Abstract
Pyrroloquinoline quinone (PQQ) is one of the important coenzymes in living organisms. In acetic acid bacteria (AAB), it plays a crucial role in the alcohol respiratory chain, as a coenzyme of alcohol dehydrogenase (ADH). In this work, the PQQ biosynthetic genes were overexpressed in Acetobacter pasteurianus CGMCC 3089 to improve the fermentation performance. The result shows that the intracellular and extracellular PQQ contents in the recombinant strain A. pasteurianus (pBBR1-p264-pqq) were 152.53% and 141.08% higher than those of the control A. pasteurianus (pBBR1-p264), respectively. The catalytic activity of ADH and aldehyde dehydrogenase increased by 52.92% and 67.04%, respectively. The results indicated that the energy charge and intracellular ATP were also improved in the recombinant strain. The acetic acid fermentation was carried out using a 5 L self-aspirating fermenter, and the acetic acid production rate of the recombinant strain was 23.20% higher compared with the control. Furthermore, the relationship between the PQQ and acetic acid tolerance of cells was analyzed. The biomass of recombinant strain was 180.2%, 44.3%, and 38.6% higher than those of control under 2%, 3%, and 4% acetic acid stress, respectively. After being treated with 6% acetic acid for 40 min, the survival rate of the recombinant strain was increased by 76.20% compared with the control. Those results demonstrated that overexpression of PQQ biosynthetic genes increased the content of PQQ, therefore improving the acetic acid fermentation and the cell tolerance against acetic acid by improving the alcohol respiratory chain and energy metabolism.
One Sentence Summary
The increase in PQQ content enhances the activity of the alcohol respiratory chain of Acetobacter pasteurianus, and the increase in energy charge enhances the tolerance of cells against acetic acid, therefore, improving the efficiency of acetic acid fermentation.
Keywords: Acetobacter pasteurianus, Acetic acid fermentation, pyrroloquinoline quinone, Acetic acid tolerance, Energy charge
Graphical Abstract
Graphical Abstract.

Introduction
Acetic acid bacteria (AAB) are renowned for their exceptional ethanol oxidation capabilities and high acetic acid tolerance (Wang et al., 2015; Zheng et al., 2017). The process involves pyrroloquinoline quinone (PQQ)-dependent alcohol dehydrogenase (ADH), which catalyzes the oxidation of ethanol to aldehyde, and then aldehyde dehydrogenase (ALDH) oxidizes aldehyde into acetic acid, which is all located at the cell membrane (Miah et al., 2021). PQQ serves as the catalytic site of ADH, facilitating the oxidation of ethanol (Matsushita et al., 2008). During this process, the enzyme removes electrons, which are subsequently through the prosthetic group PQQ into the electron transport chain; this chain ultimately utilizes these electrons in the reduction of oxygen, generating water, and releasing energy as a byproduct (Chinnawirotpisan et al., 2003; Gomez-Manzo et al., 2015; Gullo & Giudici, 2008; Yakushi & Matsushita, 2010). The alcohol respiratory chain uses ethanol to produce ATP and acetic acid. These ATP provide energy for bacterial growth, which is beneficial for increasing bacterial stress resistance (Anthony, 1996). Strains of Acetobacter and Gluconobacter are widely used in acetic acid fermentation, which can tolerate acetic acid even up to 60 g L−1 (Azuma et al., 2009; Prust et al., 2005). The acetic acid fermentation and tolerance of Acetobacter and Gluconobacter are highly positively correlated with the efficiency of ADH and ALDH activity (Krusong et al., 2015; Nakano & Fukaya, 2008). Wu et al. (2017) study showed that enhanced expression of ADH subunits, particularly AdhA and AdhB, show increased acetic acid production. And Gao et al. (2021) study showed that constructing two modules, ethanol oxidation pathway and PQQ biosynthesis pathway, improves acetic acid production and strain tolerance. Therefore, PQQ is related to the production of acetic acid and strain tolerance.
PQQ has been identified as another important cofactor after NADH and NADPH (Misra et al., 2012), which can improve the adaptability of certain microorganisms to extreme environments such as radiation, high acidity, and high temperatures (Gao et al., 2021). And the production of PQQ is more prevalent in Gram-negative than Gram-positive bacteria, with numerous studies highlighting the ability of PQQ (Mi et al., 2020; Shen et al., 2012). The Pqq operon (Pqq A-E) encodes the enzymes necessary for PQQ biosynthesis. In Gluconobacter, the PQQ synthases are encoded by the pqqABCDE gene cluster (Holscher & Gorisch, 2006; Liu et al., 2020). The synthesis process initiates with the glutamic acid and tyrosine residues in pqqA, which form the intermediate compound acid hydroquinoline (Trcek et al., 2006). The intermediate product is transformed into PQQ by interacting with pqqC and pqqD proteins, during which pqqB is responsible for the transport of PQQ to the periplasmic space (Chinnawirotpisan et al., 2003). Lastly, pqqE catalyzes the formation of carbon-carbon bonds in the precursor peptide pqqA (Zhu et al., 2018).
The energy charge (EC) of bacterial cells serves as an indicator of metabolic demand during growth and product synthesis (Ano et al., 2008; Tan et al., 2015; Xia et al., 2016). And during the acetic acid fermentation, AAB utilize glucose and ethanol in the environment to produce energy for growth and metabolism. One mole glucose releases approximately 2870 kJ Gibbs free energy, whereas 1 mol ethanol to acetic acid releases 493 kJ/mol Gibbs free energy (Zheng, Chang et al., 2018). Energy changes during acetic acid fermentation are visualized by these indicators. Additionally, energy is intricately linked to the tolerance against acidity stress (Croes et al., 1981). Song et al. (2022) have analyzed the energy and substance metabolism of AAB during the acetic acid fermentation process, indicating that the conversion of ethanol to acetic acid is the main source for energy generation. And PQQ is essential in the process of ethanol conversion to acetic acid. Nevertheless, the relationship between PQQ and energy metabolism is less studied.
