Abstract
Over the past decade, there has been a growing interest in ferritin-based vaccines due to their enhanced antigen immunogenicity and favorable safety profiles, with several vaccine candidates targeting various pathogens advancing to phase I clinical trials. Nevertheless, challenges associated with particle heterogeneity, improper assembly and unanticipated immunogenicity due to the bulky protein adaptor have impeded further advancement. To overcome these challenges, we devise a universal ferritin-adaptor delivery platform based on structural insights derived from the natural ferritinophagy complex of the human ferritin heavy chain (FTH1) and the nuclear receptor coactivator 4 (NCOA4). The engineered ferritinophagy (Fagy)-tag peptide demonstrate significantly enhanced binding affinity to the 24-mer ferritin nanoparticle, enabling efficient antigen presentation. Subsequently, we construct a self-assembling rabies virus (RABV) vaccine candidate by noncovalently conjugating the Fagy-tagged glycoprotein domain III (GDIII) of RABV to the ferritin nanoparticle, maintaining superior homogeneity, stability and immunogenicity. This vaccine candidate induces potent, rapid, and durable immune responses, and protects female mice against the authentic RABV challenge after single-dose administration. Furthermore, this universal, ferritin-based antigen conjugating strategy offers significant potential for developing vaccine against diverse pathogens and diseases.
Subject terms: Protein vaccines, Structural biology, Viral infection
The study by Fu and colleagues presents a ferritin-adaptor platform for vaccine development, featuring a rabies virus vaccine candidate that enhances antigen stability and provides potent, durable protection after a single-dose administration.
Introduction
Polymeric ferritin forms 24 copies of protein complex, which is ubiquitous in diverse organisms, including animals, plants, bacteria, and fungi1. In vertebrates, ferritin contains 24 subunits of ferritin heavy chain (FTH) and ferritin light chain (FTL) in different ratios that vary between different cell types2. Its primary physiological function is to sequester iron in an insoluble, nontoxic state while preserving its intracellular bioavailability. This process is crucial for maintaining iron homeostasis3. In the past decade, interest in nanoparticle vaccines has increased owing to their ability to present antigenic repetitive arrays analogous to viral surfaces. Nanoparticle vaccines are characterized by high safety and biocompatibility, rapid production, broad applicability, excellent stability, and the capacity to elicit enduring and robust immune responses, even at relatively low doses4–8. Its exceptional antigen presentation attributes make ferritin extremely versatile for the development of subunit nanoparticle vaccines. This versatility is demonstrated through its capacity to present diverse disease-associated antigens through mechanisms such as chemical conjugation, fusion protein design, or Tag/Catcher interactions9–17. Nevertheless, several crucial challenges persist during the preparation of ferritin-based nanoparticle vaccines. For instance, surface antigen cross-linking through chemical conjugation leads to particle heterogeneity and low efficiency of B-cell activation18,19. Moreover, as for the antigen-ferritin gene fusion approach, inappropriate antigen folding and interference in antigen–antigen interactions can result in either the inhibition of scaffold assembly or the loss of antigen activity20,21. Furthermore, antigen labeling using Tag/Catcher technology can elicit redundant immune responses against proteins encoding Tag/Catcher11,16,22,23. Addressing these challenges is therefore crucial for the continued development of effective ferritin-based nanoparticle vaccines.
To develop a universal ferritin-based nanoparticle vaccine platform, we drew inspiration from the physiological process to design a novel adapter tag for the FTH1, providing guidance by its natural interaction with a receptor. One well-known receptor of FTH1 is the human transferrin receptor 1 (hTfR1, also known as CD71), a widely expressed and versatile carrier responsible for importing iron in response to intracellular variations of this essential element24–26. The complex structure reveals that FTH1 binds to CD71 receptor through four specific contact regions on the apical domain, covering an overall area of ~1900 Å2 27,28. This extensive interaction makes CD71 an unsuitable candidate for adapter design of FTH1. Interestingly, prior research has indicated that the nuclear receptor coactivator 4 (NCOA4) serves as a selective cargo receptor that facilitates the delivery of ferritin to the lysosome via autophagosomes29–32. The direct interaction occurs between a crucial surface arginine in FTH1 and a C-terminal element (residues 383–522) in NCOA4. However, the structural basis of this interaction has not been elucidated32,33. Here, we solved the complex structure of the FTH1 and NCOA4 C-terminal regions at atomic resolution by utilizing cryogenic electron microscopy (cryo-EM), which provided structural insight into the regulatory mechanisms of ferritinophagic flux and the modulation of intracellular iron bioavailability. Moreover, we computationally designed a 16-amino acid peptide adapter based on the NCOA4/FTH1 protein complex structure, which has a binding affinity of 34 nM to FTH1. The engineered Fagy tag, which bound to the same site on FTH1, exhibited a substantial improvement in binding affinity to FTH1. Subsequently, the rabies nanoparticle vaccine was chosen as the starting point for evaluating the immune responses and in vivo protective efficacy of this versatile delivery platform.
Rabies, a historically significant disease that dates back to the beginning of human–canine interactions nearly 40,000 years ago, is a notably neglected disease associated with an annual toll of ~60,000 human fatalities, predominantly occurred in developing regions of Africa and Asia34–36. The most common causative agent of human rabies is the rabies virus (RABV), which belongs to the Lyssavirus genus of the Rhabdoviridae family. The virus is readily transmitted through the bite of an infected animal, frequently a dog, and is capable of infecting and replicating within the central nervous system, ultimately causing severe neurological disease. Clinical features of RABV infection typically include multiple neuronal dysfunctions, with a mortality rate as high as 100%. There is currently no treatment available for patients exhibiting neurological clinical signs37. Rabies vaccines, administered for pre-exposure immunization or as post-exposure prophylaxis, have been proven to be the most effective means of preventing infection from this deadly viral zoonosis36,38,39. Substantial advances have been achieved since Louis Pasteur’s pioneering work on nerve tissue inactivated vaccines in 188540, encompassing developments such as cell culture vaccines41,42, modified live vaccines43, adjuvanted vaccines44,45, genetically modified vaccines46, recombinant vaccines47, nucleic acid-based vaccines48–50, and oral vaccines51, several of which are either in clinical use or under investigation. The Global Strategic Plan, introduced in 2018, seeks to eliminate worldwide human rabies deaths caused by dogs by the year 2030, and it places significant emphasis on preventing canine rabies through annual mass vaccination campaigns, targeting at least 70% of dog populations52. The demand for vaccines with heightened safety, stability, cost-effectiveness, and optimized immunogenicity has led to the development of next-generation vaccines, such as novel recombinant vaccines. The RABV glycoprotein (RABV-G) belongs to Class III of viral membrane fusion proteins and shares domain topology with the fusion proteins found in vesicular stomatitis virus and Chandipura virus, as well as baculoviruses and herpesviruses53–56. RABV-G is the sole exposed spike protein on the surface of RABV and serves as a critical antigen for eliciting neutralizing antibodies57. Previous work has proven that domain III of RABV-G (GDIII), also known as the pleckstrin homology domain (PHD domain), is highly conserved among the Lyssavirus genus and the Rhabdovirus family58. Numerous well-characterized neutralizing antibodies have been found to recognize this specific region, which effectively blocks the conformational changes in the G protein induced by the acidic environment required for membrane fusion58–60.
In this study, we engineered and synthesized a recombinant RABV vaccine by utilizing the innovative Fagy tag delivery platform for optimal GDIII antigen presentation on ferritin nanoparticles. Encouragingly, GDIII-Ferritin nanoparticle vaccine demonstrated increased antigen stability, and elicited sustained and broad-spectrum humoral immune responses in vivo, along with a heightened Th1-biased CD4+ T-cell immune responses. Importantly, after the administration of either two doses or one dose, the GDIII-Ferritin nanoparticle vaccine achieved full protection against intracerebral RABV challenge in mice, revealing significant immunological advantages and developmental potential.
Results
Structural analysis of NCOA4/FTH1 complex and structure-guided engineering of the Fagy-tag
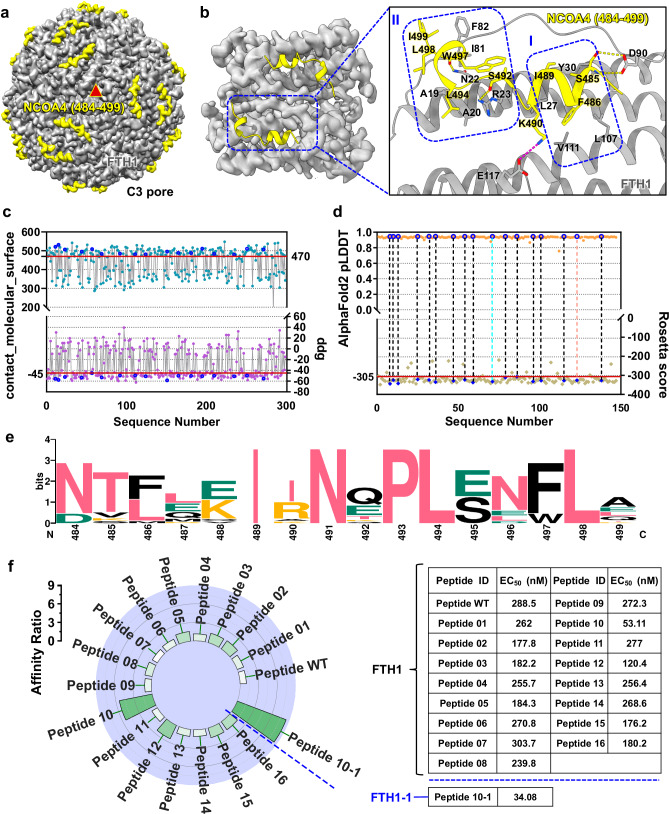
The C-terminus of the NCOA4 is implicated in ferritin binding and the regulation of cellular iron homeostasis31. However, the binding mechanism has not been definitively elucidated. To examine the structural basis of this binding mode, we prepared the C-terminus of NCOA4 (475–511) and the human ferritin heavy chain (FTH1) complex, which has exceptional purity and monodispersity (Supplementary Fig. 4a–c). Employing single-particle cryo-EM, the atomic structure of the complex was solved at 2.2 Å (Fig. 1a; Supplementary Fig. 5; Supplementary Table 1). In this structure, 16 residues from the C-terminus of NCOA4 (484–499) were built into the electron density map and shown to play pivotal roles in the interaction with FTH1. NCOA4 (484–499) comprised two short consecutive α-helices that predominantly interacted with Helix_A, Helix_C, and the BC_loop of FTH1. This binding interface was further fortified by hydrogen bonds, salt bridges, and two substantial hydrophobic cores. Hydrophobic core I encompassed residues L27 and Y30 of the FTH1 Helix_A, residues L107 and V111 of Helix_C, and residues F486 and I489 of NCOA4. Moreover, hydrophobic core II comprised residues A19 and A20 on the FTH1 Helix_A, residues I81 and F82 on the BC_loop, and residues L494, W497, L498, and I499 of NCOA4. The interactions between FTH1 and NCOA4 were further enhanced by salt bridges connecting E117 and K490 and hydrogen bonds between N22 and W497, R23 and S492, and D90 and S485 (Fig. 1b and Supplementary Fig. 2a).
Fig. 1. Engineering, characterization, and screening of peptide adapters based on the structure of the NCOA4/FTH1 complex.
a Front view of the 24-mer single-particle cryo-EM density map of the NCOA4 (484–499)/FTH1 complex at the central point of threefold symmetry axes. NCOA4 (484–499) colored yellow and FTH1 colored gray. b Structural analysis of the NCOA4 (484–499)/FTH1 complex. Residues comprising hydrophobic cores I and II are enclosed. Interface residues are labeled and represented as sticks. Hydrogen bonds are depicted with yellow dashed lines, and salt bridges are depicted with magenta dashed lines. c After designing sequences using ProteinMPNN, Rosetta score, Rosetta binding energy (ddg) values and packing (contact_molecular_surface) were calculated by Rosetta. Red lines serve as baselines, indicating the median values of ddg and contact_molecular_surface. Sequences falling within the threshold defined by the two red lines are excluded. The blue circles represent the values of ddg and contact_molecular_surface for sequences that have undergone subsequent experimental characterization. d Precise sequence screening based on the Rosetta score and the AlphaFold2 pLDDT. The red line represents the baseline, indicating the median value of the Rosetta score. The blue circles highlight the evaluation values of the Rosetta score and the AlphaFold2 pLDDT for sequences that have undergone subsequent experimental characterization. e Amino acid conservation analysis of all peptide sequences utilized for experimental characterization using WebLogo. f Analysis of the binding affinity between the engineered peptide and ferritin. The affinity of various designed peptides was compared to that of the wild-type peptide, with the affinity of the wild-type peptide for FTH1 (EC50 = 288.5 nM) set as the baseline value of 1. The increase in affinity of the designed peptides relative to the baseline value is depicted, with a color gradient from white to green indicating an increase in affinity from low to high. The table on the right displays specific EC50 values characterizing the binding affinities of various peptides. The EC50 values were obtained through nonlinear regression dose-response stimulation analysis of ELISA data using GraphPad Prism 8.0.2 software with the log(agonist) vs. Response-variable slope (four parameters) model. Each value represents the average of three repeated experiments.
In order to design and screen peptide adapters with higher binding affinity, we performed sequence optimization based on the structure of the NCOA4/FTH1 complex described above. The backbone of NCOA4 and the amino acids at the interface (within 4 Å) with FTH1 were selected, and 298 sequences were generated with ProteinMPNN. Additionally, structure prediction confidence metrics, such as Rosetta scores and other additional biophysical properties were computed. The statistical analyses were conducted on the binding energy (ddg), contact_molecular_surface, and score parameters for each predicted structure. Subsequently, 146 sequences with superior characterization parameters were selected (Fig. 1c and Supplementary Fig. 1a). Ultimately, after a comprehensive assessment of multiple key parameters, the 16 sequences with the highest AlphaFold2 pLDDT scores > (0.944) were selected for further analysis (Fig. 1d). This multistep approach of sequence design and selection aimed to establish a robust foundation for subsequent analyses and experimental characterizations. The focus was on predicting conserved features and optimizing mutant combinations (Fig. 1e and Supplementary Fig. 1b). First, the modified 16 peptides were fused to the C-terminus of the glutathione S-transferase (GST) tag for individual display (Supplementary Fig. 3b). Then, enzyme-linked immunosorbent assay (ELISA) was used to screen for FTH1-specific peptides (Supplementary Fig. 3c, d). Among these peptides, Peptide 10 exhibited an affinity of 53.11 nM, representing an ~5.5-fold increase in binding affinity compared to that of NCOA4 (484–499) (the Peptide WT) (Fig. 1f). Subsequently, we performed a restricted computational design of Peptide 10 and FTH1 simultaneously. The combination with the highest confidence, Peptide 10-1/FTH1-1, was selected for recombinant expression and ELISA affinity measurements (Supplementary Figs. 1b, c and 3a–c). The analysis revealed an increase in binding affinity by nearly one order of magnitude, reaching 34.08 nM for Peptide 10-1/FTH1-1 compared to that for Peptide WT/FTH1 (Fig. 1f).
