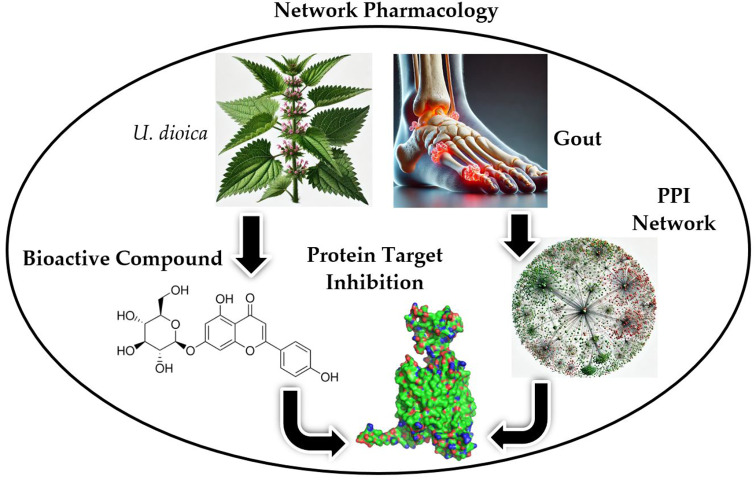
Abstract
Urtica dioica (stinging nettle) has been traditionally used in Chinese medicine for the treatment of joint pain and rheumatoid arthritis. This study aims to elucidate the active compounds and mechanisms by which it acts against gout arthritis (GA). Gout-related genes were identified from the DisGeNet, GeneCards, and OMIM databases. These genes may play a role in inhibiting corresponding proteins targeted by the active compounds identified from the literature, which have an oral bioavailability of ≥ 30% and a drug-likeness score of ≥ 0.18. A human protein-protein interaction network was constructed, resulting in sixteen clusters containing plant-targeted genes, including ABCG2, SLC22A12, MAP2K7, ADCY10, RELA, and TP53. The key bioactive compounds, apigenin-7-O-glucoside and kaempferol, demonstrated significant binding to SLC22A12 and ABCG2, suggesting their potential to reduce uric acid levels and inflammation. Pathway enrichment analysis further identified key metabolic pathways involved, highlighting a dual mechanism of anti-inflammatory and urate-lowering effects. These findings underscore the potential of U. dioica in targeting multiple pathways involved in GA, combining traditional medicine with modern pharmacology. This integrated approach provides a foundation for future research and the development of multi-target therapeutic strategies for managing gout arthritis.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-024-00254-9.
Keywords: Urtica dioica, Gout arthritis, Network pharmacology, OMIM, Leiden algorithm
Introduction
Gout is one of the most persistent arthritic disorders and is characterized by ongoing severe acute inflammation (Wang et al. 2023a). Significant comorbid conditions, such as metabolic syndrome and heart failure, are also associated with gout (Choi et al. 2007; Annemans et al. 2008). Gout affects 1–4% of the global population and is projected to increase significantly, leading to more disability, health issues, and economic burdens. Current treatments, such as urate-lowering therapies (ULTs), are not always effective and can have side effects, highlighting the need for novel treatments. Urtica dioica, or stinging nettle, has traditionally been used for treating joint pain and rheumatoid arthritis. Its anti-inflammatory properties and bioactive compounds, such as citric acid, sitosterol-b-D-glucoside, and gentisic acid, show promise in treating gout by targeting specific pathways involved in its development. Thus, U. dioica could be a valuable addition to gout treatment strategies based on its traditional use and preliminary evidence. Gout (arthritis) is a condition that falls within the category of rheumatic illnesses. Gout is a metabolic ailment spurred by purine metabolism instability. Monosodium urate crystals (Enomoto et al. 2002) build within joints, leading to gout. When the blood uric acid level is habitually increased (hyperuricemia, HUA) and exceeds the saturation threshold, MSU crystals are produced. Gout is most common in men and often affects adults and elderly individuals. One to four% of the population worldwide develops it, and those individuals tend to be younger. By 2060, it is anticipated that 55% more people will die from gout. Urtica dioica, commonly known as the stinging nettle, is a perennial flowering plant that has been used in traditional medicine for centuries. It is widely distributed in temperate regions across the world and is known for its therapeutic properties. The plant contains various bioactive compounds, including flavonoids, phenolic acids, and lignans, which contribute to its anti-inflammatory and antioxidant activities. U. dioica has been traditionally used to treat conditions such as arthritis, gout, and other inflammatory disorders. (Singh and Gaffo 2020; Man et al. 2017). Hyperuricemia is a necessary condition for the emergence of the painful, incapacitating disease known as gout (Punzi et al. 2012). Due to its widespread occurrence across the world, gout causes significant disability, health loss, and economic stress (Wang et al. 2023a) (See Fig. 1).
Fig. 1.
Gout-affected Big Toe (https://www.biorender.com/). This figure illustrates a big toe affected by gout, highlighting the typical symptoms including inflammation, swelling, and the presence of urate crystal deposits. The visual representation emphasizes the characteristic redness and swelling around the joint, which are common indicators of gout. The detailed depiction provides a clear view of the affected area, making it a useful reference for understanding the clinical manifestations of gout in the big toe
Primary gout
Primary gout is always caused by cooccurring diseases, such as arterial blood hypertension, and other disorders of carbohydrate and lipid metabolism, which are caused by genetic predisposition, an improper diet high in protein and red meat, excessive alcohol consumption, and obesity. In cases of certain ailments, such as hemolytic failure and tumor conditions, a rise and disintegration of nucleotides concurrent with an elevated level of cytolysis results in secondary gout (Kozub et al. 2012).
Second phase of hyperuricemia
The second phase of hyperuricemia, which manifests as permanent tophaceous gout and frequently comprises polyarticular segments, signs between attacks, and crystal deposition known as “tophi” in soft tissues or joints, can transition to untreated hyperuricemia. It results in renal tophi and the formation of tophi and joint discomfort (Punzi et al. 2012; Hainer et al. 2014).
Gout is an apparent drastic phase of progressive arthritis because the crystals may lodge in a joint, leading primarily to a rapid aggressive reaction, or in soft tissues, such as cartilage, triggering no swelling. One of the oldest illnesses affecting men is likely gout (Punzi et al. 2012). The most common symptom of gout is the abrupt development of intense clinical monoarticular rheumatism in a peripheral leg joint (Emmerson 1996).
Association of hyperuricemia with diabetes
An increased risk of diabetes is linked to hyperuricemia. By preventing nitric oxide’s biological activities and boosting the release of inflammatory substances such as adipocytokines, which can influence insulin-stimulated glucose uptake, uric acid may contribute to insulin resistance. Many studies have revealed a complex relationship between hyperuricemia and diabetes (Jiang et al. 2023).
History of gout
Over 4,000 years ago, Egyptians gave the earliest historical narrative of the podagra. Despite being widespread, the “disease of kings” is nonetheless fascinating due to its link to dietary intake, alcohol intake, social status, and lifestyle. The condition is one of the very few to be diagnosed so promptly and simultaneously happens to be the one that has triggered complications with treatment ever since (Kolasinski 2014). The accumulation of uric acid crystals in tissues and organs occurs as an outcome of several metabolic diseases (Hainer et al. 2014).
Monosodium urate crystal deposition
Purine is a substance that promotes the metabolic disorder gout. When the concentration of urate in the blood exceeds the dissolution limits, a phase transition occurs in the presence of sodium, and monosodium urate (Enomoto et al. 2002) crystals are produced (Martillo et al. 2014). Due to the accumulation of MSU crystals in bones and surrounding tissues, gout often affects the big toe, although it may also influence other joints and can cause acute bouts of severe arthritis (Richette et al. 2015).
