Abstract
Oral squamous cell carcinoma (OSCC) is the sixth most common type of cancer globally. While smoking is a key risk factor, rising cases in non-smokers highlight the need to explore other factors like diet. This scoping review aims to deepen the evidence on the relationship between OSCC and diet, following PRISMA-ScR guidelines, and was registered on Open Science Framework. Searches were performed in four electronic databases: MEDLINE, Embase, Web of Science, and Lilacs, without date or language restrictions. Studies were evaluated, extracted, and compiled in a narrative table. Seventeen studies with 10,954 patients were analyzed. Most patients were male (74.63%), aged 18–89 (average 50.62). Studies were mainly from high (82%) and medium (17%) Human Development Index (HDI) countries. Dietary surveys included a Food Frequency Questionnaire (FFQ) (58.8%), interviews/questionnaires (17.6%), and an FFQ with a photographic atlas (5.9%). Certain foods in excess like fruits, vegetables, and tea were inversely associated with OSCC, while salty meats, dairy, coffee, sausages, and fried and spicy foods were positively associated. Due to the heterogeneity of the tools used to obtain food frequency data, the results should be interpreted cautiously. New standardized studies and randomized trials are essential to advance understanding and control confounding factors in this field.
Keywords: diet, squamous cell carcinoma of head and neck, mouth neoplasms, public health
1. Introduction
Oral squamous cell carcinoma (OSCC) is the most prevalent head and neck cancer, with approximately 350,000 new cases each year worldwide [1,2]. Alcohol and tobacco cause irreversible DNA damage in the squamous cells lining the oral mucosa [3,4], representing the main etiological factors for OSCC initiation [5]. Other environmental risk factors such as oral microbiome imbalance [6,7,8], chronic mechanical trauma [9], and dental implants have also been studied in the OSCC context, but their etiopathogenic role is still unclear [10,11]. Recent studies have associated eating patterns with several malignant neoplasms’ oncogenesis [9,10] and show that a healthy diet, rich in fresh fruits and vegetables and bioactive compounds, offers a synergy of antioxidant, anti-inflammatory, anti-angiogenic, and anti-proliferative properties [12,13,14]. These properties can potentially control cancer cells, preventing or treating the disease [13].
For OSCC, the high heterogeneity among studies assessing food consumption prevents a strong substantial association between eating patterns and tumor development. Therefore, synthesizing the literature regarding eating patterns in OSCC patients is vital to characterize the key most consumed food in this group of patients. Providing a broad perspective on this subject may aid the inclusion of preventive nutritional advice in the existing OSCC prevention recommendations [15]. None of the previous literature has encompassed such a wide range of foods and food groups for this type of cancer in a single study. In this scoping review, we evaluated the published literature on the eating patterns of OSCC patients and performed a detailed descriptive analysis of their nutritional landscape.
2. Materials and Methods
This study aimed to deepen the evidence on the relationship between OSCC and diet through a scoping review, mapping the existing literature on the relationship between diet and OSCC and conducting a detailed descriptive analysis of the findings to identify research gaps on this topic. It was developed according to the Joanna Briggs Institute tool (JBI) [16] and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [17]. This study was registered on the Open Science Framework (https://osf.io/f4ndm/, accessed on 1 March of 2024) [18].
2.1. Data Source and Search Strategy
The searches were performed in four electronic databases MEDLINE, Embase, Web of Science, and Lilacs, with no date or language restrictions. The studies were evaluated for eligibility, extracted, and compiled in a narrative form. Additionally, we hand-searched the reference lists of the included studies. The last search was conducted in March 2024.
Descriptors were identified in Medical Subject Headings (MeSH), Descritores em Ciências da Saúde (DeCS), and Embase Subject Headings (Emtree). These were combined with the Boolean operator AND, while their synonyms were combined with the Boolean operator OR. The following MeSH terms formed the search strategy used, which was adapted based on descriptors in each database, presented in Table S1.
The acronym PCC (P—population; C—concept; C—context) was used to formulate the central question. The guiding question was “What are the different associations of this type of cancer with diet?” (Table 1). The research strategy was developed with the support of a collaborator experienced in review studies (N.S.G.).
Table 1.
PCC acronym and question components.
| Abbreviation | Description | Question Components |
|---|---|---|
| P | Population | Patients diagnosed with OSCC (experimental group) and without OSCC (control group) |
| C | Concept | Association between food and eating patterns and the disease |
| C | Context | Food and eating patterns |
2.2. Eligibility Criteria
We included quantitative and qualitative observational studies that evaluated individuals aged ≥ 18 years with OSCC and used questionnaires that carried out a descriptive assessment of dietary patterns, whether validated or not. Reviews, preclinical studies, interventional studies, ecological studies, cost-effectiveness analyses, letters, and editorials were excluded. Studies that evaluated pregnant or lactating women, children, or adolescents and studies involving individuals who used chronic supplementation or carcinogenic herbs (areca nut derivate and betel quid), alcohol, or tobacco were excluded.
2.3. Study Selection and Data Extraction
The studies found in the electronic search of the databases were exported in “ris” format to the Rayyan Qatar Computing Research Institute application for systematic reviews [19]. The title and abstracts were independently screened by two reviewers (LCL and ABAMAS) to determine whether they met the inclusion criteria. After this stage, the textual analysis of the studies was carried out. Three independent reviewers (NSG, RRMC, and MGR) analyzed any discrepancies. To create the extraction table in an Excel® 2013 spreadsheet, the following data were collected: reference (author, year, and title), study location, study design, follow-up period (weeks), age, gender, lesion location, foods analyzed (type or group), eating patterns evaluated, and main results for the outcomes assessed. In addition, we used it to calculate the mean and standard deviation (SD).
2.4. Data Description
Different studies employ varying methods to categorize the same food groups. Therefore, to understand the relationship between the pattern of consumption of each group and OSCC compared to the control group, we analyzed individual consumption within each study and frequency of consumption. This approach enabled us to determine the frequency of consumption for each food analyzed in both the OSCC and the control group without OSCC. Then, we calculated average frequencies to discern the eating patterns by food group between patients with OSCC and the control group. Finally, we conducted individual assessments of the eating patterns of OSCC patients to identify the most and least frequently consumed food types.
Fruit consumption was categorized into various subgroups, including fruit, fresh fruit, citrus fruits, oranges, apples, bananas, tomatoes, and all fruit. Leafy vegetables were categorized as green vegetables, yellow vegetables, cruciferous vegetables, lettuce, group A greens (green leaves), group B greens (others), and group C greens (roots and tubers). Red meat, chicken, and fish were categorized into red meat, chicken, fish, fresh meat, salted meat, and barbecue. Dairy and dairy products were categorized into dairy products and milk. Cold cuts were categorized as bacon and sausages. The results regarding the frequency of culinary preparations revealed that most OSCC patients consume fried and spicy/peppery foods, and hot or very hot beverages. The beverages assessed included infusions and teas categorized into various types, including general tea, coffee, mate tea, green tea, and black tea.
