Abstract
Porcine organs and human induced pluripotent stem cell (iPSC)-derived organoids as alternative organs for human transplantation have garnered attention, but both face technical challenges. Interspecies chimeric organ production using human iPSCs shows promise in overcoming these challenges. Our group successfully generated chimeric renal organoids using human iPSC-derived nephron progenitor cells (NPCs) and fetal mouse kidneys. However, the current technology is limited to rodents. Therefore, this study focused on producing human-pig chimeric renal organoids, as pigs are the most promising species for xenotransplantation. Modification of existing culture systems enables continuous renal development in both species, resulting in the successful creation of human-pig chimeric renal organoids. Moreover, this method can be applied to generate humanized xenogeneic kidneys for future clinical applications. This study provides evidence that optimizing culture conditions enables the early-stage kidney development beyond species barriers, thus laying the foundation for accelerating research on humanized xenogeneic kidney fabrication for clinical purposes.
Subject terms: Stem-cell biotechnology, Regenerative medicine, Tissue engineering
Creation of chimeric renal organoids using human iPSC technology with fetal pig kidneys demonstrates inter-species coexistence and codevelopment in early kidney development, paving the way for humanized xenogeneic kidney creation for clinical use.
Introduction
In recent years, porcine organs have garnered attention as alternative grafts for human organ transplantation, with several pioneering clinical studies utilizing porcine kidneys having been conducted1–3. However, it has become evident that immunological challenges persist in achieving long-term engraftment and functionality4. On the other hand, studies on organogenesis utilizing human induced pluripotent stem cells (iPSCs) have also been actively pursued in recent years5–7. Nonetheless, the complete establishment of requisite cell types and environments for organogenesis is not yet achieved, necessitating further research to create functional organs8. The other method of chimera organogenesis using blastocyst complementation, in which human pluripotent stem cells are injected into blastocyst stage embryos genetically engineered not to develop specific organs, has also garnered attention9–14. However, there remain ethical issues about the resulting animals, as it is difficult to selectively engraft human cells exclusively into the targeted organs within interspecies organisms14.
Interspecies chimeric organogenesis by injecting human iPSC-derived cells into developing organs of different animal species is envisioned as a promising approach to address these challenges in organ production15,16. Indeed, in our previous studies, we achieved the partial creation of heterologous, including human, chimeric kidneys by utilizing the prenatal kidney development environment of a different species16,17. Additionally, our xenotransplantation research demonstrated that heterologous transplantation of porcine fetal kidneys to adult monkeys resulted in lower immune rejection compared to adult organ transplantation18. Furthermore, our study using rodents demonstrated that when creating an interspecies chimeric kidney by injecting the recipient’s renal cells into the kidney of a different species, the resulting kidney transplanted into another recipient individual has lower immunogenicity compared to conventional interspecies transplantation19.
As mentioned above, we have obtained evidence suggesting the future clinical utility and potential of the humanized xeno-kidney, combining human renal cells with embryonic kidneys from a different species. However, the chimerism rate and maturity of human cells are still insufficient, necessitating the identification of a specific environment that enables coexistence and co-development regardless of species. Therefore, as a new chimera model aimed at exploring culture conditions that promote the coexistence and co-development of cells from humans and other animal species, “chimeric renal organoids” were successfully generated by co-culturing dissociated fetal kidneys and human NPCs at the single-cell level20.
However, with the current culture techniques, even the chimeric renal organoids combining human and mouse cells did not exhibit sufficient competency to coexist and co-develop. Furthermore, studies utilizing pluripotent stem cells from different species have suggested the presence of a barrier inhibiting interspecies chimera formation21, along with reported variations in the rate of organogenesis among interspecies animals22. These suggest that there are remaining challenges in effectively achieving interspecies chimera formation. Additionally, it remained unclear whether the generation of such chimeric kidneys or chimeric renal organoids could be achieved using human-derived renal progenitor cells in large animals such as pigs, as previous studies were limited to rodent models.
Pigs are considered the most ideal organ xenograft donor and have several advantages for chimeric kidney production due to their similar organ size, physiological functions resembling humans, rapid growth, and ease of organ procurement23. In xenotransplantation of pig organs, challenges in immune rejection continue to be addressed, and our ultimate goal is to overcome immunological barriers by creating humanized pig kidneys. Thus, the generation of chimeric renal organoids using human NPCs and porcine kidney cells represents a crucial step toward the future development of humanized pig kidney organogenesis for clinical application. Nevertheless, no methods for chimeric renal organoid creation using human renal cells and porcine renal cells, including the culture of porcine fetal kidney cells, have been reported to date. Furthermore, recent studies employing blastocyst complementation have revealed that human iPSCs and their derivatives are not highly committed to pig blastocysts14, highlighting the need for establishing cultivation techniques for efficient human-pig chimera formation.
In this study, to construct a culture system enabling cross-species renal development, we initially modified existing human-mouse mixed organoid culture systems to prolong survival and facilitate renal development in their component species. We then validated the applicability of this method to porcine fetal kidney cells, ultimately achieving the creation of human-pig chimeric organoids. Importantly, the identified culture conditions were suitable not only for human-xenogeneic chimeric renal organoids but also for human-xenogenic chimeric kidneys for future clinical purposes.
Results
Optimization of culture conditions for chimeric renal organoid composition
The production of fetal mouse kidney organoids, in which fetal kidneys are enzymatically processed, aggregated from a single-cell state, and subsequently reconstructed to recapitulate renal structures, has been documented in previous studies24,25. Leveraging the self-organization of fetal kidney progenitor cells, we demonstrated the incorporation of human iPSC-derived NPCs during fetal kidney organoid formation, resulting in the formation of interspecies human-mouse chimeric nephrons20. However, while we initially considered the survival and organoid formation of cell types constituting the renal organoids to be crucial, it became evident in our approach that the survival and organoid formation of human NPCs were not sufficient even when utilizing human NPCs with confirmed differentiation potential (Supplementary Fig. 1a, b). Consequently, it was confirmed that the proportion of human cells within chimeric renal organoids and the chimera formation rate diminished in a time-dependent manner (Supplementary Fig. 1c, d).
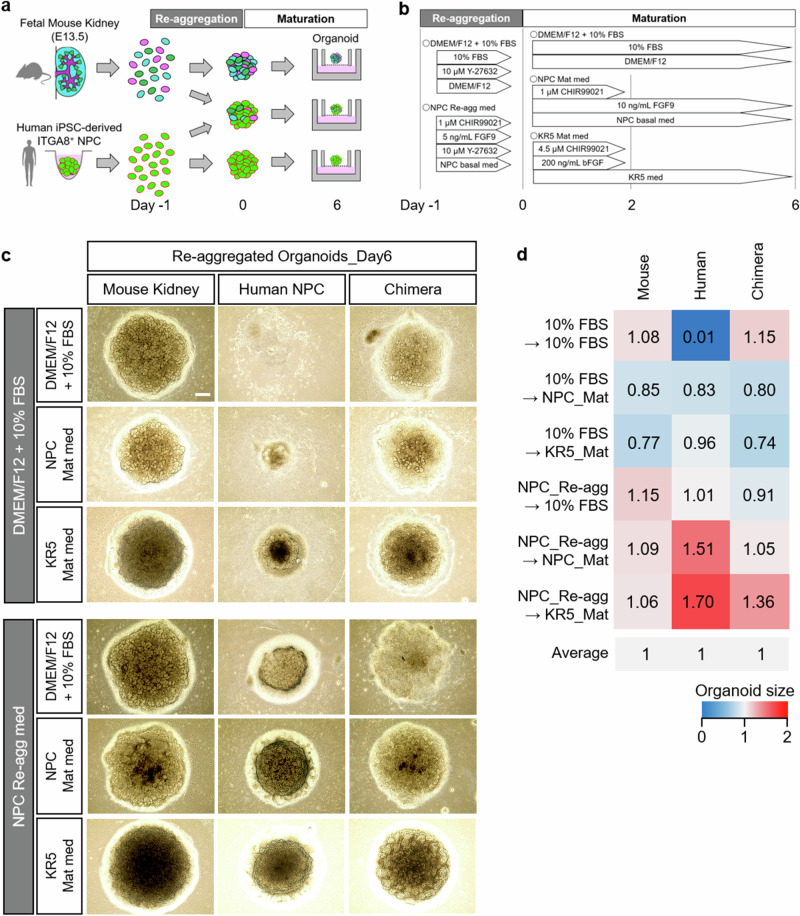
Therefore, we further explored culture conditions that would enable the survival, differentiation, and maturation of three organoid models: human-animal chimeric renal organoids and their constituent species-specific renal organoids (e.g., human renal organoids and mouse or pig renal organoids). We initiated an investigation into the culture conditions using human-mouse chimeric renal organoids as a starting point (Fig. 1a, b). During the formation of chimeric renal organoids, the reaggregation medium and maturation medium have conventionally been FBS-supplemented DMEM/F1220. In this study, we selected previously employed media for differentiation and maturation of NPCs, specifically “NPC_Re-agg” medium for the reaggregation medium, and “NPC_Mat” medium and “KR5_Mat” medium15,16 for the maturation medium26–29, and conducted a comparative investigation (Fig. 1a, b). Initially, using DMEM/F12 with 10% FBS for reaggregation and either NPC_Mat or KR5_Mat for maturation, organoid formation was observed in all species/conditions; however, the reaggregated products from human NPC did not exhibit a distinct organoid morphology (Fig. 1c).
