Abstract
Many studies have shown an association of obstructive sleep apnea (OSA) with incident cardiovascular diseases, particularly when comorbid with insomnia, excessive sleepiness, obesity hypoventilation syndrome, and chronic obstructive pulmonary disease. Randomized controlled trials (RCTs) have demonstrated that treatment of OSA with positive airway pressure devices (CPAP) improves systemic hypertension, particularly in those with resistant hypertension who are adherent to CPAP. However, large RCTs have not shown long-term benefits of CPAP on hard cardiovascular outcomes, but post hoc analyses of these RCTs have demonstrated improved hard outcomes in those who use CPAP adequately. In theory, low CPAP adherence and patient selection may have contributed to neutral results in intention-to-treat analyses. Only by further research into clinical, translational, and basic underlying mechanisms is major progress likely to continue. This review highlights the various treatment approaches for sleep disorders, particularly OSA comorbid with various other disorders, the potential reasons for null results of RCTs treating OSA with CPAP, and suggested approaches for future trials.
Keywords: CPAP, hypoventilation syndrome, noninvasive ventilation, obesity, OSA
In part 1 of the State-of-the-Art Review, we reviewed various sleep disorders including insomnia and obstructive sleep apnea (OSA) and associated cerebrocardiovascular diseases. In part 2, we focus on current therapeutic options for sleep disorders including insomnia, OSA comorbid with insomnia (COMISA), with morbid obesity (obesity hypoventilation syndrome [OHS]), and OSA with chronic obstructive pulmonary disease (overlap syndrome), and their impact on incident cardiovascular disease (CVD).
TREATMENT OF CHRONIC INSOMNIA AND INCIDENT CVD
Multiple studies have suggested that chronic insomnia is often associated with increased sympathetic activity, up-regulation of the inflammatory cascade, and incident CVD (see part 1). Further, extension of wakefulness is associated with activation of the elevated orexin pathway neurohormones,1 which are known to also promote sympathetic hyperactivity. It is therefore plausible that treatment of insomnia with short sleep duration by improving sleep quality could lead to improved CV outcomes. It is plausible that treatment of insomnia by improving sleep quantity could lead to improved CV outcomes.
There are limited randomized controlled trials (RCTs) evaluating the impact of insomnia treatment on CVD outcomes. Cognitive behavioral therapy for insomnia (CBTi) is considered first-line therapy. Two pilot studies have been performed. The first study2 randomized 29 patients with insomnia to CBTi or sleep hygiene and found a clinically, but not statistically, significant reduction in insomnia severity. Additionally, there was a 3 mm Hg reduction in systolic blood pressure (BP) in the treatment arm compared with a 0.5-mm Hg increase in the control arm (P = 0.5, Cohen’s D = 0.22). There was a small reduction (1.1 mm Hg) in diastolic BP in the treatment arm compared with an increase of 0.8 mm Hg in the control arm (P = 0.4, Cohen’s D = 025).
Another randomized study3 enrolling 23 heart failure (HF) patients found clinically significant improvement in insomnia symptoms, quality of life, and mood in the CBTi group compared with the control group. Long-term studies are needed to determine whether treatment of insomnia could improve CV function in HF.
Although CBTi is the recommended first-line treatment of insomnia, pharmacologic agents are often used in clinical practice. Benzodiazepine receptor agonists have been commonly used to treat insomnia. These medications exert their effects on gamma amino butyric acid (GABA), a widely distributed inhibitory neurotransmitter in the brain that promotes sleep. In 1 RCT with a benzodiazepine,4 402 patients with insomnia and hypertension were randomized to either estazolam (n = 202) or placebo (n = 200) for 4 weeks. The reductions in systolic and diastolic BP were significantly greater in the active arm compared with the control arm: 10.5 mm Hg vs 2.5 mm Hg and 8.1 mm Hg vs 2.7 mm Hg, respectively (P < 0.001).
Although chronic insomnia can occur in isolation, when associated with OSA (COMISA),5 it conveys a poorer prognosis compared with either condition alone.6 At least 3 RCTs have used CBTi in patients with COMISA. In 1 of these RCTs, 145 patients with COMISA (untreated moderate-to-severe OSA, Apnea-Hypopnea Index [AHI] ≥15 events/h of sleep) were randomized to CBTi or to a no-treatment control group. Overnight sleep studies were completed pretreatment and posttreatment. With CBTi, there was a 7.5 event/h greater AHI difference (mean decrease 5.5 events/h [95% CI: 1.3–9.7 events/h) compared with the control arm (mean increase 2.0 events/h [95% CI: −2.0 to 6.1 events/h]). The CBTi group also had a greater reduction in total number (P = 0.029) and duration (P = 0.031) of awakenings. This study suggests that insomnia disorder may exacerbate OSA, providing support for treating insomnia in the presence of OSA. Several other RCTs 6–9 have assessed the combination of CBTi and positive airway pressure (PAP), and found that concurrent CBTi and PAP use is superior to PAP alone on insomnia outcomes, which in the long run may improve CV outcomes, particularly if CBTi improves PAP adherence.
Regarding benzodiazepines used commonly to treat insomnia, there are concerns for the potential to compromise breathing, particularly in those with moderate-to-severe OSA.10 More recently, dual-orexin receptor antagonists (DORAs) have been approved for treatment of chronic insomnia. These medications exert sleep promotion by antagonizing wake-promoting endogenous orexin neuropeptides at orexin receptors and should not cause respiratory depression.11–14 The impact of DORAs on CV outcomes in people with insomnia or with COMISA is unclear but worthy of future investigation.
TREATMENT OF OSA AND INCIDENT CVD
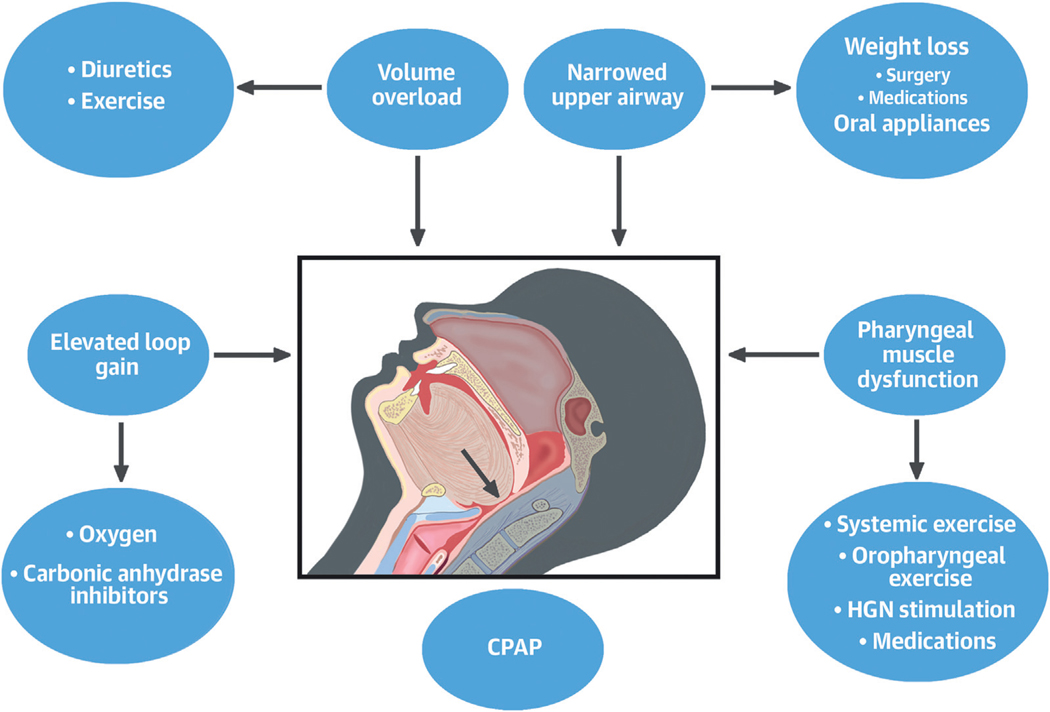
While asleep, the final common pathway causing an obstructive apnea or hypopnea is relaxation of the genioglossus muscle, with the tongue falling backward and closing (apnea) or narrowing (hypopnea) the pharyngeal airway. Nonetheless, multiple other factors can cause or contribute to OSA pathogenesis, and these phenotypes/endotypes are depicted in the Central Illustration and discussed in the following text.
CENTRAL ILLUSTRATION. Phenotypic Therapeutic Options in Obstructive Sleep Apnea.
The figure depicts various mechanisms which could be target of therapy in obstructive sleep apnea. Although continuous positive airway pressure (CPAP) device is the most effective therapeutic option, other variables could also be targeted as depicted. HGN = hypoglossal nerve.
POSITIVE AIRWAY PRESSURE.
PAP devices are the mainstay of therapy for OSA. These devices increase intrapharyngeal pressure, widening the pharyngeal airway in a dose-dependent manner, thereby preventing upper airway occlusion. In the following text, we discuss RCTs for treatment of OSA with a continuous positive airway pressure (CPAP) device on 5 OSA-specific CV consequences: 1) systemic hyper-tension; 2) pulmonary hypertension; 3) HF; 4) OHS; and 5) overlap syndrome.
Impact of treatment of OSA with CPAP on systemic hypertension.
Hypertension is highly prevalent and is the leading risk factor causing incident CVD such as stroke, HF, and premature death.15 Many studies, reviewed in part 1, have shown OSA contributing to hypertension and resistant hypertension.
