Abstract
Background:
Catheter-associated urinary tract infections (CA-UTIs) pose a significant challenge in intensive care unit (ICU) patients with COVID-19.
Objective:
The study aims to assess the prevalence of CA-UTIs, identify the causative pathogens and their resistance profiles, and determine the risk factors and outcomes associated with CA-UTIs in ICU patients with COVID-19.
Design:
Single-center, retrospective cohort study.
Methods:
The study included 201 adult ICU patients diagnosed with COVID-19 between March 2020 and July 2021. Patients were categorized into CA-UTI (n = 56) and non-CA-UTI (n = 145) groups. Data on demographic characteristics, clinical course, treatment, and outcomes were collected. Logistic regression analysis was used to identify risk factors for CA-UTI.
Results:
CA-UTIs developed in 28% of patients (n = 56). Incidence density of 15.8 episodes per 1000 catheter days. The average onset occurrence is 7.2 days after ICU admission. Patients with CA-UTI had longer ICU stays (18.8 days vs 10.5 days, p < 0.001) and more elevated mortality rates (75.0% vs 54.5%, p = 0.010), higher mechanical ventilation (MV) usage (98.2% vs 88.3%, p = 0.027), a longer average duration of MV (16.6 days vs 9.1 days, p < 0.001). Longer ICU and hospital stays were significant risk factors for CA-UTI. Other factors, such as the use of corticosteroids, chronic organ insufficiency or immunocompromized status, female sex, age, diabetes mellitus, and the duration of urinary catheterization, did not show significant associations with CA-UTI risk in this cohort. Gram-negative bacteria, particularly Klebsiella pneumoniae (28 cases), was the most common pathogen, with a high prevalence of multidrug resistance (38.8%) with type ESBL, MBL, NDM, and OXA-48. The occurrence of multidrug resistant (MDR) organisms was 68.8%.
Conclusion:
The findings of this study underscore the prevalence of CA-UTIs in ICU patients with COVID-19, significantly impacting patient outcomes. Effective infection control and targeted antimicrobial therapy are crucial to managing these infections.
Keywords: catheter-associated urinary tract infections, COVID-19, ICU, multidrug-resistant organisms
Introduction
Catheter-associated urinary tract infections (CA-UTIs) are among the most common healthcare-associated infections, posing a significant challenge in intensive care units (ICUs).1,2 Critically ill patients are particularly susceptible to these infections due to the frequent use of urinary catheters, prolonged hospital stays, and compromised immune systems. 3 The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has imposed unprecedented challenges on healthcare systems globally. 4 The emergence of COVID-19 has further complicated the management of CA-UTIs, as patients with severe COVID-19 often require extended ICU care and invasive procedures, increasing their risk of secondary infections. 3
The decision to conduct this research was influenced by a previous study on bloodstream infections (BSIs), which indicated that 9.4% of BSI cases were attributed to CA-UTIs. 5 Given the significant role of CA-UTIs in contributing to BSIs 6 and their impact on patient outcomes, understanding the prevalence, causative organisms, risk factors, and clinical outcomes of CA-UTIs in COVID-19 ICU patients was deemed essential.
Recent studies have highlighted various prognostic factors influencing outcomes in critically ill patients with COVID-19. For instance, a comprehensive analysis conducted by Bartoszewicz et al. 7 identified critical markers associated with ICU mortality, including elevated levels of interleukin-6 (IL-6) and white blood cells (WBCs) within the first 48 h of ICU admission. These findings underscore the importance of monitoring inflammatory markers to improve patient management and outcomes.
The study’s objective is to determine the characteristics of CA-UTIs in ICU patients with COVID-19, including identifying the prevalence, causative organisms and their resistance profiles, risk factors, and the impact of CA-UTIs on clinical outcomes. Understanding these elements can significantly improve antibiotic stewardship programs, implement targeted infection control, and improve patient outcomes and resource utilization in critical care settings.
Materials and methods
Study design and population
Following the STROBE guidelines 8 (STROBE checklist is available as Supplemental Material), this retrospective cohort study was conducted at the University Clinical Hospital’s ICU in Bialystok, Poland. The study included patients admitted to the ICU between March 3, 2020 and July 1, 2021. Data collection covered the period from ICU admission to discharge or death.
The study included 201 adult patients diagnosed with COVID-19. Eligibility requires patients to be older than 18, have a confirmed acute COVID-19 infection verified by reverse transcription polymerase chain reaction, and be admitted to the ICU due to SARS-CoV-2 infection. Exclusion criteria included pregnant women and individuals admitted to the ICU for non-COVID-19 reasons, such as elective surgeries or other emergencies. Patients were categorized into two groups: those who developed CA-UTI (n = 56) and those who did not (n = 145).
