Abstract
Dendrobium is a precious Chinese herbal medicine, which belongs to the genus Orchidaceae. Ancient records and modern pharmacological research show that Dendrobium has pharmacological effects such as anti-tumor, antioxidant regulating immunity and blood glucose, and anti-aging. Dendrobium contains polysaccharides, alkaloids, bibenzyl, sesquiterpenes, phenanthrene, polyphenols and other types of chemicals. Its pharmacological activity is closely related to these chemical components. For example, dendrobium extracts can achieve anti-tumor effects by inhibiting tumor cell proliferation and metastasis, promoting cell apoptosis and ferroptosis, or increasing cell sensitivity to chemotherapy drugs. It enhances immunity by regulating immune cell activity or cytokine release. In addition, it can alleviate neurodegenerative diseases by protecting nerve cells from apoptotic damage. In recent years, research reports on biologically active compounds in Dendrobium have shown a blowout growth, which makes us realize that it is meaningful to continuously update the research progress on the components and pharmacological regulatory mechanism of this traditional Chinese medicine. By classifying the collected chemical components according to different chemical structures and summarizing their pharmacological mechanisms, we investigated the current research progress of Dendrobium and provide a more comprehensive scientific foundation for the further development and clinical transformation of Dendrobium in the future.
Keywords: Dendrobium, Chemical composition, Pharmacological mechanism, Dendrobium polysaccharides
1. Introduction
Dendrobium belongs to the Orchidaceae family. It is found all over Asia, including China, Thailand, Laos, and Vietnam, among others. There are various strains of Dendrobium. According to statistics, there are more than 1,000 species worldwide, among which as many as 80 species of Dendrobium have been recorded in China [1,2]. Chinese Pharmacopoeia 2020 Edition (Part One) defines Dendrobium as the newly harvested or dehydrated stems of cultivated products of Dendrobium huoshanense C.Z.Tang et S.J.Cheng, Dendrobium nobile Lindl., Dendrobium fimbriatum Hook or. Dendrobium chrysotoxum Lindl., and similar botanical herbs of the same origin. It is widely used because of its high ornamental, edible, and medicinal value. Among them, Dendrobium officinale has been included in the list of both medicine and food by the National Health and Family Planning Commission of China [3]. In China, Dendrobium has a medicinal history of thousands of years, which can be dated back to ShenNongBenCaoJing. It is known as the first of the nine herbs with miraculous effects and is known as soft gold. Among the many strains of Dendrobium chrysotoxum Lindl., the strains with medicinal value account for about 65% [4].
Studies have shown that main chemical parent structural in Dendrobium include polysaccharides, flavonoids, bibenzyls, polyphenols, alkaloids, and sesquiterpenes. However, the chemical composition and structure of different strains of Dendrobium are also different [5]. In recent decades, Dendrobium and its extracts have been used to treat tumors, hyperglycemia, hyperlipidemia, decreased immunity, and neurological diseases caused by aging, etc [6]. In order to fully understand the regulation mechanism of this precious Chinese medicinal material on the human body and the main regulation pathways of various compounds, this review summarizes the chemical composition of Dendrobium and its pathways in vivo and in vitro reported in existing studies. In addition, we also focus on the clinical transformation of dendrobium. Therefore, in this review, we have made statistics and sorted out the dendrobium preparations currently on the pharmaceutical market in China.
2. Materials and methodology
A search strategy was used to extract available literature from the PubMed database and China National Knowledge Infrastructure (CNKI). The term “Dendrobium” was used to search in the database. After carefully screening the titles and abstracts of all identified literature to confirm that they contained extracts from Dendrobium and their pharmacological effects to ensure that they met the inclusion criteria, nearly 200 articles that met these criteria were included. In addition, we searched the PubMed and CNKI databases using the compound names in Table 1 as search terms to screen literature related to pharmacological effects and structural analysis. We searched literature in CNKI using the search terms “Shihu Yeguang Wan”, “Shihu Mingmu Wan”, “Shihu Yeguang Keli”, “Fufang Xianshihu Keli”, “Fufang Xianshihu Jiaonang” and “Fufang Shihu Pian”, the one which unrelated to disease or pharmacological effects was excluded. A total of 20 articles meeting these criteria were included. Original studies, including prospective and retrospective studies and review papers, were included and cross-referenced. The ingredients and indications of Chinese patent medicines were queried through the China Medical Information Platform (https://www.dayi.org.cn/), and the Latin names of herbal medicines were confirmed through the China Species Library. Flora (https://species.sciencereading.cn/biology/v/biologicalIndex/122.html), and database of systems pharmacology for drug discovery from herbal medicines (TCMSP) (https://old.tcmsp-e.com/index.php).
Table 1.
Types of compounds in dendrobium extracts, representative compound structures and their pharmacological effects.
| Compound type | Compound name | Compound structure | Pharmacological effects |
|---|---|---|---|
| Polysaccharides | Dendrobium huoshanense stem polysaccharide | Anti-diabetic [7,8], anti-fibrotic [9], skin protection [10,11], anticancer [12,13,14], improve cognitive dysfunction [15], regulate intestinal flora [16], anti-viral [17,18], regulate lipid metabolism [19], anti-arthritis [20,21], neuroprotection [20], anti-hypertensive [22], regulate immunity and anti-inflammatory [23,24], anti-aging [25,26], relieve dry eye syndrome [27], and antioxidant activity [28]. | |
| D. officinale polysaccharide |  |
||
| A low-molecular polysaccharide from Dendrobium huoshanense | 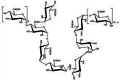 |
||
| D. huoshanense polysaccharide 1 [29] | |||
| D. huoshanense polysaccharide 2 [29] | |||
| D. huoshanense polysaccharide 3 [29] |  |
||
| D. huoshanense polysaccharide 4 [29] |  |
||
| D. huoshanense polysaccharide 5 [29] |  |
||
| Alkaloids | Dendrobine |  |
Neuroprotective and anti-nerve damage effects [30,[31], [32], [33], [34]], reduce cognitive dysfunction [35], improve abnormal liver lipid metabolism and intestinal metabolic function [36], regulate body metabolism [37], reduces acute liver injury [[38], [39], [40], [41]], relieves acute lung injury [42], reduces bone ablation and bone destruction [43]. |
| Dendrobine-N-oxide | 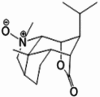 |
||
| Nobilonine |  |
||
| Dendroxine | 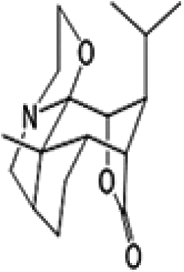 |
||
| 6-Hydroxy-nobilonine | 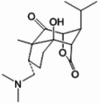 |
||
| 13-Hydroxy-14-oxodendrobine |  |
||
| Dendrocrepidine A | 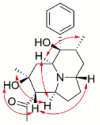 |
||
| Dendrocrepidine B |  |
||
| Dendrocrepidine C | 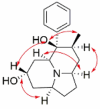 |
||
| Dendrocrepidine D | 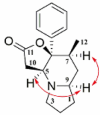 |
||
| Dendrocrepidine E | 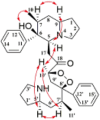 |
||
| (±)-Dendrocrepidine F | 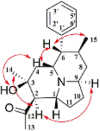 |
||
| Flavonoids | Naringenin | 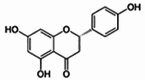 |
Antitumor [44,45], immunomodulatory and cardiovascular protective effects [46,47], neurodegenerative mitigation [48], antiviral and anti-inflammatory [49], antidiabetic [50], and osteoprotective effects [51]. |
| Apigenin | 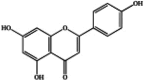 |
Anti-cancer [52,53], anti-diabetic [54,55], anti-inflammatory [56], anti-viral [57], anti-depressant [58], anti-neurodegenerative diseases [59], cardioprotective [60], bone-protective [51], immune modulation [61], and treatment of skin diseases [62,63]. | |
| Anthocyanin |  |
Anti-neurodegeneration [64,65], cardiovascular protection [66], anti-inflammatory and anti-obesity [67,68], anti-cancer [69,70], antioxidant and free radical scavenger [71]. | |
| Baicalein |  |
Anti-cancer [72], neurological and cardioprotective effects [73,74,75], antibacterial [76,77], anti-acute liver and kidney injury [78,79], joint protection [80], and anti-inflammatory effects [81]. | |
| Hesperidin | 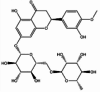 |
Anti-pulmonary fibrosis [82], neuroprotection [83], anti-osteoporosis [84], anti-inflammatory and anti-oxidative stress [85,86], anti-cancer [87]. | |
| Bibenzyl | Erianin | 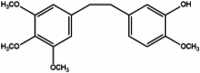 |
Anti-tumor [88,89], anti-diabetic retinopathy [90], anti-angiogenic [91,92,93], Anti-arthritic and osteoprotective effects [94,95], immune modulation [96], anti-inflammatory [97,98], antibacterial [99,100], antiviral [101], alleviating neurodegeneration and cognitive dysfunction [102], anti-cataract [103,104]. |
| Moscatilin |  |
||
| Gigantol | 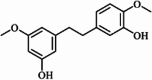 |
||
| Chrysotobibenzyl |  |
||
| Chrysotoxine | 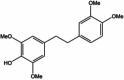 |
||
| Phenanthrenes | Dendropalpebrone | 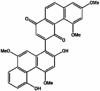 |
Anti-oxidize effect [105], immunomodulatory effect [106], antibacterial [107], anti-inflammatory [108], anti-tumor [109,110,111]. |
| Dendrocrumenol B | 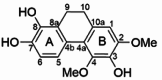 |
||
| Dendrocrumenol D | 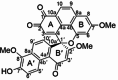 |
||
| Dendroscabrol A | 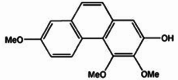 |
||
| 2,5-Dihydroxy-4-methoxy-phenanthrene 2-O-a-L rhamnopyranosyl-(1–6)-b-D-glucopyranoside | 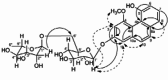 |
||
| 5-Methoxy-2,4,7,9S-tetrahydroxy-9,10-dihydrophenanthrene | 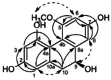 |
||
| 4-Methoxy-2,5,7,9S-tetrahydroxy-9,10-dihydrophenanthrene | 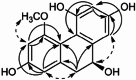 |
||
| 5-Methoxy-4,7,9S-trihydroxy-9,10-dihydrophenanthrene | 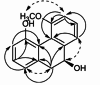 |
||
| 4-Methoxy-2,5,9R-trihydroxy-9,10-dihydrophenanthrene 2-O b-D-glucopyranoside | 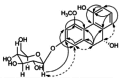 |
||
| 1,2,5,9R-Tetrahydroxy-9,10-dihydrophenanthrene 5-O-b-D glucopyranoside | 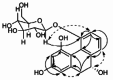 |
||
| Nudol | 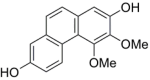 |
||
| Denbinobin |  |
||
| Polyphenols | Tannins (proanthocyanidins) |  |
Anti-tumor [112], antioxidant, antibacterial and anti-infective [113,114], antidiabetic [115], antiviral [116], fights urinary tract infections [117], bone protection [118], regulate biological rhythms [119], regulate intestinal flora [120,121], cardioprotection [122,123], neuroprotection [51,124], Prevent liver damage and skin cancer [125,126], and anti-inflammatory [127]. |
| Syringic acid | 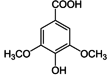 |
||
| Ellagic acid | 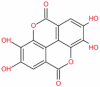 |
||
| Sesquiterpenes | Dendroterpene A |  |
Antidiabetic [128,129], neuroprotective effect [130], anti-tumor [129], cataract inhibition [131], and angiogenesis inhibition [131]. |
| Dendroterpene B | 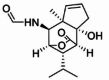 |
||
| Dendroterpene C |  |
||
| Dendroterpene D | 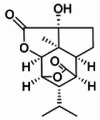 |
||
| Dendroterpene E | 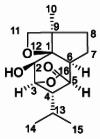 |
||
| Dendroaduoid A |  |
||
| Dendroaduol | 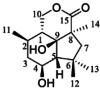 |
||
| (+)-(1R,2S,3R,4S,5R,6S,9R)-3,11,12-trihydroxypicrotoxane-2(15)-lactone |  |
||
| (−)-(1S,2R,3S,4R,5S,6R,9S,12R)-3,11,13-trihydroxypicrotoxane-2(15)-lactone | 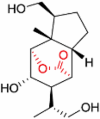 |
||
| (+)-(1R,5R,6S,8R,9R)-8,12-dihydroxy-copacamphan-3-en-2-one |  |
||
| Dendrowardin A | 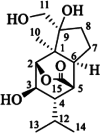 |
||
| Dendrowardin B | 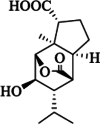 |
||
| Dendrowardin C |  |
||
| Dendrowardin D |  |
||
| Dendrowardin E | 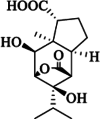 |
||
| Dendrowardin F | 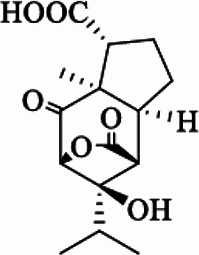 |
||
| Dendrowardin G |  |
||
| Dendrowardin H | 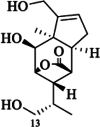 |
||
| Dendrowardin I |  |
||
| Dendrowardin J |  |
||
| (−)-(1R,2S,3R,4S,5R,6S,9S,11R)-11-Carboxymethyldendrobine | 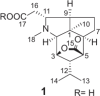 |
||
| (+)-(1R,2S,3S,4R,5R,6S,9R)-2,4,11-Trihydroxypicrotoxane-3(15) lactone | 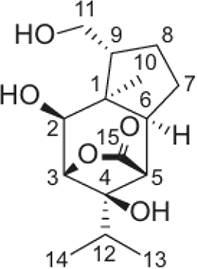 |
||
| (+)-(1R,2S,3R,4S,5R,6S,9R)-2,11,12-Trihydroxypicrotoxane-3(15) lactone | 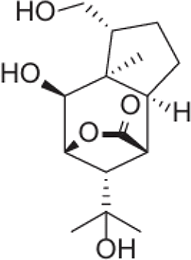 |
||
| Findlayanin | 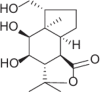 |
||
| Amino acids | Alanine |  |
By affecting genes related to bacterial membrane transport and amino acid metabolism pathways, it has an anti-Escherichia coli, Pseudomonas aeruginosa and Candida albicans effect [132,133]. |
| Cysteine |  |
||
| Aspartate | 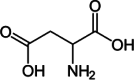 |
||
| Glutamate |  |
||
| Phenylalanine | 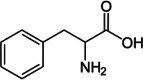 |
||
| Glycine |  |
||
| Histidine | 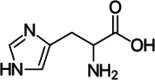 |
||
| Isoleucine |  |
||
| Lysine | 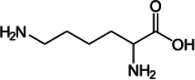 |
||
| Leucine | 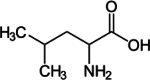 |
||
| Methionine |  |
||
| Asparagine | 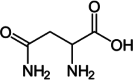 |
||
| Proline | 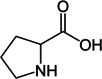 |
||
| Glutamine | 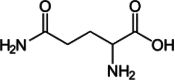 |
||
| Arginine |  |
||
| Serine | 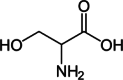 |
||
| Threonine | 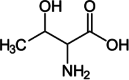 |
||
| Valine |  |
||
| Tryptophan | 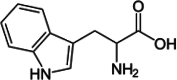 |
||
| Tyrosine |  |
||
| Coumarins | Ayapin |  |
Antibacterial [134], anti-cancer, anti-diabetic, anti-inflammatory [135], cardiac, hepatic and neuroprotective effects [136,137]. |
| Scopoletin | 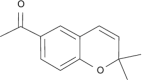 |
||
| Scopolin | 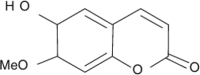 |
||
| Demethoxyencecalin |  |
||
| 3-acetyl-4-acetoxyacetophenone | 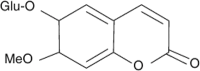 |
||
| 3-acetyl-4-hydroxyacetophenone |  |
3. The overall regulation of Dendrobium to human health
It is recorded in the Chinese Pharmacopoeia that the effects of Dendrobium candidum are benefiting the stomach and promoting body fluid, nourishing yin and clearing away heat. It is used to treat symptoms such as dry mouth and polydipsia, fluid injuries in febrile diseases, insufficient stomach yin, loss of appetite, lingering heat after illness, hyperactivity of fire due to yin deficiency, bone steaming fever, blurred vision, and weakness or flaccidity of the muscles and bones [138]. Modern pharmacological studies proved that Dendrobium can regulate the immune system and affect multiple organs in the human body (Fig. 1).
Fig. 1.
Schematic diagram of the effect of the traditional Chinese medicine Dendrobium on the human body (created with BioRender).
3.1. Immune and nervous system modulatory effects
Dendrobium and its bioactive compounds have the ability to regulate the immune system through immune cells and cytokines. Dendrobium polysaccharides (DP) stimulate immune activity by activating the extracellular signal-regulated kinase 1/2 (ERK 1/2) and nuclear factor-kappa B (NF-κB) signaling pathways in human leukemic monocytic cell line THP-1 cells [139]. Additionally, some subfractions of DP exhibit immunomodulatory activities in vivo and enhance immune responses by increasing proliferation of immune cells, the secretion of cytokines such as TNF-α, the production of nitric oxide (NO), and phagocytosis [140]. Among these compounds, 2,3-O-acetylated-1,4-β-D-glucomannan can target chemokines such as chemokine CC motif ligand 4 (CCL 4) and interferon gamma-inducible protein 10 (IP-10) to trigger immune responses in human leukemia monocytic cell line THP-1 cells. These effects are primarily related to the activation of NF-κB, which is regulated by the Toll-like receptor 4 (TLR 4) signaling pathway [141]. Another major regulatory system influenced by Dendrobium is the central nervous system. Dendrobine has protective effects against amyloid-β (Aβ)(25-35)-induced neuronal damage [142]. Further research into the mechanism found that the effect is to inhibit Aβ(25-35)-induced axonal degeneration by activating the autophagy process, which may be the pharmacological basis of dendrobium in preventing Alzheimer's disease (AD) [143]. Other reported anti-AD mechanisms include the regulation of hyperphosphorylation of microtubule-associated tau protein, reduction of extracellular amyloid plaques, activation of autophagy, enhancement of synaptic connections, suppression of neuroinflammation and inhibition of neuronal apoptosis, etc [[30], [35], [144], [145], [146], [147], [148]]. In addition, Dendrobium extract can also alleviate symptoms of chronic unpredictable mild stress depression [[149], [150], [151]], and improve the health of sub-healthy mice caused by neuroendocrine and immune system disorders [152].
