Abstract
Background/objectives:
In the USA, diabetes disproportionately affects Hispanics/Latinx, continuing to contribute to health disparities. To address the diabetes epidemic, separate programs for pre-diabetes and diabetes are promoted nationwide. However, engagement by Hispanics/Latinx in either program is lagging. Recent evidence suggests that offering a single community health worker delivered intervention that includes both groups and allows family members to participate may be more effective and in harmony with Latino cultural values, especially if offered to Latino women (Latinas) who traditionally are in charge of food preparation. Our objective was to explore the results of an intervention delivered to low-income Latinas at various dysglycemic levels (diabetic and pre-diabetic).
Methods:
In this quasi-experimental mixed-methods cohort study we longitudinally assessed biometric outcomes and health behaviors among obese Latinas at risk for—and with—diabetes, participating in the same intervention. Data were collected at baseline and 3 months post-intervention. Focus group discussions and interviews provided qualitative data to help contextualize findings.
Results:
Participants at different levels of the dysglycemic spectrum benefited equally from the intervention across most measures. Among participants whose relatives had diabetes, weight loss exceeded that of participants without diagnosed relatives. Domestic partners’ support, attending the program in a group setting, and previous diagnoses from a healthcare professional were associated with better results.
Conclusions:
Our findings indicate that a community health worker-delivered intervention for Hispanics/Latinx with—and at-risk for—diabetes is feasible and could be more effective in reducing Hispanics/Latinx’ diabetes burden. Health educators and clinicians should consider tapping into the collective nature of the Latinx/Hispanic culture to encourage healthy behaviors among individuals whose family members have diabetes, regardless of their dysglycemic status. We recommend replicating this study with a more rigorous randomized design, a larger number of participants and longer-term follow-up.
Keywords: Latinx/Hispanics, familismo, (pre)diabetes, community health workers, mixed methods design
Plain Language Summary
Encouraging similar habits around eating for all family members - whether at risk of or with diabetes - is in keeping with one of the key latino values: putting family first. Preliminary results point to more success for everyone when a “family first” strategy - instead of promoting individual treatment plans - is applied among latinas with diabetes or at risk of developing diabetes
In the USA, Latinos are among the groups with the highest rates of obesity and diabetes. People with obesity often have diabetes too. We know that, when a person in the family has diabetes, almost always other family members will be on the path to developing diabetes, if they do not yet have it. We also know that exercise and healthy eating habits can help prevent and control diabetes. In the USA, instead of offering the entire family (those on their way to diabetes and those with diabetes) the same program - “eat healthier and exercise” - there are separate programs or options based on each diagnosis: a program for those with diabetes, a program for those who qualify as having prediabetes, and no program for those who do not qualify but are on their way to having pre-diabetes due to obesity. Offered programs have not been successful among Latinos for several reasons in part because they just don’t attend as many classes as other groups. For Latinos, family - and specially having mealtimes together - is extremely important (“familismo” concept). This is even more critical for women preparing meals for their families. Separate diets and mealtimes goes against, and may sometimes interfere with, that sense of unity. Methods: In this study, participants were Latino women (Latinas) from Southern California who either had diabetes or were on the path to having diabetes because of their weight or lab results. They all enrolled in a program where community health workers encouraged them to eat better and to exercise. We checked weight, labs and behaviors before and after the program and interviewed some after they completed it. Results: all benefited from the program. But those whose relatives had diabetes, those whose doctors told them they had prediabetes/diabetes, and those attending in groups did better. Conclusion: using the proposed family-based approach among Latinos may lead to better diabetes prevention and management in clinical settings.
The diabetes epidemic is on the rise worldwide affecting more than half a billion adults (IDF). 1 Of these, more than 37 million live in the USA. Overall incidence (new cases) of diabetes has reportedly decreased but upward trends continue among minorities and low-income populations.2 -4 Hispanics/Latinx (H/L) as a group have one of the highest rates. Indeed, Latino women, who happen to have the lowest median income in the USA, are more likely to develop diabetes and to experience worse complications.5 -8
To address the diabetes epidemic, the Centers for Disease Control and Prevention established and promotes 2 different evidence-based programs: the National Diabetes Prevention Program (NDPP) for those at risk of diabetes, and the Diabetes Self-Management Education and Support (DSMES) program to help those with diabetes better manage their condition.9,10 Both programs are led by trained diabetes educators and promote behavior modification: healthy eating, regular physical activity and self-monitoring/self-management.
While diabetes is known to be inversely correlated with poverty and low literacy, and despite reported minimal success among low-income participants during the first decade of program implementation, updated qualification requirements for both programs continue to place low-income individuals at a disadvantage.11,12
In the USA, the NDPP—an adaptation of the Diabetes Prevention Program (a landmark study where 5% weight loss through lifestyle changes resulted in diabetes risk reduction) 13 —lasts 1 year and consists of 16 weekly classes offered online or at a community center or clinic, followed by monthly meetings. The goal is to lose at least 5% of one’s initial weight. To qualify, one must be overweight or obese with blood sugar levels indicating pre-diabetes. Another way to qualify is to be referred by a physician based on being “high risk” (having enough “points” based on age, gender, weight, having hypertension, and having a relative with diabetes). However, the qualifying threshold is higher for individuals on public assistance (Medicaid recipients). In addition, NDPP reimbursement—and therefore individual programs’ sustainability—is dependent on participants reaching the 5% weight loss goal, a threshold less likely to be reached by low-income participants.14 -16 As a result, the program is often not accessible to low-income H/L at risk of diabetes who do not meet the cut-off hemoglobin A1C—or the “high risk” threshold referral—making programs offered in communities with low-income populations not sustainable. Furthermore, Latinos who have attended the NDPP struggle to retain healthy behaviors after program completion.
As for the DSMES programs, they consist of 6 to 10 classes and must be offered in a clinical setting to be reimbursable. Goals are customized for each participant, and diabetes self-management—monitoring of glucose and feet, medication adherence and regular visits to healthcare providers—is emphasized. For individuals on public assistance (e.g. Medicare) to qualify, they must have been diagnosed with diabetes within the past year, and no more than 10 hours are reimbursed. 17
In both the NDPP and the DSMES programs, low-income participants tend to report more barriers to success.18 -20 Most of these are associated with social determinants of health (childcare, transportation, language, cost of food) including frustration that family and others in their friendship circle cannot attend due to program restrictions. On average, Hispanics/Latinos, especially Hispanic women (Latinas), tend to have lower income and less formal education, enroll and attend less, and—not surprisingly—are less likely to achieve the target weight loss goal when compared to non-Hispanic Whites (nHWs), especially if receiving public assistance.21 -24 With diabetes rates more than double among Hispanics since the dissemination of these programs, it is necessary to seriously consider alternative approaches.
Several core values of the Hispanic/Latino families have been described as having a strong impact on health behaviors: machismo (men needing to appear strong), fatalismo (the inevitability of certain life conditions), marianismo (the duty to sacrifice for others even at one’s own expense—an attribute of “virtual” Latino women), familismo (putting family before everything else). 25 More specifically, the last 2 relate more to Latinas. Of these, familismo has emerged as one of the most impactful regarding diabetes self-management among Hispanics/Latinos. 26
Familismo has been associated with family dynamics that impact food choices and preparation, social support, and motivation to adopt new behaviors, sharing of health information and decision-making relating to diet and treatments. Somewhat related to marianismo and familismo is the importance of women as those who prepare meals27,28 It would therefore seem important to capitalize on these core values when addressing a lifestyle-based intervention that requires changes in eating habits. Indeed, several studies among Hispanics/Latinos have suggested that core Latino values such as familismo be taken more into consideration in interventions and healthcare services for this group.25,29,30
Having a family member with diabetes, as well as being overweight or obese are known to be independent risk factors for diabetes, as is implied in the risk assessment of CDC for inclusion in the NDPP. 9 The more obese, the higher the risk for a later diagnosis of diabetes: 7.6% for those with class I obesity, 20.1% for individuals with class II obesity, 38.8% for those with class III obesity.31,32 Therefore, it seems reasonable to presume that overweight/obese Latinos who have a family member with diabetes could benefit from a prediabetes intervention, even in the absence of abnormal glycemic levels. Yet, based on the criteria for inclusion of covered programs they are often not able to attend any program.
We previously explored the feasibility of a CHW-led culturally adapted joint program for Latinos along the dysglycemic spectrum (at risk of/diagnosed with diabetes) as an alternative approach to diabetes prevention/self-management. In that intervention (brief summary included below), participants with prediabetes and those with diabetes had similar results and benefited equally from attending the same program. Results showed that physician referral and attending with family and friends were associated with better results. Based on these outcomes, we proposed a novel approach to the diabetes problem among Latinos: more of a family-based emphasis with physicians using the diabetes diagnosis of a Latino patient as a cue to refer family members to a single diabetes prevention (as opposed to enrollment into different interventions based on individual blood measurements cut-offs). 33
The purpose of this paper is to expand on our previous findings by exploring if study outcomes for Latinas at risk of developing diabetes (overweight/obese with or without prediabetes) and with diabetes enrolled in the same program present with significant changes across both groups when they attend a CHW-led program. Specifically, we explore if outcomes improved by a previous diagnosis of diabetes from a physician, their A1C at study enrollment, or when they had a family member with diabetes. We also explored the impact of social support and previous diagnosis (either pre-diabetes or diabetes) on outcomes.
