Abstract
The role of incretin-based therapies, including glucagon-like peptide-1 receptor agonists (GLP1RAs) and dual GLP-1/glucose-dependent insulinotropic polypeptide (GIP) receptor agonists, in the management of type 2 diabetes mellitus (T2DM) and obesity has been increasingly recognized, along with significant cardiovascular (CV) benefits. Despite the clinical efficacy of incretin-based therapies, high costs, suboptimal access, limited insurance coverage, and therapeutic inertia present substantial barriers to widespread adoption. Overcoming these obstacles is essential for the equitable initiation, access, and utilization of incretin-based therapies. Clinicians must make targeted efforts to ensure health equity in the use of these and other advanced therapies.
Keywords: Incretin-based therapy, Obesity, Diabetes, Hypertension, Cardiovascular disease, Racial/ethnic disparities
1. Introduction
Over 70 % of United States (US) adults have been identified with overweight or obesity, and >10 % have type 2 diabetes mellitus (T2DM), increasing their risk for stroke, coronary artery disease (CAD), heart failure (HF), chronic kidney disease (CKD), cancer, and premature death [1]. Although race is not a true biologic or scientific category, it is a social construct that may affect health outcomes. Racial/ethnic groups in the US, including Black, Hispanic, and Asian populations, experience a disproportionately higher burden of T2DM, compounded by increased rates of diabetes-related complications [2]. These sequelae include adverse cardiovascular (CV) and renal outcomes [2]. In an effort to address the complex interplay between cardiac, renal, and metabolic health in primary prevention, the American Heart Association has developed the Predicting Risk of cardiovascular disease EVENTs (PREVENT) risk calculator [3]. This updated calculator eliminates the use of race in the calculation of CV risk and incorporates zip code, a social determinant of health (SDOH), to estimate 10- and 30-year risk of cardiovascular disease (CVD), atherosclerotic cardiovascular disease (ASCVD), and HF.
These SDOH include the conditions in which people are born, grow, live, work, and age, all of which impact health and well-being. Factors contributing to inequities in access to new and emerging treatments, such as incretin-based therapies, include limited healthcare access, low socioeconomic status (SES), therapeutic inertia by clinicians, inadequate insurance coverage, and lower levels of education and health literacy. Addressing these societal barriers is not only a clinical necessity, but a moral imperative to eliminate disparities in CV health outcomes across all populations [4].
Incretin-based therapies, including glucagon-like peptide-1 receptor agonists (GLP1RAs) and glucose-dependent insulinotropic polypeptide receptor and GLP-1 receptor (GIPR/GLP1R) dual agonists, have been shown to effectively treat T2DM and obesity, significantly reducing weight and adverse CV events [1]. These various pharmacologic agents demonstrate advantages which continue to emerge across various patient populations, improving renal outcomes, obstructive sleep apnea (OSA), and exercise tolerance in individuals with heart failure with preserved ejection fraction (HFpEF) [5]. Despite the benefits of these novel agents, aspects of cost and access remain significant barriers, particularly in the US, where many insurance plans do not offer coverage [6]. Addressing these and other obstacles is critical to ensuring broader and more equitable use of these new and emerging therapies. This commentary highlights the growing indications, concerns, and challenges in prescribing and implementing incretin-based therapies, as well as actions to reduce treatment inequities.
2. The role of incretin-based therapies in cardiovascular management
Based on the preponderance of growing evidence, GLP1RAs are now a cornerstone in the treatment of T2DM [7]. Incretin hormones are crucial for glucose homeostasis, mediating nutrient absorption and pancreatic hormone secretion. Furthermore, GLP1RAs enhance insulin secretion, suppress glucagon, slow gastric emptying, reduce post-prandial glycemic spikes, and decrease appetite, energy intake, and body weight. Recent CV outcome trials have underscored the efficacy of GLP1RAs in reducing major adverse cardiovascular events (MACE) in patients with T2DM [8]. Several major studies, such as the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) (2016), Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) (2019), and the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) (2016), have elucidated the CV impact of GLP1RAs in patients with T2DM. These clinical studies have demonstrated benefits for patients both with and at risk for ASCVD [8].
Although originally developed for utilization mainly by primary care clinicians and endocrinologists, CV specialists should be aware of several key developments for the use of GIP and GLP1RAs and recognize the broader advantages of these agents beyond glycemic control, including enhanced weight management, and CV and renal benefits [7]. In consideration of these concepts, the Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT) (2023) trial demonstrated that semaglutide reduced the risk of MACE (CV death, non-fatal MI, and stroke) in patients with overweight or obesity with established CVD, but without diabetes, to 6.5 % compared to 8 % in the placebo group [9]. Semaglutide is now approved as an adjunct to a reduced calorie diet and increased physical activity to improve CV outcomes in patients with overweight and obesity. However, some studies have shown significant rebound weight gain suggesting long-term adherence to the medication is likely required for stable weight loss and cardiometabolic benefits [8]. In addition, in A Study of Tirzepatide versus Semaglutide Once Weekly as Add-on Therapy to Metformin in Participants with Type 2 Diabetes (SURPASS-2) trial (2021), tirzepatide (GIP/GLP1RA) showed superior outcomes and cost-effectiveness compared to semaglutide, including lower rates of diabetes-related complications and improved life expectancy [10]. Nevertheless, in recently published data, substantial regain of lost weight was noted with discontinuing tirzepatide in patients with obesity or overweight [11].
