Abstract
Long coronavirus disease 2019 (COVID-19)—a postacute consequence of severe acute respiratory syndrome coronavirus 2 infection—manifests with a broad spectrum of relapsing and remitting or persistent symptoms as well as varied levels of organ damage, which may be asymptomatic or present as acute events such as heart attacks or strokes and recurrent infections, hinting at complex underlying pathogenic mechanisms. Central to these symptoms is vascular dysfunction rooted in thrombotic endothelialitis. We review the scientific evidence that widespread endothelial dysfunction (ED) leads to chronic symptomatology. We briefly examine the molecular pathways contributing to endothelial pathology and provide a detailed analysis of how these cellular processes underpin the clinical picture. Noninvasive diagnostic techniques, such as flow-mediated dilation and peripheral arterial tonometry, are evaluated for their utility in identifying ED. We then explore mechanistic, cellular-targeted therapeutic interventions for their potential in treating ED. Overall, we emphasize the critical role of cellular health in managing Long COVID and highlight the need for early intervention to prevent long-term vascular and cellular dysfunction.
Keywords: postacute COVID-19 syndrome, SARS-CoV-2, reinfection, endothelium, vascular, thrombosis
The ongoing global health crisis caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a significant burden on health care systems worldwide. 1 Long coronavirus disease 2019 (COVID-19) or postacute sequelae of COVID-19 (PASC) is a chronic, systemic disease state and classified as an infection-associated chronic condition linked to SARS-CoV-2 infection presenting with characteristic symptoms of Long COVID, lasting for a minimum duration of 3 months. 2 Long COVID also encompasses a wide variety of newly diagnosed or worsened preexisting conditions and objectively detectable functional impairments. 3 Evidence has shown that symptoms as well as systemic pathology can last for several years, and the effects can accumulate with reinfections. 4 5 6 7 8 The aim of this review is to shed light on the fundamental aspects of Long COVID pathobiology, thus providing pointers for diagnosis, potential clinical intervention, and further research.
To gain a full understanding of the disease mechanisms in Long COVID, it is important to acknowledge the primary vasculopathic distribution and characteristics of acute COVID-19 visible radiologically 9 10 11 12 and histologically. 13 14 Acute COVID-19 lung disease manifests primarily through thrombotic and congestive abnormalities, predominantly affecting the pulmonary vascular network. 9 10 11 13 14 15 16 17 18 The macroscopic radiological lung damage is matched by histological microangiopathic findings and endothelial dysfunction (ED). 19 20 21 22 23 Taking into account this primary vasculopathic basis of acute COVID-19 is essential for understanding the varied vascular pathologies observed in Long COVID, highlighting the central role of vascular injury in both phases of the disease. Among these, persistence of thrombotic endothelialitis has been proposed as a possible primary pathology driving the chronicity of Long COVID, 24 25 with dysfunctional vascular endothelium acting as a source of “fibrinaloid microclots.” 26 27 28 Fibrinaloid molecules refer to fibrin(ogen) molecules that have undergone a structural transformation to an amyloid structure, in contrast to the fibrin monomers or polymers that result from the cleavage of fibrinogen by thrombin. These fibrinaloid microclots are small blood clots that contain fibrinaloid molecules, along with other trapped proteins and other molecules. One additional consequence of this thrombotic endothelialitis is a reduction in the density or number of capillaries (referred to as capillary rarefication). 29 30 This capillary rarefication, 31 when combined with circulating fibrinaloid microclots, can result in an imbalance between reduced blood supply and increased demand, especially during exercise, and potentially explains much of the symptoms and pathology of Long COVID. 26 32
In this review, we explore the potential role of persistent thrombotic endothelialitis in the symptoms of Long COVID and discuss the methods available to clinicians for assessing ED and its physiological consequences. These methods include endothelium-dependent flow-mediated dilation (FMD), 33 the use of instruments such as the EndoPAT, 34 35 which is a device approved by the U.S. Food and Drug Administration to diagnose ED, and capillaroscopy. 29 36 37 38 These methods may prove valuable in the diagnosis of Long COVID and provide tools to monitor therapeutic response to targeted treatments for endothelialitis. We also discuss the potential role of viral persistence and oral/gut dysbiosis in ongoing endothelial inflammation in Long COVID.
The Interplay between Endothelial Dysfunction and Coagulation
Modulation of the Functional Phenotype of Endothelial Cells Leading to Dysfunction
Endothelial cells are involved in the regulation of hemostasis, thrombosis, and inflammation within blood vessels. The occurrence of endothelial pathology and dysfunction within microvessels leads to a comprehensive disruption of vascular function and capillary rarefication. The pathological processes involved in ED are well-known (examples are found in diabetes mellitus, cardiometabolic disease, and kidney disease). Endothelial pathology encompasses barrier impairment, compromised vasodilation, increased vessel rigidity, aberrant blood flow, and the occurrence of thrombotic phenomena. (See Supplementary Table S1 for glossary of terminology, available in online version only).
Activation of endothelial cells is categorized into two phases: “stimulation” (an initial occurrence) and “activation” (a subsequent occurrence), which are termed Type I endothelial cell activation and Type II endothelial cell activation, respectively 39 40 ( Fig. 1 ). The process leading to ED is characterized by a release of stored proteins and/or a production of specific proteins. Type I endothelial activation or dysfunction is a rapid, reversible event, in which endothelial cells release prestored proteins such as von Willebrand factor (VWF), P-selectin, thrombin, and histamine (released from the granules of nearby mast cells that can interact with the endothelium to exacerbate inflammatory responses and vascular permeability).
Fig. 1.
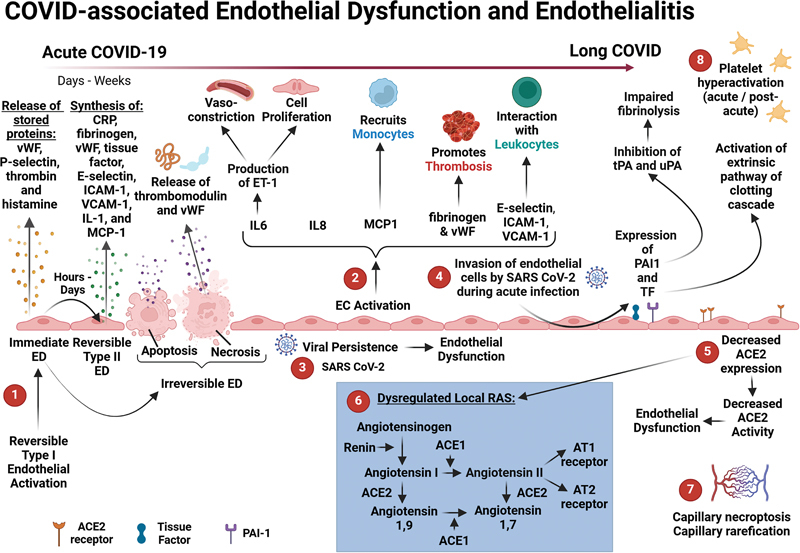
COVID-associated endothelial dysfunction and endothelialitis. Schematic representation of COVID-associated ED and endothelialitis. (1) Under pathological conditions, Type I and Type II endothelial activation—together with endothelial cell injury, such as endothelial cell apoptosis and necrosis—are all involved in ED. 39 (2) Endothelial activation promotes the production of various cytokines, chemokines, and other biomolecules that are involved in coagulation and the immune response. 40 (3) Viral persistence may contribute to ongoing endothelial inflammation or ED in Long COVID. 87 88 (4) After invasion of the endothelial cells by the virus, P-selectin, TF, ICAM1, VCAM1, and PAI-1 are expressed on these cells and ultra-large VWF multimers are also released. 58 (5) The downregulation of ACE2 favors ED 39 135 136 137 and leads to (6) a dysregulated local RAS with increased levels of Angiotensin II. 55 (7) Capillary rarefication (indicating an inflammatory response) has also been noted in patients suffering from Long COVID. 30 (8) Platelet hyperactivation is a key feature of a hypercoagulable state and has also been noted as a prominent feature in the pathobiology of acute infection 68 69 and Long COVID. 71 Created with BioRender.com. ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; ED, endothelial dysfunction; ICAM1, intercellular adhesion molecule 1; PAI-1, plasminogen activator inhibitor 1; RAS, renin–angiotensin system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TF, tissue factor; VCAM1, vascular cell adhesion molecule 1; VWF, Von Willebrand factor.
Type II endothelial activation or dysfunction (which is reversible) can occur over extended periods, such as hours or days, and involves the production of proteins including C-reactive protein, fibrinogen, VWF, tissue factor (TF), E-selectin, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and monocyte chemoattractant protein-1 (MCP-1). During type II endothelial activation/dysfunction, proteins are synthesized in specific cell compartments within the endothelial cells (including the endoplasmic reticulum, nucleus, or ribosomes). 39 40
Importantly, Type I endothelial cell activation/dysfunction is associated with the loss of anticoagulant molecules, whereas Type II endothelial cell activation/dysfunction results in procoagulant molecules being synthesized. 41 The imbalance (reduced levels of anticoagulant molecules and increased synthesis of procoagulant molecules) therefore promotes a prothrombotic state. 41 The endothelial pathological processes discussed above occur during acute COVID-19 infection 39 and may persist during Long COVID. 42
Widespread Endothelial and Coagulation Dysfunction during Acute COVID-19 Infection
The acute stage of COVID-19 disease is characterized by widespread ED, platelet hyperactivation, microthrombosis, and impairment of the microcirculation. 20 21 43 44 Autopsies of COVID-19 cases have also demonstrated endothelial inflammation and dysfunction as well as thrombotic events (notably microinfarcts and microthrombi in the cerebral neocortex). 45 46 47 48 Widespread microthrombi disseminated throughout the pulmonary vasculature, further support the argument that vasculopathy is important in COVID-19 pathogenesis. 13 20 While a detailed examination of the physiological pathways leading to ED during acute COVID-19 infection is beyond the scope of this study, we will briefly discuss several key processes.
