Abstract
Background
Accumulating data implicate interleukin (IL)-33, a proinflammatory cytokine released locally upon epithelial cell damage, in the pathogenesis of COPD. In a phase 2 study, itepekimab, a human monoclonal antibody against IL-33, reduced exacerbations and improved lung function in a subgroup analysis of former smokers with COPD with an acceptable safety profile.
Methods
The study designs of AERIFY-1 and AERIFY-2 are described in this article.
Discussion
The primary objective of AERIFY-1/2 (NCT04701983/NCT04751487), two phase 3 randomised, double-blind, placebo-controlled trials, is to assess the efficacy and safety of itepekimab versus placebo in a population of former smokers with moderate-to-severe COPD over up to 52 weeks. An additional secondary population of current smokers are being enrolled in AERIFY-2. These two studies will enrol patients (aged 40–85 years) with COPD and chronic bronchitis who had ≥2 moderate or ≥ 1 severe exacerbations within the previous year despite standard-of-care triple or double background therapy. All participants are required to have ≥10-pack-year smoking history, and ≥6 months since smoking cessation for former smokers. The primary end-point is the annualised rate of moderate or severe acute exacerbation of COPD. Secondary end-points include change from baseline in pre- and post-bronchodilator forced expiratory volume in 1 s, and annualised frequency of severe exacerbations. Symptomatic end-points include Evaluating Respiratory Symptoms in COPD and St. George's Respiratory Questionnaire, safety and anti-drug antibody responses.
Shareable abstract
Two phase 3, randomised, placebo-controlled trials will evaluate the efficacy and safety of anti-interleukin-33 therapy with itepekimab in former smokers with moderate-to-severe COPD up to 52 weeks https://bit.ly/3JmARLU
Introduction
COPD is a heterogeneous lung condition characterised by chronic respiratory symptoms (dyspnoea, cough, sputum production and/or exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction [1, 2]. The natural history of COPD involves progressive decline in lung function, even in the absence of ongoing exposure to causal factors such as cigarette smoke. Acute exacerbations are associated with significant morbidity, more-rapid lung function decline and increased mortality, particularly following exacerbations that require hospitalisation [3, 4].
Main COPD treatment strategies include, among others, smoking cessation, long-acting bronchodilators and inhaled corticosteroids (ICS); however, only minor incremental advances in treatment have been made in the past 50 years [1]. Smoking cessation slows the rate of lung function decline and reduces the risk of hospitalisation and mortality in COPD [1]. Despite this clinical benefit, former smokers with COPD are still at high risk for exacerbations and have substantial residual morbidity. Globally, the prevalence of COPD in former smokers has been reported as 16.33%, compared with 18.36% in current smokers and 7.20% in never-smokers, with smoking accounting for over 70% of cases in high-income countries [5]. Despite the efficacy of standard-of-care therapies, such as bronchodilators, in reducing exacerbations and improving lung function and symptoms, a substantial proportion of patients with COPD continue to experience frequent exacerbations and symptoms, with associated morbidity [6, 7]. There is a need for novel therapies to further reduce the risk of exacerbations and symptoms, and improve other COPD outcomes such as patient quality of life [6–11].
A number of small-molecule and biologic therapies aimed at inflammatory mediators of COPD have been tested in clinical trials, including those targeting tumour necrosis factor-α, interleukin (IL)-1, IL-5 and IL-5Rα, IL-8, IL-8R (CXCR2) and IL-17 [12]; so far none have demonstrated efficacy in a confirmatory trial, and some have demonstrated safety concerns.
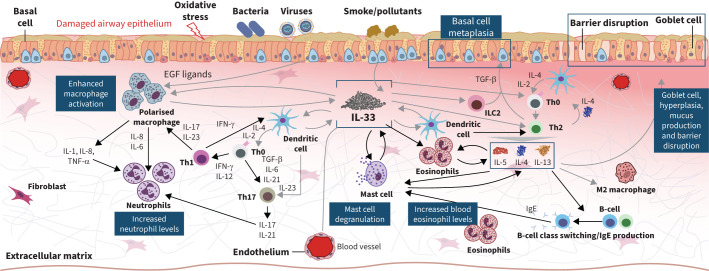
IL-33 is a proinflammatory cytokine localised to the nuclei of a variety of cell types, including epithelial and endothelial cells [13]. When airway epithelial cells are exposed to airborne allergens, viruses, cigarette smoke or air pollutants, IL-33 is released and acts as a short-lived alarmin molecule, which is proposed to initiate and amplify both type 1 and type 2 inflammation [13]. Interestingly, single-cell transcriptomics identify high levels of ST2 expression on mast cells in bronchial biopsies, and these cells have been known to promote release of type 1 and non-type 2 cytokines. In a preclinical model of chronic airway inflammation and exacerbation, IL-33 levels increased and induced a prolonged state of neutrophilic, eosinophilic and CD4+ T-cell airway inflammation and tissue remodelling that was reversed by blockade with an IL-33 neutralising antibody. Furthermore, inhibition of IL-33 in these models induced amelioration of pathogenic transcriptomic signature and a reversion towards a healthy ciliated epithelium [14]. Indeed IL-33 has been associated with increased mucus production and airway remodelling by promoting production of fibroblasts [13].
