Abstract
Controlling and reducing plaque formation plays a pivotal role in preventing and treating periodontal disease, often utilizing antibacterial drugs to enhance therapeutic outcomes. Mesoporous silica nanoparticles (MSN), an FDA-approved inorganic nanomaterial, possess robust physical and chemical properties, such as adjustable pore size and pore capacity, easy surface modification, and high biosafety. Numerous studies have exploited MSN to regulate drug release and facilitate targeted delivery. This study aimed to synthesize an MSN-tetracycline (MSN-TC) complex and investigate its inhibitory potential on Porphyromonas gingivalis (P. gingivalis)-induced bone resorption. The antibacterial efficacy of MSN-TC was evaluated through bacterial culture experiments. A P. gingivalis-induced bone resorption model was constructed by subcutaneously injecting P. gingivalis around the cranial bone of rats. Micro-computed tomography was employed to assess the inhibitory impact of MSN and MSN-TC on bone resorption. Furthermore, the influence of MSN and MSN-TC on osteoclast differentiation was examined in vitro. The MSN exhibited optimal pore size and particle dimensions for effective loading and gradual release of TC. MSN-TC demonstrated significant bacteriostatic activity against P. gingivalis. MSN-TC-treated rats showed significantly reduced cranial bone tissue destruction compared to MSN or TC-treated rats. Additionally, both MSN and MSN-TC exhibited inhibitory effects on the receptor activator of nuclear factor kappa-Β ligand-mediated osteoclast differentiation. The MSN-TC complex synthesized in this study demonstrated dual efficacy by exerting antibacterial effects on P. gingivalis and by resisting osteoclast differentiation, thereby mitigating bone resorption induced by P. gingivalis.
Graphical Abstract

Introduction
Periodontitis comprises a group of chronic infectious diseases that provoke the degradation of periodontal supporting tissues and is the primary contributor to adult tooth loss [1–3]. Current therapeutic strategies for periodontitis involve mechanical plaque removal, supplemented by antibiotics to enhance efficacy [4, 5]. Tetracycline (TC), a broad-spectrum antibiotic, exhibits inhibitory effects on a wide range of periodontal pathogenic bacteria [6, 7]. However, oral administration of TC faces challenges, such as low local concentrations and brief duration of effectiveness [8–10]. Therefore, there is a growing focus on local drug delivery as a research target for antibiotics [11]. Mucoadhesive-loaded TC formulations, with varied ratios of hydroxyethylcellulose, polyvinylpyrrolidine, and polycarbonate, have demonstrated enhanced pathogen clearance, improving periodontal health [12]. Furthermore, TC-nanoparticle calcium sulfate composite microspheres reduced bacterial load at periodontal infection sites through localized TC release and promoted local tissue regeneration due to the inherent characteristics of sulfuric acid [13]. Nanofiber preparations, using polylactic acid glycolic acid, gum yarrow, and TC hydrochloride, showed sustained TC release of up to 75 days [14].
In recent years, the widespread utilization of nanomaterials has opened a novel avenue for researching drug resistance and targeted drug delivery. Mesoporous silica nanoparticles (MSN) exhibit advantages, such as stable physicochemical properties and good biosafety [15]. Moreover, MSN demonstrates the capacity to enhance drug solubility [16–18], regulate drug release [19, 20], and facilitate targeted drug delivery [21–23], positioning it as a promising drug carrier [24, 25].
The purpose of this study was to investigate the inhibitory effect of MSN-TC on Porphyromonas gingivalis (P. gingivalis) and assess the therapeutic effect of MSN-TC in periodontitis by loading the insoluble drug TC in the MSN matrix.
Materials and methods
MSN synthesis
Cetyltrimethyl ammonium bromide (0.200 g) was dissolved by stirring in double-distilled water (100 ml). Subsequently, 720 µl of 2 M NaOH was added to the solution and warmed to 80 °C. Next, 1 ml of tetraethyl orthosilicate was added gradually over 2 h. The resulting MSN were collected by centrifugation at 14,000 rpm for 10 min. Ethanol containing 1% hydrochloric acid was then used for reflux at 40 °C for 24 h to eliminate the template agent. Following additional centrifugation, the products were washed three times alternately with ultrapure water and absolute ethanol. Finally, the MSN were obtained through freeze-drying. TC was bound to MSN by dispersing 20 mg of MSN into 5 ml of distilled water and reacting this with 30 mg of TC in the dark for 48 h. After centrifugation, MSN-TC was obtained by washing three times with double-distilled water, followed by freeze-drying.
TC loading and release
During the preparation of MSN-TC, the supernatant obtained after centrifugation and washing was collected. The absorbance at 276 nm was measured using a Nanodrop one ultra-micro ultraviolet spectrophotometer (Thermofisher, Waltham, USA). The linear regression equation correlating TC concentration and optical density were compared to quantify the concentration of TC in the supernatant, and the encapsulation rate of MSN was calculated. The drug loading rate of TC was calculated as the percentage mass reduction after heating MSN and MSN-TC using a thermogravimetric analyzer.
