Abstract
The trunks of elephants have prominent wrinkles from their base to the very tip. But neither the obvious differences in wrinkles between elephant species nor their development have been studied before. In this work, we characterize the lifelong development of trunk wrinkles in Asian and African elephants. Asian elephants have more dorsal major, meaning deep and wide, trunk wrinkles (approx. 126 ± 25 s.d.) than African elephants (approx. 83 ± 13 s.d.). Both species have more dorsal than ventral major trunk wrinkles and a closer wrinkle spacing distally than proximally. In Asian elephants, wrinkle density is high in the ‘trunk wrapping zone’. Wrinkle numbers on the left and right sides of the distal trunk differed as a function of trunk lateralization, with frequent bending in one direction causing wrinkle formation. Micro-computed tomography (microCT) imaging and microscopy of newborn elephants’ trunks revealed a constant thickness of the putative epidermis, whereas the putative dermis shrinks in the wrinkle troughs. During fetal development, wrinkle numbers double every 20 days in an early exponential phase. Later wrinkles are added slowly, but at a faster rate in Asian than African elephants. We discuss the relationship of species differences in trunk wrinkle distribution and number with behavioural, environmental and biomechanical factors.
Keywords: morphology, ageing, ontogeny, development, Proboscidea
1. Introduction
Animals have developed different morphologies for navigating, manipulating and interacting with their environments. These morphologies are often driven to accomplish a specific functional use or benefit, such as a wrinkle on the elephant trunk allows the trunk to stretch while maintaining protection [1]. Elephant trunks are their primary tool of manipulation and interaction with their environment and are essential for sensory perception such as olfaction and somatosensation [2,3]. The elephant trunk is described as one of the three prominent examples of muscular hydrostats along with octopus arms and mammalian tongues [4], but elephants are unique in that their hydrostat is covered in thick outer skin [5]. This skin has a protective function, but elephants also utilize it to assist in gripping objects when they wrap [6] or sweep food using the wrinkled ventral portion at the tip of the trunk [7]. Elephant skin is known for some simple gross mechanical properties, such as a cracked epidermis for thermoregulation [8] in African elephants and entangled collagen in the dermis of the trunk for added protection and extension [9].
The trunk’s mobility and flexibility are enabled by a highly complex musculature [10–12], controlled by a very elaborate motor nucleus [13]. The muscles are used when an elephant reaches for objects or food [1] and enable impressive fine motor control, enabling them to perform precise tasks such as peeling bananas [14]. They can also be controlled to manipulate air flow, allowing complex object manipulations, such as lifting a tortilla chip without breaking it using fluid suction [15]. We see functional mechanical differences along the trunk. Specifically, the distal parts of the trunk are very dexterous and form pseudo-joints for grasping objects [7]. In contrast, proximal trunk regions play a lesser role in manipulation and are more important for support and muscular force [1]. Trunk function becomes lateralized during elephant post-natal development, and adult elephants split into left- or right-trunkers according to their grasping preferences [16–18]. Functional differences also appear when comparing different elephant species.
African savannah elephants (Loxodonta africana, from here on called African elephants) and African forest elephants (Loxodonta cyclotis, not subject of this publication) differ from Asian elephants (Elephas maximus) with regards to their trunk morphology and behaviour. African elephants have two finger-like protrusions on their trunk tips and tend to pinch objects with their two fingers. Asian elephants, in contrast, have only one dorsal trunk finger and tend to wrap their trunk around objects [19].
Although these behavioural and morphological differences are well known, there is little understanding of the developmental factors that play a role in the functionality of the elephant trunk. Additionally, even though elephants have prominent trunk wrinkles from birth, the development of the skin of this hydrostat remains a mystery. Understanding how these wrinkles develop and change over time can help provide valuable insight into biological wrinkling and the impact of the environment and behaviour on it [20]. In this study, we seek to understand the form-function ontogeny of the wrinkled trunk skin both pre-natal and through adulthood. In our analysis, we aimed to elucidate the functional and developmental characteristics of elephant trunk wrinkles. Specifically, we ask: (i) what is the number and distribution of trunk wrinkles in adult elephants, baby elephants/fetuses and across elephant species? (ii) Are the elephant’s trunk wrinkles affected by trunk use and lateralization? (iii) Do the skin layers differ along a single wrinkle in the trunk? (iv) How do elephant trunks and trunk wrinkles develop?
2. Material and methods
2.1. Elephant specimens
All post-mortem specimens used in this study came from zoo elephants and were collected by the Leibniz Institute for Zoo and Wildlife Research, Berlin (IZW) over the last three decades in agreement with the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) regulations. Specimen reports and CITES documentation for all animals included are held at the IZW. All of these elephants had died of natural causes or were euthanized by experienced zoo veterinarians for humanitarian reasons, because of insurmountable health complications. Most of the trunks used were either fixed in 4% formaldehyde solution or frozen at −20°C. Electronic supplementary material, table S1 gives an overview of the post-mortem specimens of Asian elephants (E. maximus) and African elephants (L. africana), along with their age. In addition to the post-mortem samples, photographs of living elephants in zoos were also analysed. Electronic supplementary material, table S2 gives an overview of these elephants.
2.2. Photography of trunks
Post-mortem specimens at the laboratory and elephants at the zoos were photographed using a Sony α 7R III camera or a Sony α 7S II with a Sony FE 16–35 mm F2.8 GM E-Mount objective, a Sony FE 90 mm/2.8 Macro G OSS objective or a Sony FE 4.5–5.6/100−400 GM OSS zoom objective. Cameras were used handheld or mounted on a Hama ‘Star 62’ tripod or a Manfrotto ‘MT190CXPRO4’ carbon tripod. Photographs were either taken by one of the authors at the Berlin Zoo, and the Zoo Schönbrunn, Vienna, or provided by zoo employees, collaborators or photographers.
2.3. Elephant wrinkle measurements
The trunks were divided into zones: base, lateral shaft, dorsal shaft, ventral shaft and tip (electronic supplementary material, figure S1). The base is determined as the most proximal part of the trunk, above the tusk pouch. The tip is considered the most distal portion of the trunk from the split into the two fingers in African or finger and cartilage in Asian elephants to the end of the fingers (electronic supplementary material, figure S1A–B). The shaft is the rest of the trunk between the distal tip and the proximal base, as described. Wrinkles were identified as either ‘major’ or ‘minor’ wrinkles, with the ‘major’ being deeper, mostly regularly spaced, and traversing the whole dorsal or ventral part of the shaft (figure 2a,b). The ‘minor’ wrinkles are shallow wrinkles that partially cross the trunk with uneven spacing. In African elephants, the proximal trunk has been described to have deep wrinkles termed ‘folds’ [1], which, for our analysis, were counted as ‘major’ wrinkles. For the lateralization (figure 3c–e), we looked at the most distal 15 cm of the trunk shaft and quantified major wrinkles; trunk fingers were excluded from this analysis. This section was examined as the distal non-finger portion of the trunk is primarily used for lateral wrapping around objects [21]. The number of wrinkles was normalized by dividing the number of wrinkles on one side by the total number of wrinkles on this 15 cm trunk shaft. The fraction of wrinkles is reported in per cent. Wrinkles were counted using the multi-point tool in ImageJ. Wrinkle position, including wavelength, was determined using ImageJ.
The wavelength of the trunk wrinkles was taken as the distance between any two wrinkles. The wrinkles were sketched as lines that run transverse to the trunk length (figure 2a,b). To calculate the wavelength and the wrinkle number, perpendicular lines were drawn from the left side to the right side of the trunk. We then drew a line across the centreline of the trunk from the proximal base of the trunk to the distal tip. The number of intersections was described as a wrinkle number for that segment, and the distance between each intersection is the wavelength between those wrinkles. We analysed all the zones previously described for the average wavelength between wrinkles and the wrinkle number along the trunk.
2.4. Fetal development
To study trunk wrinkle and trunk development, we studied five fetal elephant specimens (n = 3 African elephant fetuses from the Naturkundemuseum Berlin, and n = 2 Asian elephant fetuses from our collection). We also made a major effort to collect photographs or drawings of elephant fetuses (n = 50 African, n = 12 Asian) from published work [22–48]. We assigned presumed embryonic ages, denoted as En where n is the number of days according to the formulas for embryonic length [37] or mass [37] in early fetuses. In older fetuses (greater than E200) we used the mass-age formula developed by Craig [30].
