Abstract
The World Health Organization has emphasized the importance of consuming small fruits for the prevention of chronic health problems, including diabetes, cardiovascular diseases, cancer, and obesity, which are named chronic non-communicable diseases (NCDs). Azara serrata Ruiz & Pav., commonly called “aroma de Castilla”, is a shrub endemic to Chile from the Salicaceae family that produces an underutilized blue-grey berry that grows wild in southern Chile. The species is widely used as a medicinal plant by the Andean communities of southern Chile. In this work, a high-resolution mass spectrometric analysis of the methanolic extract revealed several phenolic compounds for the first time in the edible berry of this endemic species. Furthermore, several glycosylated anthocyanins were detected and quantified using UHPLC coupled with UV/Vis detection and trapped ion mobility mass spectrometry (UHPLC-DAD-TIMS-TOF) for the anthocyanin-rich extract, which was prepared using an optimized anthocyanin extraction protocol. The extract proved to be active in the inhibition of several enzymes linked to NCDs, such as acetylcholinesterase, tyrosinase, amylase, lipase, and glucosidase (IC50 = 3.92 ± 0.23, 12.24 ± 0.03, 11.12 ± 0.10, 32.43 ± 0.0, and 371.6 ± 0.0 μg/mL, respectively). Furthermore, the extract concentrated in anthocyanins showed good antioxidant activity evidenced by the bleaching of the radicals DPPH and ABTS, ferric-reducing antioxidant power (FRAP), and oxygen radical absorbance capacity (ORAC). The results show that these neglected endemic small berries can be a source of healthy phytochemicals. These Chilean berries can be used as functional food and their extracts are candidates for use as functional ingredients in naturally healthy products.
Keywords: corcolen, salicaceae, endemic berries, enzyme inhibition, antioxidants, anthocyanins, pyrano anthocyanins, analytical membrane chromatography, collision cross-section, TIMS-TOF
1. Introduction
The Valdivian Forest has diverse endemic species with interesting compounds that are at risk of extinction including edible small berries. Several phenolic compounds and glyco-sylated anthocyanins for some representative endemic species berries have been reported previously [1,2]. With ten species of flowering plants belonging to the Salicaceae family, the genus Azara is native to temperate to subtropical parts of South America [3]. They are primarily found in Chile from the towns of Coquimbo to Valdivia, usually along lakeshores and the edges of forests. The leaves of the shrubs and small trees are alternating; however, in some species, they appear paired, measuring 1–4 cm long by 0.5–5 cm broad. The plants can reach heights of 1–8 m. They have black to red berries that are 3–10 mm broad. The plants are common in Chile, but there are not many research articles from Chile on this genus. A new myricetin 3-O-α-l-dirhamnoside was reported from Azara microphylla (local name corcolén) a long time ago [4]. Subsequently, a variety of phenolic compounds, including caffeic and coumaric acids, and three flavonoids (pinocembrin, chrysin, and luteolin) have been reported recently in honey and propolis made of A. petiolaris and A. integrifolia [5]. For Azara dentata Ruiz & Pav., local name “corcolen blanco”, cytotoxic effects and the presence of coumarins, sterols, and other components in the methanolic extract of leaves by GC-MS have been reported [6]. More recently, Ramos et al. (2023) also reported the antioxidant activity, enzymatic inhibition, and relaxation effects in rat aorta induced by the ethanolic extract of these fruits [7]. These neglected and understudied endemic Chilean species can offer novel substances or bioactivities that merit further study, especially the antifeedant compounds known as salicin glycoside derivatives [8]. However, due to the large structural variability of compounds in the Salicaceae family, which is mainly due to polar phenolic compounds, and the high number of different structures discovered so far, a thorough analysis and separation of phenolic compounds in their edible fruits is necessary [9]. Azara serrata Ruiz & Pav. (local name Aromo de Castilla) produces a white-blue-grey berry (Figure 1). In Chile, this plant grows in the regions of Coquimbo, Valparaíso, Santiago, O’Higgins, Maule, Biobío, Araucanía, and Los Ríos and is reported in the ancient literature to be used in Mapuche medicine as an antirheumatic and antitussive [3].