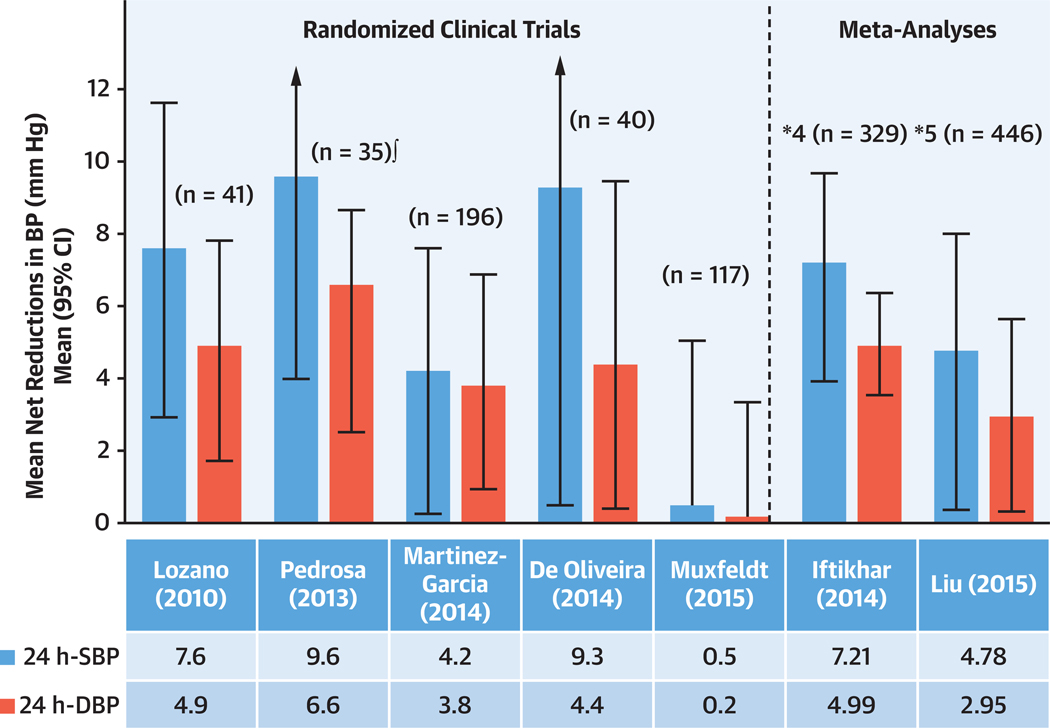
Multiple RCTs with 24-hour ambulatory BP monitoring have shown a modest effect of use of CPAP on reducing BP, equivalent to 2.5 mm Hg in 24-hour systolic and diastolic BP.16 By contrast, the effect of CPAP in reducing BP is more pronounced in resistant hypertension (Figure 1). In these patients, RCTs show significant reduction in systolic BP ranging between 4.7 to 7.2 mm Hg and diastolic BP ranging from 2.9 to 4.9 mm Hg, with large SDs (Figure 1).16 This large scatter implies that some patients may benefit with a large drop in BP, whereas others may have more modest reduction. The reasons for the differing responses have been discussed previously.16 In general, those who respond the most to CPAP experience more severe OSA, have higher baseline BP, and have higher adherence to CPAP.16,17 The drop in BP is dose-dependent, that is, there is a greater BP drop with increasing CPAP usage.
FIGURE 1. Effect of CPAP Therapy on BP in Patients With Resistant Hypertension.
The figure includes the results of the randomized controlled trials published to date. Positive figures mean improvement in blood pressure (BP) level with continuous positive airway pressure (CPAP) treatment (net changes). From Javaheri et al.17 DBP = diastolic blood pressure; SBP = systolic blood pressure.
Impact of treatment of OSA with CPAP on pulmonary hypertension.
OSA and PH could coexist together. OSA could be a cause of pulmonary hypertension (PH), and pulmonary arterial hypertension (PAH) could be comorbid with OSA. As discussed in part 1, OSA can be a cause of mild PH, though PH can be severe if OSA is comorbid with other disorders such as OHS. In 1 small (n = 23) crossover RCT18 comparing CPAP to sham CPAP, 12 weeks of CPAP therapy led to a 5 mm Hg mean pulmonary arterial pressure decrease (P < 0.0001). A second RCT19 enrolled subjects with OHS, the Pickwick trial discussed in part 1. The results of this study, discussed in the following text under “OHS,” also showed significant improvement in PH with PAP therapy.
In regard to PAH, it has been shown to be associated with nocturnal hypoxemia and sleep apnea. In a prospective study, Lowery et al19 examined the relationships between sleep-related hypoxemia and AHI with cardiac function and survival. Time below saturation of 90% was associated with right ventricular (RV) systolic pressure, RV ejection fraction, mean PAP, RV hypertrophy on electrocardiography, and transplant-free survival. It remains to be determined whether nocturnal supplemental oxygen therapy could improve survival of patients with PAH.
Impact of treatment of OSA with PAP devices on HF.
Studies have used CPAP and adaptive servoventilation (ASV) to treat OSA in patients with HF. An early large observational study of Medicare beneficiaries showed that treatment of OSA with CPAP was independently associated with decreased hospital readmission, health care cost, and mortality.20 Small RCTs in patients with heart failure with reduced ejection fraction (HFrEF) have shown that CPAP treatment has beneficial cardiac effects, decreasing awake sympathetic activity,21 myocardial sympathetic nerve function and energetics in the case of severe OSA, and increases in left ventricular ejection fraction by about 5% after 1 month of treatment.22 This latter finding has not been confirmed by another study.23
The results of the ADVENT-HF (Effect of Adaptive Servo Ventilation [ASV] on Survival and Hospital Admissions in Heart Failure) trial, an open RCT using ASV vs usual care (UC), has been published recently.24 Overall, 731 patients with HFrEF comorbid with severe OSA or CSA were randomly assigned to ASV or UC (n = 375). Patients enrolled were primarily nonsleepy, and more had OSA than CSA. Compared with UC, the use of ASV did not lead to a significant benefit in primary (the cumulative incidence of the composite of all-cause mortality, first admission to hospital for a CV reason, new-onset atrial fibrillation or flutter, and delivery of an appropriate cardioverter-defibrillator shock) or secondary (all-cause mortality) endpoints.
Notably, however, both the quality of sleep and the quality of life improved significantly with ASV, findings that will require corroboration in subsequent controlled studies. We should note that design of the ADVENT-HF trial was different from the SERVE-HF (Treatment of Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients With Heart Failure) trial25 in which patients with predominantly OSA were excluded.
Both CPAP and ASV have been used in patients with heart failure with preserved ejection fraction (HFpEF) comorbid with OSA. One study was performed with CPAP in stable ambulatory patients discussed in the preceding text, showing significant improvement in pulmonary artery pressure.18 Additionally, compared with sham CPAP, treatment with CPAP resulted in significant increases in the E/A ratio (P < 0.01) and reductions in mitral deceleration (P < 0.01) and isovolumic relaxation (P < 0.05) times.26
The study with ASV27 was executed in hospitalized patients with moderate-to-severe sleep apnea, comorbid with predominantly OSA or CSA. It showed potential beneficial effects of ASV in acute congestive heart failure (CHF) patients, particularly those with HFpEF. This study was terminated prematurely due to safety concerns from a chronic CHF study; thus, additional studies are required regarding ASV in acute CHF.
Recent real-world evidence studies suggest that adherence to PAP therapy is associated with a reduction in health care resource utilization, emergency room visits, and hospitalizations in patients with OSA and HFpEF, as well as HFrEF.28,29
Impact of treatment of OSA with CPAP on OHS.
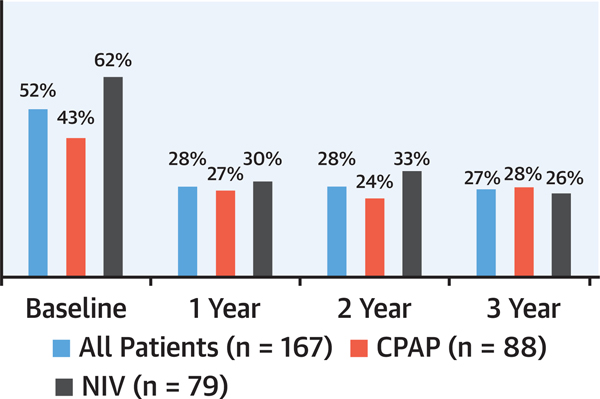
In the Pickwick trial discussed in part 1, patients with OHS and severe OSA who had echocardiographic evidence of PH at baseline (defined as pulmonary artery systolic pressure ≥40 mm Hg) were randomized to CPAP or noninvasive ventilation (NIV) for 3 years.30 The NIV device provides ventilation in an attempt to lower PaCO2, this in contrast to CPAP. In 52% of participants who had echocardiographic evidence of PH at baseline, both CPAP and NIV were equally effective in decreasing pulmonary artery systolic pressure (by approximately 8 to 10 mm Hg) and improving left ventricular diastolic dysfunction. The prevalence of PH also decreased (Figure 2). During the follow-up, both CPAP and NIV led to significant improvements in daytime PaO2 and PaCO2, forced expiratory volume over 1 second (FEV1), forced vital capacity (FVC), daytime sleepiness, dyspnea, quality of life, and BP. Based on these data, the American Thoracic Society guidelines recommend CPAP as the initial treatment of stable ambulatory adult patients with OHS and concurrent severe OSA (AHI ≥30 events/h) presenting with chronic stable respiratory failure.31 Because more than 70% of patients with OHS have severe OSA, this recommendation is applicable to most OHS patients.30 The impact of weight loss on OHS32 will be discussed later.
FIGURE 2. Prevalence and Evolution of PH in the Pickwick Trial.
This figure shows a significant drop in the prevalence of pulmonary hypertension (PH), which was sustained through the 3-year follow-up. Adapted from Masa et al19 and Mokhlesi et al.31 CPAP = continuous positive airway pressure; NIV = noninvasive ventilation.
