Abstract
Intracranial hypertension (IH) is a critical neurological emergency that requires prompt intervention because failure to treat it properly can lead to severe outcomes, including secondary brain injury. Traditionally, mannitol (MNT) has been the cornerstone of hyperosmolar therapy. However, the use of hypertonic saline (HTS) has become increasingly important because of its unique advantages. Both HTS and MNT effectively reduce intracranial pressure by creating an osmotic gradient that draws fluid from brain tissue. However, unlike MNT, HTS does not induce diuresis or significantly lower blood pressure, making it more favorable for maintaining cerebral perfusion. Additionally, HTS does not cause rebound edema and carries a lower risk of renal injury than MNT. However, it is important to note that the use of HTS comes with potential risks, such as hypernatremia, hyperchloremia, and fluid overload. Due to its unique properties, HTS is a crucial agent in the management of IH, and understanding its appropriate use is essential to optimize patient outcomes.
Keywords: Intracranial hypertension/drug therapy; Brain edema; Saline solution, hypertonic; Mannitol
GRAPHICAL ABSTRACT

INTRODUCTION
Despite significant advances in neurocritical care, intracranial hypertension (IH) remains a critical and potentially life-threatening neurological condition. IH was defined as intracranial pressure (ICP) exceeding 22 mmHg sustained for more than 5 minutes. Persistent IH can lead to a reduction in cerebral perfusion pressure (CPP), resulting in secondary brain injury or even death. Osmotic therapy is one of the fundamental approaches for treating IH, with mannitol (MNT) being the most commonly used agent since the 1970s.26) In the 1990s, hypertonic saline (HTS) was introduced,16) and while both agents are currently in use, the global adoption of HTS has increased.1)
Recent pediatric traumatic brain injury (TBI) guidelines29) recommend HTS over MNT. However, there is an ongoing debate on its use in adults, and the level of evidence remains low. Additionally, in Korea, osmotic therapy is predominantly centered on the MNT. Therefore, this review aims to summarize the effects and best usage of HTS, as well as its differences from MNT. Through this analysis, we aim to identify the most suitable osmotic agents for patients with IH in various clinical settings to ensure the best possible outcomes.
HYPEROSMOLAR THERAPY
Typically, brain tissue osmolality is approximately 3 mOsm/L higher than that of the serum. When TBI occurs, the resulting damage and inflammation in brain cells lead to ionic imbalance and increased osmolality. This, in turn, worsens the cerebral edema and contributes to IH development. Administering hyperosmolar agents significantly increases serum osmolality, reversing this osmotic gradient and causing water to move from the brain parenchyma into the blood vessels, thereby reducing cerebral edema and lowering ICP.
IDEAL TARGET OSMOLARITY AND SODIUM CHLORIDE LEVEL
There is no consensus on a clear threshold for hyperosmolar therapy. Generally, the maximum safe osmolarity is recommended to be below 320 mOsm/L, as exceeding this level can impair cardiac, immune, and renal function.11) Serum sodium levels above 155–160 mEq/L define severe hypernatremia, which can lead to potential complications. To avoid renal injury, serum chloride levels should be maintained below 110–115 mEq/L.42)
ICP GUIDED VS. EMPIRICAL USE OF HTS
Research on the efficacy of ICP-guided hyperosmolar therapy in improving patient outcomes has produced inconsistent findings, with ongoing controversies preventing the establishment of Class 1 evidence to support its recommendations. Despite this, ICP monitoring-guided treatment offers several advantages such as ensuring consistent therapeutic strategies, alleviating the burden of individual clinician decision-making, and demonstrating potential improvements in both patient mortality and prognosis. Routine infusion of hyperosmotic therapy (e.g., every 4 or 6 hours) is not recommended without clear evidence of IH. With the evidence of IH without ICP monitoring, scheduled hyperosmolar therapy is reasonable.
MECHANISM OF ACTION OF HTS
HTS refers to solutions with sodium chloride concentrations higher than 0.9% (154 mEq/L of sodium and chloride). Various concentrations of HTS are used clinically, ranging from 1.8% to 30% saline,53) with most typical preparations being of 3%, 5%, 7.5%, 14.6%, and 23.4%. Sodium and chloride are combined in a 1:1 ratio to form sodium chloride, which has a molecular weight of 58.44 g/mol (TABLE 1).36) They are primarily distributed in the 3 major body fluid compartments, the plasma and interstitial fluid, with minimal presence in the intracellular fluid, thus contributing to maintaining blood tonicity.60) The osmotic reflection coefficient (RQ) of sodium chloride is 1.0.50) They are excreted by the kidneys, with most reabsorbed in the proximal tubules (FIGURE 1).
TABLE 1. Comparing osmolarity of osmotic agents.
| Agent | Osmolarity (mOsm/L) | Sodium concentrations (mEq/L) |
|---|---|---|
| Lactate ringers | 275 | 130 |
| 0.9% NaCl | 308 | 154 |
| 1.7% NaCl | 582 | 291 |
| 3.0% NaCl | 1,026 | 513 |
| 7.5% NaCl | 2,566 | 1,283 |
| 10% NaCl | 3,424 | 1,713 |
| 20% NaCl | 6,848 | 3,426 |
| 23.4% NaCl | 8,008 | 4,004 |
| 20% Mannitol | 1,098 | n/a |
n/a: not available.