2.5. Micro-computed tomography scanning
All samples for micro-computed tomography (microCT) scanning were taken from trunks that were fixed in 4% formaldehyde for several months. To characterize wrinkles from different trunk regions, an Asian baby elephant trunk was cut in half sagittal and stained in 1% iodine solution for 33 days to enhance tissue contrast. The half trunk was then stained for 84 days in a lower concentration of iodine solution. For the African baby elephant trunk, the sample was first put for 30 days in a 1% iodine solution, 30 days in a 2% iodine solution and finally 30 days in a 3% iodine solution.
All iodine solutions were prepared by diluting 5% Lugol’s iodine in distilled water. The scans for the Asian baby elephant trunk were performed using the YXLON FF20 CT scanner (YXLON International GmbH, Hamburg, Germany) at the Humboldt University of Berlin. The African baby elephant trunk was scanned at the Museum für Naturkunde Berlin with a YXLON FF85 CT (YXLON International GmbH, Hamburg, Germany).
2.6. Micro-computed tomography and histology determining baby trunk wrinkle and amplitude and skin thickness
The amplitudes of the trunk wrinkles from the baby trunks that we had microCTs of were taken as a trough-to-crest measurement as in a sinusoidal wave. This is a simple estimation to differentiate the wrinkled pattern in the post-mortem baby specimens. The amplitude was calculated using side views of transversely dissected trunks allowing crest-to-crest calculation of various segments along the trunk’s surface.
To compare the African and Asian elephant microCTs (figure 4a,e) we normalized the positional information using the total trunk length (63 cm for African elephant baby, 36 cm for Asian elephant baby). Therefore, the trunk wrinkle amplitude and wavelength are plotted on the same axis of African and Asian elephants by dividing the position along the trunk by total length, giving a dimensionless length. This means the trunk position is unitless, and a trunk position of 0 is at the proximal base of the trunk, and near the distal tip, it would have a value of 1. To perform statistical comparisons along the normalized length of the African and Asian elephants, we performed zone-wise comparisons between three primary zones: the proximal, mid-section and distal sections. Each section has a normalized length of 0.3 of the normalized trunk length, with the proximal section ranging from 0 to 0.3, the mid-section 0.3–0.6 and the distal section 0.6–0.9. We did not analyse the normalized trunk length of 0.9–1.0 as this portion of the microCTs did not have enough resolution to get amplitude or wavelength measurements. The three sections were averaged for each specimen and compared. Lack of resolution at the tip was because of the resolution of the microCT for the overall size of the sample as well as movement artefacts.
We also show histological sections from an Asian baby elephant’s trunk tip finger (electronic supplementary material, figure S2A; originally done for Deiringer et al. [49] but not shown there) to determine the histological differences between the two primary skin layers (electronic supplementary material, figure S2B–C). The section taken has presence of three complete wrinkles along the dorsal portion of the finger. Samples were stained using a standard haematoxylin-eosin stain for elephant tissue [49] and imaged using an Olympus BX51 microscope (Olympus, Japan) with an MBFCX9000 camera (MBF Bioscience, Williston, USA).
2.7. Statistical analysis of wrinkle numbers
We used a confidence interval of 95% throughout the analyses. A priori, we detected outliers (Tukey–Fence, k = 1.5), checked normality (Shapiro–Wilk test) and checked equality of variances (two-tailed F test). Statistical methods were chosen accordingly, possible dependence of measurements was taken into account as well. Descriptive statistics, specific tests used, effect sizes and p-values are reported in the Results.
3. Results
We studied wrinkles on the trunk of Asian (E. maximus) and African (L. africana) elephants (figure 1). We analysed photographs of live elephants from Zoos (electronic supplementary material, table S2) and post-mortem samples that were collected in a decade-long effort by the IZW (electronic supplementary material, table S1). We looked at skin structure in relation to wrinkles using post-mortem specimens and microCT scans. To examine the early development of wrinkles, we studied post-mortem material from fetuses and newborns.
Figure 1.
Asian (E. maximus) and African (L. africana) elephants differ in trunk morphology, including trunk wrinkles. (a) Drawing of the trunk of an adult Asian elephant. Note the larger number of transversal wrinkles in the Asian elephant compared with the African elephant trunk in (h). (b) Proximal trunk base wrinkles of an Asian elephant. (c) Same as (b) but for the middle part of the trunk. (d) Same as (b) but for the distal part of the trunk. (e–g) Same as (b–d) but for an African elephant. (h) Drawing of the trunk of an adult African elephant. Note the major wrinkles on the proximal half of the trunk that fold over each other and how they transition to the tightly packed distal trunk wrinkles. Illustrations (a,h): Cindy Ritter. Photo credit (b–d): Lena Kaufmann, Humboldt Universität zu Berlin; Zoologischer Garten Berlin, Berlin, Germany. Photo credit (e–g): Lena Kaufmann, Humboldt Universität zu Berlin; Zoo Schönbrunn, Vienna, Austria.
3.1. Asian and African elephants differ in trunk wrinkles and overall morphology
Asian (figure 1a ) and African (figure 1h ) elephant trunks differ in their morphology. The coloration of the trunk and skin texture differs between species, with Asian elephant trunk skin looking lighter, having pinkish pigmentation and smoother skin (figure 1b–d ). African elephant trunk skin appears greyer and more cracked (figure 1e–g ). Another obvious difference lies in the form and arrangement of wrinkles that can be found on the whole trunk from the base until the very tip. In both species, transversal trunk wrinkles are more clear and deeper than longitudinal trunk wrinkles and there are few or no oblique (non-transversal or longitudinal) wrinkles (figure 1). In this work, we only report numbers of transversal trunk wrinkles on the dorsal or ventral trunk. Dorsal trunk wrinkles can be major or minor wrinkles. Major wrinkles are deeper and more regularly spaced than minor wrinkles. For details, see §2 and figure 4, where microCT scans very clearly show the difference between the deep major wrinkles and the shallower minor wrinkles, often lying in between two major ones. We found that the number of total dorsal trunk wrinkles (major + minor) in adult Asian elephants (n = 7, out of these 5 females and 2 males; x̄ = 155, s.d. = 26) is larger than in African elephants (n = 7, all females; x̄ = 109, s.d. = 14; Mann–Whitney U test, U = 0.94, z = 2.69, p = 0.007). In both species, we can see the distance between wrinkles (wavelength) decreasing towards the distal end of the trunk (figure 1). In Asian elephants, wrinkles of the proximal trunk appear shallower than in African elephants and more irregularly spaced (figure 1b,e ). Wrinkles of the medial (figure 1c,f ) and distal shaft (figure 1d,g ) appear more densely packed in Asian than in African elephants. For a visualization of the partition of the trunk in base, shaft and tip zones, see electronic supplementary material, figure S1A. In both elephant species studied, we also noticed numerous partial wrinkles both on the proximal base and distal tip of the trunks. These partial, ‘broken’ wrinkles wrap around half of the trunk from one lateral side and after a gap often continuing shifted a little bit proximally or distally. We conclude that Asian and African elephants have visually distinct patterns of trunk wrinkles.