It has been proven that the high catalytic activity and acid tolerance of PQQ-ADH contribute to the growth and metabolic activity of AAB under acidic conditions (Masud et al., 2010). And PQQ serves as the catalytic site of ADH, thereby it plays an important role in acetic acid tolerance and production of acetic acid. However, the specific role of PQQ in acetic acid tolerance and production of acetic acid, particularly its connection to energy metabolism through the alcohol respiratory chain, remains unclear. Therefore, this study investigated the impact of PQQ on acetic acid fermentation by overexpressing the PQQ biosynthetic genes in Acetobacter pasteurianus, with the aim of elucidating the relationship between the alcohol respiratory chain and energy metabolism.
Materials and Methods
Strains, Plasmids, and Primers
A. pasteurianus CGMCC 3089 is registered in the Chinese General Microbiological Culture Collection Center (Zheng et al., 2015). Escherichia coli JM109 was used for the construction of recombinant vectors. Plasmid pBBR1-p264 that was kindly provided by Professor Uwe Deppenmeier (University of Bonn, Bonn, Germany) was used to overexpress PQQ biosynthetic genes in A. pasteurianus. Recombinant strain of E. coli BL21 (pET-28a-gcd) overexpressing PQQ-dependent glucose dehydrogenase was used to determine the concentration of PQQ in A. pasteurianus, which was donated as a gift by Professor Long Liu (Jiangnan University, China). Primers were used in this research, and are listed in Tables S1 and S2.
Media and Culture Conditions
E. coli was grown on Luria–Bertani broth at 37°C and 200 r min−1. GY medium (3% glucose, 1.5% yeast extract) and GY plate (3% glucose, 1.5% yeast extract, and 1.7% agar) were used for DNA manipulation of A. pasteurianus. GYA medium (3% glucose, 1.5% yeast extract, and an appropriate amount of acetic acid) was used for analyzing the acetic acid tolerance of A. pasteurianus. GYE medium (3% glucose, 1.5% yeast extract, and 3.5% ethanol) was used as the seed medium of A. pasteurianus. The acetic acid fermentation was performed with GPAE medium (2% glucose, 2% peptone, 1% acetic acid, and 8% ethanol).
For acetic acid fermentation, cells were incubated with 100 mL of seed medium in 500 mL Erlenmeyer flasks, and cultured at 30°C and 180 r min−1. When the optical density (OD) at 610 nm was approximately 1.2, the cells were transferred into fermentation medium with 10% inoculum. Acetic acid fermentation was performed in a 5 L self-aspirating fermenter (Nanjing Huike Bioengineering Equipment Corporation, Nanjing, China) containing 3.5 L of GPAE medium at 30°C, and the aeration was of 0.15 vvm (volume air per volume media per minute).
Construction of Strain Overexpressing PQQ Biosynthetic Genes
The gene cluster of pqqABCDE was amplified using A. pasteurianus CGMCC 3089 genomic DNA as the template using primers pqq F/R as listed in Table S1. Plasmid pBBR1-p264 was used for constructing the vector pBBR1-p264-pqq containing the pqqABCDE gene cluster. The recombinant plasmid was transferred into A. pasteurianus CGMCC 3089 by electroporation.
Acetic Acid Tolerance Analysis
Strains of A. pasteurianus (pBBR1-p264) and A. pasteurianus (pBBR1-p264-pqq) were cultured in Erlenmeyer flasks with GY medium under initial acetic acid concentrations of 0%, 1%, 2%, 3%, and 4% (v/v) at 30°C and 180 r min−1. Then, OD at 610 nm was compared after 48 hr cultivation.
To test the tolerance of the strains toward higher acetic acid stresses, shock experiments were performed according to the previously reported method (Zheng, Wang et al., 2018).
Assay of Genes Transcription
For quantitative real-time PCR (RT-PCR) experiments, the strains cultured in GPAE medium were collected when the OD reached about 0.6. Total RNA was isolated using RNA Plus Kit (Takara Biotechnology, Dalian, China) following the manufacturer's procedure. RNA samples were reverse-transcribed with RevertAid™ First Strand cDNA Synthesis Kit (Takara Biotechnology, Dalian, China) according to the manufacturer's instructions. Subsequently, the quantitative gene analysis was performed on an ABI Step-One Plus Real-Time PCR System (StepOnePlus, Applied Biosystems, Foster city, CA, USA) using the primers listed in Table S1. Results were expressed by 2−△△Ct with the 16S rRNA as the internal standard gene.
For each gene, the sample of A. pasteurianus (pBBR1-p264) was defined as the expression level of 1.0, and results were expressed as the fold increase of mRNA over the control samples.
Analytical Methods
The cell growth was monitored based on the OD value by a spectrophotometer (UVmini-1240, Shimadzu, Kyoto, Japan) at 610 nm. The acidity of the fermentation broth was titrated by 0.1 M NaOH using phenolphthalein as an indicator. The concentrations of ethanol and glucose in the broth were measured using a biosensor (SBA-40C, Shandong Academy of Sciences, Jinan, China). Before the measurement, the instrument was standardized with a standard solution containing 0.5 g/L ethanol and 1 g/L glucose, and then the concentrations of ethanol and glucose in the sample were measured.
The average acid production rate (Racid) equation was as follows:
 |
The specific production rate (SR) of acetic acid equation was as follows:
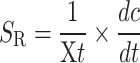 |
where SR is the specific rate of acetic acid production. dc/dt presents the rate of acetic acid production, ethanol consumption, or glucose consumption. Xt is the biomass at time t.
