Abstract
The protein epidermal growth factor (EGF), which plays a crucial role in promoting cell proliferation and survival, has recently demonstrated potential in reducing inflammation. In this study, we examined the impact of EGF on the anti-inflammatory and anti-proliferative properties of pterygium, a prevalent hypervascular proliferative disease affecting the ocular surface. In surgically excised tissues, markers for fibrotic and inflammatory signals, including VIM, ACTA2, FAP, MMP2, VCAM1, ICAM1, CD86, IL6, and IL1B were upregulated in the pterygium body stroma compared to the normal conjunctival stroma. EGF exerted anti-inflammatory and anti-vasculogenic effects on pterygial fibroblasts when co-cultured with M1 macrophages. Moreover, exosomes derived from EGF-preconditioned M1 macrophages suppressed the heightened inflammatory and vasculogenic signals in pterygial fibroblasts induced by exosomes from M1 macrophages. Paradoxically, the proliferation of pterygial fibroblasts was inhibited by EGF in the in vitro microenvironment with M1 macrophages, despite EGF being known as a growth factor. EGF-preconditioning of M1 macrophages rescued the increased proliferation of pterygial fibroblasts induced by exosomes from M1 macrophages. In conclusion, our findings demonstrate that EGF effectively mitigates inflammation and proliferation in pterygial fibroblasts within a microenvironment containing M1 macrophages.
Keywords: EGF, Exosome, Inflammation, Proliferation, Pterygial fibroblast, Pterygium
Subject terms: Inflammation, Conjunctival diseases
Introduction
Pterygium is a prevalent ocular surface condition identified by the development of a triangular growth that consists of fibrous subconjunctival connective tissue and thickening of the overlying conjunctival epithelium1. The development of pterygia is commonly acknowledged to be linked to the responsive generation of wounds triggered by oxidative stress caused by exposure to ultraviolet (UV) light, ocular irritation, and/or inflammation on the ocular surface2. UV exposure induces pro-inflammatory changes in the cornea, characterized by the increased expression of cytokines such as interleukin (IL)-1, IL-6, IL-83, and tumor necrosis factor (TNF)-α4. This is consistent with the heightened presence of inflammatory cells observed in pterygium specimens.
While the specific involvement of macrophage subtypes in pterygium development is not fully understood, a previous investigation found cyclooxygenase-2-expressing macrophages that simultaneously express vascular endothelial growth factor in the pterygial stromal layer5. Additionally, it is known that the density of lymphatic vessels is higher in pterygium compared to the conjunctiva, and this increased density is associated with pterygium recurrence and greater severity6–8. According to a previous study, conditioned media from UV type B (UVB)-irradiated corneal limbal epithelial cells less inhibited lymphatic endothelial cell proliferation. Moreover, macrophage-recruiting cytokines, such as monocyte chemoattractant protein-1, were significantly upregulated following the irradiation of corneal limbal fibroblasts. Although it is unclear which subset of macrophages is relevant to the lymphangiogenesis in pterygium, this suggests that UVB irradiation in pterygium may be linked to the upregulation of indirect macrophage-recruiting cytokines after UVB exposure9.
Epidermal growth factor (EGF) is a widely recognized protein known for its ability to stimulate cell proliferation, differentiation, and survival10. Its involvement in tissue recovery post-injury through EGF receptor (EGFR) signaling has been extensively researched, particularly in the context of skin regeneration11. The potential role of EGF in the inflammatory process and immune system is not uniformly agreed upon. For example, the EGFR signaling pathway leads to airway mucin production, neutrophil recruitment, and airway epithelial repair, producing innate immune responses to infectious and noxious stimuli12. However, EGFR initiates anti-inflammatory responses in vascular endothelial cells from the aorta, while mediating pro-inflammatory responses in coronary arterial endothelial cells, suggesting that the role of EGF in inflammation regulation varies depending on the cell type13. Fortunately, heparin-binding EGF-like growth factor (HB-EGF) has been shown to decrease M1 polarization induced by lipopolysaccharide (LPS) and promote M2 polarization while protecting human fetal small intestinal epithelial FHs-74 cells14.
Considering pterygium is characterized by proliferating fibrovascular tissue, contemplating the use of EGF in pterygial cells to mitigate the associated innate immune activity, potentially involved in pterygium pathogenesis, may seem paradoxical. Therefore, this study aims to explore whether the anti-inflammatory and anti-angiogenic effects of EGF on induced M1 macrophages could serve as a potential therapeutic strategy for reducing inflammation and proliferation in pterygial fibroblasts.
Results
Activation of fibrotic and inflammatory signals in pterygium body stromal tissues
First, to investigate the activation of fibroblasts, myeloid cells, and their related inflammations, we compared gene expressions for the activation of fibroblasts, adhesion molecules, and M1 macrophages between conjunctival stroma and pterygium body stroma using surgically obtained tissues. The expressions of VIM (i.e., vimentin), ACTA2 (i.e., alpha-smooth muscle actin), FAP (i.e., fibroblast activation protein, alpha), and MMP2(i.e., matrix metalloproteinase 2), which are known to be involved in epithelial-mesenchymal transition15, activation of stromal fibroblasts16, or pathologic fibrosis17, were upregulated in pterygium body stroma compared to conjunctival stroma (Fig. 1). Additionally, adhesion molecules, including VCAM1 and ICAM1, which mediate the adhesion of immune cells, as well as M1 macrophage marker CD86 and its secreting cytokine IL6 and IL1B, were upregulated in pterygium body stroma. However, the expression of the anti-inflammatory M2 macrophage marker MRC1 (also known as Cd206) was not different between the conjunctival stroma and pterygium body stroma (Fig. 2).