3. Results
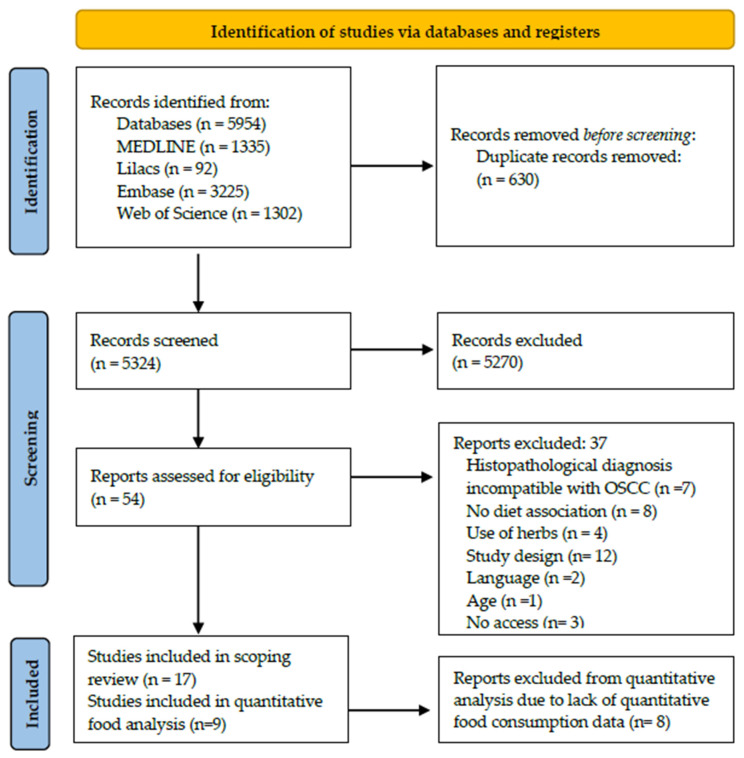
Our initial search retrieved 5954 studies in MEDLINE (via PubMed), Embase, Web of Science, and Lilacs. After excluding 630 duplicates, 5324 titles and abstracts were screened. Full-text articles for the remaining 54 records were retrieved, of which 37 were excluded according to the eligibility criteria described in Table 2 and Table 3. No records were included through hand-searching. Thus, 17 studies were included in the scoping review, 8 of which were excluded from the quantitative consumption analysis due to a lack of data (Figure 1).
Table 2.
General characteristics of the studies analyzed.
| Food Group | Participants in Total (n) | Participants with OSCC (n) | Studies Analyzed | Mean Frequency of OSCC (%) | Mean Frequency of Control Group (%) |
|---|---|---|---|---|---|
| Fruits | 5227 | 2256 | 5 | 46.88 | 53.13 |
| Vegetables | 592 | 296 | 1 | 52.06 | 47.94 |
| Leafy Vegetables | 4132 | 1616 | 5 | 47.83 | 52.17 |
| Red Meat, Chicken, and Fish | 2567 | 1198 | 2 | 48.66 | 51.34 |
| Dairy Products | 924 | 433 | 2 | 45.98 | 54.02 |
| Infusions | 1701 | 3698 | 4 | 30.30 | 69.70 |
| Cold Cuts | 1061 | 542 | 2 | 53.30 | 46.61 |
| Fried Preparations | 427 | 296 | 1 | 50.84 | 49.16 |
| Spicy Preparations | 592 | 187 | 1 | 41.34 | 41.34 |
| Beverages—Temperature | 427 | 187 | 1 | 41.79 | 58.21 |
Legend: OSCC—oral squamous cell carcinoma.
Table 3.
Qualitative and quantitative description of food and beverage consumption frequencies in OSCC patients.
| Food Group | Lowest Frequency Reports of Food Consumption in OSCC Patients | Highest Frequency Reports of Food Consumption in OSCC Patients | ||||
|---|---|---|---|---|---|---|
| Type of Food | Quantitative Consumption | Frequency (%) | Type of Food | Quantitative Consumption | Frequency (%) | |
| Fruits | Fruits | Three or more times a week | 13.74% | Fruits | Less than three times a week | 34.46% |
| Fresh Fruit | One or more times a week | 20.00% | Fresh Fruit | Never | 46.88% | |
| Citrus Fruits | One or more times a week | 30.15% | Citrus Fruits | Less than once a week | 55.70% | |
| Orange | Two or more times a day | 30.77% | Orange | Less than once a week | 57.58% | |
| Apple | Two or more times a day | 35.09% | Apple | Less than once a week | 57.52% | |
| Banana | Two or more times a day | 36.25% | Banana | Less than once a week | 58.51% | |
| All fruits | Two or more times a day | 37.58% | All fruits | Less than once a week | 61.15% | |
| Tomato | Two or more times a day | 41.52% | Tomato | Less than once a week | 100.00% | |
| Vegetables | Carrot | Two or more times a day | 33.33% | Carrot | Less than once a day | 56.20% |
| Leafy Vegetables | Green Vegetables | Once a day or more | 18.94% | Green Vegetables | Less than once a week | 56.70% |
| Yellow Vegetables | One or more times a week | 35.86% | Yellow Vegetables | Less than once a week | 63.41% | |
| Cruciferous Vegetables | One or more times a week | 37.01% | Cruciferous Vegetables | Less than once a week | 56.85% | |
| Vegetable Group C (Roots and Tubers) | Three or more times a week | 37.50% | Vegetable Group C (Roots and Tubers) | Less than three times a week | 54.87% | |
| Lettuce | Two or more times a day | 41.41% | Lettuce | Less than once a week | 63.75% | |
| Vegetable Group A (Green Leaves) | Three or more times a week | 45.71% | Group A Vegetables (Green Leaves) | Less than three times a week | 58.33% | |
| Vegetables | One or more times a week | 45.76% | Vegetables | Never | 62.50% | |
| Vegetable Group B (Others) | Never | 50.00% | Vegetable Group B (Other) | One or more times a day | 50.89% | |
| Red Meat, Poultry, and Fish | Red Meat | Never | 35.97% | Red Meat | One or more times a week | 58.22% |
| Chicken | Less than once a week | 37.06% | Chicken | Never | 55.77% | |
| Fish | Less than once a week | 38.22% | Fish | One or more times a week | 52.38% | |
| Fresh Meat | Less than once a day | 41.36% | Fresh Meat | Once a day or more | 53.14% | |
| Salted Meat | Never | 46.05% | Salted Meat | Once a week or more | 75.00% | |
| Barbecue | Less than once a month | 47.33% | Barbecue | Three or more times a month | 50.00% | |
| Dairy Products | Dairy Products | Never | 25.00% | Dairy Products | One or more times a week | 47.37% |
| Milk | Two or more times a day | 45.04% | Milk | Less than once a day | 54.50% | |
| Cold Cuts | Bacon | Never | 30.19% | Bacon | Two or more times a day | 68.32% |
| Sausages | Never | 46.67% | Sausages | Two or more times a month | 38.71% | |
| Fried Preparations | Fried Food | Never | 30.87% | Fried Food | Two or more times a week | 80.77% |
| Spicy Preparations | Spicy and Peppery Foods | Less than once a week | 33.56% | Spicy and Peppery Foods | One or more times a week | 49.11% |
| Beverages—Temperature | Hot | Two or more times a day | 31.21% | Very Hot | Three or more times a week | 52.36% |
| Infusions and teas | Coffee | Two or more times a day | 14.35% | Coffee | Three or more times a day | 29.92% |
| Mate Tea | Never | 36.00% | Mate Tea | Once a day or more | 55.63% | |
| Green Tea | Two or more times a day | 42.98% | Green Tea | Less than once a day | 50.60% | |
| Black Tea | One or more times a day | 44.87% | Black Tea | Less than once a day | 46.69% | |
Figure 1.
