMed] [Google Scholar]
- 66.Mendonça MCP, Soares ES, de Jesus MB, Ceragioli HJ, Ferreira MS, Catharino RR, da Cruz-Höfling MA. Reduced graphene oxide induces transient blood–brain barrier opening: an in vivo study. J Nanobiotechnol. 2015;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mustafa G, Hassan D, Zeeshan M, Ruiz-Pulido G, Ebrahimi N, Mobashar A, Pourmadadi M, Rahdar A, Sargazi S, Fathi-karkan S, et al. Advances in Nanotechnology versus stem cell therapy for the theranostics of Huntington’s disease. J Drug Deliv Sci Technol. 2023;87:104774. [Google Scholar]
- 68.He X, Zhu Y, Ma B, Xu X, Huang R, Cheng L, Zhu R. Bioactive 2D nanomaterials for neural repair and regeneration. Adv Drug Deliv Rev. 2022;187:114379. [DOI] [PubMed] [Google Scholar]
- 69.Han J, Kim YS, Lim M-Y, Kim HY, Kong S, Kang M, Choo YW, Jun JH, Ryu S, Jeong H. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano. 2018;12:1959–77. [DOI] [PubMed] [Google Scholar]
- 70.Davodabadi F, Mirinejad S, Malik S, Dhasmana A, Ulucan-Karnak F, Sargazi S, Sargazi S, Fathi-Karkan S, Rahdar A. Nanotherapeutic approaches for delivery of long non-coding RNAs: an updated review with emphasis on cancer. Nanoscale. 2024;16:3881–914. [DOI] [PubMed] [Google Scholar]
- 71.Fusco L, Gazzi A, Peng G, Shin Y, Vranic S, Bedognetti D, Vitale F, Yilmazer A, Feng X, Fadeel B. Graphene and other 2D materials: a multidisciplinary analysis to uncover the hidden potential as cancer theranostics. Theranostics. 2020;10:5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao H, Sun C, Shang S, Sun B, Sun M, Hu S, Yang H, Hu Y, Feng Z, Zhou W. Wireless electrical signals induce functional neuronal differentiation of BMSCs on 3D graphene framework driven by magnetic field. ACS Nano. 2023;17:16204–20. [DOI] [PubMed] [Google Scholar]
- 73.Kim T-H, Lee K-B, Choi J-W. 3D graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials. 2013;34:8660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma Q, Yang L, Jiang Z, Song Q, Xiao M, Zhang D, Ma X, Wen T, Cheng G. Three-dimensional stiff graphene scaffold on neural stem cells behavior. ACS Appl Mater Interfaces. 2016;8:34227–33. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Gu J, Zhang Y, Guo H, Zhang S, Song J, Liu C, Wang L, Li D, Dai B. Graphene quantum dots modulate stress granule assembly and prevent abnormal phase transition of fused in sarcoma protein. ACS Nano. 2023;17:10129–41. [DOI] [PubMed] [Google Scholar]
- 76.Chen X, Pandit S, Shi L, Ravikumar V, Køhler JB, Svetlicic E, Cao Z, Garg A, Petranovic D, Mijakovic I. Graphene oxide attenuates toxicity of amyloid-β aggregates in yeast by promoting disassembly and boosting cellular stress response. Adv Funct Mater. 2023;33:2304053. [Google Scholar]
- 77.Mohebichamkhorami F, Faizi M, Mahmoudifard M, Hajikarim-Hamedani A, Mohseni SS, Heidari A, Ghane Y, Khoramjouy M, Khayati M, Ghasemi R. Microfluidic synthesis of ultrasmall chitosan/graphene quantum dots particles for intranasal delivery in Alzheimer’s disease treatment. Small. 2023;19:2207626. [DOI] [PubMed] [Google Scholar]
- 78.Kaliyaperumal P, Renganathan S, Arumugam K, Aremu BR. Engineered graphene quantum dot nanocomposite triggers α-synuclein defibrillation: therapeutics against Parkinson’s disease. Nanomedicine. 2023;47:102608. [DOI] [PubMed] [Google Scholar]
- 79.Xiong S, Luo J, Wang Q, Li Z, Li J, Liu Q, Gao L, Fang S, Li Y, Pan H. Targeted graphene oxide for drug delivery as a therapeutic nanoplatform against Parkinson’s disease. Biomater Sci. 2021;9:1705–15. [DOI] [PubMed] [Google Scholar]
- 80.Kang I, Yoo JM, Kim D, Kim J, Cho MK, Lee S-E, Kim DJ, Lee B-C, Lee JY, Kim J-J. Graphene quantum dots alleviate impaired functions in niemann-pick disease type C in vivo. Nano Lett. 2021;21:2339–46. [DOI] [PubMed] [Google Scholar]
- 81.Jia R, Li T, Jiang W, Wang J, Li X, Qu Q, Dang J, Li P. Smart milli-capsules manipulated by NIR irradiation for controllable drug delivery in-vivo for renal cell carcinoma and neurodegenerative diseases. Mater Des. 2022;224:111287. [Google Scholar]
- 82.Li X, Li K, Chu F, Huang J, Yang Z. Graphene oxide enhances β-amyloid clearance by inducing autophagy of microglia and neurons. Chem Biol Interact. 2020;325:109126. [DOI] [PubMed] [Google Scholar]
- 83.Askari N, Askari MB, Shafieipour A. Investigation the molecular structure of novel graphene hybrid scaffold in nerve regeneration. J Mol Struct. 2019;1186:393–403. [Google Scholar]