3.2. Antioxidant and photodamage effects
It is well known that ultraviolet (UV) A irradiation can damage the proliferative capacity and lifespan of cells. Research has shown that DP can effectively increase the activity of antioxidant enzymes, while inhibiting the activation of JNK pathway and the expression of matrix metalloproteinase (MMPs), thereby protecting human foreskin fibroblasts (HFF-1) from UVA-induced damage and preventing cell aging [4]. Acute exposure to large amounts of UV radiation and overproduction of reactive oxygen species (ROS) are major causes of ocular pathologies. Dendrobium extract protects ARPE-19 cells from oxidative stress induced by UV through mitogen-activated protein kinase (MAPK) and Nrf2/HO-1 signaling pathways [153]. Another aspect of photodamage is damage to the epidermis of the skin caused by acute UVB exposure. Studies have shown that DP mitigates oxidative damage and apoptosis in human immortal keratinocyte line HaCaT cells induced by UVB mediated by MAPK [154]. Another possible regulatory mechanism is to reduce the release of inflammatory factors by inhibiting the transcriptional activity of NF-κB (p65), and then alleviation occurrence of inflammatory reaction and oxidative stress irradiated by UVB [155]. At the animal level, DP can protect hairless mouse skin from photodamage by regulating the process of oxidative stress, mainly manifested as significantly reducing erythema and preventing skin dryness [156].
3.3. Visual protection
Dendrobium has beneficial effects on eye health. Dry eye disease is a common clinical condition characterized by eye irritation, dryness, vision loss, and corneal damage, often caused by abnormalities in the tear film and ocular surface. Studies have shown that Dendrobium officinale extract can make gland secretion function better in patients with dry eye syndrome. The specific mechanism may involve the upregulation of AQP5 and mucin 5ac proteins in the lacrimal gland by Dendrobium water extract, which subsequently inhibits the activation of the MAPK and NF-κB pathway. Thus, the effect of inhibiting eye inflammation is exerted [27]. This effect was also verified in mouse models [51]. Diabetic retinopathy is a microvascular complication associated with diabetes. Erianin, an active ingredient in Dendrobium nodules, inhibits retinal inflammation induced by microglia by reducing cellular glucose uptake and activation of ERK1/2-NF-κB pathway, accordingly attenuating diabetic retinal inflammation, and blood-retinal barrier disruption during disease progression [90]. In vivo experiments have shown that Dendrobium extracts can inhibit the expression of inflammation-related factors and overexpressing tight junction proteins occludin and claudin-1 to relieve diabetic retinopathy [157]. Regarding ocular damage caused by excessive UV exposure, as mentioned previously, dendrobium extract can protect ARPE-19 from UV-induced visual damage through related pathways [153]. In addition to retinal epithelial cells, DP inhibits H2O2-induced apoptosis in human lens epithelial (HLE) cells by inhibiting MAPK signaling pathway [158]. In a diabetic cataract model simulated by glucose-induced HLE cells, Dendrobium extract not only inhibited aldose reductase, but also inhibited aldose reductase gene expression. It was also proved in the rat model that gigantol can inhibit the formation of cataract induced by galactose by inhibiting the expression and activity of aldose reductase and inducible NO synthase (iNOS) gene [103]. In addition, Moscatilin, a bibenzyl compound of Dendrobium, also inhibited the progression of developmental retinal vascular diseases caused by insistent ischemia/hypoxia [159].
Some dendrobium Chinese patent medicines currently used in China are also mainly developed for dry eye disease, such as Shihu Yeguang Wan. Based on their good effects during clinical use, the future development of dendrobium preparations more suitable for topical use in the eyes may have important clinical significance and economic value.
3.4. Improving lung function
Dendrobium extract also showed excellent effects in improving lung function. Studies have shown that the effect of DP on improving airway inflammation in chronic obstructive pulmonary disease (COPD) model mice can be achieved by inhibiting oxidative stress and inflammatory responses in mice [160]. Ferroptosis is a groundbreaking discovery for a form of programmed cell death, one of its manifestations is iron-dependent lipid peroxidation. Ferroptosis has been shown to be closely connected with a variety of diseases, such as tumorigenesis, arthritis, heart disease, etc. Erianin inhibits liver cancer cell activity by inducing ferroptosis and cell migration. This process may be dependent on Ca2+/CaM pathway [88]. In vivo, Dendrobium officinale has a strong tumor-inhibitory effect on lung cancer xenograft tumor models in nude mice [161]. The mechanism of dendrobium anti-lung tumors revealed by existing studies mainly involves cycle regulation [162,163], inhibition of proliferation [164], promotion of apoptosis [165,166], inhibition of migration and invasion [167,168], inhibition of epithelial-mesenchymal transformation [169,170], inhibition of angiogenesis [91], and improvement of chemosensitivity [171,172]. In addition, Dendrobium extracts also showed the potential in targeting lung cancer stem cells [[173], [174], [175], [176]]. Among them, 4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl and Lusianthridin inhibit the cell phenotype of human lung cancer stem cells via the Akt/GSK3β/β-catenin and Src-STAT3-c-Myc signaling pathways, and improve the ability of tumor cells to drug sensitivity [174,175].
At present, researchers have conducted more extensive and in-depth research on the gastrointestinal regulation and anti-gastrointestinal tumors of dendrobium polysaccharides than other types of compounds. However, compared with monomeric compounds, the structural units of polysaccharides are more complex and variable. Therefore, we may need to do more work in the future to study the anti-gastric cancer characteristics and mechanisms of polysaccharides under specific structures.
3.5. Regulate gastrointestinal function
In terms of regulating gastrointestinal function, DP can prevent the accumulation of inflammatory factors caused by excessive activity of intestinal macrophages, restore the homeostasis of the intestinal microenvironment, and thus alleviate inflammatory bowel disease [177]. For malignant colonic lesions, DP can inhibit colorectal cancer by inhibiting autophagy and enhancing immune response [12,178]. In a rat model of chronic atrophic gastritis, Dendrobium candidum can improve the histopathology of gastric mucosa in rats, reverse gastric atrophy and intestinal metaplasia, and relieve gastritis symptoms [179]. Polysaccharides of Dendrobium huoshanense C.Z.Tang et S.J.Cheng also have protective effects on gastric mucosa [180]. As the disease progresses, in the malignant lesions of the digestive tract, Dendrobium polysaccharides can not only inhibit the precancerous lesions of gastric cancer [[181], [182], [183]], but also enhance T cell-mediated immune response, inhibit tumor angiogenesis and induce tumor cell apoptosis to play an anti-gastric cancer role [184,185]. Dendrobium officinale extract can prevent gastric cancer by regulating DNA damage, oxidative stress and cytokines related to canceration and inducing apoptosis [186].
3.6. Antiviral, reproductive system protection and anti-tumor effects
In addition to the regulation of Dendrobium on different human systems listed above, Dendrobium extracts also have antiviral effects, such as H1N1 influenza virus [17], influenza A virus [187] and herpes simplex virus [188]. It is interesting to note that the water extract of Dendrobium officinale has the potential to prevent, treat and recover from adverse reactions of COVID-19 at the moment when the new coronavirus epidemic is fully open. However, its anti-inflammatory activity can affect antibody production, possibly reducing the body's production of antibody levels [189]. Therefore, it should be used with caution in practical applications. For the reproductive system, DP has protective effects on diabetes-induced testicular damage by increasing the proliferation of testicular germ cells, inhibiting apoptosis and activating the glycolytic pathway in testis [190,191]. On the other hand, DP can also improve polycystic ovary syndrome [192,193], and premature ovarian failure in naturally aging mice [194]. Dendrobium has a broad anti-tumor spectrum. In addition to the tumor types listed above, Dendrobium is also effective against human liver cancer [195,196], breast cancer [183,197,198], bladder cancer [[199], [200], [201]], prostate cancer [109], kidney cancer [202], pancreatic cancer [203], leukemia [204], and melanoma [205], ect.
4. Composition of compounds in Dendrobium and their regulatory mechanisms
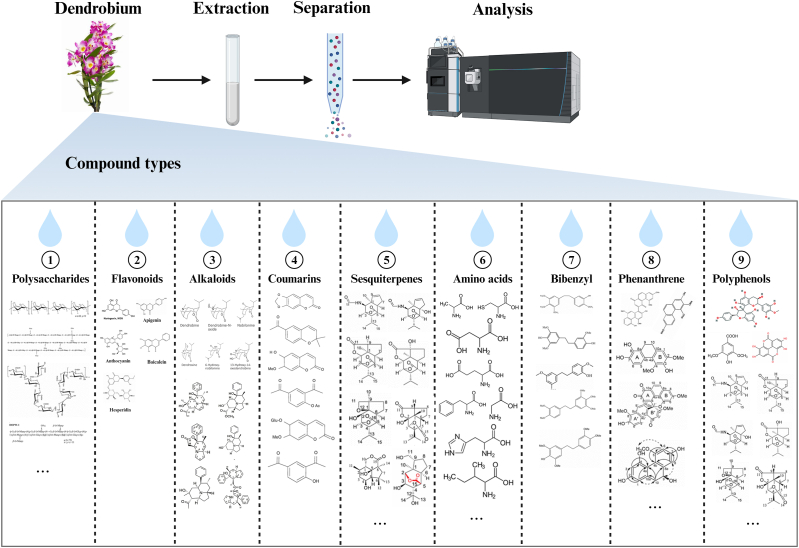
Phytochemical research data have shown that Dendrobium officinale is an excellent source of biologically active substances such as alkaloids, bibenzyls, flavonoids, and polysaccharides [206,207]. The pharmacological effects of different types of compounds are also different. The main compound categories in Dendrobium are listed in Fig. 2.
Fig. 2.
The main compound types obtained by separation and extraction of Dendrobium (created with BioRender).
4.1. Dendrobium polysaccharide
Polysaccharides refer to chemical polymers composed of more than 10 types of monosaccharides assemble, which are important active ingredients in most Chinese herbal extracts and have significant pharmacological activity. DP are mainly enriched in flowers, stems, leaves and roots of Dendrobium. Among them, the polysaccharide content in the stem is significantly higher than other parts [208]. The molecular weight, different chemical structures and molecular conformations of polysaccharides have a significant impact on their pharmacological effects and activities in vivo and in vitro. DP is mainly composed of mannose, glucose and galactose, with small amounts of rhamnose, arabinose and xylitol [209]. DP has positive effects of anti-tumor, promotion of immune response, anti-oxidation and anti-inflammation (Fig. 3). DP can inhibit the accumulation of ROS due to UV irradiation. ROS can affect and regulate a variety of cellular enzymes, such as AP-1 and NF-κB. Once activated, AP-1 and NF-κB further regulate the transcription of matrix metalloproteinases (MMPs), thereby exerting the biological effect of degrading collagen and elastin. On the other hand, as far as NF-κB itself is concerned, it is a major activator of inflammatory cell infiltration. Studies have shown that it stimulates the further expression of MMPs by inducing the release of pro-inflammatory cytokines such as TNF-α and IL [210,10]. ROS can also participate in autophagy by activating the AMPK-mTOR signaling pathway, which further activates the Bclin1 and LC3β-p65 complex [211], and DP has a blocking effect on this process [12]. In another study, UVA can rapidly activate the phosphorylation of JNK/c-Fos/c-Jun protein and up-regulate the expression of MMPs by acting on fibroblasts to generate ROS. DP can block this process [4]. In addition, DP reduced the generation of apoptotic bodies in epidermal cells, up-regulated the expression levels of cleaved-caspase9 and cleaved-caspase3, and down-regulated the activation of MAPK pathway and p53, indicating that DP has an strong protective effect against UVB-induced acute photodamage [154]. In terms of ovarian protection in female animals, DP treatment increased the levels of PCNA mRNA and protein in polycystic ovary syndrome (PCOS) ovarian tissues, and inhibited PCOS ovaries by regulating the expression and ratio of apoptosis-related proteins Bax, Bcl-2 and caspase-3 [193]. Similarly, in male animals, after DP intervention, the pathological changes of seminiferous tubules were significantly improved with the upregulation of PCNA and SIRT1 and the inhibition of apoptosis [190]. In the central nervous system, DP markedly down-regulated the expression of miR-134 in vivo and in vitro after ischemic injury, and affected the release of inflammatory factors and the expression of apoptotic proteins by changing the level of MCL-1 protein, thereby alleviating ischemic brain injury [212]. In the treatment of AD, inhibiting the hyperphosphorylation of tau protein is an important strategy, and DP has certain advantages in the treatment of AD. Studies have shown that in the lipopolysaccharide (LPS)-induced AD rat model, the phosphorylation of tau protein Ser396, Ser199-202, Ser404, Thr231, and Thr205 sites were overexpressed, along with increased GSK-3β expression, which inducd neuronal apoptosis. DP significantly improved these changes. Dendrobium nobile polysaccharide, in combination with vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor 2 (VEGFR2), can inhibit the phosphorylation of VEGFR2 and reduce the expression of VEGF and its transcription factor Activator protein-1 (AP-1) [213]. This effect blocks angiogenesis, suggesting that DP may be a potential anti-angiogenic drug. DP has also been shown to mitigate macrophage injury induced by uropathogenic Escherichia coli (UPEC) by inhibiting the activation of the NLRP3 inflammasome, reducing the induction and activation of caspase-1/GSDMD, and decreasing the secretion of pro-inflammatory cytokines, such as IL-1β [214]. In addition, the pathways regulated by DP include TLR4 signaling pathway [215], Nrf2/HO-1 signaling pathway [216], BDNF-TrkB-CREB pathway [217], fat metabolism-related pathways [19], cAMP-PKA and Akt/FoxO1 liver metabolism-related pathway [218], tumor-related Wnt/β-catenin signaling pathway [219], etc. In a gastric cancer model exposed to 1-methyl-3-nitro-1-nitrosoguanidine (MNNG), DP can regulate the NRF2 signaling pathway. The Nrf2/HO-1 signaling pathway serves as a double-edged sword, which can not only prevent normal cells from progressing into tumor cells, but also protect tumor cells from excessive oxidative stress, thereby improving the growth rate of tumor cells. In this study, the effect of DP on this pathway was specifically manifested by inhibiting NRF2 downstream heme oxygenase-1 (HO-1) and NADPH quinone oxidoreductase-1 (NQO-1), thereby preventing the progression of gastric cancer [185].
Fig. 3.
Related pathways of Dendrobium polysaccharides regulating cellular biological processes (created with BioRender).
4.2. Alkaloids
Alkaloids are a class of natural products with broad pharmacological activity, including anti-inflammatory, cardiovascular protective, anti-tumor and anti-viral. In addition to its medicinal activity, it is also a class of toxic plant active ingredients that can be used to kill insects. Up to now, dozens of alkaloids with different structures have been separated and extracted from Dendrobium [220], mainly enriched in the rhizome of Dendrobium [221]. The content of alkaloids is not only related to the species of Dendrobium, but also to the growth age and distribution parts of the plants [222]. Studies have shown that the total alkaloids in Dendrobium regulate hepatic lipids and gluconeogenesis, reduce high blood pressure and exhibit effects on the nervous system, as well as anti-inflammatory, anti-diabetic, anti-tumor and anti-viral properties [223]. Dendrobium alkaloids play a prominent role in neuroprotection. They can repair synaptic and mitochondrial damage mediated by Aβ protein, inhibit amyloidosis, and protect integral synapses by activating the Wnt/β-catenin pathway in vitro and in vivo [30]. Dendrobine can inhibit neurotoxic Mn-induced cytotoxicity by regulating autophagic flux and improving mitochondrial function mediated by PINK1/parkin. Dendrobium alkaloids can also prevent nerve cell damage caused by oxidative stress [31,32], apoptosis or pyroptosis [145,33], and DNA methylation [34]. They can alleviate cognitive dysfunction caused by the formation of extracellular amyloid plaques, regulate tau protein hyperphosphorylation, activate autophagy, enhance synaptic connections, and inhibit neuroinflammation and neuronal apoptosis [143,35,146]. In terms of hepatic lipid metabolism, Dendrobium alkaloids ameliorate abnormal lipid metabolism in the liver through two ways: strengthening the strong hydrophilic taurine-coupled bile acid to promote the elimination of cholesterol to stabilize metabolic balance, and reducing the ratio of cholic acid/chenodeoxycholic acid to regulate intestinal metabolic function [36]. In addition, Dendrobium alkaloids can promote the expression of genes related to liver glucose and lipid metabolism, and can increase the expression of Nrf2-antioxidant pathway genes, comprehensively regulating disordered body metabolism [37]. Studies have shown that Dendrobium alkaloids can attenuate acute liver injury induced by CCl4 [[38], [39], [40]], improve hepatocyte degeneration and steatosis, and normalize alcohol-induced abnormal gene expression patterns [41]. In the respiratory system, Dendrobium alkaloids restrain the emancipation of NO in macrophages induced by LPS and protect against acute lung injury caused by LPS stimulation in mice by downgrading the MyD88/MAPK signaling pathway [42]. In terms of bone protection, Dendrobine inhibited the formation of osteoclasts by inhibiting p38-c-Fos, ROS and NFATc1-MMP9 in vitro, thereby alleviating inflammatory bone ablation in vivo [43].
In general, similar to the pharmacological effects of most alkaloids, the alkaloid compounds in dendrobium have shown good medicinal activity in different disease models and are a class of compounds with development value.
