ABSTRACT
Cell growth in mycobacteria involves cell wall expansion that is restricted to the cell poles. The DivIVA homolog Wag31 is required for this process, but the molecular mechanism and protein partners of Wag31 have not been described. In this study of Mycobacterium smegmatis, we identify a connection between wag31 and trehalose monomycolate (TMM) transporter mmpl3 in a suppressor screen and show that Wag31 and polar regulator PlrA are required for MmpL3’s polar localization. In addition, the localization of PlrA and MmpL3 is responsive to nutrient and energy deprivation and inhibition of peptidoglycan metabolism. We show that inhibition of MmpL3 causes delocalized cell wall metabolism but does not delocalize MmpL3 itself. We found that cells with an MmpL3 C-terminal truncation, which is defective for localization, have only minor defects in polar growth but are impaired in their ability to downregulate cell wall metabolism under stress. Our work suggests that, in addition to its established function in TMM transport, MmpL3 has a second function in regulating global cell wall metabolism in response to stress. Our data are consistent with a model in which the presence of TMMs in the periplasm stimulates polar elongation and in which the connection between Wag31, PlrA, and the C-terminus of MmpL3 is involved in detecting and responding to stress in order to coordinate the synthesis of the different layers of the mycobacterial cell wall in changing conditions.
IMPORTANCE
This study is performed in Mycobacterium smegmatis, which is used as a model to understand the basic physiology of pathogenic mycobacteria such as Mycobacterium tuberculosis. In this work, we examine the function and regulation of three proteins involved in regulating cell wall elongation in mycobacterial cells, which occurs at the cell tips or poles. We find that Wag31, a regulator of polar elongation, works partly through the regulation of MmpL3, a transporter of cell wall constituents and an important drug target. Our work suggests that, beyond its transport function, MmpL3 has another function in controlling cell wall synthesis broadly in response to stress.
KEYWORDS: Mycobacterium, polar growth regulation, peptidoglycan, mycolic acid, MmpL3, PlrA, DivIVA, murJ, TMMs, stress
INTRODUCTION
Bacterial cell walls are important for shape maintenance and protection from stress, and cell wall construction requires careful coordination by numerous enzymes and regulatory proteins. The mycobacterial cell wall is distinctive in two ways: first, it has a three-tiered structure (1), and second, it elongates through polar rather than lateral wall expansion (1, 2). While many of the enzymes that build the cell wall have been described, little is known about how their activity is regulated.
The mycobacterial cell wall is made of peptidoglycan, arabinogalactan, and mycolic acids, which are covalently linked together (3, 4). The peptidoglycan layer is a network of sugar chains and peptides. Arabinogalactan is composed of a galactan stem with arabinan branches (5) and anchors the mycolic acid layer to the peptidoglycan (6). The mycolic acids form a lipid bilayer. In the inner leaflet, many of the mycolic acid lipids are covalently attached to the arabinogalactan layer by ester linkages (7). The outer leaflet is made of free lipids, including free mycolic acids, trehalose dimycolates (TDMs), trehalose monomycolates (TMMs), and other lipids (8). Permeability of the cell wall is greatly restricted by the mycolic acid layer because of its tight packing of lipids, thickness, and hydrophobicity (7). MmpL3 (MSMEG_0250, Rv0206c) is an essential transporter of TMM from the cytoplasm to the periplasm (9–11). MmpL3 has a large cytoplasmic C-terminal domain, which is required for its localization at the poles and interaction with some other proteins but is dispensable for growth (12–14).
Mechanisms of polar growth are not well understood, although, in Actinobacteria, DivIVA homologs are critical for this process. In mycobacteria, Wag31 (AKA DivIVA, MSMEG_4217, Rv2145c) is essential for localizing synthesis to the poles, but it is not clear how. Based on the elongasome model in lateral growers, the assumption was that Wag31 recruits peptidoglycan transglycosylase enzymes to the elongation site. This model works in Corynebacterium, where the DivIVA homolog recruits RodA to the pole (15). However, in mycobacteria, the transglycosylases are not restricted to the poles (16).
Despite circumstantial studies (17), there is no evidence that Wag31 directly regulates any factors in the peptidoglycan synthesis pathway. Co-immunoprecipitation studies indicate that Wag31 interacts with fatty acid synthase enzymes (18, 19), MmpL3, and other factors involved in lipid metabolism (18). While it has not been shown to co-localize with enzymes involved in peptidoglycan metabolism, Wag31 does co-localize with MmpL3 and other enzymes in the mycolic acid synthesis pathway (14, 20). Wag31 also co-localizes with PlrA, a putative regulatory protein that has the same depletion phenotype as Wag31, resulting in short, bulged cells with delocalized peptidoglycan metabolism (21).
In this work, we show that Wag31, PlrA, and MmpL3 have connected functions in polar growth and that MmpL3’s recruitment to the poles depends on Wag31 and PlrA. We show that the localization of Wag31, PlrA, and MmpL3 correlates with changes in cell wall metabolism and that the localization of MmpL3 is sensitive to perturbations of peptidoglycan metabolism but not inhibition of MmpL3. We show that inhibition of MmpL3 de-activates both peptidoglycan and mycolic acid metabolism. Our data show that de-localization of MmpL3 through truncation of its C-terminal domain results in morphological changes, defective responses to starvation, and decreased elongation at the old pole but does not result in de-localized cell wall metabolism. Our data support a model in which transport of TMMs by MmpL3 activates polar peptidoglycan and mycolic acid metabolism irrespective of their location, and Wag31, PlrA, and the C-terminus of MmpL3 help the cell sense and respond to environmental changes and coordinate the synthesis of the three-tiered cell wall.
RESULTS
wag31 and mmpL3 are genetically linked
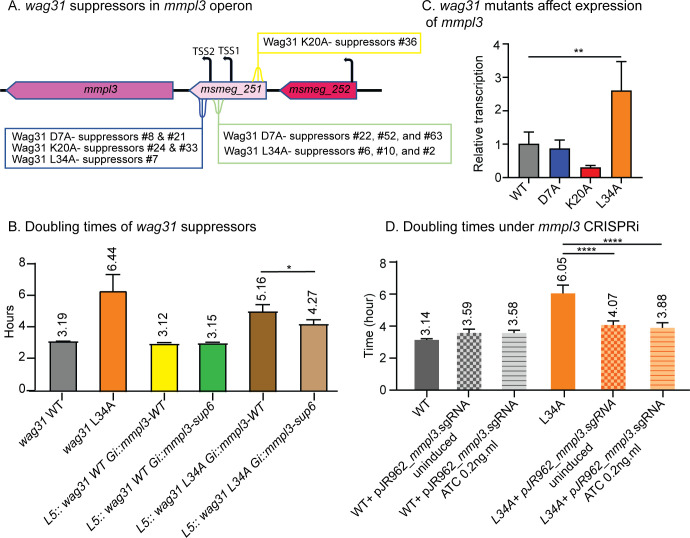
We looked for genes functionally linked to wag31 using a suppressor screen. We passaged several slow-growing wag31 point mutants—D7A, K20A, and L34A (22)—for several days, isolated fast-growing suppressors, and used whole-genome sequencing to map the mutations (Fig. 1A; Fig. S1B). We found numerous point mutations in the MSMEG_0251 gene, which is in the mmpl3 operon and contains the promoter and transcriptional start sites for mmpl3 (Fig. 1A) (23). Many of the mutations we found were near the transcriptional start sites of mmpl3.
Fig 1.
wag31 and mmpL3 are genetically connected. (A) Diagram of mmpl3 operon with positions of wag31 suppressor mutations indicated. TSS, transcriptional start sites from reference (23). (B) Doubling times of Msmeg cells expressing the suppressor #6 allele of the MSMEG_0251-mmpl3 operon in wag31 WT and wag31 L34A backgrounds. Each bar graph represents an average of three biological replicates. Error bars represent standard deviation. (C) Q-RT-PCR of mmpl3 transcript levels in wag31 WT, wag31 D7A, wag31 K20A, and wag31 L34A strains. The graph represents the relative expression of mmpl3 normalized to the housekeeping gene sigA. (D) Doubling times of Msmeg cells repressing mmpl3 transcription by CRISPRi in wag31 WT and wag31 L34A backgrounds. Three biological replicates of each strain were used for calculating the doubling time. Error bars represent standard deviation. *P ≤ 0.05; **P ≤ 0.005; and ****P ≤ 0.0001. All P-values are calculated by one-way ANOVA and Dunnett’s multiple comparisons test.
