Abstract
The study investigates the impact of steam explosion pretreatment on the distribution of free and combined phytosterols within rapeseed and its derived products. Utilizing solid phase extraction-gas chromatography (SPE-GC) analysis, we elucidated the composition and distribution of phytosterols in five rapeseed varieties and their corresponding processed oils and cakes. The results indicated that Zhongyou 516 and Xiwang 988 are richer in combined phytosterols, whereas Dadi 199, Zhongyouza 501, and Xiwang 291 have a greater concentration of free phytosterols. Steam explosion pretreatment significantly increased the extraction proportion of combined phytosterols in rapeseeds. Throughout the oil process, more than half of the total phytosterol content, specifically 57.0%, was transferred from the steam explosion-treated rapeseed into the rapeseed oil. The variety Xiwang 291 showed the highest efficiency in this transfer, achieving a rate of 61.7%. The study provides crucial data for the enhancement of rapeseed processing techniques and the efficient utilization of phytosterols. Moreover, the study highlights the potential use of the ratio of free to combined phytosterols as a discriminator for different rapeseed oil varieties, offering valuable insights for quality assurance and product differentiation in the industry.
Keywords: Steam explosion pretreatment, Phytosterol, SPE-GC analysis, Rapeseed oil processing, Dynamic change
Graphical abstract
Highlights
-
•
Determination of free and combined phytosterols by SPE-GC.
-
•
Steam explosion increased the extraction proportion of phytosterols in rapeseed.
-
•
More than 57% of phytosterols transfers to rapeseed oil after steam explosion.
-
•
Dadi 199 rapeseed has the highest total phytosterol content after steam explosion.
-
•
Free/combined phytosterols have the potential to serve as markers for identifying rapeseed oil varieties.
1. Introduction
Rapeseed (Brassica napus L.) is a traditional crop that holds great significance in agricultural development (Chew, 2020; Vigneron et al., 2006). Phytosterols are characteristic compounds found exclusively in plants, which have physiological activities such as lowering cholesterol, anticancer, and antioxidant effect (Amiot et al., 2011; Ash et al., 2011; Feng et al., 2022; Ferguson, Stojanovski, MacDonald-Wicks and Garg, 2016; Furlan et al., 2013; W. S. He et al., 2016; Kritchevsky and Chen, 2005; R. Yang et al., 2019). Rapeseed contains approximately 3000.0 mg/kg of phytosterols, making it an important source of dietary phytosterols. (Raboanatahiry et al., 2021; Szydlowska-Czerniak, 2013). Brassicasterol, campesterol, and β-sitosterol are the primary phytosterols found in rapeseed, accounting for more than 85% of the total phytosterols (M. Yang et al., 2013). During the oil extraction process, phytosterols from rapeseed are transferred into both the oil and the residual cake, respectively, thereby influencing the nutritional quality of the processed product (Bruhl and Matthaus, 2008; Ortiz et al., 2020; Zafar et al., 2019). The composition and distribution of phytosterols serve as vital indicators of the nutritional value in rapeseed oil and constitute a key feature for traceability of the oil's origin. These factors can be employed as a tool for verifying the authenticity of rapeseed oil and play a significant role in determining its source (Ramon Aparicio, 2000). However, the biological activity of phytosterols is closely related to their existing forms (D. Wang et al., 2023). For example, free phytosterols have limited practical effects in the human body due to their lower lipid solubility (W. S. He et al., 2018; Santos et al., 2019). Phytosterol ester exhibit high lipid-solubility and constitute the predominant form of phytosterol in phytosterol-fortified foods (Zheng et al., 2012). Therefore, studying the composition and content of phytosterols within rapeseed, alongside their fluctuations throughout the oil processing stages, is crucial for optimizing phytosterols utilization and for crafting high-value rapeseed oil (Can-Cauich et al., 2019; Liu et al., 2012).
It is widely recognized that the thermal pretreatment of rapeseed is a crucial step that enhances the extraction rate of lipid-soluble components, significantly impacting the flavor and quality of edible oil (Azadmard-Damirchi et al., 2010; McDowell et al., 2017; Zhou et al., 2013). This process offers two main advantages: it imparts a desirable roasted aroma to the oil and inactivates certain enzymes present in the rapeseed, including lipoxygenase and lipase (Rezkas et al., 2015). By diminishing the enzymatic activity, the detrimental effects on the quality of the oil are curtailed, leading to improved oxidative stability (McDowell et al., 2017; Siger et al., 2017). Additionally, thermal pretreatment disrupts the cell wall structure of the rapeseed, facilitating the pressing and extraction process. This enhanced extraction efficiency not only improves the yield of rapeseed oil but also contributes to the overall quality (Rekas et al., 2017; Y. J. Xu et al., 2020).
As an economical and efficient thermal pretreatment technology, steam explosion has seen widespread application across various fields in recent years. including processing agricultural by-products, producing animal feed, and preparing substances for food and pharmaceutical industries. (Wan et al., 2022; W. Wang et al., 2021). Steam explosion pretreatment primarily disrupts the plant cell wall and alters the structure of chemical substances within a high temperature and high pressure environment. It offers several advantages, such as the elimination of the need for additional chemicals, environmental friendliness, low cost, and ease of scalability (X. He et al., 2022; C. Li et al., 2023). Based on the above advantages, steam explosion pretreatment method has been used for the pretreatment of various materials to study its influence on the extraction effect of bioactive substances (Silveira et al., 2015). In a comprehensive evaluation by Yu and colleagues, the effectiveness of steam explosion was compared against conventional high-temperature roasting and microwave pretreatment methods. Their findings revealed that rapeseed oil processed via steam explosion exhibited superior quality attributes (Yu et al., 2020). Similarly, Seçmeler and colleagues demonstrated that steam explosion pretreatment significantly boosted the extraction efficiency of phytosterols, polyphenols, and other bioactive compounds from olive pomace (Secmeler et al., 2018). Further research by Zhang et al. applied steam explosion pretreatment to camellia seed extraction, highlighting its beneficial effects on extraction rate, physicochemical properties, and oxidation stability (Zhang et al., 2019). Wang and colleagues also provided evidence that this pretreatment method not only enhances extraction yield but also improves the antioxidant and α-glucosidase inhibitory effects of Java tea (J. D. Wang et al., 2023). In a word, the steam explosion pretreatment technology is a highly promising technique in the food industry.