The pharmacokinetics of TC in MSN-TC were tested using phosphate-buffered saline (PBS, PH 7.4) as an in vitro environment simulation. First, 10 mg of MSN-TC was resuspended in 20 ml of PBS, it was vigorously shaken, and 5 ml of the resulting suspension was sampled at predefined time intervals (1, 2, 4, 6, 8, 12, 24, and 72 h). After centrifugation, the supernatant was collected, and its concentration was assessed by measuring the absorbance at 276 nm. Thereafter, 5 ml PBS was added to resuspend the centrifugal precipitation, ensuring consistent release conditions within the system.
MSN and MSN-TC performance characterization
The morphology and porosity of MSN were evaluated using field emission gun scanning electron microscopy (FEG-SEM; SU8010; Hitachi, Tokyo, Japan) and transmission electron microscopy (HT7700 EXALENS; Hitachi). Specific surface area and pore size distribution were determined through Brunauer–Emmett–Teller and the Barrett–Joyner–Halenda analyses, employing nitrogen adsorption–desorption isotherms (ASAP 2020; Micromeritics, Norcross, GA, USA). Particle size and zeta potential were determined using a zeta sizer (Nano ZS; Malvern Instruments, UK). The MSN infrared absorption spectrum was detected by a Nicolet iS10 Fourier Transform infrared spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Physical states of MSN, TC, and MSN-TC were tested with an SDT Q600 thermogravimetric analyzer (Waters Corp., Milford, MS, USA) to detect weight changes in the temperature range of 0–800 °C.
MSN-TC antimicrobial activity in vitro
MSN-TC suspensions were prepared at concentrations of 12.5, 25, 50, 100, 125, 200, 250, 500, and 1000 μg/ml using BHI liquid medium; P. gingivalis (ATCC 33277) was adjusted to a concentration of 107 colony-forming units [CFU]/ml. The negative control group comprised 100 µl medium without the bacterial solution, mixed with 100 µl MSN-TC suspension at various concentrations. A mixture of bacterial solution (100 µl) and MSN-TC suspension (100 µl) at the specified concentrations was incubated for 24 h under anaerobic conditions. The minimum inhibitory concentration (MIC) of MSN-TC was determined as the minimum concentration of MSN-TC that clarified the medium, compared with the corresponding control group [26]. Subsequently, 10 µl of bacteria with concentrations higher than the MIC were inoculated into Columbia Blood Agar. The lowest administered concentration without colony growth was the minimum bactericidal concentration (MBC) [26].
Effect of MSN on osteoclast differentiation
The experiment followed all animal ethics protocols provided by Jianghan University’s ethical committee (No. JHDXLL2024--043). Mice of 6–8 weeks old were sacrificed, and all tibia and femur specimens were surgically extracted on a sterile table. The bone ends were excised, and the bone marrow was thoroughly washed with PBS. After centrifugation at 1000 rpm for 5 min, the supernatant was discarded, and 2 ml of erythrocyte lysate (1×) was added for lysis at room temperature for 5 min. Lysis reactions were halted by introducing 10 ml of complete medium (α-MEM medium + 10% FBS + double antibody), and cells were resuspended in complete medium containing 30 ng/ml macrophage colony-stimulating factor (M-CSF) post-centrifugation. After 24 h, the cells underwent another centrifugation, and the resuspension was adjusted to 5 × 106 cells/ml. Cell suspension (10 ml) was plated in 10 cm cell culture dishes, and the medium was refreshed every other day. When the cells reached 80–90% density, they were collected into a 15 ml centrifuge tube. The cells were then resuspended to appropriate concentrations in a complete medium, with 30 ng/ml M-CSF, for subsequent experiments.
For bone marrow-derived macrophage (BMM) cells, seeding was done in 24-well plates at the designated density. After 24 h TC, MSN, and MSN-TC were added to a complete medium containing 30 ng/ml M-CSF and 100 ng/ml receptor activator of nuclear factor kappa-Β ligand (RANKL). The following day’s medium was a complete medium containing 30 ng/ml M-CSF and 100 ng/ml RANKL, without the addition of TC, MSN, and MSN-TC. After 7 days of induction, cells were rinsed twice with PBS. Subsequently, the cells underwent treatment with the TRAP assay kit (Solarbio, Beijing, China) and Phalloidin-iFluor™ 488 conjugate staining kit (Antgene, Wuhan, China). Osteoclasts were observed under a light microscope, while F-actin rings and multinucleated cell formation were examined under a fluorescence microscope and subjected to quantitative analysis.
Effect of MSN-TC on P. gingivalis-induced bone damage
Pathogen-free male Sprague–Dawley rats, weighing 200–300 g, were purchased from the Laboratory Animal Center of Jianghan University. Rats were acclimatized to living in the cages for 1 week before use. Experimental procedures were performed in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals and all protocols involving animal research were approved and supervised by the ethics committee of Jianghan University (No. JHDXLL2024--043).