Impact of treatment of OSA with CPAP on overlap s yndrome.
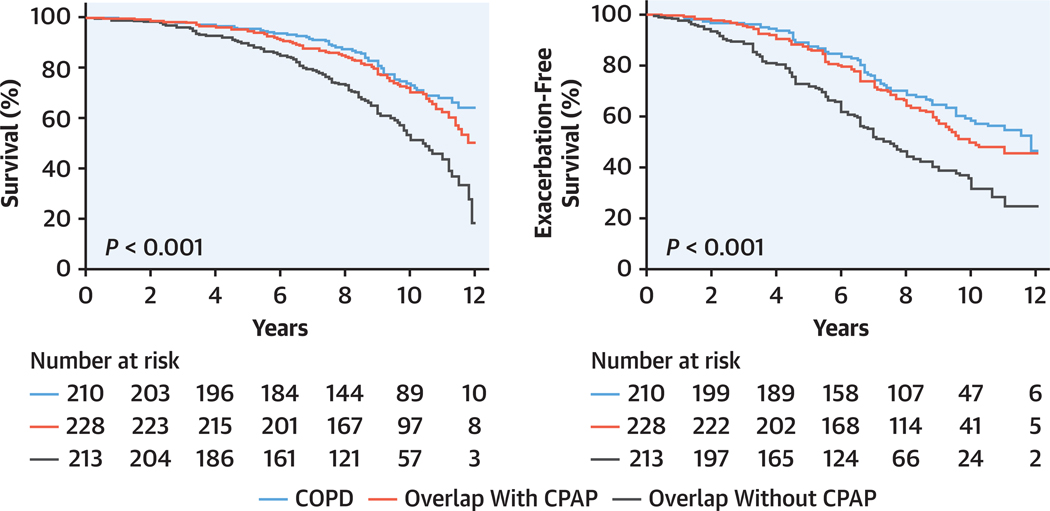
Effect of CPAP and NIV have been reviewed elsewhere.33–36 A retrospective observational study found a clinically significant reduction in health care utilization and costs in patients with chronic obstructive pulmonary disease (COPD)-OSA overlap syndrome who were adherent vs nonadherent with CPAP therapy.33,34 Long-term outcome studies have reported that patients with moderate or severe OSA treated with CPAP had similar survival rates to those with COPD alone, whereas those patients with overlap syndrome not treated with CPAP had higher mortality, especially from CVD (Figure 3), and were more likely to experience a severe COPD exacerbation leading to hospitalization.33
FIGURE 3. CPAP Treatment of OSA in the Overlap Syndrome Improves Survival.
Long-term outcome study showing that patients with overlap syndrome and moderate or severe obstructive sleep apnea (OSA) treated with continuous positive airway pressure (CPAP) have similar survival rates to those with chronic obstructive pulmonary disease (COPD) alone, whereas those patients with overlap syndrome (COPD with OSA) not treated with CPAP had higher mortality, especially from cardiovascular disease. Adapted with permission of the American Thoracic Society.34
Interim summary:
CPAP is the most efficacious treatment of OSA when tolerated, but when long-term adherence is low, other therapeutic options depicted in the Central Illustration should be considered.
THERAPEUTIC OPTIONS ADDRESSING NARROWED UPPER AIRWAY.
As depicted in the Central Illustration, weight loss and mandibular devices could expand the pharyngeal space and prevent upper airway closure. In the following text, we discuss these options and their impact on OSA and CVD.
Ma ndibular advancement devices.
These devices protrude the mandible thereby opening the pharyngeal airway and reducing airway collapsibility. A mandibular advancement device (MAD) is best used in individuals in whom OSA is due primarily to anatomically narrowed upper airway (Central Illustration).36,37
Currently, MADs are the main alternative to CPAP, particularly for patients who are intolerant of or refuse CPAP. The most recent American Academy of Sleep Medicine update suggests the role of MAD across the range of OSA severity.38 A recent meta-analysis of subjects with severe OSA consisting of 3 crossover RCTs and 1 parallel-group RCT pooling data from 151 patients (125 in the CPAP treatment arm and 124 in the MAD treatment arm) concluded that titratable MAD had a similar impact to CPAP on major patient-centered outcomes (sleepiness and quality of life). The treatment adherence and preference were largely in favor of MAD, though CPAP was more efficacious in reducing AHI and oxygen desaturation index.39 Another meta-analysis including 51 studies and 4,888 patients reported that both CPAP and MADs were associated with reductions in BP, without any differences between these 2 therapies in terms of BP outcomes.40 In terms of CV effects, a systematic review of MAD consisting of 16 studies, including 11 RCTs, reported significant reductions in daytime systolic and diastolic BP compared with baseline.41
From a phenotypic approach, MAD should be most effective in those in whom the underlying mechanism of upper airway collapse is due to altered anatomical structure with a narrow upper airway.36 In the aforementioned studies, there was no phenotypic preselection. Hence, there is a need for studies exploring the impact of MAD treatment on CV outcomes in this subgroup.42
Weight loss.
Obesity affects approximately 42% of U.S. adults and is associated with increased rates of OSA and other comorbidities including hypertension, CVD, type 2 diabetes, and premature death.43 Obesity is associated with fat deposition in the tongue44 and within the throat, narrowing the pharyngeal space and facilitating upper airway closure during sleep when the genioglossus muscle relaxes and the togue falls backward (Central Illustration). With weight loss, one loses upper body fat, including tongue, throat, and visceral fat, collectively improving OSA. In addition, weight loss provides an incremental reduction in insulin resistance, serum triglyceride levels, and BP (particularly when combined with CPAP).45
Both bariatric surgery and antiobesity medications are commonly recommended for weight loss. Among the various bariatric interventions, Roux-en-Y gastric bypass (RYGB) is among the most effective.46 One long-term RCT47 comparing UC with RYGB showed that 3 years later, body mass index (BMI) increased with UC (1.7 kg/m2), whereas it decreased significantly (11 kg/m2) in the RYGB group. Consistent with changes in BMI, AHI increased by 5 events/h in the UC group who gained weight, whereas AHI decreased by 13 events/h with weight loss.
In a prospective study48 that included 150 patients undergoing laparoscopic RYGB, 111 patients (73%) had OSA diagnosed by home sleep apnea test with respiratory polygraphy. After 5 years, weight decreased from 130 kg to 101 kg and AHI from 29 to 9 events/h of recording. There was a significant correlation between weight loss and reduction in AHI. Similarly, oxygen desaturation and heart rate decreased, and quality of life improved significantly. Of note, moderate or severe OSA persisted in 14 patients (20%).
A recent study49 using registry-based nationwide data in Sweden followed patients with sleeve gastrectomy or RYGB with OSA (n = 5,892) and 11,552 matched patients without OSA for a median of 6.8 years. Remission of OSA (defined as discontinuation of CPAP) was seen for 4,334 patients (74%). Patients in remission had a lower risk for overall mortality (cumulative incidence 6.0% vs 9.1%; P < 0.001) and major adverse cardiac events (cumulative incidence 3.4% vs 5.8%; P < 0.001) at 10 years after operation compared with those who did not reach remission. The risk was similar to that of the control group without OSA at baseline.
Most recently, weight loss medications have become of major interest,50–63 receiving considerable media coverage and widespread use. Given their success in promoting weight loss, they are also effective at treating OSA.50,51
Glucagon-like peptide-1 (GLP-1) (such as liraglutide and semaglutide), and glucose-dependent insulino-tropic peptide (GIP) and glucagon-like peptide-1 receptor agonist (tirzepatide) have been approved by the U.S. Food and Drug Administration (FDA) and have been used for treatment of obesity with or without diabetes mellitus type 2. GIP and GLP-1 are incretin hormones that are released in the intestine in response to nutrient intake and stimulate pancreatic beta cell activity, secreting insulin while delaying gastric emptying to reduce food intake.50,51 They have been approved by the FDA for long-term use in conjunction with lifestyle changes.
Multiple RCTs have shown that incretin-based therapy can promote weight loss and lower hemoglobin A1c.52–54
In an RCT enrolling 1,961 adults, the mean change in body weight from baseline to week 68 was −15% (equivalent to 15 kg) with semaglutide once weekly at a dose of 2.4 mg, compared with −2.4% with placebo (P < 0.001).53
Two incretin-based therapy studies have been reported in patients with OSA. The SCALE Sleep Apnea (Effect of Liraglutide in Obese Subjects With Moderate or Severe Obstructive Sleep Apnoea) trial evaluated liraglutide intervention vs placebo over 32 weeks in moderate-to-severe OSA subjects and reported a small, but statistically significant, improvement in AHI, systolic BP, and HbA1c.55 The largest and most systematic study has been reported with tirzepatide. Tirzepatide was first approved for type 2 diabetes treatment under the name Mounjaro (Eli Lilly). According to the results of the 72-week SURMOUNT-1 (A Study of Tirzepatide [LY3298176] in Participants With Obesity or Overweight) phase 3 randomized clinical trial, tirzepatide 15 mg weekly led to a 22-kg weight loss after 72 weeks.54 Most recently, tirzepatide has been approved by the FDA under the trade name of Zepbound56 (Eli Lilly), and the results for SURMOUNT-OSA (Obstructive Sleep Apnea Master Protocol GPIF: A Study of Tirzepatide [LY3298176] in Participants With Obstructive Sleep Apnea)57 were recently published. The investigators conducted 2 randomized trials for OSA patients with obesity: study 1 included patients not using CPAP and study 2 included patients using CPAP. In both study arms, tirzepatide vs placebo led to improvement in the AHI. Investigators observed improvement in secondary outcomes that were prespecified and controlled for multiple comparisons, including: improved systolic BP, improved C-reactive protein levels, improved hypoxic burden, and improved patient-reported outcomes.