The average equivalent yields (AEY) equation was as follows:
 |
Activities of ADH and ALDH were measured colorimetrically at 660 nm (UVmini-1240, Shimadzu) using potassium ferricyanide as an electron acceptor following the previously reported method (Ameyama & Adachi, 1982; Wu et al., 2017) with minor modification. Cells were harvested at the mid-logarithmic growth phase, then washed twice with 10 mM potassium phosphate buffer (pH 6.0). The suspension was then broken by a sonication (SB-5200DTD, Ningbo Scientz Biotechnology Co., Ltd. Ningbo, China) in an ice bath for 3 s at 200 W, followed by a 5 s pause (90 cycles). Fractured fluid was centrifuged at 10 000 ×g for 30 min at 4°C to yield the crude enzyme. One unit of enzyme activity is defined as the amount of enzyme that catalyzes the consumption of 1 μmol of the substrate per minute. The enzyme activity is expressed as units per mg of crude enzyme.
The intracellular ATP/ADP/AMP concentrations were determined using the Microorganism ATP/ADP/AMP ELISA Kit (Ruishuo Biotechnology, Shanghai, China) according to the manufacturer's instructions. The EC value of the cell was calculated according to the formula: [EC = (ATP + ½ADP)/(ATP + ADP + AMP). Besides, the content of PQQ was detected according to the reported method using strain of E. coli BL21 (pET-28a-gcd) (Si et al., 2016).
All experiments were performed in triplicate. The results were expressed as mean values with standard deviation. Using SPSS 20.0 (SPSS Inc., Chicago, IL) to analyze differences between categories, calculated with a 95% confidence interval.
Results
The Effect of Overexpressing PQQ Biosynthetic Genes on PQQ Content and Activities of ADH and ALDH
The recombinant plasmid pBBR1-p264-pqq was constructed and transferred into A. pasteurianus CGMCC 3089. The transcription of pqq gene cluster was detected by using RT-PCR, when the strains were cultured in GPAE medium to 0.6 OD (24 hr of acetic acid fermentation). The transcription of the pqq gene cluster is 37.92 times in A. pasteurianus (pBBR1-p264-pqq) than that of control. The intracellular and extracellular PQQ contents of the recombinant strain are 152.53% and 141.08% higher than the control, respectively (Fig. 1A). The results indicated that overexpression of the pqq gene cluster led to enhanced PQQ synthases. Furthermore, the catalytic activities of ADH and ALDH of the recombinant strain are 52.92% and 67.04% higher than the control, respectively (Fig. 1B). It can be speculated that the activity of PQQ-ADH was improved due to the enhanced PQQ content, which helps with the electron transfer, resulting the improved acetaldehyde formation. And Chinnawirotpisan et al. (2003) also have proved that ALDH is induced by acetaldehyde. Therefore, overexpression of the pqq gene cluster increased ADH activity, therefore producing more acetaldehyde. The acetaldehyde will help in enhancing ALDH activity. The results indicated that the overexpression of PQQ biosynthetic genes led to increased PQQ concentrations, and subsequently increased the activity of key enzymes (ADH and ALDH) in the alcohol respiratory chain.
Fig. 1.
Effects of overexpressing pqq gene clusters on pyrroloquinoline quinone (PQQ) formation and enzymes catalytic activity. (A) The intracellular and extracellular PQQ contents. (B) Catalytic activities of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). Harvesting cells during the logarithmic growth phase to detect ADH and ALDH enzyme activity.
The Effect of Enhanced PQQ on Acetic Acid Fermentation and EC
The ability of acetic acid fermentation by AAB is closely related to its energy metabolism (Li et al., 2024). Therefore, the improved
catalytic activity of the alcohol respiratory chain might affect the energy metabolism and fermentation efficiency of A. pasteurianus. A 5 L self-priming fermentor was used to study the effect of over expression PQQ biosynthetic genes on acetic acid fermentation and energy metabolism.
As shown in Fig. 2A, after 9 hr of fermentation, the growth and acid production of the recombinant strain are significantly higher than those of the control, leading to the shorter fermentation period. The acid production rate of the recombinant strain is 1.67 g/(L·hr) with an increase of 23.20% than the control of 1.36 g/(L·hr). As shown in Table 1, the average equivalent yield of the recombinant strain is 0.016 mol/100 mL/hr which is 23.08% higher than that of the control. As described in Fig. 2A, both the highest concentration of acetic acid and the fermentation efficiency are improved due to the overexpression of the pqq gene cluster. In the early and middle stages of fermentation, the specific acid production rate of the recombinant strain is higher than that of the control (Fig. 2D). At 9 hr of fermentation, the specific acid production rate of the recombinant strain is 0.78 g/100 mL/hr/OD that is 13.02 times of the control of 0.06 g/100 mL/hr/OD. And as listed in Table 2, at 6–9 hr, the Gibbs free energy generated by oxidizing ethanol in the recombinant strain is 3.636 kJ/100 mL/hr, while the control is 0.311 kJ/100 mL/hr. The sufficient energy in the recombinant strain allowed the bacterial cells to produce more acetic acid, which confirmed that the higher specific acid production rate of the recombinant strain than that of the control after 9 hr of fermentation. This improvement in acetic acid production rate was attributed to the increase in catalytic activity in the alcohol respiratory chain, accelerating the rate of ethanol oxidation (Fig. 2C). However, in the later stage of fermentation, ethanol was gradually consumed, the acid production rate of the recombinant strain decreased and was lower than that of the control. This result confirmed that the increase in catalytic activity of the alcohol respiratory chain improved the fermentation performance of A. pasteurianus.
Fig. 2.
Effect of enhanced pyrroloquinoline quinone (PQQ) formation on acetic acid fermentation. (A) Time curves of acetic acid production and cell growth. (B) Time curves of catalytic activities of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). (C) Time curves of ethanol and glucose consumption. (D) The specific production rate of acetic acid. (E) Time curves of ATP and energy charge (EC).
Table 1.
