Abstract
Currently, wide local excision is recommended after the primary excision of cutaneous melanomas. The definition of margins for wide local excision indicated by the guidelines has remained unchanged over the years, although the reported indications are derived from fairly dated studies in which melanomas tended to be thicker or in advanced stages at diagnosis. This study aimed to retrospectively evaluate the usefulness of wide local excision for local and general control of the disease and to identify patients who had benefited from the wide local excision procedure in terms of prognosis improvement. This retrospective observational study was conducted on patients who had undergone surgery for melanoma at a single institution. The primary endpoint was progression-free survival after wide local excision in patients with or without residual melanoma. The secondary endpoint was to evaluate which patients’ demographic features and melanoma histological data were associated with residual melanoma after wide local excision. In the univariate model, melanoma-positive wide local excision resulted in the worst progression-free survival; however, this association was not confirmed in the multivariate model. The results also showed that Breslow thickness was the only factor associated with an increased risk of metastasis to the wide local excision area. According to the receiver operating characteristic analysis, the optimum cutoff value of Breslow’s thickness to predict a tumor-positive wide local excision was 2.31 mm for males and 2.4 mm for females.
Keywords: early detection of cancer, esthetics, margins of excision, melanoma, microsatellite repeats, skin neoplasms
Introduction
The incidence and prevalence of malignant melanoma (MM) are constantly increasing owing to numerous screening campaigns that have led to prompt diagnosis and earlier surgical treatment [1]. Consequently, the prognosis of melanoma has improved, leading to reduced recurrence and mortality rates [2]. Improvements in melanoma prognosis are also due to surgical therapies in the early stages of the disease and the development of increasingly effective oncological protocols [3].
As suggested by scientific guidelines, surgical excision of the primary tumor with a histological diagnosis of malignant melanoma is followed by wide local excision (WLE) of the surgical scar, ensuring the removal of adequate margins of neighboring healthy tissue relative to the initial Breslow thickness [4].
The two-step approach, including WLE, is almost exclusively used in the treatment of melanoma. For other solid tumors, such as breast or colorectal cancer, complete surgical resection with tumor-free margins is considered sufficient [5].
This practice was established empirically in the 1950s, lacking proper Level 1 prospective clinical trial evidence, which is now deemed necessary to support treatment guidelines [6]. The rationale behind WLE is to eliminate potential microsatellites surrounding the melanoma, with the aim of preventing locoregional recurrence and subsequently enhancing survival.
However, when this procedure was proposed, the populations involved in the supporting studies suffered from melanomas that tended to be thicker or in advanced stages of the disease, and the definition of margins was established by analyzing recurrences and mortality in a different era with different melanoma prognoses [7,8]. Moreover, WLE is not a procedure without risks and complications, such as infections, skin dehiscence, hematomas, and seromas. Additionally, specific anatomical areas, such as the head, neck, or hands, can lead to significant aesthetic and functional deficits [9].
Over the years, WLE has been the subject of scientific research, leading to progressive changes in surgical procedures. Since the initially advocated 5 cm margins, excision margins have decreased, and current guidelines now recommend an excision margin of 1 cm for T1–T2 melanoma and 2 cm for T3–T4 melanoma [10]. This decreasing trend in margins led to the recently launched phase III MelMarT trial (NCT02385214), which compared WLE safety margins of 1 and 2 cm for stage II melanomas (pT2b-pT4b, AJCC 8th edition) [11].
The present study is part of this line of research. We hypothesized that the WLE procedure may not bring clinical benefits to patients affected by melanoma in its early stages, exposing them to overtreatment and consequent surgical complications.
The present study aimed to retrospectively evaluate the usefulness of WLE for local and general control of the disease and to identify a cohort of patients who benefited from the WLE procedure in terms of prognosis improvement.
Materials and methods
Patients and procedures
This retrospective observational study was conducted using data collected from the hospital and outpatient medical records of patients who had undergone surgery for melanoma at the Plastic and Reconstructive Surgery Unit of the Bari General Hospital. All patients with a histological diagnosis of melanoma who underwent excision of the primary lesion and subsequent WLE from 1 January 2013 to 31 December 2019, were included. Patients with less than 3 years of follow-up and those who declined consent for the use of their data for scientific purposes were excluded. The main clinical symptoms, anamnestic conditions, histological exams, and follow-up diagnostic exams were recorded: date of excision of melanoma, date of histological diagnosis, length, width, and thickness of WLE, type of melanoma, site of melanoma, side of melanoma, Breslow thickness, Clark level, presence of ulceration, number of mitoses, presence of peritumoral or intratumoral lymphocytic infiltrate, percentage of regression, tumor node metastasis stage, microscopic distance from the margins, date of WLE, length of WLE, width of WLE, thickness of WLE, date of sentinel lymph node biopsy (SLNB), site of SLNB, number of tumor-positive SLNB, date of lymphadenectomy, site of lymphadenectomy, number of lymph nodes removed, and number of lymph nodes with metastasis.
The results of clinical visits and instrumental investigations during follow-up were also collected. Instrumental investigations included chest radiography, ultrasound of the surgical wound and lymph node basin, abdominal and pelvic ultrasound, head computed tomography (CT), chest CT, abdominal and pelvic CT, head MRI, chest MRI, abdominal and pelvic MRI, and PET.
When referring to the WLE procedure, the following surgical margins were considered in relation to the primary lesion Breslow thickness, according to the current European Union (EU) guidelines [3,12]:
In situ melanomas: 5 mm;
Breslow thickness <2 mm: 1 cm;
Breslow thickness >2 mm: 2 cm.
