Abstract
Triple-negative breast cancer (TNBC) poses a significant challenge in clinical oncology due to its aggressive nature and limited targeted therapeutic options. Neoadjuvant chemotherapy (NACT) has emerged as a promising strategy in the management of early-stage TNBC. This literature review aims to provide an in-depth analysis of the role of NACT in TNBC, focusing on its impact on early-stage disease and associated outcomes. The review synthesizes evidence from recent studies, clinical trials, and meta-analyses to present a comprehensive overview of the current landscape of NACT in early-stage TNBC.
Keywords: TNBC, Breast cancer, NACT, Immunotherapy, BCS, Early breast cancer
Introduction
Triple-negative breast cancer (TNBC) is characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression, making it a subtype with limited targeted therapy options. Early-stage TNBC is associated with a higher risk of recurrence and poorer outcomes compared to other breast cancer subtypes. Neoadjuvant chemotherapy (NACT) has gained prominence as a treatment approach in early-stage TNBC, allowing for downstaging of tumors, increased rates of breast-conserving surgery, and improved overall survival.
Numerous clinical trials have investigated the role of NACT in reshaping the treatment landscape for TNBC. The landmark GeparTrio trial demonstrated that patients with TNBC who achieved a pathological complete response (pCR) after neoadjuvant treatment had significantly improved long-term outcomes, emphasizing the potential of NACT in enhancing survival rates [1]. Building on these findings, the CREATE-X trial investigated the role of postoperative capecitabine in patients with residual disease after neoadjuvant therapy, revealing a significant improvement in disease-free survival [2].
Additionally, the success of the CALGB 40603 trial in establishing the superiority of incorporating carboplatin into neoadjuvant regimens for TNBC has influenced contemporary treatment strategies, showcasing the evolving landscape of NACT in optimizing outcomes [3]. The presence of BRCA mutations has also been explored as a potential predictive marker for response to neoadjuvant platinum-based chemotherapy, as highlighted in the GeparSixto trial [4].
However, challenges persist in accurately assessing treatment response, with debates surrounding the optimal imaging modalities and the definition of pCR. The SWOG S0800 trial has contributed valuable insights into the variability in pCR rates when assessed using different imaging techniques, emphasizing the need for standardized criteria to evaluate treatment response accurately [5].
The TMH study by Gupta S et al. investigated the efficacy of platinum-based chemotherapy in patients with triple-negative breast cancer (TNBC). This study was conducted to determine whether incorporating platinum agents, such as cisplatin or carboplatin, could improve treatment outcomes for this aggressive subtype of breast cancer. Study provides strong evidence for the effectiveness of platinum-based chemotherapy in improving response rates and survival outcomes in TNBC patients, with particular benefit observed in those with BRCA mutations. This supports the integration of platinum agents into treatment protocols for this challenging breast cancer subtype5a.
In light of these trials, this literature review aims to provide a comprehensive overview of the current state of evidence regarding the role of NACT in early-stage TNBC. By synthesizing data from pivotal trials and incorporating recent advancements, we aim to elucidate the impact of NACT on early-stage TNBC and its implications for patient outcomes.
Mechanisms of Action of NACT in TNBC
Neoadjuvant chemotherapy (NACT) serves as a cornerstone in the management of early-stage triple-negative breast cancer (TNBC), employing a multifaceted approach to combat the aggressive nature of the disease. The mechanisms underlying the action of NACT are complex, involving interactions at the cellular and molecular levels. The following flowchart outlines the key mechanisms of action of NACT in early-stage TNBC, supported by relevance from clinical trials.
- Administration of chemotherapy agents:
- NACT involves the systemic administration of cytotoxic chemotherapy agents.
- Common agents include anthracyclines (e.g., doxorubicin) and taxanes (e.g., paclitaxel) [6].
- Commonly used platinum agents (TNBC patients with BRCA1/2 mutations are particularly sensitive to platinum-based therapies due to their compromised DNA repair mechanisms)
- Cisplatin
- Carboplatin
- Modulation of tumor microenvironment:
- NACT influences the tumor microenvironment, altering the composition of stromal and immune cells.
- Immune responses may be stimulated, contributing to anti-tumor effects [7].
- Downstaging of tumors:
- NACT aims to reduce the size of primary tumors.
- Downstaging facilitates surgical interventions, potentially enabling breast-conserving surgery [8].
