Abstract
Duck plague virus (DPV) is the only herpes virus known to be transmissible among aquatic animals, leading to immunosuppression in ducks, geese and swans. Long noncoding RNAs (LncRNA) are known to participate in viral infections, acting as either immune defenders or viral targets to evade the host response, but their precise roles in waterfowl virus infections are yet to be fully understood. This study aimed to investigate the role of LncRNA in DPV-induced innate immune responses. Results showed that DPV infection greatly upregulated Lnc BTU expression in duck embryo fibroblasts (DEF) and Lnc BTU promoted DPV replication. Mechanically, 4 DPV proteins, namely UL46, UL42, VP22 and US10, interacted with Lnc BTU, leading to its upregulation. Specifically, Lnc BTU facilitated the production of DNA polymerase by enhancing UL42 expression, thereby promoting DPV replication. Additionally, Lnc BTU suppressed STAT1 expression by targeting the DNA binding domain (DBD) and promoting STAT1 degradation through the proteasome pathway. Furthermore, Lnc BTU inhibited the production of key antiviral factors such as IFN-α, IFN-β, MX and OASL during DPV infection. Treatment with 2 JAK-STAT pathway activators in DEFs resulted in the inhibition of Lnc BTU expression and DPV replication. Interestingly, DPV infection led to a decrease in STAT1 levels, which was reversed by Si-Lnc BTU. These findings suggest that DPV relies on Lnc BTU to inhibit the activation of the JAK-STAT pathway and limit the production of type 1 interferons (IFN) to complete immune evasion. Our study highlights the novel role of DPV proteins UL46, UL42, VP22, US10 as RNA-binding proteins in modulating the innate antiviral immune response, and discover the role of a new host factor, Lnc BTU, in DPV immune evasion, Lnc BTU and STAT1 can be used as a potential therapeutic target for DPV infection and immune evasion.
Key words: Lnc BTU, Duck plague virus, STAT1, JAK-STAT pathway, innate immunity
INTRODUCTION
Duck plague (DP), also known as duck viral enteritis, is a highly lethal disease caused by the Duck plague virus (DPV) (Ning et al., 2022; Liang et al., 2022). Belonging to the herpesviridae family and α-herpesvirus subfamily, DPV is a double-stranded DNA virus with an envelope (Shen et al., 2010; Khan et al., 2021). Infected birds exhibit clinical symptoms such as high mortality, vascular damage, lymphoid organ atrophy, and digestive tract mucosal herpes (Dhama et al., 2017). Ducks of all ages, geese, and swans are susceptible, with mallard ducks being natural hosts due to their resistance. DPV infection triggers antiviral immunity through pattern recognition receptors (PRR). Research indicates that Toll-like receptors play a more significant role than RIG-1-like receptors in the immune response to DPV, with varying expression levels across different organs (Kumar et al., 2022). DPV primarily replicates in the digestive tract mucosa before spreading to other organs like the bursa, thymus, spleen, and liver (Dhama et al., 2017). The Chinese virulent strain genome is 162,175 bp and consists of long unique sequences (UL), short unique sequences (US), and internal and terminal repeats (IRS and TRS), forming the UL-IRS-US-TRS genome structure. These genes are expressed in immediate early, early, and late stages. Despite existing knowledge on the antigenic and genomic diversity of DPV isolates (Apinda et al., 2022a, b; Wu et al., 2022b), understanding DPV's persistent infection and immunosuppression in ducks remains a complex molecular challenge that requires further exploration (Kong et al., 2022).
Long noncoding RNAs (LncRNA) play a significant role in innate immunity by modulating antiviral innate responses through different targets and pathways. For example, the nucleus-enriched lncRNA Malat1 functions as a negative regulator of antiviral type I interferon (IFN) production (Liu et al., 2020). It is possible that host LncRNAs could be targeted by viruses to establish interactions that help evade immune clearance, although this area remains understudied. LncRNAs are RNA molecules with a length exceeding 200 nucleotides that do not encode proteins (Janakiraman et al., 2018; Nojima and J.Proudfoot, 2022). LncRNAs can function at various levels, such as DNA (Canzio et al., 2019), transcription (McHugh et al., 2015), pretranscriptional (Gao et al., 2022), translational (Perez et al., 2021) and post-translational (Lv and Zhang, 2022), participating in a wide range of biological processes (Sui et al., 2022). They play a role in regulating antiviral pathways and viral replication by influencing the expression of Interferon-Stimulated Genes (ISG) and immune genes. Some LncRNAs enhance antiviral innate immunity by suppressing viral replication, for instance, influenza A virus (IAV) infection upregulates IVRPIE in the host A549 cells, IVRPIE promotes the expression of type 1 interferons (IFNs) and ISGs including IRF1, IFIT1, IFIT3, Mx1, ISG15, and IFI44L, by affecting histone modification of these genes (Zhao et al., 2020). Conversely, certain LncRNA promote viral infection and replication, for instance, RNA polymerase PB1 associated noncoding RNA (IPAN) in influenza virus, which stabilizes viral binds to and stabilizes viral RNA polymerase PB1(Wang et al., 2019). Heat shock protein 90α (Hsp90α) correlates with the nuclear export of Herpes simplex virus 1 (HSV-1), LncRNA-MAMDC2-AS1 interacts with Hsp90α, facilitating the nuclear transport of viral tegument protein VP16 (Wang et al., 2020a). Despite some understanding of LncRNAs in viral infections and innate immunity, their versatile roles and mechanisms, including viral exploitation of LncRNAs, warrant further exploration.
The innate immune system relies on PRRs to detect pathogen-related molecular patterns and damage-related molecular patterns. Host cells respond to changes in tissue homeostasis by activating various immune mechanisms to counteract virus-mediated immune evasion, a process closely associated with interferons. IFNs are potent cytokines with antiviral and immunomodulatory properties, serving as a critical link between the innate and adaptive immune systems. Specifically, STAT1 plays a pivotal role in regulating the innate immune response during viral infections mediated by IFNs (Jung et al., 2020). Upon sensing a viral intrusion, the genetic material of the virus (DNA or RNA) triggers signaling pathways in the host cell through PRRs, leading to the activation of interferon regulatory factors (IRFs) and STAT proteins. The IFN-α/β signaling pathway relies on the formation of STAT1 and STAT2 heterodimers, which are activated by JAK kinases and phosphorylated STAT proteins (Cai et al., 2015). Studies have shown that STAT1 plays a vital role in the immune response against viral infections. For instance, the African swine fever virus pF778R does not impact STAT1 phosphorylation or dimerization but can impede IFN signaling by reducing nuclear accumulation of activated STAT1 (Chen et al., 2023). The Lb leader protease (Lbpro) of foot-and-mouth disease virus (FMDV) co-localizes with STAT1/STAT2, inhibiting their nuclear translocation, Lbpro can also cleave STAT1/STAT2 to inhibit IFN-β expression, thereby promoting FMDV replication (Ma et al., 2023). Moreover, Growing studies indicate that the interaction between STAT1 and LncRNA can influence viral replication, propagation, and the host immune response. For instance, MicroRNA miR-1 degrades LncRNA-Sros1 during Listeria monocytogenes infection, stabilizing STAT1 mRNA and improving IFN-γ-STAT1-mediated innate immunity (Xu et al., 2019). Mice lacking Lncrna-155 were more susceptible to influenza virus and pseudorabies virus infections, as Lncrna-155 can boost STAT1 activation, increasing IFN-β production to enhance the host's antiviral responses (Rai et al., 2022). The various through which LncRNA interacts with STAT1 have heightened our interest in pursuing further studies.
Previous research identified 218 LncRNA with significantly different expression in duck embryo fibroblasts (DEFs) after DPV infection (Wu et al., 2022b), some of which are known to be immune-related molecules such as macrophage mannose receptors (MR), interleukin family members, and other factors. This study aims to investigate the potential role of Lnc BTU in regulating the immune response during DPV infection. The mechanism of action of LncRNA in viral infection is complex and diverse, serving as important factors for host defense against viruses and potential targets for viruses to evade host immune responses. Further exploration of the function of LncRNA in viral infection can contribute to the development of new antiviral strategies and therapeutic methods.
MATERIALS AND METHODS
Ethics Statement
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Sichuan Agriculture University in Sichuan, China(Protocol Permit Number: SYXK(川)2019-187).Experiments were conducted in accordance with guidelines and regulations of the Institutional Animal Care and Use Committee of Sichuan Agriculture University. This study was carried out in compliance with the ARRIVE guidelines.
Cell Culture and Virus Strains
Freshly isolated DEFs were obtained from 9- to 11-day-old specific pathogen free (SPF) duck embryos (purchased from the campus of Sichuan Agricultural University in Ya'an). DEFs were cultured in Dulbecco's modified eagle's medium (DMEM, 12800-058; Gibco) supplemented with 10% newborn calf serum (NBS, Gibco), 2% penicillin and streptomycin at 37°C with 5% CO2. We used RPMI 1640(31800-014; Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) to cultivate human embryonic kidney (HEK) 293T cells for 12 to 24 h at 37°C and 5% CO2.
The strong strain CHv of DPV was isolated, identified and preserved by the Institute of Preventive Veterinary Medicine at Sichuan Agricultural University. The viral titer of the DPV CHv strain was 104.75 50% tissue culture infective does (TCID50) /100μl.
Reagents
Antibodies including Mouse-anti-Myc, mouse-anti-Flag, mouse-anti-HA, anti-β-actin, anti-GAPDH, anti-β-tubulin, HRP goat antimouse IgG antibody all purchased from (Proteintech, China), SYBR Green Premix Ex Taq II (Takara, Japan); Hieff Trans Liposomal Transfection Reagent (Yeasen, China). Cidofovir (HY-17438), MG132, Bafilomycin A1, Chloroquine (CQ), RO8191 (HY-W063968) and 2-NP (HY-W013523) all purchased from (MCE, China).
RNA Extraction and Quantitative Real-Time PCR
RNA was extracted from DEFs using the RNA-easy Isolation Reagent (Vazyme, China) in accordance with the manufacturer's protocol. Briefly, the extraction process was conducted at 4°C, beginning with the lysis of DEFs using the reagent, followed by the collection of the supernatant after centrifugation. Isopropanol was then added to precipitate the RNA, which was subsequently washed with 75% ethanol, resuspended in RNase-free water, and stored at −80°C.
Primers were designed based on reference sequences utilizing NCBI primer BLAST, and all primers are listed in Table 1. cDNA synthesis was performed using Prime Script RT Master Mix (Takara, Japan) following the manufacturer's instructions. Quantitative real-time PCR (QRT-PCR) was carried out on a Light Cycler 480Ⅱ instrument (Roche, Switzerland) employing TB Green Premix Ex Taq II (Takara, Japan). Each reaction mixture contained 5 μL of TB Green Premix Ex Taq II, 0.4 μL of forward primer (10 μM), 0.4 μL of reverse primer (10 μM), 1 μL of cDNA template, and 3.2 μL of ddH2O. The qRT-PCR program consisted of an initial step at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. Melting curves were generated to confirm the specificity of the amplification, and each sample was analyzed in triplicate. The relative expression of target genes was determined using the 2−ΔΔCt method, with duck β-actin serving as the endogenous reference gene. Statistical analysis was conducted using Student's t-test, with P-values <0.05 considered statistically significant.