The findings of incretin trials have important implications for CVD and OHS.58–62 In an RCT enrolling 17,604 patients, 8,803 received semaglutide, and 8,801 placebo.58 The mean duration of exposure to semaglutide or placebo was 34 months. The primary CV endpoint event (a composite of death from CV causes, nonfatal myocardial infarction, or nonfatal stroke in a time-to-first-event analysis) occurred in 6.5% of the patients on semaglutide compared with 8.0% in the placebo group, a 20% reduction (HR: 0.80; 95% CI: 0.72–0.90; P < 0.001). On average, semaglutide treatment resulted in a 9.4% reduction in body weight. In this trial, polysomnography was not performed; thus, it remains unclear to what degree the trial findings were dependent on weight loss, perhaps improving concomitant OSA. Notably, adverse events leading to discontinuation of the trial product occurred in 17% in patents on semaglutide group and 8% with placebo (P < 0.001), mostly gastrointestinal side effects.
The most advantageous consequences of therapeutic weight loss occur in the most obese individuals such as those with OHS. In such individuals, weight loss can potentially lead to reversal of hypoventilation and improvement in oxygenation. It has been shown that excess weight is associated with excessive CO261 production contributing to hypercapnia. This has an important clinical implication, suggesting that weight loss by itself could potentially eliminate hypercapnia in OHS.60
Last, but not least, weight loss also improves the burden of atrial fibrillation (AF), which may be in part related to improvement in OSA severity.61,62 In a trial designed to examine the effect of weight reduction vs general lifestyle advice on AF outcomes (n = 150), both arms also received intensive management of CV risk factors, which included OSA diagnosis and management. A reduction in AF burden (defined by cumulative duration and number of episodes) and improvement in AF symptom severity scores concordant with weight reduction was observed. Moreover, weight loss was experienced in both intervention and control groups, and both groups also had improvement in cardiac structure, that is, reduction in interventricular septal thickness and left atrial area.
THERAPEUTIC OPTIONS ADDRESSING VOLUME OVERLOAD.
Upper airway edema might contribute to pharyngeal collapsibility, further facilitating upper airway closure. Multiple studies from the Toronto group have shown that in the supine position, there is rostral translocation of fluid from lower extremities resulting in vascular congestion and narrowing of the pharyngeal space, promoting upper airway narrowing.64–68 Individuals with OSA and obesity frequently have lower extremity edema, particularly if comorbid with HF. Diuretic therapy has been shown to increase the pharyngeal space and improve OSA.67 In a 2-arm (17 patients in each arm) RCT68 in patients with coronary artery disease (CAD), after 4 weeks of aerobic exercise, compared with the control group, measurement of leg, neck, and thoracic fluid volumes and upper-airway cross-sectional area improved significantly with exercise. In conjunction with these changes, polysomnography revealed reduction in AHI from 31 to 21 events/h in the exercise group (P = 0.04); AHI did not change in the control group. Exercise may also improve OSA by strengthening the tongue muscle and ventilatory control as discussed in related sections.
THERAPEUTIC OPTIONS TO IMPROVE PHARYNGEAL MUSCLE DYSFUNCTION.
With removal of the wakefulness drive to breathe, sleep onset reduces dilatory muscle activity, promoting upper airway collapse. This compromise during sleep may account for development of OSA in about 30% of patients.69 Therefore, targeting stimulation of the genioglossus muscle should be effective in this subgroup of patients; to this end, hypoglossal nerve stimulation (HNS), exercise, and medications have been studied and have shown efficacy (Central Illustration).
HNS to improve muscle dysfunction.
The FDA has approved HNS for treatment of OSA for CPAP-intolerant individuals with at least moderate OSA (AHI ≥15 events/h). HNS relies on electrical stimulation of the hypoglossal nerve, which innervates the upper airway dilator muscles including the genioglossus muscle.70,71 However, there are responders and nonresponders, presumably, the former group with predominantly muscle dysfunction causing OSA. So far, no systematic studies have been performed to determine the impact of HNS intervention on CVD outcomes, and a large RCT is needed. Physiologically, individuals with impaired genioglossus muscle function determined at baseline may be most amenable to HNS intervention.
Exercise to improve muscle dysfunction.
Exercise has beneficial effects on OSA.72,73 Aside from reducing lower extremity edema, both systemic and oropharyngeal exercises have been shown to improve snoring and severity of OSA, presumably by strengthening the tongue.74 In an observational study, playing the didgeridoo, an Australian Aboriginal wind instrument in the form of a long wooden tube, improved OSA.75 This result was followed by RCTs in the general population, in those with HF or CAD. There are 3 RCTs, 1 in patients with CAD discussed in the preceding text68 and 2 in patients with HF.75,76 In a 4-arm, parallel design RCT75 compared with the control and CPAP arms, only aerobic exercise was associated with significant collective improvement in subjective daytime sleepiness, quality of life (Minnesota Living with Heart Failure Questionnaire and Short Form [36] Health Survey), and NYHA functional class (P < 0.05), even though CPAP was most effective in reducing in AHI.
There are also 2 meta-analyses, 1 with oropharyngeal and 1 with systemic exercise, confirming the results of the aforementioned RCTs. In the first meta-analysis77 involving 120 adults, selective oropharyngeal exercises reduced snoring and AHI by 50% (25 to 12 events/h of sleep). Similar results were observed with systemic exercise.78
Based on the aforementioned studies, in the management of patients with OSA, exercise should routinely be recommended, when applicable. Further, exercise could be a therapeutic option for OSA patients with CVD who refuse CPAP or are intolerant to it. The beneficial effects of exercise are multifactorial and could also be related to decreased rostral fluid redistribution increasing pharyngeal air space discussed earlier in the text, weight loss (if it occurs), and improvement in ventilatory control if up-regulated.78
Medications to improve muscle dysfunction.
Multiple medications79–83 have been studied in search of discovering a pharmacological therapy that would be appealing from the patient’s point of view. To date, a noradrenergic agent, atomoxetine, combined with an antimuscarinic agent (oxybutynin), has been the most extensively studied. This combination is based on the premise that noradrenergic activity is reduced with sleep onset leading to reduced pharyngeal muscle firing and withdrawal of excitatory amines, an inhibitory muscarinic pathway occurring during REM sleep. In OSA patients, the combination increases genioglossus muscle by 3-fold while asleep.80 In a recent RCT incorporating 209 patients with OSA, the combination of atomoxetine with oxybutynin81 was quite effective in improving OSA (AHI improvement of 6 events/h in the intention-to-treat analyses). However, there was a small rise in diastolic BP. Two phase III, multisite, RCTs (Parallel Arm Trial of AD109 and Placebo With Patients With OSA [LunAIRo], NCT05811247; Parallel-Arm Study to Compare AD109 to Placebo With Patients With OSA [SynAIRgy Study], NCT05813275) in which patients are randomized to the combination therapy or placebo over 6 to 12 months are ongoing. In a subset, 24-hour ambulatory blood pressure will be monitored to determine any potential beneficial effect.
THERAPEUTIC OPTIONS ADDRESSING ELEVATED LOOP GAIN.
Loop gain (LG) is an engineering term that defines the gain of the negative feedback loop and regulates ventilation in response to a ventilatory disturbance (Central Illustration).84,85 In the context of OSA, and consequent on a reduction in ventilation, if the magnitude of the compensatory increase in ventilation is greater than the magnitude of the preceding apnea or hypopnea, then the system is highly unstable and will fluctuate between under- and overventilation, promoting both OSA85 and central sleep apnea.86 This situation of overshoots and undershoots is characteristic of a high LG, and down-regulating these oscillations (ie, lowering gain) should improve OSA. Pharmacologically, carbonic anhydrase inhibitors79,87–92 and nocturnal supplemental oxygen93 have been used in OSA trials to lower LG.
Breathing supplemental oxygen decreases the hypoxic and hypercapnic ventilatory response, down-regulating an elevated chemical LG mediated by the chemoreceptors. Given that OSA-related hypoxemic burden is a main determinant of incident CVD, it is expected that treatment of OSA with oxygen could down-regulate a high LG and improve OSA-related CV outcomes. However, in 1 RCT of 3 months’ duration, Gottlieb et al93 randomized patients with OSA to either nocturnal supplemental oxygen or CPAP. At 3 months, compared with CPAP, oxygen was ineffective in lowering the BP, but these patients had mild nocturnal desaturation, and only severe OSA is associated with excess CV mortality. Of note, the patients with high LG had the greatest reduction in BP in a recent post hoc analysis.92
Both short- and long-acting acetazolamide improve LG and have been shown to improve OSA.79,87–92 In a cross-over RCT comparing CPAP, acetazolamide, and CPAP plus acetazolamide, contrary to CPAP, acetazolamide induced a significant reduction of systolic BP and vascular stiffness. Notably, in contrast to 1 meta-analysis,89 a second one91 did not show an improvement in OSA treated with acetazolamide.
IMPACT OF TREATMENT OF OSA WITH CPAP IN WOMEN
The sex-specific differences and considerations in the treatment of OSA in relation to CVD remain under-studied. That said, a retrospective cohort study of over 1,000 women over a 5-year follow-up period showed that severe OSA was associated with a 3-fold increased likelihood of CV death, whereas effective CPAP therapy reduced CV-specific mortality.94 Notably, women with moderate OSA have greater benefit in profiles of circulating biomarkers of inflammation and oxidative stress in post hoc clinical trial data with PAP intervention,95 findings not observed in men, thereby providing unique biological insights.
IMPACT OF TREATMENT OF OSA WITH CPAP AND OTHER OPTIONS IN CHILDREN
In children, both enlarged tonsils and obesity are intimately associated with OSA. Several studies over the last 2 decades have indicated that OSA in children is associated with increased risk of systemic hypertension, dyslipidemia, and endothelial dysfunction.96 The increased risk for these morbid consequences may not only impose an immediate adverse effect on CV health during childhood but may also promote the accelerated development of adverse CV outcomes in adulthood.