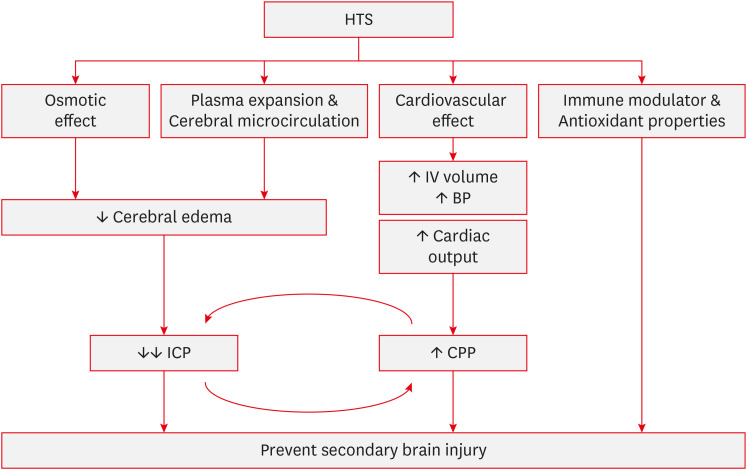
FIGURE 1. Mechanism of actions of hypertonic saline. HTS reduces cerebral edema and decreases ICP through its osmotic effect, as well as plasma expansion and improved cerebral microcirculation. Additionally, the cardiovascular effects of HTS increase intravascular volume and cardiac output, leading to an elevation in CPP. Moreover, HTS possesses immune-modulating and antioxidant properties. Together, these mechanisms work synergistically to contribute to the prevention of secondary brain injury.
HTS: hypertonic saline, IV: Intravenous, BP: blood pressure, ICP: intracranial pressure, CPP: cerebral perfusion pressure.
Osmotic effect
Cerebral edema is a response to various forms of brain injury and is defined as an increase in brain water content within either the brain cells or the extracellular space.36,45) In healthy individuals, the major cations (sodium and potassium), plasma glucose, and blood urea nitrogen determine serum osmolarity. As urea easily diffuses across the cell membrane, serum sodium is the primary molecule influencing serum osmolarity.45) The main mechanism of HTS is the osmotic shift of the fluid. After HTS, an osmolar gradient is established, causing a shift in cerebral water from the interstitial and intracellular spaces to the vascular system.15) The osmotic RQ of the endothelial membrane is 0.1, whereas that of the cell membrane, maintained by sodium/potassium ATPase, is 1.0. Consequently, most water movement occurs within the intracellular component.39,47,53) Furthermore, Sodium chloride has an RQ of 1.0, indicating that it is almost entirely excluded from crossing the intact blood-brain barrier (BBB).15) Conversely, if the BBB is disrupted, a sufficient osmotic shift may not occur due to leakage of osmotic substances into the brain tissue. Wisner et al. 59) reported in 1990 that brain water content was not reduced in the injured hemisphere.33)
Plasma expansion and cerebral microcirculation
During World War I, HTS has gained attention as a resuscitative fluid. Later, in the 1980s, researchers demonstrated successful resuscitation using 7.5% HTS in a patient with hemorrhagic shock who did not respond to standard fluids or dopamine.12) This finding highlights the fact that HTS can achieve effective plasma volume expansion with a relatively small volume. Supporting this, a 2005 study by Rocha-e-Silva and Poli de Figueiredo47) showed that the administration of 7.5% HTS did not lead to the expected increase in plasma sodium concentration, suggesting that the effect of HTS involves more than a simple sodium increase. Mazzoni et al. 38) illustrated a model of plasma volume increase through redistribution into extravascular compartments, including shifts from red blood cells to endothelial cells and the interstitium, eventually affecting tissue cells. These shifts lead to 3 significant physiological changes: reduced red blood cell diameter, increased endothelial lumen size, and hemodilution, all of which contribute to improved cerebral blood flow (CBF) and have a direct relaxant effect on the vascular smooth muscle.47) This vasodilatory effect suggests a potential therapeutic use in treating vasospasm in patients with hyponatremic subarachnoid hemorrhage patients, and some studies have explored this possibility.54)
Although the osmotic effect is the primary mechanism by which HTS lowers ICP, it is important to note that it takes at least 20–30 minutes for an osmotic gradient to form and cause dehydration in uninjured brain tissue. Therefore, the immediate reduction in ICP observed after HTS administration cannot be fully explained by the osmotic effects alone. This rapid reduction is likely due to the rapid expansion of the plasma volume.
In patients with intact cerebral autoregulation, this rapid plasma expansion leads to an autoregulatory reduction in intracerebral blood volume (venoconstriction and reduced venous blood),53) resulting in an immediate decrease in ICP. This process gradually diminishes, allowing sustained control of ICP through the osmotic gradient that forms later.36)
Cardiovascular effects
HTS facilitates the mobilization of fluid from the intracellular space to the extracellular space, thereby increasing the effective circulatory volume and suppressing renin activity.37) Consequently, there is an increase in the cardiac preload, whereas systemic vasodilation leads to a reduction in afterload. These effects highlight the cardiovascular implications of HTS, particularly for the management and resuscitation of patients with cardiac conditions.43) HTS has been shown to directly enhance the cardiac performance, including the cardiac output, by mitigating myocyte edema.55) Additionally, in patients with sepsis, HTS contributes to the restoration of cellular calcium transmembrane potential, thereby preserving cardiac contractile function.58) Moreover, in cases of decompensated heart failure refractory to diuretics, where sodium excretion and body water plateau due to prolonged diuretic use, the co-administration of HTS and loop diuretics has demonstrated improvements in weight reduction, promotion of diuresis, and preservation of renal function.10,22)
Immune modulator and antioxidant properties
The immunomodulatory effects of HTS extend across the inflammatory cascade. HTS primarily influences the function of neutrophils and lymphocytes and can shift macrophages from a proinflammatory to an anti-inflammatory state.46) HTS is involved in the regulation of adhesion molecule expression and cytokine production.