Endpoints
The primary endpoint was progression-free survival (PFS) after WLE in patients with or without residual melanoma.
PFS was defined as the time interval between the date of the histological diagnosis of melanoma and the occurrence of one of the following events, whichever occurred first, configuring a composite endpoint as follows:
Occurrence of distant metastases.
Occurrence of metastases in the locoregional lymph node basin.
Malignant melanoma-specific mortality.
The secondary endpoint was to evaluate which patients’ demographic features and melanoma histological data were associated with residual melanoma after WLE.
Statistical analysis
Quantitative data are shown as means and SD if normally distributed and as median and interquartile range (IQR) if the assumption of normality was not acceptable. Shapiro–Wilk statistics were used to test normality. Differences in continuous variables between groups were compared using Student’s t-test for normally distributed parameters or the nonparametric Mann–Whitney U test. Categorical data are expressed as frequencies and percentages, and the Chi-square test or Fisher’s exact test, as appropriate, was used to compare the groups. Statistical significance was set at P < 0.05. significant.
PFS was defined as the time interval between the date of the histological diagnosis of melanoma and the occurrence of the composite endpoint. Univariate and multivariate Cox regression models were used to evaluate the associations between 3-year PFS and other parameters. The proportional hazard assumptions for the Cox model were tested, and the results were expressed as hazard ratios (HR) and their 95% confidence intervals (CI). HRs were adjusted for sex, age at melanoma diagnosis, type of melanoma, residual melanoma at WLE, length of WLE, width of WLE, thickness of WLE, Breslow thickness (categorized into four classes: <1, 1–2, 2, and >4 mm), and presence of intratumoral and peritumoral infiltrates. Survival was described using Kaplan–Meier curves.
Univariate and multivariate logistic models were used to explore the risk of residual melanoma in WLE; the dependent variable was the presence of metastases at the margin. The variables included in the model were sex; age at melanoma diagnosis; type of melanoma; time from diagnosis to WLE; length, width, and thickness of WLE; Breslow thickness; presence of intratumoral infiltrate; presence of peritumoral infiltrate; and presence of ulcerations. The results of the logistic model are presented as odds ratios and 95% CI for each variable.
The optimal cutoff point for melanoma Breslow thickness to predict the presence or absence of residual melanoma at WLE was determined using the package cutpointr [13]. The optimal cutoff points were estimated by choosing the Youden index as a metric and selecting the optimal cutoff point to empirically maximize the Youden index in the sample [14]. Out-of-sample performance was estimated to evaluate the optimal cutoff point using the bootstrap method [15]. To calculate CI, 1500 bootstrap repeats were chosen [16]. The measures of accuracy were based on the area under the ROC curve (AUC) and the following indicators: sensitivity (true positives divided by all events), specificity (true negatives divided by all nonevents), accuracy (the sum of true positives and true negatives divided by the whole sample), and Cohen’s kappa coefficient.
Software
Data management, descriptive statistics, and modeling were performed using SAS/STAT version 9.4 for PC (SAS Institute, Cary, North Carolina, USA). To choose the cutoff value, the cutpointr package was run in RStudio version 1.4.1106 (RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA. 2021. Available online: http://www.rstudio.com/).
Results
There were 624 melanoma patients registered during the analysis period, but only 460 were included in the study according to the inclusion criteria. Indeed, 120 patients had less than 3 years of follow-up, 15 were lost to follow-up, and in 29 patients, at least one of the clinical, surgical, or histological variables under analysis was missing. The mean age was 55 years (IQR 45–66), with male patients being older (59, IQR 48–69.5) than females (51, IQR 42.5–63.5). The mean follow-up was 87.4 months (IQR 51–118).
The study population was divided into two groups. Group A included patients with melanoma disease progression, whereas group B included patients without disease progression. Group A included 72 patients (14.2%), while the remaining 388 patients belonged to group B. Demographic and melanoma data are summarized in Table 1.
Table 1.