Adenotonsillectomy, CPAP, and gastric bypass surgery (when concurrent morbid obesity is present) have been used to treat OSA in children. Adenotonsillectomy and weight loss improve OSA by increasing pharyngeal volume (Central Illustration). Adenotonsillectomy is the first-line intervention in the management of children diagnosed with OSA. Improvements in cardiac function and structure, BP, some biomarkers of CV risk, and endothelial dysfunction have all been reported, albeit not consistently, requiring further RCTs.97
Prevalence of obesity, a major risk factor for OSA, is on the rise in children. A systematic review98 incorporating 29 cohort studies with a total population of 4,970 obese children who underwent sleeve gastrectomy or RYGB surgery, along with a follow-up study at least 5 years or beyond, showed improvement in BMI, OSA severity, as well as significant remission of type 2 diabetes mellitus, dyslipidemia, and hypertension.
CPAP is recommended for children with OSA if adenotonsillectomy is not performed or if OSA remains unresolved after this surgery. A prospective study99 in children with OSA reported that CPAP improved OSA over a 1-year period. Epworth Sleepiness Scale scores improved significantly at 1 year from baseline (14 to 8; P = 0.001). However, CPAP is also more effective than adenotonsillectomy in lowering BP in hypertensive children.
Like adults, CPAP adherence rate in children with OSA is variable.100 Young age, lower BMI, and high AHI are associated with acceptable CPAP adherence. Nevertheless, implementation of behavioral modifications of both child and caretakers as part of the initiation of CPAP is usually associated with improved short-term and long-term adherence. In certain cases where CPAP is poorly tolerated, use of high-flow air via a nasal cannula101 has been highly effective. There are several mechanisms by which high-flow air via a nasal cannula could improve clinical outcomes in children101: 1) reduced anatomical dead space and consequently improved gas mixing in large airways; 2) heating and humidification of inhaled gas; 3) decreased nasal resistance and high nasal inspiratory flow; 4) generation of positive airway pressure (2 to 4 cm H2O) that results in increased end-expiratory lung volume; and 5) increased alveolar PO2.
FUTURE DIRECTIONS BASED ON THE LESSONS LEARNED FROM THE RCTs USING CPAP FOR PREVENTION OF INCIDENT CVD
Although large observational studies have reported that CPAP treatment has a beneficial effect on both primary and secondary prevention of major CVD,17 recent RCTs treating OSA with CPAP have been neutral. In 3 RCTs,102–104 with the primary outcome being the composite of all-cause mortality, CV mortality, stroke, unstable angina, new-onset AF, myocardial infarction, and HF, the combined, fully adjusted HRs were 0.81 (95% CI: 0.45–1.46), 1.05 (95% CI: 0.84–1.33), and 0.90 (95% CI: 0.70–1.16), respectively.
A similar conclusion was reached in another RCT in participants with acute coronary syndrome.105
In 2024, a critical question facing the field of cardiology and sleep is whether treatment of OSA with CPAP is effective in preventing incident primary or secondary CVD, or whether OSA is simply a bystander. It has been proposed that it has been the design/implementation of the studies, and particularly the low CPAP adherence, that led to neutral results.106
We106 and others107 have proposed that several factors may have contributed to the null results. First, for ethical reasons, many subjects with severe OSA and hypoxemia were excluded even though only the severity of OSA (AHI ≥30 events/h of sleep)108,109 and the severity of hypoxemia are associated with CV mortality. Another ethical exclusion criterion was presence of excessive sleepiness. Notably, it has been shown that sleepy OSA patients are at increased risk for CV mortality as compared with nonsleepy patients (for details, see references106,107). Perhaps, the most important reason for the null result has been poor adherence with PAP devices. In these trials, the average nightly use of the device was <4 hours, less than the minimal accepted criterion. Because intention-to-treat analysis has been used to determine outcomes, lack of adherence by some participants could have contributed to the null results; a post hoc analysis of RCTs comparing PAP users with nonadherent participants could shed light on this supposition. Indeed, the critical role of adequate adherence has been highlighted in 2 meta-analyses: In the first meta-analysis110 of 3 RCTs including 4,186 patients with individual participant data showed that on intention-to-treat analysis, comparing the CPAP group with the UC group had no effect on the first major adverse cardiocerebrovascular event (HR: 1.01 [95% CI: 0.87–1.17]). This finding is consistent with the intention-to-treat analysis of individual RCTs. However, an on-treatment analysis revealed a reduced risk of major adverse cardiocerebrovascular events associated with good adherence to CPAP (HR: 0.69 [95% CI: 0.52–0.92]).
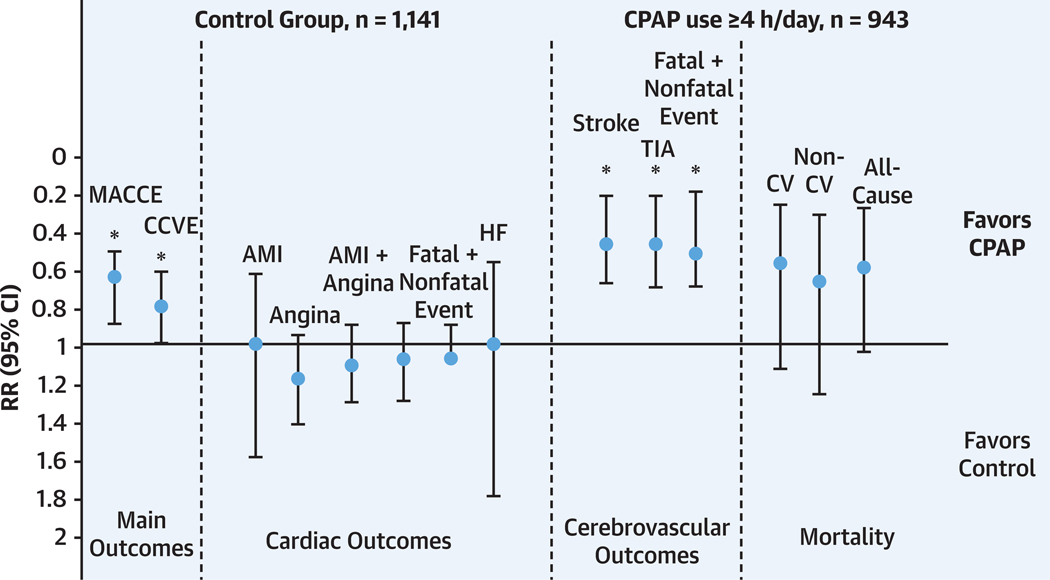
In the second meta-analysis111 of 5 RCTs using per protocol use of CPAP (ie, use of CPAP (≥4 h/d, good CPAP adherence), we compared 943 OSA patients who were CPAP adherent to 1,114 patients with UC alone, the control group. At least 4 h/night, compared with no CPAP therapy, improved the primary composite outcome (HR: 0.68 [95% CI: 0.50–0.92]; P = 0.01), with no significant heterogeneity between the included studies. In this meta-analysis, the effect was primarily on cerebrovascular composite outcome (Figure 4). One difference between the 2 meta-analyses is that we included 2 RCTs that addressed patients with cerebrovascular disease. In the other meta-analysis, the investigators did not stratify between CV and cerebrovascular events. Here, we must emphasize, in sensitivity analyses of the aforementioned RCTs, healthy user bias could have influenced the beneficial outcome, as people that were adherent might have had other differences from those that are nonadherent.
FIGURE 4. Effective Use of CPAP Improves Cerebrocardiovascular Outcomes and All-Cause Mortality.
The y-axis shows the risk ratio (RR) with 95% CI; the x-axis shows the different types of individual and composite cardiovascular (CV) events. *P < 0.05. Reprinted with permission of the American Thoracic Society.111 AMI = acute myocardial infarction; CCVE = cardiocerebrovascular event; CPAP = continuous positive airway pressure; HF = heart failure; MACCE = major adverse cardiocerebrovascular events; TIA = transient ischemic attack.
To go forward, several possibilities have been suggested. One alternative is to enroll sleepy patients with OSA and treat them with approved wake-promoting medications.106 This is done routinely in the management of sleepy OSA patients, given that close to 15% of patients with OSA who are very adherent to CPAP remain sleepy.112 Another alternative approach is propensity matching.107 Comparative effectiveness approaches and adaptive trial designs may be used to overcome some ethical concerns of long-term trials of patients not receiving active treatment.
Finally, as discussed in the preceding text, 2 meta-analyses have concluded that the key reason explaining the lack of protective effect of CPAP in RCTs has been the low adherence. One way to overcome this is to conduct a trial with a design similar to cardiology RCTs that have used a running-in period and only enrolled those who were adherent or showed no side effects of a particular medication used in the trial.113 Therefore, a long run-in period with sham CPAP and exclusion of nonadherent subjects would allow enrollment of patients with adequate CPAP adherence. CPAP adherence could be further enhanced taking advantage of educational, supportive, and behavioral interventions.