Criteria for CA-UTIs
Clinical criteria
Urine cultures were collected based on specific clinical criteria. The diagnosis of CA-UTI required the presence of symptoms or signs indicative of a urinary tract infection, such as fever, discomfort in the suprapubic region or at the costovertebral angle, and other systemic symptoms such as changes in mental state, low blood pressure, or evidence of systemic inflammatory response syndrome without other recognizable sources for these symptoms. These indicators were observed in individuals with an indwelling urethral catheter. All patients had catheters in place for at least 48 h before diagnosing CA-UTI.
Microbiological criteria
Urine samples for microbiological investigation were collected after ICU admission, ensuring catheter replacement before sample collection to avoid contamination from the catheter’s biofilm. Positive urine samples had ⩾103 colony-forming units (cfu)/mL of one or two bacterial species in a single catheter urine specimen. Cultures with three or more microorganisms were considered contaminants and excluded from the analysis.
Interpretation according to Infectious Diseases Society of America (IDSA) criteria
The diagnosis of CA-UTI necessitates that the patient demonstrates the above clinical signs and symptoms along with a positive microbiological result. The definition of CA-UTI was according to the International Clinical Practice Guidelines from the Infectious Diseases Society of America. 2
The above criteria guided the decision to start antibiotics. All patients diagnosed with CA-UTI received antibiotic treatment. The microbiological examination was established before initiating antibiotic therapy to circumvent the potential for false-negative outcomes.
Demographic data, clinical characteristics, and outcomes were collected from electronic medical records. Variables (with potential risk factors according to literature) included age, sex, body mass index (BMI), immunosuppression due to other medical conditions, history of previous urological pathology, comorbidities (e.g., diabetes mellitus,9,10 hypertension, and chronic heart failure), ICU admission scores (e.g., APACHE II), laboratory values (e.g., C-reactive protein (CRP), D-dimer, and International Normalized Ratio (INR)), and interventions (e.g., mechanical ventilation (MV), corticosteroid use, prone positioning, and duration of urinary catheterization [a primary risk factor]). 11
Statistical analysis
Data were analyzed using R software version 4.1 12 and jamovi. 13 Continuous variables were expressed as means and standard deviations (SD), while categorical variables were presented as frequencies and percentages. Comparisons between groups were made using t-tests for continuous variables and chi-square tests for categorical variables. Logistic regression analysis was used to identify risk factors for CA-UTI, with odds ratios (OR) and 95% confidence intervals (CI) calculated. A p Value of < 0.05 was considered statistically significant.
Results
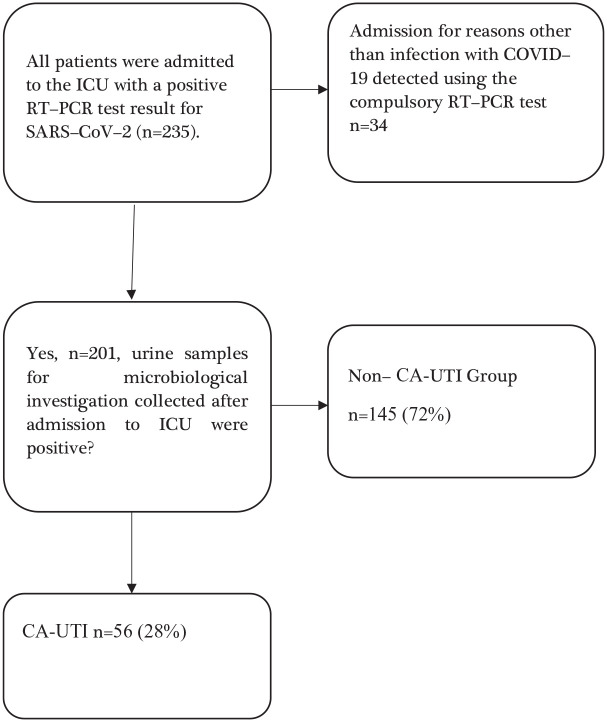
Table 1 shows the baseline characteristics, clinical course, treatment, and outcomes of ICU patients with COVID-19. A total of 235 adult patients diagnosed with COVID-19 were admitted to the ICU. After applying the exclusion criteria, 201 patients were included in the study, with 56 (28%) developing CA-UTI and 145 (72%) not developing CA-UTI (Figure 1).
Table 1.