Flowchart for the selection of studies, 2024.
3.1. Study Characterization
Regarding design, 14/17 papers were case–control studies (82%), two were cross-sectional studies (11%), and one was a cohort study (5%). The studies were conducted in South America (n = 7 studies; 41%), Asia (n = 6 studies; 35%), North America, and Europe (with 2 studies each; 11%). Regarding the Human Development Index (HDI), only high (n = 14 studies; 82%) and medium (n = 3 studies; 17%) HDI countries were analyzed, with the highest-ranking country being the United Kingdom (15th position) and the lowest being India (134th position).
3.2. Sample Description
The seventeen included studies described a total of 10,954 patients, of whom 8175 (74.63%) were male and 2059 (18.79%) female. Two studies did not identify the sex of their participants. The age ranged from 18 to 89 years (50.62 ± 3.0). Tumor location was only reported by five studies. The tongue was the most common anatomic location (n = 182 patients; 4.43%; 36.40 ± 31.0), followed by floor of the mouth (n = 27 patients; 0.65%; 6.75 ± 4.7) and the hard palate (n = 8 patients; 0.19%; 2.67 ± 1.15).
The average follow-up time was 221.44 ± 152.12 weeks, as described in 16 studies. We categorized the studies into three groups, studies with a high follow-up time (≥105 weeks), studies with an intermediate follow-up time (53–104 weeks), and studies with a low follow-up time (≤52 weeks). Eleven studies (68.8%) presented a high follow-up time, two studies (12.5%) had an intermediate follow-up, and three (18.7%) studies had a low period of follow-up.
Schooling was categorized according to the length of study in years, with the highest proportion of study participants having completed between 0 and 5 years (n = 4342 individuals; 65.49%), considered to be low schooling, followed by medium schooling, with 6 to 12 years of study (n = 1283 individuals; 19.35%) and, finally, completion of more than 13 years (n = 1005 individuals; 15.15%), equivalent to high schooling. Other socio-demographic information such as income, social vulnerability index, occupation, and marital status, were not described in the studies evaluated.
3.3. Quantitative and Qualitative Eating Patterns Description
Regarding the type of dietary survey, ten studies (58.8%) used only the Food Frequency Questionnaire (FFQ), three studies used interviews and pre-structured questionnaires, another three studies used both questionnaires and self-reporting (17.6%, respectively), and only one study (5.9%) approached patients with the FFQ plus a photographic atlas for further instruction.
From each food group analyzed, we obtained a categorization of the overall average and specifically for each type of food, depending on the frequency of food consumption. This makes a description of each of the groups necessary. The general characteristics of the studies analyzed and the individual frequency of food consumption for the OSCC group are described in Table 2 and Table 3, respectively.
3.4. Fruits and Vegetables
Consumption of fruit three or more times a week was correlated with a lower frequency of OSCC. Conversely, the absence of fresh fruit consumption was correlated with a higher frequency of OSCC. In the vegetable food group, only carrots were evaluated. A minority of OSCC patients reported carrot consumption of two or more times a day (33.33%), whereas the majority reported consuming carrots less than once a day (56.20%).
3.5. Leafy Vegetables
The frequency of consumption of all these leafy vegetables was low for the majority of OSCC patients, who reported consuming them less than once or three times a week.
3.6. Red Meat, Chicken, and Fish
Interestingly, 36% of OSCC participants reported never consuming red meat, while 58% ate red meat one or more times a week. Accordingly, consumption of salted meat once a week or more was reported by 75% of participants, while 46% never consumed it. In general, the habit of never consuming red meat, or consuming it less than once a day, week, or month, was less frequently reported by OSCC individuals. Therefore, in the population of individuals with OSCC, a high frequency of red meat consumption was observed, with the sole exception of chicken, for which lack of consumption was predominant.
3.7. Dairy Products
Among OSCC patients, 45% reported consuming milk two or more times a day, while 54.5% consumed it less than once a day. Moreover, only 25% of participants indicated never consuming dairy products, while 47% consumed them one or more times a week.
3.8. Cold Cuts
Approximately 70% of patients with OSCC consume bacon two or more times a day, whereas 30% abstain from this food entirely. Conversely, the frequency of sausage consumption was more evenly distributed, with 46% of patients reporting no consumption and 39% consuming it two or more times a month.
3.9. Culinary Preparations and Beverages
Notably, 80.7% of the surveyed OSCC patients reported eating fried foods two or more times a week, while 30.8% indicated that they never consume it. Furthermore, 49% of participants with OSCC reported consuming spicy/peppery foods one or more times a week, and 33% stated that they consume it less than once a week. Finally, 52% of OSCC patients consume very hot beverages three or more times a week, and 31% consume hot foods two or more times a day.
Mate tea was the most frequently consumed beverage among OSCC participants, with 55.6% reporting that they drank it once a day or more, in contrast to 36% who did not consume it. Green and black tea frequency was evenly distributed. Finally, nearly 44% of OSCC participants stated consuming coffee three or more (29.9%) or two or more (14.3%) times a day.