Methods
Study participants and recruitment
Participants (N = 94) were mono and bilingual mostly low-income Latinas residing in the Inland Empire (Southern California), an area with one of the highest rates of diabetes-related mortality in the state.34,35 and home to one of the largest Hispanic/Latino populations in the USA.
Women were recruited via flyers posted throughout the community (schools, community centers, stores), through word of mouth, at health fairs and by personal invitation from the CHWs who facilitated the program.
To qualify for the intervention, program participants were required to be at least 18 years of age, overweight or obese (BMI) ⩾ 25 kg/m2) and self-identify as a Latino woman or “Latina.” Inclusion criteria for quantitative analyses included being a Latino woman and having attended at least 80% of the program—and willingness to answer the survey questions. Exclusion criteria included being pregnant or breastfeeding.
In-person classes included up to 30 individuals each and were held at churches or community centers. During the COVID-19 pandemic, participants were given the option to attend online classes (via zoom) which were smaller (no more than 12 participants per class).
Eligibility criteria for our contextual post program qualitative study included (1) having attended the intervention—to qualify for focus group discussions (FGDs); or (2) being a CHW who taught the intervention—to qualify for a key informant interview (KII). Focus group participants (N = 91) were recruited from all cohorts immediately following program completion. CHWs (N = 3) completed KIIs for triangulation reasons within 10 days of course completion.
Study procedures and data collection
This study was a 3 months mixed-method, non-equivalent, quasi-experimental design with dependent pre-test and 3 months’ post-test sample undertaken in the years 2021 to 2022. CHWs and research assistants recorded participants’ weight and height, body fat percentage, and measured waist circumference. Average blood glucose level over the past 3 months (A1C) was determined from a finger prick. Questionnaires—available in both English and Spanish—were printed or sent via Qualtrics and included basic demographic information, dietary questions, known pre-diabetes or diabetes status and whether participants had family members with diabetes.
For the qualitative data collection, 8 focus groups with participants, and 3 KIIs (with CHWs who taught the program), each lasting between 20 and 73 minutes, were conducted upon program completion in Spanish by 3 trained bilingual interviewers (researchers) at 2 community centers or via zoom. The semi-structured interview guides for the KIIs and FGDs were created based on Charmaz’s 36 grounded theory approach. Questions explored reasons for enrolling in the program, behavioral changes, and family dynamics. Probes were used to expand exploration and to help identify new issues. Concurrent analyses helped researchers determine that data gathered from the KIIs and FGDs had reached saturation.
Each participant provided active written informed consent in Spanish or English—based on their language preference—before data collection. A $10 gift certificate to a local store was given as an incentive for each data collection and for participation in the FGDs. Ethical approval for the study was obtained from the Loma Linda University Institutional Review Board (IRB #518068) in accordance with the principles of the Declaration of Helsinki.
Measures
Setting—whether the participant attended classes in person physically near others (group), or attended alone online from home (alone). This information was gathered from the CHWs field notes and records. Level of measurement: dichotomous.
Glycosylated hemoglobin (A1C)—Average blood glucose measured by collecting blood from a finger prick using Alere Afinion HbA1c (Axis-Shield, Oslo, Norway) assay tests and Alere Afinion AS100 Analyzer.
Anthropometric Measures
Weight (lbs) and height (inches) were measured with an InBody 270 scale (InBody USA, Cerritos, CA) and Seca stadiometer (Seca North America, Chino, CA). Participants were instructed to stand up without shoes, headwear, metal or heavy clothing. Level of measurement: continuous.
Body mass index (kg/m 2 ) was calculated using the following formula: weight × 703/(height × height). Level of measurement: continuous.
Waist measurement (inches). Using a measuring tape wrapped around the narrowest part of the participant’s abdomen (approximately 2 inches above the navel) a line was recorded where tape met and marked after participant exhaled. Level of measurement: continuous.
Body fat percentage (%) was read from the InBody 270 scale output and was recorded. Level of measurement: continuous.
Self-Reported Measures
Demographics: participants reported age (years), marital status (4 categories), children (6 categories), household size (5 categories), whether or not participant is main cook for household (no/yes), highest educational level either in the USA or in country of origin (7 categories), employment status (merged and reported as no/yes), annual family income (4 categories), birth country (responses merged into 3 categories—USA, Mexico or Central/South America), years living in the USA and acculturation (using the validated Short Acculturation Scale for Hispanics). 37
Food insecurity was determined using a validated scale. 38 Category was determined based on participants’ level of agreement with the following statement “Within the past 12 months—we worried whether our food would run out before we got money to get more”—and—“The food we bought just didn’t last, and we didn’t have money to get more.” Possible responses were “never true” (0), “sometimes true” (1), and “often true” (2). This scale has been translated and used to assess food insecurity among Latinos. 39
Consumption of sugar-sweetened beverages. After identifying beverages on a flipchart with pictures and names of locally available sugar-sweetened beverages, participants self-reported the quantity and type of beverages consumed in response to the question “Over the last 2 weeks, how many times did you drink the following?” Answer choices used a 6-point Likert-type scale where 1 is “never” and 6 is “several times a day.”
Consumption of unhealthy food. After identifying food items on a flipchart depicting pictures and names of locally available unhealthy foods, participants self-reported the quantity and type of food consumed in response to the question “Over the last 2 weeks, how many times did you eat the following?” using a 6-point Likert-type scale where 1 is “never” and 6 is “several times a day.”
Pre-diabetes diagnosis. Based on the participant’s response to the question: “Do you currently have any of the following?” Check all that apply (prediabetes, high blood pressure, high cholesterol levels).
Diabetes diagnosis. Upon enrollment, the participant was asked whether or not she had been told by a healthcare provider that she had diabetes. The answer was recorded on the enrollment sheet (No/yes).
Family member with diabetes (FMWD) was assessed based on the response to the question: “Do any of your relatives have diabetes?” (No/yes).
Perceived partner support for healthy eating. Based on the response to “If you have a spouse or partner, which one of the following behaviors best describes the way he/she behaves toward you when it comes to weight loss/control?” Options included: “Discourages me from eating healthy” (−1), “Neither discourages nor encourages me” (0), “Encourages me to eat healthy” (1) or “This doesn’t apply to me; I don’t have a spouse or partner at this time.” If participant chose the latter answer indicating she had no spouse or partner, the answer was labeled as “missing.” This measure was developed by our team and has not been validated.
Perceived partner support for physical activity. Based on the response to “If you have a spouse or partner, which one of the following behaviors best describes the way he/she behaves toward you when it comes to exercising?” Options included: “Discourages me from exercising” (−1), “Neither discourages nor encourages me to walk or exercise” (0), “Encourages me to exercise or to walk” (1), or “This doesn’t apply to me; I don’t have a spouse or partner at this time.” If a participant had no spouse or partner, the answer was labeled as missing.
Data Analyses
Quantitative data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 27. The primary outcomes were changes in hemoglobin A1C, weight, body fat percentage and waist circumference, whereas secondary outcomes were health behaviors including less consumption of unhealthy foods and sugar-sweetened beverages. Chi-square tests were used to assess baseline and 3-months differences between groups. To evaluate biometric measurements, an independent t (or Mann-Whitney) test was performed. Pre-post changes were assessed using sample paired t (or Wilcoxon) tests. Before performing analyses, data were inspected for inconsistencies and outliers.
A priori power analyses were conducted using G*Power 40 to determine the minimum sample size needed to detect within-group pre-post differences with small to medium effect sizes and a significance criterion of α = .05. To achieve 80% power, the minimum sample size needed was N = 75. Thus, the analyses for our primary study outcomes had sufficient power.
Key informant interviews and FGDs were audio-taped and transcribed verbatim by bilingual CHWs and research assistants after ensuring participants’ permission. The transcripts were then analyzed for emergent themes supported by critical quotes using the 2017 version of computer software program MaxQDA® (v.12, Berlin, GDR) 41 to code them. An a priori code book was used but coding was intentionally conducted to allow new codes to emerge. The codebook was developed by 3 research assistants and expanded later as emergent themes were identified and organized. All analyses were conducted in Spanish, and relevant quotes were translated to English before inclusion in this manuscript.
