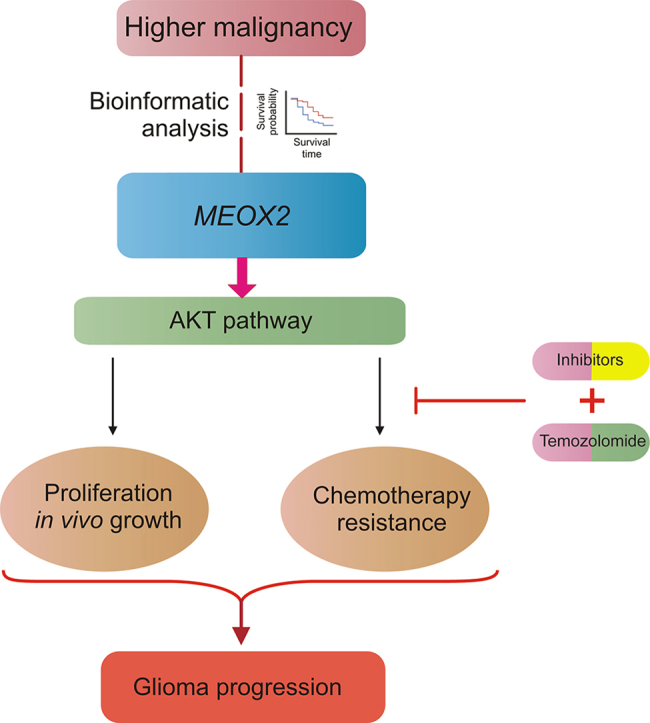
Graphical abstract
Highlights
-
•
MEOX2 correlates with glioma malignancy and poor prognosis.
-
•
MEOX2 promotes glioma growth and temozolomide chemoresistance.
-
•
MEOX2 drives glioma chemoresistance by activating AKT signaling.
-
•
MEOX2 serves as a therapeutic target for glioma chemotherapeutic intervention.
Glioblastoma (GBM) is a primary and fatal subtype of adult brain tumors. Despite standard treatments, including surgical resection and temozolomide (TMZ) chemotherapy, overall survival is only 16 months [1]. Profound genomic heterogeneity and altered transcriptional profiles drive chemoresistance, leading to tumor recurrence and a poor prognosis. Gain of chromosome 7 is a pivotal event in initiation and recurrence [2]. Our previous studies highlighted that engrailed 2 (EN2), a homeobox transcription factor at 7q36.3, is negatively correlated with glioma malignancy [3]. This led us to explore additional genes associated with GBM chemoresistance. At the 7p21.2 locus, mesenchyme homeobox 2 (MEOX2), another homeobox factor, has emerged as a candidate gene. Recent studies have yielded conflicting results regarding the influence of MEOX2 on glioma, underscoring the need to understand its intricate expression patterns and biological functions in GBM [4,5]. In this study, we comprehensively investigated the expression patterns and functions of MEOX2 in gliomas, particularly in terms of chemoresistance. Our findings reveal a positive correlation between MEOX2 expression and glioma malignancy. Increased MEOX2 expression is concomitant with accelerated glioma growth and decreased sensitivity to TMZ chemotherapy. Mechanistically, MEOX2 exerts oncogenic effects by modulating protein kinase B (AKT) activity, thereby orchestrating glioma chemoresistance.
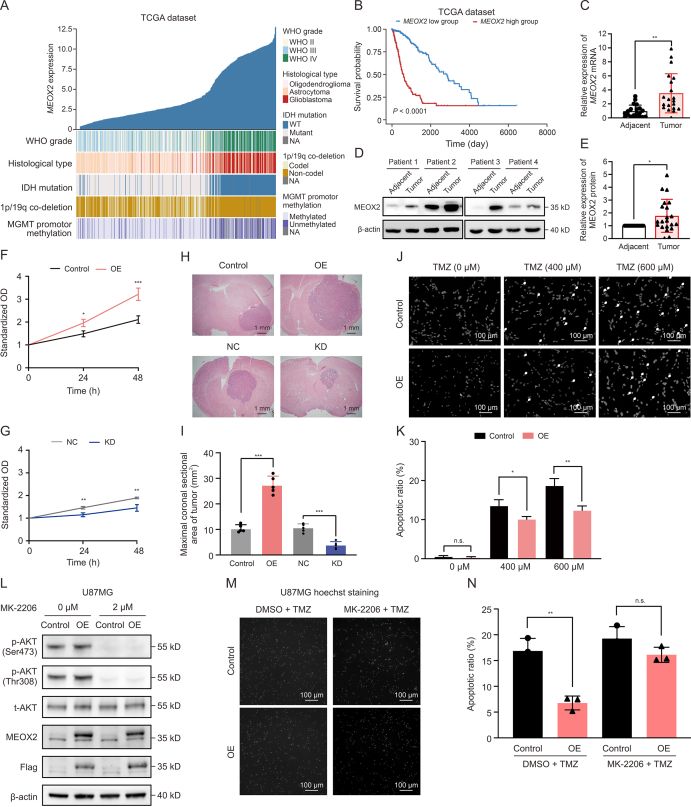
To evaluate the clinical significance of MEOX2 in gliomas, we analyzed its expression profile in The Cancer Genome Atlas (TCGA) glioma dataset. All the in silico and following experiment methods are shown in the Supplementary data. The results showed that MEOX2 expression increased in parallel with World Health Organization (WHO) grades and histological features (Figs. 1A, S1A, and S1B). Elevated MEOX2 expression was relevant to crucial molecular biomarkers, including wild-type isocitrate dehydrogenase 1 (IDH1), 1p/19q non-co-deletion, and methylguanine DNA methyltransferase (MGMT) promoter unmethylation, which are associated with high malignancy and poor prognosis (Fig. 1A). MEOX2 was significantly upregulated in GBM (WHO grade IV) in TCGA cohort (Fig. S1C). Moreover, MEOX2 expression was upregulated in wild-type IDH gliomas (Fig. S1D). Glioma patients with high MEOX2 expression exhibited significantly shorter overall survival (OS) than those with low MEOX2 expression (P < 0.0001) (Figs. 1B, S2A, and S2B). To validate the correlation between MEOX2 and glioma malignancy, we analyzed MEOX2 expression in the Chinese Glioma Genome Atlas (CGGA) dataset. Similarly, the expression pattern of MEOX2 in CGGA was consistent with that in TCGA (Figs. S2C and D). We then examined the MEOX2 expression profiles in the glioma cohort of our institution and found that the expression levels of MEOX2 were higher in tumor tissues than in paired adjacent tissues (Figs. 1C−E, S3A, and S3B), suggesting that MEOX2 expression is a predictive biomarker of glioma (area under the curve (AUC) = 0.840) (Fig. S3C).