To validate that the mutations in MSMEG_0251 suppress the mutations in wag31, we built an Msmeg strain where both wag31 and msmeg-0251-mmpl3 were complemented at exchangeable integrating vectors (24) and deleted from their native loci (S1C). We performed growth curves to determine if the slow growth of the wag31 L34A mutant was suppressed by the missense mutation (D127G) in MSMEG_0251-mmpl3 found in suppressor #6 (Fig. 1B; Fig. S1A). In the wag31 L34A background, the strain with the suppressor #6 allele of MSMEG_0251-mmpl3 grows faster than the strain with the MSMEG_0251-mmpl3 wild-type allele, which indicates that the mutation in MSMEG_0251 partially suppresses the wag31 L34A slow growth phenotype (Fig. 1B).
MSMEG_0251 has been shown to interact with MmpL3 (13), so we explored whether the loss of the MSMEG_0251 function could account for the suppression of the wag31 mutants, but our results suggest that this was unlikely to be the mechanism of suppression (Fig. S2).
We hypothesized that the mutations in MSMEG_0251 might suppress the defects of the wag31 mutants due to altered transcription of mmpl3 (23). We isolated mRNA from wag31 WT and three of the strains from which we isolated suppressors that mapped to the mmpl3 locus—wag31 D7A, wag31 K20A, and wag31 L34A—and performed Q-RT-PCR (Fig. 1C). Our data show that transcription of mmpl3 is altered in the wag31 K20A and wag31 L34A mutants and was significantly increased in wag31 L34A (Fig. 1C). To determine if increased mmpl3 transcription causes the slow growth of the wag31 L34A strain, we mildly repressed mmpl3 using CRISPRi (25, 26) with no or very low induction. We found that a slight reduction of mmpl3 transcription suppressed the growth rate defect in the wag31 L34A strain (Fig. 1D). This result confirms a genetic connection between wag31 and mmpl3 and indicates that regulation of MmpL3 activity is connected to Wag31 function.
Wag31, PlrA, and MmpL3 work in the same pathway to control polar growth
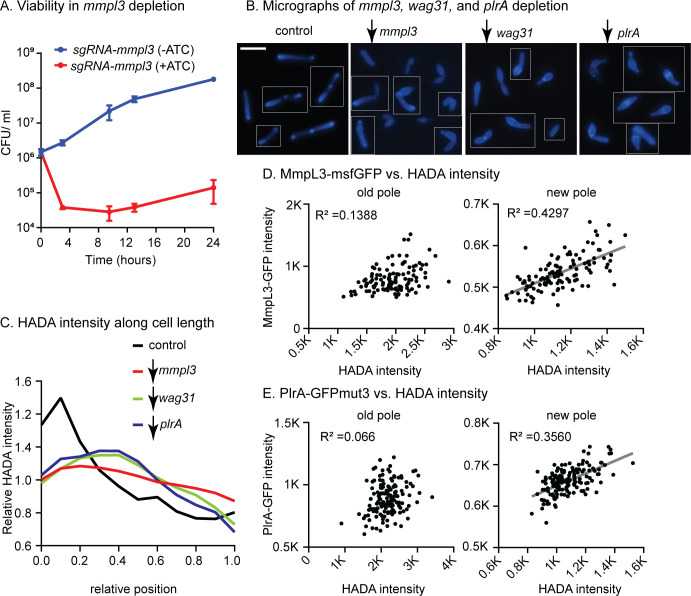
Wag31 and MmpL3 co-localize at the poles and the septa (14, 20), but a direct connection between these proteins is undescribed. To determine whether Wag31 and MmpL3 work in the same pathway in regulating polar growth, we characterized the phenotype of mmpl3-depleted cells (Fig. 2A and B). Our results show that depletion of mmpl3 causes cells to become short and bulgy (Fig. 2B) with delocalized peptidoglycan metabolism (Fig. 2C) as measured by staining with the fluorescent D-amino acid HADA (27). This same phenotype has also been observed in wag31 (28) and plrA (21) depletion (Fig. 2B and C). These data suggest that Wag31, PlrA, and MmpL3 work together in restricting cell wall metabolism to the cell poles. We note that FDAA dyes in mycobacteria are incorporated largely by LD-transpeptidases and that their staining directly reports on peptidoglycan remodeling (29). However, because FDAA staining correlates strongly with new cell wall incorporation (16, 29, 30), we use it throughout as a proxy of polar cell wall metabolism.
Fig 2.
Wag31, PlrA, and MmpL3 work in the same pathway to regulate polar growth. (A) Colony-forming units (CFUs) of Msmeg carrying the mmpl3 CRISPRi transcriptional repression construct in uninduced (blue, mmpl3 expressed) and induced (red, mmpl3 repressed) conditions. (B) Fluorescent micrographs of HADA-stained cells in WT (control) and during depletion of mmpl3, wag31, or plrA. Fluorescent signal shows the incorporation of the fluorescent D-amino acid HADA, which reports on peptidoglycan metabolism. The scale bar is 5 µm. (C) Relative averaged HADA intensity across the length of 300+ cells from experiments in panel B. Data from each cell is oriented so the signal from the pole that is brighter by HADA, presumed to be the old pole, is at position 0 on the X axis, and the new pole is at position 1. Data are normalized such that the mean of each trace is set to 1 to call attention to differences in distribution of signal. (D) Maximum intensity of the MmpL3-msfGFP signal per cell pole in 300+ cells is plotted against the maximum intensity of the HADA signal at the same pole. Data are separated by pole; the pole of each cell that is brighter by HADA is presumed to be the old pole, and the dimmer pole is presumed to be the new pole. (E) Maximum intensity of PlrA-GFPmut3 signal per cell pole in 300+ cells is plotted against the maximum intensity of HADA signal at the same pole, as in panel D. R2 values in panels D and E were calculated by linear regression analysis. The linear fit (gray line) is only shown where there is a correlation.
After division, Wag31 focus size increases as the new cell pole matures from an elongation-inactive to an elongation-active state (21, 31). We hypothesized that if MmpL3, Wag31, and PlrA work together to regulate cell wall metabolism at the poles, then the size of PlrA and MmpL3 foci might also increase as peptidoglycan metabolism at the new pole increases. We quantified the maximum intensity of MmpL3-msfGFP, PlrA-GFPmut3, and HADA for individual cell poles and plotted PlrA-GFPmut3 and MmpL3-msfGFP intensities as functions of HADA intensity for each pole. Our data show that MmpL3-msfGFP and PlrA-GFPmut3 signals moderately correlate with peptidoglycan metabolism at the new poles but not at the old poles (Fig. 2D and E). Thus, Wag31, MmpL3, and PlrA accumulate together as the new pole matures.
Wag31 and PlrA localize MmpL3 to the pole, but Wag31 does not regulate MmpL3’s transport activity
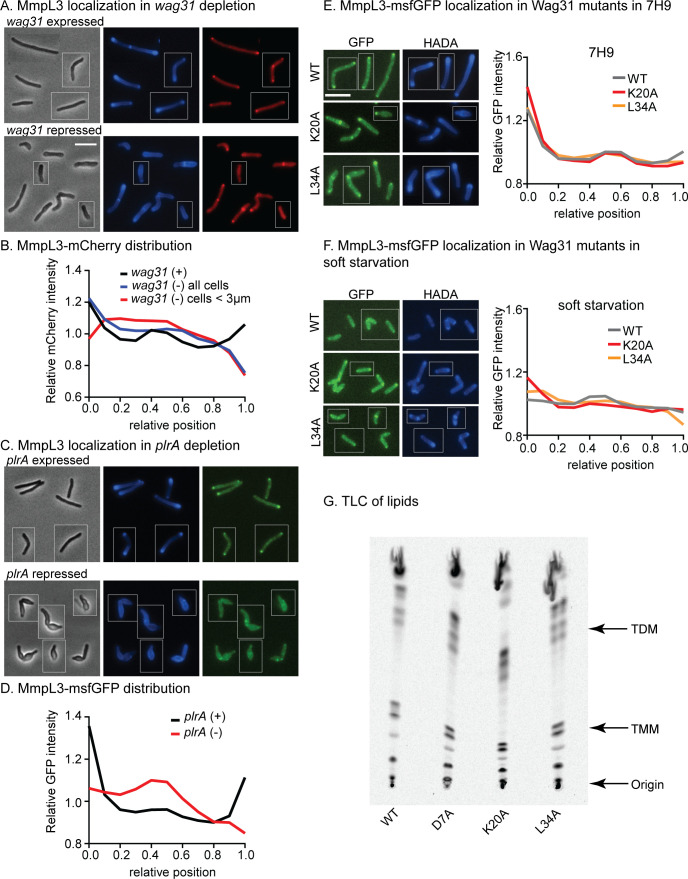
MmpL3 co-localizes with Wag31 (14, 20). To test if Wag31 is required for MmpL3’s polar localization, we depleted wag31 through CRISPRi (26) and looked at MmpL3-mCherry localization. In all cells averaged, the MmpL3-mCherry signal is still high at the old poles, likely due to the persistence of Wag31 foci there in partial depletion conditions, but is delocalized from new poles, where we would expect no Wag31 to accumulate after the induction of the CRISPRi system (Fig. 3). In cells under 3 µm in length, which we conclude have very low Wag31 levels, as in references (21, 28, 32), we observed that the MmpL3-mCherry signal was delocalized from both poles and re-distributed to the side walls (Fig. 3B). These data indicate that MmpL3’s polar localization is dependent on Wag31.