Numerous studies have suggested that IL-33 may be a key pathophysiological driver of COPD [15–18], including a phase 2 study identifying associations between functional variants in IL33 and IL1RL1 (the gene for the IL-33 receptor) and COPD risk (NCT03546907) [19] (figure 1). The totality of data suggests that IL-33 inhibition is predicted to be impactful for preventing exacerbations and improving lung function, and may yield symptomatic benefit in patients with moderate-to-severe COPD [19].
FIGURE 1.
Itepekimab, a novel fully human anti-IL-33 monoclonal antibody, blocks the effects of IL-33, an alarmin implicated in type 1 and type 2 inflammation in COPD. IL: interleukin; Th: T helper; IFN: interferon; EGF: epidermal growth factor; TGF: transforming growth factor; TNF: tumour necrosis factor.
Itepekimab is a fully human monoclonal antibody against IL-33 (figure 1). A phase 2 proof-of-concept trial in patients with COPD did not meet the primary end-point in the overall population, and efficacy was not observed in the subgroup of current smokers. However, treatment with itepekimab resulted in a striking reduction of moderate and severe exacerbation rates and improved lung function in a pre-specified subgroup of former smokers during the treatment period [20]. It also demonstrated an acceptable safety profile.
The objective of this report is to describe the study designs of the phase 3 AERIFY-1 and AERIFY-2 clinical trials, which aim to assess the efficacy and safety of itepekimab in former smokers with moderate-to-severe COPD in replicate pivotal trials.
Materials and methods
Study objectives
In both AERIFY-1 and AERIFY-2, the primary objective is to evaluate the efficacy of itepekimab compared with placebo on the annualised rate of moderate or severe acute exacerbations of COPD (AECOPD) in former smokers with moderate-to-severe COPD. Secondary objectives include evaluation of the efficacy of itepekimab on lung function, severe exacerbations, corticosteroid-treated exacerbations, respiratory symptoms and health-related quality of life; and the safety/tolerability, pharmacokinetics and immunogenicity of itepekimab in former smokers with moderate-to-severe COPD.
For AERIFY-2, a secondary population of current smokers, in which no efficacy of itepekimab was observed in the phase 2 COPD study, will also be enrolled to further evaluate itepekimab in smokers or confirm phase 2 study findings. The secondary objectives are to estimate the efficacy of itepekimab compared with placebo on the annualised rate of acute moderate or severe COPD exacerbation and lung function, and to evaluate the safety/tolerability, pharmacokinetics and immunogenicity of itepekimab.
Study design
Schematics of the study designs are shown in figure 2. AERIFY-1 and AERIFY-2 are global, multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 3 studies designed to assess the efficacy, safety and tolerability of two dosing regimens of itepekimab in patients with moderate-to-severe COPD who are former smokers and are on an established triple or double controller therapy. Former smokers will be randomised 1:1:1 to itepekimab 300 mg every 2 weeks (q2w), itepekimab 300 mg every 4 weeks (q4w) with alternating matching placebo at the 2-week interval between itepekimab dosing, or matching placebo (q2w) administered subcutaneously (SC) during the treatment period, and will be followed for safety for an additional 20-week post-treatment follow-up period. In AERIFY-2, a separate cohort of current smokers with moderate-to-severe COPD on an established triple or double controller therapy will be randomised 1:1 to SC itepekimab 300 mg q2w or matching placebo during the up to 52-week treatment period, with a 20-week post-treatment follow-up period.
FIGURE 2.
Itepekimab phase 3 study design: a) AERIFY-1; b) AERIFY-2. R: randomisation; SC: subcutaneous; q2w: every 2 weeks; q4w: every 4 weeks.
AERIFY-1 was initiated on 16 December 2020 with a planned completion date of 28 November 2025. AERIFY-2 was initiated on 12 February 2021 with a planned completion date of 17 October 2025.
These global trials are being conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guideline and applicable regulatory requirements. The protocol and informed consent forms were approved by Independent Ethics Committees and Institutional Review Boards. Patients provide written informed consent before participation.
Study subjects
Selected patient eligibility criteria for AERIFY-1 and AERIFY-2 are presented in table 1. The AERIFY-1 and AERIFY-2 primary populations are patients with COPD who are former smokers, defined as a smoking history of ≥10 pack-years with smoking cessation (abstinence) occurring ≥6 months prior to screening, a threshold chosen because of the 87% lower odds of smoking resumption in patients who have achieved this milestone compared with those with <6 months of abstinence [21, 22]. The decision to focus on this population was informed by: 1) the efficacy observed in the pre-specified subgroup analysis by smoking status in the proof-of-concept study ACT15104 discussed above; 2) a safety profile observed in this population; and 3) the large unmet need in former smokers with COPD [5].
TABLE 1.
