Abstract
Abnormal sensory processing is core to neuropsychiatric and neurodevelopmental disorders, such as schizophrenia and autism spectrum disorders. Developing efficient therapies requires understanding the basic sensory pathways and identifying circuit abnormalities during early development. Auditory brainstem responses (ABRs) are well-established biomarkers for auditory processing on the brainstem level. Beyond their advantage of being easily applicable in clinics (given their non-invasive nature), ABRs have high reproducibility in rodents and translate well to humans (e.g. wave identity), despite species differences (e.g. wave features). We hypothesized that ABRs would reveal sensory abnormalities in neurodevelopmental models with construct validity, such as Neurexin1α knockout (Nrxn1α KO) rats during their development. In a previous study, adult Nrxn1α KO rats showed altered cortical auditory-evoked potentials and impaired prediction error to auditory stimuli (Janz in Transl Psychiat, 12:455, 2022 ). This study used ABR measurements to assess brainstem physiology during auditory processing in Nrxn1α KO rats and their wild-type littermates. Therefore, we followed the development trajectories of ABRs from the age of 3 weeks to 12 weeks longitudinally. We found that juvenile Nrxn1α KO rats (3 weeks of age) show altered ABRs, which normalized during further development. This alteration was confined to increased latency in waves II, III, and IV of the ABRs, suggesting impaired auditory processing on the level of the superior olivary complex and inferior colliculus. In conclusion, our results suggest that early but transient deficits in the processing of auditory information on the level of the brainstem are present in Nrxn1α KO rats, which may contribute to later cortical auditory processing deficits observed in adulthood. Our study emphasizes the value of ABRs as a functional readout of auditory brainstem circuit function with potential value as a translational biomarker.
Keywords: Biomarkers, Auditory brainstem responses, Non-invasive brain technology, Neurodevelopmental disorders, Autism spectrum disorders, Schizophrenia, Neurexins
Subject terms: Neural circuits, Autism spectrum disorders
Introduction
Neurodevelopmental disorders are a class of brain disorders that start as early as brain prenatal stages1. In the current DSM-5, the section “Neurodevelopmental disorders” describes abnormal sensory processing as one of the symptoms of autism spectrum disorders (ASDs)2. Auditory brainstem responses (ABRs), also known as brainstem auditory evoked potentials, are a non-invasive electrophysiological measurement of neuronal signal transmission along the auditory pathway. In the last 30 years, a large amount of literature has been published on the link between impaired auditory brainstem functions and sensory abnormalities in ASD patients3,4, and ABR measurements have been considered a promising translational biomarker to predict ASDs3,4. In humans and rodents, ABR measurements result in a characteristic waveform, with specific response waves (ABR waves), that speak to the activation of specific neuronal populations along the auditory pathway5–7. ABRs measure the evoked potentials from the auditory nerve to the mesencephalon, following acoustic stimulation in the first 10 ms time window. In ABR measurements, we can differentiate between four to five positive response waves5–7. These waves represent synchronized neural activity and signal transmission from the auditory nerve, through the brainstem, the thalamus, and lastly to the auditory cortex8–10. Wave I is generated due to activation of the distal part of the auditory nerve (AN). Wave II is considered to be generated due to the activation of cochlear nucleus (CN) neurons. Wave III is generated by the superior olivary complex (SOC), wave IV by the lateral lemniscus and inferior colliculus (IC), and wave V represents the signal transmission to higher thalamic and cortical circuitry8–10. Depending on methodological and experimental conditions, rat ABRs may vary in shape and amplitude, with either wave I11, wave II12 or wave IV13,14 identified as the most prominent wave. Studies in ASD individuals showed impaired ABR signals, such as delay in the wave I responses15,16, or at wave V17,18 compared to healthy controls. Two meta-analyses confirm differences in ABR wave latencies, between ASD individuals and typically developed controls17,19. Mutations in the neurexins (NRXN) gene family lead to an increase in the risk of schizophrenia and are strongly associated with other neurodevelopmental disorders, including ASD20–22. Nrxn1α is part of the neurexins presynaptic cell adhesion proteins, which play essential roles in shaping synapse physiological properties, maturation, and organization 20,21.