Earlier SARS-CoV-2 variants such as the wild type COVID-19 virus (Wuhan strain), the Alpha variant (also known as B.1.1.7), Beta and Delta variants were associated with more severe acute disease and clinical presentations in comparison to Omicron. The variants prior to Omicron were also more vasculopathic. 49 50 51 52 During acute infection, the virus infects and replicates in multiple oral epithelial cell types. Intravascular viral delivery from the upper respiratory tract to the pulmonary vasculature serves to explain the vasculopathic characteristics and gravity-dependent vascular distribution of the acute phase lung disease, including the total lack of airway inflammation. 53 Apart from direct viral/endothelial angiotensin-converting enzyme 2 (ACE2) interaction triggering increases of angiotensin-II, the virus also invades endothelial cells via this receptor, disturbing vascular function. 54 55
One of the first papers on ED in acute COVID-19 was from Varga and coworkers, who demonstrated features of widespread ED with associated apoptosis in lung tissue obtained from a 69-year-old hypertensive patient who succumbed to the acute disease. 21 Zhang and coworkers suggest that endothelial necrosis leads to the release of thrombomodulin, as well as VWF, triggering further endothelial damage with subsequent vasculitis and thrombosis. 39
Cellular interaction between SARS-CoV-2 and endothelial ACE2 receptors has been proposed as a mechanism responsible for the phenomenon of immunothrombosis (inflammatory-mediated clotting in situ) in the acute phase of the lung disease. 19 54 56 ACE2 is utilized by SARS-CoV-2 to gain entry into host cells (including endothelial cells) and is involved in the renin–angiotensin–aldosterone system (RAAS) 57 ; as well as the renin–angiotensin system (RAS). 55 Endothelial cells exhibit activity for both RAS and RAAS, with both systems playing crucial roles in vascular function and homeostasis.
After invasion of endothelial cells by the virus, P-selectin, TF, ICAM-1, VCAM-1, and plasminogen activator inhibitor-1 (PAI-1) are expressed on these cells and ultra-large VWF multimers are also released. 58 A study by Montezano and coworkers demonstrated that the S1 subunit of the SARS-CoV-2 spike protein promotes the production of interleukin 6 (IL-6), MCP-1, ICAM-1, as well as PAI-1 in endothelial cells and in turn leads to the activation of nuclear factor kappa B (NF-κB) via ACE2 that is independent of the enzymatic activity of ACE2. 59 It is also known that NF-κB activation may result in oscillations in nuclear NF-κB abundance, 60 and therefore, its dynamics may suggest a significant inflammatory response to SARS-CoV-2 infection. 61 The S1 subunit of the SARS-CoV-2 spike protein causes acute lung injury and activation of the NF-κB inflammatory pathway 72 hours after exposure (as demonstrated in a COVID-19 murine model). 62 The aforementioned molecules together with NF-κB activation all contribute to endothelial inflammation ( Fig. 2 ).
Fig. 2.
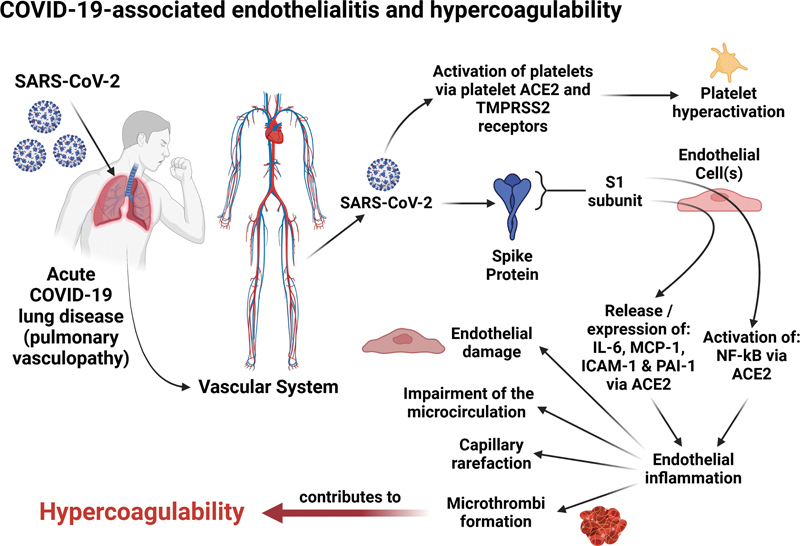
COVID-19-associated endothelialitis and hypercoagulability. Factors contributing to COVID-19-induced endothelialitis and hypercoagulability including platelet activation, cytokine release by endothelial cells from SARS-CoV-2 S1 subunit stimulation, and NF-κB activation leading to inflammation. Long-term effects include capillary rarefication and persistent fibrinaloid microclots, which may impair microcirculation and oxygen delivery. 27 30 32 59 72 Created with BioRender.com. ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; ICAM1, intercellular adhesion molecule 1; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; NF-κB, nuclear factor kappa B; PAI-1, plasminogen activator inhibitor 1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane protease serine 2 receptors; VCAM1, vascular cell adhesion molecule 1.
Upregulated inflammatory mediators can also indirectly contribute to endothelial cell injury by altering vessel barrier integrity, 63 causing the layers of the endothelium to develop a procoagulant phenotype. Increased levels of PAI-1 have been shown to be associated with increased COVID-19 disease severity and mortality. 64 65 Levels of tissue-type plasminogen activator (t-PA) and PAI-1 in patients with severe COVID-19 have been found to be significantly elevated and also demonstrate a positive correlation with neutrophil count and neutrophil activation. 66 PAI-1, including its cofactor vitronectin, are significantly increased in patients requiring hospitalization due to COVID-19 in comparison to non-COVID-19-related respiratory disease and healthy controls. 67 In addition, clot lysis times were increased in samples obtained from individuals with COVID-19. This can be attributed to inhibition of fibrinolysis secondary to increased levels of PAI-1. 67 Therefore, elevated levels of PAI-1 can contribute to a suboptimal fibrinolytic response in individuals with COVID-19. 67
Platelet hyperactivation and clotting pathology are key features of a hypercoagulable state and have also been noted as a prominent feature in the pathobiology of acute infection. 68 69 70 71 Direct binding of SARS-CoV-2 to platelets through ACE2 and transmembrane protease serine 2 receptors may also promote platelet activation, aggregation, adhesion, and platelet complex formation contributing to platelet hyperactivation. 72
The acute phase of the disease is thus marked by a significant dysregulation of molecules that may lead to endothelial disruption resulting in microthrombosis and compromised microcirculation. Several mechanisms contribute to profound ED during an acute COVID-19 infection, including the direct interaction of the virus with endothelial ACE2 receptors, increased levels of proinflammatory and procoagulant cytokines, and elevated endothelial damage molecules. This dysfunction can continue, evolving into ongoing dysregulation in Long COVID cases.
Widespread Endothelial and Coagulation Dysfunction Associated with Long COVID
In Long COVID, persistent endothelial cell dysfunction, clotting abnormalities (persistent microclot formation), and platelet hyperactivation 71 have been noted as significant and ongoing pathological processes ( Fig. 2 ), thought to be accompanied by viral (or spike protein) persistence, immunological factors, and latent virus reactivation. 6 7 58 73 74 Platelet complex formation has been noted between platelets (also known as platelet–platelet complexes) and between platelets and circulating blood cells 70 71 and they can also bind to endothelial cells. These interactions happen because of ongoing vascular ED that concurrently promotes platelet adhesion and hyperactivation through upregulation of inflammatory and adhesion molecules. 71
In Long COVID, endothelial cells exhibit apoptotic tendencies several months following the initial COVID-19 infection, resulting in impaired intercellular signaling between connexin channels in endothelial cells and vascular smooth muscle cells. 7 75 76 The protective glycocalyx matrix in the capillary endothelium, acting as a fluid barrier, may undergo shedding due to elevated inflammatory mediators, leading to significant alterations in microvascular resistance and capillary hemodynamics. 75 Syndecan-1 (SDC-1) might be another marker of importance, as it is an established parameter for measuring endothelial glycocalyx injury. 77 SDC-1 may serve as a reliable marker for measuring glycocalyx injury in Long COVID patients. 77 In their 2021 cross-sectional study, Vollenberg and coworkers examined SDC-1 levels in convalescent COVID-19 patients who experienced a mild disease course without hospitalization. 77 Comparisons were made with healthy individuals and hospitalized COVID-19 patients with mild acute disease. The results revealed significantly elevated SDC-1 levels in convalescent COVID-19 patients around 88 days after symptom onset in comparison to healthy controls. However, no significant difference was observed when compared with SDC-1 levels in hospitalized patients with acute disease. This study suggests ongoing endothelial damage in convalescent COVID-19 patients with mild disease progression, indicating persistent effects even without prior severe disease. 77
Recovering COVID-19 patients often exhibit elevated levels of endothelial cell biomarkers such as VWF and factor VIII. 78 Vascular transformation blood biomarkers are also of particular significance. Angiopoietin-1 (AGP-1) promotes blood vessel maturation and stability and has a protective function. AGP-1 and P-selectin levels together have been shown to have high accuracy in identifying those with Long COVID when compared to healthy controls and individuals with acute COVID-19. 79 Endothelin-1 (ET-1) is another peptide molecule produced by endothelial cells; it plays a significant role in regulating vascular tone and blood pressure. 80 It acts as a potent vasoconstrictor, causing blood vessels to narrow and leading to an increase in blood pressure. Elevated levels of ET-1 contribute to a harmful cycle of increased vasoconstriction, inflammation, oxidative stress, and vascular remodeling; all of which can lead to further ED and vascular disease. Both ET-1 and Angiopoietin-2 have been found to be dysregulated in Long COVID patients up to eight months after mild to moderate infection (the study population included patients with Long COVID and a subset of patients fulfilling criteria for myalgic encephalomyelitis/chronic fatigue syndrome). 73 Elevated levels of ET-1 were also observed in Long COVID patients at 5 months after acute infection, contrasting with those who had recovered from COVID-19 as well as healthy individuals. 73
Serum vascular endothelial growth factor-A (VEGF-A) has also been identified as a potential biomarker to identify individuals suffering from Long COVID. 81 The SARS-CoV-2 spike protein has the ability to bind to Neuropilin-1 (NRP-1), which acts as a coreceptor for VEGF-A. 81 Therefore, the binding of the spike protein to the NRP-1 receptor counteracts the binding of VEGF-A leading to increased levels of VEGF-A in circulation. Elevated levels of VEGF-A cause an imbalance in the pathways that play a role in angiogenesis and nociception, leading to microvascular and neurological damage. 81 These findings provide an explanation for the clinical manifestations and ongoing vasculopathy in individuals with Long COVID.