The Intervention
The content of our CHW-led intervention and of the NDPP are similar in program targets in that they both emphasize exercise, healthy eating—including tips for shopping, cooking, and eating out, redirected thinking, stress management, heart health, and garnering social support for healthy behaviors. However, our intervention differs from the NDPP by the intentional use of CHWs with similar backgrounds, and also by deliberately simplifying the material especially when it comes to the reading of food labels and of participant’s understanding of food categories. In addition, participants were not asked to keep track of their physical activity or weight at home as we helped them monitor their weight during program days (many participants did not have a scale). Finally, we used active adult education approaches and intentionally aligned all materials and presentations with participants’ Latino culture including using colors, acrostics and known Latino sayings throughout the program to emphasize and help cement key concepts in a way that validates the culture. Furthermore, diabetes complications and how to navigate the healthcare system were emphasized. Moreover, a subset of participants was referred by a physician.
Results
Quantitative results
Baseline characteristics of participants are in Table 1 below.
Table 1.
Baseline population characteristics.
| Measures | Overall group | No relative with DM a | Yes, relative with DM a | ||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) or N (%) | n | Mean (SD) or N (%) | n | Mean (SD) or N (%) | P-value | |
| Demographic | |||||||
| Age | 136 | 50.27 (12.02) | 56 | 50.34 (12.30) | 80 | 50.23 (11.90) | .96 |
| Marital status | 136 | 56 | 80 | .61 | |||
| Single | 11 (8.1) | 5 (8.9) | 6 (7.5) | ||||
| Married/With partner | 102 (75) | 40 (71.4) | 62 (77.5) | ||||
| Divorced/separated | 21 (15.4) | 9 (16.1) | 12 (15.0) | ||||
| Widow | 2 (1.5) | 2 (3.6) | 0 (0) | ||||
| Children | 136 | 56 | 80 | .42 | |||
| 0 | 7 (5.) | 3 (5.4) | 4 (5.0) | ||||
| 1 | 9 (6.6) | 5 (8.9) | 4 (5.0) | ||||
| 2 | 39 (28.7) | 14 (25.0) | 25 (31.3) | ||||
| 3 | 42 (30.9) | 13 (23.2) | 29 (36.3) | ||||
| 4 | 23 (16.9) | 13 (23.2) | 10 (12.5) | ||||
| 5 | 16 (11.8) | 8 (14.3) | 8 (10.0) | ||||
| Educational level | 135 | 55 | 80 | .66 | |||
| No formal education | 4 (3) | 2 (3.6) | 2 (2.5) | ||||
| Elementary | 29 (21.5) | 14 (25.5) | 15 (18.8) | ||||
| Secondary | 29 (21.5) | 11 (20) | 18 (22.5) | ||||
| High school | 24 (17.8) | 6 (10.9) | 18 (22.5) | ||||
| Vocational or some College | 17 (12.6) | 10 (18.2) | 7 (8.8) | ||||
| Some College | 18 (13.3) | 6 (10.9) | 12 (15) | ||||
| University | 14 (10.4) | 6 (10.9) | 8 (10) | ||||
| Employment status | 135 | 56 | 79 | .70 | |||
| Unemployed | 94 (69.6) | 40 (71.4) | 54 (68.4) | ||||
| Employed (self or for wages) | 41 (30.4) | 16 (28.6) | 25 (31.6) | ||||
| Health insurance | 133 | 55 | 78 | .77 | |||
| None | 53 (39.8) | 22 (40.0) | 31 (39.7) | ||||
| Federal/government | 55 (41.4) | 23 (41.9) | 32 (41.1) | ||||
| Private | 25 (18.8) | 10 (18.2) | 15 (19.2) | ||||
| Birth country | 136 | 56 | 80 | .73 | |||
| USA | 9 (6.6) | 3 (5.4) | 6 (7.5) | ||||
| Mexico | 120 (88.2) | 50 (89.3) | 70 (87.5) | ||||
| Central or South America | 7 (5.1) | 3 (5.4) | 4 (5.0) | ||||
| Acculturation | 134 | 56 | 1.67 (0.87) | 78 | 1.69 (0.87) | .91 | |
| Low | 121 (90.3) | 50 (89.3) | 71 (91.0) | ||||
| High | 13 (9.7) | 6 (10.7) | 7 (9.0) | ||||
| Annual family income | 102 | 45 | 57 | .77 | |||
| Less than $21 000 | 38 (37.3) | 17 (37.8) | 21 (36.8) | ||||
| Between $20 000 and $49 999 | 52 (51) | 21 (46.7) | 31 (54.4) | ||||
| Between $50 000 and $75 000 | 8 (7.8) | 5 (11.1) | 3 (5.3) | ||||
| More than $75 000 | 4 (3.9) | 2 (4.4) | 2 (3.5) | ||||
| Food insecurity | 136 | 56 | 80 | .06 | |||
| Yes, food insecure | 54 (39.7) | 17 (30.4) | 37 (46.3) | ||||
| Not food insecure | 82 (60.3) | 39 (69.6) | 43 (53.8) | ||||
| Biometric | |||||||
| Hemoglobin A1C | 134 | 5.9 (1.22) | 55 | 5.58 (0.70) | 79 | 6.13 (1.44) | .004** |
| Body mass index (BMI) | 136 | 32.39 (5.07) | 56 | 32.77 (4.81) | 80 | 32.13 (5.26) | .47 |
| Body fat (percentage) | 136 | 44.70 (5.71) | 56 | 44.37 (6.25) | 79 | 44.86 (5.33) | .62 |
| Waist circumference (inches) | 129 | 39.43 (4.40) | 53 | 39.94 (4.22) | 76 | 39.08 (4.52) | .28 |
| Health behaviors | |||||||
| Consumption of sugar-sweetened beverages | 134 | 56 | 78 | .33 | |||
| Never | 31 (23.1) | 11 (19.6) | 20 (25.6) | ||||
| Once a week | 44 (32.8) | 20 (35.7) | 24 (30.8) | ||||
| 2-3 times/week | 39(29.1) | 12 (21.4) | 27 (34.6) | ||||
| Almost every day | 11 (8.2) | 9 (16.1) | 2 (2.6) | ||||
| Every day | 8 (6.0) | 3 (5.4) | 5 (6.4) | ||||
| Several times a day | 1 (0.7) | 1 (1.8) | 0 (0) | ||||
| Consumption of unhealthy foods (times/week) | 134 | 56 | 78 | .34 | |||
| Never | 16 (11.9) | 6 (10.7) | 10 (12.8) | ||||
| Once a week | 50 (37.3) | 24 (42.9) | 26 (33.3) | ||||
| 2-3 times/week | 51 (38.1) | 22 (39.3) | 29 (37.2) | ||||
| Almost every day | 10 (7.5) | 2 (3.6) | 8 (10.3) | ||||
| Every day | 5 (3.7) | 1 (1.8) | 4 (5.1) | ||||
| Several times a day | 2 (1.5) | 1 (1.8) | 1 (1.3) | ||||
DM: diabetes mellitus.
Significant differences between “no relative with DM” and “yes, relative with DM” groups, P < .005.
All participants were female and mean age was 50.27 (±12.02) years. The majority (75%) lived with a spouse or partner, had 2 to 4 children (77%) and were unemployed (70%). Formal education was relatively low (46% had not completed high school and only 10% had a college degree). Nearly half (40%) reported being food insecure and having no health insurance. Of those who were aware of their family’s annual income, 88% reported family income of less than $50 000, which is considered low income in this region. 42 Lastly, most (88%) were born in Mexico and 90% had a low acculturation level despite having lived in the USA for several years. Not reported on the table, all participants except for 3, were the main cooks at home and 64% of households included at least 4 individuals. Baseline glycosylated hemoglobin was positively correlated with waist circumference (rs = 0.25, P = .005).
When comparing those who reported having relatives with diabetes (FMWD) and those who had no relatives with diabetes (no FMWD), there were no baseline group differences except in that glycosylated hemoglobin (A1C) was higher (6.13% ± 1.44% vs 5.58% ± 0.70%) among those reporting FMWD (P = .004). Women who did not cook at home all belonged to the latter group. Of note, those who did not know about their diagnosis before enrolling into the program had a lower mean A1C both at baseline (P = .002) and at the end of the intervention (P = .02).
After analyzing overall pre-post weight change overall, the authors explored outcomes based on having family members with diabetes, being previously diagnosed by a physician with either pre-diabetes or diabetes and based on participants’ baseline A1C, biometric measurements and behaviors. Results are reported in Table 2 below.
Table 2.