4.3. Flavonoids
Flavonoids are one of the remarkable abundant groups of chemical products of natural plant origin. Across all dendrobium parts, studies have shown that the flavonoid content in leaves is significantly higher than in stems [224]. In dendrobium leaves, flavonoids and polysaccharides are the most important active substances. Naringenin is a flavonoid compound found only in the stems of Dendrobium officinale [225]. In addition, apigenin, anthocyanin, baicalein, and hesperidin are also important components of Dendrobium flavonoids [226]. Studies have shown that naringenin has a broad anti-cancer spectrum, whether it is naringenin alone, embedded and loaded in drug delivery systems, or used in combination with chemotherapy drugs [44]. They can be used to treat tumors at various sites, such as brain cancer, oral squamous cell carcinoma, colon cancer, lung cancer, breast cancer, pancreatic cancer, liver cancer [45], brain cancer, skin cancer, prostate cancer, cervical cancer, ovarian cancer, bladder cancer, gastric cancer and osteosarcoma. Its effect in controlling tumor progression may involve the regulation of multiple mechanisms [44], such as inducing apoptosis, arresting cell cycle, inhibiting angiogenesis, modifying Wnt/β-catenin, NLRP3/NF-ĸB [45], PI3K/Akt, TGF- β and other signaling pathways. In addition, naringenin also exhibits immunomodulatory and cardiovascular protective effects [46,47], and also has certain research and development value in the treatment of aging-related neurodegeneration such as AD and Parkinson's disease [48]. Besides, it has confirmed pharmacological effects in antiviral and anti-inflammatory [49], antidiabetic [50], and bone protection [51]. Apigenin is an important flavonoid widely found in herbs and vegetables. Studies have shown that it has significant effects in anti-cancer [52,53], anti-diabetic [54,55], anti-inflammatory [56], anti-viral [57], anti-depressant [58], anti-neurodegenerative diseases [59], cardioprotective [60], bone-protective [51], immune modulation [61], and treatment of skin diseases [62,63]. Anthocyanin inhibits oxidative stress and reactive astrogliosis, inhibits nerve cell apoptosis and tau protein hyperphosphorylation, resists neuroinflammation, restores cholinergic and amyloidosis pathway dysfunction, and regulates membrane potential abnormalities to achieve the anti-neurodegenerative results [64,65]. In addition, it inhibits inflammation and obesity by regulating the release of inflammatory mediators and the activation of Toll-like receptor signaling pathways, as well as immune activation and the connection between transcription factors in adipose tissue [67,68]. Unsurprisingly, anthocyanin also exhibits excellent pharmacological activity in some serious human diseases, such as cancer. It has shown significant anti-cancer activity against lung cancer, breast cancer, prostate cancer, gastric cancer, liver cancer and colorectal cancer by exhibiting antioxidant, anti-inflammatory, anti-tumor cell proliferation, interfering with tumor cell cycle, inhibiting metastasis, and modulating immune responses [69,70]. In addition, anthocyanin has powerful antioxidant and free radical scavenging activities [71]. Baicalein is an emerging anti-cancer natural compound. Studies have shown that Baicalein resensitizes lung adenocarcinoma cells to cisplatin by inhibiting the EMT properties of tumor cells through the PI3K/Akt/NF-κB pathway [227]. It induces apoptosis and autophagy of tumor cells, inhibits angiogenesis, regulates signal transduction and transcriptional activators, blocks normal cell cycle, and activates PI3K/Akt and other pathways to achieve a wide range of anti-cancer purposes. Baicalein improves ulcerative colitis through the AhR/IL-22 pathway [228]. Baicalein exerts neuroprotective effects by inhibiting oxidative stress and the activity of the ERK pathway [73,74]. In addition, Baicalein also exhibits excellent antibacterial [76,77], anti-acute liver and kidney injury [78,79], joint protection [80], and anti-inflammatory effects [81]. As a bioflavonoid, hesperidin is present in high concentrations in citrus fruits in addition to its presence in dendrobium. Hesperidin has shown effectiveness in anti-pulmonary fibrosis [82], neuroprotection [83], anti-osteoporosis [84], anti-inflammatory and anti-oxidative stress [85,86], and anti-cancer [87].
4.4. Bibenzyl
Bibenzyl compounds are a class of compounds composed of two benzyl units. The benzyl groups are connected to the methyl groups through a single C-C bond. Bibenzyl compounds are widely found in a variety of plants in nature, including ferns, mosses and angiosperms. As a biologically active compound, they are abundant in Dendrobium, mainly in the stems [229]. These compounds have shown excellent antioxidant and anticancer activities, and has become an important compound type in the current study of Dendrobium extract. For example, three new bibenzyl compounds isolated from Dendrobium nobile showed good activity in the free radical scavenging experiment in vitro [230]. In addition, Moscatilin [205], Gigantol [164,201], Erianin [231,89], Chrysotobibenzyl [232,233] and Chrysotoxine [176] in Dendrobium are the star anticancer compounds of Dendrobium bibenzyl compounds in recent years. Among them, Erianin is more prominent in antitumor activity than other bibenzyl compounds. Erianin is effective against lung cancer [88,165], bladder cancer [199,200], breast cancer [234], gastric cancer [181], liver cancer [196], kidney cancer [202], cervical cancer [235], prostate cancer [236], oral squamous cell carcinoma [237], nasopharyngeal carcinoma [163], esophageal squamous cell carcinoma [238], osteosarcoma [239], melanoma and colorectal cancer [240]. The currently revealed anti-tumor mechanisms include promoting ferroptosis of tumor cells, inhibiting tumor cell migration [88,202], inducing oxidative stress [199], inhibiting tumor cell proliferation [165], promoting cell apoptosis [234], causing cell cycle arrest [181,200], inducing DNA damage and abnormal mitosis [241], promoting mitochondrial apoptosis [235], promoting autophagy [242], inhibiting angiogenesis [91], and regulating immunity [96]. With the gradual deepening of research, scientists discovered the huge medicinal value of Erianin. Currently, pharmacological research on Erianin is continuing. Moreover, in view of the poor water solubility of this compound, researchers are still exploring ways to make up for this shortcoming of Erianin, such as using nanomaterials to encapsulate it to build a drug delivery system, or loading it on functional materials to give it additional Functional characteristics [243].
4.5. Phenanthrenes
Phenanthrene compounds are structurally polycyclic aromatic hydrocarbons composed of three benzene rings. Phenanthrene has a wide variety of biological activities, and Orchidaceae is the most important source of natural phenanthrene [244]. The phenanthrene compounds extracted from Dendrobium have effects of antitumor [245,246], anti-inflammatory [108,247], immune regulation [106], antibacterial [107], and antioxidant [105]. Studies have shown that Dendropalpebrone significantly reduces ROS in H2O2-stimulated macrophages, and increases the activeness of superoxide dismutase (SOD), glutathione peroxidase and catalase (CAT) enzymes, thereby playing a role in scavenging free radicals [105]. Two Dendrocrumenol compounds have forceful immune regulation effect on CD3+ T cells and CD14+ monocytes. It is shown to reduce the production of IL-2 and TNF in T cells and monocytes treated with PMA/Iono, and reduce the activation of T cells [106]. In in vitro antibacterial experiments, dendroscabrol B showed strong α-glucosidase inhibitory activity [107]. Phenanthrene compounds extracted from Dendrobium candidum can inhibit the production of NO by LPS-activated mouse macrophage RAW264.7. The underlying mechanism may involve inhibiting the expression of iNOS induced by LPS, as well as the phosphorylation of p38, JNK, MAPK, and IκBα [108]. In terms of antitumor activity, Dendrobium extract Nudol exhibited antiproliferative effects against osteosarcoma cells, with the mechanism likely involving cell cycle arrest and apoptosis through caspase-dependent pathways [110]. In the reproductive system, Denbinobin can prevent CXCL12-induced migration of prostate cancer cells by inhibiting the activity of Rac1 [109]. In terms of digestion and metabolism, Dendrobium phenanthrene compounds exhibited inhibitory effects on pancreatic lipase activity. In addition, Denbinobin inhibits the proliferation activity and invasion ability of gastric cancer cells through MAPK-related pathways [111].
4.6. Polyphenols
By analyzing the content and tendency analysis of current research reports on Dendrobium extracts, that while most studies focus on common bioactive components (such as polysaccharides and alkaloids) in Dendrobium orchid, another important secondary metabolite polyphenols have been overlooked [[248], [249], [250]]. The biological activities of polyphenols can be awakened with the help of microbial fermentation, such as the anti-inflammatory and antioxidant stress effects of polyphenols [251]. It is clear that the gut microbiota plays a key role in regulating the production of polyphenols in the body, increasing their bioavailability and bioactivity [252]. Conversely, polyphenols can also modulate the composition or activity of colonic microbiome [120,121]. Previous studies have also reported several polyphenolic compounds in Dendrobium, such as rutin, hyperoside, and quercetin. But they were later classified as flavonoids. In addition, Dendrobium also contains phenolic acids and their derivatives, including syringic acid, ellagic acid, and gallotannins. These polyphenolic compounds have been shown to possess various biological activities, such as hypoglycemic, antioxidant, antimicrobial, anticancer, and immunity-boosting potential [253,127]. Due to the lack of research on the mechanism of Dendrobium polyphenols, their deep regulation mechanism on the body remains to be elucidated.
4.7. Sesquiterpenes
Dendrobium contains many sesquiterpenes, including picridane, isovanillin, cyclodiocamphenes, codiocamphores, and juniperane sesquiterpenes [254]. These compounds are reported to have the effects of neuroprotection, immune regulation, anti-diabetes, anti-tumor and improvement of acute cerebral ischemia. Terpenoids extracted from Dendrobium showed the inhibitory activity of α-glucosidase, suggesting that they may be potential drugs for diabetes treatment [128,129]. (+)-(1R,2S,3R,4S,5R,6S,9R)-3,11,12-trihydroxypicrotoxane-2(15)-lactone, (+)-(1R,5R,6S,8R,9R)-8,12-dihydroxy-copacamphan-3-en -2-one, and (−)-(1S,2R,3S,4R,5S, 6R,9S,12R)-3,11,13-trihydroxypicrotoxane-2(15)-lactone have obvious protective effect on neural damage caused by H2O2-induced oxidative damage in pheochromocytoma cells [130]. In terms of anti-tumor, terpenoids extracted from Dendrobium have shown inhibitory effects on multiple tumor cell lines such as colorectal cancer, lung cancer, pancreatic cancer and gastric cancer [129]. Sesquiterpenoids extracted from Dendrobium stems are inhibitory to D-galactose-induced proliferation of HLECs cells, providing new candidate compounds for cataract therapy [131]. In addition, in the zebrafish model, the terpenoid extract of Dendrobium nobile also showed an influence on the generation of injured blood vessels [255]. Recently, it also showed preliminary antioxidative [256], and immunomodulatory activities [[257], [258], [259]].
4.8. Amino acids
After extraction and analysis, it is found that there are more than 20 kinds of basic amino acids in Dendrobium [260,261]. The amino acid content in leaves of Dendrobium was substantially higher than that in stems. There are also substantial differences in the amount of amino acids contained in different parts of a plant. In Dendrobium officinale, the total amino acid content in leaves was 6.76–7.97 g/100 g, and that in stems was 1.61–2.44 g/100 g [262]. The free amino acids and trace elements in Dendrobium officinale are one of the necessary nutritional elements for the human body, including a variety of essential and non-essential amino acids [263]. This is an important material basis for its pharmacological effects. For example, 12 trace elements and 20 amino acids were detected in Shihu Yeguang Pills [264]. Due to the complex regulatory mechanism of amino acids, the pathways of amino acids in Dendrobium have not yet been elucidated.
4.9. Others
In addition to the above main types of compounds, Dendrobium also contains tannin compounds. Tannins are a unique class of phenolic metabolites, with a molecular weight between 500 and 30,000 Da. The content of tannins in Dendrobium is related to the species of Dendrobium [116]. There is literature indicating that among different strains of Dendrobium, Dendrobium nobile Lindl. has the highest tannin content, about 14.28%, followed by Dendrobium chrysotoxum Lindl., about 3.61% [265]. The content of tannins in Dendrobium candidum is only less than 0.4% [266]. Modern pharmacological studies have shown that tannins have various biological activities such as regulating gastrointestinal motility [267], antitumor [268], antioxidation [116], antibacterial [269], and antiviral [270]. However, the specific pharmacological mechanism of tannins in Dendrobium has not been reported so far, and further exploration is needed.
In addition, Dendrobium extract also contains coumarins, Ayapin, scopoletin, marsartene, and 6,7-dimethoxycoumarin [271]. Studies have shown that Ayapin has antibacterial, anti-infectious and anti-inflammatory activity [134,272]. Scopolamine has protective effects on various chronic diseases, such as anti-cancer, anti-diabetic, anti-inflammatory, neuroprotective, cardioprotective and hepatoprotective effects [136,137]. Scopoletin modulates multiple tumor molecular signals, including EGFR, AMPK, NF-κB, MAPK/ERK, STAT3and PI3K/Akt/mTOR. In addition, it is also involved in the regulation of key markers of metabolic diseases aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC) and triglyceride (TG) levels. In neurological diseases, it participates in the regulation of inflammatory factors, such as ILs and TNF, thereby reducing the symptoms and severity of these diseases [135]. The reported compound types, representative compound structures, and their pharmacological effects in dendrobium are shown in Table 1.
Since researchers continue to optimize the extraction and separation process of the compounds in Dendrobium, we are gradually unraveling the mystery of this ancient Chinese herbal medicine. Both ancient medical records and modern pharmacological research have revealed its extensive pharmacological activities. Whether it is in vivo or in vitro studies, or the study of metabolic characteristics, it is an important step to achieve the transformation of dendrobium extracts. This is the key bottleneck that we urgently need to solve.
5. Dendrobium preparations in China
In order to understand the marketing and application of dendrobium products in China, we have reviewed the approved dendrobium-related domestic drugs, imported drugs and traditional Chinese medicine formulas information on the website of the National Medical Products Administration (NMPA) (https://www.nmpa.gov.cn/). It was found that there were a total of 178 approval information, among which Dendrobium preparations were divided into 6 types, including Shihu Yeguang Wan, Shihu Mingmu Wan, Shihu Yeguang Keli, Fufang Xianshihu Keli, Fufang Xianshihu Jiaonang, and Fufang Shihu Pian. Their compatibility composition and functional indication information are shown in Table 2.
Table 2.
The composition, indications and pharmacological research progress of the dendrobium proprietary Chinese medicine compound approved by NMPA.
| Drug Name | Compatibility (Latin name) | Indications supported by drug inserts | Indications supported by literature |
|---|---|---|---|
| Shihu Yeguang Wan | Asparagus cochinchinensis, Ophiopogon japonicus, Rehmannia glutinosa, Rehmanniae Radix Praeparata, Panax Ginseng C. A. Mey., Poria Cocos(Schw.) Wolf., Rhizoma Dioscoreae, Lycii Fructus, Achyranthis Bidentatae Radix, Dendrobium nobile, Cassia mimosoides, Dendranthema lavandulifolium, Amygdalus Communis Vas, Cuscutae Semen, Antelope Horn, Cistanches Herba, Schisandra chinensis, Saposhnikoviae Radix, Glycyrrhiza uralensis, Astragalus complanatus, Coptis chinensis, Citrus aurantium L., Chuanxiong Rhizoma, Gleditsia sinensis Lam., Celosia argentea. | It is suitable for liver and kidney deficiency, yin deficiency and fire exuberance, cataracts, dark eyes, and dim vision. | Relieve dry eye syndrome [[273], [274], [275], [276]], vitreous opacity [277,278], central serous chorioretinopathy [279,280], glaucoma [281], visual fatigue [282], cataract [283], and low intraocular pressure [284]. |
| Shihu Mingmu Wan | Dendrobium nobile, Celosia argentea, Cassia mimosoides, Tribulus terrester, Rehmannia glutinosa, Rehmanniae Radix Praeparata, Lycium chinense, Cuscuta chinensis, Cistanche deserticola, Panax ginseng, Rhizoma Dioscoreae, Poria Cocos(Schw.) Wolf., Asparagi Radix, Ophiopogon japonicus, Schisandra chinensis, licorice, Aurantii Fructus, Chrysanthemi Flos, Saposhnikoviae Radix, Coptidis Rhizoma, Achyranthis Bidentatae Radix, Chuanxiong Rhizoma, Amygdalus Communis Vas, Gypsum Fibrosum, Magnetitum, Bubali Cornu. | It is suitable for patients with liver and kidney deficiency, dilated pupils, night blindness, blurred vision, cataract pain, dizziness, and mental fatigue caused by rising deficiency fire. | Effective against diabetic retinopathy [285,286], macular degeneration [287], and dry eye syndrome [288]. |
| Shihu Yeguang Keli | Dendrobium nobile, Rehmanniae Radix Praeparata, Lycii Fructus, Cuscutae Semen, Achyranthis Bidentatae Radix, Chrysanthemi Flos, Tribulifructus, Celosiae Semen, Cassiae Semen, Bubali Cornu, Antelope Horn, licorice, Panax Ginseng C. A. Mey., Rhizoma Dioscoreae, Poria Cocos(Schw.) Wolf., Cistanches Herba, Rehmannia glutinosa, Schisandra chinensis, Asparagi Radix, Ophiopogon japonicus, Amygdalus Communis Vas, Saposhnikoviae Radix, Chuanxiong Rhizoma, Aurantii Fructus, Coptidis Rhizoma. | It is suitable for liver and kidney deficiency, yin deficiency and fire exuberance, cataracts, dark eyes, and dim vision. | Effective against cataract [289,290]. |
| Fufang Xianshihu Keli | Dendrobium nobile, Radix Puerariae, Panax Notoginseng (Burk.) F. H. Chen Ex C. Chow. | It is suitable for dry mouth and throat, hunger, inability to eat, and polydipsia caused by insufficient stomach yin. | Treat alcoholic liver disease [291], and enhance immunity [292]. |
| Fufang Xianshihu Jiaonang | Dendrobium nobile, Radix Puerariae, Panax Notoginseng (Burk.) F. H. Chen Ex C. Chow. | It is suitable for symptoms such as insufficient stomach yin, dry mouth and throat, hunger and inability to eat, red tongue and lack of fluid, dryness and deficiency of fluid after drinking, and polydipsia after drinking. | NA |
| Fufang Shihu Pian | Panax Ginseng C. A. Mey., Antelope Horn, Schisandra chinensis, Lycii Fructus, Chuanxiong Rhizoma, Rhizoma Dioscoreae, Rehmannia glutinosa, Angelicae Sinensis Radix, Bubali Cornu, Scutellariae Radix, Gardeniae Fructus, Saposhnikoviae Radix, Dendrobium nobile, Aurantii Fructus, Ophiopogon japonicus, Eucommiae Cortex, Cassiae Semen, licorice, Asparagi Radix, Achyranthis Bidentatae Radix, Cuscutae Semen, Rehmanniae Radix Praeparata, Poria Cocos(Schw.) Wolf., Amygdalus Communis Vas, Tribulifructus, Chrysanthemi Flos, Celosiae Semen, Anemarrhenae Rhizoma. | It is suitable for symptoms such as dizziness and cataracts, vision loss, mydriasis, round nebula and cataracts, blurred vision caused by clouds and fog, and tears in the wind. | NA |
6. Summary and outlook
Dendrobium has been extensively employed as a functional food and herbal medicine for the prevention and treatment of many diseases. Dendrobium has a long history of folk application, but in-depth and multi-center clinical research is still needed to confirm its pharmacological effects and mechanism of action in the human body. This review summarizes the conditioning and therapeutic effects of Dendrobium on different organs of the human body, and classifies and describes the pharmacological effects of different types of compounds in Dendrobium. We found that there are many studies on the polysaccharides and alkaloids in Dendrobium extracts. These studies have verified the pharmacological effects of Dendrobium from different mechanisms, but there are still great limitations in promoting the transformation and medicinal use of Dendrobium. In addition, more investigation evidence is still needed for the development of the medicinal potential of Dendrobium. By combining modern technology, better control of the quality and safety of Dendrobium, and research and development of the series of products of Dendrobium, the development of traditional Chinese medicine Dendrobium can be promoted.