- 84.Guo R, Li J, Chen C, Xiao M, Liao M, Hu Y, Liu Y, Li D, Zou J, Sun D. Biomimetic 3D bacterial cellulose-graphene foam hybrid scaffold regulates neural stem cell proliferation and differentiation. Colloids Surf B Biointerfaces. 2021;200:111590. [DOI] [PubMed] [Google Scholar]
- 85.Pradhan K, Das G, Khan J, Gupta V, Barman S, Adak A, Ghosh S. Neuro-regenerative choline-functionalized injectable graphene oxide hydrogel repairs focal brain injury. ACS Chem Neurosci. 2018;10:1535–43. [DOI] [PubMed] [Google Scholar]
- 86.Feng W, Han X, Hu H, Chang M, Ding L, Xiang H, Chen Y, Li Y. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nat Commun. 2021;12:2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang H, Xia L, Ye X, Xu J, Liu T, Wang L, Zhang S, Feng W, Du D, Chen Y. Ultrathin niobium carbide MXenzyme for remedying hypertension by antioxidative and neuroprotective actions. Angew Chem Int Ed. 2023;62:e202303539. [DOI] [PubMed] [Google Scholar]
- 88.Du C, Feng W, Dai X, Wang J, Geng D, Li X, Chen Y, Zhang J. Cu2+-chelatable and ROS-scavenging MXenzyme as NIR-II-triggered blood–brain barrier-crossing nanocatalyst against Alzheimer’s disease. Small. 2022;18:2203031. [DOI] [PubMed] [Google Scholar]
- 89.Hu H, Huang H, Xia L, Qian X, Feng W, Chen Y, Li Y. Engineering vanadium carbide MXene as multienzyme mimetics for efficient in vivo ischemic stroke treatment. Chem Eng J. 2022;440:135810. [Google Scholar]
- 90.Seitak A, Shanti A, Al Adem K, Farid N, Luo S, Iskandarov J, Karanikolos GN, Liao K, Chan V, Lee S. 2D MXenes for controlled releases of therapeutic proteins. J Biomed Mater Res A. 2023;111:514–26. [DOI] [PubMed] [Google Scholar]
- 91.Wei H, Gu Y, Li A, Song P, Liu D, Sun F, Ma X, Qian X. Conductive 3D Ti3C2Tx MXene-matrigel hydrogels promote proliferation and neuronal differentiation of neural stem cells. Colloids Surf B Biointerfaces. 2024;233:113652. [DOI] [PubMed] [Google Scholar]
- 92.Tang Z, Liu Y, Xiang H, Dai X, Huang X, Ju Y, Ni N, Huang R, Gao H, Zhang J. Bifunctional MXene-augmented retinal progenitor cell transplantation for retinal degeneration. Adv Sci. 2023;10:2302747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Y, Hu Y, Wei H, Cao W, Qi Y, Zhou S, Zhang P, Li H, Li G-L, Chai R. Two-dimensional Ti3C2Tx MXene promotes electrophysiological maturation of neural circuits. J Nanobiotechnol. 2022;20:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Driscoll N, Erickson B, Murphy BB, Richardson AG, Robbins G, Apollo NV, Mentzelopoulos G, Mathis T, Hantanasirisakul K, Bagga P. MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci Transl Med. 2021;13:eabf8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiu Z, Cao G, Lv S, Yu D, Fu J, Yan H, Li Y, Zhou P, Wu X, Liu Z. A novel AD theranostic platform with NIR-II laser controlled drug release and real-time monitoring of therapeutic outcomes. Chem Eng J. 2023;469:143882. [Google Scholar]
- 96.Ren C, Li D, Zhou Q, Hu X. Mitochondria-targeted TPP-MoS2 with dual enzyme activity provides efficient neuroprotection through M1/M2 microglial polarization in an Alzheimer’s disease model. Biomaterials. 2020;232:119752. [DOI] [PubMed] [Google Scholar]
- 97.Qi X, Ye P, Xie M. MoS2 quantum dots based on lipid drug delivery system for combined therapy against Alzheimer’s disease. J Drug Deliv Sci Technol. 2023;82:104324. [Google Scholar]
- 98.Qi X, Li L, Ye P, Xie M. Macrophage membrane-modified MoS2 quantum dots as a nanodrug for combined multi-targeting of Alzheimer’s disease. Adv Healthc Mater. 2023;13:2303211. [DOI] [PubMed] [Google Scholar]
- 99.Wang X, Han Q, Liu X, Wang C, Yang R. Multifunctional inhibitors of β-amyloid aggregation based on MoS2/AuNR nanocomposites with high near-infrared absorption. Nanoscale. 2019;11:9185–93. [DOI] [PubMed] [Google Scholar]
- 100.Tan H, Huang Y, Dong S, Bai Z, Chen C, Wu X, Chao M, Yan H, Wang S, Geng D. A chiral nanocomplex for multitarget therapy to alleviate neuropathology and rescue Alzheimer’s cognitive deficits. Small. 2023;19:2303530. [DOI] [PubMed] [Google Scholar]
- 101.Alamri OA, Qusti S, Balgoon M, Ageeli AA, Al-Marhaby FA, Alosaimi AM, Jowhari MA, Saeed A. The role of MoS2 QDs coated with DSPE-PEG-TPP in the protection of protein secondary structure of the brain tissues in an Alzheimer’s disease model. Int J Biol Macromol. 2024;255:128522. [DOI] [PubMed] [Google Scholar]
- 102.Feng M, Bell DR, Wang Z, Zhang W. Length-dependent structural transformations of huntingtin PolyQ domain upon binding to 2D-nanomaterials. Front Chem. 2020;8:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.