Pre-post differences between baseline and 3 months by participant characteristic and study outcome.
| Primary outcomes | ||||
|---|---|---|---|---|
| Biometric measurements | N | Baseline mean (SD) | 3-Months mean (SD) | P-value |
| Hemoglobin A1C (only if ≥5.7%) | 36 | 6.82 (1.46) | 6.72 (1.74) | .59 |
| Family member with diabetes | ||||
| No family member with diabetes | 10 | 6.37 (1.27) | 6.49 (1.71) | .48 |
| Yes, family member with diabetes | 26 | 7.00 (1.52) | 6.81(1.77) | .46 |
| Previous knowledge of diagnosis | ||||
| Did not know about diagnosis before | 14 | 6.03 (0.65) | 6.00 (0.93) | .79 |
| Yes, previously knew about diagnosis | 22 | 7.33 (1.62) | 7.18 (1.98) | .63 |
| Diagnosis category based on baseline A1C | ||||
| No prediabetes, no diabetes (less than 5.7%) | — | —— | ——– | ——– |
| Pre-diabetes (between 5.7% and 6.4%) | 22 | 5.84 (0.18) | 5.73 (0.27) | .02* |
| Diabetes (more than 6.5%) | 14 | 8.37 (1.22) | 8.28 (1.94) | .85 |
| Weight (BMI ⩾ 25 kg/m2) | 95 | 171.99 (30.91) | 169.19 (30.72) | <.001** |
| Family member with diabetes | ||||
| No family member with diabetes | 37 | 168.19 (24.34) | 166.68 (24.86) | .006* |
| Yes, family member with diabetes | 58 | 174.41(34.45) | 170.79 (34.05) | <.001** |
| Previous knowledge of diagnosis | ||||
| Did not know about diagnosis before | 65 | 170.35 (31.07) | 167.63 (31.07) | <.001** |
| Yes, previously knew about diagnosis | 30 | 175.55 (30.78) | 172.56 (30.18) | <.001** |
| Diagnostic category based on baseline A1C | ||||
| No prediabetes, no diabetes (less than 5.7%) | 59 | 172.55 (32.53) | 169.28 (32.82) | <.001** |
| Pre-diabetes (between 5.7% and 6.4%) | 21 | 163.47 (20.97) | 161.18 (20.07) | .03* |
| Diabetes (more than 6.5%) | 14 | 183.79 (34.87) | 182.21 (32.94) | .29 |
| Body fat % (only if >31%) | 92 | 45.18 (4.81) | 44.31 (5.06) | <.001** |
| Family member with diabetes | ||||
| No family member with diabetes | 36 | 44.84 (4.27) | 44.56 (4.41) | .24 |
| Yes, family member with diabetes | 56 | 45.41 (5.15) | 44.16 (5.47) | <.001** |
| Previous knowledge of diagnosis | ||||
| Did not know about diagnosis before | 63 | 45.05 (4.90) | 44.34 (4.99) | <.001** |
| Yes, previously knew about diagnosis | 29 | 45.48 (4.69) | 44.25 (5.30) | .02* |
| Diagnosis category based on baseline A1C | ||||
| No prediabetes, no diabetes (less than 5.7%) | 58 | 45.02 (4.75) | 44.19 (5.01) | <.001** |
| Pre-diabetes (between 5.7% and 6.4%) | 21 | 45.47 (5.48) | 44.49 (5.43) | .10 |
| Diabetes (more than 6.5%) | 13 | 45.46 (4.25) | 44.60 (5.06) | .29 |
| Waist circumference (only if >35 inches) | 71 | 40.65 (3.66) | 38.13 (4.12) | <.001** |
| Primary outcomes | ||||
| Biometric measurements | N | Baseline mean (SD) | 3-Months mean (SD) | P-value |
| Family member with diabetes | ||||
| No family member with diabetes | 28 | 40.74 (3.44) | 38.53 (3.62) | <.001** |
| Yes, family member with diabetes | 43 | 40.59 (3.87) | 38.87 (4.44) | <.001** |
| Previous knowledge of diagnosis | ||||
| Did not know about diagnosis before | 48 | 40.22 (3.67) | 37.76 (4.22) | <.001** |
| Yes, previously knew about diagnosis | 23 | 41.57 (3.62) | 38.90 (3.89) | <.001** |
| Diagnosis category based on baseline A1C | ||||
| No prediabetes, no diabetes (less than 5.7%) | 44 | 40.19 (3.55) | 37.77 (4.05) | <.001** |
| Pre-diabetes (between 5.7% and 6.4%) | 15 | 40.70 (3.33) | 37.96 (3.77) | .002** |
| Diabetes (more than 6.5%) | 12 | 42.5 (4.20) | 39.92 (4.74) | .003** |
| Secondary outcomes health behaviors | N | Baseline mean (SD) | 3-Months mean (SD) | P-value |
| Consumption of sugar-sweetened beverages | ||||
| Family member with diabetes | 96 | 1.50 (1.14) | 0.88 (0.86) | <.001** |
| No family member with diabetes | 36 | 1.72 (1.16) | 1.08 (1.03) | .001** |
| Yes, family member with diabetes | 60 | 1.37 (1.12) | 0.75 (0.73) | <.001** |
| Previous knowledge of diagnosis | ||||
| Did not know about diagnosis before | 67 | 1.37(1.09) | 0.84 (0.73) | <.001** |
| Yes, previously knew about diagnosis | 29 | 1.76 (1.24) | 0.97 (1.12) | <.001** |
| Diagnosis category based on baseline A1C | ||||
| No prediabetes, no diabetes (less than 5.7%) | 59 | 1.58 (1.12) | 0.97 (0.93) | <.001** |
| Pre-diabetes (between 5.7% and 6.4%) | 23 | 1.35 (1.19) | 0.87 (0.69) | .02* |
| Diabetes (more than 6.5%) | 13 | 1.54 (1.20) | 0.54 (0.78) | .002** |
| Consumption of unhealthy foods | 94 | 1.64 (1.08) | 1.14 (0.67) | <.001** |
| Family member with diabetes | ||||
| No family member with diabetes | 36 | 1.56 (1.03) | 1.19 (0.67) | .62 |
| Yes, family member with diabetes | 58 | 1.69 (1.11) | 1.10 (0.67) | <.001** |
| Consumption of unhealthy foods | ||||
| Previous knowledge of diagnosis | ||||
| Did not know about diagnosis before | 65 | 1.60 (1.09) | 1.15 (0.71) | .002* |
| Yes, previously knew about diagnosis | 29 | 1.72 (1.07) | 1.10 (0.56) | .004* |
| Diagnosis category based on baseline A1C | ||||
| No prediabetes, no diabetes (less than 5.7%) | 58 | 1.71 (1.08) | 1.14 (0.69) | <.001** |
| Pre-diabetes (between 5.7% and 6.4%) | 22 | 1.59 (1.26) | 1.32 (0.57) | .32 |
| Diabetes (more than 6.5%) | 13 | 1.54 (0.66) | 0.92 (0.64) | .005* |
Statistically significant differences between pre-test and post-test within group, P < .05.
Statistically significant differences between pre-test and post-test within group, P < .005.
Intervention effects—overall study sample. Overall, participants’ weight, consumption of sugar-sweetened beverages (SSB) and unhealthy foods were statistically significantly lower at 3 months (P = .000). Body fat percentage and waist circumference for those at higher risk (>31% and >35 inches, respectively) were also lower at 3 months (P = .000). However glycosylated hemoglobin improvement among those with abnormal baseline A1C (5.7%), only showed a non-statistically significant decrease (P = .59).
Intervention effects according to baseline diagnosis (A1C). Upon comparing weight loss between those at risk of diabetes due to overweight, those with pre-diabetes (glycosylated hemoglobin between 5.7% and 6.4%) and those with diabetes (glycosylated hemoglobin above 6.4%), the former group lost the most weight (3.27 ± 4.80 lbs, P = .000) followed by those with prediabetes (2.29 ± 4.35 lbs, P = .03), while those with diabetes only had a non-statistically significant weight loss of 1.58 ± 5.32 lbs (P = .29) at 3 months.
However, all 3 groups had a significant reduction in waist circumference: those within normal glycosylated hemoglobin lost 2.08 (±2.24) inches (P = .000), those with prediabetes, 2.13 (±2.64) inches (P = .002), and those with diabetes, 2.63 (±2.40) inches (P = .003). Regarding body fat percentage, only those with normal baseline glycosylated hemoglobin had a statistically significant loss (P = .000) whereas the trend toward less body fat was not statistically significant in the other 2 groups. All 3 groups reported a statistically significant reduction in consumption of sugar-sweetened beverages (0.61 ± 1.02, P = .000; 0.48 ± 0.95, P = .02 and 1.00 ± 0.91, P = .002, respectively), but only those with diabetes and those with normal glycosylated hemoglobin reported a statistically significant reduction in consumption of unhealthy foods (P = .005 and .000, respectively). Those with prediabetes had a non-statistically significant downward trend in unhealthy foods consumption (P = .32).
Lastly, among those with prediabetes, the reduction in glycosylated hemoglobin from 5.84 (±0.18)% to 5.73 (±0.27)% was statistically significant (P = .02), whereas only a trend was seen among participants with diabetes (from 8.37% ± 1.22% to 8.28% ± 1.94%, P = .85).