Characteristics of patients with COVID-19 at ICU admission, risk factors for developing CA-UTI disease course, treatment, and outcomes.
| Headcount | Non-CA-UTI | CA-UTI | All n = 201 | p Value |
|---|---|---|---|---|
| n1 = 145 (72%) | n2 = 56 (28%) | |||
| Baseline and demographic | ||||
| Average BMI (±SD) | 33.6 (19.8) | 31.3 (5.3) | 33.0 (17.0) | 0.389 |
| Female—no. (%) | 61 (42.1) | 26 (46.4) | 87 (43.3) | 0.635 |
| Mean age (±SD)—years | 66.6 (12.5) | 64.9 (10.7) | 66.1 (12.1) | 0.388 |
| Diabetes mellitus | 43 (29.7) | 17 (30.4) | 60 (29.9) | 1.000 |
| Atrial fibrillation | 20 (13.8) | 8 (14.3) | 28 (13.9) | 1.000 |
| Hypertension | 87 (60.0) | 34 (61.8) | 121 (60.5) | 0.872 |
| Obesity | 30 (20.7) | 13 (24.1) | 43 (21.6) | 0.699 |
| Chronic heart failure | 37 (25.5) | 9 (16.4) | 46 (23.0) | 0.192 |
| History of previous urological pathology | 13 (9.0) | 7 (12.5) | 20 (10.0) | 0.441 |
| On arrival in the ICU | ||||
| Mean APACHE II (±SD) | 29.1 (8.6) | 29.0 (6.6) | 29.1 (8.0) | 0.948 |
| Mean PaO2/FiO2 (±SD)—mmHg | 128.5 (80.5) | 123 (66.6) | 126.9 (76.7) | 0.646 |
| Acute kidney failure—no. (%) | 56 (38.6) | 13 (23.2) | 69 (34.3) | 0.047 |
| CRP (±SD) mg/L | 90.9 (98.3) | 65.2 (61.5) | 83.7 (90.2) | 0.070 |
| D-dimer (±SD) | 4.6 (5.0) | 2.6 (3.0) | 3.9 (4.4) | 0.026 |
| INR (±SD) | 1.4 (0.3) | 1.3 (0.2) | 1.4 (0.3) | 0.010 |
| Interleukin 6 (±SD) pg/mL | 504.1 (920.6) | 349.7 (785) | 451 (876.9) | 0.340 |
| Absolute neutrophils (×103/µL) | 10.9 (7.3) | 9.7 (6.0) | 10.5 (6.9) | 0.421 |
| Neutrophils percent (±SD) | 73.4 (27.7) | 78.8 (23.6) | 75.2 (26.4) | 0.349 |
| Procalcitonin (±SD) ng/mL | 2.6 (9.4) | 1.3 (5.2) | 2.3 (8.4) | 0.342 |
| White blood cells (×103/µL) (±SD) | 13.9 (10.5) | 10.1 (5.7) | 12.9 (9.5) | 0.010 |
| FIO2 mean (SD) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.462 |
| During hospitalization | ||||
| Mean hospital LOS (±SD)—days | 15.6 (12.3) | 24.2 (19.2) | 18.0 (15.0) | <0.001 |
| Duration of urinary catheterization (±SD)—days | 15.7 (54.9) | 22.5 (11.2) | 17.6 (47.1) | 0.359 |
| Antibiotics—no. (%) | 127 (87.6) | 56 (100) | 182 (90.5) | 0.028 |
| Mean LOS at ICU (±SD)—days | 10.5 (8.8) | 18.8 (11.1) | 12.8 (10.1) | <0.001 |
| Mechanical ventilation—no. (%) | 128 (88.3) | 55 (98.2) | 183 (91.0) | 0.027 |
| MV duration (±SD)—days | 9.1 (8.0) | 16.6 (9.1) | 11.2 (9.0) | <0.001 |
| Infusion of neuromuscular blocking agents (NMBAs) at least one day (%) | 85 (58.6) | 50 (89.3) | 135 (67.2) | <0.001 |
| Corticosteroids—no. (%) | 126 (86.9) | 51 (91.1) | 177 (88.1) | 0.477 |
| Prone Position—no. (%) | 55 (37.9) | 24 (42.9) | 79 (39.3) | 0.524 |
| Chronic organ insufficiency or immune compromise—no. (%) | 93 (64.1) | 35 (62.5) | 128 (63.7) | 0.871 |
| Ventilator-associated pneumonia—no. (%) | 37 (25.5) | 30 (53.6) | 67 (33.3) | <0.001 |
| Bloodstream infection—no. (%) | 21 (14.5) | 22 (39.3) | 43 (21.4) | <0.001 |
| Average of ICU hospitalization | ||||
| CRP (±SD) mg/L | 96.8 (79.4) | 104.2 (72.1) | 98.9 (77.3) | 0.543 |
| D-dimer (±SD) | 3.1 (3.9) | 2.7 (1.9) | 3.0 (3.5) | 0.502 |
| INR (±SD) | 1.3 (0.3) | 1.2 (0.2) | 1.2 (0.2) | 0.101 |
| Interleukin 6 (±SD) pg/mL | 488.9 (823.8) | 457.9 (767.7) | 480.1 (806.5) | 0.808 |
| Absolute neutrophils (×103/µL) | 9.1 (8.3) | 9.0 (5.8) | 9.1 (7.6) | 0.892 |
| Neutrophils percent (±SD) | 59.6 (37.1) | 69.2 (31.5) | 62.3 (35.8) | 0.089 |
| Procalcitonin (±SD) ng/mL | 3.5 (9.1) | 2.8 (5.6) | 3.3 (8.3) | 0.601 |
| White blood cells (×103/µL) (±SD) | 16.3 (9.7) | 12.7 (4.3) | 15.3 (8.7) | 0.007 |
| Outcome | ||||
| Death—no. (%) | 79 (54.5) | 42 (75.0) | 121 (60.2) | 0.010 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; BSI, bloodstream infection; CA-UTI, Catheter-associated urinary tract infection; ICU, intensive care unit; LOS, length of stay; NMBAs, neuromuscular blocking agents; VAP, ventilator-associated pneumonia.