The conventional method for establishing an animal model of periodontal inflammation involves inducing periodontitis by ligating the tooth neck with silk threads [27–29]. However, the progression of the disease can be affected by factors such as the loss of silk during ligation and different ligation sites. In addition, the small space of the periodontal pocket makes it difficult to administer the drug, and to ensure an accurate dose. Therefore, we used skull infection, induced by subcutaneous injection of P. gingivalis, to simulate the periodontitis model [30–32]. Twenty-five male Sprague–Dawley rats were randomly divided into five groups (n = 5 each). The rats were securely fixed on the operating table under inhalation anesthesia, and 0.5 ml of PBS was injected into the periosteum through the sagittal suture of the calvarium for 8 days in the negative control group. In the experimental groups, rats were injected with 1.0 × 1010 CFU/ml of P. gingivalis (0.5 ml) for the first 3 days. Twelve hours after P. gingivalis injection on day 4, the experimental groups received injections of PBS, MSN (50 mg/kg), TC (12.5 mg/kg), or MSN-TC (50 mg/kg) for the subsequent 5 days.
Twelve hours after the final injection, the rats were euthanized and the calvaria bones were isolated and fixed at room temperature for 2 days in 4% paraformaldehyde. Following PBS washing, the samples were scanned using a micro-computed tomography machine with a resolution of 1024 × 1024 pixels, slice thickness of 20 μm, voltage of 70 kV, and current of 114 μA. Subsequently, three-dimensional models of the calvarium were constructed to analyze bone microstructure and calculate bone volume fraction and trabecular bone indexes. The scanned skulls were then transformed into paraffin specimens, observed under a microscope, and subjected to quantitative analysis after hematoxylin and eosin (HE) and tartrate-resistant acid phosphatase (TRAP) staining.
Statistical analysis
Experimental data were statistically analyzed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA) and presented as mean ± standard deviation. A significant difference was found at P < 0.05. The normality distribution was investigated by applying the Kolmogorov-Smirnov test and the equal variance of the compared groups was tested by applying the f test. One-way analyses of variance (ANOVA) with Tukey’s test were used to analyse the significance of differences among groups.
Results
Characterization of the MSN and MSN-TC
The prepared MSN exhibited a spherical shape with relatively uniform particle size and minimal aggregation under FEG-SEM (Fig. 1a). Moreover, the surface of the MSN was rough, suggesting numerous pore structures within the mesoporous silica. Transmission electron microscopy revealed a distinct mesoporous structure in MSN. The highly ordered hexagonal pores measured ~150 nm in diameter (Fig. 1b).
Fig. 1.
Performance characterization of MSN. a FEG-SEM image of MSN, scale bar = 500 nm. b Transmission electron microscopy image of MSN, scale bar = 100 nm (Scale of local magnification = 50 nm). c MSN nitrogen adsorption-desorption isotherms. d MSN pore size distribution. e MSN particle size distribution. f MSN FTIR spectra. FEG-SEM field emission gun scanning electron microscopy
The nitrogen adsorption-desorption isotherm analysis of MSN exhibited type IV isotherms with H1-type hysteresis loops. Utilizing the BET and BJH theoretical models, the specific surface area of the MSN was calculated to be 500.5764 m2/g, the pore volume was 0.4417 cm³/g, and the pore size was 4.5169 nm (Fig. 1c, d). Dynamic light scattering determined the particle size of MSN to be ~341.9 ± 51 nm (Fig. 1e). This measurement, larger than that obtained via transmission electron microscopy, is attributed to the hydrated diameter of the suspended particle in the liquid during dynamic light scattering.
The FTIR spectra (Fig. 1f) illustrated a broad band between 3765 and 2800 cm−1, corresponding to the stretching vibration frequency of the silyl group. The distinct and robust peak at 1100 cm−1 represented an asymmetric Si–O–Si antisymmetric stretching vibration, while the absorption band at 790 cm−1 indicated a symmetric Si-O-Si stretching vibration.
TC loading and release trends
The zeta potential during TC loading onto MSN (Fig. 2a) revealed a surface rich in hydroxyl groups with a negative potential after ionization. The initial MSN potential was −18.7 mV, while the TC potential was −6.53 mV. Following TC adsorption through diffusion, the potential became −27.6 mV. The results of the thermogravimetric analysis are illustrated in Fig. 2b. The MSN sample demonstrated stability after experiencing a mass loss of ~19.41% at around 100 °C, potentially attributed to water loss. The complete reaction of the TC sample occurred at ~620 °C. In contrast, the MSN-TC sample, at around 620 °C, exhibited a complete reaction of loaded TC, reaching stability after a mass loss of about 44.58%. Consequently, the calculated drug loading rate of TC was determined to be 25.17%. The concentration of all supernatants during MSN-TC synthesis was determined using a Nanodrop 2000 spectrometer to calculate the TC encapsulation efficiency. The linear regression equation of the standard concentration curve was determined as y = 0.0292x + 0.0614, resulting in an encapsulation TC efficiency at 16.78%.
Fig. 2.