Intervention effects based on having a family member with diabetes. In both groups—those who have a family member with diabetes (FMWD) and those who had no family members with diabetes (no-FMWD)—waist circumference and weight were statistically significantly lower at 3 months (P = .000 and .006). However, these changes were more pronounced among the FMWD group (2.66 ± 2.48 inches and 3.62 ± 5.41 lbs less) than among those not reporting FMWD (2.22 ± 2.25 inches and 1.52 ± 3.14 lbs less). Mean percentage of weight lost was 0.95 (±1.88)% and 2.06 (±3.22)%, respectively and group differences were statistically significant (P = .04). Body fat loss was statistically significant among the FMWD group (1.25% ± 2.23%, P = .000) but not in the no-FMWD group (0.28% ± 1.40%, P = .24). Although changes among those with abnormal baseline glycosylated hemoglobin were not statistically significant, those in the no-FMWD group had a trend toward worsening (from 6.37 ± 1.27 to 6.49 ± 1.71, ns) whereas those with FMWD showed a trend toward improvement (from 7.00 ± 1.52 to 6.81 ± 1.77, ns).
When comparing dietary behaviors, both groups reduced their consumption of SSB (P = .000). These changes were slightly more among the no-FMWD (0.64 ± 1.10) than among the FMWD (0.62 ± 0.92). However, only the FMWD group had a statistically significant reduction in consumption of unhealthy foods (0.59 ± 1.06, P = .000). The reduction among the no-FMWD group was not statistically significant (0.36 ± 1.13, P = .62).
Intervention effects based on awareness of a previous physician diagnosis of prediabetes or diabetes. When looking at individuals who were aware of their diagnosis, whether prediabetes or diabetes, both groups had statistically significant improvements in biometric measurements and dietary patterns, but those with a previous diagnosis consistently showed more improvement than those who had not been diagnosed prior to enrollment: they lost 2.99 (±4.16) lbs less and 1.23 (±2.73)% body fat, and reduced their waist circumference by 2.67 (±1.91) inches (cf 2.72 ± 5.03 lbs weight loss, 0.70% ± 1.55% less body fat, 2.40 ± 2.59 inches less waist circumference). Also, consumption of sugar-sweetened beverages and unhealthy foods dropped more among those previously diagnosed (0.79 ± 0.90 and 0.55 ± 1.02, respectfully) than among those not previously diagnosed (0.62 ± 1.05 and 0.45 ± 1.10, respectfully). Among those with abnormal baseline glycosylated hemoglobin pre-post changes were not statistically significant but there was a trend toward improvement. Group differences remained statistically significant (P = .002 at baseline and P = .02 at 3 months).
Intervention effects based on perceived social support. Table 3 describes the effects of attending as part of a group and of perceived social support from domestic partner for healthy behaviors. Participants who felt that their domestic partners encouraged them to eat healthy or to engage in physical activity lost more weight (2.64 ± 5.61 lbs, P = .01 and 2.03 ± 4.24 lbs, P = .008 respectively) whereas those who felt that their partners discouraged healthy eating and physical activity experienced less weight loss (1.20 ± 4.62 lbs, P = .52 and 1.30 ± 4.94, P = .51, respectively). Not included in Table 3, those who felt that their partners were neutral toward their adoption of healthy eating and physical activity lost the most weight (3.02 ± 4.46, P = .000 and 3.28 ± 5.21, P = .000, respectively). Comparing participants who attended in a group setting (physically near each other) and those who attended alone via zoom, there were also differences. While they all had statistically significant weight loss, those attending in a group setting lost more weight compared to those who attended online alone (2.88±4.98lbs, P = .000 versus 2.37±3.21lbs, P = .02, respectively).
Table 3.
Pre-post group differences at baseline and at 3 months for weight by perceived partner support for healthy eating and physical activity and by intervention delivery modality (online versus group / in-person).
| PERCEIVED PARTNER SUPPORT | N | Baseline mean (SD) |
3 mo mean (SD) |
p value |
|---|---|---|---|---|
| Partner’s attitude toward healthy eating | 80 | |||
| Discourages healthy eating | 7 | 179.51 (20.03) | 178.31 (16.80) | .52 |
| Neither encourages nor discourages | 40 | 165.41 (27.90) | 162.39 (26.65) | <.001** |
| Encourages healthy eating | 33 | 178.50 (36.39) | 175.87 (36.66) | .01* |
| Partner’s attitude toward physical activity | 79 | |||
| Discourages physical activity | 7 | 162.04 (27.07) | 160.74 (24.33) | .51 |
| Neither encourages nor discourages | 37 | 170.92 (27.28) | 167.64 (26.60) | <.001** |
| Encourages physical activity | 35 | 175.00 (36.93) | 172.98 (36.85) | .008* |
| Setting in which participant attended | 95 | |||
| Attended in-person, in a group | 81 | 170.45 (26.81) | 167.59 (26.44) | <.001** |
| Attended online alone | 14 | 180.27 (49.49) | 177.90 (49.76) | .016* |
Statistically significant differences between pre-test and post-test within group, P <.05.
Statistically significant differences between pre-test and post-test within group, P <.005.
Qualitative results
FGDs were conducted with 91 participants, and the KIIs with 3 bilingual CHWs who led the program. Three themes emerged: (1) a diagnosis was a strong cue to enroll and to remain engaged in the program; (2) as primary meal preparers, women felt that maintaining family unity around mealtimes was paramount; (3) family members’ and friends’ support and engagement was an important source of motivation. Table 4 describes the themes in more details and includes supporting quotes for each of the themes.
Table 4.
Themes derived from focus group discussions.
| Themes | Quotes |
|---|---|
| 1. A diagnosis was a strong cue to enroll and remain engaged. | |
| Almost all participants enrolled because of a recent diagnosis or health problem – pre-diabetes, diabetes or other chronic disease – that led them to seek an intervention: “I went to the consulate. I took the exam, and the doctor told me I had fatty liver. I got scared that day.” “The main reason I signed up is that I was talking to a friend, and I told her that at the yearly physical exam the doctor told me that my cholesterol was very high, and I didn’t know what was happening. Then my friend mentioned the program to me and I signed up.” “I joined this group because it caught my attention because I am pre-diabetic” Having a diagnosis also served to motivate them to take more seriously the program: “Since I have diabetes, I became more conscientious about having to take care of myself.” “My motivation was that I had already gained a lot of weight. . .. besides that, my diabetes was out of control.” |
|
| 2. As primary meal preparers, women felt that maintaining family unity around mealtimes was paramount | |
|
“For me the most significant obstacle was my family who did not accept the changes, I was willing to do it more than anything for my health, but my problem was always that I wanted to have my family together.”
“My husband didn’t like vegetables and. . . I would sit down and eat differently from the others. We would eat at the same time, but they’d eat their different dishes. . . My husband has heart problems and when he saw me eating that way, he told me that his doctor had told him that it [what I was eating] was healthier. . . I told him I’m willing to cook whatever. . . Now we all get together and we sit together to eat at the dining table.” “When my mom comes to visit me, I can’t say no because she does it with so much love and everything and I don’t know how long I’m going to have my mom. . . and I have to eat in front of her; she sits down and I have to eat everything. . . and if she eats something, I have to eat it with her. It is difficult for me to separate that. . . when it comes to food.” Some suggested having a family program, to engage men and promote more family unity as they sought to improve their health: “I think if you make it [the program] for men, in my opinion it will be a hard sell. I think it’s easier to have a family program because men always theorize ‘you’re the one cooking, not me; you go!’ So it you make a family program then they’d be forced to attend.” |
|
| 3. Family members’ support and engagement was an important source of motivation | |
| Participants shared how family members motivated and helped them succeed: “When we go out with other people, my children speak up for me saying: my mom does not eat these anymore. And when I don’t feel like cooking, now they fix a salad and only a few quesadillas” “In my household, my children would say “you cook what you can eat and we’ll eat what you cook so we too can be healthy. . . everything I cook, they eat; and if I’m on a diet, then my entire household is too.” On the contrary, when asked about obstacles, several of the women - especially those who did not lose weight- attributed their lack of motivation and inability to succeed to lack of family support (for healthy behaviors): “I’d attend the classes and leave highly motivated. . . but once I got home it was not the same. I have children of all ages and they want to eat differently and I’m the only one eating alone and it’s not the same.” “I started to cook more vegetables and more fiber and put these on the table, at first my daughter didn’t want to eat them. . .that was a bit of discouragement for me.” “I would try to exercise a little but my spouse would tell me ‘why are exercising? You’re not fat’” |
|
Discussion
This study sought to explore if, for overweight and/or obese low-income Latinas, a CHW-led intervention could be successfully delivered to those at risk of diabetes and with diabetes. Biometric and behavior modifications were also assessed using cultural and contextual factors: whether or not participants had family members with diabetes and whether there was a previous physician diagnosis of prediabetes or diabetes. Furthermore, the impact of a formal diagnosis (prediabetes or diabetes) and perceived social support were explored.