3.2. Trunk major and minor wrinkles differ in counts and distribution between Asian and African elephants
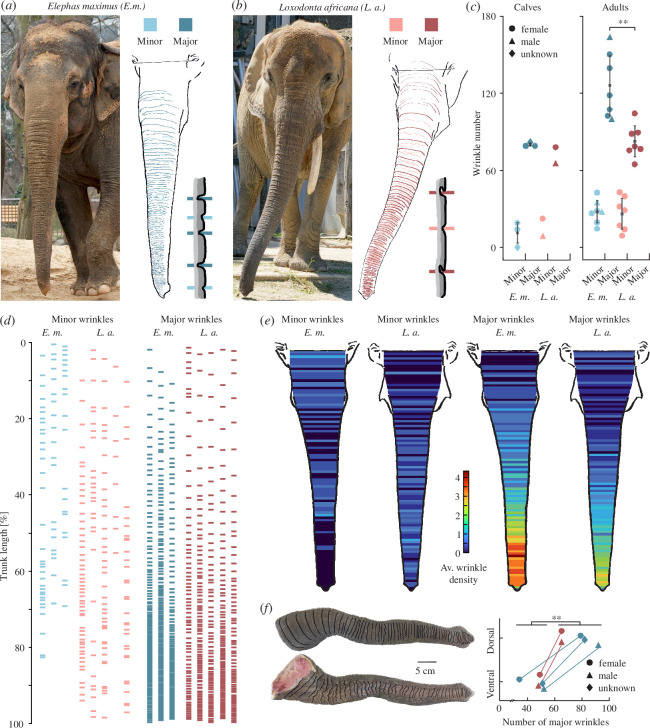
Wrinkles were traced on photographs of elephant trunks and colour-coded according to wrinkle type, which could be major or minor wrinkles (figure 2a,b ; note the schematics depicting major/minor wrinkles in both species). In elephant babies, we did not find differences in major or minor wrinkle numbers. The Asian baby elephants (n = 3) have on average 80 (s.d. = 2) major and 11 (s.d. = 10) minor dorsal trunk wrinkles, whereas in the African baby elephants (n = 2), we counted on average 72 (s.d. = 9) major and 15 (s.d. = 10) minor dorsal trunk wrinkles (figure 2c ). Adult Asian elephants (n = 7), however, have significantly more dorsal major trunk wrinkles (x̄ = 126, s.d. = 25) than African elephants (n = 7, x̄ = 83, s.d. = 13; two-sample t‐test t(12) = 4.05, d = 2.16, p = 0.002) but similar numbers of minor trunk wrinkles (figure 2c ). Adult Asian elephants have on average 28 (s.d. = 9) minor trunk wrinkles and African elephants on average 26 (s.d. = 13) minor trunk wrinkles. When controlling for sex and comparing only female elephants, the difference in major wrinkle numbers between Asian (n = 5, x̄ = 124, s.d. = 21) and African elephants is even stronger (n = 7, x̄ = 83, s.d. = 13; two-sample t‐test t(10) = 4.28, d = 2.51, p = 0.002). In Asian elephants, wrinkle numbers increase from on average 91 (s.d. = 8) total wrinkles in the babies (n = 3) to 155 (s.d. = 26) total wrinkles in the adults (n = 7). This change is mostly due to the increase in major wrinkles from 80 (s.d. = 2) in the babies to 126 (s.d. = 25) in the adult Asian elephants. For the African elephants, on the other hand, the change in total wrinkle number from on average 87 (s.d. = 19) in the babies (n = 2) to 109 (s.d. = 14) in the adults is a bit smaller. Major wrinkles increase in African elephants from 72 (s.d. = 9) in the babies to on average 83 (s.d. = 13) in the adults (figure 2c ).
Figure 2.
Transversal trunk major and minor wrinkles differ in counts and distribution between Asian and African elephants. (a) Female Asian elephant Carla (Zoo Berlin) next to a tracing of minor and major wrinkles with a schematic of what minor/major wrinkles look like in Asian elephants. Major wrinkles in contrast to minor wrinkles are deeper, more regularly occurring, and regularly spaced, and transverse the whole dorsal or ventral trunk. Note the zero-line between the eyes, wrinkles proximal to this were not included in the analysis. (b) Same as (a) but for female African elephant Drumbo (at the time of photograph Zoo Schönbrunn). (c) Comparison of trunk wrinkle numbers between Asian and African elephants in babies (left) and adults (right). Asian elephant babies (n = 3) and African elephant babies (n = 2) have similar numbers of minor and major wrinkles. Asian adult elephants (n = 7) have more major trunk wrinkles (x̄ = 126, s.d. = 25) than African adult elephants (n = 7, x̄ = 83, s.d. = 13; two-sample t‐test t(12) = 4.05, d = 2.16, p = 0.003). Circle = female, triangle = male, diamond = unknown. (d) Positions of minor (left) and major (right) wrinkles on trunks normalized to total trunk length in individual female Asian (n = 3) and African (n = 5) elephants. (e) Heatmaps showing the distribution of minor (left) and major (right) trunk wrinkles in Asian (n = 3) and African (n = 5) elephants, based on (d). Heatmaps show average density of wrinkles, wrinkle positions are normalized to trunk length. Asian elephants have on average more minor wrinkles in the proximal part of the trunk, whereas in African elephants minor wrinkles are more spread over the rest of the trunk. Asian elephants have on average more major wrinkles in the distal half of the trunk with a particularly high density in the region where they bend when wrapping objects. Blue shows a low average density of wrinkles and red a high average density of wrinkles at this position of the trunk. (f) On the left, a photograph of an African elephant baby trunk showing tracings of major wrinkles on the dorsal (upper) and ventral (lower) sides of the trunk. On the right, a comparison of major wrinkles on the dorsal and ventral sides of the same trunk in Asian (n = 3) and African (n = 2) elephants. There are significantly more wrinkles on the dorsal (x̄ = 77, s.d. = 10) than on the ventral sides of the trunks (x̄ = 47, s.d. = 7; two-tails paired t‐test t(4) = 5.07, d = 2.27, p = 0.007). Circle = female, triangle = male, diamond = unknown. The line between symbols represents both symbols being part of the same trunk, one being the winkle number on the dorsal and one the wrinkle number on the ventral side. Photo credit (a): Lena Kaufmann, Humboldt Universität zu Berlin; Zoologischer Garten Berlin, Berlin, Germany. Photo credit (b): Lena Kaufmann, Humboldt Universität zu Berlin; Zoo Schönbrunn, Vienna, Austria. Photo credit (f): Lena Kaufmann, Humboldt Universität zu Berlin.
The minor wrinkles are denser in the Asian elephant’s proximal part of the trunk, whereas in African elephants, they are more spread out than in the Asian and denser in the distal part of the trunk (figure 2d,e ). In both species, the density of major wrinkles increases towards the trunk tip, with the average density of major wrinkles at the distal third of the trunk being even higher in Asian elephants than in African elephants (figure 2d,e ). We found a significantly greater number of major wrinkles dorsally (x̄ = 77, s.d. = 10) than ventrally (x̄ = 47, s.d. = 7; paired t‐test t(4) = −5.07, d = 2.27, p = 0.007; figure 2f ) in both Asian (n = 3, all less than 5 years old) and African (n = 2, one adult and one baby) elephants.
A more in-depth analysis of wrinkle numbers separated in different trunk zones (base, shaft or tip; see electronic supplementary material, figure S1A and B) revealed a difference in major wrinkles on the trunk shaft, with adult Asian elephants (n = 7) having a greater number of major trunk shaft wrinkles (x̄ = 115, s.d. = 26) than adult African elephants (n = 7, x̄ = 70, s.d. = 13; two-sample t‐test t(12) = 4.09, d = 2.18, p = 0.002; electronic supplementary material, figure S1C). No differences between species were found in the number of wrinkles on the trunk base or tip, independently of pooling or not pooling major and minor wrinkles or female and male elephants. There is also no significant difference between the two species in minor trunk wrinkle numbers of the trunk shaft. In babies of both species, we found comparable numbers of major and minor wrinkles, so it is notable that Asian elephants gain more wrinkles during their life than African elephants (electronic supplementary material, figure S1C). The differences in wrinkle numbers between the two species reflect differences in the number of major wrinkles, but not minor wrinkles (figure 2c and electronic supplementary material, figure S1C).
3.3. Trunk wrinkle number is lateralized
Almost all adult elephants show marked left–right asymmetries in trunk whisker length as shown by Deiringer et al. [49] (figure 3a–c ). Elephants use the distal third of their trunk to wrap food or other objects and the whiskers are longer on the side they prefer wrapping towards, designating the ‘trunkedness’ (figure 3a,b ). This side preference and, as a function of it, whisker asymmetry is age- and use-dependent as elephant babies are born without it and will develop a favoured side along with their trunk control which takes around two months [50]. Whisker abrasion appears on the opposite side of the one wrapped towards, as it is more often in contact with the ground. We identified the ‘trunkedness’ of all our specimens and counted lateral wrinkles, as illustrated with the trunk tip of an African elephant in figure 3c . Whiskers were longer on its right trunk side; thus, it presumably was a right-trunker, preferentially wrapping towards its right side.
Figure 3.
Left- and right-trunkers have more wrinkles on the left and right trunk sides, respectively (a) Image of a left-trunker with the trunk tip and distal trunk shaft of an Asian elephant. Note the shorter whiskers on the right side and the longer whiskers on the left side of the distal trunk shaft. Modified from Deiringer et al. [49]. (b) Image showing a left-trunker behaviour of an Asian elephant reaching for a watermelon. The image displays a trunk wrapping to the left with the right-side of the distal shaft of the trunk in contact with the ground which results in whisker abrasion. Modified from Deiringer et al. [49]. (c) African elephant trunk tip and distal trunk shaft, in red the major wrinkles on the trunk shaft that were counted. The red line crossing the tip is the last wrinkle counted. (d) Univariate plot of the fraction of wrinkles (normalized to the total count on both trunk sides) on the trunk side with shorter or longer whiskers. Trunk function is lateralized in elephants and so-called left-trunkers, who preferentially grasp towards the left side, have shorter whiskers of the right side of the distal trunk shaft [49]; the reverse is true for right-trunkers. We observed approximately 10% more wrinkles on the longer whisker side. In yellow, the wrinkles fraction on the longer whisker side and in blue, the wrinkles on the shorter whisker side. One dot is one animal, yellow and blue for the same animal plotted on the same axis. Wrinkle counts are reported as percentage of total wrinkles on the respective trunk shaft specimen. Paired sample t‐test t(14) = 2.59, d = 0.67, p = 0.022. n = 5 adult African elephants, 9 adult Asian elephants. (e) Univariate plot of the fraction of wrinkles (normalized to the total count on both trunk sides) on the left or right side of the trunk. The full dots are the wrinkles fraction on the left side and the empty dots are the wrinkles on the right side. One dot is one animal, for the same animal the dots are plotted on the same axis. n = 5 adult African elephants, 9 adult Asian elephants. Photo credit (a,b,c): Lena Kaufmann, Humboldt Universität zu Berlin.