To date, only a limited number of studies have focused on investigating auditory brainstem processing deficits in ASD rodent’ models13,23. In a previous study, we showed that the Neurexin1α knockout (Nrxn1α KO) rat exhibited altered auditory cortical evoked potential in their adult life phase1. In this study, we used ABR measurements to study the subcortical auditory processing during different time points of development. We hypothesized that if auditory brainstem function is compromised in Nrxn1α KO rats, this should be reflected in ABR abnormalities compared to wild-type littermates at a certain point during development and this finding could be linked to disruptions in auditory signal processing in neurodevelopmental and neuropsychiatric disorder, such as schizophrenia or ASDs.
Materials and methods
Animals
Experiments were conducted on Nrxn1α KO rats and wild-type littermates (strain: Sprague Dawley-Nrxn1 < tm1sage > bred by Charles River, France)1,24,25. Rats were housed in groups of two, in a temperature-controlled room on a 12 h light/dark cycle with ad libitum food and water. Only male rats were used, given that behavioral abnormalities are most pronounced in Nrxn1α KO males24,26. In this study, one cohort of animals WT (N = 15), and KO (N = 15) was recorded at different time points at 3, 4, and 6 weeks of age. For the recordings at weeks 8 and 12, we used WT (N = 15), and KO (N = 14). All procedures were approved by the Federal Food Safety and Veterinary Office of Switzerland (Basel-Nr.2857) and conducted in adherence to the Swiss federal ordinance on animal protection and welfare, as well as according to the rules of the Association for Assessment and Accreditation of Laboratory Animal Care International and the ARRIVE guidelines27.
Electrophysiological recording and acoustic stimulation
We applied click sound ABR measurement protocols, that we previously published1,25. In short, 512 click sound responses, generated at a 200 kHz sampling rate, were applied in a sound-attenuating and electrostatically grounded chamber. Each click sound is a broadband mono-phasic square wave signal. Click sounds were presented at a rate of 21 clicks/s, at different sound levels (90, 80, 70, 60, 50, 40, 30, 20, and 10 dB SPL), starting with the highest stimulus intensities. The clicks were generated via a multi-field speaker (MF1, Tucker-Davis Technologies, FL, USA), which was positioned 10 cm from the animal’s right ear. The ABR signals were recorded with 13 mm subdermal needle electrodes (Cat. no.: NS-s83018-r9-10, Rochester, Coral Springs, FL, USA). The signal electrode was placed on the vertex, the reference electrode under the right ear, and the ground electrode under the left ear. During the measurement, animals were under isoflurane-based anesthesia; induction at 5%, and maintenance at 2.5% (MiniHUB-V3, TemSega, FR). Body temperature was maintained at 37 °C using a thermic heating pad (Kent Scientific Corporation, CN, USA).
Euthanasia
At the end of the experiments, animals received terminal anesthesia; 150 mg/kg pentobarbital (Eskonarkon, Switzerland), i.p.,1:20 dilution with NaCl, followed by decapitation, after confirming a lack of reflexes by paw pinching.
Data processing and analysis
Data analysis was performed as previously described1,25. In brief, in a pre-processing step, ABR data were normalized to its pre-stimulus baseline. The resulting ABR waveforms were statistically tested for differences between genotypes (see Statistical testing). Individual wave amplitudes and latencies were detected manually from each ABRs signal post 90 dB click sound stimulations. Quantitative assessment of hearing thresholds was performed according to Bogaerts et al.28, considering 10 dB as a baseline signal and quantifying the signal-to-noise ratios (SNR). SNRs higher than 4 were considered a hearing response. In addition, qualitative assessments of hearing thresholds were also performed by a blinded expert evaluator, who identified the SPL at which a stimulus-related deflection was evident for each animal.
Statistical testing
Statistical testing of ABR waveforms between genotypes was performed with an unpaired cluster-based permutation test (CBPT), using custom Python scripts1,25. In summary, the CBPT starts with individual t-tests for each data point, employing a two-tailed approach (significance level set to p < 0.05). After this, the resultant clusters undergo a significance assessment by comparing the aggregated t-values of the original clusters and those derived from clusters obtained via permutation. This comparison is conducted over a considerable number of iterations (N = 1000 permutations), with a significance threshold maintained at p < 0.05. We visualized both cluster types (with permutation testing: full-colored bars above graphs; w/o permutation: brighter bars of the same color). Normal distribution of data was assumed, but not tested. The Area-under-the-curve and ABR waves analysis were tested with Prism 8 software (GraphPad, CA, USA), performing two-tailed unpaired Student’s t-test or repeated-measures (RM) two-way ANOVA with Tukey’s or Sidak’s post-test (significance level set to p < 0.05).