A more extensive interaction network increases the affinity and specificity of engineered peptides
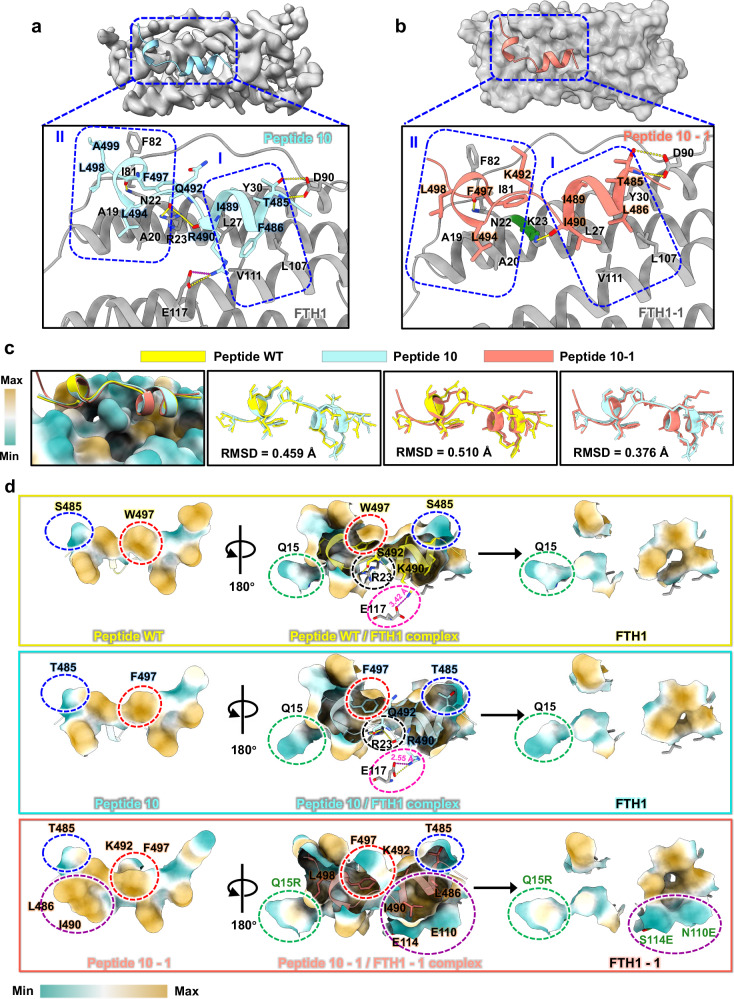
To elucidate the structural basis of the heightened binding affinity of Peptide 10/FTH1 and Peptide 10-1/FTH1-1, we conducted comprehensive in-depth structural investigations. The protein complexes were prepared as NCOA4/FTH1, and their purity and stability were determined through size-exclusion chromatography (SEC) (Supplementary Fig. 4a). The hydrodynamic radius was determined by dynamic light scattering (DLS) (Supplementary Fig. 4b), and the particle dispersity was verified by negative stain electron microscopy (negative stain EM) (Supplementary Fig. 4c). Subsequently, we solved the high-resolution structure of Peptide 10/FTH1 at 2.02 Å using the Cryo-EM single-particle method (Supplementary Fig. 6 and Supplementary Table 1). Structural analysis revealed that the binding mode of Peptide 10 and the peptide WT to FTH1 were a close match, consistent with our primary design intention of maintaining the main binding site. Specifically, hydrophobic core I is composed of residues L27 and Y30 of Helix_A, residues L107 and V111 of Helix_C on FTH1, and residues F486 and I489 on Peptide 10. Hydrophobic core II consists of residues A19 and A20 of Helix_A, residues I81 and F82 of the BC_loop on FTH1, and residues L494, F497, L498, and A499 on Peptide 10. Interactions within the hydrophobic core are further stabilized by salt bridges between E117 and R490, as well as by the complicated hydrogen bond networks between R23 and Q492, R23 and R490, D90 and T485, and N22 and F497 (Fig. 2a and Supplementary Fig. 2b). In addition, we solved the crystal structure of Peptide 10-1/FTH1-1 at 2.3 Å. In this structure, the binding mode remained virtually unchanged as NCOA4/FTH1 and Peptide 10/FTH1 complexes. Hydrophobic core I consists of residues L27 and Y30 of Helix_A, residues L107 and V111 of Helix_C on FTH1, and residues L486, I489, and I490 on Peptide 10-1. Additionally, hydrophobic core II includes residues A19 and A20 of Helix_A, residues I81 and F82 of the BC_loop on FTH1-1, and residues L494, F497, and L498 on Peptide 10-1. Hydrogen bonds between K23 and L490, D90 and T485, and N22 and F497 play significant roles in strengthening the binding affinities, while salt bridge between E117 and R490 in Peptide 10/FTH1 no longer exist here because of the replacement of R490 with I490 (Fig. 2b and Supplementary Fig. 2c, d).
Fig. 2. Structural analysis of high-affinity peptide adapter models and evaluation of engineering precision.
a Structural analysis of the Peptide 10/FTH1 complex. Specific details of the interactions at the binding interface are presented in the enlarged panel below. Residues contributing to the formation of hydrophobic cores I and II are enclosed. Peptide 10 is colored pale turquoise, and FTH1 is colored gray. b Structural analysis of the Peptide 10-1/FTH1-1 complex. The specific details of these interactions are further elucidated in the enlarged panel below. Residues contributing to the formation of hydrophobic cores I and II are enclosed. Peptide 10-1 is colored salmon, and FTH1-1 is presented as an opaque surface in gray. The mutated residues on FTH1-1 that are involved in these interactions are colored in deep green. c Structure alignment between the initial models and the high-affinity peptide models. The RMSD values for the alignment of different peptides are displayed. Ferritin is presented on the surface, and the binding interface is color coded according to hydrophobic properties (from weak to strong: cyan–white–maroon). Peptide WT is colored yellow, Peptide 10 is colored pale turquoise, and Peptide 10-1 is colored salmon. d The impact of key residue mutations on the binding affinity of the peptide for ferritin. Specific details of the critical interactions involved in stabilizing the complex structure are highlighted with dashed outlines in different colors. Residues involved in hydrophobic interactions are displayed on the surface. The sequence is colored according to hydrophobic properties (weak to strong: cyan–white–maroon). The residues that were mutated in FTH1-1 and participate in interactions are highlighted in deep green. Hydrogen bonds are represented by yellow dashed lines, and salt bridges are represented by magenta dashed lines.
Structure alignment revealed that Peptide WT, Peptide 10, and Peptide 10-1 exhibit almost identical folds, with root mean square deviation (RMSD) values ≤0.5 for all atoms (Fig. 2c). The increased affinity for engineered peptides was derived from tiny changes in their sequences and interactions with FTH1 or FTH1-1. For Peptide 10/FTH1, the Ser residue at position 485 was replaced by a Thr residue, and the side chain of this residue increased the size of hydrophobic core II. At the same time, Arg at position 490 forms a strong salt bridge with E117, with a distance of 2.55 Å, which is notably shorter than the 3.42 Å in the Peptide WT/FTH1. Moreover, Phe is more hydrophobic than Trp, and the replacement at position 497 increases the hydrophobic interaction in hydrophobic core I. This replacement also induces a slight conformational change in R23 because of its shorter side chain, resulting in a more extensive hydrogen bonding network with the carbonyl oxygens R490 and Q492. Hence, this engineering design did not alter the intrinsic mode of interaction with FTH1 but greatly increases the binding affinity (Fig. 2d). Peptide 10-1/FTH1-1 was developed from Peptide 10/FTH1, and the replacements at positions 485 and 497 were retained. The structure reveals that residue changes at positions 486 (Leu) and 490 (Ile) in Peptide WT/FTH1 and Peptide 10-1/FTH1 eliminate the original salt bridge with Glu117 but contribute to the formation of a more stable and compact hydrophobic core I. Additionally, mutations in FTH1, including S114E and N110E, enhance this hydrophobic core by lengthening side chains. Moreover, the introduction of a methylene group from K492 provides supplementary hydrophobic surface area, contributing to the formation of a larger hydrophobic core II. The Q15R mutation in FTH1-1 creates another hydrophobic pocket centered on L498 (Fig. 2d). This engineered adapter Peptide 10-1, originated from the C-terminus of NCOA4, was selected and referred to as the “ferritinophagy (Fagy) tag” hereafter.
The design of a rabies nanoparticle vaccine based on the Fagy tag delivery platform
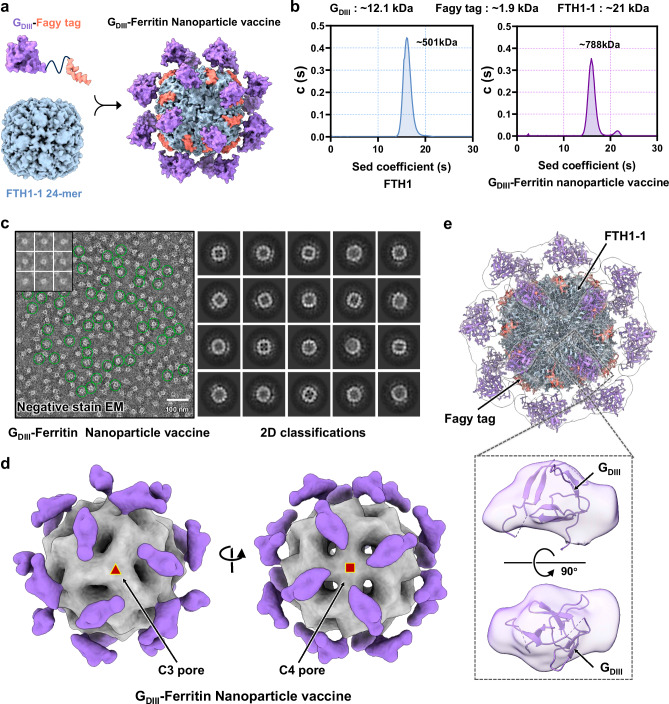
To assess the practicality of the Fagy tag delivery platform, we rationally designed a nanoparticle vaccine utilizing the domain III of rabies virus glycoprotein (RABV-GDIII) antigen. At first, GDIII was fused with the Peptide WT, Peptide 10, as well as the Fagy tag, respectively (Supplementary Fig. 3d). Then, the binding affinities of the combinations of GDIII-Peptide WT/FTH1, GDIII-Peptide 10/FTH1, and GDIII-Fagy tag/FTH1-1 were measured using surface plasmon resonance (SPR). The results clearly showed an approximately sevenfold increase in binding affinity between the GDIII-Fagy tag and FTH1-1 (2.900E − 08 M) compared to that between the GDIII-Peptide WT and FTH1 (1.913E − 07 M) (Supplementary Fig. 3e). The nanoparticle vaccine formulation involved a fusion protein comprising multiple copies of Fagy-tagged GDIII antigens, a 24-mer structural scaffold of ferritin. The spontaneous assembly of the GDIII antigen proteins was presented onto the nanoparticle scaffold surface through the Fagy tag. The display of multiple homologous antigens on the ferritin nanoparticle surface could exhibit immunological advantages, increasing the immunogenicity of the antigen (Fig. 3a). Next, the efficiency of antigen presentation and potential modes of action were investigated. We produced GDIII-Ferritin nanoparticle vaccines with high purity and monodispersity, evaluated their average molecular weight using analytical ultra-centrifugation (AUC), and performed geometric calculations. The findings revealed that each nanoparticle displayed ~19 GDIII antigens on the surface, achieving an efficiency of up to 80% and establishing a foundation for high immunogenicity (Fig. 3b). Subsequently, negative stain EM data for the GDIII-Ferritin nanoparticle vaccine were collected and analyzed, further confirming its monodispersity. By employing single-particle picking and 2D classification on the obtained movies, we identified a significant correlation with antigen presentation efficiency, as determined by AUC calculations (Fig. 3c). To assess the genuine antigenic performance of GDIII, 3D reconstruction was conducted on single-particle stacks of nanoparticles exhibiting O symmetry (Fig. 3d). The reconstructed results suggested the spatial distribution of GDIII on the ferritin surface and demonstrated that the density of each antigen monomer was uniform with that of ferritin (Fig. 3e). Collectively, these data provided additional confirmation of the accurate assembly and effective antigen delivery capabilities of this innovative nanoparticle platform.
Fig. 3. The antigen presentation efficiency and performance mode of GDIII-Ferritin nanoparticle vaccine.
a Schematic representation of a fusion protein comprising multiple copies of Fagy-tagged GDIII antigens, an FTH1-1-based 24-meric nanoparticle, and a GDIII-Ferritin nanoparticle vaccine complex. b The states of ferritin and GDIII-Ferritin in solution were confirmed by analytical ultra-centrifugation (AUC) assay. The calculated molecular weights corresponding to each peak in AUC, where they are labeled above the curve. “Sed” stands for sedimentation. Experiments were conducted independently in triplicates. c Negative stain EM image of the GDIII-Ferritin nanoparticle vaccine. Raw image (left panels) and 2D classifications (right panels). The top left corner of the left panel is a representative image of particle picking. The green circles highlight representative vaccine particles. The 644 negative stain EM images of GDIII-Ferritin nanoparticle vaccine show similar antigen delivery efficiency and particle dispersion. d 3D reconstruction electron density map of the GDIII-Ferritin nanoparticle vaccine from negative stain EM data. The left panel shows a map of positive projection with threefold symmetry axes at the center. The right panel shows a map of positive projection with fourfold symmetry axes at the center. The entire map is displayed on an opaque surface, the delivery core is shaded in gray, and the spikes are colored in medium purple. e Fitting of the GDIII-Ferritin density map to the RABV GDIII structure (PDB: 6TOU) model and the Peptide 10-1/FTH1-1 structure model (PDB: 8WIE). The positions of GDIII, ferritin, and the Fagy tag are all indicated. The lower panel provides an enlarged view of the spike region of the GDIII-Ferritin density map fitted to the GDIII structure model. The map is displayed in transparent surface form.