Fig. 1. Screening of culture conditions using human-mouse chimeric renal organoids.
a Experimental scheme for the generation and examination of chimeric renal organoids. b Experimental scheme depicting the tested culture conditions and time course for chimeric renal organoid fabrication. c Bright-field images of organoids formed under each culture condition. Scale bar represents 200 μm. d Quantitative analysis of the sizes of fetal mouse kidney organoids, human NPC organoids, and chimeric renal organoids composed of human NPC and fetal mouse kidney, under various culture conditions.
On the other hand, when utilizing NPC_Re-agg as the reaggregation medium, organoid formation was observed for all maturation conditions. Among the investigated maturation culture conditions, organoids cultured with NPC_Mat or KR5_Mat exhibited a particularly distinct three-dimensional structure (Fig. 1c). From the morphological analysis, it was quantitatively confirmed that all three organoid models exhibited larger organoid sizes when NPC_Re-agg was used as a reaggregation medium, followed by NPC_Mat or KR5_Mat as a maturation medium (Fig. 1d). These studies elucidated novel culture conditions for establishing the three renal organoid models: the mouse, human, and chimeric human-mouse renal organoids. It was implied that both reaggregation and maturation media strongly influence renal organoid formation as well as chimeric renal organoid formation.
Evaluation of heterogeneous chimeric renal organoids formed under different culture conditions
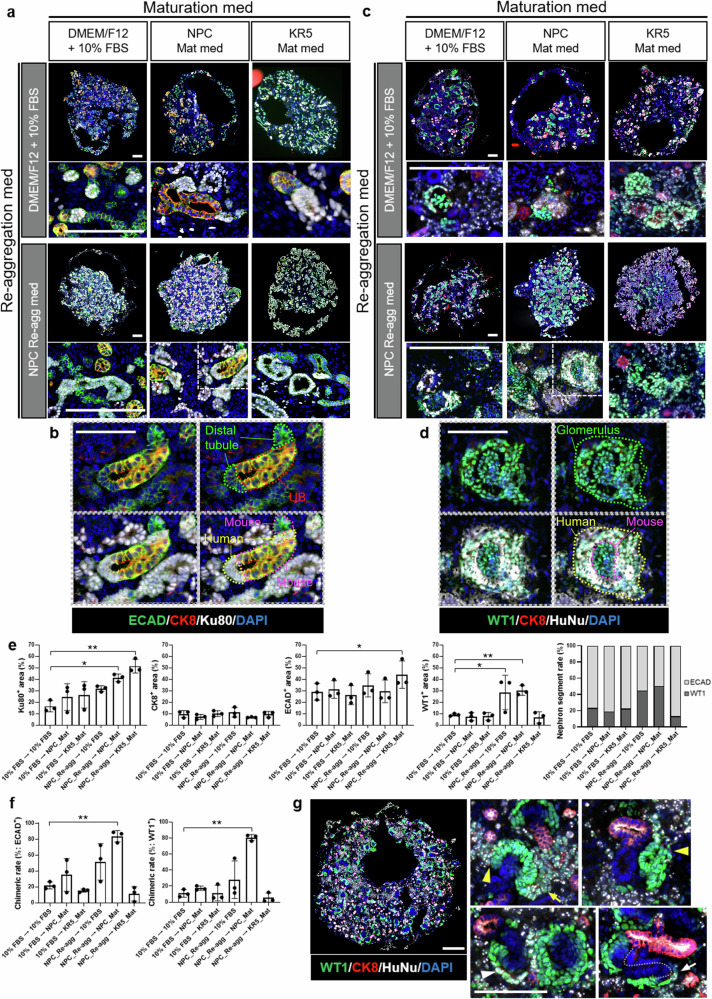
We conducted an immunostaining evaluation of six combinations of reaggregation and maturation media to investigate the conditions. Immunostaining was conducted on human-mouse chimeric renal organoids using representative markers of nephron segments, including WT1 (expressed in differentiating NPCs and glomeruli) and E-cadherin-1 (ECAD specific to distal tubules), as well as the representative marker of ureteric buds (UBs), CK8. Regardless of the culture conditions, the formed human-mouse chimeric renal organoids exhibited WT1-positive glomerular structures, ECAD-positive distal tubule structures, and CK8-positive UB structures (Fig. 2a–d). No significant differences were observed in the CK8 positivity rate under any of the culture conditions (Fig. 2e). However when NPC_Re-agg was used as the reaggregation medium, organoids exhibited a higher proportion of Ku80- or HuNu-positive human cells and a greater density of kidney structures compared to organoids cultured in DMEM/F12 with 10% FBS (Fig. 2a–d). In the groups where KR5_Mat was used as the maturation medium, irrespective of the reaggregation medium used, numerous tubular structures were observed (Fig. 2a–d). Images of organoids costained for Ku80 and HuNu, human cell markers, demonstrated the formation of a “chimeric structure” including nephron structures that are part human and part mouse (Fig. 2a–d). These data suggest that the formed organoids are not mere mixtures of heterologous kidney cells but have common structures that develop in a coordinated manner.
Fig. 2. Evaluation of human-mouse chimeric renal organoids formed under different culture conditions.
a, b Immunostaining images of chimeric renal organoids at Day 6 that were stained using antibody against a distal tubule marker, a ureteric bud marker, and a human cell marker. Scale bars represent 200 μm (a: original images) and 100 μm (b: magnified images). c, d Immunostaining images of chimeric renal organoids at Day 6 that were stained using antibody against a glomerulus marker, a ureteric bud marker, and a human cell marker. Scale bars represent 200 μm (c: original images) and 100 μm (d: magnified images). e Cellular composition analysis based on immunostaining images (n = 3 independent experiments; mean ± s.d.; *P < 0.05, **P < 0.01; one-way ANOVA followed by the Tukey test). f Quantitative analysis of chimera formation rate. The left graph indicates the chimera rate in distal tubule staining images, while the right graph indicates the chimera rate in glomerulus staining images (n = 3 independent experiments; mean ± s.d.; **P < 0.01; one-way ANOVA followed by the Tukey test). g Immunostaining images of chimeric renal organoids at Day 2 of culture in the combination of NPC_Re-agg and NPC_Mat media. White arrowhead indicates cap structure, yellow arrowheads indicate RVs, yellow arrow indicates comma-shaped body, and white arrow indicates S-shaped body. Scale bars represent 200 μm.
Chimeric renal organoid formation was demonstrated under all the investigated culture conditions. On the other hand, cell population analysis conducted on the 6th day of maturation culture revealed variations in the % area of human cells, ECAD-positive cells, and WT1-positive cells depending on the culture conditions (Fig. 2e). Particularly, using NPC_Re-agg as the reaggregation medium maintained the human cell content at a high level on the 6th day of maturation culture (Fig. 2e). This result emphasized the importance of the surrounding environment during reaggregation, and in contrast to the previously reported chimeric renal organoid reaggregation medium, NPC_Re-agg contained CHIR99021 and FGF9, suggesting that the activation of Wnt and FGF9 signals significantly contributed to human cell survival during chimeric renal organoid reaggregation.
Furthermore, using NPC_Re-agg as the reaggregation medium and NPC_Mat as the maturation medium, the highest nephron segment rate was achieved (Fig. 2e). On the other hand, even with NPC_Re-agg as the reaggregation medium, maturation with KR5_Mat resulted in a significant predominance of ECAD-positive cells (Fig. 2e), implying an influence on the differentiation and orientation of each nephron segment of NPCs.