Fig. 1.
Mesenchyme homeobox 2 (MEOX2) is positively associated with glioma malignancy, and regulates temozolomide (TMZ) chemoresistance. (A) Correlation between MEOX2 expression and glioma histological features and prognostic biomarkers in The Cancer Genome Atlas (TCGA) dataset (n = 681). (B) Kaplan-Meier survival analysis using clinical information from the TCGA dataset. Patients are divided into low and high MEOX2 groups by median expression level (n = 681). (C) Real-time quantitative polymerase chain reaction (RT-qPCR) analysis of messenger RNA (mRNA) expression in paired glioblastoma (GBM) tissues. Results are shown as mean ± standard deviation (SD) (n = 20). (D) Western blots of representative 4 pairs of GBM and adjacent tissues. (E) Quantifications of MEOX2 expression in 21 pairs of GBM and peritumor tissues (mean ± SD). (F, G) Cell Counting Kit-8 (CCK-8) assay showing that MEOX2 overexpression (OE) increased cell numbers compared with control (F), and MEOX2 knockdown (KD) decreased cell numbers in KNS89 cells (G) (n = 3). (H, I) Hematoxylin and eosin (H&E) staining of intracranial xenograft tumor in nude mice with KNS89 cells (H) and the maximal coronal sectional area of tumors (I) (n = 5 per group). (J, K) Image (J) and quantification (K) of Hoechst staining showing that MEOX2 OE decreased TMZ-induced apoptosis of KNS89 cells (400 or 600 μM, 48 h) (n = 3). (L) Western blots indicated that phosphorylation of protein kinase B (AKT) at both Ser473 and Thr308 were inhibited in MEOX2 OE/control cells by 2 μM MK-2206 in U87MG cells. (M, N) Hoechst staining (M) and quantification (N) of MEOX2 OE/control cells treated by combination of TMZ and vehicle (dimethyl sulfoxide (DMSO)) or TMZ and an AKT inhibitor MK-2206 showed that TMZ resistance effect of MEOX2 OE was antagonized by MK-2206 (n = 3). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.0001, based on paired Student's t-test. n.s.: not significant. WHO: World Health Organization; IDH: isocitrate dehydrogenase; MGMT: methylguanine DNA methyltransferase; WT: wildtype; NA: not available; NC: negative control; p-AKT: phospho-AKT; t-AKT: total AKT.
We investigated whether changes in MEOX2 expression affect the chemoresistance of glioma cells. Using a lentivirus-based strategy to manipulate MEOX2 expression in glioma cell lines (Figs. S4A−C), we found that MEOX2 overexpression (OE) increased cell growth, whereas MEOX2 knockdown (KD) decreased the growth of glioma cells (Figs. 1F, 1G, and S4D−G). Wound healing and Transwell assays further showed that MEOX2 enhanced the migration/invasion of glioma cells (Fig. S5). Next, we examined whether MEOX2 regulated glioma growth in vivo, by transplanting MEOX2-OE and KD cells into the cortex of immunocompromised nude mice. As anticipated, MEOX2 OE significantly increased tumor progression, as indicated by the maximal coronal sectional area of the tumor and the Ki67 index. Reciprocally, MEOX2 KD decreased the growth of glioma xenografts (Figs. 1H, 1I, and S6).