Evaluation of the stability and antigenic effectiveness of the GDIII-Ferritin rabies nanoparticle vaccine
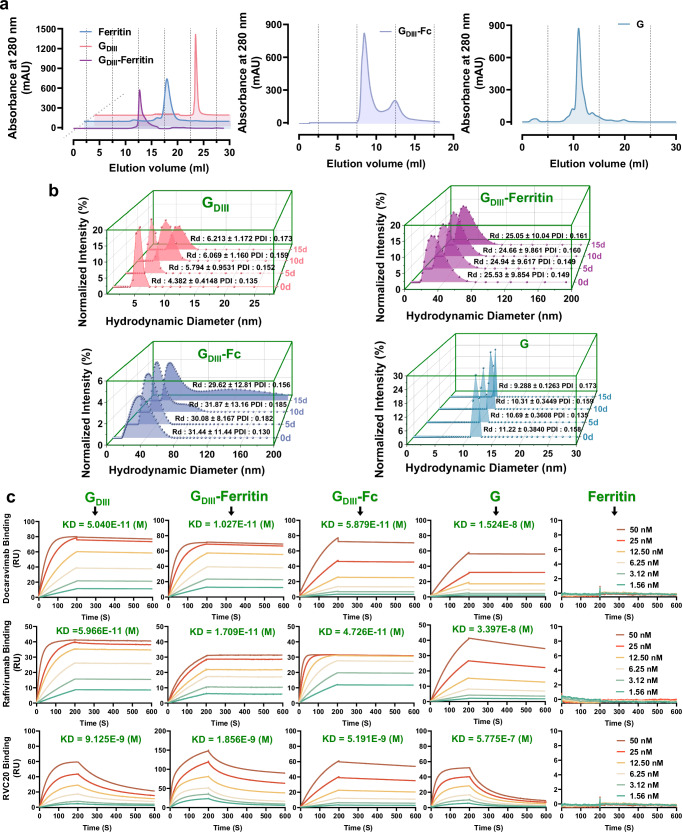
To explore the potential immunological advantages of the GDIII-Ferritin nanoparticle vaccine, three alternative rabies subunit vaccines were also prepared for parallel immunogenicity assessment. These included the monomeric form of GDIII protein (GDIII), a fusion protein of GDIII linked to the human antibody Fc domain (GDIII-Fc), and the full-length G monomeric protein (G) (Fig. 4a). During the vaccine preparation process, it was unexpectedly discovered that GDIII-Fc assembled into ~30 nm particles utilizing negative stain EM and DLS (Fig. 4b and Supplementary Fig. 4c). While monitoring the dynamic size changes of the four rabies vaccines stored at 4 °C for 20 days using DLS, we noted that, unlike the other three vaccines that showed particle aggregation or protein degradation, the GDIII-Ferritin nanoparticle vaccine exhibited superior stability (Fig. 4b). Furthermore, assessment of protein degradation under different temperature storage conditions also revealed that the GDIII-Ferritin nanoparticle vaccine maintains stability without discernible degradation, and the assembly of nanoparticles significantly enhanced the structural stability of the GDIII antigen (Supplementary Fig. 4e). Additionally, repeated freeze–thaw cycles did not compromise the stability of the GDIII-Ferritin nanoparticle vaccine (Supplementary Fig. 4f). Thermal shift assay also demonstrates its robust resistance to a pH range of 5.0–10.0, as well as to denaturing agents such as 1 M urea and 20% fetal bovine serum (FBS) (Supplementary Fig. 4g).
Fig. 4. Stability and antigenic evaluation of the rabies subunit vaccines.
a Chromatographic purification profiles of the four vaccines. Ferritin is shown as a steel blue curve, GDIII is shown as a rose-red curve, GDIII-Ferritin in a purple curve, GDIII-Fc is shown as a conch curve, and G is shown as a blue-gray curve. b Changes in the hydrodynamic particle size distribution of the four vaccines over 15 days of storage at 4 °C. c SPR kinetic tests for the binding affinity between the four vaccines, scaffold ferritin protein and three GDIII epitope-specific neutralizing antibodies (Docaravimab, Rafivirumab, and RVC20). Sensor grams were obtained using a Biacore T200 instrument at six different concentrations (ranging from 50 to 1.56 nM for each analyte, using twofold dilution). KD: dissociation equilibrium constant calculated as Kd/Ka; smaller values generally indicate stronger binding, as indicated in the respective graphs. All the curves were best fitted using GraphPad Prism 8.0.2.
The effective presentation of antigenic neutralization epitopes is essential for optimal vaccine efficacy. Consequently, we expressed and purified three GDIII-specific neutralizing antibodies, namely, Docaravimab and Rafivirumab, which are currently undergoing clinical studies (Thera-SAbDab; http://opig.stats.ox.ac.uk/webapps/therasabdab), RVC20, which was previously shown to possess perfect neutralization activity58. The binding affinities of these antibodies for diverse GDIII antigenic epitopes were evaluated using SPR. The results conclusively demonstrated that the GDIII-Ferritin nanoparticle vaccine effectively highlighted the antigenic neutralization epitopes (Supplementary Fig. 4d). Notably, the anticipated binding profiles indicated a minimal risk of antigenic epitope shielding, revealing an augmented ability to cross-link and activate B-cell receptors specific to the GDIII protein of RABV (Fig. 4c). Taken together, these comprehensive analyses proved that, in comparison to other types of rabies subunit vaccines tested in this work, the GDIII-Ferritin nanoparticle vaccine demonstrated distinctively superior stability and antigenic effectiveness.
The GDIII-Ferritin nanoparticle vaccine elicited potent humoral immune responses in mice
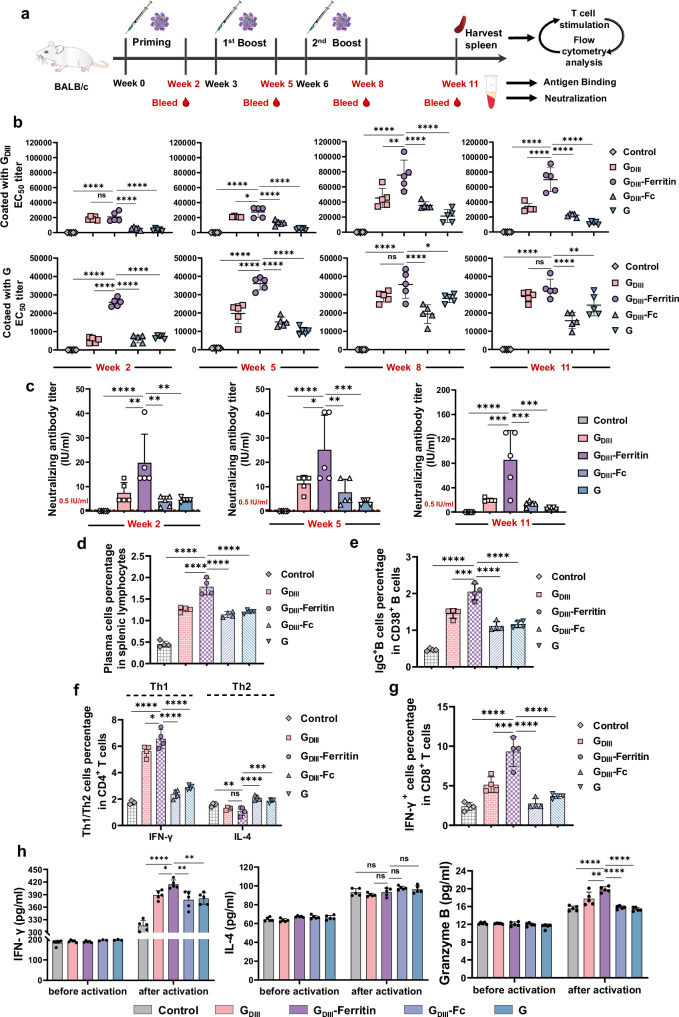
To compare the efficacy of the GDIII-Ferritin nanoparticle vaccine with that of other rabies subunit vaccines, BALB/c mice were subcutaneously immunized with a 50 µg dose of the vaccine formulated with Freund’s adjuvant. The same mass dose was used in the GDIII group, GDIII-Ferritin group, GDIII-Fc group, and G group. Equal volumes of adjuvant-vaccinated mice were used as the control group. The vaccinations utilized a prime-boost-booster approach at weeks 0, 3, and 6. Serum samples were collected at weeks 2, 5, 8, and 11 and subjected to ELISA to measure the titers of antigen-specific IgG antibodies (Fig. 5a). After the priming immunization, the GDIII-Ferritin nanoparticle vaccine rapidly elicited increased titers of binding antibodies that specifically targeted GDIII and G antigens in mice. The immune response exhibited a persistent and increasing trend, consistently exceeding the serum-binding antibody titers observed in the other subunit vaccine groups throughout all observation periods. These findings emphasized the heightened immunogenicity of the GDIII-Ferritin nanoparticle vaccine (Fig. 5b and Supplementary Figs. 8, 9a, b). Moreover, we found that vaccination with an equal mass dose of the GDIII monomer also led to a significantly increased level of IgG-binding antibody. When the mass dose was normalized to the molar dose of the GDIII antigen, it became evident that, despite an ~50% reduction in the molar dose of GDIII, the GDIII-Ferritin nanoparticle vaccine still maintained a substantial titer of binding antibodies. This finding suggested a potential correlation between antigen valency and the prompt induction of antigen-specific antibodies. In addition, the GDIII-Ferritin nanoparticle vaccine induced markedly higher titers of specific serum-binding antibodies than the GDIII-Fc vaccine. Which emphasized the advantageous adaptability of the innovative Fagy-tagged nanoparticle delivery platform for enhancing antigen efficacy. Additionally, the titer of the GDIII-specific IgG antibody elicited by the GDIII-Ferritin nanoparticle vaccine was greater than that elicited by the G monomer, demonstrating a notable advantage in epitope focusing. The higher serum antigen-specific binding antibody titer in the GDIII monomer immunization group compared to the G monomer group may be attributed to several factors. First, the molar dose of GDIII antigen was approximately three times higher than that of the G and GDIII-Fc groups when administered at the same dose. Additionally, the highly glycosylated nature and pH-sensitive feature of the G protein likely contributed to its lower immunogenicity. Furthermore, the heterogeneous polymerization state of GDIII-Fc, as observed in negative-stained EM, likely shielded the GDIII antigen epitopes, thereby reducing its immunogenicity (Fig. 5b). Additionally, comparisons of the immunogenicity induced by the Fagy tag/ferritin scaffold with the Helicobacter pylori ferritin further demonstrate a significant advantage in minimizing the introduction of non-essential immunogens (Supplementary Fig. 9c, d).
Fig. 5. Immunogenicity assessment of the rabies subunit vaccines.
a Schematic representation of the mouse immunization process (n = 5). b Titers of GDIII/G-specific serum-binding antibodies induced by different vaccines at 2, 5, 8, and 11 weeks after priming immunization. The upper panel shows the antigen GDIII. The lower panel shows the antigen G (n = 5). c Titers of neutralizing antibodies in mouse sera from different immunization groups at 2, 5, and 11 weeks after priming immunization (n = 5). The titers plotted were from rabies live virus (CVS-11) neutralization experiments. d Statistical analysis of the frequency of antibody-producing cells (CD138+ plasma cells) in splenic lymphocytes induced by vaccines (n = 4). The gating strategy is shown in Supplementary Fig. 10a. e Statistical analysis of the frequency of IgG-specific memory B cells located in the spleen germinal centers (GCs) induced by the vaccines (n = 4). The gating strategy is shown in Supplementary Fig. 10a. f Statistical analysis of the frequency of CD4+ IFN-γ+ Th1 and CD4+ IL-4+ Th2 cells, presented as a percentage of total CD4+ T cells, reflecting the activation status of different functional T-cell subsets (n = 4). The gating strategy is shown in Supplementary Fig. 12a. g Statistical analysis of the frequency of CD8+ IFN-γ+ cytotoxic T lymphocytes, presented as a percentage of total CD8+ T cells, reflecting the activation status of cytotoxic effector T cells (n = 4). The gating strategy is shown in Supplementary Fig. 12a. h Determination of characteristic cytokines secreted by T cells isolated from the spleens of experimental mice before and after activation (n = 5). The concentrations of serum cytokines were calculated by fitting the OD450 values obtained from ELISA to the regression line equation. All spleen lymphocytes used for cell typing were derived from mouse spleens 5 weeks after the second booster immunization. In (b–h), all experiments were conducted independently in triplicates, all the data represented as mean ± SEM and were analyzed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test. ns (not significant), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
Next, to evaluate the neutralizing antibody titers induced by these vaccine candidates, live virus (CSV-11) neutralization assays were performed. All the subunit vaccines consistently produced serum neutralizing antibodies that surpassed the World Health Organization (WHO)-defined prophylactic RABV vaccination threshold of virus neutralization titers (VNTs)61 2 weeks after the prime immunization. Remarkably, the serum neutralizing antibody titers peaked at the 11th week after priming, revealing substantial differences among the different vaccine groups. The titers of neutralizing antibodies induced by the GDIII-Ferritin nanoparticle vaccine were ~4.5-fold greater than those induced by GDIII, 6-fold greater than those induced by GDIII-Fc, and 47-fold greater than those induced by the G protein. This result further supported the superior immunogenicity of the GDIII-Ferritin nanoparticle vaccine. Therefore, we hypothesize that the heightened immunogenicity can be attributed to the superior antigenic valency on the surface of the GDIII-Ferritin nanoparticles, which leads to more extensive and easily activated downstream signaling pathways in B cells (Fig. 5c).