Fig. 1.
Activation of fibrosis-related signals in pterygium body stroma compared to normal conjunctival stroma. (A-D) Real-time RT-PCR analysis of VIM, ACTA2, FAP, and MMP2 in conjunctival stromal (CJ) and pterygium body stromal (PT) tissues. Wilcoxon signed-rank test (A and C) or paired t-test (B and D). N = 8 to 11. *p 0.05 and **p
0.05 and **p 0.01.
0.01.
Fig. 2.
Activation of gene expressions for adhesion molecules and M1 macrophage in pterygium body stroma compared to normal conjunctival stroma. (A-F) Real-time RT-PCR analysis of adhesion molecules including VCAM1 and ICAM1, M1 macrophage marker CD86 and its secreting cytokine IL6 and IL1B, and M2 macrophage marker MRC1 in conjunctival stromal (CJ) and pterygium body stromal (PT) tissues. Wilcoxon signed-rank test. N = 14 to 16. *p 0.05, **p
0.05, **p 0.01, ***p
0.01, ***p 0.001 and ****p
0.001 and ****p 0.0001.
0.0001.
EGF reduces inflammatory signals in pterygial fibroblasts during co-culture with M1 macrophages
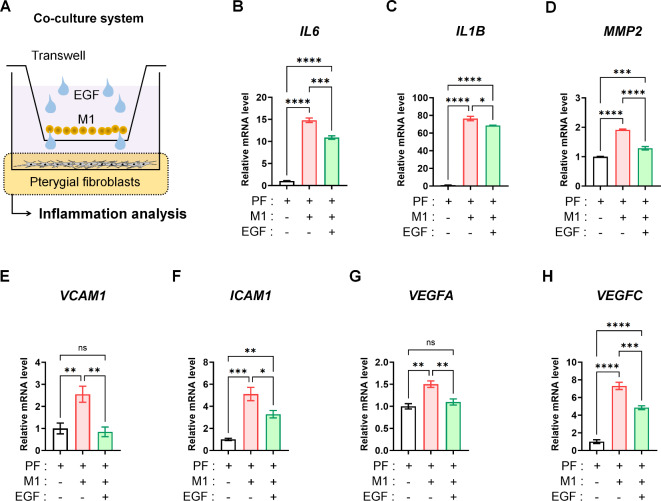
Based on the activation of inflammatory and M1 macrophage-related signals observed in pterygium stromal tissues, we subsequently investigated the anti-inflammatory effect of EGF on pterygial fibroblasts coexisting with M1 macrophages. In clinical settings involving the ocular surface, resident cells in ocular tissues are not isolated from immune cells; they coexist. To simulate this situation, we co-cultured pterygial fibroblasts and M1 macrophages in a transwell co-culture system, treating both cell types with EGF (10 ng/mL for 24 h) (Fig. 3A).
Fig. 3.
EGF-induced attenuation of inflammatory, fibrotic, and vasculogenic signals in pterygial fibroblasts during co-culture with M1 macrophages. (A) Scheme of co-culture of M1 macrophages and pterygium fibroblasts using transwell system with EGF treatment (10 ng/mL for 24 h). (B-H) Real-time RT-PCR analysis for IL6, IL1B, MMP2, VCAM1, ICAM1, VEGFA and VEGFC expressions in pterygial fibroblast with and without EGF (10 ng/mL) during co-culture with M1 macrophages (24 h). Analysis of variance followed by Tuckey’s post-hoc test. N = 4. *p 0.05, **p
0.05, **p 0.01, ***p
0.01, ***p 0.001, ****p
0.001, ****p 0.0001, and ns: not significant. EGF, epidermal growth factor; PF, pterygial fibroblast.
0.0001, and ns: not significant. EGF, epidermal growth factor; PF, pterygial fibroblast.
When co-cultured with M1 macrophages, pterygial fibroblasts showed upregulated expression of inflammatory markers IL6 and IL1B, the fibrotic marker MMP2, adhesion molecules VCAM1 and ICAM1, and vasculogenic factors VEGFA and VEGFC. However, applying EGF (10 ng/mL for 24 h) in the co-culture system significantly decreased the expression of all these markers and factors in pterygial fibroblasts (Fig. 3B-H).
EGF-primed M1 macrophage exosomes alleviate inflammatory signals in pterygial fibroblasts
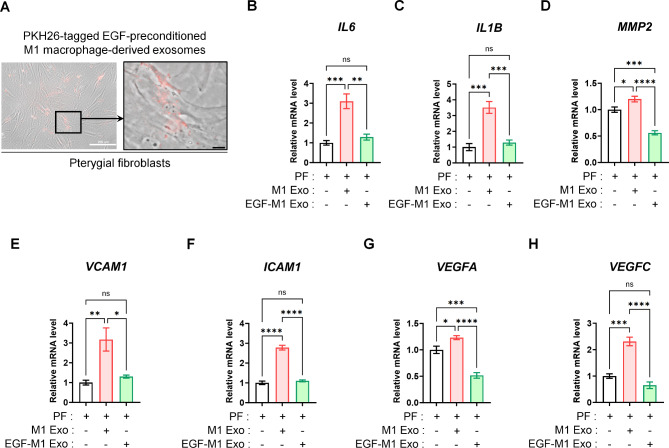
Exosomes, ranging in size from 40 to 160 nm, play a role in cell-to-cell communication by transporting diverse signals18. The direct blockade of the EGF receptor (EGFR) may not be favored due to possible downregulation mechanisms19. However, according to our previous findings, exosomes derived from EGF-treated M1 macrophages demonstrated enriched proteomic profiles related to immune system regulation and inflammation inhibition5. Therefore, for a more in-depth investigation into M1 macrophage-dependent alterations of inflammatory signals in pterygial fibroblasts, we obtained excreted exosomes from EGF-preconditioned M1 macrophages (EGF-M1 Exos) or exosomes from control M1 macrophages (M1 Exos). Remarkably, M1 Exos effectively penetrated cultured pterygial fibroblasts (Fig. 4A). As observed in the co-culture system (Fig. 3), the elevated expressions of the inflammatory markers IL6 and IL1B, the fibrotic marker MMP2, adhesion molecules VCAM1 and ICAM1, and vasculogenic factors VEGFA and VEGFC in M1 Exo-treated (20 µg/mL for 24 h) pterygial fibroblasts were suppressed by the treatment with EGF-M1 Exos (20 µg/mL for 24 h) (Fig. 4B to H).
