Abstract
Background and Purpose:
Ultra-high dose-rate radiotherapy (FLASH) has been shown to mitigate normal tissue toxicities associated with conventional dose rate radiotherapy (CONV) without compromising tumor killing in preclinical models. A prominent challenge in preclinical radiation research, including FLASH, is validating both the physical dosimetry and the biological effects across multiple institutions.
Materials and Methods:
We previously demonstrated dosimetric reproducibility of two different electron FLASH devices at separate institutions using standardized phantoms and dosimeters. In this study, tumor-free adult female mice were given 10 Gy whole brain FLASH and CONV irradiation at both institutions and evaluated for the reproducibility and temporal evolution of multiple neurobiological endpoints.
Results:
FLASH sparing of behavioral performance on novel object recognition (4 months post-irradiation) and of electrophysiologic long-term potentiation (LTP, 5 months post-irradiation) was reproduced between institutions. Differences between FLASH and CONV on the endpoints of hippocampal neurogenesis (Sox2, doublecortin), neuroinflammation (microglial activation), and electrophysiology (LTP) were not observed at early times (48 h to 2 weeks), but recovery of immature neurons by 3 weeks was greater with FLASH.
Conclusion:
In summary, we demonstrated reproducible FLASH sparing effects on the brain between two different beams at two different institutions with validated dosimetry. FLASH sparing effects on the endpoints evaluated manifested at later but not the earliest time points.
Keywords: Radiotherapy, FLASH, Intercomparison, Neurobehavior, Electrophysiology, Neuroinflammation, Neurogenesis
Introduction
Ultra-high dose rate FLASH has been shown repeatedly to spare radiation injury to normal tissue while maintaining uncompromised tumoricidal efficacy compared to the same doses of conventional dose rate irradiation (CONV) [1,2]. Whole-brain FLASH in mice spares long-term cognitive function evaluated with multiple neurobehavioral assessments [3-9]. Similar sparing effects have been observed on electrophysiological assessments of long-term potentiation (LTP), cerebral vasculature, and neuronal morphology, as have reductions in neuroinflammation [5,6,8-10].
An important challenge in preclinical FLASH radiobiology is reproducing consistent beam parameters and biological results between institutions [11]. Validated physical beam parameters are a prerequisite for obtaining reproducible and robust biological effects. A cross-institutional dosimetric comparison study was previously conducted between electron linacs at Centre Hospitalier Universitaire Vaudois (CHUV) and Stanford using a standardized phantom [12]. More recently, another cross-institutional dosimetric comparison was conducted across three institutes using a 3-D printed anatomically realistic mouse phantom [13]. In parallel, a dosimetric and biological cross-institutional comparison was performed between the eRT6 and the proton-FLASH beam at the Paul Scherrer Institute (PSI) [14]. In all studies, the ability to generate consistent and accurate delivery of FLASH and CONV doses was replicated across all institutions.
Having completed cross-validated physical dosimetry at these institutions, this study aimed to confirm the biological equivalence on brain sparing in tumor-free mice of electron FLASH beams with distinct temporal structure. Animals were irradiated at both CHUV and Stanford replicating as closely as possible the same dose, dosing regimen, and treatment field in FLASH and CONV. The electron FLASH irradiators at these institutions have previously been described and characterized in detail [12,13].
For endpoints, we selected two functional CNS outcomes that have proven reliable indicators of the FLASH effect on normal brain function. Neurocognitive function remains a gold standard for CNS outcomes and the FLASH effect, and when coupled with assessments of LTP, a readout of synaptic plasticity, provides two independent and unequivocal markers of neurological sparing. However, we also sought to complement these studies to assess the early radiation response of the brain to dose rate modulation. Thus, in a secondary goal, we sought to link early (<1 month) radiation-induced sequelae in the brain to the adverse late functional outcomes that take many months to manifest. Early studies finding cyclical waves of secondary reactive mediators involving reactive oxygen and nitrogen species and inflammatory molecules gave rise to the concept that the irradiated brain may never return to baseline [15,16]. We therefore sought to analyze selected functional, inflammatory, and neurogenic outcomes at times up to three weeks post-irradiation.
Materials and methods
Animals
Female C57BL/6J mice (n = 16/group) were purchased from Jackson Laboratories (Sacramento, CA), allowed to acclimate, and were 11–12 weeks of age at irradiation. Animal procedures were conducted in accordance with NIH guidelines and Institutional Animal Care and Use Committees (IACUCs: APLAC-27939, AUP-21–025) for animal experimentation and follow ARRIVE guidelines and address the 10 essential criteria described therein.
A separate cohort of female C57BL/6J mice (n = 8–16/group) were purchased from Charles River Laboratories (France), allowed to acclimate, and were 12 weeks of age at irradiation. Animal procedures were conducted in accordance with the ethics committees (VD2920, VD3241, VD3603 and AUP-21–025). All animals were housed in standard housing conditions, maintained on a 12 h light:dark cycle and provided with ad libitum access to food and water.
As prior work demonstrated FLASH brain sparing in both male and female mice, for this inter-institutional reproducibility study we focused on female mice.