Figure 1.
Azara serrata Ruiz & Pav. fruits and its leaves, collected in February 2022 from the Botanical Garden, UACh, Valdivia.
Chronic or noncommunicable diseases (NCDs) are long-lasting conditions that often progress slowly. The primary forms are diabetes, cancer, chronic respiratory diseases (such as asthma and chronic obstructive lung disease), and cardiovascular diseases (such as heart attacks and stroke) (WHO) [10]. Together with neurodegenerative diseases (Alzheimer’s, Parkinson’s, and others; Alzheimer’s accounts for roughly 70% of dementia cases), these illnesses are the primary causes of premature deaths globally [10]. Berries are the primary source of phenolic compounds in diets, and health-promoting phenolics have been found to be present in several South American berries [11,12,13,14]. Indeed, berries and native fruits from unique habitats, like the Chilean terroir, can yield intriguing and complex phytochemicals that might serve as models for exploring potential new uses for them as food additives, nutraceuticals, or herbal remedies. In this study, through UHPLC-DAD-TIMS-TOF analyses, we offer a variety of novel approaches to the chemical and biological activity of underappreciated Azara serrata fruits regarding antioxidant properties and the inhibition of enzymes related to NCDs.
Anthocyanins, as secondary plant metabolites, prominently occur in berries, vegetables, and fruits of a large variety of plant families [15]. As for the plant family of salicaceae, it has been reported that anthocyanins can occur in the bark of Salix purpurea and some of its hybrids [16]. Another representative of the anthocyanins found in the Salicaceae plant family is said to be various species of Populus L. [17]. The species Populus nigra L. is reported to contain anthocyanins such as cyanidin, delphinidin, petunidin, and pelargonidin [17]. Only a limited number of salicaceae plants produce fruits such as Azara serrata Ruits & Pav. The plant Idesia polycarpa produces orange-red berries, which are likely to contain carotenoids due to their color and high oil content, which has been reported to be up to 26.26% in seeds and 43.6% in the pulp (dry weight) [18]. Other investigated salicaceaes that produce fruits containing anthocyanins are Dovyalis hebecarpa native to Sri Lanka and southern India and Flacourtia jangomas, which is cultivated in the southeast of Asia [19,20].
A large-scale review has pointed out that anthocyanins show positive effects on cardiovascular health and vision. They also possess antidiabetic, neuroprotective, antimicrobial, and anti-inflammatory properties and are related to chemopreventive effects against cancer [21]. The anthocyanins in red raspberries, mainly containing different glycosides of cyanidin and some pelargonidins, for example, have been shown to modulate proteins involved in antioxidant, anticancer, and anti-inflammatory effects in cultured cells [22]. However, the authors of the review emphasized that in vitro models cannot be directly translated to in vivo studies without concerns, especially regarding the concentration levels used in in vitro studies compared to bioavailability and metabolism in vivo. Therefore, it is crucial to maintain a critical perspective regarding the potential health benefits of anthocyanin-rich foods [22].
In this study, we present the results of an analysis of berries from Azara serrata Ruiz & Pav. by UHPLC-DAD-TIMS-TOF, as well as results from different assays regarding the content of various substance classes. Additionally, docking studies were performed for a selection of compounds occurring in the berries.