Multiple recent studies have suggested that OSA is a heterogeneous disorder (Central Illustration) such that OSA heterogeneity may have contributed to the null effect of CPAP, diluting its effect. Aside from AHI that has been used as the main criterion for participation in previous RCTs, new polysomnographic biomarkers have evolved heralding poor prognosis and predictors of OSA-reflated CV mortality.114
We already have alluded to hypoxic burden, which has been associated with hard CV outcomes,108 and notably such individuals were excluded in previous RCTs. But in 1 study,92 administration of nocturnal supplemental oxygen failed to decrease 24-hour BP, this in contrast to CPAP. However, these individuals had very mild oxygen desaturation, and the results are not surprising. Any future study should not exclude patients who experience severe hypoxemia during sleep due to OSA, and the appropriate dose of oxygen needs to be determined during sleep studies. Another polysomnographic biomarker of OSA is the autonomic response to obstructive events. Although during apnea, heart rate decreases (similar to the diving reflex), post-event, there is an abrupt rise in heart rate, which is further augmented if the respiratory event is also associated with electroencephalographic cortical arousal.115 This rise in the heart rate has been associated with CV mortality.116
Another biomarker, which has been shown to predict BP response to CPAP treatment in patients with resistant hypertension and OSA, is a singular cluster of 3 plasma microRNAs.117 An RCT using this biomarker in a placebo design could determine the critical role (or absence thereof) of this biomarker in resistant hypertension.
FUTURE DIRECTIONS FOR NON-PAP THERAPY OF INSOMNIA AND OSA
Given the high prevalence of insomnia and its adverse CV consequences, either insomnia alone or with OSA (COMISA [Comorbid Insomnia and Sleep Apnea]), the field needs large systematic placebo-controlled RCTs to treat insomnia with approved DORAS to determine potential improvement in hard CV outcomes and neurocognitive dysfunction.
Exercise has been shown to strengthen the tongue and, based on the current literature, could be potentially curative for treatment of mild-to-moderate OSA. This needs to be determined in a long-term trial that should include measures of CV biomarkers and 24-hour BP monitoring as the first approach.
There is also a need for more robust studies for comparative effectiveness of treatment of OSA relative to CPAP for CV outcomes. Examples include MAD devices and HNS. Although CPAP is more effective than MADs in reducing the AHI, there is a growing body of evidence demonstrating comparable benefits between MADs and CPAP in ameliorating OSA associated CV risk. The repeated failure to demonstrate the CV benefits of CPAP due to poor adherence, and the emergence of MADs as a viable alternative, suggest it is time to reconsider the relative merits of CPAP and MADs. So far, most of the studies on the effects of MADs on CV risk have been small-scale and have focused on BP reduction. Further evaluation of the potential of MADs in improving CV outcomes are urgently needed.
Similarly, HNS has been used for treatment of OSA in the CPAP intolerant or those who refuse CPAP, but there are no systematic studies to demonstrate its effectiveness on any hard outcomes of CVD, bio-markers of CVD or hypertension. Therefore, a large-scale RCT is needed with hard outcomes as the primary endpoint.
As mentioned throughout this review, different pathophysiological mechanisms in addition to the classical anatomical burden have been shown to contribute to the obstruction of the upper airway. In fact, up to two-thirds of OSA patients may have 1 or more nonanatomic pathophysiologic mechanisms involved in upper airway collapse.69 If the mechanisms involved in upper airway obstruction (Central Illustration) were easy to identify in clinical practice using data obtained from routine sleep studies without specialized equipment or interventions, a tailored, personalized OSA therapy could be provided, including some non-CPAP therapies, discussed earlier, as first-line treatments. However, further research is needed to define phenotypic subgroups of patients who are most amenable to the different available OSA therapies.
Last, manipulation of gut microbiota could have a therapeutic role.118–125 OSA has been implicated in inducing adverse changes in the gut microbiome in both animal models and epidemiologic studies. Considering the relatively modest or even disappointing results reported by RCTs with CPAP treatment of OSA and prevention of CV adverse outcomes, interventions aimed at reversing the gut dysbiosis have been advanced as viable adjuvant therapy to PAP118 At this time, no prospective RCT has been conducted to examine this issue. However, some preliminary findings from murine models are encouraging. Indeed, Lactobacillus rhamnosus GG administration in a rodent model of high-fat diet and OSA resulted in increased expression of nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant pathways along with reduced myocardial remodeling as well as mitigation of systemic hypertension.119 Similarly, exogenous acetate administration in a rodent model of OSA ameliorated the systemic hypertension attributable to gut dysbiosis.120 Probiotic treatment consisting of a mixture of 8 different probiotic microorganisms markedly attenuated or even abolished the adverse effects of fecal microbiota transfer from intermittent hypoxia exposed mice to naive mice on systemic BP and coronary artery vascular function. Thus, expanded exploration of the putative microorganisms underlying protective CV functions in the context of sleep-disordered breathing, and randomized trials incorporating individualized pre- and probiotic strategies as adjuvant management strategies of PAP-treated OSA patients seem warranted.117,118
WOMEN AND OSA
Future investigations should focus on sex-specific endophenotypes indicative of PAP responsivity; the effect of different types of OSA treatment including HNS and oral appliances on CV outcomes in women vs men; and the benefit of treatment of REM-related OSA given its predilection to women and sex-specific differences in CV outcome type.
CONCLUSIONS
Considerable progress has been made regarding our understanding of sleep health and its impact on the CV system. Although the mechanisms linking sleep apnea to cardiometabolic risk are well established, definitive randomized trials showing improvements in hard outcomes with interventions such as CPAP are still lacking. Such studies are needed in children and adults including women, as well as those with OHS, overlap syndrome and OSA comorbid with diabetes mellitus type 2, and with HFpEF. Studies in women, particularly during pregnancy, are also lacking. This review has discussed the pathophysiologic mechanisms underlying the relationship between OSA and cardiocerebrovascular disease, potential reasons for null results of RCTs treating OSA with CPAP, and suggested approaches for future trials.
HIGHLIGHTS.
Chronic insomnia is a common disorder and could lead to CVD and neurocognitive dysfunction.
Limited number of studies suggest that treatment of insomnia, with or without comorbid OSA, could improve sleep and potential CV outcomes. Large RCTs are needed.
OSA plays a major role causing or contributing to the progression of CVD.
Post hoc analyses of RCTs treating OSA with CPAP show that adequate CPAP users benefit from a reduction in incident CVD.
The field is in need of large RCTs with CPAP and other devices with hard CV endpoints.
FUNDING SUPPORT
ResMed provided a philanthropic donation to UC San Diego. Dr Shahrokh Javaheri has received income related to medical education from Res Med, Jazz, Idorsia, Eli Lilly, and Avadel Pharmaceutical; and is a consultant to Zoll-Respicardia. Dr Sogol Javaheri has received grant funding from Zoll and the Massachusetts Technology Collaborative; and is supported by an internal health equity grant from Harvard Medical School. Dr Mehra has received an honorarium from the American Academy of Sleep Medicine; has received funds for service on the American Board of Internal Medicine writing group; has received NIH funding; and has received royalties from Up to Date. Dr Somers is supported by National Institutes of Health (NIH) grant HL65176; has served as a consultant for Bayer, Jazz Pharmaceuticals, Huxley, Apnimed, ResMed, Lilly, and Respicardia; and is on the Sleep Number Scientific Advisory Board. Dr Zee is a consultant to Eisai, Idorsia, Jazz, and Harmony; and has received institutional grants from Vanda and Sleep Number. Dr Cistulli has an appointment to an endowed academic Chair at the University of Sydney that was established from ResMed funding; has received research support from ResMed and SomnoMed; is a consultant to ResMed, SomnoMed, Signifier Medical Technologies, Bayer, and Sunrise Medical; and has a pecuniary interest in SomnoMed related to a role in research and development (2004). Dr Malhotra is funded by the NIH; and has received income related to medical education from Powell Mansfield, Livanova, Eli Lilly, and Zoll.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- AHI
Apnea-Hypopnea Index
- ASV
adaptive servoventilation
- BMI
body mass index
- BP
blood pressure
- CAD
coronary artery disease
- CBTi
cognitive behavioral therapy for insomnia
- CHF
congestive heart failure
- COMISA
obstructive sleep apnea comorbid with insomnia
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure
- CV
cardiovascular
- CVD
cardiovascular disease
- DORA
dual-orexin receptor antagonist
- FDA
U.S. Food and Drug Administration
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- HNS
hypoglossal nerve stimulation
- LG
loop gain
- MAD
mandibular advancement device
- NIV
noninvasive ventilation
- OHS
obesity hypoventilation syndrome
- OSA
obstructive sleep apnea
- PAH
pulmonary arterial hypertension
- PAP
positive airway pressure
- PH
pulmonary hypertension
- RCT
randomized controlled trial
- RV
right ventricular
- RYGB
Roux-en-Y gastric bypass
- UC
usual care
Footnotes
AUTHOR DISCLOSURES
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23(8):3555–3560. 10.1523/JNEUROSCI.23-08-03555.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javaheri S, Reid M, Drerup M, Mehra R, Redline S. Reducing coronary heart disease risk through treatment of insomnia using web-based cognitive behavioral therapy for insomnia: a methodological approach. Behav Sleep Med. 2020;18:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris KM, Schiele SE, Emery CF. Pilot randomized trial of brief behavioral treatment for insomnia in patients with heart failure. Heart Lung. 2019;48:373–380. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Yang Y, Li Q, et al. The impact of the improvement of insomnia on blood pressure in hypertensive patients. J Sleep Res. 2017;26:105–114. [DOI] [PubMed] [Google Scholar]
