Abstract
Background
Severe fever with thrombocytopenia syndrome (SFTS) is a rapidly progressing infectious disease with a high fatality rate caused by a novel bunyavirus (SFTSV). The role of lipids in viral infections is well-documented; however, the specific alterations in lipid metabolism during SFTSV infection remain elusive. This study aims to elucidate the lipid metabolic dysregulations in the early stages of SFTS patients.
Methods
This study prospectively collected peripheral blood sera from 11 critical SFTS patients, 37 mild SFTS patients, and 23 healthy controls during the early stages of infection for lipidomics analysis. A systematic bioinformatics analysis was conducted from three aspects integrating lipid differential expressions, lipid differential correlations, and lipid-clinical indices correlations to reveal the serum lipid metabolic dysregulation in SFTSV-infected individuals.
Results
Our findings reveal significant lipid metabolic dysregulation in SFTS patients. Specifically, compared to healthy controls, SFTS patients exhibited three distinct modes of lipid differential expression: increased levels of lipids including phosphatidylserine (PS), hexosylceramide (HexCer), and triglycerides (TG); decreased levels of lipids including lysophosphatidylcholine (LPC), acylcarnitine (AcCa), and cholesterol esters (ChE); and lipids showing “dual changes” including phosphatidylcholine (PC) and phosphatidylethanolamine (PE). Finally, based on lipid metabolic pathways and literature analysis, we systematically elucidated the potential mechanisms underlying lipid metabolic dysregulation in the early stage of SFTSV infection.
Conclusions
Our study presents the first global serum lipidome profile and reveals the lipid metabolic dysregulation patterns in the early stage of SFTSV infection. These findings provide a new basis for the diagnosis, treatment, and further investigation of the disease.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03672-w.
Keywords: SFTS, Novel bunyavirus, Lipidomics, Lipid
Background
Severe fever with thrombocytopenia syndrome (SFTS) is a rapidly progressing infectious disease caused by a novel bunyavirus (SFTS virus, SFTSV) with a high fatality rate exceeding 10% [1, 2]. The virus was first identified by Chinese scholars in 2010 [3] and subsequently reported in South Korea, Japan, Thailand, and other places [4–6]. The virus is primarily transmitted through tick bites. It was included in the list of World Priority Diseases for Research by the World Health Organization in 2017 [7]. According to the National Guidelines for Diagnosis and Treatment of SFTS 2023 (guidelines 2023) published by the National Health Commission of the People’s Republic of China (NHC) [8], SFTS patients are classified into mild, moderate, severe, and critical cases, with critical patients exhibiting features such as septic shock, multiple organ failure, and severe consciousness impairment. However, to date, there is no specific drug available for treating SFTS patients.
Numerous studies have reported the crucial role of lipids in viral infection and cell-mediated immune response [9–15]. However, research on the relationship between lipid metabolism and SFTS is limited. Lipids play pivotal roles in the viral infection process through three main aspects: (a) Cellular membrane Remodeling. Lipids are involved in remodeling host cell membranes to facilitate viral entry, replication, and budding [9]. Viruses often exploit host lipid metabolism pathways to alter the composition and structure of cellular membranes, creating specialized microenvironments conducive to viral replication [10]. (b) Host lipid metabolism manipulation. Viruses can manipulate host lipid metabolism to support their replication and propagation [11]. They may modulate lipid synthesis, storage, and transport pathways to meet their metabolic demands. This manipulation can involve alterations in lipid droplet formation, fatty acid metabolism, and cholesterol biosynthesis. (c) Immune response modulation. Lipid mediators, such as prostaglandins, leukotrienes, and sphingolipids, regulate inflammation, immune cell recruitment, and cytokine production [12–14]. Viruses can exploit these lipid mediators to evade host immune surveillance, promote immune evasion, or enhance viral pathogenesis. Additionally, lipid rafts on the cell membrane serve as platforms for immune cell signaling and antigen presentation, influencing the activation and function of immune cells during viral infection [15].
There have been numerous reports documenting changes in blood lipid composition during viral infections: In patients infected with the Zika virus, elevated levels of serum phosphatidylethanolamine (PE) were observed [16]. Mice infected with respiratory syncytial virus exhibited significant increases in PE content in both lung tissue and plasma [17]. Patients diagnosed with human immunodeficiency virus (HIV) infection showed increases in saturated fatty acids and decreases in polyunsaturated fatty acids (PUFA) [18]. Patients with acute respiratory distress syndrome (ARDS) demonstrated a decrease in plasma levels of PUFA-phosphatidylcholine (PC), PUFA-PE, and PUFA-triacylglycerol (TG) [19], while an increase in C18 unsaturated free fatty acids in serum was associated with ARDS development [20]. Influenza virus infection has been linked to the identification of certain bioactive derivatives of PUFA, such as 13-S-hydroxyoctadecadienoic acid and 9-S-hydroxyoctadecadienoic acid, which induce and reduce inflammation, respectively [21]. Deceased individuals with Ebola virus disease (EVD) exhibited reductions in plasma levels of PC, lysophosphatidylcholine (LPC), and phosphatidylinositol, alongside elevations in phosphatidylserine (PS), PE, and diacylglycerol (DG) compared to healthy controls [22]. In coronavirus disease 2019 (COVID-19) infection, a potential correlation was discovered between ganglioside GM3-rich exosomes and the pathogenesis of COVID-19 infection [23].
These observations underscore the significant and profound alterations in lipid metabolism during viral infections, offering a promising avenue for exploring the mechanisms of viral infection. However, regrettably, there have been no studies focusing on serum lipidome to uncover the dysregulation of lipids in SFTSV infection. Therefore, our study aims to address this research gap. Our study provides the first comprehensive analysis of the serum lipidome in SFTSV infection, revealing notable dysregulation of lipid metabolism. By filling this gap in the field of lipid research in SFTSV infection, we hope to pave the way for future studies to elucidate the mechanisms underlying human lipid dysregulation during SFTSV infection. Such investigations hold the potential to yield new breakthroughs in understanding the pathological mechanisms of SFTSV infection.
Methods
Study design and participants
This study prospectively collected peripheral blood sera from 11 critical SFTS patients, 37 mild SFTS patients, and 23 healthy controls during the early stages of infection for lipidomics analysis. SFTS patients were recruited from the Department of Infectious Diseases at Shandong Public Health Clinical Center from April 2022 to September 2022, while healthy controls were recruited from the Center of Health Management at Shandong Provincial Hospital affiliated to Shandong First Medical University in May 2022. Inclusion Criteria: According to the diagnostic criteria of guidelines 2023 published by the NHC, patients diagnosed with SFTS by clinical physicians with at least one positive serum SFTSV-PCR result were included. Exclusion Criteria: (a) hospital stays of less than 72 h; (b) concomitant infections of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), hepatitis C virus, Brucella, Mycobacterium tuberculosis, suspected human immunodeficiency virus (HIV), or syphilis that could affect our study results; (c) times from symptom onset to admission > 7 days; (d) recent use of lipid-lowering drugs such as statins, etc.
According to Gai et al., SFTS can be divided into three phases: febrile stage (3–7 days), multi-organ dysfunction stage (7–13 days), and recovery stage (11–17 days) [24]. To study early-stage changes in serum lipidomics of SFTS patients, we collected serum samples only from patients in the febrile phase.
Subsequently, SFTS patients were further categorized into mild and critical groups based on clinical symptoms. According to the 2023 guidelines [8], SFTS is classified into mild, moderate, severe, and critical categories based on clinical symptoms. Patients with mild SFTS typically experience slight fatigue or discomfort and have a self-limiting course, usually recovering within a week. Moderate SFTS is characterized by noticeable general discomfort, poor appetite, nausea, vomiting, and other gastrointestinal symptoms, but without neurological symptoms. Severe SFTS patients present with neurological symptoms such as drowsiness, confusion, stupor, pulmonary infections, and gastrointestinal bleeding. Critical SFTS patients exhibit coma (Glasgow Coma Scale ≤ 8), shock, or other organ failures necessitating intensive care unit (ICU) management. To simplify the study, in this research, the mild group encompasses mild, moderate, and severe SFTS cases, while the critical group comprises patients with critical SFTS [25]. This study was approved by the Ethics Review Committee of Shandong Provincial Hospital. This work was conducted following the guidelines of the Declaration of Helsinki of the World Medical Association.