TC loading and release trends. a Zeta-potentials of MSN, TC, and MSN-TC. b Total amount of TC loaded by MSN. c The cumulative percentage release of TC from MSN-TC. d Linear relationship of tetracycline concentration
The in vitro drug release pattern of MSN-TC was investigated in a simulated environment (pH 7.4 PBS). As depicted in Fig. 2c, a burst release of TC within the initial 8 h led to a cumulative release of 40%, followed by a gradual, sustained release extending up to 72 h. The relationship between the drug release rate (Q) and time (t) can be expressed as Q = 30.35(1-e−0.26t). This equation indicates that the drug release kinetics follow a first-order kinetic model, with a goodness of fit R2 = 0.9938. The drug release curve initially rises sharply and then gradually approaches the maximum drug release over time.
MSN-TC in vitro antimicrobial activity
P. gingivalis colonies on Columbia Blood Agar exhibited smooth-surfaced, raised, round morphology, measuring ~1–2 mm in diameter, with blackened colonies evident after 7–10 days of culture. The MIC of MSN-TC against P. gingivalis was 100 μg/ml, whereas the MBC was 200 μg/ml.
Ability of MSN to inhibit osteoclast differentiation in vitro
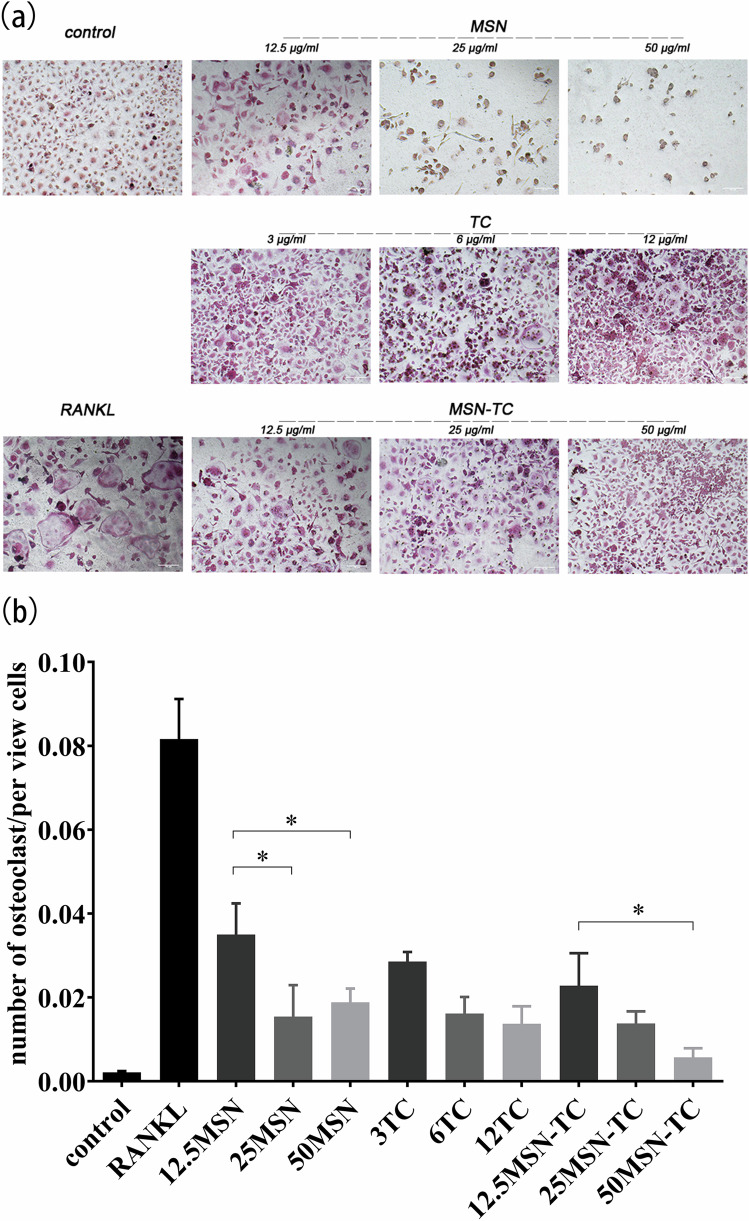
Concentrations of TC, MSN, and MSN-TC were determined based on the preceding experimental results, and their impact on osteoclast formation was investigated. BMM cells were induced to differentiate with RANKL (Fig. 3a). In the RANKL group, numerous osteoclasts, with diameters from 20 to 200 μm, with 2–50 nuclei, were observed. However, at concentrations of 12.5, 25, and 50 μg/ml, the number and size of osteoclasts decreased with increasing concentrations of MSN, TC, and MSN-TC. Considering that osteoclasts result from the fusion of multiple cells and the total cell count is relatively low, the ratio of the number of osteoclasts to the total number of cells in the field of view was calculated for statistical analysis (Fig. 3b). All the experimental groups exhibited a statistically significantly lower ratio than the RANKL group did (P < 0.01). At the same concentration, the MSN-TC group exhibited a lower ratio than the MSN and TC groups, which was not statistically significant. With increasing concentration, the ratio decreased in all three groups. A statistical difference was observed in the MSN group, but not in the TC group. Additionally, a statistically significant difference was found between the concentrations of 50 μg/ml and 12.5 μg/ml in the MSN-TC group.
Fig. 3.