Results
Altered auditory brainstem processing during the early life stage of Nrxn1α KO rats
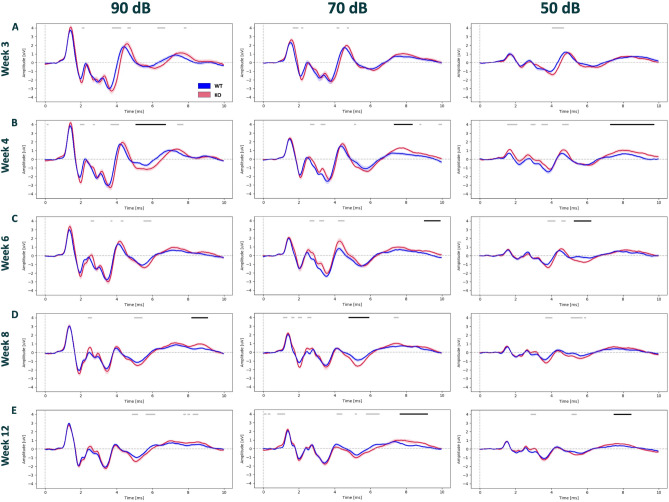
To study auditory brainstem processing during the development of Nrxn1α KO rats, we compared ABRs of Nrxn1α KO rats to their wild-type littermates at distinct time-points: after weaning (3 weeks old), their early juvenile phase (4 and 6 weeks old), and during adolescence/early adulthood (8 and 12 weeks old). We conducted the ABR measurements at different sound levels from 90 to 10 dB, in 10 dB decrements, for all measured time points. As in already established click ABR protocols1,13,25, higher stimulus intensities (90 dB) resulted in larger physiological responses. Importantly, the lowest identifiable ABR responses were evident at 40 dB for both Nrxn1α KO and wildtype littermates. This observation suggests similar hearing sensitivity between genotypes. More subtle differences in amplitude and/or latency at distinct ABR waves may reflect functional differences in specific nodes of the auditory brainstem circuitry. Therefore, we compared ABRs at different sound pressure levels and time points between genotypes directly using CBPT, Fig. 1 shows ABR waveforms only depicted for 90, 70, and 50 dB for easier visualization; analysis for all sound pressure levels can be found in Supplementary Figs. 1, 2. Starting with week 3 of age, Nrxn1α KO rats display a delay in parts of the ABRs in comparison to their wild-type littermates, these differences were qualitatively evident for ABRs elicited at 90 dB (between 3.1 and 5.3 ms), but not statistically significant (Fig. 1A). However, CBPT showed significant differences between the genotypes at week 4 of age (Fig. 1B); at 90 dB stimulus intensity (between 5.1 and 6.8 ms, d = − 1.05, p < 0.01), at 70 dB (between 7.3 and 8.3 ms, d = 1.24, p < 0.05), and at 50 dB (between 7.3 and 9.6 ms, d = 0.97, p < 0.01). Intriguingly, the identified genotypic differences at 3 and 4 weeks of age seem to normalize during further development, with no apparent differences already at the age of 6 weeks and onwards, indicating a transient impairment of auditory processing in the early life stage of Nrxn1α KO rats.
Fig. 1.
Comparison of ABR waveforms between Nrxn1α KO and wild-type littermate rats during development. ABR waveforms across the stimulus intensities 90, 70, and 50 dB from each tested time point; starting with week 3 (A), week 4 (B), week 6 (C), (WT: N = 15, KO: N = 15), week 8 (D), and week 12 (E); (WT: N = 15, KO: N = 14). Recordings from the WT are in blue and Nrxn1α KO in red. Unpaired CBPT revealed significant differences between clusters following 1000 permutations (p < 0.05) and displayed in Black bars above the graphs. The Gray bars indicate clusters that have not reached a significant threshold post-permutation. Data displayed as mean ± SEM.