Figure 1.
Flowchart of patient screening and inclusion.
COVID-19, coronavirus disease 2019; ICU, intensive care unit; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CA-UTI, catheter-associated urinary tract infection.
The incidence density was calculated as the number of CA-UTI episodes per 1000 catheter days. The total number of catheter days was 3536 days. Therefore, the incidence density of CA-UTI was 15.8 episodes per 1000 catheter days (56 episodes/3536 catheter days * 1000).
The mean age was similar between groups (CA-UTI: 64.9 years vs non-CA-UTI: 66.6 years), with a slightly higher proportion of females in the CA-UTI group (46.4% vs 42.1%). There were no significant differences in comorbidities like diabetes mellitus and hypertension. Acute kidney failure was infrequently in the CA-UTI group (23.2% vs 38.6%, p = 0.047). The average BMI was slightly lower in the CA-UTI group (31.3) compared to the non-UTI group (33.6), with no significant difference (p = 0.389). The average APACHE II score was similar between the CA-UTI group (29.0) and the non-UTI group (29.1), showing comparable initial severity of illness (p = 0.948).
The duration of urinary catheterization was also longer in the CA-UTI group (22.5 days) compared to the non-UTI group (15.7 days), although this difference was not statistically significant (p = 0.359). The average time to onset of CA-UTI from ICU admission was 7.2 days. All patients in the CA-UTI group received antibiotics (100%) compared to 87.6% in the non-UTI group (p = 0.028). The mean ICU LOS was significantly longer for the CA-UTI group (18.8 days) compared to the non-UTI group (10.5 days, p < 0.001). MV was required for a higher proportion of patients in the CA-UTI group (98.2%) compared to the non-UTI group (88.3%, p = 0.027), and the duration of MV was longer in the CA-UTI group (16.6 days vs 9.1 days, p < 0.001). The use of NMBAs for at least 1 day was more common in the CA-UTI group (89.3%) compared to the non-UTI group (58.6%, p < 0.001). Notably, only one patient belonging to a non-CA-UTI group was a kidney transplant recipient. A history of previous urological pathology was slightly more common in the CA-UTI group (12.5%) compared to the non-CA-UTI group (9.0%), but this difference was not statistically significant (p = 0.441). Similarly, the prevalence of chronic organ insufficiency or immune compromise was comparable between the groups (CA-UTI: 62.5% vs non-CA-UTI: 64.1%, p = 0.871).
C-reactive protein (CRP) levels were slightly lower in the CA-UTI group (65.2 mg/L) compared to the non-UTI group (90.9 mg/L), though this was not statistically significant (p = 0.070). D-dimer levels were significantly lower in the CA-UTI group (2.6 mg/L) compared to the non-UTI group (4.6 mg/L, p = 0.026). The INR was also significantly lower in the CA-UTI group (1.3) than the non-UTI group (1.4, p = 0.010). The WBC count at different time points (after 24, 48, and an average of 72 hours post-ICU admission) was generally lower in UTI patients, with the difference after 72 h being statistically significant (p = 0.005). The two groups had no significant differences in neutrophil percentages at these time points. The overall ICU hospitalization averages for neutrophil percentage and WBC count were lower in the UTI group, with the latter reaching statistical significance (p = 0.007).