Subsequently, the duration of protection elicited by those vaccines was evaluated. The mice were euthanized 5 weeks after the second boost vaccination. Lymphocytes, which were isolated from the spleen, were used to evaluate the phenotypes of the plasma cells and IgG-specific memory B cells. The GDIII-Ferritin nanoparticle vaccine induced a significantly greater percentage of plasma cells, as substantiated by robust support from the humoral immune response, as indicated by antibody titers (Fig. 5d and Supplementary Fig. 10a, b). The excellent activation ratio of IgG-specific memory B cells in the spleen further confirmed the considerable advantage of the GDIII-Ferritin nanoparticle vaccine in inducing the formation of long-lasting immunological memory (Fig. 5e and Supplementary Fig. 10a, c). In summary, the GDIII-Ferritin nanoparticle vaccine significantly promoted plasma cell differentiation, the generation of IgG-specific memory B cells, and enduring protective humoral immune responses. The advantages of eliciting potent humoral immune responses further substantiated the potential of the GDIII-Ferritin nanoparticle vaccine as a promising candidate for rabies virus infection.
The GDIII-Ferritin nanoparticle vaccine elicited Th1-biased cellular immune responses in mice
Considering the previously demonstrated advantages of nanoparticle vaccines in cellular immunology12,22,62,63, we specifically analyzed the differences in four cytokines, including IFN-γ, IFN-λ, IL-4, and Granzyme B, in the serum of vaccinated mice. The changes in IFN-γ levels in the serum indicated significant differences in the magnitude of cellular immune activation induced by the different subunit vaccines. Furthermore, the increased secretion level of IFN-λ indicates the vigorous stimulation of immune cells, confirming the stable presentation of antigenic proteins in vivo and the activation of the intracellular viral clearance mechanism by the GDIII-Ferritin nanoparticle vaccine. This suggests potential effects on antiviral defense and the regulation of innate immune activity. The consistent serum IL-4 concentration in both the GDIII and GDIII-Ferritin vaccine groups, as well as in the control group, demonstrated the absence of substantial activation effects on Th2-biased immune responses, ensuring safety (Supplementary Fig. 11a).
Based on the above results, we performed a statistical analysis of cell population frequencies and activation patterns within distinct T-cell subsets. This study aimed to comprehensively characterize vaccine-induced cellular immunity. Our observations revealed the considerable advantage of the GDIII-Ferritin nanoparticle vaccine in promoting T-cell proliferation (Supplementary Fig. 11b, d, e). This discovery was consistent with the findings for the serum Granzyme B concentration (Supplementary Fig. 11c, d, f). CD4+ T cells that produced IFN-γ play a crucial role in eliciting optimal antibody responses and activating antiviral cellular immunity64,65. To further evaluate the effect on T helper cells and confirm the specificity of T-cell activation, we concurrently stimulated isolated T cells with the GDIII antigen and PMA and employed flow cytometry (FCM) to determine the percentage of active T-cell subsets in the presence of a Golgi apparatus blocker. The results indicated that both the GDIII-Ferritin nanoparticle vaccine and the GDIII monomer elicited effective CD4+ IFN-γ+ Th1 cell responses, and the efficacy of the GDIII-Ferritin nanoparticle vaccine was superior (Fig. 5f and Supplementary Fig. 12a, c). However, there was no significant difference in the activation of CD4+ IL-4+ Th2 cells induced by the GDIII-Ferritin nanoparticle vaccine compared to that in the control group. This Th1-biased immune response could offer enhanced protection against viral infection (Fig. 5f and Supplementary Fig. 12a, d). These findings indicated that the nanoparticle vaccine was also able to activate stronger T-cell immune responses to eliminate possibly infected cells (Fig. 5g and Supplementary Fig. 12a, b). Moreover, changes in key cytokines (IFN-γ, IL-4, and Granzyme B) in the cell culture supernatant both before and after T-cell activation also verified the advantages of the GDIII-Ferritin nanoparticle vaccine for inducing robust and safe T-cell immune responses apart from B-cell responses (Fig. 5h).
The GDIII-Ferritin nanoparticle vaccine potently protected mice against RABV infection
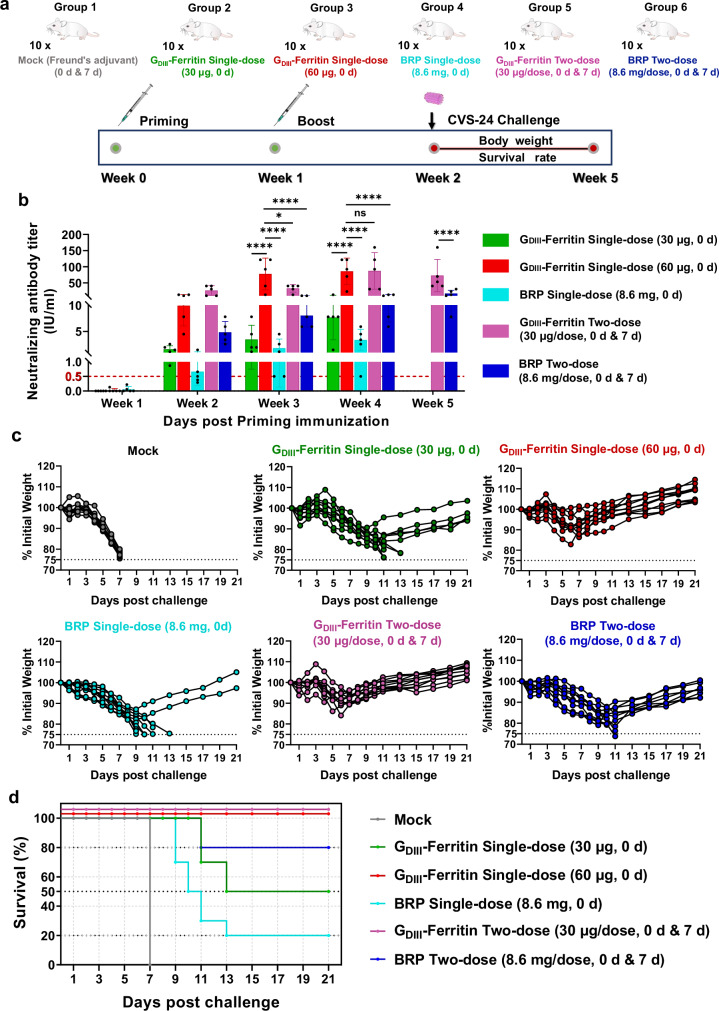
To investigate the correlation between higher VNTs and specific T-cell immune responses induced by the GDIII-Ferritin nanoparticle vaccine and its in vivo protective efficacy, we conducted an experimental study using a RABV intracerebral challenge model in BALB/c mice. Currently marketed rabies vaccines are mostly inactivated, requiring individuals to receive 4–5 doses to achieve effective protection post-exposure66, while animals need immunizations annual. To explore the optimal immunization strategy and dosing regimen for the novel GDIII-Ferritin rabies vaccine, six parallel immunization groups were established to assess the induction of immune responses triggered by vaccination before intracerebral challenge in the experimental animals (Fig. 6a, b and Supplementary Fig. 13). At the 2nd week post priming immunization, the neutralizing antibody levels in the GDIII-Ferritin single-dose (60 μg, 0 d) and GDIII-Ferritin two-dose (30 μg/dose, 0 d and 7 d) groups were >10 IU/ml, approximately tenfold increase compared to the other groups (Fig. 6b). Therefore, each mouse in the virus challenge study was intracerebrally injected with a high lethal dose of the rabies CVS-24 strain (50-fold LD50) on the 14th day following the priming immunization (Fig. 6a). Survival rate, body weights, and clinical signs were monitored until 21 days after infection. As shown in Fig. 6c, overall body weight loss was evident in all challenged mice, no matter whether they received vaccination. Clinical sign of ruffled fur appeared on the 5th day post challenged in all the mice. Due to acute viral infection, by the 7th day post challenge, all the mice in the Mock group showed clinical signs of trembling, arched back, and hind limb paralysis, and were euthanized simultaneously. In the single-dose group of the standard Biological Reference Preparation (BRP) (8.6 mg, 0 d), eight mice exhibited severe clinical signs of rabies, including trembling, arched back, and hind limb paralysis ~11 days post challenge, and were euthanized. Only two mice recovered from the infection. In the BRP two-dose (8.6 mg/dose, 0 d and 7 d) group, two mice exhibited typical rabies symptoms, such as trembling and arched back on the 11th day post challenge and were subsequently euthanized. The surviving mice began to regain weight on the 10th day and gradually recovered from the infection and no other clinical signs were observed in the following days. Three mice in the GDIII-Ferritin single-dose (30 μg, 0 d) group died of infection on the 11th day post challenge, and two mice were euthanized for obvious clinical signs of hind limb paralysis on the 13th day post challenge. Surviving mice in this group only showed ruffled fur and gradually regain body weight after the 10th day without clinical signs in the following days. Surprisingly, both the GDIII-Ferritin single-dose (60 μg, 0 d) group and the GDIII-Ferritin two-dose (30 μg/dose, 0 d and 7 d) group showed 100% survival rate, and the clinical sign of ruffled fur in the mice was very mild, and gradual recovery of the body weight and disappearance of ruffled fur sign on the 6th day post challenge (Fig. 6c, d). All the surviving mice in the experimental groups remained healthy 21 days post challenge and exhibited no clinical signs.
Fig. 6. Evaluation of the protective efficacy of the GDIII-Ferritin nanoparticle vaccine.
a Schematic representation of the vaccination strategies and rabies virus challenge protocols for different groups of experimental mice. (n = 10/group). Group 1 (Mock) served as the negative control and received two subcutaneous immunizations with Freund’s adjuvant; Group 2 was the GDIII-Ferritin nanoparticle vaccine single-dose (30 μg, 0 d) group that received a single subcutaneous immunization; Group 3 was the GDIII-Ferritin nanoparticle vaccine single-dose (60 μg, 0 d) group that received a single subcutaneous immunization; Group 4 was the commercial rabies vaccine BRP single-dose (8.6 mg, 0 d) group that received a single subcutaneous immunization. Group 5 was the GDIII-Ferritin nanoparticle vaccine two-dose (30 μg/dose, 0 d and 7 d) group that received two subcutaneous immunizations; Group 6 was the commercial rabies vaccine BRP two-dose (8.6 mg/dose, 0 d and 7 d) group that received two subcutaneous immunizations. All the experimental mice underwent intracranial rabies virus challenge on day 14 post priming immunization. b Virus neutralizing antibody titers (VNATs) in the sera of mice within 28 days full-dose vaccine immunization (n = 5). Experiments were conducted independently in triplicates. Data represented as mean ± SEM and were analyzed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test. ns (not significant), *p < 0.05, **p < 0.01, ****p < 0.0001. c Weight changes in mice during challenge with the rabies virus CVS-24 strain were recorded for 21 days. d The survival rates of the infected mice were calculated during 21 days post challenge. Source data are provided as a Source Data file.
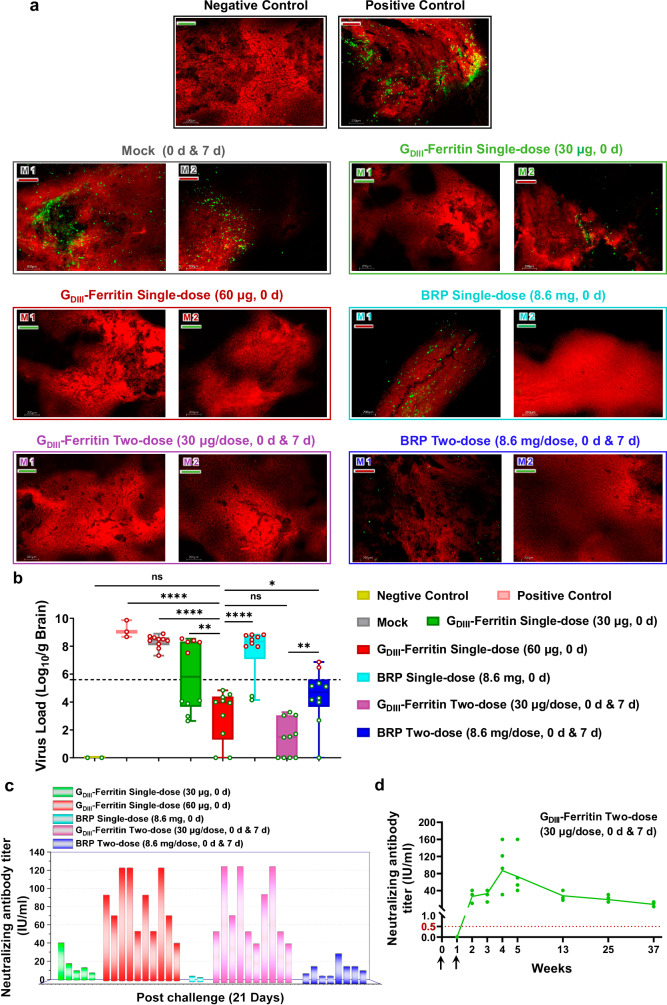
Remarkably, the sera from mice that survived 21th day post challenge showed exceptionally high titers of RABV neutralizing antibodies in the GDIII-Ferritin single-dose (60 μg, 0 d) group and the GDIII-Ferritin two-dose (30 μg/dose, 0 d and 7 d) group, which demonstrates a significant correlation between the survival rate and the titer of specific neutralizing antibodies of mice immunized with the GDIII-Ferritin nanoparticle vaccine (Fig. 7c). Moreover, we also harvested brain tissue samples from mice that succumbed to infection and from mice that remained alive 21 days post challenge. All the brain tissues from the dead mice were shown to be positive for RABV antigen by direct fluorescent antibody (DFA) analysis, which indicated that these mice died from the RABV infection. Notably, RABV-specific fluorescent spots were observed in the brain tissue of five deceased mice from the GDIII-Ferritin single-dose (30 μg, 0 d) group and eight deceased mice from the BRP single-dose (8.6 mg, 0 d) group. In contrast, the positive control and mock groups exhibited even stronger virus-specific fluorescence spots. The distribution of virus-specific fluorescent spots in the brain tissue of the two dead mice in the BRP two-dose (8.6 mg/dose, 0 d and 7 d) group was significantly reduced, and no virus was detected in the brain tissue of the surviving mice (Fig. 7a and Supplementary Fig. 14). It was evident that vaccination with one dose of the GDIII-Ferritin nanoparticle vaccine (60 μg, 0 d) or two doses of the GDIII-Ferritin nanoparticle vaccine (30 μg, 0 d and 7 d) completely inhibited viral infection and eliminated rabies virus in the brain following the RABV challenge, which was consistent with their full protective efficacy. The RABV loads in the brain tissue of mice receiving either two doses of the GDIII-Ferritin nanoparticle vaccine (30 μg/dose, 0 d and 7 d) or one dose of the GDIII-Ferritin nanoparticle vaccine (60 μg, 0 d) were significantly lower than those in the BRP two-dose (8.6 mg/dose, 0 d and 7 d) group, as determined by quantitative real-time reverse transcription PCR (qRT-PCR) targeting the viral nucleoprotein gene. Compared to the positive control, mice in the BRP two-dose (8.6 mg/dose, 0 d and 7 d) group exhibited a significant decrease in viral load. In contrast, there was no significant reduction in viral load in the deceased mice from the single-dose BRP group (8.6 mg, 0 d). This indicates that the protective effect of the BRP vaccine is closely related to the dosage and frequency of immunizations (Fig. 7b). Combined with the recovery of the body weight of the test mice, it was obvious that the neutralizing antibody titers induced by the GDIII-Ferritin nanoparticle vaccine effectively promoted the clearance of the rabies virus. In addition, we observed sustained levels of protective neutralizing antibodies in the serum of mice immunized with two doses of the GDIII-Ferritin nanoparticle vaccine (30 μg/dose) for more than 9 months. This finding underscores the significant advantage of the strong and long-lasting protective effect elicited by these self-assembling nanoparticle vaccine candidates (Fig. 7d).