TC, MSN, and MSN-TC suppress RANKL-stimulated osteoclastogenesis from BMM. a Mature osteoclasts, labeled with TRAP staining, scale bar = 200 μm. b TRAP-positive multinucleated cells with multiple nuclei (three or more) were called osteoclasts and quantitatively analyzed in ratio to the total number of cells in the field of vision. **P < 0.01, *P < 0.05 vs. RANKL group
The results of F-actin staining are depicted in Fig. 4. The RANKL group showed multiple mature osteoclasts with F-actin rings and multiple nuclei, while the control group exhibited a decrease in the number of mature osteoclasts and the size of F-actin rings. Moreover, compared with the RANKL group, all the experimental groups showed an inhibitory effect on F-actin formation (P < 0.05). Additionally, increases in MSN concentration led to statistically significant decreases in the number and size of F-actin rings. This may be attributed to MSN’s inhibition of both osteoclast differentiation and bone resorption. An MSN-TC concentration of 50 μg/ml exhibited a stronger inhibitory effect on F-actin ring formation than those at 12.5 and 25 μg/ml, with a statistically significant difference. However, there was no statistically significant difference among the concentrations in the TC group. When comparing the effect of the three drugs at the same concentration, the inhibitory effect of MSN and MSN-TC at 12.5 μg/ml and 50 μg/ml was better than that of TC at the same concentration, and 25 μg/ml MSN was better than TC and MSN-TC.
Fig. 4.
TC, MSN, and MSN-TC inhibit RANKL-induced formation of the F-actin ring in osteoclasts. a The nucleus was labeled with blue DAPI and green Phalloidin-Ifluor™ 488 Conjugate F-actin ring, scale bar = 200 μm. b Cells with green F-actin were labeled as mature osteoclasts and quantitatively analyzed in percentage comparison with the number of mature osteoclasts in the RANKL group. **P < 0.01, *P < 0.05 ; ####P < 0.0001, ###P < 0.0009, ##P < 0.01, #P < 0.05
Micro-CT cranial imaging and section results
Micro-CT scanning is illustrated in Fig. 5. The Pg group displayed multiple bone defects, with significantly decreased bone volume density (bone volume [BV]/total volume [TV]), trabecular bone thickness (Tb.Th), and trabecular bone number (Tb.N). Conversely, the other three groups exhibited an upward trend in these parameters post-treatment, with the MSN-TC group demonstrating superior efficacy compared to the TC and MSN groups. The BV/TV, Tb.Th, and Tb.N of the MSN-TC group closely resembled those of the blank control group. Additionally, trabecular bone space (Tb.Sp) in the calvaria widened in the Pg group and decreased with MSN and TC treatments. The MSN-TC group also decreased Tb.Sp after treatment, although it did not reach the level of the blank group; the difference was statistically significant.
Fig. 5.
Reconstruction analysis of bone tissue in different experimental groups. Morphometric indices: a BV/TV, b Tb.N, c Tb.Sp, and d Tb.Th were analyzed by micro-CT. **P < 0.01, *P < 0.05 vs. control group; ##P < 0.01, #P < 0.05 vs. Pg group
HE staining of rat calvarial bone tissue sections are presented in Fig. 6a, c. In the Pg group, the bone tissue structure experienced significant damage, with reduced thickness and the infiltration of numerous inflammatory cells. The MSN and TC groups exhibited less damage to bone structure than the Pg group did, yet inflammatory cell infiltration persisted. The MSN-TC group displayed a relatively intact bone structure, with the least inflammatory cell infiltration. However, there was no significant difference in the number of osteoblasts among the groups. TRAP staining mirrored the bone structure observed in HE staining (Fig. 6b, d). TRAP-positive cells were abundant in the Pg group, less so in the MSN and TC groups, and notably reduced in the MSN-TC group. Moreover, the decrease in the number of osteoclasts in the MSN-TC group was statistically significant compared to that in the MSN group and TC group. However, the number of osteoclasts in each group remained significantly higher than that in the control group.
Fig. 6.
Paraffin section of rat calvarial bone. a, b Calvarial bones are stained with HE and TRAP histology. c, d Osteoblasts and osteoclasts are quantified. **P < 0.01, *P < 0.05 vs. control group; ##P < 0.01, #P < 0.05 vs. Pg group
Discussion
In this study, the synthesized MSN exhibited a clearer pore structure, smaller particle size, and larger pore size and volume by improving the synthesis method [32]. The drug loading rate of MSN-loaded TC was 25.17%, slightly surpassing the results of Bhuvaneswari et al.’s study on MCM-41 mesoporous silica-loaded TC [33]. Following an initial burst release of ~20% in the first 8 h, MSN-TC achieved a plateau state after 72 h of sustained release. The sustained release effect was better than that of Lin et al. [34]. These variations can be attributed to factors such as molecular size, surface charge, mesoporous silica pore size, pore volume, and surface-modified molecules of the drug. Jia et al. [35] prepared three kinds of MSN aperture to verify the importance of the aperture on the load capacity and drug release performance of MSN. In addition, a mesoporous structure not only affects the crystal morphology and molecular dynamics of loaded drugs, but also changes the method of drug release. For example, the drug release rate of two-dimensional mesoporous silicon with straight pore channels is usually faster than that of three-dimensional cubic pore channels [36]. Moreover, Samaneh et al.’s in vitro release study showed that amino-modified SBA-15 had a higher load rate and a slower release rate for TC [37]. These factors influence the loading and release processes by affecting the interaction between drug molecules and mesoporous silica [34, 38–40].