4. Discussion
The World Cancer Research Fund International and the American Institute for Cancer Research state that eating fruits and vegetables is associated with a lower risk of various types of cancer, including oral cancer [20,21]. This can be explained by the properties of several bioactive compounds found in these foods, such as lycopene, resveratrol, flavonoids, isothiocyanates, and other minerals like magnesium and folate. These compounds have antitumor, antioxidant, and antiproliferative properties that enhance the immune system. They can also influence cellular mechanisms related to cell cycle regulation, DNA repair, and reactive oxygen species elimination [14].
Our research findings synthesize and illustrate the eating patterns adopted by OSCC patients. It was observed that fruits and vegetables were less frequently consumed than red meat, dairy products, cold cuts, fried or spicy foods, and hot or very hot beverages among OSCC patients.
The increased consumption of meat, especially salted and red meat, may lead to excessive iron storage or oxidative stress resulting from the free radicals related to red meat digestion [20,22]. In addition to the digestive metabolic alterations, red meat carcinogens can also be generated or increased according to the preparation/preservation method adopted. Interestingly, our results revealed a high frequency of cold cut, particularly bacon, and fried food consumption among OSCC patients. The relationship between the frequency of cancer in general and dairy products is still not well understood in the literature. However, it is known that dairy products are rich in calcium, vitamin D, and conjugated linoleic acid, which can influence cell specificity and differentiation, providing anti-cancer effects [23]. On the other hand, their high levels of fats and potential contaminants can make them pro-carcinogenic [24]. Therefore, it is important to consider that, like most foods, excessive consumption of dairy products does not provide benefits, while inadequate consumption fails to capture their important and nutritious properties [23]. While the frequency of milk consumption was evenly distributed among the OSCC patients included in our review, 47% consume other dairy products one or more times a week, and 25% indicated never consuming dairy products.
Daily coffee consumption may be beneficial due to its variety of biologically active compounds, known as antioxidants, and its ability to modulate apoptotic response and reverse cell cycle checkpoint function [25]. However, despite some studies, such as those cited in the systematic review by Li et al., 2016 [26], suggesting that high coffee consumption reduces the risk of oral cancer, it is noteworthy that any excesses are not safe. In our review, nearly 44% of OSCC participants stated consuming coffee three or more (29.9%) or two or more (14.3%) times a day. Similarly, with coffee, the literature supports the protective effect of tea against OSCC through the action of its antioxidant compounds, such as polyphenols and catechins, which act as scavengers of reactive oxygen species and can influence transcription factors and enzymatic activities [27]. We retrieved the OSCC patients’ consumption of green, black, and mate tea. Although green and black tea were evenly consumed by this population, 55.6% of the sample reported a daily consumption of one or more portions of mate tea, while 36% stated that they did not consume it [27].
The higher consumption of spicy and hot foods, as well as very hot temperatures, has been linked to a greater frequency of OSCC [28,29]. These subgroups should be considered based on their ability to cause mechanical and chemical sensitivity of the local mucosa. An interesting finding of our work was that 80.7% of the OSCC patients surveyed reported consuming fried food at least twice a week, while 30.8% stated they never eat fried food. We also found that 52% of OSCC patients consume very hot beverages three or more times a week, and 31% consume hot beverages two or more times a day. A controversial finding is that capsaicin, a compound found in peppers and spicy foods, may act as a cancer suppressant through its antioxidant and anti-inflammatory effects; in addition, it is one of the groups of crops that receives the largest amount of pesticides. Given its benefits, consuming spicy foods may lead to a lower risk of OSCC [30]. However, paradoxically, the low consumption of spicy food by the OSCC population may be associated with the symptoms of the disease [28,29]. In our review, 49% of the OSCC participants reported eating spicy or peppery food at least once a week, while 33% mentioned consuming it less than once a week.
Validity, Limitations, and Prospects
The main limitation of this scoping review is the high heterogeneity among the included studies. To mitigate this, we categorized the results based on the type of foods and frequency of consumption for a descriptive analysis. While this approach enabled us to overcome this limitation for the current data, it is imperative to interpret our results with caution. We strongly advocate for further efforts to develop a standardized nutritional assessment tool for future observational studies that will help in elucidating the relationship between diet and OSCC risk.
The assessment of eating patterns represents a challenging task due to several errors in the application and interpretation of the different dietary survey protocols. Despite the absence of a gold-standard protocol, the Food Frequency Questionnaire (FFQ) is a low-cost and minimally participant-dependent survey method. Therefore, this approach has been used in various epidemiological and dietary investigative studies. Some other surveys include 24 h dietary recall (R24H) and self-reporting. However, these methods tend to omit foods/groups and rely solely on memory bias, being affected by patients’ memory issues due to aging or misunderstandings caused by educational limitations [31,32]. Since old age and low levels of schooling were predominant among our included population, this might affect the reliability of our results.
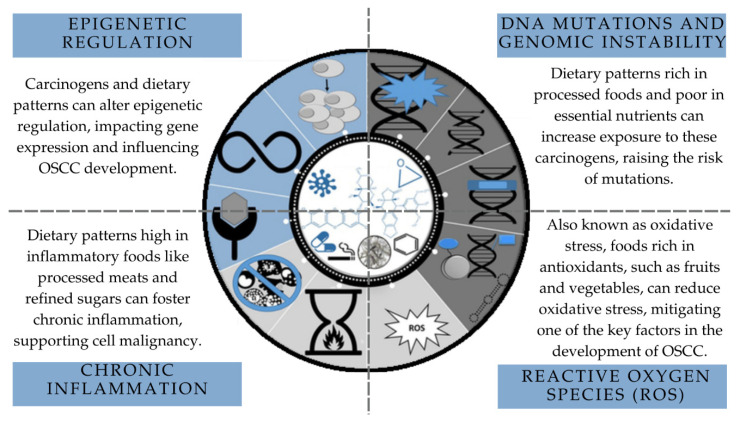
Investigating whether a specific dietary pattern can cause and sustain molecular alterations until the development of OSCC is challenging due to the complexity of molecular events associated with oral carcinogenesis (Figure 2) [33]. To understand how diet influences the genetic and epigenetic mechanisms associated with oral cancer, it would be necessary to select a sample of individuals whose confounding variables could be controlled so that molecular assays could be carried out. Furthermore, the standardization of the methods used to collect data on dietary patterns is fundamental for a more consistent characterization of the dietary profile of individuals affected by OSCC.
Figure 2.
Mechanistic view of cancer modulation and associations with dietary patterns. Source: adapted from Guyton et al., 2018, Chemical Research in Toxicology [33].