Fig. 4.
The relative attenuation of inflammatory, fibrotic, and vasculogenic signals in pterygial fibroblasts by the delivery of EGF-preconditioned M1 macrophage-derived exosomes. (A) The low- and high-magnified images of the delivered PKH26 dye-labeled M1 macrophage-derived exosomes delivered and penetrated into the cultured pterygial fibroblasts. Scale bars: 200 μm (white) and 20 μm (black). (B-H) Real-time RT-PCR analysis for IL6, IL1B, MMP2, VCAM1, ICAM1, VEGFA, and VEGFC expressions in pterygial fibroblasts treated with M1 macrophage-derived exosome (M1 Exo, 20 µg/mL for 24 h) or EGF-preconditioned M1 macrophage-derived exosome (EGF-M1 Exo, 20 µg/mL for 24 h). Analysis of variance followed by Tuckey’s post-hoc test. N = 4. *p 0.05, **p
0.05, **p 0.01, ***p
0.01, ***p 0.001, ****p
0.001, ****p 0.0001, and ns: not significant. EGF, epidermal growth factor; PF, pterygial fibroblast.
0.0001, and ns: not significant. EGF, epidermal growth factor; PF, pterygial fibroblast.
Anti-proliferative effect of EGF on pterygial fibroblasts with M1 macrophages
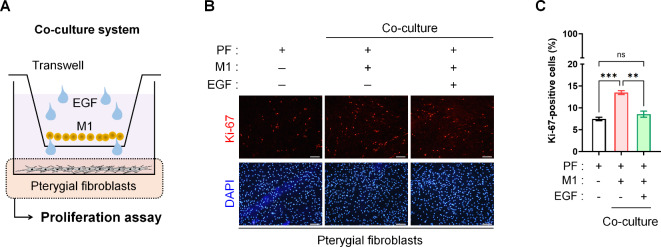
While we have newly discovered the anti-inflammatory effect of EGF on pterygial fibroblasts adjacent to M1 macrophages, we also investigated whether EGF treatment induces the proliferation of pterygial fibroblasts exposed to M1 macrophages, given the well-established role of EGF as a growth factor. As in Fig. 3, we treated both M1 macrophages and pterygial fibroblasts in a transwell co-culture system with EGF and then performed a proliferation assay on pterygial fibroblasts (Fig. 5A). The population of proliferating Ki-67+ pterygial fibroblasts increased significantly when co-cultured with M1 macrophages. However, EGF (10 ng/mL) inhibited M1-induced proliferation, reducing the proliferation of pterygial fibroblasts to the level observed in control conditions (Fig. 5B and C).
Fig. 5.
Effect of EGF on the anti-proliferation of cultured pterygial fibroblasts during during co-culture with M1 macrophages. (A) Representative images of Ki-67 staining of cultured pterygial fibroblasts with or without EGF treatment (10 ng/mL) during co-culture with M1 macrophages. Scale bars: 200 μm. (B) Comparison of proportion of Ki-67+ cells with or without EGF treatment (10 ng/mL) during co-culture with M1 macrophages. Analysis of variance followed by Tuckey’s post-hoc test. N = 3. **p 0.01, ***p
0.01, ***p 0.001, and ns: not significant. EGF, epidermal growth factor; PF, pterygial fibroblast.
0.001, and ns: not significant. EGF, epidermal growth factor; PF, pterygial fibroblast.
In addition, the alteration in the growth velocity of pterygial fibroblasts in the presence of M1-derived exosomes (20 µg/mL) versus EGF (10 ng/mL)-preconditioned M1-derived exosomes (20 µg/mL) was evaluated to simulate the impact of adjacent M1 macrophage regulation on pterygial fibroblast proliferation. According to the daily growth curves of pterygial fibroblasts, the treatment of pterygial fibroblasts with M1 Exos or EGF-M1 Exos did not affect proliferation on day 1. However, on day 2, the number of pterygial fibroblasts was higher with EGF-M1 Exo treatment compared to with M1 Exo treatment, while M1 Exo even enhanced proliferation of pterygial fibroblasts compared to M1 Exo treatment or vehicle on day 3. There was no difference in cell numbers between the vehicle group and the EGF-M1 Exo treatment group (Fig. 6).
Fig. 6.