The formation and accumulation of plaque biofilm is a major cause of periodontal disease. This biofilm exhibits a structured and intricate architecture, with diverse bacteria creating a three-dimensional ecological structure [41]. It can not only resist the host defense components or drugs but also secrete various virulence factors, which can cause inflammatory changes in gingival connective tissue and induce the formation of a large number of osteoclasts, leading to bone resorption [42, 43]. In this study, a model of RANKL-mediated osteoclast differentiation of BMM cells [44] was employed to investigate the effect of MSN-TC on osteogenesis and bone resorption. TRAP-positive multinucleated cells are markers of osteoclast progenitor cell differentiation, and TRAP-positive cells with 3 or more nuclei are called mature osteoclasts [45]. An actin ring is characteristic of terminal differentiation of osteoclast progenitor cells and the necessary condition for osteoclasts to perform bone resorption. The formation of bone resorption lacunae indicated that the osteoclasts had differentiated and matured, with bone resorption activity [46, 47]. By using TRAP staining to observe the differentiation of BMM cells into osteoclasts, and F-actin staining to observe the bone resorption function of mature osteoclasts, we found that TC, MSN, and MSN-TC treatment inhibited osteoclast formation and bone resorption in a concentration-dependent manner. Previous studies have reported that TC drugs and MSN can block bone resorption [7, 48, 49]. Furthermore, Franco et al. [50] found that TC drugs inhibited osteoclast generation by blocking the activity of MMP-9. As indicated in the F-actin staining experiment, 25 μg/ml and 50 μg/ml MSN almost completely inhibited the bone resorption function of osteoclasts. This suggests that MSN has a strong inhibitory effect on the differentiation of BMM to form osteoclasts under RANKL stimulation, consistent with previous reports [51, 52]. However, MSN-TC treatment had a weaker inhibitory effect on bone resorption than MSN treatment. We hypothesize that the reason is probably related to the surface of MSN-TC being covered by TC, which only showed a very gentle inhibitory effect on bone resorption.
In the simulated periodontitis animal model, compared with the Pg group, the degree of bone destruction in the TC group and MSN-TC group was significantly reduced, with the treatment effect of the MSN-TC group notably stronger than that of the TC group. The number of bone trabeculae in the MSN-TC group essentially returned to the level of the sham operation group. Some studies have shown that MSN and TC have the potential to stimulate osteogenesis [53, 54]. However, there was no significant difference in the number of osteoblasts among the animal samples of each group in this experiment; this may require further experimental verification. Additionally, compared with the Pg group, the number of osteoclasts decreased in all experimental groups, with the MSN-TC group showing the most significant decrease. According to the results shown in Figs. 4, 5, and 6, MSN-TC exhibited weaker bone resorption inhibition in vitro and stronger bone resorption inhibition in vivo than MSN did. Because MSN-TC combined the inhibitory effect of MSN on osteoclasts and the antibacterial effect of TC, it showed the strongest effect in reducing bone damage in the simulated periodontitis animal model.
Conclusion
The results of this study demonstrate that MSN-TC has antibacterial activity, a high TC loading rate of 25.17%, and a long release time of 72 h in vitro. Furthermore, MSN-TC exhibited the capacity to reduce the destruction of bone tissue in the skull infection model after subcutaneous injection of P. gingivalis. We hypothesize that the mechanism of action of MSN-TC is dependent on the antibacterial effect of TC and the inhibitory effect of MSN and TC on the differentiation of osteoclasts. Our current findings on the antibacterial and anti-inflammatory effects of MSN-TC provide a potential approach for further development of antibacterial encapsulated MSN for the treatment of periodontitis.
Acknowledgements
The authors would like to thank Sun Bin-lian (Jianghan University) for technical assistance.
Author contributions
All authors have made substantial contributions to the conception and design of the study. Meng-ya Li and Qing-an Xu contributed to the conception, design, and data interpretation, as well as drafted and critically revised the manuscript. Jian Sun was involved in data analysis and data interpretation. Dong Zhao and Wen Zhang were involved in the critical review of the manuscript.
Funding
This study was funded by the Guiding Project of the Hubei Provincial Health and Wellness Committee (Grant No. WJ2023F040).