The analysis of phytosterol content encompasses various methods such as thin-layer chromatography, enzyme assays, spectrophotometry, liquid chromatography, and gas chromatography (GC) (Garcia-Llatas et al., 2021; Yuan, 2022). Among these, GC is particularly favored for the separation and quantification of phytosterols due to its advantages, including short detection time, high sensitivity, selectivity, excellent separation efficiency, accurate results, and cost-effectiveness (Chen et al., 2015; Schlag et al., 2022; Tan et al., 2019). Currently, GC has been employed to reveal the free phytosterols in edible oils (B. C. Xu et al., 2020; Xu et al., 2014), the total phytosterols in vegetable oils (Schlag et al., 2022), the phytosterol oxidation products in edible oils (Hu et al., 2015), the total phytosterol in rice products (Islam et al., 2021). The primary focus in the field is on the analysis of total phytosterols in oil crops, with less emphasis on the individual components in different forms. Our research group has successfully developed a straightforward, expedient, and efficient SPE-GC analytical procedure specifically for assessing the levels of free and combined phytosterols in rapeseed (D. Li et al., 2022). This innovative method has laid a solid foundation for investigating the dynamics of free and combined phytosterols throughout various stages of oil processing.
Thermal pretreatment of the rapeseed has been shown to enhance the yield and nutritional value of the oil produced. At present, research into the impact of steam explosion pretreatment on the migration of phytosterols during rapeseed processing is still in its early stages. In this study, we initially subjected the rapeseed to steam expansion pretreatment, followed by the subsequent oil processing steps. We then investigated the impact of steam explosion on the phytosterol content in rapeseed and its derived products using SPE-GC analysis. Lastly, to understand the behavior of phytosterols during the oil production process, we conducted multiple statistical analysis of their migration patterns in the rapeseed.
2. Materials and methods
2.1. Materials and reagents
Five varieties of rapeseed, including Zhongyouza 501, Zhongyou 516, Xiwang 988 and Xiwang 291 and Dadi 199, were provided by the Oil Crops Research Institute of Chinese Academy of Agricultural Sciences (Wuhan, China). n-hexane, ethyl acetate, chloroform were chromatographic grade and purchased from Fisher company, London, UK. Anhydrous sodium sulfate, H2SO4 and NaCl were analytical grade and purchased from CNW (Shanghai, China). Silica SPE cartridge with 500 mg sorbent per cartridge (Sep-Pak) was purchased from Waters (Milford, CT, USA); β-cholestanol and methyl heptadecanoate was chromatographic grade and purchased from Shanghai Anpu Cui Shi Standard Technology Service Co., Ltd. (Shanghai, China). N,O-bis(trimethylsilyl)trifluoroacetamide (containing 1% trimethylchlorosilane) was analytical grade and purchased from ixiai (Shanghai) Chemical Industry Development Co., LTD (Shanghai, China).
2.2. Instruments
We utilized a variety of specialized equipment in our study: a multifunctional airflow explosion apparatus (XSS-QPD) from Wuhan Xin Shi Shang Food Machinery Co., Ltd.; an Agilent 6890 gas chromatograph equipped with a flame ionization detector (FID); an LTP-205 oil extraction press from Liangtai, Dongguan Xiangju Intelligent Co., Ltd.; an MTV-100 multi-tube vortex mixer by Hangzhou Aosheng Instrument Co., Ltd.; a DC-24 nitrogen evaporator from Shanghai Anpu Experiment Technology Co., Ltd.; a magnetic stirrer for constant temperature water baths (Model HH-6D) manufactured by Changzhou Zhongcheng Instrument Manufacturing Co., Ltd.; an electronic analytical balance (ML204) by Mettler Toledo Instruments (Shanghai) Co., Ltd.; a refrigerated centrifuge from Thermo Fisher Scientific, USA; an electric heating constant temperature oven (Model SHFG-01); and a YTLG-10A vacuum freeze dryer by Shanghai Yetuo Technology Co., Ltd.; XSS-QPD multifunctional air expander (Xinshishang Food Machinery Co., Ltd., Wuhan, China)
2.3. Methods and procedures
2.3.1. Steam explosion pretreatment and press oil of rapeseed (Yu et al., 2020)
Steam explosion pretreatment was conducted using an XSS-QPD multifunctional air expander (Xinshishang Food Machinery Co., Ltd., Wuhan, China). This apparatus primarily comprises an air expansion tank, a pressure gauge, a heating device, a rotating device, a safety protection device and a vibration damping device. The steam explosion was realized by the pressure release within a very short time (0.0875 s) after heating (B. Li et al., 2019). 400 g of each rapeseed variety (Zhongyouza 501, Zhongyou 516, Xiwang 988 and Xiwang 291 and Dadi 199) was adjusted to a moisture content of 10 % and treated with steam explosion at 1.0 MPa. A set of rapeseed that was not subjected to the steam explosion served as a baseline control group. Following the pretreatment, the moisture content of the rapeseed was subsequently modified to reach 6%. The rapeseed was then refrigerated at 4 °C for an additional 12 h period. Finally, the rapeseed was processed using an LTP-205 oil expeller to extract the oil and separate it from the rapeseed cake.