Untargeted lipidomics analysis
Serum sample collection and processing
Serum samples from SFTS patients were collected as fasting blood on the morning of the second day after admission. Serum samples from healthy volunteers were collected as fasting blood in the morning at the health check-up center. The blood collection process was carried out by a team of professional nurses using standardized commercial serum collection tubes. Collected blood samples were initially left to stand at 4 °C for 30 min, followed by centrifugation at 2500 rpm for 15 min using a high-speed centrifuge. After centrifugation, the supernatant was transferred to Eppendorf tubes and stored at – 80 °C. Once all samples were collected, they were packaged with dry ice and transported to Hangzhou Calibra Lab for further lipidomics analysis.
LC–MS/MS method for lipid analysis
MS-grade methanol, MS-grade acetonitrile, and HPLC-grade 2-propanol were purchased from Thermo Fisher. HPLC-grade formic acid and HPLC-grade ammonium formate were purchased from Sigma. The internal lipid standard chosen was Avanti’s SPLASH LipidoMIX™ internal standard product [26]. Lipids were extracted according to the methyl tert-butyl ether (MTBE) method. Briefly, 40 μL serum samples were first spiked with 20 μL internal lipid standards and then homogenized with 200 μL water and 240 μL methanol. After that, 800 μL of MTBE was added and the mixture was ultrasound 20 min at 4 ℃ followed by sitting still for 30 min at room temperature. The solution was centrifuged at 14,000 g for 15 min at 10℃ and the upper organic solvent layer was obtained and dried under nitrogen. Reverse phase chromatography was selected for LC separation using CSH C18 column (1.7 μm, 2.1 mm × 100 mm, Waters). The lipid extracts were re-dissolved in 200 μL 90% isopropanol/acetonitrile, centrifuged at 14,000 g for 15 min, and finally, 3 μL of the sample was injected. Solvent A was acetonitrile–water (6:4, v/v) with 0.1% formic acid and 10 mM ammonium formate, and solvent B was acetonitrile–isopropanol (1:9, v/v) with 0.1% formic acid and 10 mM ammonium formate. The initial mobile phase was 30% solvent B at a flow rate of 300 μL/min. It was held for 2 min, and then linearly increased to 100% solvent B in 23 min, followed by equilibrating at 5% solvent B for 10 min. Mass spectra was acquired by Q-Exactive Plus in positive and negative mode, respectively. Electrospray ionization (ESI) parameters were optimized and preset for all measurements as follows: Source temperature, 300 °C; Capillary Temp, 350 °C, the ion spray voltage was set at 3000 V, S-Lens RF Level was set at 50% and the scan range of the instruments was set at m/z 200–1800 [27, 28].
Identification of lipid species
The lipid species were identified using LipidSearchTM database. LipidSearchTM is a search engine for the identification of lipid species based on MS/MS math. LipidSearch contains more than 30 lipid classes and more than 1,500,000 fragment ions in the database. The mass tolerance for precursor and fragment were set to 5 ppm.
After peak annotation, unreliable lipids were excluded when the relative standard deviation (RSD, %) of the quality control (QC) samples was > 25%. Annotated lipids were identified putatively based on matching precursor ion m/z values and the product ion pattern of the data to the LipidSearch database. Next, the identified lipid list was confirmed by searching the Human Metabolome Database, Lipidmaps, and Lipidblast databases, including accurate mass and MS/MS spectrum, to improve the confidence in lipid identification.
Data processing and statistical analysis
Data preprocessing
Missing data in the clinical laboratory data were addressed using group median interpolation. The lipidomics data underwent a log(x + 1) transformation and were normalized through Z-scores to improve adherence to a normal distribution.
Quality control (QC) analysis of lipidomics
QC samples, obtained from an average mixture of all samples, were used to assess the consistency of analytical samples subjected to the same treatment methodology. Principal component analysis (PCA) and Pearson correlation analysis were conducted on the QC samples to evaluate the stability and repeatability of lipidomics data.
Lipid clustering analysis
Unsupervised PCA and uniform manifold approximation and projection (UMAP) analysis can analyze the patterns of lipid separation among different groups. Supervised orthogonal partial least squares-discriminant analysis (OPLS-DA) can evaluate the extent of lipid dissimilarity between groups and can be ascertained whether the presence of overfitting exists through 1000 permutation tests. The Metaboanalyst website (https://www.metaboanalyst.ca/) is utilized for conducting PCA and OPLS-DA analyses.
Lipid differential expression analysis
Pairwise comparison of lipidome among the three groups was performed using the PerMANOVA method [29] after adjusting baseline information. P-values obtained from statistical tests were converted to q-values according to Storey-Tibshirani distribution. Fold change (FC) values were calculated as the ratio of mean values between groups. 2000 bootstrap resamples and bias correction through the BCA method were used to estimate 95% confidence intervals (95% CI) for inter-group FC. Only lipids with FC > 1.5 or FC < 0.67 and q-value < 0.05 are included for further analysis. Then, Mfuzz cluster analysis was used to correlate the lipids with clinical disease severity. All analysis results were obtained using R (version 4.3.1).
Lipid differential correlation analysis
This section can be referenced from Song et al. [23]. First, the Spearman coefficients and correlation p-values for all lipids between healthy controls group (H group) and mild patients group (M group) were calculated. The p-values were then converted to q-values using the Storey-Tibshiran method. Lipids with q < 0.05 and Spearman coefficient > 0 were labeled as “ + ,” while those with q < 0.05 and Spearman coefficient < 0 were labeled as “ − ,” and those with q ≥ 0.05 were labeled as “0.” Next, the Fisher z-transform method was used to convert the Spearman coefficients to z-scores. Spearman coefficients greater than 0.99 were replaced with 0.99. The specific formula for the Fisher z-transform is:
Afterwards, the inter-group difference in z-scores(dz) can be calculated using the formula:
The p-value of dz can be determined by utilizing the standard normal distribution and the two-tailed p-value. The “DiffCorr” package in R is capable of efficiently executing this procedure. Finally, using Storey-Tibshiran method transformed the p-value into q-value, and only those differential lipid pairs with q-value < 0.05 were selected. The absolute values of dz were converted to a scale ranging from 0 to 1 and were utilized as edge weights in an undirected graph. “MEGENA” package in R was used to compute the planar filtered network and examine the differential subnetwork through module calculation. Subsequently, the network graph was visualized using Cytoscape software (version 3.9.1), with the Compound Spring Embedder Layout selected as the appropriate method for representing the intricate networks.
Lipid-clinical indices correlation analysis
Spearman coefficients between differential lipids and clinical laboratory indicators in SFTS patients were calculated. P-value is corrected by Storey-Tibshiran distribution to obtain q-value. Only correlations with q-value < 0.05 are presented in the correlation heat map.
Results
Sera lipidome was dramatically altered by SFTSV infection
A total of 37 mild patients (M group), 11 critical patients (C group), and 23 healthy controls (H group) participated in this study. Baseline information, sampling temporal information, clinical symptoms, and laboratory results are presented in Table 1, Additional file 1: Fig. S1, Additional file 1: Table S1, and Additional file 1: Table S2, respectively.
Table 1.