Fig 3.
Both Wag31 and PlrA are required for MmpL3’s polar localization. (A) Phase (left), HADA (middle), and Mmpl3-mCherry (right) images of Msmeg expressing MmpL3-mCherry in untreated (top) and CRISPRi repression of wag31 transcription. Scale bar is 5 µm and applies to all images in the figure. (B) Relative averaged MmpL3-mCherry intensity across the length of 300+ cells from experiments in panel A. Data from each cell are oriented so the signal from the pole that is brighter by HADA, presumed to be the old pole, is at position 0 on the X axis, and the new pole is at position 1. Data are normalized such that the mean of each trace is set to 1 to call attention to differences in distribution of signal. Relative intensity alongside the cell in wag31 depletion. Traces are the average of n = 353 wag31-expressed cells or n = 572 wag31-repressed cells. (C) Phase (left), HADA (middle), and MmpL3-msfGFP (right) images of Msmeg expressing MmpL3-msfGFP during transcriptional depletion of plrA. (D) Relative averaged MmpL3- msfGFP intensity across the length of 300+ cells from experiments in panel B, as in panel C. Traces are the average of n = 319 plrA-expressed cells or n = 347 plrA-repressed cells. (E) MmpL3-msfGFP intensity across the length of 300+ cells from strains in 7H9 with the indicated wag31 alleles. Representative micrographs to the left. (F) MmpL3-msfGFP intensity across the length of 300+ cells from strains in 5-hour soft starvation with the indicated wag31 alleles. Representative micrographs to the left. (G) Thin-layer chromatography of C14-labeled lipids extracted from strains with the indicated wag31 alleles in 7H9. Lipid species that could be identified are labeled.
As PlrA is also required for polar growth (21), we determined if MmpL3’s localization is dependent on PlrA. We depleted plrA and measured MmpL3-msfGFP distribution (Fig. 3C). Our data show that MmpL3 delocalizes from the poles when PlrA is depleted (Fig. 3D). Thus, polar localization of MmpL3 is dependent on both Wag31 and PlrA.
We hypothesize that Wag31, PlrA, and MmpL3 work together in coordinating polar cell wall expansion. Previous studies showed that MmpL3 with a truncation of its C-terminal cytoplasmic domain—which is the only domain that has access to Wag31—is delocalized from the poles (13, 14) but the cells are still viable, indicating that polar localization of MmpL3 is not essential for growth. We measured the polar localization of MmpL3 in wag31 mutants that are defective for polar growth in the logarithmic phase and in minimal media with Tween80 as a carbon source (“soft starvation”). We found (Fig. 3E) that MmpL3 was slightly mis-localized in these conditions in these strains. However, polar localization of MmpL3 does not correlate with polar growth, as the wag31 K20A mutant is defective in old pole elongation (22) but has increased old pole localization of MmpL3, while the L34A mutant is defective in new pole elongation and has decreased new pole localization of MmpL3.
To determine if Wag31’s control of MmpL3’s localization regulates MmpL3’s transport activity, we extracted C14-labeled lipids from strains with different wag31 alleles to compare the relative amounts of TMM and TDM: increased relative levels of TMM/TDM indicate decreased MmpL3 transport activity (12, 33). The relative levels of TMM/TDM were not changed in strains with wag31 alleles with different polar growth (22) and MmpL3 localization patterns (Fig. 3F). These data indicate that mis-localization of MmpL3 does not affect MmpL3 transport activity and suggest that Wag31 does not regulate MmpL3 transport activity. This conclusion is corroborated by previous work, which showed that truncation of the C-terminal domain of MmpL3, which causes complete de-localization of MmpL3 away from the poles, had no obvious effect on cell growth (12–14).
Our results indicate that the connection between Wag31, PlrA, and MmpL3 at the cell poles does not regulate MmpL3’s transport activity. We hypothesize that this connection could be involved in sensing changes in metabolism and coordinating the regulation of other cell wall layers at the pole.
Localization of MmpL3, PlrA, and Wag31 responds to energy stresses
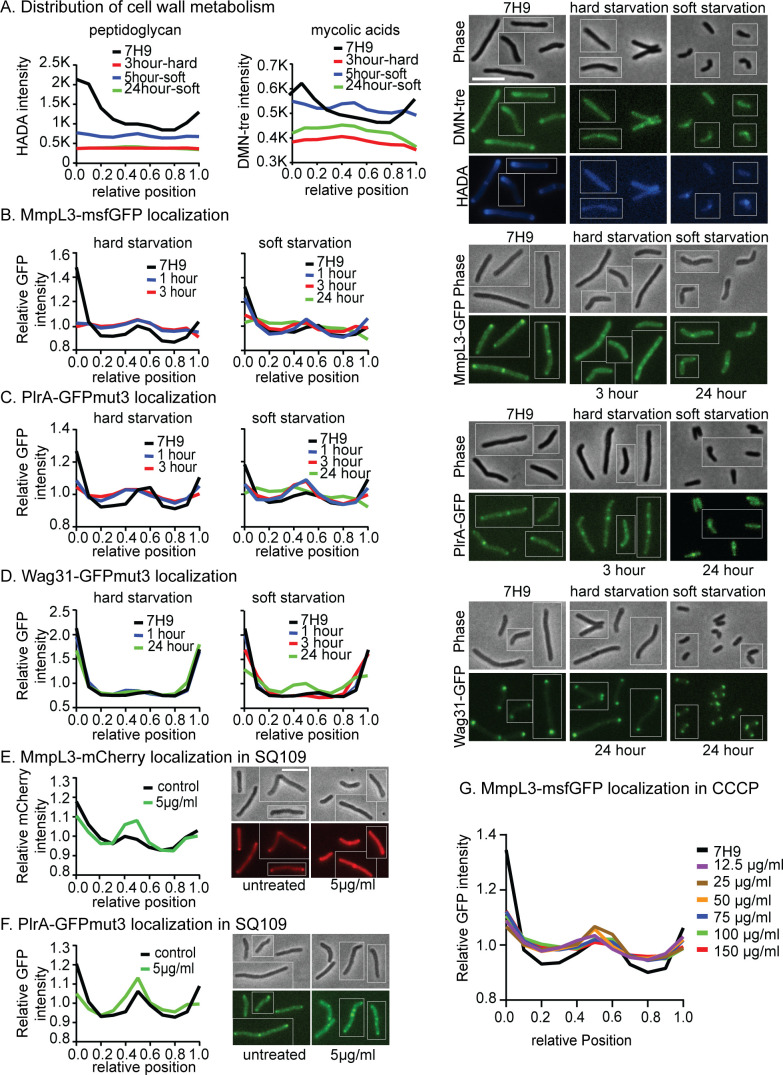
Polar growth in mycobacteria only occurs in nutrient-rich and low-stress conditions: in starvation and other stresses, cell wall metabolism is downregulated and re-distributed along the lateral walls (16, 29, 34) (Fig. 4A). When Msmeg is switched from the logarithmic phase to complete carbon starvation (“hard starvation”), it stops growing, and cell size does not change. But when it is switched to media with only Tween80 as a carbon source (“soft starvation”), polar elongation is downregulated and septation continues for a while, leading to very short cells (35, 36). We find that peptidoglycan and mycolic acid metabolism, as measured by HADA and DMN-tre (37) staining, delocalize from the poles and re-distribute to the sidewall and the septa in starvation, with slightly more septal cell wall metabolism apparent in soft starvation, especially at earlier time points when reductive division is occurring (Fig. 4A; Fig. S3A).
Fig 4.
