Abstract
Diethyl 2-(((4-methyl-2-oxo-2H-chromen-7-yl)oxy)methylene)malonate (2) was synthesized from coumarin 1 and diethyl ethoxymethylene malonate in ethanol, followed by cyclization in diphenyl ether to give chromene-9-carboxylate (3). Sugar hydrazones 5a-c were formed by reacting hydrazide 4 with D-galactose, D-mannose, and D-xylose, then acetylated to per-O-acetyl derivatives 6a-c. Heating 5a-c with acetic anhydride at 100 °C gave oxadiazolines 7a-c. Compound 8, obtained by refluxing 4 with carbon disulfide, was alkylated to 9 or reacted to give 10. Further reactions yielded acetoxy derivative 13 and hydroxy derivative 14. Compounds 17a-e and 18a-e were synthesized using thiomorpholinophenyl ureido/thioureido-s-triazine. These compounds were characterized and evaluated for antibacterial activity against Gram (+ve) bacteria (B. subtilis, S. aureus) and Gram (-ve) bacteria (E. coli, P. aeruginosa) in addition to yeast-like fungi (C. albicans). Compounds 11, 13, 15, 16, 17c-e, and 18a-e showed the highest antibacterial activity. Molecular docking was performed to study their binding with transpeptidases.
Keywords: 1,3,4-Oxadiazole; Pyrano[2,3-f]chromene; Sugars; Antimicrobial activities; Molecular docking; Transpeptidases
Graphical abstract
Highlights
-
•
Synthesis of pyrano [2,3-f]chromene derivatives bearing oxadiazole ring.
-
•
Synthesis of oxadiazole ring linked to sugar and s-triazine moieties.
-
•
This work was to study antimicrobial efficacy of newly synthesized compounds and docking studies.
-
•
Some of the newly synthesized compounds showed promising antimicrobial activity.
1. Introduction
Health issues are increasingly serious clinical concerns. To address these challenges, medicinal chemists are actively developing innovative treatments [1]. Heterocyclic compounds, especially those with five- or six-membered rings containing nitrogen, oxygen, or sulfur, are widely used in therapy [2]. Compounds like oxadiazoles, which contain nitrogen atoms, are of particular interest in medical and pharmaceutical research [3]. Over the past 20 years, significant advancements have been made in discovering natural products based on chromene, with 2H-chromenes and 2-oxo-2H-chromenes (coumarins) being the most studied.
Chromene and its derivatives are important organic compounds widely found in nature [4], known for their biological and pharmacological properties, including anti-HIV [5], anti-cancer [6,7], antibacterial [[8], [9], [10]], and anti-neurodegenerative activities [[11], [12], [13]]. They are also used as organic pesticides [14] and intermediates [15] in synthesizing natural and synthetic compounds. For organic and medicinal chemists, the challenge lies in developing new, environmentally friendly synthetic techniques. Current trends focus on one-pot, one-step, solvent-free, and catalyst-free methods [[16], [17], [18]], which offer benefits like reduced reaction times and simplified purification processes [19].
The ring of 1,3,4-oxadiazole is a crucial heterocyclic compound due to its broad biological activity [20]. This ring structure, characterized by the substitution of two nitrogen atoms for methylene groups, reduces aromaticity and introduces conjugated diene characteristics, enhancing its therapeutic potential [21]. Among its isomers, the 1,3,4-oxadiazole isomer is particularly noted for its diverse therapeutic applications [[22], [23], [24], [25], [26], [27], [28], [29]].
The potent pharmacological actions of 1,3,4-oxadiazole may stem from its toxophoric –N=C–O– bond [30]. Among them, substituted 1,3,4-oxadiazoles are quite interesting from a medicinal standpoint [31]. Specifically, 2,5-diaryl-1,3,4-oxadiazoles have greater stability in comparison to their corresponding 2,5-dialkyl derivatives.
The 1,3,4-oxadiazole ring is present in a variety of drugs (Fig. 1), including Furamizole, a nitrofuran derivative with potent antibacterial activity [32]. Anti-arrhythmic therapy uses the medications Nesapidil and Raltegravir, which are antiviral drugs. The FDA-approved anticancer medication Zibotentan has the most distinctive derivatives available on the market in its 1,3,4-oxadiazole nucleus [33]. Tiodazosin is a medication used to treat hypertension [34].
Fig. 1.
Some drugs containing 1,3,4-oxadiazole moiety.
Additionally, sugar-based 1,3,4-oxadiazoles are a vital class of heterocyclic compounds [35] known for their broad spectrum of biological activities, particularly as antimicrobial agents against bacteria and fungi. These compounds also exhibit properties such as anti-epileptic activity [36], apoptosis induction [37], antimycobacterial effects [38], and antifungal [39], and serve as inhibitors for various biological targets.
In previous work [40,41], The main goal of our present work is to synthesize some heterocyclic compounds that are characterized by multi-directional biological activity, such as antibiotics, antimicrobials, and anticancer agents. Hence the importance of this work, in which we constructed some different Oxadiazole compounds and evaluated them biologically as antibacterial agents.
2. Results and discussion
2.1. Chemistry
The Von Pechmann reaction provides coumarins when phenols condense with ethyl acetoacetate in an acidic media. When sulfuric acid is present, resorcinol and ethyl acetoacetate produce 7-hydroxy-4-methylcoumarin (1) [42].
Compound 2 was produced in high yield by refluxing the starting coumarin 1 with diethyl ethoxymethylene malonate in ethanol (Scheme 1). Compound 2 showed the molecular ion (M+) peak at m/z 346 (M+, 48 %) in its mass spectrum, while the infrared spectrum revealed the carbonyl ester group absorption band at 1695 cm−1. After boiling in diphenyl ether, 2 underwent cyclization to get compound 3 (Scheme 1). Compound 3's 1H NMR spectrum showed ethyl group signals as a quartet at δ 4.31 ppm (J = 5.5 Hz) and a triplet at δ 1.28 ppm (J = 5.5 Hz), addition to four aromatic protons at δ 6.27 (singlet signal due to C3-H coumarin), 7.02, 7.84 (two doublets AB system, J = 18 Hz due to C5-H and C6-H coumarin), and 8.57 ppm (singlet signal due to C8-H pyran ring) beside CH3 protons at δ 2.42 ppm as singlet signal. Compound 3 displayed characteristic bands at 1710 (COOEt), and 1641 cm−1 (C=O) groups in its IR spectrum. The equivalent acid, hydrazide 4, was given by treating 3 with hydrazine hydrate (Scheme 1). In hydrazide derivative 4, the ethyl group has vanished, and the protons of the CONHNH2 group are visible at δ 4.65 (NH2) and 8.65 ppm (NH).
Scheme 1.
Synthesis of compounds 5a-c, 6a-c and 7a-c.
The corresponding sugar hydrazones 5a–c were produced in 90–95 % yields when 4 interacted with D-galactose, D-mannose, and/or D-xylose in an aqueous ethanolic solution with a catalytic quantity of acetic acid (Scheme 1). Characteristic bands were visible in the IR spectra of samples 5a–c. These bands corresponded to the hydroxyl groups around 3451–3420 and 3310 cm−1 for NH. The sugar chain protons at δ 3.45–5.61 ppm, the aromatic protons at δ 6.40 (singlet), 7.00, 7.75 (two doublets AB system, J = 18 Hz), and 8.35 ppm (singlet), the N=CH proton at δ 8.85 ppm, and the proton for NH at δ 11.15 ppm were all detected in 5a′s 1H NMR spectrum. The per-O-acetyl derivatives, 6a-c, were obtained in 71–86 % yield by agitating in pyridine acetic anhydride with the sugar hydrazones 5a-c at 25 °C (Scheme 1). The infrared spectra of samples 6a–c displayed distinct absorption bands at ν 3310 cm−1 for NH and v 1737 cm−1 corresponding to the acetyl carbonyl groups. Compound 6b′s 1H NMR spectrum revealed signals at δ 1.82–2.43 ppm due to O-acetyl group protons, while the remaining sugar chain protons were detected in the δ 4.26–5.68 ppm range. Conversely, oxadiazoline derivatives 7a-c were obtained by heating sugar hydrazone 5a-c with acetic anhydride to 100 °C (Scheme 1). The carbonyl groups and the disappearance of the NH peak, respectively, were represented by distinctive bands in the infrared spectra of samples 7a–c, which were located at ν 1740-1640 cm−1. The oxadiazoline proton appeared within the aromatic proton range at δ 6.24 ppm, while the proton signals of acetyl groups were observed in the region of δ 1.18–2.10 ppm in the 1H NMR spectrum of sample 7a.
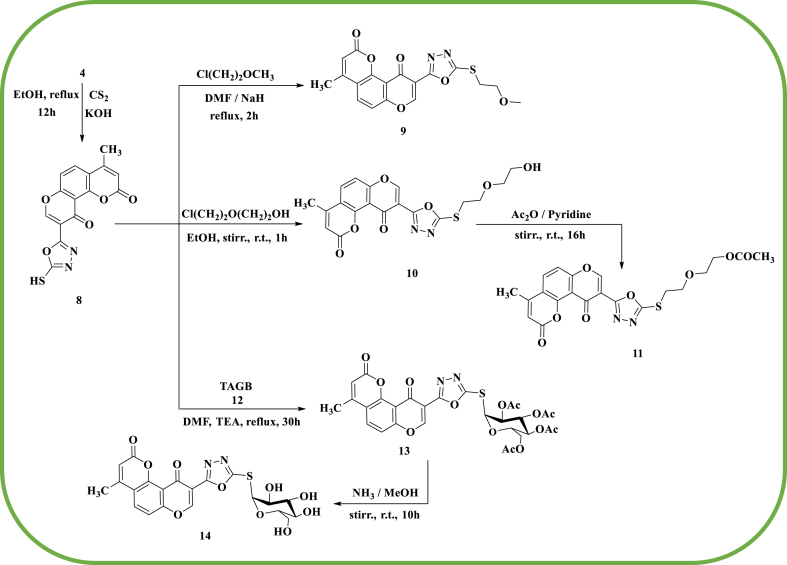
With anhydrous KOH methanol, 4 was refluxed with carbon disulfide to obtain compound 8 with an 83 % yield (Scheme 2). In its 1H NMR spectrum, the aromatic protons were represented by four signals at δ 6.30 (singlet), 7.00, 7.90 (two doublets AB system, J = 18 Hz), and 8.70 ppm (singlet), and the SH protons appeared at δ 13.52 ppm as a singlet signal. The M+ peak was visible in the MS at m/z 328 (M+, 38 %). Compound 8 yielded 9 when it reacted with 2-chloroethyl methyl ether in DMF and sodium hydride at 25 °C (Scheme 2). The 1H NMR spectra revealed triplet signals for CH2O at δ 3.65 ppm (J = 5.5 Hz), the two S-CH2 groups at δ 3.78 ppm (J = 5.5 Hz), and a singlet signal of O-methyl protons at δ 3.49 ppm.