Main characteristics of the patients according to the composite outcome
| Group Aa (n = 72) |
Group Bb (n = 388) |
P-value | |
|---|---|---|---|
| Sex | |||
| Male | 49 (68.1) | 185 (51) | 0.0094 |
| Female | 23 (31.9) | 178 (49) | |
| Age at diagnosis (years) median (IQR) | 54 (42–63) | 55 (45–66) | 0.745 |
| Hospital | |||
| Policlinico | 51 (70.8) | 257 (71.6) | 0.8871 |
| Other | 21 (29.2) | 102 (28.4) | |
| Melanoma site | |||
| Face | 6 (8.3) | 18 (5) | 0.4775 |
| Head | 1 (1.4) | 14 (3.9) | |
| Arm – medial | 3 (4.2) | 30 (8.4) | |
| Arm – dorsal | 2 (2.8) | 9 (2.5) | |
| Hand – palmar | 1 (1.4) | 2 (0.6) | |
| Hand – dorsal | 0 (0) | 6 (1.7) | |
| Chest | 8 (11.1) | 42 (11.7) | |
| Abdomen and anterior groin | 3 (4.2) | 21 (5.8) | |
| Back | 30 (41.7) | 117 (32.6) | |
| Buttocks | 1 (1.4) | 19 (5.3) | |
| Front thigh | 6 (8.3) | 41 (11.4) | |
| Front leg | 5 (6.9) | 17 (4.7) | |
| Rear thigh | 1 (1.4) | 2 (0.6) | |
| Rear leg | 0 (0) | 8 (2.2) | |
| Foot – dorsal | 1 (1.4) | 8 (2.2) | |
| Foot – plantar | 4 (5.6) | 4 (1.1) | |
| Ear | 0 (0) | 1 (0.3) | |
| Type of melanoma | |||
| In situ | 1 (1.4) | 47 (13.2) | <0.0001 |
| Superficial diffusion | 14 (19.4) | 167 (46.8) | |
| Epithelioid | 11 (15.3) | 35 (9.8) | |
| Nodular | 42 (58.3) | 80 (22.4) | |
| Malignant lentigo | 4 (5.6) | 26 (7.3) | |
| Other | 0 (0) | 2 (0.6) | |
| Clark stage | |||
| I | 1 (1.5) | 47 (13.9) | <0.0001 |
| II | 0 (0) | 55 (16.3) | |
| III | 11 (16.4) | 134 (39.8) | |
| IV | 44 (65.7) | 92 (27.3) | |
| V | 11 (16.4) | 9 (2.7) | |
| Ulceration | 27 (58.7) | 63 (31.3) | 0.0011 |
| Peritumoral lymphocytic infiltrate | 36 (70.6) | 164 (70.7) | >0.99 |
| Intratumoral lymphocytic infiltrate | 40 (80) | 214 (86.3) | 0.275 |
| Breslow class | |||
| <1 mm | 7 (11.3) | 126 (42.3) | <0.0001 |
| 1–2 mm | 16 (25.8) | 80 (26.8) | |
| 2.1–4 mm | 19 (30.6) | 60 (20.1) | |
| >4 mm | 20 (32.3) | 32 (10.7) | |
| Residual melanoma at wide local excisionc | 7 (10.4) | 15 (4.3) | 0.0653 |
Data are shown as N (%).
IQR, interquartile range; WLE, wide local excision.
Patients with composite endpoint.
Patients without composite endpoint.
Residual melanoma at WLE at the time of WLE.
Male participants were more frequent in Group A (68.06 vs. 50.96%, P = 0.0094). Patients in Group A principally had nodular melanoma with a Breslow thickness >2.1 mm (62.91 vs. 30.87%, respectively). No significant differences were observed between the two groups with respect to melanoma sites. Furthermore, no significant difference was observed for the presence of residual melanoma at WLE (10.45 vs. 4.29%, P = 0.0653). The histological characteristics related to the primary tumor and WLE of the patients in whom the presence of residual melanoma at WLE was found are shown in Table 1.
Survival curves for sex, type of melanoma, and presence of residual melanoma at WLE were plotted using the Kaplan–Meier method (Fig. 1). Patients with residual melanoma at WLE showed a lower survival rate, with a mean (±standard error) survival rate of 6.99 (0.67) and 24.86 (0.53) months, respectively (P = 0.039). Male patients survived less than females: mean survival (standard error) was 16.22 (0.52) and 25.97 (0.66) months, respectively (P = 0.006).
Fig. 1.
Survival curves drawn with the Kaplan–Meier method for sex, presence of a residual melanoma at WLE, and Breslow class. WLE, wide local excision.
Univariate and multivariate Cox regression models were used to assess the factors associated with the composite PFS endpoint. All hazard ratios and their confidence ranges are presented in Table 2.
Table 2.
Factors associated with progression-free survival toward composite endpoints
| Univariate model | Multivariate model | |||
|---|---|---|---|---|
| HRa | 95% CI | HRa | 95% CI | |
| Presence of metastases at wide local excision | 2.23 | 1.02–4.89 | 1.62 | 0.63–4.17 |
| Time from diagnosis to wide local excision | 1 | 0.99–1 | 0.99 | 0.97–1.01 |
| Sex | 1.99 | 1.2–3.3 | 1.49 | 0.82–2.7 |
| Age at diagnosis | 1 | 0.98–1.02 | 0.99 | 0.97–1 |
| Type of melanoma | ||||
| In situ vs. surface diffusion | 0.24 | 0.03–1.85 | ||
| In situ vs. epithelioid | 0.08 | 0.01–0.67 | ||
| In situ vs. nodular | 0.05 | 0.01–0.34 | ||
| In situ vs. lentigo maligna | 0.14 | 0.02–1.21 | ||
| Superficial spreading vs. epithelioid | 0.35 | 0.15–0.8 | ||
| Superficial spreading vs. nodular | 0.19 | 0.1–0.35 | ||
| Superficial spreading vs. lentigo maligna | 0.55 | 0.18–1.68 | ||
| Epithelioid vs. nodular | 0.55 | 0.27–1.13 | ||
| Epithelioid vs. lentigo maligna | 1.6 | 0.49–5.19 | ||
| Nodular vs. lentigo maligna | 2.93 | 1.05–8.18 | ||
| WLE length | 1.08 | 0.98–1.19 | ||
| WLE width | 1.65 | 1.34–2.04 | 1.46 | 1.03–2.06 |
| WLE thickness | 1.78 | 1.1–2.88 | ||
| Breslow class | ||||
| 1 vs. 2 | 0.24 | 0.1–0.61 | 0.23 | 0.09–0.64 |
| 1 vs. 3 | 0.17 | 0.07–0.42 | 0.17 | 0.06–0.47 |
| 1 vs. 4 | 0.09 | 0.04–0.23 | 0.11 | 0.04–0.32 |
| 2 vs. 3 | 0.7 | 0.37–1.33 | 0.74 | 0.37–1.48 |
| 2 vs. 4 | 0.37 | 0.19–0.71 | 0.48 | 0.23–1.03 |
| 3 vs. 4 | 0.53 | 0.28–0.99 | 0.66 | 0.32–1.34 |
| Intratumoral infiltrate | 0.97 | 0.52–1.8 | ||
| Peritumoral infiltrate | 0.64 | 0.32–1.28 | ||
| Melanoma site | ||||
| Head-neck vs. limbs | 1.20 | 0.49–2.96 | ||
| Head-neck vs. torso | 0.94 | 0.40–2.22 | ||
| Head-neck vs. other | 3.98 | 0.48–33.09 | ||
| Limbs vs. torso | 0.78 | 0.46–1.32 | ||
| Limbs vs. other | 3.32 | 0.45–24.66 | ||
| Torso vs. other | 4.24 | 0.58–30.88 | ||
Breslow class: 1 (<1 mm); 2 (1–2 mm); 3 (2.1–4 mm); 4 (>4 mm).