The Average Equivalent Yield of Different Strains
| A. pasteurianus (pBBR1-p264) | A. pasteurianus (pBBR1-p264-pqq) | |
|---|---|---|
| Fermentation period (h) | 54 | 47 |
| Acetic acid generation (mol/100 mL) | 0.116 ± 0.005 | 0.123 ± 0.002 |
| Ethanol consumption (mol/100 mL) | 0.161 ± 0.006 | 0.163 ± 0.008 |
| Glucose consumption (mol/100 mL) | 0.002 ± 0.000 | 0.002 ± 0.000 |
| Average equivalent yield (mol/100 mL/h) | 0.013 ± 0.001 | 0.016 ± 0.001 |
Note. Each value in the table is expressed as mean ± S.D. (n = 3).
Table 2.
The Energy Production During Different Stages of Acetic Acid Fermentation
| Hglucose (kJ/100 mL/h) | HEthanol (kJ/100 mL/h) | |||
|---|---|---|---|---|
| Stage(h) | (pBBR1-p264) | (pBBR1-p264-pqq) | (pBBR1-p264) | (pBBR1-p264-pqq) |
| 0–3 | 0.527 ± 0.026 | 0.638 ± 0.040 | 0.439 ± 0.012 | 0.439 ± 0.022 |
| 3–6 | 0.432 ± 0.027 | 0.500 ± 0.030 | 0.134 ± 0.007 | 0.223 ± 0.011 |
| 6–9 | 0.115 ± 0.005 | 0.287 ± 0.014 | 0.311 ± 0.016 | 3.636 ± 0.200 |
| 9–21 | 0.078 ± 0.004 | 0.070 ± 0.004 | 0.890 ± 0.035 | 1.373 ± 0.060 |
| 21–33 | 0.146 ± 0.006 | 0.101 ± 0.006 | 0.825 ± 0.031 | 2.281 ± 0.150 |
| 33–45 | 0.039 ± 0.002 | 0.021 ± 0.001 | 2.235 ± 0.112 | 1.558 ± 0.070 |
| 45–47 | 0.059 ± 0.003 | 0.032 ± 0.002 | 4.143 ± 0.227 | 2.337 ± 0.117 |
| 47–54 | 0.007 ± 0.001 | 0.005 ± 0.001 | 3.145 ± 0.150 | 0.464 ± 0.030 |
Note. Each value in the table is expressed as mean ± S.D. (n = 3).
This study investigated the effect of enhancing PQQ content on the catalytic activities of ADH and ALDH. As shown in Fig. 2B, before 9 hr of fermentation, the ADH and ALDH activities of the recombinant strain are higher than those of the control, resulting in higher growth and acid production. And then, due to the adaptation to the fermentation environment, the expression and catalytic activity of ADH and ALDH increased, and the ethanol was quickly oxidized. However, after 20 hr when the acetic acid was more than about 3 g/100 mL, the catalytic activity of ADH began to decrease due to the acetic acid inhibition (Trcek et al., 2007), and then the ALDH (after 32 hr).
Besides, the ATP and EC during the fermentation process were detected. As listed in Fig. 2E, before 6 hr there is no significant difference in ATP and EC between the recombinant strain and the control. Ethanol consumption is slow, and glucose serves as the main source of production capacity for bacterial growth. At 9 hr, the early stage of logarithmic phase, the ATP in the recombinant strain and the control are 0.01 and 0.02 μmol/g DCW, respectively. And after 9 hr of fermentation, the main metabolic pathway for energy supply is the alcohol respiratory chain (Table 2). The activities of ADH and ALDH and the specific acid production rate of the recombinant strain are also higher than those of the control. At 21 and 33 hr, in the mid-logarithmic phase, the ATP and EC of the recombinant strain are also higher than those of the control. However, after 49 hr due to the almost complete utilization of alcohol (less than 5 g/L), the levels of ATP and EC become similar at 47 hr for both strains. Therefore, it can be speculated that the improved activity of the alcohol respiratory chain, which was due to the enhanced PQQ content, improved the acetic acid fermentation, and the increased ATP and EC of cells.
The Effect of Enhanced PQQ on Acetic Acid Tolerance
In this study, the increased biomass in acetic acid fermentation by overexpressing PQQ biosynthetic genes suggested that the tolerance of cells against acetic acid might be improved. Therefore, the effect of overexpressing PQQ biosynthetic genes on acetic acid tolerance was analyzed. As shown in Fig. 3A, there is no significant difference (p > .05) between the growth of two strains under low stress conditions of 0% and 1% acetic acid. The growth of the control strain is almost inhibited by the 2% acetic acid, whereas, the biomass of A. pasteurianus (pBBR1-p264-pqq) is 180.2%, 44.3%, and 38.6% higher than that of control under 2%, 3%, and 4% acetic acid stress, respectively. Furthermore, an acetic acid shock experiment was performed to determine the effect of enhanced PQQ on acetic acid tolerance. As shown in Fig. 3B, there is no difference between the two strains when treated with 2% acetic acid. However, the survival rate of A. pasteurianus (pBBR1-p264-pqq) is 30.70% and 76.20% higher than those of the control strain under 4% and 6% acetic acid conditions. Therefore, overexpression of PQQ biosynthetic genes enhanced acetic acid tolerance and improves cell growth under acetic acid stress conditions.
Fig. 3.
The effect of enhanced pyrroloquinoline quinone (PQQ) on acetic acid tolerance. (A) Cell growth. Cultivate the strain in GY medium with initial acetic acid concentrations of 0%, 1%, 2%, 3%, and 4% (v/v) for 48 hr. (B) Cell survival rate. The cells were treated with GYA media containing 2%, 4%, and 6% of acetic acid for 40 min.
The acetic acid tolerance is one of the most significant properties for AAB, which contributes to withstanding this stress with multiple mechanisms. These mechanisms include the TCA cycle, ATP-binding cassette transporter (ABC transporter), the alcohol respiratory chain, amino acid metabolism, cell morphology and membrane composition alterations, and acid induced protein (Song et al., 2022). In this work, the alcohol respiratory chain was enhanced by overexpressing PQQ biosynthetic genes (Fig. 1), thereby improving the acetic acid tolerance of A. pasteurianus. Besides, the intracellular EC and ATP were enhanced (Fig. 2E). The improvement of energy metabolism might be helpful for acetic acid tolerance by providing ATP for the ABC transporter.