Drinking alcohol may be related to various diseases, including gout recurrence. Alcohol is known to increase uric acid levels in blood serum and cause gout flares (Nieradko-Iwanicka 2022). The sudden emergence of a painful, swollen, heated, and red joint is known as gout, which is a therapeutically obvious event of acute inflammation (Nutmakul 2022).
Causes of gout
The fundamental cause of hyperuricemia is aberrant uric acid production. The two main sources of uric acid in the human body are endogenous and exogenous. Approximately 80% of the total amount of uric acid in the body comes from endogenous sources, primarily the breakdown of nucleotides, while the other 20% is primarily derived from exogenous sources, primarily food (Wang et al. 2023b). Exogenous uric acid can be found in a variety of foods, including deep-sea fish, fish eggs, pork, sausages, mushrooms, baijiu, beer, and broth (Chen et al. 2023).
Materials and methods
Data preparation
Gout-related target collection and prediction of bioactive compounds of Uritica dioica
Gout-related targets were collected from the Disgenet, GeneCards (Stelzer et al. 2011) and OMIM databases (Hamosh et al. 2000) with “gout” as the keyword, while information about the chemical constituents of Urtica dioica was obtained from published literature. Then, the canonical SMILESs for the chemical compounds were obtained from the PubChem database (Kim et al. 2016). After that, the SwissADME web server was used to calculate the oral bioavailability of the obtained constituents, and the Molsoft web server was used to calculate the drug likeness of active compounds with an oral bioavailability ≥ 30 and a drug likeness ≥ 0.18 (Quah et al. 2023). In the next step, the targets of these compounds that might or might not be related to gout were collected using the SwissTargetPrediction web server (Gfeller et al. 2013), in where the selected species was Homo sapiens.
Cluster formation
Generate and visualize clusters
This study employed a Python-based pipeline to generate and visualize clusters of genes related to gout arthritis within the human protein‒protein interaction (Stelzer et al. 2011) network. The methodology involves several key steps, including data preparation, network construction, cluster formation, and visualization.
Initially, we imported essential Python libraries, including `pandas` for data manipulation, `networkx` for network analysis, `graphistry` for network visualization, `leidenalg` for clustering, and `matplotlib` for plotting. The input gene data and the human PPI network were loaded from the respective CSV files. The gene data included genes of interest, while the PPI network provided information on protein interactions. Using NetworkX, we constructed a PPI network from the loaded data. The input genes were then mapped to this PPI network to create a subgraph consisting only of the relevant genes and their interactions. This subgraph represents the subset of the PPI network pertinent to our study.
To facilitate efficient clustering, the NetworkX graph was converted into an iGraph object. The Leiden algorithm, known for its robustness and accuracy in detecting communities within large networks, was applied to this iGraph subgraph. The algorithm identified clusters of genes, with each node (gene) assigned a cluster membership. This step enabled the identification of functionally related groups of genes within the network.
For visualization, we utilized graphistry, an interactive visualization tool that allows for intuitive exploration of complex networks. The network, along with its clusters, was visualized to provide clear insights into the gene interactions and cluster formations. The nodes in the visualizations were color-coded based on their cluster memberships, enhancing the interpretability of the results.
The files generated from the latter analysis (3.1, 3.2) were used to make these clusters. The network and clusters were generated using a python-based in-house-developed pipeline. The network maps the input genes to the whole PPI network (interactome) of humans. Then, the protein network was extracted and visualized using Graphistry software. In the next step, the gene network is constructed, and clusters are generated using the Leiden algorithm (hierarchical clustering algorithm).
Clusters with disease-related genes
To check the disease relevance, the Enrichr software was used. Clusters of genes were pasted in the gene entry box and clicked on for submission. After that, the disease data were clicked on, and the DisGeNet and OMIM data were downloaded.
Acquisition of intersection targets
To identify common genes, the Venny 2.1 software was used to construct a Venn diagram by using a plant target file with disease-relevant clustered genes.
Construction of the PPI network
The intersecting targets obtained were imported into the STRING 11.5 database (Szklarczyk et al. 2021). The species was set to “Homo sapiens”, and a confidence level greater than 0.4 was used. The free targets in the networks were then hidden, and protein‒protein interaction (Stelzer et al. 2011) networks were constructed. Using Network Analysis in the Cytoscape software (version 3.10.1) (Otasek et al. 2019), a PPI network was subsequently constructed using the obtained network representations. Lines represent relationships, and nodes represent associated targets. The nodes in this network represent significant targets, and the lines indicate links between them.
Construction of the drug-component-target-disease network
Using the software Cytoscape (version 3.10.1), we inserted the previously determined targets for U. dioica and gout to create a network representing the link between drugs and diseases. The nodes represent pertinent targets, while the lines illustrate any possible connections that may exist between them.
Pathway enrichment analysis
The Enrichr database is an online tool for gene annotation, classification, transcription, drug/disease, and pathway analysis (Evangelista et al. 2023). To further identify the related effects of U. dioica in treating gout, we used Reactome and KEGG metabolic pathway enrichment analysis to define the main metabolic pathway of U. dioica in treating gout. We constructed key protein and core pathway interaction data that were analyzed in Cytoscape 3.10.1 to construct a target-pathway network.
Molecular docking verification
First, the structures of 6 proteins encoded by identified core targets, SLC22A12 urate transporter 1 (URAT1) (ID: 4ZW9, gene: SLC22A12), ABCG2 (ID: 8BHT, gene: EGFR), RELA (ID: 2VEG, gene: RELA), MAP2K7 (ID: 6QHO, gene: MAP2K7), ADCY10 (ID: 7OVD, gene: ADCY10), and TP53 (ID: 5O1F, gene: TP53), were downloaded from the Protein Data Bank. PyRx software, which is a downloadable source, was used to carry out the molecular docking of these genes.
In the next steps, the structures of the ligands were downloaded from the PubChem database and then converted to pdbqt files. Then, the small molecules were docked into the active site of the targets using PyRx software. Freely available Discovery Studio Visualizer software was used to analyze the docking results. ChimeraX software is also freely available and was used for visualization of the docking results (See Fig.2) Schematic chart of Network Pharmacology using In House pipeline to investigate Neighboring Clusters, Genes relevancy with Gout (https://www.biorender.com/).
Fig. 2.
The workflow explores the therapeutic potential of Urtica dioica L. (stinging nettle) in gout arthritis (GA). Gout-related targets were collected from DisGeNet, GeneCards, and OMIM, while U. dioica chemical constituents were sourced from literature and PubChem. SwissTargetPrediction predicted targets of these constituents, selecting compounds with oral bioavailability ≥ 30% and drug-likeness ≥ 0.18 using SwissADME and Molsoft. A human PPI network was constructed with NetworkX, mapping relevant genes. The Leiden algorithm identified gene clusters, visualized with Graphistry. Disease relevance was assessed using Enrichr, and common genes were identified using Venny 2.1. Intersecting genes were analyzed with STRING, and PPI networks were visualized with Cytoscape. Molecular docking studies were performed using PyRx and visualized by ChimeraX. Key bioactive compounds identified were apigenin-7-O-glucoside and kaempferol. Apigenin-7-O-glucoside showed significant binding to SLC22A12 and ABCG2, potentially reducing uric acid levels and inflammation. Kaempferol also effectively binds to these targets, suggesting its role in modulating uric acid excretion and inflammation. Targeting SLC22A12 can decrease uric acid levels, alleviating gout symptoms, while enhancing ABCG2 function can improve uric acid elimination, reducing gout attacks
Results
Data preparation
A total of 192 chemicals were initially retrieved. However, only the targets of 9 chemicals were selected for further analysis. This selection was based on the criteria of oral bioavailability (OB) and drug-likeness (DL). Specifically, the SwissADME web server was used to calculate the oral bioavailability, and the Molsoft web server was used to determine the drug-likeness of these chemicals. Only those chemicals with an oral bioavailability ≥ 30% and a drug-likeness score ≥ 0.18 were chosen for further investigation, resulting in a focused analysis on the most promising compounds.