Selected patient eligibility criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Primary population of former smokers (AERIFY-1 and AERIFY-2) | |
| • 40 to 85 years of age • Physician diagnosis of COPD for at least 1 year based on the GOLD definition • Moderate-to-severe COPD with post-BD FEV1/FVC ratio <0.70 and a post-BD FEV1 % predicted ≥30% and <80% at screening (Visit 1A) and at baseline/randomisation (Visit 2) • Smoking history of ≥10 pack-years • Participant-reported history of signs and symptoms of chronic bronchitis (chronic productive cough for at least 3 months in the year prior to screening in a participant in whom other causes of chronic cough (e.g., inadequately treated gastro-oesophageal reflux or chronic rhinosinusitis; or clinical diagnosis of bronchiectasis) has been excluded) • Participants with a documented history of high exacerbation risk defined as having had ≥2 moderate or ≥1 severe exacerbations within the year prior to screening (Visit 1A), with at least one exacerbation treated with systemic corticosteroids; at least one exacerbation must have occurred while participants were on their current controller therapy • Participants with SoC controller therapy, including triple (LAMA+LABA+ICS) or double therapy (ICS+LABA or LAMA+LABA) • BMI ≥18.0 kg·m−2 or ≥16.0 kg·m−2 for participants enrolled in East-Asian countries • Female participant not pregnant, not breastfeeding, not of child-bearing potential or following contraceptive guidance • Informed consent • Patients who are not currently smoking; smoking cessation must have occurred ≥6 months prior to screening |
• Current diagnosis of asthma according to the GINA guidelines, or documented history of asthma unless asthma resolved before 18 years of age and has not recurred • Clinically significant new abnormal electrocardiogram within 6 months prior to screening • Clinically significant and current pulmonary disease other than COPD • Diagnosis of cor pulmonale, evidence of right cardiac failure or moderate-to-severe pulmonary hypertension • Hypercapnia requiring bilevel positive airway pressure • Moderate or severe AECOPD within 4 weeks prior to screening • Prior history of or planned: lung pneumonectomy or lung volume reduction procedures • Unstable ischaemic heart disease, cardiac arrythmias, uncontrolled hypertension, tuberculosis, human immunodeficiency virus • Suspicion of, or confirmed, COVID-19 infection or in contact with known exposure to COVID-19 at screening; known history of COVID-19 infection within 4 weeks prior to screening; history of requiring mechanical ventilation or extracorporeal membrane oxygenation secondary to COVID-19 within 3 months prior to screening; participants who have had a COVID-19 infection who have not yet sufficiently recovered to participate in the procedures of a clinical trial • Evidence of acute or chronic infection requiring systemic treatment with antibacterial, antiviral, antifungal, antiparasitic or antiprotozoal medications within 4 weeks before screening • Participants with active autoimmune disease or participants using immunosuppressive therapy • History of malignancy within 5 years before screening, except completely treated in situ carcinoma of the cervix, completely treated and resolved nonmetastatic squamous or basal cell carcinoma of the skin • Previous use of itepekimab • Active smoking or vaping of any products (e.g., nicotine, tetrahydrocannabinol) within 6 months prior to screening • Participants with a confirmed positive cotinine test at screening or baseline/randomisation, in the absence of nicotine replacement therapy with negative anabasine test at screening |
| Secondary population of current smokers (AERIFY-2 only) | |
| • 40 to 85 years of age • Physician diagnosis of COPD for at least 1 year based on the GOLD definition • Moderate-to-severe COPD with post-BD FEV1/FVC ratio <0.70 and a post-BD FEV1% predicted ≥30% and <80% at screening (Visit 1A) and at baseline/randomisation (Visit 2) • Smoking history of ≥10 pack-years • Participant-reported history of signs and symptoms of chronic bronchitis (chronic productive cough for at least 3 months in the year prior to screening in a participant in whom other causes of chronic cough (e.g., inadequately treated gastro-oesophageal reflux or chronic rhinosinusitis; or clinical diagnosis of bronchiectasis) has been excluded) • Participants with a documented history of high exacerbation risk defined as having had ≥2 moderate or ≥1 severe exacerbations within the year prior to screening (Visit 1A), with at least one exacerbation treated with systemic corticosteroids; at least one exacerbation must have occurred while participants were on their current controller therapy • Participants with SoC controller therapy, including triple (LAMA+LABA+ICS) or double therapy (ICS+LABA or LAMA+LABA) • BMI ≥18.0 kg·m−2 or ≥16.0 kg·m−2 for participants enrolled in East-Asian countries • Female participant not pregnant, not breastfeeding, not of child-bearing potential or following contraceptive guidance |
• Current diagnosis of asthma according to the GINA guidelines, or documented history of asthma unless asthma resolved before 18 years of age and has not recurred • Clinically significant new abnormal electrocardiogram within 6 months prior to screening • Clinically significant and current pulmonary disease other than COPD • Diagnosis of cor pulmonale, evidence of right cardiac failure or moderate-to-severe pulmonary hypertension • Hypercapnia requiring bilevel positive airway pressure • Moderate or severe AECOPD within 4 weeks prior to screening • Prior history of or planned: lung pneumonectomy or lung volume reduction procedures • Unstable ischaemic heart disease, cardiac arrythmias, uncontrolled hypertension, tuberculosis, human immunodeficiency virus • Suspicion of, or confirmed, COVID-19 infection or in contact with known exposure to COVID-19 at screening; known history of COVID-19 infection within 4 weeks prior to screening; history of requiring mechanical ventilation or extracorporeal membrane oxygenation secondary to COVID-19 within 3 months prior to screening; participants who have had a COVID-19 infection who have not yet sufficiently recovered to participate in the procedures of a clinical trial • Evidence of acute or chronic infection requiring systemic treatment with antibacterial, antiviral, antifungal, antiparasitic or antiprotozoal medications within 4 weeks before screening |
| • Informed consent • Patients who are currently smoking ≥1 cigarette/day on average in the week prior to screening |
• Participants with active autoimmune disease or participants using immunosuppressive therapy • History of malignancy within 5 years before screening, except completely treated in situ carcinoma of the cervix, completely treated and resolved nonmetastatic squamous or basal cell carcinoma of the skin • Previous use of itepekimab • Vaping of any products (e.g., nicotine, tetrahydrocannabinol) within 6 months prior to screening |
GOLD: Global Initiative for Chronic Obstructive Lung Disease; BD: bronchodilator; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; SoC: standard of care; LABA: long-acting β2-agonist; LAMA: long-acting muscarinic antagonist; ICS: inhaled corticosteroids; BMI: body mass index; GINA: Global Initiative for Asthma; AECOPD: acute exacerbation of COPD.