Scheme 2.
Synthesis of compounds 8-14.
In an ethanolic NaOH solution, compound 8 reacted with 2-chloroethoxy ethanol to give compound 10 in a 94 % yield (Scheme 2). The Mass, IR, and 1H NMR spectroscopy were used to assert its structure. The M+ peak appeared in MS of 10 at m/z 416 (M+, 30 %). With acetic anhydride in pyridine, compound 10 was acetylated to yield compound 11. The IR spectrum revealed a band at 1735 cm−1 that corresponds to COCH3 moiety, confirming its structure.
Furthermore, compound 13 was obtained when 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (TAGB) (12) reacted with 8 in DMF at 25 °C, which was catalyzed by TEA (Scheme 2). The acetyl group appeared as a band at 1751 cm−1 in the infrared spectra of 13. The acetyl protons' signal at δ 1.99 ppm, at δ 6.11 (singlet) due to aromatic protons, 7.30, 7.70 (two doublets AB system, J = 18 Hz), and 8.32 ppm (singlet), and the CH3 singlet signal at δ 2.38 ppm were all appeared in the 1H NMR spectrum. At 25 °C, 13 reacted with a methanolic ammonia solution to produce compound 14 (Scheme 2). The typical band at 3373 cm−1, which corresponds to the hydroxyl groups, appeared in the IR spectrum of compound 14.
Furthermore, compound 15 was obtained by treating compound 8 with s-triazine trichloride (Scheme 3). This was followed by condensation with morpholine, which produced 16, which was transformed into compounds 17a-e and 18a-e by reacting with aryl urea and/or aryl thiourea (Scheme 3). IR and 1HNMR were used to describe the compounds 17a-e and 18a-e. Compound 17a′s IR spectrum exhibits a peak at ν 1685 cm−1, indicating the existence of an amidic carbonyl group. Compound 17a′s 1HNMR spectrum reveals a signal at δ 9.35 and 10.20 ppm of two NH groups.
Scheme 3.
Synthesis of compounds 15-18.
2.2. Antimicrobial evaluation
The antibacterial activity of thirty newly synthesized compounds was tested in vitro against yeast-like fungi (C. albicans), Gram-positive bacteria (S. aureus and B. subtilis), and Gram-negative bacteria (P. aeruginosa and E. coli).
The Agar-diffusion method was used in this experiment. Cephalothin, chloramphenicol, and cycloheximide were the reference drugs. Minimum Inhibitory Concentration (MIC) was determined by the two-fold serial dilution method [43]. The MIC is shown in Table 1 (μg/mL).
Table 1.
Antimicrobial activity of the newly synthesized compounds.
| MIC (μg/mL) | |||||
|---|---|---|---|---|---|
| Gram Positive bacteria |
Gram Negative bacteria |
Fungi |
|||
| Compound No. | S. aureus | B. subtilis | P. aeruginosa | E. coli | C. albicans |
| 2 | 50 | 50 | 100 | 100 | 100 |
| 3 | 50 | 50 | 100 | 100 | 100 |
| 4 | 25 | 25 | 100 | 50 | 50 |
| 5a | 25 | 12.5 | 25 | 50 | 50 |
| 5b | 25 | 12.5 | 25 | 50 | 100 |
| 5c | 25 | 12.5 | 50 | 50 | 100 |
| 6a | 12.5 | 12.5 | 50 | 100 | 100 |
| 6b | 12.5 | 12.5 | 50 | 100 | 100 |
| 6c | 12.5 | 12.5 | 50 | 100 | 100 |
| 7a | 6.25 | 6.25 | 25 | 50 | 50 |
| 7b | 6.25 | 6.25 | 25 | 50 | 100 |
| 7c | 6.25 | 6.25 | 25 | 50 | 100 |
| 8 | 6.25 | 12.5 | 25 | 50 | 50 |
| 9 | 6.25 | 6.25 | 25 | 50 | 50 |
| 10 | 6.25 | 6.25 | 25 | 50 | 50 |
| 11 | 3.125 | 6.25 | 12.5 | 25 | 12.5 |
| 13 | 3.125 | 6.25 | 12.5 | 25 | 12.5 |
| 14 | 6.25 | 6.25 | 50 | 25 | 50 |
| 15 | 3.125 | 3.125 | 25 | 25 | 50 |
| 16 | 3.125 | 3.125 | 25 | 50 | 25 |
| 17a | 6.25 | 6.25 | 25 | 50 | 50 |
| 17b | 6.25 | 6.25 | 50 | 50 | 100 |
| 17c | 3.125 | 6.25 | 25 | 25 | 50 |
| 17d | 3.125 | 6.25 | 25 | 25 | 25 |
| 17e | 3.125 | 6.25 | 25 | 25 | 25 |
| 18a | 3.125 | 6.25 | 12.5 | 25 | 12.5 |
| 18b | 3.125 | 6.25 | 12.5 | 25 | 12.5 |
| 18c | 3.125 | 6.25 | 12.5 | 25 | 6.25 |
| 18d | 3.125 | 6.25 | 12.5 | 25 | 6.25 |
| 18e | 3.125 | 6.25 | 12.5 | 25 | 6.25 |
| Chloramphenicol | 3.125 | 3.125 | 6.25 | 6.25 | – |
| Cephalothin | 6.25 | 6.25 | 6.25 | 6.25 | – |
| Cycloheximide | – | – | – | – | 3.125 |
According to the information in Table 1, a large number of the compounds under investigation exhibited a variety of inhibitory effects on the spread of the tested Gram + ve and Gram-ve bacterial strains as well as against fungal strains. In general, a large number of the compounds studied proved to be more efficient against Gram + ve bacteria than against Gram-ve bacteria. It is noticed that compounds belonging to 1,3,4-oxadiazolyl pyrano chrome 11 and 13, and oxadiazolyl thio s-triazine 16–18 exhibited better anti-bacterial potentials than the rest of the other compounds.
Based on the data, compounds 11, 13, 15, 16, 17c-e, and 18a-e showed broad-spectrum antibacterial activity against the studied species. In this perspective, these compounds were demonstrated to be equipotent to chloramphenicol, as appeared by their capacity to inhibit B. subtilis growth (MIC 3.125 μg/mL) and their 50 % reduced efficacy against S. aureus in comparison to chloramphenicol, with the exception of compounds 15 and 16, where it is likewise 3.125 μg/mL. Compounds 7a–c, 8–10, 14, and 17a–b showed 50 % of chloramphenicol's growth inhbtion of B. subtilis and S. aureus (MIC 6.25 μg/mL). Compounds 2-6a-c, on the other hand, showed modest growth inhibitory activity against Gram + ve bacteria based on their MIC values (12.5–50 μg/mL). Compounds 11, 13, and 18a-e showed significant growth suppression in their antibacterial activities against the tested Gram-ve bacteria (MIC 12.5 μg/mL), whereas the rest compounds displayed moderate growth inhibition against the same organism (MIC 25–100 μg/mL). In relation to the pyranochromene compound's oxadiazole ring's antifungal strain activity, the findings showed that compounds 18c–e inhibited the growth of C. albicans 50 % lower than cycloheximide (MIC 6.25 μg/mL).
In combination with oxadiazole and oxadiazolothio s-triazine rings, pyranochromene compound 4 exhibits a robust and high antibacterial activity value. This implies that the antibacterial activities were enhanced to be equipotent to a pharmaceutical reference by the inclusion of sulfur and nitrogen atoms.
Compounds with CS and CO groups showed stronger antibacterial activity than those with electron-donating groups. The presence and position of the -NHCSNH- group and polar nitro and chloro substitutions at the C2 and C4 positions of the phenyl ring are key factors for optimizing compounds 17a-e and 18a-e.
The compounds showed greater effectiveness against Gram-positive bacteria compared to Gram-negative. This suggests their antibacterial activity is linked to the bacteria's cell wall. Gram-positive bacteria have a thick peptidoglycan-rich wall, making them more susceptible to certain antibiotics that target cell wall synthesis, while Gram-negative bacteria, with their thinner, lipopolysaccharide-rich wall, are less affected [44].
2.3. Molecular docking study
In comparison to the reference drug (cephalothin), the newly synthesized compounds demonstrated significant action against Gram-positive bacteria S. aureus and B. subtilis, as confirmed by the results of the antimicrobial activity. As a result, the potential for these substances to prevent the growth of Gram-positive bacteria was investigated under the name cephalothin.
Cefalotin or cephalothin is a first-generation semi-synthetic cephalosporin with a broad antimicrobial activity [45]. The bactericidal activity of cephalothin results from inhibition of cell wall synthesis by affinity for penicillin-binding proteins (PBPs) [46]. The PBPs are transpeptidases which are vital in peptidoglycan biosynthesis [47,48].
Transpeptidase is an enzyme that catalyzes the transpeptidation reaction between pentapeptide chains and adjacent peptide chains in the bacterial cell wall. It forms a domain in class A and B of penicillin-binding proteins peptidoglycan [49]. Therefore, the inhibition of one of these enzymes that has a significant impact on the microbial life cycle could influence protein synthesis, nucleic acid cleavage, assembly, and replication, or by altering the components and function of the cell wall [49]. Thus, to pre-assess the anti-bacterial behavior of the active compounds 11, 13, 15, 16, 17c-e, and 18a-e towards S. aureus and B. subtilis, the docking study was carried out to predict the scoring function, the binding affinity, and the orientation of the active compounds 11, 13, 15, 16, 17c-e, and 18a-e at the active sites of transpeptidases enzyme, PDB: 5TW8 (Auth A, with ceftaroline (AI8) as inhibitor) of S. aureus.