Various autoantibodies have been detected in Long COVID. 28 82 Angiotensin II type 1 receptor (AT1R) and endothelin A receptor (ETAR) autoantibodies are known to be associated with vascular abnormalities. 83 These autoantibodies have been shown to contribute to pronounced vasoconstriction and proinflammatory endothelial signaling because of their stimulatory effects. Both AT1R and ETAR have been found to be elevated in COVID-19 patients with poor outcomes. 84 Wallukat and coworkers identified autoantibodies in the sera of 31 patients who had recovered from COVID-19. 85 Twenty-nine patients were suffering from lingering symptoms and two were asymptomatic. In this study, AT1R and ETAR autoantibodies were also found to be present in some of these patients. Therefore, these autoantibodies may contribute to the vascular pathologies seen in individuals with Long COVID, 86 and the presence of autoantibodies may point to an autoimmune etiology in individuals with Long COVID.
Viral persistence may play a role in ongoing endothelial inflammation in Long COVID. 87 88 The latest and most current evidence clearly demonstrates prolonged viral persistence following infection with SARS-CoV-2. 89 A study by Peluso et al evaluated SARS-CoV-2 antigen positivity in the plasma of individuals during the post-acute phase of COVID-19, comparing it to plasma samples collected from individuals prior to the pandemic (prior to 2020). 90 Their data provide supporting evidence that SARS-CoV-2 persists within the body in some form or at a distant site for up to 14 months after acute infection. The implications of these findings would suggest that the consequences of endothelial injury are likely ongoing.
Oral dysbiosis may be of particular significance as it promotes gum disease, which in turn leads to the breakdown of the physical immune barrier of the oral cavity. 91 92 This in turn creates a potential vascular viral entry pathway for SARS-CoV-2; 93 oral dysbiosis is known to be associated with ED. 94 Oral capillary rarefication has also been noted in Long COVID patients using sublingual video microscopy. 30 This study of Long COVID patients with persistent symptoms, healthy volunteers, and critically ill COVID-19 patients demonstrated capillary rarefication even 18 months after infection. The study indicated reduced vascular density and microvascular health scores, suggesting potential long-term vascular impacts irrespective of disease severity in the acute phase and concluded that microvascular impairment plays a crucial role in both acute COVID-19 and post-acute sequelae. 30 The studies reviewed in the preceding paragraphs have shown that sustained, widespread, and persistent vascular dysfunction is present in Long COVID. 95 Microclots might be of importance in a subset of Long COVID patients where clotting abnormalities, accompanied by persistent vascular pathology, have been noted.
The Role of Microclots
Notably, the S1 subunit of the spike protein is both amyloidogenic 96 and proinflammatory. 97 98 99 Direct interactions between SARS-CoV-2, fibrin(ogen), and also platelets, can induce modifications in fibrinogen structure and promote a state of hypercoagulability, 100 accompanied by widespread ED. Even the lipid membrane of SARS-CoV-2 has been shown to be procoagulant in vitro. 101
Plasma protein pathology (resulting in fibrinaloid microclot formation) may be interlinked with the development of widespread ED and may contribute to a complex interplay between endothelialitis and a dysregulated coagulation system. Microclots have been observed in both acute COVID-19 68 102 and Long COVID 28 103 104 and can be identified using thioflavin T, an amyloid protein marker traditionally employed to detect amyloid protein in Alzheimer's dementia and other established amyloidosis. 105 Interestingly, the Omicron variant causes fewer microclots than earlier variants—thereby implying that microclots are on the disease pathway. 52 Although that is the case, the role of the Omicron variant in disease causation should not be underestimated. A study published by Xie and coworkers determined that the cumulative incidence of PASC within the first year after SARS-CoV-2 infection declined as the pandemic progressed; however, the risk of PASC remained significant, even among vaccinated individuals infected during the period where the Omicron variant was more prevalent. 106
In the context of Long COVID, a key question has been the extent to which widespread endothelialitis, the presence of persistent circulating microclots, and platelet hyperactivation may continue beyond the acute infection, and whether these phenomena are associated with or contribute to persistent symptoms. 7 58 Numerous studies have consistently demonstrated and discussed ongoing pathological coagulation in Long COVID. 43 58 107 108 109 110 Disturbances in the vasculature and the presence of microclots as well as platelet hyperactivation have the potential to impede the delivery of oxygen to tissues, accounting for many Long COVID symptoms. 26 32
Proteomics methodologies, as well as analysis of the content of microclots, have provided novel insight into the characteristics of microclots. Microclots exhibit resistance to dissolution (by trypsin) and entrap various inflammatory molecules including VWF, serum amyloid A and α2-antiplasmin (α2AP). 27 28 103 α2AP plays a crucial role in the lysis pathway, preventing the breakdown of clots; it is also a well-known inhibitor of plasmin, which degrades fibrin. Factor XIIIa (FXIIIa) can incorporate α2AP into fibrin. 111 Hence, α2AP is an effective inhibitor of fibrinolysis after incorporation into fibrin by FXIIIa, and t-PA-induced fibrinolysis is inhibited by unbound α2AP. 112 This inhibition of fibrinolysis is dependent on the amounts of α2AP cross-linked to fibrin, and therefore, higher amounts of α2AP incorporated into microclots readily explain their resistance to fibrinolysis.
Long COVID is also characterized by impaired oxygen delivery at cellular level, 113 and the presence of ubiquitous clotting pathology and endothelialitis thus provide a ready explanation. 32 Specifically, it is proposed that microclots block (or at least partially block) the microcirculation, inducing ischemia and hypoxia in affected tissues. This may, in turn, lead to hypoxia-dependent reactivation of latent viruses. 114 115
Antibodies have also been found entrapped inside microclots 28 as well as detected in the circulation of individuals. 85 Antibodies may also mediate endothelial cell activation, assessed by increased expression of molecules like VWF, complement activation, E-selectin, VCAM-1, and ICAM-1. 58 Sustained endotheliopathy, increased VWF and plasma FVIII:C levels, increased thrombin generation time, 108 and VWF/ADAMTS-13 axis imbalance have also been noted. 78 Furthermore, the formation of autoantibodies—specifically antiphospholipid antibodies—contributes to the heightened activation of endothelial cells, complement, and coagulation pathways, further enhancing the propensity for microclot formation. 58 82 Microclot presence (and in particular their proinflammatory content, including antibodies), as well as hyperactivated platelets, and widespread ED, provide a plausible explanation for the various manifestations of widespread tissue-specific dysfunction observed in Long COVID. 26 32 82
Radiology in Acute COVID-19 and Long COVID
The radiological features of acute COVID-19 lung disease help establish an understanding of pathophysiology in Long COVID ( Fig. 3 ). Acute COVID-19 lung disease is not a conventional pneumonia characterized by airway inflammation visible on computed tomography (CT) scans. 12 116 Rather, radiological features indicate vasculopathic phenomena, which act as surrogates of endothelial disruption and microangiopathic thromboembolic events. 9 10 11 The term “pulmonary vasculopathy” is preferred to “airways pneumonia” because of the dominant features of pulmonary vascular congestion secondary to microangiopathic processes, including immunothrombosis. 10 11 12 16 117 Thrombotic/immunothrombotic processes occur in a different distribution from that seen in conventional pulmonary thromboembolic disease. 9 15 Dual-energy CT (DECT) reveals vascular perfusion defects as a universal finding in patients hospitalized with respiratory symptoms. 9 11 Importantly, these acute phenomena are also found in the postacute phase with macroscopic thrombi (7.5%) and perfusion defects (87%), indicating persistent hypercoagulability and unresolved microangiopathy. 118 Low-field strength magnetic resonance imaging also shows reduced lung perfusion in Long COVID. 119 In the context of suspected pulmonary sequelae of COVID-19, imaging modalities, which can detect pulmonary perfusion defects—ventilation/perfusion scans, single-photon emission computed tomography, or DECT—are proposed as methods to evaluate residual clot burden and microvascular injury. 120
Fig. 3.
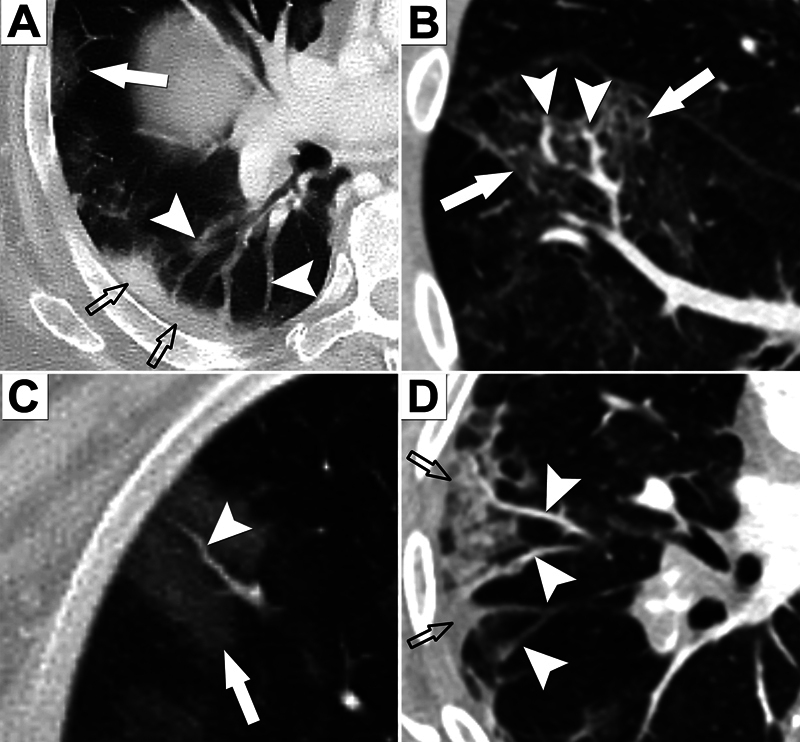
CT scans in acute COVID-19 lung disease. The scans are characterized by vascular phenomena in a vascular-dependent distribution. The airways are not inflamed (no bronchial wall thickening or mucous secretion plugging). ( A ) Peripheral GGOs (arrows) and consolidation (open arrows) accompanied by dilated pulmonary vessels (arrowheads). ( B ) GGOs accompanied by dilated vessels. ( C ) Peripheral lung GGO and dilated vessel. ( D ) Peripheral wedge-shaped areas of GGO/consolidation with dilated vessels indicating pulmonary vascular congestion. CT, computed tomography; COVID-19, coronavirus disease 2019; GGO, ground-glass opacification(s).