In addition, the lack of data in the studies may have introduced some confounding factors, such as not describing whether the vegetables are canned or fresh, whether they are organic or grown with pesticides, and how they are prepared (for example, if they are fried). There was also a lack of control for risk factors already well established in the literature that can influence diet, such as the use of areca nut derivatives, chronic smoking, and alcohol consumption.
5. Conclusions
Our results comprehensively synthesize the nutritional landscape of OSCC. We found a high frequency of red meat and cold cut consumption and a low frequency of fruit, vegetable, and leafy vegetable consumption among OSCC patients. Although this eating pattern has been described as oncogenic for other malignant neoplasms, oral carcinogenesis is a multifactorial process. Therefore, further studies are needed to confirm the carcinogenic potential of dietary habits for OSCC initiation and development. It is also noteworthy that most foods are influenced by the quantity of consumption and not just by their quality. However, due to the heterogeneity of the tools used to obtain food frequency data, the results should be interpreted with caution. Standardized studies are essential to advance understanding in this field.
The development of preventive randomized clinical trials or prospective cohort studies on this subject is crucial to control for confounding factors related to this hypothesis. In addition, it is essential to understand and control risk factors and confounding variables, adjusting them appropriately for this specific population, given the lack of robust data that provide reliable and clear information to the scientific community and professionals, from prevention to diagnosis.
Abbreviations
| OSCC | Oral squamous cell carcinoma |
| HDI | Human Development Index |
| FFQ | Food Frequency Questionnaire |
| PRISMA-ScR | Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews |
| JBI | Joanna Briggs Institute |
| MeSH | Medical Subject Headings |
| DeCS | Descritores em Ciências da Saúde |
| Emtree | Embase Subject Headings |
| CAPES | 24 h dietary recall (R24H); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior |
| PDPG | Postgraduate Development Program |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph21091199/s1, Table S1: Indexers used to select publications; Table S2: Full-text excluded articles and reasons. References [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] are cited in the Supplementary Materials.
Author Contributions
R.R.M.-C. and N.S.G. developed the study’s concept and planned it. M.G.R., R.R.M.-C. and N.S.G. conducted data verification and analysis. M.G.R. and R.R.M.-C. wrote the first paragraph of the manuscript. M.G.R., R.R.M.-C., N.S.G., L.C.L. and A.B.A.M.D.A.S., contributed to the interpretation and investigation of the data. R.R.M.-C. and N.S.G., reviewing and editing the manuscript and oversaw the research process. All of the authors had complete access to all of the study’s data and were ultimately responsible for the decision to submit them for publication. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study used data available on public websites and electronic data banks. The Brazilian government gained access to the Embase platform (via the CAPES website).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
Supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Funding Code 001. In addition, we thank CAPES for providing the scholarship of the Postgraduate Development Program (PDPG), linked to the Faculty of Medical Sciences of Minas Gerais for author MGR.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Howard A., Agrawal N., Gooi Z. Lip and Oral Cavity Squamous Cell Carcinoma. Hematol. Oncol. Clin. N. Am. 2021;35:895–911. doi: 10.1016/j.hoc.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Cancer Today. [(accessed on 9 April 2024)]. Available online: https://gco.iarc.fr/en.
- 3.Dholam K., Chouksey G. Squamous Cell Carcinoma of the Oral Cavity and Oropharynx in Patients Aged 18–45 Years: A Case–Control Study to Evaluate the Risk Factors with Emphasis on Stress, Diet, Oral Hygiene, and Family History. Indian J. Cancer. 2016;53:244–251. doi: 10.4103/0019-509X.197725. [DOI] [PubMed] [Google Scholar]
- 4.Don J., Secchi D.G., Galíndez M.F., Aballay L.R., Pasqualini M.E., Brunotto M. The Association among TP53 Rs1042522, Pri-MiR 34b/c Rs4938723 Polymorphisms and Daily Dietary Fatty Acids in Patients with Premalignant and Malignant Oral Lesions. Hum. Gene. 2022;34:201082. doi: 10.1016/j.humgen.2022.201082. [DOI] [Google Scholar]
- 5.Johnson D.E., Burtness B., Leemans C.R., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and Neck Squamous Cell Carcinoma. Nat. Reviews. Dis. Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-Moles M.Á., Ramos-García P. An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis. Cancers. 2024;16:608. doi: 10.3390/cancers16030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan Q., Zhang C., Hua H., Hu X. Compositional and Functional Changes in the Salivary Microbiota Related to Oral Leukoplakia and Oral Squamous Cell Carcinoma: A Case Control Study. BMC Oral. Health. 2023;23:1021. doi: 10.1186/s12903-023-03760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukmana B.I., Saleh R.O., Najim M.A., AL-Ghamdi H.S., Achmad H., Al-Hamdani M.M., Taher A.A.Y., Alsalamy A., Khaledi M., Javadi K. Oral Microbiota and Oral Squamous Cell Carcinoma: A Review of Their Relation and Carcinogenic Mechanisms. Front. Oncol. 2024;14:1319777. doi: 10.3389/fonc.2024.1319777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piemonte E.D., Lazos J.P., Belardinelli P., del Castillo G.V., Talavera A.D., Secchi D.G., Lanfranchi Tizeira H.E., Brunotto M.N. Efecto de La Acumulación de Factores de Riesgo En El Riesgo de Carcinoma de Células Escamosas Bucal. Rev. Fac. Cienc. Médicas Córdoba. 2021;78:158–165. doi: 10.31053/1853.0605.v78.n2.31247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A.A., Kheur S., Varadarajan S., Parveen S., Dewan H., Alhazmi Y.A., Raj T.A., Testarelli L., Patil S. Chronic Mechanical Irritation and Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Bosn. J. Basic Med. Sci. 2021;21:647–658. doi: 10.17305/bjbms.2021.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos J.C., dos Santos E.S., Normando A.G.C., Alves F.A., Kowalski L.P., Santos-Silva A.R., Vargas P.A., Lopes M.A. Oral Squamous Cell Carcinoma around Dental Implants: A Systematic Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021;131:660–674. doi: 10.1016/j.oooo.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Key T.J., Bradbury K.E., Perez-Cornago A., Sinha R., Tsilidis K.K., Tsugane S. Diet, Nutrition, and Cancer Risk: What Do We Know and What Is the Way Forward? BMJ. 2020;368:m511. doi: 10.1136/bmj.m511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-Molinero J., del Migueláñez-Medrán B.C., Puente-Gutiérrez C., Delgado-Somolinos E., Martín Carreras-Presas C., Fernández-Farhall J., López-Sánchez A.F. Association between Oral Cancer and Diet: An Update. Nutrients. 2021;13:1299. doi: 10.3390/nu13041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scully C., Bedi R. Ethnicity and Oral Cancer. Lancet Oncol. 2000;1:37–42. doi: 10.1016/S1470-2045(00)00008-5. [DOI] [PubMed] [Google Scholar]
- 15.Heller M.A., Nyirjesy S.C., Balsiger R., Talbot N., VanKoevering K.K., Haring C.T., Old M.O., Kang S.Y., Seim N.B. Modifiable Risk Factors for Oral Cavity Cancer in Non-Smokers: A Systematic Review and Meta-Analysis. Oral Oncol. 2023;137:106300. doi: 10.1016/j.oraloncology.2022.106300. [DOI] [PubMed] [Google Scholar]
- 16.Joanna Briggs Institute (JBI) Scoping Reviews Resources|Joanna Briggs Institute. [(accessed on 20 March 2024)]. Available online: https://jbi.global/scoping-review-network/resources.