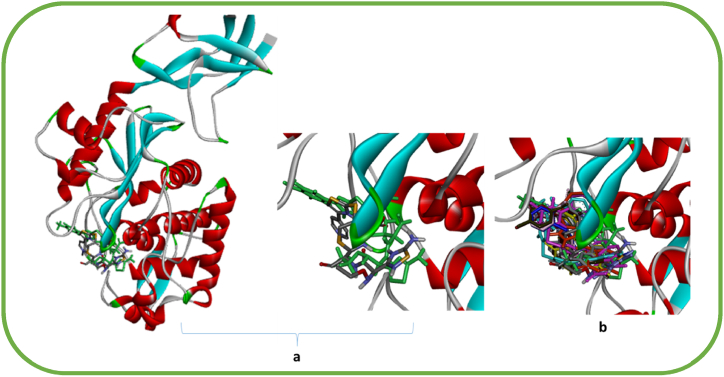
Accordingly, the X-ray crystallographic structure of transpeptidases enzyme, PDB: 5TW8 with the native inhibitor, ceftaroline (AI8) of S. aureus, was recovered from the protein data bank https://www.rcsb.org/structure/5TW8 (accessed on 09-02-2024). To validate the molecular docking process, the native ligand (AI8) was first re-docked into the active pocket of the enzyme. The ligand that was re-docked exhibited a docking score (S) of −8.4 kcal/mol. It was able to recreate all of the important connections, including alkyl and Pi-alkyl interactions, with the active amino acids of the active pocket, which include SER75, SER139, SER262, TYR291, and GLU114, through hydrogen bond interaction (Fig. 2a and b).
Fig. 2.
a): The 3D orientation of original ligand AI8 of transpeptidases enzyme (elemental colored), the re-docked ligand (green), b): The 3D orientation of the re-docked ligand (green), 11 (dark blue); 13; (orang); 15 (faint purple); 16 (pink); 17c (yellow); 17d (maroon); 17e (silver metallic); 18a (pink metallic); 18b (greenish brown); 18c (blue); 18d (dark red); 18e (blue sky) inside the binding pocket of transpeptidases enzyme (PDB: 5TW8).
Every molecule under study exhibited super-impossibility to AI8. Similar to AI8, they demonstrated a strong binding mode with the active pocket and restored the contacts with the essential amino acids of the transpeptidases enzyme (Fig. 2, Fig. 3l, and 4a-l). Additionally, they showed greater binding energy values (−8.7 to −11.2 kcal/mol) than the original ligand AI8 against the transpeptidases enzyme's binding site (PDB: 5TW8) (Table 2) (see Fig. 4).
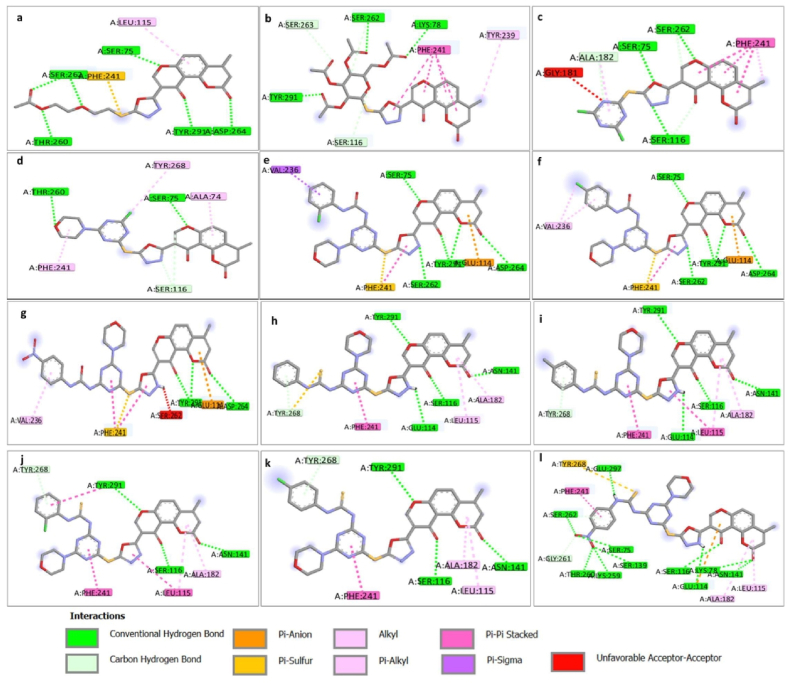
Fig. 3.
a-l. The 2D interaction of compounds 11, 13, 15, 16, 17c-e, and 18a-e inside the binding pocket of transpeptidase enzyme, PDB: 5TW8, illustrating the formed hydrogen bonds, attractive charge, pi-pi bond, and pi-alkyl.
Table 2.
The binding energy values of compounds 11, 13, 15, 16, 17c-e, and 18a-e against transpeptidase enzyme, PDB: 5TW8 (with AI8 as inhibitor) of S. aureus.
| Compound no. | Binding energy Kcal/mol |
Compound no. | Binding energy Kcal/mol |
|---|---|---|---|
| AI8 (ceftaroline) | −8.4 | AI8 (ceftaroline) | −8.4 |
| 11 | −8.7 | 17e | −10.7 |
| 13 | −9.2 | 18a | −10.2 |
| 15 | −10.1 | 18b | −10.4 |
| 16 | −10.5 | 18c | −10.4 |
| 17c | −11.2 | 18d | −10.4 |
| 17d | −11.1 | 18e | −10.7 |
Fig. 4.
a-l. The 3D configurations of compounds 11, 13, 15, 16, 17c-e, and 18a-e inside the binding pocket of transpeptidase enzyme (PDB: 5TW8).
Remarkably, all studied compounds revealed the same hydrogen interaction with the key amino acids to AI8. Especially with the key amino acid SER75, SER262, TYR291, except compounds 18a, 18b, 18c, and 18d. They showed hydrogen interaction only with TYR291.
Compound 18e was the only one that showed superior interaction with the active pocket of the transpeptidase, PDB: 5TW8. It restored the same interactions with all the main amino acids identical to the original compound AI8, including SER75, SER139, SER262, TYR291, and GLU114 via hydrogen interactions, besides PHE241 (Pi-Pi-stacked), ALA74 (Pi-alkyl) interactions. In addition, compound 18e showed additional hydrogen interactions with GLU297, GLY261, LYS259, SER116, LYS78, and ASN141 (Fig. 5a–d).
Fig. 5.
a), b) The 2D interactions of AI8 and 18e inside the binding pocket of transpeptidase enzyme (PDB: 5TW8), c) and d) clarifying the 3D configurations of AI8 and 18e inside the binding pocket of transpeptidase enzyme (PDB: 5TW8).
It might be concluded that the activity of compound 18e proved to be able to inhibit transpeptidase, a bacterial enzyme that cross-links the peptidoglycan chains to form rigid cell walls, confirming the importance of the presence of the fused pyrano [2,3-f] chromene next to oxadiazole moiety and besides 3-(4-nitrophenyl-urea) 1,3,5-triazine in agreement with the mentioned clarification described by Refs. [[50], [51], [52]].
3. Experiment
3.1. Instruments
The melting point is measured and left uncorrected when using the Gallenkamp electric melting point device. At the Mansoura University, Faculty of Science, we recorded the IR spectra at v/cm-1 (KBr) using a PerkinElmer Infrared Spectrophotometer Model 157, Grating. The 1H NMR spectra were obtained using a Varian spectrophotometer at 300 MHz and a Bruker spectrophotometer at 400 MHz (Cairo University, Faculty of Science and Mansoura University, Faculty of Pharmacy, respectively). Tetramethylsilane (TMS) was used as the internal reference, and DMSO-d6 was the solvent. Joel ECA-500 ll (Mansoura University, Faculty of Science) is where 13C NMR was conducted. The mass spectra (EI) at 70 eV were recorded at the Micro Analytical Unit, Faculty of Science, Cairo University, and Al-Azhar University, Cairo, Egypt, using the Kratos MS instrument and/or a Varian MAT 311 A Spectrometer. In Giza, Egypt, at the microanalytical center of Cairo University, elemental studies (C, H, and N) were conducted.
3.2. Chemistry
3.2.1. Synthesis of 7-hydroxy-4-methyl-2H-chromen-2-one (1)
It was prepared according to previously reported work [42].
3.2.2. Synthesis of diethyl 2-(((4-methyl-2-oxo-2H-chromen-7-yl)oxy)methylene)malonate (2)
Diethyl ethoxymethylene malonate (2.16 g, 0.01 mol) was dropped to a solution of coumarin 1 (1.76 g, 0.01 mol) in abs. ethanol (25 mL). After 5 h of reflux to the mixture of reaction, remove the solvent under reduced pressure. The semisolid derivative 2 was recrystallized from ethanol after being dried.
Yield, 91 %; m.p. = 110–112 °C; IR (KBr): νmax, cm−1: 1695 (C=O), 1640 (C=C); 1H NMR (DMSO-d6): δ = 1.23 (t, 6H, 2CH3), 2.40 (s, 3H, CH3), 4.20 (q, 4H, 2CH2), 6.27 (s, 1H, Ar-H), 6.95 (d, 1H, J = 18 Hz, Ar-H), 7.05 (s, 1H, Ar-H), 7.57 (d, 1H, J = 18 Hz, Ar-H), 8.13 (s, 1H, Ar-H); 13C NMR (DMSO-d6): δ = 14.6 (2C), 20.8, 61.2 (2C), 105.9, 110.8, 112.8, 114.0 (2C), 125.8, 152.5, 154.5 (2C), 160.9, 163.0, 165.5 (2C). MS (m/z, %): 346 (M+, 48). Anal. Calcd for C18H18O7 (346.34): C, 62.42; H, 5.24 %. Found: C, 62.21; H, 5.19 %.
3.2.3. Synthesis of ethyl 4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromene-9-carboxylate (3)
Refluxing compound 2 (3.46 g, 0.01 mol) for an hour in boiling diphenyl ether, the reaction mixture was cooled and filtered. The isolated precipitate was washed and dried with diethyl ether. Following the addition of 70 % ethanol, the resulting product recrystallized, yielding 3.
Yield, 79 %; m.p. = 221–223 °C; IR (KBr): νmax, cm−1: 1710 (COOEt), 1641 (C=O); 1H NMR (DMSO-d6): δ = 1.28 (t, 3H, J = 5.5 Hz, CH3), 2.42 (s, 3H, CH3), 4.31 (q, 2H, J = 5.5 Hz, CH2), 6.27 (s, 1H, Ar-H), 7.02 (d, 1H, J = 18 Hz, Ar-H), 7.84 (d, 1H, J = 18 Hz, Ar-H), 8.57 (s, 1H, Ar-H); 13C NMR (DMSO-d6): δ = 14.5 19.9, 61.6, 110.6, 111.8, 113.0, 114.9, 118.9, 131.8, 149.2, 152.8, 156.9, 160.5, 163.8, 165.1, 177.7. Anal. Calcd for C16H12O6 (300.27): C, 64.00; H, 4.03 %. Found: C, 63.88; H, 3.96 %.