Other Special Investigations to Consider in Patients with Long COVID
Flow-Mediated Dilation
FMD refers to the change in conduit artery diameter as a result of shear-stress-induced release of endothelial-derived vasoactive mediators. 121 FMD was initially described by Celermajer et al in 1992. 122 The authors demonstrated a difference in FMD values for young children and adults with risk factors for coronary artery disease, in the absence of anatomical evidence of plaque formation. The finding was seminal in suggesting that early endothelial abnormality likely precedes the evolution to vasculopathy. The essence of this metric is the use of noninvasive ultrasound to determine the magnitude of vasodilation in response to reactive hyperemia. FMD is undertaken by cuff inflation (5 minutes) and deflation. The maneuver induces endothelial-dependent dilation. To contrast with endothelial-independent dilation, the test is repeated with a standard dose of sublingual nitroglycerine (at an anti-anginal dose of 400 µg). Scanning is performed at 30 and 90 seconds postdeflation. Patients are studied when fasting to avoid changes induced by a high-fat meal. Measurement of the target artery diameter is obtained through analysis of B-mode ultrasound images captured at the focal point of the artery, which is identified based on achieving optimal visualization of the anterior and posterior intimal layers. For reasons of responsiveness and access to an appropriate arterial diameter, the brachial artery is most desirable ( Fig. 4 ). Raitakari and Celermajer 121 defined this technique in a later paper and described the outcomes of pharmacological interventions, namely statins, ACE inhibitors, antioxidant vitamins and folic acid, and estrogens.
Fig. 4.
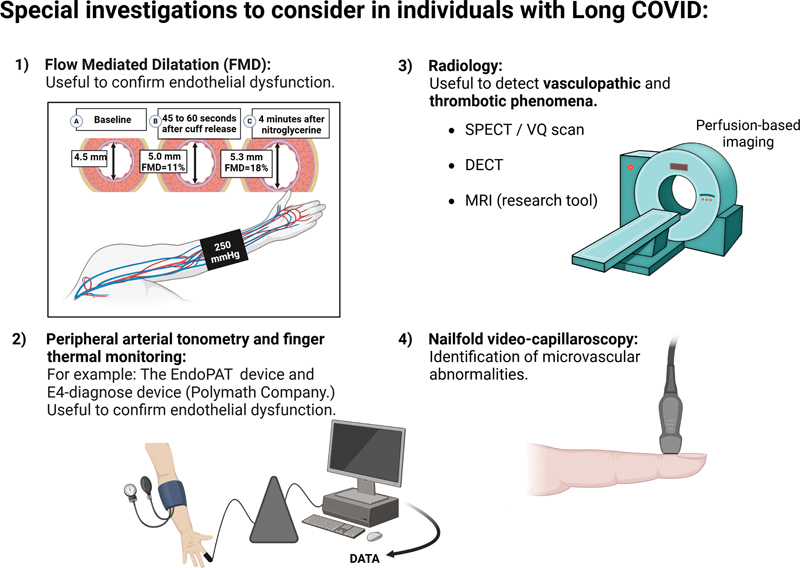
Special investigations to consider in individuals with Long COVID. Techniques such as (1) flow-mediated dilation (FMD) can be used to confirm ED in subjects suffering from Long COVID. (The brachial artery is measured during three conditions [A] at baseline, after at least 10 minutes supine rest; [B] during reactive hyperemia—induced by inflation of a sphygmomanometer cuff to 250 mm Hg for 5 minutes around the forearm and then deflation—and [C] after administration of sublingual nitroglycerin. A linear array, high resolution ultrasound transducer is used to provide B-mode images of the target vessel, proximal to the forearm cuff.) (2) Peripheral arterial tonometry can also be used to confirm ED. Perfusion-based imaging, such as SPECT, VQ, or DECT (3) can detect vasculopathic and thrombotic phenomena in individuals with persistent respiratory symptoms. (4) Nailfold video capillaroscopy is a useful technique to detect microvascular abnormalities. Created with Biorender.com. COVID, coronavirus disease; DECT, dual-energy computed tomography; ED, endothelial dysfunction; FMD, flow-mediated dilation; MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography; VQ, ventilation/perfusion scans.
Raitakari and coworkers reviewed the data for interventions that were supportive of improved endothelial function, including statins, ACE inhibitors, antioxidant vitamins and folic acid, and estrogens. 121 Encouraging results were seen in many of these interventions in terms of improved FMD, suggesting a role for these agents in endothelial repair. Because of the variability of the test according to factors such as age and sex, there is considerable variation between laboratories, making comparisons between laboratories problematic for purposes of cross-refencing results. Consequently, there have been two consensus papers published to standardize the methodology. 123 124 Based upon these two consensus papers, 123 124 Holder and coworkers 125 published reference intervals for FMD and the relationship to cardiovascular risk factors. They were able to determine a negative curvilinear relationship with FMD and age, and that there is an age-related sex difference, which may relate to the brachial artery diameter in women. It is notable that there is a range of FMD reference intervals between the sexes and older patients. There is also the need to normalize for preexisting hypertension and/or dyslipidemia.
To further refine the value of FMD, Maruhashi and coworkers examined normative FMD values, seeking to establish cutoff values for those without known risk factors and those with known cardiovascular disease risks. 126 Using pooled data from multiple sites with strict controls, the researchers successfully published receiver operating characteristics for FMD, allowing them to calculate the optimum cutoff. For the “no-risk” group, the determined cutoff was 15.6%, resulting in an area under the curve of 0.77. This corresponds to a sensitivity of 0.64 and a specificity of 0.77. A study by Heiss et al also published age-adjusted reference ranges for FMD in apparently healthy individuals, designed to serve as a biomarker for cardiovascular health. 127
There is at least one meta-analysis of FMD in Long COVID. 128 Twelve studies with 622 control and 644 convalescent patients were pooled and analyzed. FMD values were considerably lower in the Long COVID group (mean difference [MD]: −2.31%; 95% confidence interval [CI]: −3.19, −1.44; p < 0.0001). To further define this risk, the analysis was reevaluated to control for known risk factors. The results were significantly different (MD: −1.73%; 95% CI: −3.04, −0.41; p = 0.010). The authors subsequently ran a meta-regression analysis to classify Long COVID after 3 months and long-term follow-up. The modelling clearly demonstrated that there was a persistent difference in FMD between cases and controls (Z-score: −2.09; p = 0.037). 128
There is persistent endothelial injury in Long COVID patients still demonstrable at 12 months, and targeted therapies for endothelial injury (statins and blood pressure control) are critical to improving outcomes. A similar finding was confirmed in 86 COVID-19 survivors compared with 28 age- and sex-matched controls and 30 risk factor-matched controls in a study published by Gao et al. 129 Brachial artery FMD was considerably lower in COVID-19 survivors than risk-matched controls 6.9% (5.5–9.4%) and healthy controls 7.7% (5.1–10.7%). Notably, this study included a measure of tumor necrosis factor-α (TNF-α). There was an inverse correlation with serum TNF-α and FMD ( r = − 0.237, p = 0.007), suggesting that persistent serologic markers of inflammation were a concurrent feature of vascular endothelial injury.
From these methods, it is evident that FMD is a reliable marker of ED. It has been demonstrated to be an early and important marker for future cardiovascular disease. The ED seen in Long COVID is associated with markers of persistent inflammation, and the early presence of abnormal FMD, which is still demonstrable at 12 months is likely to be a factor in a significant number of early cardiovascular and cerebrovascular events within the first 3 months of COVID-19 infection. Furthermore, the evidence of ongoing abnormal vascular function as demonstrated by markedly abnormal FMD at 12 months would suggest that early intervention is warranted, and that further studies of statins and other endothelial protective therapies could be critical in preventing substantial long-term vascular disease and sequelae of infection.
Peripheral Arterial Tonometry and Finger Thermal Monitoring
Alternative methods of noninvasive determinants of endothelial injury include peripheral arterial tonometry. Commercially available tools include the EndoPAT devices (Itamar Medical, Caesarea, Israel; https://www.itamar-medical.com/professionals/endopat/ ). While this is considered a metric of endothelial function, the pulse amplitude after the reactive hyperemia is complex and the microcirculation of the fingers is partially dependent on nitric oxide. 130 Although it has been validated to correlate with microvascular function and as a predictor of cardiovascular events, it is yet to be evaluated in more substantial numbers and a broader range of Long COVID subjects.
There is at least one publication from Cimino and coworkers evaluating ED in COVID-19 patients with the Endo-PAT 2000. 130 This study had a small sample (6 patients including 5 females with a mean age of 75.8 years) and there was no control group; however, the authors do suggest that ED was demonstrated. More studies are required to define the value of this technology in Long COVID.
Charfeddine and coworkers used finger thermal monitoring as a noninvasive measurement of endothelial function in patients suffering from Long COVID. 74 They were able to demonstrate an association between Long COVID symptoms and ED; however, this requires further investigation. They also highlighted the value of sulodexide as a therapy targeting the ED. The instrument used to evaluate finger thermal monitoring was the E4-diagnose device (Polymath Company, https://www.polymath.company/E4-Diagnose.html ).
Nailfold Video Capillaroscopy
Nailfold video capillaroscopy is a noninvasive imaging technique used to examine the capillaries in the nailfold area. 131 The method involves the use of a microscope equipped with a video camera to magnify and capture images of the capillaries. Detailed observation of capillary structure, density, and blood flow allows for the diagnosis and monitoring of diseases that affect the microcirculation. This technique may be useful in individuals suffering from Long COVID. Assessment of the microvasculature through nailfold video capillaroscopy in COVID-19 patients indicates that microvascular abnormalities are observed. 37 Presently symptomatic and convalescent individuals exhibit distinct patterns of elementary capillaroscopic changes, mirroring acute and post-acute microvascular impairment. Additional research is required to determine the clinical significance of capillaroscopy in the context of COVID-19.
Potential Therapeutic Agents that Could be Useful in the Context of Long COVID
In our current understanding of Long COVID pathophysiology we consider persistent ED, platelet hyperactivation and fibrinaloid microclots to be central in the persistent manifestations and symptoms associated with widespread organ damage seen in Long COVID. However, it should be acknowledged that much more remains to be understood. For a discussion on the manifestations of organ damage in Long COVID, see https://whn.global/scientific/spectrum-of-covid-19-from-asymptomatic-organ-damage-to-long-covid-syndrome/ . If the exact pathological mechanisms involved in Long COVID could be unraveled, more targeted therapy would be possible. Several therapeutics have shown promise in the treatment of Long COVID. Anticoagulants such as non-vitamin K antagonist oral anticoagulants and antiplatelet agents, for example aspirin and clopidogrel, have demonstrated potential in individuals with Long COVID, 132 although the drug combinations need be investigated in a trial. Clinical trials have been initiated to find possible treatments for subjects suffering from Long COVID; unfortunately, no standard of care currently exists for the condition. Agents such as statins, known for their anti-inflammatory 133 and endothelial protective effects, 134 may be promising in individuals with Long COVID. An important consideration is the necessity to develop a standardized rubric for clinical diagnosis that includes consensus diagnostic criteria that covers the current spectrum of symptoms and manifestations of Long COVID. See Table 1 for a summary of Long COVID manifestations and promising, possible and adjunctive therapeutic agents that may be of value in the treatment of Long COVID.