CI, confidence interval; HR, hazard ratio; WLE, wide local excision.
Adjusted by Wald methods.
In multivariate analysis, after adjustment for age at diagnosis, sex, and the time between the diagnosis of melanoma and the WLE, a larger width of WLE (i.e. larger primary lesion) was associated with a higher risk of event (HR 1.46, 95% CI 1.03–2.06), while Breslow thickness <1 mm compared with all other Breslow classes was associated with a lower risk of event (<1 vs. 1.1–2 mm: HR 0.23, 95% CI 0.09–0.64; <1 vs. 2.1–4 mm: HR 0.17, 95% CI 0.06–0.47; <1 vs. >4 mm: HR 0.11, 95% CI 0.04–0.32). Further, it was found an association between PFS and the presence of residual melanoma at WLE in the univariate model (HR 2.23, 95% CI 1.02–4.89), but it was not confirmed in the multivariable model (HR 1.62, 95% CI 0.63–4.17).
In relation to the second endpoint, univariate and multivariable logistic models were applied to estimate the probability of finding residual melanoma at WLE in relation to the demographic data of the patients and histologic information of the melanoma. In the univariate model, a greater risk was found for each one-year increase in age (OR 1.06, 95% CI 1.02–1.1), WLE width (OR 1.5, 95% CI 1.02–2.2), and Breslow thickness (OR 1.36, 95% CI 1.15–1.61). Conversely, a reduction in risk was generally found for the histological diagnosis of superficial spreading melanoma compared to the other (superficial spreading vs. epithelioid: OR 0.07, 95% CI 0.01–0.36; 0.28–0.66; superficial versus lentigo maligna: OR 0.1, 95% CI 0.02–0.65). Furthermore, an increased risk has been observed generally for melanomas located in the head-neck area compared to the other (head-neck vs. limb: OR 4.2, 95% CI 1.41–12.47; head-neck vs. torso: OR 8.17, 95% CI 2.57–25.94). In the multivariate model adjusted for patient sex and age, only Breslow thickness was associated with an increased risk of residual melanoma in the WLE (OR 1.41, 95% CI 1.06–1.88). All ORs are reported in Table 3 (Supplementary Tables, Supplemental digital content 1, http://links.lww.com/MR/A402).
Table 3.
Odds ratios and CI for the risk of residual melanoma at WLE
| Univariate model | Multivariate model | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Time from diagnosis to wide local excision (+1 day) | 1 | 1–1 | ||
| Sex (male vs. female) | 0.98 | 0.42–2.28 | 0.14 | 0.02–0.85 |
| Age at diagnosis (+1 year) | 1.06 | 1.02–1.1 | 1.02 | 0.96–1.09 |
| Type of melanoma | ||||
| In situ vs. surface diffusion | 6.7 | 1.2–37.6 | ||
| In situ vs. epithelioid | 0.47 | 0.12–1.78 | ||
| In situ vs. nodular | 0.92 | 0.26–3.18 | ||
| In situ vs. lentigo maligna | 0.69 | 0.41–3.31 | ||
| Superficial spreading vs. epithelioid | 0.07 | 0.01–0.36 | ||
| Superficial spreading vs. nodular | 0.14 | 0.28–0.66 | ||
| Superficial spreading vs. lentigo maligna | 0.1 | 0.02–0.65 | ||
| Epithelioid vs. nodular | 1.95 | 0.64–6 | ||
| Epithelioid vs. lentigo maligna | 1.47 | 0.34–6.41 | ||
| Nodular vs. lentigo maligna | 0.75 | 0.19–3.03 | ||
| WLE length (+1 cm) | 1.04 | 0.85–1.27 | ||
| WLE width (+1 cm) | 1.5 | 1.02–2.2 | ||
| WLE thickness (+1 cm) | 2.01 | 1.11–3.65 | ||
| Breslow thickness (+1 mm) | 1.36 | 1.15–1.61 | 1.41 | 1.06–1.88 |
| Intratumoral infiltrate (Yes vs. No) | 0.92 | 0.23–3.67 | ||
| Peritumoral infiltrate (Yes vs. No) | 0.39 | 0.1–1.56 | ||
| Ulcerations (Yes vs. No) | 18.86 | 2.35–151.7 | ||
| Site of melanoma | ||||
| Head-neck vs. limbs | 4.2 | 1.41–12.47 | ||
| Head-neck vs. torso | 8.17 | 2.57–25.94 | ||
| Head-neck vs. other | 4.67 | 0.53–40.89 | ||
| Limbs vs. torso | 1.94 | 0.66–5.72 | ||
| Limbs vs. other | 1.11 | 0.13–9.36 | ||
| Torso vs. other | 0.57 | 0.06–4.98 | ||
CI, confidence interval; OR, odds ratio; WLE, wide local excision.