3.2.4. Synthesis of 4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromene-9-carbohydrazide (4)
Compound 3 (3.00 g, 0.01 mol) in 50 mL of ethanol was mixed with 1.50 g of hydrazine hydrate (0.03 mol), and the mixture was refluxed for 12 h. The mixture was left to cool to room temperature, filtering, drying, and recrystallizing it from ethanol, the pyranochromene derivative 4 was produced.
Yield, 83 %; m.p. = 270–272 °C; IR (KBr): νmax, cm−1: 3340 (NH2), 3279 (NH), 3178 (NH), 1677 (CONH), 1654 (C=O), 1633 (C=C); 1H NMR (DMSO-d6): δ = 2.43 (s, 3H, CH3), 4.65 (s, 2H, NH2), 6.29 (s, 1H, Ar-H), 7.00 (d, 1H, J = 18 Hz, Ar-H), 7.71 (d, J = 18 Hz, 1H, Ar-H), 8.31 (s, 1H, Ar-H), 8.65 (s, 1H, NH); 13C NMR (DMSO-d6): δ = 19.6, 110.5, 112.3, 113.9 (2C), 118.9, 131.9, 149.8, 152.9, 156.5, 160.3, 162.9, 166.0, 177.8. MS (m/z, %): 286 (M+, 37). Anal. Calcd for C14H10N2O5 (286.24): C, 58.75; H, 3.92; N, 9.79 %. Found: C, 58.63; H, 3.44; N, 9.66 %.
3.3. Synthesis of compound 5a-c
3.3.1. General procedure
A well-stirred solution of compound 4 (2.86 g, 0.01 mol) in ethanol (10 mL) and the equivalent monosaccharide (0.01 mol) in water (2 mL) was added to 0.5 mL of glacial acetic acid. The reaction mixture was refluxed for an hour. The resulting solution was concentrated, cooled, and then filtered off. After drying and washing with ethanol, the precipitate recrystallized from ethanol.
3.3.2. 4-Methyl-2,10-dioxo-N'-((3R,4S,5R,E)-2,3,4,5,6-pentahydroxyhexylidene)-2H,10H-pyrano [2,3-f]chromene-9-carbohydrazide (5a)
Yield, 90 %, m.p. = 210–212 °C, IR (KBr): νmax, cm−1: 3451-3420 (OH), 3310 (NH), 1659 (CONH), 1645 (C=O), 1580 (C=N); 1H NMR (DMSO-d6): δ 2.42 (s, 3H, CH3), 3.45–5.61 (m, 11H, 5OH, CH2, 4CH), 6.40 (s, 1H, Ar-H), 7.00 (d, 1H, J = 18 Hz, Ar-H), 7.75 (d, 1H, J = 18 Hz, Ar-H), 8.35 (s, 1H, Ar-H), 8.85 (s, 1H, =CH), 11.15 (s, 1H, NH); 13C NMR (DMSO-d6): δ = 20.5, 64.5, 66.6, 71.0 (2C), 72.5, 110.8, 112.9, 115.0 (2C), 118.3, 131.6, 149.5, 152.9 (2C), 156.6, 160.4, 162.2, 168.1, 177.0. Anal. Calcd for C20H20N2O10 (448.38): C, 53.57; H, 4.50; N, 6.25 %. Found: C, 53.44; H, 4.38; N, 6.18 %.
3.3.3. 4-Methyl-2,10-dioxo-N'-((3R,4R,5R,E)-2,3,4,5,6-pentahydroxyhexylidene)-2H,10H-pyrano [2,3-f]chromene-9-carbohydrazide (5b)
Yield, 93 %, m.p. = 220–222 °C, IR (KBr): νmax, cm−1: 3402 (OH, NH), 1663 (CONH), 1639 (C=O), 1610 (C=N). Anal. Calcd for C20H20N2O10 (448.38): C, 53.57; H, 4.50; N, 6.25 %. Found: C, 53.50; H, 4.35; N, 6.17 %.
3.3.4. 4-Methyl-2,10-dioxo-N'-((3R,4R,E)-2,3,4,5-tetrahydroxypentylidene)-2H,10H-pyrano [2,3-f]chromene-9-carbohydrazide (5c)
Yield, 95 %, m.p. = 195–197 °C, IR (KBr): νmax, cm−1: 3416 (OH), 3303 (NH), 1642 (CONH), 1615 (C=O). Anal. Calcd for C19H18N2O9 (418.36): C, 54.55; H, 4.34; N, 6.70 %. Found: C, 54.48; H, 4.29; N, 6.66 %.
3.4. Synthesis of 6a-c
3.4.1. General procedure
After adding 1.2 mL (0.01 mol) of acetic anhydride to a 7 mL solution of 5a–c (0.01 mol) in pyridine, the mixture was stirred for 15 h at 25 °C. Chloroform was used to collect the product after the resulting solution was added to crushed ice.
3.4.2. (2R,3S,4R,E)-6-(2-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromene-9-carbonyl) hydrazineylidene)hexane-1,2,3,4,5-pentayl pentaacetate (6a)
Yield, 77 %, m.p.: semi-solid, IR (KBr): νmax, cm−1: 3310 (NH), 1737 (COCH3), 1666 (CONH), 1639 (C=O); 1H NMR (DMSO-d6): δ 2.06 (s, 12H, 4COCH3), 2.20 (s, 3H, COCH3), 2.39 (s, 3H, CH3), 4.31 (d, 2H, CH2), 5.24–5.70 (m, 4H, 4CH), 6.29 (s, 1H, C3-H pyran), 7.11 (d, 1H, J = 18 Hz, Ar-H), 7.43 (s, 1H, C2-H pyran), 7.77 (d, 1H, J = 18 Hz, Ar-H), 8.09 (d, 1H, CH=N), 12.10 (s, 1H, NH); 13C NMR (DMSO-d6): δ = 19.8, 21.7 (5C), 62.1, 65.5, 68.0, 71.0, 72.3, 110.0, 112.8, 114.9 (2C), 118.0, 131.4, 149.4, 152.4 (2C), 156.3, 160.0, 162.0, 168.0, 171.5 (5C), 177.0. Anal. Calcd for C30H30N2O15 (658.57): C, 54.71; H, 4.59; N, 4.25 %. Found: C, 54.68; H, 4.47; N, 4.19 %.
3.4.3. (2R,3R,4R,E)-6-(2-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromene-9-carbonyl) hydrazineylidene)hexane-1,2,3,4,5-pentayl pentaacetate (6b)
Yield, 86 %, m.p. = 78–80 °C, IR (KBr): νmax, cm−1: 3270 (NH), 1739 (COCH3), 1671 (CONH), 1635C=O), 1612 (C=N); 1H NMR (DMSO-d6): δ 1.82–2.43 (m, 18H, 5COCH3 + CH3), 4.26 (d, 2H, CH2), 5.13–5.68 (m, 4H, 4CH), 6.40 (s, 1H, Ar-H), 7.02 (d, 1H, J = 18 Hz, Ar-H), 7.51 (s, 1H, Ar-H), 7.79 (d, 1H, J = 18 Hz, Ar-H), 7.90 (d, 1H, CH=N), 12.20 (s, 1H, NH); 13C NMR (DMSO-d6): δ = 19.0, 20.9 (5C), 61.9, 64.9, 67.7, 70.7, 71.9, 110.0, 112.1, 114.0 (2C), 117.6, 130.7, 148.5, 152.0 (2C), 155.9, 159.7, 161.6, 167.5, 170.7 (5C), 176.6. Anal. Calcd for C30H30N2O15 (658.57): C, 54.71; H, 4.59; N, 4.25 %. Found: C, 54.66; H, 4.45; N, 4.11 %.
3.4.4. (2R,3R,E)-5-(2-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromene-9-carbonyl)hydrazineylidene)pentane-1,2,3,4-tetrayl tetraacetate (6c)
Yield, 71 %, m.p. = 115–117 °C, IR (KBr): νmax, cm−1: 3270 (NH), 1740 (COCH3), 1685 (CONH), 1631 (C=O), 1610 (C=N); 1H NMR (DMSO-d6): δ 2.10 (s, 9H, 3COCH3), 2.25 (s, 3H, COCH3), 2.40 (s, 3H, CH3), 4.44 (d, 2H, CH2), 5.30–5.56 (m, 3H, 3CH), 6.40 (s, 1H, C3-H pyran), 7.24 (d, 1H, J = 18 Hz, Ar-H), 7.43 (s, 1H, C2-H pyran), 7.89 (d, 1H, J = 18 Hz, Ar-H), 8.20 (d, 1H, CH=N), 12.20 (s, 1H, NH); 13C NMR (DMSO-d6): δ = 20.1, 22.0 (4C), 60.5, 62.8, 66.0, 68.4, 110.8, 113.4, 115.5 (2C), 118.5, 131.9, 149.8, 153.2 (2C), 157.0, 160.6, 162.3, 168.2, 171.9 (4C), 177.7. Anal. Calcd for C27H26N2O13 (586.51): C, 55.29; H, 4.47; N, 4.78 %. Found: C, 55.18; H, 4.38; N, 4.61 %.
3.5. Synthesis of 7a-c
3.5.1. General procedure
The sugar hydrazone derivatives 5a (4.48 g, 0.01 mol), 5b (4.48 g, 0.01 mol), and 5c (4.10 g, 0.01 mol) were subjected to reflux for 3 h in a solution of acetic anhydride (15 mL). After adding the resultant solution to crushed ice, the product was removed by using chloroform.
3.5.2. (2S,3S,4R)-1-(3-acetyl-5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)pentane-1,2,3,4,5-pentayl pentaacetate (7a)
Yield, 63 %, m.p. = 90–92 °C, IR (KBr): νmax, cm−1: 1740 (COCH3), 1642 (C=O), 1615 (C=N); 1H NMR (DMSO-d6): δ 1.90 (s, 3H, COCH3), 2.04 (s, 15H, 5COCH3), 2.45 (s, 3H, CH3), 4.24 (d, 2H, CH2), 5.09–6.00 (m, 4H, 4CH), 6.25 (d, 1H, oxadiazol-CH), 6.49 (s, 1H, C3-H pyran), 6.66 (s, 1H, C2-H pyran), 7.15 (d, 1H, J = 18 Hz, Ar-H), 7.80 (d, 1H, J = 18 Hz, Ar-H); 13C NMR (DMSO-d6): δ = 18.9, 20.0, 20.1 (4C), 23.1, 60.3, 61.5, 67.1, 68.0, 74.0, 75.0, 110.1, 112.7 (2C), 114.6, 118.0, 131.2, 149.1 (2C), 152.5, 156.2 (2C), 159.9, 167.0, 169.0 (5C), 177.0. Anal. Calcd for C32H32N2O16 (700.61): C, 54.86; H, 4.60; N, 4.00 %. Found: C, 54.77; H, 4.56; N, 3.88 %.