Polymorphonuclear (PMN) cells are rapidly recruited to inflammatory sites within minutes of the onset of inflammation. This activation of PMNs can exacerbate inflammation in cases of organ injury, leading to unnecessary tissue damage. For instance, in patients with TBI, it contributes to secondary brain injury.51) HTS inhibits PMN cell activation by activating the cAMP-mediated pathway, which is associated with the suppression of cell activation processes. Furthermore, HTS modulates the immune response by regulating the TLR-4 signaling pathway.27,44) Although HTS inhibits the activation of PMN cells and production of cytokines, it remains unclear whether this effect significantly increases the risk of infection in clinical settings.8)
Lymphocytes, including B and T cells, play crucial roles in the immune system. T cell function is often suppressed because of impaired cellular immune defense. Experimental evidence suggests that HTS reduces lymphocyte apoptosis, thereby downregulating inflammation and enhancing immune responses.35,46) This effect is potentially mediated by increased IL-2 expression facilitated by the release of cellular ATP, which activates T cells. ATP release is associated with pannexin-1 channels and CBX-sensitive gap junctions that are critical for T cell function.44)
Laboratory evidence suggests that HTS attenuates inflammatory processes through its antioxidative effects. As mentioned earlier, inhibition of neutrophil activation modulates the release of adhesion molecules, reactive oxygen species, endothelin, and eicosanoids, thereby regulating oxidative stress and influencing vasomotor tone.4)
COMPARISON WITH MNT
Mechanism of action of MNT
MNT, a hyperosmolar agent traditionally used in clinical settings, is derived from mannose sugars. It predominantly resides in the extracellular fluid and exhibits an osmotic RQ of 0.9, thereby limiting its permeability across the BBB. This restricted permeability facilitates the creation of an osmotic gradient that promotes the mobilization of water from the cerebral tissues into the vascular compartment, thereby reducing cerebral edema.
Upon administration, the MNT were filtered through the glomeruli in the kidneys. However, it is neither reabsorbed nor secreted along urinary tubules, leading to osmotic diuresis. Consequently, approximately 80% of MNT is excreted in the urine.
MNT exerts its therapeutic effects primarily through 3 mechanisms: osmotic gradient effect, reduced blood viscosity, and cerebral vasoconstriction.28) Given that MNT has an osmolarity of 1,098 mOsm/L and a RQ of 0.9, its administration significantly increases the serum osmolarity. This increase effectively creates an osmotic gradient, which in turn causes fluid redistribution from the brain and alleviates perilesional edema.14) Second, MNT administration leads to an immediate reduction in hematocrit and mean corpuscular volume, enhancing red blood cell deformability.5) Consequently, CBF within the cerebral microvasculature improves. Lastly, if autoregulation is intact, cerebral arteries undergo vasoconstriction, which contributes to a reduction in ICP.52)
Efficacy and safety comparison
MNT is commonly used in clinical practice to manage ICP; however, it has drawbacks including the potential for blood pressure reduction, decreased cardiac output and blood volume, and the risk of renal failure. HTS has recently garnered significant attention as a potential alternative to MNT. As outlined in the mechanism of action section above, HTS is advantageous for controlling ICP as it increases the cardiac output, does not cause a reduction in blood pressure, and lacks diuretic effects, thereby maintaining blood volume, which supports CPP and CBF. Additionally, it has been associated with improved brain-tissue oxygenation in animal studies52) and exerts immunomodulatory effects.
However, according to current randomized clinical trials and meta-analyses, the clinical benefits of HTS compared with other hyperosmolar agents are still not clearly established. The 2016 Brain Trauma Foundation guidelines state that there is “insufficient evidence available from comparative studies to support formal recommendations.”6) The 2019 Cochrane review also reported weak evidence suggesting that HTS has little to no significant impact on long-term neurological outcomes compared with MNT.7) The Continuous Hyperosmolar Therapy for Traumatic Brain-Injured Patients (COBI) trial, published in 2021, also reported that, in a study involving 370 patients with moderate-to-severe TBI, there was no evidence that HTS infusion had an impact on long-term neurological outcomes compared to standard care.48) In a 2022 meta-analysis conducted by Gharizadeh et al. 19) on HTS for TBI, while HTS significantly reduced ICP and was advantageous in preventing secondary brain injury, it did not show differences in neurological outcomes, mortality rates, or duration of ICU and hospital stays. In a 2024 meta-analysis presented by Bernhardt et al. 2) at the Neurocritical Care Society, HTS did not show significant differences in long-term neurological outcomes (measured using the Glasgow Outcome Scale), all-cause mortality, uncontrolled ICP, or length of hospital or ICU stay. However, given that the evidence from underlying randomized clinical trials is of low or exceptionally low certainty, it remains challenging to elucidate clinically meaningful differences. Additionally, HTS is associated with an increased risk of severe hypernatremia, which is likely due to the continuous administration of high concentrations of HTS for at least 48 hours.2,48) Pulmonary edema and rebound phenomena are potential complications associated with HTS; however, they were not reported in any of the trials included in this meta-analysis. (TABLE 2).9,17)
TABLE 2. Comparison between HTS and MNT.