Table 1.
qRT-PCR primers used in the present study.
| Primer names | Sequences (5′–3′) |
|---|---|
| Lnc BTU F | ATGATGGGAAGTGTGACGG |
| Lnc BTU R | GGGGAGATAGCAGGGTTTT |
| UL46 F | TGGCTCACGCTCGCTCAATTATTAC |
| UL46 R | CTGTGTCATCGTCGTCGGAGTTATC |
| UL42 F | CAACAGCGTGATGCAGAAAGTGAAG |
| UL42 R | ATGTGCGGCTTGGTTCGTCTAAG |
| VP22 F | CTGAGCACTCTGGTAGGAATCATCG |
| VP22 R | GCTGCGTCGTCTTGCTTCTCC |
| US10 F | CTGTTTCCGACCTGGCTCTCAAG |
| US10 R | ACGGGAGCAACTGGATGGAATAAAG |
| UL30 F | AAGCAATACGAGCCAAGATTCCAAC |
| UL30 R | AGCCATTACTCACTCCACAGAACC |
| β-actin F | TTCCAGCCATCTTTCTTGGGTA |
| β-actin R | AGCGTTTACAACCTAACACCA |
| STAT1 F | AACGCAGGAAGCAGAACGAATG |
| STAT1 R | CGTGGTCTCAAGGTCTATTACTAAGC |
| IL-6 F | AAGCATCTGGCAACGACGATAAGG |
| IL-6 R | TGTGAGGAGGGATTTCTGGGTAGC |
| IFN-α F | TCCTCCAACACCTCTTCGAC |
| IFN-α R | GGGCTGTAGGTGTGGTTCTG |
| IFN-β F | CTTCTGAAAAGCAAGGACAAGAAG |
| IFN-β F | GATCTGAAGTATTTGTTGATGCTGA |
| MX F | TCCCCTGGAACTTAAACTGAAA |
| MX R | TTCTCCACTAATGTTGCCCTTT |
| OASL F | AAGAAGACGGTGCAGCAGAT |
| OASL R | GGAGAAGCAGCTGAGGAAGA |
| IRF7 F | GCCTGAAGAAGTGCAAGGTC |
| IRF7 R | TTGCAGTTGGAGAAGCACTG |
Plasmid Construction and Transfection
According to the sequence published in GenBank and the plasmid of our laboratory, we designed specific primers for the overexpression vectors of duck STAT1, Lnc BTU, β-actin, and STAT1 domain deletion mutants (Table 2). The duck template cDNA was used to amplify the corresponding gene using KOD Plus Neo (Toyobo, Japan) with the following PCR program: 98°C for 2 min, followed by 35 cycles of 98°C for 10 s, 55°C for 10 s, and 68°C for 2 min, and a final extension at 68°C for 5 min. PCR products were purified using a Universal DNA purification kit (Tiangen, China). The purified PCR products were subcloned into the pCAGGS plasmid vector using the restriction enzymes EcoRI, XhoI and SmaI (Takara, Japan). The ligation reaction was performed using T4 DNA Ligase (Takara, Japan) at 16°C overnight. The ligation mixture was transformed into Escherichia coli DH5α competent cells using the heat shock method. Positive clones were selected by blue-white screening and confirmed by colony PCR. The positive recombinant plasmids were purified using an endotoxin-free plasmid miniprep kit (Omega, China) and sequenced by a biotechnology company (YouKang, China). The sequencing results were analyzed using DNAMAN software (Lynnon Biosoft, Canada).
Table 2.
PCR primer used in this study.
| Primer names | Sequences (5′–3′) |
|---|---|
| pCAGGS-Lnc BTU F | CATCATTTTGGCAAAGAATTCGAAGGTTCAAAAAGCTCACTGAAGA |
| pCAGGS-Lnc BTU R | AAAAAGATCTGCTAGCTCGAGTGAGGGGCTGGGCTGGTA |
| pCAGGS-UL42-Flag F | GCAAAGAATTCGAGCTCAATGGCTACGCCCAAA |
| pCAGGS-UL42-Flag R | AGCAGATCTTTTTCCCTCGATTAGTCATCGTCGTCCTTGTAATCTAGGTAGGGCTTCAT |
| β-acting F | ATGGATGATGATATTGCTGCG |
| β-acting R | TTAGAAGCATTTGCGGTGGAC |
| pCAGGS-F2-Lnc BTU F | CATCATTTTGGCAAAGAATTCGGCGCTGACAAAGCGCCGAAGGTTCAAAAAGCTCACT |
| pCAGGS-F2-Lnc BTU R | AAAAAGATCTGCTAGCTCGAGTGAGGGGCTGGGCTGGTA |
| F2-pCAGGS F | GGCGCTGCAAAGCGCCGTCGACATTGATTATTG |
| F2-pCAGGS R | CAGGTGGCACTTTTCGGG |
| pCAGGS-STAT1-Myc F | AAAGAATTCGAGCTCATCGATATGGCCGCGGCCCGGCGG |
| pCAGGS-STAT1-Myc R | TTGGCAGAGGGAAAAAGATCTTTACAGATCCTCTTCAGAGATGAGTTTCTGCTCAGTTGAATATGCTGAACACATCATC |
| pCAGGS-STAT1-△SH2-Myc F | CAAAAATTTCCCTTTTTGGCTGTCTGTTTCTGAAGTCCACCC |
| pCAGGS-STAT1-△SH2-Myc R | GGGTGGACTTCAGAAACAGACAGCCAAAAAGGGAAATTTTTG |
| pCAGGS-STAT1-△DBD-Myc F | CAGCTTATCCAGAGCTCCTCTACTGACCCCAAGAACTTG |
| pCAGGS-STAT1-△DBD-Myc R | CAAGTTCTTGGGGTCAGTAGAGGAGCTCTGGATAAGCTG |
| pCAGGS-STAT1-△CCD-Myc F | CCGTGATTGGGATGCTGGACCAGAGCTCCTTCGTGGTAG |
| pCAGGS-STAT1-△CCD-Myc R | CTACCACGAAGGAGCTCTGGTCCAGCATCCCAATCACGG |
The recombinant plasmids pCAGGS-UL46-Flag, pCAGGS-VP22-HA, pCAGGS-US10-Flag and pCAGGS-UL30-HA were saved by the Institute of Preventive Veterinary Medicine, Sichuan Agricultural University.
The recombinant plasmids were transfected into DEFs or HEK293T cells using Hieff Trans Liposomal Transfection Reagent according to the manufacturer's instructions. Transfection efficiency was assessed by qRT-PCR. Each experiment was performed in triplicate. Statistical analysis was performed using Student's t-test, and P-values<0.05 were considered statistically significant.
Small RNA-Mediated Interference
DEFs were transfected with small interference RNA (siRNA) targeting Lnc BTU or STAT1, as well as negative control siRNAs, which were obtained from Ruibobio (Guangzhou, China). Transfection was performed using Hieff Trans Liposomal Transfection Reagent according to the manufacturer's instructions.
Briefly, a titration assay was conducted to determine the optimal siRNA concentration for effective knockdown. Different concentrations of siRNAs (20 nM, 40 nM, 60 nM, 80nM, and 100 nM) were tested in separate transfections, and cells were incubated for 36 h post-transfection. Knockdown efficiency was assessed through qRT-PCR analysis. The siRNA sequence that exhibited the highest knockdown efficiency with minimal cytotoxicity was chosen as the optimal concentration for subsequent experiments. The siRNA sequences used in the study are detailed in Table 3. The negative control siRNA was a scramble siRNA from RiboBio, with a sequence that does not target any known genes in the duck genome.
Table 3.
siRNA sequence of Lnc BTU and STAT1.
| Names | Sequences (5′–3′) |
|---|---|
| Lnc BTU-siRNA-1 | CTCACTGAAGACCTAATGA |
| Lnc BTU-siRNA-2 | CCTGGGATTATCAACGTAA |
| Lnc BTU-siRNA-3 | CCTCAGGATTATCAATGTA |
| STAT1-siRNA-1 | CCATTGTCGTGATCTCAAA |
| STAT1-siRNA-2 | GCCTTAATGCAGATCAGTT |
| STAT1-siRNA-3 | GAACTGGTTCACCATTGTT |
Viral Titer Determination
DEFs transfected with pCAGGS-Lnc BTU plasmid, or Si-Lnc BTU by using Hieff Trans Liposomal Transfection Reagent. Viral titer assays were performed 24 h after transfection. Serially dilute the DPV stock solution 10 times to obtain serial dilutions from 101 to 108. Add the diluted DPV to different wells of the 96-well plate, 100 μL per well. Place the 96-well plate in an incubator at 37°C and 5% CO2 for 72 h. Cell morphological changes were observed daily under an inverted microscope, and cytopathic effect (CPE) scores were recorded. The control cells were treated only with maintenance medium DMEM. The TCID50 was calculated by using the Karber method.
RNA Pull-Down and Mass Spectrometry
The RNA pull-down analysis was performed using the in vivo F2-RNA pull-down kit (Huijun, Guangzhou) with the F2-tagged sequence GGCGCTGACAAAGCGCC. The experimental design included 4 groups: pCAGGS-F2-LncBTU, pCAGGS-F2 (control), pCAGGS-F2-LncBTU infected with DPV, and pCAGGS-F2 infected with DPV (DPV control). For protein extraction, around 2 × 107 cells from each group were harvested, washed twice with cold PBS to remove culture medium components. The cell pellet was resuspended in 300 μL of lysis buffer, thoroughly mixed, and sonicated or incubated on ice for 30 min with periodic manual mixing. The resulting lysate was centrifuged to remove cellular debris, and the supernatant containing the protein extract was collected. 30μL of the supernatant was kept as input group, while the rest was stored at −80°C for subsequent RNA pull-down experiments. In the RNA pull-down procedure, each group was provided with 45 μL of magnetic beads, which were mixed with 500 μL of NT2 buffer, 20μL of the ligand was added to the beads and incubated at room temperature for 30 min on a shaker. The protein extract was then combined with the beads and incubated at 4°C overnight. Subsequently, 50μL of elution buffer was added to the beads, incubated at room temperature for 10 to 15 min on a shaker. The eluate was subjected to a magnetic separator to collect the supernatant containing the RNA pull-down products. The protein products obtained from the RNA pull-down can be utilized for further Western blot, SDS-PAGE, or Mass Spectrometry (MS) experiments. The protein bands were stained with Coomassie brilliant blue and destained using a destaining solution.
For MS analysis, a portion of the protein products from each group was sent to Guangzhou Huijun Biological Company. The samples underwent protein digestion to peptides and were subsequently analyzed using MS. The data obtained were compared among the 4 groups to identify proteins interacting with Lnc BTU and evaluate the influence of DPV infection on these interactions.
Western Blot
Total proteins were extracted from DEFs using Radio Immunoprecipitation Assay (RIPA) lysis buffer (Beyotime, China) containing protease inhibitor (PMSF, solarbio). Subsequently, 10% SDS-PAGE gels were prepared, and the protein samples were loaded for electrophoresis. The separated proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane, and the membrane was incubated in a blocking solution containing 5% skim milk for 1 h at room temperature. The membrane was then incubated with the primary antibody (anti-HA or anti-Flag) at 4°C overnight, diluted to 1:5000. After 3 rounds of washing with PBST, the membrane was incubated with the secondary antibody (HRP-conjugated antimouse IgG) at room temperature for 1 h, diluted to 1:5000. The membrane was washed again with PBST. Image Lab Software was used for Western blot analysis. Image J software was used for band quantification analysis.