Additional cardiometabolic benefits have been demonstrated and are presently under investigation. In the recent Semaglutide Treatment Effect in People With Obesity and HFpEF (STEP-HFpEF) trial (2023), semaglutide (2.4 mg) resulted in significantly improved symptoms, exercise capacity, and weight loss and reduced physical constraints compared to placebo among individuals diagnosed with HFpEF [12]. A major cause of complications in T2DM is chronic kidney disease (CKD). Despite advancements with sodium-glucose cotransporter 2 (SGLT2) inhibitors and finerenone improving kidney and CV outcomes, patients with diabetic kidney disease still face progression to kidney failure and early CV death [13]. As CKD rates continue to rise, it is predicted to be the fifth leading cause of death worldwide by 2040 [14]. Recent clinical trial evidence has emerged demonstrating improved outcomes in patients with CKD. The Evaluate Renal Function with Semaglutide Once Weekly (FLOW) (2024) trial (N = 30,533 across 28 countries) evaluated the combined effect of semaglutide and SGLT2 inhibitors in people with T2DM and CKD [13]. Primary outcomes, kidney failure, significant eGFR reduction, or kidney/cardiovascular death, were 24 % lower with semaglutide compared to placebo. The FLOW trial has demonstrated that semaglutide provides significant kidney, CV, and mortality benefits in patients with albuminuric CKD with T2DM, filling a crucial gap in treatment for high-risk patients [15]. Moreover, the ongoing Renal Mode of Action of Semaglutide in Patients with Type 2 Diabetes and Chronic Kidney Disease (REMODEL) study (NCT04865770) aims to further evaluate the renal-protective mechanisms of semaglutide by using advanced techniques like functional kidney MRI and kidney biopsies in patients with T2DM and albuminuric CKD [16].
Most recently, the A Study of Tirzepatide in Participants With Obstructive Sleep Apnea (SURMOUNT-OSA) trial (2024) demonstrated in patients with moderate-to-severe OSA and obesity, tirzepatide decreased apnea-hypopnea index (AHI), body weight, hypoxic burden, high-sensitivity C-reactive protein (hsCRP) concentration, systolic blood pressure (BP), and improved sleep-related patient-reported outcomes [17]. Additionally, ongoing clinical research is exploring expanded indications for these therapies, ranging from addiction-related behaviors to peripheral vascular disease, type 1 diabetes, metabolic liver disease, and neurodegenerative disorders. These investigations have the potential to further broaden therapeutic options beyond the established uses in T2DM, obesity and CVD indications [18].
New and emerging incretin-based therapies continue to develop. These include orally administered small-molecule agents, unimolecular coagonists combining glucagon receptor (GCGR) and GLP1RAs (survodutide, pemvidutide), and triagonists combining GCGR, GIP, and GLP1RAs (retatrutide). Other advances include distinct GIPR-GLP1R coagonists, oral agents such as orforglipron, higher doses of established agents, and combinations of long-acting amylin receptor agonists (cagrilintide) with GLP1RA [18].
With the introduction of several new agents reported in peer-reviewed literature and awaiting Food and Drug Administration (FDA) approval, the landscape for incretin-based therapies continues to evolve. Therefore, clinicians must recognize medicine is a dynamic field, characterized by a continuous influx of scientific studies and clinical observations, necessitating ongoing education and awareness of new developments and pharmacotherapy.
3. Concerns regarding incretin-based therapy adverse effects
The GLP1RAs are increasingly being utilized for weight loss; however, these therapies may be associated with potential adverse events (AEs). Whereas gastrointestinal (GI) AEs have been observed in as much as 40 %–65 % of patients, gallbladder AEs are less often reported, in as little as 3 % of patients [18]. Common GI AEs include nausea, diarrhea, constipation, and vomiting and are especially noted during dose initiation and escalation. These effects usually decrease over time and are potentially less common with long-acting GLP1RAs [19]. Despite early unconfirmed reports of an observed increase in retinopathy events with semaglutide, ongoing safety studies continue [18]. For example, one study (N = 16,827 patients, 710 with T2DM) found that patients with T2DM or obesity prescribed semaglutide had a significantly higher risk of developing nonarteritic anterior ischemic optic neuropathy compared to those on non-GLP1RA medications. However, further investigation is needed to establish causality [20].
Clinicians have justified underutilization of incretin-based therapies due to a concern of muscle mass loss. There were early concerns regarding side effects such as sarcopenia and deleterious muscle wasting, potentially associated with the profound weight loss seen with the use of incretin-based therapies. Nevertheless, intentional weight loss in people with obesity primarily reduces body fat, and less so skeletal muscle mass. Exercise, particularly resistance training, can significantly mitigate the loss of fat-free mass during weight loss. Despite some loss of muscle strength, the overall improvement in body weight enhances physical function and mobility after weight loss [21].
From the early prescribed use of these drugs, there have been serious concerns regarding pancreatitis and potentially pancreatic cancer accompanying incretin-based therapies. However, a recent analysis (N = 29,325) revealed that while GI AEs and gallbladder-related disorders were higher in the GLP1RA group compared to placebo, rates of malignant neoplasms and acute pancreatitis were similar between groups [22]. Additionally, a review of 43 studies found no significant association between GLP1RAs and pancreatitis or pancreatic cancer and another analysis (N = 543,595) of adults with T2DM indicated GLP1RA users had a 50 % lower risk of developing pancreatic cancer compared to insulin users, without an increase in pancreatitis [23]. Recent evidence has diminished previous concerns regarding serious complications, such as pancreatitis, thyroid cancer, and muscle wasting [21]. Therefore, clinicians should not use these concerns as justification to underutilize incretin-based therapies.
4. Incretin-based therapy discontinuation and nonadherence
Consistent adherence to incretin-based therapies poses a challenge for patients and is central for maximizing the health benefits of these treatments. Studies indicate significant discontinuation rates: 58 % of people do not achieve at least a 5 % reduction in weight before discontinuing their medications, with 30 % discontinuing within the first month of treatment [24]. Persistent use remains low, with only 40 % of obese patients continuing semaglutide-based GLP1RA treatments after one year in clinical settings, and 68 % discontinuing after one year in real-world scenarios [25]. Furthermore, GI AEs can cause temporary or permanent discontinuation of GLP1RA treatment, with up to 12 % of patients experiencing temporary interruptions and 1.6–6 % permanently discontinuing the treatment, compared to <1 % with placebo [19].