| Variables | Hypertonic saline | Mannitol |
|---|---|---|
| Primary mechanism | ↑ gradient across BBB | ↑ gradient across BBB |
| Immediate reduction ICP | Rapid reduction of ICP | |
| Duration of effect: 4–5 hours | Duration of effect: 4–6 hours | |
| Secondary mechanism | Mixed immunomodulatory and anti-inflammatory effects | Rheological effect |
| Hemodynamic effect | ↑ IV volume | Transient ↑ IV volume |
| ↑ MAP | Osmotic diuresis → Hypovolemia & hypotension | |
| Reflection coefficient | 1.0 = completely impermeable | 0.9 = mostly impermeable |
| Adverse effects | Fluid overload → Pulmonary edema | AKI |
| Hyperchloremic metabolic acidosis | Dehydration | |
| Hyperoncotic hemolysis | Hypotension | |
| Pontine myelinolysis, less likely | Electrolyte imbalance | |
| Phlebitis | Rebound IICP/reverse osmotic shift |
HTS: hypertonic saline, MNT: mannitol, BBB: blood-brain barrier, ICP: intracranial pressure, IV: intravenous, MAP: mean arterial pressure, AKI: acute kidney injury, IICP: increased intracranial pressure.
Clinical situations where on may be preferred over the other
In the context of elevated ICP management, where timely intervention is critical for mitigating secondary brain injury, the selection of a therapeutic agent should be based on the available route of administration and a formulation that allows for the most rapid delivery of the therapeutic agent. However, considering the distinct characteristics of HTS and MNT, the choice of agent should be tailored to the patient's specific clinical situation.
For long-term use
Rebound IH is a significant adverse effect associated with MNT administration and requires careful consideration. MNT's RQ is 0.9, indicating that approximately 10% of the administered MNT could leak into the brain parenchyma, leading to its accumulation. This process ultimately establishes a reverse osmotic gradient, thereby exacerbating brain edema.30) This accumulation is particularly pronounced when the BBB is disrupted, impeding the establishment of an effective osmotic gradient.41,59) Although this rebound phenomenon can be mitigated by close monitoring of the osmolar gap, prolonged MNT administration (beyond 2–3 days) should be avoided.36) In contrast, HTS has an RQ of 1.0, allowing complete exclusion from the brain parenchyma when the BBB remains intact. Thus, HTS may be a more advantageous choice than MNT for patients requiring extended hyperosmolar therapy. However, when MNT is tapered off slowly, the incidence of rebound effects is rare and should be factored into the therapeutic decision-making process.56)
Hypovolemic and hypotensive status
MNT exert an osmotic diuretic effect that can reduce the effective circulating volume.56) This reduction in volume may precipitate hypotension, subsequently decreasing the CPP and increasing the risk of secondary brain injury. Administering MNT at a rate not exceeding 0.1 g/kg/min over a period of 15 to 30 minutes minimizes the risk of hypotension, although caution is advised in patients with hypovolemic status.28)
Since de Felippe et al. 12) first demonstrated the hemodynamic benefits of administering 50 mL of 7.5% HTS to patients with refractory shock, over 60 clinical trials have been conducted to investigate its use in scenarios such as cardiogenic shock, hemorrhagic shock, septic shock, and volume-expanding solutions during major surgery.31,47) These studies have shown that HTS not only facilitates plasma volume expansion but also rapidly restores mean arterial pressure (MAP) and increases the cardiac output. Consequently, HTS may be advantageous in maintaining MAP and cerebral CPP in patients with TBI, where volume loss due to polytrauma is common, thereby preventing secondary brain injury. While there were initial concerns that the rapid volume expansion induced by HTS could lead to coagulopathy in patients with significant blood loss, such as those with trauma, extensive laboratory investigations of posttraumatic hypotension have shown no alterations in coagulation profiles.57) Furthermore, HTS has not been associated with increased blood loss or the need for additional blood products, even in cardiovascular surgery where hemostatic alterations are common.13)
Hypervolemic status
Volume expansion is a recognized effect of HTS administration. For example, 7.5% HTS infusion can increase the intravascular volume by up to 4 times the infused volume within minutes. This rapid expansion may carry risks, including acute pulmonary edema and decompensated heart failure, although these risks remain a subject of debate. Therefore, MNT may be preferred for patients with hypervolemia because of its osmotic diuretic properties, which facilitate fluid reduction and mitigate the risk of pulmonary edema.
Risk of renal injury
With an aging population and rising prevalence of chronic kidney disease (CKD), renal complications have become critical considerations in hyperosmolar treatment.40) MNT is known for its potential to induce acute kidney injury (AKI), which is a risk that must always be considered. The incidence of MNT-induced AKI is estimated to be around 6%–12%.21,34) While this condition is typically transient and reversible upon discontinuation of the drug, it is primarily caused by hypovolemia due to excessive osmotic diuresis and constriction of the afferent arterioles. The risk of renal injury increases with high doses of MNT or hypovolemic.28) Therefore, it is crucial to monitor the osmolar gap closely during MNT administration. When the osmolar gap is 20–55 mOsm/L, MNT should be used cautiously to avoid renal damage, and dosing is not recommended when the osmolar gap exceeds 55 mOsm/L.
Although HTS is considered to carry a lower risk of renal injury than MNT, it is not completely safe. This risk is primarily associated with excessive chloride, which can reduce the vascular tone of renal afferent arterioles and decrease glomerular filtration rate, leading to potential renal function impairment.23,25,32)
Thus, HTS should be preferred in patients with a considerable risk of renal injury, such as those who are elderly, have CKD, or are hypovolemic. However, caution is necessary when HTS is used in cases of hyperchloremia.