Calculating the chimera formation rate of human-mouse chimeras, focusing on both glomeruli and distal tubules in the chimera evaluation, the combination of NPC_Re-agg and NPC_Mat significantly improved the chimera formation rate (Fig. 2f). Chimeric renal organoids cultured in the combination of NPC_Re-agg and NPC_Mat media exhibited chimeric organization, with human NPCs contributing to mouse cap structures at the early stage of maturation culture, and chimeric structures characteristic of the renal vesicle (RV) (Fig. 2g and Supplementary Fig. 2), comma-shaped body, and S-shaped body stages (Fig. 2g).
The combination of NPC_Re-agg and NPC_Mat improved the formation of human, mouse, and human-mouse chimeric renal organoids, compared to the previous report20. Additionally, the composition of the medium was shown to influence the ratio of components in the chimera.
Evaluation of fetal pig kidney development and formation of fetal pig kidney organoids
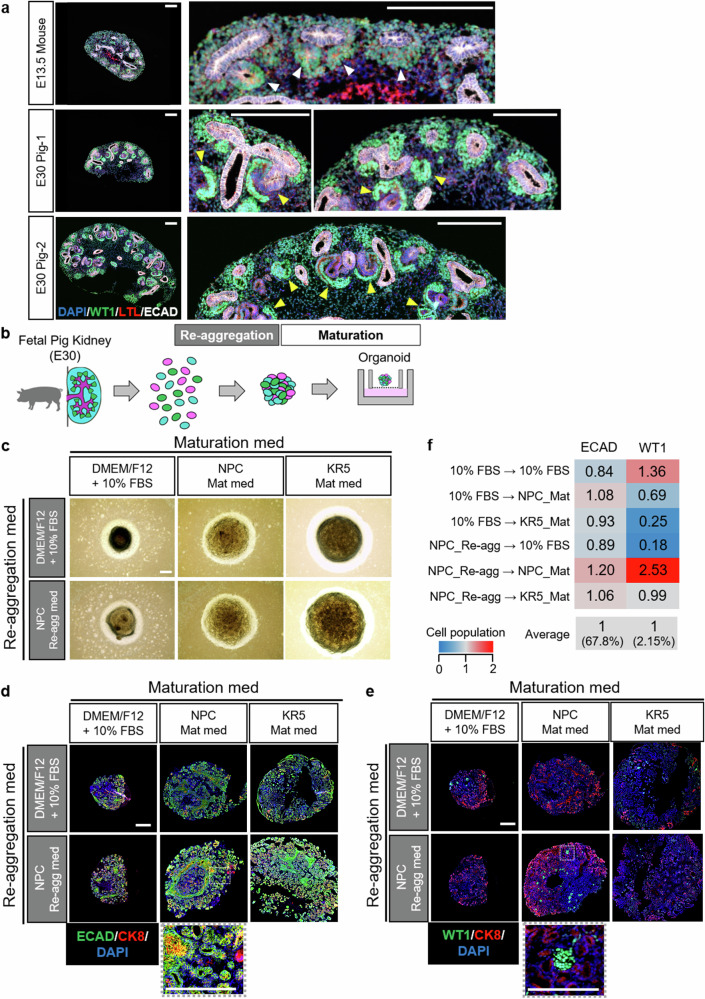
Next, we evaluated whether the selected culture conditions were suitable for forming organoids using fetal pig kidney cells. Initially, fetal pig kidneys were selectively collected through microscopic surgery of E30 porcine fetuses immediately after their removal from the uterus. The size and morphology of the harvested E30 porcine kidneys were similar to those of conventionally used E13.5 mouse kidneys. Multiple cap structures were observed beneath the renal capsule in the porcine E30 kidneys (Fig. 3a). Morphological analysis indicated that while the E13.5 mouse kidneys used in this study showed nephron development up to the RV stage, the E30 porcine kidneys exhibited structures progressing up to the comma-shaped and S-shaped body stages, albeit with some variation among individual porcine fetuses (Fig. 3a). These data suggest a slightly more advanced stage of development in E30 porcine kidneys compared to E13.5 mouse kidneys used in this study (Fig. 3a).
Fig. 3. Generation and evaluation of fetal pig kidney organoids.
a Histological comparative analysis of E13.5 mouse kidney and E30 pig kidney. Individual variation in fetal pig kidney size was observed, and representative examples of larger (E30 Pig-1) and smaller (E30 Pig-2) kidneys were presented. White arrowheads indicate RVs and yellow arrowheads indicate comma-shaped bodies or S-shaped bodies. Scale bars represent 200 μm. b Experimental scheme of organoid formation using fetal pig kidneys. c Bright-field images of pig kidney organoids formed under different culture conditions. Scale bar represents 200 μm. d Immunostaining images of pig kidney organoids at Day 6 that were stained by antibody against a distal tubule marker and a ureteric bud marker. Scale bar represents 200 μm. e Immunostaining images of pig kidney organoids at Day 6 that were stained by antibody against a glomerular marker and a ureteric bud marker. Scale bar represents 200 μm. f Quantitative analysis of cell composition ratio for fetal pig kidney organoids under various culture conditions.
Similar to the study in mice, single-cell dissociation was performed on porcine E30 kidneys, and reaggregation and maturation media were compared. Organoids were formed under all culture conditions (Fig. 3b, c). However, the organoid sizes varied with each culture condition. Regardless of the reaggregation medium used, larger porcine fetal organoids were formed when the maturation medium was either NPC_Mat or KR5_Mat, compared to the conventional medium (Fig. 3c).
Immunostaining analysis conducted on the 6th day of maturation revealed differences in the sizes of porcine fetal kidney organoids based on the culture medium (Fig. 3d, e). However, all porcine fetal kidney organoids were confirmed to possess kidney structures positive for WT1, ECAD, and CK8 (Fig. 3d, e), while they were negative for human cell markers, Ku80 and HuNu (Supplementary Fig. 3a, b). Moreover, irrespective of the culture condition, ECAD-positive structures predominated over WT1-positive ones, and a bias towards the distal tubules was observed in nephron segments (Fig. 3d–f). The combination of reaggregation medium NPC_Re-agg and maturation medium NPC_Mat yielded the highest number of WT1-positive cells (Fig. 3d–f).
Formation and evaluation of human-pig chimeric renal organoids
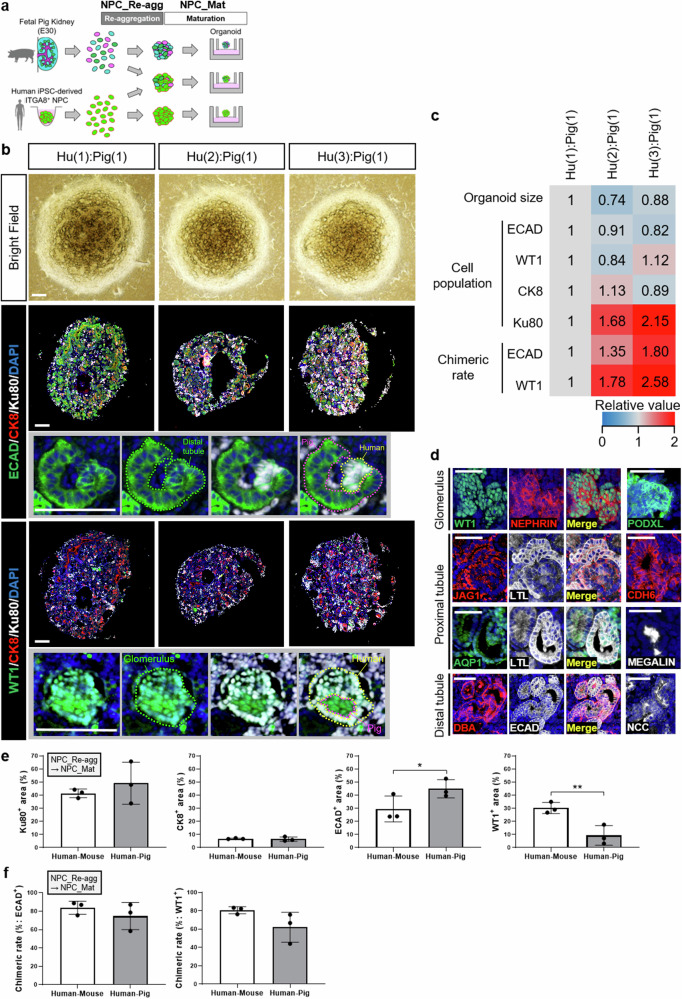
Based on the results of the porcine fetal kidney organoid culture conditions (Fig. 3b–f) and the evaluation of human-mouse chimeric renal organoids (Figs. 1c, d, 2a–f), we selected the combination of reaggregation medium NPC_Re-agg and maturation medium NPC_Mat for initiating the cultivation of human-porcine chimeric renal organoids (Fig. 4a). We proceeded with the cultivation of human-porcine chimeric renal organoids, varying the human-porcine cell mixture ratios to observe their impact on chimeric organoid formation. The cell resources included E30 porcine fetal kidneys and human iPSC-derived NPCs, the latter having been further enriched using magnetic-activated cell sorting (MACS).