Ethics approval and consent to participate
Not applicable.
Data availability statement
No data was used for the research described in the article.
Funding
This research was financially supported by the National Natural Science Foundation of China (NO.52072360).
CRediT authorship contribution statement
Xin Wei: Writing – original draft, Methodology, Investigation. Dan Wang: Writing – original draft, Investigation. Ziming Xu: Investigation. Jiajia Liu: Formal analysis. Qizhi Zhu: Formal analysis. Qi Chen: Investigation. Heng Tang: Writing – review & editing, Methodology. Weiping Xu: Writing – original draft, Supervision, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Heng Tang, Email: tangheng@mail.ustc.edu.cn.
Weiping Xu, Email: weipingx@ustc.edu.cn.
References
- 1.Bu W.C., Yu Y.N., Cheng J.M. Summary of studies on pharmacological activity of dendrobium polysaccharides. Chinese folk medicine. 2019;28(2):61–64. [Google Scholar]
- 2.Zheng S.G., Hu Y.D., Zhao R.X., Yan S., Zhang X.Q., Zhao T.M., Chun Z. Genome-wide researches and applications on Dendrobium. Planta. 2018;248(4):769–784. doi: 10.1007/s00425-018-2960-4. [DOI] [PubMed] [Google Scholar]
- 3.Lv L.Y., Zhang Z.F., Wang Q.Y., Li Y. Research progress on the safety evaluation of whole herbal medicinal and edible traditional Chinese medicine. Chinese herbal medicine. 2021;52(15):4722–4730. [Google Scholar]
- 4.Yu H L.Y., Zheng F., Chen W., Wei K. Erianin-loaded photo-responsive dendrimer mesoporous silica nanoparticles: exploration of a psoriasis treatment method. Molecules. 2022;27(19):6328. doi: 10.3390/molecules27196328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X.Q., Zhao T.M., Liu J., Zhao R.X., Zheng S.G., Chun Z., Hu Y.D. Research progress on chemical constituents and pharmacological effects of Dendrobium, Chinese herbal medicine. 2018;49(13):3174–3182. [Google Scholar]
- 6.Fan C., Sun X., Wang X., Yu H. Therapeutic potential of the chemical composition of Dendrobium nobile Lindl. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1163830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H., Zheng J., Wu Y., Zhou H., Zeng S., Li Q. Dendrobium officinale polysaccharide decreases podocyte injury in diabetic nephropathy by regulating IRS-1/AKT signal and promoting mitophagy. Aging. 2023;15(19):10291–10306. doi: 10.18632/aging.205075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Chen C., Fu X. Dendrobium officinale polysaccharide alleviates type 2 diabetes mellitus by restoring gut microbiota and repairing intestinal barrier via the LPS/TLR4/TRIF/NF-kB Axis. J. Agric. Food Chem. 2023;71(31):11929–11940. doi: 10.1021/acs.jafc.3c02429. [DOI] [PubMed] [Google Scholar]
- 9.Wang K., Yang X., Wu Z., Wang H., Li Q., Mei H., You R., Zhang Y. Dendrobium officinale polysaccharide protected CCl(4)-induced liver fibrosis through intestinal homeostasis and the LPS-TLR4-NF-kappaB signaling pathway. Front. Pharmacol. 2020;11:240. doi: 10.3389/fphar.2020.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo L., Qi J., Du D., Liu Y., Jiang X. Current advances of Dendrobium officinale polysaccharides in dermatology: a literature review. Pharm. Biol. 2020;58(1):664–673. doi: 10.1080/13880209.2020.1787470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha S.Y., Park S.Y., Lee J.S., Lee K.H., Kim J.H., Fang Y., Shin S.S. Efficacy of Dendrobium candidum polysaccharide extract as a moisturizer. J. Cosmet. Dermatol. 2022;21(7):3117–3126. doi: 10.1111/jocd.14586. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K., Zhou X., Wang J., Zhou Y., Qi W., Chen H., Nie S., Xie M. Dendrobium officinale polysaccharide triggers mitochondrial disorder to induce colon cancer cell death via ROS-AMPK-autophagy pathway. Carbohydr. Polym. 2021;264 doi: 10.1016/j.carbpol.2021.118018. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y., Lu X., Huang H., Yao Y., Liu H., Sun Y. Dendrobium officinale polysaccharide converts M2 into M1 subtype macrophage polarization via the STAT6/PPAR-r and JAGGED1/NOTCH1 signaling pathways to inhibit gastric cancer. Molecules. 2023;28(20):7062. doi: 10.3390/molecules28207062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H.Y., Ge J.C., Zhang F.Y., Zha X.Q., Liu J., Li Q.M., Luo J.P. Dendrobium officinale polysaccharide promotes M1 polarization of TAMs to inhibit tumor growth by targeting TLR2. Carbohydr. Polym. 2022;292 doi: 10.1016/j.carbpol.2022.119683. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y., Zeng X., Liu Y., Zhan S., Wu Z., Zheng X., Zhang X. Dendrobium officinale polysaccharide attenuates cognitive impairment in circadian rhythm disruption mice model by modulating gut microbiota. Int. J. Biol. Macromol. 2022;217:677–688. doi: 10.1016/j.ijbiomac.2022.07.090. [DOI] [PubMed] [Google Scholar]
- 16.Liang Y., Liu G., Xie L., Su K., Chang X., Xu Y., Chen J., Zhu Z., Yang K., Chen H., Du Z. Dendrobium candidum polysaccharide reduce atopic dermatitis symptoms and modulate gut microbiota in DNFB-induced AD-like mice. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.976421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei X., Sun W., Zhu P., Ou G., Zhang S., Li Y., Hu J., Qu X., Zhong Y., Yu W., You Z., Wang Y., Wu Y. Refined polysaccharide from Dendrobium devonianum resists H1N1 influenza viral infection in mice by activating immunity through the TLR4/MyD88/NF-kappaB pathway. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.999945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z., Xiang J., Hu D., Song B. Naturally potential antiviral agent polysaccharide from Dendrobium nobile Lindl. Pestic. Biochem. Physiol. 2020;167 doi: 10.1016/j.pestbp.2020.104598. [DOI] [PubMed] [Google Scholar]
- 19.Qu J., Tan S., Xie X., Wu W., Zhu H., Li H., Liao X., Wang J., Zhou Z.A., Huang S., Lu Q. Dendrobium officinale polysaccharide attenuates insulin resistance and abnormal lipid metabolism in obese mice. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.659626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang Z.Z., Qin D.Y., Li Q.M., Zha X.Q., Pan L.H., Peng D.Y., Luo J.P. Dendrobium huoshanense stem polysaccharide ameliorates rheumatoid arthritis in mice via inhibition of inflammatory signaling pathways. Carbohydr. Polym. 2021;258 doi: 10.1016/j.carbpol.2021.117657. [DOI] [PubMed] [Google Scholar]
- 21.Li X., Zhang Q., Zhu Y., Li Y., Mei S., Luo H., Wu K. Structural characterization of a mannoglucan polysaccharide from Dendrobium huoshanense and evaluation of its osteogenesis promotion activities. Int. J. Biol. Macromol. 2022;211:441–449. doi: 10.1016/j.ijbiomac.2022.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Li B., Wang H.Y., Huang J.H., Xu W.F., Feng X.J., Xiong Z.P., Dong Y.J., Li L.Z., He X., Wu H.S., Zhang K., Su J., Yu Q.X., Jiang N.H., Lv G.Y., Chen S.H. Polysaccharide, the active component of Dendrobium officinale, ameliorates metabolic hypertension in rats via regulating intestinal flora-SCFAs-vascular Axis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.935714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie S.Z., Shang Z.Z., Li Q.M., Zha X.Q., Pan L.H., Luo J.P. Dendrobium huoshanense polysaccharide regulates intestinal lamina propria immune response by stimulation of intestinal epithelial cells via toll-like receptor 4. Carbohydr. Polym. 2019;222 doi: 10.1016/j.carbpol.2019.115028. [DOI] [PubMed] [Google Scholar]
- 24.Xie S.Z., Hao R., Zha X.Q., Pan L.H., Liu J., Luo J.P. Polysaccharide of Dendrobium huoshanense activates macrophages via toll-like receptor 4-mediated signaling pathways. Carbohydr. Polym. 2016;146:292–300. doi: 10.1016/j.carbpol.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 25.Ye M., Liu J., Deng G., Cai X., Zhang X., Yao L., Wu J., He X., Peng D., Yu N. Protective effects of Dendrobium huoshanense polysaccharide on D-gal induced PC12 cells and aging mice, in vitro and in vivo studies. J. Food Biochem. 2022;46(12) doi: 10.1111/jfbc.14496. [DOI] [PubMed] [Google Scholar]
- 26.Chu W., Wang P., Ma Z., Peng L., Wang Z., Chen Z. Ultrasonic treatment of Dendrobium officinale polysaccharide enhances antioxidant and anti-inflammatory activity in a mouse D-galactose-induced aging model. Food Sci. Nutr. 2022;10(8):2620–2630. doi: 10.1002/fsn3.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling J., Chan C.L., Ho C.Y., Gao X., Tsang S.M., Leung P.C., Hu J.M., Wong C.K. The extracts of dendrobium alleviate dry eye disease in rat model by regulating aquaporin expression and MAPKs/NF-kappaB signalling. Int. J. Mol. Sci. 2022;23(19) doi: 10.3390/ijms231911195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian C.C., Zha X.Q., Pan L.H., Luo J.P. Structural characterization and antioxidant activity of a low-molecular polysaccharide from Dendrobium huoshanense. Fitoterapia. 2013;91:247–255. doi: 10.1016/j.fitote.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 29.He Y., Li L., Chang H., Cai B., Gao H., Chen G., Hou W., Jappar Z., Yan Y. Research progress on extraction, purification, structure and biological activity of Dendrobium officinale polysaccharides. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.965073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W., Zhang M., Wu Q., Shi J. Dendrobium nobile Lindl. alkaloids ameliorate abeta25-35-induced synaptic deficits by targeting Wnt/beta-catenin pathway in Alzheimer's disease models. J Alzheimers Dis. 2022;86(1):297–313. doi: 10.3233/JAD-215433. [DOI] [PubMed] [Google Scholar]
- 31.Liu J., Zhu T., Niu Q., Yang X., Suo H., Zhang H. Dendrobium nobile alkaloids protects against H2O2-induced neuronal injury by suppressing JAK-STATs pathway activation in N2A cells. Biol. Pharm. Bull. 2020;43(4):716–724. doi: 10.1248/bpb.b19-01083. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Pi T., Yang X., Shi J. Protective effects and mechanisms of Dendrobium nobile Lindl. Alkaloids on PC12 cell damage induced by Abeta (25-35) Behav. Neurol. 2021;2021 doi: 10.1155/2021/9990375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu D., Dong Z., Xiang F., Liu H., Wang Y., Wang Q., Rao J. Dendrobium alkaloids promote neural function after cerebral ischemia-reperfusion injury through inhibiting pyroptosis induced neuronal death in both in vivo and in vitro models. Neurochem. Res. 2020;45(2):437–454. doi: 10.1007/s11064-019-02935-w. [DOI] [PubMed] [Google Scholar]
- 34.Pi T., Lang G., Liu B., Shi J. Protective effects of Dendrobium nobile Lindl. Alkaloids on Alzheimer's disease-like symptoms induced by high-methionine diet. Curr. Neuropharmacol. 2022;20(5):983–997. doi: 10.2174/1570159X19666210809101945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D.D., Zheng C.Q., Zhang F., Shi J.S. Potential neuroprotection by Dendrobium nobile Lindl alkaloid in Alzheimer's disease models. Neural Regen Res. 2022;17(5):972–977. doi: 10.4103/1673-5374.324824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang S., Wu Q., Liu H., Ling H., He Y., Wang C., Wang Z., Lu Y., Lu Y. Alkaloids of Dendrobium nobile Lindl. Altered hepatic lipid homeostasis via regulation of bile acids. J. Ethnopharmacol. 2019;241 doi: 10.1016/j.jep.2019.111976. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y.Y., Xu Y.S., Wang Y., Wu Q., Lu Y.F., Liu J., Shi J.S. Dendrobium nobile Lindl. alkaloids regulate metabolism gene expression in livers of mice. J. Pharm. Pharmacol. 2017;69(10):1409–1417. doi: 10.1111/jphp.12778. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Zhou J., Liu J., Li S., Zhou S., Zhang C., Wang Y., Shi J., Liu J., Wu Q. RNA-Seq analysis of the protection by Dendrobium nobile alkaloids against carbon tetrachloride hepatotoxicity in mice. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111307. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J., Zhang Y., Li S., Zhou Q., Lu Y., Shi J., Liu J., Wu Q., Zhou S. Dendrobium nobile Lindl. alkaloids-mediated protection against CCl(4-)induced liver mitochondrial oxidative damage is dependent on the activation of Nrf2 signaling pathway. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110351. [DOI] [PubMed] [Google Scholar]
- 40.Li S., Zhou J., Xu S., Li J., Liu J., Lu Y., Shi J., Zhou S., Wu Q. Induction of Nrf2 pathway by Dendrobium nobile Lindl. alkaloids protects against carbon tetrachloride induced acute liver injury. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109073. [DOI] [PubMed] [Google Scholar]
- 41.Huang X., Yang S., Sun J., Li X., Zhou S.Y., Shi J.S., Liu J., Wu Q. Transcriptome analysis of protection by Dendrobium nobile alkaloids (DNLA) against chronic alcoholic liver injury in mice. Biomedicines. 2022;10(11):2800. doi: 10.3390/biomedicines10112800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu Y., Ren J., Wang L., Zhao X., Zhang M., Shimizu K., Zhang C. Protective effects of total alkaloids from Dendrobium crepidatum against LPS-induced acute lung injury in mice and its chemical components. Phytochemistry. 2018;149:12–23. doi: 10.1016/j.phytochem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Deng W., Ding Z., Wang Y., Zou B., Zheng J., Tan Y., Yang Q., Ke M., Chen Y., Wang S., Li X. Dendrobine attenuates osteoclast differentiation through modulating ROS/NFATc1/MMP9 pathway and prevents inflammatory bone destruction. Phytomedicine. 2022;96 doi: 10.1016/j.phymed.2021.153838. [DOI] [PubMed] [Google Scholar]
- 44.Motallebi M., Bhia M., Rajani H.F., Bhia I., Tabarraei H., Mohammadkhani N., Pereira-Silva M., Kasaii M.S., Nouri-Majd S., Mueller A.L., Veiga F.J.B., Paiva-Santos A.C., Shakibaei M. Naringenin: a potential flavonoid phytochemical for cancer therapy. Life Sci. 2022;305 doi: 10.1016/j.lfs.2022.120752. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q., Ou Y., Hu G., Wen C., Yue S., Chen C., Xu L., Xie J., Dai H., Xiao H., Zhang Y., Qi R. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-kappaB pathway in mice. Br. J. Pharmacol. 2020;177(8):1806–1821. doi: 10.1111/bph.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng W., Jin L., Zhang F., Zhang C., Liang W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018;135:122–126. doi: 10.1016/j.phrs.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Heidary Moghaddam R., Samimi Z., Moradi S.Z., Little P.J., Xu S., Farzaei M.H. Naringenin and naringin in cardiovascular disease prevention: a preclinical review. Eur. J. Pharmacol. 2020;887 doi: 10.1016/j.ejphar.2020.173535. [DOI] [PubMed] [Google Scholar]
- 48.Goyal A., Verma A., Dubey N., Raghav J., Agrawal A. Naringenin: a prospective therapeutic agent for Alzheimer's and Parkinson's disease. J. Food Biochem. 2022;46(12) doi: 10.1111/jfbc.14415. [DOI] [PubMed] [Google Scholar]
- 49.Tutunchi H., Naeini F., Ostadrahimi A., Hosseinzadeh-Attar M.J. Naringenin, a flavanone with antiviral and anti‐inflammatory effects: a promising treatment strategy against COVID‐19. Phytother Res. 2020;34(12):3137–3147. doi: 10.1002/ptr.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Den Hartogh D.J., Tsiani E. Antidiabetic properties of naringenin: a citrus fruit polyphenol. Biomolecules. 2019;9(3):99. doi: 10.3390/biom9030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borchers A., Pieler T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes. 2010;1(3):413–426. doi: 10.3390/genes1030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imran M., Aslam Gondal T., Atif M., Shahbaz M., Batool Qaisarani T., Hanif Mughal M., Salehi B., Martorell M., Sharifi-Rad J. Apigenin as an anticancer agent. Phytother Res. 2020;34(8):1812–1828. doi: 10.1002/ptr.6647. [DOI] [PubMed] [Google Scholar]
- 53.Xu L., Zaky M.Y., Yousuf W., Ullah A., Abdelbaset G.R., Zhang Y., Ahmed O.M., Liu S., Liu H. The anticancer potential of apigenin via immunoregulation. Curr. Pharmaceut. Des. 2021;27(4):479–489. doi: 10.2174/1381612826666200713171137. [DOI] [PubMed] [Google Scholar]
- 54.Mao X.Y., Yu J., Liu Z.Q., Zhou H.H. Apigenin attenuates diabetes-associated cognitive decline in rats via suppressing oxidative stress and nitric oxide synthase pathway. Int. J. Clin. Exp. Med. 2015;8(9):15506–15513. [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H.J., Fan Y.L., Liao H.H., Liu Y., Chen S., Ma Z.G., Zhang N., Yang Z., Deng W., Tang Q.Z. Apigenin alleviates STZ-induced diabetic cardiomyopathy. Mol. Cell. Biochem. 2017;428(1–2):9–21. doi: 10.1007/s11010-016-2913-9. [DOI] [PubMed] [Google Scholar]