Co-Immunoprecipitation Assay
The pCAGGS-UL30-HA and pCAGGS-UL42-Flag plasmids were transfected into DEFs. After 36 h of transfection, the cells were lysed by using immunol precipitation (IP) lysate (Beyotime, China) on ice for 30 min and centrifuged at 12,000 rpm for 10 min. The supernatant was collected and mixed with 1 μg of anti-HA or 1 μg anti-Flag antibody. The sample was incubated overnight at 4°C, then coupled with protein A+G agarose beads (MCE, China) at 4°C for 5 h and centrifuged. The agarose beads were collected and washed 4 times with PBST (PBS with 0.1% Tween-20). Finally, the immunoprecipitated protein was extracted from the agarose beads by boiling for 10 min in 5 × loading buffer. Image Lab Software (Bio-Rad) were used for Western blot analysis. Image J software (https://imagej.net/) was used for band quantification analysis.
RNA Binding Protein Immunoprecipitation Assay
Four viral protein plasmids (pCAGGS-UL46-Flag, pCAGGS-UL42-Flag, pCAGGS-VP22-HA, pCAGGS-US10-Flag) were separately transfected into DEFs overexpressing pCAGGS-Lnc BTU using Hieff Trans Liposomal Transfection Reagent according to the manufacturer's instructions. After 36 h of transfection, the cells were lysed using IP lysis buffer on ice for 30 min and centrifuged at 12,000 rpm for 10 min. A portion of the lysed protein solution was set aside as the input group. Protein A/G magnetic beads and flag antibodies specific for the viral proteins (anti-Flag for pCAGGS-UL46-Flag, pCAGGS-UL42-Flag, and pCAGGS-US10-Flag; anti-HA for pCAGGS-VP22-HA) were added to the remaining protein solution and incubated at 4°C overnight. The supernatant was removed using the magnetic separator to capture the immunoprecipitated complexes on the beads.
The beads were resuspended in DEPC water (Beyotime, China) to dissolve the protein complexes and were then divided into 2 portions. One portion was used for PCR experiments to amplify any RNA bound to the immunoprecipitated proteins, while the other portion was used for RNA extraction. The RNA was then subjected to reverse transcription and qPCR analysis to quantify the Lnc BTU RNA bound to the viral proteins.
Nuclear and Cytoplasmic Separation
Cytoplasmic and nuclear fractions were isolated from DEFs using a commercially available kit (Thermo Fisher Scientific, USA) according to the manufacturer's instructions. Briefly, DEFs were transfected with pCAGGS-Lnc BTU, or pCAGGS-STAT1, 10 × 106 cells per group, ice-cold CER I was added to the cell pellet and incubated on ice for 10 min, ice-cold CER II was then added to the tube and incubated on ice for 1 min, then immediately transfer the supernatant (cytoplasmic extract) to a clean prechilled tube. The pellet was then suspended in ice-cold NER for 40 min and the supernatant (nuclear extract) was harvested. Cytoplasmic and nuclear extracts were stored at −80°C until use. QRT-PCR was performed to analyze the expression of mRNA in both cellular fractions using primers for β-actin, Lnc BTU, and STAT1. The expression levels were calculated using the 2−ΔΔCT method. Protein was extracted from the cytoplasmic and nuclear fractions using RIPA lysis buffer for Western blot analysis.
Fluorescent in situ Hybridization Assay
Fluorescent in situ hybridization assay (FISH) assay is mainly used to detect Lnc BTU localization in DEFs. Fluorescent in Situ Hybridization Kit (RiboBio, China) according to the manufacturer's instructions. DEFs were infected DPV for 24 h, harvested and washed the cells 2 times with PBS, then fixed the cells with 4% paraformaldehyde for 10 min and permeated with 0.5% Triton X-100 in 4°C for 5 min; next placed the sections in 200 μl of prehybridization buffer for 30 min at 37°C and mixed the warmed hybridization solution with the Cy3-maked probe targeting Lnc BTU (red), 300 μl of probe mixture for each section incubating at 37°C for 5 to 6 h; 4 × saline sodium citrate (SSC) buffer wash the sections 15 min, 2 × SSC wash the sections 5 min, 1 × SSC wash the sections 5 min, PBS wash the sections 5min; DAPI (1 μg/mL, Thermo Fisher Scientific) was added for nuclear staining for 5min, PBS washed the sections 15 min at room temperature; dried sections covered with 50% glycerol, pictures were collected by laser confocal fluorescence microscope (Olympus, Model number: BX53F2C).
Immunofluorescence Assay
Immunofluorescence Assay (IFA) mainly detected STAT1 localization. DEFs were transfected with STAT1-Myc for 24h by using Hieff Trans Liposomal Transfection Reagent, then infected DPV at MOI = 1 for 12h, harvested and washed the cells 2 times with PBS, then fixed the cells with 4% paraformaldehyde (PFA) for 10 min and permeated with 0.5% Triton X-100 in 4 °C for 5 min. Block cells with 5% BSA for 1 h to reduce nonspecific binding. Incubate cells with mouse anti-Myc antibody overnight at 4°C. Add FITC-conjugated goat antimouse IgG and incubate at room temperature for 1h. DAPI was added for nuclear staining for 5min, dried sections covered with 50% glycerol, pictures were collected by laser confocal fluorescence microscope (Olympus, Model number: BX53F2C).
Data Analysis
Student's t-tests were used to evaluate the significance of differences between experimental groups. Relative gene expression data were obtained using the 2−ΔΔCT method, which is a commonly used approach for quantifying gene expression changes in qRT-PCR experiments. The data were analyzed using GraphPad Prism 8.0 software (La Jolla, CA) for 1-way analysis of variance (ANOVA) to compare the means of the different groups. The results are presented as the mean ± standard deviation (SD) of 3 independent experiments. Asterisks indicate the level of statistical significance (ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
RESULTS
Bioinformatics Analysis and Expression Kinetics Analysis of Lnc BTU
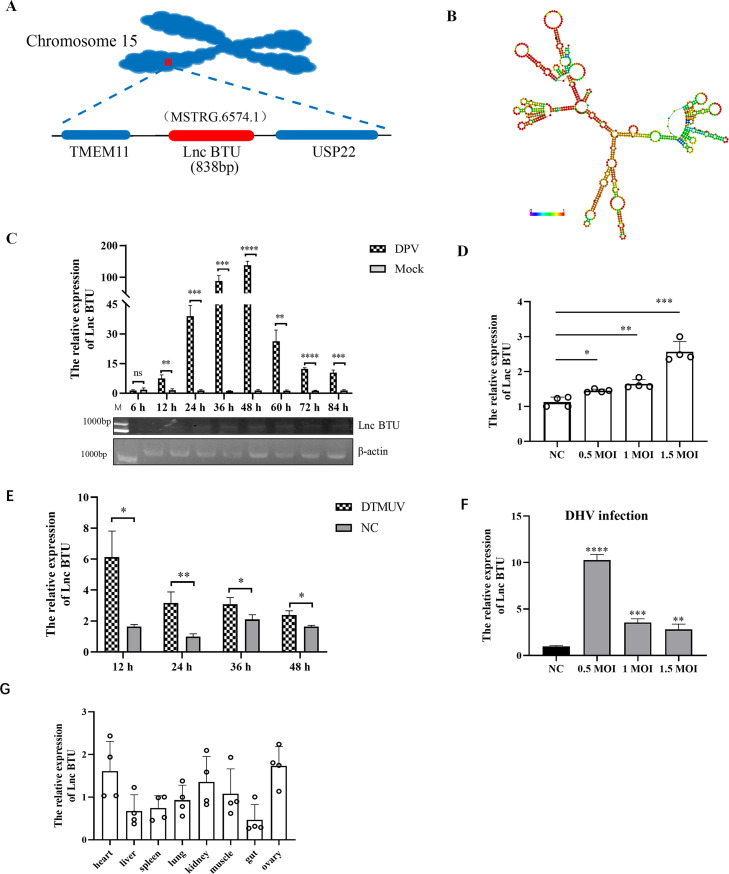
In 2022, our research team obtained a collection of novel LncRNAs by using RNA sequencing (Wu et al., 2022), and their expression levels were significantly changed after DPV infection. We found a novel LncRNA named MSTRG.6574.1 in duck. MSTRG.6574.1 is located on duck chromosome 15, positioned between the coding genes Transmembrane protein 11 (TEME11) and Ubiquitin specific peptidase 22 (USP22). To simplify future research, we named MSTRG.6574.1 as Lnc BTU (LncRNA MSTRG.6574.1 between TEME11 and USP22). The full-length sequence of Lnc BTU in ducks was obtained using single-molecule transcript sequencing, revealing a length of 838 nucleotides, a schematic diagram of the location of LncBTU on the chromosome is shown in Figure 1A. The predicted minimum free energy secondary structure of Lnc BTU was analyzed using RNA-fold software (Figure 1B). Our investigation into the induction kinetics of Lnc BTU post DPV infection in DEFs demonstrated a gradual increase in expression up to 12 h, followed by a significant spike of approximately 150-fold at 48 h (Figure 1C). Furthermore, Lnc BTU expression was found to be influenced by the infectious dose of DPV, with the highest expression observed at DPV MOI = 1.5 (Figure 1D). Additionally, infection with duck tembusu virus (DTMUV) and duck hepatitis virus (DHV) also led to a significant increase in Lnc BTU expression (Figures 1E and 1F). QRT-PCR analysis of normal duck tissues revealed that Lnc BTU is mainly enriched in the heart and ovaries (Figure 1G). In conclusion, our study has identified a novel LncRNA, Lnc BTU, in ducks, with its expression being upregulated in DEFs following DPV infection.
Figure 1.
Bioinformatics analysis and expression kinetics analysis of Lnc BTU. (A) Schematic diagram of the location of Lnc BTU on chromosomes in the duck genome. (B) RNA secondary structure prediction for Lnc BTU was analyzed using RNA-fold (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi) and the data were shown as a minimal free energy structure (MFE = −240.25 kcal/mol). Base pairing probabilities have been color-coded from 0 (blue) to 1 (red). (C) Lnc BTU mRNA expression detected by qRT-PCR and PCR in DEFs infected DPV (MOI = 1) at indicate time. (D) Lnc BTU mRNA expression detected by qRT-PCR in DEFs infected DPV (MOI = 0.5, 1, 1.5). (E, F) Lnc BTU mRNA expression detected by qRT-PCR in DEFs infected DTMUV or DHV. (G) QRT-PCR detected Lnc BTU in normal duck tissues. (Each experiment was repeated 3 times. ns, P>0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
Upregulation of Lnc BTU Promotes Duck Plague Virus Replication
To investigate the role of Lnc BTU in DPV infection, we analyzed the impact of Lnc BTU on DPV replication. Lnc BTU was knocked down in DEFs using siRNAs or overexpressed through transfection with pCAGGS-Lnc BTU. Control cells were transfected with Si-NC or pCAGGS. QRT-PCR results demonstrated that Si-1 significantly reduced Lnc BTU mRNA levels by 70-80% compared to Si-NC in DEFs (Figure 2B). To ensure the efficacy of subsequent experiments, Si-1 was selected for Lnc BTU knockdown. A standard curve for DPV replication was then established (S1 Table), and CT values were converted into copy numbers using a standard equation. Cells with knocked down or overexpressed Lnc BTU were infected with DPV at MOI = 1.5. The results indicated that overexpression of Lnc BTU led to an increase in DPV copy numbers (Figure 2C). Consistent with these findings, TCID50 results showed that Lnc BTU overexpression enhanced the viral titer of DPV (Figure 2E), while Si-Lnc BTU had the opposite effect (Figures 2D and 2E). These results suggested that Lnc BTU promotes DPV replication.
Figure 2.