- 17.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 18.Open Science Framework (OSF) Diet and Oral Squamous Cell Carcinoma: A Scoping Review. [(accessed on 1 March 2024)]. Available online: https://osf.io/f4ndm/
- 19.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Cancer Research Fund International Interactive Cancer Risk Matrix. [(accessed on 20 March 2024)]. Available online: https://www.wcrf.org/
- 21.American Institute for Cancer Research Cancer Prevention and Healthy Eating. [(accessed on 9 April 2024)]. Available online: https://www.aicr.org/cancer-prevention/healthy-eating.
- 22.Chuang S.-C., Jenab M., Heck J.E., Bosetti C., Talamini R., Matsuo K., Castellsague X., Franceschi S., Herrero R., Winn D.M., et al. Diet and the Risk of Head and Neck Cancer: A Pooled Analysis in the INHANCE Consortium. Cancer Causes Control. 2011;23:69–88. doi: 10.1007/s10552-011-9857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arafat H.M., Omar J., Shafii N., Naser I.A., Al A., Muhamad R., Damitri A., Shaqaliah A.J., Shamallakh O.M., Shamallakh K.M., et al. The Association between Breast Cancer and Consumption of Dairy Products: A Systematic Review. Ann. Med. 2023;55:2198256. doi: 10.1080/07853890.2023.2198256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y., Tao Q., Zhou F., Si Y., Fu R., Xu B., Xu J., Li X., Chen B. The Relationship between Dairy Products Intake and Breast Cancer Incidence: A Meta-Analysis of Observational Studies. BMC Cancer. 2021;21:1–12. doi: 10.1186/s12885-021-08854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tverdal A., Hjellvik V., Selmer R. Coffee Intake and Oral–Oesophageal Cancer: Follow-up of 389 624 Norwegian Men and Women 40–45 Years. Br. J. Cancer. 2011;105:157–161. doi: 10.1038/bjc.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y.-M., Peng J., Li L.-Z. Coffee Consumption Associated with Reduced Risk of Oral Cancer: A Meta-Analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016;121:381–389.e1. doi: 10.1016/j.oooo.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Kim T.S., Jeong G.W., Yang J.H., Lee K.H., Kronbichler A., van Vliet H., Grosso G., Galvano F., Aune D., Kim J.M., et al. Tea Consumption and Risk of Cancer: An Umbrella Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2020;11:1437–1452. doi: 10.1093/advances/nmaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosqueda-Solís A., Lafuente-Ibáñez De Mendoza I., Aguirre-Urizar J., Mosqueda-Taylor A. Capsaicin Intake and Oral Carcinogenesis: A Systematic Review. Med. Oral Patol. Oral E Cir. Bucal. 2021;26:261–269. doi: 10.4317/medoral.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawicki C.M., Janal M.N., Nicholson S.J., Wu A.K., Schmidt B.L., Albertson D.G. Oral Cancer Patients Experience Mechanical and Chemical Sensitivity at the Site of the Cancer. BMC Cancer. 2022;22:1–10. doi: 10.1186/s12885-022-10282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapa-Oliver A.M., Mejía-Teniente L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules. 2016;21:931. doi: 10.3390/molecules21080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Ballart J.D., Piñol J.L., Zazpe I., Corella D., Carrasco P., Toledo E., Perez-Bauer M., Martínez-González M.Á., Salas-Salvadó J., Martín-Moreno J.M. Relative Validity of a Semi-Quantitative Food-Frequency Questionnaire in an Elderly Mediterranean Population of Spain. Br. J. Nutr. 2010;103:1808–1816. doi: 10.1017/S0007114509993837. [DOI] [PubMed] [Google Scholar]
- 32.De Keyzer W., Dekkers A., Van Vlaslaer V., Ottevaere C., Van Oyen H., De Henauw S., Huybrechts I. Relative Validity of a Short Qualitative Food Frequency Questionnaire for Use in Food Consumption Surveys. Eur. J. Public Health. 2012;23:737–742. doi: 10.1093/eurpub/cks096. [DOI] [PubMed] [Google Scholar]
- 33.Guyton K.Z., Rieswijk L., Wang A., Chiu W.A., Smith M.T. Key Characteristics Approach to Carcinogenic Hazard Identification. Chem. Res. Toxicol. 2018;31:1290–1292. doi: 10.1021/acs.chemrestox.8b00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Wyk C.W., Stander I., Padayachee A., Grobler-Rabie A.F. The Areca Nut Chewing Habit and Oral Squamous Cell Carcinoma in South African Indians. A Retrospective Study. S. Afr. Med. J. 1993;83:425–429. [PubMed] [Google Scholar]
- 35.Sundermann B.V., Uhlmann L., Hoffmann J., Freier K., Thiele O.C. The Localization and Risk Factors of Squamous Cell Carcinoma in the Oral Cavity: A Retrospective Study of 1501 Cases. J. Cranio-Maxillofac. Surg. 2018;46:177–182. doi: 10.1016/j.jcms.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Llewellyn C.D., Linklater K., Bell J., Johnson N.W., Warnakulasuriya S. An Analysis of Risk Factors for Oral Cancer in Young People: A Case-Control Study. Oral Oncol. 2004;40:304–313. doi: 10.1016/j.oraloncology.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Lawal A., Kolude B., Adeyemi B., Lawoyin J., Akang E. Social Profile and Habits of Oral Cancer Patients in Ibadan. Afr. J. Med. Med. Sci. 2011;40:247–251. [PubMed] [Google Scholar]
- 38.Kune G.A., Kune S., Field B., Watson L.F., Cleland H., Merenstein D., Vitetta L. Oral and Pharyngeal Cancer, Diet, Smoking, Alcohol, and Serum Vitamin A and Β-Carotene Levels: A Case-Control Study in Men. Nutr. Cancer. 1993;20:61–70. doi: 10.1080/01635589309514271. [DOI] [PubMed] [Google Scholar]