Fig. 7. Evaluation of the protective efficacy of the GDIII-Ferritin nanoparticle vaccine.
a Representative images of direct immunofluorescence localization of the virus in animal brain tissues from control or experimental mice by DFA. Bright apple-green fluorescent spots represent the RABV. Brain tissue slices from deceased mice are labeled with a red underscore, and slices from surviving mice are labeled with a green underscore. b Quantification of rabies virus (CVS-24) nucleoprotein genomic RNA in mouse brains determined by qRT-PCR (n = 10). The virus load in the brain of deceased mice was quantified on the day of death, while in surviving mice, it was assessed on the 21st day post challenge. The brain tissue of healthy mice was used as a negative control, and the non-immune mice injecting with equal virus were used as a positive control (red spots indicate samples from deceased mice, while green spots represent samples from surviving mice, with a dotted line distinguishing between deceased and surviving mice). Experiments were conducted independently in triplicates. Data represented as mean ± SEM. Data were shown as box-and-whiskers plots (box indicates lower and upper quartiles with bar at median and whiskers spanning minimum and maximum data points) with individual data points and analyzed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test. ns (not significant), *p < 0.05, **p < 0.01, ****p < 0.0001. c Serum samples of surviving mice were collected at 21 days post challenge, and the neutralizing antibody levels were tested by FAVN. d Serum neutralizing antibody titers monitoring within 9 months after two doses of GDIII-Ferritin nanoparticle vaccine (30 μg/dose) (n = 5). To evaluate the duration of antiviral protection of the GDIII-Ferritin nanoparticle vaccine. Source data are provided as a Source Data file.
In conclusion, these findings further elucidate the high internalization efficiency and B-cell activation ability of the GDIII-Ferritin nanoparticle vaccine, even at low dose. The generation of larger amounts and higher concentrations of neutralizing antibodies facilitated the initiation of antibody-dependent cytotoxicity and complement-dependent cytotoxicity, both of which contribute to viral clearance. Additionally, compared to the high and multi-dose immunization protocols of commercial rabies vaccines, the development of the GDIII-Ferritin nanoparticle vaccine offers a promising and innovative strategy for the prevention and treatment of rabies.
Discussion
During our revision preparation, Frank et al. recently presented the cryo-EM structure of the NCOA4/FTH1 complex at a resolution of 2.9 Å, elucidating several key structural features crucial for complex formation67. Their findings provided valuable structural insights into the mechanism of NCOA4-induced ferritinophagy. The complex structure corroborated earlier biochemical evidence indicating the critical role of residue R23 in FTH1 and residues W497, I489, S492, L494, and L498 in NCOA4 for their interaction, as mutations like FTH1R23A or NCOA4I489A/W497A abolished complex formation32. In our study, we solved the cryo-EM structure of this complex at a higher resolution of 2.2 Å, offering enhanced visualization of the interaction network within the NCOA4/FTH1 complex. Specifically, R23 of FTH1 forms a hydrogen bond with S492, and its aliphatic region, along with NCOA4 residues W497, L494, and L498, contributes to the hydrophobic core II, while I489 is involved in forming hydrophobic core I. Overall, our atomic-resolution structure not only clarifies the interaction network between FTH1 and NCOA4 but also lays the groundwork for structure-guided engineering of the Fagy tag.
As noted, previous delivery systems have faced manufacturing challenges including particle heterogeneity, improper antigen folding, interference between antigens, intersubunit interactions, and immune responses to non-essential proteins20. In response to these challenges and aiming to offer an alternative ferritin-based nanoparticle vaccine platform, we engineered the natural interaction pattern found in the NCOA4/FTH1 complex proteins, and anticipated that the Fagy tag system presented here may offer several advantages over previously reported ferritin-based delivery platforms.
First, established bioconjugation technologies such as the SpyTag/SpyCatcher, Protein A/Fc, biotin/streptavidin system have proven advantageous by enabling precise and strong peptide–protein interactions, offering versatility and ease of use in various bioconjugation applications. Meanwhile, the relatively large molecular weight of SpyCatcher (~15 kDa) has also raised concerns regarding potential immunogenicity, which could limit its suitability for certain applications11,68. In our study, the Fagy-tag engineered from the natural ferritinophagy complex, provides an alternative bioconjugation approach specifically for ferritin-based delivery system. Despite its shorter length (16 amino acids), the Fagy tag enhances binding affinity and specificity through noncovalent interactions when fused to terminal ends. This design strategy aims to reduce potential antigenicity associated with larger non-antigen components. However, comprehensive studies are essential to evaluate the immunogenicity profiles of non-antigen components, including the ferritin cage and the Fagy tag itself. Moreover, it is also of great importance to systematically compare the current system with other conjugating method, such as genetically fused antigen-ferritin nanoparticles and antigen-Tag/Catcher-ferritin nanoparticles.
Second, the generation of these nanoparticle vaccines is convenient and cost-effective. Ferritin can be expressed efficiently in large quantities in Escherichia coli (E. coli), and the target antigen protein (GDIII of the rabies virus in this study) can be produced in insect cells, preserving its natural conformation and post-translational modifications. Following expression and purification, these two subunits can be simply mixed and will automatically self-assemble into nanoparticles. This process requires no specific enzymes or buffers, eliminating associated biosafety concerns.
Third, another crucial advantage lies in the compatibility of this engineered Fagy tag. Comprising two consecutive α-helices, this tag can be fused for expression at either the N-terminus or the C-terminus of the target protein. Particularly, it endows the capability to fuse two distinct antigens at both ends of the Fagy tag. This unique feature represents a substantial advantage of the Fagy-tag system, potentially allowing the presentation of two different antigens on the ferritin carrier in a 1:1 manner simultaneously. This precise control marks a notable advance, considering the previous challenges in achieving similar precision in self-assembling vaccines based on ferritin. Moreover, due to the noncompetitive compatibility of the Fagy tag binding site with the threefold apex at the ferritin N-terminus, the simultaneous fusion of a third antigen at the N-terminus allows the presentation of three different antigens in a 1:1:1 manner. This versatility and precision highlight the potential of the Fagy-tag system for advanced antigen presentation strategies in ferritin-based vaccine design, offering novel avenues for immunization research. Of course, future studies should be done to optimize the spacing restrains and orientation of fused antigens to ensure the stability of multi-component nanoparticles, these efforts will provide valuable insights into the design considerations necessary for maximizing the efficacy and stability of multi-antigen ferritin-based vaccine platforms.
The persistence of rabies continues to pose a significant and recognized challenge to global public health. A thorough examination of the clinical requirements for the rabies vaccine and the 2018 Global Rabies Prevention and Control Strategic Plan underscored the clear need for a rabies vaccine characterized by safety, stability, cost-effectiveness, high immunogenicity, reduced dosage, and ease of administration. Our novel GDIII-Ferritin nanoparticle vaccine constructed through the inventive Fagy-tag system exhibits remarkable characteristics in harmony with market needs. The Fagy tag delivery system reported here effectively presented multiple nonoverlapping neutralization epitopes of the antigen protein to reduce susceptibility to immune escape mutations. The vaccine also stimulated the differentiation of antibody-secreting cells and IgG-specific memory B cells, rapidly yielding a more robust and enduring humoral immune response. Furthermore, it induced the production of abundant GDIII-specific neutralizing antibodies, surpassing the antibody titers defined by the WHO as necessary for protective effects in animals, with a maintenance duration exceeding 9 months. Additionally, the vaccine triggered a potent protective Th1-biased CD4+ T cellular immune responses rather than a Th2-biased CD4+ T cellular responses that could lead to vaccine-associated enhanced respiratory disease69.
The vaccine demonstrated exceptional efficacy in conferring protection against intracerebral challenges in a mice model. The Fagy-tag nanoparticle delivery system here outperformed commercially available rabies vaccines by achieving comparable efficacy even at reduced doses and through a more convenient single-dose vaccination process. Noteworthy is its remarkable versatility, extending its relatively advantages beyond previous self-assembling vaccine platforms. These findings offer valuable insight for the development of universal antiviral vaccines that synergize with T-cell and B-cell immune responses, we anticipate that this platform holds promising potential for applications in developing vaccines for diverse diseases.
Methods
Cell lines, plasmids, and viruses
Hi5 (Trichoplusia ni) and Sf9 (Spodoptera frugiperda) cells were purchased from the Union Biotech (Shanghai) Co., Ltd, and cultured in ESF921 Expression Medium (Expression Systems). The 293-F cells were purchased from Gibco and cultured in the FreeStyle 293 Expression Medium (Gibco). BHK-21 cells (baby hamster kidney) were obtained from Changchun Veterinary Research Institute and cultured in Dulbecco’s modified Eagle medium (DMEM, supplemented with 10% FBS and 100 units/ml penicillin–streptomycin). DH5α and BL21 (DE3) E. coli cells were purchased from TransGen Biotech and cultured in Luria-Bertani (LB) medium.
The codon optimized wild-type cDNA of the NCOA4 (residues 475–511, Gene ID: 8031) and human ferritin heavy chain (FTH1, Gene ID: 2495) was synthesized by Tsingke Biotechnology Co., Ltd. The coding sequences (codon optimized for insect cells) for ectodomain of rabies virus glycoprotein (RABV-G, residues 20–459, Gene ID: O92284, CVS-11 strain, GenBank accession number: GQ918139.1) and human IgG crystallizable fragment (Fc, Gene ID: 3500) synthesized by GenScript. All peptides and FTH1-1 and the fusion proteins of peptide with ferritin were synthesized by Tsingke Biotechnology Co., Ltd and cloned into the pGEX-6p-1 vector and purified using a GST tag. The RABV-G, domain III of RABV-G (GDIII) was constructed joining codons for residues 50–75 and residues 201–281 with a short linker of three glycine codons, as well as the fusion proteins of GDIII with peptide were cloned into the pHBM-8His expression vector. The coding sequences for Docaravimab, Rafivirumab, and RVC20 were constructed joining the VH and VL coding regions with a glycine-serine linker of 20 codons (sequence: (G4S)3) were synthesized by Tsingke Biotechnology Co., Ltd. and cloned into the pHBM-8His expression vector. The fusion protein of GDIII with Fc (GDIII-Fc) was cloned into the pFUSE expression vector (Supplementary Table 3).
RABV strain CVS-11 and CVS-24 were obtained from the World Organization for Animal Health (WOAH) Reference Laboratory for Rabies in China, Chinese Academy of Agricultural Sciences (Changchun, China).
Protein expression and purification
The proteins of GST-peptide, ferritin and peptide–ferritin were expressed using the E. coli expression system and from BL21 (DE3) E. coli cells. Protein expression was induced 20 h using isopropyl-beta-D-thiogalactoside (IPTG) at a final concentration of 700 μM and primary purified using GST-NTA column (GE Healthcare). Subsequently, the GST-peptide proteins were further purified using Superdex 200 Increase 10/300 GL column (GE Healthcare) with equilibration buffer (50 mM Tris, 200 mM NaCl, pH 8.0), while the ferritin and peptide–ferritin proteins were further purified using Superose 6 Increase 10/300 GL column (GE Healthcare) with equilibration buffer (50 mM Tris, 200 mM NaCl, pH 8.0) after cutting off the GST tag using HRV3C protease. The purity of the target proteins was analyzed by SDS–PAGE and fractions from the single major peak were concentrated for further use.
The G, GDIII, GDIII-peptide, Docaravimab, Rafivirumab, and RVC20 proteins were expressed using the Bac-to-Bac baculovirus system. The constructs were transformed into DH10Bac component cells, and the extracted bacmids were then transfected into Sf9 cells using Cellfection II Reagent (Invitrogen). The low-titer viruses were harvested and then amplified to generate high-titer virus stock in sf9 cells. And the recombinant proteins were produced in Hi5 cells at a density of 2 × 106 cells/ml. The supernatant of cell culture containing the secreted protein was harvested ~72 h after infection, concentrated and target protein was primary captured by Ni-NTA resin (GE Healthcare). The GDIII, GDIII-peptide, Docaravimab, Rafivirumab, and RVC20 proteins were further purified on a Superdex 200 Increase 10/300 GL column (GE Healthcare) equilibrated with 50 mM Tris, 200 mM NaCl, pH 8.0 buffer. The G protein was further purified on a Q HP column (GE Healthcare). For GDIII-Fc protein large-scale expression, 1 L 293-F cells were transfected, and protein was purified from the supernatant using Ni-NTA resin (GE Healthcare) after 5 days. Further purified using a Superdex 200 Increase 10/300 GL column (GE Healthcare). SDS–PAGE analysis revealed over 98% purity of the final purified recombinant proteins, fractions were pooled and concentrated for further use.