Lnc BTU promotes DPV infection in DEFs. (A, B) QRT-PCR and PCR confirmed overexpression or knockdown Lnc BTU efficiency. (C-D) pCAGGS-Lnc BTU (pCAGGS as control) or Si-1 (Si-NC as control) transfected DEFs after DPV infection and DPV copy number in the supernatant was measured using qRT-PCR at indicate time. (E) DPV titer was detected using TCID50 assay. (Each experiment was repeated 3 times. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.)
Lnc BTU Directly Interacts With Duck Plague Virus UL46, UL42, VP22 and US10
LncRNAs can form complexes with RNA-binding proteins (RBP), which play a critical role in the infection processes of viruses (Hu et al., 2019; Sharma et al., 2019). In this study, a biotin tag F2 was added to Lnc BTU to investigate how DPV utilizes Lnc BTU for its replication. A control group with tag F2 added to pCAGGS was also included. The proteins enriched by Lnc BTU were identified using a Coomassie brilliant blue assay (Figure 3A). MS analysis revealed that the pCAGGS-F2-Lnc BTU group exhibited enrichment in 303 host proteins (Figures 3B and 3C). Interestingly, the pCAGGS-F2-Lnc BTU+DPV group showed enrichment in 110 host proteins and 5 viral proteins (Figure 3D, Table 4). Among these proteins, 4 viral proteins were selected for further investigation. Co-transfection of the pCAGGS-Lnc BTU plasmid with the 4 viral DPV plasmids (pCAGGS-UL46-Flag, pCAGGS-UL42-Flag, pCAGGS-VP22-HA, and pCAGGS-US10-Flag) into HEK293T cells or DEFs revealed interactions through RNA-pulldown and RNA Binding Protein Immunoprecipitation (RIP) assays. The results demonstrated that the 4 viral proteins interacted with Lnc BTU (Figures 3E and 3H). Moreover, Lnc BTU enrichment was observed in RNA samples immunoprecipitated against the 4 viral proteins (Figures 3I and 3L). In conclusion, Lnc BTU directly interacts with viral proteins UL46, UL42, VP22 and US10.
Figure 3.
Lnc BTU directly interacts with DPV UL46, UL42, VP22 and US10. (A) Coomassie brilliant blue assay detect Lnc BTU enriched protein. DEFs were transfected with pCAGGS-F2-LncBTU or pCAGGS-F2 (control) for 24 h, then infected with DPV at MOI = 1 for 24 h, the cells were harvested and followed by Western blot. (B, C, D) MS Analysis, host proteins in pCAGGS-F2-lnc BTU group or pCAGGS-F2-Lnc BTU+DPV group obtained by STRING software. (E–H) RNA pull-down assay was detected Lnc BTU interact with viral protein. HEK293T cells were transfected with pCAGGS-F2-LncBTU and viral protein plasmid (pCAGGS-UL46-flag, pCAGGS-UL42-Flag plasmid, pCAGGS-VP22-HA plasmid and pCAGGS-US10-Flag plasmid respectively) for 36 h, followed by Western blot with RNA pull-down kit manual. (I–L) RIP assay to detected 4 viral proteins interact with Lnc BTU. DEFs were transfected 4 viral plasmids respectively and pCAGGS-Lnc BTU for 36 h, the cells were harvested and Lnc BTU mRNA level detected by PCR and qRT-PCR. Each experiment was repeated 3 times. ns, P > 0.05; *, P < 0.05; **, P < 0.01, ***, P < 0.001; ****, P < 0.0001.
Table 4.
Lnc BTU enriches total viral proteins.
| Protein accession | Protein description | Score |
|---|---|---|
| tr|G3GQZ0|G3GQZ0_9ALPH | Anatid alphaherpesvirus 1 GN = UL49 PE = 3 SV = 1 | 105 |
| tr|G3GR55|G3GR55_9ALPH | Anatid alphaherpesvirus 1 GN = US10 PE = 3 SV = 1 | 75 |
| tr|A0A2H4G4U3|A0A2H4G4U3_9ALPH | Anatid alphaherpesvirus 1 GN = DEVCV23 PE = 3 SV = 1 | 63 |
| tr|A0A7L9VXE7|A0A7L9VXE7_9ALPH | Anatid alphaherpesvirus 1 GN = UL46 PE = 3 SV = 1 | 56 |
| tr|G3GQZ8|G3GQZ8_9ALPH | Anatid alphaherpesvirus 1 GN = UL42 PE = 3 SV = 1 | 37 |
Next, we examined how Lnc BTU affected the expression of these 4 viral proteins. Lnc BTU-overexpressed DEFs were infected with DPV at MOI = 1.5, and qRT-PCR analysis revealed that Lnc BTU significantly increased UL46, UL42, and VP22 mRNA levels (Figures 4A and 4C) while suppressing US10 mRNA expression (Figure 4D). After this, we investigated the impact of viral protein expression on DEFs. Consistent with the above, Western blot results showed that Lnc BTU enhanced the expression of UL46, UL42 and VP22 proteins (Figures 4E and 4G) while suppressing US10 expression (Figure 4H), whereas Si-Lnc BTU had an opposite effect (Figures 4I and 4L). We confirmed that Lnc BTU promoted the expression of UL46, UL42 and VP22 and inhibited US10 expression.
Figure 4.
Lnc BTU promoted UL46, UL42 and VP22 expression, inhibited US10 expression and UL46, UL42, VP22 and US10 all promoted Lnc BTU expression. (A–D) DEFs were transfected with pCAGGS-Lnc BTU for 36 h, then the cell infected with DPV at MOI = 1. QRT-PCR measured UL46, UL42, VP22 and US10 mRNA level at indicate time. (E–H) doses of pCAGGS-Lnc BTU DEFs were transfected with pCAGGS-UL46-Flag (E), pCAGGS-UL42-Flag (F), pCAGGS-VP22-HA (G) or pCAGGS-US10-Flag (H) for 36h, the cell lysates were subjected to Western blotting. (I–L) Doses Si-Lnc BTU DEFs were transfected with pCAGGS-UL46-Flag (I) or pCAGGS-UL42-Flag (J) or pCAGGS-VP22-HA (K) or pCAGGS-US10-Flag (L) for 36h, the cell lysates were subjected to Western blotting. The resulting band plot was examined for gray value by using image-J software. Bar graphs represent the mean of 3 independent experiments (±SD). (M) To explore UL46, UL42, VP22 and US10 effect on Lnc BTU expression, doses of pCAGGS-UL46-Flag, pCAGGS-UL42-Flag, pCAGGS-VP22-HA, pCAGGS-US10-Flag transfected separately to DEFs for 36 h, qRT-PCR to detect Lnc BTU mRNA level. (N) pCAGGS-Lnc BTU DEFs transfected doses of pCAGGS-UL46-Flag, pCAGGS-UL42-Flag, pCAGGS-VP22-HA or pCAGGS-US10-Flag and transfected to DEFs for 36 h, qRT-PCR to detect Lnc BTU mRNA level. Each experiment was repeated 3 times. ns, P > 0.05; *, P < 0.05; **, P < 0.01, ***, P < 0.001; ****, P < 0.0001.
To explore the effects of 4 viral proteins on Lnc BTU expression, the Lnc BTU expression by gradually increasing doses of UL46, UL42, VP22 and US10 in DEFs was examined. The qRT-PCR results showed that UL46, UL42, VP22 and US10 all promoted Lnc BTU expression (Figure 4M) regardless of exogenous Lnc BTU introduction into DEFs (Figure 4N). In the study of DPV viral proteins, it has been proved that the deletion of UL46, UL42, VP22 and US10 proteins will reduce the titer of DPV and weaken the replication of the virus (Lu et al., 2010; Burrel et al., 2012; Ma et al., 2018; Wu et al., 2020). To summarize, Lnc BTU directly interacts with DPV viral proteins UL46, UL42, VP22 and US10, and Our study uncovered a new mechanism by which DPV manipulates the host's LncRNA to achieve immune evasion, ultimately facilitating DPV replication.
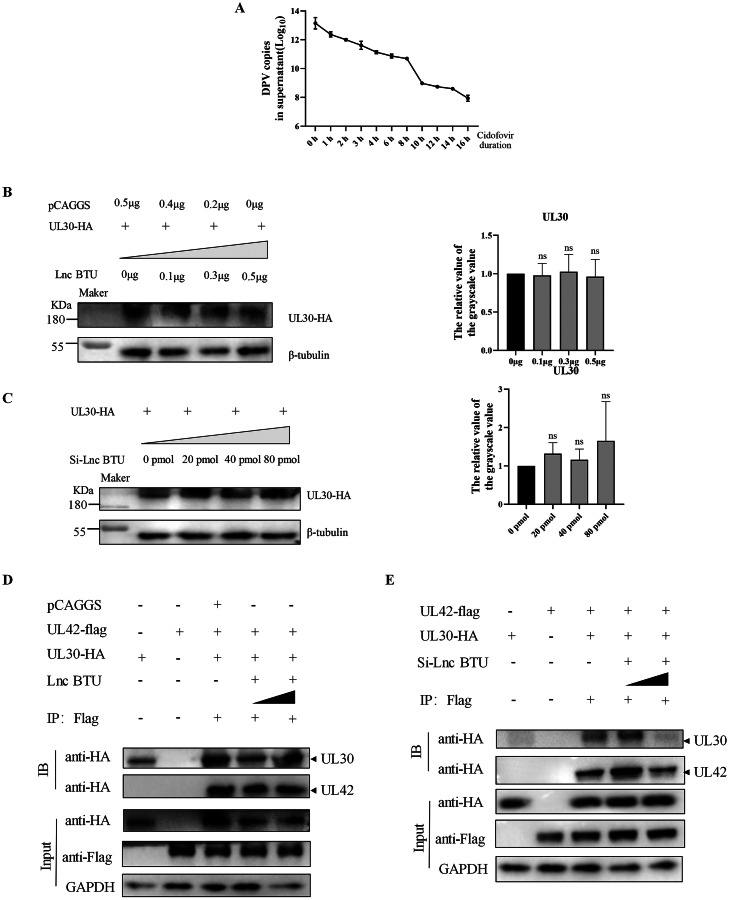
Lnc BTU Enhances DNA Polymerase Synthesis to Promote Duck Plague Virus Replication
The herpes virus DNA polymerase consists of UL42 and UL30, facilitating virus replication and proliferation (Gottlieb et al., 1990; Lin et al., 1998; Burrel et al., 2012). We treated DEFs with Cidofovir (a DNA polymerase inhibitor, 5μmol/ml) and found a significant reduction in DPV copy number (Figure 5A), suggesting that DPV replication is dependent on its DNA polymerase. Having observed that Lnc BTU can enhance the expression of UL42. Our goal was to investigate how Lnc BTU affects DPV DNA polymerase. Initially, we examined the impact of Lnc BTU on UL30 expression, revealing no significant effect based on Western blot results (Figures 5B and 5C). Subsequently, we assessed the effect of Lnc BTU on UL30-UL42 complex expression, demonstrating that Lnc BTU enhanced the formation of the UL30-UL42 complex through Co-immunoprecipitation (Co-IP) assay (Figures 5D and 5E). These findings collectively suggest that Lnc BTU promotes the synthesis of DNA polymerase, thereby facilitating DPV replication in DEFs.
Figure 5.
Lnc BTU enhances the synthesis of DPV DNA polymerase. (A) DEFs infected DPV at MOI=1, then treated with Cidofovir (10 nmol) for 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, 10 h, 12 h, 14 h and 16 h, qRT-PCR detected DPV copy numbers in the supernatant. (B, C) DEFs were transfected pCAGGS-UL30-HA and doses of pCAGGS-Lnc BTU or Si-Lnc BTU for 36 h. cell harvested for Western blotting, and the resulting band plot gray value was examined using image-J software. (D, E) DEFs co-transfected pCAGGS-UL42-Flag, pCAGGS-UL30-HA and doses of pCAGGS-Lnc BTU or Si-Lnc BTU for 36 h, Co-IP detected UL30 and UL42 protein expression.