Hypernatremia
Froelich et al. 18) compared normal saline with a continuous infusion of 3% HTS and reported that severe hypernatremia (serum sodium >160 mmol/L) occurred in 5% of the patients receiving normal saline vs. 33.6% of those receiving HTS. Similarly, a 2024 meta-analysis by Bernhardt et al. 2) found that HTS administration significantly increased the risk of adverse hypernatremia across 4 trials (relative risk, 2.13; 95% confidence interval, 1.09–4.17; p=0.03; I2=0%; 2 randomized controlled trials [RCTs], 386 participants). This analysis included a COBI RCT that used an unusually high concentration of 20% HTS continuous infusion, a factor that should be considered when interpreting the results.48)
MNT can have various effects on serum sodium concentration. Immediately following MNT administration, water rapidly increases within blood vessels owing to the osmotic gradient, potentially causing transient hyponatremia. However, without adequate volume resuscitation, continued administration can lead to hypernatremia owing to osmotic diuresis and free water loss.20)
Considering these findings, the MNT may be more favorable for patients with hypernatremia. Nonetheless, appropriate volume of resuscitation remains crucial for the management and prevention of hypernatremia during MNT administration.
Hyperglycemia
Continuous administration of MNT can lead to sustained osmotic diuresis, which may exacerbate dehydration and worsen hyperglycemia and the hyperosmolar status.28) Therefore, in elderly patients with diabetes or those with restricted fluid intake, HTS should be considered as the preferred option for hyperosmolar treatment.
ADMINISTRATION OF HTS
The choice of HTS concentration depends on the clinical scenario, with 3% being commonly used for routine management, and higher concentrations, such as 23.4%, for rapid ICP reduction. In Korea, 23.4% were not available, and the highest concentration used was 11.7%. For ICP control, the typical 23.4% dose of 30 mL (0.5 mL/kg) was equivalent to 60 mL (1 mL/kg) of 11.7%. When administering 11.7% saline, it is important to infuse it for several minutes to prevent the paradoxical lowering of blood pressure. For 3% saline, the recommended dose is 200–500 mL or 4 mL/kg infused over 15–30 minutes. If HTS does not achieve the desired ICP reduction, a combination of HTS and MNT can be used sequentially or concurrently for optimal ICP control. However, the efficacy and safety of these combination therapies are not well established.
Central venous infusion is generally recommended because of the risk of phlebitis; however, peripheral infusion may be considered during emergencies.24)
OTHER HYPERTONIC SOLUTIONS
A major drawback of HTS is hyperchloremia, prompting the exploration of alternative agents in studies, such as the ACETatE trial. This trial compared 23.4% NaCl (30 mL) with 16.4% NaCl/Na-acetate (50 mL) and found that both were equally effective in reducing ICP, the acetate group had a lower chloride load and reduced AKI rates.49) In Korea, where NaCl/Na-acetate is not available, a mixture of 11.7% NaCl (40 mL) and 20 mEq Na-acetate (60 mL) is used. Sodium bicarbonate is a practical alternative because of the rarity of sodium acetate. The osmolarity of 8.4% sodium bicarbonate is approximately 2,000 mOsm/L, which is equivalent to that of HTS, with a suitable dose being 80–100 mL. A study by Bourdeaux and Brown3) found that 85 mL of 8.4% sodium bicarbonate was as effective in reducing ICP after TBI when infused over 30 minutes (TABLE 3).
TABLE 3. Various hyperosmolar agents.
| Number | Osmotic agents | Dosage and administration | Consideration of discontinuation | Remarks |
|---|---|---|---|---|
| 1 | Hypertonic saline | 11.7% NaCl 3 ample (pediatric: 1 mL/kg, max 60 mL) | Serum Na >155 mEq/L | Preferred osmotic agents for lowering ICP, maintaining CPP (in emergency situations, administer via a peripheral line.) |
| 3% NaCl 200 mL (pediatric: 4 mL/kg, max 200 mL) | Serum Cl >109 mEq/L | |||
| Over 20 minutes via central line | Pulmonary edema | |||
| Hypervolemia | ||||
| 2 | Mannitol | >0.5 g/kg (if possible, 0.75–1 g IVS) | GFR <50 mEq/L | When serum Na is 155–159 mEq/L, consider MNT as a priority |
| Hypovolemia | Osmotic Gap = (Na × 2 + BUN/2.8 + Glucose/18) − Serum Osmolarity | |||
| Hypotension | ||||
| Osmotic gap >55 mOsm | ||||
| 3 | Sodium acetate | Na acetate 2.72 g (20 mEq) 60 mL + 11.7 NaCl 40 mL | Serum Na >155 mEq/L | Consider when, serum Na <155 mEq/L, serum Cl >109 mEq/L |
| Over 20 minutes via central line | ||||
| 4 | Sodium bicarbonate | 8.4% 80–100 mL over 20 minutes via central line | Serum Na >155 mEq/L | Consider when pH <7.2, serum Na <155 mEq/L and serum Cl >109 mEq/L |
ICP: intracranial pressure, CPP: cerebral perfusion pressure, IVS: interventricular septum, GFR: glomerular filtration rate, MNT: mannitol, BUN: blood urea nitrogen.