- 56.Zhou X.W., Wang J., Tan W.F. Apigenin suppresses innate immune responses and ameliorates lipopolysaccharide-induced inflammation via inhibition of STING/IRF3 pathway. Am. J. Chin. Med. 2024;52(2):471–492. doi: 10.1142/S0192415X24500204. [DOI] [PubMed] [Google Scholar]
- 57.Lee I.G., Lee J., Hong S.H., Seo Y.J. Apigenin's therapeutic potential against viral infection. Front. Biosci. 2023;28(10):237. doi: 10.31083/j.fbl2810237. [DOI] [PubMed] [Google Scholar]
- 58.Bijani S., Dizaji R., Sharafi A., Hosseini M.J. Neuroprotective effect of apigenin on depressive-like behavior: mechanistic approach. Neurochem. Res. 2022;47(3):644–655. doi: 10.1007/s11064-021-03473-0. [DOI] [PubMed] [Google Scholar]
- 59.Gaur K., Siddique Y.H. Effect of apigenin on neurodegenerative diseases. CNS Neurol. Disord.: Drug Targets. 2024;23(4):468–475. doi: 10.2174/1871527322666230406082625. [DOI] [PubMed] [Google Scholar]
- 60.Thomas S.D., Jha N.K., Jha S.K., Sadek B., Ojha S. Pharmacological and molecular insight on the cardioprotective role of apigenin. Nutrients. 2023;15(2):385. doi: 10.3390/nu15020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shu N., Zhang Z., Wang X., Li R., Li W., Liu X., Zhang Q., Jiang Z., Tao L., Zhang L., Hou S. Apigenin alleviates autoimmune uveitis by inhibiting microglia M1 pro-inflammatory polarization. Invest. Ophthalmol. Vis. Sci. 2023;64(5):21. doi: 10.1167/iovs.64.5.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon J.H., Kim M.Y., Cho J.Y. Apigenin: a therapeutic agent for treatment of skin inflammatory diseases and cancer. Int. J. Mol. Sci. 2023;24(2):1498. doi: 10.3390/ijms24021498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W., Liu X., Zhang Z., Yin M., Chen X., Zhao S., Wu L. Apigenin induced apoptosis by downregulating sulfiredoxin expression in cutaneous squamous cell carcinoma. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/8172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suresh S., Begum R.F., Singh S.A., V C. Anthocyanin as a therapeutic in Alzheimer's disease: a systematic review of preclinical evidences. Ageing Res. Rev. 2022;76 doi: 10.1016/j.arr.2022.101595. [DOI] [PubMed] [Google Scholar]
- 65.Zaa C.A., Marcelo A.J., An Z., Medina-Franco J.L., Velasco-Velazquez M.A. Anthocyanins: molecular aspects on their neuroprotective activity. Biomolecules. 2023;13(11):1598. doi: 10.3390/biom13111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mattioli R., Francioso A., Mosca L., Silva P. Anthocyanins: a comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules. 2020;25(17):3809. doi: 10.3390/molecules25173809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee Y.M., Yoon Y., Yoon H., Park H.M., Song S., Yeum K.J. Dietary anthocyanins against obesity and inflammation. Nutrients. 2017;9(10):1089. doi: 10.3390/nu9101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ngamsamer C., Sirivarasai J., Sutjarit N. The benefits of anthocyanins against obesity-induced inflammation. Biomolecules. 2022;12(6):852. doi: 10.3390/biom12060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J., Xu B., Sun J., Jiang X., Bai W. Anthocyanin supplement as a dietary strategy in cancer prevention and management: a comprehensive review. Crit. Rev. Food Sci. Nutr. 2022;62(26):7242–7254. doi: 10.1080/10408398.2021.1913092. [DOI] [PubMed] [Google Scholar]
- 70.de Arruda Nascimento E., de Lima Coutinho L., da Silva C.J., de Lima V., Dos Santos Aguiar J. In vitro anticancer properties of anthocyanins: a systematic review. Biochim. Biophys. Acta Rev. Canc. 2022;1877(4) doi: 10.1016/j.bbcan.2022.188748. [DOI] [PubMed] [Google Scholar]
- 71.Gabrielska J., Oszmiański J., Komorowska M., Langner M. Anthocyanin extracts with antioxidant and radical scavenging effect. Zeitschrift fur Naturforschung. C, Journal of biosciences. 1999;54(5–6):319–324. doi: 10.1515/znc-1999-5-605. [DOI] [PubMed] [Google Scholar]
- 72.Liu H., Dong Y., Gao Y., Du Z., Wang Y., Cheng P., Chen A., Huang H. The fascinating effects of baicalein on cancer: a review. Int. J. Mol. Sci. 2016;17(10):1681. doi: 10.3390/ijms17101681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song Q., Peng S., Zhu X. Baicalein protects against MPP(+)/MPTP-induced neurotoxicity by ameliorating oxidative stress in SH-SY5Y cells and mouse model of Parkinson's disease. Neurotoxicology. 2021;87:188–194. doi: 10.1016/j.neuro.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Sowndhararajan K., Deepa P., Kim M., Park S.J., Kim S. Baicalein as a potent neuroprotective agent: a review. Biomed. Pharmacother. 2017;95:1021–1032. doi: 10.1016/j.biopha.2017.08.135. [DOI] [PubMed] [Google Scholar]
- 75.Liu B.Y., Li L., Liu G.L., Ding W., Chang W.G., Xu T., Ji X.Y., Zheng X.X., Zhang J., Wang J.X. Baicalein attenuates cardiac hypertrophy in mice via suppressing oxidative stress and activating autophagy in cardiomyocytes. Acta Pharmacol. Sin. 2021;42(5):701–714. doi: 10.1038/s41401-020-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ning B., Shen J., Liu F., Zhang H., Jiang X. Baicalein suppresses NLRP3 and AIM2 inflammasome-mediated pyroptosis in macrophages infected by Mycobacterium tuberculosis via induced autophagy. Microbiol. Spectr. 2023;11(3) doi: 10.1128/spectrum.04711-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu H., Li X., Wang G., Wang C., Feng J., Lu W., Wang X., Chen H., Liu M., Tan C. Baicalein ameliorates Streptococcus suis-induced infection in vitro and in vivo. Int. J. Mol. Sci. 2021;22(11):5829. doi: 10.3390/ijms22115829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao T., Cui Y., Ji H., Yan L., Pei D., Qu S. Baicalein attenuates acute liver injury by blocking NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2021;534:212–218. doi: 10.1016/j.bbrc.2020.11.109. [DOI] [PubMed] [Google Scholar]
- 79.Yu M., Li H., Wang B., Wu Z., Wu S., Jiang G., Wang H., Huang Y. Baicalein ameliorates polymyxin B-induced acute renal injury by inhibiting ferroptosis via regulation of SIRT1/p53 acetylation. Chem. Biol. Interact. 2023;382 doi: 10.1016/j.cbi.2023.110607. [DOI] [PubMed] [Google Scholar]
- 80.Li B., Chen K., Qian N., Huang P., Hu F., Ding T., Xu X., Zhou Q., Chen B., Deng L., Ye T., Guo L. Baicalein alleviates osteoarthritis by protecting subchondral bone, inhibiting angiogenesis and synovial proliferation. J. Cell Mol. Med. 2021;25(11):5283–5294. doi: 10.1111/jcmm.16538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Z.Z., Yu X.H., Tan W.H. Baicalein inhibits macrophage lipid accumulation and inflammatory response by activating the PPARgamma/LXRalpha pathway. Clin. Exp. Immunol. 2022;209(3):316–325. doi: 10.1093/cei/uxac062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han D., Gong H., Wei Y., Xu Y., Zhou X., Wang Z., Feng F. Hesperidin inhibits lung fibroblast senescence via IL-6/STAT3 signaling pathway to suppress pulmonary fibrosis. Phytomedicine. 2023;112 doi: 10.1016/j.phymed.2023.154680. [DOI] [PubMed] [Google Scholar]
- 83.Hajialyani M., Hosein Farzaei M., Echeverria J., Nabavi S.M., Uriarte E., Sobarzo-Sanchez E. Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules. 2019;24(3):648. doi: 10.3390/molecules24030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu H.Y., Zhang Z.Z., Jiang X.Y., Duan T.H., Feng W., Wang X.G. Hesperidin anti-osteoporosis by regulating estrogen signaling pathways. Molecules. 2023;28(19) doi: 10.3390/molecules28196987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imperatrice M., Cuijpers I., Troost F.J., Sthijns M. Hesperidin functions as an ergogenic aid by increasing endothelial function and decreasing exercise-induced oxidative stress and inflammation, thereby contributing to improved exercise performance. Nutrients. 2022;14(14):2955. doi: 10.3390/nu14142955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buzdagli Y., Eyipinar C.D., Kaci F.N., Tekin A. Effects of hesperidin on anti-inflammatory and antioxidant response in healthy people: a meta-analysis and meta-regression. Int. J. Environ. Health Res. 2023;33(12):1390–1405. doi: 10.1080/09603123.2022.2093841. [DOI] [PubMed] [Google Scholar]
- 87.Aggarwal V., Tuli H.S., Thakral F., Singhal P., Aggarwal D., Srivastava S., Pandey A., Sak K., Varol M., Khan M.A., Sethi G. Molecular mechanisms of action of hesperidin in cancer: recent trends and advancements. Exp. Biol. Med. 2020;245(5):486–497. doi: 10.1177/1535370220903671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen P., Wu Q., Feng J., Yan L., Sun Y., Liu S., Xiang Y., Zhang M., Pan T., Chen X., Duan T., Zhai L., Zhai B., Wang W., Zhang R., Chen B., Han X., Li Y., Chen L., Liu Y., Huang X., Jin T., Zhang W., Luo H., Chen X., Li Y., Li Q., Li G., Zhang Q., Zhuo L., Yang Z., Tang H., Xie T., Ouyang X., Sui X. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Targeted Ther. 2020;5(1):51. doi: 10.1038/s41392-020-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Z., Liu R., Qiu M., Mei H., Hao J., Song T., Zhao K., Zou D., Wang H., Gao M. The roles of ERIANIN in tumor and innate immunity and its' perspectives in immunotherapy. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1170754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang T., Ouyang H., Mei X., Lu B., Yu Z., Chen K., Wang Z., Ji L. Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-kappaB signaling pathway. Faseb. J. 2019;33(11):11776–11790. doi: 10.1096/fj.201802614RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su C., Zhang P., Liu J., Cao Y. Erianin inhibits indoleamine 2, 3-dioxygenase -induced tumor angiogenesis. Biomed. Pharmacother. 2017;88:521–528. doi: 10.1016/j.biopha.2017.01.090. [DOI] [PubMed] [Google Scholar]
- 92.Li X., Liu X., Xing Y., Zeng L., Liu X., Shen H., Ma J. Erianin controls collagen-mediated retinal angiogenesis via the RhoA/ROCK1 signaling pathway induced by the alpha2/beta1 integrin-collagen interaction. Invest. Ophthalmol. Vis. Sci. 2022;63(1):27. doi: 10.1167/iovs.63.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong Y.Q., Fan Y., Wu D.Z., Yang H., Hu Z.B., Wang Z.T. In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent. Eur. J. Cancer. 2004;40(10):1554–1565. doi: 10.1016/j.ejca.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 94.Tsai S.W., Wang J.H., Chang Y.K., Lin C.C. Erianin alleviates collagen-induced arthritis in mice by inhibiting Th17 cell differentiation. Open Life Sci. 2023;18(1) doi: 10.1515/biol-2022-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng J., He W., Chen Y., Li L., Liang Q., Dai W., Li R., Chen F., Chen Z., Tan Y., Li X. Erianin serves as an NFATc1 inhibitor to prevent breast cancer-induced osteoclastogenesis and bone destruction. J. Adv. Res. 2024;S2090–1232(24) doi: 10.1016/j.jare.2024.03.021. 00121-8. [DOI] [PubMed] [Google Scholar]
- 96.Yang A., Li M.Y., Zhang Z.H., Wang J.Y., Xing Y., Ri M., Jin C.H., Xu G.H., Piao L.X., Jin H.L., Zuo H.X., Ma J., Jin X. Erianin regulates programmed cell death ligand 1 expression and enhances cytotoxic T lymphocyte activity. J. Ethnopharmacol. 2021;273 doi: 10.1016/j.jep.2020.113598. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X., Hu L., Xu S., Ye C., Chen A. Erianin: a direct NLRP3 inhibitor with remarkable anti-inflammatory activity. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.739953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chowdhury R., Bhuia S., Rakib A.I., Al Hasan S., Shill M.C., El-Nashar H.A.S., El-Shazly M., Islam M.T. Gigantol, a promising natural drug for inflammation: a literature review and computational based study. Nat. Prod. Res. 2024;16(Apr):1–17. doi: 10.1080/14786419.2024.2340042. [DOI] [PubMed] [Google Scholar]
- 99.Ouyang P., He X., Yuan Z.W., Yin Z.Q., Fu H., Lin J., He C., Liang X., Lv C., Shu G., Yuan Z.X., Song X., Li L., Yin L. Erianin against Staphylococcus aureus infection via inhibiting sortase A. Toxins. 2018;10(10):385. doi: 10.3390/toxins10100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang Y., Wang Z., Liu Z., Huan Q., Liu Y., Li R., Wang M., Xiao X. Gigantol restores the sensitivity of mcr carrying multidrug-resistant bacteria to colistin. Phytomedicine. 2023;117 doi: 10.1016/j.phymed.2023.154886. [DOI] [PubMed] [Google Scholar]
- 101.Meng X., Yu X., Liu C., Wang Y., Song F., Huan C., Huo W., Zhang S., Li Z., Zhang J., Zhang W., Yu J. Effect of ingredients from Chinese herbs on enterovirus D68 production. Phytother Res. 2019;33(1):174–186. doi: 10.1002/ptr.6214. [DOI] [PubMed] [Google Scholar]
- 102.Huang J.M., Huang F.I., Yang C.R. Moscatilin ameliorates tau phosphorylation and cognitive deficits in Alzheimer's disease models. J. Nat. Prod. 2019;82(7):1979–1988. doi: 10.1021/acs.jnatprod.9b00375. [DOI] [PubMed] [Google Scholar]
- 103.Fang H., Hu X., Wang M., Wan W., Yang Q., Sun X., Gu Q., Gao X., Wang Z., Gu L., Oliver Chen C.Y., Wei X. Anti-osmotic and antioxidant activities of gigantol from Dendrobium aurantiacum var. denneanum against cataractogenesis in galactosemic rats. J. Ethnopharmacol. 2015;172:238–246. doi: 10.1016/j.jep.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 104.Wu J., Li X., Wan W., Yang Q., Ma W., Chen D., Hu J., Chen C.O., Wei X. Gigantol from Dendrobium chrysotoxum Lindl. binds and inhibits aldose reductase gene to exert its anti-cataract activity: an in vitro mechanistic study. J. Ethnopharmacol. 2017;198:255–261. doi: 10.1016/j.jep.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 105.Kyokong N., Muangnoi C., Thaweesest W., Kongkatitham V., Likhitwitayawuid K., Rojsitthisak P., Sritularak B. A new phenanthrene dimer from Dendrobium palpebrae. J. Asian Nat. Prod. Res. 2019;21(4):391–397. doi: 10.1080/10286020.2018.1429416. [DOI] [PubMed] [Google Scholar]
- 106.Kongkatitham V., Dehlinger A., Wang M., Poldorn P., Weidinger C., Letizia M., Chaotham C., Otto C., Ruprecht K., Paul F., Rungrotmongkol T., Likhitwitayawuid K., Bottcher C., Sritularak B. Immunomodulatory effects of new phenanthrene derivatives from Dendrobium crumenatum. J. Nat. Prod. 2023;86(5):1294–1306. doi: 10.1021/acs.jnatprod.3c00107. [DOI] [PubMed] [Google Scholar]
- 107.Sarakulwattana C., Mekboonsonglarp W., Likhitwitayawuid K., Rojsitthisak P., Sritularak B. New bisbibenzyl and phenanthrene derivatives from Dendrobium scabrilingue and their alpha-glucosidase inhibitory activity. Nat. Prod. Res. 2020;34(12):1694–1701. doi: 10.1080/14786419.2018.1527839. [DOI] [PubMed] [Google Scholar]
- 108.Lin Y., Wang F., Yang L.J., Chun Z., Bao J.K., Zhang G.L. Anti-inflammatory phenanthrene derivatives from stems of Dendrobium denneanum. Phytochemistry. 2013;95:242–251. doi: 10.1016/j.phytochem.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 109.Lu T.L., Han C.K., Chang Y.S., Lu T.J., Huang H.C., Bao B.Y., Wu H.Y., Huang C.H., Li C.Y., Wu T.S. Denbinobin, a phenanthrene from Dendrobium nobile, impairs prostate cancer migration by inhibiting Rac1 activity. Am. J. Chin. Med. 2014;42(6):1539–1554. doi: 10.1142/S0192415X14500967. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y., Zhang Q., Xin W., Liu N., Zhang H. Nudol, a phenanthrene derivative from Dendrobium nobile, induces cell cycle arrest and apoptosis and inhibits migration in osteosarcoma cells. Drug Des. Dev. Ther. 2019;13:2591–2601. doi: 10.2147/DDDT.S180610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song J.I., Kang Y.J., Yong H.Y., Kim Y.C., Moon A. Denbinobin, a phenanthrene from Dendrobium nobile, inhibits invasion and induces apoptosis in SNU-484 human gastric cancer cells. Oncol. Rep. 2012;27(3):813–818. doi: 10.3892/or.2011.1551. [DOI] [PubMed] [Google Scholar]
- 112.Mohammadinejad A., Mohajeri T., Aleyaghoob G., Heidarian F., Kazemi Oskuee R. Ellagic acid as a potent anticancer drug: a comprehensive review on in vitro, in vivo, in silico, and drug delivery studies. Biotechnol. Appl. Biochem. 2022;69(6):2323–2356. doi: 10.1002/bab.2288. [DOI] [PubMed] [Google Scholar]
- 113.Chung K.T., Wong T.Y., Wei C.I., Huang Y.W., Lin Y. Tannins and human health: a review. Crit. Rev. Food Sci. Nutr. 1998;38(6):421–464. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- 114.Sun J., Ma X., Sun L., Zhang Y., Hao C., Wang W. Inhibitory effects and mechanisms of proanthocyanidins against enterovirus 71 infection. Virus Res. 2023;329 doi: 10.1016/j.virusres.2023.199098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ajebli M., Eddouks M. The promising role of plant tannins as bioactive antidiabetic agents. Curr. Med. Chem. 2019;26(25):4852–4884. doi: 10.2174/0929867325666180605124256. [DOI] [PubMed] [Google Scholar]