- 39.Nagle C.M., Wilson L.F., Hughes M.C.B., Ibiebele T.I., Miura K., Bain C.J., Whiteman D.C., Webb P.M. Cancers in Australia in 2010 Attributable to Inadequate Consumption of Fruit, Non-Starchy Vegetables and Dietary Fibre. Aust. N. Z. J. Public Health. 2015;39:422–428. doi: 10.1111/1753-6405.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petti S. Lifestyle Risk Factors for Oral Cancer. Oral Oncol. 2009;45:340–350. doi: 10.1016/j.oraloncology.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Stucken E., Weissman J., Spiegel J.H. Oral Cavity Risk Factors: Experts’ Opinions and Literature Support. J. Otolaryngol. Head Neck Surg. 2010;39:76–89. [PubMed] [Google Scholar]
- 42.Winn D.M. Diet and Nutrition in the Etiology of Oral Cancer. Am. J. Clin. Nutr. 1995;61:437S445S. doi: 10.1093/ajcn/61.2.437S. [DOI] [PubMed] [Google Scholar]
- 43.Amtha R., Zain R., Razak I.A., Basuki B., Roeslan B.O., Gautama W., Purwanto D.J. Dietary Patterns and Risk of Oral Cancer: A Factor Analysis Study of a Population in Jakarta, Indonesia. Oral Oncol. 2009;45:e49–e53. doi: 10.1016/j.oraloncology.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Stefani E.D., Boffetta P., Ronco A.L., Correa P., Oreggia F., Deneo-Pellegrini H., Mendilaharsu M., Leiva J.C. Dietary Patterns and Risk of Cancer of the Oral Cavity and Pharynx in Uruguay. Nutr. Cancer. 2005;51:132–139. doi: 10.1207/s15327914nc5102_2. [DOI] [PubMed] [Google Scholar]
- 45.Keshani F., Razavi S., Askari G., Zahiri Z., Heidari Z. A Comparative Analysis of Dominant Dietary Patterns in Patients with and without Oral Squamous Cell Carcinoma. Adv. Biomed. Res. 2023;12:4. doi: 10.4103/abr.abr_120_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunotto M., Secchi D.G., Aballay L.R., Shivappa N., Hebert J.R., Galíndez Costa M.F. The Inflammatory Potential of Argentinian Diet and Oral Squamous Cell Carcinoma. Nutr. Hosp. 2019 doi: 10.20960/nh.02613. [DOI] [PubMed] [Google Scholar]
- 47.Vieytes C.A.M., Rozek L.S., Wolf G.T., Arthur A.E. Associations between Diet Quality and Proinflammatory Cytokines in Newly Diagnosed Head and Neck Cancer Survivors. Curr. Dev. Nutr. 2023;7:102015. doi: 10.1016/j.cdnut.2023.102015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren J.S., Freedman N.D., Kamangar F., Dawsey S.M., Hollenbeck A.R., Schatzkin A., Abnet C.C. Tea, Coffee, Carbonated Soft Drinks and Upper Gastrointestinal Tract Cancer Risk in a Large United States Prospective Cohort Study. Eur. J. Cancer. 2010;46:1873–1881. doi: 10.1016/j.ejca.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harada K., Fujiwara R., Hisano T., Takenawa T., Mishima K. SUN-PO110: Basic Investigation on the Mechanisms of Action of Elemental Diet Elental® in Oral Cancer Treatment. Clin. Nutr. 2019;38:S100. doi: 10.1016/S0261-5614(19)32744-X. [DOI] [Google Scholar]
- 50.Dwyer J.T., Efstathion A., Palmer C., Papas A. Nutritional Support in Treatment of Oral Carcinomas. Nutr. Rev. 1991;49:332–337. [PubMed] [Google Scholar]
- 51.Abe A., Hayashi H., Ishihama T., Furuta H. Prognostic Impact of the Prognostic Nutritional Index in Cases of Resected Oral Squamous Cell Carcinoma: A Retrospective Study. BMC Oral Health. 2021;21 doi: 10.1186/s12903-021-01394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amin N., Biswas S.L., Ahmed M. Study of Squamous Cell Carcinoma in a Tertiary Level Hospital in Bangladesh. Int. J. Oral Maxillofac. Surg. 2015;44:e27. doi: 10.1016/j.ijom.2015.08.444. [DOI] [Google Scholar]
- 53.Das A., Gheena N.S., Kumar J.R. Age and Gender Predilection of Habits and Oral Cancer among an Outpatient Population Visiting a Dental Hospital. Int. J. Res. Pharm. Sci. 2020;11:1850–1854. doi: 10.26452/ijrps.v11ispl3.3547. [DOI] [Google Scholar]
- 54.Ayub M., Fayyaz M., Arshad S., Khan B.R., Bano U., Khalil F. Oral Cancer Prevalence & Finding of Alarming Consequences at Oncology Ward of Public Health Care Sector. Int. Res. J. Pharm. 2015;6:623–626. doi: 10.7897/2230-8407.069121. [DOI] [Google Scholar]
- 55.Bansal M., Gupta T.K. Dietary Risk Factors in Upper Aero-Digestive Tract Cancers. Indian J. Otolaryngol. Head Neck Surg. 2022;74:6356–6361. doi: 10.1007/s12070-022-03093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bell E.B., Reis I.M., Cohen E.R., Almuhaimid T., Smith D.H., Alotaibi F., Gordon C., Gomez-Fernandez C., Goodwin W.J., Franzmann E.J. Green Salad Intake Is Associated with Improved Oral Cancer Survival and Lower Soluble CD44 Levels. Nutrients. 2021;13:372. doi: 10.3390/nu13020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boeing H., Dietrich T., Hoffmann K., Pischon T., Ferrari P., Lahmann P.H., Boutron-Ruault M.C., Clavel-Chapelon F., Allen N., Key T., et al. Intake of Fruits and Vegetables and Risk of Cancer of the Upper Aero-Digestive Tract: The Prospective EPIC-Study. Cancer Causes Control. 2006;17:957–969. doi: 10.1007/s10552-006-0036-4. [DOI] [PubMed] [Google Scholar]