CONCLUSION
Hyperosmolar therapy plays a critical role in the management of IH, with HTS and MNT being the key agents. HTS offers not only superior ICP reduction but also helps in volume expansion and increases cardiac output, which are advantageous for maintaining CPP, thus playing a vital role in preventing secondary brain injury. Therefore, understanding the specific advantages of HTS and its appropriate use alongside MNT is essential for optimizing patient outcomes in IH treatment.
Footnotes
Funding: No funding was obtained for this study.
Conflict of Interest: The authors have no financial conflicts of interest.
Informed Consent :This type of study does not require informed consent.
Ethics Approval :This research did not require ethical approval as it does not involve human subjects, their data, or biological samples.
References
- 1.Bennett TD, Statler KD, Korgenski EK, Bratton SL. Osmolar therapy in pediatric traumatic brain injury. Crit Care Med. 2012;40:208–215. doi: 10.1097/CCM.0b013e31822e9d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernhardt K, McClune W, Rowland MJ, Shah A. Hypertonic saline versus other intracranial-pressure-lowering agents for patients with acute traumatic brain injury: a systematic review and meta-analysis. Neurocrit Care. 2024;40:769–784. doi: 10.1007/s12028-023-01771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourdeaux CP, Brown JM. Randomized controlled trial comparing the effect of 8.4% sodium bicarbonate and 5% sodium chloride on raised intracranial pressure after traumatic brain injury. Neurocrit Care. 2011;15:42–45. doi: 10.1007/s12028-011-9512-0. [DOI] [PubMed] [Google Scholar]
- 4.Bulger EM, Hoyt DB. Hypertonic resuscitation after severe injury: is it of benefit? Adv Surg. 2012;46:73–85. doi: 10.1016/j.yasu.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Burke AM, Quest DO, Chien S, Cerri C. The effects of mannitol on blood viscosity. J Neurosurg. 1981;55:550–553. doi: 10.3171/jns.1981.55.4.0550. [DOI] [PubMed] [Google Scholar]
- 6.Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury. Neurosurgery. 2017;80:6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Song Z, Dennis JA. Hypertonic saline versus other intracranial pressure-lowering agents for people with acute traumatic brain injury. Cochrane Database Syst Rev. 2020;1:CD010904. doi: 10.1002/14651858.CD010904.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Bao Y, Zhang J, Woehrle T, Sumi Y, Ledderose S, et al. Inhibition of neutrophils by hypertonic saline involves pannexin-1, CD39, CD73, and other ectonucleotidases. Shock. 2015;44:221–227. doi: 10.1097/SHK.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottenceau V, Masson F, Mahamid E, Petit L, Shik V, Sztark F, et al. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma. 2011;28:2003–2012. doi: 10.1089/neu.2011.1929. [DOI] [PubMed] [Google Scholar]
- 10.Covic A, Copur S, Tapoi L, Afsar B, Ureche C, Siriopol D, et al. Efficiency of hypertonic saline in the management of decompensated heart failure: a systematic review and meta-analysis of clinical studies. Am J Cardiovasc Drugs. 2021;21:331–347. doi: 10.1007/s40256-020-00453-7. [DOI] [PubMed] [Google Scholar]
- 11.Dabrowski W, Siwicka-Gieroba D, Robba C, Bielacz M, Sołek-Pastuszka J, Kotfis K, et al. Potentially detrimental effects of hyperosmolality in patients treated for traumatic brain injury. J Clin Med. 2021;10:4141. doi: 10.3390/jcm10184141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Felippe J, Jr, Timoner J, Velasco IT, Lopes OU, Rocha-e-Silva M., Jr Treatment of refractory hypovolaemic shock by 7.5% sodium chloride injections. Lancet. 1980;316:1002–1004. doi: 10.1016/s0140-6736(80)92157-1. [DOI] [PubMed] [Google Scholar]
- 13.de Figueiredo LF, Coselli JS. Individual strategies of hemostasis for thoracic aortic surgery. J Card Surg. 1997;12:222–228. [PubMed] [Google Scholar]
- 14.Donato T, Shapira Y, Artru A, Powers K. Effect of mannitol on cerebrospinal fluid dynamics and brain tissue edema. Anesth Analg. 1994;78:58–66. doi: 10.1213/00000539-199401000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Favre JB, Ravussin P, Chiolero R, Bissonnette B. Hypertonic solutions and intracranial pressure. Schweiz Med Wochenschr. 1996;126:1635–1643. [PubMed] [Google Scholar]
- 16.Fisher B, Thomas D, Peterson B. Hypertonic saline lowers raised intracranial pressure in children after head trauma. J Neurosurg Anesthesiol. 1992;4:4–10. doi: 10.1097/00008506-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36:795–800. doi: 10.1097/CCM.0B013E3181643B41. [DOI] [PubMed] [Google Scholar]
- 18.Froelich M, Ni Q, Wess C, Ougorets I, Härtl R. Continuous hypertonic saline therapy and the occurrence of complications in neurocritically ill patients. Crit Care Med. 2009;37:1433–1441. doi: 10.1097/CCM.0b013e31819c1933. [DOI] [PubMed] [Google Scholar]