Irradiation
Irradiation was performed at two institutions (Stanford University, California, USA and CHUV University of Lausanne, Vaud, Switzerland) with two different electron linear accelerators (linac) [12]. Comparative phantom dosimetry had been conducted as previously published [13]. The irradiation field was matched between institutions by using a collimator with a circular aperture of 1.7 cm placed in contact with the dorsal surface of the mouse head, with the rostral border just caudal to the eyes such that the whole brain was irradiated while limiting irradiation of the eyes, mouth, and rest of the body.
Irradiations at Stanford University were performed using a Varian medical linac (Trilogy, Varian Medical Systems, Inc., Palo Alto) as described previously [17]. Mice received 10 Gy whole-brain irradiation delivered in a single fraction as either CONV (0.10 Gy/s mean dose rate, 15.73 MeV) or FLASH delivered in 5 pulses (225 Gy/s mean dose rate and intra-pulse dose rate of 5.33 × 105, 16.60 MeV).
Irradiations at Lausanne University Hospital (CHUV – Centre Hospitalier Universitaire Vaudois) were performed using a prototype 6 MeV Oriatron 6e electron beam linear accelerator (PMB-Alcen, France) as described previously [7]. Mice received 10 Gy WBI delivered in a single fraction as either CONV (0.1 Gy/s mean dose rate) or FLASH delivered in a single 1.8 μs pulse (5.5 × 106 Gy/s intra-pulse dose rate).
Full beam parameters and details of both irradiation setups can be found in Supplementary Material.
Transportation
Following irradiations, mice were returned to their standard housing environment and monitored daily for body weight, appearance, and respiratory rate for the first week and every two days thereafter. Three weeks after irradiation, in accordance with institutional guidelines, the mice were transported to UC Irvine where they acclimated before conducting follow-up studies.
Cognitive testing
Novel Object Recognition (NOR)
Cognitive testing was performed at CHUV and at UCI following previously published protocols [8,18,19]. Full details regarding the testing procedures at each institute are described in the Supplementary Material.
Electrophysiology
After completion of behavioral testing, a subset of the cohort (n = 6/treatment/institution) was euthanized for electrophysiology. Further details have been described [20] and are provided in Supplementary Material.
Immunohistochemistry
Details of the immunohistochemical procedures are provided in the Supplementary Material.
Statistical analysis
Statistical analyses were performed using one-way ANOVA to confirm overall significance along with Bonferroni’s multiple comparisons test (GraphPad Prism, v8.0, San Diego, CA). For electrophysiology measurements, the fEPSP slope was measured at 10–90 % fall of the slope and data in figures on LTP were normalized to the last 10 min of baseline. All data are presented as the mean ± SEM. All analyses considered a value of P≤0.05 to be statistically significant. All functional and molecular tests were performed and scored by investigators blinded to the treatment groups of the animals.
Results
Four months after 10 Gy whole brain irradiation, animals irradiated at Stanford underwent behavioral testing. Animals receiving FLASH were statistically indistinguishable from unirradiated controls in the NOR task, whereas animals receiving CONV exhibited significant impairments (Fig. 1A, one-way ANOVA: F(2,43) = 5.1, P=0.0096, Bonferroni post-hoc: CTRL vs CONV: P=0.0067, CTRL vs FLASH: P=0.2637, CONV vs FLASH: P=0.2880).
Fig. 1. FLASH sparing of performance on the Novel Object Recognition (NOR) test late after 10 Gy single fraction whole brain irradiation is reproduced between institutions.

A) NOR scores from Stanford (4 months post-RT) were scored manually by core facility experts. CONV irradiated animals had significantly lower discrimination index than unirradiated controls, but FLASH irradiated animals were not statistically different from unirradiated controls. (n = 16/group, each datapoint represents an animal). B) NOR scores from CHUV at 3 timepoints (2,6- and 9-months post-RT) exhibit similar effects between cohorts in that a significant decrease in discrimination index was seen in the CONV irradiated animals compared to both unirradiated controls and FLASH irradiated animals. Data were analyzed using one-way ANOVA and the Bonferroni multiple comparisons test (n = 7–13/group at 2 months, n = 5–7/group at 6 months, n = 3–5/group at 9 months, each datapoint represents an animal). *,P≤0.05; **, P≤0.01; ***, P≤0.001;****, P≤0.0001; ns, not significant.
A longitudinal analysis of learning and memory with the NOR test was also performed at CHUV 2, 6- and 9-months post-RT. A statistically significant difference was observed between the CONV cohort and unirradiated controls and between CONV and FLASH cohorts at all 3 timepoints (Fig. 1B, one-way ANOVA: 2 months F(2,30) = 264.2, P<0.0001; 6 months F(2,14) = 60.56, P=<0.0001; 9 months F(2,10) = 22, P=0.0002, Bonferroni post-hoc: 2 months: CTRL vs CONV: P<0.0001, CTRL vs FLASH: P=0.1377, CONV vs FLASH: P<0.0001, 6 months CTRL vs CONV: P<0.0001, CTRL vs FLASH: P>0.9999, CONV vs FLASH: P<0.0001, 9 months: CTRL vs CONV: P=0.001, CTRL vs FLASH: P>0.9999, CONV vs FLASH: P=0.0005).