Localization of MmpL3, PlrA, and Wag31 responds to energy stresses. (A) Averaged HADA intensity (reporting on peptidoglycan) and DMN-tre intensity (reporting on mycolic acids) in both hard and soft starvation across the length of 300+ cells, representative micrographs are on the right. Data from each cell are oriented so the signal from the pole that is brighter by HADA, presumed to be the old pole, is at position 0 on the X axis, and the new pole is at position 1. Analysis was performed on the indicated number of cells, split evenly between three biological replicate cultures: 3-hour hard starvation, n = 334; 5-hour soft starvation, n = 345; 24-hour soft starvation, n = 335; and 7H9 control, n = 458. (B) Relative, averaged MmpL3-msfGFP intensity in both hard and soft starvation across the length of 300+ cells; representative micrographs are on the right. Data are oriented as in panel A. The data analysis was done on three biological replicates of the MmpL3-msfGFP strain. Analysis was performed on the indicated number of cells, split evenly between three biological replicate cultures: 1-hour hard starvation, n = 328; 3-hour hard starvation, n = 314; 1-hour soft starvation, n = 369; 3-hour soft starvation, n = 366; 24-hour soft starvation, n = 420; and 7H9 control, n = 358. (C) Relative, averaged PlrA-GFPmut3 intensity in both hard and soft starvation across the length of 300+ cells, representative micrographs are on the right, as in panel B. Analysis was performed on the indicated number of cells, split evenly between three biological replicate cultures: 1-hour hard starvation, n = 382; 3-hour hard starvation, n = 355; 1-hour soft starvation, n = 351; 3-hour soft starvation, n = 423; 24-hour soft starvation, n = 906; and 7H9 control, n = 364. (D) Relative, averaged Wag31-GFPmut3 intensity in both hard and soft starvation across the length of 300+ cells; representative micrographs are on the right, as in panel B. Analysis was performed on the indicated number of cells, split evenly between three biological replicate cultures: 1-hour hard starvation, n = 333; 24-hour hard starvation, n = 428; 1-hour soft starvation, n = 352; 3-hour soft starvation, n = 349; 24-hour soft starvation, n = 967; and 7H9 control, n = 334. (E) Relative, averaged MmpL3-mCherry intensity across the length of 300+ cells treated or untreated with 5 µg/mL of SQ109 for 1 hour, data arranged as in panel B; representative images to the right. (F) Relative, averaged PlrA-GFPmut3 intensity across the length of 300+ cells treated or untreated with 5 µg/mL of SQ109 for 1 hour, data arranged as in panel B; representative images to the right. (G) Relative, averaged MmpL3-msfGFP intensity across the length of 300+ cells treated with different concentrations of carbonyl cyanide m-chlorophenyl hydrazone (CCCP) for 1 hour, data arranged as in panel B.
We investigated whether localization of Wag31, PlrA, and MmpL3 correlates with cell wall metabolism (,Fig. 4; Fig. S3). Our data show that PlrA and MmpL3, but not Wag31, are re-distributed from the poles to the side walls in hard starvation (Fig. 4B through D; Fig. S3B through D). In soft starvation, PlrA and MmpL3 re-localize from the poles to the septa and side walls at early time points and to the sidewalls at later time points. Wag31 signal is decreased at the poles and increased at the septa after prolonged soft starvation, indicating that the Wag31 oligomers are remodeled in this condition (Fig. 4A through D; Fig. S3A through D). To determine if the observed protein re-localization is related to membrane energetics, we treated cells with SQ109. SQ109 is an MmpL3 inhibitor and protonophore, dispersing the proton motive force (33, 38). We found that PlrA and MmpL3 partially migrate from the poles to the septa upon SQ109 treatment (Fig. 4E and F; Fig. S3E and F). MmpL3 also significantly de-localizes from the poles in cells treated with carbonyl cyanide m-chlorophenyl hydrazone (CCCP), another protonophore (Fig. 4G). These results show that the distribution of PlrA and MmpL3 correlates with cell wall metabolism across nutrient conditions and that this may be connected to cell energetics (Fig. 4; Fig. S3).
MmpL3 connects the regulation of peptidoglycan and mycolic acid metabolism
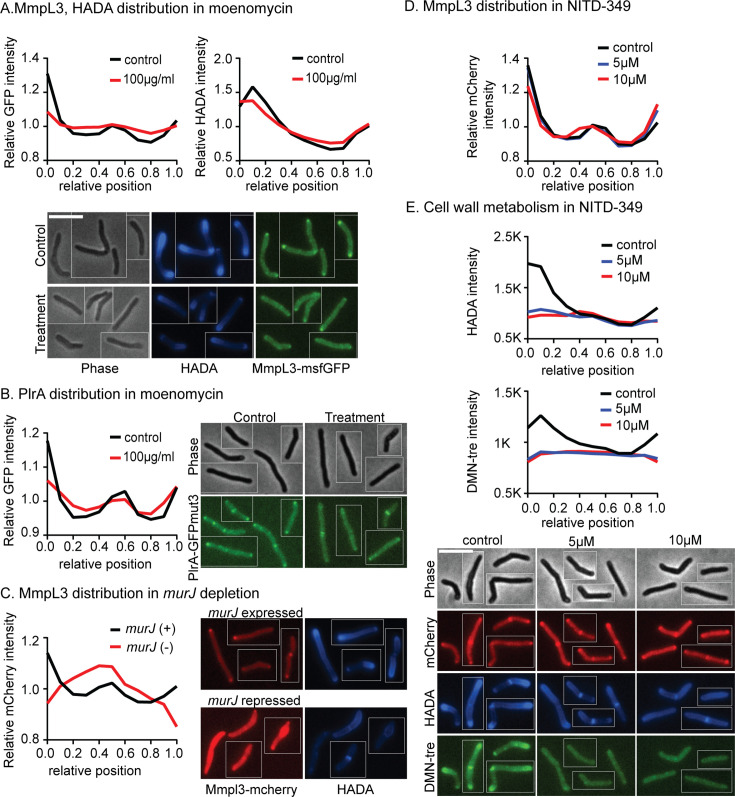
We next asked if the localization of MmpL3 and PlrA is sensitive to targeted inhibition of the cell wall. We treated the cells with moenomycin, which inhibits the transglycosylase activity of Class A penicillin-binding proteins (PBPs), and investigated MmpL3 and PlrA localization (Fig. 5A and B). We found that MmpL3 delocalizes from the poles, especially the old pole, to the side walls (Fig. 5A) and PlrA re-localizes from the old pole and the septa to the side wall (Fig. 5A and B). MmpL3 also re-localizes to the midcell when the lipidII flippase murJ is transcriptionally depleted (Fig. 5C). These data suggest that active peptidoglycan synthesis promotes the polar localization of MmpL3 and PlrA and that MmpL3 localization may be regulated by a component of the peptidoglycan synthesis pathway.
Fig 5.
MmpL3 connects the regulation of peptidoglycan and mycolic acid metabolism. (A) Relative, averaged HADA and MmpL3-msfGFP intensity across the length of 300+ cells treated or untreated with moenomycin at 100 µg/mL for 1 hour. Representative micrographs are below. Data from each cell are oriented so signal from the pole that is brighter by HADA, presumed to be the old pole, is at position 0 on the X axis, and the new pole is at position 1. (B) Relative, averaged PlrA-GFPmut3 intensity across the length of 300+ cells treated or untreated with moenomycin at 100 µg/mL for 1 hour. Data are oriented as in panel A; representative micrographs are to the right. (C) Relative, averaged MmpL3-mCherry intensity across the length of 300+ cells expressing murJ (control, black) or repressing murJ through CRISPRi for 16 hours (red). Data are oriented as in panel A; representative micrographs to the right. (D) Relative, averaged MmpL3-mCherry intensity across the length of 300+ cells treated or untreated with NITD-349 at 5 or 10 µg/mL for 1 hour. Data are oriented as in panel A; representative micrographs are below panel E. (E) Averaged HADA and DMN-tre intensity across the length of 300+ cells treated or untreated with NITD-349 at 5 or 10 µg/mL for 1 hour. Data are oriented as in panel A; representative micrographs are below.
Next, we investigated whether inhibition of MmpL3 impacts its localization and the distribution of cell wall metabolism. We used the MmpL3 inhibitor NITD-349 to disrupt trehalose monomycolate transport without affecting the proton motive force (33). MmpL3 remains at the poles when it is inhibited, though it increased at the new pole and slightly decreased at the old pole (Fig. 5D). But mycolic acid metabolism, as measured by DMN-tre staining, is inhibited all over the cell, and peptidoglycan metabolism, as measured by HADA staining, is downregulated at the poles in response to MmpL3 inhibition (Fig. 5E). Here, we show un-normalized data to emphasize the decrease in total signal. These data indicate that MmpL3’s transport activity does not affect its own localization but is required for polar peptidoglycan synthesis (Fig. 5D and E).