- 19.Gharizadeh N, Ghojazadeh M, Naseri A, Dolati S, Tarighat F, Soleimanpour H. Hypertonic saline for traumatic brain injury: a systematic review and meta-analysis. Eur J Med Res. 2022;27:254. doi: 10.1186/s40001-022-00897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gipstein RM, Boyle JD. Hypernatremia complicating prolonged mannitol diuresis. N Engl J Med. 1965;272:1116–1117. doi: 10.1056/NEJM196505272722109. [DOI] [PubMed] [Google Scholar]
- 21.Gondim FA, Aiyagari V, Shackleford A, Diringer MN. Osmolality not predictive of mannitol-induced acute renal insufficiency. J Neurosurg. 2005;103:444–447. doi: 10.3171/jns.2005.103.3.0444. [DOI] [PubMed] [Google Scholar]
- 22.Griffin M, Soufer A, Goljo E, Colna M, Rao VS, Jeon S, et al. Real world use of hypertonic saline in refractory acute decompensated heart failure: a us center’s experience. JACC Heart Fail. 2020;8:199–208. doi: 10.1016/j.jchf.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen PB, Jensen BL, Skøtt O. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension. 1998;32:1066–1070. doi: 10.1161/01.hyp.32.6.1066. [DOI] [PubMed] [Google Scholar]
- 24.Holden DN, Mucksavage JJ, Cokley JA, Kim KS, Tucker NL, Esordi MS, et al. Hypertonic saline use in neurocritical care for treating cerebral edema: a review of optimal formulation, dosing, safety, administration and storage. Am J Health Syst Pharm. 2023;80:331–342. doi: 10.1093/ajhp/zxac368. [DOI] [PubMed] [Google Scholar]
- 25.Imig JD, Passmore JC, Anderson GL, Jimenez AE. Chloride alters renal blood flow autoregulation in deoxycorticosterone-treated rats. J Lab Clin Med. 1993;121:608–613. [PubMed] [Google Scholar]
- 26.James HE, Langfitt TW, Kumar VS, Ghostine SY. Treatment of intracranial hypertension. Analysis of 105 consecutive, continuous recordings of intracranial pressure. Acta Neurochir (Wien) 1977;36:189–200. doi: 10.1007/BF01405391. [DOI] [PubMed] [Google Scholar]
- 27.Junger WG, Rhind SG, Rizoli SB, Cuschieri J, Shiu MY, Baker AJ, et al. Resuscitation of traumatic hemorrhagic shock patients with hypertonic saline-without dextran-inhibits neutrophil and endothelial cell activation. Shock. 2012;38:341–350. doi: 10.1097/SHK.0b013e3182635aca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Jeong H, Choo YH, Kim M, Ha EJ, Oh J, et al. Optimizing mannitol use in managing increased intracranial pressure: a comprehensive review of recent research and clinical experiences. Korean J Neurotrauma. 2023;19:162–176. doi: 10.13004/kjnt.2023.19.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR, et al. Guidelines for the management of pediatric severe traumatic brain injury: update of the brain trauma foundation guidelines. Pediatr Crit Care Med. 2019;20:S1–SS82. doi: 10.1097/PCC.0000000000001735. [DOI] [PubMed] [Google Scholar]
- 30.Koenig MA. Cerebral edema and elevated intracranial pressure. Continuum (Minneap Minn) 2018;24:1588–1602. doi: 10.1212/CON.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 31.Kramer GC, Elgjo GI, de Figueiredo LF, Wade CE. 7 Hyperosmotic-hyperoncotic solutions. Baillieres Clin Anaesthesiol. 1997;11:143–161. [Google Scholar]
- 32.Laragh JH, Bühler FR, Seldin DW. Frontiers in hypertension research. Berlin: Springer Science & Business Media; 2012. [Google Scholar]
- 33.Levine JM. Hypertonic saline for the treatment of intracranial hypertension: worth its salt. Crit Care Med. 2006;34:3037–3039. doi: 10.1097/01.CCM.0000248527.85721.16. [DOI] [PubMed] [Google Scholar]
- 34.Lin SY, Tang SC, Tsai LK, Yeh SJ, Shen LJ, Wu FL, et al. Incidence and risk factors for acute kidney injury following mannitol infusion in patients with acute stroke: a retrospective cohort study. Medicine (Baltimore) 2015;94:e2032. doi: 10.1097/MD.0000000000002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu YQ, Huang WD, Cai XJ, Gu LH, Mou HZ. Hypertonic saline resuscitation reduces apoptosis of intestinal mucosa in a rat model of hemorrhagic shock. J Zhejiang Univ Sci B. 2008;9:879–884. doi: 10.1631/jzus.B0820116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May CC. Treatment of elevated intracranial pressure. Brain. 2020;32:647–666. [Google Scholar]
- 37.Mazón-Ruiz J, Romero-González G, Sánchez E, Banegas-Deras EJ, Salgado-Barquinero M, la Varga LG, et al. Hypertonic saline and heart failure: “sodium-centric” or “chlorine-centric”? Nefrologia (Engl Ed) 2024;44:338–343. doi: 10.1016/j.nefroe.2024.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Mazzoni MC, Borgström P, Arfors KE, Intaglietta M. Dynamic fluid redistribution in hyperosmotic resuscitation of hypovolemic hemorrhage. Am J Physiol. 1988;255:H629–H637. doi: 10.1152/ajpheart.1988.255.3.H629. [DOI] [PubMed] [Google Scholar]
- 39.Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004;39:464–470. doi: 10.1002/hep.20056. [DOI] [PubMed] [Google Scholar]
- 40.Nitta K, Okada K, Yanai M, Takahashi S. Aging and chronic kidney disease. Kidney Blood Press Res. 2013;38:109–120. doi: 10.1159/000355760. [DOI] [PubMed] [Google Scholar]