The purified FTH1-1 and GDIII-Fagy tag proteins were co-incubated (molar ratio is 1:30) overnight at 4 °C for 15 h and subsequently the formed complex was purified using Superose 6 Increase 10/300 GL column (GE Healthcare). The purified GDIII-Ferritin nanoparticle vaccine was analyzed by SDS–PAGE. The diameters of GDIII, G, ferritin, and GDIII-Ferritin nanoparticle vaccine were characterized with a dynamic light scatter (DLS, Wyatt Technology). The dispersion and particle assembly of ferritins and GDIII-Ferritin nanoparticle vaccine were determined by negative stain EM.
Computational design and assessment of peptide adapters
Based on the analyzed structure of the NCOA4/FTH1 complex, a high-throughput collection of 298 peptide amino acid sequences was generated using ProteinMPNN. For each designed sequence, only the AlphaFold2 model with the highest pLDDT score was considered for the analysis, which were subsequently evaluated by Rosetta (beta_nov16 weights). Median values for three key assessment parameters were noted as: ddg < (−45), contact_molecular_surface > (470), and Rosetta score < (−305). Considering the relative importance of these parameters, a preliminary screening was performed to select 146 sequences that simultaneously satisfied the criteria of both contact_molecular_surface and ddg being superior to their median values. Ultimately, a comprehensive assessment based on the AlphaFold2 pLDDT (Cα confidence score) led to the selection of 16 sequences that exhibit higher reliability and superior performance for further experimental characterization. This multi-system combination approach to sequence screening provided a high-precision, high-quality amino acid sequence, supporting in-depth exploration in subsequent studies on protein structure.
Negative stain electron microscopy (negative stain EM) sample preparation
The purified ferritin, peptide–ferritin, GDIII-Fc, and GDIII-Ferritin proteins (0.1 mg/ml) of 5 μl were loaded onto a freshly glow-discharged carbon-coated grid (300 mesh, Beijing Zhongjingkeyi Technology Co., Ltd.). After incubating for 1-min, excess sample was blotted, and the grid was stained with 100 μl 3% (w/v) uranyl acetate solution for 40 s. Remove excess staining solution by aspiration and the grids were dried at room temperature for 3 min. Images were recorded in Thermo Scientific Tecnai Spirit T20 (FEI) operated at 120 kV and 4 K × 4 K Ultrascan CCD camera at 92,000× or 57,000× magnification at Nankai University.
Cryogenic electron microscopy (Cryo-EM) sample preparation and image collection
In total, 3.5 μl purified peptide–ferritin fusion proteins at the concentration of 1.7 mg/ml were loaded onto glow-discharged (40 s at 15 mA) holey carbon quantifoil Cu grids (R1.2/1.3, 300 mesh), which was blotted for 3–3.5 s using a single layer of filter paper (Ted Pella) with a humidity of 100% at 4 °C and then plunged into liquid ethane using Vitrobot Mark IV (Thermo Fisher Scientific). Recording was carried out at 300 KV on an FEI Tecnai F30 transmission electron microscope (TEM, Thermo Fisher Scientific) equipped with a K3-subunit detector under super-resolution mode at a nominal magnification of 105 K×, which yielded a pixel size of 0.345 Å. All data were automatically collected using EPU 3 software, and image was recorded into 32 frames for each movie stack and the total electron dose was set to 60 e− Å−2 and the exposure time was 1 s. The defocus range was −1.0 to −2.0 μm.
Cryo-EM data processing
For the NCOA4/FTH1 complex dataset, 1220 raw movies were subjected to motion correction using MotionCor2 (v1.3.0) implemented in RELION 3.1.170. The micrograph contrast transfer function (CTF) correction parameters were estimated using patch CTF estimation implemented in CryoSPARC (v4.0.1)71. All processes were performed using CryoSPARC unless mentioned elsewhere. At first, particles were picked out using manual-picker procedure of CryoSPARC from 50 micrographs, and then subjected to 2D classification. Good particles of 2D class averages in different views for template models were applied to select particles against entire micrographs. A total of 434,794 particles were extracted and subjected to further 2D classification. The best class-averaged particles were chosen for the initial 3D reconstruction and homogeneous refinement. Nonuniform refinement and iterative global CTF refinement were conducted, resulting in a final density map with an overall resolution of 2.19 Å, as determined by the gold-standard Fourier shell correlation (FSC) cutoff of 0.143. Finally, a contour leveling operation with a value of 0.17 was applied to achieve a map suitable for subsequent model building. The image-processing workflow is shown in Supplementary Fig. 5.
For the Peptide 10/FTH1 complex dataset, 1393 raw movies were subjected to motion correction using MotionCor2 (v1.3.0)72 implemented in RELION 3.1.170. The micrograph CTF correction parameters were estimated using patch CTF estimation implemented in CryoSPARC (v.4.0.1)71. All processes were performed using CryoSPARC unless mentioned elsewhere. At first, particles were picked out using manual-picker procedure of CryoSPARC from 30 micrographs, and then subjected to 2D classification. Good particles of 2D class averages in different views for template models were applied to select particles against entire micrographs. A total of 516,754 particles were extracted and subjected to further 2D classification. The best class-averaged particles were chosen for the initial 3D reconstruction and homogeneous refinement. Nonuniform refinement and iterative global CTF refinement were conducted, resulting in a final density map with an overall resolution of 2.02 Å, as determined by the gold-standard FSC cutoff of 0.143. Finally, a contour leveling operation with a value of 0.076 was applied to achieve a map suitable for subsequent model building. The image-processing workflow is shown in Supplementary Fig. 6.
Negative stain EM image collection and data processing
The negative stain sample of GDIII-Ferritin nanoparticles was further imaged using a 200 kV TEM equipped with a K3-subunit detector under super-resolution mode at a nominal magnification of 120,000×, resulting in a pixel size of 1.2 Å. All data were automatically collected using EPU 3 software (Thermo Fisher). Images were recorded into 16 frames for each movie stack and the total electron dose was set to 50 e− Å−2 and the exposure time was 1 s. The defocus range was −1.0 to −1.4 μm.
For the dataset, 644 raw movies of GDIII-Ferritin nanoparticle vaccine were motion-corrected using MotionCor2 (v1.3.0)72 implemented in RELION 3.1.170. All processes were performed using CryoSPARC (v4.0.1)71 unless mentioned elsewhere. At first, manual particle picking was performed, and the selected particles were subjected to 2D classification. The resulting 2D class averages were then used as templates for template picking. Subsequently, the template-picker procedure to select particles against entire micrographs, and 87,838 particles were extracted and subjected to 2D classification. The best class averages particles were selected to perform initial 3D reconstruction and homogeneous refinement. Nonuniform refinement was performed, yielding a final density map of overall 4.92 Å resolution by the gold-standard FSC cutoff of 0.143, which was sufficient for model fitting. The image-processing workflow is shown in Supplementary Fig. 7.
Model building and refinement
The atomic model of NCOA4/FTH1 complex was built based on the FTH1 structure (PDB ID: 5N27). To start model building, the FTH1 structure was docked into the cryo-EM density map using ChimeraX (https://www.cgl.ucsf.edu/chimerax/), followed by manual adjustment of main chains and side chains in Coot73 and real space refinement in PHENIX74.
The atomic model of Peptide 10/FTH1 complex was built based on the NCOA4/FTH1 complex structure (PDB ID: 8WIQ). To start model building, the NCOA4/FTH1 complex structure was docked into the cryo-EM density map using ChimeraX (https://www.cgl.ucsf.edu/chimerax/), followed by manual adjustment of main chains and side chains in Coot73 and real space refinement in PHENIX74. The majority of the images presented in the figures were created using UCSF ChimeraX (https://www.cgl.ucsf.edu/chimerax/).
Crystallization
The purified 3 and 6 mg/ml of Peptide 10-1–FTH1-1 fusion protein was screened for crystallization conditions by vapor-diffusion sitting-drop method at 16 °C, including the Index, Crystal Screen, PEG/Ion, Salt RX from Hampton Research and Wizard IIV from Emerald BioSystems. The complex crystals of Peptide 10-1/FTH1-1 appeared after 2 days under the condition of 1.5 M sodium chloride, 10% ethanol in the mother liquor. Subsequently, the conditions that yield crystals were optimized. The high-quality crystals of Peptide 10-1/FTH1-1 complex protein were obtained in an optimized condition consisting of 1.5 M sodium chloride, 15% ethanol and 20% PEG 3350 with a protein concentration of 5 mg/ml. All crystals were dehydrated and cryo-protected in 30% glycerol solution and cooled in a dry nitrogen stream at 100 K for X-ray data collection.
X-ray data collection, processing, and structure determination
Diffraction data were collected at the Shanghai Synchrotron Radiation Facility (SSRF) BL10U2 (wavelength, 0.97918 Å) at 100 K. All data sets were processed using the HKL2000 package75 for indexing, integration, and scaling. The initial phase for the Peptide 10-1/FTH1-1 complex structure was solved by molecular replacement using PHENIX74 with the NCOA4/FTH1 structure (PDB ID: 8WIQ). The initial model was fitted into the modified experimental electron density using COOT73 and further refined in PHENIX74. Model geometry was verified using the program MolProbity76. Structural figures were drawn using the program PyMOL (http://www.pymol.org). The final processing data and structure refinement statistics are summarized in Supplementary Table 2. All structural figures were generated using PyMOL (http://www.pymol.org) and UCSF ChimeraX (https://www.cgl.ucsf.edu/chimerax/).
Dynamic light scattering (DLS)
The highly purified and monodisperse proteins (FTH1, FTH1-1, NCOA4-FTH1, Peptide 10–FTH1, Peptide 10-1–FTH1-1, GDIII, GDIII-Ferritin, GDIII-Fc, and G) obtained through gel filtration chromatography, were diluted with PBS buffer to concentrations of 300, 300, 320, 320, 320, 250, 350, 250, and 270 μg/ml, respectively, for DLS analysis. The size distribution of the particles was measured using a Zetasizer Ultra instrument (Malvern Panalytical) at 25 °C in a 1 ml solvent-resistant micro cuvette. The intensity of light scattered by the particles was measured using a detector placed at a measurement angle of 173°. The samples were measured four times each, and the homogeneity and monodispersity of the particles were considered acceptable when the polydispersity index (PDI) value was below 0.2.
Surface plasmon resonance (SPR)
For assessment of the binding affinity between peptides and ferritins, purified FTH1 or FTH1-1 protein was covalently immobilized to CM5 sensor chips via amine groups in 10 mM sodium acetate buffer (pH 5.0), serial dilutions of purified GDIII-Peptide WT, GDIII-Peptide 10, and GDIII-Fagy tag were individually injected, ranging in concentration from 125 to 3.9 nM, the dilution factor was 1:2. Which were injected through the four flow cells at a flow rate of 30 µl/min for 180 s, followed by a 170 s dissociation step. After each experimental cycle, the sensor surface was regenerated by injecting 10 mM glycine (pH 2.0) at a flow rate of 30 µl/min for 30 s. Background binding from the reference flow cell was subtracted, and the binding levels were calculated using the Biacore T200 evaluation software (GE Healthcare).
The antigenic efficacy of GDIII, GDIII-Ferritin, GDIII-Fc, and G subunit vaccines were assessed using the Biacore T200 SPR system (Cytiva). All experiments were conducted at 20 °C in HBS-EP+ buffer (20 mM HEPES, 150 mM NaCl, pH 7.5, 0.005% (v/v) surfactant P20). The GDIII-specific neutralizing antibodies Docaravimab, Rafivirumab, and RVC20 were individually diluted to a concentration of 10 µg/ml in 10 mM sodium acetate (pH 5.0) and immobilized onto the active and reference flow cell surfaces of the activated CM5 sensor chip using the amine coupling method. Each antibody captured ~160 RU on the active surface of the chip. These subunit vaccines initial concentrations were, 50 nM for Docaravimab, 50 nM for Rafivirumab, and 50 nM for RVC20, with successive 1:2 dilutions, which were injected through the four flow cells at a flow rate of 30 µl/min for 200 s, followed by a 400 s dissociation step. After each experimental cycle, the sensor surface was regenerated by injecting 10 mM glycine (pH 1.5) at a flow rate of 30 µl/min for 30 s. Background binding from the reference flow cell was subtracted, and the binding levels were calculated using the Biacore T200 evaluation software (GE Healthcare).
Analytical ultra-centrifugation (AUC)
Operating under velocity sedimentation (SV) mode using an AN-50 Ti rotor with two-channel charcoal-filled centerpieces at 4 °C in a Beckman Optima XL-I. Sedimentation velocity was performed at 40,000 rpm loading 1 mg/ml protein. Data were collected at 280 nm in a continuous mode and fitted with SEDFIT.
Thermal stability shift assay
To determine the stability of GDIII-Ferritin nanoparticle vaccine, purified vaccines were made to a final concentration of 1 μg/μl in buffers of varying pH (5.0–11.0), buffers containing denaturing agents (1 M urea, 1 M guanidine-HCl), and buffers with 20% FBS. SYPRO Orange dye (Sigma-Aldrich) was added to the protein to make a final concentration of 5×. The experiments were performed in 96-well plates specific for the qRT-PCR instrument with a total volume of 25 μl/well. The assay plate was covered with a sheet of optically clear adhesive to seal each well. The assay plate was centrifuged at 700 × g for 3 min at 25 °C to remove bubbles. The assay plate was placed into the CFX96™ Real-Time System. The reaction was run from 10 °C, ramping up in increments of 0.5 °C/s to a final temperature of 95 °C with fluorescence detection throughout the experiment to generate a dataset. The melting temperature of the protein (Tm) was determined by performing nonlinear fitting of the dataset to a Boltzmann sigmoidal curve in GraphPad Prism 8.0.2 software.