We determined whether MEOX2 was involved in cell death/survival under basal and chemotherapeutic conditions. MEOX2 OE decreased the apoptotic rate of glioma cells by TMZ treatment (400 and 600 μM) indicated by Hoechst staining. Conversely, MEOX2 KD increased the tendency of TMZ-induced cell death (particularly by 600 μM TMZ) (Figs. 1J, K, and S7).
Next, we investigated the molecular mechanisms by which MEOX2 promotes glioma malignancy using transcriptomics of glioma cells infected with lentiviruses expressing either MEOX2 OE or KD. The results showed that AKT signaling was tightly regulated by MEOX2 (Fig. S8). Ectopic activation of AKT signaling occurs in various cancer types, including gliomas, and confers tumor growth and chemoresistance. Therefore, we treated MEOX2 OE/control cells with the AKT inhibitor, MK-2206, whereas MEOX2 KD/negative control (NC) cells were treated with the AKT agonist, SC-79. Results indicated that phosphorylation of AKT kinase at both Ser473 and Thr308 was inhibited in MEOX2 OE/control cells by MK-2206 (2 μM) and overactivated in MEOX2 KD/NC cells by SC-79 (5 μM) in U87MG and KNS89 cells, respectively (Figs. 1L and S9). MK-2206 abolished the proliferation, migration, and invasion of MEOX2 OE cells, whereas SC-79 restored the reduced proliferation, migration, and invasion of MEOX2 KD cells (Fig. S10). Similar effects were observed in KNS89 cells (Fig. S11). Hoechst staining showed that the TMZ resistance effect of MEOX2 OE was reversed by MK-2206 (Figs. 1M, 1N, and S12), whereas the pro-apoptotic effect of MEOX2 KD was counteracted by SC-79 (Figs. S12 and S13). These results indicated that MEOX2 enhanced glioma resistance to TMZ by activating AKT signaling.
Despite fundamental and clinical advancements in glioma treatment, glioma chemotherapy has improved slightly over the past few decades. One of the leading reasons for this is molecular heterogeneity within tumors, which contributes to chemoresistance in gliomas. Therefore, the development of precise therapeutic approaches to overcome chemotherapeutic resistance remains a pressing question. In this study, we used bioinformatics and experimental approaches to demonstrate that MEOX2 is a pro-tumor gene that enhances glioma growth and chemoresistance. We propose that MEOX2 is linked to tumor malignancy and acts as a novel prognostic predictor in glioma and that MEOX2 may promote glioma growth and TMZ resistance.
In conclusion, our study demonstrates a strong association between MEOX2 and glioma malignancy, indicating that MEOX2 positively regulates glioma growth and chemosensitivity through AKT signaling. Our findings not only provide novel insights into the functional roles of MEOX2 in glioma but also offer a promising target for future chemotherapeutic strategies.
Ethics statement
This work was approved by the Institutional Review Board of West China Hospital of Sichuan University, China. All patients provided written informed consent. The animal work was done in accordance with the Animal Care and Use Committee guidelines of West China Hospital, Sichuan University, China (Approval No.: 2021786-A).
CRediT author statement
Tengfei Li: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Funding acquisition, Writing - Original draft preparation; Kaijun Sun and Wanchun Yang: Data curation, Formal analysis, Investigation; Meiling Zhang: Formal analysis; Wentao Feng, Siliang Chen, Mingrong Zuo, and Qiuyun Yuan: Data curation; Yanhui Liu and Mina Chen: Conceptualization, Funding acquisition, Writing - Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the grants from the Science and Technology Project, Technology Innovation Research and Development Project of Chengdu (Grant No.: 2022-YF05-01456-SN), Post-Doctor Research Project, West China Hospital, Sichuan University, China (Grant No.: 2021HXBH010), and the Natural Science Foundation of Sichuan Province of China (Grant No.: 2022NSFSC1428).
Footnotes
This manuscript is linked to a preprint (https://doi.org/10.21203/rs.3.rs-1717700/v1).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.12.002.
Contributor Information
Yanhui Liu, Email: liuyh@scu.edu.cn.
Mina Chen, Email: chenmina2010@scu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Rouse C., Gittleman H., Ostrom Q.T., et al. Years of potential life lost for brain and CNS tumors relative to other cancers in adults in the United States, 2010. Neuro. Oncol. 2016;18:70–77. doi: 10.1093/neuonc/nov249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis D.N., Perry A., Wesseling P., et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro. Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li T., Yang W., Li M., et al. Engrailed 2 (EN2) acts as a glioma suppressor by inhibiting tumor proliferation/invasion and enhancing sensitivity to temozolomide. Cancer Cell Int. 2020;20:65. doi: 10.1186/s12935-020-1145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J., Chen Y., Wang Q., et al. MEOX2-mediated regulation of Cathepsin S promotes cell proliferation and motility in glioma. Cell Death Dis. 2022;13:360. doi: 10.1038/s41419-022-04845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schönrock A., Heinzelmann E., Steffl B., et al. MEOX2 homeobox gene promotes growth of malignant gliomas. Neuro. Oncol. 2022;24:1911–1924. doi: 10.1093/neuonc/noac110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.