Lnc BTU Inhibits the Activation of the JAK-STAT1 Pathway by Targeting STAT1
We found that poly(I:C) could induce the expression of Lnc BTU (Figure 6A), and Lnc BTU inhibited the expression of IFN-β in DPV infection (Figure 6B). We hypothesize that Lnc BTU is associated with the host’s innate immune response and there are host important signaling molecules associated with Lnc BTU during this process. Based on the MS data (Figures 3C and 3D), We confirmed the direct interaction between Lnc BTU and STAT1 by using RNA pull-down and RIP assays (Figures 6C and 6D). What's more, Lnc BTU overexpression significantly down-regulated STAT1 mRNA and protein levels in DEFs (Figures 6E and 6H), while Si-Lnc BTU had the opposite result. STAT1, a key player in the JAK-STAT pathway, connects cell membrane receptors to effectors and regulates interferon-dependent signaling (Xu et al., 2019; Clark et al., 2022; Tao et al., 2024). whether Lnc BTU affects the expression of genes downstream of the JAK-STAT pathway? qRT-PCR results indicated that Lnc BTU overexpression down-regulated the mRNA expression of IFN-α and IFN-β in DEFs (Figures 6K and 6L), regardless of DPV infection, as well as OASL, MX, IL-6, and IRF7 levels (Figures 6K–6S). Interestingly, DPV infection inhibits STAT1 expression at 36 to 72 h of DPV infection (Figure 7A) and Lnc BTU also inhibits STAT1 expression, whether Lnc BTU is involved in DPV infection inhibiting STAT1 expression, results showed that STAT1 levels were reversed by knockdown Lnc BTU in DPV-infected DEFs (Figure 7B). These results showed that DPV infection relies on Lnc BTU to down-regulate STAT1 expression and impact the JAK-STAT pathway.
Figure 6.
Lnc BTU inhibits the activation of the JAK-STAT signaling pathway by targeting STAT1 (A) Doses Poly (I:C) treated DEFs for 36 h, qRT-PCR and PCR detected Lnc BTU expression. (B) pCAGGS-Lnc BTU treated DEFs for 24 h, then infected DPV at MOI = 1.5 for 12 h, 24 h, 36 h and 48 h, qRT-PCR detected IFN-β level. (C) HEK293T cells were co-transfected with pCAGGS-STAT1-Myc and pCAGGS-F2-LncBTU for 36 h, cell harvested and followed by RNA pull-down kit manual. (D) RIP assay to detected STAT1 interact with Lnc BTU. DEFs co-transfected with pCAGGS-F2-LncBTU and pCAGGS-STAT1-Myc for 36 h, the cells were harvested and Lnc BTU mRNA level detected by PCR and qRT-PCR. (E, F) DEFs transfected with doses of Si-Lnc BTU (E) (0 nmol, 10 nmol, 20 nmol, 30 nmol, 50 nmol, 100 nmol) or pCAGGS-Lnc BTU (F) (0 μg, 0.5 μg, 1 μg, 1.5 μg, 2 μg, 3 μg) for 24h, infected 1.5 MOI DPV, qRT-PCR detected STAT1 mRNA level. (G, H) doses of Si-Lnc BTU (G) or pCAGGS-Lnc BTU (H) DEFs transfected with pCAGGS-STAT1-Myc for 36h, STAT1 was detected by Western blotting. (I–T) IFN-α, IFN-β, MX, OASL, IL-6, IRF7 mRNA level in doses of Si-Lnc BTU (0nmol, 10nmol, 20nmol, 30nmol, 50nmol, 100nmol) or pCAGGS-Lnc BTU (0μg, 0.5μg, 1μg, 1.5μg, 2μg, 3μg) DEFs after DPV infection (MOI = 1.5) measured by qRT-PCR, using β-actin as internal control. Each experiment was repeated 3 times. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Figure 7.
(A) QRT-PCR was used to detect STAT1 mRNA level at 6 h, 12 h, 24 h, 36 h, 48 h and 60 h after 1.5MOI DPV infection. (B) Si-Lnc BTU transfected to DEFs for 24 h, then infected DPV at MOI = 1.5 for 24 h, qRT-PCR detected STAT1 level. (C, D) DEFs were pretreated with RO8191 (5 μmol/mL), 2-NP (5 μmol/mL) or solvent DMSO for 1 h, and then infected with 1.5 MOI DPV for another 1 h, JAK1, JAK2, TYK2, STAT1, STAT2, IFN-α, IFN-β, IRF7, MX, OASL and IL-6 levels and DPV copy numbers were detected by qRT-PCR. (E) RO8191 (5 μmol/mL) and 2-NP (5 μmol/mL) treated DEFs for 2 h, then infected DPV at MOI = 1.5 for 6 h, qRT-PCR detected Lnc BTU level. (F) pCAGGS-STAT1 DEFs infected DPV at MOI = 1, qRT-PCR detected DPV copy numbers at indicate time. (G–J) DEFs transfected with pCAGGS-STAT1 or si-STAT1 for 24 h, then infected DPV at MOI = 1, qRT-PCR detected UL46, UL42, VP22 and US10 level at indicate time.
To confirm the role of the JAK-STAT pathway on DPV replication, 2 activators were utilized to stimulate the JAK-STAT pathway. DEFs underwent pretreated with RO8191 (IFN activator, 5 μmol/mL), 2-NP (STAT1 activator, 5 μmol/mL) or the solvent DMSO for 1 h, and then infected with 1.5 MOI DPV for another 1 h, DPV copy number and cytokine transcriptional levels were detected by qRT-PCR. We observed that RO8191 and 2-NP all increased the expression levels of JAK1, JAK2, TYK2, STAT1, STAT2, IFN-α, IFN-β, MX and OASL (Figure 7C), both RO8191 and 2-NP all significantly suppressed DPV replication (Figure 7D) and inhibited Lnc BTU expression regardless of the presence or absence of DPV infection (Figure 7E). These results showed that activated JAK-STAT pathway inhibited DPV replication and also inhibited Lnc BTU expression. Next, we explored how STAT1 affects DPV replication.
STAT1 overexpression in DEFs infected with DPV at MOI = 1.5 resulted in a reduction of DPV copy number in the supernatant, as shown in Figure 7F. Next, 3 siRNAs targeting STAT1 were utilized. The qRT-PCR results showed that si-STAT1 reduced the expression of STAT1 by approximately 75 to 80% in DEFs (S1 Fig). To substantiate the above finding, DEFs treated with siRNAs or transfected with STAT1-overexpressing plasmids were infected with DPV at MOI = 1.5. Cells transfected with si-NC or pCAGGS were used as controls, and the expression levels of DPV UL46, UL42, VP22, and US10 were assessed. QRT-PCR results showed that si-STAT1 significantly increased DPV UL46, UL42 and VP22 mRNA expression and decreased US10 mRNA expression (Figures 7G and 7J). These results showed that Lnc BTU targeted STAT1 and inhibited the activation of the JAK-STAT pathway, thereby promoting DPV replication.
Lnc BTU Inhibits STAT1 Expression in the Nucleus and Medicates STAT1 Degradation via the Ubiquitin-Proteasome Pathway
Studies have shown that LncRNAs interact with proteins and regulate protein localization and stability (Cai et al., 2018; Simion et al., 2020; Liu et al., 2022). To determine the specific domains of STAT1 targeted by Lnc BTU, mutations were generated in various STAT1 domains (Figure 8A). RNA pull-down and RIP assays results revealed that Lnc BTU interacted with coiled-coiled domain (CCD) and the tyrosine activation domain (SH2) but not with the DNA binding domain (DBD) deletions (Figure 8B and 8G). These findings suggest that the STAT1 DBD domain plays a crucial role in the interaction with Lnc BTU. Furthermore, our investigation into the effect of Lnc BTU on STAT1 distribution in the nucleoplasm of DEFs. FISH and Nucleoplasmic isolation assays revealed that Lnc BTU was mainly located in the cytoplasm before and after DPV infection (Figures 8H and 8K). When DPV uninfected, STAT1 was mainly located in the cytoplasm regardless of Lnc BTU overexpression or knockdown, but it was mainly located in the nucleus after DPV infection (Figure 8I). Interestingly, in Lnc BTU overexpressed DEFs, STAT1 was mainly located in the cytoplasm after DPV infection, but substantially located in the nucleus after Lnc BTU knockdown (Figures 8I and 8J). Additionally, FISH assay revealed co-localization of Lnc BTU and STAT1 in the cytoplasm before and after DPV infection (Figure 8L). These results suggest that Lnc BTU inhibits the translocation of STAT1 into the nucleus during DPV infection.
Figure 8.
Lnc BTU inhibits STAT1 expression in the nucleus and medicates STAT1 degradation via the ubiquitin-proteasome pathway. (A) STAT1 domains and truncation mutants. (B–D) HEK293T cells co-transfected with pCAGGS-F2-LncBTU and pCAGGS-STAT1-△SH2-Myc (B) or pCAGGS-STAT1-△DBD-Myc (C) or pCAGGS-STAT1-CCD-Myc (D) for 36 h, cells harvested and followed by RNA pull-down kit manual and Western blotting. (E–G) RIP assay to detected STAT1 truncation mutants interact with Lnc BTU. DEFs transfected with pCAGGS-F2-LncBTU and pCAGGS-STAT1-△SH2-Myc (E), pCAGGS-STAT1-△DBD-Myc (F) or pCAGGS-STAT1-CCD-Myc (G) for 36 h, cells harvested and used to PCR and qRT-PCR. (H) DEFs were infected with 1.5 MOI DPV for 36 h, qRT-PCR detected Lnc BTU expression obtained following nucleoplasmic isolation manual. (I, J) Si-Lnc BTU or pCAGGS-Lnc BTU DEFs infected 1.5 MOI DPV for 24 h, qRT-PCR (I) and Western blotting (J) detected STAT1 expression following nucleoplasmic isolation manual. (K, L) The colocalization between the Lnc BTU and STAT1 in DPV-infected DEFs detected by FISH assays. (M) DEFs, transfected with pCAGGS-Lnc BTU and pCAGGS-STAT1-Myc for 36 h, were treated with CHX (10 nmol/ml) at indicated times, followed by Western blotting. (N) DEFs transfected with pCAGGS-STAT1-Myc and pCAGGS-Lnc BTU for 36 h, then treated with MG132 (5 nmol/ml) or Bafilomycin A1 (10 nmol/ml) or CQ (10 nmol/ml) for 6 h, STAT1 protein expression detected by Western blotting. The resulting band plot gray value was examined by using image-J software.
Does Lnc BTU inhibit STAT1 expression by enhancing its degradation? Cycloheximide (CHX) chase assay was used to measure the steady-state protein stability of STAT1(Miao et al., 2023). After treating DEFs with CHX, we found that the Lnc BTU shorten the half-life of the STAT1 protein (Figure 8M). To explore which path mediates STAT1 degradation, proteasome inhibitor MG132 (Pehere et al., 2019) and lysosome inhibitors Bafilomycin A1 and CQ (Wang et al., 2017; Mauthe et al., 2018) were used to investigate the degradation mechanism of STAT1 in DEFs. The results showed that MG132 rescued the degradation of STAT1 mediated by Lnc BTU overexpression, but the Bafilomycin A1 and CQ did not (Figure 8N), further validating our hypothesis. Taken together, these results suggested that Lnc BTU binds to the DBD domain of STAT1 and inhibits its nuclear localization after DPV infection and very likely participates in the proteasomal degradation of STAT1.