There is also unmet need in current smokers with COPD; however, efficacy was not observed in this population in the ACT15104 proof-of-concept study. This population was included in AERIFY-2 as a separate cohort after a risk–benefit assessment considering the observed safety profile in this population and the possibility that the phase 2 findings could represent type 2 error. The sample size (n=280) of current smokers in AERIFY-2 is larger than the number of active smokers in the phase 2 study, providing an opportunity to re-assess efficacy of itepekimab in this population.
End-points
A summary of the study end-points is provided in table 2. The primary end-point in both studies is the annualised rate of moderate or severe AECOPD. Moderate exacerbations are defined as acute worsening of respiratory symptoms requiring either systemic corticosteroids and/or antibiotics. Severe exacerbations are those requiring hospitalisation, observation for >24 h in an emergency department or urgent care facility, or resulting in death. AECOPD events will be evaluated by an adjudication committee. The rate of moderate-to-severe AECOPD is an important clinical and regulatory end-point that assesses the effect of a drug on the disease [23, 24], and prevention of exacerbations is of meaningful benefit to patients. There has been no formally established clinically meaningful minimum reduction for AECOPD because of the complexity and frequency of the events [24, 25].
TABLE 2.
End-points in the former smoker population in AERIFY-1 and AERIFY-2
| End-points | |
|---|---|
| Primary end-point | Annualised rate of moderate or severe AECOPD |
| Secondary end-points | |
| Lung function | Change from baseline in pre-BD FEV1 to Week 24 (key secondary end-point) Change from baseline in post-BD FEV1 to Weeks 24 and 52 Change from baseline in pre-BD FEV1 to Week 52 Rate of change in post-BD FEV1 from baseline (post-BD FEV1 slope) to Week 52 |
| Exacerbations | Time to first moderate or severe AECOPD Annualised rate of severe AECOPD Time to first severe AECOPD Annualised rate of corticosteroid-treated AECOPD |
| Patient-reported outcomes | Change from baseline in E-RS:COPD total score to Weeks 24 and 52 Change from baseline in SGRQ total score to Weeks 24 and 52 Proportion of participants with a decrease from baseline of at least 4 points in SGRQ total score |
| Safety | Incidence of treatment-emergent adverse events, adverse events of special interest, serious adverse events and adverse events leading to permanent treatment discontinuation Incidence of potentially clinically significant laboratory test, vital signs and electrocardiogram abnormalities |
| Pharmacokinetics and immunogenicity | Functional concentrations of itepekimab in serum Incidence of treatment-emergent anti-itepekimab antibody responses |
AECOPD: acute exacerbation of COPD; BD: bronchodilator; FEV1: forced expiratory volume in 1 s; E-RS:COPD: Evaluating Respiratory Symptoms in COPD; SGRQ: St George's Respiratory Questionnaire.
The key secondary end-point is the change from baseline in pre-bronchodilator (BD) forced expiratory volume in 1 s (FEV1) at Week 24. FEV1 is a clinically relevant measure of lung function impairment and disease severity in COPD, and changes in FEV1 are widely considered to be a relevant measure of the effect of an intervention on airway mechanics [24]. In the former smoker population in the ACT15104 proof-of-concept study, the least squares mean difference for the change from baseline in pre-BD FEV1 to Week 24 versus placebo was 90 mL (95% CI 20–150 mL, nominal p=0.0076).
Additional end-points in the study will focus on patient-reported outcomes including symptom control and safety and tolerability of itepekimab treatment. The former is of particular importance as cough and sputum are prominent features of chronic bronchitis [1]. To measure symptom control, the Evaluating Respiratory Symptoms in COPD (E-RS:COPD) tool will be used to measure the effect of itepekimab on severity of treatment of respiratory symptoms in COPD; the validated 11-item E-RS:COPD assesses severity of respiratory symptoms overall and severity of individual symptoms such as breathlessness, cough and sputum, and chest symptoms [26]. Safety will be evaluated based on the incidence of treatment-emergent adverse events, adverse events of special interest, serious adverse events and adverse events leading to permanent treatment discontinuation.