The weight gain associated with therapy discontinuation can be ameliorated by addressing the combination of lifestyle, psychological, physiological factors, and the cost of continuing these medications. One review focused on the concept of “healthy weight” and explored how personal, educational, environmental, and societal factors influence health promotion. Traditionally, nutritional guidelines have emphasized reducing portion sizes or eliminating specific food groups. However, newer research highlights poor dietary quality and excessive calorie intake are major contributors to energy imbalance. Dietary counseling focused on overall dietary patterns rather than individual nutrients or food groups allows for greater flexibility and more sustainable improvements in eating habits. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets are well-researched dietary patterns, proven to effectively promote safe weight loss, prevent long-term weight gain, and reduce the risk of CVD and metabolic disorders [26]. Additionally, a study by Pastel et al. compared GLP1RA treatment with calorie restriction and dietary counseling in patients with T2DM. They found that while liraglutide more effectively lowered fasting glucose, calorie restriction also led to significant weight reduction and had beneficial effects on adipose tissue inflammation and extracellular matrix remodeling. This suggests dietary interventions may be valuable for managing weight and metabolic health even after discontinuing GLP1RAs [27]. As noted earlier, healthy lifestyles are needed to prevent cardiometabolic conditions, before and after drug therapy.
To help curtail temporary or permanent discontinuation of GLP1RAs due to GI AEs, clinicians should provide patients with specific recommendations to minimize their occurrence and severity. These recommendations include eating slowly, consuming smaller portions, increasing meal frequency, avoiding meals close to bedtime, and selecting easily digestible foods [19]. Setting realistic expectations about treatment outcomes and explaining that GI AEs are usually temporary is important. In the event of GI AEs during dose escalation, clinicians should reduce the dose to the previous dose then gradually increase again.
Finally, the need for ongoing medication adherence, thorough patient education, behavioral support, tailored treatment plans, and shared decision-making (SDM) remains crucial to optimizing outcomes.
5. Primordial prevention and the need for healthy lifestyles to enhance cardiometabolic health
Despite these developments in the application of incretin-based therapies in adults, primordial prevention in children and adolescents is required to avoid the upcoming tsunami of cardiometabolic disease and CVD. This has been evidenced by the Bogalusa Heart Study, which linked early-life risk factors to adult CVD outcomes, particularly in racial/ethnic populations. There is a growing push for legal initiatives to improve childhood health by mandating reduced youth sugar consumption, which may help mitigate this looming health crisis [28].
Additionally, school-based health promotion programs may play a role in preventing future obesity and diabetes, although a cluster randomized clinical trial of secondary schools in Spain showed only marginal long-terms benefits [30]. However, access to healthy food remains a significant barrier. For instance, a study (N = 109) in New Orleans, Louisiana, found that patients in food deserts with limited access to quality foods had significantly lower rates of glycemic control (21 %) compared to the national average (35 %). This highlights the challenges of managing T2DM and the importance of clinicians considering food access and other health disparities when setting treatment goals [31].
6. Recognizing the confusing lexicon of incretin-based therapies
Various FDA-approved indications may complicate the initiation of incretin-based therapies. For specific agents across multiple conditions within the T2DM, obesity and CV continuum, different brand names are often given for the same molecule. For instance, the different branded names for semaglutide and tirzepatide can lead to confusion for both clinicians and patients. Semaglutide is marketed under three different brand names, Ozempic, Wegovy, and Rybelsus, with brand naming based on indications for T2DM, overweight, obesity, and CV risk reduction. In addition, tirzepatide and liraglutide are marketed under different brand names depending on the approved indication (Table 1). Clinicians must be familiar with the various names and indications associated with incretin-based therapies.
Table 1.
Incretin-based therapies: generic names, brand names and adult indications.
| Generic | Brand | Indication | Year approved |
|---|---|---|---|
| Dulaglutide | Trulicity | T2DM, CV event risk reduction in pts. w/DM and CVD | 2014 |
| Exenatidea | Byetta | T2DM | 2005 |
| Exenatide | Bydureon BCise | T2DM | 2017 |
| Liraglutide | Victoza | T2DM, CV event risk reduction in pts. w/DM and CVD | 2010 |
| Liraglutide | Saxenda | Obesity/overweight | 2014 |
| Lixisenatidea | Adlyxin | T2DM | 2016 |
| Semaglutide | Ozempic | T2DM, CV event risk reduction in pts. w/DM and CVD | 2017 |
| Semaglutide | Rybelsus | T2DM | 2019 |
| Semaglutide | Wegovy | Obesity/overweight, CV event risk reduction in pts. w/overweight/obesity and CVD | 2021 |
| Tirzepatide | Mounjaro | T2DM | 2022 |
| Tirzepatide | Zepbound | Obesity/overweight | 2023 |
CV: cardiovascular, T2DM: Type 2 Diabetes, CVD = cardiovascular disease.
Discontinued, no longer available in US market.
7. Disparities in access to incretin-based therapies
Perhaps the greatest barrier to the use of incretin-based therapies is their cost, which disproportionately affects certain racial/ethnic populations and those with lower socioeconomic status (SES) and educational attainment. Nationally, wealth disparities are pronounced, with self-identified racial and ethnic groups holding significantly less wealth [32]. The median net worth is $285,000 for White, $44,900 for Black, and $61,600 for Hispanic households. Asian households have the highest median net worth of $536,000. Data are not available for American Indian and Alaska Native (AIAN) and Native Hawaiian and Pacific Islander (NHPI) populations [32]. Of greatest concern, in cities such as New Orleans, Louisiana, with a large history of racial bias and structural inequities underlying a disadvantaged population, this wealth gap may be an even greater barrier for use of newer and developing therapies [33].
As a result, socioeconomic disparities may limit the widespread benefits of incretin-based therapies. Research indicates decreased utilization among people residing in socioeconomically disadvantaged areas, those with limited income and educational attainment, and Non-Hispanic Black and Hispanic populations, despite their disproportionate burden of T2DM and CVD [34]. Contributing factors include inadequate health insurance coverage, limited access to healthcare, financial constraints, low health literacy, and clinician awareness and bias [34]. Furthermore, in the US, access to these medications can be hindered by health insurance coverage, exacerbating disparities linked to race, ethnicity, and SES.