Receiver operating characteristic (ROC) curve analysis was performed to determine the optimum cutoff value for differentiating patients with and without residual melanoma at WLE. A cutoff value was chosen that simultaneously achieved high sensitivity and specificity. An aid to choosing the cutoff comes from the Youden index (first column of Table 4), which had the highest value at 2.31 mm. This criterion allowed us to achieve the highest results in terms of sensitivity (0.75) and specificity (0.75), with the lowest false-positive and false-negative error rates (Table 4).
Table 4.
Fitting parameters of optimal cutoff points
| Cutoff point (mm) | Youden | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|
| 0.5 | 0.1 | 0.14 | 1 | 0.1 |
| 0.9 | 0.21 | 0.36 | 0.87 | 0.34 |
| 1.3 | 0.33 | 0.53 | 0.81 | 0.51 |
| 1.5 | 0.34 | 0.6 | 0.75 | 0.59 |
| 1.9 | 0.39 | 0.65 | 0.75 | 0.64 |
| 2.1 | 0.43 | 0.68 | 0.75 | 0.68 |
| 2.31 | 0.5 | 0.75 | 0.75 | 0.75 |
| 2.4 | 0.44 | 0.75 | 0.69 | 0.75 |
| 2.5 | 0.40 | 0.76 | 0.63 | 0.77 |
The 95% bootstrap CI for the optimum cutoff level was 2.31–2.4 mm. The model-fitting parameters are listed in Table 5.
Table 5.
Fitting parameters of the cutoff model for the Bag set and the Out of bag bootstrap set
| Bag | Out of bag | |||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| AUC | 0.76 | 0.72–0.80 | 0.76 | 0.71–0.81 |
| Accuracy | 0.76 | 0.74–0.78 | 0.75 | 0.72–0.77 |
| Sensitivity | 0.78 | 0.71–0.86 | 0.67 | 0.51–0.80 |
| Specificity | 0.76 | 0.73–0.78 | 0.75 | 0.73–0.78 |
| Kappa Cohens | 0.15 | 0.12–0.19 | 0.11 | 0.07–0.15 |
AUC, area under the ROC curve; IQR, interquartile range.
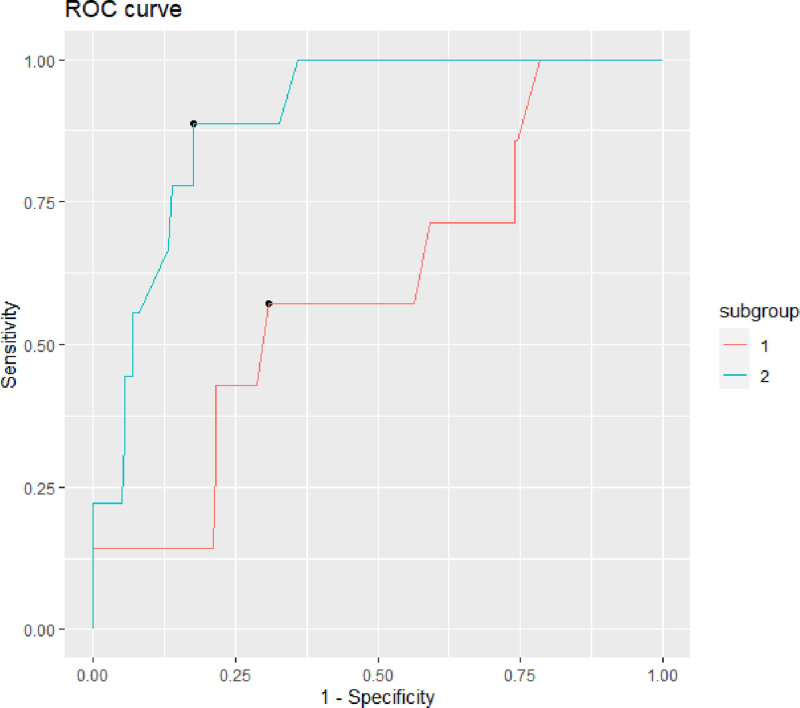
Stratified analysis by sex was conducted to verify the cutoff levels in males and females. The optimum threshold of Breslow thickness to discriminate metastasis was 2.31 mm (IQR 2.31–2.90) in women and 2.4 mm (IQR 0.87–3.2) in men. Figure 2 shows the ROC curves.
Fig. 2.
ROC to compare the model fit for males and females. ROC, receiver operating characteristic curve.
The model for women showed a higher AUC respect to that for men, respectively 0.9 vs. 0.6, and a higher Youden index, which resulted in 0.71 in women and 0.26 in men. The fitting parameters of the models are listed in Table 6.
Table 6.
Fitting parameters of the cutoff model for the Bag set and the Out of bag bootstrap set stratified by sex
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Bag | Out of bag | Bag | Out of Bag | |||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| AUC | 0.6 | 0.52–0.60 | 0.61 | 0.50–0.71 | 0.9 | 0.87–0.92 | 0.91 | 0.86–0.94 |
| Accuracy | 0.69 | 0.29–0.59 | 0.67 | 0.29–0.57 | 0.83 | 0.8–0.86 | 0.82 | 0.77–0.85 |
| Sensitivity | 0.8 | 0.62–1 | 0.5 | 0–0.76 | 1 | 0.89–1 | 0.8 | 0.6–1 |
| Specificity | 0.68 | 0.26–0.76 | 0.68 | 0.26–0.76 | 0.83 | 0.79–0.86 | 0.83 | 0.77–0.86 |
| Kappa Cohens | 0.07 | 0.03–0.12 | 0 | 0.0–0.02 | 0.31 | 0.23–0.38 | 0.21 | 0.14–0.29 |
AUC, area under the ROC curve; IQR, interquartile range.