3.5.3. (2S,3R,4R)-1-(3-acetyl-5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)pentane-1,2,3,4,5-pentayl pentaacetate (7b)
Yield, 86 %, m.p. = 74–76 °C, IR (KBr): νmax, cm−1: 3425 (NH), 1743 (COCH3), 1630 (C=O); 1H NMR (DMSO-d6): δ 1.98 (s, 3H, COCH3), 2.11 (s, 15H, 5COCH3), 2.32 (s, 3H, CH3), 4.30 (d, 2H, CH2), 5.12–6.06 (m, 4H, 4CH), 6.30 (d, 1H, oxadiazol-CH), 6.55 (s, 1H, C3-H pyran), 6.70 (s, 1H, C2-H pyran), 7.20 (d, 1H, J = 18 Hz, Ar-H), 7.83 (d, 1H, J = 18 Hz, Ar-H); 13C NMR (DMSO-d6): δ = 18.1, 19.6, 19.9 (4C), 22.5, 60.7, 61.8, 67.4, 68.3, 74.2, 75.7, 110.5, 113.0 (2C), 115.1, 118.1, 131.7, 149.5 (2C), 153.0, 156.7 (2C), 160.2, 167.4, 169.5 (5C), 177.5. Anal. Calcd for C32H32N2O16 (700.61): C, 54.86; H, 4.60; N, 4.00 %. Found: C, 54.79; H, 4.58; N, 3.98.
3.5.4. (2S,3R)-1-(3-acetyl-5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)butane-1,2,3,4-tetrayl tetraacetate (7c)
Yield, 25 %, m.p. = oily (viscous), IR (KBr): νmax, cm−1: 3250 (NH), 1746 (COCH3), 1636 (C=O), 1538 (C=N); 1H NMR (DMSO-d6): δ 2.00 (s, 3H, COCH3), 2.15 (s, 12H, 4COCH3), 2.36 (s, 3H, CH3), 4.20 (d, 2H, CH2), 5.30–5.80 (m, 3H, 3CH), 6.34 (d, 1H, oxadiazol-CH), 6.60 (s, 1H, C3-H pyran), 6.75 (s, 1H, C2-H pyran), 7.24 (d, 1H, J = 18 Hz, Ar-H), 7.88 (d, 1H, J = 18 Hz, Ar-H); 13C NMR (DMSO-d6): δ = 19.9, 20.9, 21.2 (3C), 24.0, 61.4, 64.2, 66.7, 75.0, 76.0, 111.4, 113.8 (2C), 115.9, 118.0, 132.3, 150.1 (2C), 153.8, 157.8 (2C), 161.1, 168.0, 170.0 (4C), 178.4. Anal. Calcd for C29H28N2O14 (628.54): C, 55.42; H, 4.49; N, 4.46 %. Found: C, 55.37; H, 4.41; N, 4.40 %.
3.5.5. Synthesis of 9-(5-mercapto-1,3,4-oxadiazol-2-yl)-4-methyl-2H,10H-pyrano [2,3-f]chromene-2,10-dione (8)
Compound 4 (2.80 g, 0.01 mol) was mixed with 5 mL of carbon disulphide, along with potassium hydroxide (0.56 g, 0.01 mol) in 2 mL of water. The reaction mixture was refluxed for 20 h. After the solvent evaporated, the residue was filtered off, diluted in water, and acidified with diluted hydrochloric acid. The precipitate was extracted from the ethanol and recrystallized after being filtered off and cleaned with water.
Yield, 83 %; m.p. = 290–292 °C; IR (KBr): νmax, cm−1: 1640 (C=O), 1376 (C=S); 1H NMR (DMSO-d6): δ = 2.30 (s, 3H, CH3), 6.30 (s, 1H, Ar-H), 7.00 (d, 1H, Ar-H), 7.90 (d, 1H, Ar-H), 8.70 (s, 1H, Ar-H), 13.52 (s, 1H, SH); 13C NMR (DMSO-d6): δ = 18.5, 110.9, 112.1, 114.4, 118.0 (2C), 131.4, 145.0, 150.1, 152.2, 156.5, 159.6, 160.5 (2C), 175.1. MS (m/z, %): 328 (M+, 22). Anal. Calcd for C15H8N2O5S (328.30): C, 54.88; H, 2.46; N, 8.53 %. Found: C, 54.80; H, 2.33; N, 8.41 %.
3.5.6. Synthesis of 9-(5-((2-methoxyethyl)thio)-1,3,4-oxadiazol-2-yl)-4-methyl-2H,10H-pyrano [2,3-f]chromene-2,10-dione (9)
A solution of 8 (3.28 g, 0.01 mol) in DMF (15 mL) was mixed with anh. NaH (0.24 g, 0.01 mol). The mixture was stirred at 25 °C for 2 h. Chloroethyl methyl ether (0.95 g, 0.01 mol) was then added. The reaction mixture was added to ice-cold water after being agitated for 30 h at 25 °C. The reaction mixture was extracted using ethyl acetate. After that, at reduced pressure, the solvent evaporated to produce 9.
Yield, 48 %; m.p. = 180–182 °C; IR (KBr): νmax, cm−1: 1643 (C=O); 1H NMR (DMSO-d6): δ = 2.43 (s, 3H, CH3), 3.49 (s, 3H, CH3), 3.65 (t, 2H, J = 5.5 Hz, O-CH2), 3.78 (t, 2H, J = 5.5 Hz, S-CH2), 6.20 (s, 1H, Ar-H), 7.05 (d, 1H, J = 18 Hz, Ar-H), 7.40 (d, 1H, J = 18 Hz, Ar-H), 8.31 (s, 1H, Ar-H); 13C NMR (DMSO-d6): δ = 19.4, 37.9, 58.8, 74.4, 116.5, 112.6, 114.9, 118.5 (2C), 131.8, 145.5, 150.4, 152.3, 156.9, 159.8, 160.9 (2C), 175.5. Anal. Calcd for C18H14N2O6S (386.38): C, 55.96; H, 3.65; N, 7.25 %. Found: C, 55.88; H, 3.56; N, 7.11 %.
3.5.7. Synthesis of 9-(5-((2-(2-hydroxyethoxy)ethyl)thio)-1,3,4-oxadiazol-2-yl)-4-methyl-2H,10H-pyrano [2,3-f]chromene-2,10-dione (10)
A solution of 8 (3.28 g, 0.01 mol) in abs. ethanol (15 mL) was mixed with NaOH (0.4 g, 0.01 mol), and the mixture was agitated for an hour at 25 °C. The mixture was refluxed for 30 h after 1.24 g (0.01 mol) of 2-(2-Chloroethoxy)ethanol was added. The solvent was removed under reduced pressure. Ethanol was used to recrystallize the resultant solid.
Yield, 94 %; m.p. = 102–104 °C, IR (KBr): νmax, cm−1: 3400 (OH, NH), 1639 (C=O), 1588 (C=N), 1484 (C-O-C); 1H NMR (DMSO-d6): δ = 2.35 (s, 3H, CH3), 3.60 (t, 2H, S-CH2), 3.71 (t, 2H, O-CH2), 3.88 (t, 2H, CH2-OH), 3.99 (t, 2H, O-CH2), 5.10 (s, 1H, OH), 6.40 (s, 1H, Ar-H), 7.24 (d, 1H, J = 19 Hz, Ar-H), 7.59 (d, 1H, J = 19 Hz, Ar-H), 8.51 (s, 1H, Ar-H). MS (m/z, %): 416 (M+, 30). Anal. Calcd for C19H16N2O7S (416.40): C, 54.80; H, 3.87; N, 6.73 %. Found: C, 54.71; H, 3.74; N, 6.66 %.
3.5.8. Synthesis of 2-(2-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)ethoxy)ethyl acetate (11)
After adding 1.02 g (0.01 mol) of acetic anhydride to the 10.4 g (0.01 mol) solution in 7 ml of pyridine, the reaction mixture was stirred for 16 h at 25 °C. After adding the resultant solution to the crushed ice, the mixture was filtered, washed with water, and allowed to dry.
Yield, 54 %; m.p. = 198–200 °C; IR (KBr): νmax, cm−1: 1735 (COCH3), 1633 (C=O), 1583 (C=N). 1H NMR (DMSO-d6): δ = 2.23 (s, 3H, CH3), 2.39 (s, 3H, CH3), 3.55 (t, 2H, S-CH2), 3.68 (t, 2H, O-CH2), 3.82 (t, 2H, CH2-OH), 3.92 (t, 2H, O-CH2), 6.11 (s, 1H, Ar-H), 7.30 (d, 1H, J = 19 Hz, Ar-H), 7.62 (d, 1H, J = 19 Hz, Ar-H), 8.26 (s, 1H, Ar-H); 13C NMR (DMSO-d6): δ = 19.1, 20.9, 37.4, 66.9, 68.9, 72.7, 110.5, 112.0, 114.0, 117.9 (2C), 131.0, 145.0, 150.0, 152.1, 156.3, 159.5, 160.4 (2C), 170.6, 174.9. Anal. Calcd for C21H18N2O8S (458.44): C, 55.02; H, 3.96; N, 6.11 %. Found: C, 55.00; H, 3.87; N, 6.02 %.
3.5.9. Synthesis of (2R,3R,5R,6R)-2-(acetoxymethyl)-6-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)tetrahydro-2H-pyran-3,4,5-triyl triacetate (13)
TEA (0.5 mL) was used to catalyze a solution of 8 (3.28 g, 0.01 mol) in DMF (15 mL), (TAGB) (12) (4.11 g, 0.01 mol) was added. The reaction mixture was agitated at 25 °C for 30 h. The mixture was filtered off, cleaned with water, and let to dry after being added to the crushed ice.