Table 1. Long COVID manifestations, affected organ systems, and therapeutic agents that may be effective in the treatment of Long COVID.
| Long COVID manifestations https://whn.global/scientific/spectrum-of-covid-19-from-asymptomatic-organ-damage-to-long-covid-syndrome/ | ||||
|---|---|---|---|---|
| Affected organ system | Manifestations | |||
| Endothelial cells and blood vessels | Formation of microthrombi, effects on endothelial layers of all organ systems, including lungs, heart, kidneys, liver, muscle | |||
| Heart | Reduced blood oxygen, inflamed atherosclerotic plaques, infection of coronary vessels, ischemia, reports of heart attacks | |||
| Brain | Loss of white and gray matter, disruption of the blood–brain barrier, autonomic nervous system disruptions, ischemic stroke, intracranial hemorrhage, early onset of dementia, Alzheimer's disease, and Parkinson's disease | |||
| Endocrine system | Effects on tissues via ACE2 binding, Type I and II diabetes, thyroid disease, reproductive hormone dysfunction, fertility issues, disrupted menstrual function, adrenal insufficiency | |||
| Immune system | Effects on T-cells, B-cells, dendritic cells, monocytes, platelets | |||
| Therapeutics that may be effective in the treatment of Long COVID | ||||
| Drug class | Known action | Usual indication | Level of evidence | Possible uses in Long COVID (pending trial data) |
| Promising therapies | ||||
| Non-vitamin K antagonist oral anticoagulants (NOACs) | Anticoagulant | AF/ PE | Standard of care | Possibly useful as future clinical trial agents as anticoagulant treatment Clinician-initiated treatment regimens suggest it to be useful. Clinical trials should be done |
| Aspirin | Anti-platelet activity | IHD; Post NOAC for PE | Standard of care |
Possibly useful in platelet antagonist.
70
71
Found to be useful in acute COVID-19
71
Clinician-initiated treatment regimens suggest it to be useful. Clinical trials should be done |
| Clopidogrel | Reduced platelet aggregation | Unstable angina; IHD with stent | Standard of care |
Possibly useful in platelet antagonist.
70
71
Found to be useful in acute COVID-19 Clinician-initiated treatment regimens suggest it to be useful. Clinical trials should be done |
| Selective serotonin reuptake inhibitors | Antidepressant | Depression | Excellent | Possibly useful for anxiety associated with Long COVID. Possible anti-platelet effect 135 |
| Statins | Cholesterol lowering | Dyslipidemia | Standard of care | Essential for COVID-related dyslipidemia. Known endothelial protective effects |
| Probiotics (AB21) | Gut dysbiosis | Supplement | Good | Possibly useful in Long COVID. Proven to reduce colonic COVID-19 and increase fecal shedding. 136 |
| Colchicine | Anti-inflammatoryAnti-mitotic | Serositis | Good |
Demonstrated value in pericarditis
137
and pleurisy
Possibly useful in pericarditis and pleurisy associated with Long COVID |
| Budesonide | Steroid | Asthma | Good | No evidence for benefit outside of asthma but could be useful 138 |
| Ivabradine | Negative chronotrope | NYHA Class 3–4 CCF | Good | Proven benefit in POTS. 139 (possibly helpful in Long COVID-associated POTS) |
| Midodrine | Pressor agent | POTS | Established | Possibly useful in Long COVID-associated POTS 140 |
| Metformin | Oral hypoglycemic | Type II diabetes | Established | Benefit in diabetes related to COVID-19 Reduced risk of developing Long COVID 141 Benefit in diabetes related to COVID-19 under evaluation 142 |
| Biologics | Various | Sero-positive/negative arthropathy | Established | Possibly useful in Long COVID arthritis Useful for CRS in COVID-19 143 |
| ACE Inhibitors | ACE1 antagonists | Hypertension | Standard of care | Possibly useful in treating hypertension secondary to Long COVID. 144 No independent effect |
| Modafinil/armodafinil | Dopaminergic wakefulness promoter | Narcolepsy | Standard of care | Possibly useful excessive somnolence 145 Off-label usage need to be established |
| Melatonin | Regulates circadian rhythm Established evidence in jetlag and insomnia |
Insomnia/sleep rhythm regulation REM behavior disorder |
Established | Possibly useful to for sleep in Long COVID. Possibly useful as endothelial protection in Long COVID Attenuates ox-LDL-induced ED by reducing ER stress and inhibiting JNK/Mff signaling 146 May inhibit apoptosis, increase mitochondrial membrane potential, and increase autophagy of myocardial microvascular endothelial cells under hypertensive state 147 Helpful for sleep and REM sleep behavior disorder 148 |
| Possible therapies | ||||
| Warfarin | Anticoagulant | AF/pulmonary embolus | Standard of care | Possibly useful as anticoagulation treatment, however, not tested 149 |
| Nattokinase | Fibrinolytic | Supplement | N/A | Many positive reports from patients—not tested |
| 5HT-1 antagonists, mast cell stabilizers and leukotriene receptor antagonists | Antihistamines, mast cell stabilization | Allergy | Established | Possibly useful in Long COVID associated with MCAS |
| Omalizumab | Anti IgE | Severe asthma with eosinophilia | Established | Likely to be valuable in Long COVID associated with MCAS 150 and in those with food allergies 151 |
| Mepolizumab | Anti IL-5 | Severe asthma with eosinophilia | Established | Possibly useful in Long COVID associated with MCAS. 152 No trial data available could be useful to investigate |
| Sulodexide | Anticoagulant and antithrombotic action | Peripheral arterial thrombosis, venous thrombosis, as well as treatment of venous leg ulcers and intermittent claudication | Established | Significantly improves ED in individuals with Long COVID 74 153 |
| Adjunctive therapies | ||||
| 5HT-2 antagonists | Acid reduction | Reflux | Standard of care | Possibly useful for GORD related to dysautonomia and vagal dysfunction in Long COVID Useful as prophylaxis when using anticoagulation and antiplatelet therapy |
| Proton pump inhibitors | Acid reduction | Reflux | Standard of care | Possibly useful for GORD related to dysautonomia and vagal dysfunction in Long COVID Useful as prophylaxis when using anticoagulation and antiplatelet therapy |
Abbreviations: 5HT-1, serotonin; ACE1, angiotensin-converting enzyme 1; ACE2, angiotensin-converting enzyme 2; AF, atrial fibrillation; CCF, congestive cardiac failure; COVID, coronavirus disease; CRS, cytokine release syndrome; ED, endothelial dysfunction; ER, endoplasmic reticulum; GORD, gastroesophageal reflux disease; IHD, ischemic heart disease; LDL, low-density lipoprotein; MCAS, mast cell activation syndrome; NOACs, non-vitamin K antagonist oral anticoagulants; NYHA, New York Heart Association; PE, pulmonary embolus; POTS, postural orthostatic tachycardia syndrome; REM sleep, rapid eye movement sleep.
Conclusion
In the light of the ongoing global health crisis due to SARS-CoV-2, the emergence of Long COVID has presented the medical community with a formidable challenge. The condition, characterized by a multitude of debilitating symptoms, functional impairment, and objectively detectable pathology, along with the cumulative effects of reinfections that persist long after the acute phase of an infection due to SARS-CoV-2, does not yet have any proven treatments.
Long COVID is a complex and multifaceted condition with mounting evidence suggesting that ED and vasculopathy lie at its core. The vascular phenomena observed, primarily driven by procoagulant microangiopathic processes and ED, underscore the pivotal role of the endothelium in the disease's pathophysiology. The present review makes a strong case that persistent thrombotic endothelialitis is one of the potential primary pathologies driving the chronicity of Long COVID. The presence of a denuded vascular endothelium as a catalyst of fibrinaloid microclot formation combined with capillary rarefication could explain a significant proportion of the symptoms and clinical manifestations associated with the condition. A dire need exists for a better understanding of Long COVID pathophysiology to make definitive treatment possible for these individuals. A summary of key take-home points for clinicians is also provided ( Table 2 ). A considerable international effort is required for all nations and economies to better understand and appreciate the vast pathophysiological mechanisms and sequelae of this disease.
Table 2. Take-home pointers for clinicians.
| Endothelial health assessment |
|---|
| Given our presentation of evidence for the central role of endothelialitis in Long COVID, clinicians should consider routine assessment of endothelial health using tools such as endothelium-dependent FMD, peripheral arterial tonometry and capillaroscopic analysis. These assessments can help identify individuals at risk for Long COVID or monitor those already affected |
| Imaging |
| Conventional tools such as chest radiographs and CT scans are not sensitive for making the diagnosis of pulmonary abnormalities associated with Long COVID. In some patients, especially those previously hospitalized with acute COVID-19, the use of perfusion-based imaging modalities may demonstrate persistent vasculopathic dysfunction in the lungs |
| Targeted therapeutic approaches |
| The recognition of persistent thrombotic endothelialitis as a potential driver of Long COVID calls for the exploration of targeted therapeutic interventions aimed at mitigating ED. Clinicians should stay informed about emerging treatment strategies in this regard |
| Genetic profiling |
| Understanding the role of host genetic polymorphisms in Long COVID symptom diversity underscores the importance of genetic profiling for personalized treatment plans. Clinicians should consider incorporating genetic testing into their diagnostic and treatment protocols |
| Long-term care and management |
| Long COVID requires long-term care and management. Clinicians should adopt a holistic approach, addressing not only the acute symptoms but also the potential long-term consequences of ED |
| Key takeaway |
| One crucial takeaway from our analysis is the urgent need to recognize the centrality of ED in Long COVID. Further, the intermittent character of Long COVID symptoms remains a puzzle that requires further investigation. It is evident that host genetic polymorphisms play a pivotal role in determining the diverse clinical symptomatology experienced by Long COVID sufferers, highlighting the need for personalized approaches to treatment and management. A complete understanding of factors such as oral dysbiosis and viral persistence in the upstream causation of ED is yet to be elucidated but should be an urgent focus |
Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; ED, endothelial dysfunction; FMD, flow-mediated dilation.