Fig. 4. Generation and evaluation of human-pig chimeric renal organoids.
a Experimental scheme for generating human-pig chimeric renal organoids using fetal pig kidneys and human iPSC-derived NPCs. b Bright-field images and immunostaining images of chimeric renal organoids formed at different human-pig mixing ratios and stained by a distal tubule marker, a glomerular marker, and a human cell marker. Scale bars represent 200 μm. c Quantitative analysis of size, cellular composition ratio, and chimeric structure formation rate using human-pig chimeric renal organoids at different human-pig cell mixing ratios. d Images of human(3)-pig(1) chimeric renal organoids immunostained by antibodies at various developmental stages of each nephron segment. Scale bars represent 100 μm. e Cellular composition analysis based on images of organoids after immunostaining. The quantitative data of human-pig chimeric renal organoids were obtained from chimeric organoid images with a human-pig cell mixing ratio of 3:1. The quantitative data of human-mouse chimeric renal organoids were reutilized from Fig. 2e (n = 3 independent experiments; mean ± s.d.; *P < 0.05, **P < 0.01; two-tailed t-test). f Quantitative analysis of chimera formation rate. The left graph indicates the chimera rate in images of stained distal tubules, while the right graph indicates the chimera rate in images of stained glomeruli. The quantitative data of human-pig chimeric renal organoids were obtained from chimeric organoid images with a human-pig cell mixing ratio of 3:1. The quantitative data of human-mouse chimeric renal organoids were reutilized from Fig. 2f (n = 3 independent experiments; mean ± s.d.; two-tailed t-test).
By utilizing these two cell resources and cultivating them under the selected culture conditions, we confirmed the formation of human-porcine chimeric renal organoids (Fig. 4b). Immunostaining using a distal tubule marker revealed the formation of chimeric structures in multiple locations within the human-porcine chimeric renal organoids (Fig. 4b).
Immunostaining analysis using a glomerular marker showed that glomeruli were present in very few numbers within the human-porcine chimeric renal organoids (Fig. 4b). Similar findings were observed in porcine fetal kidney organoids (Fig. 3d–f), suggesting that the origin of this phenomenon was likely the porcine fetal kidney rather than the human NPC component. However, the few glomerular structures identified had chimeric structure, indicating that human NPCs demonstrated chimera-forming ability within porcine glomeruli (Fig. 4b).
The quantitative analysis demonstrated that, regardless of variations in the mixing ratio of human and pig cells, the size of organoids remained largely consistent (Fig. 4b, c). No significant differences were observed in the composition ratio of each nephron segment or UBs. However, the composition ratio of human cells increased and interestingly the rate of chimeric renal structure formation increased in a human cell mixing ratio-dependent manner (Fig. 4b, c).
In terms of both human cell inclusion rate and chimeric structure formation rate, immunostaining analysis of chimeric renal organoids at the optimal human-pig mixing ratio of 3:1 revealed the expression of various glomerular markers, proximal tubule markers, and distal tubule markers, indicating the presence of late developmental stage in the generated chimeric renal organoids (Fig. 4d). Similar staining patterns were observed in fetal pig kidney organoids and human NPC-derived organoids cultured under the same conditions (Supplementary Fig. 4a, b). These results suggest that the selected culture conditions facilitate the coexistence and coincident development of human and pig renal cells, leading to the induction of certain levels of differentiation and maturation in the organoids.
Comparison between human-pig and human-mouse chimeric renal organoids at a 3:1 mixing ratio revealed characteristic cellular compositions, where the human-pig chimeric organoids exhibited a higher proportion of ECAD-positive distal tubules and a lower proportion of WT1-positive glomeruli compared to human-mouse chimeric organoids (Fig. 4e). However, no significant differences were observed in the rate of chimeric structure formation (Fig. 4f). These findings suggest that, from the perspective of chimeric structure formation, human-pig chimeric renal organoids are comparable to human-mouse chimeric renal organoids.
Utilization of selected culture conditions for generation of humanized xenogeneic kidneys
Targeting clinical applications, we next conducted investigations to validate the applicability of the selected culture conditions for the establishment of humanized xenogeneic kidneys. In our previous study, we confirmed that human NPCs can mature and connect with mouse UBs, reaching the RV stage when cultured in MEMα medium supplemented with FBS16. However, the engraftment efficiency of human NPCs was low, and the formation of chimeric structures was limited. Additionally, it is essential for kidney development that NPCs form a cap-like structure surrounding the UB during its early stages. Our previous reports lacked conclusive evidence of a distinct human-mouse chimeric cap-like structure, which may have hindered further progression of kidney development16. Therefore, in this study, we investigated whether the engraftment and chimera formation abilities of human NPCs could be enhanced, as well as foster the formation of chimeric cap-like structures, by culturing them in selected conditions using xenogeneic embryonic kidneys after injection (Fig. 5a).
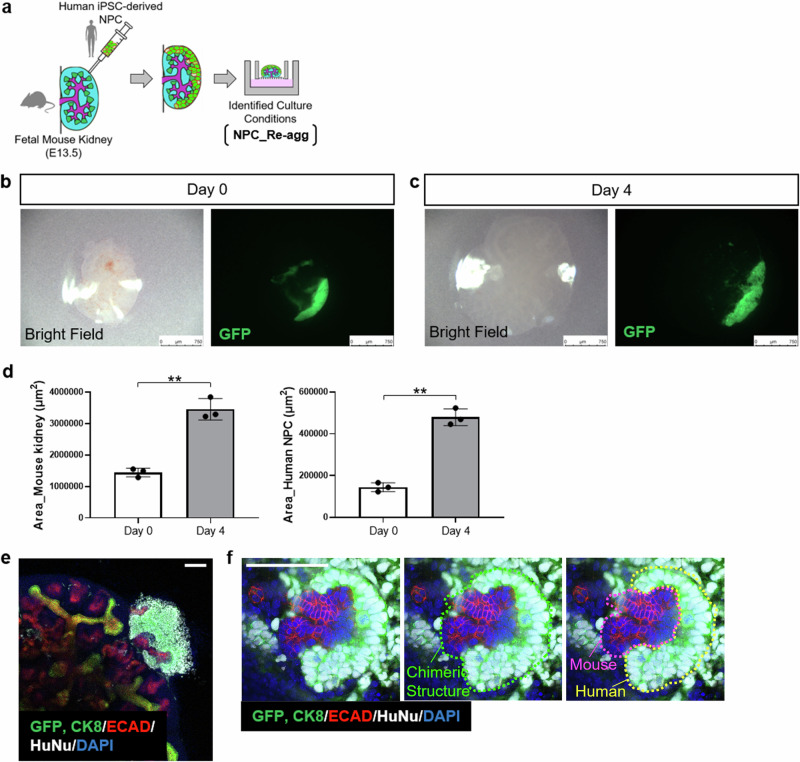
Fig. 5. Evaluation of human NPC-injected fetal mouse kidney using specific culture conditions.
a Experimental scheme for the generation and in vitro culture of human-mouse chimeric kidneys. b Fluorescence stereomicroscope images of fetal mouse kidney immediately after injection of human NPC. Scale bar represents 750 μm. c Fluorescence stereomicroscope images of human NPC-injected fetal mouse kidney cultured for 4 days under the identified culture conditions. Scale bar represents 750 μm. d Quantitative analysis of the area of human NPC-injected fetal mouse kidneys and the injected human NPC (n = 3 independent experiments; mean ± s.d.; **P < 0.01; two-tailed t-test). e, f Immunostaining images of human NPC-injected fetal mouse kidney cultured for 4 days under the identified culture conditions. Scale bar represents 100 μm.