Hearing sensitivity is similar between Nrxn1α KO and WT rats
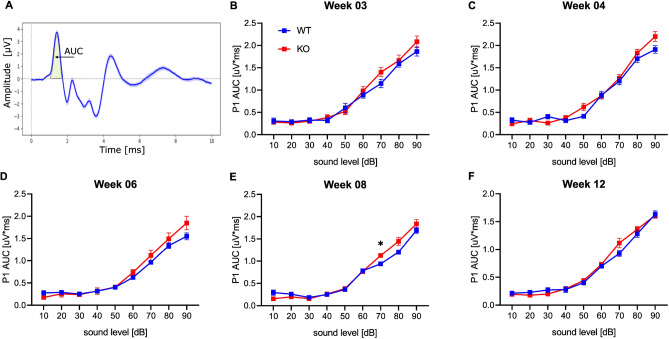
To assess hearing sensitivity, we calculated the area under the curve for the first positive wave of the ABRs (P1 AUC) across all stimulus intensities (90, 80, 70, 60, 50, 40, 30, 20, 10 dB) and at each age in our study (see Fig. 2). As expected, the increase of the P1 AUC was following the increase in the applied stimulus intensities. Interestingly, this positive relationship between stimulus intensities and response magnitudes seems to be preserved across the different developmental time points (see Fig. 2). For example, the average P1 AUC at 90 dB stimulus intensity; for week 3; (WT: 1.865 uV*ms, KO: 2.083 uV*ms), for week 6 (WT: 1.551 uV*ms, KO: 1.849 uV*ms), for week 08; (WT: 1.692 uV*ms, KO: 1.838 uV*ms), and for week 12 (WT: 1.633 uV*ms, KO: 1.614 uV*ms). In addition, hearing threshold analysis for the earliest time point of the ABR recordings (week 3 of age), suggests similar hearing sensitivity of Nrxn1α KO and WT rats (Supplementary Fig. 3). Nonetheless, the two-tailed ANOVA test with Sidak’s correction showed a significant difference between the Nrxn1α KO and their wild-type littermates, confined to the P1 AUC at 70 dB ABRs stimulus intensity and during a late phase of the young life of the animals (8 weeks old) (see Fig. 2E).
Fig. 2.
Hearing sensitivity is similar between Nrxn1α KO and wild-type littermate rats during the development. (A) Schematic illustrating the area-under-the-curve AUC of wave I (P1 AUC) used to assess hearing sensitivity. (B, C) Quantitative analysis of P1 AUC across stimulus intensities for Nrxn1α KO (in red) and wild-type (in blue) rats; at 3 weeks (B), at 4 weeks (C), at 6 weeks (D) (WT: N = 15, KO: N = 15), at 8 weeks (E), and 12 weeks of age (F) (WT: N = 15, KO: N = 14). Data displayed as mean ± SEM. Two-way mixed ANOVA, with Sidak’s post-test, *p < 0.05.
Nrxn1α KO rats show a delay in ABRs during the early stage of life
Finally, we studied the individual features of ABR waveforms at 90 dB, given that clusters of significant differences were identified, without differentiating whether this difference is due to changes in amplitudes or latencies or a combination of both. The first series of the analysis focused on ABR-wave latencies, which relates to the speed of signal transmission along the auditory brainstem (Fig. 3). Absolute latencies of wave II, III, and IV were significantly increased for Nrxn1α KO rats compared to their wild-type littermates at the age of 3 weeks (p < 0.05), see Fig. 3A. The average wave latency was for wave II (WT: 2.253 ms, KO: 2.309 ms), for wave III (WT: 3.205 ms, KO: 3.318 ms), and for wave IV (WT: 4.382 ms, KO: 4.553 ms). Additionally, interpeak latencies were inferred by subtracting the latency between two respective waves, see Fig. 3C. Here, the two-tailed ANOVA test with Sidak’s correction showed a significant difference between the Nrxn1α KO and their wild-type littermates, confined to the wave IV–I (mean diff. = − 0.1420, p = 0.0331) and wave III-I (mean diff. = − 0.08320, p = 0.0464), but not wave II-I (mean diff. = − 0.02653, p > 0.05). Following the absolute and interpeak latencies (Fig. 3B,D) over time showed that the initial genotypic differences at three weeks of age are normalized during further development. Statistical testing only revealed significant differences between the Nrxn1α KO and their wild-type littermates at 3 weeks of age. We also compared the amplitudes of individual ABR waves for all time points (see Supplementary Fig. 4). Statistical tests did not show significant differences in ABR amplitudes between the Nrxn1α KO and their wild-type littermates (Supplementary Fig. 4A and B). This supports the notion that the significant differences in ABRs between Nrxn1α KO rats and their wild-type littermates are linked to slower signal propagation within the auditory brainstem circuitry and not due to differences in hearing thresholds.