The MmpL3 C-terminal domain regulates polar elongation and stress response
MmpL3 has an N-terminal domain that performs TMM transport (39) and a poorly conserved and unstructured C-terminal cytoplasmic domain. The C-terminal domain is required for MmpL3’s localization at the poles and the septa (14), and previous studies revealed no overt phenotypes of the MmpL3 ∆CT strain, suggesting the C-terminal domain has little effect on TMM transport (13, 14). Our data also indicate that MmpL3’s TMM transport role is functionally independent of its localization and connection to Wag31, mediated by the C-terminal domain (Fig. 3E, F, and 5D). Since MmpL3’s polar localization is dependent on both its C-terminal domain and Wag31 and PlrA, the C-terminal domain must interact, directly or indirectly, with Wag31 or PlrA. We characterized the phenotypes of the MmpL3 ∆CT strain (MmpL31-749) (Fig. 6) to better understand the function of the C-terminal domain of MmpL3 and the polar recruitment of MmpL3 by Wag31 and PlrA.
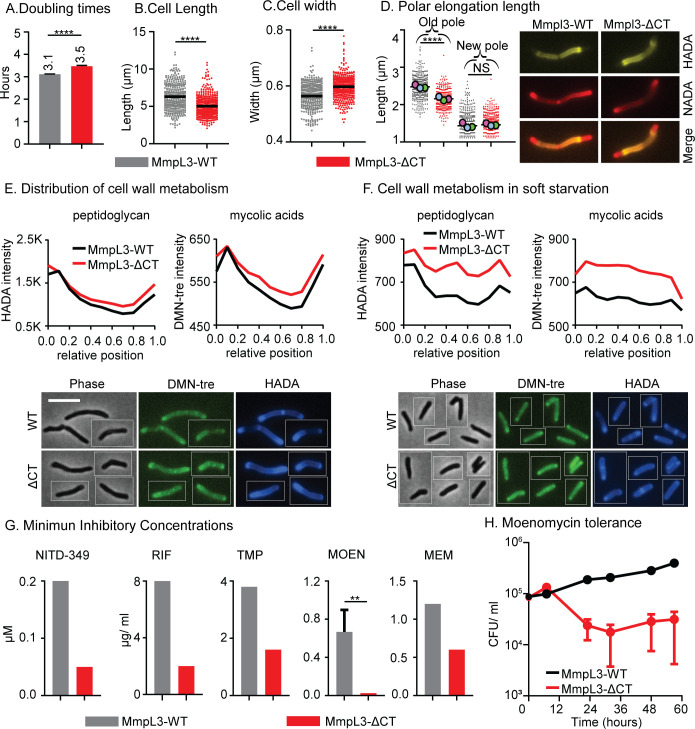
Fig 6.
The MmpL3 C-terminal domain regulates polar elongation and starvation response. (A) Doubling time of Msmeg with mmpl3 WT or mmpl3-∆CT alleles. Data represent an average of three biological replicates; error bars represent standard deviation. (B) and (C) The length and width of cells in panel A. Black bar is the mean. (D) Length of polar elongation in the mmpl3 WT and mmpl3-∆CT strains during 1.5 hours of log phase growth between initial HADA stain and later NADA stain. The length between the pole tip and the end of the HADA signal was measured for each cell pole. Black bar is at the mean, and colored balls represent the means of ~100+ cells from each biological replicate culture. The representative images show the double-stained cell, HADA (false colored yellow), and NADA (false colored red). (E) Averaged HADA and DMN-tre intensity across the length of 300+ cells of each strain in the log phase. Data from each cell are oriented so signal from the pole that is brighter by HADA, presumed to be the old pole, is at position 0 on the X axis, and the new pole is at position 1. Representative images are below. The scale bar is 5 µm and applies to all images. (F) Averaged HADA and DMN-tre intensity across the length of 300+ cells of each strain after 5 hours of soft starvation; data are oriented as in panel E, and representative images are below. Un-normalized data are shown in panels E and F to emphasize total signal changes. Imaging conditions were not comparable between panels E and F; refer to Fig. 4A to see the effects of starvation on HADA and DMN-tre staining. (G) Minimum inhibitory concentrations of indicated strains were measured using spot assay on LB Lennox plates. Three biological replicate cultures of each strain were measured; error bars are not shown when the values were identical between the replicates. Error bars represent standard deviation. NITD-349 is an MmpL3 inhibitor; RIF, rifampin; TMP, trimethoprim; MOEN, moenomycin; and MEM, meropenem. (H) CFU of indicated strains during soft starvation and treatment with 100 µg/mL of moenomycin. Error bars represent the standard error of the mean. ns, P > 0.05; **P ≤ 0.005; and ****P ≤ 0.0001.
We found that cells of the MmpL3 ∆CT strain are shorter, wider, and grow slightly slower than the WT cells (Fig. 6A through C). We measured the length of polar elongation using sequential FDAA staining (Fig. 6D) and found that MmpL3 ∆CT cells have slower elongation at the old pole (Fig. 6D). Next, we looked at peptidoglycan and mycolic acid metabolism using HADA and DMN-tre (Fig. 6E) in the logarithmic phase and found that staining of both cell wall layers is still polar in the MmpL3 ∆CT strain, though there is a slight increase of staining along the lateral walls (Fig. 6E). These data indicate that the C-terminal domain of MmpL3 is needed for maximal polar elongation at the old pole, but that polar localization of MmpL3 is not required for polar cell wall metabolism.
Due to the localization changes of MmpL3 and PlrA in starvation, we hypothesized that the connection between Wag31, PlrA, and the C-terminus of MmpL3 is involved in regulating cell wall metabolism in response to environmental changes. To test if MmpL3 ∆CT cells are defective in starvation response, we soft-starved the cells for 5 hours and observed that the MmpL3 ∆CT strains had greater HADA and DMN-tre staining than MmpL3 WT under starvation (Fig. 6F). This result indicates that the C-terminal domain of MmpL3 promotes downregulation of cell wall metabolism during stress (Fig. 6F).
To determine if the MmpL3 C-terminal domain affects antibiotic susceptibility during growth, we performed minimum inhibitory concentration (MIC) assays with antibiotics that target both cell wall and cytoplasmic enzymes. We found that MmpL3 ∆CT is more sensitive to all the antibiotics we tested, even those not connected to cell wall metabolism like rifampin and trimethoprim (Fig. 6G). We tested the hypothesis that the MmpL3 ∆CT strain may have a greater permeability than MmpL3 WT using an ethidium bromide uptake assay (40); however, we observed no difference in permeability to ethidium bromide (Fig. S3H). Thus, the C-terminal domain of MmpL3 promotes antibiotic resistance broadly, possibly through the regulation of the cell wall and other metabolic processes (Fig. 6E and F).
Since the downregulation of cell wall metabolism in starvation contributes to antibiotic tolerance (41, 42), we measured survival in moenomycin-treated cultures during soft starvation. Our results show that the MmpL3 ∆CT strain dies faster than MmpL3 WT (Fig. 6H). These data show that the MmpL3 ∆CT strain is more sensitive to moenomycin in starvation as well as growth conditions, which could be due to increased peptidoglycan metabolism in starvation (Fig. 6F).
DISCUSSION
We find that one function of Wag31 is to regulate MmpL3; mmpl3 expression is affected by Wag31, though this regulation is likely indirect (Fig. 1). Our results indicate that wag31, plrA, and mmpl3 are in the same pathway in regulating polar growth (Fig. 2) and responding to environmental stresses (Fig. 4 and 5; Fig. S3) and that Wag31 and PlrA are required for MmpL3 to localize to the pole (Fig. 3). Our characterization of an mmpl3 mutant that is not localized by Wag31 suggests that Wag31’s regulation of MmpL3 does not affect MmpL3’s transport activity but promotes the downregulation of cell wall metabolism broadly in stress (Fig. 6).
Our data are consistent with a model in which the presence of TMMs in the periplasm promotes polar elongation of all cell wall layers, irrespective of the location of their transport. When we inhibited the transport of TMMs into the periplasm with NITD-349, we saw delocalized and downregulated peptidoglycan as well as mycolic acid metabolism (Fig. 5E). Delocalization of MmpL3 through truncation of its cytoplasmic domain (14) does not significantly impact the polarity of cell wall metabolism (Fig. 6E), showing that the location of MmpL3 is not critical for polar growth but that the transport of TMMs is. We propose that TMMs may interact with other partners at the poles in the periplasm to stimulate polar cell wall synthesis. One possibility is that PgfA, which is localized at the poles, interacts with TMMs and is known to be involved in regulating polar cell wall metabolism (43). Previous work showed that MmpL3, LamA, and PgfA regulate the asymmetry of polar growth by controlling mycolic acid transport (43, 44). Polar growth requires the coordinated assembly of all the layers of the wall; thus, these results indicate that MmpL3, LamA, and PgfA are involved in regulating peptidoglycan and arabinogalactan synthesis, directly or indirectly. Our results support the model (43) that polar growth of all cell wall layers is dependent on mycolic acid transport.