EGFP-labeled human iPSC-derived NPCs were dissociated into single cells and injected under the renal capsule of E13.5 mouse embryos, which were then cultured at the air-liquid interface in NPC_Re-agg medium for four days. Microscopic examination of the injected fetal mouse kidneys immediately after injection revealed that the morphology of the recipient kidneys remained intact, and the amount of EGFP-labeled human NPCs injected beneath the renal capsule surface was observed (Fig. 5b). By the fourth day of culture, it was quantitatively confirmed that the recipient fetal mouse kidneys had increased in size, and the human NPCs injected beneath their capsule also exhibited proportional enlargement (Fig. 5c, d).
Immunostaining analysis revealed thick and layered engraftment of human cells beneath the mouse embryonic renal capsule, forming a three-dimensional structure (Fig. 5e). Furthermore, high-magnification images demonstrated the engraftment of human cells at the tip of the mouse UB-like structure, strongly indicating the formation of chimeric cap-like structure (Fig. 5f and Supplementary Movie 1). These results demonstrate that the selectively chosen culture conditions contribute to the survival and chimera formation ability of human NPCs injected into mouse fetal kidneys beneath the subrenal capsule. Furthermore, the resulting chimeric structures exhibited characteristic features of early kidney development, signifying the achievement of a milestone on the pathway to the future acquisition of renal functionality in humanized xenogeneic kidneys.
Discussion
This study established a cultivation system enabling cross-species nephrogenesis in the early stages of kidney development by refining the existing culture conditions for human-mouse chimeric renal organoids. This optimized culture system allows for the coexistence of both the animal and human cellular components at least during the early stage of nephrogenesis and facilitates more efficient interspecies chimera formation. Furthermore, the identified optimized culture conditions were found to be suitable for the formation of porcine fetal kidney organoids. Ultimately, we achieved the first successful creation of human-porcine chimeric renal organoids. The ability of human NPCs to form chimeric renal organoids with porcine cells strongly supports the potential of utilizing the developmental environment of porcine fetus, such as through fetal organ complementation, to enable the development of human renal precursor cells. In addition, we discovered that using selected conditions to culture fetal mouse kidneys, into which human NPCs were subcapsularly injected, improved the survival of human cells and enhanced chimera formation. These findings hold significant promise for future research on the production of human-xenogeneic chimeric kidneys for clinical purposes.
In order to improve cell survival and chimera formation rates in human-mouse chimeric renal organoids and achieve the formation of human-pig chimeric renal organoids, we initially explored conditions for the stable organoid maturation of the constituent cells, human NPCs, and fetal mouse (or pig) kidney cells. Through the investigation of conditions, we identified culturing conditions where cells from different animal species can survive within the chimeric renal organoids. Consequently, it is believed that the opportunities for interspecies cell-cell interactions increased, leading to a higher chimera formation rate. These findings suggest that the optimization of culture conditions can surpass the barrier mechanisms that suppress interspecies chimera formation21, indicating a significant developmental insight.
In particular, a significant improvement in the human cell ratio within chimera organoids was observed when NPC_Re-agg, containing CHIR99021 and FGF9, was used as a reaggregation culture medium (Fig. 2e). Taguchi et al. demonstrated that the addition of low-concentration CHIR99021 and FGF9 is crucial in inducing differentiation from posterior intermediate mesoderm to metanephric nephron progenitors using human iPSCs28. FGF9 has been reported to contribute to the maintenance and proliferation of human NPCs30. Previous studies on renal development using rodents have also reported that the formation of the metanephric mesenchyme critically relies on the FGF receptors, while the maintenance of the metanephric mesenchyme necessitates FGF9/FGF2030,31. Therefore, it is believed that the FGF9 signaling pathway significantly contributes to the survival and development of not only mouse renal cells but also human NPCs in chimera organoids.
Recently, Li et al. successfully induced the differentiation of renal organoids from naïve-like embryonic stem cells (ESCs) derived from pigs32. Although our study differs from theirs in terms of the original cellular resources used, they also employed FGF9 and Wnt signal activators in the process of porcine renal organoid generation. Considering that their porcine ESC-derived renal organoids were produced by appropriately controlling both signals, which is analogous to the human kidney development process33–35, it is suggested that FGF9 and Wnt signals are also important factors in pig kidney development. Thus, the observed commonalities between human and pig kidney development processes are believed to be one of the contributing factors facilitating the coexistence and chimera formation of human NPCs and pig fetal kidney cells in our study.
On the other hand, when KR5_Mat was used as the maturation medium, although the survival rate of human NPCs and fetal mouse kidney cells was maintained, the chimera formation rate was lower (Fig. 2f). KR5_Mat provides a strong Wnt signal in the early stages of maturation culture, and it has been reported that excessive Wnt signal not only accelerates nephron development but also biases differentiation towards the distal tubules36–38. Furthermore, Chen et al. demonstrated through experiments involving the injection of embryonic mouse NPCs into cap mesenchyme of mouse fetal kidneys at different developmental stages, the developmental timing difference significantly influences the survival and differentiation of injected NPCs39.
Thus, it is postulated that the strong action of the Wnt signal disrupted the balance of self-renewal and differentiation of human NPCs, leading to an increased population of epithelialized or differentiated NPCs, as well as causing a mismatch in developmental stages between human and mouse. Consequently, this perturbation is believed to have resulted in a reduction of opportunities for cell-cell interactions in the appropriate cellular state and mistiming of chimera formation. In cells cultured in NPC_Mat medium, which maintains a relatively low Wnt signal condition, no bias in differentiation was observed, and efficient chimera formation was achieved (Fig. 2f).
This suggests that not only cell survival rates but also the regulation of differentiation direction, as well as the synchronization of developmental stages between mice and humans as previously discussed40,41, may be important factors for chimera formation. Further research, considering differences among animal species, is anticipated to shed more light on this matter.
As shown in Fig. 3, differences in the composition of nephron segments were observed between porcine and murine fetal kidney organoids. In this study, E30 pig and E13.5 mouse kidneys were used as cellular resources, considering potential limitations in the accuracy of pregnancy detection methods and inter-individual variations. Nonetheless, at least in the case of the E30 porcine kidney, a greater abundance of late-stage nephron structures was observed. Furthermore, in E13.5 mouse kidney organoids, an increase in the proportion of distal tubules dependent on Wnt signaling during culture was observed, while in fetal pig kidney organoids, the composition of cell types remained relatively stable regardless of the strength of Wnt signaling (Fig. 3d–f). Considering the decrease in NPCs and the increase in tubular structures as nephron development progresses, the observed differences in the nephron segment composition in this study cannot be entirely disregarded as solely due to the differences in animal species. It is believed that at the very least, variations in the developmental origins have influenced these differences.
The chimeric renal structures observed in this study were mosaic chimeras, where some of the renal structures were replaced by the host animal components. This is likely attributed to the fact that the host animal NPCs were not excluded in this experimental system. The future development of a system that selectively excludes host pig NPCs, such as Six2-DTA systems16, is expected to further enhance the chimera formation rates and achieve complete humanization of NPCs in the porcine kidney.
NPCs are known to exist beneath the renal capsule during the fetal period. In order to generate humanized xenogeneic kidneys for future clinical applications, we endeavored to inject human NPCs beneath the renal capsule of fetal mice. In this study, we successfully identified culture conditions that improve the survival and chimera formation of human NPCs injected beneath the renal capsule, thereby improving not only the formation of chimeric renal structures but also confirming the presence of cap-like structures characteristic of early kidney development. This indicates the incorporation of human NPCs into early mouse development and holds promise for further kidney development. While a similar investigation using the human-pig combination has not been conducted in this study, these findings could serve as a pivotal milestone on the pathway toward the future generation of xenogeneic chimera kidneys with potential clinical immunological advantages compared to genetically edited xeno-kidneys that still retain inherent risks of immune rejection.
There are still remaining challenges to fully exploiting the potential of our human-xenogeneic chimeric renal models to acquire renal function. Long-term maturation of the chimeric renal organoid and the human NPC-injected chimeric kidney necessitates sufficient nutrient and oxygen supply to the internal structure; furthermore, from a physical standpoint, blood inflow to the glomeruli is necessary for them to acquire filtration capacity. In other words, we believe that transplantation into the animal body and vascular invasion are essential for functional acquisition in both renal models42. In fact, in our previous study, we confirmed the feasibility of urine production through the transplantation of mouse-rat chimeric kidney models created by injecting fetal rat renal cells into fetal mouse kidneys with endogenous NPCs that can be eliminated through drug induction and transplanting the chimeric kidneys into immunodeficient adult mice16.