2. Results and Discussion
2.1. Phenolic Profile of Azara serrata Ruiz & Pav.
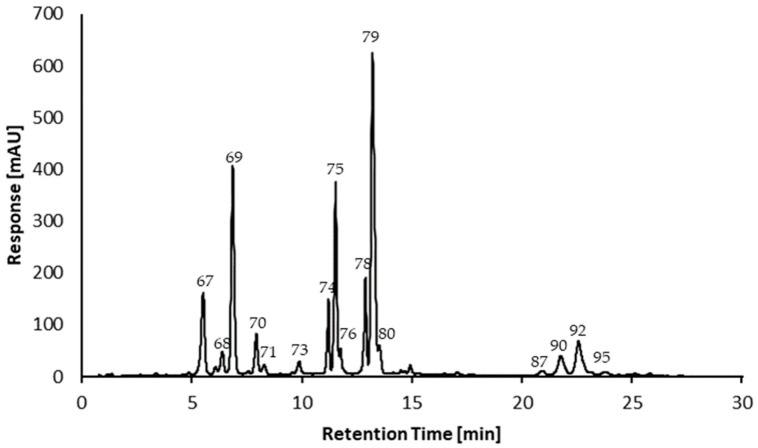
The base peak chromatograms (ESI-negative) as well as UV/Vis at wavelengths of 280 nm and 520 nm of a methanolic extract of the dried berries from Azara serrata Ruiz & Pav. are illustrated in Figure 2. The negative system operation mode was used for the elucidation of the methanolic extract and the positive mode was used for the analysis of the anthocyanins. Table 1 shows the results of the spectrometric investigation by UHPLC-DAD-TIMS-TOF analysis, which includes high-resolution mass spectral data and fragmentation and ion mobility data in the form of CCS values. The compounds were tentatively annotated. The UHPLC-DAD-TIMS-TOF-MS analysis of the purified anthocyanin extract (cf. Section 3.3.3) in positive operation mode is in the Supplementary Materials Figure S1. A detailed explanation is below.
Figure 2.
UHPLC-DAD-TIMS-TOF-MS analysis of the methanolic extract of the berries from Azara serrata Ruiz & Pav., with base peak all MS ne—gative (I), DAD signal at 280 nm (II), and DAD signal at 520 nm (III).
Table 1.
Phenolic compounds and anthocyanins of the methanolic and purified anthocyanin extract of the berries of Azara serrata Ruiz & Pav., tentatively identified (cf. Section 3.11) by UHPLC-DAD-TIMS-TOF. pyr = pyrano-type compounds (cf. Figure 4); ac = acetylated; cou = coumaroylated. Anthocyanin species of pyrano-type compounds are shown in brackets.
| Peak Number | Retention Time [min] | Compound Name | UV/Vis Max. [nm] | Molecular Formula | Theoretical Mass [m/z] | Detected Mass [m/z] | Mass Error [ppm] | Fragment Ion MS/MS [m/z] | CCS [Å2] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.20 | unknown | C12H16O8 | 287.0772 | 287.0769 | 1.3 | 125, 83 | 169.4 | |
| 2 | 4.20 | caffeoyl glucaric acid | 212–324 | C15H16O11 | 371.0620 | 371.0616 | 1.0 | 209, 191 | 185.3 |
| 3 | 4.82 | unknown | C16H19NO9 | 368.0987 | 368.0981 | 1.7 | 160, 119 | 174.9 | |
| 4 | 5.01 | gallic acid glucoside isomer | C13H16O10 | 331.0671 | 331.0668 | 0.8 | 285, 123 | 168.3 | |
| 5 | 5.68 | caffeoyl glucaric acid isomer | 198–278 | C15H16O11 | 371.0620 | 371.0614 | 1.5 | 209, 191 | 171.6 |
| 6 | 6.28 | pyrocatechuic acid glucoside | 216–284 | C13H16O9 | 315.0722 | 315.0719 | 0.9 | 153 | 171.6 |
| 7 | 6.69 | gallic acid glucoside isomer | C13H16O10 | 331.0671 | 331.0663 | 2.2 | 313, 169 | 169.3 | |
| 8 | 6.93 | idesin/salirepin isomer | 196–276 | C13H18O8 | 301.0929 | 301.0928 | 0.5 | 139 | 165.6 |
| 9 | 7.37 | unknown | C16H14O11 | 381.0463 | 381.0476 | −3.5 | 259, 241, 139 | 175.5 | |