- 5.Sweetman A, Lack L, McEvoy D. Refining the measurement of insomnia in patients with obstructive sleep apnea. J Clin Sleep Med. 2019;15: 1717–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechat B, Appleton S, Melaku YA, et al. Comorbid insomnia and sleep apnoea is associated with all-cause mortality. Eur Respir J. 2022;60(1): 2101958. 10.1183/13993003.01958-2021 [DOI] [PubMed] [Google Scholar]
- 7.Sweetman A, Lack L, McEvoy RD, et al. Cognitive behavioural therapy for insomnia reduces sleep apnoea severity: a randomised controlled trial. ERJ Open Res. 2020;6(2):00161–2020. 10.1183/23120541.00161-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechat B, Appleton S, Melaku YA, et al. The association of comorbid insomnia and sleep apnea with prevalent cardiovascular disease and incident cardiovascular events. J Sleep Res. 2022;31:e13563. [DOI] [PubMed] [Google Scholar]
- 9.Ong JC, Crawford MR, Dawson SC, et al. A randomized controlled trial of CBT-I and PAP for obstructive sleep apnea and comorbid insomnia: main outcomes from the MATRICS study. Sleep. 2020;43(9):zsaa041. 10.1093/sleep/zsaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn MA. Hypnotics and the control of breathing: a review. Br J Clin Pharmacol. 1983;16(suppl 2):245S–250S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boof ML, Dingemanse J, Lederer K, Fietze I, Ufer M. Effect of the new dual orexin receptor antagonist daridorexant on nighttime respiratory function and sleep in patients with mild and moderate obstructive sleep apnea. Sleep. 2021;44(6):zsaa275. 10.1093/sleep/zsaa275 [DOI] [PubMed] [Google Scholar]
- 12.Boof ML, Dingemanse J, Brunke M, et al. Effect of the novel dual orexin receptor antagonist daridorexant on night-time respiratory function and sleep in patients with moderate chronic obstructive pulmonary disease. J Sleep Res. 2021;30:e13248. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Palcza J, Card D, et al. Effects of suvorexant, an orexin receptor antagonist, on respiration during sleep in patients with obstructive sleep apnea. J Clin Sleep Med. 2016;12:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mignot E, Mayleben D, Fietze I, et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21:125–139. [DOI] [PubMed] [Google Scholar]
- 15.Cheng JY, Lorch D, Lowe AD, et al. A randomized, double-blind, placebo-controlled, crossover study of respiratory safety of lembor-exant in moderate to severe obstructive sleep apnea. J Clin Sleep Med. 2024;20:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilar D. Preventing heart failure in people with hypertension: the time is now. JACC Heart Fail. 2023;11:689–690. [DOI] [PubMed] [Google Scholar]
- 17.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masa JF, Mokhlesi B, Benitez I, et al. Echo-cardiographic changes with positive airway pressure therapy in obesity hypoventilation syndrome. Long-term Pickwick randomized controlled clinical trial. Am J Respir Crit Care Med. 2020;201:586–597. [DOI] [PubMed] [Google Scholar]
- 20.Lowery MM, Hill NS, Wang L, et al. Sleep-related hypoxia, right ventricular dysfunction, and survival in patients with group 1 pulmonary arterial hypertension. J Am Coll Cardiol. 2023;82: 1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183:539–546. [DOI] [PubMed] [Google Scholar]
- 22.Usui K, Bradley TD, Spaak J, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. 2005;45:2008–2011. [DOI] [PubMed] [Google Scholar]
- 23.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. [DOI] [PubMed] [Google Scholar]
- 24.Bradley TD, Logan AG, Lorenzi Filho G, et al. Adaptive servo-ventilation for sleep-disordered breathing in patients with heart failure with reduced ejection fraction (ADVENT-HF): a multi-centre, multinational, parallel-group, open-label, phase 3 randomised controlled trial. Lancet Respir Med. 2024;12:153–166. [DOI] [PubMed] [Google Scholar]
- 25.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arias MA, Garcia-Rio F, Alonso-Fernandez A, et al. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112:375–383. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol. 2017;69(12):1577–1587. 10.1016/j.jacc.2017.01.041 [DOI] [PubMed] [Google Scholar]
- 28.Cistulli PA, Malhotra A, Cole KV, et al. Positive airway pressure therapy adherence and health care resource use in patients with obstructive sleep apnea and heart failure with preserved ejection fraction. J Am Heart Assoc. 2023;12:e028733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhotra A, Cole KV, Malik AS, et al. Positive airway pressure adherence and health care resource utilization in patients with obstructive sleep apnea and heart failure with reduced ejection fraction. J Am Heart Assoc. 2023;12:e028732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masa JF, Mokhlesi B, Benitez I, et al. Long-term clinical effectiveness of continuous positive airway pressure therapy versus non-invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open-label, randomised controlled trial. Lancet. 2019;393:1721–1732. [DOI] [PubMed] [Google Scholar]
- 31.Mokhlesi B, Masa JF, Brozek JL, et al. Evaluation and management of obesity hypoventilation syndrome. an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2019;200:e6–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raphelson JR, Schmickl CN, Sonners C, et al. Obesity hypoventilation syndrome and post-surgical outcomes in a bariatric surgery cohort. Obes Surg. 2022;32:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;9:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325–331. [DOI] [PubMed] [Google Scholar]
- 35.Macrea M, Oczkowski S, Rochwerg B, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202:e74–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bamagoos AA, Cistulli PA, Sutherland K, et al. Polysomnographic endotyping to select patients with obstructive sleep apnea for oral appliances. Ann Am Thorac Soc. 2019;16:1422–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bamagoos AA, Cistulli PA, Sutherland K, et al. Dose-dependent effects of mandibular advancement on upper airway collapsibility and muscle function in obstructive sleep apnea. Sleep. 2019;42(6):zsz049. 10.1093/sleep/zsz049 [DOI] [PubMed] [Google Scholar]
- 38.Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11:773–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trzepizur W, Cistulli PA, Glos M, et al. Health outcomes of continuous positive airway pressure versus mandibular advancement device for the treatment of severe obstructive sleep apnea: an individual participant data meta-analysis. Sleep. 2021;44(7):zsab015. 10.1093/sleep/zsab015 [DOI] [PubMed] [Google Scholar]
- 40.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280–2293. [DOI] [PubMed] [Google Scholar]
- 41.de Vries GE, Wijkstra PJ, Houwerzijl EJ, Kerstjens HAM, Hoekema A. Cardiovascular effects of oral appliance therapy in obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:55–68. [DOI] [PubMed] [Google Scholar]
- 42.Dissanayake HU, Colpani JT, Sutherland K, et al. Obstructive sleep apnea therapy for cardio-vascular risk reduction–time for a rethink? Clin Cardiol. 2021;44:1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McTigue K, Larson JC, Valoski A, et al. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. [DOI] [PubMed] [Google Scholar]
- 44.Kim AM, Keenan BT, Jackson N, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37:1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quintas-Neves M, Preto J, Drummond M. Assessment of bariatric surgery efficacy on obstructive sleep apnea (OSA). Rev Port Pneumol (2006). 2016;22(6):331–336. 10.1016/j.rppnen.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 47.Furlan SF, Drager LF, Santos RN, et al. Three-year effects of bariatric surgery on obstructive sleep apnea in patients with obesity grade 1 and 2: a sub-analysis of the GATEWAY trial. Int J Obes (Lond). 2021;45(4):914–917. 10.1038/s41366-021-00752-2 [DOI] [PubMed] [Google Scholar]
- 48.Peromaa-Haavisto P, Luostarinen M, Juusela R, Tuomilehto H, Kossi J. Obstructive sleep apnea: the effect of bariatric surgery after five years–a prospective multicenter trial. Obes Surg. 2024;34:1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenberg E, Ottosson J, Naslund E. Remission of obesity-related sleep apnea and its effect on mortality and cardiovascular events after metabolic and bariatric surgery: a propensity matched cohort study. J Am Coll Surg. 2024;239(2):77–84. 10.1097/XCS.0000000000001047 [DOI] [PubMed] [Google Scholar]
- 50.Elmaleh-Sachs A, Schwartz JL, Bramante CT, et al. Obesity management in adults: a review. JAMA. 2023;330:2000–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grunstein RR, Wadden TA, Chapman JL, et al. Giving weight to incretin-based pharmacotherapy for obesity-related sleep apnea: a revolution or a pipe dream? Sleep. 2023;46(10):zsad224. 10.1093/sleep/zsad224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384: 989–1002. [DOI] [PubMed] [Google Scholar]
- 53.Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–515. [DOI] [PubMed] [Google Scholar]
- 54.Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. [DOI] [PubMed] [Google Scholar]
- 55.Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40:1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abbasi J. FDA green-lights tirzepatide, marketed as Zepbound, for chronic weight management. JAMA. 2023;330:2143–2144. [DOI] [PubMed] [Google Scholar]
- 57.Malhotra A, Grunstein RR, Fietze I, et al. , SURMOUNT-OSA Investigators. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N Engl J Med. Published online June 21, 2024. 10.1056/NEJMoa2404881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Javaheri S, Simbartl LA. The association between low CO2 production and central sleep apnea in men with heart failure. Ann Am Thorac Soc. 2023;20:721–727. [DOI] [PubMed] [Google Scholar]
- 59.Kakazu MT, Soghier I, Afshar M, et al. Weight loss interventions as treatment of obesity hypoventilation syndrome. a systematic review. Ann Am Thorac Soc. 2020;17(4):492–502. [DOI] [PubMed] [Google Scholar]
- 60.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389: 2221–2232. [DOI] [PubMed] [Google Scholar]
- 61.Reddy YNV, Anantha-Narayanan M, Obokata M, et al. Hemodynamic effects of weight loss in obesity: a systematic review and meta-analysis. JACC Heart Fail. 2019;7:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 63.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 64.Redolfi S, Arnulf I, Pottier M, Bradley TD, Similowski T. Effects of venous compression of the legs on overnight rostral fluid shift and obstructive sleep apnea. Respir Physiol Neurobiol. 2011;175:390–393. [DOI] [PubMed] [Google Scholar]
- 65.Redolfi S, Arnulf I, Pottier M, et al. Attenuation of obstructive sleep apnea by compression stockings in subjects with venous insufficiency. Am J Respir Crit Care Med. 2011;184:1062–1066. [DOI] [PubMed] [Google Scholar]