- Enhanced surgical options:
- Successful NACT may increase the feasibility of breast-conserving surgery.
- This offers cosmetic advantages and preserves breast function [9].
- Potential impact on micrometastatic disease:
- NACT addresses micrometastatic disease that may not be detectable clinically.
- Improved eradication of micrometastases may contribute to long-term disease control [10].
- Decision-making in cases of non-pCR:
- When patients do not achieve pCR (non-pCR) after neoadjuvant therapy, it indicates a higher risk of recurrence and potentially poorer prognosis. In cases of non-pCR, the information gleaned from the neoadjuvant phase guides the selection of adjuvant therapies, ensuring that residual disease is effectively targeted to improve patient outcomes. By incorporating additional chemotherapy, targeted therapies, immunotherapy, and personalized treatment approaches, clinicians can enhance the overall survival and quality of life for TNBC patients who do not achieve pCR.
Clinical Efficacy and Response Assessment of Neoadjuvant Chemotherapy (NACT) in Early-Stage Triple-Negative Breast Cancer (TNBC)
- Pathological complete response (pCR):
- One of the primary endpoints in assessing the clinical efficacy of NACT is the achievement of pathological complete response (pCR), defined as the absence of invasive cancer in both the breast and axillary lymph nodes upon surgical resection [11].
- Disease-free survival (DFS) and overall survival (OS):
- NACT’s impact on disease-free survival (DFS) and overall survival (OS) serves as crucial indicators of its long-term efficacy [12].
- Imaging modalities for response assessment:
- Various imaging modalities, including ultrasound, magnetic resonance imaging (MRI), and positron emission tomography (PET), are employed to assess tumor response to NACT [13]. Ultrasound serves as the cornerstone imaging modality for monitoring treatment response in TNBC, particularly in the early stages of the disease. Its ability to provide detailed anatomical information and measure changes in tumor size makes it indispensable in clinical practice. On the other hand, PET scan plays a crucial role in the evaluation of locally advanced disease or disease progression to metastasis.
- Molecular and genomic markers for response prediction:
- Identification of molecular and genomic markers, such as Ki-67 expression and gene signatures, aids in predicting response to NACT and stratifying patients for personalized treatment [14].
- Residual cancer burden (RCB):
- RCB is a comprehensive measure combining residual tumor size, nodal status, and biomarker expression, providing a more nuanced assessment of response [14].
Biomarkers and Predictors of Response
Identifying biomarkers and predictors of response to NACT in TNBC is crucial for optimizing treatment strategies and personalizing patient care.
- Ki-67 expression:
- Ki-67, a nuclear protein associated with cell proliferation, is a widely studied biomarker in TNBC. High Ki-67 levels correlate with increased tumor cell proliferation and are associated with a better response to chemotherapy [15].
- Tumor-infiltrating lymphocytes (TILs):
- The presence of TILs, particularly in TNBC, is associated with improved response to neoadjuvant therapy and better outcomes. High TIL levels suggest an enhanced immune response against the tumor [16].
- Gene expression signatures:
- Various gene expression profiles, such as the PAM50 intrinsic subtypes and molecular signatures like the 21-gene recurrence score, help categorize tumors and predict response to NACT [17].
- BRCA mutation status:
- TNBCs with BRCA mutations may exhibit increased sensitivity to DNA-damaging agents, such as platinum-based chemotherapy.
- PD-L1 expression:
- Programmed death-ligand 1 (PD-L1) expression on tumor cells is being explored as a potential biomarker for response to immune checkpoint inhibitors in combination with neoadjuvant chemotherapy.
- Homologous recombination deficiency (HRD) score as a biomarker:
- HRD score is a measure of the extent of genomic instability caused by defects in the DNA repair pathway, particularly in homologous recombination.
Potential in Guiding Treatment Decisions
Predictive value: The HRD score can predict which TNBC patients are more likely to respond to DNA-damaging agents, such as platinum-based chemotherapy and PARP inhibitors. Tumors with high HRD scores tend to have greater sensitivity to these treatments due to their compromised ability to repair DNA damage.
Personalized therapy: By assessing the HRD score, clinicians can tailor treatment plans more effectively. Patients with high HRD scores may benefit more from therapies targeting DNA repair mechanisms, while those with low HRD scores might be directed toward alternative treatment options.