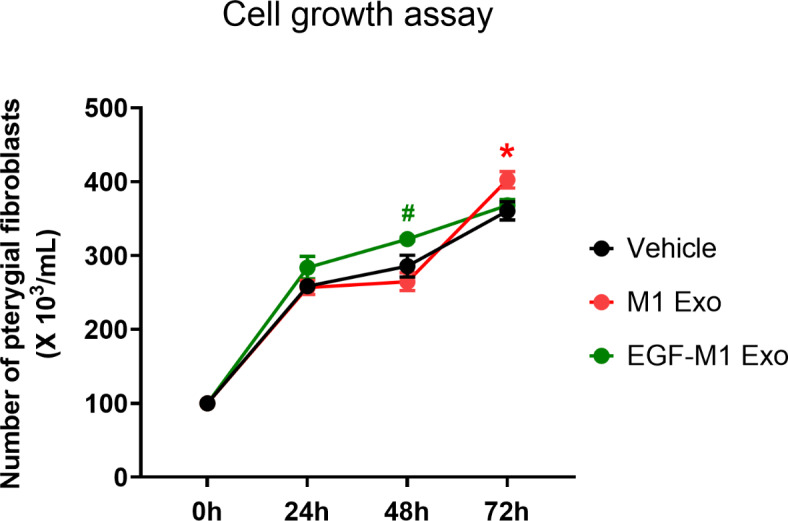
Suppression of pterygial fibroblast proliferation induced by M1 macrophage-derived exosomes using preconditioning of M1 macrophages with EGF. Growth curves of pterygial fibroblasts in the absence or presence of M1-derived exosomes (M1 Exo) or EGF (10 ng/mL)-preconditioned M1-derived exosomes (EGF-M1 Exo) over 72 h. Analysis of variance followed by Tuckey’s post-hoc test. N = 4. *p 0.05 (Vehicle vs. M1 Exo) and #p
0.05 (Vehicle vs. M1 Exo) and #p 0.05 (EGF-M1 Exo vs. M1 Exo). EGF, epidermal growth factor.
0.05 (EGF-M1 Exo vs. M1 Exo). EGF, epidermal growth factor.
These results collectively suggest that the adjacent M1 macrophage induces inflammation and proliferation of pterygial fibroblasts which can be attenuated by EGF.
Discussions
Our findings elucidate the paracrine effect of EGF-treated M1 macrophages on the anti-inflammatory and anti-proliferative responses of pterygial fibroblasts. Pterygial stromal tissues exhibited higher expressions of fibrosis- and innate immunity-related signals compared to conjunctival stroma. Specifically, the presence of M1 macrophages near pterygial fibroblasts in vitro significantly promoted their proliferation. However, co-culturing pterygial fibroblasts and M1 cells with EGF, as well as treating pterygial fibroblasts with M1-derived exosomes, effectively inhibited inflammatory signals and the proliferation of pterygial fibroblasts. These results suggest a close association between innate immunity-related inflammation and pterygial stromal growth, highlighting a potential therapeutic target for EGF in pterygium.
Fibroblasts and macrophages are ubiquitous in all tissues, and a growing body of evidence supports the notion that these cells engage in direct communication, influencing the overall tissue microenvironment and impacting disease outcomes20. In a steady state, fibroblasts and macrophages are often found in close association, responding to reciprocal growth factor signals in various tissues21. Moreover, in the etiology of fibrotic disorders, it is widely accepted that macrophages play key roles in both the initiation and resolution of fibrosis through inflammatory interactions22. While EGFR is present on human macrophages23, its role in macrophages has primarily been studied in cancer research. EGFR has been identified on rabbit peripheral monocytes and macrophages, where it promoted cell proliferation and migration24. As an example, the secretion of amphiregulin (AREG) by macrophages activates EGFR on fibroblasts, leading to the promotion of fibroblast migration and collagen synthesis in the heart and liver25,26. This effect was observed in a TGFβ1-driven genetic model of lung fibrosis, where the suppression of AREG using siRNA or the inhibition of EGFR through pharmacological means significantly reduced fibrosis27. In contrast, in lipopolysaccharide (LPS)-challenged fibroblasts from diabetic foot ulcers, EGF suppressed the relative gene expressions, including toll-like receptor (TLR)2, CD14, TNF, and IL-6, except IL-B128. However, in the present study, IL1Bwas also downregulated in pterygial fibroblasts by co-culturing with M1 macrophages with EGF or by EGF-M1 Exo treatment. We still do not fully understand this discrepancy in the effect of EGF, but we hypothesize that the modification of microenvironmental M1 macrophages using EGF might have indirectly influenced pterygial fibroblasts to attenuate inflammatory signals more broadly. In addition, our suggestion is that the effect of EGFR stimulation may vary depending on the type of macrophages and the ligands of EGFR. In this context, it has been reported that heparin-binding EGF-like growth factor (HB-EGF) mediates negative feedback inhibition of M2 polarization in Raw 264.7 cells and peritoneal macrophages29. In macrophages derived from THP-1 human monocyte cell lines, HB-EGF demonstrated a significant decrease in LPS-induced M1 polarization, while concurrently promoting M2 polarization through the activation of signal transducers and activators of transcription 314. Likewise, in our previous study, the expression of the M2 macrophage marker ARG1 in the murine cornea was significantly higher with EGF-M1 Exo eye drops compared to M1 Exo eye drops, while the M1 macrophage marker CD86was not affected by EGF-M1 Exo treatment. Although further investigation is needed to elucidate the role of EGF in human conjunctival fibroblasts and pterygial fibroblasts, the M2 polarizing effect of EGF on macrophages may be a promising area for future research30.