- 58.Bravi F., Bosetti C., Filomeno M., Levi F., Garavello W., Galimberti S., Negri E., La Vecchia C. Foods, Nutrients and the Risk of Oral and Pharyngeal Cancer. Br. J. Cancer. 2013;109:2904–2910. doi: 10.1038/bjc.2013.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casto B.C., Knobloch T.J., Galioto R.L., Yu Z., Accurso B.T., Warner B.M. Chemoprevention of Oral Cancer by Lyophilized Strawberries. Anticancer Res. 2013;33:4757–4766. [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J., Qiu Y., Cai L., Liu F., Chen F., Yan L., Wu J., Bao X., Liu F., Zheng X., et al. [Pickled Food, Fish, Seafood Intakes and Oral Squamous Cell Carcinoma: A Case-Control Study] Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51:680–685. doi: 10.3760/cma.j.issn.0253-9624.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Chatelain K., Phippen S., McCabe J., Teeters C.A., O’Malley S., Kingsley K. Cranberry and Grape Seed Extracts Inhibit the Proliferative Phenotype of Oral Squamous Cell Carcinomas. Evid.-Based Complement. Altern. Med. 2011;2011:1–12. doi: 10.1093/ecam/nen047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan L., Chen F., Liu D., Huang J., Liu F., Wu J., Liu F., Ye J., Qiu Y., Lin L., et al. [Tea, Coffee Intakes and Risk of Oral Squamous Cell Carcinoma: A Case-Control Study] Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37:1531–1535. doi: 10.3760/cma.j.issn.0254-6450.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 63.Kaokaen P., Jaiboonma A., Chaicharoenaudomrung N., Kunhorm P., Janebodin K., Noisa P., Jitprasertwong P. Cordycepin-Loaded Nanoparticles from Cassava Starch Promote the Proliferation of Submandibular Gland Cells and Inhibit the Growth of Oral Squamous Carcinoma Cells. Nutr. Cancer. 2020;73:2014–2029. doi: 10.1080/01635581.2020.1819350. [DOI] [PubMed] [Google Scholar]
- 64.Hassabou N.F., Farag A.F. Anticancer Effects Induced by Artichoke Extract in Oral Squamous Carcinoma Cell Lines. J. Egypt. Natl. Cancer Inst. 2020;32 doi: 10.1186/s43046-020-00026-4. [DOI] [PubMed] [Google Scholar]
- 65.Jagtap S.V., Tele J. Clinicohistopathological Profile of Malignant and Pre-Malignant Lesions of Oral Cavity. Int. J. Res. Pharm. Sci. 2020;11:5729–5734. doi: 10.26452/ijrps.v11i4.3217. [DOI] [Google Scholar]
- 66.Kansara S., Wang T., Koochakzadeh S., Liou N.E., Graboyes E.M., Skoner J.M., Hornig J.D., Sandulache V.C., Day T.A., Huang A.T. Prognostic Factors Associated with Achieving Total Oral Diet Following Osteocutaneous Microvascular Free Tissue Transfer Reconstruction of the Oral Cavity. Oral Oncol. 2019;98:1–7. doi: 10.1016/j.oraloncology.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kato K., Long N.K., Makita H., Toida M., Yamashita T., Hatakeyama D., Hara A., Mori H., Shibata T. Effects of Green Tea Polyphenol on Methylation Status of RECK Gene and Cancer Cell Invasion in Oral Squamous Cell Carcinoma Cells. Br. J. Cancer. 2008;99:647–654. doi: 10.1038/sj.bjc.6604521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kapila A.R., Rawal Y., Renner R.J., Schwartz S.J., Tian Q., Larsen P.E., Mallery S.R. Suppression of the Tumorigenic Phenotype in Human Oral Squamous Cell Carcinoma Cells by an Ethanol Extract Derived from Freeze-Dried Black Raspberries. Nutr. Cancer. 2006;54:58–68. doi: 10.1207/s15327914nc5401_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao C.-T., Chang J.T.-C., Wang H.-M., Ng S.-H., Hsueh C., Lee L.-Y., Lin C.-H., Chen I.-H., Huang S.-F., Cheng A.-J., et al. Analysis of Risk Factors of Predictive Local Tumor Control in Oral Cavity Cancer. Ann. Surg. Oncol. 2007;15:915–922. doi: 10.1245/s10434-007-9761-5. [DOI] [PubMed] [Google Scholar]
- 70.Liu S.-A., Tsai W.-C., Wong Y.-K., Lin J.-C., Poon C.-K., Chao S.-Y., Hsiao Y.-L., Chan M.-Y., Cheng C.-S., Wang C.-C., et al. Nutritional Factors and Survival of Patients with Oral Cancer. Head Neck. 2006;28:998–1007. doi: 10.1002/hed.20461. [DOI] [PubMed] [Google Scholar]
- 71.Trachootham D., Chingsuwanrote P., Yoosadiang P., Mekkriangkrai D., Ratchawong T., Buraphacheep N., Kijanukul S., Saekhow S., Pongpitchayadej O., Vongvachvasin K., et al. Partial Substitution of Glucose with Xylitol Suppressed the Glycolysis and Selectively Inhibited the Proliferation of Oral Cancer Cells. Nutr. Cancer. 2017;69:862–872. doi: 10.1080/01635581.2017.1339097. [DOI] [PubMed] [Google Scholar]
- 72.Turati F., Galeone C., La Vecchia C., Garavello W., Tavani A. Coffee and Cancers of the Upper Digestive and Respiratory Tracts: Meta-Analyses of Observational Studies. Ann. Oncol. 2011;22:536–544. doi: 10.1093/annonc/mdq603. [DOI] [PubMed] [Google Scholar]
- 73.Yang M., Luo Q., Chen X., Chen F. Bitter Melon Derived Extracellular Vesicles Enhance the Therapeutic Effects and Reduce the Drug Resistance of 5-Fluorouracil on Oral Squamous Cell Carcinoma. J. Nanobiotechnol. 2021;19 doi: 10.1186/s12951-021-00995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anwar N., Pervez S., Chundriger Q., Awan S., Moatter T., Ali T.S. Oral Cancer: Clinicopathological Features and Associated Risk Factors in a High Risk Population Presenting to a Major Tertiary Care Center in Pakistan. PLOS ONE. 2020;15:e0236359. doi: 10.1371/journal.pone.0236359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stefani E.D., Oreggia F., Boffetta P., Deneo-Pellegrini H., Ronco A., Mendilaharsu M. Tomatoes, Tomato-Rich Foods, Lycopene and Cancer of the Upper Aerodigestive Tract: A Case-Control in Uruguay. Oral Oncol. 2000;36:47–53. doi: 10.1016/S1368-8375(99)00050-0. [DOI] [PubMed] [Google Scholar]
- 76.Shirataki Y., Kawase M., Saito S., Kurihara T., Tanaka W., Satoh K., Sakagami H., Motohashi N. Selective Cytotoxic Activity of Grape Peel and Seed Extracts against Oral Tumor Cell Lines. Anticancer Res. 2000;20:423–426. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study used data available on public websites and electronic data banks. The Brazilian government gained access to the Embase platform (via the CAPES website).