Improved outcomes: Utilizing the HRD score as a biomarker allows for a more personalized approach to therapy, potentially improving overall response rates and survival outcomes by ensuring patients receive the most appropriate treatments based on their tumor biology.
Adverse Effects and Quality of Life
The administration of chemotherapy and immunotherapy in triple-negative breast cancer (TNBC) is crucial for improving outcomes but can also lead to various adverse effects, impacting both physical health and psychosocial well-being. It is essential to consider these factors comprehensively in treatment planning and patient management.
Chemotherapy
Short-Term Adverse Effects
Immediate reactions: Nausea, vomiting, hair loss (alopecia), fatigue, and peripheral neuropathy are common immediate side effects of chemotherapy.
Hematologic effects: Bone marrow suppression leading to neutropenia, anemia, and thrombocytopenia may occur, necessitating close monitoring and supportive care.
Long-Term Adverse Effects
Cardiotoxicity: Some chemotherapy agents, such as anthracyclines, may cause long-term cardiac complications.
Neurotoxicity: Persistent neuropathies and cognitive changes can affect quality of life post-treatment.
Secondary malignancies: There is a potential risk of developing secondary cancers years after chemotherapy, which requires ongoing surveillance.
Immunotherapy
Short-Term Adverse Effects
Immune-related adverse events (irAEs): These can include skin rash, colitis, pneumonitis, and endocrine dysfunction, resulting from the activation of the immune system against normal tissues.
Long-Term Adverse Effects
Autoimmune disorders: Rarely, immunotherapy can trigger autoimmune diseases that may manifest long after treatment completion.
Prolonged treatment effects: Some patients may experience lingering fatigue or other symptoms due to prolonged immune system activation.
Psychosocial Impacts
Fear and anxiety: Patients may experience fear of progression or recurrence, which can significantly impact mental health and quality of life.
Psychological distress: Coping with the diagnosis, treatment-related changes in appearance, and uncertainty about the future can lead to anxiety, depression, and emotional distress.
Social and practical challenges: Treatment schedules, financial burdens, and changes in daily routines can strain relationships and affect overall wellbeing.
Cognitive Function and Chemobrain
Some patients undergoing NACT may experience cognitive impairment, commonly referred to as “chemobrain.” This can manifest as difficulties in memory, attention, and concentration.
Addressing adverse effects related to chemotherapy and immunotherapy goes beyond managing physical symptoms; it involves comprehensive supportive care to mitigate long-term complications and address psychosocial impacts. Multidisciplinary approaches that integrate medical oncologists, psychologists, and support services are essential in providing holistic care for TNBC patients.
These trials (Table 1) collectively contribute valuable insights into the role of neoadjuvant chemotherapy in early-stage TNBC. GeparSixto and KEYNOTE-522, in particular, suggest potential benefits in terms of increased pCR rates when incorporating carboplatin and immunotherapy, respectively. However, the lack of significant differences in pCR rates in CALGB 40603 and the absence of an improvement in EFS in TNT highlight the complexity of optimizing treatment strategies for TNBC, indicating the need for further research and individualized approaches (Table 2).
Table 1.
Comprehensive summary of four notable trials investigating neoadjuvant chemotherapy (NACT) in early-stage triple-negative breast cancer (TNBC)
| Study | Design | Intervention | Sample Size | Primary endpoint | Key Findings |
|---|---|---|---|---|---|
| GeparSixto (2013) [1] | Phase II randomized controlled trial | Anthracycline/taxane + carboplatin vs. anthracycline/taxane | 595 | Pathological complete response (pCR) rate | The addition of carboplatin resulted in a significant increase in the pCR rate (53.2% vs. 36.9%) |
| CALGB 40603 (2014) [2] | Phase II randomized controlled trial | Paclitaxel + carboplatin vs. paclitaxel | 443 | Pathological complete response (pCR) rate | No significant difference in pCR rates between the two treatment arms |
| TNT (2018) [3] | Phase III randomized controlled trial | (AC-T) (standard) vs. docetaxel/carboplatin (TC) | 582 | Event-free survival (EFS) | No significant difference in EFS was observed between the two arms |
| KEYNOTE-522 (2019) [4] | Phase III randomized controlled trial | Pembrolizumab + chemotherapy vs. placebo + Chemotherapy | 1,174 | Pathological complete response (pCR) rate | The addition of pembrolizumab led to a significantly higher pCR rate (64.8% vs. 51.2%) |
Table 2.