2.3.2. Extraction of phytosterols from rapeseed, rapeseed cake and rapeseed oil (Folch, 1957)
A 10 g sample of either rapeseed or rapeseed cake was subjected to freeze-drying under a vacuum at −60 °C for 24.0 h, after which it was ground into a fine powder. A 0.2 g portion of this powder was then combined with 2.2 mL methanol and 4.4 mL chloroform. Subsequently, 100 μL of β-cholestanol (1.0 mg/mL), serving as an internal standard, was introduced to the mixture. This blend was vortexed at 1800 rpm for 5.0 min, followed by ultrasonication at 30 °C for 1.0 h. An addition of 1.65 mL deionized water was made, and the mixture was again vortexed for 10 min before being centrifuged at 5000 rpm for 5.0 min to separate the organic layer. To the remaining aqueous layer, 2.0 mL of chloroform were added, and the extraction was performed twice more. The organic phases obtained were pooled, dried with nitrogen gas, and then brought up to volume with 5 mL of n-hexane.
For rapeseed oil sample, 100 μL of 1.0 mg/mL β-cholestanol was added to 50.0 mg rapeseed oil sample as an internal standard and diluted by 5.0 mL n-hexane.
2.3.3. Separation of free/combined phytosterols by SPE (Esche et al., 2012)
200.0 mg of anhydrous sodium sulfate were layered on top of the SPE column, which contains 500.0 mg of sorbent material per cartridge, followed by the activation with two aliquots of 5.0 mL each of n-hexane, after which the flow-through was discarded. Subsequently, the sample solution from step 2.3.2 was loaded onto the SPE column. The combined phytosterols were then eluted in two stages, first with two portions of 5.0 mL each of a n-hexane/ethyl acetate mixture in a 96.0:4.0 vol ratio, designated as fraction I; next, the free phytosterols were collected using the same volume of a 5.0:95.0 n-hexane/ethyl acetate mixture, known as fraction II. Nitrogen gas was utilized to evaporate the solvents from both fractions I and II.
2.3.4. Derivatization of free/combined phytosterols
-
(1)
Derivatization of combined phytosterols (Azadmard-Damirchi et al., 2010)
To fraction I, introduce 3.0 mL KOH/CH3CH2OH (2.0 mol/L) solution, then blend thoroughly with a vortex mixer. Proceed to saponify in a water bath set at 90 °C for 20.0 min. Subsequently, incorporate an additional 2.0 mL water and 1.5 mL n-hexane, followed by vigorous vortex mixing at 2500 rpm for 3 min to separate the organic phase. This extraction process is to be repeat three times, with the organic phases pooled together afterward. Utilize nitrogen to evaporate any residual solvent, then introduce 100 μL N,O-bis(trimethylsilyl)trifluoroacetamide (containing 1% trimethylchlorosilane) derivatization reagent. After vortex mixing, subject the mixture to an oven at 105 °C for 15.0 min to facilitate the derivatization reaction. Once completed, allow the mixture to cool down to ambient temperature and reconstituted by 100 μL n-hexane.
-
(2)
Derivatization of free phytosterols
Add 100 μL of N,O-bis(trimethylsilyl)trifluoroacetamide, which includes 1% trimethylchlorosilane, as a derivatization reagent to fraction II. Mix the solution thoroughly using a vortex mixer, then subject it to derivatization by placing it in an oven at 105 °C for a duration of 15.0 min. After the derivatization process, allow the mixture to cool down to room temperature before reconstituting it with 100 μL of n-hexane.
2.3.5. GC analysis of phytosterols
The derivatized phytosterols were examined using GC equipped with a DB-5HT capillary column. The injection parameters were as follows: a volume of 1.0 μL was injected; the injection port was set at 320 °C with a pressure of 9.9 psi and a split ratio of 25 : 1; helium was used as the carrier gas. For the column oven, the temperature profile started at 60 °C, held for 1.0 min, then ramped up at a rate of 10 °C per minute to reach 260 °C, where it was maintained for 14 min. The flame ionization detector (FID) settings included a heater temperature of 320 °C, with air, hydrogen, and helium flow rates set at 400.0 mL/min, 40.0 mL/min, and 30.0 mL/min, respectively.
2.3.6. Quantitative analysis of phytosterols is calculated as follows:
Ax represent the chromatographic peak area of the phytosterol; Ms denotes the mass of the internal standard; As signifies the chromatographic peak area of the internal standard; M is used to express the weight of the sample.
2.3.7. Analysis of oil content in rapeseed, residual oil content in rapeseed cake and oil yield in rapeseed oil processing (Cong et al., 2020; Y. Li et al., 2006)
-
(1)
Sample preparation method
Add 2.0 mL of 5% H2SO4/CH3OH solution and 300 μL of toluene to a 15.0 mg sample of rapeseed or rapeseed cake. Subsequently, introduce 50 μL of a 5.0 mg/mL methyl heptadecanoate standard solution as the internal standard. Then, proceed with saponification in a water bath at 95 °C for 1.5 h. After saponification, add 2.0 mL of a 0.9% NaCl aqueous solution and 1.0 mL of n-hexane. Mix the solution by vortexing at 1800 rpm for 5.0 min, followed by centrifugation at 5000 rpm for 5.0 min. Finally, collect the organic phase for subsequent GC analysis.
-
(2)
GC analysis of fatty acids
The determination of oil content in rapeseed and the residual oil content in rapeseed cake was conducted using GC equipped with a DB-Fast FAME capillary column. The injection parameters were as follows: a volume of 1.0 μL was injected; the injection port temperature was set at 250 °C with a pressure of 9.9 psi and a split ratio of 20:1, using helium as the carrier gas. For the column oven temperature program: it started at 80 °C for 0.5 min, then ramped up at 40 °C/min to 165 °C, followed by an increase at 4 °C/min to 230 °C, where it was held for 6.0 min. The FID settings included a heater temperature of 260 °C, with hydrogen, air, and helium flow rates of 40.0 mL/min, 350.0 mL/min, and 20.0 mL/min, respectively.
-
(3)
Oil content in rapeseed or residual oil content in rapeseed cake is calculated as follows:
S1 is the peak areas of fatty acids in the sample; S2 is the peak area of internal standard; N is the quality of internal standard; M is the quality of sample.