The GeneCards, DisGeNet and OMIM databases were searched to identify the possible targets of gout. These three databases produced a total of 1690 results from GeneCard, 206 from DisGeNet and 49 from OMIM. When duplicates were removed, the prospective mapping of 864 targets of active components with disease targets (from three different databases) revealed 84 similar targets. The literature search resulted in the retrieval of 192 chemicals. Following the discovery, filtering, and elimination of duplicates, a group of approximately 113 genes was selected for further processing. Using the SwissTargetPrediction database, 864 targets were obtained from the 9 chemical components of the U. dioica plant (Table 1).
Table 1.
The 10 most biologically active chemical components of U. dioica
Cluster formation
To justify the cluster search with sixteen containing plant-targeted genes, we identified the most significant targets for gout treatment, a series of intersecting genes between U. dioica targets and disease-related clusters by using Venny 2.1. The STRING database was then utilized to analyze these intersecting genes, leading to the construction of PPI networks. The analysis highlighted key genes with a high degree of relevance to gout, including ABCG2, SLC22A12, MAP2K7, ADCY10, RELA, and TP53.
Molecular docking studies were conducted to predict the binding affinity of U. dioica’s bioactive compounds to these key gout-related targets. Using PyRx software for docking and ChimeraX for visualization, apigenin-7-O-glucoside and kaempferol emerged as compounds with strong binding potential. Specifically, apigenin-7-O-glucoside showed a significant binding affinity to the SLC22A12 gene product, while kaempferol demonstrated strong binding to the ABCG2 gene product. These findings suggest that these compounds can modulate the activity of these critical targets, potentially reducing uric acid levels and alleviating inflammation, thereby offering a promising therapeutic strategy for managing gout arthritis.
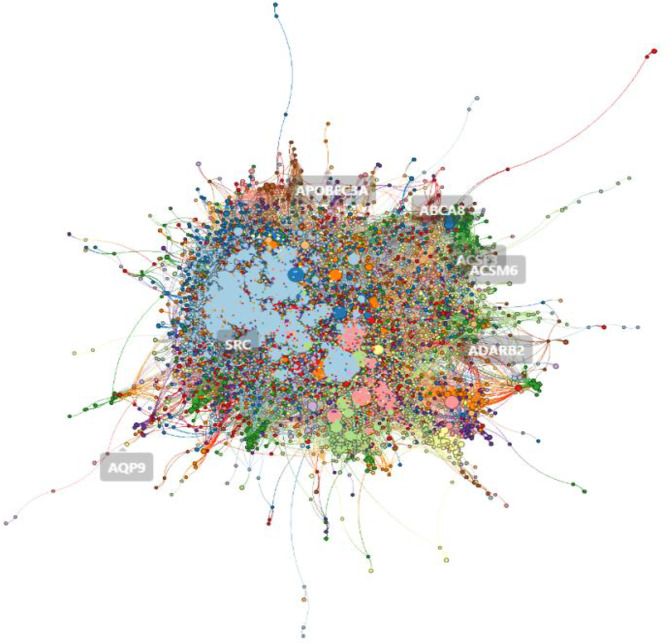
The initial network construction resulted in a comprehensive PPI subgraph that included only the genes of interest and their interactions. This subgraph served as the basis for clustering. The Leiden algorithm effectively identified clusters within this network, revealing functional groupings of genes potentially involved in gout arthritis. The visualization of these clusters was accomplished using graphistry, which provides an interactive platform for exploring complex networks. The overall visualization revealed 9684 nodes and 665,444 edges, illustrating the extensive interactions within the PPI network (Fig. 3). After the clusters were created, 20 clusters were formed.
Fig. 3.
Overall cluster visualization of 9684 nodes and 665,444 edges
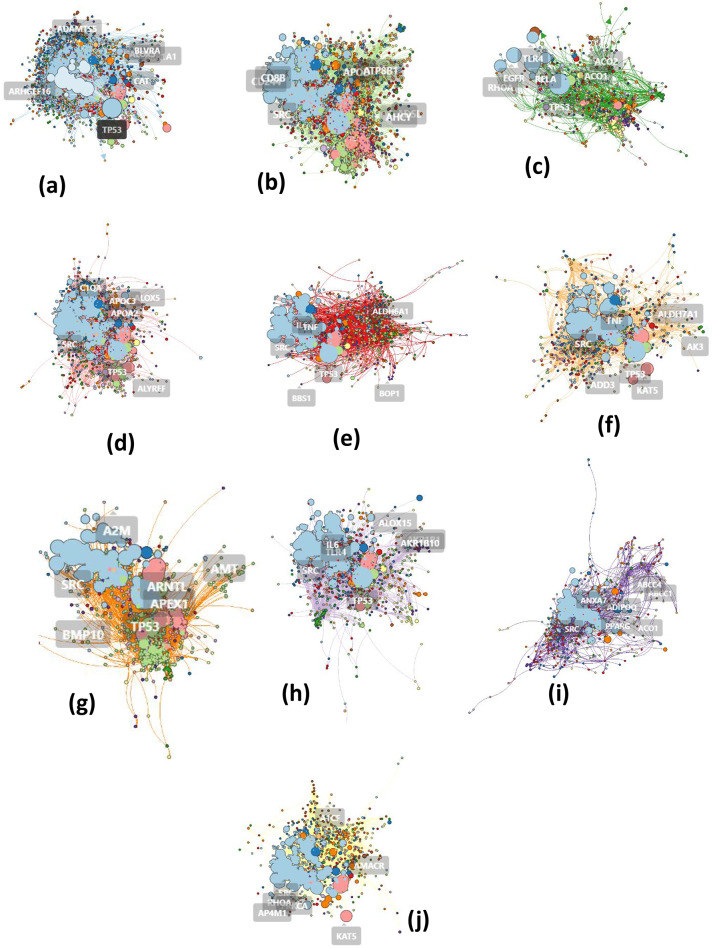
Visualization of the clusters was performed by graphing. Cluster 1 had 3164 nodes and 21,053 edges, Cluster 2 had 2662 nodes and 10,813 edges, Cluster 3 had 1182 nodes and 2748 edges, Cluster 4 had 1875 nodes and 6936 edges, Cluster 5 had 1274 nodes and, 3966 edges, Cluster 6 had 1149 nodes and 2323 edges, Cluster 7 had 1069 nodes and 2993 edges, Cluster 8 had 1321 nodes and 3082 edges, Cluster 9 had 815 nodes and 1594 edges, Cluster 10 had 1003 nodes and 2736 edges, Cluster 11 had 895 nodes and 2011 edges, Cluster 12 had 605 nodes and 2222 edges, Cluster 13 had 597 nodes and 1178 edges, Cluster 14 had 458 nodes and 980 edges, Cluster 15 had 361 nodes and 866 edges, Cluster 16 had 341 nodes and 705 edges, Cluster 17 had 109 nodes and 197 edges, Cluster 18 had 45 nodes and 74 edges, Cluster 19 had 38 nodes and 49 edges and Cluster 20 had 19 nodes and 18 edges in Figs. 4 and 5 provided detailed visual representations of the clusters, highlighting the node and edge composition of each cluster, for the gene interactions and the potential biological significance of each cluster in the context of gout.