Itepekimab dose regimens and background therapy
Itepekimab demonstrated a long terminal half-life of 30.0 to 31.6 days following single intravenous or SC doses in healthy subjects or multiple SC doses in patients with asthma, indicative of potential for extended dosing intervals [27]. A population pharmacokinetic model was developed to describe concentrations of functional itepekimab (molecules with one or two free binding sites) in serum of healthy subjects and patients with COPD or asthma and used to simulate potential dose regimens. Itepekimab 300 mg q2w and 300 mg q4w regimens are expected to achieve mean trough concentrations at steady-state ≥2-fold of concentrations associated with maximum in vivo efficacy in a murine model of asthma induced by chronic house dust mite exposure [14].
In the ACT15104 proof-of-concept study, itepekimab 300 mg administered SC q2w during the 24- to 52-week treatment period was well tolerated, safe and efficacious in reducing the rate of exacerbations and improving lung function in the subgroup of former smokers with COPD [19]. Itepekimab 300 mg SC q2w administered over 12 weeks had an acceptable safety profile and was efficacious in a phase 2 study of patients with moderate-to-severe asthma [28]. Based on these data and phase 1 data, a regimen of itepekimab 300 mg SC q2w is being studied in both AERIFY-1 and AERIFY-2 to replicate the findings of the phase 2 ACT15104 study; in addition, a regimen of itepekimab 300 mg q4w is being studied in former smokers in both studies (figure 2).
Participants in AERIFY-1/2 must be on a stable regimen of background controller therapy for COPD prior to enrolment and throughout the up to 52-week treatment period with either triple therapy (ICS+long-acting β2-agonist (LABA)+long-acting muscarinic antagonist (LAMA)) or double therapy (ICS+LABA, or LAMA+LABA).
Sample size estimation and analysis
Taking into account the results of the phase 2 study [20], the sample size was determined based on the following assumptions for the primary end-point of annualised rate of moderate or severe AECOPD: 1) mean treatment duration per participant of 0.94 years; 2) an annualised placebo rate of 1.5; and 3) a dispersion parameter of 1.0 for the negative binomial distribution. With a total of 930 former smokers, each study will have a 90% power to detect a 30% relative reduction in the primary end-point based on a two-sided significance level of 0.05. As a result of the geopolitical situation that began in Ukraine on 24 February 2022, which has led to disruptions in the conduct of the study in that world region, the sample size of AERIFY-1 was increased to n=960 to compensate for the shorter than expected observation duration in participants in Ukraine; no new participants were recruited in Ukraine or Russia, due to the uncertainties of the impact and unfolding dynamics of the situation at the time. In addition, during the COVID-19 pandemic, overall a lower than expected rate of exacerbations has been reported in COPD patients. This is likely the result of social distancing and/or mask wearing leading to fewer respiratory infections, a known trigger for COPD exacerbations [29]. The sample size of n=930 patients may therefore be increased to compensate for a lower than expected end-point event rate, with an additional 24–52 weeks treatment period for potential additional participants.
For AERIFY-2, the targeted enrolment for the secondary current smoker cohort is n=280 (140 per arm) in addition to the n=930 former smokers with COPD. Assuming the rate of moderate-to-severe AECOPD is similar between itepekimab and placebo within current smokers, as was seen in the proof-of-concept study [19], a sample size of 280 participants yields an 80% probability to observe a risk reduction of <15% at the end of the study. The precision of the estimate of risk reduction is 30% (95% CI half-width). The original sample size of n=240 for current smokers was increased to n=280 because of the same reason as mentioned before (lower than expected rate of AECOPD due to the COVID-19 pandemic).
Efficacy analyses will be performed in former smokers in the intention-to-treat population, defined as all randomised participants according to the treatment group as randomised. Similar efficacy analyses will be performed in the secondary population of current smokers in AERIFY-2. Safety analyses will be performed in the safety population, defined as all participants exposed to treatment, analysed according to the treatment actually received.
The primary estimand will be a treatment policy estimand for AERIFY-2. This estimand compares the treatment effect on efficacy for the participants randomised to an itepekimab regimen versus placebo, regardless of what treatment participants actually received or treatment adherence status [30]. For AERIFY-1 the primary estimand will be a treatment policy estimand except for participants enrolled in Ukraine, for whom the primary estimand will be a “while-on-treatment” policy estimand (as defined by ICH-E9-R1). This policy estimand considers the response to treatment prior to the occurrence of a relevant intercurrent event, in this case the Ukraine/Russia military conflict, since treatment responses as determined by this study's end-points occurring during a military conflict may not be adequately captured.
The efficacy end-points of annualised rates of moderate or severe AECOPD and severe AECOPD will be analysed using a negative binomial regression model, with the total number of events as the response variable and log-transformed observation duration as the offset variable. Time-to-event end-points will be analysed using a Cox regression model. The change from baseline in continuous variables such as FEV1 and patient-reported outcomes will be analysed using a mixed-effect model with repeated measures approach.
Discussion
AERIFY-1 and AERIFY-2 are randomised, double-blind, placebo-controlled, 52-week phase 3 trials designed to confirm the efficacy and safety of itepekimab in former smokers with moderate-to-severe COPD observed in a phase 2 study [19].