Nevertheless, even with apparent insurance coverage, there may be racial/ethnic disparities in incretin therapy utilization. For example, a study among Veterans (N = 209,460 across 130 Veteran Affairs (VA) facilities) found only 9.4 % of patients were prescribed a GLP1RA. Those more likely to be prescribed the medication were younger and self-identified White patients. Black and Hispanic patients were less likely to be prescribed GLP1RAs, a trend also seen in patients with CKD, despite the VA's wider access to care and insurance, and lack of medication copays [35].
One study (N = 195,915) demonstrated patients had significantly higher odds of discontinuing GLP1RAs at 12 months if they were Black or Hispanic, male, and Medicare or Medicaid enrollees, lived in areas with very high levels of social needs, and had obesity only, HF, or other CVD conditions besides HF at baseline [36]. Furthermore, Medicare data from 2016 to 2019 (N = 4,057,725 individuals aged >65 years with T2DM and either incident ASCVD or CHF, 10.28 % Non-Hispanic Black patients) demonstrated that medication initiation of GLP1RAs was less common among Black patients, patients identifying as other racial/ethnic groups, and those with higher socioeconomic deprivation [5].
Moreover, the type of insurance coverage impacts the distribution of treatments. One study (N = 382,574) found Medicare beneficiaries were less likely to receive newer glucose-lowering medications, including GLP1RAs and SGLT2 inhibitors, compared to commercially insured beneficiaries, with even greater disparities among lower-income patients [37]. A retrospective cohort analysis of commercially insured patients with T2DM found while GLP1RA use increased, it remained generally low, particularly among those with ASCVD who could benefit most. Those identified as Asian, Black, and Hispanic patients, as well as those with low income, were less likely to receive GLP1RA treatment [34]. In contrast, female sex and higher median household incomes (>$100,000) were associated with higher GLP1RA use [34].
Educational attainment may also contribute to disparities in GLP1RA treatment. One study (N = 4777 adults with T2DM) suggested that use of GLP1RAs/SGLT2 inhibitors was higher among individuals who completed some college or higher education compared to those with a high school education or less [38]. A retrospective cohort study (N = 125,636) indicated patients with CV comorbidities and those identifying as Black, Hispanic, or Asian were less likely to be prescribed SGLT2 inhibitors or GLP1RAs [39]. Additionally, individuals with a high school level education had lower odds of receiving GLP1RA or SGLT2 inhibitors compared to those with a postgraduate degree [39].
8. Limitations in incretin-based therapy access by insurance coverage and availability
The Congressional Budget Office has projected an increase in the uninsured rate from an all-time low of 7.2 % in 2023 to 8.9 % by 2034. The largest rise in uninsured individuals is expected among adults aged 19–44, largely due to the end of Medicaid's continuous eligibility provisions in 2023 and 2024 and the end of enhanced Marketplace subsidies after 2025 [40]. Without intervention, the uninsured rate is expected to rise next year [40].
Despite some improvements in health coverage across racial and ethnic groups over time, disparities persist. Nonelderly AIAN (19 %), Hispanic (18 %), NHPI (13 %), and Black (10 %) individuals continue to experience higher uninsured rates, whereas nonelderly White (7 %) and Asian (6 %) individuals have the lowest uninsured rates [32]. These disparities in coverage may potentially increase if previous efforts to expand access to affordable health care are not sustained.
Although health plans generally cover incretin-based therapies for the treatment of diabetes, rarely are these drugs approved to treat obesity [6]. For instance, semaglutide for obesity is included in only 1 % of Affordable Care Act Marketplace prescription drug plans, whereas semaglutide for diabetes is covered by 82 % of these plans [6]. Moreover, Marketplace plans that do cover GLP1RA drugs for obesity require prior authorization and this potentially limits access despite cost-offsetting rebates. The high list prices of incretin-based therapies will likely raise premiums as a result of expanded coverage and the growing demand for these medications [6].
Currently, Medicare is legally prohibited from covering obesity medications, with only 20 % of state Medicaid agencies permitting coverage of weight loss drugs [41]. However, the recent addition of a CV indication for semaglutide may lead to coverage under Medicare Part D for patients with overweight or obesity and CV disease [42].
In addition, the shortage of incretin-based therapies creates barriers to continuing treatment and maximizing therapy. There is uncertainty about what doses will be available and which pharmacies will have the medication in stock. Shortages are being caused by increased demand and off-label use [43].
9. High costs of incretin-based therapy
While incretin-based therapy offers significant CV benefits across all populations, cost remains a major concern for patients. Physician-patient discussions about affordability are critical as these medications become more popular, with out-of-pocket costs potentially reaching hundreds or even more than $1000 per month. Programs such as patient assistance programs, Medicare's Low Income Subsidy or “Extra Help” program, and manufacturer savings cards offer relief for some patients. However, these options may have limitations and temporary availability, potentially exacerbating disparities in access and affordability [44].
Until June 24, 2024, no generic incretin-based therapy was available. The launch of generic liraglutide injection marks the first authorized non-branded GLP1RA in the US for treating T2DM and obesity. This launch is a step forward in expanding access, lowering costs, and fostering market competition, all of which enhance healthcare outcomes and patient satisfaction [45].
10. Suboptimal care created by barriers to access
Patients are increasingly seeking access to weight loss medications, such as semaglutide or tirzepatide, through a variety of channels, including pharmacies and online platforms. Typically prescribed for T2DM, approval for individuals without T2DM depends on medical assessment by a prescribing clinician. There are several options for obtaining these prescriptions, ranging from traditional in-person clinic visits, which are often covered by health insurance, to online services. These online platforms may offer consultations with different fee structures, such as a fee for the initial visit and a monthly subscription fee for ongoing access.