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- 2.Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75:7–23. [DOI] [PubMed] [Google Scholar]
- 3.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. [DOI] [PubMed] [Google Scholar]
- 4.Herrera D, Sanz M, Jepsen S, Needleman I, Roldán S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002;29:136–62. [DOI] [PubMed] [Google Scholar]
- 5.Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, et al. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol. 2020;47:4–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson ML, Levy SB. Antimicrobial therapeutics reviews: antibiotics that target the ribosome. In: Bush K, editor. Blackwell Science Publ: Oxford; 2011. [DOI] [PubMed]
- 8.Addy M, Martin MV. Systemic antimicrobials in the treatment of chronic periodontal diseases: a dilemma. Oral Dis. 2003;9:38–44. [DOI] [PubMed] [Google Scholar]
- 9.Miller CS, McGarity GJ. Tetracycline-induced renal failure after dental treatment. J Am Dent Assoc. 2009;140:56–60. [DOI] [PubMed] [Google Scholar]
- 10.Wright J, Paauw DS. Complications of antibiotic therapy. Med Clin N Am. 2013;97:667–79. [DOI] [PubMed] [Google Scholar]
- 11.HR R, Dhamecha D, Jagwani S, Rao M, Jadhav K, Shaikh S, et al. Local drug delivery systems in the management of periodontitis: a scientific review. J Control Release. 2019;307:393–409. [DOI] [PubMed] [Google Scholar]
- 12.Jones DS, Woolfson AD, Brown AF, Coulter WA, McClelland C, Irwin CR. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J Control Release. 2000;67:357–68. [DOI] [PubMed] [Google Scholar]
- 13.Sindhura Reddy N, Sowmya S, Bumgardner JD, Chennazhi KP, Biswas R, Jayakumar R. Tetracycline nanoparticles loaded calcium sulfate composite beads for periodontal management. Biochim Biophys Acta. 2014;1840:2080–90. [DOI] [PubMed] [Google Scholar]
- 14.Ranjbar-Mohammadi M, Zamani M, Prabhakaran MP, Bahrami SH, Ramakrishna S. Electrospinning ofPLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater Sci Eng C Mater Biol Appl. 2026;58:521–31. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Zhou X, He C. Mesoporous silica nanoparticles for tissue-engineering applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11:e1573. [DOI] [PubMed] [Google Scholar]
- 16.Thomas MJ, Slipper I, Walunj A, Jain A, Favretto ME, Kallinteri P, et al. Inclusion of poorly soluble drugs in highly ordered mesoporous silica nanoparticles. Int J Pharm. 2010;387:272–7. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Wang J, Bai X, Jiang T, Zhang Q, Wang S. Mesoporous silica nanoparticles for increasing the oral bioavailability and permeation of poorly water soluble drugs. Mol Pharm. 2012;9:505–13. [DOI] [PubMed] [Google Scholar]
- 18.Biswas N. Modified mesoporous silica nanoparticles for enhancing oral bioavailability and antihypertensive activity of poorly water soluble valsartan. Eur J Pharm Sci. 2017;99:152–60. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Xu X, Zhang K, Sun B, Wang L, Meng L, et al. Codelivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles-polymerpolyethylenimine to improve oral squamous carcinoma treatment. Int J Nanomed. 2017;13:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu MM, Ge Y, Qiu J, Shao D, Zhang Y, Bai J, et al. Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int J Nanomed. 2018;13:7697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Zhou B, Du X, Wang Y, Zhang J, Ai Y, et al. Folic acid (FA)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed Pharmacother. 2020;125:109561. [DOI] [PubMed] [Google Scholar]
- 22.Juneja R, Vadarevu H, Halman J, Tarannum M, Rackley L, Dobbs J, et al. Combination of nucleic acid and mesoporous silica nanoparticles: optimization and therapeutic performance in vitro. ACS Appl Mater Interfaces. 2020;12:38873–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Dai D, Lou X, Ma L, Wang B, Yang YW. Supramolecular nanomaterials based on hollow mesoporous drug carriers and macrocycle-capped CuS nanogates for synergistic chemo-photothermal therapy. Theranostics. 2020;10:615–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Quan G, Wu Q, Zhang X, Niu B, Wu B, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm Sin B. 2018;8:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Zhang Y, Feng N. Mesoporous silica nanoparticles: synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin Drug Deliv. 2019;16:219–37. [DOI] [PubMed] [Google Scholar]
- 26.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48:5–16. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Gong Z, Lin Q, Wang W, Liu S, Li S. Denervation effectively aggravates rat experimental periodontitis. J Periodontal Res. 2017;52:1011–20. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Jia Z, Almoshari Y, Lele SM, Reinhardt RA, Wang D. Local application of pyrophosphorylated simvastatin prevents experimental periodontitis. Pharm Res. 2018;35:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao X, YU XJ, Xie JL, Liu S, Li S. Protective effect and related mechanisms of curcumin in rat experimental periodontitis. Head Face Med. 2018;35:164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li ZF, Cao LH, Wang Y, Zhang Z, Fan MW, Xu QA. Inhibitory effect of 1,25-dihydroxyvitamin D3 on Porphyromonas gingivalis-induced inflammation and bone resorption in vivo. Arch Oral Biol. 2016;72:146–56. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Zhu L, Zhang J, Yu J, Cheng X, Peng B. Anti-osteoclastogenic activity of isoliquiritigenin via inhibition of NF-κB-dependent autophagic pathway. Biochem Pharmacol. 2016;106:82–93. [DOI] [PubMed] [Google Scholar]