Two weeks after completion of behavioral testing and 5 months post-RT, Theta Burst Stimulation (TBS) was applied to hippocampal slices from a subset of the cohort to induce LTP. Five theta bursts to Schaffer collaterals induced fEPSP (Fig. 2). After this initial short-term potentiation, a gradual decay in the fEPSP was observed in all cohorts at both institutes as shown in Fig. 2 A and B. The stable potentiation value in the fEPSP slope was significantly lower in CONV groups than unirradiated controls but not in FLASH groups. This result is quantified by mean potentiation measured 50–60 min after TBS (Fig. 2). The mean potentiation results showed the same effect in CONV cohorts with the same level of significance. Stanford cohort one-way ANOVA: F(2,30) = 54.39, P<0.0001, Bonferroni post-hoc: CTRL vs CONV: P<0.0001; FLASH vs CONV: P<0.0001. CHUV cohort one-way ANOVA: F(2,33) = 30.11, P<0.0001, Bonferroni post-hoc: CTRL vs CONV: P<0.0001; FLASH vs CONV: P<0.0001.
Fig. 2. FLASH sparing of Long Term Potentiation (LTP) in late (5 month) mice after 10 Gy single fraction whole brain irradiation is reproduced between institutions.

A) The LTP measurement results from cohorts irradiated at Stanford. (left) Following a stable 20 min baseline recording, the slope of the field Excitatory Postsynaptic Potential (fEPSP) as a percentage of baseline shows an immediate increase in potentiation after delivering Theta Burst Stimulation (TBS). The combined slope of the CONV irradiated cohort fails to stabilize unlike the unirradiated control or the FLASH irradiated group. (middle) The mean potentiation 50–60 min post-TBS for each treatment group. The mean potentiation is significantly lower in the CONV group compared to both the unirradiated controls and the FLASH group. (right) Representative traces collected during baseline (black line) and 50–60 min post-TBS (red line) for each group. Scale = 1 mV/5ms. B) The fEPSP slope as percentage of baseline, mean potentiation and electrophysiological traces for the cohorts irradiated at CHUV. The group differences and levels of significance are the same between each institute. Data were analyzed using one-way ANOVA and the Bonferroni multiple comparisons test (n = 10–11 slices/group). ****, P≤0.0001.
For selected CNS endpoints, much less is known of how FLASH might differentially impact acute responses. Measurements of LTP as described above were repeated on adult mice 2 weeks after 10 Gy CONV and FLASH irradiation at CHUV. Interestingly, and in marked contrast to our past publications conducted at > 4 months post-irradiation (8–10), there was no observable difference between the irradiation groups in mean potentiation at an early time point (Fig. 3, one-way ANOVA: F(2,31) = 0.3822, P=0.6856, Bonferroni post-hoc: CTRL vs CONV: P=0.8566; CTRL vs FLASH: P=0.9727, FLASH vs CONV: P=0.9810). There also was no deviation in the fEPSP slope from the unirradiated control group for either FLASH or CONV treatment groups as shown in Fig. 3.
Fig. 3. LTP is not altered acutely (2 weeks) after 10 Gy single fraction whole brain irradiation by either FLASH or CONV.

The fEPSP slope as percentage of baseline is indistinguishable between unirradiated controls, CONV, and FLASH groups irradiated at CHUV. No significant difference between mean potentiation 50–60 min post-TBS was observed in either irradiation group. Data were analyzed using one-way ANOVA and the Bonferroni multiple comparisons test (n = 10–12 slices/group). ns, not significant. This contrasts with the decrement in LTP observed to emerge later after CONV but not FLASH (see Fig. 2).
Immunohistochemical staining for IBA-1 (microglial marker) and CD-68 (reactive microglia marker) indicated a significant increase in neuroinflammation 48 h after irradiation. However, both FLASH and CONV induced similarly increased reactive microglia compared to unirradiated controls (Fig. 4, one-way ANOVA: F(2,71) = 4.964, P=0.0096, Bonferroni post-hoc: CTRL vs CONV: P=0.0420; CTRL vs FLASH: P=0.0019, FLASH vs CONV: P=0.5710). Previous studies have shown persistently elevated neuroinflammation at both acute and chronic timepoints [21], but the marked attenuation of these effects reported long after FLASH [5,6] was not evident at this acute timepoint.
Fig. 4. Neuroinflammation in the hippocampus increases acutely (48 h) after 10 Gy single fraction whole brain irradiation by either FLASH or CONV.