Baseline information of participants
| Variables | Health group (n = 23) | SFTS | p-value | |||
|---|---|---|---|---|---|---|
| Non-critical group (n = 36) | Critical group (n = 11) | H/M | H/C | M/C | ||
| Age, years, mean ± SD | 68.0 ± 4.3 | 64.3 ± 6.8 | 71.5 ± 7.3 | 0.013* | 0.163 | 0.010* |
| Gender | > 0.999 | > 0.999 | > 0.999 | |||
| Female | 9 (39.1%) | 13 (36.1%) | 4 (36.4%) | |||
| Male | 14 (60.9%) | 23 (63.9%) | 7 (63.6%) | |||
| BMI, kg/m2, mean ± SD | 25.65 ± 3.81 | 21.83 ± 3.12 | 22.03 ± 4.03 | < 0.001*** | 0.022* | 0.880 |
| Smoking history | 0.749 | 0.388 | 0.435 | |||
| N | 19 (82.61%) | 28 (77.78%) | 7 (63.64%) | |||
| Y | 4 (17.39%) | 8 (22.22%) | 4 (36.36%) | |||
| Drinking history | 0.540 | 0.388 | 0.467 | |||
| N | 19 (82.61%) | 27 (75.00%) | 7 (63.64%) | |||
| Y | 4 (17.39%) | 9 (25.00%) | 4 (36.36%) | |||
| Diabetes history | 0.292 | 0.638 | > 0.999 | |||
| N | 18 (78.26%) | 32 (88.89%) | 10 (90.91%) | |||
| Y | 5 (21.74%) | 4 (11.11%) | 1 (9.09%) | |||
| Cardiovascular Disease history | > 0.999 | > 0.999 | 0.417 | |||
| N | 22 (95.65%) | 35 (97.22%) | 10 (90.91%) | |||
| Y | 1 (4.35%) | 1 (2.78%) | 1 (9.09%) | |||
| Hypertension history | 0.007** | 0.066 | > 0.999 | |||
| N | 8 (34.78%) | 26 (72.22%) | 8 (72.73%) | |||
| Y | 15 (65.22%) | 10 (27.78%) | 3 (27.27%) | |||
| Cerebralvascular disease history | 0.072 | 0.002** | 0.097 | |||
| N | 23 (100.00%) | 30 (83.33%) | 6 (54.55%) | |||
| Y | 0 (0.00%) | 6 (16.67%) | 5 (45.45%) | |||
| Times from onset to admission (d) | 6.0 (5.0–7.0) | 5.0 (4.5–5.5) | 0.073 | |||
| Outcome: death | 0 (0.0%) | 8 (72.7%) | ||||
The p-values are presented with three significant digits and marked for significance with asterisks: *, p < 0.05; **, p < 0.01; ***, p < 0.001
Serum samples from 71 participants, along with 9 quality control (QC) samples, underwent analysis using liquid chromatography-tandem mass spectrometry (LC–MS/MS). The chromatograms of QC samples are shown in Additional file 1: Fig. S2. After comprehensive screening, a total of 1362 lipids were identified and quantified. Subsequently, 1091 lipids, covering 32 subclasses, with a quality control relative standard deviation (QCRSD) value below 0.25 were selected for subsequent analysis. Principal component analysis (PCA) within QC samples showed a high degree of aggregation (Additional file 1: Fig. S3A), and Pearson correlation analysis revealed that the majority of correlation coefficients between QC samples exceeded 0.99 (Additional file 1: Fig. S3B), indicating the high stability and repeatability of the lipidomic data. To ensure reliability, only lipids with a QCRSD value below 0.25 were chosen for subsequent data analysis.
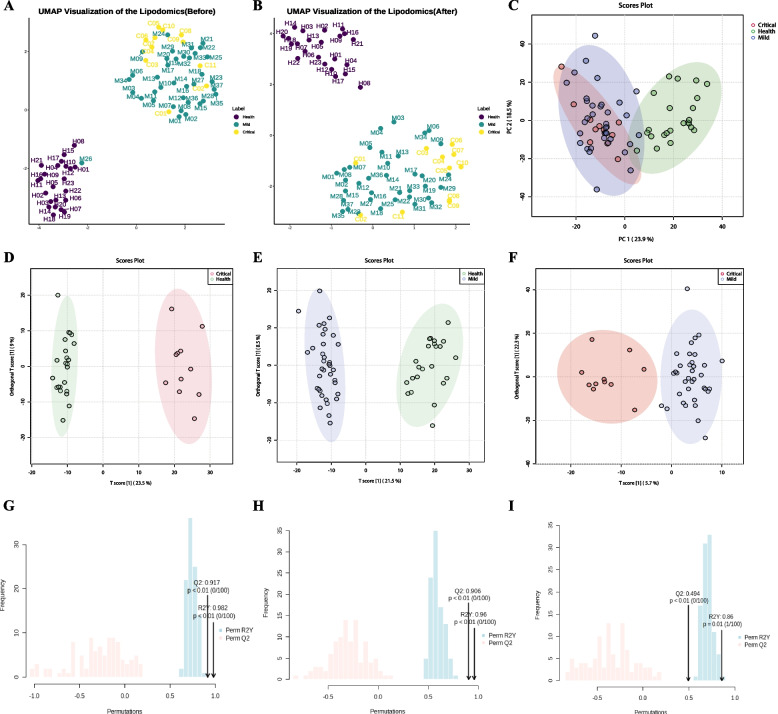
Uniform manifold approximation and projection (UMAP) analysis indicated that sample M26 was a significant outlier in classification and resembled a healthy individual (Fig. 1A). The clinical record of patient M26 showed a positive SFTSV test during outpatient treatment, with mild symptoms experienced. However, SFTSV-PCR tests conducted upon admission yielded two negative results. This discrepancy may be attributed to a low viral load rendering it undetectable by PCR. To ensure data validity, sample M26 was excluded from subsequent data analysis in this study (Fig. 1B).
Fig. 1.
Clustering analysis of lipidomics between different groups. A–B Uniform Manifold Approximation and Projection (UMAP) was utilized for the analysis of lipidomic profiles. In A, sample M26 emerges as a notable outlier. Subsequently, B displays the UMAP landscape after the removal of the outlier, sample M26, illustrating the normalized distribution of the remaining samples. C The PCA score plot, depicted in a two-dimensional format, compares lipidomic profiles across three distinct groups, offering insights into their variance and similarity. D–F The OPLS-DA score plot further delineates the lipidomic differences among the three groups, emphasizing the discriminatory power of the lipid profiles in distinguishing between health states. G–I The robustness and predictive accuracy of the OPLS-DA models are validated through 1000 permutation tests. Specifically: G demonstrates a Q2 of 0.906 and an R2Y of 0.960, indicating a strong model performance between groups H versus M. H reveals a Q2 of 0.917 and an R2Y of 0.982, indicating a stronger model performance between groups H versus C. I shows a Q2 of 0.494 and an R2Y of 0.86, indicating moderate model efficacy between groups M versus C
Finally, unsupervised classification within the serum lipidome using UMAP and PCA revealed a distinct separation between SFTS patients and healthy controls (Fig. 1B–C). To further verify differences in the serum lipidome between the three groups, supervised orthogonal partial least squares-discriminant analysis (OPLS-DA) was conducted with 1000 permutation tests. The results indicated a significant separation in the serum lipidome between groups H versus M (Q2 = 0.917, R2Y = 0.982), H versus C (Q2 = 0.906, R2Y = 0.960)), and M versus C (Q2 = 0.494, R2Y = 0.860) (Fig. 1D–I). These findings demonstrate that SFTSV infection can dramatically alter the serum lipidome of patients, with also existing differences between groups M and C in SFTS patients.
Differential expression analysis revealed three distinct modes of lipid differential expression
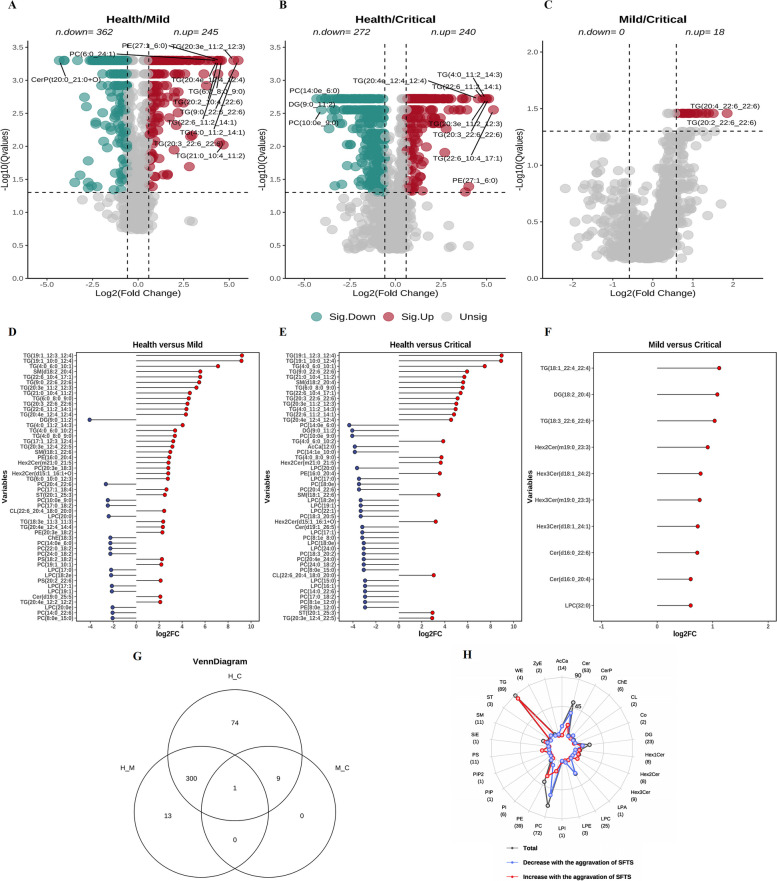
Differential lipids between groups were presented on volcano plots (Fig. 2A–C). Subsequently, Mfuzz cluster analysis was employed to correlate lipids with clinical disease severity (Additional file 1: Fig. S4). Only lipids exhibiting a common trend with disease severity were included in the final analysis. This process is illustrated in the Mfuzz cluster diagram, where lipids in clusters 5, 9, and 11 showed a positive correlation trend, and lipids in clusters 3, 6, 7, and 10 displayed a negative correlation trend.