In the absence of supportive science and validated studies of various therapeutic modalities, the scale and severity of the problem will continue to mount without efforts to mitigate and reduce infections and repeated infections.
Acknowledgments
D.B.K. thanks the Balvi Foundation (grant number: 18) and the Novo Nordisk Foundation for funding (grant number: NNF20CC0035580). The content and findings reported and illustrated are the sole deduction, view, and responsibility of the researchers and do not reflect the official position and sentiments of the funders.
Funding Statement
Funding E.P.: Funding was provided by National Research Foundation of South Africa (grant number: 142142) and South African Medical Research Council (self-initiated research [SIR] grant), and Balvi Foundation (grant number: B31).
Footnotes
Conflict of Interest E.P. is a named inventor on a patent application covering the use of fluorescence methods for microclot detection in Long COVID. G.L.J.: Director of Radiology Masterclass. The other authors have no conflict of interest to declare.
Supplementary Material
References
- 1.Global Burden of Disease Long COVID Collaborators . Wulf Hanson S, Abbafati C, Aerts J G et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–1615. doi: 10.1001/jama.2022.18931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Academies of Sciences, Engineering, and Medicine. . Washington, DC:: The National Academies Press;; 2024. A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences. [PubMed] [Google Scholar]
- 3.Ewing A G, Salamon S, Pretorius E et al. Review of organ damage from COVID and Long COVID: a disease with a spectrum of pathology. Med Rev. 2024 doi: 10.1515/mr-2024-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Clinical Case Definition Working Group on Post-COVID-19 Condition . Soriano J B, Murthy S, Marshall J C, Relan P, Diaz J V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(04):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritsche L G, Jin W, Admon A J, Mukherjee B. Characterizing and predicting post-acute sequelae of SARS CoV-2 infection (PASC) in a large academic medical center in the US. J Clin Med. 2023;12(04):1328. doi: 10.3390/jcm12041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proal A D, VanElzakker M B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12:698169. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis H E, McCorkell L, Vogel J M, Topol E J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(03):133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28(07):1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang M, Som A, Carey D et al. Pulmonary vascular manifestations of COVID-19 pneumonia. Radiol Cardiothorac Imaging. 2020;2(03):e200277. doi: 10.1148/ryct.2020200277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel B V, Arachchillage D J, Ridge C A et al. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202(05):690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridge C A, Desai S R, Jeyin N et al. Dual-energy CT pulmonary angiography (DECTPA) quantifies vasculopathy in severe COVID-19 pneumonia. Radiol Cardiothorac Imaging. 2020;2(05):e200428. doi: 10.1148/ryct.2020200428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd-Jones G, Alcock R, Oudkerk M. COVID-19 lung disease is a pulmonary vasculopathy. Clin Radiol. 2024;79(07):e975–e978. doi: 10.1016/j.crad.2024.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Carsana L, Sonzogni A, Nasr A et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox S E, Akmatbekov A, Harbert J L, Li G, Quincy Brown J, Vander Heide R S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(07):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dam L F, Kroft L JM, van der Wal L I et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddy R L, Sin D D. Computed tomography vascular tree-in-bud: a novel prognostic imaging biomarker in COVID-19? Am J Respir Crit Care Med. 2020;202(05):642–644. doi: 10.1164/rccm.202007-2833ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemec S F, Bankier A A, Eisenberg R L. Lower lobe-predominant diseases of the lung. AJR Am J Roentgenol. 2013;200(04):712–728. doi: 10.2214/AJR.12.9253. [DOI] [PubMed] [Google Scholar]
- 18.Deinhardt-Emmer S, Wittschieber D, Sanft J et al. Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and the correlation with tissue damage. eLife. 2021;10:e60361. doi: 10.7554/eLife.60361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonaventura A, Vecchié A, Dagna L et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(05):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackermann M, Verleden S E, Kuehnel M et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(02):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga Z, Flammer A J, Steiger Pet al. Endothelial cell infection and endotheliitis in COVID-19 Lancet 2020395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkel M, Weikert T, Marston K et al. Lethal COVID-19: radiologic-pathologic correlation of the lungs. Radiol Cardiothorac Imaging. 2020;2(06):e200406. doi: 10.1148/ryct.2020200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kianzad A, Meijboom L J, Nossent E J et al. COVID-19: histopathological correlates of imaging patterns on chest computed tomography. Respirology. 2021;26(09):869–877. doi: 10.1111/resp.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabretta E, Moraleda J M, Iacobelli M et al. COVID-19-induced endotheliitis: emerging evidence and possible therapeutic strategies. Br J Haematol. 2021;193(01):43–51. doi: 10.1111/bjh.17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu S W, Ilyas I, Weng J P. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2023;44(04):695–709. doi: 10.1038/s41401-022-00998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kell D B, Laubscher G J, Pretorius E. A central role for amyloid fibrin microclots in Long COVID/PASC: origins and therapeutic implications. Biochem J. 2022;479(04):537–559. doi: 10.1042/BCJ20220016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pretorius E, Vlok M, Venter C et al. Persistent clotting protein pathology in Long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20(01):172. doi: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruger A, Vlok M, Turner S et al. Proteomics of fibrin amyloid microclots in Long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol. 2022;21(01):190. doi: 10.1186/s12933-022-01623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosei C A, Gaggero A, Famà F et al. Skin capillary alterations in patients with acute SarsCoV2 infection. J Hypertens. 2022;40(12):2385–2393. doi: 10.1097/HJH.0000000000003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osiaevi I, Schulze A, Evers G et al. Persistent capillary rarefication in Long COVID syndrome. Angiogenesis. 2023;26(01):53–61. doi: 10.1007/s10456-022-09850-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wardlaw J M, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18(07):684–696. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- 32.Kell D B, Pretorius E. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, Long COVID, and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J. 2022;479(16):1653–1708. doi: 10.1042/BCJ20220154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mclaughlin M, Sanal-Hayes N EM, Hayes L D, Berry E C, Sculthorpe N F.People with Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome exhibit similarly impaired vascular function. Am J Med 2023:S0002-9343(23)00609-5 [DOI] [PubMed]
- 34.Chopoorian A H, Wahba A, Celedonio J et al. Impaired endothelial function in patients with postural tachycardia syndrome. Hypertension. 2021;77(03):1001–1009. doi: 10.1161/HYPERTENSIONAHA.120.16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakubowski M, Turek-Jakubowska A, Szahidewicz-Krupska E, Gawrys K, Gawrys J, Doroszko A. Profiling the endothelial function using both peripheral artery tonometry (EndoPAT) and laser Doppler flowmetry (LD) - complementary studies or waste of time? Microvasc Res. 2020;130:104008. doi: 10.1016/j.mvr.2020.104008. [DOI] [PubMed] [Google Scholar]
- 36.Çakmak F, Demirbuga A, Demirkol D et al. Nailfold capillaroscopy: a sensitive method for evaluating microvascular involvement in children with SARS-CoV-2 infection. Microvasc Res. 2021;138:104196. doi: 10.1016/j.mvr.2021.104196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Natalello G, De Luca G, Gigante L et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement. Microvasc Res. 2021;133:104071. doi: 10.1016/j.mvr.2020.104071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mondini L, Confalonieri P, Pozzan R et al. Microvascular alteration in COVID-19 documented by nailfold capillaroscopy. Diagnostics (Basel) 2023;13(11):1905. doi: 10.3390/diagnostics13111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Tecson K M, McCullough P A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev Cardiovasc Med. 2020;21(03):315–319. doi: 10.31083/j.rcm.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 40.Gimbrone M A, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(04):620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Defelice A F, Hanig J P, Colatsky T. Biomarkers of endothelial cell activation serve as potential surrogate markers for drug-induced vascular injury. Toxicol Pathol. 2010;38(06):856–871. doi: 10.1177/0192623310378866. [DOI] [PubMed] [Google Scholar]
- 42.Durstenfeld M S, Weiman S, Holtzman M, Blish C, Pretorius R, Deeks S G. Long COVID and post-acute sequelae of SARS-CoV-2 pathogenesis and treatment: a Keystone Symposia report. Ann N Y Acad Sci. 2024;1535(01):31–41. doi: 10.1111/nyas.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jing H, Wu X, Xiang M, Liu L, Novakovic V A, Shi J. Pathophysiological mechanisms of thrombosis in acute and Long COVID-19. Front Immunol. 2022;13:992384. doi: 10.3389/fimmu.2022.992384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huertas A, Montani D, Savale L et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56(01):2.001634E6. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conway E M, Mackman N, Warren R Q et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22(10):639–649. doi: 10.1038/s41577-022-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fahmy O H, Daas F M, Salunkhe V et al. Is microthrombosis the main pathology in coronavirus disease 2019 severity? A systematic review of the postmortem pathologic findings. Crit Care Explor. 2021;3(05):e0427. doi: 10.1097/CCE.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haberecker M, Schwarz E I, Steiger P et al. Autopsy-based pulmonary and vascular pathology: pulmonary endotheliitis and multi-organ involvement in COVID-19 associated deaths. Respiration. 2022;101(02):155–165. doi: 10.1159/000518914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanley B, Naresh K N, Roufosse C et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(06):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsakok M T, Watson R A, Saujani S J et al. Reduction in chest CT severity and improved hospital outcomes in SARS-CoV-2 omicron compared with delta variant infection. Radiology. 2023;306(01):261–269. doi: 10.1148/radiol.220533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon S H, Lee J H, Kim B N, Chest C T. Chest CT findings in hospitalized patients with SARS-CoV-2: delta versus omicron variants. Radiology. 2023;306(01):252–260. doi: 10.1148/radiol.220676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menni C, Valdes A M, Polidori Let al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study Lancet 2022399(10335):1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grobbelaar L M, Kruger A, Venter C . Research Square; 2022. Relative hypercoagulopathy of the SARS-CoV-2 Beta and Delta variants when compared to the less severe Omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. [DOI] [PubMed] [Google Scholar]