Discussion
Melanoma treatment has changed radically over the past decade, mainly because of advances in systemic treatment. Simultaneously, surgical therapy for malignant melanoma has undergone changes, especially with respect to the treatment of lymph node metastases [17]. Indeed, current guidelines recommend the increasingly selective use of surgery for the treatment of tumor-positive sentinel lymph nodes, reserving highly invasive surgical treatments, such as lymphadenectomy, only for cases of lymphatic macrometastases of melanoma [18]. Currently, nodal clinical instrumental observation is the most common modality for the management of lymphatic involvement in malignant melanoma. This approach has led to a significant reduction in surgery-related complications without worsening patient prognosis [19].
However, this change in perspective has not been observed in the surgical management of primary malignant melanoma lesions. Current guidelines suggest a two-step procedure with primary excision followed by WLE. Despite several prospective randomized trials, the optimal extent of the excision margin remains controversial, which is reflected in the persistent lack of consensus among global guidelines.
To date, there have been seven completed RCTs, including a recently published feasibility report for active enrollment in the MelMarT-II trial [6,20–25]. A further six meta-analyses, including a Cochrane review, concluded that the current evidence was insufficient to identify optimal excision margins [26–30].
The actual EU guideline states that an adequate safety margin is needed ‘to minimize the risk of local recurrence’, in line with a meta-analysis showing that the use of a wider border may be preferable, improving specific-melanoma survival and recurrence control [3,9,29]. Nevertheless, when dealing with malignant melanoma arising in cutaneous areas with aesthetic or functional relevance, the same EU guidelines suggest narrower margins for re-excision to preserve function, maintain cosmesis, and allow reconstruction, especially for facial, acral, and genital lesions [3]. Lau et al. [31] examined a group of patients with stage IA melanomas who underwent standard excision with 10-mm margins and identified self-reported postoperative complications in 25% of cases. This relatively high percentage confirms the necessity of employing 10-mm excision margins, especially for lesions situated near critical structures. Being aware of the above-mentioned criticism, Maurichi et al. [32] retrospectively investigated whether the outcomes of a narrower (5-mm) WLE were associated with local recurrence and melanoma-specific mortality in patients with T1a melanomas close to critical structures. Their findings suggest that local excision with 5-mm margins in T1a melanoma may not be associated with an increased risk of local recurrence.
Zijlker et al. [5] conducted a literature search to identify data on the outcomes after omitting WLE. No prospective or retrospective studies have been conducted. This retrospective study showed no differences in overall survival after correcting for confounding factors [33]. In addition, the incidence of melanoma tumor cells in WLE specimens varies from 0 to 4.2% in the literature [34–38]. This means that ≥96% will not benefit from routine WLE, as they do not harbor any disease.
Considering the lack of updated guidelines regarding WLE margins and the limitations related to their application, the authors aimed to deepen the focus on this topic and evaluate the efficacy of the WLE procedure through a retrospective evaluation of a single-institution large case series. To the best of our knowledge, this is the first study to highlight a possible association between the performance of WLE and patient prognosis while also identifying factors that may predict WLE positivity. The ultimate goal was to select a cohort of patients who would benefit from this surgical procedure. Indeed, along with the surgical complications that may be associated with WLE, there is criticism regarding the reliability of lymphatic mapping after the removal of suspected lesions in anatomical regions in which reconstruction with a skin graft or flap is required. Although current guidelines suggest performing definitive surgical excision simultaneously with SLNB, further studies are required to demonstrate the accuracy of SLNB in these situations [39]. Lastly, the COVID era has forced surgeons to dramatically reduce their practice due to rapidly decreasing hospital admissions for elective surgery, which has led to the need to further select patients with melanoma eligible for surgery.
This study is the first to show that the incidence of melanoma tumor cells in WLE specimens is low. Indeed, among the 460 subjects who satisfied the histological, clinical, and follow-up inclusion criteria, residual malignant melanoma tissue after WLE was found in only 22 patients (4.78%) (7 patients in group A and 15 patients in group B). Although the frequency of melanoma progression is higher in patients who undergo tumor-positive WLE, local or distant malignant melanoma metastases have been reported in patients who undergo tumor-negative WLE.
Despite the statistically significant impact on disease progression in the univariate analysis, the presence of residual melanoma in WLE lost its significance as an independent predictive variable for malignant melanoma prognosis in the Cox multivariate analysis. According to the multivariate evaluation of clinical and pathological variables favoring disease progression, only Breslow thickness and width of the WLE demonstrated predictive power. The relationship between the width of the WLE and worsening prognosis can be explained by the greater depth of the primary malignant melanoma lesion or by the lack of radicality for anatomical reasons. Therefore, Breslow thickness was the only independent predictive factor for melanoma progression.