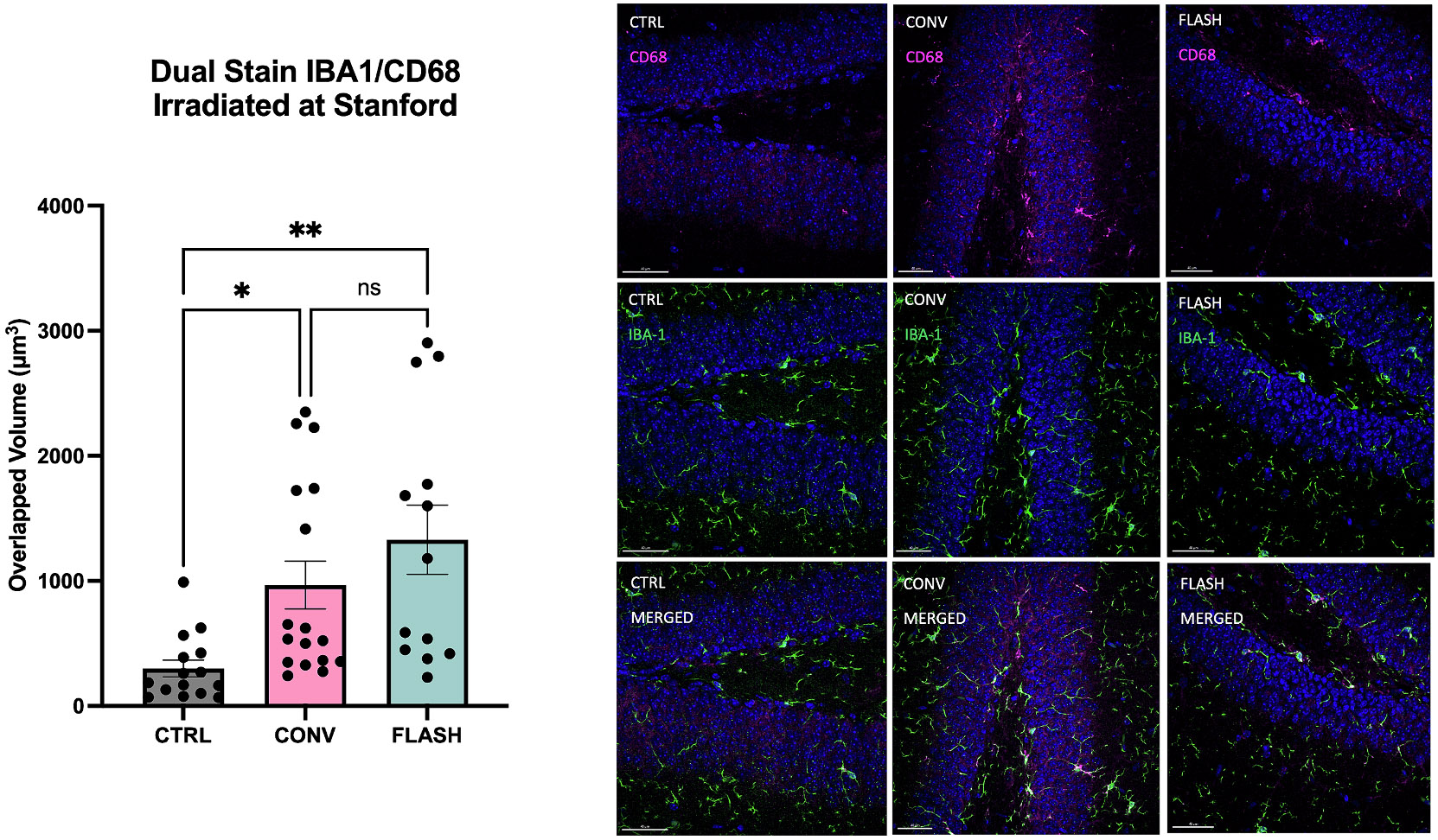
Dual Stain of IBA-1 (microglial stain) and CD68 (activated microglial stain) in the hippocampus of mice 48 h after irradiation at Stanford. (left) The volume of overlap of the two stains is plotted with each data point representing a single section. A significant increase in the volume of activated microglia was observed in both the CONV and FLASH cohorts compared to unirradiated controls. (right) Representative images of the CD68 stain alone (top) with DAPI in blue, the IBA-1 stain alone (middle) and the combined stain (bottom) for each of the three treatment groups. Data were analyzed using one-way ANOVA and the Bonferroni multiple comparisons test (n = 5–11/group, each datapoint represents an average of 2–3 sections/animal). **, P≤0.01; ns, not significant. This contrasts with the resolution of neuroinflammation observed to emerge later after FLASH but not CONV.
In the same cohort of animals stained for IBA1/CD68, Sox2 immunohistochemical staining was conducted on other hippocampal sections to evaluate the impact of 10 Gy whole brain irradiation at the acute 48 h post-irradiation time point on the population of radial glial-like neural stem/progenitor cells essential for self-renewal and differentiation [22]. Only Sox2 + cells in the subgranular zone (SGZ) were counted. The Sox2 stain revealed no significant difference between either the CONV or FLASH irradiated cohorts relative to the unirradiated controls (Fig. 5) (F(2,9) = 0.5034, P=0.6205, Bonferroni post-hoc: CTRL vs CONV: P>0.9999; CTRL vs FLASH: P>0.9999, FLASH vs CONV: P>0.9999), suggesting no significant loss of neural stem cells or neural progenitor cells in the dentate gyrus.
Fig. 5. The number of neural stem cells in the hippocampus is unchanged acutely (48 h) after 10 Gy single fraction whole brain irradiation by either FLASH or CONV.

Sox2 stain of the SGZ of the hippocampus of mice 48 h after irradiation of 10 Gy at Stanford. No significant difference between any irradiation group was observed (n = 3/group).