General overview of some key trials that have explored the impact of neoadjuvant chemotherapy (NACT) on overall survival in early-stage triple-negative breast cancer (TNBC)
| Intervention | Outcome | |
|---|---|---|
| GeparSixto (2013) [1] | Anthracycline/taxane + carboplatin vs. anthracycline/taxane | Although the primary endpoint was pathological complete response (pCR), this trial did not directly assess overall survival. It demonstrated an increase in pCR with the addition of carboplatin, which can be associated with improved long-term outcomes |
| CREATE-X (2017) | Capecitabine vs. Observation following standard adjuvant chemotherapy | While CREATE-X focused on the adjuvant setting, it is noteworthy for its impact on overall survival. Patients receiving capecitabine post-surgery showed a significant improvement in disease-free survival, and a trend towards improved overall survival was observed |
| KEYNOTE-522 (2019) | Pembrolizumab + chemotherapy vs. placebo + chemotherapy | While the primary endpoint was pCR, the trial demonstrated a potential trend toward improved event-free survival. The overall survival data may become more mature over time |
| IMpassion031 (2020) | Atezolizumab + chemotherapy vs. placebo + chemotherapy | The trial showed an improvement in invasive disease-free survival in the intention-to-treat population, which could suggest a potential benefit in overall survival. However, mature overall survival data may require further follow-up |
CALGB 150007 and EORTC 10994/BIG 1–00 (Table 3): These trials did not show a significant difference in disease-free survival (DFS) or overall survival (OS) between NACT and upfront surgery, suggesting similar long-term outcomes with both approaches.
Table 3.
Comparative overview of trials NACT vs. upfront surgery
| Study | Intervention | Sample size | Primary endpoint | Key findings |
|---|---|---|---|---|
| CALGB 150007 (2003) | NACT vs. upfront surgery | 162 | Disease-free survival (DFS) | No significant difference in DFS between the two arms |
| GeparTrio (2009) | NACT vs. upfront surgery | 2,072 | Pathological complete response (pCR) | Higher pCR rates with NACT (29.6% vs. 11.8%) |
| SWOG S0800 (2015) | NACT vs. upfront surgery | 310 | Event-free survival (EFS) | No significant difference in EFS between the two arms |
| EORTC 10994/BIG 1–00 | NACT vs. upfront surgery | 337 | Overall survival (OS) | No significant difference in OS between NACT and upfront surgery |
GeparTrio: This trial demonstrated a higher pathological complete response (pCR) rate with NACT, emphasizing the potential benefits of preoperative chemotherapy in achieving a complete response.
SWOG S0800: No significant difference in event-free survival (EFS) was observed between NACT and upfront surgery, indicating comparable efficacy in preventing disease progression.
Table 4 shows immunotherapy, particularly with pembrolizumab and atezolizumab, in combination with neoadjuvant chemotherapy, has shown promising results in increasing pathological complete response rates in early TNBC. However, ongoing research is needed to determine long-term outcomes, optimal patient selection, and potential biomarkers predictive of response to immunotherapy in the neoadjuvant setting for early TNBC.
Table 4.
Immunotherapy in neoadjuvant setting for early TNBC
| Study | Intervention | Sample size | Key findings |
|---|---|---|---|
| KEYNOTE-522 (2019) | Pembrolizumab + chemotherapy (carboplatin and paclitaxel) vs. placebo + chemotherapy | 1174 | The addition of pembrolizumab significantly increased the pCR rate compared to placebo (64.8% vs. 51.2%) |
| IMpassion031 (2020) | Atezolizumab + nab-paclitaxel vs. placebo + nab-paclitaxel | 333 | Atezolizumab combined with nab-paclitaxel demonstrated an increased pCR rate in the intention-to-treat population (58.0% vs. 41.4%) |
| NeoTRIPaPDL1 (2020) | Atezolizumab + nab-paclitaxel followed by epirubicin/cyclophosphamide vs. placebo + nab-paclitaxel followed by epirubicin/cyclophosphamide | 280 | The addition of atezolizumab led to an increased pCR rate (58.7% vs. 41.4%) |
| NeoPACT study | Carboplatin + docetaxel + pembrolizumab | 110 | ProlifSig could identify a subgroup of immune low TNBCs that can achieve substantial rates of pCR with neoadjuvant chemoimmunotherapy |
The Use of PARP Inhibitors and Bevacizumab in the Neoadjuvant Setting for TNBC
Despite initial promise, recent studies have shown mixed results. The BrighTNess trial (Geyer CE, Sikov WM, Huober J. et al. 2022) found no significant long-term benefit with the addition of carboplatin and veliparib. Similarly, the CALGB 40603 trial (Shepherd JH, Ballman K, Polley MYC et al. 2022) reported no substantial improvement in long-term outcomes with the addition of carboplatin and bevacizumab. These findings highlight the complexity of treating TNBC and underscore the need for continued research into optimizing neoadjuvant therapies
Adjuvant Therapy with Capecitabine, Olaparib, and Pembrolizumab in TNBC
In summary, capecitabine, olaparib, and pembrolizumab show promise in the adjuvant treatment of TNBC, each with specific benefits and limitations. Their use should be guided by individual patient and tumor characteristics to maximize therapeutic outcomes (Tables 5 and 6).