Notably, the mortality rate was significantly higher in the CA-UTI group (75.0%) compared to the non-UTI group (54.5%, p = 0.010). UTIs were a source of 9.4% of the BSI cases, respectively. 5
Table 2 presents the pathogens responsible for CA-UTIs in ICU patients, highlighting the prevalence and multidrug resistance (MDR) characteristics. The bacteria were classified into sensitivity, MDR, and extensive drug resistance according to the European Centre for Disease Prevention and Control criteria. 15 The table identifies nine pathogens, with Klebsiella pneumoniae being the most prevalent (28 cases, 36.36% MDR), followed by Enterococcus faecalis (17 cases, 10.39% MDR) and E. faecium (11 cases, 6.49% MDR). Gram-positive pathogens include E. faecalis and E. faecium, while the Gram-negative group comprises K. pneumoniae, E. coli, Acinetobacter baumannii, Proteus mirabilis, E. cloacae, K. aerogenes, and P. aeruginosa. K. pneumoniae exhibits the highest percentage of MDR, with resistance types including ESBL, MBL, NDM, and OXA-48. Other significant resistances observed include HLAR and Vancomycin-Resistant Enterococci (VRE) in E. faecium, and ESBL in E. coli and P. mirabilis. The table shows the varying degrees of MDR across these pathogens, with detailed annotations for each resistance type, such as HLAR, HLARG, ESBL, MBL, NDM, OXA-48, and VRE.
Table 2.
Pathogens causing CA-UTI sorted by frequency and grouped by gram staining.
| Pathogens | Frequency in patients’ samples | Gram +/− | MDR (%) | Type of MDR |
|---|---|---|---|---|
| Enterococcus faecalis | 17 | G+ | 10.39 | HLAR, HLARG |
| Enterococcus faecium | 11 | G+ | 6.49 | HLAR, VRE |
| Klebsiella pneumoniae | 28 | G− | 36.36 | ESBL, MBL, NDM, OXA-48 |
| Escherichia coli | 7 | G− | 3.90 | ESBL |
| Acinetobacter baumannii | 5 | G− | 5.19 | MDR |
| Proteus mirabilis | 3 | G− | 3.90 | ESBL |
| Enterobacter cloacae | 2 | G− | 1.30 | MDR |
| Klebsiella aerogenes | 2 | G− | 1.30 | ESBL |
| Pseudomonas aeruginosa | 2 | G− | 0.00 |
ESBL, Extended-Spectrum Beta-Lactamases; HLAR, High-Level Aminoglycoside Resistance; HLARG, High-Level Aminoglycoside Resistance with Gentamicin; MBL, Metallo-Beta-Lactamases; MDR, Multidrug Resistant; NDM, New Delhi Metallo-Beta-Lactamase; OXA-48, OXA-48 Beta-Lactamase; VRE, Vancomycin-Resistant Enterococci.
Table 3 summarizes the logistic regression analysis of factors associated with catheter-associated CA-UTIs in ICU patients. Longer ICU stays (OR: 1.085, p < 0.001) and more extended hospital stays (OR: 1.039, p = 0.001) were significantly associated with an increased risk of CA-UTI. Acute kidney failure was inversely related to CA-UTI risk (OR: 0.480, p = 0.041). MV showed a strong, although the p value indicates marginal significance, association with CA-UTI (OR: 7.300, p = 0.056). Other factors, such as the use of corticosteroids, chronic organ insufficiency or immunocompromized status, female sex, age, diabetes mellitus, and the duration of urinary catheterization, did not show significant associations with CA-UTI risk in this cohort.
Table 3.
Logistic regression of factors associated with CA-UTI occurrence.
| Independent variables | Univariate analysis |
|---|---|
| OR (95% CI), p Value | |
| Acute kidney failure | 0.480 (0.237–0.972, p = 0.041) |
| ICU LOS | 1.085 (1.0470–1.124, p = <0.001) |
| Use of corticosteroids | 1.538 (0.5450–4.340, p = 0.416) |
| Chronic organ insufficiency or immunocompromized | 0.932 (0.492–1.765, p = 0.829) |
| Female sex | 1.193 (0.642–2.219, p = 0.576) |
| Age | 0.989 (0.964–1.01, p = 0.386) |
| Diabetes mellitus | 1.034 (0.528–2.025, p = 0.922) |
| Duration of urinary catheterization (days) | 0.999 (0.96–0.99, p = 0.974) |
| Hospital LOS | 1.039 (1.015–1.063, p = 0.001) |
| Mechanical ventilation | 7.3 (0.95–56.25, p = 0.056) |
CA-UTI, Catheter-associated urinary tract infection; ICU, Intensive Care Unit; LOS, length of stay; OR, Odds Ratio.
Discussion
The study findings highlight the substantial burden of CA-UTIs among ICU patients with COVID-19. The prevalence of CA-UTI was 28%, with an incidence density of 15.8 episodes per 1000 catheter days. The average onset time of CA-UTI is 7.2 days post-ICU admission. Patients who developed CA-UTIs had significantly longer ICU stays, higher mortality rates (75% vs 54.5%), and increased MV usage compared to those who did not develop CA-UTIs. The most frequently isolated pathogens were Gram-negative bacteria, particularly K. pneumoniae, which exhibited a high rate of MDR (ESBL, MBL, NDM, and OXA-48). E. faecalis and E. faecium, Gram-positive pathogens, also showed significant MDR profiles (VRE HLAR, HLARG). The frequent occurrence of multidrug resistant organisms was 68.8%.