Fig. 4.
Cluster 1 (a) has 3164 nodes and 21,053 edges, Cluster 2 (b) has 2662 nodes and 10,813 edges, Cluster 3 (c) has 1182 nodes and 2748 edges, Cluster 4 (d) has 1875 nodes and 6936 edges, Cluster 5 (e) has 1274 nodes and 3966 edges, Cluster 6 (f) has 1149 nodes and 2323 edges, Cluster 7 (g) has 1069 nodes and 2993 edges, Cluster 8 (h) has 1321 nodes and 3082 edges, Cluster 9 (i) has 815 nodes and 1594 edges, and Cluster 10 (j) has 1003 nodes and 2736 edges
Fig. 5.
Cluster 11 (k) has 895 nodes and 2011 edges, Cluster 12 (l) has 605 nodes and 2222 edges, Cluster 13 (m) has 597 nodes and 1178 edges, Cluster 14 (n) has 458 nodes and 980 edges, Cluster 15 (o) has 361 nodes and 866 edges, Cluster 16 (p) has 341 nodes and 705 edges, Cluster 17 (q) has 109 nodes and 197 edges, Cluster 18 (r) has 45 nodes and 74 edges, Cluster 19 (s) has 38 nodes and 49 edges, and Cluster 20 (t) has 19 nodes and 18 edges
Disease relevance
After checking the disease relevance of 20 clusters and their numbering starting form (0–19), 4 clusters (0, 15, 16 and 18) were excluded because they did not have genes that cause gout. From the Enrichr disease data (DisGeNet and OMIM) downloaded in tabulated form, a p value < 0.05 was selected. These 16 clusters were subsequently subjected to further analysis of disease-related genes.
Intersecting genes
One-by-one intersections of the 16 clusters of genes with 4 plant targets (2, 14, 17 and 19) were excluded because they did not have any common genes. A Venn diagram was generated with the help of Venny 2.0 (Fig. 6), which shows the intersection of the two datasets of 12 different clusters. In other words, the components of U. dioica that could interact with those clusters included gout-causing genes (Table 2 shows the number of intersecting genes in each cluster).
Fig. 6.
Venn diagram of intersecting genes. (a) Cluster 1 has 23 genes, (b) Cluster 3 has 12 genes, (c) Cluster 4 has 17 genes, (d) Cluster 5 has 8 genes, (e) Cluster 6 has 15 genes, (f) Cluster 7 has 6 genes, (g) Cluster 8 has 4 genes, (h) Cluster 9 has 16 genes, (i) Cluster 10 has 6 genes, (j) Cluster 11 has 26 genes, (k) Cluster 12 has 19 genes, and (l) Cluster 13 has 2 genes
Table 2.
List of clusters with intersecting genes
| Cluster no | Total no of Intersected Genes | List of common Gene |
|---|---|---|
| 1 | 23 | PIM2, NR3C1, ESRRB, PIM1, JUN, NUAK1, CDK6, MAPK14, ESRRA, TP53, BRD4, PKN1, AHR, PARP1, MPG, ESR2, CREBBP, CSNK2A1, CA9, AR, HDAC2, SQLE, ESR1 |
| 3 | 12 | CSNK1A1, TNKS2, MAPKAPK5, KDM5A, MAP2K7, CFTR, MAPK10, TDP2, RELA, MAP3K14, MAP3K7, TNF |
| 4 | 17 | AKR1B10, APEX1, PFKFB3, ADORA3, NQO1, ADORA2A, TYMP, SLC29A1, PYGL, NUDT1, ODC1, ARG1, AKR1B1, ADORA1, GYS1, ADK, GLO1 |
| 5 | 8 | PDE5A, KCNA3, PRKACA, ADCY10, SIGMAR1, CA1, CAMK2B, P2RX7 |
| 6 | 15 | POLA1, AURKB, WEE1, TOP2A, TERT, TOP1, PRKDC, CHEK1, PLK1, CDK5, TNKS, NEK2, CDK1, CDK2, PLK4 |
| 7 | 6 | LRRK2, HSP90AB1, DYRK1A, ERN1, TTL, MAPT |
| 8 | 4 | GAK, ABCC1, ABCG2, SLC22A12 |
| 9 | 16 | F2, MPO, ACHE, TTR, BACE1, OPRD1, NAE1, SHBG, EDNRB, F10, F7, PLG, MERTK, SERPINE1, APP, F9 |
| 10 | 6 | MTOR, CAMKK2, MCL1, DAPK1, RPS6KA3, GSK3B |
| 11 | 26 | CYP1B1, SLC6A4, PLA2G1B, CBR1, AKR1A1, ABCB1, AKR1C4, PTGES, HSD17B1, CYP1A2, ALOX5, PLA2G2A, MAOA, AKR1C2, HTR2A, ALDH2, CYP19A1, AKR1C1, XDH, ALOX15, DRD4, ALOX12, PTGS2, AKR1C3, HSD17B2, CYP1A1 |
| 12 | 19 | DRD2, GSK3A, CA12, CA7, GRK6, SLC5A2, SLC6A2, CA4, CYP24A1, CA6, OPRM1, CA5A, CA2, CA14, GPR35, CA13, HTR1A, AVPR2, CA3 |
| 13 | 2 | MMP13, MMP8 |
Protein–protein interaction network
By using the 12 clusters with disease-related genes, PPI networks were generated by using String. Cluster 1 has 23 nodes and 76 edges; Cluster 3 has 12 nodes and 13 edges; Cluster 4 has 17 nodes and 25 edges; Cluster 5 has 8 nodes and 4 edges; Cluster 6 has 15 nodes and 62 edges; Cluster 7 has 6 nodes and 6 edges; Cluster 8 has 4 nodes and 2 edges; Cluster 9 has 16 nodes and 32 edges; Cluster 10 has 6 nodes and 6 edges; Cluster 11 has 26 nodes and 91 edges; Cluster 12 has 19 nodes and 34 edges; and Cluster 13 has 2 nodes and 1 edge (shown in Fig. 7).
Fig. 7.