Regarding the second endpoint, logistic regression showed that only sex and Breslow thickness were associated with a positive WLE. Therefore, an ROC analysis separated by sex was conducted to determine the Breslow thickness (used as continuous in mm), which could predict residual melanoma at WLE. ROC curve analysis identified a value of 2.31 mm as the optimum cutoff value of Breslow’s thickness to predict a tumor-positive WLE, showing a sensitivity of 0.75 and specificity of 0.75 (AUC, 0.76, and 0.5). A Breslow thickness value of 2.31 mm was confirmed as the optimum cutoff in women, demonstrating a high accuracy (AUC = 0.9, Youden index = 0.71) in the prediction of tumor-positive WLE; in men, the better cutoff was 2.4 mm, but with a lower accuracy (AUC = 0.6, Youden index = 0.26).
Therefore, the data suggest the need to more carefully evaluate the routine preventive use of WLE in melanomas less than 2.3 mm thick, according to the high rate of negativity after removal and the absence of a significant impact on the prognosis, even in cases of tumor-positive WLE. Therefore, overtreatment can be avoided, reducing health costs, hospital stays, and eventual complications.
The main limitation of this study was that it was a retrospective, single-center study. However, the sample size and long-term follow-up allowed us to draw conclusions from a statistical perspective. Moreover, the design of the analysis in this study may be useful for conducting research on a larger scale.
Conclusion
A preliminary study showed that only approximately 5% of patients had residual melanoma at WLE without affecting PFS. These data suggest that Breslow thickness is the only predictive factor for melanoma progression and a predictive factor for residual melanoma WLE with a cutoff of 2.3 mm, suggesting a more selective recourse to WLE in thin melanomas. However, prospective randomized trials are required to corroborate these results.
Acknowledgements
The conceptualization of the study was led by G.G. and E.N., with formal analysis conducted by M.G., M.E.M., and P.T. The investigation was carried out by F.R., V.R., and R.E. Methodology was developed by E.N., F.R., and M.G., under the supervision of G.G., P.T., and R.E. The original draft was written by E.N., F.R., M.G., and M.E.M., with review and editing by G.G. and P.T.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee (n. 6679, 13.01.2021).
Our study used healthcare data with clinical and demographic information. There are legal restrictions on the data, which are owned by Policlinico di Bari and cannot be transferred in their actual format to other entities. An anonymized dataset will be made available upon request for qualified researchers. In this case, the reference person is Giuseppe Giudice.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.melanomaresearch.com.
References
- 1.Bolick NL, Geller AC. Epidemiology of melanoma. Hematol Oncol Clin North Am. 2021; 35:57–72. [DOI] [PubMed] [Google Scholar]
- 2.Kahlon N, Doddi S, Yousif R, Najib S, Sheikh T, Abuhelwa Z, et al. Melanoma treatments and mortality rate trends in the US, 1975 to 2019. JAMA Netw Open. 2022; 5:e2245269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Basset-Seguin N, et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment - Update 2022. Eur J Cancer. 2022; 170:256–284. [DOI] [PubMed] [Google Scholar]
- 4.Swetter SM, Thompson JA, Albertini MR, Barker CA, Baumgartner J, Boland G, et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, version 2.2021. J Natl Compr Canc Netw. 2021; 19:364–376. [DOI] [PubMed] [Google Scholar]
- 5.Zijlker LP, Eggermont AMM, van Akkooi ACJ. The end of wide local excision (WLE) margins for melanoma. Eur J Cancer. 2023; 178:82–87. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Cascinelli N, Adamus J, Balch C, Bandiera D, Barchuk A, et al. Thin stage I primary cutaneous malignant melanoma. Comparison of excision with margins of 1 or 3 cm. N Engl J Med. 1988; 318:1159–1162. [DOI] [PubMed] [Google Scholar]
- 7.Urist MM, Balch CM, Soong S, Shaw HM, Milton GW, Maddox WA. The influence of surgical margins and prognostic factors predicting the risk of local recurrence in 3445 patients with primary cutaneous melanoma. Cancer. 1985; 55:1398–1402. [DOI] [PubMed] [Google Scholar]
- 8.Welvaart K, Hermans J, Zwaveling A, Ruiter DJ. Prognoses and surgical treatment of patients with stage I melanomas of the skin: a retrospective analysis of 211 patients. J Surg Oncol. 1986; 31:79–86. [DOI] [PubMed] [Google Scholar]
- 9.Giudice G, Leuzzi S, Robusto F, Ronghi V, Nacchiero E, Giardinelli G, et al. Sentinel lymph node biopsy in head and neck melanoma*. G Chir. 2014; 35:149–155. [PMC free article] [PubMed] [Google Scholar]
- 10.Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019; 30:1884–1901. [DOI] [PubMed] [Google Scholar]
- 11.Coit D, Ariyan C. MelMART Trial: it’s now or never. Ann Surg Oncol. 2018; 25:2493–2495. [DOI] [PubMed] [Google Scholar]
- 12.Ethun CG, Delman KA. The importance of surgical margins in melanoma. J Surg Oncol. 2016; 113:339–345. [DOI] [PubMed] [Google Scholar]
- 13.Thiele C, Hirschfeld G. cutpointr: improved estimation and validation of optimal cutpoints in R. J Stat Soft. 2021; 98:1–27. [Google Scholar]
- 14.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005; 47:458–472. [DOI] [PubMed] [Google Scholar]