The doublecortin (DCX) stain was performed at one week, two weeks and three weeks post-irradiation to evaluate the acute impact on immature neurons, often used as a surrogate marker for neurogenesis [23]. The number of DCX+cells was severely reduced in both irradiated groups at one and two weeks compared to unirradiated controls with no difference between FLASH and CONV (Fig. 6) 1 week: F(2,8) = 195.8, P<0.0001, Bonferroni post-hoc: 1 week: CTRL vs CONV: P<0.0001; CTRL vs FLASH: P<0.0001, FLASH vs CONV: P>0.9999). Whereas the recovery of DCX+cells plateaued at 2 weeks after CONV, levels increased significantly after FLASH at 3 weeks post-RT (unpaired t-test between FLASH and CONV at 3 weeks, t = 5.862, df = 6, P=0.0011).
Fig. 6. The number of immature neurons in the hippocampus is severely depleted acutely (1–2 weeks) after 10 Gy single fraction whole brain irradiation by either FLASH or CONV, but at 3 weeks recovers more with FLASH than CONV.
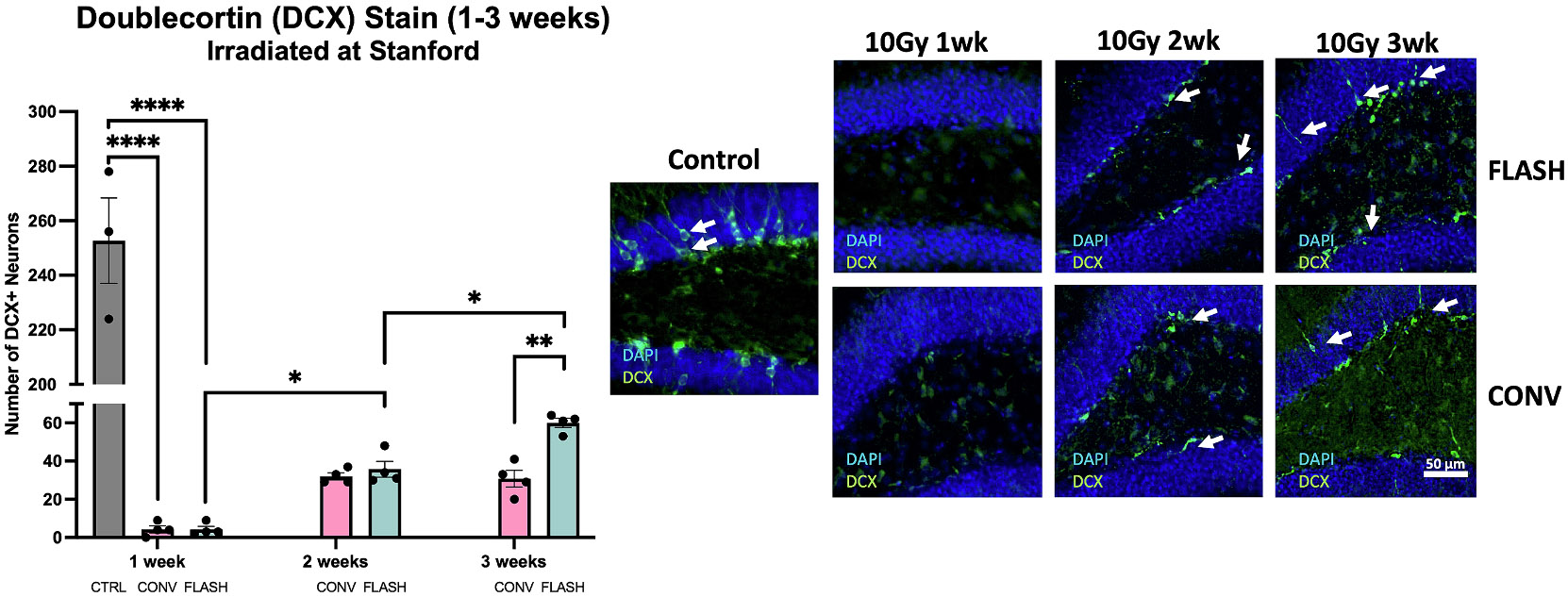
DCX staining in the hippocampus of mice 1 week, 2 weeks and 3 weeks after irradiation of 10 Gy at Stanford. A strong depletion in DCX+cells was observed in both CONV and FLASH groups to the same significance level at 1 week. At 2 weeks, some DCX+cells regenerated both in FLASH and CONV. However, the ongoing recovery of the FLASH groups reaches a significant difference compared to the CONV group at 3 weeks. Data were analyzed using one-way ANOVA and the Bonferroni multiple comparisons test (n = 4–5/group). *,P≤0.05; **,P≤0.01; ****,P≤0.0001; ns, not significant.