Schematic Diagram of Lnc BTU Targeting STAT1 in the JAK-STAT Pathway to Help DPV Evade Host Innate Immunity (Figure 9).
Figure 9.
Schematic diagram of Lnc BTU targeting STAT1 in the JAK-STAT signaling pathway to help DPV evade host innate immunity.
DISCUSSION
DPV is the only herpes virus known to be transmissible among aquatic animals, leading to immunosuppression in swans, geese and ducks. However, the exact molecular mechanism through which DPV impact the host’s innate immune system remains unclear (Niu et al., 2021; Wu et al., 2022a, 2023). Recent research has highlighted the relationship between lncRNAs and viral replication. For instance, Influenza A virus (IAV) has been shown to upregulate LncRNA-DFRV, resulting in the production of 2 transcripts. The longer form of DFRV inhibits IAV replication, while the shorter form suppresses the expression of DFRV long and promotes IAV replication (Wang et al., 2023). LncRNAs, which do not encode proteins, exhibit versatility in their functions, challenging the traditional view that they are merely genetic “noise”. Here, Lnc BTU induced by DPV infection promotes the production of DPV DNA polymerase and inhibits the JAK-STAT pathway mediated IFNs production, ultimately facilitating DPV replication.
LncRNA can regulate gene expression by functioning as a protein scaffold. We used MS to find Lnc BTU binding proteins, which included viral proteins and host proteins. Among the 5 viral proteins enriched with significant differences, 4 DPV proteins UL46, UL42, VP22 and US10 caught our attention. Herpesvirus protein is categorized into immediate early (IE), early β (early, E), and late γ (late, L) stages based on gene translation speed. UL46, a late gene in herpesvirus, has been found to interact with interferon gene protein stimulators (STING) in both Pseudorabies virus (PRV) and HSV-1, inhibiting the phosphorylation of STING and the transmission of the interferon signaling pathway (Deschamps and Kalamvoki, 2017, p. 1; Xu et al., 2020). VP22, a late gene in herpesvirus, known as UL49, is a crucial cortical protein for DPV replication, initially located in the cytoplasm during early viral infection but potentially moving to the nucleus later on. VP22 can hinder cGAS enzyme activity, impede interferon signal delivery in host cells, and evade the host innate immune response (Wu et al., 2020, Huang et al., 2018). US10 protein, primarily localized in the nucleus, undergoes phosphorylation postherpesvirus infection (Nishiyama et al., 1997). In DPV, US10 is classified as a late gene, and its removal leads to a significant reduction in DPV viral titer (Ma et al., 2018). Our study revealed that DPV 4 viral proteins UL46, UL42, VP22 and US10 interacted and enhanced Lnc BTU expression (Figs. 3E–L, 4M–N). On the other hand, doses pCAGGS-Lnc BTU promoted UL46, UL42 and VP22 expression inhibited US10 expression, doses Si-Lnc BTU obtained the opposite results (Figures 4A–L), although Lnc BTU has opposite effects on different viral proteins of DPV, the apparent result is that Lnc BTU promotes DPV replication, this also reflects the complexity of viral proteins in the interaction between virus and host. For the first time, we reported the novel functions of UL46, UL42, VP22 and US10 as RNA-binding proteins in the innate immune response. Further exploration is needed to understand the detailed mechanisms underlying the interaction between these viral proteins and Lnc BTU, including examining interaction sites and the impact of Lnc BTU on the DPV replication cycle.
In addition, the herpesvirus DNA polymerase consists of 2 subunits: UL30, stabling DNA polymerase activity, and UL42, promoting continuous DNA polymerase synthesis (Gottlieb et al., 1990; Wang et al., 2022). During Herpes simplex virus (HSV) infection, UL30 facilitates DNA synthesis, while UL42 enhances the interaction between UL30 and DNA template strands (Zhou et al., 2014). Our research group has demonstrated that DPV DNA polymerase is composed of UL42 and UL30. Inhibition of DNA polymerase with cidofovir significantly reduced the DPV copy number (Figure 5A). Our study discovered that Lnc BTU increased UL42 expression, so, whether Lnc BTU affect DNA polymerase synthesis to promote DPV replication? Co-IP results showed that Lnc BTU not affect UL30 expression (Figures 5B–C), but specifically targets DPV UL42 protein and enhances its expression (Figures 5D–E), as a consequence, DNA polymerase expression is promoted, allowing the replication of DPV. Here, for the first time, our study identifies a LncRNA which can promote DPV replication by influencing DPV DNA polymerase formation during DPV infection. There are few studies that explored the interaction between host LncRNA and viral polymerase, in the study of influenza A virus, the LncRNA-IPAN is hijacked by IAV to assist in IAV infection independently of IFN. IPAN associates with and stabilizes the RNA-dependent RNA polymerase PB1, enabling efficient synthesis (Wang et al., 2019). Unlike this article, Lnc BTU expression is dependent on the production of IFNs. We found that Poly (I:C) promoted Lnc BTU expression (Figure 6A) and Lnc BTU inhibited IFN-β expression after DPV infection (Figure 6B), these results suggest that Lnc BTU potentially plays a role in mediating intracellular signaling pathways. Additionally, MS data hints at a potential connection between Lnc BTU and STAT1.
In antiviral responses, STAT1 has been implicated in various infections. For instance, in pseudorabies virus infection, the upregulation of LNC-000641 is reported to promote PRV replication by inhibiting the JAK-STAT pathway (Fang et al., 2021). Similarly, TRIM28 is shown to facilitate porcine epidemic diarrhea virus replication by inhibiting the JAK-STAT1 pathway through mitophagy (Li et al., 2024). Additionally, porcine epidemic diarrhea virus nsp7 is found to inhibit interferon-induced JAK-STAT signaling by sequestering the interaction between KPNA1 and STAT1 (Zhang et al., 2022). Rabies virus P protein interacts with STAT1 to hinder interferon signal transduction by impeding nuclear accumulation and DNA binding of STAT1 (Vidy et al., 2005). the Foot-and-Mouth Disease Virus Lb Protease is known to cleave intracellular transcription factors STAT1 and STAT2 to counteract IFN-β-induced signaling (Ma et al., 2023). The NS2A protein of Zika virus inhibits the transmission of interferon signaling by targeting and degrading STAT1 and STAT2 (Fanunza et al., 2021). In our study, RNA pull-down and RIP assays showed that Lnc BTU interacted with STAT1, Lnc BTU inhibited STAT1 mRNA and protein expression (Figures 6C–H). STAT1 expression was down-regulated during DPV infection (Figure 7A), but can reversed by knockdown Lnc BTU in DPV-infected DEFs (Figure 7B), these results concluded that DPV infection is rely on Lnc BTU to inhibit STAT1 expression. STAT1 as one of the important factors of the JAK-STAT pathway, whether Lnc BTU associated with the JAK-STAT1 pathway by interacting STAT1? We found that Lnc BTU also inhibited the expression of IFN-α, IFN-β, MX, OASL, IRF7 and IL-6 downstream of the JAK-STAT pathway (Figures 6K–V). For DPV replication, what role does the JAK-STAT pathway play? We used 2-NP and RO8191 activators to activate the JAK-STAT pathway (Figure 7C), and found that JAK-STAT pathway activation inhibited Lnc BTU expression and DPV replication (Figures 7D–E), meanwhile, we found that STAT1 inhibited DPV replication (Figure 7F), overexpression of STAT1 down-regulated proteins UL46, UL42, VP22 expression and up-regulated US10 expression, knockdown of STAT1 has the opposite results (Figures 7G–J). In conclusion, we confirmed that DPV is rely on Lnc BTU to downregulate the expression of STAT1 and inhibit the activation of the JAK-STAT pathway, consequently limiting the activation of the JAK-STAT pathway and reducing type 1 interferon production. STAT1, known for its role as an RNA-binding protein, is commonly studied in cancer research. For example, in myocardial injury, the noncoding RNA BANCR is upregulated and BANCR targets STAT1 to enhance IFN-β expression, leading to cardiomyocyte apoptosis postinjury (Wang et al., 2020b). In diabetes, lncRNA-UC.25+ down-regulates the P2Y14 receptor expression, while STAT1 positively regulates P2Y14 receptor expression by targeting its promoter region. UC.25+ modulates P2Y14 expression through STAT1 to mitigate diabetes development (Wu et al., 2022c). However, our study is the first to reveal that STAT1 also plays a crucial role as an RNA-binding protein in the context of viral infection.
STATs have highly conserved domains, including N-terminal domains, the coiled-coiled domain (CCD), the DNA binding domain (DBD) and the tyrosine activation domain (SH2); however, the carboxy-terminal transcriptional activation domain varies among species(Hüntelmann et al., 2014; Remling et al., 2023). Our results showed that the interaction between Lnc BTU and STAT1 is dependent on STAT1 DBD domain (Fig. 8B–G), there are research showed that the DBD domain of STAT1 has a role in the nuclear export signal, which is controlled by JAK(Mowen and David, 2000), but this was not proven in ducks, we compared the DBD domain of human STAT1 and duck and found that their sequence consistency is 95.65%, this result hints that Lnc BTU may be closely related to the nuclear export of STAT1. whether Lnc BTU affect the distribution of STAT1 in the cell? We found that overexpression of Lnc BTU inhibited STAT1 expression in the nucleus during DPV infection, and STAT1 returned to the nucleus after knockdown of Lnc BTU. Therefore, we concluded that Lnc BTU inhibited STAT1 expression in the nucleus, and this process is likely related to the STAT1 DBD domain, whether the DBD domain is related to the nuclear input or output of STAT1 need to further examine. It has been reported that LncRNA can affect the stability and degradation of proteins by interacting with proteins (Munschauer et al., 2018; Liu et al., 2021), and whether Lnc BTU affects the degradation of STAT1? We found that STAT1 has self-degradation effect, and Lnc BTU has accelerated the degradation of STAT1 (Figure 8M), and this degradation is reversed by proteasome inhibitor MG132 (Figure 8N). These results suggested that STAT1 degradation occurs mainly through the proteasome pathway. In brief, our findings indicate that Lnc BTU interacts with STAT1′s DBD domain, inhibiting its nuclear localization. Meanwhile, Lnc BTU reduces the half-life of the STAT1 protein and accelerates its degradation via the ubiquitin-proteasome pathway.
In conclusion, this article reveals a new mechanism by which DPV utilizes Lnc BTU to evade the immune system and promote its own replication. DPV upregulates Lnc BTU level in DEFs, which, in turn promotes DPV replication. Mechanistically, DPV directly interacts with Lnc BTU through its 4 viral proteins (UL46, UL42, VP22, and US10) to upregulate Lnc BTU expression. Lnc BTU promotes the expression of DNA polymerase by particularly promoting UL42 expression, thereby facilitating DPV replication. What's more, Lnc BTU targets STAT1 and inhibits STAT1 expression, thereby inhibiting the activation of the JAK-STAT pathway, so that DPV can evade the innate immune response within DEFs (Figure 9). It was first reported that DPV viral proteins UL46, UL42, VP22 and US10 function as RNA-binding proteins and participate in the regulation of innate immune responses. Lnc BTU and STAT1 can be used as potential therapeutic targets for DPV infection and immune evasion.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (32473016); Sichuan Province Program (2022NSFSC0078); the Program Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (CARS-SVDIP); the earmarked fund for China Agriculture Research System (CARS-42-17), and the National Key Research and Development Program of China (2023YFD1802601-1).