- 116.Serrano J., Puupponen-Pimiä R., Dauer A., Aura A.M., Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009;53(S2):S310–S329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- 117.Jagannathan V., Viswanathan P. Proanthocyanidins-Will they effectively restrain conspicuous bacterial strains devolving on urinary tract infection? J. Basic Microbiol. 2018;58(7):567–578. doi: 10.1002/jobm.201800131. [DOI] [PubMed] [Google Scholar]
- 118.Li H., Zhang Y., Hao Y., Xu P., Wang X., Zhu B., Lu C., Xu K. Proanthocyanidins inhibit osteoblast apoptosis via the PI3K/AKT/Bcl-xL pathway in the treatment of steroid-induced osteonecrosis of the femoral head in rats. Nutrients. 2023;15(8):1936. doi: 10.3390/nu15081936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soliz-Rueda J.R., López-Fernández-Sobrino R., Bravo F.I., Aragonès G., Suarez M., Muguerza B. Grape seed proanthocyanidins mitigate the disturbances caused by an abrupt photoperiod change in healthy and obese rats. Nutrients. 2022;14(9):1834. doi: 10.3390/nu14091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Filosa S., Di Meo F., Crispi S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen Res. 2018;13(12):2055–2059. doi: 10.4103/1673-5374.241429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Etxeberria U., Fernandez-Quintela A., Milagro F.I., Aguirre L., Martinez J.A., Portillo M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013;61(40):9517–9533. doi: 10.1021/jf402506c. [DOI] [PubMed] [Google Scholar]
- 122.Han X., Bai L., Kee H.J., Jeong M.H. Syringic acid mitigates isoproterenol-induced cardiac hypertrophy and fibrosis by downregulating Ereg. J. Cell Mol. Med. 2022;26(14):4076–4086. doi: 10.1111/jcmm.17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu G., Zhang B.F., Hu Q., Liu X.P., Chen J. Syringic acid mitigates myocardial ischemia reperfusion injury by activating the PI3K/Akt/GSK-3beta signaling pathway. Biochem. Biophys. Res. Commun. 2020;531(2):242–249. doi: 10.1016/j.bbrc.2020.07.047. [DOI] [PubMed] [Google Scholar]
- 124.Ogut E., Armagan K., Gul Z. The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations. Metab. Brain Dis. 2022;37(4):859–880. doi: 10.1007/s11011-022-00960-3. [DOI] [PubMed] [Google Scholar]
- 125.Ha S.J., Lee J., Park J., Kim Y.H., Lee N.H., Kim Y.E., Song K.M., Chang P.S., Jeong C.H., Jung S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018;154:435–445. doi: 10.1016/j.bcp.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 126.Gheena S., Ezhilarasan D., Shree Harini K., Rajeshkumar S. Syringic acid and silymarin concurrent administration inhibits sodium valproate-induced liver injury in rats. Environ. Toxicol. 2022;37(9):2143–2152. doi: 10.1002/tox.23557. [DOI] [PubMed] [Google Scholar]
- 127.Gong X., Jiang S., Tian H., Xiang D., Zhang J. Polyphenols in the fermentation liquid of Dendrobium candidum relieve intestinal inflammation in zebrafish through the intestinal microbiome-mediated immune response. Front. Immunol. 2020;11:1542. doi: 10.3389/fimmu.2020.01542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang P., Chen X., Cai C.H., Kong F.D., Huang S.Z., Yuan J.Z., Xu X.L., Mei W.L., Dai H.F. A new picrotoxane-type sesquiterpene from Dendrobium nobile Lindl. Nat. Prod. Res. 2022;36(8):2112–2117. doi: 10.1080/14786419.2020.1851224. [DOI] [PubMed] [Google Scholar]
- 129.Huang L., Meng Y.F., Wang G., Yang J.W., Zhang M.S., Dong M.J., Sun C.X., Xiao S.J. Complete (1) H and (13) C assignments of two new sesquiterpenoids from Dendrobium aduncum. Magn. Reson. Chem. 2023;61(6):386–391. doi: 10.1002/mrc.5345. [DOI] [PubMed] [Google Scholar]
- 130.Ma C., Meng C.W., Zhou Q.M., Peng C., Liu F., Zhang J.W., Zhou F., Xiong L. New sesquiterpenoids from the stems of Dendrobium nobile and their neuroprotective activities. Fitoterapia. 2019;138 doi: 10.1016/j.fitote.2019.104351. [DOI] [PubMed] [Google Scholar]
- 131.Fan W.W., Yang D., Cheng Z.Q., Xu F.Q., Dong F.W., Wei X.Y., Hu J.M. Ten picrotoxane-type sesquiterpenoids from the stems of Dendrobium wardianum Warner. Phytochemistry. 2021;190 doi: 10.1016/j.phytochem.2021.112858. [DOI] [PubMed] [Google Scholar]
- 132.Zhuang S., Bao Y., Zhang Y., Zhang H., Liu J., Liu H. Antibacterial mechanism of the Asp-Asp-Asp-Tyr peptide. Food Chem. X. 2022;13 doi: 10.1016/j.fochx.2022.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu H., Zhang H., Wang Q., Li S., Liu Y., Ma L., Huang Y., Stephen Brennan C., Sun L. Mechanisms underlying the antimicrobial actions of the antimicrobial peptides Asp-Tyr-Asp-Asp and Asp-Asp-Asp-Tyr. Food Res. Int. 2021;139 doi: 10.1016/j.foodres.2020.109848. [DOI] [PubMed] [Google Scholar]
- 134.Prats E., Galindo J.C., Bazzalo M.E., León A., Macías F.A., Rubiales D., Jorrín J.V. Antifungal activity of a new phenolic compound from capitulum of a head rot-resistant sunflower genotype. J. Chem. Ecol. 2007;33(12):2245–2253. doi: 10.1007/s10886-007-9388-9. [DOI] [PubMed] [Google Scholar]
- 135.Sakthivel K.M., Vishnupriya S., Priya Dharshini L.C., Rasmi R.R., Ramesh B. Modulation of multiple cellular signalling pathways as targets for anti-inflammatory and anti-tumorigenesis action of Scopoletin. J. Pharm. Pharmacol. 2022;74(2):147–161. doi: 10.1093/jpp/rgab047. [DOI] [PubMed] [Google Scholar]
- 136.Parama D., Girisa S., Khatoon E., Kumar A., Alqahtani M.S., Abbas M., Sethi G., Kunnumakkara A.B. An overview of the pharmacological activities of scopoletin against different chronic diseases. Pharmacol. Res. 2022;179 doi: 10.1016/j.phrs.2022.106202. [DOI] [PubMed] [Google Scholar]
- 137.Zhang W.Z., Zhao W.M., Ge C.Y., Li X.W., Sun Z.S. Scopoletin attenuates intracerebral hemorrhage-induced brain injury and improves neurological performance in rats. Neuroimmunomodulation. 2021;28(2):74–81. doi: 10.1159/000505731. [DOI] [PubMed] [Google Scholar]
- 138.Song X.Y., Feng X., Zhao X.M. A survey of research on Dendrobium officinale. Journal of Liaoning University of Traditional Chinese Medicine. 2015;17(8):118–120. [Google Scholar]
- 139.He T.B., Huang Y.P., Yang L., Liu T.T., Gong W.Y., Wang X.J., Sheng J., Hu J.M. Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016;83:34–41. doi: 10.1016/j.ijbiomac.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 140.Wei W., Feng L., Bao W.R., Ma D.L., Leung C.H., Nie S.P., Han Q.B. Structure characterization and immunomodulating effects of polysaccharides isolated from Dendrobium officinale. J. Agric. Food Chem. 2016;64(4):881–889. doi: 10.1021/acs.jafc.5b05180. [DOI] [PubMed] [Google Scholar]
- 141.Huang Y.P., He T.B., Cuan X.D., Wang X.J., Hu J.M., Sheng J. 1,4-beta-d-Glucomannan from Dendrobium officinale activates NF-small ka, CyrillicB via TLR4 to regulate the immune response. Molecules. 2018;23(10):2658. doi: 10.3390/molecules23102658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang W., Wu Q., Lu Y.L., Gong Q.H., Zhang F., Shi J.S. Protective effects of Dendrobium nobile Lindl. alkaloids on amyloid beta (25-35)-induced neuronal injury. Neural Regen Res. 2017;12(7):1131–1136. doi: 10.4103/1673-5374.211193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li L.S., Lu Y.L., Nie J., Xu Y.Y., Zhang W., Yang W.J., Gong Q.H., Lu Y.F., Lu Y., Shi J.S. Dendrobium nobile Lindl alkaloid, a novel autophagy inducer, protects against axonal degeneration induced by Abeta(25-35) in hippocampus neurons in vitro. CNS Neurosci. Ther. 2017;23(4):329–340. doi: 10.1111/cns.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feng C.Z., Cao L., Luo D., Ju L.S., Yang J.J., Xu X.Y., Yu Y.P. Dendrobium polysaccharides attenuate cognitive impairment in senescence-accelerated mouse prone 8 mice via modulation of microglial activation. Brain Res. 2019;1704:1–10. doi: 10.1016/j.brainres.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 145.Li D.D., Fan H.X., Yang R., Li Y.Y., Zhang F., Shi J.S. Dendrobium nobile Lindl. Alkaloid suppresses NLRP3-mediated pyroptosis to alleviate LPS-induced neurotoxicity. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.846541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu B., Huang B., Liu J., Shi J.S. Dendrobium nobile Lindl alkaloid and metformin ameliorate cognitive dysfunction in senescence-accelerated mice via suppression of endoplasmic reticulum stress. Brain Res. 2020;1741 doi: 10.1016/j.brainres.2020.146871. [DOI] [PubMed] [Google Scholar]
- 147.Huang J., Huang N., Zhang M., Nie J., Xu Y., Wu Q., Shi J. Dendrobium alkaloids decrease Abeta by regulating alpha- and beta-secretases in hippocampal neurons of SD rats. PeerJ. 2019;7 doi: 10.7717/peerj.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang C.C., Kong Y.L., Zhang M.S., Wu Q., Shi J.S. Two new alkaloids from Dendrobium nobile Lindl. exhibited neuroprotective activity, and dendrobine alleviated Abeta(1-42) -induced apoptosis by inhibiting CDK5 activation in PC12 cells. Drug Dev. Res. 2023;84(2):262–274. doi: 10.1002/ddr.22030. [DOI] [PubMed] [Google Scholar]
- 149.Jiang N., Fan L.X., Yang Y.J., Liu X.M., Lin H.Y., Gao L., Wang Q. Antidepressant effects of the extract of Dendrobium nobile Lindl on chronic unpredictable mild stress-induced depressive mice. Sheng Li Xue Bao. 2017;69(2):159–166. [PubMed] [Google Scholar]
- 150.Xiong T.W., Liu B., Wu Q., Xu Y.Y., Liu P., Wang Y., Liu J., Shi J.S. Beneficial effects of Dendrobium nobile Lindl. Alkaloids (DNLA) on anxiety and depression induced by chronic unpredictable stress in rats. Brain Res. 2021;1771 doi: 10.1016/j.brainres.2021.147647. [DOI] [PubMed] [Google Scholar]
- 151.Zhu Y., Liu M., Cao C., Qu S., Zheng J., Zhu Z., Chen Z., Wang Z., Zhu Z., Huang F., Duan J.A. Dendrobium officinale flos increases neurotrophic factor expression in the hippocampus of chronic unpredictable mild stress-exposed mice and in astrocyte primary culture and potentiates NGF-induced neuronal differentiation in PC12 cells. Phytother Res. 2021;35(5):2665–2677. doi: 10.1002/ptr.7013. [DOI] [PubMed] [Google Scholar]
- 152.Shi M.L., Yan M.Q., Su J., Yu J.J., Ye S.Y., Fu M., Hu X.L., Niu Z.W., Wu W.Y., Chen S.M., Chen S.H., Chen J.Z., Lv G.Y. Effects of Dendrobium officinale ultrafine powder on sub-health mice induced by unhealthy lifestyle based on neuroendocrine immune system. Food Funct. 2022;13(23):12436–12450. doi: 10.1039/d2fo02158g. [DOI] [PubMed] [Google Scholar]
- 153.Hsu W.H., Chung C.P., Wang Y.Y., Kuo Y.H., Yeh C.H., Lee I.J., Lin Y.L. Dendrobium nobile protects retinal cells from UV-induced oxidative stress damage via Nrf2/HO-1 and MAPK pathways. J. Ethnopharmacol. 2022;288 doi: 10.1016/j.jep.2021.114886. [DOI] [PubMed] [Google Scholar]
- 154.Long Y., Wang W., Zhang Y., Du F., Zhang S., Li Z., Deng J., Li J. Photoprotective effects of Dendrobium nobile Lindl. Polysaccharides against UVB-induced oxidative stress and apoptosis in HaCaT cells. Int. J. Mol. Sci. 2023;24(7):6120. doi: 10.3390/ijms24076120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Quan W.Y., Fan L.H., Li Q., Lin C.M., Liang Y.Y., Zhang Q., Ye H., Li X.J., Wu K.F., Zhu Y.Z. DHPW1 attenuation of UVB-induced skin photodamage in human immortalized keratinocytes. Exp. Gerontol. 2022;166 doi: 10.1016/j.exger.2022.111897. [DOI] [PubMed] [Google Scholar]
- 156.Mai Y., Niu Z., He W., Lai X., Huang S., Zheng X. The reparative effect of Dendrobium officinale protocorms against photodamage caused by UV-irradiation in hairless mice. Biol. Pharm. Bull. 2019;42(5):728–735. doi: 10.1248/bpb.b18-00901. [DOI] [PubMed] [Google Scholar]
- 157.Yu Z., Gong C., Lu B., Yang L., Sheng Y., Ji L., Wang Z. Dendrobium chrysotoxum Lindl. alleviates diabetic retinopathy by preventing retinal inflammation and tight junction protein decrease. J. Diabetes Res. 2015;2015 doi: 10.1155/2015/518317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zha X.Q., Deng Y.Y., Li X.L., Wang J.F., Pan L.H., Luo J.P. The core structure of a Dendrobium huoshanense polysaccharide required for the inhibition of human lens epithelial cell apoptosis. Carbohydr. Polym. 2017;155:252–260. doi: 10.1016/j.carbpol.2016.08.087. [DOI] [PubMed] [Google Scholar]
- 159.Chao W.H., Lai M.Y., Pan H.T., Shiu H.W., Chen M.M., Chao H.M. Dendrobium nobile Lindley and its bibenzyl component moscatilin are able to protect retinal cells from ischemia/hypoxia by dowregulating placental growth factor and upregulating Norrie disease protein. BMC Compl. Alternative Med. 2018;18(1):193. doi: 10.1186/s12906-018-2256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu Z., Huang L., Sun L., Nie H., Liang Y., Huang J., Wu F., Hu X. Ecust004 suppresses breast cancer cell growth, invasion, and migration via EMT regulation. Drug Des. Dev. Ther. 2021;15:3451–3461. doi: 10.2147/DDDT.S309132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pang C., Zhang X., Huang M., Xie G., Liu S., Ye X., Zhang X. Dendrobium officinalis inhibited tumor growth in non-small cell lung cancer. Transl. Cancer Res. 2020;9(4):2683–2691. doi: 10.21037/tcr.2020.02.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Petpiroon N., Sritularak B., Chanvorachote P. Phoyunnanin E inhibits migration of non-small cell lung cancer cells via suppression of epithelial-to-mesenchymal transition and integrin alphav and integrin beta3. BMC Compl. Alternative Med. 2017;17(1):553. doi: 10.1186/s12906-017-2059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu Y.T., Hsieh M.J., Lin J.T., Chen G., Lin C.C., Lo Y.S., Chuang Y.C., Hsi Y.T., Chen M.K., Chou M.C. Erianin induces cell apoptosis through ERK pathway in human nasopharyngeal carcinoma. Biomed. Pharmacother. 2019;111:262–269. doi: 10.1016/j.biopha.2018.12.081. [DOI] [PubMed] [Google Scholar]
- 164.Losuwannarak N., Roytrakul S., Chanvorachote P. Gigantol targets MYC for ubiquitin-proteasomal degradation and suppresses lung cancer cell growth. Cancer Genomics Proteomics. 2020;17(6):781–793. doi: 10.21873/cgp.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang H.Q., Xie X.F., Li G.M., Chen J.R., Li M.T., Xu X., Xiong Q.Y., Chen G.R., Yin Y.P., Peng F., Chen Y., Peng C. Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytother Res. 2021;35(8):4511–4525. doi: 10.1002/ptr.7154. [DOI] [PubMed] [Google Scholar]
- 166.Boonjing S., Pothongsrisit S., Wattanathamsan O., Sritularak B., Pongrakhananon V. Erianthridin induces non-small cell lung cancer cell apoptosis through the suppression of extracellular signal-regulated kinase activity. Planta Med. 2021;87(4):283–293. doi: 10.1055/a-1295-8606. [DOI] [PubMed] [Google Scholar]
- 167.Hlosrichok A., Sumkhemthong S., Sritularak B., Chanvorachote P., Chaotham C. A bibenzyl from Dendrobium ellipsophyllum induces apoptosis in human lung cancer cells. J. Nat. Med. 2018;72(3):615–625. doi: 10.1007/s11418-018-1186-x. [DOI] [PubMed] [Google Scholar]
- 168.Chaotham C., Chanvorachote P. A bibenzyl from Dendrobium ellipsophyllum inhibits migration in lung cancer cells. J. Nat. Med. 2015;69(4):565–574. doi: 10.1007/s11418-015-0925-5. [DOI] [PubMed] [Google Scholar]
- 169.Luo Y., Ren Z., Du B., Xing S., Huang S., Li Y., Lei Z., Li D., Chen H., Huang Y., Wei G. Structure identification of ViceninII extracted from Dendrobium officinale and the reversal of TGF-beta1-induced Epithelial(-)Mesenchymal transition in lung adenocarcinoma cells through TGF-beta/Smad and PI3K/Akt/mTOR signaling pathways. Molecules. 2019;24(1):144. doi: 10.3390/molecules24010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Unahabhokha T., Chanvorachote P., Sritularak B., Kitsongsermthon J., Pongrakhananon V. Gigantol inhibits epithelial to mesenchymal process in human lung cancer cells. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/4561674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wattanathamsan O., Treesuwan S., Sritularak B., Pongrakhananon V. Cypripedin, a phenanthrenequinone from Dendrobium densiflorum, sensitizes non-small cell lung cancer H460 cells to cisplatin-mediated apoptosis. J. Nat. Med. 2018;72(2):503–513. doi: 10.1007/s11418-018-1176-z. [DOI] [PubMed] [Google Scholar]