Several factors can influence the activation and proliferation of pterygial fibroblasts, with prolonged inflammatory processes posing a potential harm to surrounding tissues. This can result in the continuation of tissue-damaging inflammatory cascades and subsequent scarring1. For instance, TNF-α activates NF-kB pathways in pterygial fibroblasts, leading to the up-regulation of NF-kB target genes, including RANTES, MCP-1, ENA-78, and MMPs31. Despite the known association of the relative overexpression of growth factors such as HB-EGF32, VEGF33, platelet-derived growth factor (PDGF)34, and insulin-like growth factor binding protein-2 (IGFBP-2)35with pterygium pathogenesis, our results intriguingly revealed that EGF induces anti-proliferative and partial anti-growth effects on pterygial fibroblasts. Pterygial fibroblasts reside within the microenvironment of immune cells and inflammatory molecules in pterygium tissues. In our co-culture and exosome delivery systems, EGF doesn’t solely influence pterygial fibroblasts but also affects the microenvironment of pterygial fibroblasts with macrophages. Previous studies have suggested that pro-inflammatory cytokines like IL-6 and IL-8, in conjunction with VEGF, fibroblast growth factor-2, EGF, and PDGF, stimulate the mitosis of pterygium epithelial cells and fibroblasts34,36–39. Considering the interplay between inflammation and the action of growth factors in pterygium, our suggestion is that EGF treatment surprisingly exerts an anti-proliferative effect on pterygial fibroblasts. Nevertheless, a lower concentration (1 ng/mL) of EGF treatment in the co-culture system unexpectedly triggered the inflammatory and vasculogenic signals of pterygial fibroblasts (data not shown), in contrast to the suppression of these signals observed with 10 ng/mL of EGF treatment as depicted in Fig. 3. We hypothesize that an ample quantity of EGF is essential to effectively inhibit inflammation in the microenvironment, thereby overpowering potential M1 activation and vasculogenic activation of pterygial fibroblasts induced by EGF as a growth factor.
Although EGFR expression is limited or minimal in the superficial conjunctival and limbal epithelial cells40, it is expressed in corneal epithelial cells41, where it plays a critical role in corneal epithelial wound healing. EGF, produced by the lacrimal glands and secreted into the tears42, may easily influence the ocular surface. Based on the anti-inflammatory role of EGF in ocular surface cells demonstrated in this study and our previous research30, investigating the secretion of EGF and the aberrant expression of EGFR in chronic inflammatory diseases of the ocular surface would be an interesting topic for future research.
The present study has several limitations. We utilized the THP-1 human monocyte cell line to examine phenotypical alterations with EGF, which may not fully reflect the conditions of macrophages in the ocular surface. In addition, experiments with EGF or exosomes were conducted only at the specified time and with the specified concentration. In in vitro experiments using differentiated immune cells, the culture time and drug treatment time can induce unintended differentiation or dedifferentiation of the immune cells, leading to inherent limitations in accuracy when conducting in vitro experiments under various conditions. To address this issue, pharmacodynamic and -kinetic analyses of EGF using a pterygium animal model will be necessary in the future. Additionally, we did not investigate the EGFR-dependent action of EGF on macrophages and pterygial fibroblasts, limiting our ability to provide a mechanistic explanation for the anti-inflammatory role of EGF on macrophages and its subsequent effects on pterygial fibroblasts. Nonetheless, there have been no previous studies investigating the novel role of EGF for the therapeutic strategy as a future medical treatment in pterygium.
In conclusion, our findings reveal that EGF effectively mitigates inflammation and proliferation in pterygial fibroblasts within a microenvironment containing M1 macrophages. This unexpected and novel role of EGF emphasizes the importance of considering pterygium as a disease triggered by inflammation and immunity, rather than merely a cellular growth disorder.
Methods
Study approval
Informed consent
was obtained from all subjects. We obtained approval from the Institutional Review Boards at Chung-Ang University Hospital (Approval No. 2106-007-465) to perform this study, which includes utilizing surgically obtained pterygium and conjunctival tissues, reviewing clinical data of the subjects, and performing in vitro experiments using the obtained tissues, adhering to the principles outlined in the Declaration of Helsinki.
Harvest of the pterygial and conjunctival tissues
The serial experiments were performed using human tissues among a total of 17 subjects obtained during pterygium surgery. The average age of the subjects was 55.1 ± 8.0 years (range: 36 to 70). There were 10 male and 7 female subjects. All subjects had primary pterygium, and all cases were located in the nasal area. When the tissues were surgically obtained enough, they were cut half to be used for tissue analysis as well as primary culture for cell analysis. All subjects. Although it was not possible to clearly assess the presence or extent of chronic inflammation, given that pterygium is an inflammation-related disease, none of the subjects had pseudopterygium, and acute infection was excluded in all pterygium patients at the time of surgery. Furthermore, none of the subjects experienced recurrence after surgery.
Pterygium body stromal tissue and normal conjunctival tissue from the superior bulbar conjunctiva were obtained during routine pterygium excision combined with conjunctivolimbal autograft surgery. To prevent contamination by epithelial cells, only the stromal tissue was harvested after removing the epithelium. Additionally, to completely separate normal conjunctival tissue from pterygium tissue, the normal conjunctival tissue was harvested from the superior 12 o’clock limbal region, far from the pterygium tissue. The tissue excisions were carried out by a single surgeon (KWK) to maintain consistency in the harvesting technique. Patients with a history of ocular trauma and systemic immunological disorders were excluded from the study.