The concern is that increasing widespread use of medical spas and online services may offer medicines which are often compounded, with the potential for unsuspected quality related complications. Drug compounding combines or alters ingredients to customize medications for individual patient needs, potentially blending multiple drugs. Notably, compounded drugs lack FDA approval [46]. Patients should be aware these products may not contain the same active ingredient as FDA-approved semaglutide and could be salt formulations like semaglutide sodium or semaglutide acetate, which have not been proven safe or effective [47]. These online sources may fail to promote the use of a safe, integrated, team-based approach to prescribing incretin-based therapies, leading to delays in the recognition of serious side effects or monitoring of associated cardiometabolic diseases.
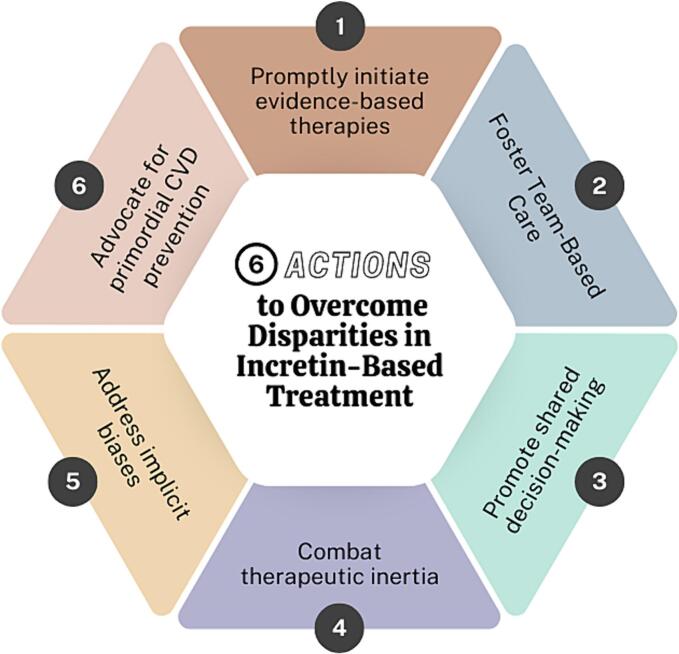
11. Actions to overcome disparities in incretin-based treatment
Addressing disparities in incretin-based treatment involves several actionable strategies: prompt initiation of evidence-based therapies, fostering team-based care, promoting SDM, combating therapeutic inertia, addressing implicit bias, and advocating for primordial CVD prevention (Fig. 1). Evidence-based therapies should be initiated in patients with T2DM, obesity, CKD, and CVD. This requires clinicians remain current on treatment guidelines and recommendations and make appropriate and timely diagnoses.
Fig. 1.
(Central Illustration): 6 Actions to Overcome Disparities in Incretin-Based Treatment.
Team-based care is pivotal in overcoming barriers to incretin-based therapy use. This model of care places the patient and their support system at the center of a dynamic healthcare team, which can include physicians, advanced practice nurses, physician assistants, pharmacists, social workers, clinic staff (medical assistants, nurses, care coordinators), and others. This collaborative approach empowers patients and enhances adherence through interdisciplinary communication. Clinicians can proactively educate nurses and medical assistants on the clinical information needed to complete prior authorizations. Thus, reducing the direct administrative burden on the prescriber and decreasing the need for peer-to-peer consultations. The use of manufacturer checklists and tip sheets should also be encouraged to avoid unnecessary appeals which delay treatment. Pharmacists play a critical role by assisting with medication administration education, navigating the prior authorization process, and applying manufacturer coupons and discounts. In addition, social workers play a key role in referring patients to foundations and community resources that offer financial assistance.
Shared decision-making and patient-centered care are integral to the operationalization of a team-based approach to prescribing incretin-based treatment. During the SDM process, the patient and clinician engage in the exchange of information to reach a mutual decision about the patient's plan of care. It is the clinician's responsibility to inform the patient and their support system about the mechanism of action of incretin-based therapies, potential side effects, and potential barriers to access. Clinicians can also address lifestyle changes that will help to decrease side effects and ensure successful treatment with incretin-based therapies.
In addition, therapeutic inertia must be addressed to reduce disparities in incretin-based treatment. This lack of timely adjustment to therapy when the patient's treatment goals are not met can result in decline in the patient's status and failure to reach treatment optimization. Actions to combat therapeutic inertia include adherence to evidence-based guidelines and the use of clinical decision supporthealth tools to guide treatment. Moreover, racial bias and discrimination, coupled with poor access to quality care and socioeconomic disparities, are closely tied to therapeutic inertia [48].
Although clinicians may not be aware of their personal biases, it is important to recognize implicit bias as a driver of health care disparities. To address implicit bias, clinicians must first acknowledge it exists because of past experiences and what has been taught, and take responsibility for identifying and understanding individual biases [49]. Following these initial steps, clinicians can undergo training on implicit bias and access assessment tools and resources to reduce its occurrence. Valid implicit bias training may benefit all clinicians, especially those who serve diverse patient populations, which include recent immigrants, those with English as a second language, and members of socially disadvantaged populations.
Despite increasing evidence related to the benefits of incretin-based therapy in addressing the growing burden of obesity, diabetes, and CVD, addressing the SDOH is essential for effectively curtailing this growing US public health crisis. Health and elected officials must adopt positive measures to eliminate food deserts and swamps, improve access to safe areas for physical activity, and reduce barriers to equitable healthcare [29]. The time for advocating for primordial CVD prevention is now, especially for minority populations and children from families living in adverse socioeconomic environments [50]. Clinicians should become active in their respective professional organizations to support and facilitate advocacy efforts to address the SDOH and barriers to incretin-based therapies.
By implementing these six actions, healthcare teams can enhance the delivery of incretin-based treatments and reduce disparities in patient outcomes.
12. Conclusion
The role of incretin-based therapies in the management of T2DM has expanded to include effective therapies for weight loss in patients with overweigh or obesity, in addition to improving CVD outcomes in patients with T2DM. However, the underutilization of these therapies is driven by clinician bias, high costs, low adherence rates, and limited access to medications, which could unintentionally widen health disparities.