The choice of primary end-point for this study is informed by the course of COPD disease, which demonstrates rapid disease progression and increased mortality following exacerbations, particularly severe exacerbations that require hospitalisation [3, 4], with preventing exacerbations as a key goal of COPD management [1]. In addition, despite a subset of patients achieving exacerbation reduction with standard-of-care therapy, a proportion of patients with moderate-to-severe COPD continue to experience exacerbations [6, 7].
The study designs presented here will help to further identify the patient population most likely to benefit from itepekimab treatment. These studies were designed with an enrichment strategy [30, 31], intended to confirm data from the phase 2 study, where efficacy was observed in the former smoker subgroup. Current smokers were also included to ascertain the efficacy observed in this subgroup, as well as to provide additional safety data in this population to clinicians. The inclusion of both study populations may provide further insight into differences in disease mechanism between current and former smokers with COPD. These studies will also be a resource for future post hoc analyses aimed at identifying predictors of response.
For current smokers, smoking cessation is a critical first step in modifying the clinical course of COPD [1]. Smoking cessation slows the rate of lung function decline and reduces the risk of hospitalisation and mortality; however, former smokers with COPD continue to suffer from lung function decline and remain at high risk for exacerbations and substantial morbidity and mortality [1, 8, 11]. The differential efficacy of itepekimab observed in the phase 2 study may provide additional motivation for smoking cessation [19].
The differential efficacy observed between current and former smokers in the phase 2 study is incompletely understood [19]. Recent data provide a specific biological rationale for the observed difference in the efficacy of itepekimab between former and current smokers. In the airway epithelium, IL-33 expression is limited to basal airway epithelial cells, a cell population that is reduced in number in patients who smoke [32]. These data suggest that itepekimab may not be efficacious in patients who are actively smoking, due to a diminished number of IL-33-containing basal cells in the airway epithelium. If the efficacy of itepekimab in former but not current smokers with COPD is confirmed in the AERIFY phase 3 trials, these data would suggest that IL-33 released from airway basal cells is an important contributor to COPD exacerbation in former smokers, and that other pathways may continue to drive progression of COPD in current smokers.
Differential efficacy by smoking status has been observed with several other COPD therapies, including ICS and macrolides, as shown in figure 3 [19, 33–39]. In the SUMMIT study, ICS+LABA reduced exacerbations by 36% compared with placebo among former smokers and by 19% compared with placebo among current smokers [34]. In the InforMing the PAthway of COPD Treatment (IMPACT) study, the addition of ICS to LAMA+LABA resulted in a 30% reduction in exacerbations in former smokers compared with a 14% reduction in current smokers [34]. A subgroup analysis of a randomised trial of macrolide therapy in COPD demonstrated that exacerbation risk reduction with azithromycin was strongest among former smokers, with comparatively little effect among current smokers [33].
FIGURE 3.
Current evidence for differential treatment effects between former and current smokers with COPD. BD: bronchodilator; CXCL2: chemokine (C-X-C motif) ligand 2; FEV1: forced expiratory volume in 1 s; ICS: inhaled corticosteroids; IL: interleukin; LABA: long-acting β2-agonist; LAMA: long-acting muscarinic antagonist. #: results from ICS/LABA of former smokers versus placebo compared with ICS/LABA of current smokers versus placebo. ¶: no p-value was reported.
Dupilumab has been demonstrated to significantly reduce exacerbations in populations of former smokers with eosinophilic COPD [40]. However, no pivotal studies on biologics have demonstrated any efficacy outside of this subpopulation with high circulating eosinophil levels. Mepolizumab, an anti-IL-5 antibody, as add-on therapy to ICS+LABA, demonstrated a 2% reduction in exacerbations in the overall COPD population, and an 18–20% reduction in exacerbations in those with high circulating eosinophils [9]. Benralizumab, an anti-IL-5 receptor antibody, as add-on therapy to ICS+LABA, showed mixed results in patients with COPD and high circulating eosinophils, with reduction of exacerbations ranging from 4% worsening to 17% improvement, depending on dose [10]. The improvement in both exacerbation rate and lung function observed with itepekimab in former smokers with COPD supports the hypothesis that IL-33 might be important in both acute and chronic inflammatory airway responses [20, 28].
In summary, the AERIFY-1 and -2 trials, two phase 3, randomised, double-blind, placebo-controlled trials, are designed to comprehensively evaluate the efficacy and safety data for a novel, first-in-class compound that targets both type 2 and type 1 inflammatory pathways in COPD.
Acknowledgements
Medical writing/editorial assistance was provided by Jennifer L.F. Port and Luke Ray of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals Inc., according to the Good Publication Practice guideline.
Provenance: Submitted article, peer reviewed.
Ethics statement: The protocol and informed consent forms were approved by Independent Ethics Committees and Institutional Review Boards. Patients provide written informed consent before participation.
AERIFY-1 and AERIFY-2 are registered at www.clinicaltrials.gov with identifier numbers NCT04701983 and NCT04751487, respectively.
This article has an editorial commentary: https://doi.org/10.1183/23120541.00433-2024
Conflict of interest: K.F. Rabe reports consultancy, speaker fees’ and advisory board membership for AstraZeneca, Boehringer Ingelheim, Chiesi, Gilead, GSK, Novartis, Pearl, Sanof and Teva; and is cofounder of rnatics, Germany.