We propose that the regulation of MmpL3 by Wag31 and PlrA is important in detecting and responding to stresses, in order to coordinate cell wall synthesis in changing conditions. The polar localization of MmpL3 through Wag31 and PlrA is not significantly regulating MmpL3’s transport activity since the MmpL3 ∆CT mutant has a minimal growth defect (Fig. 6A), and wag31 mutants that affect MmpL3 localization (Fig. 3E) do not affect its transport activity (Fig. 3F). MmpL3 and PlrA are de-localized in starvation (Fig. 4B and C; Fig. S3B and C), disruption of the proton motive force (Fig. 4E and F; Fig. S3F through H), and inhibition of peptidoglycan metabolism (Fig. 5A through C). However, delocalization of MmpL3 alone is not sufficient to downregulate polar growth since the MmpL3 ∆CT mutant is completely delocalized from the poles (13, 14) but maintains the polarity of cell wall metabolism (Fig. 6E). However, regulation of MmpL3 through its C-terminus, which we presume occurs through Wag31 and PlrA, becomes essential in antibiotic stresses (Fig. 6G and H) and helps downregulate cell wall metabolism under starvation (Fig. 6F). Thus, it seems that delocalization of MmpL3 occurs when polar growth is downregulated, but that this delocalization itself does not downregulate MmpL3’s activity but may instead be an indication that MmpL3 is sensing changes in cell metabolism and helping downregulate cell wall metabolism globally in response (Fig. 6F), likely through regulatory interactions of its C-terminal domain.
In this work, we find that Wag31, PlrA, and MmpL3 work together to regulate cell wall metabolism. We show that their cooperation is important for regulating polar growth and polar asymmetry (Fig. 2 and 6D). We propose that periplasmic TMMs are a key signal to stimulate polar peptidoglycan metabolism (Fig. 5E and 6E). Finally, we propose that the C-terminal domain of MmpL3 has a function in sensing and responding to stress through its interactions with Wag31 and PlrA and that the functioning of the C-terminal regulatory domain and the TMM transport domain is separate. While these studies were performed in Msmeg, the proteins and their domain structures are all conserved in Mycobacterium tuberculosis; therefore, these signaling events may be conserved in the pathogen as well. The C-terminal domain of MmpL3 is also conserved in several Corynebacteria species.
MATERIALS AND METHODS
Bacterial strain and growth conditions
All mycobacterial strains were grown in 7H9 (BD, Sparks, MD, USA) medium, as described (22). Hdb media were made as described (45) or with glycerol omitted for the soft starvation experiments. For plating the Msmeg strain, LB Lennox plates were used. Three different E. coli strains were used for cloning including DH5a, TOP10, and XL1-Blue. Antibiotic concentrations for mycobacterial strain were kanamycin, 25 µg/mL; hygromycin, 50 µg/mL; nourseothricin, 20 µg/mL; and zeocin, 20 µg/mL. For E. coli, kanamycin, 50 µg/mL; nourseothricin, 40 µg/mL; and zeocin, 25 µg/mL were used.
Growth curves
Logarithmic phase cultures were diluted to OD600 = 0.1. A Synergy Neo2 linear multi-Mode Reader was used to shake the 96-well plates at 37°C, and OD600 was read every 15 min.
Strain construction
The mmpl3 allele swap strain was made in a strain with wag31 knocked out at its native locus and complemented at the L5 phage integrase site (22). The msmeg_0251-mmpl3 operon was complemented at the Giles phage integrase site, and then a knockout of both genes at the native locus was made using double-stranded recombineering (46). Protein localization constructs were cloned using Gibson cloning (47). Primers are listed in Table S1. The MSMEG_0251 knockout and the CRISPRI constructs to deplete MSMEG_0250 (mmpl3) and MSMEG_4217 (wag31) and MSMEG_6929 (murJ) and MSMEG_4227 (murG) were from the Mycobacterial Systems Resource collection (26).
Cell staining
All cells were grown to the logarithmic phase, then incubated with HADA at 10 µM (27) for 15 min and DMN-tre at 1 µg/mL for 30 min (37) at 37°C. Then, HADA and DMN-tre were washed out and the pellets were resuspended in either 7H9, HdB + 0.05% tween 80 with no glycerol (soft starvation) or in PBS tyloxapol (hard starvation) before taking images. All cells were imaged live within 20 min of mounting.
Microscopy
Microscopy was done on live cells mounted on HdB agarose pads for growth conditions or on PBS agarose pads for starvation experiments. Cells were observed on a Nikon Ti-2 widefield fluorescence microscope with a Plan Apo 100×, 1.45 NA objective. Images were collected with a Photometrics Prime 95B camera. GFPmut3, msfGFP, and DMN-tre were imaged using a filter cube with a 470/40 nm bandpass excitation filter, a 495 nm dichroic mirror, and a 525/50 nm emission filter. mCherry was imaged using a filter cube with a 5,600/40 nm bandpass excitation filter, a 585 nm dichroic mirror, and a 630/70 nm emission filter. HADA was imaged a using filter cube with a 350/50 nm bandpass excitation filter, a 400 nm dichroic mirror, and a 460/50 nm emission filter. The fluorescence data were extracted from microscope images using MicrobeJ (48), and MATLAB code in reference (22) was used to do further evaluation.
Elongation assays
To measure the polar length within 1.5 hours, logarithmic phase cells were used. The cells were stained with HADA at 10 µM (27) for 15 min, then washed with 7H9, and the pellet was resuspended in 5 mL of 7H9 and outgrown for 1.5 hours at 37°C. Then, the cells were stained with NADA at 10 µM for 5 min, washed, mounted, and imaged.
Starvation assays
In starvation experiments, cultures were grown in 7H9 to the logarithmic phase and then pelleted, washed, and resuspended in either HdB 0.05% tween 80 with no glycerol (soft starvation) or in PBS with 0.05% tyloxapol (hard starvation).
Drug treatment of cells
All drug treatments were performed on logarithmic phase cultures in 7H9 at the following concentrations: SQ109, 5 µg/mL; CCCP, 12.5, 25, 50, 75, 100, and 150 µg/mL; moenomycin, 100 µg/mL; and NITD349, 5 and 10 µM. The cells were incubated for 1 hour at 37°C before taking images.
Antibiotic tolerance assay
The biological replicates of MmpL3 WT and MmpL3 ∆CT strains were grown in 7H9 with proper antibiotics to the logarithmic phase. Then, 7H9 was removed and the cell pellets were washed with HdB 0.05% tween 80 with no glycerol (soft starvation media). The washed pellets were resuspended in 3 mL of HdB 0.05% tween80 with no glycerol to an OD of 0.05, then they were treated with moenomycin at 100 µg/mL. Colony-forming unit counts were done on LB Lennox plates with zeocin 20 µg/mL.
Minimum inhibitory concentration assay
MmpL3 WT and MmpL3 ∆CT strains were grown in 7H9 to the logarithmic phase, then serial dilutions (10−1–10−6) of three biological replicates of each strain were plated on LB Lennox plates with different antibiotic concentrations. The MIC was determined by finding the lowest concentration of the drug in which there was at least a 10-fold difference in colony number compared to the no-drug control.
RNA extraction and Q-RT-PCR
The cells were grown up to the logarithmic phase (OD600 = 0.6–0.7) and then the RNA was extracted from three biological replicates of each strain. Approximately 20–30 mL of culture was pelleted, resuspended in 1,000 µL of TRIzol (Zymo Research), and lysed by bead beating. Zymogen Direct-zol RNA Miniprep Plus (catalog no. 2070) kit and the manufacturer’s protocol were used to purify RNA. RNA samples were normalized before DNase 1 treatment (ThermoFisher). RT-PCR was conducted with the Kapa Biosystems Sybr Fast one-step qRT-PCR kit. The QPCR primers were designed by Primer3 (Table S3). SigA transcript levels were used as an internal control. RT-PCR was done as described (49) in a Bio-Rad CFX Connect system. To analyze the data, we used the ΔΔCT method as described (50).
Thin layer chromatography of lipid extracts
The lipids were extracted as previously described in reference (12) with some modifications. Cells were incubated with sodium [1–14C]-acetate (final concentration: 0.5 µCi·mL; Perkin Elmer) for 3 hours at 37°C. The cells were pelleted and washed two times with PBS 1×. Then, 6 mL of chloroform, methanol, and water (1:2:0.2) was added and shaken at 25°C with 180 rpm overnight to get the final two-phase separation. The samples were centrifuged at 4,400 rpm for 15 min to separate the phases; then, the organic phase was transferred to a new tube. This step was repeated twice until all organic phases were collected from each sample, and then they were air-dried using N2. The dried samples were washed with chloroform-methanol-NaCl (0.9%) (4:2:1) to remove extra lipids, and the new organic phase was transferred to a new tube and air-dried using N2.