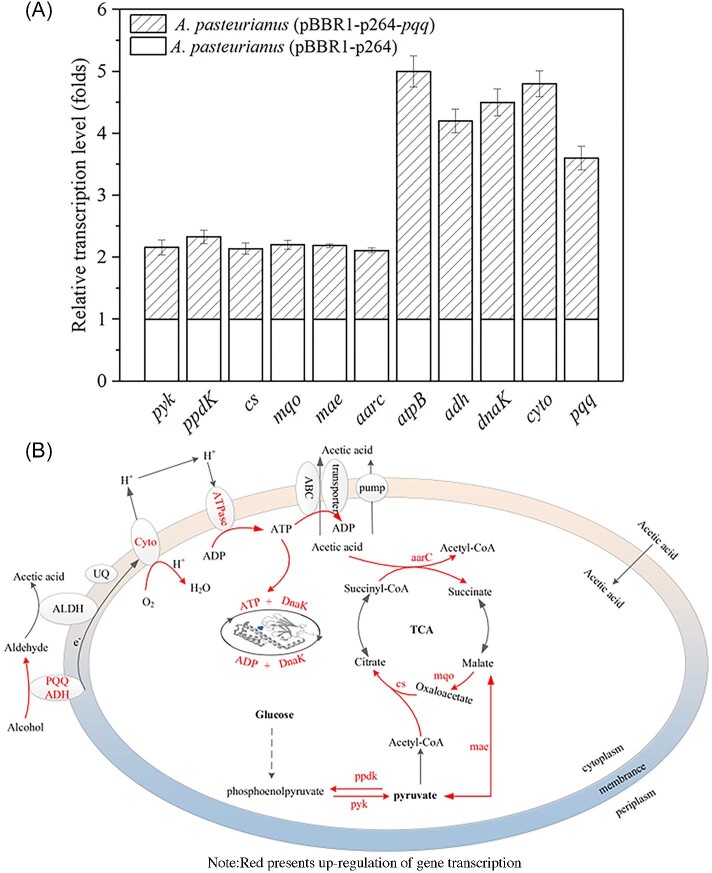
To reveal the effect of enhanced PQQ formation on the acetic acid tolerance, transcriptions of genes that have been proven responsible for energy metabolism and acetic acid tolerance were analyzed. Among them, ADH (encoding by adh), PQQ (pqq gene cluster), and cytochrome oxidase (cyto) are responsible for the alcohol respiratory chain. Pyruvate kinase (pyk) and pyruvate phosphate dikinase (ppyk) are related to the glycolysis pathway. Citrate synthase (cs), malate: quinone oxidoreductase (mqo), malic enzyme (mae), and acetyl-CoA hydrolase (aarC) are related to the TCA cycle. ATPase (atpB) is related to ATP production, and molecular chaperone DnaK (dnak) is related to acetic acid tolerance. As shown in Fig. 4A, in A. pasteurianus (pBBR1-p264-pqq) the relative transcript levels of genes atpB, adh, dnak, and cyto are 4.0, 3.2, 3.5, and 3.8 times of the control, respectively. Furthermore, genes related to glucose metabolism and TCA cycle transcription, including pyk, ppdk, cs, mqo, mae, and aarc are also improved, indicating that the glucose consumption of the recombinant strain was faster than that of the control (Fig. 2C). These results indicated that the enhanced PQQ formation improved the alcohol respiratory chain and the upregulation of genes related to glucose metabolism to increase the energy metabolism (as described in Fig. 4B). These results were agreed with the EC and Gibbs free energy in acetic acid fermentation, as shown in Fig. 2E and Table 2.
Fig. 4.
The regulation of genes transcription by overexpressing pyrroloquinoline quinone (PQQ). (A) Transcription levels of genes related to acetic acid tolerance and energy metabolism. The cells were collected when the OD reached about 0.6; (B) Schematic diagram of metabolic network.
Discussion
PQQ-ADH and ALDH are strictly related to the alcohol respiratory chain, and are responsible for the AAB tolerance against acetic acid. It has been reported that enhancing ADH activity by overexpressing the adhA gene can improve acetic acid tolerance (Wu et al., 2017). PQQ, as a new important cofactor was discovered after the discovery of NADH and NADPH. In this work, overexpression of PQQ biosynthetic genes improved the acetic acid fermentation, the acidity tolerance, and energy metabolism of cells. Therefore, manipulating cofactors to coordinate product and energy demands is an effective strategy to increase target product production (Wang et al., 2017). Fine-tuning the balance between the alcohol respiratory chain and PQQ regeneration can alleviate the contradiction between suitability and acetic acid production, and enable A. pasteurianus cells to grow in a high-concentration acid environment and acetic acid fermentation (Gao et al., 2021). In this study, overexpression of the pqqABCDE gene cluster in A. pasteurianus significantly increased the intracellular and extracellular PQQ content, proving that pqqABCDE gene cluster encodes PQQ (Holscher & Gorisch, 2006). PQQ plays an important role for the catalytic activity of ADH, facilitating the oxidation of ethanol (Matsushita et al., 2008). Furthermore, Gao et al. (2021) also has proved that cofactor PQQ levels are positively correlated to PQQ-ADH activity and overexpression of the pqq gene cluster is found to directly improve the catalytic activity of PQQ-ADH. Therefore, enhancing PQQ is an effective strategy to improve acetic acid fermentation.