Our findings are consistent with previous studies that have documented high rates of CA-UTIs in ICU settings, particularly among critically ill patients.16,17 The high prevalence of Gram-negative bacteria and the significant presence of MDR organisms align with global trends observed in nosocomial infections.6,18 Similar pathogens species were reported in this research.10,17,18 The early onset of CA-UTI after ICU admission indicates a window of opportunity for intervention that may mitigate the risk of CA-UTI and its consequences. This trend complicates treatment options and raises alarms about the growing challenge of antibiotic resistance in healthcare settings, especially in a global pandemic. 19
The extended ICU and hospital stay as risk factors for CA-UTI development underscore the findings of prior research that prolonged hospitalization increases the risk of nosocomial infections. These findings suggest that the duration of hospitalization and ICU admission, which often correlate with the severity of illness and the need for invasive interventions, are critical factors in developing CA-UTIs. MV usage is strongly associated with CA-UTI occurrence, reflecting the complexity and severity of care required for these patients. The potential risk posed by MV often necessitates prolonged catheterization and increases the risk of secondary infections. 20
Inadequacies in catheter care, particularly deviations from sterile protocols, failure to uphold a closed drainage system, and bacterial colonization of the drainage bag, are critical determinants in developing CA-UTIs. 21 During the COVID-19 pandemic, these issues may be exacerbated due to the increased workload and reduced staff-to-patient ratios in ICUs. 20 The necessity of stringent infection control measures for COVID-19 patients can lead to difficulties maintaining optimal catheter care practices. Personal protective equipment (PPE) requirements and isolation protocols can limit the frequency and thoroughness of catheter maintenance, increasing the risk of contamination and infection. 22
Interestingly, traditional risk factors for CA-UTI, such as diabetes mellitus and the duration of urinary catheterization, did not significantly correlate with CA-UTI in our study. The severity and management of COVID-19 itself may overshadow the influence of traditional risk factors. The acute illness and intensive care required for COVID-19 patients could be the primary determinants of CA-UTI risk, making other factors less significant. The high prevalence of comorbidities, such as diabetes mellitus, across both CA-UTI and non-CA-UTI groups might have diluted the effect of these conditions as distinguishing risk factors. Both groups might have received similar levels of care due to these comorbidities. ICU patients may have received more vigilant care and monitoring, potentially mitigating the increased risk of CA-UTI. Similarly, monitoring and managing urinary catheters may have been equally rigorous in all patients due to the critical care environment.
The high mortality rate associated with CA-UTI patients underscores the severe impact of these infections on critically ill patients. Implementing targeted infection control strategies, such as regular catheter care protocols, timely removal of unnecessary catheters, and stringent hand hygiene practices, is crucial in reducing CA-UTI rates. Additionally, antibiotic stewardship programs are essential to ensure appropriate antibiotic use, minimize the development of resistance, and optimize patient outcomes. Findings underscore the profound impact of CA-UTIs on patient morbidity and mortality, emphasizing the need for effective infection control measures and timely antimicrobial interventions. Enhanced infection control measures, including closed drainage systems and strict adherence to sterile techniques, are crucial in reducing the incidence of CA-UTIs in ICU settings.
The study also revealed laboratory test features of UTI patient’s lower WBC counts. The significantly lower initial WBC counts and subsequent measurements in CA-UTI patients may indicate a different inflammatory response or a potential immunosuppressive state that predisposes these patients to secondary infections. 23 Results suggest a distinct clinical profile for CA-UTI patients upon ICU admission, which could have implications for early diagnosis and management.
The increased mortality level in patients with CA-UTIs can be interpreted as a consequence of the additional burden imposed by the infection on already critically ill patients. 24 CA-UTIs act synergistically with COVID-19 to exacerbate patient conditions, leading to poor outcomes.
The strength of this study is the comprehensive data collection and analysis, providing a clear picture of CA-UTI prevalence, risk factors, and outcomes in ICU patients with COVID-19. While our study provides valuable insights into the CA-UTIs among ICU patients with COVID-19, it is not without limitations. The retrospective, single-center design may limit the generalizability of our findings. Additionally, the reliance on existing medical records introduces the potential for biases and incomplete data.
The sample size for this study was not predetermined through formal calculation. Instead, it was based on the available population of COVID-19 patients admitted to the ICU during the specified study period. This approach, while practical, limits the ability to ensure statistical power and precision in estimating the study’s effect sizes. This limitation should be addressed in future studies with prospectively calculated sample sizes to enhance the robustness of the findings.