Macrophage polarization from THP-1 monocytes
We induced M1 macrophages from the THP-1 cell line (TIB-202, ATCC, Manassas, VA, USA) using a well-established protocol43. THP-1 cells were cultured in RPMI 1640 (WelGENE, Daegu, South Korea) with supplements. After seeding them in 6-well plates and initiating differentiation into M0 macrophages with 50 nM phorbol 12-myristate 13-acetate (PMA; #P8139, Sigma-Aldrich, St Louis, MO, USA) for 24 h, we subsequently polarized them into M1 macrophages by exposing them to LPS (O111:B4, #L2630, 100 ng/ml, Sigma-Aldrich) and recombinant human interferon-gamma (IFN-γ, #570216, 20 ng/ml, BioLegend, San Diego, CA, USA) for an additional 24 h.
Culture of pterygial and conjunctival fibroblasts
All pterygium and conjunctival stromal samples were placed in sterile tubes containing Dulbecco’s phosphate-buffered saline (DPBS; Gibco, Invitrogen, Carlsbad, CA, USA) and promptly transported to the laboratory within 1 h of excision for culture. These specimens were utilized for explant cultures to generate human conjunctival fibroblasts and pterygial fibroblasts. For fibroblast isolation, each specimen was cut into explants (3 mm2) and positioned in 6-well plates. After a 10-minute adhesion period, each explant was covered with alpha-MEM (Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco) and 100 units/mL penicillin/streptomycin (Gibco) before being placed in a humidified incubator (37 °C, 5% CO2). The medium was subsequently changed every 2 to 3 days.
Co-culture of pterygial fibroblasts with M1 macrophages
For the co-culture of pterygial fibroblasts and M1 macrophages, pterygial fibroblasts were initially seeded in 6-well plates at a density of 1.2 × 105 cells per well and cultured for 24 h. The co-culture was performed using transwell inserts with a pore size of 0.4 μm (SPL Life Sciences, Pocheon-si, Gyeonggi-do, Korea) positioned in 6-well plates. M1 macrophages were resuspended in RPMI 1640 at a density of 3 × 105 cells per insert. The co-cultures of M1 macrophages and pterygial fibroblasts were then left undisturbed, incubating at 37 °C in a humidified 5% CO2 incubator for 24 h. RNA extraction from pterygial fibroblasts was subsequently carried out for analysis.
Extraction and verification of exosomes
M1 macrophages were cultured in RPMI 1640 medium containing exosome-depleted FBS (#EXO-FBS-250 A-1, System Biosciences, Palo Alto, CA, USA). Exosomes were precipitated from the cultured medium using the Total Exosome Isolation Reagent (#4478359, Invitrogen, CA, USA). Briefly, the medium underwent centrifugation at 2,500 rpm for 30 min to eliminate cells and debris. The supernatant, devoid of particles, was collected and filtered through a 0.22-µm-pore filter (#SLGVR33RS, Merck Millipore, Burlington, MA, USA). Following this, the medium was combined with the Total Exosome Isolation Reagent and left to incubate overnight at 4 °C. Exosomes were then pelleted by centrifugation at 13,000 rpm for 1 h at 4 °C, re-suspended in PBS, and stored at -80 °C. Quantification of exosome protein content was achieved using a Pierce™ BCA protein assay kit (#23227, Thermo Fisher Scientific).
The exosomes used in this study were verified by examining isolated exosomes based on their size and the presence of exosomal markers like Hsp70, CD63, and CD81 as we previously established30. The size distribution and concentration of purified exosomes were determined through nanoparticle tracking analysis with a Malvern NanoSight NS300 (Malvern Instruments Company, Malvern, England) and the corresponding NanoSight NTA 3.4 Analytical software.
Fluorescence labeling of exosomes and verification of intracellular delivery
M1 macrophage-derived exosomes were labeled with PKH26 red fluorescent cell linker dye (#MINI26, Sigma-Aldrich) following the manufacturer’s instructions. The labeling process involved adding 1 µL of PKH26 dye to 200 µL of Diluent C fluid from the kit and incubating them for 5 min at room temperature. To stop the labeling process, an equal volume of 10% BSA was added, and the exosomes were re-purified using the total exosome isolation reagent precipitation method.
For exosome treatment, the culture medium of pterygial fibroblasts was replaced with medium containing PKH26-labeled M1 macrophage-derived exosomes. The cells were incubated with these exosomes for 16 h and then fixed with 4% paraformaldehyde after washing with PBS. Subsequent analysis of the cells was conducted using an inverted fluorescence microscope (DMi8, Leica).
Treatment of pterygial fibroblasts with EGF and M1 macrophage-derived exosomes
We treated M1 macrophages with recombinant human EGF (#E9644, Sigma-Aldrich) at concentrations of 10 ng/mL for 24 h for RNA isolation. The concentration of EGF in this study was based on our previous research, in which 1 ng/mL or 10 ng/mL of EGF was successfully used to downregulate inflammatory signals in M1 macrophages in in vitro experiments30. For exosome treatment, cultured pterygial fibroblasts were exposed to either 20 µg/mL of M1 macrophage-derived exosomes or 20 µg/mL of EGF-preconditioned (10 ng/mL) M1 macrophage-derived exosome for 24 h.
Purification of total RNA and real-time qrt-PCR
Total RNA was extracted from pterygial fibroblasts, conjunctival, and pterygial stromal tissues using the RiboEx total RNA isolation solution (GeneAll Biotechnology, Seoul, Korea). Single-strand complementary DNA (cDNA) was synthesized from 1 µg of total RNA using cDNA synthesis kits (#K1622, Thermo Fisher Scientific, Waltham, MA, USA). Quantitative RT-PCR was performed using a CFX96TM Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) in a total volume of 20 µL, containing 10 µL SYBR Premix Ex Taq (Takara Bio Inc., Otsu, Japan). Relative gene quantities were determined using the comparative cycle threshold (Ct) method after normalization to a reference gene, 18 S ribosomal RNA.