-
(4)
Oil yield in rapeseed oil processing is calculated as follows:
Ro is the oil content of rapeseed; Co is the residual oil content of rapeseed cake.
2.3.8. Data statistics and analysis
Excel 2016, Origin 2019 and SPSS-20 were used for data statistics and analysis. Phytosterols in rapeseed were subjected to PCA through loading plots, VIP scores plots, and heatmap for evaluating the results using MetaboAnalyst 5.0 platform (Pang et al., 2024). Among them, the structure of input data for PCA analysis includes sample name, compound name, and compound content.
3. Results and discussion
3.1. Effects of steam explosion pretreatment on the composition and content of phytosterols in rapeseed, rapeseed oil and rapeseed cake
3.1.1. Rapeseed
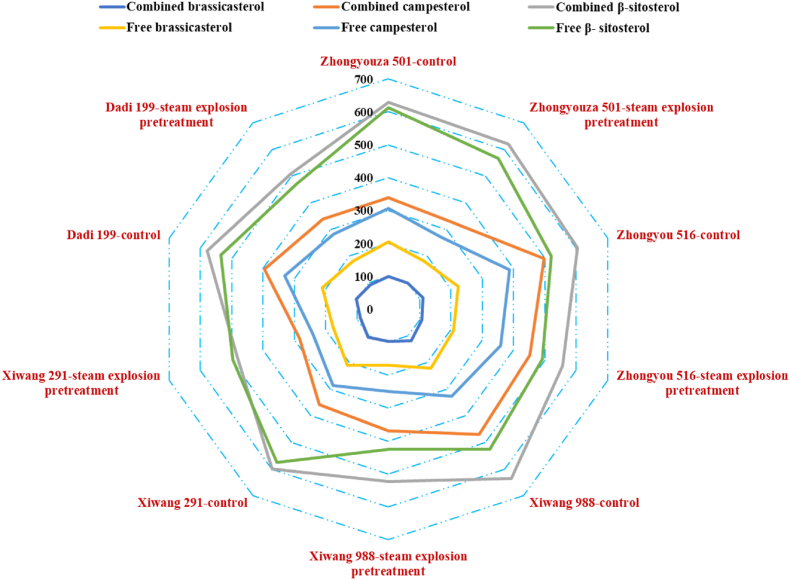
Among the five rapeseed varieties studied, β-sitosterol was found to have the highest content of free and combined phytosterols, with campesterol in second place, and brassicasterol being the lowest (as shown in Fig. 1 and Table S1). The total phytosterol content of untreated rapeseed varieties, ranked from highest to lowest, is as follows: Zhongyou 516 (3385.1 mg/kg), Dadi 199 (3157.3 mg/kg), Zhongyouza 501 (3154.2 mg/kg), Xiwang 988 (2941.9 mg/kg), and Xiwang 291 (2894.2 mg/kg).
Fig. 1.
Effects of steam explosion pretreatment on the composition and content of phytosterols in rapeseed (mg/kg). Values are means±standard deviations, n = 3 parallel determinations.
The total phytosterol content in rapeseed after steam explosion pretreatment is ranked as follows: Dadi 199 (3522.4 mg/kg, an increase of 365.1 mg/kg), Zhongyou 516 (3497.8 mg/kg, an increase of 112.7 mg/kg), Xiwang 291 (3492.5 mg/kg, an increase of 598.3 mg/kg), Zhongyouza 501 (3404.9 mg/kg, an increase of 250.8 mg/kg), and Xiwang 988 (3275.7 mg/kg, an increase of 333.8 mg/kg). Notably, Xiwang 291 consistently showed the highest total phytosterol content, irrespective of pretreatment. Specifically, for Zhongyouza 501, the total phytosterol content significantly increased from 3154.2 to 3404.9 mg/kg (P < 0.05), with both combined and free phytosterols showing significant increases. In contrast, for Zhongyou 516, the total phytosterol content only slightly increased without reaching statistical significance, but there was a notable increase in combined brassicasterol. For Xiwang 988, the total phytosterol content significantly rose, with no significant changes in combined and free phytosterols except for free campesterol. Xiwang 291 showed a significant increase in both total combined and free phytosterols, with all individual phytosterols showing significant increases. Dadi 199 also exhibited significant increases in both total combined and free phytosterols, with all individual phytosterols affected. The steam explosion pretreatment likely disrupted the microstructure and cell structure of the rapeseed, facilitating the extraction of chemical components and leading to an overall increase in phytosterol content across the varieties studied.
3.1.2. Rapeseed oil
The analysis of phytosterols compositions and contents in rapeseed oil from various rapeseed varieties after steam explosion pretreatment (Fig. 2 and Table S2). Initially, Xiwang 291 had the highest total phytosterol content at 6874.9 mg/kg, with other varieties following in descending order. Post-pretreatment, Xiwang 291 maintained the highest content at 7035.3 mg/kg, with other varieties showing varying increases, particularly Zhongyou 516, Xiwang 988, and Dadi 199. Specifically, Zhongyouza 501 and Zhongyou 516 oils exhibited significant increases in combined phytosterols, with Xiwang 988 showing notable increases in certain specific components. Xiwang 291 oil displayed significant enhancements across all phytosterol forms. In contrast, Dadi 199 experienced a slight increase in free phytosterols and a more pronounced rise in combined forms. The differential effects of steam explosion pretreatment on these varieties can be attributed to genetic and environmental factors. This pretreatment method not only varied in its impact but also improved the extraction efficiency of phytosterols, thereby benefiting the production of enriched rapeseed oil.
Fig. 2.
Effects of steam explosion pretreatment technology on the composition and content of phytosterols in rapeseed oil (mg/kg). Values are means±standard deviations, n = 3 parallel determinations.