Yield, 63 %; m.p. = 222–224 °C; IR (KBr): νmax, cm−1: 1751 (COCH3), 1635 (C=O), 1554 (C=N); 1H NMR (DMSO-d6): δ = 1.99 (s, 12H, 4COCH3), 2.38 (s, 3H, CH3), 4.25–4.41 (m, 2H, CH2), 4.80–5.60 (m, 5H, CH-sugar), 6.11 (s, 1H, Ar-H), 7.30 (d, 1H, Ar-H), 7.70 (d, 1H, Ar-H), 8.32 (s, 1H, Ar-H). Anal. Calcd for C29H26N2O14S (658.59): C, 52.89; H, 3.98; N, 4.25 %. Found: C, 52.76; H, 3.83; N, 4.21 %.
3.5.10. Synthesis of 4-methyl-9-(5-(((2R,3R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)thio)-1,3,4-oxadiazol-2-yl)-2H,10H-pyrano [2,3-f]chromene-2,10-dione (14)
A solution containing 13 (6.58 g, 0.01 mol) was obtained in a methanolic ammonia solution (30 mL methanol, 34 % ammonia, 50 mL) during a 10-h incubation period at 25 °C. The solvent evaporated under reduced pressure, resulting in a solid powder as the end product.
Yield, 92 %; m.p. = 228–230 °C; IR (KBr): νmax, cm−1: 3373 (OH), 1642 (C=O), 1589 (C=N); 1H NMR (DMSO-d6): δ = 2.40 (s, 3H, CH3), 3.59–3.85 (m, 6H, 4CH + CH2), 4.10–4.30 (m, 4H, 4OH), 5.00 (d, 1H, S-CH-O), 6.35 (s, 1H, C3-H coumarin), 7.22 (d, 1H, AB system, Ar-H), 7.92 (d, 1H, AB system, Ar-H), 8.57 (s, 1H, C2-H pyran); 13C NMR (DMSO-d6): δ = 19.3, 61.9, 70.6, 74.6, 80.1, 86.6, 92.9, 110.0, 111.6, 113.5, 117.4 (2C), 130.2, 144.2, 149.5, 151.5, 155.9, 158.5, 159.9 (2C), 174.1. Anal. Calcd for C21H18N2O10S (490.44): C, 51.43; H, 3.70; N, 5.71 %. Found: C, 51.36; H, 3.61; N, 5.59 %.
3.5.11. Synthesis of 9-(5-((4,6-dichloro-1,3,5-triazin-2-yl)thio)-1,3,4-oxadiazol-2-yl)-4-methyl-2H,10H-pyrano [2,3-f]chromene-2,10-dione (15)
At 0–5 °C, a stirred solution of cyanuric chloride (1.84 g, 0.01 mol) in D.M.F. (92 mL) was mixed with oxadiazole 8 (3.28 g, 0.01 mol) in D.M.F. (17 mL). The solution was neutralized by adding 10 % Na2CO3 solution. For 4 h, the stirring was maintained at 0–5 °C. Toluene: ethyl acetate (80:20) was used as the eluent in TLC, which was used to monitor the reaction's progression. The process was stopped, and the mixture produced was put into crushed ice. Following filtration, the product was washed with water and left to crystallize the ethanol, yielding 15.
Yield, 72 %; m.p. = 180–182 °C; IR (KBr): νmax, cm−1: 1640 (C=O), 1610 (C=N), 750 (C-Cl). 1H NMR (DMSO-d6): δ = 2.39 (s, 3H, CH3), 6.40 (s, 1H, Ar-H), 7.11 (d, 1H, J = 18 Hz, Ar-H), 7.70 (d, 1H, J = 18 Hz, Ar-H), 8.85 (s, 1H, Ar-H); 13C NMR (DMSO-d6): δ = 19.0, 111.7, 112.6, 115.0, 118.7 (2C), 131.9, 145.8, 150.5, 152.5, 157.0, 159.8, 161.2 (2C), 169.8 (2C), 175.7, 198.0. MS (m/z, %): 476 (M+, 60), 474 (M+ − 2, 28). Anal. Calcd for C18H7Cl2N5O5S (476.24): C, 45.40; H, 1.48; N, 14.71 %. Found: C, 45.33; H, 1.41; N, 14.66 %.
3.5.12. Synthesis of 9-(5-((4-chloro-6-morpholino-1,3,5-triazin-2-yl)thio)-1,3,4-oxadiazol-2-yl)-4-methyl-2H,10H-pyrano [2,3-f]chromene-2,10-dione (16)
Morpholine (0.87 mL, 0.01 mol) dissolved in D.M.F. (5 mL) was gradually added to a well-stirred solution of 15 (4.76 g, 0.01 mol) in D.M.F. (10 mL) while keeping the temperature at 35 °C. The pH was balanced by adding 10 % sodium carbonate solution. The temperature was gradually increased to 45 °C in 2 h. The reaction was monitored using TLC with benzene:acetone (95:5) as the eluent. The mixture was poured into crushed ice, to produce solid needles, the substance was filtered, washed with water, and allowed to crystallize from the ethanol.
Yield, 68 %; m.p. = 184–186 °C; IR (KBr): νmax, cm−1: 1645 (C=O), 1615 (C=N), 760 (C-Cl). 1H NMR (DMSO-d6): δ = 2.45 (s, 3H, CH3), 3.48 (t, 4H, 2CH2), 3.61 (t, 4H, 2CH2), 6.39 (s, 1H, Ar-H), 7.25 (d, 1H, J = 18 Hz, Ar-H), 7.61 (d, 1H, J = 18 Hz, Ar-H), 8.51 (s, 1H, Ar-H). MS (m/z, %): 526 (M+, 58), 524 (M+ − 2, 31). Anal. Calcd for C22H15ClN6O6S (526.91): C, 50.15; H, 2.87; N, 15.95 %. Found: C, 50.01; H, 2.73; N, 15.88 %.
3.5.13. Synthesis of compounds (17a-e and 18a-e)
A solution of 16 (5.26 g, 0.01 mol) and appropriate phenyl urea and/or phenyl thiourea derivatives (0.01 mol) in DMF (18 mL) was refluxed for 3 h using a water bath set at 80–90 °C. The reaction was monitored by TLC using toluene: ethylacetate (80:20) as eluent. The mixture was then poured into crushed ice once the procedure was stopped. The result was separated from the ethanol by filtering, washing with water, and recrystallizing.
3.5.14. Synthesis of 1-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)-3-phenylurea (17a)
Yield, 71 %; m.p. 185–187 °C; IR (KBr): νmax, cm−1: 3150 (NH), 1685 (CO, amidic), 1645 (CO), 1610 (C=N), 1590 (C=C), 1040 (C-O-C); 1H NMR (DMSO-d6): δ = 2.25 (s, 3H, CH3), 3.74 (t, 4H, 2CH2), 3.86 (t, 4H, 2CH2), 6.15 (s, 1H, Ar-H), 7.10–7.50 (m, 7H, Ar-H), 8.41 (s, 1H, Ar-H), 9.35 (s, 1H, NH), 10.20 (s, 1H, NH); 13C NMR (DMSO-d6): δ = 20.1, 49.0 (2C), 66.5 (2C), 109.8, 110.5, 114.9, 118.1 (2C), 121.2 (2C), 128.5 (3C), 130.4, 139.0, 145.1, 148.0, 151.0 (2C), 156.0, 159.8, 168.0 (2C), 171.0, 175.5 (2C), 197.0. Anal. Calcd for C29H22N8O7S (626.60): C, 55.59; H, 3.54; N, 17.88 %. Found: C, 55.50; H, 3.48; N, 17.80 %.
3.5.15. Synthesis of 1-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)-3-(p-tolyl)urea (17b)
Yield, 76 %; m.p. 210–212 °C; IR (KBr): νmax, cm−1: 3155 (NH), 1680 (CO, amidic), 1650 (CO), 1615 (C=N), 1585 (C=C), 1045 (C-O-C); 1H NMR (DMSO-d6): δ = 2.15 (s, 3H, CH3), 2.33 (s, 3H, CH3), 3.68 (t, 4H, 2CH2), 3.80 (t, 4H, 2CH2), 6.25 (s, 1H, Ar-H), 7.05 (d, 1H, J = 18 Hz, Ar-H), 7.25 (d, 2H, J = 19 Hz, Ar-H), 7.45 (d, 2H, J = 18 Hz, Ar-H), 7.77 (d, 1H, J = 19 Hz, Ar-H), 8.55 (s, 1H, Ar-H), 9.45 (s, 1H, NH), 10.31 (s, 1H, NH). Anal. Calcd for C30H24N8O7S (640.63): C, 56.25; H, 3.78; N, 17.49 %. Found: C, 56.16; H, 3.69; N, 17.40 %.
3.5.16. Synthesis of 1-(2-chlorophenyl)-3-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)urea (17c)
Yield, 69 %; m.p. 215–217 °C; IR (KBr): νmax, cm−1: 3160 (NH), 1675 (CO, amidic), 1660 (CO), 1620 (C=N), 1575 (C=C), 1035 (C-O-C), 785 (C-Cl); 1H NMR (DMSO-d6): δ = 2.41 (s, 3H, CH3), 3.74 (t, 4H, 2CH2), 3.86 (t, 4H, 2CH2), 6.27 (s, 1H, Ar-H), 7.05–7.48 (m, 6H, Ar-H), 8.51 (s, 1H, Ar-H), 9.32 (s, 1H, NH), 10.20 (s, 1H, NH). Anal. Calcd for C29H21ClN8O7S (661.05): C, 52.69; H, 3.20; N, 16.95 %. Found: C, 52.61; H, 3.15; N, 16.88 %.
3.5.17. Synthesis of 1-(4-chlorophenyl)-3-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f] chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)urea (17d)
Yield, 70 %; m.p. 185–187 °C; IR (KBr): νmax, cm−1: 3160 (NH), 1675 (CO, amidic), 1660 (CO), 1620 (C=N), 1575 (C=C), 1035 (C-O-C), 785 (C-Cl); 1H NMR (DMSO-d6): δ = 2.40 (s, 3H, CH3), 3.55 (t, 4H, 2CH2), 3.70 (t, 4H, 2CH2), 6.45 (s, 1H, Ar-H), 7.30 (d, 1H, J = 18 Hz, Ar-H), 7.50 (d, 2H, J = 19 Hz, Ar-H), 7.70 (d, 2H, J = 18 Hz, Ar-H), 7.85 (d, 1H, J = 19 Hz, Ar-H), 8.50 (s, 1H, Ar-H), 9.25 (s, 1H, NH), 10.11 (s, 1H, NH). Anal. Calcd for C29H21ClN8O7S (661.05): C, 52.69; H, 3.20; N, 16.95 %. Found: C, 52.59; H, 3.13; N, 16.90 %.