| 10 | 7.52 | unknown | C18H24O12 | 431.1187 | 431.1195 | 1.9 | 137, 93 | 187.5 | |
| 11 | 7.80 | idesin/salirepin isomer | C13H18O8 | 301.0929 | 301.0929 | 0.1 | 121, 59 | 164.3 | |
| 12 | 8.06 | pyrocatechuic acid glucoside | C13H16O9 | 315.0722 | 315.0719 | 0.8 | 153, 109 | 166.1 | |
| 13 | 8.54 | unknown | C14H20O9 | 331.1035 | 331.1031 | 1.0 | 285, 123 | 173.0 | |
| 14 | 8.71 | caffeoyl glucaric acid isomer | 218–324 | C15H16O11 | 371.0620 | 371.0617 | 0.8 | 209, 191 | 174.2 |
| 15 | 8.97 | chlorogenic acid isomer | 216–324 | C16H18O9 | 353.0878 | 353.0878 | 1.5 | 191, 135 | 170.9 |
| 16 | 9.36 | unknown | C18H24O13 | 447.1144 | 447.1140 | 1.0 | 152, 108 | 190.1 | |
| 17 | 9.74 | caffeoyl threonic acid isomer | 216–326 | C13H14O8 | 297.0616 | 297.0615 | 0.2 | 179, 135 | 159.3 |
| 18 | 9.85 | arbutin | 194–272 | C12H16O7 | 271.0823 | 271.0823 | 0.3 | 109 | 156.5 |
| 19 | 10.07 | idesin/salirepin isomer | 196–274 | C13H18O8 | 301.0929 | 301.0928 | 0.2 | 139, 121 | 162.6 |
| 20 | 11.18 | caffeoyl threonic acid isomer | 218–328 | C13H14O8 | 297.0616 | 297.0615 | 0.5 | 179, 135 | 159.3 |
| 21 | 11.62 | coumaroylquinic acid | C16H18O8 | 337.0929 | 337.0925 | 1.3 | 163, 119 | 179.3 | |
| 22 | 12.31 | caffeic acid glucoside | C15H18O9 | 341.0878 | 341.0877 | 0.2 | 179, 135 | 177.8 | |
| 23 | 12.78 | chlorogenic acid isomer | 216–326 | C16H18O9 | 353.0878 | 353.0874 | 1.0 | 191, 127 | 184.4 |
| 24 | 13.67 | feruloylquinic acid isomer | 218–324 | C17H20O9 | 367.1035 | 367.1030 | 1.3 | 191, 134, 117 | 179.1 |
| 25 | 14.04 | chlorogenic acid isomer | 216–326 | C16H18O9 | 353.0878 | 353.0877 | 0.2 | 191, 135, | 184.4 |
| 26 | 15.96 | caffeoyl threonic acid isomer | 218–324 | C13H14O8 | 297.0616 | 297.0617 | −0.5 | 179, 135, 75 | 158.2 |
| 27 | 16.47 | unknown | C20H32O10 | 431.1923 | 431.1917 | 1.3 | 299, 71, 59 | 199.9 | |
| 28 | 16.68 | unknown | C18H28O9 | 387.1661 | 387.1663 | −0.6 | 369, 207, 163 | 186.8 | |
| 29 | 18.62 | feruloylquinic acid isomer | 218–322 | C17H20O9 | 367.1035 | 367.1032 | 0.7 | 191, 134, 93 | 197.2 |
| 30 | 19.05 | verbasoside | C20H30O12 | 461.1664 | 461.1670 | −1.1 | 191, 131, 89 | 205.8 | |
| 31 | 20.00 | unknown | C15H16N2O6 | 319.0936 | 319.0938 | −0.7 | 142, 130, 116 | 181.5 | |
| 32 | 20.32 | unknown | C19H26O10 | 413.1453 | 413.1445 | 2.0 | 161, 133 | 200.7 | |
| 33 | 21.78 | idescarpin/HCH-salirepin isomer | C20H24O11 | 439.1246 | 439.1249 | −0.7 | 301, 283, 139 | 193.1 | |
| 34 | 22.50 | idescarpinol/HCH-salirepinol | C20H26O11 | 441.1402 | 441.1400 | 0.4 | 301, 283 157, 139 | 194.3 | |