We discovered that, through mechanical usage, elephants develop wrinkles on their lateral distal trunk. To quantify the differences in wrinkle numbers between the trunk sides, we looked at the first 15 cm of the shaft, i.e. right after the trunk tip, of 15 elephant trunks. In both, Asian (n = 10) and African (n = 5) elephants, there are approximately 10% more wrinkles on the distal trunk shaft side with the longer whiskers. Here, we report the fraction of wrinkles on the left or right side of this part of the trunk in per cent of total wrinkles found on both sides. This bias of having more wrinkles on the trunk side with longer whiskers (x̄ = 53%, s.d. = 4%) than on the shorter whiskers side (x̄ = 47%, s.d. = 4%) was systematic and significant (paired sample t‐test t(14) = 2.59, d = 0.67, p = 0.022; figure 3d ). In contrast, we did not observe a systematic difference in trunk side wrinkle numbers as a function of species difference or of left (x̄ = 52%, s.d. = 5%) versus right trunk side (x̄ = 48%, s.d. = 5%; figure 3e ). This corresponds with the distribution of ‘trunkedness’ being approximately 50/50 in elephant populations [16]. Taken together, this indicates that differences in trunk side wrinkle numbers are due to the individual’s ‘trunkedness’, being a ‘left-’ or ‘right-trunker’. In other words, behavioural preferences shape the morphology or the trunk.
3.4. Skin layer anisotropy along the wrinkled skin of elephant baby trunks
We performed microCT scans of iodine-stained trunks of Asian and African elephant babies to visualize trunk wrinkles and underlying skin structure. These scans provided high-resolution images of entire elephant trunks (figure 4). Major and minor wrinkles were readily visible in volume renderings (figure 4a ) and parasagittal sections (figure 4b ) of an Asian baby elephant trunk. As we noted before in adult elephant trunks, wrinkle frequency increased from proximal to distal (figure 4c,d ).
Figure 4.
Visualization of trunk wrinkles in microCT scans of an Asian and an African baby elephant trunk. (a) Volume rendering of a microCT-scanned Asian baby elephant trunk. (b) Sagittal slice of an Asian baby elephant trunk. (c) High magnification view of proximal dorsal wrinkles in the Asian baby elephant trunk. Note the constant thickness of the putative epidermis, while the putative dermis is getting thinner in the troughs of the wrinkles. The two primary load-bearing layers of skin are highlighted; the epidermis in blue, and the dermis in pink. (d) High magnification view of distal dorsal wrinkles in the Asian baby elephant trunk with highlights of the two primary load-bearing skin layers. Note the constant thickness of the putative epidermis, while the putative dermis is getting thinner in the troughs of the wrinkles. (e) Volume rendering of a microCT-scanned African baby elephant trunk. (f) Sagittal slice of an African baby elephant trunk. (g) High magnification view of dorsal wrinkles in the African baby elephant trunk. Note the constant thickness of the putative epidermis, while the putative dermis is getting thinner in the troughs of the wrinkles. The two primary load-bearing layers of skin are highlighted similarly to (c) and (d).
It appears that the morphology of the different skin layers differs along the arc length of a wrinkle. In the Asian baby elephant, the outer layer of the skin, the thickness of the epidermis (blue highlight), appears to be constant throughout a wrinkle; however, the dermis (pink highlight) becomes thicker in between the troughs and is thinner in the trough of a wrinkle (figure 4c,d ). Thus, trunk major wrinkles are not mere creases of the skin but show clearly non-homogeneous skin layers along their arc length. In particular, because of the reduction of parts of dermis in the trough, the skin is quite thin and presumably also more flexible. Minor wrinkles appeared to be slight indentions in the epidermis, like folding of the major wrinkle crest on itself. Very similar observations were made on volume renderings (figure 4e ) and parasagittal sections (figure 4f ) of an African baby elephant trunk. Similar to the Asian baby elephant, the epidermis of the African baby elephant was observed to be of near-constant thickness throughout major wrinkles. At the same time, we found the dermis layer to change between the crests and troughs of the major wrinkles (figure 4g ).
We performed a more detailed histological analysis of skin layers in the trunk tip of an Asian baby elephant and found that the dermis changes from approximately 0.9 mm in the crest to 0.6 mm in the trough. For the epidermis, we observed a constant thickness of approximately 0.4 mm (electronic supplementary material, figure S2). We conclude that the morphology of the major wrinkles in African and Asian baby elephants differs along the arc length of the wrinkle. We observed a thinner dermis in the trough of the wrinkle and a thicker dermis at the crest.
3.5. Wrinkle and amplitude species differentiation in micro-computed tomography
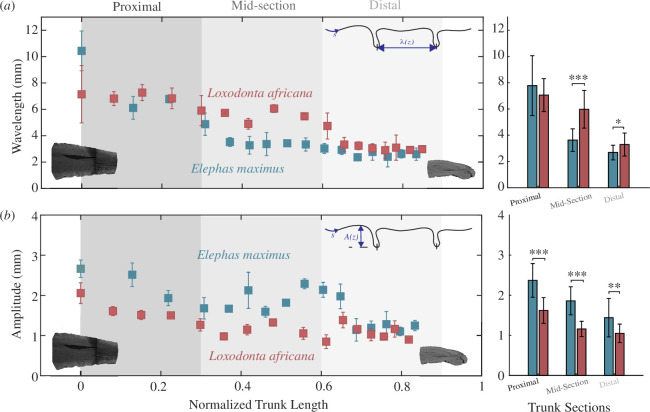
We used measurements of individual wrinkles of the microCT-scanned specimens to perform a more in-depth analysis of wrinkle wavelengths, the distances between two major wrinkle troughs, and amplitudes or depths. In both Asian and African baby elephants, the wavelength between dorsal trunk wrinkles was highest at the proximal portion of the trunk and decreased towards the tip (figure 5a ). In comparing the zones of the African and Asian baby elephants, we see the African baby elephant has significantly larger wavelengths (figure 5a ) in the mid-section (x̄ = 5.98 mm, s.d. = 1.43 mm; one-way ANOVA F = 32.93, p < 0.001) and distal section (x̄ = 3.29 mm, s.d. = 0.88 mm; one-way ANOVA F = 6.98, p = 0.01) compared with the Asian baby elephant’s mid-section (x̄ = 3.64 mm, s.d. = 0.86 mm) and distal section (x̄ = 2.68, s.d. = 0.56). This is consistent with other results, as larger wavelengths indicate fewer wrinkles along the trunk. At the distal tip, the wavelengths are nearly equivalent, with both elephants having wavelengths around 3 mm (figure 5a ). In analysis of the amplitude differences between the species, we see that the Asian baby elephant has significantly higher amplitudes along the whole trunk (figure 5b ) including proximal (x̄ = 2.71 mm, s.d. = 0.42 mm; one-way ANOVA F = 22.5, p < 0.001), mid-section (x̄ = 1.86 mm, s.d. = 0.35 mm; one-way ANOVA F = 44.68, p < 0.001), and distal (x̄ = 1.45 mm, s.d. = 0.48 mm; one-way ANOVA F = 11.87, p = 0.001) sections. This is compared with the African baby elephant with amplitudes decreasing by nearly 35% from the proximal section (x̄ = 1.62 mm, s.d. = 0.32 mm), mid-section (x̄ = 1.12 mm, s.d. = 0.22 mm), to the distal portion (x̄ = 1.05 mm, s.d. = 0.22 mm).
Figure 5.