Ki-67 staining and ki-67 proliferation index
Primarily cultured pterygial fibroblasts were cultivated on sterile coverslips. The cells were fixed with 100% MeOH at -20 °C for 10 min and permeabilized using 0.1% Triton-X100 for 10 min at room temperature. Subsequently, the cells were incubated with anti-rabbit Ki-67 polyclonal antibody (1:300; Abcam, Cambridge, UK) overnight at 4 °C. Following this, the cells were treated with anti-rabbit IgG Alexa Fluor 594 (Thermo Fisher Scientific) for 30 min at room temperature. The cells on coverslips were mounted on slides using Vectashield with DAPI. Immediately after treatment, the cells were observed under a fluorescence microscope (IX71, Olympus Optical Ltd., London, UK). Manual counting of the digitized cell images was performed using ImageJ software (https://imagej.net/Fiji). The Ki-67 proliferation index was then calculated by dividing the number of Ki-67-positive cells by the total number of counted cells: (number of Ki-67-stained nuclei/number of all nuclei) × 100 (%). The total number of cells analyzed for cell counting were 562.3 ± 10.6 in the control group, 607.3 ± 11.9 in the pterygial fibroblasts co-cultured with the M1 macrophage group, and 572.7 ± 46.5 in the pterygial fibroblasts co-cultured with the M1 macrophages and treated with EGF (10 ng/mL) group. These differences were not statistically significant among the groups.
Cell growth assay: Cell counting kit 8 (CCK-8) assay
Pterygial fibroblasts were seeded at a density of 1 × 104 cells/mL in a 96-well plate. Subsequently, 10 µL of CCK-8 reagent (Enzo Life Sciences, Farmingdale, NY, USA) was added to each well, and the culture plates were incubated in 5% CO2 at 37 °C for 2 h. Following this, 100 µL of each culture solution was transferred to a separate 96‐well plate, and the optical density (OD) was measured at 450 nm using a Spectramax™ 340PC384 microplate photometer (Molecular Devices, Sunnyvale, CA, USA). Cell counts were obtained at baseline and after 24 h and 72 h.
Statistical analyses
Statistical analysis was performed using GraphPad Prism (ver. 10, GraphPad Software Inc., La Jolla, CA, USA). The choice between parametric or non-parametric tests was determined by assessing the homogeneity of variance through a Levene test. For comparing gene expressions between conjunctival and pterygial tissues, either the parametric paired t-test or the non-parametric Wilcoxon signed-rank test was employed. Analysis of alterations in gene expression in pterygial fibroblasts after treatment with M1 macrophage-derived exosomes or co-culture with M1 macrophages, the differences in proportional Ki-67-positive cells among groups, and the variations in cell growth velocity among different time sets or groups were conducted through analysis of variance followed by Tuckey’s post-hoc test. Graphical representation of data within graphs was presented as the mean ± standard error. All experiments in this study were repeated in three or more separate trials. Statistical significance was considered when P < 0.05.
Data availability
The datasets generated and analyzed in the current study are available from the corresponding author on reasonable request.
Author contributions
K.W.K designed the study. S.J.L and S.H.L. collected data. S.J.L and A.K. analyzed the data. S.J.L, S.H.L., and K.W.K. wrote the manuscript. K.W.K. revised the manuscript.
Funding statement
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number RS-2023-00209498) and was supported by the Chung-Ang University Research Scholarship Grants in 2024.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim, K. W., Park, S. H. & Kim, J. C. Fibroblast biology in pterygia. Exp. Eye Res. 142, 32–39 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Di Girolamo, N. Signalling pathways activated by ultraviolet radiation: role in ocular and cutaneous health. Curr. Pharm. Des. 16, 1358–1375 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Di Girolamo, N., Kumar, R. K., Coroneo, M. T. & Wakefield, D. UVB-mediated induction of interleukin-6 and – 8 in pterygia and cultured human pterygium epithelial cells. Invest. Ophthalmol. Vis. Sci. 43, 3430–3437 (2002). [PubMed] [Google Scholar]
- 4.Kennedy, M. et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest. Ophthalmol. Vis. Sci. 38, 2483–2491 (1997). [PubMed] [Google Scholar]
- 5.Park, C. Y. et al. Cyclooxygenase-2-expressing macrophages in human pterygium co-express vascular endothelial growth factor. Mol. Vis. 17, 3468–3480 (2011). [PMC free article] [PubMed] [Google Scholar]
- 6.Ling, S. et al. Comparative evaluation of lymphatic vessels in primary versus recurrent pterygium. Eye (Lond). 26, 1451–1458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling, S., Liang, L., Lin, H., Li, W. & Xu, J. Increasing lymphatic microvessel density in primary pterygia. Arch. Ophthalmol. 130, 735–742 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Qi, C. X. et al. Relationship between angiogenesis and lymphangiogenesis in recurrent pterygium. Int. J. Ophthalmol. 5, 655–660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Notara, M. et al. Short-term uvb-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem Cell. Res. 15, 643–654 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Herbst, R. S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 59, 21–26 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Zieske, J. D., Takahashi, H., Hutcheon, A. E. & Dalbone, A. C. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest. Ophthalmol. Vis. Sci. 41, 1346–1355 (2000). [PubMed] [Google Scholar]