Fig. 2.
Differential lipidome profiling across various groups. A–C showcase volcano plots that illustrate the differential lipid species between groups, where the x-axis denotes the log2 fold change (log2FC) and the y-axis represents the negative logarithm (base 10) of the Q-value (-log10[Qvalue]). Green dots denote lipid species that are significantly decreased, whereas red dots indicate those that are significantly increased. D–F presents the top 50 differential lipids identified across three groups, with blue marking the down-regulated and red highlighting the up-regulated significantly differential lipids. G displays a Venn diagram that delineates the lipid species selection process following mfuzz clustering. H employs a radar map to demonstrate the numbers of lipid subclasses that undergo significant changes as the disease progresses. The overall count of significantly altered lipid species within each subclass is traced by a dark gray line and annotated in parentheses alongside each subclass label around the radar map’s perimeter. Increases and decreases in lipid numbers are illustrated with red and blue lines, respectively
The top 50 lipids that changed significantly between groups are shown in Fig. 2D–F. The Venn diagram displays the final set of screened lipids, totaling 397 filtered lipids (Fig. 2G). Heatmaps were employed to visually illustrate the expression patterns of differential lipids. Two clustering modes were implemented for the heatmaps: one at the lipid species level (Additional file 1: Fig. S5A), to reveal the distinct differential expression patterns as a whole between SFTS patients and healthy controls; the other at the lipid subclass level, to showcase the differential expression patterns within different lipid subclasses between groups (Additional file 1: Fig. S5B).
Comparing healthy individuals with all SFTS-infected patients, clustering on the lipid subclass level revealed three distinct modes of differential lipid expression in SFTSV infection: an increased mode, a decreased mode, and a “dual changed” mode, wherein some lipids in the subclass increased while others decreased. Detailed depictions of all the filtered differential lipids were presented in the forest plot (Additional file 1: Fig. S6).
Finally, a radar map was employed to illustrate the detailed changes of lipids in each subclass (Fig. 2H). The radar map highlighted significant changes in lipids during SFTSV infection, including elevation in PS, TG, and HexCer (including HexCer1,HexCer2, and HexCer3), reduction in LPC, AcCa, and ChE, and a “dual change” in PC and PE.
Differential correlation analysis revealed dysregulated interactions between lipid pairs
Co-regulated lipid pairs characterized by robust correlations between their expressions commonly imply their involvement in a common metabolic pathway and are subject to shared regulatory mechanisms [23]. If correlations between certain lipid pairs are altered during SFTSV infection compared to healthy states, these lipid pairs may signify specific patterns of metabolic disturbances.
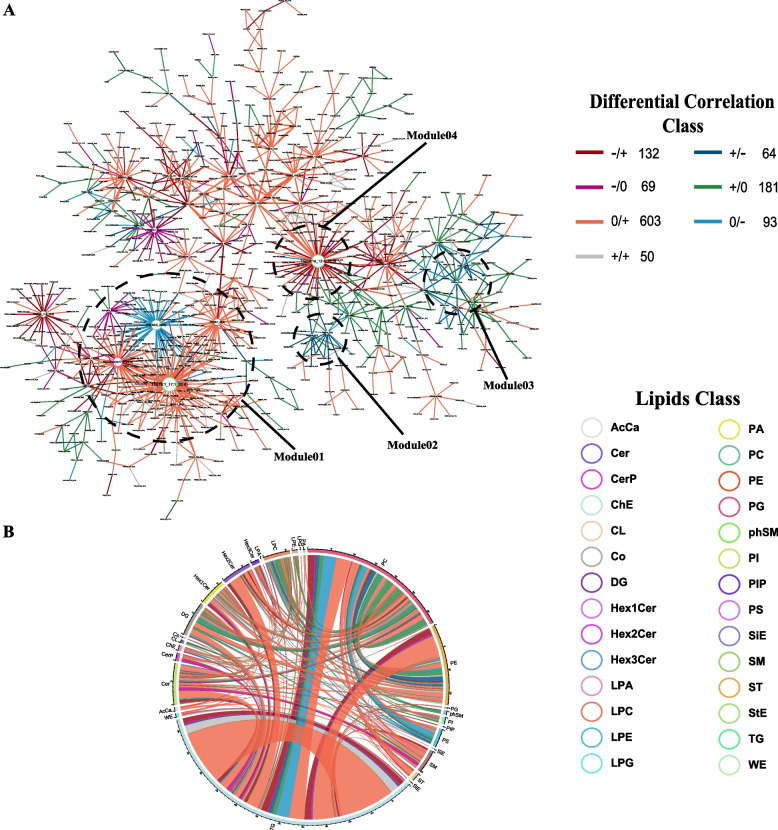
To elucidate the initial lipid metabolic disturbances induced by SFTSV infection, we performed differential co-expression analysis on all 1091 lipids. Based on the analysis results, we classified the patterns of differential lipid pairs into 7 distinct categories and assigned them diverse color labels. Of particular significance are two patterns depicted by dark red lines and dark blue lines, representing the transition from negative correlation to positive correlation and from positive correlation to negative correlation from health to disease conditions. This phenomenon, termed “switching mechanism” [30], plays a pivotal role in elucidating intricate biological systems and warrants heightened attention.
Four modules deserving attention were circled in the differential correlation network (Fig. 3A). Module I comprises four hub lipids: TG (18:1_17:1_22:6), Hex2Cer (m17:0_22:6), PE (18:1_22:2), and PC (18:0_22:5). Module II comprises two hub lipid species: PS (20:2_22:6) and DG (18:1e_22:2). Module III comprises two hub lipids: PC (8:1e_12:0) and PE (20:1_14:0). Module IV comprises one hub lipid species: TG (14:1e_12:4_18:3). These four modules play important roles in the connectivity of the network, suggesting that these hub lipids may serve as key lipids co-regulated during SFTSV infection.
Fig. 3.
Differential correlation analysis. Lipid pairs with significant differential correlations (q-value < 0.05) were included. Sign/sign indicates the direction in control/mild SFTS, and the number that follows indicates the number of lipid pairs in the global networks exhibiting this pattern of change. For instance, the dark red line − / + 132 in the upper legend of the global networks indicates that the correlation between two connected lipid pairs is negative ( −) in health, and becomes positive ( +) in mild SFTS patients. A total of 132 lipid pairs connected by dark red lines in the global network displayed this pattern of change (− / +). There is a total of 7 differential correlation patterns marked with different colors. A Differential Correlation Network. Four modules (module 1–module 4) of biological interest are circled for emphatical discussion. B Chord diagram. The chord diagram displayed all lipid pairs from the differential correlation network on the subclass level
A chord diagram was used to visualize the differential correlation on the subclass level (Fig. 3B). The most significant alterations showed in the chord diagram were the significantly augmented correlations between most TGs in infected individuals (depicted by dark red lines or orange lines). This finding, coupled with the increase in TGs, implies that the increased TGs likely originate from a common pathway. The chord diagram revealed the most intricate alterations in PC, PE, and TG, suggesting their potential significance in the infection process. Within the human body, PC, PE, and TG follow a common synthetic pathway, with DG serving as a crucial intermediate product. However, both positive and negative correlations between PCs and PEs, TGs, and DGs were observed, suggesting the existence of at least two distinct regulated patterns of PCs during SFTSV infection. This phenomenon accords with the “dual changed” expression mode within PC. Additionally, the positive correlation between PC and LPC disappeared, which may be attributed to the inhibition of the conversion from PC to LPC. A significantly enhanced negative correlation between PS and PC indicated a potential inhibition of the conversion process from PC to PS.