Wrinkle amplitude and wavelength of African and Asian elephant baby trunks. (a) Wavelength of an Asian baby elephant (blue) trunk and an African baby elephant (maroon) trunk, taken from the microCT-scans in figure 4. Averages of three different proximal, mid-section and distal sections are shown with shaded sections where the average of the wrinkle amplitude and wavelength are compared statistically in each section. Statistics for the three sections are shown on the right. The African baby elephant has significantly larger wavelengths in the mid-section (x̄ = 5.98 mm, s.d. = 1.43 mm; one-way ANOVA F = 32.93, p < 0.001) and distal section (x̄ = 3.29 mm, s.d. = 0.88 mm; one-way ANOVA F = 6.98, p = 0.01) compared with the Asian baby elephant’s mid-section (x̄ = 3.64 mm, s.d. = 0.86 mm) and distal section (x̄ = 2.68 mm, s.d. = 0.56 mm). (b) Crest-to-crest amplitude of an Asian baby elephant (blue) trunk and an African baby elephant (maroon) trunk, taken from the microCT scans in figure 4. The average of different zones is shown similarly to (a) with comparisons between the species. Statistics for the three sections are shown on the right. The Asian baby elephant has significantly higher amplitudes along the whole trunk including proximal (x̄ = 2.71 mm, s.d. = 0.42 mm; one-way ANOVA F = 22.5, p < 0.001), mid-section (x̄ = 1.86 mm, s.d. = 0.35 mm; one-way ANOVA F = 44.68, p < 0.001), and distal (x̄ = 1.45 mm, s.d. = 0.48 mm; one-way ANOVA F = 11.87, p = 0.001) sections. This is compared with the African baby elephant with amplitudes decreasing by nearly 35% along the trunk from the proximal section (x̄ = 1.62 mm, s.d. = 0.32 mm), to the mid-section (x̄ = 1.12 mm, s.d. = 0.22 mm) and to the distal portion (x̄ = 1.05 mm, s.d. = 0.22 mm).
3.6. Fetal trunk and trunk wrinkle development
We examined how trunk wrinkles develop and how they relate to trunk development in general. To address this issue, we studied Asian (n = 2) and African elephant (n = 3) fetal specimens, as well as published photographs or drawings of elephant fetuses (n = 12 Asian, n = 50 African). We then assigned embryonic (E) ages to these specimens, as detailed in §2.
Figure 6a shows schematic drawings of different stages of fetal African elephant heads, their trunks (black), their trunk wrinkles (grey) and their upper (red) and lower (green) lip. The elephant trunk develops from a large nose primordium. Wrinkles are initially added rapidly and then gradually. A schematic overview is given for wrinkle development (figure 6b ) and lip development (figure 6c ). The upper lip–nose fusion occurs rapidly between embryonic days E100 and E130.
Figure 6.
Fetal trunk and trunk wrinkle development. (a) Schematic drawings of African elephant fetuses. Grey, outline of the head and wrinkles; grey dashed, putative wrinkles; black, outline of the trunk; green, lower lip; red, upper lip. Fetuses were redrawn from references following references [22–48]. E# indicates the embryonic age of the photo with the number indicating days. (b) Schematic of stages of fetal wrinkle development in African and Asian elephants. (c) Schematic of stages of fetal lip development in African elephants. (d) Relative length growth of various body parts in African elephants. Length was normalized to the length of the respective body part in E80 fetuses and is given in per cent. The trunk grows more than other body parts and the accelerated growth occurs mainly between E60 and E150. (e) Trunk length growth in African and Asian elephants is similar. (f) Wrinkle development in African and Asian elephants. Wrinkle number increases in sharply different phases: Between E80 and E130, there is an exponential increase in wrinkle number with a doubling time of approximately 20 days. After E130 addition of wrinkles is slow, but slightly faster in Asian than in African elephants. Note that wrinkles could only be counted in a subset of fetuses.
The trunk shows more length growth than other elephant body parts; this faster growth occurs early (E60–E150, figure 6d ). The fetal trunk length growth pattern is similar in Asian and African elephants (figure 6e ). A log plot of wrinkle numbers against fetal age reveals that wrinkles develop in two sharply different phases (figure 6f ). Between E80 and E150, there is an exponential growth of wrinkle numbers with a doubling time of about 20 days, after that addition of wrinkles is slow, and slightly faster in Asian elephant fetuses than in African ones. The number of wrinkles on fetal elephant trunks between E200 and birth in Asian (n = 8, x̄ = 64, s.d. = 12) and African elephants (n = 11, x̄ = 44, s.d. = 6) is plausibly continued in the total number of wrinkles we found in Asian (n = 3, x̄ = 91, s.d. = 8) and African (n = 2, x̄ = 87, s.d. = 19) baby elephants, based on our laboratory specimen as well as photographs from zoos (figure 2c ).
Adult African elephants have two fingers at the tip of their trunk a dorsal (top) and a ventral (bottom) finger. In contrast, Asian elephants only have a dorsal finger and a ventral cartilage stump. We characterized the development of the trunk tip and the fingers in a schematic (figure 7a ). The trunk first grows as a stump. Then around embryonic day 130, the ventral finger grows out in African elephants, whereas Asians grow out a bulbous ventral trunk tip structure. The fact that the ventral finger grows out first is shown in figure 7b for African elephants. Specifically, we observed that the ventral finger tends to be longer between E120 (before that there are no fingers) and E200. Dorsal finger development follows a slight delay in both species and differs in time course between Asian and African elephants. In African elephants, finger growth goes through a brief initial exponential length increase, after which the finger grows slower and more gradually (figure 7c ), our data were insufficient for a detailed assessment of finger growth patterns in Asian elephants.
Figure 7.
Fetal trunk finger development in Asian and African elephants. (a) Schematic of stages of trunk tip development in African and Asian elephants. (b) Length difference of dorsal and ventral trunk finger in African elephants throughout fetal development. Pre-E120, there are no fingers, then the ventral finger is longer (highlighted as red dots) and after E200, the dorsal finger takes over. (c) Fetal dorsal and ventral finger growth in African elephants. Finger growth goes through a brief exponential phase (E130–E180), after which finger growth slows down. The length of both the dorsal and ventral fingers could not be determined in all specimens. Note that the ventral finger (circles) tends to be longer than the dorsal finger (triangles) in early fetuses and shorter than the dorsal finger in older fetuses.
We conclude that the trunk is the fastest growing body part of elephants and that wrinkles are added in two steps, a first exponential growth step, and a second slower addition step, which differs between Asian and African elephants.
4. Discussion
We assessed elephant trunk wrinkles and their pre-, as well as post-natal, development by reviewing published literature across developmental time, photography, microCT imaging and analysis of post-mortem specimens in samples of fetal, newborn and adult African and Asian elephants. We find the trunk wrinkles of African and Asian elephants to be different in several aspects.
4.1. Differences in wrinkles are potentially tied to genetic, behavioural and environmental factors
Specifically, adult Asian elephants have about 1.5 times more trunk wrinkles than adult African elephants, due to an extensive addition of major wrinkles in Asian elephants during their lifetime development from baby to adult. This trend begins already at fetal stages and continues throughout post-natal development, turning into a significant difference in adult elephants. Additionally, even though we find a closer spacing of wrinkles in the distal than in the proximal trunk in both species, the density of major wrinkles on the distal third of the trunk is much higher in adult Asian than in adult African elephants.
Taken together, these results could indicate that species differences in trunk wrinkles and trunk wrinkle morphology might have a genetic component—we already see slight differences in fetal and early post-natal stages, and differences in adults could very well also be partially genetically determined. We would also like to propose, however, that specific behavioural adaptations of Asian and African elephants contribute to the effects we see here. Asian elephants have only one finger at the tip of the trunk and a cartilage bulb on the ventral side of the tip; their preferred trunk behaviour, e.g. when feeding, is to wrap with the distal third of the trunk [19]. This ‘trunk wrapping zone’ in Asian elephants is also the trunk region in which we found the highest density of major wrinkles, as described above. It has been shown before that elephants form pseudo-joints with their trunks, at the very same trunk region [7]. As hydrostats do not have joints in their trunk, the term pseudo-joint indicates a section on their trunk made functionally equivalent to a joint, such as an elbow or a knee, by muscle contractions, making grasping or manoeuvring with the trunk possible [51]. We are suggesting that the wrinkles in the distal third of the trunk facilitate bending and wrapping and are making the formation of pseudo-joints possible. African elephants have two fingers at the tip of their trunks and prefer to pinch with their trunk tip when feeding or picking up objects within a certain range of size and form [19]. Over the course of the last years, our behavioural observations at various zoos confirmed these differences in trunk behaviour.
Another factor influencing trunk wrinkling might be environmental conditions. African savannah elephants and Asian elephants are adapted to distinct environmental niches, with African elephants primarily living in dry environments [52] and Asian elephants living in more humid environments. Humidity has been shown to have an impact on human skin, with humans developing more wrinkles after transitioning from a high- to a low-humidity environment [53]. However, there is no indication of humid environments leading to species being generally more wrinkled. Further exploration is needed to test the morphological and mechanical differences in skin across mammalian taxa in relation to humidity of an animal’s natural environment.