- 32.Cai X, Li Z, Zhao Y, Katz J, Michalek SM, Feng X, et al. Enhanced dual function of osteoclast precursors following calvarial Porphyromonas gingivalis infection. J Periodontal Res. 2020;55:410–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia L, Shen J, Li Z, Zhang D, Zhang Q, Duan C, et al. Successfully tailoring the pore size of mesoporous silica nanoparticles: exploitation of delivery systems for poorly water-soluble drugs. Int J Pharm. 2012;439:81–91. [DOI] [PubMed] [Google Scholar]
- 34.Khademi AA, Amini K, Ghodsian B, Zahed SM, Teymori F, Shadmehr E. Removal efficiency of calcium hydroxide intracanal medicament with RinsEndo system in comparison with passive ultrasonic irrigation, an in vitro study. Dent Res J. 2015;12:157–60. [PMC free article] [PubMed] [Google Scholar]
- 35.Lin CX, Qiao SZ, Yu CZ, Ismadji S, Lu GQ. Periodic mesoporous silica and organosilica with controlled morphologies as carriers for drug release. Microporous Mesoporous Mater. 2009;117:213–9. [Google Scholar]
- 36.Jia L, Shen J, Li Z, Zhang D, Zhang Q, Liu G, et al. In vitro and in vivo evaluation of paclitaxel-loaded mesoporous silica nanoparticles with three pore sizes. Int J Pharm. 2013;445:12–9. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Wang J, Zhi Z, Jiang T, Wang S. Facile synthesis of 3D cubic mesoporous silica microspheres with a controllable pore size and their application for improved delivery of a water-insoluble drug. J Colloid Interface Sci. 2011;363:410–7. [DOI] [PubMed] [Google Scholar]
- 38.Hashemikia S, Hemmatinejad N, Ahmadi E, Montazer M. Optimization of tetracycline hydrochloride adsorption on amino modified SBA-15 using response surface methodology. J Colloid Interface Sci. 2015;443:105–14. [DOI] [PubMed] [Google Scholar]
- 39.Rakhshaei R, Namazi H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater Sci Eng C Mater Biol Appl. 2017;73:456–64. [DOI] [PubMed] [Google Scholar]
- 40.Butler KS, Durfee PN, Theron C, Ashley CE, Carnes EC, Brinker CJ. Protocells: modular mesoporous silica nanoparticle-supported lipid bilayers for drug delivery. Small. 2016;12:2173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2:1599–607. [DOI] [PubMed] [Google Scholar]
- 42.Koo H, Falsetta ML, Klein MI. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 2013;92:1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. 2015;2015:615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Sapkota M, Kim SW, Soh Y. Herbacetin inhibits RANKL-mediated osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Eur J Pharmacol. 2016;777:17–25. [DOI] [PubMed] [Google Scholar]
- 45.Tevlin R, McArdle A, Chan CKF, Pluvinage J, Walmsley GG, Wearda T. et al. Osteoclast derivation from mouse bone marrow. J Vis Exp. 2014;93:e520566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ono T, Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018;149:325–41. [DOI] [PubMed] [Google Scholar]
- 47.Shen Y, Wang Z, Tan J, Zhong J, Chen L. TRAF6/ERK/p38 pathway is involved in interleukin-17-mediated autophagy to promote osteoclast precursor cell differentiation. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021;50:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams S, Wakisaka A, Zeng QQ, Barnes J, Martin G, Wechter WJ, et al. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone. 1996;19:637–44. [DOI] [PubMed] [Google Scholar]
- 49.Sun X, Zhang J, Wang Z, Liu B, Zhu S, Zhu L, et al. Licorice isoliquiritigenin-encapsulated mesoporous silica nanoparticles for osteoclast inhibition and bone loss prevention. Theranostics. 2019;9:5183–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franco GC, Kajiya M, Nakanishi T, Ohta K, Rosalen PL, Groppo FC, et al. Inhibition of matrix metalloproteinase-9 activity by doxycycline ameliorates RANK ligand-induced osteoclast differentiation in vitro and in vivo. Exp Cell Res. 2011;317:1454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnusson C, Uribe P, Jugdaohsingh R, Powell JJ, Johansson A, Ransjö M. Inhibitory effects of orthosilicic acid on osteoclastogenesis in RANKL-stimulated RAW264.7 cells. J Biomed Mater Res A. 2021;109:1967–78. [DOI] [PubMed] [Google Scholar]
- 52.Beck GR Jr, Ha SW, Camalier CE, Yamaguchi M, Li Y, Lee JK, et al. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomedicine. 2012;8:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin SH, Kweon H, Park JB, Kim CH. The effects of tetracycline-loaded silk fibroin membrane on proliferation and osteogenic potential of mesenchymal stem cells. J Surg Res. 2014;192:e1–e9. [DOI] [PubMed] [Google Scholar]
- 54.Rasool N, Negi D, Singh Y. Thiol-functionalized, antioxidant, and osteogenic mesoporous silica nanoparticles for osteoporosis. ACS Biomater Sci Eng. 2023;9:3535–45. [DOI] [PubMed] [Google Scholar]