Enzyme-linked immunosorbent assay (ELISA)
For the screening of binding affinity between ferritin and peptides, each well of an ELISA 96-well assay plate (Corning) was first coated with 100 μl of PBS solution containing ferritin at a concentration of 2 μg/ml. The plates were incubated overnight at 4 °C. Then, they were washed five times with washing buffer composed of PBS and 0.05% (v/v) Tween 20. Subsequently, each well was coated with 250 μl of blocking buffer containing PBS and 5% milk. The plates were incubated with the blocking buffer for 2 h at room temperature, followed by another five washes with the washing buffer. GST-peptides were diluted in blocking buffer starting from a detection concentration of 1 μM and serially diluted by a factor of 2, were added to each well at a volume of 100 μl. Then, the plates were incubated at room temperature for 1 h and subsequently washed five times. Next, the anti-GST tag antibody (Abcam) conjugated with horseradish peroxidase (HRP) was diluted in blocking buffer at a ratio of 1:10,000, and 100 μl of the diluted antibody was added to each well. The plates were incubated with the antibody for 1 h at room temperature and then washed eight times. Finally, 100 μl of tetramethylbenzidine (TMB, Life Sciences) was added to each well, and after 10–15 min, the reaction was stopped with 50 μl of 2 N sulfuric acid. The resulting plate readouts were measured at a wavelength of 450 nm. The ELISA data were analyzed using GraphPad Prism 8.0.2 software to do best fit and calculate EC50 values.
For the determination of serum-binding antibody titers, each well of an ELISA 96-well assay plate (Corning) was initially coated with 100 μl of PBS solution containing the GDIII or G antigens at a concentration of 2 μg/ml. The plates were incubated overnight at 4 °C. Subsequently, they were washed five times with washing buffer composed of PBS and 0.05% (v/v) Tween 20. Afterward, each well was coated with 250 μl of blocking buffer containing PBS and 5% milk. The plates were incubated with the blocking buffer for 2 h at room temperature, followed by another five washes with the washing buffer. Mouse serum, diluted in blocking buffer starting from a 1/100 concentration and serially diluted by a factor of 3, were added to each well at a volume of 100 μl. Then, the plates were incubated at room temperature for 1 h and subsequently washed five times. Next, HRP-conjugated goat anti-mouse IgG secondary antibody (Abcam) was diluted in blocking buffer at a ratio of 1:10,000, and 100 μl of the diluted secondary antibody was added to each well. The plates were incubated with the secondary antibody for 1 h at room temperature and then washed eight times. Finally, 100 μl of TMB was added to each well, and after 6–8 min, the reaction was stopped with 70 μl of 2 N sulfuric acid. The resulting plate readouts were measured at a wavelength of 450 nm. The ELISA data were analyzed using GraphPad Prism 8.0.2 software to do best fit and calculate EC50 values. The quantification of serum cytokines was conducted following the operational procedures provided by the commercial assay kit (mlbio).
Immunization and sample collection
All animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC). Six-week-old female BALB/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., and housed in ventilated cages under environmentally controlled conditions at Nankai University (12-h light/dark cycle, 21;°C, 50% humidity). Female mice were selected due to their docile nature for handling, rather than any gender-specific factors. For the immunogenicity study, the mice were divided into five groups (Control, GDIII, GDIII-Ferritin, GDIII-Fc, and G), each comprising five mice. The prime vaccination at week 0 entailed subcutaneous injection of 50 μg vaccine formulated with Freund’s complete adjuvant (FCA, equal volumes of FCA vaccinated mice were set as the mock group). Subsequent booster immunizations were administered at weeks 3 and 6, involving 50 μg vaccine formulated with Freund’s incomplete adjuvant (FIA, the Control group vaccinated by equal volumes of FIA). Blood samples were collected from the mice’s eye in the 2nd, 5th, 8th, and 11th weeks post-prime immunization. After standing at room temperature for 30 min, the blood samples were centrifuged at 4 °C at 300 × g/min for 10 min to separate serum and blood cells. The serum samples were heat-inactivated at 56 °C for 30 min and utilized to determine the titers of GDIII/G protein antigen-specific IgG-binding antibodies and virus neutralizing antibodies. In the 5th week after the second boost vaccination, all mice underwent euthanasia, blood collection, and isolation of splenic lymphocytes. Subsequently, FCM was employed to analyze the phenotypic characteristics of lymphocytes. And the secretion levels of representative cytokines were quantified both before and after T-cell activation.
Splenocytes isolation and flow cytometry (FCM)
The mouse spleen was collected in RPMI Medium 1640 basic and passed through a 70 μm filter to remove tissue fragments and blood vessels. The spleen PBMC separation reagent kit (Life Sciences) was used for Ficoll gradient centrifugation to isolate PBMCs. Subsequently, the cells were incubated in ACK lysis buffer (Life Sciences) to remove red blood cells, followed by passing through a 40 μm filter to obtain single splenocytes. For surface marker staining, the cells were incubated with fluorochrome-conjugated monoclonal antibodies in PBS containing 1% BSA on ice for 30 min. At first, the nonspecific binding of Fc receptors was blocked using anti-CD16/32 antibody (BD). Then, LIVE/DEAD Fixable Viability Dyes (FVS780, BD) were used to gate live cells. The following indicated antibodies were used: percp-cy5.5 anti-mouse CD45 antibody (BD), APC anti-mouse CD45R/B220 antibody (BD), FITC anti-mouse CD3 antibody (BD), PE anti-mouse CD4 antibody (BD), eFluor 450 anti-mouse CD8 antibody (eBioscience), PE anti-mouse CD138 antibody (BioLegend), FITC anti-mouse CD38 antibody (BD) and Brilliant Violet 421 anti-mouse IgG antibody (BioLegend). After washing cells with PBS containing 1% BSA solution following antibody staining, the samples were acquired and analyzed by a 5-laser LSRFortessa flow cytometer (BD Biosciences, San Jose, CA, USA) with BD FACS Diva 6 software at Nankai University. The data were further processed using FlowJo_v10.8.1_CL software.
T-cell culture, activation, and intracellular cytokine staining (ICS)
The isolated mouse splenic PBMCs were stimulated with 10 μg/ml GDIII antigen and T-cell activation reagent (a mixture of PMA and ionomycin, BD) for 10 h to promote cytokine expression. BFA (BD) was used to prevent cytokine secretion and promote intracellular retention. After incubation, the cells were collected and the nonspecific binding of Fc receptors was blocked using anti-mouse CD16/32 antibody (BD). LIVE/DEAD Fixable Viability Dyes (FVS780, BD) were used to exclude dead cells. FITC anti-mouse CD3 antibody (BD) was used for cell surface staining to isolate the T-cell population. PE anti-mouse CD4 antibody (BD) and eFluor 450 anti-mouse CD8 antibody (eBioscience) were used to isolate specific T-cell subsets. Then, the cells were fixed with formaldehyde and permeabilized with saponin. ICS was performed using percp-cy5.5 anti-IFN-γ antibody (BioLegend) and APC anti-IL-4 antibody (BioLegend). Finally, using the LSRFortessa flow cytometer (BD Biosciences, San Jose, CA, USA) and FlowJo_v10.8.1_CL software for data collection and analysis, respectively.
Virus neutralization test
The determination of rabies virus neutralizing antibodies titer (RVNT) was conducted through the fluorescent antibody virus neutralization test (FAVN) employing the Challenge virus standard strain CVS-11, according to WOAH manual77. Serum samples collected at the 2nd, 5th, and 11th weeks were measured, and results were expressed in international units (IU/ml) following the standard procedure77. Initially, 100 μl of DMEM medium were added to each well, and 50 μl of serum sample as well as standard serum and negative control serum were added to the first well of the cell culture plate. Subsequently, the serum samples underwent a threefold dilution series with four replicates for each dilution, while the 100 TCID50 of virus in 50 µl is added to each serum-filled well. Following dilution, the virus was introduced into the wells and incubated with serum samples at 37 °C for 60 min to facilitate neutralization. Next, 20,000 BHK-21 cells in 50 µl were added to each well, and the culture plate was incubated at 37 °C with 5% CO2 for 48 h. After incubation, the cell culture plate was transferred to a biosafety cabinet, and the wells were rinsed once in PBS, then fixed in 80% acetone at room temperature for 30 min. The fixed cells were stained using FITC anti-RABV nucleoprotein fluorescent antibody (FDI, Fujirebio Diagnostics, Inc., USA). The reading evaluation is qualitative (plus or minus) under a fluorescence microscope and recorded. The neutralizing antibody titer (IU/ml) of the tested serum was calculated by substituting the observed parameters into the Spearman–Kärber formula (WHO, 2023)77.
Mouse immunization and viral challenge
The 60 six-week-old female BALB/c mice were divided into six groups, each comprising 10 female mice (12-h light/dark cycle, 21 °C, 50% humidity). While previous studies have shown that both male and female mice can be infected by RABV, female mice were selected for this study due to their docile nature for handling, rather than any gender-specific factors. These groups were set as follows. Group 1: Mock (0 d and 7 d), mice were immunized by subcutaneous injection with 100 μl emulsion formulated using 50% (v/v) Freund’s adjuvant and 50 μl of PBS buffer. Priming and boost immunization were performed on days 0 and 7. Group 2: GDIII-Ferritin single-dose (30 μg, 0 d), mice were immunized by subcutaneous injection with 100 μl emulsion formulated using 50% (v/v) Freund’s adjuvant and 30 μg of GDIII-Ferritin nanoparticle vaccine. A single immunization was given on day 0. Group 3: GDIII-Ferritin single-dose (60 μg, 0 d), mice were immunized by subcutaneous injection with 100 μl emulsion formulated using 50% (v/v) Freund’s adjuvant and 60 μg of GDIII-Ferritin nanoparticle vaccine. A single immunization was given on day 0. Group 4: commercial inactivated rabies vaccine BRP Single-dose (8.6 mg, 0 d), the 5th batch of the BRP was produced by the European Directorate for the Quality of Medicines (EDQM, Cat. code: R0100000). It consists of freeze-dried rabies vaccine (inactivated) with an assigned titer of 10 IU/vial. BRP powder (86 mg) was reconstituted with 1 ml PBS buffer and 0.1 dose (1 dose = 10 IU) of BRP was subcutaneously injected to mice. A single immunization was given on day 0. Group 5: GDIII-Ferritin two-dose (30 μg/dose, 0 d and 7 d), mice were immunized by subcutaneous injection with 100 μl emulsion formulated using 50% (v/v) Freund’s adjuvant and 30 μg of GDIII-Ferritin nanoparticle vaccine. Priming and boost were performed on days 0 and 7. Group 6: commercial inactivated rabies vaccine BRP two-dose (8.6 mg/dose, 0 d and 7 d). BRP powder (86 mg) was reconstituted with 1 ml PBS buffer and 0.1 dose (1 dose = 10 IU) of BRP was subcutaneously injected to mice. Priming and boost were performed on days 0 and 7. On the 14th day post priming immunization, each test mouse was intracerebrally injected with 50-fold LD50 of the virulent RABV CVS-24 strain as previously described78. All animals were challenged for 21 days and weight change and clinical signs were observed daily, as well as the percent survival was calculated. Blood samples of surviving mice were collected on day 21th post challenge for neutralization antibody titer test. Quantitative and qualitative analyses of viral load in mouse brain tissue were conducted through qRT-PCR77 and direct immunofluorescence77 using half of the brain tissue respectively. The titers of neutralizing antibodies in the serum of surviving mice were determined by FAVN test according to WOAH manual77. The exploratory experiment investigating the optimal timepoint for pathogenic challenge was conducted in accordance with the aforementioned grouping and immune procedures. Blood samples were collected commencing from the 1st week after full-dose immunization, aiming to assess the efficacy of neutralizing antibodies and observe the physiological performance of mouse spleens. The measurements were conducted four times in total, with a weekly interval.
RNA extraction and quantitative real-time reverse transcription PCR (qRT-PCR)
To quantify viral genome copies in brain tissue, RNA was isolated with TRIzol® Reagent (Invitrogen) according to the manufacturer’s instructions, and qRT-PCR was performed using the Agilent-Stratagene Mx3000P Q-PCR System according to a previously described protocol79. Briefly, RNA extracted from 0.2 g brain tissue (half of brain tissue) was transcribed into cDNA in a reaction mixture with a total volume of 30 µl using M-MuLV reverse transcriptase (TAKARA). The qRT-PCR reaction mixture comprised 2 μl of cDNA combined with 12.5 µl of 2 × SYBR Green Mix (TIANGEN, China), 9 µl of treated water, and 0.75 µl of specific primer for the RABV N genomic sequence (the primer sequences as follows, forward: 5′-ATGTAACACCYCTACAATG-3′ and reverse: 5′-GCAGGGTAYTTRTACTCATA-3′). A standard curve was generated from serially diluted plasmids carrying a RABV N gene to calculate RABV N RNA levels in brain tissue.
Direct fluorescent antibody (DFA) test
The WHO recommended gold standard for rabies diagnosis is the DFA test77. This test used a composite sample of half brain tissue, that includes the brain stem and the cerebellum directly impressed onto the slide. All the impression slides are fixed in 80% high-grade cold acetone at −20 °C for 20 min. Then rinsed in PBST for three time and air dried. The slides were incubated with 25% FITC Anti-rabies Monoclonal Globulin contained Evans blue (FDI Fujirebio Diagnostics, Inc., USA) as directed by the manufacturer for 45 min at 37 °C in a humid chamber. Finally, the slides were examined for specific apple-green fluorescence indicating the presence of viral antigen by fluorescence microscope. Brain tissue appears dark red under a fluorescence microscope. Dull green or red/green auto-fluorescent granules should not be counted as positive antigens.
Quantification and statistical analysis
All statistical analyses were conducted using GraphPad Prism 8.0.2 or OriginPro 2023b. Detailed information regarding the methods employed for calculating statistical significance can be found in the figure legends corresponding to each analysis. The number of experimental replicates (including replicate wells) or biological replicates (representing the number of subjects in each group) was documented within the relevant figure legends, “Results”, or “Methods” sections. The determination of statistical significance was indicated in each figure legend.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We would like to thank X.L. at the cryo-EM Center, Shanxi Academy of Advanced Research and Innovation, for her technical support of the cryo-EM data collection, and BL10U2 of Shanghai Synchrotron Radiation Facility (Shanghai) for X-ray crystallography data collection, Changchun Veterinary Research Institute (Changchun, Jilin Province) for neutralization assay and in vivo rabies virus challenge assay. This work was supported by the Natural Science Foundation of Jilin Province (20230101368JC to Y.Z.), the National Natural Science Foundation of China (32471263 to Y.G., 32273098 to Y.Z., 8227130865 to L.Z., 82373072 to Z.N.), the Jilin Provincial Science and Technology Development Planning (20220204136YY to Y.Z.), the Fundamental Research Funds for the Central Universities (030-63233042 to Y.G.), the R&D Program of Guangzhou Laboratory (SRPG22-002 to Z.S., SRPG22-003 to Z.R.), the Natural Science Foundation of Hebei Province (H2022201067 to Z.N.), and the Central Government Guides Local Science and Technology Development Special Project (236Z7744G to Z.N.).