Lipid-clinical indices correlation analysis revealed pathologically relevant interactions between differential lipids and clinical indices
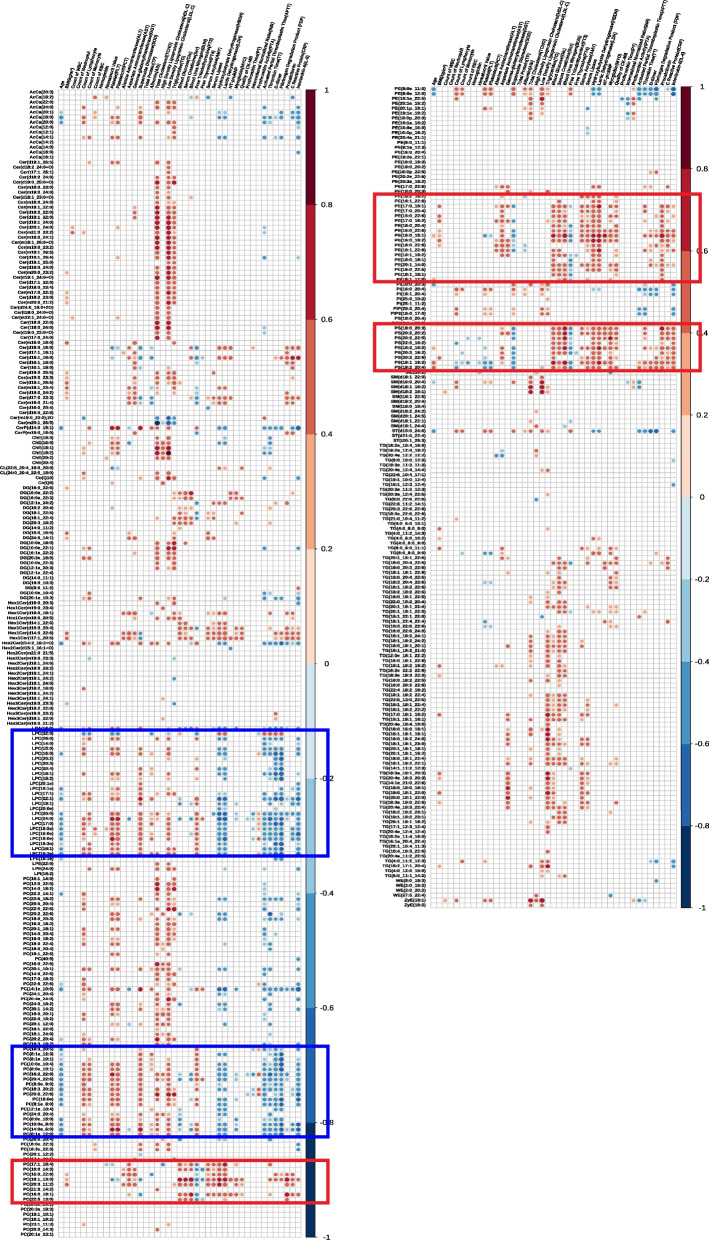
To associate differential lipids with clinical significance, we conducted a correlation analysis between differential lipids and clinical laboratory indicators (Fig. 4). The plots revealed that LPCs displayed a positive correlation with platelet indices (platelet (PLT), plateletcrit (PCT)) and liver function (superoxide dismutase (SOD)), while showing a negative correlation with myocardial enzymes (hydroxybutyrate-dehydrogenase (HBDH), lactate dehydrogenase (LDH)), coagulation function indices (activated partial thromboplastin time (APTT), thrombin time (TT), D-dimer, fibrin degradation products (FDP)), and inflammatory indices (interleukin 6 (IL-6)). Similarly, certain PCs exhibited comparable associations with clinical indices to those of LPCs.
Fig. 4.
Lipids-clinical indices correlations in SFTS patients. The plots illustrate Spearman correlations between lipids and clinical indices. The p-value is corrected by Storey-Tibshiran method to obtain the q-value. Only correlations with q-value < 0.05 are indicated with colored circles. Negative correlations are shown in blue and positive correlations were shown in red, with color intensity indicating the magnitude of correlations. Two boxes with different colors are framed to show lipid species with similar clinical indicators correlations. The blue box contains LPC and some PCs, while the red box contains PS, some PEs, and some PCs
PSs demonstrated a significant positive correlation with renal function indices (serum creatinine (Scr), blood urea nitrogen (BUN)), myocardial enzymes (HBDH, LDH), and inflammatory indices (procalcitonin, C-reactive protein (CRP), IL-6). Additionally, some PCs and PEs showed similar associations with clinical indices as PSs.
Moreover, there was a significant positive correlation between ChEs and total cholesterol, high-density lipoproteincholesterol (HDL-C), and low-density lipoproteincholesterol (LDL-C), alongside a positive correlation between the majority of TGs and triglyceride indices, demonstrating the congruity of lipidomic results with clinical laboratory testing results.
Discussion
Lam et al. extensively reviewed the existing approaches for functional analysis of lipidomic [31]. In this study, we analyzed the lipid metabolic dysregulation from four aspects: lipid clustering analysis, lipid differential expression analysis, lipid differential correlation analysis, and lipid-clinical indices correlation analysis. Our study demonstrated that SFTSV infection induced significant and profound alterations in the serum lipidome, including elevation in PS, HexCer, TG, reduction in LPC, AcCa, ChE, and dual changes in PC, PE, Cer, and DG.
Three distinct modes of differential lipid expression
Lipids that increased during SFTSV infection
PS levels in serum significantly increased during SFTSV infection. PS is not associated with the composition of lipoproteins, so plasma PS levels are usually low [32]. Therefore, during SFTSV infection, the elevated PS may primarily originate from two sources: release from platelet activation and apoptosis of blood cells. Extracellular vesicles, including exosomes and microvesicles (MVs), serving as important components of the lipoprotein-free fraction in plasma, are known to increase in circulation under conditions of inflammation and infection [33]. MVs are in particular enriched in PS lipids [34]. Platelet is a prominent source of MVs [35]. Platelet activation triggers a mechanism where PS is exposed on the outer leaflet of the platelet membrane, leading to the generation and release of MVs into the bloodstream [36]. PS-rich MVs play a critical role in blood coagulation and thrombosis induction [37]. Recent studies have shown that SFTSV replicates within platelets, causing increased platelet activation, PS exposure, and subsequent thrombus formation [38]. Additionally, PS is a common marker of cell apoptosis. During apoptosis, PS translocates from the inner to the outer leaflet of the plasma membrane [39]. Therefore, the apoptosis of platelets and other blood cells also contributes to the increased PS levels in the blood circulation.
The majority of triglycerides also exhibited increased levels. Infection and inflammation can accelerate the breakdown of adipose tissue, leading to the release of fatty acids. This, in turn, stimulates the liver to synthesize and release triglycerides into the bloodstream [40]. Therefore, despite potential reductions in dietary intake during illness, TG levels may rise or remain within normal ranges. Consistent with our lipidomic findings, blood biochemical tests also showed increases in triglyceride levels.
HexCers, another class of lipids, demonstrated significant increases during SFTSV infection. HexCer is a crucial component of cell membranes involved in intracellular signal transduction, although its specific function remains unclear [41]. One study suggested that HexCer serves as a marker for hepatic necroinflammation in patients with chronic hepatitis C [42]. In our study, notable increases in Hex2Cer and Hex3Cer were observed with escalating disease severity. Further investigations are warranted to elucidate the role of HexCer during SFTSV infection.
Lipids that decreased during SFTSV infection
LPC levels exhibited significant decreases in SFTSV infection. LPC is synthesized through two pathways in the human body: one occurs in the liver, where phospholipase A2 (PLA2) converts PC to LPC; the other takes place in the bloodstream, where lecithin-cholesterol acyltransferase (LCAT) esterifies cholesterol on the surface of high-density lipoproteins (HDL) and simultaneously converts PC to LPC [43]. Therefore, the reduction in serum HDL during SFTSV infection may contribute to decreased LPC synthesis through the LCAT pathway in the bloodstream. Additionally, LPC is closely related to inflammation [44]. Previous studies have shown that decreased LPC levels are associated with high inflammation states such as sepsis [45, 46]. In this study, we also found a significant negative correlation between LPC and inflammatory mediators such as IL-6. Therefore, the reduced LPC levels in SFTS patients can reflect the inflammatory status of their bodies to some extent.
Additionally, AcCa showed significant decreases. AcCa are crucial intermediate products in mitochondrial metabolism, formed during the metabolic process of long-chain fatty acids (LCFA). AcCa are involved in the transportation of LCFA into mitochondria for oxidation, serving as a rate-limiting step in LCFA oxidation [47]. Therefore, the decrease in AcCa suggests a potential inhibition in LCFA metabolism, indicating a possible deficiency in energy supply during SFTSV infection.