- 53.Lloyd-Jones G, Molayem S, Pontes C, Chapple I. The COVID-19 pathway: a proposed oral-vascular-pulmonary route of SARS-CoV-2 infection and the importance of oral healthcare measures. J Oral Med Dent Res. 2021;2(01):1–25. [Google Scholar]
- 54.Lloyd-Jones G, Oudkerk M. COVID-19: angiotensin II in development of lung immunothrombosis and vasculitis mimics. Lancet Rheumatol. 2021;3(05):e325–e326. doi: 10.1016/S2665-9913(21)00068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel S K, Juno J A, Lee W S et al. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: implications for COVID-19 pathogenesis and consequences. Eur Respir J. 2021;57(05):2.00373E6. doi: 10.1183/13993003.03730-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loo J, Spittle D A, Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76(04):412–420. doi: 10.1136/thoraxjnl-2020-216243. [DOI] [PubMed] [Google Scholar]
- 57.Beyerstedt S, Casaro E B, Rangel E B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40(05):905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altmann D M, Whettlock E M, Liu S, Arachchillage D J, Boyton R J. The immunology of Long COVID. Nat Rev Immunol. 2023;23(10):618–634. doi: 10.1038/s41577-023-00904-7. [DOI] [PubMed] [Google Scholar]
- 59.Montezano A C, Camargo L L, Mary S et al. SARS-CoV-2 spike protein induces endothelial inflammation via ACE2 independently of viral replication. Sci Rep. 2023;13(01):14086. doi: 10.1038/s41598-023-41115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashall L, Horton C A, Nelson D E et al. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science. 2009;324(5924):242–246. doi: 10.1126/science.1164860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasanvand A. COVID-19 and the role of cytokines in this disease. Inflammopharmacology. 2022;30(03):789–798. doi: 10.1007/s10787-022-00992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colunga Biancatelli R ML, Solopov P A, Sharlow E R, Lazo J S, Marik P E, Catravas J D. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2021;321(02):L477–L484. doi: 10.1152/ajplung.00223.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teuwen L A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(07):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baycan O F, Barman H A, Bolen F et al. Plasminogen activator inhibitor-1 levels as an indicator of severity and mortality for COVID-19. North Clin Istanb. 2023;10(01):1–9. doi: 10.14744/nci.2022.09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Z, Dai W, Zhu W et al. Plasma tissue-type plasminogen activator is associated with lipoprotein(a) and clinical outcomes in hospitalized patients with COVID-19. Res Pract Thromb Haemost. 2023;7(06):102164. doi: 10.1016/j.rpth.2023.102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zuo Y, Warnock M, Harbaugh A et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci Rep. 2021;11(01):1580. doi: 10.1038/s41598-020-80010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whyte C S, Simpson M, Morrow G B et al. The suboptimal fibrinolytic response in COVID-19 is dictated by high PAI-1. J Thromb Haemost. 2022;20(10):2394–2406. doi: 10.1111/jth.15806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pretorius E, Venter C, Laubscher G J, Lourens P J, Steenkamp J, Kell D B. Prevalence of readily detected amyloid blood clots in 'unclotted' type 2 diabetes mellitus and COVID-19 plasma: a preliminary report. Cardiovasc Diabetol. 2020;19(01):193. doi: 10.1186/s12933-020-01165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venter C, Bezuidenhout J A, Laubscher G J et al. Erythrocyte, platelet, serum ferritin, and P-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int J Mol Sci. 2020;21(21):8234. doi: 10.3390/ijms21218234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner S, Khan M A, Putrino D, Woodcock A, Kell D B, Pretorius E. Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol Metab. 2023;34(06):321–344. doi: 10.1016/j.tem.2023.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C, Yu C, Jing H et al. Long COVID: the nature of thrombotic sequelae determines the necessity of early anticoagulation. Front Cell Infect Microbiol. 2022;12:861703. doi: 10.3389/fcimb.2022.861703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang S, Liu Y, Wang X et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13(01):120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haffke M, Freitag H, Rudolf G et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) J Transl Med. 2022;20(01):138. doi: 10.1186/s12967-022-03346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Charfeddine S, Ibn Hadj Amor H, Jdidi J et al. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front Cardiovasc Med. 2021;8:745758. doi: 10.3389/fcvm.2021.745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jarrott B, Head R, Pringle K G, Lumbers E R, Martin J H. “Long COVID”-a hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol Res Perspect. 2022;10(01):e00911. doi: 10.1002/prp2.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vijayakumar B, Boustani K, Ogger P P et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55(03):542–5.56E7. doi: 10.1016/j.immuni.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vollenberg R, Tepasse P R, Ochs K et al. Indications of persistent glycocalyx damage in convalescent COVID-19 patients: a prospective multicenter study and hypothesis. Viruses. 2021;13(11):2324. doi: 10.3390/v13112324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Irish COVID-19 Vasculopathy Study (iCVS) Investigators . Fogarty H, Ward S E, Townsend L et al. Sustained VWF-ADAMTS-13 axis imbalance and endotheliopathy in Long COVID syndrome is related to immune dysfunction. J Thromb Haemost. 2022;20(10):2429–2438. doi: 10.1111/jth.15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel M A, Knauer M J, Nicholson M et al. Elevated vascular transformation blood biomarkers in long-COVID indicate angiogenesis as a key pathophysiological mechanism. Mol Med. 2022;28(01):122. doi: 10.1186/s10020-022-00548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Banecki K MRM, Dora K A. Endothelin-1 in health and disease. Int J Mol Sci. 2023;24(14):11295. doi: 10.3390/ijms241411295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Talotta R. Impaired VEGF-A-mediated neurovascular crosstalk induced by SARS-CoV-2 spike protein: a potential hypothesis explaining Long COVID-19 symptoms and COVID-19 vaccine side effects? Microorganisms. 2022;10(12):2452. doi: 10.3390/microorganisms10122452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kell D B, Pretorius E. Are fibrinaloid microclots a cause of autoimmunity in Long COVID and other post-infection diseases? Biochem J. 2023;480(15):1217–1240. doi: 10.1042/BCJ20230241. [DOI] [PubMed] [Google Scholar]
- 83.Philogene M C, Johnson T, Vaught A J, Zakaria S, Fedarko N. Antibodies against angiotensin II type 1 and endothelin A receptors: relevance and pathogenicity. Hum Immunol. 2019;80(08):561–567. doi: 10.1016/j.humimm.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miedema J, Schreurs M, van der Sar-van der Brugge S et al. Antibodies against angiotensin II receptor type 1 and endothelin A receptor are associated with an unfavorable COVID19 disease course. Front Immunol. 2021;12:684142. doi: 10.3389/fimmu.2021.684142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wallukat G, Hohberger B, Wenzel K et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. doi: 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Civieri G, Iop L, Tona F. Antibodies against angiotensin II type 1 and endothelin 1 type A receptors in cardiovascular pathologies. Int J Mol Sci. 2022;23(02):927. doi: 10.3390/ijms23020927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yao Q, Doyle M E, Qing-Rong L et al. Long-term dysfunction of taste papillae in SARS-CoV-2. NEJM Evid. 2023;2(09) doi: 10.1056/evidoa2300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lima T M, Martins R B, Miura C S et al. Tonsils are major sites of prolonged SARS-COV-2 infection in children. Microbiol Spectr. 2023;11(05):e0134723. doi: 10.1128/spectrum.01347-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Omidvari N, Jones T, Price P M et al. First-in-human immunoPET imaging of COVID-19 convalescent patients using dynamic total-body PET and a CD8-targeted minibody. Sci Adv. 2023;9(41):eadh7968. doi: 10.1126/sciadv.adh7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peluso M J, Swank Z N, Goldberg S A et al. Plasma-based antigen persistence in the post-acute phase of COVID-19. Lancet Infect Dis. 2024;24(06):e345–e347. doi: 10.1016/S1473-3099(24)00211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vuuren M JV, Nell T A, Carr J A, Kell D B, Pretorius E. Iron dysregulation and inflammagens related to oral and gut health are central to the development of Parkinson's disease. Biomolecules. 2020;11(01):30. doi: 10.3390/biom11010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soffritti I, D'Accolti M, Fabbri C et al. Oral microbiome dysbiosis is associated with symptoms severity and local immune/inflammatory response in COVID-19 patients: a cross-sectional study. Front Microbiol. 2021;12:687513. doi: 10.3389/fmicb.2021.687513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lloyd-Jones G, Pontes C C, Molayem S, Chapple I LC. The oral-vascular-pulmonary infection route: a pathogenic mechanism linking oral health status to acute and post-acute COVID-19. Curr Oral Health Rep. 2023;10:163–174. [Google Scholar]
- 94.Gualtero D F, Lafaurie G I, Buitrago D M, Castillo Y, Vargas-Sanchez P K, Castillo D M. Oral microbiome mediated inflammation, a potential inductor of vascular diseases: a comprehensive review. Front Cardiovasc Med. 2023;10:1.250263E6. doi: 10.3389/fcvm.2023.1250263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eberhardt N, Noval M G, Kaur R et al. SARS-CoV-2 infection triggers pro-atherogenic inflammatory responses in human coronary vessels. Nat Cardiovasc Res. 2023;2(10):899–916. doi: 10.1038/s44161-023-00336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nyström S, Hammarström P. Amyloidogenesis of SARS-CoV-2 spike protein. J Am Chem Soc. 2022;144(20):8945–8950. doi: 10.1021/jacs.2c03925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parry P I, Lefringhausen A, Turni C et al. 'Spikeopathy': COVID-19 spike protein is pathogenic, from both virus and vaccine mRNA. Biomedicines. 2023;11(08):2287. doi: 10.3390/biomedicines11082287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cano-Mendez A, García-Larragoiti N, Damian-Vazquez M et al. Platelet reactivity and inflammatory phenotype induced by full-length spike SARS-CoV-2 protein and its RBD domain. Int J Mol Sci. 2022;23(23):15191. doi: 10.3390/ijms232315191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perico L, Morigi M, Galbusera M et al. SARS-CoV-2 spike protein 1 activates microvascular endothelial cells and complement system leading to platelet aggregation. Front Immunol. 2022;13:827146. doi: 10.3389/fimmu.2022.827146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grobbelaar L M, Venter C, Vlok M et al. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci Rep. 2021;41(08):BSR20210611. doi: 10.1042/BSR20210611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saud Z, Tyrrell V J, Zaragkoulias A et al. The SARS-CoV2 envelope differs from host cells, exposes procoagulant lipids, and is disrupted in vivo by oral rinses. J Lipid Res. 2022;63(06):100208. doi: 10.1016/j.jlr.2022.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laubscher G J, Lourens P J, Venter C, Kell D B, Pretorius E. TEG ® , microclot and platelet mapping for guiding early management of severe COVID-19 coagulopathy . J Clin Med. 2021;10(22):5381. doi: 10.3390/jcm10225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pretorius E, Venter C, Laubscher G J et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/post-acute sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 2022;21(01):148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turner S, Naidoo C A, Usher T J et al. Increased levels of inflammatory and endothelial biomarkers in blood of Long COVID patients point to thrombotic endothelialitis. Semin Thromb Hemost. 2023;50(02):288–294. doi: 10.1055/s-0043-1769014. [DOI] [PubMed] [Google Scholar]