Thin layer chromatography (TLC) was used to analyze the extracted lipids. The chamber was equilibrated with chloroform, methanol, and water (40:8:1) for 1 hour. Dried radiolabeled lipid samples were suspended in chloroform: methanol (2:1), measured by a Perkin Elmer BetaScout Liquid Scintillation Counter, and normalized to spot an equal amount of C14 on a TLC Silica gel 60 F254 plate (Sigma-Aldrich). TLC plate was developed, then air dried for 2 hours and visualized by Storm 860 scanner. The ImageQuant software was used to view the images.
Chemical synthesis
SQ109 was synthesized as previously reported (51) with minimal modification. Briefly, N-isoprenyl ethylene diamine (9.8 mg, 0.05 mmol, 1.0 equiv) and 2-adamantanone (9.0 mg, 0.06 mmol, 1.2 equiv) were stirred in methanol (1.5 mL) for 2 hours at 23°C. Then, sodium borohydride (3.8 mg, 0.1 mmol, 2.0 equiv) was added slowly over 15 min. The headspace of the reaction was purged with argon gas, and the reaction was stirred at 23°C for 18 hours. The reaction was diluted to 15 mL with total methanol, and water (20 mL) was added to the reaction to quench the remaining reducing agent. The solution was extracted with ethyl acetate (3 × 50 mL), and the organic phases were combined and evaporated in vacuo. The crude material was purified via flash chromatography (SiO2) using an isocratic eluent of 88:10:2 CHCl3:CH3OH:NH4OH. The purified material was evaporated in vacuo and converted to its HCl salt with an etherial solution of HCl gas bubbled into Et2O to yield a white solid, 10.8 mg (53%) yield. NMR data (CDCl3, 500 MHz) matched those of the previous methods (51).
Ethidium bromide uptake assay
The assay was performed as described previously with some modifications (40). Three biological replicates of MmpL3 WT and MmpL3 ∆CT strains were grown in 7H9 with proper antibiotics to the late logarithmic phase. Then, 7H9 was removed, and the cell pellets were resuspended with HdB 0.05% tween 80 with glycerol and diluted to an OD600 of 0.5. A volume of 100 µL of cells was added per well of black, clear-bottomed 96-well microplates, and the ethidium bromide was just added to a final concentration of 20 µM before measuring the fluorescence intensity. The fluorescence was measured by Synergy Neo2 linear multi-Mode Reader with an excitation wavelength of 530 nm and an emission wavelength of 590 nm.
ACKNOWLEDGMENTS
We thank Allison Fay and Michael Glickman for the mcherry-MmpL3 strains. We thank Celena Gwin and Hesper Rego for the msfGFP-MmpL3 construct. We thank Ei Phoo Phoo Aung, Joseph Wade, and Keith Derbyshire for the generous gift of the MSRdb collection.
This work was funded by NIH grant R01AI148917 to C.C.B. and a STARs award from the University of Texas System to J.A.B.
Contributor Information
Cara C. Boutte, Email: cara.boutte@uta.edu.
Patricia A. Champion, University of Notre Dame, Notre Dame, Indiana, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jb.00204-24.
Fig. S1 to S3.
Tables S1 to S3.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Thanky NR, Young DB, Robertson BD. 2007. Unusual features of the cell cycle in Mycobacteria: polar-restricted growth and the snapping-model of cell division. Tuberculosis (Edinb) 87:231–236. doi: 10.1016/j.tube.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 2. Aldridge BB, Fernandez-Suarez M, Heller D, Ambravaneswaran V, Irimia D, Toner M, Fortune SM. 2012. Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science 335:100–104. doi: 10.1126/science.1216166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minnikin DE. 1991. Chemical principles in the organization of lipid components in the mycobacterial cell envelope. Res Microbiol 142:423–427. doi: 10.1016/0923-2508(91)90114-p [DOI] [PubMed] [Google Scholar]
- 4. Daffé M, Draper P. 1997. The envelope layers of Mycobacteria with reference to their pathogenicity, p 131–203. In Advances in Microbial Physiology. Elsevier. [DOI] [PubMed] [Google Scholar]
- 5. McNeil M, Wallner SJ, Hunter SW, Brennan PJ. 1987. Demonstration that the galactosyl and arabinosyl residues in the cell-wall arabinogalactan of Mycobacterium leprae and Mycobacterium tuberculosis are furanoid. Carbohydr Res 166:299–308. doi: 10.1016/0008-6215(87)80065-4 [DOI] [PubMed] [Google Scholar]
- 6. Hett EC, Rubin EJ. 2008. Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev 72:126–156, doi: 10.1128/MMBR.00028-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crick DC, Mahapatra S, Brennan PJ. 2001. Biosynthesis of the arabinogalactan-peptidoglycan complex of Mycobacterium tuberculosis. Glycobiology 11:107R–118R. doi: 10.1093/glycob/11.9.107r [DOI] [PubMed] [Google Scholar]
- 8. Marrakchi H, Lanéelle M-A, Daffé M. 2014. Mycolic acids: structures, biosynthesis, and beyond. Chem Biol 21:67–85. doi: 10.1016/j.chembiol.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 9. Jain M, Chow ED, Cox JS.. 2008. The MmpL protein family, p. 201–210. In The Mycobacterial Cell Envelope. [Google Scholar]
- 10. Tseng TT, Gratwick KS, Kollman J, Park D, Nies DH, Goffeau A, Saier MH. 1999. The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J Mol Microbiol Biotechnol 1:107–125. [PubMed] [Google Scholar]
- 11. Yang Y, Kulka K, Montelaro RC, Reinhart TA, Sissons J, Aderem A, Ojha AK. 2014. A hydrolase of trehalose dimycolate induces nutrient influx and stress sensitivity to balance intracellular growth of Mycobacterium tuberculosis. Cell Host Microbe 15:153–163. doi: 10.1016/j.chom.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belardinelli JM, Yazidi A, Yang L, Fabre L, Li W, Jacques B, Angala SK, Rouiller I, Zgurskaya HI, Sygusch J, Jackson M. 2016. Structure-function profile of MmpL3, the essential mycolic acid transporter from Mycobacterium tuberculosis. ACS Infect Dis 2:702–713. doi: 10.1021/acsinfecdis.6b00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Belardinelli JM, Stevens CM, Li W, Tan YZ, Jones V, Mancia F, Zgurskaya HI, Jackson M. 2019. The MmpL3 interactome reveals a complex crosstalk between cell envelope biosynthesis and cell elongation and division in mycobacteria. Sci Rep 9:10728. doi: 10.1038/s41598-019-47159-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carel C, Nukdee K, Cantaloube S, Bonne M, Diagne CT, Laval F, Daffé M, Zerbib D. 2014. Mycobacterium tuberculosis proteins involved in mycolic acid synthesis and transport localize dynamically to the old growing pole and septum. PLoS ONE 9:e97148. doi: 10.1371/journal.pone.0097148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sieger B, Bramkamp M. 2014. Interaction sites of DivIVA and RodA from Corynebacterium glutamicum. Front Microbiol 5:738. doi: 10.3389/fmicb.2014.00738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melzer ES, Kado T, García-Heredia A, Gupta KR, Meniche X, Morita YS, Sassetti CM, Rego EH, Siegrist MS. 2022. Cell wall damage reveals spatial flexibility in peptidoglycan synthesis and a nonredundant role for RodA in mycobacteria. J Bacteriol 204:e0054021. doi: 10.1128/jb.00540-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jani C, Eoh H, Lee JJ, Hamasha K, Sahana MB, Han J-S, Nyayapathy S, Lee J-Y, Suh J-W, Lee SH, Rehse SJ, Crick DC, Kang C-M. 2010. Regulation of polar peptidoglycan biosynthesis by Wag31 phosphorylation in mycobacteria. BMC Microbiol 10:327. doi: 10.1186/1471-2180-10-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu W, Zhang L, Mai J, Peng R, Yang E, Peng C, Wang H. 2014. The Wag31 protein interacts with AccA3 and coordinates cell wall lipid permeability and lipophilic drug resistance in Mycobacterium smegmatis. Biochem Biophys Res Commun 448:255–260. doi: 10.1016/j.bbrc.2014.04.116 [DOI] [PubMed] [Google Scholar]