Addressing these issues demands a collaborative approach from both patients and clinicians. Regular assessment of treatment efficacy, coupled with the timely incorporation of new therapies, is essential to achieving treatment goals. To overcome disparities in incretin-based therapies, clinicians should prioritize the initiation of evidence-based medicine, integration of health-care disciplines, promotion of SDN, and advocacy for primordial CVD prevention. Additionally, it is imperative to combat therapeutic inertia and address implicit biases. In conclusion, targeted interventions are crucial for identifying and eliminating disparities in the treatment of cardiometabolic and CV disease, including with the use of incretin-based therapies. Clinicians must take purposeful actions to treat our patients with the best available care, regardless of race/ethnicity, sex/gender, geography, SES, and ability or disability.
Funding/disclosures
There was no funding associated with this manuscript.
Ethical statement
No patient data or information was used when drafting this manuscript.
Credit authorship contribution statement
Tina K. Reddy: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. Chloé D. Villavaso: Writing – review & editing, Writing – original draft, Conceptualization. Anuhya V. Pulapaka: Writing – review & editing, Writing – original draft. Keith C. Ferdinand: Writing – review & editing, Writing – original draft, Visualization, Supervision, Conceptualization.
Declaration of competing interest
Keith C. Ferdinand is a consultant for Medtronic, Lilly, Amgen, Novartis, Boehringer-Ingelheim
TR – None
CV – None
AP – None
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2024.100455.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
Supplementary Fig. 1
References
- 1.Emanuel E.J., Dellgren J.L., McCoy M.S., Persad G. Fair allocation of GLP-1 and dual GLP-1-GIP receptor agonists. N. Engl. J. Med. 2024;390(20):1839–1842. doi: 10.1056/NEJMp2400978. [DOI] [PubMed] [Google Scholar]
- 2.Lavalle Cobo A., Masson W., Lobo M., Barbagelata L., Forte E., Corral P., Nogueira J.P. Ethnic/racial and geographic disparities on major cardiovascular events in glucagon like Peptide-1 receptor agonists trials: a Meta-analysis. Curr. Probl. Cardiol. 2023;48(11) doi: 10.1016/j.cpcardiol.2023.101940. [DOI] [PubMed] [Google Scholar]
- 3.Larkin H. What to know about PREVENT, the AHA’s new cardiovascular disease risk calculator. JAMA. 2024;331(4):277–279. doi: 10.1001/jama.2023.25115. [DOI] [PubMed] [Google Scholar]
- 4.Ferdinand K.C. The case for eliminating racial and ethnic cardiovascular disparities in the USA. Nat. Rev. Cardiol. 2024;21(2):65–66. doi: 10.1038/s41569-023-00971-2. [DOI] [PubMed] [Google Scholar]
- 5.Cromer S.J., Lauffenburger J.C., Levin R., Patorno E. Deficits and disparities in early uptake of glucagon-like peptide 1 receptor agonists and SGLT2i among Medicare-insured adults following a new diagnosis of cardiovascular disease or heart failure. Diabetes Care. 2023;46(1):65–74. doi: 10.2337/dc22-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costly GLP-1 Drugs are Rarely Covered for Weight Loss by Marketplace Plans. Kaiser Family Foundation. https://www.kff.org/affordable-care-act/press-release/costly-glp-1-drugs-are-rarely-covered-for-weight-loss-by-marketplace-plans/#:∼:text=While%20insurers%20receive%20rebates%20to,put%20upward%20pressure%20on%20premiums Accessed June 22, 2024.
- 7.Committee A.D.A.P.P. Summary of revisions: standards of Care in Diabetes—2024. Diabetes Care. 2023;47(Supplement_1):S5–S10. doi: 10.2337/dc24-SREV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillai P, Modarressi T. GLP1RAs in clinical practice: therapeutic advances and safety perspectives. Am. Coll. Cardiol. Accessed June 5, 2024. https://www.acc.org/Latest-in-Cardiology/Articles/2024/04/15/11/19/GLP1RAs-in-Clinical-Practice.
- 9.Lincoff A.M., Brown-Frandsen K., Colhoun H.M., Deanfield J., Emerson S.S., Esbjerg S., Hardt-Lindberg S., Hovingh G.K., Kahn S.E., Kushner R.F., Lingvay I., Oral T.K., Michelsen M.M., Plutzky J., Tornoe C.W., Ryan D.H., Investigators S.T. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 2023;389(24):2221–2232. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 10.Valentine W.J., Hoog M., Mody R., Belger M., Pollock R. Long-term cost-effectiveness analysis of tirzepatide versus semaglutide 1.0 mg for the management of type 2 diabetes in the United States. Diabetes Obes. Metab. 2023;25(5):1292–1300. doi: 10.1111/dom.14979. [DOI] [PubMed] [Google Scholar]
- 11.Aronne L.J., Sattar N., Horn D.B., Bays H.E., Wharton S., Lin W.Y., Ahmad N.N., Zhang S., Liao R., Bunck M.C., Jouravskaya I., Murphy M.A., Investigators S-. Continued treatment with Tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA. 2024;331(1):38–48. doi: 10.1001/jama.2023.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosiborod M.N., Abildstrøm S.Z., Borlaug B.A., Butler J., Rasmussen S., Davies M., Hovingh G.K., Kitzman D.W., Lindegaard M.L., Møller D.V., Shah S.J., Treppendahl M.B., Verma S., Abhayaratna W., Ahmed F.Z., Chopra V., Ezekowitz J., Fu M., Ito H., Lelonek M., Melenovsky V., Merkely B., Núñez J., Perna E., Schou M., Senni M., Sharma K., Van der Meer P., von Lewinski D., Wolf D., Petrie M.C. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 2023;389(12):1069–1084. doi: 10.1056/NEJMoa2306963. [DOI] [PubMed] [Google Scholar]
- 13.Perkovic V., Tuttle K.R., Rossing P., Mahaffey K.W., Mann J.F.E., Bakris G., Baeres F.M.M., Idorn T., Bosch-Traberg H., Lausvig N.L., Pratley R. Effects of Semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 2024 doi: 10.1056/NEJMoa2403347. [DOI] [Google Scholar]
- 14.Gallagher A. Results from the FLOW trial show benefits of Semaglutide on chronic kidney disease. Pharm. Times Accessed June 29, 2024. https://www.pharmacytimes.com/view/results-from-the-flow-trial-show-benefits-of-semaglutide-on-chronic-kidney-disease?s=01.