Discussion
The primary goal of this study was to validate from a biological perspective that FLASH neurological sparing after whole brain irradiation could be reproduced with different electron beams at different institutions when delivering dose and dose rates previously demonstrated to produce the FLASH effect in independent experiments. We directly compared FLASH irradiation platforms between two electron linacs that have been used extensively to examine the FLASH effect, but in this case matching the mouse model, irradiation field, and dosing regimen between them. This was followed by uniform assessment of cognition and electrophysiology at the central independent reference facility. This evaluation was preceded by a thorough dosimetric comparison of both irradiation platforms for FLASH-relevant ultra-high dose-rate and CONV dose rates [12,13], confirming agreement between measured and planned doses sufficient for preclinical studies (within ± 3 % agreement in [12] and differences from the prescribed dose in [13] ranged from 2.5-3.6 % in CONV and 2.7–6.4 % in FLASH). The current study confirms that equivalent biological FLASH effects are achieved for one sex at a particular dose regimen by both institutions under harmonized conditions, with equivalent preservation of cognition as well as synaptic plasticity. Similar comparative studies have been conducted to evaluate the equivalence of electron and proton FLASH beams [14] and for sparing of gastrointestinal toxicities after electron FLASH [24]. In the former case, neurocognitive function was spared after both FLASH modalities while tumor control and anti-tumor immunity was maintained equally between dose rates and modality. For the latter case, two electron beams were validated dosimetrically and shown to spare survival and intestinal crypt cell regeneration.
The FLASH sparing of learning, memory, attention, mood, social interaction and fear memory have been found repeatedly after electron FLASH at different doses and fractionation regimens [3,5-9]. To focus our objectives, the novel object recognition task was chosen as a logical and robust endpoint to compare outcomes after CONV and FLASH between the Trilogy and eRT6 linacs. Exposure of animals to CONV from either institute resulted in cognitive impairments, with statistically significant reductions in the discrimination index observed compared to unirradiated control mice. No difference between the FLASH irradiated animals and unirradiated controls was observed, indicating that each electron linac was able to spare radiation-induced learning and memory impairment when delivering FLASH dose rates.
The prolonged sparing of learning and memory deficits after FLASH suggests a preservation of synaptic elements involved in neurotransmission. In three recent studies, FLASH delivery of hypofractionated dosing regimens (2 × 10 Gy, 3 × 10 Gy) and standard of care fractionation (10 × 3 Gy) was shown to preserve LTP compared to CONV months after irradiation [8-10]. Therefore, we sought to replicate this finding between institutions at this single dose of 10 Gy. We found that the fESPS slope was reduced significantly for the hour post theta burst stimulation only in the CONV irradiated animals. The mean potentiation over this period was significantly inhibited in CONV cohorts from both institutions but not statistically different from unirradiated controls in FLASH cohorts from either electron linac. LTP remains a reliable standard to assess synaptic plasticity and these data further demonstrate that FLASH does not perturb the firing of Schaffer collaterals in the hippocampus, thereby preserving neurotransmission and synaptic integrity. The similarity of the LTP results between the cohorts irradiated at each institute validates the equivalence of the FLASH parameters generated by each linac and supports evidence for electrophysiological assessments as a reliable biomarker of the FLASH effect when assessed at late timepoints.
The functional equivalence of NOR and LTP outcomes between each electron linac combined with the recent comparison between the eRT6 and the proton beam Gantry1/PSI [14] establish certain consistent benefits of FLASH to critical functional outcomes in the CNS with various beams. However, we also sought to determine whether studies conducted at earlier times might provide more information and perhaps predictive value.
Past efforts linking the onset, progression, and severity of late radiation effects in the brain to early changes in blood brain barrier permeability, apoptosis, inflammation and neurogenic cell kill have proven difficult [25,26]. There has been considerable difficulty uncovering specific biomarkers of neurocognitive decline, highlighted by the rich literature derived from space radiation studies on the brain that have failed to identify specific signatures of cognitive decline, albeit under much different exposure conditions [27,28]. Therefore, in efforts to address the identification of an early biomarker and temporal response of the FLASH effect, a secondary objective of this study was to investigate the impact of FLASH at earlier timepoints up to three weeks post-irradiation. Numerous past FLASH studies have demonstrated its ability to spare the CNS from late radiation toxicity [3,6-9,18], and the results of this study support these findings. What is much less understood is if or how FLASH might prevent early radiation responses leading to toxicity in the brain.
To investigate the potential for FLASH to modulate early toxicities in the brain, we employed one of our most reliable late markers of the FLASH effect, namely LTP. When assessed at late times (>1 month) CONV irradiated cohorts exhibit significant and persistent reductions in slope of the fEPSP, effects not evident after FLASH [8-10]. While this finding was replicated after a single dose of 10 Gy at five months post-irradiation, no such change was found at two weeks post-irradiation. All cohorts exhibited identical LTP firing activity, clearly indicating that the radiation-induced inhibition of LTP manifests at times later than two weeks after CONV, an effect that never manifests after FLASH.
Elevated neuroinflammation is involved in perpetuating a host of radiation-induced and other neurological complications and the ability of FLASH to suppress the levels of reactive microglia has proven to be another robust marker of the FLASH effect [6,8,9,29]. Thus, follow-up immunohistochemical investigations were undertaken to analyze reactive microglial levels two days post-irradiation. While the levels of reactive microglia showed an increase in both irradiated cohorts, no difference between FLASH and CONV was observed. Clearly, inflammation is an immediate response of the brain to radiation damage, but the signature of radiation injury does not persist in FLASH irradiated cohorts suggesting that these incipient processes can be resolved.