- 15.Altman DG, Royston P. What do we mean by validating a prognostic model. Stat Med. 2000; 19:453–473. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000; 19:1141–1164. [DOI] [PubMed] [Google Scholar]
- 17.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014; 370:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pathak S, Zito PM. Clinical guidelines for the staging, diagnosis, and management of cutaneous malignant melanoma. StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 19.Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017; 318:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ringborg U, Andersson R, Eldh J, Glaumann B, Hafström L, Jacobsson S, et al. Resection margins of 2 versus 5 cm for cutaneous malignant melanoma with a tumor thickness of 0.8 to 2.0 mm: randomized study by the Swedish Melanoma Study Group. Cancer. 1996; 77:1809–1814. [DOI] [PubMed] [Google Scholar]
- 21.Khayat D, Rixe O, Martin G, Soubrane C, Banzet M, Bazex JA, et al. Surgical margins in cutaneous melanoma (2 cm versus 5 cm for lesions measuring less than 2.1-mm thick). Cancer. 2003; 97:1941–1946. [DOI] [PubMed] [Google Scholar]
- 22.Balch CM, Urist MM, Karakousis CP, Smith TJ, Temple WJ, Drzewiecki K, et al. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial. Ann Surg. 1993; 218:262–7; discussion 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas JM, Newton-Bishop J, A’Hern R, Coombes G, Timmons M, Evans J, et al. Excision margins in high-risk malignant melanoma. N Engl J Med. 2004; 350:757–766. [DOI] [PubMed] [Google Scholar]
- 24.Moncrieff MD, Gyorki D, Saw R, Spillane AJ, Thompson JF, Peach H, et al. 1 versus 2-cm excision margins for pT2-pT4 primary cutaneous melanoma (MelMarT): a feasibility study. Ann Surg Oncol. 2018; 25:2541–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillgren P, Drzewiecki KT, Niin M, Gullestad HP, Hellborg H, Månsson-Brahme E, et al. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2 mm: a randomised, multicentre trial. Lancet. 2011; 378:1635–1642. [DOI] [PubMed] [Google Scholar]
- 26.Sladden MJ, Balch C, Barzilai DA, Berg D, Freiman A, Handiside T, et al. Surgical excision margins for primary cutaneous melanoma. Cochrane Database Syst Rev. 2009; 4:CD004835. [DOI] [PubMed] [Google Scholar]
- 27.Lens MB, Nathan P, Bataille V. Excision margins for primary cutaneous melanoma: updated pooled analysis of randomized controlled trials. Arch Surg. 2007; 142:885–91; discussion 891. [DOI] [PubMed] [Google Scholar]
- 28.Haigh PI, DiFronzo LA, McCready DR. Optimal excision margins for primary cutaneous melanoma: a systematic review and meta-analysis. Can J Surg. 2003; 46:419–426. [PMC free article] [PubMed] [Google Scholar]
- 29.Wheatley K, Wilson JS, Gaunt P, Marsden JR. Surgical excision margins in primary cutaneous melanoma: a meta-analysis and Bayesian probability evaluation. Cancer Treat Rev. 2016; 42:73–81. [DOI] [PubMed] [Google Scholar]
- 30.Hanna S, Lo SN, Saw RP. Surgical excision margins in primary cutaneous melanoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2021; 47:1558–1574. [DOI] [PubMed] [Google Scholar]
- 31.Lau KL, Bradish T, Rannan-Eliya S. ‘Primum non nocere’: how harmless is routine wide local excision for AJCC stage IA melanoma. Ann R Coll Surg Engl. 2020; 102:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurichi A, Barretta F, Patuzzo R, Sala L, Miceli R, Gallino G, et al. Association of excision margin size with local recurrence and survival in patients with T1a melanoma at critical structures. JAMA Dermatol. 2023; 159:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haniff J, de Vries E, Claassen AT, Looman CW, van Berlo C, Coebergh JW. Non-compliance with the re-excision guidelines for cutaneous melanoma in The Netherlands does not influence survival. Eur J Surg Oncol. 2006; 32:85–89. [DOI] [PubMed] [Google Scholar]
- 34.McGoldrick RB, Ng D, Sawyer A, Mackey S, Vadodaria S, Powell BW. Malignant melanoma re-excision specimens: is there a need for histopathological analysis. J Plast Reconstr Aesthet Surg. 2008; 61:983–984. [DOI] [PubMed] [Google Scholar]
- 35.de Waal AC, Vossen R, Aben KK, Kiemeney LA, van Rossum MM, Blokx WA. Limited role for histopathological examination of re-excision specimens of completely excised melanomas. Virchows Arch. 2014; 465:225–231. [DOI] [PubMed] [Google Scholar]
- 36.Bolshinsky V, Lin MJ, Serpell J, Leung M, Wolfe R, McLean C, Kelly JW. Frequency of residual melanoma in wide local excision (WLE) specimens after complete excisional biopsy. J Am Acad Dermatol. 2016; 74:102–107. [DOI] [PubMed] [Google Scholar]
- 37.Hocevar M, Dragonja Z, Pilko G, Gazic B, Zgajnar J. Residual melanoma after an excisional biopsy is an independent prognostic factor for local recurrence and overall survival. Eur J Surg Oncol. 2014; 40:1271–1275. [DOI] [PubMed] [Google Scholar]
- 38.Molenkamp BG, Sluijter BJ, Oosterhof B, Meijer S, van Leeuwen PA. Non-radical diagnostic biopsies do not negatively influence melanoma patient survival. Ann Surg Oncol. 2007; 14:1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giudice G, Robusto F, Vestita M, Annoscia P, Elia R, Nacchiero E. Single-stage excision and sentinel lymph node biopsy in cutaneous melanoma in selected patients: a retrospective case-control study. Melanoma Res. 2017; 27:573–579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.