| 35 | 23.03 | unknown | 218–328 | C21H26O10 | 437.1453 | 437.1448 | 1.2 | 161, 133 | 215.9 |
| 36 | 24.90 | idescarpin/HCH-salirepin isomer | 196–278 | C20H24O11 | 439.1246 | 439.1244 | 0.4 | 301, 139 | 217.8 |
| 37 | 25.98 | trichocarposide isomer | 220–320 | C22H24O9 | 431.1348 | 431.1351 | −0.7 | 307, 145, 123 | 219.2 |
| 38 | 26.42 | unknown | 220–314 | C21H26O9 | 421.1504 | 421.1500 | 1.0 | 145, 117 | 213.1 |
| 39 | 26.61 | populoside | 220–324 | C22H24O10 | 447.1297 | 447.1295 | 0.4 | 323, 179, 161, 132 | 201.8 |
| 40 | 26.99 | unknown | 220–326 | C21H28O10 | 439.1610 | 439.1616 | −1.3 | 161, 133 | 211.5 |
| 41 | 27.48 | trichocarposide isomer | 220–312 | C22H24O9 | 431.1348 | 431.1350 | −0.5 | 161, 133 | 202.4 |
| 42 | 27.80 | unknown | 220–312 | C21H26O9 | 421.1504 | 421.1508 | −0.9 | 137 | 206.4 |
| 43 | 28.18 | salicortin isomer | 220–312 | C21H28O9 | 423.1661 | 423.1657 | 0.8 | 145, 117 | 215.1 |
| 44 | 29.48 | salicortin isomer | 220–312 | C21H28O9 | 423.1661 | 423.1654 | 1.5 | 145, 117 | 207.5 |
| 45 | 29.89 | flacourtin/salireposide | C20H22O9 | 405.1191 | 405.1189 | 0.5 | 135, 91 | 202.1 | |
| 46 | 30.18 | trichocarposide isomer | 220–312 | C22H24O9 | 431.1348 | 431.1345 | 0.7 | 161, 132 | 214.1 |
| 47 | 30.80 | salicortin isomer | 220–312 | C21H28O9 | 423.1661 | 423.1658 | 0.7 | 145, 117 | 215.4 |
| 48 | 31.22 | idescarpin/HCH-salirepin isomer | 196–276 | C20H24O11 | 439.1246 | 439.1236 | 2.3 | [2M − H] 879.2552, 301, 139 | 193.1 |
| 49 | 31.34 | HCH-idescarpin/di-HCH-salirepin isomer | C27H30O14 | 577.1563 | 577.1558 | 0.8 | 439, 421, 301, 139 | 222.4 | |
| 50 | 31.96 | cou-idesin/salirepin | C22H24O10 | 447.1297 | 447.1295 | 0.4 | 301, 139 | 201.8 | |
| 51 | 32.31 | trichocarposide isomer | 220–326 | C22H24O9 | 431.1348 | 431.1351 | −0.9 | 161, 133 | 213.8 |
| 52 | 32.52 | cou-arbutin | 220–312 | C21H22O9 | 417.1191 | 417.1189 | 0.4 | 307, 163, 145 | 208.5 |
| 53 | 32.7 | cou-salicin | 220–326 | C22H24O9 | 431.1348 | 431.1340 | 1.8 | 161, 133 | 211.9 |
| 54 | 32.96 | ac-salicortin | 220–314 | C23H30O10 | 465.1766 | 465.1763 | 0.8 | 423, 405, 163, 145 | 220.7 |
| 55 | 33.62 | ac-cou-idesin/salirepin isomer | 220–312 | C24H26O11 | 489.1402 | 489.1401 | 0.3 | 343, 301, 139 | 210.2 |
| 56 | 34.51 | unknown coumaroyl species | 222–310 | C22H24O8 | 415.1398 | 415.1398 | 0.1 | 163, 145, 117 | 211.9 |
| 57 | 34.79 | ac-cou-idesin/salirepin isomer | C24H26O11 | 489.1402 | 489.1402 | 0.0 | 343, 301, 139 | 217.5 | |
| 58 | 35.02 | unknown coumaroyl species | C22H24O8 | 415.1398 | 415.1395 | 0.7 | 163, 145, 117 | 209.8 | |