AAB oxidize alcohol to form acetic acid while producing energy which is important for cell growth especially in high-acidity environments (Song et al., 2022). In this study, the transcription of genes confirmed to be related to ethanol oxidation and acetic acid tolerance of AAB was detected. It was found that the enhanced PQQ formation not only improved the alcohol respiratory chain, but also improved energy metabolism through glucose metabolism and TCA cycle, as shown in Fig. 4B. Additionally, the intracellular EC and ATP were improved by enhancing PQQ formation, which can reflect the energy demand of A. pasteurianus during acetic acid fermentation. In acetic acid fermentation, ATP is consumed by ATP binding cassette transporter which is an important mechanism to endow AAB with an acetic acid tolerance. The TCA cycle cannot provide enough energy in a high acetic acid environment, and the main energy-supplying metabolic pathway is the alcohol respiratory chain (Qi et al., 2013). High ATP production rate makes the strain highly acid tolerant and also enables the strain to have a high acetic acid production rate (Song et al., 2022). As described in Figs. 2 and 3, the EC and ATP concentrations of PQQ enhanced strains were higher than those of the control, and the tolerance of PQQ enhanced strains was also higher than that of the control. This result confirmed that high concentrations of EC and ATP could provide energy for the strain to consume ATP binding cassette transporters, improving better acetic acid tolerance than the control. In this work, the transcription levels of genes related to alcohol respiratory chain and ATP production were significantly increased, which improved the acetic acid fermentation ability and energy metabolism of the recombinant strain. This also confirms the close correlation between alcohol respiratory chain and energy metabolism (Li et al., 2024). Dnak, as a stress protein, is beneficial for the correct folding of proteins, allowing microorganisms to survive under environmental stress (Zheng et al., 2015). In this work, due to the increase of acetic acid production, the transcription level of molecular chaperone Dnak also greatly increased, improving the acetic acid tolerance of the strain. Of course, this is only one aspect of increasing tolerance, and the increase in energy metabolism also provides sufficient energy for the energy consumption of ATP binding cassette transporters, allowing acetic acid to transfer outside the membrane and alleviate the stress of acetic acid (Song et al., 2022). In conclusion, the increased ADH activity and energy were responsible for the improved acetic acid tolerance of recombinant strain.
In summary, the enhanced PQQ formation improved the alcohol respiratory chain, TCA cycle, and ATPase which are all related to the energy metabolism during acetic acid fermentation, and these might be the main reasons for the improved specific production rate of acetic acid and average equivalent yields (Fig. 2D, Table 1). Therefore, overexpression the PQQ biosynthetic genes in Acetobacter pasteurianus improved the alcohol respiratory chain, resulting in more energy production from the alcohol respiratory chain. These energies could improve the tolerance of cells against acetic acid and the efficiency of acetic acid fermentation. In the future, we will expand our research on the other species of Acetobacter to further evaluate the effects of PQQ enhancement on energy metabolism and product formation.
Supplementary Material
Acknowledgments
We thank Professor Uwe Deppenmeier (University of Bonn, Bonn Germany) for the generous donation of plasmid pBBR1-p264. We thank Professor Long Liu (Jiangnan University, China) for the generous donation Recombinant strain of E. coli BL21 (pET-28a-gcd) overexpressing PQQ-dependent glucose dehydrogenase is used to determine the concentration of PQQ in A. pasteurianus.
Contributor Information
Wenqing Zhang, State Key Laboratory of Food Nutrition and Safety, Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China.
Chen Feng, State Key Laboratory of Food Nutrition and Safety, Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China.
Chunxue Zhang, State Key Laboratory of Food Nutrition and Safety, Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China.
Jia Song, State Key Laboratory of Food Nutrition and Safety, Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China.
Li Li, College of Biotechnology Engineering, Sichuan University of Science and Engineering, Yibin 644000, China.
Menglei Xia, State Key Laboratory of Food Nutrition and Safety, Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China.
Wei Ding, Shanxi Province Key Laboratory of Vinegar Fermentation Science and Engineering, Shanxi Zilin Vinegar Industry Co., Ltd., Taiyuan 030400, China.
Yu Zheng, State Key Laboratory of Food Nutrition and Safety, Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China; Shanxi Province Key Laboratory of Vinegar Fermentation Science and Engineering, Shanxi Zilin Vinegar Industry Co., Ltd., Taiyuan 030400, China; Haihe Laboratory of Synthetic Biology, Tianjin 300308, China.
Min Wang, State Key Laboratory of Food Nutrition and Safety, Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China.
Author contributions
W. Z. performed the experiments and substantially contributed to the acquisition, analysis, and interpretation of data. C. F. and C. Z. were involved in the experiments. J. S., L. L., M. X., and W. D. were involved in discussed. Y. Z. and M. W. designed the study and were involved in drafting and revising. All authors have read and approved the manuscript.
Funding
This work was supported by the Key Research and Development Projects of Shanxi Province (202202140601018), Innovation Fund of Haihe Laboratory of Synthetic Biology (22HHSWSS00013), Shanxi Provincial Department of Science and Technology (202204010931002), Key Research and Development Program of Ningxia (2022BBF02010), and the Taishan Industrial Experts Program.