- 15.Georgianos P.I., Leivaditis K., Liakopoulos V. The glucagon-like peptide-1 receptor agonist semaglutide in diabetic kidney disease: a new kid on the block to afford maximal kidney protection. Eur. J. Clin. Investig. 2024;e14284 doi: 10.1111/eci.14284. [DOI] [PubMed] [Google Scholar]
- 16.Bjornstad P., Cherney D., Lawson J., Møntegaard C., Pruijm M., Tuttle K., Vrhnjak B., Kretzler M. MO399: Remodel: a mechanistic trial evaluating the effects of Semaglutide on the kidneys in people with type 2 diabetes and chronic kidney disease. Nephrol. Dial. Transplant. 2022;37(Supplement_3) doi: 10.1093/ndt/gfac070.013. [DOI] [Google Scholar]
- 17.Malhotra A., Grunstein R.R., Fietze I., Weaver T.E., Redline S., Azarbarzin A., Sands S.A., Schwab R.J., Dunn J.P., Chakladar S., Bunck M.C., Bednarik J., Investigators S.-O. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N. Engl. J. Med. 2024 doi: 10.1056/NEJMoa2404881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drucker D.J. Efficacy and safety of GLP-1 medicines for type 2 diabetes and obesity. Diabetes Care. 2024 doi: 10.2337/dci24-0003. [DOI] [PubMed] [Google Scholar]
- 19.Gorgojo-Martínez J.J., Mezquita-Raya P., Carretero-Gómez J., Castro A., Cebrián-Cuenca A., de Torres-Sánchez A., García-de-Lucas M.D., Núñez J., Obaya J.C., Soler M.J., Górriz J.L., Rubio-Herrera M. Clinical recommendations to manage gastrointestinal adverse events in patients treated with Glp-1 receptor agonists: a multidisciplinary expert consensus. J. Clin. Med. 2022;12(1) doi: 10.3390/jcm12010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hathaway J.T., Shah M.P., Hathaway D.B., Zekavat S.M., Krasniqi D., Gittinger J.W., Jr., Cestari D., Mallery R., Abbasi B., Bouffard M., Chwalisz B.K., Estrela T., Rizzo J.F., III Risk of Nonarteritic anterior ischemic optic neuropathy in patients prescribed Semaglutide. JAMA Ophthal. 2024 doi: 10.1001/jamaophthalmol.2024.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conte C., Hall K.D., Klein S. Is weight loss-induced muscle mass loss clinically relevant? JAMA. 2024 doi: 10.1001/jama.2024.6586. [DOI] [PubMed] [Google Scholar]
- 22.Singh S., Garg A., Tantry U.S., Bliden K., Gurbel P.A., Gulati M. Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients. Curr. Probl. Cardiol. 2024;49(3) doi: 10.1016/j.cpcardiol.2024.102403. [DOI] [PubMed] [Google Scholar]
- 23.Dankner R., Murad H., Agay N., Olmer L., Freedman L.S. Glucagon-like Peptide-1 receptor agonists and pancreatic Cancer risk in patients with type 2 diabetes. JAMA Netw. Open. 2024;7(1) doi: 10.1001/jamanetworkopen.2023.50408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. 58% of patients discontinue use of obesity meds before reaching meaningful weight loss, Study Shows. Forbes Accessed June 22, 2024. https://www.forbes.com/sites/joshuacohen/2024/06/20/study-shows-58-of-patients-discontinue-use-of-obesity-meds-before-reaching-meaningful-weight-loss/.
- 25.Real-world analysis of GLP-1a drugs for weight loss finds low adherence and increased cost in first year. Prime Therapeutics. https://www.primetherapeutics.com/news/real-world-analysis-of-glp-1a-drugs-for-weight-loss-finds-low-adherence-and-increased-cost-in-first-year/ Accessed June 22, 2024.
- 26.Lavie C.J., Laddu D., Arena R., Ortega F.B., Alpert M.A., Kushner R.F. Healthy weight and obesity prevention: JACC health promotion series. J. Am. Coll. Cardiol. 2018;72(13):1506–1531. doi: 10.1016/j.jacc.2018.08.1037. [DOI] [PubMed] [Google Scholar]
- 27.Pastel E., McCulloch L.J., Ward R., Joshi S., Gooding K.M., Shore A.C., Kos K. GLP-1 analogue-induced weight loss does not improve obesity-induced AT dysfunction. Clin. Sci. (Lond.) 2017;131(5):343–353. doi: 10.1042/cs20160803. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen V., Ferdinand K.C. Primordial prevention: reducing consumption of sugar-sweetened beverages in racial/ethnic populations. Am. Heart J. Plus. 2023;27 doi: 10.1016/j.ahjo.2023.100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh J.I., Lee K.J., Hipp A. Food deserts exposure, density of fast-food restaurants, and park access: exploring the association of food and recreation environments with obesity and diabetes using global and local regression models. PLoS One. 2024;19(4) doi: 10.1371/journal.pone.0301121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos-Beneit G., Fernandez-Alvira J.M., Tresserra-Rimbau A., Bodega P., de Cos-Gandoy A., de Miguel M., Ramirez-Garza S.L., Laveriano-Santos E.P., Arancibia-Riveros C., Carral V., Orrit X., Rodriguez C., Carvajal I., Haro D., Peyra C., Martinez-Gomez J., Alvarez-Benavides A., Estruch R., Lamuela-Raventos R.M., Fernandez-Jimenez R., Fuster V. School-based cardiovascular health promotion in adolescents: a cluster randomized clinical trial. JAMA Cardiol. 2023;8(9):816–824. doi: 10.1001/jamacardio.2023.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delk J.A., Singleton B.A., Al-Dahir S., Kirchain W., Bailey-Wheeler J. The effect of food access on type 2 diabetes control in patients of a New Orleans, Louisiana, clinic. J. Am. Pharm. Assoc. 2003;62(5) doi: 10.1016/j.japh.2022.05.001. (2022) 1675-1679. [DOI] [PubMed] [Google Scholar]
- 32.Ndugga N, Hill L, Artiga S. Key data on health and health care by race and ethnicity. Kaiser Family Foundation. Accessed June 23, 2024. https://www.kff.org/key-data-on-health-and-health-care-by-race-and-ethnicity/?entry=executive-summary-introduction.