- 41.Palma L, Bruni G, Fiaschi AI, Mariottini A. Passage of mannitol into the brain around gliomas: a potential cause of rebound phenomenon. A study on 21 patients. J Neurosurg Sci. 2006;50:63–66. [PubMed] [Google Scholar]
- 42.Pfortmueller CA, Uehlinger D, von Haehling S, Schefold JC. Serum chloride levels in critical illness-the hidden story. Intensive Care Med Exp. 2018;6:10. doi: 10.1186/s40635-018-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfortmueller CA, Kindler M, Schenk N, Messmer AS, Hess B, Jakob L, et al. Hypertonic saline for fluid resuscitation in ICU patients post-cardiac surgery (HERACLES): a double-blind randomized controlled clinical trial. Intensive Care Med. 2020;46:1683–1695. doi: 10.1007/s00134-020-06132-0. [DOI] [PubMed] [Google Scholar]
- 44.Quiñones-Ossa GA, Shrivastava A, Perdomo WAF, Moscote-Salazar LR, Agrawal A. Immunomodulatory effect of hypertonic saline solution in traumatic brain-injured patients and intracranial hypertension. Indian J Neurotrauma. 2020;17:74–78. [Google Scholar]
- 45.Raslan A, Bhardwaj A. Medical management of cerebral edema. Neurosurg Focus. 2007;22:1–12. doi: 10.3171/foc.2007.22.5.13. [DOI] [PubMed] [Google Scholar]
- 46.Rocha e Silva M. Hypertonic saline for treatment of shock: have we looked for everything? MedicalExpress (São Paulo) 2014;1:14–21. [Google Scholar]
- 47.Rocha-e-Silva M, Poli de Figueiredo LF. Small volume hypertonic resuscitation of circulatory shock. Clinics (Sao Paulo) 2005;60:159–172. doi: 10.1590/s1807-59322005000200013. [DOI] [PubMed] [Google Scholar]
- 48.Roquilly A, Moyer JD, Huet O, Lasocki S, Cohen B, Dahyot-Fizelier C, et al. Effect of continuous infusion of hypertonic saline vs standard care on 6-month neurological outcomes in patients with traumatic brain injury: the COBI randomized clinical trial. JAMA. 2021;325:2056–2066. doi: 10.1001/jama.2021.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadan O, Singbartl K, Kraft J, Plancher JM, Greven AC, Kandiah P, et al. Low-chloride- versus high-chloride-containing hypertonic solution for the treatment of subarachnoid hemorrhage-related complications: the ACETatE (a low chlorie hypertonic solution for brain edema) randomized trial. J Intensive Care. 2020;8:32. doi: 10.1186/s40560-020-00449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schizodimos T, Soulountsi V, Iasonidou C, Kapravelos N. An overview of management of intracranial hypertension in the intensive care unit. J Anesth. 2020;34:741–757. doi: 10.1007/s00540-020-02795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shields CJ, O’Sullivan AW, Wang JH, Winter DC, Kirwan WO, Redmond HP. Hypertonic saline enhances host response to bacterial challenge by augmenting receptor-independent neutrophil intracellular superoxide formation. Ann Surg. 2003;238:249–257. doi: 10.1097/01.sla.0000080827.77985.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soustiel JF, Vlodavsky E, Zaaroor M. Relative effects of mannitol and hypertonic saline on calpain activity, apoptosis and polymorphonuclear infiltration in traumatic focal brain injury. Brain Res. 2006;1101:136–144. doi: 10.1016/j.brainres.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 53.Strandvik GF. Hypertonic saline in critical care: a review of the literature and guidelines for use in hypotensive states and raised intracranial pressure. Anaesthesia. 2009;64:990–1003. doi: 10.1111/j.1365-2044.2009.05986.x. [DOI] [PubMed] [Google Scholar]
- 54.Suarez JI, Qureshi AI, Parekh PD, Razumovsky A, Tamargo RJ, Bhardwaj A, et al. Administration of hypertonic (3%) sodium chloride/acetate in hyponatremic patients with symptomatic vasospasm following subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1999;11:178–184. doi: 10.1097/00008506-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Thompson R, Greaves I. Hypertonic saline--hydroxyethyl starch in trauma resuscitation. J R Army Med Corps. 2006;152:6–12. doi: 10.1136/jramc-152-01-02. [DOI] [PubMed] [Google Scholar]
- 56.Torre-Healy A, Marko NF, Weil RJ. Hyperosmolar therapy for intracranial hypertension. Neurocrit Care. 2012;17:117–130. doi: 10.1007/s12028-011-9649-x. [DOI] [PubMed] [Google Scholar]
- 57.Wade CE, Kramer GC, Grady JJ, Fabian TC, Younes RN. Efficacy of hypertonic 7.5% saline and 6% dextran-70 in treating trauma: a meta-analysis of controlled clinical studies. Surgery. 1997;122:609–616. doi: 10.1016/s0039-6060(97)90135-5. [DOI] [PubMed] [Google Scholar]
- 58.Wang YL, Lam KK, Cheng PY, Kung CW, Chen SY, Chao CC, et al. The cardioprotective effect of hypertonic saline is associated with inhibitory effect on macrophage migration inhibitory factor in sepsis. BioMed Res Int. 2013;2013:201614. doi: 10.1155/2013/201614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wisner DH, Schuster L, Quinn C. Hypertonic saline resuscitation of head injury: effects on cerebral water content. J Trauma. 1990;30:75–78. doi: 10.1097/00005373-199001000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Crit Care. 2010;14:226. doi: 10.1186/cc9052. [DOI] [PMC free article] [PubMed] [Google Scholar]