Conflict of Interest
The authors declare no competing interests.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
- Ameyama M., Adachi O. (1982). Alcohol dehydrogenase from acetic acid bacteria, membrane-bound. Methods in Enzymology, 89, 450–457. 10.1016/S0076-6879(82)89078-26214691 [DOI] [Google Scholar]
- Ano Y., Toyama H., Adachi O., Matsushita K. (2008). Energy metabolism of a unique acetic acid bacterium, Asaia bogorensis, that lacks ethanol oxidation activity. Bioscience, Biotechnology, and Biochemistry, 72(4), 989–997. 10.1271/bbb.70740 [DOI] [PubMed] [Google Scholar]
- Anthony C. (1996). Quinoprotein-catalysed reactions. Biochemical Journal, 320(3), 697–711. 10.1042/bj3200697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y., Hosoyama A., Matsutani M., Furuya N., Horikawa H., Harada T., Hirakawa H., Kuhara S., Matsushita K., Fujita N., Shirai M. (2009). Whole-genome analyses reveal genetic instability of Acetobacter pasteurianus. Nucleic Acids Research, 37(17), 5768–5783. 10.1093/nar/gkp612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnawirotpisan P., Theeragool G., Limtong S., Toyama H., Adachi O. O., Matsushita K. (2003). Quinoprotein alcohol dehydrogenase is involved in catabolic acetate production, while NAD-dependent alcohol dehydrogenase in ethanol assimilation in Acetobacter pasteurianus SKU1108. Journal of Bioscience and Bioengineering, 96(6), 564–571. 10.1016/S1389-1723(04)70150-4 [DOI] [PubMed] [Google Scholar]
- Croes A. F., Oord J. F. V. D., Tromp A. G. G. M. (1981). Changes in the adenylate energy charge and the induction of sporulation in Saccharomyces diastaticus. Archives of Microbiology, 129(1), 47–48. 10.1007/BF00417178 [DOI] [Google Scholar]
- Gao L., Wu X., Xia X., Jin Z. (2021). Fine-tuning ethanol oxidation pathway enzymes and cofactor PQQ coordinates the conflict between fitness and acetic acid production by Acetobacter pasteurianus. Microbial Biotechnology, 14(2), 643–655. 10.1111/1751-7915.13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Manzo S., Escamilla J. E., Gonzalez-Valdez A., Lopez-Velazquez G., Vanoye-Carlo A., Marcial-Quino J., de la Mora-de la Mora I., Garcia-Torres I., Enriquez-Flores S., Contreras-Zentella M. L., Arreguin-Espinosa R., Kroneck P. M., Sosa-Torres M. E. (2015). The oxidative fermentation of ethanol in Gluconacetobacter diazotrophicus is a two-step pathway catalyzed by a single enzyme: Alcohol-aldehyde dehydrogenase (ADHa). International Journal of Molecular Sciences, 16(1), 1293–1311. 10.3390/ijms16011293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullo M., Giudici P. (2008). Acetic acid bacteria in traditional balsamic vinegar: Phenotypic traits relevant for starter cultures selection. International Journal of Food Microbiology, 125(1), 46–53. 10.1016/j.ijfoodmicro.2007.11.076 [DOI] [PubMed] [Google Scholar]
- Holscher T., Gorisch H. (2006). Knockout and overexpression of pyrroloquinoline quinone biosynthetic genes in Gluconobacter oxydans 621H. Journal of Bacteriology, 188(21), 7668–7676. 10.1128/JB.01009-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusong W., Yaiyen S., Pornpukdeewatana S. (2015). Impact of high initial concentrations of acetic acid and ethanol on acetification rate in an internal Venturi injector bioreactor. Journal of Applied Microbiology, 118(3), 629–640. 10.1111/jam.12715 [DOI] [PubMed] [Google Scholar]
- Li Y.-N., Peng M.-Y., Lu Z.-M., Dong Y.-L., Chai L.-J., Shi J.-S., Zhang X.-J., Xu Z.-H. (2024). Lactiplantibacillus plantarum and komagataeibacter europaeus enhance energy metabolism, acetic acid and aromatic amino acids catabolism flux in cider vinegar fermentation. Lwt, 198, 115968. 10.1016/j.lwt.2024.115968 [DOI] [Google Scholar]
- Liu D., Ke X., Hu Z. C., Zheng Y. G. (2020). Combinational expression of D-sorbitol dehydrogenase and pyrroloquinoline quinone increases 6-(N-hydroxyethyl)-amino-6-deoxy-alpha-L-sorbofuranose production by gluconobacter oxydans through cofactor manipulation. Enzyme and Microbial Technology, 141, 109670. 10.1016/j.enzmictec.2020.109670 [DOI] [PubMed] [Google Scholar]
- Masud U., Matsushita K., Theeragool G. (2010). Cloning and functional analysis of adhS gene encoding quinoprotein alcohol dehydrogenase subunit III from Acetobacter pasteurianus SKU1108. International Journal of Food Microbiology, 138(1-2), 39–49. 10.1016/j.ijfoodmicro.2009.12.027 [DOI] [PubMed] [Google Scholar]
- Matsushita K., Kobayashi Y., Mizuguchi M., Toyama H., Adachi O., Sakamoto K., Miyoshi H. (2008). A tightly bound quinone functions in the ubiquinone reaction sites of quinoprotein alcohol dehydrogenase of an acetic acid bacterium, gluconobacter suboxydans. Bioscience, Biotechnology, and Biochemistry, 72(10), 2723–2731. 10.1271/bbb.80363 [DOI] [PubMed] [Google Scholar]
- Mi Z., Cheng J., Zhao P., Tian P., Tan T. (2020). Improved production of pyrroloquinoline quinone by simultaneous augmentation of its synthesis gene expression and glucose metabolism in Klebsiella pneumoniae. Current Microbiology, 77(7), 1174–1183. 10.1007/s00284-020-01918-3 [DOI] [PubMed] [Google Scholar]
- Miah R., Nina S., Murate T., Kataoka N., Matsutani M., Matsushita K., Yakushi T. (2021). Major aldehyde dehydrogenase AldFGH of gluconacetobacter diazotrophicus is independent of pyrroloquinoline quinone but dependent on molybdopterin for acetic acid fermentation. Applied Microbiology and Biotechnology, 105(6), 2341–2350. 10.1007/s00253-021-11144-x [DOI] [PubMed] [Google Scholar]
- Misra H. S., Rajpurohit Y. S., Khairnar N. P. (2012). Pyrroloquinoline-quinone and its versatile roles in biological processes. Journal of Biosciences, 37(2), 313–325. 10.1007/s12038-012-9195-5 [DOI] [PubMed] [Google Scholar]