- 66.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–246. [DOI] [PubMed] [Google Scholar]
- 67.Bucca CB, Brussino L, Battisti A, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132:440–446. [DOI] [PubMed] [Google Scholar]
- 68.Mendelson M, Lyons OD, Yadollahi A, Inami T, Oh P, Bradley TD. Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur Respir J. 2016;48: 142–150. [DOI] [PubMed] [Google Scholar]
- 69.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–149. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz AR, Jacobowitz O, Eisele DW, et al. Targeted hypoglossal nerve stimulation for patients with obstructive sleep apnea: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2023;149:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Awad KM, Malhotra A, Barnet JH, et al. Exercise is associated with a reduced incidence of sleep-disordered breathing. Am J Med. 2012;125: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guimaraes KC, Drager LF, Genta PR, et al. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179:962–966. [DOI] [PubMed] [Google Scholar]
- 74.Puhan MA, Suarez A, Lo Cascio C, et al. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. BMJ. 2006;332:266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Servantes DM, Javaheri S, Kravchychyn ACP, et al. Effects of exercise training and CPAP in patients with heart failure and OSA: a preliminary study. Chest. 2018;154:808–817. [DOI] [PubMed] [Google Scholar]
- 76.Ueno LM, Drager LF, Rodrigues AC, et al. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep. 2009;32: 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camacho M, Certal V, Abdullatif J, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38:669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hedner J, Zou D. Drug therapy in obstructive sleep apnea. Sleep Med Clin. 2018;13:203–217. [DOI] [PubMed] [Google Scholar]
- 80.Schmickl CN, Edwards BA, Malhotra A. Drug therapy for obstructive sleep apnea: are we there yet? Am J Respir Crit Care Med. 2022;205(12): 1379–1381. 10.1164/rccm.202202-0301ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taranto-Montemurro L, Messineo L, Sands SA, et al. The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity. A randomized, placebo-controlled, double-blind crossover trial. Am J Respir Crit Care Med. 2019;199:1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schweitzer PK, Taranto-Montemurro L, Ojile JM, et al. The combination of aroxybutynin and atomoxetine in the treatment of obstructive sleep apnea (MARIPOSA): a randomized controlled trial. Am J Respir Crit Care Med. 2023;208(12): 1316–1327. 10.1164/rccm.202306-1036OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taranto-Montemurro L, Messineo L, Azarbarzin A, et al. Effects of the combination of atomoxetine and oxybutynin on OSA endotypic traits. Chest. 2020;157:1626–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khoo M. Determinants of ventilatory instability and variability. Respir Phsiol. 2000;122:167–182. [DOI] [PubMed] [Google Scholar]
- 85.Khoo MC. Using loop gain to assess ventilatory control in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1044–1045. [DOI] [PubMed] [Google Scholar]
- 86.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141–163. 10.1002/cphy.c110057 [DOI] [PubMed] [Google Scholar]
- 87.Edwards BA, Connolly JG, Campana LM, et al. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep. 2013;36:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590(5):1199–1211. 10.1113/jphysiol.2011.223925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmickl CN, Landry S, Orr JE, et al. Effects of acetazolamide on control of breathing in sleep apnea patients: mechanistic insights using meta-analyses and physiological model simulations. Physiol Rep. 2021;9:e15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmickl CN, Landry SA, Orr JE, et al. Acetazolamide for OSA and central sleep apnea: a comprehensive systematic review and meta-analysis. Chest. 2020;158:2632–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ni YN, Yang H, Thomas RJ. The role of acetazolamide in sleep apnea at sea level: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(6):1295–1304. 10.5664/jcsm.9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmickl CN, Orr JE, Sands SA, et al. Loop gain as a predictor of blood pressure response in patients treated for obstructive sleep apnea. Ann Am Thorac Soc. 2024;21(2):296–307. 10.1513/AnnalsATS.202305-437OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campos-Rodriguez F, Queipo-Corona C, Carmona-Bernal C, et al. Continuous positive airway pressure improves quality of life in women with OSA. A randomized-controlled trial. Am J Respir Crit Care Med. 2016;194(10):1286–1294. 10.1164/rccm.201602-0265OC [DOI] [PubMed] [Google Scholar]
- 95.May AM, Wang L, Strohl KP, Walia H, Hazen SL, Mehra R. Sex-specific differential responses of circulating biomarkers in obstructive sleep apnea treatment. A post hoc analysis of a randomized controlled trial. Ann Am Thorac Soc. 2020;17(5):605–613. 10.1513/AnnalsATS.201908-593OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borreguero D, Izquierdo JL, Mediano O. Impact of obstructive sleep apnea in cardiovascular risk in the pediatric population: a systematic review. Sleep Med Rev. 2023;71:101818. 10.1016/j.smrv.2023.101818 [DOI] [PubMed] [Google Scholar]
- 97.Barlow SE. Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 98.Wu Z, Gao Z, Qiao Y, et al. Long-term results of bariatric surgery in adolescents with at least 5 years of follow-up: a systematic review and meta-analysis. Obes Surg. 2023;33(6):1730–1745. 10.1007/s11695-023-06593-4 [DOI] [PubMed] [Google Scholar]
- 99.Sudarsan SS, Paramasivan VK, Arumugam SV, et al. Comparison of treatment modalities in syndromic children with obstructive sleep apnea–a randomized cohort study. Int J Pediatr Otorhinolaryngol. 2014;78(9):1526–1533. 10.1016/j.ijporl.2014.06.027 [DOI] [PubMed] [Google Scholar]
- 100.Sawunyavisuth B, Ngamjarus C, Sawanyawisuth K. Adherence to continuous positive airway pressure therapy in pediatric patients with obstructive sleep apnea: a meta-analysis. Ther Clin Risk Manag. 2023;19:143–162. 10.2147/TCRM.S358737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arora B, Mahajan P, Zidan MA, Sethuraman U. Nasopharyngeal airway pressures in bronchiolitis patients treated with high-flow nasal cannula oxygen therapy. Pediatr Emerg Care. 2012;28:1179–1184. [DOI] [PubMed] [Google Scholar]
- 102.McEvoy RD, Antic NA, Heeley E, et al. , SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 103.Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbé F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1(1):61–72. 10.1016/S2213-2600(12)70051-6 [DOI] [PubMed] [Google Scholar]
- 104.Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- 105.Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, et al. , Spanish Sleep Network. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8(4):359–367. 10.1016/S2213-2600(19)30271-1 [DOI] [PubMed] [Google Scholar]
- 106.Javaheri S, Martinez-Garcia MA, Campos-Rodriguez F. CPAP treatment and cardiovascular prevention: we need to change the design and implementation of our trials. Chest. 2019;156:431–437. [DOI] [PubMed] [Google Scholar]
- 107.Pack AI, Magalang UJ, Singh B, Kuna ST, Keenan BT, Maislin G. Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: understanding and overcoming bias. Sleep. 2021;44(2):zsaa229. 10.1093/sleep/zsaa229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardio-vascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40(14):1149–1157. 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6: e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanchez-de-la-Torre M, Gracia-Lavedan E, Benitez ID, et al. Adherence to CPAP treatment and the risk of recurrent cardiovascular events: a meta-analysis. JAMA. 2023;330:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Javaheri S, Martinez-Garcia MA, Campos Rodriguez F, Muriel A, Peker Y. Continuous positive airway pressure adherence for prevention of major adverse cerebrovascular and cardiovascular events in obstructive sleep apnea. Am J Respir Crit Care Med. 2020;201:607–610. [DOI] [PubMed] [Google Scholar]
- 112.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 114.Redline S, Azarbarzin A, Peker Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat Rev Cardiol. 2023;20:560–573. [DOI] [PubMed] [Google Scholar]
- 115.Azarbarzin A, Ostrowski M, Moussavi Z, Hanly P, Younes M. Contribution of arousal from sleep to postevent tachycardia in patients with obstructive sleep apnea. Sleep. 2013;36:881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Azarbarzin A, Sands SA, Younes M, et al. The sleep apnea-specific pulse rate response predicts cardiovascular morbidity and mortality. Am J Respir Crit Care Med. 2021;203(12):1546–1555. 10.1164/rccm.202010-3900OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: blood pressure response to continuous positive airway pressure treatment. J Am Coll Cardiol. 2015;66:1023–1032. [DOI] [PubMed] [Google Scholar]
- 118.Ko CY, Fan JM, Hu AK, et al. Disruption of sleep architecture in Prevotella enterotype of patients with obstructive sleep apnea-hypopnea syndrome. Brain Behav. 2019;9:e01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ko CY, Liu QQ, Su HZ, et al. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci (Lond). 2019;133:905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ko CY, Su HZ, Zhang L, Zeng YM. Disturbances of the gut microbiota, sleep architecture, and mTOR signaling pathway in patients with severe obstructive sleep apnea-associated hypertension. Int J Hypertens. 2021;2021:9877053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cai Y, Juszczak HM, Cope EK, Goldberg AN. The microbiome in obstructive sleep apnea. Sleep. 2021;44(8):zsab061. 10.1093/sleep/zsab061 [DOI] [PubMed] [Google Scholar]
- 122.Badran M, Mashaqi S, Gozal D. The gut microbiome as a target for adjuvant therapy in obstructive sleep apnea. Expert Opin Ther Targets. 2020;24:1263–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu H, Wang J, Cai J, et al. Protective effect of Lactobacillus rhamnosus GG and its supernatant against myocardial dysfunction in obese mice exposed to intermittent hypoxia is associated with the activation of Nrf2 pathway. Int J Biol Sci. 2019;15:2471–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ganesh BP, Nelson JW, Eskew JR, et al. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension. 2018;72:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Badran M, Khalyfa A, Ericsson AC, et al. Gut microbiota mediate vascular dysfunction in a murine model of sleep apnoea: effect of probiotics. Eur Respir J. 2023;61(1):2200002. 10.1183/13993003.00002-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]