Future research should focus on prospective, multicenter studies to validate these findings and explore additional risk factors for CA-UTIs. Studies are also needed to investigate the effectiveness of various infection control interventions and antimicrobial stewardship programs in reducing CA-UTI rates and improving patient outcomes.
Conclusion
The predominance of MDR Gram-negative bacteria, particularly K. pneumoniae, complicates treatment and highlights the need for infection control measures and targeted antimicrobial therapy. Addressing these challenges is crucial for improving outcomes.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361241278218 for Catheter-associated urinary tract infections in critically Ill patients with COVID-19: a retrospective cohort study by Paulina Dąbrowska, Mateusz Bartoszewicz, Klaudia Bartoszewicz, Juliusz Kosel, Samuel Stróż, Jerzy Robert Ładny and Sławomir Lech Czaban in Therapeutic Advances in Infectious Disease
Supplemental material, sj-docx-2-tai-10.1177_20499361241278218 for Catheter-associated urinary tract infections in critically Ill patients with COVID-19: a retrospective cohort study by Paulina Dąbrowska, Mateusz Bartoszewicz, Klaudia Bartoszewicz, Juliusz Kosel, Samuel Stróż, Jerzy Robert Ładny and Sławomir Lech Czaban in Therapeutic Advances in Infectious Disease
Acknowledgments
None.
Appendix
Abbreviations
BSI bloodstream infection
VAP ventilator-associated pneumonia
MDR Multidrug resistant
MDRo Multidrug Resistant organisms
ICU intensive care unit
DM Diabetes Mellitus
LOS length of stay
MV mechanical ventilation
APACHE II Acute Physiology and Chronic Health Evaluation II
ARDS acute respiratory distress syndrome
NMB neuromuscular block
ESBL Extended-Spectrum Beta-Lactamases
HLAR High-Level Aminoglycoside Resistance
HLARG High-Level Aminoglycoside Resistance with Gentamicin
MBL Metallo-Beta-Lactamases
NDM New Delhi Metallo-Beta-Lactamase
OXA-48 OXA-48 Beta-Lactamase
VRE Vancomycin-Resistant Enterococci
SARS-CoV-2 Severe Acute Respiratory Syndrome Coronavirus 2
COVID-19 Coronavirus Disease 2019
RT-PCR Reverse Transcription Polymerase Chain Reaction
CA-UTI Catheter-Associated Urinary Tract Infection
PPE Personal protective equipment
Footnotes
ORCID iD: Mateusz Bartoszewicz  https://orcid.org/0000-0001-6455-6962
https://orcid.org/0000-0001-6455-6962
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Paulina Dąbrowska, Department of Anaesthesiology and Intensive Care, Medical University of Bialystok, Bialystok, Poland.
Mateusz Bartoszewicz, Department of Anaesthesiology and Intensive Care, Medical University of Bialystok, Bialystok, Poland.
Klaudia Bartoszewicz, Department of Clinical Immunology, Medical University of Bialystok, Bialystok, Poland.
Juliusz Kosel, Department of Anaesthesiology and Intensive Care, Medical University of Bialystok, Bialystok, Poland.
Samuel Stróż, Department of Clinical Immunology, Medical University of Bialystok, Bialystok, Poland.
Jerzy Robert Ładny, Department of Emergency Medicine, Medical University of Bialystok, Bialystok, Poland.
Sławomir Lech Czaban, Department of Anaesthesiology and Intensive Care, Medical University of Bialystok, Marii Skłodowskiej Curie 24A Bialystok 15-276, Poland.
Declarations
Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Bialystok, Poland (protocol code: APK.002.242.2021, date of approval:29.04.2021). The requirement for patient consent was waived due to the study’s retrospective nature.
Consent for publication: Not applicable.
Author contributions: Paulina Da˛browska: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing – original draft; Writing – review & editing.
Mateusz Bartoszewicz: Conceptualization; Data curation; Methodology; Resources; Software; Visualization.
Klaudia Bartoszewicz: Validation.
Juliusz Kosel: Supervision.
Samuel Stróż: Writing – review & editing.
Jerzy Robert Ładny: Supervision.
Sławomir Lech Czaban: Project administration.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received partial funding from the Medical University of Bialystok. The funders had no role in study design, data collection and analysis, publication decisions, or manuscript preparation.
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to the hospital’s policies.