- Nakano S., Fukaya M. (2008). Analysis of proteins responsive to acetic acid in Acetobacter: Molecular mechanisms conferring acetic acid resistance in acetic acid bacteria. International Journal of Food Microbiology, 125(1), 54–59. 10.1016/j.ijfoodmicro.2007.05.015 [DOI] [PubMed] [Google Scholar]
- Prust C., Hoffmeister M., Liesegang H., Wiezer A., Fricke W. F., Ehrenreich A., Gottschalk G., Deppenmeier U. (2005). Complete genome sequence of the acetic acid bacterium gluconobacter oxydans. Nature Biotechnology, 23(2), 195–200. 10.1038/nbt1062 [DOI] [PubMed] [Google Scholar]
- Qi Z., Wang W., Yang H., Xia X., Yu X. (2013). Mutation of acetobacter pasteurianus by UV irradiation under acidic stress for high-acidity vinegar fermentation. International Journal of Food Science & Technology, 49(2), 468–476. 10.1111/ijfs.12324 [DOI] [Google Scholar]
- Shen Y. Q., Bonnot F., Imsand E. M., RoseFigura J. M., Sjolander K., Klinman J. P. (2012). Distribution and properties of the genes encoding the biosynthesis of the bacterial cofactor, pyrroloquinoline quinone. Biochemistry, 51(11), 2265–2275. 10.1021/bi201763d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Z., Zhu J., Wang W., Huang L., Wei P., Cai J., Xu Z. (2016). Novel and efficient screening of PQQ high-yielding strains and subsequent cultivation optimization. Applied Microbiology and Biotechnology, 100(24), 10321–10330. 10.1007/s00253-016-7739-6 [DOI] [PubMed] [Google Scholar]
- Song J., Wang J., Wang X., Zhao H., Hu T., Feng Z., Lei Z., Li W., Zheng Y., Wang M. (2022). Improving the acetic acid fermentation of acetobacter pasteurianus by enhancing the energy metabolism. Frontiers in Bioengineering and Biotechnology, 10, 815614. 10.3389/fbioe.2022.815614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. M., Lee S. M., Dykes G. A. (2015). Acetic acid induces pH-independent cellular energy depletion in Salmonella enterica. Foodborne Pathogens and Disease, 12(3), 183–189. 10.1089/fpd.2014.1853 [DOI] [PubMed] [Google Scholar]
- Trcek J., Jernejc K., Matsushita K. (2007). The highly tolerant acetic acid bacterium gluconacetobacter europaeus adapts to the presence of acetic acid by changes in lipid composition, morphological properties and PQQ-dependent ADH expression. Extremophiles, 11(4), 627–635. 10.1007/s00792-007-0077-y [DOI] [PubMed] [Google Scholar]
- Trcek J., Toyama H., Czuba J., Misiewicz A., Matsushita K. (2006). Correlation between acetic acid resistance and characteristics of PQQ-dependent ADH in acetic acid bacteria. Applied Microbiology and Biotechnology, 70(3), 366–373. 10.1007/s00253-005-0073-z [DOI] [PubMed] [Google Scholar]
- Wang B., Shao Y., Chen F. (2015). Overview on mechanisms of acetic acid resistance in acetic acid bacteria. World Journal of Microbiology and Biotechnology, 31(2), 255–263. 10.1007/s11274-015-1799-0 [DOI] [PubMed] [Google Scholar]
- Wang M., Chen B., Fang Y., Tan T. (2017). Cofactor engineering for more efficient production of chemicals and biofuels. Biotechnology Advances, 35(8), 1032–1039. 10.1016/j.biotechadv.2017.09.008 [DOI] [PubMed] [Google Scholar]
- Wu X., Yao H., Cao L., Zheng Z., Chen X., Zhang M., Wei Z., Cheng J., Jiang S., Pan L., Li X. (2017). Improving acetic acid production by over-expressing PQQ-ADH in Acetobacter pasteurianus. Frontiers in Microbiology, 8, 1713. 10.3389/fmicb.2017.01713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia K., Zang N., Zhang J., Zhang H., Li Y., Liu Y., Feng W., Liang X. (2016). New insights into the mechanisms of acetic acid resistance in Acetobacter pasteurianus using iTRAQ-dependent quantitative proteomic analysis. International Journal of Food Microbiology, 238, 241–251. 10.1016/j.ijfoodmicro.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Yakushi T., Matsushita K. (2010). Alcohol dehydrogenase of acetic acid bacteria: Structure, mode of action, and applications in biotechnology. Applied Microbiology and Biotechnology, 86(5), 1257–1265. 10.1007/s00253-010-2529-z [DOI] [PubMed] [Google Scholar]
- Zheng Y., Chang Y., Zhang R., Song J., Xu Y., Liu J., Wang M. (2018). Two-stage oxygen supply strategy based on energy metabolism analysis for improving acetic acid production by Acetobacter pasteurianus. Journal of Industrial Microbiology & Biotechnology, 45(9), 781–788. 10.1007/s10295-018-2060-2 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wang J., Bai X., Chang Y., Mou J., Song J., Wang M. (2018). Improving the acetic acid tolerance and fermentation of acetobacter pasteurianus by nucleotide excision repair protein UvrA. Applied Microbiology and Biotechnology, 102(15), 6493–6502. 10.1007/s00253-018-9066-6 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Zhang K., Su G., Han Q., Shen Y., Wang M. (2015). The evolutionary response of alcohol dehydrogenase and aldehyde dehydrogenases of Acetobacter pasteurianus CGMCC 3089 to ethanol adaptation. Food Science and Biotechnology, 24(1), 133–140. 10.1007/s10068-015-0019-x [DOI] [Google Scholar]
- Zheng Y., Zhang R., Yin H., Bai X., Chang Y., Xia M., Wang M. (2017). Acetobacter pasteurianus metabolic change induced by initial acetic acid to adapt to acetic acid fermentation conditions. Applied Microbiology and Biotechnology, 101(18), 7007–7016. 10.1007/s00253-017-8453-8 [DOI] [PubMed] [Google Scholar]
- Zhu W., Martins A. M., Klinman J. P. (2018). Methods for expression, purification, and characterization of PqqE, a radical SAM enzyme in the PQQ biosynthetic pathway. Methods in Enzymology, 606, 389–420. 10.1016/bs.mie.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.