The authors had previously shown the feasibility and relative success of a joint program for low-income Latinos on the dysglycemic spectrum 33 and had proposed that healthcare providers use diabetes diagnosis as a cue or motivator to encourage lifestyle changes among other family members regardless of their dysglycemic profile.
Participants, with diabetes and those who were at risk for diabetes, benefited from the intervention: participants improved their dietary habits (reduced consumption of sugar sweetened beverages and unhealthy foods), reduced abdominal fat and weight, factors that are known to reduce the risk of diabetes. More than half (53%) lost at least 2.2 lbs—a weight loss known to reduce diabetes and cardiovascular disease risk 43 —over the span of 3 months. A smaller percentage (10%) had 5% of initial weight loss, which is generally considered a clinically significant weight loss.44,45
When looking at our culturally aligned subcategories, having a family member with diabetes, having a previous physician diagnosis for prediabetes or diabetes, or even having glycosylated hemoglobin results shared at study baseline were associated with more weight loss and body fat loss, except among participants with diabetes.
In our previous study, participants with prediabetes and those with diabetes lost a comparable amount of weight. But in this study, participants with normal ranges of glycosylated hemoglobin lost more weight than those with pre-diabetes, while those with diabetes only had a trend toward weight loss. Similarly, only a trend toward lower glycosylated hemoglobin and body fat reduction were seen among those with diabetes.
However, waist circumference, a measure of central adiposity seemed to indicate that the program worked equally across all 3 groups. Waist circumference is considered a more reliable predictor of diabetes and—in the case of those with diabetes—an indicator of cardiovascular disease or diabetes kidney disease.46 -48
The lesser effect on glycosylated hemoglobin, weight and body fat percentage of those with diabetes may be due to several factors: it is well documented that weight loss is more difficult among individuals with diabetes for a variety of reasons and that, even when weight loss is present changes may not be reflected in the glycosylated hemoglobin among individuals with advanced diabetes.49,50 Furthermore, we cannot rule out the possibility that the use of obesogenic diabetes medications (insulin or some oral diabetes drugs) among some participants may have attenuated intervention effects. 51 Unfortunately, we were unable to control for the effect of these medications or for length of time with diabetes. Future research with longer follow-up periods could help identify whether or not these factors played a role in the results.
According to our findings, low-income overweight and obese Latinas with low to medium formal education whose relatives have diabetes benefit more from attending a lifestyle program regardless of their glycemic profile. The only instance in which those with FMWD did not seem to experience better results is with regards to reduction in consumption of sugar-sweetened beverages. However, this may be due to an already lower baseline consumption.
When comparing participants whose family members had diabetes with those who did not have family members with diabetes, those whose family members had diabetes (and are therefore at higher risk for diabetes) lost more abdominal fat and more than twice the weight (2.06% ± 3.22% vs 0.95% ± 1.88% of initial weight). This is despite starting out with higher baseline glycosylated hemoglobin, which is not surprising, since they are expected to be at higher risk by family association. 52
Indeed, a higher percentage of this FMWD group (13.8%) lost at least 5% of their weight, a clinically significant weight loss, and 60% experienced weight loss that leads to a diabetes risk reduction of at least 16% and at least 4% to 8% for cardiovascular disease53,54—compared to those without FMWD (2.7% and 41%, respectively). Weight loss among the former group (3.62 ± 5.41 lbs) was more than that reported in 2 studies among mostly Latinos living in Southern California: a 12-week study implemented among Latinos with diabetes (2.07 lbs mean weight loss) 55 and another NDPP study among mostly Latinos with prediabetes (2.15% ± 2.96% of initial weight loss at 16 weeks). 56 In keeping with the health belief model, having a member of the family with diabetes could be a powerful cue for overweight or obese (i.e., at risk) Latinas to benefit more from a preventive/self-management intervention, even without a physician encounter. This could avoid a delay in care, especially in view of the high rates of uninsured in this population, 57 and Latinos’ reluctance to seek medical care in the absence of acute symptoms, as is the case with pre-diabetes.
Both quantitative and qualitative results indicate that having previously been diagnosed with pre-diabetes or diabetes prior to enrollment into the program is associated with more motivation and better results as opposed to enrolling with no previous diagnosis. This was also seen in the differing trends in glycosylated hemoglobin between those previously diagnosed and those not diagnosed. Our previous study indicated a positive association between success and physician referral. The impact of both physician referral and physician clearly providing a diagnosis has been reported by others, especially among monolingual Spanish-speaking Latinos.58,59 The ramification is that it is imperative for healthcare providers to clearly articulate to patients a diagnosis as it can have an important role in motivating them to seek enrollment in/and to succeed in a lifestyle-based program, even if they do not “qualify” for a pre-diabetes diagnosis. It may be that defining patients’ “risk levels” on the dysglycemic spectrum—as is the case with the Finnish diabetes risk score (FINDRISC)—would accomplish a similar effect. 60
As has been demonstrated previously,61,62 social support for healthy behaviors is associated with better results, especially in less individualistic cultures: in our study those who perceived support for healthy behaviors from spouses tended to lose more weight (more than double the weight when the behavior in question was food consumption). In our qualitative analyses, participants who perceived more support expressed how much this helped them. On the contrary, several of those who experienced lack of support from their spouses and other family members benefited less from the program. Interestingly, those reporting a neutral attitude from spouses lost the most weight. It could be that some of the “positive” feedback may have backfired as it may have had the impact of “nagging,” a phenomenon reported in other studies.63,64
Lifestyle changes for diabetes prevention and self-management revolve primarily around food and physical activity, behaviors bound to impact family dynamics. Besides sharing how they benefited from the support of their domestic partners and social network, participants expressed how important preserving the family unit intact and cohesive at mealtime was for them. Having separate dietary habits and schedules within one family was viewed as undesirable and stressful. Implied is the concept that providing a distinct mealtime experience to one family member (presumably following medical advice) would not only be a burden for the meal preparer,65,66 more importantly it would disrupt the sense of family unity. This was so important that a participant suggested creating programs for families as a means of “selling” the new lifestyle changes to the men in the family. Other studies confirm the fact that familismo influences Latinas’ behaviors and affects their success in adopting healthy behaviors.61,67 -69 These could be the reason why individual-based programs have so little success among Latinos.
Figure 1 below illustrates the proposed familismo-compatible alternative.
Figure 1.
Depiction of current model of diabetes prevention and self-management and proposed “familismo” model.
This type of familismo-compatible approach would ensure that as many as possible family members engage in similar behaviors. One exception being, that for the person with diabetes, one could add supplemental diabetes self-management education (medications, self-monitoring). This approach would have the advantage of providing education to both those at risk and those with diabetes, in a way that builds on the core values of the Latino culture.
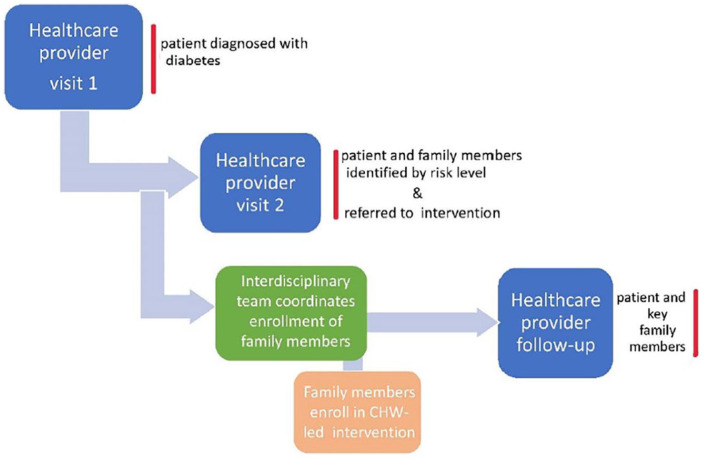
Extending the lifestyle portion of the “treatment” to the immediate family at risk by having one intervention for all offers a simpler, more feasible option without increasing the stress level—a factor known to worsens diabetes 70 - nor decreasing the much-needed social support that is so critical for Latinas. We suspect that such a familismo-compatible diabetes intervention would be financially and culturally more viable even in the larger households. 71 Patient-provider contact could also be most effective since the intervention would fit within cultural norms. Furthermore, with CHWs becoming a more integral part of clinical interdisciplinary teams, they too could help facilitate coordination and referrals to the appropriate programs (see Figure 2 below).
Figure 2.
Proposed familismo-compatible approach to diabetes prevention and self-management.
Lastly, our findings seem to indicate that—in addition to having a strong family motivation (someone diagnosed with diabetes) and working in collaboration with physicians, community-based programs such as this one hold great promise for the prevention of diabetes among Latinas.