3.5.18. Synthesis of 1-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)-3-(4-nitrophenyl)urea (17e)
Yield, 65 %; m.p. 190–192 °C; IR (KBr): νmax, cm−1: 3145 (NH), 1675 (CO, amidic), 1645 (CO), 1600 (C=N), 1595 (C=C), 1530 (sym. NO2), 1350 (asym. NO2), 1045 (C-O-C); 1H NMR (DMSO-d6): δ = 2.41 (s, 3H, CH3), 3.74 (t, 4H, 2CH2), 3.86 (t, 4H, 2CH2), 6.27 (s, 1H, Ar-H), 7.05 (d, 1H, J = 18 Hz, Ar-H), 7.17 (d, 2H, J = 19 Hz, Ar-H), 7.84 (d, 3H, Ar-H), 8.56 (s, 1H, Ar-H), 9.32 (s, 1H, NH), 10.20 (s, 1H, NH). Anal. Calcd for C29H21N9O9S (671.60): C, 51.86; H, 3.15; N, 18.77 %. Found: C, 51.77; H, 3.07; N, 18.70 %.
3.5.19. Synthesis of 1-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)-3-phenylthiourea (18a)
Yield, 65 %; m.p. 160–162 °C; IR (KBr): νmax, cm−1: 3160 (NH), 1650 (CO), 1615 (C=N), 1595 (C=C), 1350 (C=S), 1051 (C-O-C); 1H NMR (DMSO-d6): δ = 2.45 (s, 3H, CH3), 3.70 (t, 4H, 2CH2), 3.80 (t, 4H, 2CH2), 6.30 (s, 1H, Ar-H), 7.00–7.41 (m, 7H, Ar-H), 8.59 (s, 1H, Ar-H), 11.00 (s, 1H, NH), 13.50 (s, 1H, NH). Anal. Calcd for C29H22N8O6S2 (642.67): C, 54.20; H, 3.45; N, 17.44 %. Found: C, 54.11; H, 3.40; N, 17.33 %.
3.5.20. Synthesis of 1-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)-3-(p-tolyl)thiourea (18b)
Yield, 68 %; m.p. 155–157 °C; IR (KBr): νmax, cm−1: 3145 (NH), 1645 (CO), 1610 (C=N), 1580 (C=C), 1355 (C=S), 1040 (C-O-C); 1H NMR (DMSO-d6): δ = 2.35 (s, 3H, CH3), 2.45 (s, 3H, CH3), 3.70 (t, 4H, 2CH2), 3.80 (t, 4H, 2CH2), 6.20 (s, 1H, Ar-H), 7.10 (d, 1H, J = 19 Hz Ar-H), 7.42 (s, 4H, Ar-H), 7.80 (d, 1H, J = 19 Hz, Ar-H), 8.60 (s, 1H, Ar-H), 11.36 (s, 1H, NH), 13.35 (s, 1H, NH); 13C NMR (DMSO-d6): δ = 18.0, 21.9, 49.5 (2C), 67.0 (2C), 110.1, 111.3, 114.4, 118.6 (2C), 126.0 (2C), 129.0 (2C), 130.8, 135.0, 137.0, 145.6, 148.2, 151.5, 156.2, 160.0, 162.2 (2C), 171.8, 175.9 (2C), 180.0, 196.9. Anal. Calcd for C30H24N8O6S2 (656.69): C, 54.87; H, 3.68; N, 17.06 %. Found: C, 54.79; H, 3.59; N, 16.98 %.
3.5.21. Synthesis of 1-(2-chlorophenyl)-3-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)thiourea (18c)
Yield, 70 %; m.p. 205–207 °C; IR (KBr): νmax, cm−1: 3160 (NH), 1660 (CO), 1620 (C=N), 1575 (C=C), 1350 (C=S), 1035 (C-O-C), 780 (C-Cl); 1H NMR (DMSO-d6): δ = 2.37 (s, 3H, CH3), 3.57 (t, 4H, 2CH2), 3.70 (t, 4H, 2CH2), 6.37 (s, 1H, Ar-H), 7.25–7.69 (m, 6H, Ar-H), 8.66 (s, 1H, Ar-H), 13.46 (s, 1H, NH), 14.30 (s, 1H, NH). Anal. Calcd for C29H21ClN8O6S2 (677.11): C, 51.44; H, 3.13; N, 16.55 %. Found: C, 51.39; H, 3.05; N, 16.49 %.
3.5.22. Synthesis of 1-(4-chlorophenyl)-3-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)thiourea (18d)
Yield, 68 %; m.p. 161–163 °C; IR (KBr): νmax, cm−1: 3155 (NH), 1641 (CO), 1615 (C=N), 1570 (C=C), 1335 (C=S), 1035 (C-O-C), 780 (C-Cl); 1H NMR (DMSO-d6): δ = 2.39 (s, 3H, CH3), 3.75 (t, 4H, 2CH2), 3.88 (t, 4H, 2CH2), 6.31 (s, 1H, Ar-H), 7.20 (d, 1H, J = 18 Hz, Ar-H), 7.40 (d, 2H, J = 19 Hz, Ar-H), 7.59 (d, 2H, J = 19 Hz, Ar-H), 7.73 (d, 1H, J = 18 Hz, Ar-H), 8.40 (s, 1H, Ar-H), 11.55 (s, 1H, NH), 13.21 (s, 1H, NH). Anal. Calcd for C29H21ClN8O6S2 (677.11): C, 51.44; H, 3.13; N, 16.55 %. Found: C, 51.37; H, 3.09; N, 16.50 %.
3.5.23. Synthesis of 1-(4-((5-(4-methyl-2,10-dioxo-2H,10H-pyrano [2,3-f]chromen-9-yl)-1,3,4-oxadiazol-2-yl)thio)-6-morpholino-1,3,5-triazin-2-yl)-3-(4-nitrophenyl)thiourea (18e)
Yield, 76 %; m.p. 131–193 °C; IR (KBr): νmax, cm−1: 3145 (NH), 1645 (CO), 1600 (C=N), 1595 (C=C), 1530 (sym. NO2), 1350 (asym. NO2), 1333 (C=S), 1045 (C-O-C); 1H NMR (DMSO-d6): δ = 2.45 (s, 3H, CH3), 3.50 (t, 4H, 2CH2), 3.60 (t, 4H, 2CH2), 6.35 (s, 1H, Ar-H), 7.15 (d, 1H, J = 18 Hz, Ar-H), 7.75 (d, 2H, J = 19 Hz, Ar-H), 7.90 (d, 1H, J = 18 Hz, Ar-H), 8.07 (d, 2H, J = 19 Hz, Ar-H), 8.65 (s, 1H, Ar-H), 11.95 (s, 1H, NH), 13.43 (s, 1H, NH). Anal. Calcd for C29H21N9O8S2 (687.66): C, 50.65; H, 3.08; N, 18.33 %. Found: C, 50.58; H, 3.00; N, 18.27 %.
3.6. Biological activity
It was carried out in accordance with the protocols documented in earlier research [53].
3.7. Molecular docking
Using PyRx tools Autodock Vina (version 1.1.2), a molecular docking study of the compounds under inquiry was carried out in conjunction with: a: the crystal structure of the transpeptidase enzyme, PDB: 5WT8 of S. aureus [54]. The transpeptidase enzyme's crystal structure, PDB: 5WT8, was obtained from the protein data bank at https://www.rcsb.org. Using the VEGA ZZ 2.3.2 tool, the native ligand and water molecules were extracted from the proteins. Polar hydrogen and Kollman charges were then added, and Autodock Vina tools were used to convert the proteins into PDBQT format. Every designed chemical is recorded as a mol file, which Open Babel software uses to protonate, minimize, and convert to a pdb file. In order to specify the number of torsions and to construct a pdbqt file, the generated pdb file was uploaded to Autodock Vina tools. The grid map was made using AutoGrid and a grid box. Each chemical produced a certain number of docked positions, and these were graded based on the binding energy. The most complicated and fitting pose for the receptor under study was determined to have the lowest binding energy and a 0 root-mean-square deviation (RMSD). With BIOVIA Discovery Studio 2021, the molecular interactions and binding mechanisms of the top postures were graphically analyzed.
4. Conclusion
We present here a series of (1,3,4-oxadiazol-2-yl)pyrano [2,3-f]chromene, containing galactose, mannose, and xylose hydrazones that were synthesized via hydrazides reaction with monosaccharides, trying to find new candidates with better antibacterial properties. Acetic anhydride-induced cyclization of sugar hydrazones provided derivatives of substituted oxadiazolines. Additionally, 17a–e and 18a–e were synthesized using 1,3,4-oxadiazoly thiomorpholinophenyl ureido and/or (phenyl thioureido)-s-triazine. Selected Gram (+ve) (B. subtilis, S. aureus) and Gram (-ve) bacteria (E. coli, P. aeruginosa) were used in addition to a yeast-like fungi (C. albicans) to test the newly synthesized compounds' antimicrobial activities. Compounds 11, 13, 15, 16, 17c-e, and 18a-e showed the highest antibacterioal activity. Furthermore, a molecular docking study has been studied to assess the binding behavior of the active compounds with the target crystal structure of transpeptidases (a bacterial enzyme that cross-links the peptidoglycan chains to form rigid cell walls)
Ethical approval
Not applicable.
Consent to participate
All authors participated directly in the current research work.
Consent to publish
The authors agree to publish the article under the Creative Commons Attribution License.
Availability of data and materials
All relevant data are within the manuscript and available from the corresponding author upon request.