Table 5.
The roles of capecitabine, olaparib, and pembrolizumab in the adjuvant therapy of TNBC, examining both supporting and opposing trial data
| Therapy | Supporting trials | Findings | Opposing trials | Concerns |
|---|---|---|---|---|
| Capecitabine | CREATE-X | Improved DFS and OS in residual disease post-neoadjuvant chemotherapy | Various smaller studies | Inconsistent benefits in different patient subsets |
| Olaparib | OlympiA | Improved DFS and OS in germline BRCA-mutated patients | None explicitly opposing efficacy | Long-term toxicity, cost-effectiveness, limited to BRCA mutations |
| Pembrolizumab | KEYNOTE-522 | Improved pCR rates and EFS when combined with neoadjuvant chemotherapy | Long-term benefit unclear | Significant immune-related adverse events |
Table 6.
Summary of key randomized studies on chemotherapy and immunotherapy in early-stage triple-negative breast cancer (TNBC)
| Study | Therapy | pCR rate | Overall survival (OS) | Progression-free survival (PFS) |
|---|---|---|---|---|
| CREATE-X | Adjuvant capecitabine | Significantly higher in capecitabine group | Improved OS with capecitabine | Improved PFS with capecitabine |
| OlympiA | Adjuvant olaparib (gBRCA mutation) | Not applicable | Improved OS in olaparib group | Improved PFS in olaparib group |
| KEYNOTE-522 | Neoadjuvant pembrolizumab + chemotherapy | Higher pCR in pembrolizumab group | Data still maturing, early signs of improvement | Improved EFS with pembrolizumab |
| CALGB 40603 | Neoadjuvant carboplatin and/or bevacizumab | Increased with Carboplatin addition | No significant difference observed | Improved with Carboplatin addition |
| BrighTNess | Neoadjuvant carboplatin + veliparib + paclitaxel | Increased with carboplatin | No significant improvement observed | No significant improvement observed |
| GeparSixto | Neoadjuvant carboplatin + chemotherapy | Higher pCR with carboplatin addition | OS data not significant | Improved PFS with carboplatin addition |
| TNT | Docetaxel vs. carboplatin | Not a primary endpoint | No significant difference observed | No significant difference observed |
Future Directions and Challenges
- Personalized treatment approaches:
- Future directions involve refining neoadjuvant therapy through personalized approaches, incorporating biomarkers, genomics, and molecular profiling.
- Targeted therapies, such as PARP inhibitors for BRCA-mutated TNBC or immunotherapies based on tumor immune profiles, are areas of active investigation.
- Integration of immunotherapy:
- Expanding the role of immunotherapy by exploring novel checkpoint inhibitors, combination strategies, and identifying predictive biomarkers.
- Investigating the long-term impact of immunotherapy on disease-free and overall survival in the neoadjuvant setting.
- Innovations in targeted therapies:
- Evaluating novel targeted therapies, including agents targeting specific signaling pathways or resistance mechanisms identified through molecular studies.
- Ongoing research in the development of new drugs and exploring their efficacy in early-stage TNBC.
- Optimizing chemotherapy regimens:
- Exploring alternative chemotherapy regimens, dose intensification, and sequencing to maximize effectiveness and minimize toxicity.
- Studying the potential benefits of adaptive treatment strategies based on early response assessments during neoadjuvant therapy.
- Role of radiomics and imaging:
- Advancing the role of radiomics and imaging technologies for early response prediction, allowing for timely treatment modifications.