Furthermore, ChE also significantly decreased. Consistent with the lipidomic findings, blood biochemical tests showed significant decreases in total cholesterol, HDL-C, and LDL-C. Notably, similar changes in ChE and triglycerides have been observed in COVID-19 infection, where total cholesterol, HDL-C, and LDL-C decrease, and triglycerides increase or remain “inappropriately normal” due to malnutrition. The underlying mechanisms for this phenomenon are multifaceted, while Professor Feingold has provided an in-depth explanation [48] Despite being in the context of COVID-19 infection, it also applies to SFTSV infection because many of the mechanisms are common. Additionally, liver damage frequently leads to a metabolic disturbance in cholesterol and triglyceride. For example, reduction in serum cholesterol levels is a prevalent indication of chronic hepatitis C [49]. Recent studies have indicated that decreased cholesterol levels can serve as a predictor for mortality during SFTSV infection [50], which corroborates our findings.
Lipids that showed “dual changed” during SFTSV infection
PC and PE exhibited “dual changed” in SFTSV infection, with most PCs decreasing while most PEs increasing during SFTSV infection. PC plays a critical role in hepatic synthesis and secretion of lipoproteins [51]. Approximately 75% of the total phospholipid content in HDL and 65% in LDL are composed of PC [52]. Hence, the decrease in HDL and LDL levels during SFTSV infection can account for the majority of PC decreases. Moreover, PC, PE, and other phospholipids are integral components of the cellular membrane system [53, 54]. Viral infections have the potential to induce cell necrosis or pyroptosis, leading to disruption of membrane integrity and subsequent release of cellular contents. Consequently, the release of PC, PE, and other phospholipids into the bloodstream occurs due to necrosis or pyroptosis of infected cells, resulting in elevated levels of these phospholipids in the blood. Additionally, necrosis and pyroptosis of cells induce macrophages to engulf cellular debris while releasing various inflammatory molecules, including IL-6 and TNF-α, which trigger an inflammatory state in the body.
Similar lipidome changes between SFTSV and Ebola virus infections
Our lipidomic findings during SFTSV infection exhibit notable similarities to those observed in Ebola virus disease (EVD), particularly regarding lipid PS [22, 55] (Additional file 1: Fig. S7). Kyle et al. extensively discussed PS functions and potential mechanisms in EVD, which are highly relevant to SFTSV infection. SFTSV demonstrates a preference for various cell types, such as intermediate monocytes and plasma cells [56–58], while the Ebola virus primarily targets macrophages and dendritic cells in peripheral blood [59]. Moreover, thrombocytopenia, a common manifestation in both SFTS and EVD, underscores the significance of platelets as a prominent source of serum MVs enriched in PS. This commonality suggests that despite differences in viral infections, shared targets can result in analogous dysregulated lipid metabolic patterns, highlighting the commonality of lipid metabolism in viral infections.
Kyle et al. also observed elevated levels of Cer containing C16:0 and C18:0 in both EVD fatalities and survivors compared to healthy controls. After examining their research, we found that Cer containing C16:0 and C18:0 refers to Cer (d18:0_16:0), Cer (d18:0_18:0), Cer (d18:1_16:0), Cer (d18:1_18:0), and Cer (d18:2_16:0). In our study, except for Cer (d18:2_16:0), the other four Cer species also showed an increase in SFTS patients compared to healthy controls. However, other Cer species containing C16:0 and C18:0 in our study did not exhibit significant patterns, possibly due to the identification of more Cer species in our non-targeted lipidomics approach.
Summary of lipid metabolic dysregulation during SFTSV infection
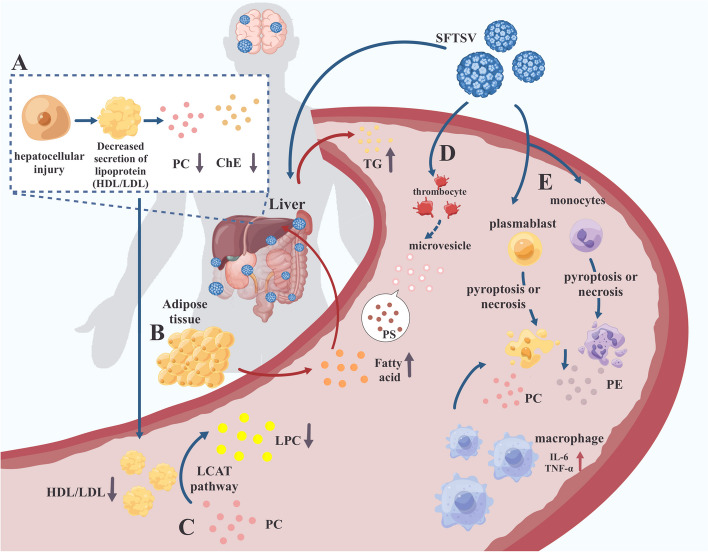
Finally, we summarized the major changes in serum lipidomics during SFTSV infection in Fig. 5, along with brief summaries of possible mechanisms. (a) SFTSV infection induces hepatic dysfunction, resulting in the reduction of lipoprotein and then leading to a decrease in PC and ChE. (b) SFTSV infection accelerates the mobilization of adipose tissue, resulting in the release of a quantity of fatty acids. The excessive fatty acids stimulate the liver to synthesize TG and secrete very low-density lipoprotein (VLDL) to transport TG into the bloodstream. (c) The decrease in serum HDL levels may result in a corresponding decrease in the synthesis of LPC through the LCAT pathway. (d) SFTSV infection induces platelet activation, resulting in the release of micro-vesicles which possess high PS levels on their surface. (e) Pyroptosis or necrosis of infected cells comprises the membrane integrity, leading to the release of membrane lipids (mainly PC and PE) and cellular contents. This event subsequently triggers an inflammatory response. These explanations are only theoretical. We hope that future studies can delve deeper into the causes of changes in serum lipidome during SFTSV infection, unraveling the mystery of human lipid metabolism disorders triggered by SFTSV infection and providing new avenues for studying the mechanism of SFTSV infection.
Fig. 5.
Summary of lipid metabolic dysregulation during SFTSV infection. A SFTSV infection triggers hepatic dysfunction, leading to a reduction in lipoprotein levels, which subsequently causes a decrease in phosphatidylcholine (PC) and cholesteryl esters (ChE) in serum. B The infection accelerates the mobilization of adipose tissue, releasing a significant amount of fatty acids. These excessive fatty acids prompt the liver to synthesize triglycerides (TG) and secrete very-low-density lipoproteins (VLDL) into the bloodstream for TG transport. C A decrease in serum high-density lipoprotein (HDL) levels results in a reduced synthesis of lysophosphatidylcholine (LPC) via the lecithin-cholesterol acyltransferase (LCAT) pathway. D SFTSV infection leads to platelet activation, which in turn releases microvesicles with high levels of phosphatidylserine (PS) on their surface. E Pyroptosis or necrosis of infected cells compromises membrane integrity, leading to the release of membrane lipids, mainly PC and phosphatidylethanolamine (PE), and cellular contents. This event triggers an inflammatory response
Limitations
This study has several limitations. First, the sample size is relatively small, primarily due to low disease incidence, a single sampling centers, and short sampling period throughout the study duration. Even though, we rigorously controlled sampling quality and excluded ineligible samples in the methodology description to ensure sample representativeness and result reliability. Second, patients may be at different stages of illness. SFTS is an acute infectious disease with short windows of time for each period. To address this issue, we initially excluded patients with symptom onset to hospitalization times exceeding 7 days to include those in the early stages of illness and minimize interference from different disease stages. Third, lipidomics studies inherently have limitations. Unlike metabolomics with clear targets, lipidomic analysis detects lipid molecules that may not universally match across different lipid databases. Our study utilized the LipidSearchTM database, a comprehensive and authoritative database of lipids. Nonetheless, there remains a need for a database similar to KEGG for transcriptomics and metabolomics, which could integrate lipid molecules with metabolic pathways for broader biological information analysis.
Conclusions
Our study presented the first global serum lipidome profile and revealed the lipid metabolic dysregulation patterns in the early stage of SFTSV infection. These findings provide a new basis for the diagnosis, treatment, and further investigation of the disease.
Supplementary Information
Additional File 1: Tables S1-S2, Figures S1-S7. Table S1. Clinical Manifestations of SFTS Patients. Table S2. Laboratory Examination Results of Participants. Figure S1. Sampling time information for SFTS patients. Figure S2. Chromatograms of QC samples. Figure S3. Repeatability Test of Lipidomics Results. Figure S4. Mfuzz Clustering for Lipids Screened after Volcano Plot. Figure S5. Heatmap Analysis. Figure S6. Forest Plot. Figure S7. Comparison of Differential Lipid Subclasses between SFTS and EVD.