(a) Cluster 1 has 23 nodes, 76 edges, (b) Cluster 3 has 12 nodes, 13 edges, (c) Cluster 4 has 17 nodes, 25 edges, (d) Cluster 5 has 8 nodes, 4 edges, (e) Cluster 6 has 15 nodes, 62 edges, (f) Cluster 7 has 6 nodes, 6 edges, (g) Cluster 8 has 4 nodes, 2 edges, (h) Cluster 9 has 16 nodes, 32 edges, (i) Cluster 10 has 6 nodes, 6 edges, (j) Cluster 11 has 26 nodes, 91 edges, (k) Cluster 12 has 19 nodes, 34 edges, and (l) Cluster 13 has 2 nodes, 1 edge
Construction of the drug-component-target-disease network
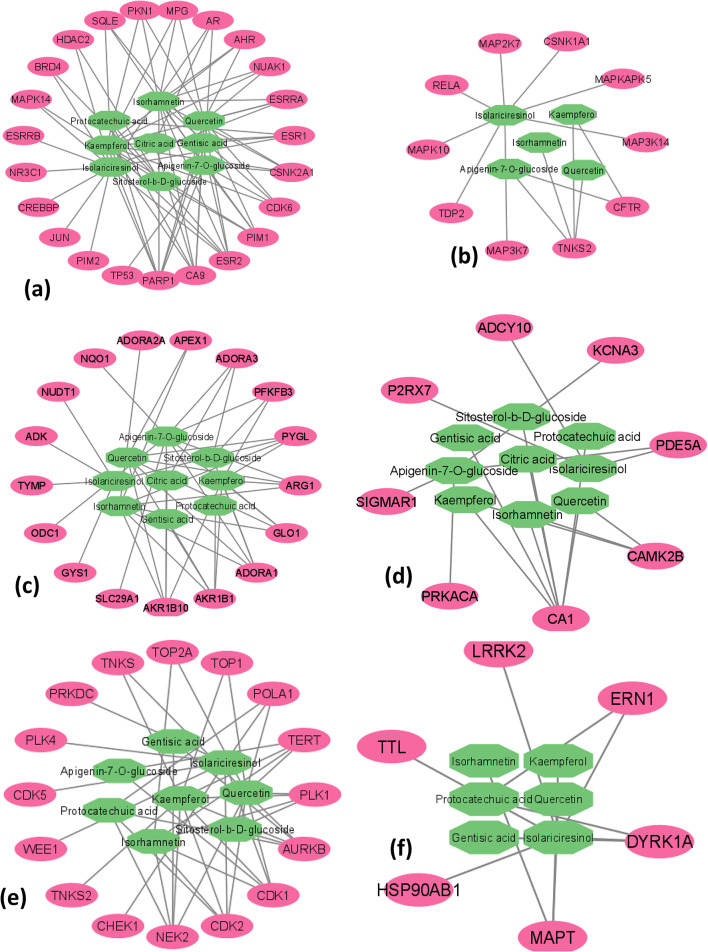
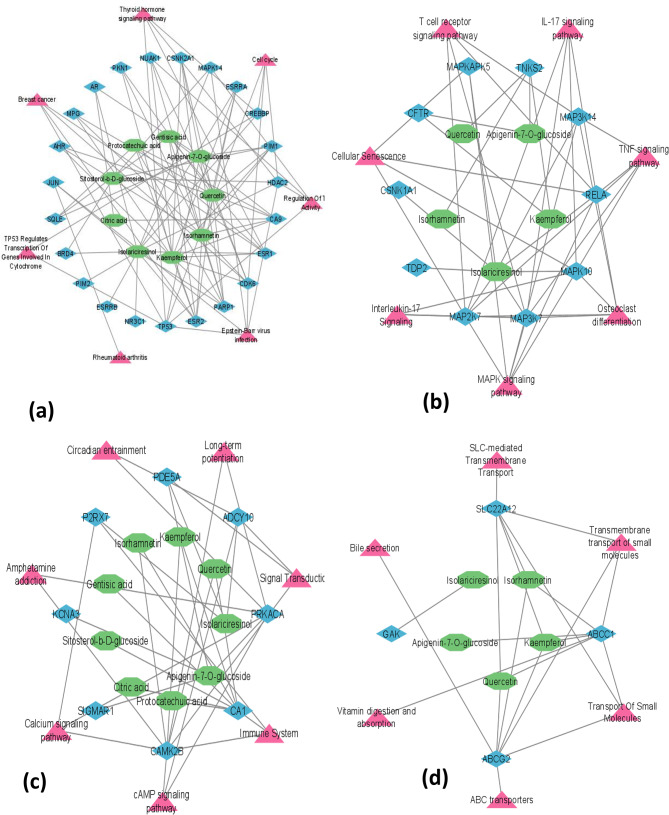
The topological analysis and network diagram of compound targets were made by using Cytoscape (version 3.10.1). The degree values of the compound targets of each cluster were calculated separately. We selected only those genes that had the highest degree of correlation, among others, and were relevant to our disease. The pink ellipse nodes represent targets, and the green octagon nodes represent compounds (Figs. 7 and 8).
Fig. 8.
(a) Cluster (b) Cluster 3, (c) Cluster 4, (d) Cluster 5, (e) Cluster 6 and (f) Cluster 7
Therefore, only 6 genes were selected for further docking analysis because they had a relatively high degree, SLC22A12 and ABCG2 were from cluster 8, RELA and MAP2K7 were from cluster 3, ADCY10 was from cluster 5, and TP53 was from cluster 1. These 4 clusters were used for pathway analysis, and genes in the other 8 clusters were excluded.
Pathway enrichment analysis
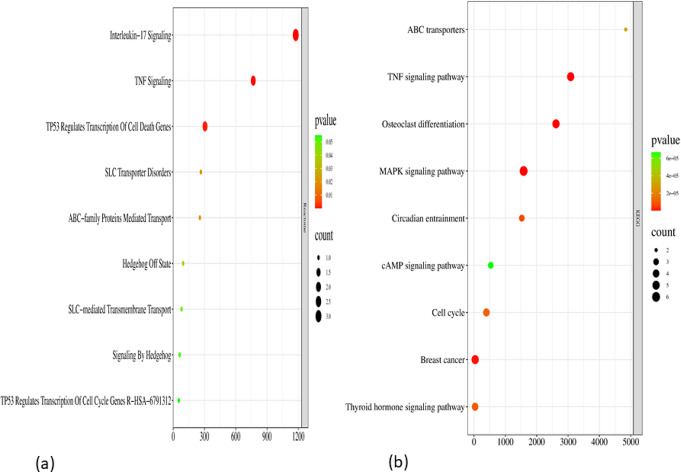
We imported potential targets into Enrichr for analysis to investigate the biological mechanism of gout treatment with U. dioica. We then filtered the results to the top 7 pathway enrichments based on P values. We constructed key protein and main pathway interaction data that were analyzed in Cytoscape 3.7.2 to construct a target-pathway network (shown in Fig. 9).
Fig. 9.
(g) Cluster 8, (h) Cluster 9, (i) Cluster 10 (j) Cluster 11, (k) Cluster 12 and (l) Cluster 13
The KEGG and Reactome pathways of 4 clusters with disease-related genes were downloaded from Enrichr to identify pathways involved in the pharmacological effects of U. dioica in the treatment of gout, of which 10 pathways from KEGG and 10 from Reactome are shown in Fig. 10. The findings indicated that the pharmacological effects of U. dioica on the management of gout are based on two main mechanisms: the inflammatory response and uric acid excretion. These factors had the most notable impact on the inflammatory process. These outcomes achieved two gout treatment objectives: lowering uric acid and reducing inflammation. The study’s findings supported the claims that U. dioica has anti-inflammatory and uric acid-lowering properties (Han et al. 2020; Upton 2013; Nayak et al. 2020). One may also conclude that the effect of U. dioica on gout was not exerted by a single substance but rather through the combined action of numerous components with various targets.
Fig. 10.