Both Asian and African elephants have more major wrinkles on the dorsal side of the trunk than on the ventral side. It should be noted that most of the specimens analysed here were less than 5 years old, so we believe the difference between dorsal and ventral wrinkles is predetermined/already present at birth, and trunk use over time might add additional wrinkles, but this increase is negligible compared with the dorsoventral difference at birth. This could be related to a different function of dorsal and ventral trunk wrinkles. It has been shown that the distal dorsal part of the trunk contributes the most to trunk stretching and that the ventral side stretches comparably little when the trunk is extended [1]. The distal ventral trunk has been described to be used in sweeping food together [9] and most trunk manipulation movements are accomplished with gripping and grabbing on the ventral side [21]. Specifically, the trunk section just before the trunk tip is used in holding food or other objects, often between the lateral skin ridges that go along the ventral trunk and that have a very high density of whiskers in this distinct trunk part [49]. We are suggesting that the dorsoventral difference in trunk wrinkles can be explained by the dorsal wrinkles contributing strongly to the trunk’s ability to stretch, while the ventral wrinkles are especially important for improved grip.
4.2. Trunk lateralization drives wrinkle differences
When looking at the most distal 15 cm of the trunks of adult Asian and African elephants, we found a difference of 10% in wrinkle numbers between the left/right side of the trunk shaft correlating with the individual’s ‘trunkedness’. Both Asian [17] and African [18] elephants exhibit lateralization, or ‘handedness’/‘trunkedness’, with their trunks, meaning they will prefer a direction when executing complex motion tasks. This lateralization means the trunk is contacting the ground more often with one side, causing additional force and abrasions, e.g. of the whiskers, at this side of the distal trunk. Additionally, lateralization in elephants indicates that they curve the trunk to wrap and pick up objects to a specific side, left or right, making them ‘left-’ or ‘right-trunkers’ [7]. Our results show that in adult elephants, there are more wrinkles on the side of the distal trunk the elephant is preferentially bending or wrapping the trunk towards. To give an example, a ‘left-trunker’ would preferably wrap their trunk towards the left of their body and perform left-oriented behaviours with the trunk, thereby frequently compressing the left side of their distal trunk and stretching the right side at the same time. The fact that there are more wrinkles on the trunk side towards which elephants preferentially wrap and that is, therefore, more often compressed points to the increase in wrinkles being a result of long-term lateralized use of the trunk.
The differences in wrinkle numbers between sides of the distal trunk are independent of species and there is no overall difference between the left and right side when we look at our samples taken together. The absence of such a left/right difference is in line with the fact that in elephants, in contrast to humans, there is no overall population-wide bias toward one side in trunk lateralization [11]. We had one case of an ambidextrous African elephant with no whisker or wrinkle difference, reinforcing our theory of the modification of the wrinkle pattern based on a user-dependent experience. If the elephant does not favour its left or right trunk side, there would not be an abrasion of whiskers nor a more frequent compression on its trunk skin on one side. Without this frequent compression, the trunk would not get more wrinkles. In the elephant babies that we looked at, the numbers of trunk wrinkles were comparable for both sides of the trunk. Because trunk lateralization emerges with the functional ability of the trunk [50], and it takes nearly 2 years to gain full control of the trunk [54], our data indicate that trunk wrinkle patterns are affected by use. Elephants use their trunks daily to grab objects and eat nearly 200 kg of food [55], potentially leading to around a million compression cycles a year due to lateralization. It has been shown that compression of film-substrate systems leads to a mismatching of the modulus, similar to the skin modulus, and creases and wrinkles form [56]. Therefore, millions of cycles of lateralized compression could easily lead to an increase in wrinkles.
4.3. Changes in skin layer thickness might contribute to the functionality of wrinkles
In the microCT scans of the Asian elephant baby and the African elephant baby and the histology of the Asian baby elephant trunk tip, we see that trunk skin layer thickness shifts, with the dermis shrinking in the troughs of the wrinkles. To our knowledge, these skin layer-specific differences in thickness along a wrinkle have not been described in other species before. The total skin thickness decreases by coarsely a factor of two in the troughs of the wrinkles.
Previously, it has been described that the troughs of the wrinkles on African elephant trunks are the primary stress concentration zone when stretching [1]. This is due to the fact the troughs of the wrinkles provide additional arc length for the skin to stretch, allowing after stretching the wrinkle to become smooth. We find these changes of skin thickness already in a newborn baby, so it is possible that they are creating the wrinkles. It could also be, however, that pre-natal stress on the trunk is playing a part in developing skin layers and wrinkles. Wrinkle formation is often described as an instability that develops from stress, displacement or bending that acts on a non-wrinkled surface [57]. In humans, wrinkling is related to the skin elasticity and weakening of the upper dermis [58]. In the case of elephants, it appears they have wrinkles that form as instabilities from lateralization, but as we show, they also develop wrinkles before birth. Therefore, in these elephant trunks, instead of a mechanical instability forming these wrinkles before birth, another explanation is morphological instabilities from skin layer differences along the arc length of a wrinkle. Having surface morphological instabilities in materials produces different types of wrinkling behaviour in bilayer tubes [59]. We show that the biological composite of elephant skin has morphological instabilities of skin layer thicknesses along the arc length potentially causing additional wrinkles to form. Further study is needed to understand exactly how skin layer differences could cause instabilities in a complex muscular hydrostat like the elephant trunk. We also see partial wrinkles, which we denote as ‘broken wrinkles’ that are mostly spanning from the lateral trunk towards the middle of the dorsal trunk shaft where they either just have a gap or a gap and a shift up or down. They do not continue to the ventral portions. These broken wrinkles may operate to allow additional flexibility in lateral manipulation events, assisting with enlarging the surface area of interaction with objects, similar to how specialized wrinkles in the intestine provide an enlarged surface through a broken wrinkled type of mechanism [60].
In the microCT scans, it is visible that the African baby elephant trunk has larger absolute wavelengths than the Asian baby elephant trunk in the medial towards distal part. This is consistent with the rest of our findings, as larger absolute wavelengths along the trunk indicate fewer wrinkles. We also found significant differences in wrinkle amplitude between the two specimens, with the Asian baby elephant trunk having deeper wrinkles than the African baby elephant trunk. The amplitude differences are already seen around birth, so they seem to mark a predetermined difference between the two species, and they could impact the ability to stretch out to reach far objects. With Asian elephants having smaller absolute wavelengths and larger amplitudes, it is possible that Asian elephants could reach further than African elephants as they would have more arc length of skin to flatten for reaching far away objects; however, no controlled comparisons have been made to see maximum reaching distances of the individual elephant species. Since the analyses made here are all based on data from elephant babies, an expansion of these observations to specimens from adult elephants would be necessary to see if our results can be extrapolated.
4.4. Elephant trunk wrinkles develop early in fetal ontogeny
When analysing trunk development, we found that the trunk shows exceptional growth in early pregnancy (E60–E150) which exceeds that of other body parts. These findings align with earlier conclusions on trunk development from transrectal ultrasound imaging [37]. Prior developmental studies have not observed the connections to wrinkles, but we see that the earliest elephant (approx. E60) fetuses we examined have a large nose with periodic irregularities, which could be a precursor of the trunk wrinkling. We found the development of wrinkles occurs in a bipartite pattern beginning for the first approximately E130 as an exponential growth every 20 days. This coincides with the time of trunk length growth (figure 6d ) as well as the head, and these wrinkles could serve as a way to discretize this complex hydrostat into sub-sections, which has been shown in portions of the elephant brain [2].
At the approximately E130, the increase in wrinkle number slows down sharply with the trunk length continuing to grow, which would probably increase the distance between wrinkles along the trunk until birth. Our observations also provide a staging/dating of the upper lip nose fusion [33,34], which appears to occur between postnatal E100 and E130, i.e. approximately in the fourth month of pregnancy. Finally, we find that trunk tip development trails trunk extension and that the trunk first grows out as a stump. Then, after E120 the fingers develop, whereby the ventral finger extends first. The bulbous ventral trunk tip of Asian elephants is present at an early point (approx. E130).
5. Conclusion
Wrinkles and creases improve the ability of soft biological materials to bend [61], which might explain many of our findings, in particular the proximal–distal, dorsoventral, lateralized and species differences in wrinkle distribution. The high density of wrinkles in the Asian elephants’ ‘trunk wrapping zone’ shows how trunk wrinkles and their unique form in African and Asian elephants could contribute to the phenomenally flexible actuation of trunks. Our analysis extends earlier work on the wrinkle structure of elephant skin [1] and gives insights into the development of the largest extant land mammals.