- 105.Kell D B, Pretorius E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Prog Biophys Mol Biol. 2017;123:16–41. doi: 10.1016/j.pbiomolbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 106.Xie Y, Choi T, Al-Aly Z. Postacute sequelae of SARS-CoV-2 infection in the pre-delta, delta, and omicron Eras. N Engl J Med. 2024;391(06):515–525. doi: 10.1056/NEJMoa2403211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fan B E, Ng J, Chan S SW et al. COVID-19 associated coagulopathy in critically ill patients: a hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis. J Thromb Thrombolysis. 2021;51(03):663–674. doi: 10.1007/s11239-020-02318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Irish COVID-19 Vasculopathy Study (iCVS) investigators . Fogarty H, Townsend L, Morrin H et al. Persistent endotheliopathy in the pathogenesis of Long COVID syndrome. J Thromb Haemost. 2021;19(10):2546–2553. doi: 10.1111/jth.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuin M, Barco S, Giannakoulas G et al. Risk of venous thromboembolic events after COVID-19 infection: a systematic review and meta-analysis. J Thromb Thrombolysis. 2023;55(03):490–498. doi: 10.1007/s11239-022-02766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patell R, Bogue T, Koshy A et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rijken D C, Abdul S, Malfliet J J, Leebeek F W, Uitte de Willige S. Compaction of fibrin clots reveals the antifibrinolytic effect of factor XIII. J Thromb Haemost. 2016;14(07):1453–1461. doi: 10.1111/jth.13354. [DOI] [PubMed] [Google Scholar]
- 112.Mosesson M W, Siebenlist K R, Hernandez I, Lee K N, Christiansen V J, McKee P A. Evidence that alpha2-antiplasmin becomes covalently ligated to plasma fibrinogen in the circulation: a new role for plasma factor XIII in fibrinolysis regulation. J Thromb Haemost. 2008;6(09):1565–1570. doi: 10.1111/j.1538-7836.2008.03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nalbandian A, Sehgal K, Gupta A et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(04):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang J H, Wang N, Li A et al. Hypoxia can contribute to the induction of the Epstein-Barr virus (EBV) lytic cycle. J Clin Virol. 2006;37(02):98–103. doi: 10.1016/j.jcv.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 115.Huang R, Huestis M, Gan E S, Ooi E E, Ohh M. Hypoxia and viral infectious diseases. JCI Insight. 2021;6(07):e147190. doi: 10.1172/jci.insight.147190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ufuk F, Savaş R. Chest CT features of the novel coronavirus disease (COVID-19) Turk J Med Sci. 2020;50(04):664–678. doi: 10.3906/sag-2004-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kerchberger V E, Bastarache J A. Pulmonary vasculopathy in COVID-19 acute respiratory distress syndrome: a step closer to the full picture. Am J Respir Crit Care Med. 2022;206(07):809–810. doi: 10.1164/rccm.202205-1019ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mohamed I, de Broucker V, Duhamel A et al. Pulmonary circulation abnormalities in post-acute COVID-19 syndrome: dual-energy CT angiographic findings in 79 patients. Eur Radiol. 2023;33(07):4700–4712. doi: 10.1007/s00330-023-09618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heiss R, Tan L, Schmidt S et al. Pulmonary dysfunction after pediatric COVID-19. Radiology. 2023;306(03):e221250. doi: 10.1148/radiol.221250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dhawan R T, Gopalan D, Howard L et al. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med. 2021;9(01):107–116. doi: 10.1016/S2213-2600(20)30407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raitakari O T, Celermajer D S. Flow-mediated dilatation. Br J Clin Pharmacol. 2000;50(05):397–404. doi: 10.1046/j.1365-2125.2000.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Celermajer D S, Sorensen K E, Gooch V M et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 123.International Brachial Artery Reactivity Task Force . Corretti M C, Anderson T J, Benjamin E J et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(02):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 124.Thijssen D H, Black M A, Pyke K E et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(01):H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holder S M, Bruno R M, Shkredova D A et al. Reference intervals for brachial artery flow-mediated dilation and the relation with cardiovascular risk factors. Hypertension. 2021;77(05):1469–1480. doi: 10.1161/HYPERTENSIONAHA.120.15754. [DOI] [PubMed] [Google Scholar]
- 126.Maruhashi T, Kajikawa M, Kishimoto S et al. Diagnostic criteria of flow-mediated vasodilation for normal endothelial function and nitroglycerin-induced vasodilation for normal vascular smooth muscle function of the brachial artery. J Am Heart Assoc. 2020;9(02):e013915. doi: 10.1161/JAHA.119.013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heiss C, Rodriguez-Mateos A, Bapir M, Skene S S, Sies H, Kelm M. Flow-mediated dilation reference values for evaluation of endothelial function and cardiovascular health. Cardiovasc Res. 2023;119(01):283–293. doi: 10.1093/cvr/cvac095. [DOI] [PubMed] [Google Scholar]
- 128.Ambrosino P, Sanduzzi Zamparelli S, Mosella M et al. Clinical assessment of endothelial function in convalescent COVID-19 patients: a meta-analysis with meta-regressions. Ann Med. 2022;54(01):3234–3249. doi: 10.1080/07853890.2022.2136403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao Y P, Zhou W, Huang P N et al. Persistent endothelial dysfunction in coronavirus disease-2019 survivors late after recovery. Front Med (Lausanne) 2022;9:809033. doi: 10.3389/fmed.2022.809033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cimino G, Vizzardi E, Calvi E et al. Endothelial dysfunction in COVID-19 patients assessed with Endo-PAT2000. Monaldi Arch Chest Dis. 2022;92(04) doi: 10.4081/monaldi.2022.2213. [DOI] [PubMed] [Google Scholar]
- 131.Smith V, Ickinger C, Hysa E et al. Nailfold capillaroscopy. Best Pract Res Clin Rheumatol. 2023;37(01):101849. doi: 10.1016/j.berh.2023.101849. [DOI] [PubMed] [Google Scholar]
- 132.Laubscher G J, Khan M, Venter Cet al. Treatment of Long COVID symptoms with triple anticoagulant therapy Res Square 2023 10.21203/rs.3.rs-2697680/v1[Preprint]. [DOI] [Google Scholar]
- 133.Kell D B. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wolfrum S, Jensen K S, Liao J K. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23(05):729–736. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- 135.Amraei R, Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9(07):1652. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lei Y, Zhang J, Schiavon C R et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128(09):1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fraga-Silva R A, Costa-Fraga F P, Murça T M et al. Angiotensinconverting enzyme 2 activation improves endothelial function. Hypertension. 2013;61(06):1233–1238. doi: 10.1161/HYPERTENSIONAHA.111.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.NICE guideline 2021. COVID-19 rapid guideline: managing COVID-19. https://www.nice.org.uk/guidance/ng191 https://www.nice.org.uk/guidance/ng191
- 139.Taub P R, Zadourian A, Lo H C, Ormiston C K, Golshan S, Hsu J C. Randomized trial of ivabradine in patients with hyperadrenergic postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2021;77(07):861–871. doi: 10.1016/j.jacc.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 140.Ross A J, Ocon A J, Medow M S, Stewart J M. A double-blind placebo-controlled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond) 2014;126(04):289–296. doi: 10.1042/CS20130222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.COVID-OUT Study Team . Bramante C T, Buse J B, Liebovitz D M et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis. 2023;23(10):1119–1129. doi: 10.1016/S1473-3099(23)00299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ma Z, Patel N, Vemparala P, Krishnamurthy M. Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci Rep. 2022;12(01):5553. doi: 10.1038/s41598-022-09639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jung S M, Kim W-U. Targeted immunotherapy for autoimmune disease. Immune Netw. 2022;22(01):e9. doi: 10.4110/in.2022.22.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pathangey G, Fadadu P P, Hospodar A R, Abbas A E. Angiotensin-converting enzyme 2 and COVID-19: patients, comorbidities, and therapies. Am J Physiol Lung Cell Mol Physiol. 2021;320(03):L301–L330. doi: 10.1152/ajplung.00259.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pliszka A G. Modafinil: a review and its potential use in the treatment of Long COVID fatigue and neurocognitive deficits. Am J Psychiatry Resid J. 2022;17:5–7. [Google Scholar]
- 146.Li P, Xie C, Zhong J, Guo Z, Guo K, Tu Q. Melatonin attenuates ox-LDL-induced endothelial dysfunction by reducing ER stress and inhibiting JNK/Mff signaling. Oxid Med Cell Longev. 2021;2021:5.589612E6. doi: 10.1155/2021/5589612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang L, Wang W, Han R, Liu Y, Wu B, Luo J. Protective effects of melatonin on myocardial microvascular endothelial cell injury under hypertensive state by regulating Mst1. BMC Cardiovasc Disord. 2023;23(01):179. doi: 10.1186/s12872-023-03159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McGrane I R, Leung J G, St Louis E K, Boeve B F. Melatonin therapy for REM sleep behavior disorder: a critical review of evidence. Sleep Med. 2015;16(01):19–26. doi: 10.1016/j.sleep.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.OpenSAFELY Collaborative . Wong A YS, Tomlinson L A, Brown J P et al. Association between warfarin and COVID-19-related outcomes compared with direct oral anticoagulants: population-based cohort study. J Hematol Oncol. 2021;14(01):172. doi: 10.1186/s13045-021-01185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abdelmaksoud A, Goldust M, Vestita M. Omalizumab and COVID-19 treatment: could it help? Dermatol Ther. 2020;33(04):e13792. doi: 10.1111/dth.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wood R A, Togias A, Sicherer S H et al. Omalizumab for the treatment of multiple food allergies. N Engl J Med. 2024;390(10):889–899. doi: 10.1056/NEJMoa2312382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pala D, Pistis M. Anti-IL5 drugs in COVID-19 patients: role of eosinophils in SARS-CoV-2-induced immunopathology. Front Pharmacol. 2021;12:622554. doi: 10.3389/fphar.2021.622554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Charfeddine S, Ibnhadjamor H, Jdidi J et al. Sulodexide significantly improves endothelial dysfunction and alleviates chest pain and palpitations in patients with long-COVID-19: insights from TUN-EndCOV study. Front Cardiovasc Med. 2022;9:866113. doi: 10.3389/fcvm.2022.866113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