- Integration of artificial intelligence (AI) and machine learning in analyzing imaging data to refine treatment strategies.
- Minimizing residual disease:
- Investigating strategies to eradicate minimal residual disease after neoadjuvant therapy, aiming to reduce the risk of relapse.
- Developing targeted therapies to eliminate micrometastatic disease not detected by conventional imaging.
- Enhanced biomarker discovery:
- Identifying and validating novel biomarkers associated with treatment response and long-term outcomes.
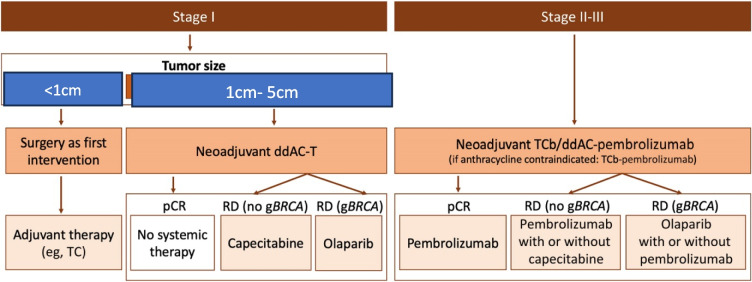
- Incorporating multi-omics data (genomics, transcriptomics, proteomics) to unravel the molecular landscape of TNBC and guide therapeutic decisions (Fig. 1).
Fig. 1.
Treatment algorithm for early-stage triple-negative breast cancer. ddAC-T, dose-dense doxorubicin and cyclophosphamide followed by paclitaxel; gBRCA, germline BRCA mutation; pCR, pathologic complete response; RD, residual disease; TC, docetaxel and cyclophosphamide; TCb, paclitaxel and carboplatin
Conclusion
Neoadjuvant therapy has emerged as a valuable and evolving approach in the management of early-stage triple-negative breast cancer (TNBC), significantly impacting both treatment strategies and patient outcomes. In summary, neoadjuvant therapy has transformed the landscape of TNBC management in the early stage, offering opportunities for tailored treatment, improved surgical options, and increased pCR rates. The integration of immunotherapy adds a promising dimension to these advancements. Ongoing research endeavors and a comprehensive, multidisciplinary approach are crucial to further refine neoadjuvant strategies and optimize outcomes for individuals with early-stage TNBC.
Declarations
Conflict of Interest
The author declares no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.von Minckwitz G, Schneeweiss A, Loibl S et al (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 15(7):747–756 [DOI] [PubMed] [Google Scholar]
- 2.Masuda N, Lee SJ, Ohtani S et al (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 376(22):2147–2159 [DOI] [PubMed] [Google Scholar]
- 3.Sikov WM, Berry DA, Perou CM et al (2015) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 33(1):13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey LA, Rugo HS, Marcom PK et al (2012) TBCRC 001: Randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 30(21):2615–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hylton NM, Blume JD, Bernreuter WK et al (2012) Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I-SPY TRIAL. Radiology 263(3):663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2018) Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. The Lancet 371(9606):29–40 [DOI] [PubMed] [Google Scholar]
- 7.Schmid P et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121 [DOI] [PubMed] [Google Scholar]
- 8.Cortazar P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 384(9938):164–172 [DOI] [PubMed] [Google Scholar]
- 9.Mieog JS et al (2007) Preoperative chemotherapy for women with operable breast cancer. Cochrane Database of Systematic Reviews 2:CD005002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarneri V et al (2014) Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 30(15):1747–1752 [DOI] [PubMed] [Google Scholar]
- 11.von Minckwitz G et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804 [DOI] [PubMed] [Google Scholar]
- 12.Rastogi P et al (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26(5):778–785 [DOI] [PubMed] [Google Scholar]
- 13.Groheux D et al (2013) Early monitoring of response to neoadjuvant chemotherapy in breast cancer with 18F-FDG PET/CT: defining a clinical aim. Eur J Nucl Med Mol Imaging 40(2):206–211 [DOI] [PubMed] [Google Scholar]
- 14.Symmans WF et al (2017) Genomic index of sensitivity to endocrine therapy for breast cancer. J Clin Oncol 28(27):4111–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viale G et al (2008) Ki-67 as a prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96(10):1504–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denkert C et al (2018) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 36(9):830–837 [DOI] [PubMed] [Google Scholar]
- 17.Lehmann BD et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig 121(7):2750–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]