Author contributions
Y.G., Z.R., and L.Z. conceived the project. D.F., P.Y., J.J., and Z.S. performed gene construction, protein expression, and purification. D.F. and W.W. performed the collection of Cryo-EM and X-ray data, as well as structure determination. L.Z., F.Z., and R.Y. performed a structure-based engineering design of peptide adapters. D.F., P.Y., and N.Z. performed the preparation and immunological evaluation of the vaccines. D.F., W.W., and P.Y. performed the in vitro binding assays, flow cytometry analysis, and structural analysis. Y.Z., Y.T., N.Z., and Z.N. performed animal rabies virus challenge experiments. Y.Z. and C.Y. performed authentic fluorescent antibody virus neutralization (FAVN) test, qRT-PCR assay, and direct fluorescent antibody (DFA) test. Y.Z., C.Y., and D.F. performed animal immunization and analyzed the results. D.F., W.W., and Y.Z. performed comprehensive analysis of all data and was responsible for the preparation of figures. Y.G., D.F., W.W., Y.Z., and L.Z. designed the experiments and wrote the manuscript. All authors read and approved the contents of the manuscript.
Peer review
Peer review information
Nature Communications thanks Ling Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Density maps and structure coordinates have been deposited into the Electron Microscopy Data Bank (EMDB) and the Protein Data Bank (PDB) with accession codes EMD-37566 and PDB ID 8WIQ for NCOA4/FTH1 complex, EMD-37578 and PDB ID 8WJF for Peptide 10/FTH1 complex. The atomic model generated from X-ray crystallographic studies of the Peptide 10-1/FTH1-1 complex has been deposited at the Protein Data Bank (PDB, http://www.rcsb.org/) under accession codes PDB ID 8WIE. Source data are provided with this paper.
Competing interests
Y.G., D.F., and P.Y. declare the following competing interests: patent has been filed for the Fagy-tag and self-assembly nanoparticle system presented here. All other authors declare no competing interests.
Ethics statements
The rabies virus challenge experiment has obtained approval by the Animal Ethics Committee of Changchun Veterinary Research Institute, Chinese Academy of Agricultural Sciences (IACUC of AMMS-11-2024-044). The mice immunization experiment has obtained approval by the Animal Ethics Committee of the College of Life Sciences, Nankai University (2024-SYDWLL-000177).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dan Fu, Wenming Wang, Yan Zhang.
Contributor Information
Zihe Rao, Email: raozh@mail.tsinghua.edu.cn.
Lei Zhao, Email: jackyzhao010@126.com.
Yu Guo, Email: guoyu@nankai.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-52908-z.
References
- 1.Munro, H. N. & Linder, M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol. Rev.58, 317–396 (1978). [DOI] [PubMed] [Google Scholar]
- 2.Harrison, P. M. & Arosio, P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta (BBA) Bioenerg.1275, 161–203 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Bhaskar, S. & Lim, S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater.9, e371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chattopadhyay, S., Chen, J. Y., Chen, H. W. & Hu, C. J. Nanoparticle vaccines adopting virus-like features for enhanced immune potentiation. Nanotheranostics1, 244–260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes, A. C., Mohsen, M. & Bachmann M. F. Harnessing nanoparticles for immunomodulation and vaccines. Vaccines5, 6 (2017). [DOI] [PMC free article] [PubMed]
- 6.Pati, R., Shevtsov, M. & Sonawane, A. Nanoparticle vaccines against infectious diseases. Front. Immunol.9, 2224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith, D. M., Simon, J. K. & Baker, J. R. Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol.13, 592–605 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lung, P., Yang, J. & Li, Q. Nanoparticle formulated vaccines: opportunities and challenges. Nanoscale12, 5746–5763 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Kanekiyo, M. et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature499, 102–106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanekiyo, M. et al. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell162, 1090–1100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma, X. et al. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses. Immunity53, 1315–1330.e1319 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He, L. et al. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv.7, eabf1591 (2021). [DOI] [PMC free article] [PubMed]
- 13.Wang, W. et al. Ferritin nanoparticle-based SpyTag/SpyCatcher-enabled click vaccine for tumor immunotherapy. Nanomedicine16, 69–78 (2019). [DOI] [PubMed] [Google Scholar]
- 14.He, L. et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun.7, 12041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houser, K. V. et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nat. Med.28, 383–391 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai, W. et al. Development of a ferritin-based nanoparticle vaccine against the SARS-CoV-2 Omicron variant. Signal Transduct. Target. Ther.7, 173 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders, K. O. et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature594, 553–559 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caldeira, J. C. & Peabody, D. S. Thermal stability of RNA phage virus-like particles displaying foreign peptides. J. Nanobiotechnol.9, 22 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssens, M. E. et al. Folding properties of the hepatitis B core as a carrier protein for vaccination research. Amino Acids38, 1617–1626 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues, M. Q., Alves, P. M. & Roldão, A. Functionalizing ferritin nanoparticles for vaccine development. Pharmaceutics13, 1621 (2021). [DOI] [PMC free article] [PubMed]
- 21.Nguyen, B. & Tolia, N. H. Protein-based antigen presentation platforms for nanoparticle vaccines. NPJ Vaccines6, 70 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, Y. N. et al. Mechanism of a COVID-19 nanoparticle vaccine candidate that elicits a broadly neutralizing antibody response to SARS-CoV-2 variants. Sci. Adv.7, eabj3107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang, Y. F. et al. Rapid development of SARS-CoV-2 spike protein receptor-binding domain self-assembled nanoparticle vaccine candidates. ACS Nano15, 2738–2752 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Daniels, T. R., Delgado, T., Rodriguez, J. A., Helguera, G. & Penichet, M. L. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol.121, 144–158 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Kleven, M. D., Jue, S. & Enns, C. A. Transferrin receptors TfR1 and TfR2 bind transferrin through differing mechanisms. Biochemistry57, 1552–1559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med.133, 46–54 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Cheng, Y., Zak, O., Aisen, P., Harrison, S. C. & Walz, T. Structure of the human transferrin receptor-transferrin complex. Cell116, 565–576 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Montemiglio, L. C. et al. Cryo-EM structure of the human ferritin-transferrin receptor 1 complex. Nat. Commun.10, 1121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chasteen, N. D. & Harrison, P. M. Mineralization in ferritin: an efficient means of iron storage. J. Struct. Biol.126, 182–194 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Asano, T. et al. Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells. Mol. Cell. Biol.31, 2040–2052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancias, J. D., Wang, X., Gygi, S. P., Harper, J. W. & Kimmelman, A. C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature509, 105–109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancias, J. D. et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife4, e10308 (2015). [DOI] [PMC free article] [PubMed]
- 33.Gryzik, M. et al. Expression and characterization of the ferritin binding domain of nuclear receptor coactivator-4 (NCOA4). Biochim. Biophys. Acta Gen. Subj.1861, 2710–2716 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Amarasinghe, G. K. et al. Taxonomy of the order Mononegavirales: update 2018. Arch. Virol.163, 2283–2294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fooks, A. R. et al. Renewed public health threat from emerging lyssaviruses. Viruses13, 1769 (2021). [DOI] [PMC free article] [PubMed]
- 36.Ugolini, G. & Hemachudha, T. Rabies: changing prophylaxis and new insights in pathophysiology. Curr. Opin. Infect. Dis.31, 93–101 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Sriwijitalai, W. & Wiwanitkit, V. Rabies, rabies vaccine, and renal failure: clinical issues. Saudi J. Kidney Dis. Transplant.30, 560–563 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Hemachudha, T. et al. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol.12, 498–513 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Parize, P. et al. Systematic booster after rabies pre-exposure prophylaxis to alleviate rabies antibody monitoring in individuals at risk of occupational exposure. Vaccines9, 309 (2021). [DOI] [PMC free article] [PubMed]
- 40.Bordenave, G. Louis Pasteur (1822-1895). Microbes Infect.5, 553–560 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Kissling, R. E. Growth of rabies virus in non-nervous tissue culture. Proc. Soc. Exp. Biol. Med.98, 223–225 (1958). [DOI] [PubMed] [Google Scholar]
- 42.Kondo, A. Growth characteristics of rabies virus in primary chick embryo cells. Virology27, 199–204 (1965). [DOI] [PubMed] [Google Scholar]
- 43.Wiktor, T. J. et al. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc. Natl. Acad. Sci. USA81, 7194–7198 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, Y. et al. A novel rabies vaccine based-on toll-like receptor 3 (TLR3) agonist PIKA adjuvant exhibiting excellent safety and efficacy in animal studies. Virology489, 165–172 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Wijaya, L. et al. An accelerated rabies vaccine schedule based on toll-like receptor 3 (TLR3) agonist PIKA adjuvant augments rabies virus specific antibody and T cell response in healthy adult volunteers. Vaccine35, 1175–1183 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Liang, M. et al. A single dose of recombinant VSV-RABV(G) vaccine provides full protection against RABV challenge. Virol. Sin.37, 455–458 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faber, M. et al. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc. Natl. Acad. Sci. USA106, 11300–11305 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaur, M., Saxena, A., Rai, A. & Bhatnagar, R. Rabies DNA vaccine encoding lysosome-targeted glycoprotein supplemented with Emulsigen-D confers complete protection in preexposure and postexposure studies in BALB/c mice. FASEB J.24, 173–183 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Garg, R., Kaur, M., Saxena, A., Prasad, R. & Bhatnagar, R. Alum adjuvanted rabies DNA vaccine confers 80% protection against lethal 50 LD(50) rabies challenge virus standard strain. Mol. Immunol.85, 166–173 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Hellgren, F. et al. Unmodified rabies mRNA vaccine elicits high cross-neutralizing antibody titers and diverse B cell memory responses. Nat. Commun.14, 3713 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, D. K. et al. Oral immunization of mice with recombinant rabies vaccine strain (ERAG3G) induces complete protection. Clin. Exp. Vaccine Res.4, 107–113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. Food, Agriculture Organization of the United Nations, World Organisation for Animal Health. Zero By 30: The Global Strategic Plan to End Human Deaths from Dog-mediated Rabies by 2030 (World Health Organization, 2018).
- 53.Roche, S., Rey, F. A., Gaudin, Y. & Bressanelli, S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein G. Science315, 843–848 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Baquero, E. et al. Structure of the low pH conformation of Chandipura virus G reveals important features in the evolution of the vesiculovirus glycoprotein. PLoS Pathog.11, e1004756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kadlec, J., Loureiro, S., Abrescia, N. G. A., Stuart, D. I. & Jones, I. M. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat. Struct. Mol. Biol.15, 1024–1030 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Vollmer, B. et al. The prefusion structure of herpes simplex virus glycoprotein B. Sci. Adv.6, eabc1726 (2020). [DOI] [PMC free article] [PubMed]
- 57.de Melo, G. D., Hellert, J., Gupta, R., Corti, D. & Bourhy, H. Monoclonal antibodies against rabies: current uses in prophylaxis and in therapy. Curr. Opin. Virol.53, 101204 (2022). [DOI] [PubMed] [Google Scholar]
- 58.Hellert, J. et al. Structure of the prefusion-locking broadly neutralizing antibody RVC20 bound to the rabies virus glycoprotein. Nat. Commun.11, 596 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, F. et al. Structural analysis of rabies virus glycoprotein reveals pH-dependent conformational changes and interactions with a neutralizing antibody. Cell Host Microbe27, 441–453.e447 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Albertini, A. A., Baquero, E., Ferlin, A. & Gaudin, Y. Molecular and cellular aspects of rhabdovirus entry. Viruses4, 117–139 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. WHO Expert Consultation on Rabies: Second Report (World Health Organization, 2013). [PubMed]
- 62.Lazear, H. M. et al. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci. Transl. Med.7, 284ra259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donnelly, R. P. & Kotenko, S. V. Interferon-lambda: a new addition to an old family. J. Interferon Cytokine Res.30, 555–564 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenberg, E. S. et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science278, 1447–1450 (1997). [DOI] [PubMed] [Google Scholar]
- 65.Zhu, J., Yamane, H. & Paul, W. E. Differentiation of effector CD4 T cell populations*. Annu. Rev. Immunol.28, 445–489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ertl, H. C. J. New rabies vaccines for use in humans. Vaccines7, 54 (2019). [DOI] [PMC free article] [PubMed]
- 67.Hoelzgen, F. et al. Structural basis for the intracellular regulation of ferritin degradation. Nat. Commun.15, 3802 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zakeri, B. et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA109, E690–E697 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graham, B. S. Rapid COVID-19 vaccine development. Science368, 945–946 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife7, e42166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr.66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr.66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Otwinowski, Z. & Minor, W. [20] Processing of X-ray diffraction data collected in oscillation mode. in Methods in Enzymology (Academic Press, 1997). [DOI] [PubMed]
- 76.Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr.66, 12–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.World Organisation for Animal Health (WOAH). WOAH terrestrial manual 2023. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (ed Health) (World Organisation for Animal Health, 2023).
- 78.Li, J. et al. An mRNA-based rabies vaccine induces strong protective immune responses in mice and dogs. Virol. J.19, 184 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tu, Z. et al. Inhibition of rabies virus by 1,2,3,4,6-penta-O-galloyl-β-d-Glucose involves mTOR-dependent autophagy. Viruses10, 201 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Density maps and structure coordinates have been deposited into the Electron Microscopy Data Bank (EMDB) and the Protein Data Bank (PDB) with accession codes EMD-37566 and PDB ID 8WIQ for NCOA4/FTH1 complex, EMD-37578 and PDB ID 8WJF for Peptide 10/FTH1 complex. The atomic model generated from X-ray crystallographic studies of the Peptide 10-1/FTH1-1 complex has been deposited at the Protein Data Bank (PDB, http://www.rcsb.org/) under accession codes PDB ID 8WIE. Source data are provided with this paper.