- 172.Song T.H., Chen X.X., Lee C.K., Sze S.C., Feng Y.B., Yang Z.J., Chen H.Y., Li S.T., Zhang L.Y., Wei G., Shi J., Xu K., Ng T.B., Zhu L.L., Zhang K.Y. Dendrobine targeting JNK stress signaling to sensitize chemotoxicity of cisplatin against non-small cell lung cancer cells in vitro and in vivo. Phytomedicine. 2019;53:18–27. doi: 10.1016/j.phymed.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 173.Bhummaphan N., Chanvorachote P. Gigantol suppresses cancer stem cell-like phenotypes in lung cancer cells. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/836564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bhummaphan N., Petpiroon N., Prakhongcheep O., Sritularak B., Chanvorachote P. Lusianthridin targeting of lung cancer stem cells via Src-STAT3 suppression. Phytomedicine. 2019;62 doi: 10.1016/j.phymed.2019.152932. [DOI] [PubMed] [Google Scholar]
- 175.Taweecheep P., Khine H.E.E., Hlosrichok A., Ecoy G.A.U., Sritularak B., Prompetchara E., Chanvorachote P., Chaotham C. Stemness-suppressive effect of bibenzyl from Dendrobium ellipsophyllum in human lung cancer stem-like cells. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/5516655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bhummaphan N., Pongrakhananon V., Sritularak B., Chanvorachote P. Cancer stem cell-suppressing activity of chrysotoxine, a bibenzyl from Dendrobium pulchellum. J. Pharmacol. Exp. Therapeut. 2018;364(2):332–346. doi: 10.1124/jpet.117.244467. [DOI] [PubMed] [Google Scholar]
- 177.Liu H., Liang J., Zhong Y., Xiao G., Efferth T., Georgiev M.I., Vargas-De-La-Cruz C., Bajpai V.K., Caprioli G., Liu J., Lin J., Wu H., Peng L., Li Y., Ma L., Xiao J., Wang Q. Dendrobium officinale polysaccharide alleviates intestinal inflammation by promoting small extracellular vesicle packaging of miR-433-3p. J. Agric. Food Chem. 2021;69(45):13510–13523. doi: 10.1021/acs.jafc.1c05134. [DOI] [PubMed] [Google Scholar]
- 178.Liang J., Li H., Chen J., He L., Du X., Zhou L., Xiong Q., Lai X., Yang Y., Huang S., Hou S. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol. Res. 2019;148 doi: 10.1016/j.phrs.2019.104417. [DOI] [PubMed] [Google Scholar]
- 179.Wu Y., Li Y., Jin X.M., Dai G.H., Chen X., Tong Y.L., Ren Z.M., Chen Y., Xue X.M., Wu R.Z. Effects of Granule Dendrobii on chronic atrophic gastritis induced by N-methyl-N'-nitro-N-nitrosoguanidine in rats. World J. Gastroenterol. 2022;28(32):4668–4680. doi: 10.3748/wjg.v28.i32.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xie H., Hu M., Yu J., Yang X., Li J., Yu N., Han L., Peng D. Mass spectrometry-based metabolomics reveal Dendrobium huoshanense polysaccharide effects and potential mechanism of N-methyl-N'-nitro-N-nitrosoguanidine -induced damage in GES-1 cells. J. Ethnopharmacol. 2023;310 doi: 10.1016/j.jep.2023.116342. [DOI] [PubMed] [Google Scholar]
- 181.Wang Y., Chu F., Lin J., Li Y., Johnson N., Zhang J., Gai C., Su Z., Cheng H., Wang L., Ding X. Erianin, the main active ingredient of Dendrobium chrysotoxum Lindl, inhibits precancerous lesions of gastric cancer (PLGC) through suppression of the HRAS-PI3K-AKT signaling pathway as revealed by network pharmacology and in vitro experimental verification. J. Ethnopharmacol. 2021;279 doi: 10.1016/j.jep.2021.114399. [DOI] [PubMed] [Google Scholar]
- 182.Zhao Y., Huang H.X., Tang S.Y., Sun Y.Z. RNA-Seq analysis reveals Dendrobium officinale polysaccharides inhibit precancerous lesions of gastric cancer through PER3 and AQP4. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/3036504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zheng Q., Qiu D., Liu X., Zhang L., Cai S., Zhang X. Antiproliferative effect of Dendrobium catenatum Lindley polypeptides against human liver, gastric and breast cancer cell lines. Food Funct. 2015;6(5):1489–1495. doi: 10.1039/c5fo00060b. [DOI] [PubMed] [Google Scholar]
- 184.Liu B., Li Q.M., Shang Z.Z., Zha X.Q., Pan L.H., Luo J.P. Anti-gastric cancer activity of cultivated Dendrobium huoshanense stem polysaccharide in tumor-bearing mice: effects of molecular weight and O-acetyl group. Int. J. Biol. Macromol. 2021;192:590–599. doi: 10.1016/j.ijbiomac.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 185.Zhao Y., Sun Y., Wang G., Ge S., Liu H. Dendrobium officinale polysaccharides protect against MNNG-induced PLGC in rats via activating the NRF2 and antioxidant enzymes HO-1 and NQO-1. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/9310245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhao Y., Liu Y., Lan X.M., Xu G.L., Sun Y.Z., Li F., Liu H.N. Effect of Dendrobium officinale extraction on gastric carcinogenesis in rats. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/1213090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li R., Liu T., Liu M., Chen F., Liu S., Yang J. Anti-influenza A virus activity of dendrobine and its mechanism of action. J. Agric. Food Chem. 2017;65(18):3665–3674. doi: 10.1021/acs.jafc.7b00276. [DOI] [PubMed] [Google Scholar]
- 188.Sukphan P., Sritularak B., Mekboonsonglarp W., Lipipun V., Likhitwitayawuid K. Chemical constituents of Dendrobium venustum and their antimalarial and anti-herpetic properties. Nat. Prod. Commun. 2014;9(6):825–827. [PubMed] [Google Scholar]
- 189.Gao X., Ye T., Lei Y., Zhang Q., Luo Y., Yang H., Zeng X., Zhou W., Wen Q., Liu J., Xiong H., Wan R. Dendrobium officinale aqueous extract influences the immune response following vaccination against SARS-CoV-2. Biomed. Pharmacother. 2023;162 doi: 10.1016/j.biopha.2023.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lei X., Huo P., Xie Y.J., Wang Y., Liu G., Tu H., Shi Q., Mo Z.C., Zhang S. Dendrobium nobile Lindl polysaccharides improve testicular spermatogenic function in streptozotocin-induced diabetic rats. Mol. Reprod. Dev. 2022;89(4):202–213. doi: 10.1002/mrd.23556. [DOI] [PubMed] [Google Scholar]
- 191.Luo M., Liao B., Ma D., Wang J., Wang J., Liu J., Lei X., Cai Y., Tang L., Zhao L., Long S., Yang F., Lei X. Dendrobium nobile-derived polysaccharides ameliorate spermatogenic disorders in mice with streptozotocin-induced diabetes through regulation of the glycolytic pathway. Int. J. Biol. Macromol. 2022;216:203–212. doi: 10.1016/j.ijbiomac.2022.06.193. [DOI] [PubMed] [Google Scholar]
- 192.Feng X., Wang D., Hu L., Lu H., Ling B., Huang Y., Jiang Q. Dendrobium officinale polysaccharide ameliorates polycystic ovary syndrome via regulating butyrate dependent gut-brain-ovary axis mechanism. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.962775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang S., Tu H., Zhu J., Liang A., Huo P., Shan K., He J., Zhao M., Chen X., Lei X. Dendrobium nobile Lindl. polysaccharides improve follicular development in PCOS rats. Int. J. Biol. Macromol. 2020;149:826–834. doi: 10.1016/j.ijbiomac.2020.01.196. [DOI] [PubMed] [Google Scholar]
- 194.Wu Y.Y., Liang C.Y., Liu T.T., Liang Y.M., Li S.J., Lu Y.Y., Liang J., Yuan X., Li C.J., Hou S.Z., Lai X.P. Protective roles and mechanisms of polysaccharides from Dendrobium officinal on natural aging-induced premature ovarian failure. Biomed. Pharmacother. 2018;101:953–960. doi: 10.1016/j.biopha.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 195.Guo Z., Zhou Y., Yang J., Shao X. Dendrobium candidum extract inhibits proliferation and induces apoptosis of liver cancer cells by inactivating Wnt/beta-catenin signaling pathway. Biomed. Pharmacother. 2019;110:371–379. doi: 10.1016/j.biopha.2018.11.149. [DOI] [PubMed] [Google Scholar]
- 196.Hong J., Xie Z., Yang F., Jiang L., Jian T., Wang S., Guo Y., Huang X. Erianin suppresses proliferation and migration of cancer cells in a pyruvate carboxylase-dependent manner. Fitoterapia. 2022;157 doi: 10.1016/j.fitote.2022.105136. [DOI] [PubMed] [Google Scholar]
- 197.Xie H., Feng S., Farag M.A., Sun P., Shao P. Synergistic cytotoxicity of erianin, a bisbenzyl in the dietetic Chinese herb Dendrobium against breast cancer cells. Food Chem. Toxicol. 2021;149 doi: 10.1016/j.fct.2020.111960. [DOI] [PubMed] [Google Scholar]
- 198.Su W., Zeng L., Chen W. Moscatilin suppresses the breast cancer both in vitro and in vivo by inhibiting HDAC3. Dose Response. 2021;19(1) doi: 10.1177/15593258211001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xiang Y., Chen X., Wang W., Zhai L., Sun X., Feng J., Duan T., Zhang M., Pan T., Yan L., Jin T., Gao Q., Wen C., Ma W., Liu W., Wang D., Wu Q., Xie T., Sui X. Natural product erianin inhibits bladder cancer cell growth by inducing ferroptosis via NRF2 inactivation. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.775506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhu Q., Sheng Y., Li W., Wang J., Ma Y., Du B., Tang Y. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits bladder cancer cell growth via the mitochondrial apoptosis and JNK pathways. Toxicol. Appl. Pharmacol. 2019;371:41–54. doi: 10.1016/j.taap.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 201.Zhao M., Sun Y., Gao Z., Cui H., Chen J., Wang M., Wang Z. Gigantol attenuates the metastasis of human bladder cancer cells, possibly through Wnt/EMT signaling. OncoTargets Ther. 2020;13:11337–11346. doi: 10.2147/OTT.S271032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shen H., Geng Z., Nie X., Liu T. Erianin induces ferroptosis of renal cancer stem cells via promoting ALOX12/P53 mRNA N6-methyladenosine modification. J. Cancer. 2023;14(3):367–378. doi: 10.7150/jca.81027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang L., Fang Y., Xu X.F., Jin D.Y. Moscatilin induces apoptosis of pancreatic cancer cells via reactive oxygen species and the JNK/SAPK pathway. Mol. Med. Rep. 2017;15(3):1195–1203. doi: 10.3892/mmr.2017.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhu Y., Kong Y., Hong Y., Zhang L., Li S., Hou S., Chen X., Xie T., Hu Y., Wang X., Huoshanmycins A.‒C. New polyketide dimers produced by endophytic Streptomyces sp. HS-3-L-1 from Dendrobium huoshanense. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.807508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cardile V., Avola R., Graziano A.C.E., Russo A. Moscatilin, a bibenzyl derivative from the orchid Dendrobium loddigesii, induces apoptosis in melanoma cells. Chem. Biol. Interact. 2020;323 doi: 10.1016/j.cbi.2020.109075. [DOI] [PubMed] [Google Scholar]
- 206.Wang Y.H. Traditional uses, chemical constituents, pharmacological activities, and toxicological effects of Dendrobium leaves: a review. J. Ethnopharmacol. 2021;270 doi: 10.1016/j.jep.2021.113851. [DOI] [PubMed] [Google Scholar]
- 207.Xu X., Zhang C., Wang N., Xu Y., Tang G., Xu L., Feng Y. Bioactivities and mechanism of actions of Dendrobium officinale: a comprehensive review. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/6293355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang L.C., Xiang J., Hong K., Yi Y., Wang Y.Z., Lin J.Q. Accumulation of alkaloids and water-soluble polysaccharides in Dendrobium nobile. Shizhen Traditional Chinese Medicine. 2010;21(11):2864–2865. [Google Scholar]
- 209.Jiang Y.L., Luo J.P. Research progress on pharmacological activity and chemical structure of polysaccharides from Dendrobium officinale. Shizhen Traditional Chinese Medicine. 2011;22(12):2986–2988. [Google Scholar]
- 210.Ahmadinejad F., Geir Møller S., Hashemzadeh-Chaleshtori M., Bidkhori G., Jami M.S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants. 2017;6(3):51. doi: 10.3390/antiox6030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ke Y., Zhan L., Lu T., Zhou C., Chen X., Dong Y., Lv G., Chen S. Polysaccharides of Dendrobium officinale Kimura & Migo leaves protect against ethanol-induced gastric mucosal injury via the AMPK/mTOR signaling pathway in vitro and vivo. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.526349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu J., Han Y., Zhu T., Yang Q., Wang H., Zhang H. Dendrobium nobile Lindl. polysaccharides reduce cerebral ischemia/reperfusion injury in mice by increasing myeloid cell leukemia 1 via the downregulation of miR-134. Neuroreport. 2021;32(3):177–187. doi: 10.1097/WNR.0000000000001562. [DOI] [PubMed] [Google Scholar]
- 213.Wang Z., Jin C., Li X., Ding K. Sulfated polysaccharide JCS1S2 inhibits angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Carbohydr. Polym. 2019;207:502–509. doi: 10.1016/j.carbpol.2018.11.091. [DOI] [PubMed] [Google Scholar]
- 214.Zhang X., Yan Y., Lv Y., Li X., Chen L., Huang Z., Zhou J., Wang Y., Wang X., Wang X., Gu H. Dendrobium officinale polysaccharides attenuate uropathogenic Escherichia coli (UPEC)-induced pyroptosis in macrophage cells. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113098. [DOI] [PubMed] [Google Scholar]
- 215.Wen Y., Xiao H., Liu Y., Yang Y., Wang Y., Xu S., Huang S., Hou S., Liang J. Polysaccharides from Dendrobium officinale ameliorate colitis-induced lung injury via inhibiting inflammation and oxidative stress. Chem. Biol. Interact. 2021;347 doi: 10.1016/j.cbi.2021.109615. [DOI] [PubMed] [Google Scholar]
- 216.Liang J., Wu Y., Yuan H., Yang Y., Xiong Q., Liang C., Li Z., Li C., Zhang G., Lai X., Hu Y., Hou S. Dendrobium officinale polysaccharides attenuate learning and memory disabilities via anti-oxidant and anti-inflammatory actions. Int. J. Biol. Macromol. 2019;126:414–426. doi: 10.1016/j.ijbiomac.2018.12.230. [DOI] [PubMed] [Google Scholar]
- 217.Yang Y., Fan L., Peng Y., Peng C., Li X. Alcohol-soluble polysaccharides from Dendrobium officinale flowers as an antidepressant by regulating the gut-brain axis. Int. J. Biol. Macromol. 2022;216:836–849. doi: 10.1016/j.ijbiomac.2022.07.220. [DOI] [PubMed] [Google Scholar]
- 218.Liu Y., Yang L., Zhang Y., Liu X., Wu Z., Gilbert R.G., Deng B., Wang K. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J. Ethnopharmacol. 2020;248 doi: 10.1016/j.jep.2019.112308. [DOI] [PubMed] [Google Scholar]
- 219.Zhao Y., Li B., Wang G., Ge S., Lan X., Xu G., Liu H. Dendrobium officinale polysaccharides inhibit 1-methyl-2-nitro-1-nitrosoguanidine induced precancerous lesions of gastric cancer in rats through regulating Wnt/beta-catenin pathway and altering serum endogenous metabolites. Molecules. 2019;24(14):2660. doi: 10.3390/molecules24142660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Duan H., Er-Bu A., Dongzhi Z., Xie H., Ye B., He J. Alkaloids from Dendrobium and their biosynthetic pathway, biological activity and total synthesis. Phytomedicine. 2022;102 doi: 10.1016/j.phymed.2022.154132. [DOI] [PubMed] [Google Scholar]
- 221.Shi B.S., Shao Y.T., Chen L.J., Zhang L., Pu D., Wen X.L., Chen Y.P., Li Y.P. Research progress on chemical constituents and pharmacological effects of Dendrobium nobile. Journal of Kunming Medical University. 2017;38(10):124–129. [Google Scholar]
- 222.Yao R., Gao Q.J., Yu Y., Peng J.C., Zheng C. Comparison of polysaccharide and total alkaloid contents in Dendrobium officinale at different harvesting stages. Chinese Journal of Veterinary Medicine. 2021;40(3):10–12. [Google Scholar]
- 223.Mou Z., Zhao Y., Ye F., Shi Y., Kennelly E.J., Chen S., Zhao D. Identification, biological activities and biosynthetic pathway of dendrobium alkaloids. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.605994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liu B., Shang Z.Z., Li Q.M., Zha X.Q., Wu D.L., Yu N.J., Han L., Peng D.Y., Luo J.P. Structural features and anti-gastric cancer activity of polysaccharides from stem, root, leaf and flower of cultivated Dendrobium huoshanense. Int. J. Biol. Macromol. 2020;143:651–664. doi: 10.1016/j.ijbiomac.2019.12.041. [DOI] [PubMed] [Google Scholar]
- 225.Wang Z., Jiang W., Liu Y., Meng X., Su X., Cao M., Wu L., Yu N., Xing S., Peng D. Putative genes in alkaloid biosynthesis identified in Dendrobium officinale by correlating the contents of major bioactive metabolites with genes expression between Protocorm-like bodies and leaves. BMC Genom. 2021;22(1):579. doi: 10.1186/s12864-021-07887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wu Q., Chai Y.H., Liu C.Y., Niu J.J., Ma S.B. Research progress on chemical constituents and pharmacological effects of Dendrobium. Journal of Guizhou University of Traditional Chinese Medicine. 2021;43(5):99–103. [Google Scholar]