Acknowledgements
We thank the Berlin Zoological Garden and in particular Rolf Becker, Rouven Schulze, Konstantin Becker and Lucas Baum. Petra Prager with her extensive collection of photographs of elephants in zoos, as well as several zoological institutions contributed. In particular, the Berlin Zoo (Germany) and the Zoo Schönbrunn (Vienna, Austria), as well as Zoo Augsburg (Germany), Opel Zoo Kronberg (Germany), Zoo Poznan (Poland), Tierpark Hagenbeck (Germany), the Elefantenhof Platschow (Germany) and the Tbilisi Zoo (Georgia). We also thank our collaborators Ani Shubitidze, Lennart Eigen, Undine Schneeweiss, Luke Longren and Eduard Maier for their precious help in different parts of this project. A.K.S. thanks the Alexander von Humboldt Foundation, Max Planck Society and the International Max Planck Research School for Intelligent Systems for the Support.
Contributor Information
Andrew K. Schulz, Email: aschulz@is.mpg.de.
Lena V. Kaufmann, Email: lena.kaufmann@bccn-berlin.de.
Noemie Reveyaz, Email: noemie.reveyaz@bccn-berlin.de.
Cindy Ritter, Email: cindy.ritter@bccn-berlin.de.
Thomas Hildebrandt, Email: hildebrandt@izw-berlin.de.
Michael Brecht, Email: michael.brecht@bccn-berlin.de.
Ethics
All material and morphology measurements were taken from photos of previously dissected species. Specific information about each of the individuals analysed is included in the supplement.
Data accessibility
The data from the paper is included in a Dryad repository [62] for specific wrinkle numbers for each animal.
Supplementary material is available online [63].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
A.K.S.: conceptualization, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft, writing—review and editing; L.V.K.: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft, writing—review and editing; N.R.: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft, writing—review and editing; C.R.: resources, visualization, writing—original draft, writing—review and editing; T.H.: data curation, project administration, resources, supervision, writing—review and editing; M.B.: conceptualization, data curation, funding acquisition, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Supported by BCCN Berlin, Humboldt-Universität zu Berlin, and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany´s Excellence Strategy – EXC-2049 – 390688087.
References
- 1. Schulz AK, et al. 2022. Skin wrinkles and folds enable asymmetric stretch in the elephant trunk. Proc. Natl Acad. Sci. USA 119 , e2122563119. ( 10.1073/pnas.2122563119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reveyaz N, et al. 2024. A myelin map of trunk folds in the elephant trigeminal nucleus. Elife 13 , RP94142. ( 10.7554/eLife.94142.3) [DOI] [Google Scholar]
- 3. Purkart L, et al. 2022. Trigeminal ganglion and sensory nerves suggest tactile specialization of elephants. Curr. Biol. 32 , 904–910. ( 10.1016/j.cub.2021.12.051) [DOI] [PubMed] [Google Scholar]
- 4. Kier WM. 2012. The diversity of hydrostatic skeletons. J. Exp. Biol. 215 , 1247–1257. ( 10.1242/jeb.056549) [DOI] [PubMed] [Google Scholar]
- 5. Schulz AK, Schneider N, Zhang M, Singal K. 2023. A year at the forefront of hydrostat motion. Biol. Open 12 , bio059834. ( 10.1242/bio.059834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulz AK, Reidenberg JS, Ning Wu J, Ying Tang C, Seleb B, Mancebo J, Elgart N, Hu DL. 2023. Elephant trunks use an adaptable prehensile grip. Bioinspir. Biomim. 18 , 026008. ( 10.1088/1748-3190/acb477) [DOI] [PubMed] [Google Scholar]
- 7. Wu J, Zhao Y, Zhang Y, Shumate D, Braccini Slade S, Franklin SV, Hu DL. 2018. Elephant trunks form joints to squeeze together small objects. J. R. Soc. Interface 15 , 20180377. ( 10.1098/rsif.2018.0377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martins AF, Bennett NC, Clavel S, Groenewald H, Hensman S, Hoby S, Joris A, Manger PR, Milinkovitch MC. 2018. Locally-curved geometry generates bending cracks in the African elephant skin. Nat. Commun. 9 , 3865. ( 10.1038/s41467-018-06257-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulz AK, Plotczyk M, Sordilla S, Boyle M, Singal K, Reidenberg JS, Hu DL, Higgins CA. 2023. Second harmonic generation imaging reveals entanglement of collagen fibers in the elephant trunk skin dermis. bioRxiv ( 10.1101/2023.08.11.553031) [DOI] [Google Scholar]
- 10. Cuvier G, MacGillivray W. 1893. The animal kingdom of the Baron Cuvier. Edinb. J. Nat. Hist. Phys. Sci. 31 . [Google Scholar]
- 11. Shoshani J, et al. 1982. On the dissection of a female Asian elephant (Elephas maximus maxiums Linnaeus, 1758) and data from other elephants. Elephant 2 , 3–93. ( 10.22237/elephant/1521731887) [DOI] [Google Scholar]
- 12. Longren LL, Eigen L, Shubitidze A, Lieschnegg O, Baum D, Nyakatura JA, Hildebrandt T, Brecht M. 2023. Dense reconstruction of elephant trunk musculature. Curr. Biol. 33 , 4713–4720. ( 10.1016/j.cub.2023.09.007) [DOI] [PubMed] [Google Scholar]
- 13. Kaufmann LV, Schneeweiß U, Maier E, Hildebrandt T, Brecht M. 2022. Elephant facial motor control. Sci. Adv. 8 , eabq2789. ( 10.1126/sciadv.abq2789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaufmann LV, Becker R, Ochs A, Brecht M. 2023. Elephant banana peeling. Curr. Biol. 33 , R257–R258. ( 10.1016/j.cub.2023.02.076) [DOI] [PubMed] [Google Scholar]
- 15. Schulz AK, Ning Wu J, Ha SYS, Kim G, Braccini Slade S, Rivera S, Reidenberg JS, Hu DL. 2021. Suction feeding by elephants. J. R. Soc. Interface 18 , 20210215. ( 10.1098/rsif.2021.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giljov A, de Silva S, Karenina K. 2017. Context-dependent lateralization of trunk movements in wild Asian elephants. BioComm. 62 , 82–92. ( 10.21638/11701/spbu03.2017.204) [DOI] [Google Scholar]
- 17. Haakonsson JE, Semple S. 2009. Lateralisation of trunk movements in captive Asian elephants (Elephas maximus). Laterality 14 , 413–422. ( 10.1080/13576500802572442) [DOI] [PubMed] [Google Scholar]
- 18. Lefeuvre M, Gouat P, Mulot B, Cornette R, Pouydebat E. 2022. Analogous laterality in trunk movements in captive African elephants: a pilot study. Laterality 27 , 101–126. ( 10.1080/1357650X.2021.1999253) [DOI] [PubMed] [Google Scholar]
- 19. Racine RN. 1980. Behavior associated with feeding in captive African and Asian elephants. Elephant 1 , 57–71. ( 10.22237/elephant/1521731845) [DOI] [Google Scholar]
- 20. Surapaneni VA, et al. 2022. Groovy and gnarly: surface wrinkles as a multifunctional motif for terrestrial and marine environments. Integr. Comp. Biol. 62 , 749–761. ( 10.1093/icb/icac079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dagenais P, Hensman S, Haechler V, Milinkovitch MC. 2021. Elephants evolved strategies reducing the biomechanical complexity of their trunk. Curr. Biol. 31 , 4727–4737. ( 10.1016/j.cub.2021.08.029) [DOI] [PubMed] [Google Scholar]
- 22. Gaeth AP, Short RV, Renfree MB. 1999. The developing renal, reproductive, and respiratory systems of the African elephant suggest an aquatic ancestry. Proc. Natl Acad. Sci. USA 96 , 5555–5558. ( 10.1073/pnas.96.10.5555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allen WR, Stansfield FJ. 2021. Placentation in the African elephant (Loxodonta africana). In Placentation in mammals: tribute to EC Amoroso’s lifetime contributions to viviparity (eds Geisert RD, Spencer T), pp. 181–204. Cham, Switzerland: Springer International Publishing. ( 10.1007/978-3-030-77360-1_9) [DOI] [Google Scholar]
- 24. Amoroso EC, Perry JS. 1964. The foetal membranes and placenta of the African elephant (Loxodonta africana). Phil. Trans. R. Soc. Lond. B 248 , 1–34. ( 10.1098/rstb.1964.0007) [DOI] [PubMed] [Google Scholar]