Green octagons show compounds, blue diamonds show targets, and pink triangles show pathways
Molecular docking verification
Molecular docking was used to investigate potential targets for biologically active components that have the capacity to lower the incidence of gout. Docking analysis allowed accurate prediction of the considerable binding affinity between component binding pockets and target protein binding pockets. The top 6 protein targets, ABCG2, SLC22A12, MAP2K7, ADCY10, RELA, and TP53, were chosen for molecular docking. The selection of these protein targets was based on their significance within the identified gene clusters during network analysis, as these proteins demonstrated a high degree of connectivity and relevance to gout. On the other hand, the top 5 drug-like molecules, such as, sorhamnetin, quercetin, isolariciresinol, kaempferol and apigenin-7-O-glucoside, derived from U. dioica were successfully docked with one of the six predicted targets of gout (Figs. 11 and 12) (See Fig. 13; Table 3). The selection of compounds for molecular docking was based on stringent criteria to ensure their potential efficacy and suitability as therapeutic agents. Specifically, compounds were chosen if they demonstrated an oral bioavailability of 30% or higher and a drug-likeness score of at least 0.18.
Fig. 11.
(a) Reactome pathway and (b) KEGG pathway analysis of gout patients treated with U. dioica
Fig. 12.
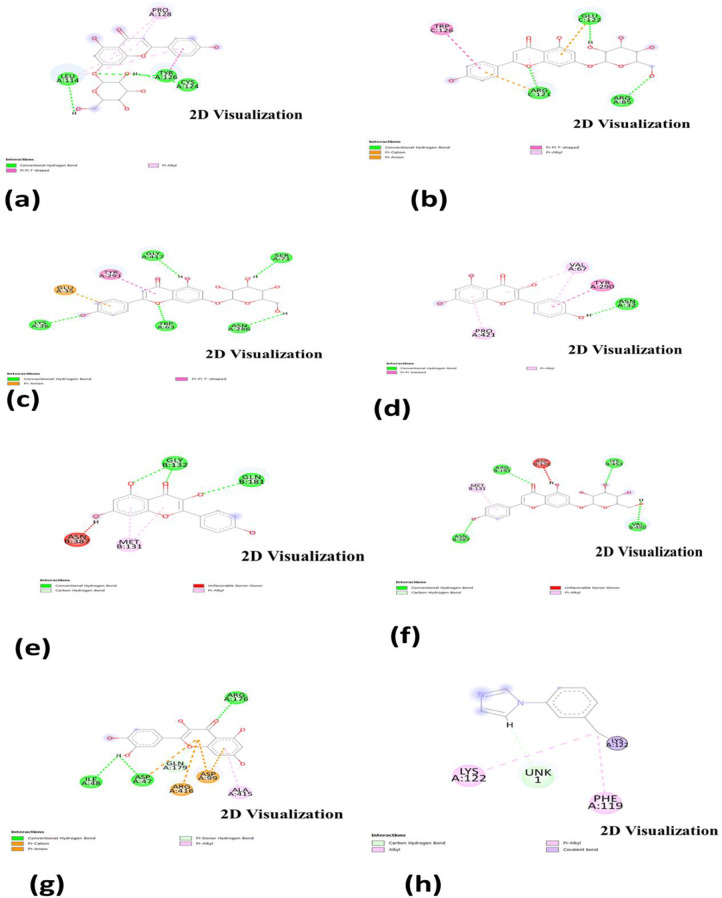
Diagram of the binding of compounds with (a) TP53, (b) MAP2K7, (c) SLC22A12, (d) SLC22A12, (e) ABCG2, (f) ABCG2, (g) ADCY10 and (h) RELA
Fig. 13.
The binding modes of (a) TP53, (b) MAP2K7, (c) SLC22A12, (d) SLC22A12, (e) ABCG2, (f) ABCG2, (g) ADCY10 and (h) RELA. (visualized by drug discovery tool and drugs are obtained from PubChem Database: https://pubchem.ncbi.nlm.nih.gov/)
Table 3.
Binding energy, RMSD and interactions of bioactive constituents and their target proteins (The docking results are obtained through PyRx Tool https://pyrx.sourceforge.io/)
| Protein name | Compound name | Binding affinity (kcal/mol) | RMSD (Å) | Hydrogen bonds and other interacting residues |
|---|---|---|---|---|
| ABCG2 | Apigenin-7-O-glucoside | -7.1 | 2.37 | ASN B:387, MET B:131, ARG B:191, ASP B:128, LYS B:453, VAL B:450 |
| ABCG2 | Kaempferol | -6.3 | 2.88 | ASN B:387, MET B:131, GLY B:132, GLN B:181 |
| ADCY10 | Apigenin-7-O-glucoside | -6.5 | 2.28 |
ASP A:47, ARG A:416, GLN A:179, ASP A:99, PHE A336 |
| ADCY10 | Quercetin | -6.9 | 2.46 | ILE A:48, ASP A:47, ARG A:416,ASP A:99, GLN A:179, ALA A:415, ARG A:176 |
| MAP2K7 | Apigenin-7-O-glucoside | -8.3 | 2.08 | TRP C:126, ARG C:121, GLU C:122, ARG A:85 |
| MAP2K7 | Kaempferol | -6.8 | 2.27 | ARG C:121, GLU C:122, SER B:90, ASP A:206 |
| RELA | Apigenin-7-O-glucoside | -6.8 | 2.22 | LYS A:122, PHE A:119 |
| RELA | Kaempferol | -6.2 | 2.25 | LYS A:122, UNK 1, PHE A:119, LYS A:122 |
| SLC22A12 | Apigenin-7-O-glucoside | -8.9 | 2.61 | LYS A:36, GLU A:35, TRY A:291, GLY A:417, TRP A:63, ASN A:286, SER A:71 |
| SLC22A12 | Kaempferol | -7.4 | 2.80 | PRO A:421,VAL A:67, TYR A:290, ASN A:32 |
Discussion
Gout is a prevalent disorder that is becoming more common everywhere. Gout, in addition to intense arthritic pain, is linked to early mortality, which is conventionally explained by a high incidence of comorbidities, particularly renal and cardiovascular disorders. Comorbidities must be taken into account in gout since they complicate therapy of the condition and may affect the patients’ critical prognosis (Bardin and Richette 2017).
Gout and hyperuricemia are both clinical conditions linked to a higher risk of developing metabolic, renal, and cardiovascular (heart failure, myocardial infarction, stroke) consequences (Burnier 2023).
The synovial fluid of the knees had visible MSU crystals in 58% of the asymptomatic individuals with nontophaceous gout. No clinical or laboratory factors, such as blood uric acid level, medication use, or time since most recent gout episode, were sufficient to distinguish the MSU crystal positive group (Bomalaski et al. 1986).
A notable green vegetable in this region is derived from the perennial plant Urtica dioica, commonly known as stinging nettle. It is called as “Sisnu” in the local dialect and is eaten with vegetables, sour soup, and curries. U. dioica has traditionally been used as a diuretic and to treat gout, arthritis, joint pain, and other rheumatic conditions (Hall and Bravo-Clouzet 2013; Sharma et al. 2022).
To investigate the inner workings of U.dioica, we took a networking pharmacology strategy in this particular study. Isorhamnetin, quercetin, isolariciresinol, kaempferol, and apigenin-7-O-glucoside are the major active components of U. dioica, as determined by degree value. Kaempferol and quercetin have the potential to be utilized as an adjuvant treatment to treat inflammatory illnesses and oxidative stress because of their substantial anti-inflammatory and antioxidant effects (Tian et al. 2021).
Quercetin is able to be utilized as an auxiliary to boost the anti-rheumatic monotherapy’s insufficient response. Quercetin antagonism decreased the anti-inflammatory efficacy of diclofenac in arthritic gout-pain model rats. Consequently, it is not advised to use this combination to treat gout that is rheumatic (Ventura-Martínez et al. 2021; Haleagrahara et al. 2018).