Fig. 3.
Nrxn1α KO rats show a delay in ABR latency during the early juvenile phase. (A) Absolute latencies for individual ABR waves in 3 week old rats. Nrxn1α KO (in red) and wild-type (in blue) rats. (B) Absolute latencies for individual ABR waves across all measured time points (Week 3–6 WT: N = 15, KO: N = 15)) (Week 8–12 (WT: N = 15, KO: N = 14)), each data point represents the averaged ABR wave latency. (C) Interpeak latencies for individual ABR waves in 3 week old rats. (D) Interpeak latencies for individual ABR waves across all measured time points (week 3–6 WT: N = 15, KO: N = 15)) (week 8–12 (WT: N = 15, KO: N = 14)), each data point represents the averaged relative ABR wave latency. Two-way mixed ANOVA, with Tukey’s post-test, revealed statistically significant differences between the Nrxn1α KO and WT rats. *p < 0.05.
Discussion
The present study represents the first longitudinal investigation of auditory brainstem function in Nrxn1α KO rats. Here, we sought to determine developmental changes of ABRs in Nrxn1α KO rats, and their wild-type littermates. ABRs have been widely used to assess auditory brainstem processing impairments among different rodent models29–31. In a previous study, we showed that adult Nrxn1α KO rats have altered cortical auditory-evoked potentials and impaired auditory prediction error1. However, it is unknown if auditory processing deficits are present at the brainstem level during the development of Nrxn1α KO rats. Therefore, we assessed the ABR of Nrxn1α KO rats at different ages compared to age-matched wild-type littermates. Our study reveals that juvenile Nrxn1α KO rats at three weeks of age have increased latencies of waves II, III, and IV, while amplitudes were unaltered. These differences were, however, not evident anymore after three weeks of age, suggesting normalization for the early auditory deficit. This phenotype was found despite a similar hearing threshold for both, Nrxn1α KO and wild-type littermate rats. Our study is in line with previous findings on altered auditory brainstem processing in other rat models linked to neurodevelopmental disorders13. Similar to Nrxn1α KO rats, rats lacking Cntnap2 (another member of the neurexin gene family) showed increased latencies in waves II, III, and IV without changed hearing thresholds compared to wild-type littermates13. This phenotypic convergence suggests an important role of neurexin genes in auditory brainstem circuit function and its association with neurodevelopmental disorders. Indeed, both Nrxn1α and Cntnap2 are expressed in many auditory brainstem nuclei, such as the ventral CN, SOC, and IC13,32,33. A recent functional study has shown that neurexins, which are highly expressed in the medial nucleus of the trapezoid body (MNTB), control the strength and kinetics of glycinergic neurotransmission from the MNTB to the lateral superior olive34. Moreover, neurexins were shown to regulate GABABR-mediated neurotransmission at the calyx of Held within the MNTB35. While the previous studies did not focus specifically on Nrxn1α, but they used KO mouse lines for all three neurexin isoforms, our data obtained in rats lacking only Nrxn1α demonstrate the importance of Nrxn1α in early-life synaptic transmission within the ascending auditory pathway. Considering that both Nrxn1α and Cntnap2 KO rodents show impaired ultrasonic vocalization26,36, in which the auditory brainstem plays an important role37, our data expands the mechanistic understanding of how deficiencies in neurexins may impair early social communication. Such developmentally early deficits may manifest as altered social behavior in later life, as reported for Nrxn1α KO24,26,38 and Cntnap2 KO39 resembling a key feature in various neuropsychiatric and neurodevelopmental disorders2.
While our ABRs display all the features of typical ABRs (Waveforms I-IV), one has to note that the direct comparison to previously published data is complicated by the use of different experimental settings across studies. Nonetheless, a limitation of the current study is the use of click sounds only, for acoustic stimulation. Further studies may explore whether tones at different frequencies (most importantly between 8 and 32 kHz) would provide a more granular characterization of auditory differences. Moreover, other methodologies to assess the function of the auditory systems, such as Distortion Product Otoacoustic Emissions measurements could enrich the characterization of Nrxn1α KO-related auditory abnormalities. Also, expanding the age range to pre-weaning timepoints in future studies could reveal more extensive developmental deficits of auditory circuit formation. Considering that our study focused on male rats, future studies may investigate sex differences in ABRs of Nrxn1α KO rats. Taken together, the identified ABR phenotype in Nrxn1α KO rats suggests an early impairment of auditory brainstem function, which is compensated during further development. These results expand the current knowledge of auditory processing deficits associated with Nrxn1α deficiency, which is an important genetic risk factor for ASDs and other neurodevelopmental or neuropsychiatric disorders.