3.1.3. Rapeseed cake
Subsequently, the study investigated the phytosterol levels in rapeseed cake from various varieties of rapeseed following steam explosion pretreatment (Fig. 3 and Table S3). Notably, the total combined phytosterols in Zhongyouza 501, Zhongyou 516, Xiwang 988, Xiwang 291, and Dadi 199 exhibited significant decreases (P < 0.01), with the most pronounced reduction observed in Xiwang 291. A similar pattern was evident for total free phytosterols, which also significantly declined across all varieties (P < 0.01). Additionally, the overall total phytosterol content showed a decrease, reaching statistical significance (P < 0.05) for Zhongyou 516, Xiwang 988, and Dadi 199. The reduction in phytosterol content in rapeseed cake is likely due to the pretreatment's disruption of the rapeseed's microstructure and cell integrity, facilitating easier extraction of chemical components into the rapeseed oil. These findings suggest that steam explosion pretreatment effectively concentrates phytosterols in the oil, while concurrently reducing their presence in the residual cake.
Fig. 3.
Effects of steam explosion pretreatment technology on the composition and content of phytosterols in rapeseed cake (mg/kg). Values are means±standard deviations, n = 3 parallel determinations.
3.2. Effect of steam explosion pretreatment on oil content in rapeseed, residual oil content in rapeseed cake and oil yield in rapeseed oil processing
As shown in Fig. 4 and Table S4, the oil extraction of four rapeseed varieties increased after steam explosion pretreatment (Zhongyouza 501 increased by 0.8%; Zhongyou 516 increased by 6.6%; Xiwang 988 increased by 5.3%; Xiwang 291 increased by 2.7%). After the steam explosion pretreatment, the oil yield of different rapeseed varieties also increased (Zhongyouza 501 increased by 3.9%; Zhongyou 516 increased by 18.2%; Xiwang 988 increased by 15.5%; Xiwang 291 increased by 8.1%; Dadi 199 increased by 5.7%). As for the residual oil rate of rapeseed cake after steam explosion pretreatment, the residual oil rate of various rapeseed cakes decreased (Zhongyouza 501 rapeseed cake decreased from 26.8% to 23.7%, reducing by 3.1%; Zhongyou 516 rapeseed cake decreased from 33.9% to 26.1%, reducing by 7.8%; Xiwang 988 rapeseed cake decreased from 28.8% to 20.7%, reducing by 8.1%; Xiwang 291 rapeseed cake decreased from 24.2% to 19.3%, reducing by 4.9%; Dadi 199 rapeseed cake decreased from 32.0% to 23.4%, reducing by 8.6%). These experimental results may be attributed to the steam explosion that occurs during the pressure release phase of the steam explosion pretreatment. This process leads to more extensive cell destruction, thereby facilitating the extraction of active ingredients, such as lipids, from the cells. Consequently, this improves the oil content of rapeseed and enhances the oil yield during processing, ultimately reducing the residual oil content in the rapeseed cake (Niu et al., 2015).
Fig. 4.
Analysis of the oil content in rapeseed, the residual oil content in rapeseed cake and the oil yield in oil processing with/without steam explosion pretreatment of rapeseed. Values are means±standard deviations, n = 3 parallel determinations. ∗means significant difference (P < 0.05), ∗∗indicates extremely significant difference (P < 0.01).
3.3. Effect of steam explosion pretreatment on migration of phytosterols during oil processing
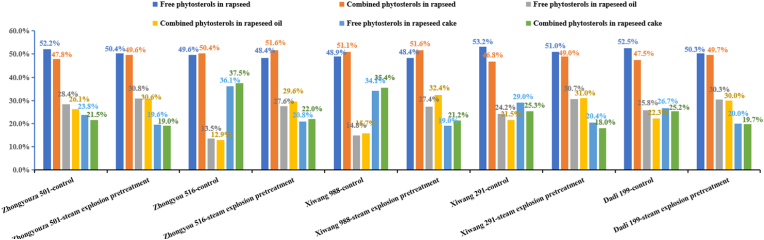
Fig. 5 and Table S5 present a comprehensive analysis of steam explosion pretreatment's impact on phytosterol migration in five rapeseed varieties. Pretreatment altered the distribution of free and combined phytosterols, generally increasing the proportion of combined forms. For instance, Zhongyouza 501 rapeseed saw a shift from 52.2% free phytosterols and 47.8% combined phytosterols to 50.4% and 49.6%, respectively. Across varieties, steam explosion pretreatment consistently drove more phytosterols into the oil, with Xiwang 291 showing the highest transfer rate to oil at 61.7%. Therefore, steam explosion pretreatment effectively releases phytosterols, enriches the oil's phytosterol profile, and is influenced by factors such as rapeseed variety and cultivation conditions, which in turn affect the final oil quality.
Fig. 5.
The dynamic changes of combined and free phytosterols in rapeseed during oil processing with/without steam explosion pretreatment of rapeseed. Values are means±standard deviations, n = 3 parallel determinations.
After investigating the impact of steam explosion pretreatment on the migration and transformation of phytosterols during rapeseed processing, we employed statistical analysis to visually characterize phytosterols in rapeseed oil, using the steam explosion pretreatment as the original variable. The results are shown in Fig. 6. According to the VIP scores, it is evident that both combined and free brassicasterol exhibit VIP values exceeding 1, indicating they are the two phytosterols with the most pronounced differences across the five rapeseed oil varieties. The PCA 2D Scores Plot reveals distinct differences in phytosterol content across these rapeseed oil varieties, suggesting that phytosterols could serve as a discriminative tool for variety identification. The heat map results indicate good sample repeatability and demonstrate that the five rapeseed oil varieties can be categorized into two distinct groups based on their phytosterol compositions. The phytosterol profiles of Zhongyouza 501 and Xiwang 291 rapeseed oils are relatively similar, while those of Xiwang 988, Zhongyou 516, and Dadi 199 rapeseed oils are also closely related. Consequently, the unique phytosterol distribution in oils from different rapeseed varieties provides a valuable foundation for their identification and differentiation.
Fig. 6.