- 19. Meniche X, Otten R, Siegrist MS, Baer CE, Murphy KC, Bertozzi CR, Sassetti CM. 2014. Subpolar addition of new cell wall is directed by DivIVA in mycobacteria. Proc Natl Acad Sci U S A 111:E3243–51. doi: 10.1073/pnas.1402158111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thouvenel L, Rech J, Guilhot C, Bouet J-Y, Chalut C. 2023. In vivo imaging of MmpL transporters reveals distinct subcellular locations for export of mycolic acids and non-essential trehalose polyphleates in the mycobacterial outer membrane. Sci Rep 13:7045. doi: 10.1038/s41598-023-34315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quintanilla SY, Arejan NH, Patel PB, Boutte CC. 2023. PlrA (MSMEG_5223) is an essential polar growth regulator in Mycobacterium smegmatis. PLoS ONE 18:e0280336. doi: 10.1371/journal.pone.0280336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Habibi Arejan N, Ensinck D, Diacovich L, Patel PB, Quintanilla SY, Emami Saleh A, Gramajo H, Boutte CC. 2022. Polar protein Wag31 both activates and inhibits cell wall metabolism at the poles and septum. Front Microbiol 13:1085918. doi: 10.3389/fmicb.2022.1085918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martini MC, Zhou Y, Sun H, Shell SS. 2019. Defining the transcriptional and post-transcriptional landscapes of Mycobacterium smegmatis in aerobic growth and hypoxia. Front Microbiol 10:591. doi: 10.3389/fmicb.2019.00591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pashley CA, Parish T. 2003. Efficient switching of mycobacteriophage L5-based integrating plasmids in Mycobacterium tuberculosis. FEMS Microbiol Lett 229:211–215. doi: 10.1016/S0378-1097(03)00823-1 [DOI] [PubMed] [Google Scholar]
- 25. Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, Pritchard JR, Church GM, Rubin EJ, Sassetti CM, Schnappinger D, Fortune SM. 2017. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol 2:16274. doi: 10.1038/nmicrobiol.2016.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Judd JA, Canestrari J, Clark R, Joseph A, Lapierre P, Lasek-Nesselquist E, Mir M, Palumbo M, Smith C, Stone M, et al. 2021. A mycobacterial systems resource for the research community. MBio 12:e02401-20. doi: 10.1128/mBio.02401-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuru E, Hughes HV, Brown PJ, Hall E, Tekkam S, Cava F, Pedro MA, Brun YV, VanNieuwenhze MS. 2012. In situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent D -amino acids. Angew Chem Int Ed 51:12519–12523. doi: 10.1002/anie.201206749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Melzer ES, Sein CE, Chambers JJ, Siegrist MS. 2018. DivIVA concentrates mycobacterial cell envelope assembly for initiation and stabilization of polar growth. Cytoskeleton (Hoboken) -> Cytoskeleton 75:498–507. doi: 10.1002/cm.21490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baranowski C, Welsh MA, Sham L-T, Eskandarian HA, Lim HC, Kieser KJ, Wagner JC, McKinney JD, Fantner GE, Ioerger TR, Walker S, Bernhardt TG, Rubin EJ, Rego EH. 2018. Maturing Mycobacterium smegmatis peptidoglycan requires non-canonical crosslinks to maintain shape. Elife 7:e37516. doi: 10.7554/eLife.37516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. García-Heredia A, Pohane AA, Melzer ES, Carr CR, Fiolek TJ, Rundell SR, Lim HC, Wagner JC, Morita YS, Swarts BM, Siegrist MS. 2018. Peptidoglycan precursor synthesis along the sidewall of pole-growing mycobacteria. Elife 7:e37243. doi: 10.7554/eLife.37243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hannebelle MTM, Ven JXY, Toniolo C, Eskandarian HA, Vuaridel-Thurre G, McKinney JD, Fantner GE. 2020. A biphasic growth model for cell pole elongation in mycobacteria. Nat Commun 11:452. doi: 10.1038/s41467-019-14088-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang C-M, Nyayapathy S, Lee J-Y, Suh J-W, Husson RN. 2008. Wag31, a homologue of the cell division protein DivIVA, regulates growth, morphology and polar cell wall synthesis in mycobacteria. Microbiology (Reading) 154:725–735. doi: 10.1099/mic.0.2007/014076-0 [DOI] [PubMed] [Google Scholar]
- 33. Li W, Stevens CM, Pandya AN, Darzynkiewicz Z, Bhattarai P, Tong W, Gonzalez-Juarrero M, North EJ, Zgurskaya HI, Jackson M. 2019. Direct inhibition of MmpL3 by novel antitubercular compounds. ACS Infect Dis 5:1001–1012. doi: 10.1021/acsinfecdis.9b00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayashi JM, Richardson K, Melzer ES, Sandler SJ, Aldridge BB, Siegrist MS, Morita YS. 2018. Stress-induced reorganization of the mycobacterial membrane domain. MBio 9:e01823-17. doi: 10.1128/mBio.01823-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu M-L, Gengenbacher M, Dick T. 2016. Mild nutrient starvation triggers the development of a small-cell survival morphotype in mycobacteria. Front Microbiol 7:947. doi: 10.3389/fmicb.2016.00947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu M-L, Chan CL, Dick T. 2016. Rel Is required for morphogenesis of resting cells in Mycobacterium smegmatis. Front Microbiol 7:1390. doi: 10.3389/fmicb.2016.01390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamariza M, Shieh P, Ealand CS, Peters JS, Chu B, Rodriguez-Rivera FP, Babu Sait MR, Treuren WV, Martinson N, Kalscheuer R, Kana BD, Bertozzi CR. 2018. Rapid detection of Mycobacterium tuberculosis in sputum with a solvatochromic trehalose probe. Sci Transl Med 10:eaam6310. doi: 10.1126/scitranslmed.aam6310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodriguez-Rivera FP, Zhou X, Theriot JA, Bertozzi CR. 2018. Acute modulation of mycobacterial cell envelope biogenesis by front-line tuberculosis drugs. Angew Chem Int Ed Engl 57:5267–5272. doi: 10.1002/anie.201712020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Su C-C, Klenotic PA, Bolla JR, Purdy GE, Robinson CV, Yu EW. 2019. MmpL3 is a lipid transporter that binds trehalose monomycolate and phosphatidylethanolamine. Proc Natl Acad Sci U S A 116:11241–11246. doi: 10.1073/pnas.1901346116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Purdy GE, Niederweis M, Russell DG. 2009. Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol Microbiol 73:844–857. doi: 10.1111/j.1365-2958.2009.06801.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boutte CC, Baer CE, Papavinasasundaram K, Liu W, Chase MR, Meniche X, Fortune SM, Sassetti CM, Ioerger TR, Rubin EJ. 2016. A cytoplasmic peptidoglycan amidase homologue controls mycobacterial cell wall synthesis. Elife 5:e14590. doi: 10.7554/eLife.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dulberger CL, Rubin EJ, Boutte CC. 2020. The mycobacterial cell envelope - a moving target. Nat Rev Microbiol 18:47–59. doi: 10.1038/s41579-019-0273-7 [DOI] [PubMed] [Google Scholar]
- 43. Gupta KR, Gwin CM, Rahlwes KC, Biegas KJ, Wang C, Park JH, Liu J, Swarts BM, Morita YS, Rego EH. 2022. An essential periplasmic protein coordinates lipid trafficking and is required for asymmetric polar growth in mycobacteria. Elife 11:e80395. doi: 10.7554/eLife.80395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rego EH, Audette RE, Rubin EJ. 2017. Deletion of a mycobacterial divisome factor collapses single-cell phenotypic heterogeneity. Nature 546:153–157. doi: 10.1038/nature22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berney M, Weimar MR, Heikal A, Cook GM. 2012. Regulation of proline metabolism in mycobacteria and its role in carbon metabolism under hypoxia. Mol Microbiol 84:664–681. doi: 10.1111/j.1365-2958.2012.08053.x [DOI] [PubMed] [Google Scholar]
- 46. van Kessel JC, Hatfull GF. 2007. Recombineering in Mycobacterium tuberculosis. Nat Methods 4:147–152. doi: 10.1038/nmeth996 [DOI] [PubMed] [Google Scholar]
- 47. Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- 48. Ducret A, Quardokus EM, Brun YV. 2016. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Nat Microbiol 1:16077. doi: 10.1038/nmicrobiol.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. César Hunt-Serracín Augusto, Kazi Misha I., Boll Joseph M., Boutte Cara C.. 2022. In Mycobacterium abscessus, the stringent factor rel regulates metabolism but is not the only (p)ppGpp synthase. Journal of Bacteriology 204:e00434-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 51. Onajole OK, Govender P, van Helden PD, Kruger HG, Maguire GEM, Wiid I, Govender T. 2010. Synthesis and evaluation of SQ109 analogues as potential anti-tuberculosis candidates. Eur J Med Chem 45:2075–2079. doi: 10.1016/j.ejmech.2010.01.046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S3.
Tables S1 to S3.