Strengths and limitations
Several limitations of this study need to be acknowledged. Since this was a small sample size and participants were mostly women of Mexican descent with low acculturation, our findings cannot be generalized to all Latinos/Hispanics. Furthermore, although it was obvious from the focus group statements that the great majority of participants had financial challenges, we could not confirm participants’ income. Thus, applicability to other low-income Latinas may be limited though our high rate of self-reported food insecurity is a strong proxy for our participants’ low-income status. Also, we did not control for some of the key variables such as mental health, obesogenic medication use, access to healthy foods and time since diagnosis as our study was mostly exploratory and descriptive in nature and therefore had limited power to include these variables. Therefore, more research controlling for these variables may help to confirm whether or not our results remain valid. This program focused on social support from spouse and group attendance as proxy for social support. Therefore, the social support of other family members and friends, also critical to Latino culture may not have been comparable between all participants and should be explored in future studies.
One strength of this study is that we were able to compare individuals attending the same program taught by CHWs. Other strengths included having CHWs corroborating participants’ statements from the qualitative phase of the research. Lastly, because participants attended in a variety of settings (in person or online) our results occurred despite the usual lack of programing during COVID-19 pandemic era.
Conclusions and Implications for Practice
For low-income Latinas enrolled in a lifestyle-based intervention, maintaining family unity especially around mealtimes is of utmost importance and having family members diagnosed with diabetes is associated with better results among those on the dysglycemic spectrum. Furthermore, a previous diagnosis from a healthcare provider is associated with higher motivation and better outcomes.
Based on these findings, behavioral recommendations for Latinos on the dysglycemic spectrum should be promoted to occur within a unified family intervention. Policymakers should consider providing coverage to support familismo sensitive approaches and incentives to healthcare providers who adopt culturally compatible programs for low-income Latinos with a high risk for diabetes. Applying our recommendations on a larger scale among low-income Latinas may help confirm whether our findings are generalizable. This study expands the existing literature by providing alternative approaches to health educators and healthcare professionals seeking to reduce the diabetes burden among those most affected.
Supplemental Material
Supplemental material, sj-docx-1-end-10.1177_11795514241274696 for Making the Most of Familismo to Curb the Diabetes Epidemic: Early Evidence of Success Delivering the Same Intervention to Latinas at Risk for and With Diabetes by Maud Joachim-Célestin and Susanne B Montgomery in Clinical Medicine Insights: Endocrinology and Diabetes
Supplemental material, sj-docx-2-end-10.1177_11795514241274696 for Making the Most of Familismo to Curb the Diabetes Epidemic: Early Evidence of Success Delivering the Same Intervention to Latinas at Risk for and With Diabetes by Maud Joachim-Célestin and Susanne B Montgomery in Clinical Medicine Insights: Endocrinology and Diabetes
Acknowledgments
The authors would like to first thank community health workers Maria Elena Cháirez, Lizbeth Hernandez, Marisa Aguero, and Maggie Medina for their hard work and dedication. We are also grateful to San Manuel Gateway College Promotores Academy and to El Sol Neighborhood and Educational Center for their continuous support of this project and of the community health workers. Lastly, this program would not have been possible without the input and detailed work of research assistants Marisol Lara and Raveena Chara.
Additional Note: Both authors are submitting this original manuscript for first publication in this journal and the manuscript is not being considered for publication elsewhere nor has it been published anywhere else.
ORCID iD: Maud Joachim-Célestin  https://orcid.org/0000-0001-5424-4666
https://orcid.org/0000-0001-5424-4666
Supplemental Material: Supplemental material for this article is available online.
Declarations
Ethics Approval and Consent to Participate: This study was performed in line with the principles of the Declaration of Helsinki. All study procedures were approved by the Loma Linda University Institutional Review Board (IRB #518068) in accordance with the principles of the Declaration of Helsinki. Each participant provided active written informed consent in Spanish or English—based on their language preference—before data collection.
Consent for Publication: There was no need to obtain consent for publication as participant’s names or personal information were de-identified in this study.
Author contributions: Maud Joachim-Célestin: Conceptualization; Data curation; Formal analysis; Investigation; Project administration; Software; Supervision; Visualization; Writing—original draft; Writing—review & editing. Susanne B Montgomery: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—original draft; Writing—review & editing.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: a grant from the Ardmore Institute of Health [Grant # 2170480].
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of Data and Materials: The datasets generated during the current study are not publicly available, but de-identified data will be made available upon request where such requests are compliant with receipt of ethical approval from the sending and receiving hosts’ Institutional Ethics Review Boards.
References
- 1. International Diabetes Federation IDF Diabetes Atlas 10th Edition. International Diabetes Federation; 2021. [Google Scholar]
- 2. Centers for Disease Control and Prevention. Diabetes home. Data and statistics. Diabetes report card. State diabetes trends. Published May 15, 2024. Accessed June 30, 2024. https://www.cdc.gov/diabetes/library/reports/reportcard/national-state-diabetes-trends.htmland [Google Scholar]
- 3. Magliano DJ, Chen L, Islam RM, et al. Trends in the incidence of diagnosed diabetes: a multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol. 2021;9:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu C, He L, Li Y, et al. Diabetes risk among US adults with different socioeconomic status and behavioral lifestyles: evidence from the national health and nutrition examination survey. Front Public Health. 2023;11:1-12. 10.3389/fpubh.2023.1197947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. US Department of Labor. Women’s Bureau. Data and Statistics. Earnings. Median annual earnings by sex, race and Hispanic ethnicity (dol.gov).
- 6. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020; 36:e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karter AJ, Laiteerapong N, Chin MH, et al. Ethnic differences in geriatric conditions and diabetes complications among older, insured adults with diabetes: the diabetes and aging study. Aging Health. 2015;27:894-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marquez I, Calman N, Crump C. A framework for addressing diabetes-related disparities in US Latino populations. Community Health. 2019;44:412-422. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Diabetes. Referring Patients to DSMES. Atlanta, GA: Centers for Disease Control and Prevention, US. Department of Health and Human Services. Published May 15, 2024. 2020. Accessed June 30, 2024. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf [Google Scholar]
- 10. U.S. Health and Human Services. National Diabetes Prevention Program. What is the National DPP. Published May 15, 2024. Accessed June 30, 2024. https://www.cdc.gov/diabetes/professional-info/employers.html [Google Scholar]
- 11. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44:258-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ritchie ND, Sauder KA, Phimphasone-Brady P, Amura CR. Rethinking the national diabetes prevention program for low-income whites. Diabetes Care. 2018;41:e56-e57. [DOI] [PubMed] [Google Scholar]
- 13. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med. 2002;346:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delahanty LM, Trief PM, Cibula DA, Weinstock RS. Barriers to weight loss and physical activity, and coach approaches to addressing barriers, in a real-world adaptation of the DPP lifestyle intervention: a process analysis. Diabetes Educ. 2019;45:596-606. [DOI] [PubMed] [Google Scholar]
- 15. Ritchie ND, Gutiérrez-Raghunath S, Durfee MJ, Fischer H. Supplemental text message support with the national diabetes prevention program: pragmatic comparative effectiveness trial. JMIR Mhealth Uhealth. 2020;8:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Apolzan J, Venditti E, Edelstein S, et al. Long-term weight loss with metformin or lifestyle intervention in the diabetes prevention program outcomes study. Ann Intern Med. 2019;170:682-690. Erratum in: Ann Intern Med. 2020;173:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Formagini T, Brooks JV, Jacobson LT, Roberts AW. Reimbursement policies for diabetes prevention program (DPP): implications for racial and ethnic health disparities. Kans J Med. 2021;14:234-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aguayo-Mazzucato C, Diaque P, Hernandez S, et al. Understanding the growing epidemic of type 2 diabetes in the Hispanic population living in the United States. Diabetes Metab Res Rev. 2019;35:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delahanty LM, Peyrot M, Shrader PJ, et al. Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the diabetes prevention program (DPP). Diabetes Care. 2013;36:34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baucom KJW, Bauman T, Gutierrez Chavez M, et al. Barriers to participation and lifestyle change among lower versus higher income participants in the national diabetes prevention program: lifestyle coach perspectives. Transl Behav Med. 2022;12:860-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association. Economic costs of diabetes in the U.S. In 2017. Diabetes Care. 2018;41:917-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chambers EC, Rehm CD, Correra J, et al. Factors in placement and enrollment of primary care patients in YMCA’s diabetes prevention program, Bronx, New York, 2010–2015. Prev Chronic Dis. 2017;14:E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin AL, Warren JP, Lipman RD. The landscape for diabetes education: results of the 2012 AADE national diabetes education practice survey. Diabetes Educ. 2013;39:614-622. [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. The National Diabetes Prevention Program. Lifestyle change program provider resources. US Department of Health and Human Services. Published May 15, 2024. Accessed June 30, 2024. https://www.cdc.gov/diabetes/prevention/lcp-details.html [Google Scholar]