However, despite our recent attempts to transplant human-mouse chimeric renal organoids into the subcapsular region of host immunodeficient mice, differentiation and maturation of the grafts pose challenges to the full realization of our chimeric renal organoids. Sharmin et al. have demonstrated that even human NPCs, when transplanted into the subcapsular region of mouse kidneys, can form functional glomeruli with Bowman’s space. They co-cultured human NPCs with mouse embryonic spinal cord to promote differentiation and maturation, followed by transplantation of these two components together. Furthermore, they co-transplanted agarose rods soaked in VEGF solution to induce host vascularization43. The implementation of such techniques for post-transplantation renal development and host vascularization promotion may offer valuable solutions to the problem of generating chimeric renal organoids that are functional. Another suitable approach may involve transplanting chimeric renal organoids with vascular networks obtained through pre-cultivation in artificially scaffolded environments such as microphysiological systems utilizing fluidic dynamics.
Similar technical challenges are anticipated for the acquisition of renal function in xenogeneic kidney models with human NPC injection. In particular, the introduction of the system for removing endogenous NPCs in fetal kidneys, as mentioned earlier, would be a crucial element to expedite research toward the creation of functional humanized xenogeneic kidneys.
In summary, this study provides evidence showing that the cross-species renal development can be achieved by optimizing cultivation conditions, using human, mouse, and pig renal cells. While further advancement of renal development to acquire kidney function remains a future challenge, the established cultivation technique holds great potential for accelerating future research on humanized pig kidney generation, aimed at clinical applications.
Methods
Human iPSC culture
The human iPSC line, iPSC-S09, and EGFP-labeled human iPSC lines, iPSC-S09 and 317-12-Ff 44, were used in this study. The iPSC-S09 line was established by Sumitomo Pharma Co., Ltd. from peripheral blood cells using Sendai virus vectors. The cells were maintained on iMatrix-511 (Nippi. Inc., Osaka, Japan) in StemFit medium (Ajinomoto Co., Inc., Tokyo, Japan), and cultured in a humidified atmosphere of 5% CO2 at 37 °C. The cell culture medium was changed every 1–2 days and passaged every 7 days by treatment with TrypLE Select Enzyme (Thermo Fisher Scientific Inc., MA, USA).
The experimental protocols using clinical-grade human iPSCs were approved by the Research Ethics Committee of Sumitomo Pharma Co., Ltd., Japan.
Induction of NPCs from human iPSCs
NPCs were induced from human iPSCs according to previously established methods28,45 with slight modification. Briefly, on the day of induction (day 0), 10,000 iPSCs per well were seeded in a PrimeSurface 96 Slit-Well plate (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) containing basal medium for human NPCs (NPC basal medium)28,45, which was supplemented with 3 ng/mL human activin A (R&D Systems, Inc., Minneapolis, MN, USA), 1 ng/mL human bone morphogenetic protein 4 (Bmp4) (R&D Systems, Inc.), 20 ng/mL human basic fibroblast growth factor (bFGF) (R&D Systems, Inc.), and 10 μM Y27632 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). After 24 h of incubation (day 1), the culture medium was changed to the NPC basal medium with 10 μM CHIR99021 (FUJIFILM Wako Pure Chemical Corporation). Every other day thereafter (days 3 and 5), half of the medium was replaced with NPC basal medium containing 10 μM CHIR99021 (FUJIFILM Wako Pure Chemical Corporation) and 10 μM Y27632 (FUJIFILM Wako Pure Chemical Corporation). On day 7, the medium was switched to the NPC basal medium, which was supplemented with 10 ng/mL human activin A (R&D Systems, Inc.), 5 ng/mL human Bmp4 (R&D Systems, Inc.), 3 μM CHIR99021, 0.1 μM retinoic acid (Sigma–Aldrich, MO, USA), and 10 μM Y27632 (FUJIFILM Wako Pure Chemical Corporation). On day 10, the medium was changed to the NPC basal medium, which was supplemented with 1 μM CHIR99021 (FUJIFILM Wako Pure Chemical Corporation), 5 ng/mL fibroblast growth factor 9 (FGF9) (Abcam plc., Cambridge, United Kingdom), and 10 μM Y27632 (FUJIFILM Wako Pure Chemical Corporation). On day 13, the spheres were collected for experimental use.
The NPCs used for injection into the fetal mouse kidney were induced following a previously reported method46.
Cell sorting
For the selection of integrin subunit alpha 8 (ITGA8)-positive NPCs, anti-Biotin MicroBeads UltraPure (Miltenyi Biotec B.V. & Co. KG, Bergisch Gladbach, Germany), MS Columns (Miltenyi Biotec B.V. & Co. KG), and a MiniMACS Separator (Miltenyi Biotec B.V. & Co. KG) were used according to the manufacturer’s protocols.
Research animals
The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments involving the collection of fetuses from pregnant pigs were conducted in the laboratory of IVTeC Co., Ltd. (Tokyo, Japan) with the approval of the Animal Experiment Ethics Committee of IVTeC Co., Ltd. (approval numbers: IVT22-191 and IVT23-95). Experiments related to organoids were conducted in the laboratory of Sumitomo Pharma Co., Ltd. with the approval of the Institutional Animal Care and Use Committee of Sumitomo Pharma Co., Ltd. (approval number: AN14292). Experiments related to humanized xenogeneic kidneys were conducted in the laboratory of the Jikei University School of Medicine with the approval of the Institutional Animal Care and Use Committee of the Jikei University School of Medicine (approval numbers: 2018-066 and D2021-070). We have complied with all relevant ethical regulations for animal use. Every effort was made to minimize animal suffering. Pregnant female C57BL/6NCrSlc mice on embryonic day 13.5 were purchased from Japan SLC, Inc. (Shizuoka, Japan). Pregnant female Microminipigs on embryonic day 30 were purchased from Fuji Micra, Inc. (Shizuoka, Japan).
Collection and single-cell dissociation of fetal kidneys
Pregnant pigs and mice were subjected to cesarean sections to excise the uterus. The excised uteri were rinsed with PBS (Thermo Fischer Scientific, Inc.), after which they were incised with surgical scissors to expose the fetuses. The fetuses were then separated from the placenta, and transferred to culture dishes containing PBS. Under microscopic observation, the head of each fetus was removed, and further dissection was performed to separate the spinal cord from the dorsal aspect of the trunk. The exposed fetal kidneys were grasped with forceps, detached, and subsequently collected into a 1.5 mL tube containing a minimum essential medium (MEMa; Thermo Fisher Scientific, Inc.).
Single cells from pig and mouse fetal kidneys were obtained following a previously reported method20,25,47. In brief, the tube containing fetal kidneys was centrifuged at 700 g for 3 min, the supernatant was removed, and 1 mL of accutase (AT104, Innovative Cell Technologies, Inc. CA, USA) was dispensed. The sample was vortexed and incubated at 37 °C for 5 min (repeated twice), and further manual pipetting and incubation were performed again for 5 min. The cell suspension was then centrifuged at 300 g for 5 min to remove the subsequent accutase supernatant. The pellet was resuspended in 1 mL of PBS with 10 μM Y27632 (FUJIFILM Wako Pure Chemical Corporation). In addition, the cells were passed through a 40 μm cell strainer (Corning Incorporated, NY, USA) to remove clumps of cells and obtain a single-cell suspension of mouse or pig cells.
Chimeric renal organoids culture
In accordance with the methods described above, ITGA8-positive human NPCs, as well as dissociated fetal mouse kidney cells or fetal pig kidney cells, were prepared.
The cell density for all samples was adjusted to 1 × 106 cells/mL in DMEM/F12 medium (Thermo Fisher Scientific Inc.) with 10% FBS and 10 μM Y27632 (FUJIFILM Wako Pure Chemical Corporation) or NPC basal medium with 1 μM CHIR99021 (FUJIFILM Wako Pure Chemical Corporation), 5 ng/mL FGF9 (Abcam plc.), and 10 μM Y27632 (FUJIFILM Wako Pure Chemical Corporation), referred to as “NPC_Re-agg”.
The human-mouse mixture was prepared in a ratio of 1:3, and the human-pig mixtures were prepared at ratios of 1:1, 2:1, or 3:1, respectively. The mixtures were seeded into a V-bottomed 96-well low-binding plate (Sumitomo Bakelite Co., Ltd.) to achieve a total of 2 × 105 cells/well containing 200 µL of culture medium. The 96-well plate was centrifuged at 1000 rpm for 4 min and cultured overnight in a 5% CO2 incubator at 37 °C.