Author contributions: Ning Luo: Writing—original draft, Conceptualization, Methodology, Software, Formal analysis, Data curation. Anchun Chen: Resources; Mingshu Wang: Resources; Sun Chen: Resources, Dekang Zhu: Resources; Mafeng Liu: Resources; Zhen Wu: Resources; Ying Wu: Resources; Juan Huang: Resources; Bin Tian: Resources; Xumin Ou: Resources; Zhongqiong Yin: Resources, Software; Renyong Jia: Supervision, Data curation, Funding acquisition, Resources, Writing—review and editing.
Availability of data and material: All data and material included in this study are available upon request by contact with the corresponding author.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.104238.
Appendix. Supplementary materials
REFERENCES
- Apinda N., Muenthaisong A., Chomjit P., Sangkakam K., Nambooppha B., Rittipornlertrak A., Koonyosying P., Yao Y., Nair V., Sthitmatee N. Simultaneous protective immune responses of Ducks against Duck plague and fowl cholera by recombinant duck enteritis virus vector expressing pasteurella multocida OmpH gene. Vaccines. 2022;10:1358. doi: 10.3390/vaccines10081358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apinda N., Yao Y., Zhang Y., Reddy V.R.A.P., Chang P., Nair V., Sthitmatee N. CRISPR/Cas9 editing of Duck enteritis virus genome for the construction of a recombinant vaccine vector expressing omph gene of pasteurella multocida in two novel insertion sites. Vaccines. 2022;10:686. doi: 10.3390/vaccines10050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrel S., Aït-Arkoub Z., Agut H., Boutolleau D. Genotypic characterization of herpes simplex virus DNA polymerase UL42 processivity factor. Antiviral. Res. 2012;93:199–203. doi: 10.1016/j.antiviral.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Cai B., Cai J., Luo Y., Chen C., Zhang S. The Specific roles of JAK/STAT signaling pathway in sepsis. Inflammation. 2015;38:1599–1608. doi: 10.1007/s10753-015-0135-z. [DOI] [PubMed] [Google Scholar]
- Cai R., Sun Y., Qimuge N., Wang G., Wang Y., Chu G., Yu T., Yang G., Pang W. Adiponectin AS lncRNA inhibits adipogenesis by transferring from nucleus to cytoplasm and attenuating adiponectin mRNA translation. Biochim. Biophys. Acta. Mol. Cell. Biol. Lipids. 2018;1863:420–432. doi: 10.1016/j.bbalip.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Canzio D., Nwakeze C.L., Horta A., Rajkumar S.M., Coffey E.L., Duffy E.E., Duffié R., Monahan K., O'Keeffe S., Simon M.D., Lomvardas S., Maniatis T. Antisense lncRNA transcription mediates DNA demethylation to drive stochastic protocadherin α promoter choice. Cell. 2019;177:639–653.e15. doi: 10.1016/j.cell.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Li L., Liu L., Liu Z., Guo S., Tan C., Chen H., Wang X. African swine fever virus pF778R attenuates type I interferon response by impeding STAT1 nuclear translocation. Virus. Res. 2023;335 doi: 10.1016/j.virusres.2023.199190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.N., Begg L.R., Filiano A.J. Unique aspects of IFN -γ/STAT1 signaling in neurons. Immunol. Rev. 2022;311:187–204. doi: 10.1111/imr.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps T., Kalamvoki M. Evasion of the STING DNA-sensing pathway by VP11/12 of herpes simplex virus 1. J. Virol. 2017;91 doi: 10.1128/JVI.00535-17. e00535-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Kumar N., Saminathan M., Tiwari R., Karthik K., Kumar M.A., Palanivelu M., Shabbir M.Z., Malik Y.S., Singh R.K. Duck virus enteritis (duck plague)—A comprehensive update. Vet. Q. 2017;37:57–80. doi: 10.1080/01652176.2017.1298885. [DOI] [PubMed] [Google Scholar]
- Fang L., Gao Y., Liu X., Bai J., Jiang P., Wang X. Long non-coding RNA LNC_000641 regulates pseudorabies virus replication. Vet. Res. 2021;52:52. doi: 10.1186/s13567-021-00922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanunza E., Carletti F., Quartu M., Grandi N., Ermellino L., Milia J., Corona A., Capobianchi M.R., Ippolito G., Tramontano E. Zika virus NS2A inhibits interferon signaling by degradation of STAT1 and STAT2. Virulence. 2021;12:1580–1596. doi: 10.1080/21505594.2021.1935613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Jiang M., Guo F., Liu X., Zhang Q., Yang S., Yeung Y.T., Yang R., Wang K., Wu Q., Zhang D., Zhang C., Laster K.V., Ge M., Nie W., Liu K., Dong Z. A novel lncRNA MTAR1 promotes cancer development through IGF2BPs mediated post-transcriptional regulation of c-MYC. Oncogene. 2022;41:4736–4753. doi: 10.1038/s41388-022-02464-x. [DOI] [PubMed] [Google Scholar]
- Gottlieb J., Marcy A.I., Coen D.M., Challberg M.D. The herpes simplex virus type 1 UL42 gene product: A subunit of DNA polymerase that functions to increase processivity. J. Virol. 1990;64:5976–5987. doi: 10.1128/jvi.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Huang P., Yan Y., Zhou Z., Wang J., Wu G. Hepatitis B virus X protein related lncRNA WEE2-AS1 promotes hepatocellular carcinoma proliferation and invasion. Biochem. Biophys. Res. Commun. 2019;508:79–86. doi: 10.1016/j.bbrc.2018.11.091. [DOI] [PubMed] [Google Scholar]
- Huang J., You H., Su C., Li Y., Chen S., Zheng C. Herpes simplex virus 1 tegument protein VP22 abrogates cGAS/STING-mediated antiviral innate immunity (RM Sandri-Goldin, Ed.) J. Virol. 2018;92 doi: 10.1128/JVI.00841-18. e00841-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüntelmann B., Staab J., Herrmann-Lingen C., Meyer T. A conserved motif in the linker domain of STAT1 transcription factor is required for both recognition and release from high-affinity DNA-binding sites. PLoS. ONE. 2014;9:e97633. doi: 10.1371/journal.pone.0097633. (AP Costa-Pereira, ed.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiraman H., House R.P., Gangaraju V., Diehl J.A., Howe P.H., Palanisamy V. The long (lncRNA) and short (miRNA) of it: TGFβ-mediated Control of RNA-binding proteins and non-coding RNAs. Mol. Cancer. Res. 2018;16:567–579. doi: 10.1158/1541-7786.MCR-17-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.R., Ashhurst T.M., West P.K., Viengkhou B., King N.J.C., Campbell I.L., Hofer M.J. Contribution of STAT1 to innate and adaptive immunity during type I interferon-mediated lethal virus infection. PLOS. Pathog. 2020;16 doi: 10.1371/journal.ppat.1008525. (M Suthar, ed.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K.A., Islam Md.A., Sabuj A.A.M., Bashar Md.A., Islam Md.S., Hossain Md.G., Hossain M.T., Saha S. Molecular characterization of duck plague virus from selected HAOR areas of Bangladesh. Open. Vet. J. 2021;11:42–51. doi: 10.4314/ovj.v11i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Wu X., Liao L., Xie Z., Feng K., Chen F., Zhang X., Xie Q. Duck plague virus infection alter the microbiota composition and intestinal functional activity in Muscovy ducks. Poult. Sci. 2022;102 doi: 10.1016/j.psj.2022.102365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J., Dandapat S., Panickan S., Kumar A., Singh M., Bindu S., Dhama K. Expression profiles of toll like receptors, MHC and cytokine genes along with viral load in organs of ducklings infected with an Indian isolate of duck enteritis virus. Microb. Pathog. 2022;165 doi: 10.1016/j.micpath.2022.105502. [DOI] [PubMed] [Google Scholar]
- Li X., Yan Z., Ma J., Li G., Liu X., Peng Z., Zhang Y., Huang S., Luo J., Guo X. TRIM28 promotes porcine epidemic diarrhea virus replication by mitophagy-mediated inhibition of the JAK-STAT1 pathway. Int. J. Biol. Macromol. 2024;254 doi: 10.1016/j.ijbiomac.2023.127722. [DOI] [PubMed] [Google Scholar]
- Liang Z., Guo J., Yuan S., Cheng Q., Zhang X., Liu Z., Wang C., Li Z., Hou B., Huang S., Wen F. Pathological and molecular characterization of a Duck plague outbreak in Southern China in 2021. Animals. 2022;12:3523. doi: 10.3390/ani12243523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Dai C.Y., Ricciardi R.P. Cloning and functional analysis of Kaposi's sarcoma-associated herpesvirus DNA polymerase and its processivity factor. J. Virol. 1998;72:6228–6232. doi: 10.1128/jvi.72.7.6228-6232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Wang Z., Liu L., Yang Z., Liu S., Ma Z., Liu Y., Ma Y., Zhang L., Zhang X., Jiang M., Cao X. LncRNA Malat1 inhibition of TDP43 cleavage suppresses IRF3-initiated antiviral innate immunity. Proc. Natl. Acad. Sci. 2020;117:23695–23706. doi: 10.1073/pnas.2003932117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Chen Y., Huang Y., Cao K., Liu T., Shen H., Cui J., Li B., Cai J., Gao F., Yang Y. Long non-coding RNA ANRIL promotes homologous recombination-mediated DNA repair by maintaining ATR protein stability to enhance cancer resistance. Mol. Cancer. 2021;20:94. doi: 10.1186/s12943-021-01382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.-T., Zou Y.-X., Zhu W., Sen-Liu G.Zhang, Ma R.-R., Guo X., Gao P. lncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell. Death. Differ. 2022;29:627–641. doi: 10.1038/s41418-021-00879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Cheng A., Wang M., Jiang J., Zhu D., Jia R., Luo Q., Liu F., Chen Z., Chen X., Yang J. Polyclonal antibody against the DPV UL46M protein can be a diagnostic candidate. Virol. J. 2010;7:83. doi: 10.1186/1743-422X-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X., Zhang Q. LncRNA RP11-214F16.8 drives breast cancer tumorigenesis via a post-translational repression on NISCH expression. Cell. Signal. 2022;92 doi: 10.1016/j.cellsig.2022.110271. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zeng Q., Wang M., Cheng A., Jia R., Yang Q., Wu Y., Zhao X.-X., Liu M., Zhu D., Chen S., Zhang S., Liu Y., Yu Y., Zhang L., Chen X. US10 Protein is crucial but not indispensable for duck enteritis virus infection in vitro. Sci. Rep. 2018;8:16510. doi: 10.1038/s41598-018-34503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Luo Z., Song R., Nian X., Choudhury S.M., Ru Y., Yang F., Zhang Y., Zeng Z., Cao W., Pei J., Liu X., Zheng H. The foot-and-mouth disease virus Lb protease cleaves intracellular transcription factors STAT1 and STAT2 to antagonize IFN-β–induced signaling. J. Immunol. 2023;210:283–296. doi: 10.4049/jimmunol.2101042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauthe M., Orhon I., Rocchi C., Zhou X., Luhr M., Hijlkema K.-J., Coppes R.P., Engedal N., Mari M., Reggiori F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh C.A., Chen C.-K., Chow A., Surka C.F., Tran C., McDonel P., Pandya-Jones A., Blanco M., Burghard C., Moradian A., Sweredoski M.J., Shishkin A.A., Su J., Lander E.S., Hess S., Plath K., Guttman M. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature. 2015;521:232–236. doi: 10.1038/nature14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y., Du Q., Zhang H.G., Yuan Y., Zuo Y., Zheng H. Cycloheximide (CHX) chase assay to examine protein half-life. Bio. protocol. 2023;13(11):e4690. doi: 10.21769/BioProtoc.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowen K., David M. Regulation of STAT1 nuclear export by Jak1. Mol. Cell. Biol. 2000;20:7273–7281. doi: 10.1128/mcb.20.19.7273-7281.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munschauer M., Nguyen C.T., Sirokman K., Hartigan C.R., Hogstrom L., Engreitz J.M., Ulirsch J.C., Fulco C.P., Subramanian V., Chen J., Schenone M., Guttman M., Carr S.A., Lander E.S. The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nature. 2018;561(7721):132–136. doi: 10.1038/s41586-018-0453-z. [DOI] [PubMed] [Google Scholar]