- 25. Perez-Brescia M, Beck CT, Alicea Planas J, et al. Famalismo Primero and Puerta Cerrada in self-managing diabetes among Hispanics: a qualitative meta-synthesis. J Transcult Nurs. 2022;33:666-674. [DOI] [PubMed] [Google Scholar]
- 26. Caballero AE. Understanding the Hispanic/Latino patient. Am J Med. 2011;124:S10-S15. [DOI] [PubMed] [Google Scholar]
- 27. Corona K, Campos B, Rook KS, Biegler K, Sorkin DH. Do cultural values have a role in health equity? A study of Latina mothers and daughters. Cultur Divers Ethnic Minor Psychol. 2019;25:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Villegas E, Hammons AJ, Wiley AR, Fiese BH, Teran-Garcia M. Cultural influences on family mealtime routines in Mexico: focus group study with Mexican mothers. Children. 2022;9:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marrero DG. An article in two parts: my Dinners with Richard and addressing diabetes disparities in Hispanic populations. Diabetes Spectr. 2022;35:252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vidal TM, Williams CA, Ramoutar UD, Haffizulla F. Type 2 diabetes mellitus in Latinx populations in the United States: a culturally relevant literature review. Cureus. 2022;14:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. New Engl J Med. 2001;345:790-797. [DOI] [PubMed] [Google Scholar]
- 32. Astrup A, Finer N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes Rev. 2000;1:57-59. [DOI] [PubMed] [Google Scholar]
- 33. Joachim-Célestin M, Gamboa-Maldonado T, Dos Santos H, Montgomery SB. Delivering the same intervention to Hispanic/Latinos with pre-diabetes and diabetes. Early evidence of success in a longitudinal mixed method study. Inquiry. 2021;58:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. U.S. Census Bureau. Quick Facts, San Bernardino County, California; Riverside County, California; California. U.S. Department of Commerce. Accessed July 1, 2024. https://www.census.gov/quickfacts/fact/table/sanbernardinocountycalifornia,riversidecountycalifornia,CA/PST045219 [Google Scholar]
- 35. San Bernardino County. Community indicators. Chronic disease. Accessed July 10, 2024. https://indicators.sbcounty.gov/wellness/chronic-disease/ [Google Scholar]
- 36. Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. Sage Publications; 2006. [Google Scholar]
- 37. Marin G, Sabogal F, Marin BV, Otero-Sabogal R, Perez-Stable EJ. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9:183-205. [Google Scholar]
- 38. Hager ER, Quigg AM, Black MM, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126:e26-e32. [DOI] [PubMed] [Google Scholar]
- 39. Nagao-Sato S, Druziako S, Baltaci A, et al. Differences in reporting food insecurity and factors associated with differences among Latino fathers and mothers. BMC Public Health. 2021;21:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149-1160. [DOI] [PubMed] [Google Scholar]
- 41. Verbi Software Consult Sozialforschung GmbH. MAXQDA plus [Computer Software]. Version 12. 2015. [Google Scholar]
- 42. State of California Business, Consumer and Housing Agency. California income limits. 2022. https://www.documentcloud.org/documents/23070404-california-income-limits-2022
- 43. Morris E, Jebb SA, Oke J, et al. Effect of weight loss on cardiometabolic risk: observational analysis of two randomised controlled trials of community weight-loss programmes. Br J Gen Pract. 2021;71:e312-e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity. 2015;23:2319-2120. [DOI] [PubMed] [Google Scholar]
- 45. Ross R. Is setting a criterion for ‘clinically significant weight loss’ necessary? Obesity. 2016;24:791. [DOI] [PubMed] [Google Scholar]
- 46. Wan H, Wang Y, Xiang Q, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol. 2020;19:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Franek E, Pais P, Basile J, et al. General versus central adiposity as risk factors for cardiovascular-related outcomes in a high-risk population with type 2 diabetes: a post hoc analysis of the REWIND trial. Cardiovasc Diabetol. 2023;22:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16:177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wing RR, Marcus MD, Epstein LH, Salata R. Type II diabetic subjects lose less weight than their overweight nondiabetic spouses. Diabetes Care. 1987;10:563-566. [DOI] [PubMed] [Google Scholar]
- 50. Franz MJ. The dilemma of weight loss in diabetes. Diabetes Spectr. 2007;20:133-136. [Google Scholar]
- 51. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Centers for Disease Control and Prevention. Diabetes. Diabetes risk factors. Published May 15, 2024. Accessed July 7, 2024. https://www.cdc.gov/diabetes/basics/risk-factors.html [Google Scholar]
- 53. Froom P, Goldbourt U. Secular decrease in blood pressure and reduction in mortality from cardiovascular disease in Israeli workers. J Hum Hypertens. 2004;18:113-118. [DOI] [PubMed] [Google Scholar]
- 54. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878-884. [DOI] [PubMed] [Google Scholar]
- 55. Metghalchi S, Rivera M, Beeson L, et al. Improved clinical outcomes using a culturally sensitive diabetes education program in a Hispanic population. Diabetes Educ. 2008;34:698-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. DeFosset AR, Sivashanmugam M, Mosst J, Kuo T. Clinic- and community-based national diabetes prevention programs in Los Angeles. Health Educ Behav. 2022;49:647-657. [DOI] [PubMed] [Google Scholar]
- 57. Health and Human Services Office of Minority Health. Profile: Hispanic/Latino Americans. US Department of Health and Human Services. Published February 24, 2023. Accessed August 25, 2023. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=64 [Google Scholar]
- 58. Ragsdale C, Wright J, Shokar G, Salaiz R, Shokar NK. Hispanic patient perspectives of the physician’s role in obesity management. J Clin Med Res. 2017;9:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Toney AM, Pineros-Leano M, Pérez-Flores NJ, Gomez D, Aguayo L. ‘It is in our hands-why wait until you are sick?’: perceptions about diabetes prevention of Latina mothers in Mexico and the United States. Diabet Med. 2023;40:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nieto-Martinez R, Barengo NC, Restrepo M, et al. Large scale application of the Finnish diabetes risk score in Latin American and Caribbean populations: a descriptive study. Front Endocrinol. 2023;14:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Joachim-Célestin M, Gamboa-Maldonado T, Dos Santos H, Montgomery SB. A qualitative study on the perspectives of Latinas enrolled in a diabetes prevention program: is the cost of prevention too high? J Prim Care Community Health. 2020;11:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen M, Yun Q, Lin H, et al. Factors related to diabetes self-management among patients with type 2 diabetes: A Chinese cross-sectional survey based on self-determination theory and social support theory. Patient Prefer Adherence. 2022;16:925-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sullivan KT, Pasch LA, Schreier M, Healy M. Responses to intimate partners’ attempts to change health behavior: the role of readiness. J Soc Pers Relat. 2018;35:1356-1380. [Google Scholar]
- 64. Kiernan M, Moore SD, Schoffman DE, et al. Social support for healthy behaviors: scale psychometrics and prediction of weight loss among women in a behavioral program. Obesity. 2012;20:756-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Walker EA, Weiss L, Gary-Webb TL, et al. Power up for health: pilot study outcomes of a diabetes prevention program for men from disadvantaged neighborhoods. Am J Mens Health. 2018;12:989-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Isasi CR, Parrinello CM, Jung MM, et al. Psychosocial stress is associated with obesity and diet quality in Hispanic/Latino adults. Ann Epidemiol. 2015;25:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Im EO, Lee B, Hwang H, et al. “A waste of Time”: Hispanic women’s attitudes toward physical activity. Women Health. 2010;50:563-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Austin JL, Smith JE, Gianini L, Campos-Melady M. Attitudinal familism predicts weight management adherence in Mexican-American women. J Behav Med. 2013;36:259-269. [DOI] [PubMed] [Google Scholar]
- 69. McLaughlin EA, Campos-Melady M, Smith JE, et al. The role of familism in weight loss treatment for Mexican American women. J Health Psychol. 2017;22:1510-1523. [DOI] [PubMed] [Google Scholar]
- 70. Hammons A, Olvera N, Teran-Garcia M, Villegas E, Fiese B. Mealtime resistance: Hispanic mothers’ perspectives on making healthy eating changes within the family. Appetite. 2021;159:1-6. [DOI] [PubMed] [Google Scholar]
- 71. Zhuo X, Zhang P, Barker L, et al. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes Care. 2014;37:2557-2564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-end-10.1177_11795514241274696 for Making the Most of Familismo to Curb the Diabetes Epidemic: Early Evidence of Success Delivering the Same Intervention to Latinas at Risk for and With Diabetes by Maud Joachim-Célestin and Susanne B Montgomery in Clinical Medicine Insights: Endocrinology and Diabetes
Supplemental material, sj-docx-2-end-10.1177_11795514241274696 for Making the Most of Familismo to Curb the Diabetes Epidemic: Early Evidence of Success Delivering the Same Intervention to Latinas at Risk for and With Diabetes by Maud Joachim-Célestin and Susanne B Montgomery in Clinical Medicine Insights: Endocrinology and Diabetes