Conflict of interest: F.J. Martinez has been on steering committees for Afferent/Merck, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Nitto Pharma, Patara Pharma/Respivant Sciences, Pearl Pharmaceuticals, ProMedior/Roche, ProMetic Life Sciences, Stromedix/Biogen and Veracyte; has been an advisory board member for AstraZeneca, BioScale/ProTerrix Bio, Boehringer Ingelheim, Chiesi, CSL Behring, Gala Therapeutics, Genentech, GlaxoSmithKline, Novartis, Pearl Pharmaceuticals, Physicians Education Resource, Sunovion, Teva and Zambon; has been a consultant for Bridge Biotherapeutics, Bristol Myers Squibb and twoXR; reports continuing medical education presentation support from the Canadian Respiratory Network, Chiesi, CME Outfitters, Dartmouth University, France Foundation, Inova Fairfax, MD Magazine, Methodist Hospital, Miller Communications, National Association for Continuing Education/Haymarket, New York University, PeerView, Prime Education, Rare Diseases Healthcare Communication, Rockpointe, University of Alabama at Birmingham, UpToDate, Vindico Pharmaceuticals, WebMD/MedScape and Zambon; and has been on the data and safety monitoring board for Boehringer Ingelheim and GlaxoSmithKline.
Conflict of interest: S.P. Bhatt has served on advisory boards for Boehringer Ingelheim and Regeneron, received consulting fees from Sanofi, and reports fees for continuing medical education from IntegrityCE.
Conflict of interest: T. Kawayama reports support from GSK, AstraZeneca, Novartis Pharma and Healios.
Conflict of interest: B.G. Cosio has served as a consultant and received speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Menarini, GSK, Sanofi and Teva.
Conflict of interest: R.M. Mroz reports support from AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, MSD, Gilead, GSK, Novartis, Pearl Pharmaceuticals, Sanofi and Teva.
Conflict of interest: M.M. Boomsma, H. Goulaouic, M. Djandji, Y. Liu, C.R. Xu and H. Staudinger are Sanofi employees and may hold stock and/or stock options in the company.
Conflict of interest: M.C. Nivens, X. Soler, M.P. Kosloski and D.J. Lederer are Regeneron Pharmaceuticals Inc. employees and shareholders.
Conflict of interest: N. Amin is a prior employee and shareholder for Regeneron Pharmaceuticals Inc.
Conflict of interest: R.M. Abdulai is a prior employee and stock holder for Sanofi.
Support statement: This research was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Chronic Obstructive Lung Diseases (GOLD). G lobal Strategy for Diagnosis, Management and Prevention of COPD 2023 Report . Date last updated: 17 February 2023. Date last accessed: 3 April 2023. https://goldcopd.org/wp-content/uploads/2024/02/GOLD-2024_v1.2-11Jan24_WMV.pdf
- 2.Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med 2019; 381: 1257–1266. doi: 10.1056/NEJMra1900500 [DOI] [PubMed] [Google Scholar]
- 3.Suissa S, Dell'Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012; 67: 957–963. doi: 10.1136/thoraxjnl-2011-201518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 195: 324–330. doi: 10.1164/rccm.201605-1014OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varmaghani M, Dehghani M, Heidari E, et al. Global prevalence of chronic obstructive pulmonary disease: systematic review and meta-analysis. East Mediterr Health J 2019; 25: 47–57. doi: 10.26719/emhj.18.014 [DOI] [PubMed] [Google Scholar]
- 6.Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med 2018; 6: 747–758. doi: 10.1016/S2213-2600(18)30327-8 [DOI] [PubMed] [Google Scholar]
- 7.Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 2017; 389: 1919–1929. doi: 10.1016/S0140-6736(17)30188-5 [DOI] [PubMed] [Google Scholar]
- 8.Kramarow EA. Health of former cigarette smokers aged 65 and over: United States, 2018. Natl Health Stat Rep 2020; 145: 1–12. [PubMed] [Google Scholar]
- 9.Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. doi: 10.1056/NEJMoa1708208 [DOI] [PubMed] [Google Scholar]
- 10.Criner GJ, Celli BR, Brightling CE, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med 2019; 381: 1023–1034. doi: 10.1056/NEJMoa1905248 [DOI] [PubMed] [Google Scholar]
- 11.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994; 272: 1497–1505. doi: 10.1001/jama.1994.03520190043033 [DOI] [PubMed] [Google Scholar]
- 12.van Haarst A, McGarvey L, Paglialunga S. Review of drug development guidance to treat chronic obstructive pulmonary disease: US and EU perspectives. Clin Pharm Ther 2019; 106: 1222–1235. doi: 10.1002/cpt.1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol 2014; 31: 31–37. doi: 10.1016/j.coi.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 14.Allinne J, Scott G, Lim WK, et al. IL-33 blockade affects mediators of persistence and exacerbation in a model of chronic airway inflammation. J Allergy Clin Immunol 2019; 144: 1624–1637.e10. doi: 10.1016/j.jaci.2019.08.039 [DOI] [PubMed] [Google Scholar]
- 15.Byers DE, Alexander-Brett J, Patel AC, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 2013; 123: 3967–3982. doi: 10.1172/JCI65570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorska K, Nejman-Gryz P, Paplinska-Goryca M, et al. Comparative study of IL-33 and IL-6 levels in different respiratory samples in mild-to-moderate asthma and COPD. COPD 2018; 15: 36–45. doi: 10.1080/15412555.2017.1416074 [DOI] [PubMed] [Google Scholar]