- 33.Habans R., Tomlin H. A Profile of Wealth in the New Orleans Metro. 2024. https://www.datacenterresearch.org/reports_analysis/a-profile-of-wealth-in-the-new-orleans-metro/?s=01
- 34.Eberly L.A., Yang L., Essien U.R., Eneanya N.D., Julien H.M., Luo J., Nathan A.S., Khatana S.A.M., Dayoub E.J., Fanaroff A.C., Giri J., Groeneveld P.W., Adusumalli S. Racial, ethnic, and socioeconomic inequities in glucagon-like Peptide-1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum. 2021;2(12) doi: 10.1001/jamahealthforum.2021.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregg L.P., Worsley M.L., Ramsey D.J., Segar M.W., Matheny M.E., Virani S.S., Navaneethan S.D. Racial and ethnic disparities and facility-level variation in GLP-1 RA prescription among US veterans with CKD. Clin. J. Am. Soc. Nephrol. 2023;18(11):1479–1482. doi: 10.2215/cjn.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Do D., Lee T., Peasah S.K., Good C.B., Inneh A., Patel U. GLP-1 receptor agonist discontinuation among patients with obesity and/or type 2 diabetes. JAMA Netw. Open. 2024;7(5) doi: 10.1001/jamanetworkopen.2024.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCoy R.G., Van Houten H.K., Deng Y., Mandic P.K., Ross J.S., Montori V.M., Shah N.D. Comparison of diabetes medications used by adults with commercial insurance vs Medicare advantage, 2016 to 2019. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.35792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittman B.G., Le P., Payne J.Y., Ayers G., Rothberg M.B. Sociodemographic disparities in GLP-1RA and SGLT2i use among US adults with type 2 diabetes: NHANES 2005-march 2020. Curr. Med. Res. Opin. 2024;40(3):377–383. doi: 10.1080/03007995.2024.2303413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasti E.C., Basina M., Calma J., Maron D.J., Rodriguez F., Sandhu A.T. Disparities in adoption of new diabetic therapies with cardiovascular benefits. Diabetes Res. Clin. Pract. 2023;196 doi: 10.1016/j.diabres.2022.110233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hale J., Hong N., Hopkins B., Lyons S., Molloy E. 2024. The Congressional Budget Office Coverage T. Health Insurance Coverage Projections For The US Population And Sources Of Coverage, By Age, 2024-34, Health Aff (Millwood) 101377hlthaff202400460. [DOI] [PubMed] [Google Scholar]
- 41.Cohen J. Passage of treat and reduce obesity act faces an uphill Battle. Forbes Accessed June 22, 2024. https://www.forbes.com/sites/joshuacohen/2024/01/04/passage-of-treat-and-reduce-obesity-act-faces-an-uphill-battle/?sh=11417fd310f8.
- 42.Awan O. Medicare will now cover weight-loss drug Wegovy. Forbes Accessed June 22, 2024. https://www.forbes.com/sites/omerawan/2024/04/01/medicare-will-now-cover-weight-loss-drug-wegovy-heres-what-this-means-for-healthcare/?sh=436a8ea319ef.
- 43.Mahase E. GLP-1 agonist shortage will last until end of 2024, government warns. Bmj. 2024;384 doi: 10.1136/bmj.q28. [DOI] [PubMed] [Google Scholar]
- 44.Weber S. Helping Patients Navigate High Costs of GLP-1 Medications. Accessed June 22. 2024. https://www.medscape.com/viewarticle/helping-patients-navigate-high-costs-glp-1-medications-2024a1000ac4?form=fpf
- 45.Teva Announces Launch of Authorized Generic of Victoza® (liraglutide injection 1.8mg), in the United States. Business Wire. Accessed June 29. 2024. https://finance.yahoo.com/news/teva-announces-launch-authorized-generic-135000447.html?guccounter=1
- 46.Compounding and the FDA . 2024. Questions and Answers. U.S. Food and Drug Administration. Accessed June 22. [Google Scholar]
- 47.Medications Containing Semaglutide Marketed for Type 2 Diabetes or Weight Loss. U.S. Food and Drug Administration. Accessed June 23, 2024. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/medications-containing-semaglutide-marketed-type-2-diabetes-or-weight-loss#:∼:text=What%20should%20patients%20know%20about,outsourcing%20facilities%20registered%20with%20FDA.
- 48.Gavin J.R., Abaniel R.M., Virdi N.S. Therapeutic inertia and delays in insulin intensification in type 2 diabetes: a literature review. Diabetes Spectr. 2023;36(4):379–384. doi: 10.2337/ds22-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Combating implicit bias and stereotypes Think Cultural Health. Accessed June 29. 2024. https://thinkculturalhealth.hhs.gov/maternal-health-care/assets/pdfs/Combating_implicit_bias_and_stereotypes.pdf
- 50.Ferdinand K.C. Primordial prevention of cardiovascular disease in childhood: the time is now. J. Am. Coll. Cardiol. 2019;73(16):2022–2024. doi: 10.1016/j.jacc.2019.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1