The formed mixture aggregates were transferred to the air-liquid interface and cultured for a maximum of 6 days to promote maturation. As the maturation medium, we used DMEM/F12 medium (Thermo Fisher Scientific Inc.) with 10% FBS, NPC basal medium with 1 μM CHIR99021 (FUJIFILM Wako Pure Chemical Corporation) and 10 ng/mL FGF9 (Abcam plc.), referred to as “NPC_Mat”, or KR5 medium26,27 with 4.5 μM CHIR99021 (FUJIFILM Wako Pure Chemical Corporation) and 200 ng/mL bFGF (R&D Systems, Inc.), referred to as “KR5_Mat”. After 48 h of maturation culture, NPC_Mat medium without CHIR99021 and KR5_Mat medium without CHIR99021 and bFGF were prepared and exchanged.
The same method as described above was employed even in the case of non-chimeric renal organoid cultures.
Injection of human NPCs into the subrenal capsule of fetal mice
Human iPSC-derived NPCs, without cell sorting, were injected under the capsule of the embryonic kidney according to a previously reported method17. In detail, C57BL/6NCrSlc mice were euthanized by cervical dislocation at 13.5 days of gestation. The fetuses were then removed with the uterus and placed in a 10-cm dish containing Hank’s Balanced Salt Solution. Thereafter, the fetuses were separated from the uterus and euthanized via decapitation. First, a cut was made along the spinal cord from the base of the hindlimb of the fetus, and a similar cut was made on the other side to take out the vertebral column. After the kidneys on both sides of the fetus were visible, the fetus was fixed with micro-tweezers. Subsequently, progenitor cells were placed in glass needles. Afterward, the glass needles were inserted into the renal capsule through the renal hilus under a fluorescent stereomicroscope (Leica M205FA, Leica Microsystems GmbH, Wetzlar, Germany), and the human NPCs were injected under the renal capsule by mouth pipetting. The injected kidneys were detached from the fetus and then transferred to the air-liquid interface for 4 days of in vitro culture. As the organ culture medium, we used NPC basal medium with 1 μM CHIR99021, 5 ng/mL FGF9, and 10 μM Y27632, referred to as “NPC_Re-agg”. After 24 h of culture, NPC_Re-agg medium without Y27632 was prepared and exchanged.
Frozen section immunostaining
In vitro cultured organoids, fetal mouse kidneys, and fetal pig kidneys were fixed with 4% paraformaldehyde (FUJIFILM Wako Pure Chemical Corporation) and sectioned with a cryostat (Leica Microsystems GmbH) to prepare frozen sections. Frozen sections were treated with antigen retrieval reagent (RM102-H, LSI Medience Corporation, Tokyo, Japan) at 121 °C for 5 min. The primary antibodies used were as follows: anti-Wilms’ tumor-1 (WT1; 1:400; ab89901, Abcam plc./1:100; sc-7385, Santa Cruz Biotechnology Inc., TX, USA), anti-cytokeratin 8 (CK8 (TROMA-I) ; 1:100, DSHB, IA, USA), anti-human nuclei (HuNu; 1:100; MAB4383, Merck Millipore, MA, USA), anti-Ku80 (1:100; 2180S, Cell Signaling Technology, Inc., MA, USA), anti-lotus tetragonolobus lectin (LTL; 1:500; B-1325, Vector Laboratories, Inc., CA, USA), anti-E-cadherin-1 (ECAD; 1:500; 610181, BD Biosciences, CA, USA), anti-nephrin (1:500; GP-N2, Vector Laboratories, Inc.), anti-podocalyxin (PODXL; 1:40; AF1658, R&D Systems, Inc.), anti-Jagged 1 (JAG1; 1:300; sc-390177, Santa Cruz Biotechnology Inc.), anti-cadherin 6 (CDH6; 1:50; AF1658, R&D Systems, Inc.), anti-aquaporin 1 (AQP1; 1:100; ab9566, Abcam plc.), anti-megalin (1:300; sc-515772, Santa Cruz Biotechnology Inc.), anti-Dolichos biflorus agglutinin (DBA; 1:500; B-1035, Vector Laboratories, Inc.), and anti-sodium-chloride cotransporter (NCC; 1:1000; AB3553, Merck Millipore). Nuclear counterstaining was performed with DAPI (Nacalai Tesque, Inc., Kyoto, Japan). Stained sections were analyzed with the imaging cytometer, IN Cell Analyzer 6000 (Cytiva, Tokyo, Japan). Image analyses were performed with the imaging software, IN Cell Developer Toolbox (Cytiva).
Whole-mount immunostaining
Cultured fetal mouse kidneys injected with human NPCs were fixed with 4% paraformaldehyde in PBS for 15 min at 4 °C and washed three times with PBS. Samples were blocked using 1% donkey serum, 0.2% skimmed milk, and 0.3% Triton X/PBS for 1 h at room temperature, and incubated overnight at 4 °C with primary antibodies. The primary antibodies used were as follows: anti-cytokeratin 8 (CK8 (TROMA-I); 1:100, DSHB), anti-human nuclei (HuNu; 1:100; MAB4383, Merck Millipore), and anti-E-cadherin-1 (ECAD; 1:500; 610181, BD Biosciences). After washing three times with PBS, the samples were incubated with secondary antibodies for 1 h at room temperature. Samples were mounted with ProLong Gold Antifade Mountant containing DAPI (P36931, Thermo Fisher Scientific Inc.). Each sample was examined under an LSM880 confocal laser scanning microscope (Carl Zeiss AG, Baden-Württemberg, Germany).
Measurement of the chimeric rate
Immunostained sections of frozen organoids were captured using a fluorescence microscope. Chimeric structures were defined as renal structures with ECAD-positive cells containing Ku80-positive cells and renal structures with WT1-positive cells containing HuNu- or Ku80-positive cells. The number of chimeric structures and the total number of renal structures were quantified. The chimera formation rate was calculated by dividing the number of chimeric structures by the total number of renal structures. For chimera rate calculation, three sections from the central slice of the organoid specimen and two additional sections located 50 µm apart from anterior and posterior directions were used. Three independent experiments were conducted.
Area-based cell composition analysis
Immunostained sections of frozen organoids were captured using a fluorescence microscope. Using the IN Cell Developer Toolbox (Cytiva), the fluorescence intensity of the organoid slice images was used to calculate the positive areas of ECAD, WT1, CK8, Ku80, and DAPI. The positive area of each marker was divided by the DAPI-positive area to calculate the proportion of each constituent cell type within the organoid. For cell composition analysis, three sections from the central slice of the organoid specimen and two additional sections located 50 µm apart from anterior and posterior directions were used.
The fetal mouse kidneys injected with human NPCs expressing EGFP were subjected to whole-tissue imaging using the EVOS FL Cell Imaging System (Thermo Fisher Scientific Inc.) to acquire brightfield and fluorescence images. The image analysis software, ImageJ, was utilized to quantitatively determine the total area of the fetal kidneys in the bright-field images, as well as to quantify the regions of EGFP-positive human NPC in the fluorescence images.
All analyses were conducted through three independent experiments.
Morphological analysis of organoids
The bright-field images of the organoids were acquired using the EVOS FL Cell Imaging System (Thermo Fisher Scientific Inc.). The ImageJ analysis software was utilized to quantitatively determine the area of the organoids in the bright-field images.
Flow cytometry
Biotinylated anti-ITGA8 (R&D Systems, Inc.), allophycocyanin-labeled streptavidin (BioLegend, Inc., CA, USA), and phycoerythrin-labeled anti-platelet-derived growth factor receptor A (PDGFRA) (BioLegend, Inc.) were used for cell staining. Data were acquired and analyzed using BD FACSAria IIu (BD Biosciences) and BD FACSDiva software (BD Biosciences), respectively.
Statistics and reproducibility
Statistical analyses were performed with R version 3.6.0 (The R Foundation for Statistical Computing). A two-tailed Student t-test was carried out for two-group comparisons, and one-way analysis of variance (ANOVA) followed by a Tukey test was performed for multiple-group comparisons. To ensure the reproducibility of the quantitative data, independent experiments were conducted three times. The screening experiments for cell culture conditions were conducted once, but after selecting the conditions, independent experiments were performed three times to confirm their reproducibility.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Yuna Yakura and Mizuki Otake for their experimental and technical assistance. This work was supported by the Japan Agency for Medical Research and Development (AMED; grant no. 24bm1223003h0003 and 24bm1123036h0002) and the JST FOREST Program (grant no. JPMJFR2011).