Kaempferol has urate-lowering and anti-inflammatory properties in gouty arthritis and combination hyperuricemia. By lowering infiltration of inflammatory cells and inflammatory cytokine release, kaempferol successfully reduced inflammation in the kidneys and ankles of mice (Huang et al. 2023).
The primary active ingredient used for the management of hyperuricemia is apigenin 7-O-glucoside (Zhang et al. 2023). It has become well-known that protocatechuic acid variants have antioxidant and hepatoprotective properties (Semaming et al. 2015).
The network analysis suggests that the key gout targets are inhibited by Urtica dioica, which may explain its therapeutic effects for gout. This study clarifies the active compounds, their predicted targets, and the related pathways involved in the treatment of gout, all within the context of network pharmacology. By identifying active substances, their potential targets, and associated pathways, the study provides an analytical framework for future investigation.
In the context of the current study on Urtica dioica for the treatment of gout arthritis, there are some valuable examples of how network pharmacology and LC-MS/MS analysis can be used to understand the therapeutic potential of herbal medicines. For instance, Banerjee et al. (2019) utilized LC-MS/MS and network pharmacology to explore the effects of Trigonella foenum-graecum on hyperlipidemia and hyperglycemia, highlighting the combination synergy and biochemical impacts of the plant’s compounds (Banerjee et al. 2019). Similarly, Banerjee et al. (2021) demonstrated the immunoprotective potential of Andrographis paniculata against respiratory viral infections through detailed pharmacological analysis (Banerjee et al. 2021). Lastly, Banerjee et al. (2022) combined LC-MS/MS profiles with network pharmacology to predict the molecular mechanisms underlying the hyperlipidemic activity of Lagenaria siceraria, showcasing the utility of these methods in identifying bioactive compounds and their targets (Banerjee et al. 2022).
These examples support the current study’s methodology by demonstrating how similar approaches can effectively identify and validate the therapeutic effects of herbal compounds. By integrating these examples, the manuscript not only situates U. dioica within a broader context of herbal medicine research but also reinforces the robustness and relevance of using network pharmacology and biochemical analysis to uncover the mechanisms of action of traditional medicinal plants.
The transmembrane proteins identified as ABC transporters exist in almost all tissues and are required for the release of a wide range of molecules or substrates, such as organic and inorganic anions, amino acids, and metals (Fujita and Ichida 2018). Drug transporter ABCG2, often referred to as the breast cancer-resistant protein, was found (An and Morris 2011). The nature of intestinal secretion of uric acid has been recently reviewed. It seems that ABCG2, which eliminates up to one third of all uric acid and is frequently exhibited in intestinal tissue is the primary extra renal location of uric acid elimination (Xu et al. 2016). Furthermore, a large portion of early-onset gout may be caused by ABCG2 malfunction (Matsuo et al. 2014).
SLC22A12 gene solute carrier family 22 member 12 encodes urate transporter 1 (URAT1) was first described in mouse as Rst. Among these, the well-known urate transporter gene URAT1 has been found to be the root cause of renal hypouricemia type 1. It has been determined that URAT1 is a urate-anion converter that controls SUA levels because it is essential for urate reabsorption in human kidney (Enomoto et al. 2002). Gout and SUA concentrations were found to be substantially correlated with SLC22A12 (Zhou et al. 2015).
Conclusion
The most common form of inflammatory arthritis worldwide is gout. The elucidation of pharmaceutical action methods is greatly facilitated by network pharmacology. Over the last two decades, the incidence of gout has climbed globally by 63.44%, and the number of years spent with a handicap has increased globally by 51.12%. The male to female sex ratio remained constant at 3:1, while the overall incidence of gout increased with time in both sexes. The pharmacological properties of U. dioica include analgesic, antiandrogenic, antihyperglycemic, and anti-inflammatory effects. The current study suggested that the combination of multiple compounds in U. dioica is beneficial for treating gout. This study identified additional therapeutic targets for gout treatment and established a foundation for demonstrating the effectiveness of multicomponent, multitargeted chemical regimens. Through the application of network pharmacology and a molecular docking approach, the molecular basis of the action of U. dioica against gout has been identified. A network study revealed that U. dioica has multiple targeting molecules, indicating that these substances operate simultaneously on many gout pathways. The ABCG2 and SLC22A12 genes are involved in the spike of uric acid, and the phytochemicals of U. dioica selected for this study have been proven to have therapeutic efficacy for the disease gout.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgment
We would like to express our gratitude to project No. № FSER-2024-0003 for their support of the chemoinformatic aspects of this research. Their contributions have been invaluable in advancing our work.
Abbreviations
- NPs
Natural product
- TCM
Traditional Chinese medicine
- U. dioica
Urtica dioica
- MSU
Monosodium urate crystals
- HU
Hyperuricemia
- SUA
Serum uric acid
- ULT
Urate-lowering therapies
- OMIM
Online Mendelian Inheritance in Man
- DEGs
Differentially expressed genes
- CDs
Cyclodextrins
- PPi
Protein protein interatcion
- DL
Drug likiness
- OB
Oral bioavailability
- GO
Gene ontology
- BP
Biological Processes
- CC
Cellular Component
- MF
Molecular Functions
- RMSD
Root Mean Square Deviation
- SMILES
Simplified molecular-input line-entry system
- URAT1
Urate Anion Exchanger 1
- SLC22A12
Solute Carrier Family 22 Member 12
- ABCG2
ATP-binding cassette superfamily G member 2
Author contributions
M.Q. and Z.K. conducted the initial literature review and identified the active constituents of Urtica dioica. M.M.F. and H.A. designed and developed the in-house pipeline for clustering and network visualization. S.S. supervised the project, provided critical insights, and oversaw the molecular docking studies. M.Q., M.M.F., and H.A. analyzed the data, including databases such as GeneCards, OMIM, and DisGeNet for target identification. Z.K. prepared the figures and visualizations, including the protein-protein interaction networks using STRING and the target–compound–pathway networks in Cytoscape. M.Q. and S.S. wrote the main manuscript text. All authors reviewed and approved the final manuscript.