The VIP scores, PCA 2D Scores Plot and Heatmap of rapeseed oil after steam explosion pretreatment. Values are means±standard deviations, n = 3 parallel determinations. C-brassicasterol: Combined brassicasterol; C-campesterol: Combined campesterol; C-sitosterol: Combined β-sitosterol; F-brassicasterol: Free brassicasterol; F-campesterol: Free campesterol; F-sitosterol: Free β-sitosterol; Total CPS: Total combined phytosterols; Total FPS: Total free phytosterols; Total PS: Total phytosterols.
4. Conclusions
In this work, we analyzed the distribution of both combined and free phytosterols across five rapeseed varieties following steam explosion pretreatment employing the SPE-GC technique. Our results showed a significant enhancement in phytosterol content in rapeseed oil post-pretreatment, with over 57.0% of the total phytosterols migrating to the oil. These findings elucidate the impact of steam explosion pretreatment on phytosterol composition and distribution in both rapeseed and its derived products, particularly during oil processing. Furthermore, we observed that the phytosterol distribution varies among different rapeseed varieties and is affected differently by the pretreatment process. Therefore, the distinct distribution patterns of phytosterols could serve as potential markers for identifying and differentiating between rapeseed varieties. This research provides significant insights for evaluating rapeseed processing technology and enhancing the value of its processed products.
CRediT authorship contribution statement
Dan Wang: Conceptualization, Methodology, Visualization, Investigation, Writing – original draft. Dong Li: Conceptualization, Methodology, Visualization, Investigation, Writing – original draft. Qiuhui Xu: Methodology, Investigation. Xin Lv: Methodology, Investigation. Hong Chen: Investigation. Fang Wei: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge financial support for this work from National Key R&D Program Key Special Project, China (Grant No. 2021YFD1600103), National Natural Science Foundation of China, China (Grant No. U21A20274, 22301313 and 32472446), Technology Innovation Project of Hubei Province, China (Grant No. 2021BEC021), Innovation group project of Hubei Province, China (2023AFA042), and Agricultural Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences, China (CAAS-ASTIP-2013-OCRI).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2024.100869.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Amiot M.J., Knol D., Cardinault N., Nowicki M., Bott R., Antona C., Borel P., Bernard J.P., Duchateau G., Lairon D. Phytosterol ester processing in the small intestine: impact on cholesterol availability for absorption and chylomicron cholesterol incorporation in healthy humans. JLR (J. Lipid Res.) 2011;52(6):1256–1264. doi: 10.1194/jlr.M013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash M.M., Hang J., Dussault P.H., Carr T.P. Phytosterol stearate esters elicit similar responses on plasma lipids and cholesterol absorption but different responses on fecal neutral sterol excretion and hepatic free cholesterol in male Syrian hamsters. Nutr. Res. 2011;31(7):537–543. doi: 10.1016/j.nutres.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Azadmard-Damirchi S., Habibi-Nodeh F., Hesari J., Nemati M., Achachlouei B.F. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010;121(4):1211–1215. [Google Scholar]
- Bruhl L., Matthaus B. Sensory assessment of virgin rapeseed oils. Eur. J. Lipid Sci. Technol. 2008;110(7):608–610. [Google Scholar]
- Can-Cauich C.A., Sauri-Duch E., Moo-Huchin V.M., Betancur-Ancona D., Cuevas-Glory L.F. Effect of extraction method and specie on the content of bioactive compounds and antioxidant activity of pumpkin oil from Yucatan, Mexico. Food Chem. 2019;285:186–193. doi: 10.1016/j.foodchem.2019.01.153. [DOI] [PubMed] [Google Scholar]
- Chen Y.Z., Kao S.Y., Jian H.C., Yu Y.M., Li J.Y., Wang W.H., Tsai C.W. Determination of cholesterol and four phytosterols in foods without derivatization by gas chromatography-tandem mass spectrometry. J. Food Drug Anal. 2015;23(4):636–644. doi: 10.1016/j.jfda.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew S.C. Cold-pressed rapeseed (Brassica napus) oil: chemistry and functionality. Food Res. Int. 2020;131:108997–109009. doi: 10.1016/j.foodres.2020.108997. [DOI] [PubMed] [Google Scholar]
- Cong Y., Zheng M., Huang F., Liu C., Zheng C. Sinapic acid derivatives in microwave-pretreated rapeseeds and minor components in oils. J. Food Compos. Anal. 2020;87:103394–103402. [Google Scholar]
- Esche R., Barnsteiner A., Scholz B., Engel K.H. Simultaneous analysis of free phytosterols/phytostanols and intact phytosteryl/phytostanyl fatty acid and phenolic acid esters in cereals. J. Agric. Food Chem. 2012;60(21):5330–5339. doi: 10.1021/jf300878h. [DOI] [PubMed] [Google Scholar]
- Feng S., Wang L., Shao P., Lu B., Chen Y., Sun P. Simultaneous analysis of free phytosterols and phytosterol glycosides in rice bran by SPE/GC-MS. Food Chem. 2022;387:132742–132751. doi: 10.1016/j.foodchem.2022.132742. [DOI] [PubMed] [Google Scholar]
- Ferguson J.J., Stojanovski E., MacDonald-Wicks L., Garg M.L. Fat type in phytosterol products influence their cholesterol-lowering potential: a systematic review and meta-analysis of RCTs. Prog. Lipid Res. 2016;64:16–29. doi: 10.1016/j.plipres.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Folch J. A simple method for the isolation and purification of total lipids from animal Tissues. J. Biol. Chem. 1957;226(1):24–36. [PubMed] [Google Scholar]