CRediT authorship contribution statement
Kahdr Alatawi: Writing – original draft, Visualization, Resources, Methodology. Ahmad Fawzi Qarah: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation. Haifa Alharbi: Writing – review & editing, Writing – original draft, Software, Methodology, Formal analysis. Ali Alisaac: Writing – review & editing, Writing – original draft, Software, Resources, Formal analysis. Matokah M. Abualnaja: Writing – original draft, Resources, Methodology, Formal analysis. Roba M.S. Attar: Writing – review & editing, Validation, Methodology, Formal analysis, Data curation. Amerah Alsoliemy: Writing – original draft, Visualization, Software, Methodology, Formal analysis. Nashwa M. El-Metwaly: Writing – review & editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38294.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Wang P.Y., Shao W.B., Xue H.T., Fang H.S., Zhou J., Wu Z.B., Song B.A., Yang S. Res. Chem. Intermed. 2017;43:6115–6130. [Google Scholar]
- 2.Kaur M., Singh S., Kaur M. Eur. J. Pharm. Med. Res. 2018;5:277–282. [Google Scholar]
- 3.Ahsan M.J. Tur. J. Chem. 2018;42:1334–1343. [Google Scholar]
- 4.Pratap R., Ram V.J. Chem. Rev. 2014;114:10476–10526. doi: 10.1021/cr500075s. [DOI] [PubMed] [Google Scholar]
- 5.Kang Y., Mei Y., Du Y., Jin Z. Org. Lett. 2003;5:4481–4484. doi: 10.1021/ol030109m. [DOI] [PubMed] [Google Scholar]
- 6.Puppala M., Zhao X., Casemore D., Zhou B., Aridoss G., Narayanapillai S., Xing C. Bioorg. Med. Chem. 2016;24:1292–1297. doi: 10.1016/j.bmc.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil S.A., Patil R., Pfeffer L.M., Miller D.D. Future Med. Chem. 2013;5:1647–1660. doi: 10.4155/fmc.13.126. [DOI] [PubMed] [Google Scholar]
- 8.Shah N.K., Shah N.M., Patel M.P., Patel R.G. Chem. Sci. J. 2013;125:525–530. [Google Scholar]
- 9.Sangani C.B., Shah N.M., Patel M.P., Patel R.G. Med. Chem. Res. 2013;22:3831–3842. [Google Scholar]
- 10.Jardosh H.H., Patel M.P. Med. Chem. Res. 2013;22:2954–2963. [Google Scholar]
- 11.Fridén-Saxin M., Seifert T., Landergren M.R., Suuronen T., Lahtela-Kakkonen M., Jarho E.M., Luthman K. J. Med. Chem. 2012;55:7104–7113. doi: 10.1021/jm3005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar M.S.L., Singh J., Manna S.K., Maji S., Konwar R., Panda G. Bioorg. Med. Chem. Lett. 2018;28:778–782. doi: 10.1016/j.bmcl.2017.12.065. [DOI] [PubMed] [Google Scholar]
- 13.Madhu G., Sudhakar M., Kumar K.S., Reddy G.R., Sravani A., Ramakrishna K., Rao C.P. Russ. J. Gen. Chem. 2017;87:2421–2428. [Google Scholar]
- 14.Dintzner M.R., Wucka P.R., Lyons T.W. J. Chem. Educ. 2006;83:270–272. [Google Scholar]
- 15.Balalaie S., Ashouriha M., Rominger F., Bijanzadeh H.R. Mol. Divers. 2013;17:55–61. doi: 10.1007/s11030-013-9423-4. [DOI] [PubMed] [Google Scholar]
- 16.Patel D.M., Vala R.M., Sharma M.G., Rajani D.P., Patel H.M. ChemistrySelect. 2019;4:1031–1041. [Google Scholar]
- 17.Patel D.M., Sharma M.G., Vala R.M., Lagunes I., Puerta A., Padrón J.M., Rajani D.P., Patel H.M. Bioorg. Chem. 2019;86:137–150. doi: 10.1016/j.bioorg.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Patel H.M., Rajani D.P., Sharma M.G., Bhatt H.G. Lett. Drug Des. Discov. 2019;16:119–126. [Google Scholar]
- 19.Dinparast L., Hemmati S., Zengin G., Alizadeh A.A., Bahadori M.B., Kafil H.S., Dastmalchi S. Rapid. ChemistrySelect. 2019;4:9211–9215. [Google Scholar]
- 20.Salahuddin M.A., Yar M.S., Mazumder R., Chakraborthy G.S., Ahsan M.J., Rahman M.U. Synth. Commun. 2017;47:1805–1847. [Google Scholar]
- 21.Patel K.D., Prajapati S.M., Panchal S.N., Patel H.D. Synth. Commun. 2014;44:1859–1875. [Google Scholar]
- 22.Srinivas M., Satyaveni S., Ram B. J. Pharm. Res. 2018;12:758–763. [Google Scholar]
- 23.Ahsan M.J., Bhandari L., Makkar S., Singh R., Hassan M.Z., Geesi M.H., Bakht M.A., Jadav S.S., Balaraju T., Riadi Y., Rani S., Khalilullah H., Gorantla V., Hussain A. Lett. Drug. Des. Dis. 2020;17:145–154. [Google Scholar]
- 24.Ahsan M.J., Choupra A., Sharma R.K., Jadav S.S., Padmaja P., Hassan M., Al-Tamimi A., Geesi M.H., Bakht M.A. Anti Cancer Agents Med. Chem. 2018;18:121–138. doi: 10.2174/1871520617666170419124702. [DOI] [PubMed] [Google Scholar]
- 25.Ahsan M.J., Meena R., Dubey S., Khan V., Manda S., Jadav S.S., Sharma P., Geesi M.H., Hassan M.Z., Bakht M.A., Riadi Y. Med. Chem. Res. 2018;27:864–883. [Google Scholar]
- 26.Ahsan M.J., Yadav R.P., Saini S., Hassan M., Bakht M.A., Jadav S.S., Al-Tamimi S., Bin A., Geesi M.H., Ansari M.Y., Khalilullah H. Lett. Org. Chem. 2018;15:49–56. [Google Scholar]
- 27.Ahsan M.J., Hassan M., Jadav S.S., Geesi M.H., Bakht M.A., Riadi Y., Akhtar M., Mallick M.N., Akhter M. Lett. Org. Chem. 2020;17:133–140. [Google Scholar]
- 28.Yatam S., Jadav S.S., Gundla R., Gundla K.P., Reddy G.M., Ahsan M.J., Chimakurthy J. ChemSelect. 2018;3:10305–10310. [Google Scholar]
- 29.Rathore A., Sudhakar R., Ahsan M.J., Ali A., Subbarao N., Jadav S.S., Umar S., Yar M.S. Bioorg. Chem. 2017;70:107–117. doi: 10.1016/j.bioorg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Manjunatha K., Poojary B., Lobo P.L., Fernandes J., Kumari N.S. Eur. J. Med. Chem. 2010;45:5225–5233. doi: 10.1016/j.ejmech.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Iyer V.B., Gurupadayya B., Koganti V.S., Inturi B., Shivanna Chandan R. Med. Chem. Res. 2017;26:190–204. [Google Scholar]
- 32.Singh R., Chauhan A., Adv Int J. Biol. Res. 2013;3:140–149. [Google Scholar]
- 33.Sudeesh K., Gururaja R. Org. Chem. Curr. Res. 2017;6:2–5. [Google Scholar]
- 34.Nimavat B., Mohan S., Saravanan J., Deka S., Talukdar A., Sahariah B.J., Dey B.K., Sharma R.K. Int. J. Res. Pharm. Chem. 2012;2:594–602. [Google Scholar]
- 35.Rigo A., Couturier D., Hetero J. Chem. 2011;22:925–930. [Google Scholar]
- 36.Yamada N., Kataoka Y., Nagami T., Hong S., Kawal S., Kuwano E. J. Pestic. Sci. 2004;29:205–208. [Google Scholar]
- 37.Katayoun A., Jessen N.M., Wang J.Y., Maliartchouk S., Kasibhatla S. Mol. Cancer. Ther. 2005;4:761–771. doi: 10.1158/1535-7163.MCT-04-0333. [DOI] [PubMed] [Google Scholar]
- 38.Maria G.M., Daniele Z., Luciano V., Maurizio F., Marco F., Pricl S., Scialino G., Banfi E. Bioorg. Med. Chem. 2005;13:3797–3809. doi: 10.1016/j.bmc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Guang F., Yang Z., Ming L., Xiang H.Q. Chin. Chem. Lett. 2001;12:877–880. [Google Scholar]
- 40.Abumelha H.M., Alqahtani A.M., Alharbi H., Alalawy A.I., Attar R.M., Abualnaja M.M., El-Metwaly N.M. J. Saudi Chem. Soc. 2024;101884 [Google Scholar]
- 41.Abdula A.M., Qarah A.F., Alatawi K., Qurban J., Abualnaja M.M., Katuah H.A., El-Metwaly N.M. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Von Pechmann H., Duisberg C. Dtsch. Chem. Ges. 1883;16:2119–2128. [Google Scholar]
- 43.Bondock S., Ramy R., Etman H.A., Fadda A.A. Eur. J. Med. Chem. 2008;43:2122–2129. doi: 10.1016/j.ejmech.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Abuo-Melha H., Fadda A.A. Spectrochim. Acta Mol. Biomol. Spectrosc. 2012;89:123–128. doi: 10.1016/j.saa.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 45.Vardanyan R.S., Hruby V.J. vol. 32. Elsevier; Amsterdam: 2006. pp. 425–498. (Antibiotics in Synthesis Of Essential Drugs). ISBN 978-0-444-52166-8. [Google Scholar]
- 46.a) X. Sáez-Llorens, G.H. McCracken, Chapter 37 - clinical pharmacology of antibacterial agents. In Infectious Diseases of the Fetus and Newborn Infant (Sixth Edition);.; b) Remington J.S., Klein J.O., Wilson C.B., Baker C.J., editors. W.B. Saunders, Philadelphia. 2006. pp. 1223–1267. ISBN 978-0-7216-0537-1. [Google Scholar]
- 47.Chen W., Zhang Y.-M., Davies C. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.01651-16. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauvage E., Kerff F., Terrak M., Ayala J.A., Charlier P. FEMS Microbio. Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 49.Cochrane S.A., Lohans C.T. Eur. J. Med. Chem. 2020;194 doi: 10.1016/j.ejmech.2020.112262. [DOI] [PubMed] [Google Scholar]
- 50.Kassab R.M., Zaki M.E.A., Abo Dena A.S., Al-Hussain S.A., Abdel-Aziz M.M., Muhammad Z.A. Future Med. Chem. 2022;14:1881–1897. doi: 10.4155/fmc-2022-0196. [DOI] [PubMed] [Google Scholar]
- 51.Khanna A., Dubey P., Sagar R. Curr. Org. Chem. 2021;25:2378–2456. [Google Scholar]
- 52.Duskaev G., Rakhmatullin S., Kvan O. Vet. World. 2020;13:2484–2492. doi: 10.14202/vetworld.2020.2484-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Refat H.M., Fadda A.A. Eur. J. Med. Chem. 2013;70:419–426. doi: 10.1016/j.ejmech.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Dallakyan S., Olson A.J. Methods Mol. Biol. 2015;1263:243–250. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and available from the corresponding author upon request.