Funding
The authors thank the Russian Science Foundation (grant no. 22-65-00022) for the financial support of this project.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- An G, Morris ME (2011) The sulfated conjugate of biochanin A is a substrate of breast cancer resistant protein (ABCG2). Biopharm Drug Dispos 32(8):446–457 [DOI] [PubMed] [Google Scholar]
- Annemans L et al (2008) Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis 67(7):960–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Bhattacharjee P, Kar A, Mukherjee PK (2019) LC-MS/MS analysis and network pharmacology of trigonella foenum-graecum- a plant from ayurveda against hyperlipidemia and hyperglycemia with combination synergy. Phytomedicine 152944. 10.1016/j.phymed.2019.152944 [DOI] [PubMed]
- Banerjee S, Kar A, Mukherjee PK, Haldar PK, Sharma N, Katiyar CK (2021) Immunoprotective potential of ayurvedic herb Kalmegh (Andrographis paniculata) against respiratory viral infections – LC–MS/MS and network pharmacology analysis. Phytochem Anal 32:629–639. 10.1002/pca.3011 [DOI] [PubMed] [Google Scholar]
- Banerjee S, Tiwari A, Kar A, Chanda J, Biswas S, Ulrich-Merzenich G, Mukherjee P (2022) Combining LC-MS/MS profiles with network pharmacology to predict molecular mechanisms of the hyperlipidemic activity of Lagenaria siceraria stand. J Ethnopharmacol 300:115633. 10.1016/j.jep.2022.115633 [DOI] [PubMed] [Google Scholar]
- Bardin T, Richette P (2017) Impact of comorbidities on gout and hyperuricemia: an update on prevalence and treatment options. BMC Med 15:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomalaski JS, Lluberas G, Schumacher HR Jr (1986) Monosodium urate crystals in the knee joints of patients with asymptomatic nontophaceous gout. Arthritis Rheumatism: Official J Am Coll Rheumatol 29(12):1480–1484 [DOI] [PubMed] [Google Scholar]
- Burnier M (2023) Gout and hyperuricemia: modifiable cardiovascular risk factors? Front Cardiovasc Med 10:1190069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J et al (2023) Mechanisms of theaflavins against gout and strategies for improving the bioavailability. Phytomedicine, 154782 [DOI] [PubMed]
- Choi HK et al (2007) Prevalence of the metabolic syndrome in patients with gout: the third national health and nutrition examination survey. Arthritis Care Research: Official J Am Coll Rheumatol 57(1):109–115 [DOI] [PubMed] [Google Scholar]
- Emmerson BT (1996) The management of gout. N Engl J Med 334(7):445–451 [DOI] [PubMed] [Google Scholar]
- Enomoto A et al (2002) Molecular identification of a renal urate–anion exchanger that regulates blood urate levels. Nature 417(6887):447–452 [DOI] [PubMed] [Google Scholar]
- Evangelista JE et al (2023) Enrichr-KG: bridging enrichment analysis across multiple libraries. Nucleic Acids Res, gkad393 [DOI] [PMC free article] [PubMed]
- Fujita K, Ichida K (2018) ABCG2 as a therapeutic target candidate for gout. Expert Opin Ther Targets 22(2):123–129 [DOI] [PubMed] [Google Scholar]
- Gfeller D, Michielin O, Zoete V (2013) Shaping the interaction landscape of bioactive molecules. Bioinformatics 29(23):3073–3079 [DOI] [PubMed] [Google Scholar]
- Hainer BL, Matheson EM, Wilkes RT (2014) Diagnosis, treatment, and prevention of gout. Am Family Phys 90(12):831–836 [PubMed] [Google Scholar]
- Haleagrahara N et al (2018) Flavonoid quercetin–methotrexate combination inhibits inflammatory mediators and matrix metalloproteinase expression, providing protection to joints in collagen-induced arthritis. Inflammopharmacology 26:1219–1232 [DOI] [PubMed] [Google Scholar]
- Hall J, Bravo-Clouzet R (2013) Anti-inflammatory herbs for arthritis. In: Bioactive food as dietary interventions for arthritis and related inflammatory diseases. Elsevier, pp. 619–631
- Hamosh A et al (2000) Online mendelian inheritance in man (omim). Hum Mutat 15(1):57–61. 10.1002/(SICI)1098-1004(200001)15:1%3C57::AID-HUMU12%3E3.0.CO;2-G [DOI] [PubMed]
- Han S et al (2020) Hypouricemic effects of extracts from Urtica hyperborea Jacq. ex Wedd. in hyperuricemia mice through XOD, URAT1, and OAT1. BioMed Res Int [DOI] [PMC free article] [PubMed]
- Huang Y et al (2023) Kaempferol suppresses inflammation in mice suffering from both hyperuricemia and gouty arthritis through inhibiting NLRP3 inflammasome and NF-κB pathway
- Jiang J et al (2023) Prevalence of diabetes in patients with hyperuricemia and gout: a systematic review and meta-analysis. Curr Diab Rep, 1–15 [DOI] [PubMed]
- Kim S et al (2016) PubChem substance and compound databases. Nucleic Acids Res 44(D1):D1202–D1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinski SL (2014) Food, drink, and herbs: alternative therapies and gout. Curr Rheumatol Rep 16:1–7 [DOI] [PubMed] [Google Scholar]
- Kozub A et al (2012) Capsella bursa-pastoris-a common weed and little-known medicinal plant. Postępy Fitoterapii
- Man SM, Karki R, Kanneganti TD (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277(1):61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martillo MA, Nazzal L, Crittenden DB (2014) The crystallization of monosodium urate. Curr Rheumatol Rep 16:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H et al (2014) ABCG2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci Rep 4(1):3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak C et al (2020) Individualized homeopathic medicines and Urtica urens mother tincture in treatment of hyperuricemia: an open, randomized, pragmatic, pilot trial. J Complement Integr Med 18(3):599–608 [DOI] [PubMed] [Google Scholar]
- Nieradko-Iwanicka B (2022) The role of alcohol consumption in pathogenesis of gout. Crit Rev Food Sci Nutr 62(25):7129–7137 [DOI] [PubMed] [Google Scholar]
- Nutmakul T (2022) A review on benefits of quercetin in hyperuricemia and gouty arthritis. Saudi Pharmaceutical J [DOI] [PMC free article] [PubMed]
- Otasek D et al (2019) Cytoscape automation: empowering workflow-based network analysis. Genome Biol 20:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzi L et al (2012) Gout as autoinflammatory disease: new mechanisms for more appropriated treatment targets. Autoimmun rev 12(1):66–71 [DOI] [PubMed] [Google Scholar]
- Quah Y et al (2023) In silico investigation of Panax ginseng lead compounds against COVID-19 associated platelet activation and thromboembolism. J Ginseng Res 47(2):283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richette P et al (2015) Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis 74(1):142–147 [DOI] [PubMed] [Google Scholar]
- Semaming Y et al (2015) Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evidence-Based Complementary and Alternative Medicine [DOI] [PMC free article] [PubMed]
- Sharma S et al (2022) Antioxidant potential of selected wild edible leafy vegetables of sikkim himalayan region: effects of cooking methods and gastrointestinal digestion on activity. Front Nutr 9:861347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JA, Gaffo A (2020) Gout epidemiology and comorbidities. In: Seminars in arthritis and rheumatism. Elsevier [DOI] [PubMed]
- Stelzer G et al (2011) In-silico human genomics with GeneCards. Hum Genomics 5(6):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D et al (2021) The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 49(D1):D605–D612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C et al (2021) Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South Afr J Bot 137:257–264 [Google Scholar]
- Upton R (2013) Stinging nettles leaf (Urtica dioica L.): extraordinary vegetable medicine. J Herb Med 3(1):9–38 [Google Scholar]
- Ventura-Martínez R et al (2021) Quercetin decreases the antinociceptive effect of diclofenac in an arthritic gout-pain model in rats. J Pharm Pharmacol 73(10):1310–1318 [DOI] [PubMed] [Google Scholar]
- Wang Y et al (2023a) Global status and trends in gout research from 2012 to 2021: a bibliometric and visual analysis. Clin Rheumatol, 1–18 [DOI] [PMC free article] [PubMed]
- Wang Y et al (2023b) Natural products in attenuating renal inflammation by inhibiting the NLRP3 inflammasome in diabetic kidney disease. Front Immunol 14:1196016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X et al (2016) Uric acid transporters hiding in the intestine. Pharm Biol 54(12):3151–3155 [DOI] [PubMed] [Google Scholar]
- Zhang Y et al (2023) Paeonia× Suffruticosa Andrews leaf extract and its main component apigenin 7-O-glucoside ameliorate hyperuricemia by inhibiting xanthine oxidase activity and regulating renal urate transporters. Phytomedicine 118:154957 [DOI] [PubMed] [Google Scholar]
- Zhou Z-W et al (2015) Polymorphisms in GCKR, SLC17A1 and SLC22A12 were associated with phenotype gout in Han Chinese males: a case–control study. BMC Med Genet 16:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.