Supplementary Information
Acknowledgements
We would like to acknowledge Marie Bainier, Damian Roqueiro, Simon Gross and Eva Maria Amen for their excellent support. Furthermore, we would like to thank the Roche Innovation Centre Graduate Students Internship Program for the funding of Samuel Marashli.
Author contributions
SM: Conceptualization, Methodology, Data acquisition, Formal analysis, Investigation, Software, Visualization, Writing-Original draft. PJ: Conceptualization, Methodology, Supervision, Methodology, Software, Validation, Writing—Review & Editing. RR: Conceptualization, Supervision, Project administration, Funding acquisition, Writing—Review & Editing.
Funding
F. Hoffmann-La Roche (Roche).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
SM received a graduate student internship from F. Hoffmann-La Roche (Roche). PJ and RR were under employment by the company F. Hoffmann-La Roche (Roche). The funder provided support in the form of salaries for authors but did not have any additional role in the study design, data collection, analysis, decision to publish, or manuscript preparation. This does not alter the authors’ adherence to all the journal policies on sharing data and materials.
Ethics approval and consent to participate
All procedures were approved by the ethics committee of the Federal Food Safety and Veterinary Office of Switzerland and conducted in adherence to the Swiss federal ordinance on animal protection and welfare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73920-9.
References
- 1.Janz, P. et al. Neurexin1α knockout rats display oscillatory abnormalities and sensory processing deficits back-translating key endophenotypes of psychiatric disorders. Transl. Psychiat.12, 455 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Association, A. P. Diagnostic and Statistical Manual of Mental Disorders. 5th (DSM-5). American Psychiatric Association (2013).
- 3.Talge, N. M., Adkins, M., Kileny, P. R. & Frownfelter, I. Click-evoked auditory brainstem responses and autism spectrum disorder: A meta-analytic investigation of disorder specificity. Pediatr. Res.92, 40–46 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miron, O., Beam, A. L. & Kohane, I. S. Auditory brainstem response in infants and children with autism spectrum disorder: A meta-analysis of wave V. Autism Res.11, 355–363 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popelar, J., Grecova, J., Rybalko, N. & Syka, J. Comparison of noise-induced changes of auditory brainstem and middle latency response amplitudes in rats. Hear. Res.245, 82–91 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Alvarado, J. C., Fuentes-Santamaría, V., Jareño-Flores, T., Blanco, J. L. & Juiz, J. M. Normal variations in the morphology of auditory brainstem response (ABR) waveforms: A study in wistar rats. Neurosci. Res.73, 302–311 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Eggermont, J. J. Auditory brainstem response. Handb Clin. Neurol.160, 451–464 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Picton, T. W., Stapells, D. R. & Campbell, K. B. Auditory evoked potentials from the human cochlea and brainstem. J. Otolaryngol. Suppl.9, 1–41 (1981). [PubMed] [Google Scholar]
- 9.Melcher, J. R., Guinan, J. J., Knudson, I. M. & Kiang, N. Y. S. Generators of the brainstem auditory evoked potential in cat. II. Correlating lesion sites with waveform changes. Hearing Res.93, 28–51 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Møller, A. R., Jannetta, P. J. & Jho, H. D. Click-evoked responses from the cochlear nucleus: A study in human. Electroencephalogr Clin. Neurophysiol. Evoked Potentials Sect.92, 215–224 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Johne, M. et al. Processing of auditory information in forebrain regions after hearing loss in adulthood: Behavioral and electrophysiological studies in a rat model. Front. Neurosci.16, 966568 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overbeck, G. W. & Church, M. W. Effects of tone burst frequency and intensity on the auditory brainstem response (ABR) from albino and pigmented rats. Hear. Res.59, 129–137 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Scott, K. E. et al. Altered auditory processing, filtering, and reactivity in the Cntnap2 knock-out rat model for neurodevelopmental disorders. J. Neurosci.38, 8588–8604 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch, L., Gaese, B. H. & Nowotny, M. Strain comparison in rats differentiates strain-specific from more general correlates of noise-induced hearing loss and tinnitus. J. Assoc. Res. Otolaryngol.23, 59–73 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azouz, H. G., Kozou, H., Khalil, M., Abdou, R. M. & Sakr, M. The correlation between central auditory processing in autistic children and their language processing abilities. Int. J. Pediatr. Otorhi.78, 2297–2300 