Author contributions
K.F., S.Y., K.S., K.M., and T.K. designed the study. K.F., S.Y., K.S., K.M., S.K., and T.K. carried out experiments and analyzed the data. K.F. wrote the manuscript. S.Y., K.S., K.M., T.K, A.I., M.I., E.K., and T.Y. interpreted the data and revised the manuscript. A.I., M.I., E.K., and T.Y. supervised the project. All authors have approved the final version of the manuscript.
Peer review
Peer review information
Communications Biology thanks Philip O’Connell, Ryuji Morizane, and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editor: Christina Karlsson Rosenthal. A peer review file is available.
Data availability
All data are available from the corresponding authors upon reasonable request. All reasonable requests will be promptly reviewed by the corresponding authors to determine whether the request is subject to any intellectual property or confidentiality obligations. The source data for the graphs are available in Supplementary Data 1.
Competing interests
S.Y., K.M., S.K., and T.Y. declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. K.F., K.S., T.K., A.I., and M.I. are the employees of Sumitomo Pharma Co., Ltd. E.K. has received a research fund from Sumitomo Pharma Co., Ltd. as a result of the Collaborative Research Agreement between The Jikei University School of Medicine and Sumitomo Pharma Co., Ltd. E.K. is the Chief Information Officer of Kobayashi Regenerative Research Institute, LLC. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Koki Fujimori, Email: koki.fujimori@sumitomo-pharma.co.jp.
Shuichiro Yamanaka, Email: shu.yamanaka@jikei.ac.jp.
Takashi Yokoo, Email: tyokoo@jikei.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-06986-w.
References
- 1.Porrett, P. M. et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant.22, 1037–1053 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Montgomery, R. A. et al. Results of two cases of pig-to-human kidney xenotransplantation. N. Engl. J. Med.386, 1889–1898 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Locke, J. E., Kumar, V., Anderson, D. & Porrett, P. M. Normal graft function after pig-to-human kidney xenotransplant. JAMA Surg.10.1001/jamasurg.2023.2774 (2023). [DOI] [PMC free article] [PubMed]
- 4.Loupy, A. et al. Immune response after pig-to-human kidney xenotransplantation: a multimodal phenotyping study. Lancet 10.1016/S0140-6736(23)01349-1 (2023). [DOI] [PubMed]
- 5.Tang, X.-Y. et al. Human organoids in basic research and clinical applications. Sig. Transduct. Target Ther.7, 168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim, J., Koo, B.-K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol.21, 571–584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsia, G. S. P., Esposito, J., Da Rocha, L. A., Ramos, S. L. G. & Okamoto, O. K. Clinical application of human induced pluripotent stem cell-derived organoids as an alternative to organ transplantation. Stem Cells Int.2021, 1–16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen, K. B. & Little, M. H. Organoids are not organs: sources of variation and misinformation in organoid biology. Stem Cell Rep.18, 1255–1270 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng, C., Ballard, E. B. & Wu, J. The road to generating transplantable organs: from blastocyst complementation to interspecies chimeras. Development148, dev195792 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi, T. et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell142, 787–799 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Rashid, T., Kobayashi, T. & Nakauchi, H. Revisiting the flight of Icarus: making human organs from PSCs with large animal chimeras. Cell Stem Cell15, 406–409 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Tan, T. et al. Chimeric contribution of human extended pluripotent stem cells to monkey embryos ex vivo. Cell184, 2020–2032.e14 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Goto, T. et al. Generation of pluripotent stem cell-derived mouse kidneys in Sall1-targeted anephric rats. Nat. Commun.10, 451 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu, J. et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell168, 473–486.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarmah, H. et al. Towards human organ generation using interspecies blastocyst complementation: challenges and perspectives for therapy. Front. Cell Dev. Biol.11, 1070560 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto, T. et al. Generation of human renal vesicles in mouse organ niche using nephron progenitor cell replacement system. Cell Rep.32, 108130 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Yamanaka, S. et al. Generation of interspecies limited chimeric nephrons using a conditional nephron progenitor cell replacement system. Nat. Commun.8, 1719 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takamura, T. et al. In vivo development of fetal pig kidneys in mature monkeys under clinically approved immunosuppressant drugs. Engineering10, 65–73 (2022). [Google Scholar]
- 19.Saito, Y. et al. Generation of functional chimeric kidney containing exogenous progenitor-derived stroma and nephron via a conditional empty niche. Cell Rep.39, 110933 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto, N. et al. Evaluation of the ability of human induced nephron progenitor cells to form chimeric renal organoids using mouse embryonic renal progenitor cells. Biochem. Biophys. Res. Commun.662, 18–25 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Zheng, C. et al. Cell competition constitutes a barrier for interspecies chimerism. Nature592, 272–276 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardoso-Moreira, M. et al. Gene expression across mammalian organ development. Nature571, 505–509 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper, DavidK. C., Gollackner, Bernd & Sachs, DavidH. Will the pig solve the transplantation backlog? Annu. Rev. Med.53, 133–147 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Xinaris, C. et al. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J. Am. Soc. Nephrol.23, 1857–1868 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito, Y., Matsumoto, N., Yamanaka, S., Yokoo, T. & Kobayashi, E. Beneficial impact of interspecies chimeric renal organoids against a xenogeneic immune response. Front. Immunol.13, 848433 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Z. et al. 3D culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell19, 516–529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsujimoto, H. et al. A modular differentiation system maps multiple human kidney lineages from pluripotent stem cells. Cell Rep.31, 107476 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell14, 53–67 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Tanigawa, S. et al. Activin is superior to BMP7 for efficient maintenance of human iPSC-derived nephron progenitors. Stem Cell Rep.13, 322–337 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barak, H. et al. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev. Cell22, 1191–1207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poladia, D. P. et al. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev. Biol.291, 325–339 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Li, M. et al. Porcine kidney organoids derived from naïve-like embryonic stem cells. IJMS25, 682 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature526, 564–568 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Khoshdel Rad, N., Aghdami, N. & Moghadasali, R. Cellular and molecular mechanisms of kidney development: from the embryo to the kidney organoid. Front. Cell Dev. Biol.8, 183 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnell, J., Achieng, M. & Lindström, N. O. Principles of human and mouse nephron development. Nat. Rev. Nephrol.18, 628–642 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Low, J. H. et al. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell25, 373–387.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown, A. C., Muthukrishnan, S. D. & Oxburgh, L. A synthetic niche for nephron progenitor cells. Dev. Cell34, 229–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bugacov, H., Der, B., Kim, S., Lindström, N. O. & McMahon, A. P. Canonical Wnt transcriptional complexes are essential for induction of nephrogenesis but not maintenance or proliferation of nephron progenitors. Dev. Biol.10.1101/2023.08.20.554044 (2023). [Google Scholar]
- 39.Chen, S. et al. Intrinsic age-dependent changes and cell-cell contacts regulate nephron progenitor lifespan. Dev. Cell35, 49–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Little, M. H. The life cycle of the nephron progenitor. Dev. Cell35, 5–6 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Thomas, J. et al. Running the full human developmental clock in interspecies chimeras using alternative human stem cells with expanded embryonic potential. npj Regen. Med.6, 25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies, J. A., Holland, I., Gül, H. Kidney organoids: steps towards better organization and function. Biochem. Soc. Trans. 10.1042/BST20231554 (2024). [DOI] [PMC free article] [PubMed]
- 43.Sharmin, S. et al. Human induced pluripotent stem cell–derived podocytes mature into vascularized glomeruli upon experimental transplantation. JASN27, 1778–1791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oceguera-Yanez, F. et al. Engineering the AAVS1 locus for consistent and scalable transgene expression in human iPSCs and their differentiated derivatives. Methods101, 43–55 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Taguchi, A. & Nishinakamura, R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell21, 730–746.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Matsui, K. et al. Long-term viable chimeric nephrons generated from progenitor cells are a reliable model in cisplatin-induced toxicity. Commun. Biol.6, 1097 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto, N. et al. Techniques of fragile renal organoids transplantation in mice. Acta Cir. Bras.36, e361102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding authors upon reasonable request. All reasonable requests will be promptly reviewed by the corresponding authors to determine whether the request is subject to any intellectual property or confidentiality obligations. The source data for the graphs are available in Supplementary Data 1.