- 227.Yu M., Qi B., Xiaoxiang W., Xu J., Liu X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-kappaB pathway. Biomed. Pharmacother. 2017;90:677–685. doi: 10.1016/j.biopha.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 228.Li Y.Y., Wang X.J., Su Y.L., Wang Q., Huang S.W., Pan Z.F., Chen Y.P., Liang J.J., Zhang M.L., Xie X.Q., Wu Z.Y., Chen J.Y., Zhou L., Luo X. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol. Sin. 2022;43(6):1495–1507. doi: 10.1038/s41401-021-00781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xiao S.J., Liu Z., Zhang M.S., Chen Y.Z., Nie X.Q., Zhang J.Y., He Y.Q., Shi J.S. A new bibenzyl compound from Dendrobium nobile. Yao Xue Xue Bao. 2016;51(7):1117–1120. [PubMed] [Google Scholar]
- 230.Zhang X., Xu J.K., Wang N.L., Su Y.B., Yao X.S., Wang Z. Antioxidant activities of bibenzyls and phenolic acids in Dendrobium nobile. Chinese Journal of Pharmaceutical Sciences. 2008;(11):829–832. [Google Scholar]
- 231.Zhang Y., Zhang Q., Wei F., Liu N. Progressive study of effects of erianin on anticancer activity. OncoTargets Ther. 2019;12:5457–5465. doi: 10.2147/OTT.S200161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Petpiroon N., Bhummaphan N., Tungsukruthai S., Pinkhien T., Maiuthed A., Sritularak B., Chanvorachote P. Chrysotobibenzyl inhibition of lung cancer cell migration through Caveolin-1-dependent mediation of the integrin switch and the sensitization of lung cancer cells to cisplatin-mediated apoptosis. Phytomedicine. 2019;58 doi: 10.1016/j.phymed.2019.152888. [DOI] [PubMed] [Google Scholar]
- 233.Chanvorachote P., Kowitdamrong A., Ruanghirun T., Sritularak B., Mungmee C., Likhitwitayawuid K. Anti-metastatic activities of bibenzyls from Dendrobium pulchellum. Nat. Prod. Commun. 2013;8(1):115–118. [PubMed] [Google Scholar]
- 234.Xu Y., Fang R., Shao J., Cai Z. Erianin induces triple-negative breast cancer cells apoptosis by activating PI3K/Akt pathway. Biosci. Rep. 2021;41(6) doi: 10.1042/BSR20210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li M., He Y., Peng C., Xie X., Hu G. Erianin inhibits human cervical cancer cell through regulation of tumor protein p53 via the extracellular signal-regulated kinase signaling pathway. Oncol. Lett. 2018;16(4):5006–5012. doi: 10.3892/ol.2018.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Trapika L., Liu X.T., Chung L.H., Lai F., Xie C., Zhao Y., Cui S., Chen J., Tran C., Wang Q., Zhang S., Don A.S., Li G.Q., Hanrahan J.R., Qi Y. Ceramide regulates anti-tumor mechanisms of erianin in androgen-sensitive and castration-resistant prostate cancers. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.738078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chen Y.T., Hsieh M.J., Chen P.N., Weng C.J., Yang S.F., Lin C.W. Erianin induces apoptosis and autophagy in oral squamous cell carcinoma cells. Am. J. Chin. Med. 2020;48(1):183–200. doi: 10.1142/S0192415X2050010X. [DOI] [PubMed] [Google Scholar]
- 238.Deng X., Wu Q., Li D., Liu Y. Erianin exerts antineoplastic effects on esophageal squamous cell carcinoma cells by activating the cGMP-PKG signaling pathway. Nutr. Cancer. 2023;75(6):1473–1484. doi: 10.1080/01635581.2023.2205047. [DOI] [PubMed] [Google Scholar]
- 239.Wang H., Zhang T., Sun W., Wang Z., Zuo D., Zhou Z., Li S., Xu J., Yin F., Hua Y., Cai Z. Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016;7(6) doi: 10.1038/cddis.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang P., Jia X., Lu B., Huang H., Liu J., Liu X., Wu Q., Hu Y., Li P., Wei H., Liu T., Zhao D., Zhang L., Tian X., Jiang Y., Qiao Y., Nie W., Ma X., Bai R., Peng C., Dong Z., Liu K. Erianin suppresses constitutive activation of MAPK signaling pathway by inhibition of CRAF and MEK1/2. Signal Transduct. Targeted Ther. 2023;8(1):96. doi: 10.1038/s41392-023-01329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dong H., Wang M., Chang C., Sun M., Yang F., Li L., Feng M., Zhang L., Li Q., Zhu Y., Qiao Y., Xie T., Chen J. Erianin inhibits the oncogenic properties of hepatocellular carcinoma via inducing DNA damage and aberrant mitosis. Biochem. Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114266. [DOI] [PubMed] [Google Scholar]
- 242.Sun Y., Li G., Zhou Q., Shao D., Lv J., Zhou J. Dual targeting of cell growth and phagocytosis by erianin for human colorectal cancer. Drug Des. Dev. Ther. 2020;14:3301–3313. doi: 10.2147/DDDT.S259006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wei X., Liu J., Xu Z., Wang D., Zhu Q., Chen Q., Xu W. Research progress on the pharmacological mechanism, in vivo metabolism and structural modification of Erianin. Biomed. Pharmacother. 2024;173 doi: 10.1016/j.biopha.2024.116295. [DOI] [PubMed] [Google Scholar]
- 244.Chu L.H., Gu R.H., Qin L.K. Research progress on chemical constituents and pharmacological effects of Dendrobium nobile, Chinese herbal medicine. 2021;52(24):7693–7708. [Google Scholar]
- 245.Zhou W., Zeng Q.F., Xia J., Wang L., Tao L., Shen X.C. Study on the antitumor active ingredients of phenanthrene in Dendrobium nobile. Chinese Journal of Pharmaceutical Sciences. 2018;53(20):1722–1725. [Google Scholar]
- 246.Zhou X.M., Zheng C.J., Gan L.S., Chen G.Y., Zhang X.P., Song X.P., Li G.N., Sun C.G. Bioactive phenanthrene and bibenzyl derivatives from the stems of Dendrobium nobile. J. Nat. Prod. 2016;79(7):1791–1797. doi: 10.1021/acs.jnatprod.6b00252. [DOI] [PubMed] [Google Scholar]
- 247.Yang L., Qin L.H., Bligh S.W., Bashall A., Zhang C.F., Zhang M., Wang Z.T., Xu L.S. A new phenanthrene with a spirolactone from Dendrobium chrysanthum and its anti-inflammatory activities. Bioorg. Med. Chem. 2006;14(10):3496–3501. doi: 10.1016/j.bmc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 248.Wang Y., Tong Y., Adejobi O.I., Wang Y., Liu A. Research advances in multi-omics on the traditional Chinese herb Dendrobium officinale. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.808228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tang H., Zhao T., Sheng Y., Zheng T., Fu L., Zhang Y. Dendrobium officinale Kimura et Migo: A Review on Its Ethnopharmacology. Phytochemistry, Pharmacology, and Industrialization, Evid Based Complement Alternat Med. 2017 doi: 10.1155/2017/7436259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chen W., Lu J., Zhang J., Wu J., Yu L., Qin L., Zhu B. Traditional Uses, Phytochemistry, Pharmacology, and Quality Control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.726528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Monagas M., Urpi-Sarda M., Sanchez-Patan F., Llorach R., Garrido I., Gomez-Cordoves C., Andres-Lacueva C., Bartolome B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1(3):233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 252.Cardona F., Andres-Lacueva C., Tulipani S., Tinahones F.J., Queipo-Ortuno M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24(8):1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 253.Wang H., Li J., Liang X., Tao S., Wu Z., Wei G. Taxonomic and functional diversity of Dendrobium officinale microbiome in Danxia habitat. J. Appl. Microbiol. 2022;132(5):3758–3770. doi: 10.1111/jam.15488. [DOI] [PubMed] [Google Scholar]
- 254.Wang P., Chen X., Wang H., Huang S.Z., Cai C.H., Yuan J.Z., Zhu G.L., Xu X.L., Mei W.L., Dai H.F. Four new picrotoxane-type sesquiterpenes from Dendrobium nobile Lindl. Front. Chem. 2019;7:812. doi: 10.3389/fchem.2019.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Meng C.W., He Y.L., Peng C., Ding X.J., Guo L., Xiong L. Picrotoxane sesquiterpenoids from the stems of Dendrobium nobile and their absolute configurations and angiogenesis effect. Fitoterapia. 2017;121:206–211. doi: 10.1016/j.fitote.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 256.Zhang X., Xu J.K., Wang J., Wang N.L., Kurihara H., Kitanaka S., Yao X.S. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J. Nat. Prod. 2007;70(1):24–28. doi: 10.1021/np060449r. [DOI] [PubMed] [Google Scholar]
- 257.Ye Q., Qin G., Zhao W. Immunomodulatory sesquiterpene glycosides from Dendrobium nobile. Phytochemistry. 2002;61(8):885–890. doi: 10.1016/s0031-9422(02)00484-3. [DOI] [PubMed] [Google Scholar]
- 258.Zhao W., Ye Q., Tan X., Jiang H., Li X., Chen K., Kinghorn A.D. Three new sesquiterpene glycosides from Dendrobium nobile with immunomodulatory activity. J. Nat. Prod. 2001;64(9):1196–1200. doi: 10.1021/np0102612. [DOI] [PubMed] [Google Scholar]
- 259.Zhao C., Liu Q., Halaweish F., Shao B., Ye Y., Zhao W. Copacamphane, picrotoxane, and alloaromadendrane sesquiterpene glycosides and phenolic glycosides from Dendrobium moniliforme. J. Nat. Prod. 2003;66(8):1140–1143. doi: 10.1021/np0301801. [DOI] [PubMed] [Google Scholar]
- 260.Hao J.W., Zhu A.L., Chen N.D., Liu X.Q., Li Q., Xu H.M., Yang W.H., Qin C.F., Wu J.D. Simultaneous analysis of 20 free amino acids by a single marker combined with an HPLC fingerprint evaluation of Dendrobium huoshanense. J. Food Sci. 2021;86(11):4828–4839. doi: 10.1111/1750-3841.15931. [DOI] [PubMed] [Google Scholar]
- 261.Chen Y.Q., Hu Y.L., Bai Y.B., Zhou H.G., Li Z.S. Amino acid composition and analysis of dendrobium. Journal of Tropical Crops. 2019;40(9):1823–1830. [Google Scholar]
- 262.Liu Z.P., Guo Y.Y., Liu J.J., Si J.P., Wu L.S., Zhang X.F. Effects of strains and parts of Dendrobium officinale on amino acid content. Chinese Journal of Traditional Chinese Medicine. 2015;40(8):1468–1472. [PubMed] [Google Scholar]
- 263.Wang Z.D., Dai Y.F., Luo S.J., Li Y.W. Research progress on chemical constituents and pharmacological effects of Dendrobium officinale. West China J. Pharm. Sci. 2022;37(4):472–476. [Google Scholar]
- 264.Jia Y.Y., Zhao B.N. Determination of trace elements and amino acids in Shihu Yeguang pills. Shandong Pharmaceutical Industry. 1996;(1):9–10. [Google Scholar]
- 265.Chen J.J., Guo L., Xu L., Wei L., Luo F.L., Zhang T.M. Comparison of tannin content between Dendrobium sheathes and Dendrobium varieties recorded in Pharmacopoeia. Chinese Journal of Experimental Formulas. 2013;19(2):61–63. [Google Scholar]
- 266.Wen B.J., Xia H.L., Hu P., Li R.Y., Zhang H., Yang M.N., Zhang T.M. Effects of different extraction methods on the content of tannins in Dendrobium dendrobii. Journal of Chengdu University of Traditional Chinese Medicine. 2015;38(4):16–18. [Google Scholar]
- 267.Mattioli L.B., Corazza I., Micucci M., Pallavicini M., Budriesi R. Tannins-Based extracts: effects on gut chicken spontaneous contractility. Molecules. 2023;28(1):395. doi: 10.3390/molecules28010395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zeng X.Q., Jiang W.B., Du Z.J., Kokini J.L. Encapsulation of tannins and tannin-rich plant extracts by complex coacervation to improve their physicochemical properties and biological activities: a review. Crit. Rev. Food Sci. Nutr. 2022;63(18):3005–3018. doi: 10.1080/10408398.2022.2075313. [DOI] [PubMed] [Google Scholar]
- 269.Chung K.T., Wong T.Y., Wei C.I., Huang Y.W., Lin Y. Tannins and human health: a review. Crit. Rev. Food Sci. Nutr. 1998;38(6):421–464. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- 270.Wang S.C., Chou I.W., Hung M.C. Natural tannins as anti-SARS-CoV-2 compounds. Int. J. Biol. Sci. 2022;18(12):4669–4676. doi: 10.7150/ijbs.74676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wrigley T.C. Ayapin, scopoletin and 6,7-dimethoxycoumarin from Dendrobium thyrsiflorum (Reichb. f.) Nature. 1960;188:1108. doi: 10.1038/1881108a0. [DOI] [PubMed] [Google Scholar]
- 272.Fan X.N., Lin S., Zhu C.G., Liu Y., Hu J.F., Chen X.G., Wang W.J., Chen N.H., Shi J.G. Aromatic chemical constituents and their activities in diversia Florida. Chinese Journal of Traditional Chinese Medicine. 2011;36(1):48–56. [PubMed] [Google Scholar]
- 273.Ji Y., Jin H., Fang N. Clinical and safety evaluation of Dendrobium Night Light Pills combined with sodium hyaluronate eye drops in the treatment of dry eye. Chinese Journal of Traditional Chinese Medicine. 2019;37(8):1970–1973. [Google Scholar]
- 274.Zhu X.M., Tang S.J., Xi C. Clinical evaluation of Dendrobium luminous pills combined with sodium hyaluronate eye drops and vitamin AD soft capsules in the treatment of dry eye. Chinese Pharmaceutical Industry. 2020;29(8):100–102. [Google Scholar]
- 275.Wang J.D., Liu Y., Cao C.H., Zong B.T., Yao J. Clinical study on Dendrobium Night Light Pills combined with polyethylene glycol eye drops in the treatment of dry eye. Modern Drugs and Clinics. 2024;39(2):432–437. [Google Scholar]
- 276.Yu M.E. Observation on the efficacy of Shihu Yeguang Pills combined with polyvinyl alcohol eye drops in the treatment of dry eye syndrome due to liver and kidney yin deficiency. Shanxi Traditional Chinese Medicine. 2016;32(5):24–25. [Google Scholar]
- 277.He Y.Z., Yu J., Su Y.Y. Clinical observation of dendrobium night light pills in the treatment of vitreous opacity. Chinese Minkang Medicine. 2011;23(9):1127–1128. [Google Scholar]
- 278.Cheng K.Y. Shihu Yeguang Pills and Xuesaitong Tablets combined with amioiodine eye drops in the treatment of vitreous degeneration and opacity. Jiangxi Medicine. 2008;(4):334–335. [Google Scholar]
- 279.Huang J.L. Shihu Yeguang pills combined with compound xueshuantong capsules in the treatment of central serous chorioretinopathy. Guangming Traditional Chinese Medicine. 2008;23(12):1934–1935. [Google Scholar]
- 280.Peng Z.H. Observation on the efficacy of Shihu Yeguang Pills combined with low-dose photodynamic therapy in the treatment of 38 cases of chronic persistent central serous chorioretinopathy. Chinese Journal of Laser Medicine. 2012;21(5):341. [Google Scholar]
- 281.Q L.F., Gong H., Liu Z.Y., Gong J. Summary of 28 cases of postoperative treatment of advanced glaucoma with Dendrobium Night Light Pills. Hunan Journal of Traditional Chinese Medicine. 2005;(4):22–25. [Google Scholar]
- 282.Qu Y.L., Zhang W. Clinical observation of Dendrobium luminous pills combined with massage in the treatment of visual fatigue. Modern Chinese Medicine. 2017;37(5):63–64. [Google Scholar]
- 283.Gao L.Y., Ren R., Ding M. Study on the molecular mechanism of Shihu Yeguang pill in treating cataract. Journal of Nankai University (Natural Science Edition). 2023;56(2):67–76. [Google Scholar]
- 284.Wang H.M., Du M.H. Clinical observation of 32 cases of Dendrobium Night Light Pills treating hypotonia caused by various chronic uveitis. Journal of Industrial and Enterprise Medicine. 1994;(1):14–15. [Google Scholar]
- 285.Zhang J., Li G.F. Study on the effect of intravitreal injection combined with oral administration of Dendrobium Mingmu Pills on the surgical treatment effect and visual acuity of patients with proliferative diabetic retinopathy. Shanxi Medical Journal. 2022;51(10):1088–1091. [Google Scholar]
- 286.Ma R.X. Clinical study on Dendrobium Mingmu Pills combined with western medicine in the treatment of non-proliferative diabetic retinopathy. New Chinese Medicine. 2019;51(1):100–103. [Google Scholar]
- 287.Chen Y. 2019. Research on the Biological Basis of Classic Prescriptions for Improving Eyesight: Enlightenment on the Prevention and Treatment of Age-Related Macular Degeneration the 8th Professional Committee on Deficiency Syndrome and Geriatric Medicine of the Chinese Society of Integrated Traditional Chinese and Western Medicine. [Google Scholar]
- 288.Jin H.J. Clinical observation of Dendrobium Mingmu Pills combined with sodium hyaluronate eye drops in the treatment of dry eye. New Chinese Medicine. 2015;47(10):149–150. [Google Scholar]
- 289.Sun Z.Q., Peng Y.G., Shou D.W., Xiao M.Y., Ye C.Y. Effects of Dendrobium luminous granules on experimental cataract in rats and bulbar conjunctival microcirculation in rabbits. Chinese Journal of Traditional Chinese Medicine Ophthalmology. 1998;(1):1–4. [Google Scholar]
- 290.Huang L., Liu J.F., Dou H.F., Li D.F., Zhang Y. Comparison of pharmacological effects of Dendrobium luminous granules and pills. Chinese Journal of Experimental Prescriptions. 1996;(2):24–27. [Google Scholar]
- 291.Zhao M., Qiu Y.X., Hu Y., Liu Z.H., Wang P.L., Chen W.W., Pan A., Cheng J.M. Discussion on the mechanism and experimental verification of compound fresh dendrobium granules in preventing and treating alcoholic liver disease based on network pharmacology. Journal of Nanjing University of Traditional Chinese Medicine. 2023;39(9):895–909. [Google Scholar]
- 292.Wu Y.G., Zhao Z.R., Zhang P., Xin Y.F., Liu H. Study on the effects of Dendrobium officinale compound granules on immune function in mice. Chinese Journal of Traditional Chinese Medicine. 2015;33(8):1936–1938. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.