Lastly, past studies have shown that FLASH can spare the neurogenic niche at late times post-irradiation [4,18], and other work has found intestinal crypt sparing after electron and proton FLASH at earlier times [24,30]. Interestingly, neither FLASH nor CONV significantly impacted the number of Sox2 + cells at 48 h post-irradiation. Data suggest that populations of early stage, transiently amplifying progenitor cells exhibit more resistance to irradiation, possibly because there was simply insufficient time to express radiation-induced lethality. In contrast, a severe depletion of DCX+cells was induced by both CONV and FLASH one week post-irradiation compared to unirradiated controls. However, recovery of DCX+cells began to manifest by two weeks, and by three weeks there was greater recovery of DCX+cells in FLASH compared to CONV irradiated mice. The enhanced temporal recovery of immature neurons after FLASH is interesting and may suggest that FLASH promotes a wound healing process, although the molecular targets involved remain uncertain.
The results of this study are limited to only one strain and sex of mice and only one dose regimen. While the FLASH effect has been confirmed in mice of both sexes, different ages and a variety of dose regimens at the CHUV, this is the first study to compare the biological output of the 1-pulse delivery at CHUV to the 5-pulse delivery at Stanford. In addition, only one behavioral test was conducted which is not solely dependent on intact hippocampal function. Further tests are needed to validate these results and confirm biological equivalence. While the early timepoint studies indicate that the FLASH sparing effect may only manifest in the late radiation response, the results are limited by the small number of timepoints assessed per endpoint. A longitudinal study of these endpoints would provide more temporal information on the onset of the distinction between FLASH and CONV for different cell types and functional outcomes.
Collectively, this multicenter biological intercomparison FLASH study demonstrates that FLASH sparing of cognitive function and brain electrophysiology compared to conventional dose rate brain irradiation is a robust biological phenomenon. The use of validated dosimetry on different linacs between institutions provided us with a framework to perform comparable biological investigations using different beam lines. Assessment of LTP, inflammatory and neurogenic endpoints at post-irradiation preceding three weeks yielded few dose-rate dependent changes. Data emphasize the difficulties of identifying early biomarkers of the FLASH effect in the brain but suggest that conducting longitudinal studies on more biological endpoints will be important to elucidate candidate mechanisms.
Supplementary Material
Acknowledgements
This work was supported by NCI grant P01CA244091 (M-CV, BWL, PM and CLL), philanthropic donors to the Stanford Department of Radiation Oncology and Conacyt (PZ-B). We would also like to thank Francesca Drayson for assistance to immunohistochemical staining.
Abbreviations:
- CONV
conventional dose rate
- WBI
Whole Brain Irradiation
- LTP
Long Term Potentiation
Footnotes
Disclosures
BWL is a founder and board member of TibaRay, and a consultant on a clinical trial steering committee for BeiGene.
Declaration of interest
BWL has received research support from Varian Medical Systems, is a cofounder and board member of TibaRay, and is a consultant on a clinical trial steering committee for BeiGene. The other authors have no competing interests to declare.
CRediT authorship contribution statement
Olivia G.G. Drayson: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Stavros Melemenidis: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Nikita Katila: Methodology, Investigation, Formal analysis. Vignesh Viswanathan: Methodology, Investigation. Enikö A. Kramár: Methodology, Investigation, Formal analysis. Richard Zhang: Methodology, Investigation, Formal analysis. Rachel Kim: Methodology, Investigation. Ning Ru: Methodology, Investigation. Benoit Petit: Methodology, Investigation. Suparna Dutt: Methodology, Investigation. Rakesh Manjappa: Methodology, Investigation. M. Ramish Ashraf: Methodology, Investigation. Brianna Lau: Methodology, Investigation. Luis Soto: Methodology, Investigation. Lawrie Skinner: Methodology, Investigation. Amu S. Yu: Methodology, Investigation. Murat Surucu: Methodology, Investigation. Peter G. Maxim: Methodology, Investigation, Funding acquisition, Formal analysis. Paola Zebadua-Ballasteros: Methodology, Investigation, Formal analysis. Marcelo A. Wood: Writing – original draft, Funding acquisition. Pierre Montay-Gruel: Methodology, Investigation. Janet E. Baulch: Writing – review & editing, Methodology, Investigation, Formal analysis. Marie-Catherine Vozenin: Writing – review & editing, Writing – original draft, Investigation, Funding acquisition, Formal analysis, Conceptualization. Billy W. Loo: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Conceptualization. Charles L. Limoli: Writing – review & editing, Writing – original draft, Project administration, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.radonc.2024.110534.