Acknowledgements
We thank Calibra Lab at DIAN Diagnostics for providing lipidomics technical assistance. We thank Yinuo Chen (Department of Neurology, First Affiliated Hospital of Wenzhou Medical) for providing graphic art assistance, Xixi Hu (The University of Texas at Austin) for assisting in data analysis supporting, and Xingyu Yu (Ernst & Young LLP of London) for language proofreading.
Abbreviations
- AcCa
Acylcarnitines
- Cer
Ceramides
- CerP
Ceramides phosphate
- ChE
Cholesteryl ester
- CL
Cardiolipin
- Co
Coenzyme
- DG
Diacylglycerol
- Hex1Cer
Hexosylceramides
- Hex2Cer
Dihexosylceramides
- Hex3Cer
Trihexosylceramide
- LPA
Lyso-phosphatidic acid
- LPC
Lyso-phosphatidylcholine
- LPE
Lyso-phosphatidylethanolamine
- LPI
Lyso-phosphatidylinositol
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PI
Phosphatidylinositol
- PIP
Phosphoinositides
- PIP2
Phosphatidylinositol-4,5-bisphosphate
- PS
Phosphatidylserine
- SiE
Sitosteryl ester
- SM
Sphingomyelin
- ST
Sulfatide
- TG
Triacylglycerol
- WE
Wax ester
- ZyE
Zymosteryl ester
Authors’ contributions
S.G, YJ.Y, BJ.W, CJ.W, SG.G designed research; S.G, JY.Z, ZG.Y, ZQ.K, LR.T, Q.D, SH.W, J.Z, YM.H performed research; S.G, ZG.Y, WJ.W, DQ.Q analyzed data; S.G, YJ.Y, YM.H, SG.G wrote the paper.
Funding
This study was supported by the National Natural Science Foundation of China [Grant Nos. NSFC82072079 and NSFC82001294], and Open Foundation of Key Laboratory of Digital Technology in Medical Diagnostics of Zhejiang Province [SZZD202219].
Availability of data and materials
The original data and code have been uploaded to the Science Data Bank, accessible at the following website: https://www.scidb.cn/en/s/IJzeIv.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Review Committee of Shandong Provincial Hospital, affiliated with Shandong First Medical University (approval number: SWYX:NO. 2022–246). Informed consent was obtained from all participants involved in the study.
Consent for publication
All authors have provided their consent for publication of this manuscript. We confirm that all necessary approvals have been obtained and that the manuscript does not violate any confidentiality agreements or rights.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuai Guo, Yunjun Yan and Jingyao Zhang contributed equally to this work.
Contributor Information
Yumei Hao, Email: yumeihao@zju.edu.cn.
Shougang Guo, Email: guoshougang1124@163.com.
References
- 1.Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14(8):763–72. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Lu QB, Xing B, Zhang SF, Liu K, Du J, Li XK, Cui N, Yang ZD, Wang LY, et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011–17: a prospective observational study. Lancet Infect Dis. 2018;18(10):1127–37. [DOI] [PubMed] [Google Scholar]
- 3.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YR, Yun Y, Bae SG, Park D, Kim S, Lee JM, Cho NH, Kim YS, Lee KH. Severe Fever with Thrombocytopenia Syndrome Virus Infection, South Korea, 2010. Emerg Infect Dis. 2018;24(11):2103–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rattanakomol P, Khongwichit S, Linsuwanon P, Lee KH, Vongpunsawad S, Poovorawan Y. Severe Fever with Thrombocytopenia Syndrome Virus Infection, Thailand, 2019–2020. Emerg Infect Dis. 2022;28(12):2572–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi Y, Kato H, Yamagishi T, Shimada T, Matsui T, Yoshikawa T, Kurosu T, Shimojima M, Morikawa S, Hasegawa H, et al. Severe Fever with Thrombocytopenia Syndrome, Japan, 2013–2017. Emerg Infect Dis. 2020;26(4):692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH. 2017 Annual review of diseases prioritized under the Research and Development Blueprint. In WHO Meeting report: World Health Organization; 2017. [Google Scholar]
- 8.National guidelines for diagnosis and treatment of Severe Fever with Thrombocytopenia Syndrome 2023 in China (http://www.nhc.gov.cn/cms-search/downFiles/39ddd92264f64094985fbef0439da17b.pdf). Health Commission of the People's Republic of China.
- 9.Lorizate M, Kräusslich HG. Role of lipids in virus replication. Cold Spring Harb Perspect Biol. 2011;3(10): a004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, He G, Filipowicz NA, Randall G, Belov GA, Kopek BG, Wang X. Host Lipids in Positive-Strand RNA Virus Genome Replication. Front Microbiol. 2019;10:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girdhar K, Powis A, Raisingani A, Chrudinová M, Huang R, Tran T, Sevgi K, Dogus Dogru Y, Altindis E. Viruses and Metabolism: The Effects of Viral Infections and Viral Insulins on Host Metabolism. Annu Rev Virol. 2021;8(1):373–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Hoecke H, Vandenbulcke L, Van Cauwenberge P. Histamine and leukotriene receptor antagonism in the treatment of allergic rhinitis: an update. Drugs. 2007;67(18):2717–26. [DOI] [PubMed] [Google Scholar]
- 13.Jaschonek K, Muller CP. Platelet and vessel associated prostacyclin and thromboxane A2/prostaglandin endoperoxide receptors. Eur J Clin Invest. 1988;18(1):1–8. [DOI] [PubMed] [Google Scholar]
- 14.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roncato R, Angelini J, Pani A, Talotta R. Lipid rafts as viral entry routes and immune platforms: A double-edged sword in SARS-CoV-2 infection? Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(6): 159140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Queiroz A, Pinto IFD, Lima M, Giovanetti M, de Jesus JG, Xavier J, Barreto FK, Canuto GAB, do Amaral HR, de Filippis AMB, et al. Lipidomic Analysis Reveals Serum Alteration of Plasmalogens in Patients Infected With ZIKA Virus. Front Microbiol. 2019;10:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan J, Qian W, Shen C, Lin L, Xie T, Peng L, Xu J, Yang R, Ji J, Zhao X. High-resolution lipidomics reveals dysregulation of lipid metabolism in respiratory syncytial virus pneumonia mice. RSC Adv. 2018;8(51):29368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowman ER, Kulkarni M, Gabriel J, et al. Altered Lipidome Composition Is Related to Markers of Monocyte and Immune Activation in Antiretroviral Therapy Treated Human Immunodeficiency Virus (HIV) Infection and in Uninfected Persons. Front Immunol. 2019;10:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maile MD, Standiford TJ, Engoren MC, Stringer KA, Jewell ES, Rajendiran TM, Soni T, Burant CF. Associations of the plasma lipidome with mortality in the acute respiratory distress syndrome: a longitudinal cohort study. Respir Res. 2018;19(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bursten SL, Federighi DA, Parsons P, Harris WE, Abraham E, Moore EE Jr, Moore FA, Bianco JA, Singer JW, Repine JE. An increase in serum C18 unsaturated free fatty acids as a predictor of the development of acute respiratory distress syndrome. Crit Care Med. 1996;24(7):1129–36. [DOI] [PubMed] [Google Scholar]
- 21.Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, Thomas PG, Dennis EA, Aderem A. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. 2013;154(1):213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyle JE, Burnum-Johnson KE, Wendler JP, Eisfeld AJ, Halfmann PJ, Watanabe T, Sahr F, Smith RD, Kawaoka Y, Waters KM, Metz TO. Plasma lipidome reveals critical illness and recovery from human Ebola virus disease. Proc Natl Acad Sci U S A. 2019;116(9):3919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song JW, Lam SM, Fan X, Cao WJ, Wang SY, Tian H, Chua GH, Zhang C, Meng FP, Xu Z, et al. Omics-Driven Systems Interrogation of Metabolic Dysregulation in COVID-19 Pathogenesis. Cell Metab. 2020;32(2):188–202.e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gai ZT, Zhang Y, Liang MF, Jin C, Zhang S, Zhu CB, et al. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis. 2012;206:1095–102. 10.1093/infdis/jis472. [DOI] [PubMed] [Google Scholar]
- 25.Huang T, Fan Y, Xia Y, Xu X, Chen X, Ye H, Chen Y, Wang S. Association of low HDL-c levels with severe symptoms and poor clinical prognosis in patients with severe fever and thrombocytopenia syndrome. Front Microbiol. 2023;14:1239420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SPLASH LipidoMIX™ Internal Standard Product Number 330707. (https://avantilipids.com/assets/products/attachments/330707-Mixture-Components-and-Concentrations.pdf) Avanti Polar Lipids, Inc.