- 12.Burgel, P. R. & Nadel, J. A. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur. Respir J. 32, 1068–1081 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Dubourg, V. et al. EGFR activation differentially affects the inflammatory profiles of female human aortic and coronary artery endothelial cells. Sci. Rep. 13, 22827 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei, J. & Besner, G. E. M1 to M2 macrophage polarization in heparin-binding epidermal growth factor-like growth factor therapy for necrotizing enterocolitis. J. Surg. Res. 197, 126–138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeisberg, M. & Neilson, E. G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 119, 1429–1437 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pure, E. & Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene. 37, 4343–4357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Droppelmann, C. A., Gutierrez, J., Vial, C. & Brandan, E. Matrix metalloproteinase-2-deficient fibroblasts exhibit an alteration in the fibrotic response to connective tissue growth factor/CCN2 because of an increase in the levels of endogenous fibronectin. J. Biol. Chem. 284, 13551–13561 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367 (2020). [DOI] [PMC free article] [PubMed]
- 19.Okada, Y. et al. EGFR downregulation after Anti-EGFR Therapy predicts the Antitumor Effect in Colorectal Cancer. Mol. Cancer Res. 15, 1445–1454 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Buechler, M. B., Fu, W. & Turley, S. J. Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity. 54, 903–915 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Uderhardt, S., Martins, A. J., Tsang, J. S., Lammermann, T. & Germain, R. N. Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage. Cell. 177, 541–555 e517 (2019). [DOI] [PMC free article] [PubMed]
- 22.Wynn, T. A. & Barron, L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 30, 245–257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholes, A. G., Hagan, S., Hiscott, P., Damato, B. E. & Grierson, I. Overexpression of epidermal growth factor receptor restricted to macrophages in uveal melanoma. Arch. Ophthalmol. 119, 373–377 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Lamb, D. J., Modjtahedi, H., Plant, N. J. & Ferns, G. A. EGF mediates monocyte chemotaxis and macrophage proliferation and EGF receptor is expressed in atherosclerotic plaques. Atherosclerosis. 176, 21–26 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Liu, L. et al. Amphiregulin enhances cardiac fibrosis and aggravates cardiac dysfunction in mice with experimental myocardial infarction partly through activating EGFR-dependent pathway. Basic. Res. Cardiol. 113, 12 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Perugorria, M. J. et al. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology. 48, 1251–1261 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Zhou, Y. et al. Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-beta-induced pulmonary fibrosis. J. Biol. Chem. 287, 41991–42000 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendoza-Mari, Y. et al. Epidermal growth factor effect on lipopolysaccharide-induced inflammation in fibroblasts derived from diabetic foot ulcer. Scars Burn Heal. 8, 20595131211067380 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao, G. et al. Activation of Epidermal Growth Factor Receptor in Macrophages mediates feedback inhibition of M2 polarization and gastrointestinal tumor cell growth. J. Biol. Chem. 291, 20462–20472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, S. J., Lee, S. H., Koh, A. & Kim, K. W. EGF-conditioned M1 macrophages convey reduced inflammation into corneal endothelial cells through exosomes. Heliyon 10 (2024). [DOI] [PMC free article] [PubMed]
- 31.Siak, J. J., Ng, S. L., Seet, L. F., Beuerman, R. W. & Tong, L. The nuclear-factor kappaB pathway is activated in pterygium. Invest. Ophthalmol. Vis. Sci. 52, 230–236 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Nolan, T. M., Di Girolamo, N., Coroneo, M. T. & Wakefield, D. Proliferative effects of heparin-binding epidermal growth factor-like growth factor on pterygium epithelial cells and fibroblasts. Invest. Ophthalmol. Vis. Sci. 45, 110–113 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Jin, J. et al. Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea. 22, 473–477 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Kria, L., Ohira, A. & Amemiya, T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 98, 195–201 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Solomon, A. et al. Overexpression of insulin-like growth factor-binding protein-2 in pterygium body fibroblasts. Invest. Ophthalmol. Vis. Sci. 44, 573–580 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Nishida, T., Nakamura, M., Mishima, H. & Otori, T. Interleukin 6 promotes epithelial migration by a fibronectin-dependent mechanism. J. Cell. Physiol. 153, 1–5 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Hoppenreijs, V. P., Pels, E., Vrensen, G. F., Felten, P. C. & Treffers, W. F. Platelet-derived growth factor: receptor expression in corneas and effects on corneal cells. Invest. Ophthalmol. Vis. Sci. 34, 637–649 (1993). [PubMed] [Google Scholar]
- 38.Andresen, J. L. & Ehlers, N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr. Eye Res. 17, 79–87 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Di Girolamo, N., Coroneo, M. & Wakefield, D. Epidermal growth factor receptor signaling is partially responsible for the increased matrix metalloproteinase-1 expression in ocular epithelial cells after UVB radiation. Am. J. Pathol. 167, 489–503 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, Z., Carvajal, M., Carraway, C. A., Carraway, K. & Pflugfelder, S. C. Expression of the receptor tyrosine kinases, epidermal growth factor receptor, ErbB2, and ErbB3, in human ocular surface epithelia. Cornea. 20, 81–85 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Peterson, J. L. & Ceresa, B. P. Epidermal growth factor receptor expression in the corneal epithelium. Cells 10 (2021). [DOI] [PMC free article] [PubMed]
- 42.van Setten, G. B., Tervo, T., Viinikka, L., Perheentupa, J. & Tarkkanen, A. Epidermal growth factor in human tear fluid: a minireview. Int. Ophthalmol. 15, 359–362 (1991). [DOI] [PubMed] [Google Scholar]
- 43.Genin, M., Clement, F., Fattaccioli, A., Raes, M. & Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 15, 577 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in the current study are available from the corresponding author on reasonable request.