- 17.Jiang M, Tao S, Zhang S, et al. Type 2 innate lymphoid cells participate in IL-33-stimulated Th2-associated immune response in chronic obstructive pulmonary disease. Exp Ther Med 2019; 18: 3109–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearley J, Silver JS, Sanden C, et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity 2015; 42: 566–579. doi: 10.1016/j.immuni.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 19.Rabe KF, Celli BR, Wechsler ME, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med 2021; 9: 1288–1298. doi: 10.1016/S2213-2600(21)00167-3 [DOI] [PubMed] [Google Scholar]
- 20.Riera-Martínez L, Cànaves-Gómez L, Iglesias A, et al. The role of IL-33/ST2 in COPD and its future as an antibody therapy. Int J Mol Sci 2023; 24: 8702. doi: 10.3390/ijms24108702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alboksmaty A, Agaku IT, Odani S, et al. Prevalence and determinants of cigarette smoking relapse among US adult smokers: a longitudinal study. BMJ Open 2019; 9: e031676. doi: 10.1136/bmjopen-2019-031676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herd N, Borland R, Hyland A. Predictors of smoking relapse by duration of abstinence: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction 2009; 104: 2088–2099. doi: 10.1111/j.1360-0443.2009.02732.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration (FDA) . Chronic obstructive pulmonary disease: developing drugs for treatment. Guidance for Industry. 2016. Date last accessed: 14 February 2023. www.govinfo.gov/content/pkg/FR-2016-05-20/pdf/2016-11855.pdf
- 24.European Medicines Agency . Guideline on clinical investigation of medicinal products in the treatment of chronic obstructive pulmonary disease (COPD). 2012. Date last accessed: 14 February 2023. www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-chronic-obstructive-pulmonary-disease_en.pdf
- 25.Jones PW, Beeh KM, Chapman KR, et al. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014; 189: 250–255. doi: 10.1164/rccm.201310-1863PP [DOI] [PubMed] [Google Scholar]
- 26.Leidy NK, Murray LT. Patient-reported outcome (PRO) measures for clinical trials of COPD: the EXACT and E-RS. COPD 2013; 10: 393–398. doi: 10.3109/15412555.2013.795423 [DOI] [PubMed] [Google Scholar]
- 27.Kosloski MP, Kalliolias GD, Xu CR, et al. Pharmacokinetics and pharmacodynamics of itepekimab in healthy adults and patients with asthma: Phase I first-in-human and first-in-patient trials. Clin Transl Sci 2022; 15: 384–395. doi: 10.1111/cts.13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechsler ME, Ruddy MK, Pavord ID, et al. Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. N Engl J Med 2021; 385: 1656–1668. doi: 10.1056/NEJMoa2024257 [DOI] [PubMed] [Google Scholar]
- 29.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Reduction in hospitalised COPD exacerbations during COVID-19: A systematic review and meta-analysis. PLoS One 2021; 16: e0255659. doi: 10.1371/journal.pone.0255659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eu ropean Medicines Agency . ICH E9 (R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials. 2020. Date last accessed: 14 February 2023. www.ema.europa.eu/en/documents/scientific-guideline/ich-e9-r1-addendum-estimands-sensitivity-analysis-clinical-trials-guideline-statistical-principles_en.pdf
- 31.Food and Drug Administration . Enrichment strategies for clinical trials to support approval of human drugs and biological products. 2019. Date last accessed: 14 February 2023. www.fda.gov/media/121320/download
- 32.Faiz A, Boedijono FS, Timens W, et al. The regulation of IL33 following smoking cessation. Eur Respir J 2022; 60: Suppl. 66, 3314. [Google Scholar]
- 33.Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med 2014; 189: 1503–1508. doi: 10.1164/rccm.201402-0207OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med 2018; 378: 1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 35.Bhatt SP, Anderson JA, Brook RD, et al. Cigarette smoking and response to inhaled corticosteroids in COPD. Eur Respir J 2018; 51: 1701393. doi: 10.1183/13993003.01393-2017 [DOI] [PubMed] [Google Scholar]
- 36.Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med 2019; 7: 745–756. doi: 10.1016/S2213-2600(19)30190-0 [DOI] [PubMed] [Google Scholar]
- 37.Rennard SI, Dale DC, Donohue JF, et al. CXCR2 Antagonist MK-7123. A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191: 1001–1011. doi: 10.1164/rccm.201405-0992OC [DOI] [PubMed] [Google Scholar]
- 38.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365: 689–698. doi: 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bardsley S, Criner GJ, Halpin DMG, et al. Single-inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol versus dual therapy in current and former smokers with COPD: IMPACT trial post hoc analysis. Respir Med 2022; 205: 107040. doi: 10.1016/j.rmed.2022.107040 [DOI] [PubMed] [Google Scholar]
- 40.Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. N Engl J Med 2023; 389: 205–214. doi: 10.1056/NEJMoa2303951 [DOI] [PubMed] [Google Scholar]