References
- 1. Ong CCH, Farhanah S, Linn KZ, et al. Nosocomial infections among COVID-19 patients: an analysis of intensive care unit surveillance data. Antimicrob Resist Infect Control 2021; 10: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010; 50: 625–663. [DOI] [PubMed] [Google Scholar]
- 3. Blot S, Ruppé E, Harbarth S, et al. Healthcare-associated infections in adult intensive care unit patients: changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive Crit Care Nurs 2022; 70: 103227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cypress BS. COVID-19: the economic impact of a pandemic on the healthcare delivery system in the United States. Nurs Forum 2022; 57: 323–327. [DOI] [PubMed] [Google Scholar]
- 5. Bartoszewicz M, Czaban SL, Bartoszewicz K, et al. Bacterial bloodstream infection in critically ill patients with COVID-19: a retrospective cohort study. Ther Adv Infect Dis 2023; 10: 20499361231207178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Werneburg GT. Catheter-associated urinary tract infections: current challenges and future prospects. Res Rep Urol 2022; 14: 109–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartoszewicz K, Bartoszewicz M, Gradkowski W, et al. Analysis of prognostic factors in critically ill patients with COVID-19. PLoS ONE 2024; 19: e0302248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Platt R, Polk BF, Murdock B, et al. Risk factors for nosocomial urinary tract infection. Am J Epidemiol 1986; 124: 977–985. [DOI] [PubMed] [Google Scholar]
- 10. Venkataraman R, Manuel GG, JR N, et al. Prevalence, risk factors, causative organism and antibiotic susceptibility of catheter associated urinary tract infections. Int J Res Med Sci 2023; 12: 183–187. [Google Scholar]
- 11. Patel PK, Advani SD, Kofman AD, et al. Strategies to prevent catheter-associated urinary tract infections in acute-care hospitals: 2022 update. Infect Control Hosp Epidemiol 2023; 44: 1209–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Team RC. R: A Language and environment for statistical computing (Version 4.1) [Computer software], 2021. [Google Scholar]
- 13. The jamovi p. jamovi (Version 2.3) [Computer software], 2022. [Google Scholar]
- 14. Bartoszewicz M, Kosel J, Nadolny K, et al. Ventilator-associated pneumonia among patients with COVID-19: a retrospective cohort study. Emerg Med Serv 2023; 10: 210–216. [Google Scholar]
- 15. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–281. [DOI] [PubMed] [Google Scholar]
- 16. Saleem M, Syed Khaja AS, Hossain A, et al. Catheter-associated urinary tract infection in intensive care unit patients at a Tertiary Care Hospital, Hail, Kingdom of Saudi Arabia. Diagnostics (Basel) 2022; 12: 20220712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bardi T, Pintado V, Gomez-Rojo M, et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis 2021; 40: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Despotovic A, Milosevic B, Cirkovic A, et al. The impact of COVID-19 on the profile of hospital-acquired infections in adult intensive care units. Antibiotics (Basel) 2021; 10: 20210923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Laethem J, Wuyts SCM, Pierreux J, et al. Presumed urinary tract infection in patients admitted with COVID-19: are we treating too much? Antibiotics (Basel) 2021; 10: 20211206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zand F, Vakili H, Asmarian N, et al. Unintended impact of COVID-19 pandemic on the rate of catheter related nosocomial infections and incidence of multiple drug resistance pathogens in three intensive care units not allocated to COVID-19 patients in a large teaching hospital. BMC Infect Dis 2023; 23: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Decker SG, Bosch N, Murphy J. Catheter-associated urinary tract infection reduction in critical care units: a bundled care model. BMJ Open Qual 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kubde D, Badge AK, Ugemuge S, et al. Importance of hospital infection control. Cureus 2023; 15: e50931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stróż S, Kosiorek P, Stasiak-Barmuta A. The COVID-19 inflammation and high mortality mechanism trigger. Immunogenetics 2023. [DOI] [PubMed] [Google Scholar]
- 24. Radu VD, Costache RC, Onofrei P, et al. Multidrug-resistant (MDR) urinary tract infections associated with gut microbiota in CoV and Non-CoV patients in a urological clinic during the pandemic: a single center experience. Antibiotics (Basel) 2023; 12: 20230528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361241278218 for Catheter-associated urinary tract infections in critically Ill patients with COVID-19: a retrospective cohort study by Paulina Dąbrowska, Mateusz Bartoszewicz, Klaudia Bartoszewicz, Juliusz Kosel, Samuel Stróż, Jerzy Robert Ładny and Sławomir Lech Czaban in Therapeutic Advances in Infectious Disease
Supplemental material, sj-docx-2-tai-10.1177_20499361241278218 for Catheter-associated urinary tract infections in critically Ill patients with COVID-19: a retrospective cohort study by Paulina Dąbrowska, Mateusz Bartoszewicz, Klaudia Bartoszewicz, Juliusz Kosel, Samuel Stróż, Jerzy Robert Ładny and Sławomir Lech Czaban in Therapeutic Advances in Infectious Disease