- Furlan C.P.B., y Castro Marques A., Marineli R.d.S., Maróstica M.R. Conjugated linoleic acid and phytosterols counteract obesity induced by high-fat diet. Food Res. Int. 2013;51(1):429–435. [Google Scholar]
- Garcia-Llatas G., Alegría A., Barberá R., Cilla A. Current methodologies for phytosterol analysis in foods. Microchem. J. 2021;168 [Google Scholar]
- He W.S., Hu D., Wang Y., Chen X.Y., Jia C.S., Ma H.L., Feng B. A novel chemo-enzymatic synthesis of hydrophilic phytosterol derivatives. Food Chem. 2016;192:557–565. doi: 10.1016/j.foodchem.2015.07.047. [DOI] [PubMed] [Google Scholar]
- He W.S., Li L.L., Huang Q.J., Yin J., Cao X.C. Highly efficient synthesis of phytosterol linolenate in the presence of Bronsted acidic ionic liquid. Food Chem. 2018;263:1–7. doi: 10.1016/j.foodchem.2018.04.107. [DOI] [PubMed] [Google Scholar]
- He X., Li W., Chen Y., Lei L., Li F., Zhao J., Zeng K., Ming J. Dietary fiber of Tartary buckwheat bran modified by steam explosion alleviates hyperglycemia and modulates gut microbiota in db/db mice. Food Res. Int. 2022;157:111386–111396. doi: 10.1016/j.foodres.2022.111386. [DOI] [PubMed] [Google Scholar]
- Hu Y.Z., Yang G.L., Huang W.S., Lai S.Y., Ren Y.P., Huang B.F., Zhang L.X., Li P.W., Lu B.Y. Development and validation of a gas chromatography-mass spectrometry method for determination of sterol oxidation products in edible oils. RSC Adv. 2015;5(51):41259–41268. [Google Scholar]
- Islam M.A., Jeong B.-G., Kerr W.L., Chun J. Validation of phytosterol analysis by alkaline hydrolysis and trimethylsilyl derivatization coupled with gas chromatography for rice products. J. Cereal. Sci. 2021;101 [Google Scholar]
- Kritchevsky D., Chen S.C. Phytosterols—health benefits and potential concerns: a review. Nutr. Res. 2005;25(5):413–428. [Google Scholar]
- Li B., Yang W., Nie Y.Y., Kang F.F., Goff H.D., Cui S.W. Effect of steam explosion on dietary fiber, polysaccharide, protein and physicochemical properties of okara. Food Hydrocolloids. 2019;94:48–56. [Google Scholar]
- Li C., Huang X., Xi J. Steam explosion pretreatment to enhance extraction of active ingredients: current progress and future prospects. Crit. Rev. Food Sci. Nutr. 2023:1–9. doi: 10.1080/10408398.2023.2181760. [DOI] [PubMed] [Google Scholar]
- Li D., Wang D., Xiao H.M., Lv X., Zheng C., Liu C.S., Chen H., Wei F. Simultaneous analysis of free/combined phytosterols in rapeseed and their dynamic changes during microwave pretreatment and oil processing. Foods. 2022;11(20):3219–3236. doi: 10.3390/foods11203219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Beisson F., Pollard M., Ohlrogge J. Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phytochemistry. 2006;67(9):904–915. doi: 10.1016/j.phytochem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Liu C.S., Yang M., Huang F.H. Influence of extraction processing on rheological properties of rapeseed oils. J. Am. Oil Chem. Soc. 2012;89(1):73–78. [Google Scholar]
- McDowell D., Elliott C.T., Koidis A. Pre-processing effects on cold pressed rapeseed oil quality indicators and phenolic compounds. Eur. J. Lipid Sci. Technol. 2017;119(9):1600357–1600366. [Google Scholar]
- Niu Y., Rogiewicz A., Wan C., Guo M., Huang F., Slominski B.A. Effect of microwave treatment on the efficacy of expeller pressing of Brassica napus rapeseed and Brassica juncea mustard seeds. J. Agric. Food Chem. 2015;63(12):3078–3084. doi: 10.1021/jf504872x. [DOI] [PubMed] [Google Scholar]
- Ortiz R., Geleta M., Gustafsson C., Lager I., Hofvander P., Lofstedt C., Cahoon E.B., Minina E., Bozhkov P., Stymne S. Oil crops for the future. Curr. Opin. Plant Biol. 2020;56:181–189. doi: 10.1016/j.pbi.2019.12.003. [DOI] [PubMed] [Google Scholar]
- Pang Z., Lu Y., Zhou G., Hui F., Xu L., Viau C., Spigelman Aliya F., MacDonald Patrick E., Wishart David S., Li S., Xia J. MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024;52(W1):W398–W406. doi: 10.1093/nar/gkae253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboanatahiry N., Li H., Yu L., Li M. Rapeseed (Brassica napus): processing, utilization, and genetic improvement. Agronomy. 2021;11(9):1776–1812. [Google Scholar]
- Ramon Aparicio R.A.-R. Authentication of vegetable oils by chromatographic techniques. J. Chromatogr. A. 2000;881:93–104. doi: 10.1016/s0021-9673(00)00355-1. [DOI] [PubMed] [Google Scholar]
- Rekas A., Wroniak M., Scibisz I. Microwave radiation and conventional roasting in conjunction with hulling on the oxidative state and physicochemical properties of rapeseed oil. Eur. J. Lipid Sci. Technol. 2017;119(7):1600501–1600511. [Google Scholar]
- Rezkas A., Wroniak M., Rusinek R. Influence of roasting pretreatment on high-oleic rapeseed oil quality evaluated by analytical and sensory approaches. Int. J. Food Sci. Technol. 2015;50(10):2208–2214. [Google Scholar]
- Santos V.D.S., Braz B.B., Silva A.A., Cardoso L.P., Ribeiro A.P.B., Santana M.H.A. Nanostructured lipid carriers loaded with free phytosterols for food applications. Food Chem. 2019;298 doi: 10.1016/j.foodchem.2019.125053. 125053-112063. [DOI] [PubMed] [Google Scholar]