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Chen, J. et al. Atypical longitudinal development of speech-evoked auditory brainstem response in preschool children with autism spectrum disorders. Autism Res.12, 1022–1031 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Miron, O. et al. Prolonged auditory brainstem response in universal hearing screening of newborns with autism spectrum disorder. Autism Res.14, 46–52 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borenstein-Levin, L. et al. Effects of neurodevelopmental risk factors on brainstem maturation in premature infants. Pediatr. Res.92, 168–173 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Talge, N. M., Tudor, B. M. & Kileny, P. R. Click-evoked auditory brainstem responses and autism spectrum disorder: A meta-analytic review. Autism Res.11, 916–927 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Südhof, T. C. Synaptic neurexin complexes: A Molecular code for the logic of neural circuits. Cell171, 745–769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Südhof, T. C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature455, 903–911 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tromp, A., Mowry, B. & Giacomotto, J. Neurexins in autism and schizophrenia—a review of patient mutations, mouse models and potential future directions. Mol. Psychiatry26, 747–760 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Scott, K. E. et al. Closing the species gap: Translational approaches to studying sensory processing differences relevant for autism spectrum disorder. Autism. Res.14, 1322–1331 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Esclassan, F., Francois, J., Phillips, K. G., Loomis, S. & Gilmour, G. Phenotypic characterization of nonsocial behavioral impairment in neurexin 1α knockout rats. Behav. Neurosci.129, 74–85 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Marashli, S., Janz, P. & Redondo, R. L. Auditory brainstem responses are resistant to pharmacological modulation in Sprague Dawley wild-type and Neurexin1α knockout rats. BMC Neurosci.25, 18 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kight, K. E. et al. Social behavior in prepubertal neurexin 1α deficient rats: A model of neurodevelopmental disorders. Behav. Neurosci.135, 782–803 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Plos Biol.8, e1000412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogaerts, S., Clements, J. D., Sullivan, J. M. & Oleskevich, S. Automated threshold detection for auditory brainstem responses: comparison with visual estimation in a stem cell transplantation study. Bmc Neurosci.10, 104–104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro, A. C. & Monteiro, P. Auditory dysfunction in animal models of autism spectrum disorder. Front. Mol. Neurosci.15, 845155 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seif, A., Shea, C., Schmid, S. & Stevenson, R. A. A systematic review of brainstem contributions to autism spectrum disorder. Front. Integr. Neurosci.15, 760116 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dadalko, O. I. & Travers, B. G. Evidence for brainstem contributions to autism spectrum disorders. Front. Integr. Neurosci.12, 47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchigashima, M., Cheung, A., Suh, J., Watanabe, M. & Futai, K. Differential expression of neurexin genes in the mouse brain. J. Comp. Neurol.527, 1940–1965 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon, A. et al. Expression of Cntnap2 (Caspr2) in multiple levels of sensory systems. Mol. Cell. Neurosci.70, 42–53 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Jiang, H.-H. et al. Neurexins control the strength and precise timing of glycinergic inhibition in the auditory brainstem. Elife10.7554/elife.94315.1 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo, F., Sclip, A., Merrill, S. & Südhof, T. C. Neurexins regulate presynaptic GABAB-receptors at central synapses. Nat. Commun.12, 2380 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Möhrle, D. et al. Characterizing maternal isolation-induced ultrasonic vocalizations in a gene–environment interaction rat model for autism. Genes, Brain Behav.22, 2841 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts, P. D. & Portfors, C. V. Responses to social vocalizations in the dorsal cochlear nucleus of mice. Front. Syst. Neurosci.9, 172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achterberg, E. J. M., Biemans, B. & Vanderschuren, L. J. M. J. Neurexin1α knockout in rats causes aberrant social behaviour: relevance for autism and schizophrenia. Psychopharmacology10.1007/s00213-024-06559-z (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott, K. E. et al. Loss of Cntnap2 in the rat causes autism-related alterations in social interactions, stereotypic behavior, and sensory processing. Autism Res.13, 1698–1717 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.