- Ning Y., Huang Y., Wang M., Cheng A., Jia R., Liu M., Zhu D., Chen S., Zhao X., Zhang S., Yang Q., Wu Y., Huang J., Tian B., Ou X., Mao S., Gao Q., Sun D., Yu Y., Zhang L. Evaluation of the safety and immunogenicity of Duck-plague virus gE mutants. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.882796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y., Yamada H., Yamashita Y., Daikoku T., Tsurumi T., Jiang Y.M. The product of the US10 gene of herpes simplex virus type 1 is a capsid/tegument-associated phosphoprotein which copurifies with the nuclear matrix. J. Gen. Virol. 1997;78:2923–2931. doi: 10.1099/0022-1317-78-11-2923. [DOI] [PubMed] [Google Scholar]
- Niu Y., Su S., Chen X., Zhao L., Chen H. Biological characteristic and cytokines response of passages duck plague virus in ducks. Virus. Res. 2021;295 doi: 10.1016/j.virusres.2021.198320. [DOI] [PubMed] [Google Scholar]
- Nojima T., Proudfoot N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. cell. biol. 2022;23(6):389–406. doi: 10.1038/s41580-021-00447-6. [DOI] [PubMed] [Google Scholar]
- Pehere A.D., Nguyen S., Garlick S.K., Wilson D.W., Hudson I., Sykes M.J., Morton J.D., Abell A.D. Tripeptide analogues of MG132 as protease inhibitors. Bioorg. Med. Chem. 2019;27:436–441. doi: 10.1016/j.bmc.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Perez C.A.G., Adachi S., Nong Q.D., Adhitama N., Matsuura T., Natsume T., Wada T., Kato Y., Watanabe H. Sense-overlapping lncRNA as a decoy of translational repressor protein for dimorphic gene expression. PLoS. Genet. 2021;17 doi: 10.1371/journal.pgen.1009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K.R., Liao Y., Cai M., Qiu H., Wen F., Peng M., Wang S., Liu S., Guo G., Chi X., Maarouf M., Chen Y., Huang S., Chen J.-L. MIR155HG plays a bivalent role in regulating innate antiviral immunity by encoding long noncoding RNA-155 and microRNA-155-5p. mBio. 2022;13:e02510–e02522. doi: 10.1128/mbio.02510-22. (BR Cullen and MS Miller, eds.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remling L., Gregus A., Wirths O., Meyer T., Staab J. A novel interface between the N-terminal and coiled-coil domain of STAT1 functions in an auto-inhibitory manner. Cell. Commun. Signal. 2023;21(1):170. doi: 10.1186/s12964-023-01124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G., Tripathi S.K., Das S. lncRNA HULC facilitates efficient loading of HCV-core protein onto lipid droplets and subsequent virus-particle release. Cell. Microbiol. 2019;21(10):e13086. doi: 10.1111/cmi.13086. [DOI] [PubMed] [Google Scholar]
- Shen F.X., Ma G.P., Cheng A.C., Wang M.S., Li C.F., Sun K.F., Chang H., Zhu D.K., Jia R.Y., Chen X.Y., Sun T. Development and application of an indirect immunohistochemical method for the detection of duck plague virus vaccine antigens in paraffin sections and localization in the vaccinated duckling tissues. Poult. Sci. 2010;89:1915–1923. doi: 10.3382/ps.2010-00848. [DOI] [PubMed] [Google Scholar]
- Simion V., Zhou H., Haemmig S., Pierce J.B., Mendes S., Tesmenitsky Y., Pérez-Cremades D., Lee J.F., Chen A.F., Ronda N., Papotti B., Marto J.A., Feinberg M.W. A macrophage-specific lncRNA regulates apoptosis and atherosclerosis by tethering HuR in the nucleus. Nat. Commun. 2020;11:6135. doi: 10.1038/s41467-020-19664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui N., Zhang R., Jiang Y., Yu H., Xu G., Wang J., Zhu Y., Xie Z., Hu J., Jiang S. Long noncoding RNA expression rofiles elucidate the potential roles of lncRNA- XR_003496198 in Duck hepatitis A virus type 1 infection. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.858537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q., Liu N., Wu J., Chen J., Chen X., Peng C. Mefloquine enhances the efficacy of anti-PD-1 immunotherapy via IFN-γ-STAT1-IRF1-LPCAT3-induced ferroptosis in tumors. J. Immunother. Cancer. 2024;12 doi: 10.1136/jitc-2023-008554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidy A., Chelbi-Alix M., Blondel D. Rabies virus P protein interacts with STAT1 and inhibits interferon signal transduction pathways. J. Virol. 2005;79:14411–14420. doi: 10.1128/JVI.79.22.14411-14420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., You T., Fan H., Wang Y., Chu T., Poncz M., Zhu L. Rapamycin and bafilomycin A1 alter autophagy and megakaryopoiesis. Platelets. 2017;28:82–89. doi: 10.1080/09537104.2016.1204436. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang Y., Li Q., Zhao J., Yi D., Ding J., Zhao F., Hu S., Zhou J., Deng T., Li X., Guo F., Liang C., Cen S. Influenza virus exploits an interferon-independent lncRNA to preserve viral RNA synthesis through stabilizing viral RNA polymerase PB1. Cell. Rep. 2019;27:3295–3304.e4. doi: 10.1016/j.celrep.2019.05.036. [DOI] [PubMed] [Google Scholar]
- Wang S., He F., Li Z., Hu Y., Huangfu N., Xie D. Long non-coding RNA BANCR promotes interferon-β-induced cardiomyocyte apoptosis by targeting signal transducer and activator of transcription 1 in vitro. Int. J. Clin. Exp. Pathol. 2020;13(11):2840–2852. [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Huang L., Wang Y., Luo W., Li F., Xiao J., Qin S., Wang Z., Song X., Wang Y., Jin F., Wang Y. Single-cell RNA-sequencing analysis identifies host long noncoding RNA MAMDC2-AS1 as a co-factor for HSV-1 nuclear transport. Int. J. Biol. Sci. 2020;16:1586–1603. doi: 10.7150/ijbs.42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wei Y., Wu H., Feng L., Liu C., Huang L. Specific inhibition of the interaction between pseudorabies virus DNA polymerase subunits UL30 and UL42 by a synthetic peptide. Vet. Microbiol. 2022;272 doi: 10.1016/j.vetmic.2022.109517. [DOI] [PubMed] [Google Scholar]
- Wang K., Gong M., Zhao S., Lai C., Zhao L., Cheng S., Xia M., Li Y., Wang K., Sun H., Zhu P., Zhou Y., Ao Q., Deng X. A novel lncRNA DFRV plays a dual function in influenza A virus infection. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1171423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Cheng A., Wang M., Jia R., Yang Q., Wu Y., Zhu D., Zhao X., Chen S., Liu M., Zhang S., Ou X., Mao S., Gao Q., Sun D., Wen X., Liu Y., Yu Y., Zhang L., Tian B., Pan L., Chen X. Alphaherpesvirus major tegument protein VP22: Its precise function in the viral life cycle. Front. Microbiol. 2020;11:1908. doi: 10.3389/fmicb.2020.01908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Tan S., He Q., Wang M., Chen S., Jia R., Yang Q., Zhu D., Liu M., Zhao X., Zhang S., Huang J., Ou X., Mao S., Gao Q., Sun D., Tian B. Deletion of double copies of the US1 gene reduces the infectivity of recombinant duck plague virus in vitro and in vivo. Microbiol. Spectr. 2022;10:e01140–22. doi: 10.1128/spectrum.01140-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Tian B., Wang M., Cheng A., Jia R., Zhu D., Liu M., Yang Q., Wu Y., Huang J., Zhao X., Chen S., Zhang S., Ou X., Mao S., Gao Q., Sun D., Yu Y., Zhang L., Pan L. Duck plague virus negatively regulates IFN signaling to promote virus proliferation via JNK signaling pathway. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.935454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Zeng Y., Cheng A., Sun A., Wang M., Chen S., Liu M., Zhu D., Zhao X., Wu Y., Yang Q., Zhang S., Huang J., Ou X., Gao Q., Mao S., Sun D., Tian B., Zhang L., Yin Z., Jia R. Differential expression profile and in-silico functional analysis of long noncoding RNA and mRNA in duck embryo fibroblasts infected with duck plague virus. BMC. Genomics. 2022;23:509. doi: 10.1186/s12864-022-08739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Zhou C., Xiao Z., Tang G., Guo H., Hu Z., Hu Q., Peng H., Pi L., Zhang Z., Wang M., Peng T., Huang J., Liang S., Li G. LncRNA-UC.25 + shRNA alleviates P2Y14 receptor–mediated diabetic neuropathic pain via STAT1. Mol. Neurobiol. 2022;59:5504–5515. doi: 10.1007/s12035-022-02925-0. [DOI] [PubMed] [Google Scholar]
- Wu Y., Liu L., Zhang M., Zhan H., Wang C., Wang M., Chen S., Jia R., Yang Q., Zhu D., Liu M., Zhao X., Zhang S., Huang J., Ou X., Mao S., Gao Q., Sun D., Tian B., Cheng A. A recombinant Duck plague virus containing the ICP27 deletion marker provides robust protection in Ducks (J Miranda, ed.) Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.00983-23. e00983-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Jiang Y., Xu X., Su X., Liu Y., Ma Y., Zhao Y., Shen Z., Huang B., Cao X. Inducible degradation of lncRNA Sros1 promotes IFN-γ-mediated activation of innate immune responses by stabilizing Stat1 mRNA. Nat. Immunol. 2019;20:1621–1630. doi: 10.1038/s41590-019-0542-7. [DOI] [PubMed] [Google Scholar]
- Xu J., Gao F., Wu J., Zheng H., Tong W., Cheng X., Liu Y., Zhu H., Fu X., Jiang Y., Li L., Kong N., Li G., Tong G. Characterization of nucleocytoplasmic shuttling of pseudorabies virus protein UL46. Front. Vet. Sci. 2020;7:484. doi: 10.3389/fvets.2020.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yuan S., Peng Q., Ding Z., Hao W., Peng G., Xiao S., Fang L. Porcine epidemic diarrhea virus nsp7 inhibits interferon-induced JAK-STAT signaling through sequestering the interaction between KPNA1 and STAT1. J. Virol. 2022;96:e00400–e00422. doi: 10.1128/jvi.00400-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Xia M., Wang K., Lai C., Fan H., Gu H., Yang P., Wang X. A long non-coding RNA IVRPIE promotes host antiviral immune responses through regulating interferon β1 and ISG expression. Front. Microbiol. 2020;11:260. doi: 10.3389/fmicb.2020.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Yang K., Wills E., Tang L., Baines J.D. A mutation in the DNA polymerase accessory factor of herpes simplex virus 1 restores viral DNA replication in the presence of raltegravir (RM Longnecker, ed.) J. Virol. 2014;88:11121–11129. doi: 10.1128/JVI.01540-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.