See Clinical Research on Page 2484
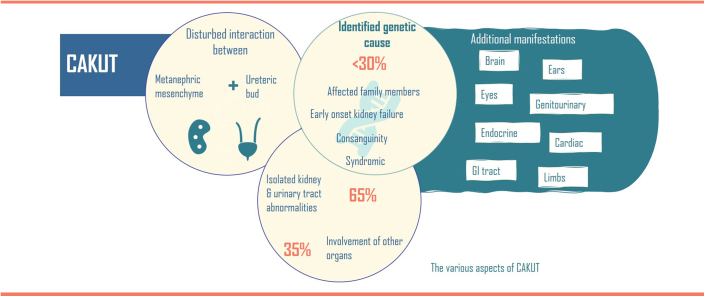
Congenital anomalies of the kidney and urinary tract (CAKUT) represent a significant health burden, affecting 1:100 to 1:500 in newborns and a leading cause of kidney failure in children.1 CAKUT result from disturbed renal and urinary tract tissue morphogenesis and patterning defects caused by impaired cell differentiation. CAKUT often result from disturbed interactions between the 2 main embryonic tissues of the kidney anlage, namely the ureteric bud (that gives rise to the collecting duct system) and the metanephric mesenchyme (a derivative of the most posterior intermediate mesoderm), containing the multipotent precursor cell population that gives rise to the glomeruli, proximal and distal tubulous systems as well as interstitial stromal cells.2 Kidney development relies on the signal-mediated, reciprocal tissue interactions between the ureteric bud and mesenchyme to guide the coordinated morphogenesis for proper organization of each nephron within the complex structure of the mature kidney. CAKUT can be mostly isolated affecting the kidney and urinary tract, or in about 35% of cases in combination with other organ malformations including cardiac, brain, vertebral and limb defects/malformations (Figure 1).3,4
Figure 1.
Summary of CAKUT aspects.
Genetic findings include chromosomal imbalances as well as dysfunction of single genes with a broad genetic heterogeneity. However, in contrast with other heritable childhood onset kidney phenotypes (such as steroid resistant nephrotic syndrome, with high detection rates of genetic causes), less than 30% of CAKUT cases are genetically explained to date, even though dysfunction, disruption, or deletions in over 200 genes have been detected in patients with CAKUT. The rate of genetic diagnoses is higher for bilateral or syndromic CAKUT phenotypes compared with unilateral or isolated cases.5 The lack of genetic diagnoses suggest unidentified epigenetic or environmental factors.
de Fallois et al.6 now describe occurrence of CAKUT in up to 15% of patients with a diagnosis of Chung-Jansen-Syndrome (OMIM 617991). Chung-Jansen-Syndrome is a very rare complex neurodevelopmental disorder, resulting from dominant pathogenic genetic variants in the Pleckstrin Homology Domain Interaction protein (PHIP) gene. Although CAKUT manifestations had been previously reported in Chung-Jansen-Syndrome cases in the literature, PHIP had not been appreciated as a bona fide CAKUT gene. Underlying pathogenic mechanisms have not been studied for PHIP. Like many other genes in which genetic variants cause CAKUT, PHIP dysfunction seems to lead to a wide range of reno-urinary tract phenotypes, from horseshoe kidney to renal hypoplasia or vesicourethral reflux. de Fallois et al.6 show high PHIP expression in the renal cortex of the developing kidney as well as in tubular progenitor structures. Localization of human disease alleles within the N-terminus or the eight β-propeller-forming WD40 repeat, could potentially be impacting PHIP chromatin binding prior to DNA replication and resulting in genomic instability. To understand how this instability translates into the diverse CAKUT manifestations in affected individuals will require further study. As clinical care in CAKUT to date remains purely symptomatic, understanding the underlying molecular pathogenic mechanisms could provide potential therapeutic entry points, especially for renal hypoplasia or dysplasia.
Given that CAKUT phenotypes not leading to impaired kidney function may go undetected, CAKUT could be underdiagnosed both in the general population and among patients with syndrome related conditions. At the same time, given the vast genetic heterogeneity underlying CAKUT, single or gene panel based genetic tests may miss rare underlying genetic causes. Hence, as de Fallois et al.6 rightly state, exome- or genome-wide testing should be considered in CAKUT, especially after a negative gene panel-based test, which may reveal novel phenotype-genotype correlations. PHIP has not been previously reported in CAKUT exome studies and would not be on commonly used CAKUT gene panels. PHIP likely does not play a large causal role in isolated CAKUT. However, PHIP could account for some unsolved syndromic CAKUT cases, especially those that have not yet been analyzed using exome or genome sequencing.
Disclosure
All the authors declared no competing interests.
Funding Statement
Miriam Schmidts is supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) (project ID 431984000–SFB 1453, project ID 499552394–SFB 1597, project ID 503306912–FOR 5547 and Excellence Initiative Centre for Integrative Biological Signalling Studies (CIBSS) (EXC-2189) (project ID 390939984).
References
- 1.Chesnaye N., Bonthuis M., Schaefer F., et al. Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERA-EDTA registry. Pediatr Nephrol. 2014;29:2403–2410. doi: 10.1007/s00467-014-2884-6. [DOI] [PubMed] [Google Scholar]
- 2.Taguchi A., Kaku Y., Ohmori T., et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Sanna-Cherchi S., Caridi G., Weng P.L., et al. Genetic approaches to human renal agenesis/hypoplasia and dysplasia. Pediatr Nephrol. 2007;22:1675–1684. doi: 10.1007/s00467-007-0479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll C., Dott B., Alembik Y., Roth M.P. Associated nonurinary congenital anomalies among infants with congenital anomalies of kidney and urinary tract (CAKUT) Eur J Med Genet. 2014;57:322–328. doi: 10.1016/j.ejmg.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaou N., Renkema K.Y., Bongers E.M., Giles R.H., Knoers N.V. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol. 2015;11:720–731. doi: 10.1038/nrneph.2015.140. [DOI] [PubMed] [Google Scholar]
- 6.de Fallois J., Sieckmann T., Schönauer R., et al. Pathogenic PHIP variants are variably associated with CAKUT. Kidney Int Rep. 2024;9:2484–2497. doi: 10.1016/j.ekir.2024.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]