- Schlag S., Huang Y., Vetter W. GC/EI-MS method for the determination of phytosterols in vegetable oils. Anal. Bioanal. Chem. 2022;414(2):1061–1071. doi: 10.1007/s00216-021-03730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secmeler O., Guclu Ustundag O., Fernandez-Bolanos J., Rodriguez-Gutierrez G. Effect of subcritical water and steam explosion pretreatments on the recovery of sterols, phenols and oil from olive pomace. Food Chem. 2018;265:298–307. doi: 10.1016/j.foodchem.2018.05.088. [DOI] [PubMed] [Google Scholar]
- Siger A., Gawrysiak-Witulska M., Bartkowiak-Broda I. Antioxidant (tocopherol and canolol) content in rapeseed oil obtained from roasted yellow-seeded Brassica napus. J. Am. Oil Chem. Soc. 2017;94(1):37–46. doi: 10.1007/s11746-016-2921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira M.H.L., Morais A.R.C., da Costa Lopes A.M., Olekszyszen D.N., Bogel-Łukasik R., Andreaus J., Pereira Ramos L. Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem. 2015;8(20):3366–3390. doi: 10.1002/cssc.201500282. [DOI] [PubMed] [Google Scholar]
- Szydlowska-Czerniak A. Rapeseed and its products--sources of bioactive compounds: a review of their characteristics and analysis. Crit. Rev. Food Sci. Nutr. 2013;53(4):307–330. doi: 10.1080/10408398.2010.529959. [DOI] [PubMed] [Google Scholar]
- Tan S., Niu Y., Liu L., Su A., Hu C., Meng Y. Development of a GC-MS/SIM method for the determination of phytosteryl esters. Food Chem. 2019;281:236–241. doi: 10.1016/j.foodchem.2018.12.092. [DOI] [PubMed] [Google Scholar]
- Vigneron P.Y., Caigniez J., Stoclin B., Bregnard J.P. Rapeseed oil: becoming a multi-purpose oil. Ocl-Oilseeds and Fats Crops and Lipids. 2006;13(5):363–367. [Google Scholar]
- Wan F., Feng C., Luo K., Cui W., Xia Z., Cheng A. Effect of steam explosion on phenolics and antioxidant activity in plants: a review. Trends Food Sci. Technol. 2022;124:13–24. [Google Scholar]
- Wang D., Xiao H., Lyu X., Chen H., Wei F. Lipid oxidation in food science and nutritional health: a comprehensive review. Oil Crop Science. 2023;8(1):35–44. [Google Scholar]
- Wang J., Zhang X., Liu J., Li R., Zhou J., Li M., Lu J., Zhao G., Li X., Sui W., Zhang M., Chen H. Steam explosion improves extractability, antioxidant activity and α-glucosidase inhibitory activity of the constituents of Java tea (Clerodendranthus spicatus) Innovat. Food Sci. Emerg. Technol. 2023;86:103350–103359. [Google Scholar]
- Wang W., Yang B., Li W., Zhou Q., Liu C., Zheng C. Effects of steam explosion pretreatment on the bioactive components and characteristics of rapeseed and rapeseed products. LWT--Food Sci. Technol. 2021;143:111172–111181. [Google Scholar]
- Xu B.C., You S.C., Zhou L., Kang H.B., Luo D.L., Ma H.Y., Han S.H. Simultaneous determination of free phytosterols and tocopherols in vegetable oils by an improved SPE-GC-FID method. Food Anal. Methods. 2020;13(2):358–369. [Google Scholar]
- Xu B.C., Zhang L.X., Wang H., Luo D.L., Li P.W. Characterization and authentication of four important edible oils using free phytosterol profiles established by GC-GC-TOF/MS. Anal. Methods. 2014;6(17):6860–6870. [Google Scholar]
- Xu Y.J., Jiang F., Song J., Yang X., Shu N., Yuan L., Tan C.P., Liu Y. Understanding of the role of pretreatment methods on rapeseed oil from the perspective of phenolic compounds. J. Agric. Food Chem. 2020;68(33):8847–8854. doi: 10.1021/acs.jafc.0c03539. [DOI] [PubMed] [Google Scholar]
- Yang M., Zheng C., Zhou Q., Huang F., Liu C., Wang H. Minor components and oxidative stability of cold-pressed oil from rapeseed cultivars in China. J. Food Compos. Anal. 2013;29(1):1–9. [Google Scholar]
- Yang R., Xue L., Zhang L., Wang X., Qi X., Jiang J., Yu L., Wang X., Zhang W., Zhang Q., Li P. Phytosterol contents of edible oils and their contributions to estimated phytosterol intake in the Chinese diet. Foods. 2019;8(8):334–345. doi: 10.3390/foods8080334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G., Guo T., Huang Q. Preparation of rapeseed oil with superhigh canolol content and superior quality characteristics by steam explosion pretreatment technology. Food Sci. Nutr. 2020;8(5):2271–2278. doi: 10.1002/fsn3.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T. A review on analysis of steroid profile in different biological matrices. Microchem. J. 2022;172:106897–106901. [Google Scholar]
- Zafar S., Li Y.L., Li N.N., Zhu K.M., Tan X.L. Recent advances in enhancement of oil content in oilseed crops. J. Biotechnol. 2019;301:35–44. doi: 10.1016/j.jbiotec.2019.05.307. [DOI] [PubMed] [Google Scholar]
- Zhang S., Pan Y.G., Zheng L., Yang Y., Zheng X., Ai B., Xu Z., Sheng Z. Application of steam explosion in oil extraction of camellia seed (Camellia oleifera Abel.) and evaluation of its physicochemical properties, fatty acid, and antioxidant activities. Food Sci. Nutr. 2019;7(3):1004–1016. doi: 10.1002/fsn3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M.M., Wang L., Huang F.H., Dong L., Guo P.M., Deng Q.C., Li W.L., Zheng C. Ultrasonic pretreatment for lipase-catalyed synthesis of phytosterol esters with different acyl donors. Ultrason. Sonochem. 2012;19(5):1015–1020. doi: 10.1016/j.ultsonch.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Yang M., Huang F., Zheng C., Deng Q. Effect of pretreatment with dehulling and microwaving on the flavor characteristics of cold-pressed rapeseed oil by GC-MS-PCA and electronic nose discrimination. J. Food Sci. 2013;78(7):C961–C970. doi: 10.1111/1750-3841.12161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.