- 27.Sarafian MH, Gaudin M, Lewis MR. Objective set of criteria for optimization of sample preparation procedures for ultra-high throughput untargeted blood plasma lipid profiling by ultra performance liquid chromatography-mass spectrometry. Anal Chem. 2014;86(12):5766–74. [DOI] [PubMed] [Google Scholar]
- 28.Cajka T, Smilowitz JT, Fiehn O. Validating Quantitative Untargeted Lipidomics Across Nine Liquid Chromatography-High-Resolution Mass Spectrometry Platforms. Anal Chem. 2017;89(22):12360–8. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z, Satten GA, Mitchell C, Hu YJ. Constraining PERMANOVA and LDM to within-set comparisons by projection improves the efficiency of analyses of matched sets of microbiome data. Microbiome. 2021;9(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayano M, Takigawa I, Shiga M, Tsuda K, Mamitsuka H. ROS-DET: robust detector of switching mechanisms in gene expression. Nucleic Acids Res. 2011;39(11): e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam SM, Wang Z, Li B, Shui G. High-coverage lipidomics for functional lipid and pathway analyses. Anal Chim Acta. 2021;1147:199–210. [DOI] [PubMed] [Google Scholar]
- 32.Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res. 2009;50(3):574–85. [DOI] [PubMed] [Google Scholar]
- 33.Ruan J, Miao X, Schlüter D, Lin L, Wang X. Extracellular vesicles in neuroinflammation: Pathogenesis, diagnosis, and therapy. Mol Ther. 2021;29(6):1946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89(2):205–12. [DOI] [PubMed] [Google Scholar]
- 35.Clark SR, Thomas CP, Hammond VJ, Aldrovandi M, Wilkinson GW, Hart KW, Murphy RC, Collins PW, O’Donnell VB. Characterization of platelet aminophospholipid externalization reveals fatty acids as molecular determinants that regulate coagulation. Proc Natl Acad Sci U S A. 2013;110(15):5875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolegowska B, Lubkowska A, De Girolamo L. Platelet lipidomic. J Biol Regul Homeost Agents. 2012;26(2 Suppl 1):23s–33s. [PubMed] [Google Scholar]
- 37.Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res. 2003;42(5):423–38. [DOI] [PubMed] [Google Scholar]
- 38.Fang L, Yu S, Tian X, Fu W, Su L, Chen Z, Yan C, He J, Hong J, Lian W, et al. Severe fever with thrombocytopenia syndrome virus replicates in platelets and enhances platelet activation. J Thromb Haemost. 2023;21(5):1336–51. [DOI] [PubMed] [Google Scholar]
- 39.Chaurio RA, Janko C, Muñoz LE, Frey B, Herrmann M, Gaipl US. Phospholipids: key players in apoptosis and immune regulation. Molecules. 2009;14(12):4892–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45(7):1169–96. [DOI] [PubMed] [Google Scholar]
- 41.Ishibashi Y, Kohyama-Koganeya A, Hirabayashi Y. New insights on glucosylated lipids: metabolism and functions. Biochim Biophys Acta. 2013;1831(9):1475–85. [DOI] [PubMed] [Google Scholar]
- 42.Li JF, Qu F, Zheng SJ, Ren JY, Wu HL, Liu M, Liu H, Ren F, Chen Y, Zhang JL, Duan ZP. Plasma sphingolipids as potential indicators of hepatic necroinflammation in patients with chronic hepatitis C and normal alanine aminotransferase level. PLoS ONE. 2014;9(4): e95095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prokazova NV, Zvezdina ND, Korotaeva AA. Effect of lysophosphatidylcholine on transmembrane signal transduction. Biochemistry (Mosc). 1998;63(1):31–7. [PubMed] [Google Scholar]
- 44.Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208(1):10–8. 10.1016/j.atherosclerosis.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 45.Park DW, Kwak DS, Park YY, Chang Y, Huh JW, Lim CM, Koh Y, Song DK, Hong SB. Impact of serial measurements of lysophosphatidylcholine on 28-day mortality prediction in patients admitted to the intensive care unit with severe sepsis or septic shock. J Crit Care. 2014;29(5):882.e885–811. [DOI] [PubMed] [Google Scholar]
- 46.Cho WH, Park T, Park YY, Huh JW, Lim CM, Koh Y, Song DK, Hong SB. Clinical significance of enzymatic lysophosphatidylcholine (LPC) assay data in patients with sepsis. Eur J Clin Microbiol Infect Dis. 2012;31(8):1805–10. [DOI] [PubMed] [Google Scholar]
- 47.Holloway GP, Bezaire V, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J Physiol. 2006;571(Pt 1):201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feingold KR. The bidirectional interaction of COVID-19 infections and lipoproteins. Best Pract Res Clin Endocrinol Metab. 2023;37(4):101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bassendine MF, Sheridan DA, Bridge SH, Felmlee DJ, Neely RD. Lipids and HCV. Semin Immunopathol. 2013;35(1):87–100. [DOI] [PubMed] [Google Scholar]
- 50.Zheng H, Geng Y, Gu C, Li M, Mao M, Wan Y, et al. A Reservoir Computing with Boosted Topology Model to Predict Encephalitis and Mortality for Patients with Severe Fever with Thrombocytopenia Syndrome: A Retrospective Multicenter Study. Infect Dis Ther. 2023;12(5):1379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cole LK, Vance JE, Vance DE. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim Biophys Acta. 2012;1821(5):754–61. [DOI] [PubMed] [Google Scholar]
- 52.Skipski VP, Barclay M, Barclay RK, Fetzer VA, Good JJ, Archibald FM. Lipid composition of human serum lipoproteins. Biochem J. 1967;104(2):340–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scherer PG, Seelig J. Structure and dynamics of the phosphatidylcholine and the phosphatidylethanolamine head group in L-M fibroblasts as studied by deuterium nuclear magnetic resonance. EMBO J. 1987;6(10):2915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morita SY, Ikeda Y. Regulation of membrane phospholipid biosynthesis in mammalian cells. Biochem Pharmacol. 2022;206:115296. [DOI] [PubMed] [Google Scholar]
- 55.Eisfeld AJ, Halfmann PJ, Wendler JP, Kyle JE, Burnum-Johnson KE, Peralta Z, Maemura T, Walters KB, Watanabe T, Fukuyama S, et al. Multi-platform ’Omics Analysis of Human Ebola Virus Disease Pathogenesis. Cell Host Microbe. 2017;22(6):817–829.e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song P, Zheng N, Liu Y, Tian C, Wu X, Ma X, Chen D, Zou X, Wang G, Wang H, et al. Deficient humoral responses and disrupted B-cell immunity are associated with fatal SFTSV infection. Nat Commun. 2018;9(1):3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Li X, Lv S, Peng X, Cui N, Yang T, Yang Z, Yuan C, Yuan Y, Yao J, et al. Single-cell landscape of peripheral immune responses to fatal SFTS. Cell Rep. 2021;37(8): 110039. [DOI] [PubMed] [Google Scholar]
- 58.Li YH, Huang WW, He WQ, He XY, Wang XH, Lin YL, Zhao ZJ, Zheng YT, Pang W. Longitudinal analysis of immunocyte responses and inflammatory cytokine profiles in SFTSV-infected rhesus macaques. Front Immunol. 2023;14:1143796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falasca L, Agrati C, Petrosillo N, Di Caro A, Capobianchi MR, Ippolito G, Piacentini M. Molecular mechanisms of Ebola virus pathogenesis: focus on cell death. Cell Death Differ. 2015;22(8):1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1: Tables S1-S2, Figures S1-S7. Table S1. Clinical Manifestations of SFTS Patients. Table S2. Laboratory Examination Results of Participants. Figure S1. Sampling time information for SFTS patients. Figure S2. Chromatograms of QC samples. Figure S3. Repeatability Test of Lipidomics Results. Figure S4. Mfuzz Clustering for Lipids Screened after Volcano Plot. Figure S5. Heatmap Analysis. Figure S6. Forest Plot. Figure S7. Comparison of Differential Lipid Subclasses between SFTS and EVD.
Data Availability Statement
The original data and code have been uploaded to the Science Data Bank, accessible at the following website: https://www.scidb.cn/en/s/IJzeIv.