- 25. Ayer AA, Mariappa D. 1950. External characters of three foetuses of the Indian elephant. Proc. Indian Acad. Sci. - Sect. B 31 , 193–209. ( 10.1007/BF03050577) [DOI] [Google Scholar]
- 26. Bolk L. 1913. Über die äuszere Körpergestalt eines Fetus von Elephas Maximus. See https://wellcomecollection.org/works/ch3ekymk/items?canvas=47. [Google Scholar]
- 27. Brauer A. 1910. Short report on an embryo of the African elephant. Ber. SitzBer. Freunde. 273–274. [Google Scholar]
- 28. Buss IO, Smith NS. 1966. Observations on reproduction and breeding behavior of the African elephant. J. Wildl. Manage. 30 , 375. ( 10.2307/3797826) [DOI] [Google Scholar]
- 29. Cappellini E, et al. 2014. Resolution of the type material of the Asian elephant, Elephas maximus Linnaeus, 1758 (Proboscidea, Elephantidae). Zool. J. Linn. Soc. 170 , 222–232. ( 10.1111/zoj12084) [DOI] [Google Scholar]
- 30. Craig G. 2023. Foetal mass and date of conception in african elephants: a revised formula. Afr. J. Sci. 80 , 512–516. [Google Scholar]
- 31. Durrieux ACR. 1912. Foetus of Eleohas africanus. Paris, France: Society of Biology. [Google Scholar]
- 32. Eales NB. 1926. XI.—the anatomy of the head of a foetal African elephant, Elephas africanus (Loxodonta africana). Trans. R. Soc. Edinb. Earth Sci. 54 , 491–551. ( 10.1017/S0080456800016082) [DOI] [Google Scholar]
- 33. Fischer MS, Trautmann U. 1987. Fetuses of African elephants (Loxodonta africana) in photographs. Elephant 2 , 40–45. ( 10.22237/elephant/1521732098) [DOI] [Google Scholar]
- 34. Fischer VMS. 1987. Die Oberlippe der Elefanten. Z. Säugetierkunde 52 , 262–263. [Google Scholar]
- 35. Gray J. 1868. Notes on the fetus of an elephant and of a hippopotamus in the collection of the British Museum. Proc. Zool. Soc. Lond. 1 , 491. [Google Scholar]
- 36. Hautier L, Stansfield FJ, Allen WRT, Asher RJ. 2012. Skeletal development in the African elephant and ossification timing in placental mammals. Proc. R. Soc. B 279 , 2188–2195. ( 10.1098/rspb.2011.2481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hildebrandt T, et al. 2007. Foetal age determination and development in elephants. Proc. R. Soc. B 274 , 323–331. ( 10.1098/rspb.2006.3738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hill WCO. 1938. The external and radiological anatomy of a foetal Asiatic elephant. Ceylon J. Sci. B. 21 , 31–43. [Google Scholar]
- 39. Lonnenberg E. 1905. Demonstration eines Fötus vom westafrikanischen elefanten. Elephas. cyclotis. Matschie. [Google Scholar]
- 40. Perry JS, Amoroso EC. 1964. The structure and development of the reproductive organs of the female African elephant. Phil. Trans. R. Soc. Lond. B 248 , 35–51. ( 10.1098/rstb.1964.0008) [DOI] [Google Scholar]
- 41. Raubenheimer EJ. Development of the tush and tusk and tusklessness in African elephant (Loxodonta africana). Koedoe 43 , 57–64. ( 10.4102/koedoe.v43i2.199) [DOI] [Google Scholar]
- 42. Stansfield FJ. 2012. Ontogeny of the ovarian follicular reserve of the African elephant (Loxodonta africana). PhD thesis, University of Pretoria, South Africa. See https://repository.up.ac.za/handle/2263/27999. [Google Scholar]
- 43. Stansfield FJ, Allen WR. 2012. Development of the germinal ridge and ovary in the African elephant (Loxodonta africana). See https://repository.up.ac.za/handle/2263/58565. [DOI] [PubMed]
- 44. Stansfield FJ, Nothling JO, Soley JT, Allen WR. 2012. Development of the germinal ridge and ovary in the African elephant (Loxodonta africana). See https://repository.up.ac.za/handle/2263/20984. [DOI] [PubMed]
- 45. Toldt K. 1913. Über die äuszere Körpergestalt eines Fetus von Elephas Maximus. See https://works.wellcomecollection.org/works/ch3ekymk/items?canvas=5.
- 46. Null T. 1881. The form and proportions of a foetal indian elephant. J. Anat. Physiol. 15 , 518–522. [PMC free article] [PubMed] [Google Scholar]
- 47. Wettstein O. 1920. External morphological differences between fetuses of Elephas africanus and E maximus. Arch. Zool. 13 , 1. [Google Scholar]
- 48. Zimmermann EAV. 1783. Description and picture of an unborn elephant. In Beschreibung eines ungeborenen Elefanten nebst beschiedenen bisher ungedruckten Nachrichten die Naturgeschichte der Elefanten betreffend, Editor and Author are: Eberhard A. W. Zimmermann, year is 1783. [Google Scholar]
- 49. Deiringer N, et al. 2023. The functional anatomy of elephant trunk whiskers. Commun. Biol. 6 , 591. ( 10.1038/s42003-023-04945-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keerthipriya P, Tewari R, Vidya TNC. 2015. Lateralization in trunk and forefoot movements in a population of free-ranging Asian elephants (Elephas maximus). J. Comp. Psychol. 129 , 377–387. ( 10.1037/com0000013) [DOI] [PubMed] [Google Scholar]
- 51. Flash T, Zullo L. 2023. Biomechanics, motor control and dynamic models of the soft limbs of the octopus and other cephalopods. J. Exp. Biol. 226 , jeb245295. ( 10.1242/jeb.245295) [DOI] [PubMed] [Google Scholar]
- 52. Wright PG, Luck CP. 1984. Do elephants need to sweat? S. Afr. J. Zool. 19 , 270–274. ( 10.1080/02541858.1984.11447892) [DOI] [Google Scholar]
- 53. Goad N, Gawkrodger DJ. 2016. Ambient humidity and the skin: the impact of air humidity in healthy and diseased states. J. Eur. Acad. Dermatol. Venereol. 30 , 1285–1294. ( 10.1111/jdv.13707) [DOI] [PubMed] [Google Scholar]
- 54. Sukumar R. 1993. The Asian elephant: ecology and management. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 55. Ullrey DE. 2014. Elephants: nutrition and dietary husbandry. AZA Nutrition Advisory Group. See https://nagonline.net/807/elephants-nutrition-dietary-husbandry/. [Google Scholar]
- 56. Budday S, Andres S, Walter B, Steinmann P, Kuhl E. 2017. Wrinkling instabilities in soft bilayered systems. Phil. Trans. R. Soc. A 375 , 20160163. ( 10.1098/rsta.2016.0163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cerda E, Mahadevan L. 2003. Geometry and physics of wrinkling. Phys. Rev. Lett. 90 , 074302. ( 10.1103/PhysRevLett.90.074302) [DOI] [PubMed] [Google Scholar]
- 58. Batisse D, Bazin R, Baldeweck T, Querleux B, Lévêque JL. 2002. Influence of age on the wrinkling capacities of skin. Skin Res. Technol. 8 , 148–154. ( 10.1034/j.1600-0846.2002.10308.x) [DOI] [PubMed] [Google Scholar]
- 59. Liu C, Du Y, Li K, Lü C. 2024. Morphological instability of geometrically incompatible bilayer tubes. Int. J. Mech. Sci. 269 , 109061. ( 10.1016/j.ijmecsci.2024.109061) [DOI] [Google Scholar]
- 60. Nagaraja S, Leichsenring K, Ambati M, De Lorenzis L, Böl M. 2021. On a phase-field approach to model fracture of small intestine walls. Acta Biomater. 130 , 317–331. ( 10.1016/j.actbio.2021.06.002) [DOI] [PubMed] [Google Scholar]
- 61. Sigaeva T, Mangan R, Vergori L, Destrade M, Sudak L. 2018. Wrinkles and creases in the bending, unbending and eversion of soft sectors. Proc. R. Soc. A 474 , 20170827. ( 10.1098/rspa.2017.0827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schulz A, Kaufmann L, Reveyaz Net al. Data from: Elephants develop wrinkles through both form and function [Dataset]. Dryad Digital Repository. ( 10.5061/dryad.zkh1893g4) [DOI]
- 63. Schulz AK, Kaufmann L, Reveyaz N, Ritter C, Hildebrandt TB, Brecht M. 2024. Data from: Elephants develop wrinkles through both form and function. Figshare. ( 10.6084/m9.figshare.c.7477925) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from the paper is included in a Dryad repository [62] for specific wrinkle numbers for each animal.
Supplementary material is available online [63].