References
- [1].Vozenin MC, Bourhis J, Durante M. Towards clinical translation of FLASH radiotherapy. Nat Rev Clin Oncol 2022;19:791–803. [DOI] [PubMed] [Google Scholar]
- [2].Limoli CL, Vozenin M-C. Reinventing radiobiology in the light of FLASH radiotherapy. Annu Rev Cancer Biol 2023;7:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol 2017;124:365–9. [DOI] [PubMed] [Google Scholar]
- [4].Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol 2018; 129:582–8. [DOI] [PubMed] [Google Scholar]
- [5].Simmons DA, Lartey FM, Schuler E, Rafat M, King G, Kim A, et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol 2019;139:4–10. [DOI] [PubMed] [Google Scholar]
- [6].Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. PNAS 2019;116:10943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Montay-Gruel P, Acharya MM, Goncalves Jorge P, Petit B, Petridis IG, Fuchs P, et al. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin Cancer Res 2021;27:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alaghband Y, Allen BD, Kramar EA, Zhang R, Drayson OGG, Ru N, et al. Uncovering the protective neurologic mechanisms of hypofractionated FLASH radiotherapy. Cancer Res Comm 2023;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Allen BD, Alaghband Y, Kramar EA, Ru N, Petit B, Grilj V, et al. Elucidating the neurological mechanism of the FLASH effect in juvenile mice exposed to hypofractionated radiotherapy. Neuro Oncol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Limoli CL, Kramar EA, Almeida A, Petit B, Grilj V, Baulch JE, et al. The sparing effect of FLASH-RT on synaptic plasticity is maintained in mice with standard fractionation. Radiother Oncol 2023;186:109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Draeger E, Sawant A, Johnstone C, Koger B, Becker S, Vujaskovic Z, et al. A dose of reality: How 20 years of incomplete physics and dosimetry reporting in radiobiology studies may have contributed to the reproducibility crisis. Int J Radiat Oncol Biol Phys 2020;106:243–52. [DOI] [PubMed] [Google Scholar]
- [12].Jorge PG, Melemenidis S, Grilj V, Buchillier T, Manjappa R, Viswanathan V, et al. Design and validation of a dosimetric comparison scheme tailored for ultra-high dose-rate electron beams to support multicenter FLASH preclinical studies. Radiother Oncol 2022;175:203–9. [DOI] [PubMed] [Google Scholar]
- [13].Ashraf MR, Melemenidis S, Liu K, Grilj V, Jansen J, Velasquez B, et al. Multi-institutional audit of FLASH and conventional dosimetry with a 3D-printed anatomically realistic mouse phantom. Int J Radiat Oncol Biol Phys 2024. 10.1016/j.ijrobp.2024.03.017. [DOI] [PubMed] [Google Scholar]
- [14].Almeida A, Togno M, Ballesteros-Zebadua P, Franco-Perez J, Geyer R, Schaefer R, et al. Dosimetric and biologic intercomparison between electron and proton FLASH beams. Radiother Oncol 2023:109953. [DOI] [PubMed] [Google Scholar]
- [15].Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. Int J Radiat Biol 1997;72:45–53. [DOI] [PubMed] [Google Scholar]
- [16].Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: A review. Int J Radiat Biol 2004;80:251–9. [DOI] [PubMed] [Google Scholar]
- [17].Schuler E, Trovati S, King G, Lartey F, Rafat M, Villegas M, et al. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int J Radiat Oncol Biol Phys 2017;97:195–203. [DOI] [PubMed] [Google Scholar]
- [18].Alaghband Y, Cheeks SN, Allen BD, Montay-Gruel P, Doan NL, Petit B, et al. Neuroprotection of radiosensitive juvenile mice by ultra-high dose rate FLASH irradiation. Cancers (Basel) 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Drayson O, Vozenin M-C, Limoli C. A rigorous behavioral platform for the assessment of radiation-induced neurological outcomes. Method Molec Biol. 2023; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vogel-Ciernia A, Matheos DP, Barrett RM, Kramar EA, Azzawi S, Chen Y, et al. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat Neurosci 2013;16:552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lumniczky K, Szatmari T, Safrany G. Ionizing radiation-induced immune and inflammatory reactions in the brain. Front Immunol 2017;8:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci 2004;26:148–65. [DOI] [PubMed] [Google Scholar]
- [23].Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci 2001;14:629–44. [DOI] [PubMed] [Google Scholar]
- [24].Valdes Zayas A, Kumari N, Liu K, Neill D, Delahoussaye A, Goncalves Jorge P, et al. Independent reproduction of the FLASH effect on the gastrointestinal tract: A multi-institutional comparative study. Cancers (Basel) 2023;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tofilon PJ, Fike JR. The radioresponse of the central nervous system: A dynamic process. Radiat Res 2000;153:357–70. [DOI] [PubMed] [Google Scholar]
- [26].Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol 2009;19:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Desai RI, Limoli CL, Stark CEL, Stark SM. Impact of spaceflight stressors on behavior and cognition: A molecular, neurochemical, and neurobiological perspective. Neurosci Biobehav Rev 2022;138:104676. [DOI] [PubMed] [Google Scholar]
- [28].Limoli C. Can a comparison of clinical and deep space irradiation scenarios shed light on the radiation response of the brain? Br J Radiol 2020;93:20200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Montay-Gruel P, Markarian M, Allen BD, Baddour JD, Giedzinski E, Jorge PG, et al. Ultra-high-dose-rate FLASH irradiation limits reactive gliosis in the brain. Radiat Res 2020;194:636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Diffenderfer ES, Verginadis II, Kim MM, Shoniyozov K, Velalopoulou A, Goia D, et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int J Radiat Oncol Biol Phys 2020;106:440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