| 59 | 35.64 | idescarpin/HCH-salirepin isomer | 196–276 | C20H24O11 | 439.1246 | 439.1249 | −0.7 | [2M − H] 879.2552, 301, 139 | 193.1 |
| 60 | 35.85 | unknown | 222–310 | C23H26O8 | 429.1555 | 429.1552 | 0.6 | 145, 117 | 219.6 |
| 61 | 36.72 | unknown | C31H34O14 | 629.1876 | 629.1864 | 1.8 | 157 | 232.2 | |
| 62 | 36.78 | idesin/salirepin salicylate | C20H22O10 | 421.1140 | 421.1135 | 1.3 | 137, 93 | 193.4 | |
| 63 | 37.44 | cou-idescarpin/HCH-salirepin | C29H30O13 | 585.1614 | 585.1613 | 0.1 | 439, 301, 139 | 220.7 | |
| 64 | 37.85 | ac-cou-idescarpin/HCH-salirepin isomer | 196–220 | C31H32O14 | 627.1719 | 627.1721 | −0.3 | 481, 343, 301 | 232.1 |
| 65 | 38.30 | ac-cou-idescarpin/HCH-salirepin isomer | 196–220 | C31H32O14 | 627.1719 | 627.1711 | 1.3 | 481, 343, 301 | 239.9 |
| 66 | 38.78 | diac-cou-idescarpin/HCH-salirepin | 222 | C33H34O15 | 669.1825 | 669.1824 | 0.2 | 523, 481, 343 | 242.8 |
| Anthocyanins and derivates | |||||||||
| 67 | 13.71 | delphinidin-glucoside | 522 | C21H21O12 | 465.1028 | 465.1023 | 1.0 | 303 | 205.2 |
| 68 | 14.07 | delphinidin-rutinoside | 522 | C27H31O16 | 611.1607 | 611.1608 | −0.3 | 303 | 236.0 |
| 69 | 15.52 | cyanidin-glucoside | 514 | C21H21O11 | 449.1078 | 449.1075 | 0.7 | 287 | 201.5 |
| 70 | 16.29 | cyanidin-rutinoside | 516 | C27H31O15 | 595.1657 | 595.1660 | −0.4 | 287 | 232.7 |
| 71 | 16.85 | delphinidin-arabinose | C20H19O11 | 435.0922 | 435.0921 | 0.3 | 303 | 200.5 | |
| 72 | 17.12 | pelargonidin-glucoside | 510 | C21H21O10 | 433.1129 | 433.1129 | 0.0 | 271 | 199.1 |
| 73 | 18.68 | cyanidin-arabinose | 514 | C20H19O10 | 419.0973 | 419.0970 | 0.7 | 287 | 196.9 |
| 74 | 20.99 | pyr-(delphinidin-rutinoside) | 372–514 | C47H51O26 | 1031.2663 | 1031.2664 | −0.1 | 723, 439, 393 | 291.7 |
| 75 | 21.48 | pyr-(delphinidin-glucoside) | 372–512 | C41H41O22 | 885.2085 | 885.2084 | −0.3 | 723, 439, 393 | 272.7 |
| 76 | 21.74 | pyr-(delphinidin-arabinoside) | 372–512 | C40H39O21 | 855.1978 | 855.1974 | 0.5 | 723, 439, 393 | 269.2 |
| 77 | 21.90 | pyr-ac-(delphinidin-glucoside) | C43H43O23 | 927.2190 | 927.2192 | −0.3 | 765, 439, 393 | 279.9 | |
| 78 | 22.71 | pyr-(cyanidin-rutinoside) | 356–508 | C47H51O25 | 1015.2174 | 1015.2713 | 0.1 | 707, 423, 377 | 289.2 |
| 79 | 23.26 | pyr-(cyanidin-glucoside) | 354–506 | C41H41O21 | 869.2137 | 869.2135 | −0.2 | 707, 423, 377 | 269.2 |
| 80 | 23.50 | pyr-(cyanidin-arabinoside) | 354–508 | C40H39O20 | 839.2029 | 839.2032 | −0.3 | 707, 423, 377 | 266.1 |
| 81 | 24.38 | pyr-(pelargonidin-glucoside) | C41H41O20 | 853.2186 | 853.2179 | 0.8 | 691, 407, 361 | 267.4 | |
| 82 | 24.64 | pyr-base-(cyanidin-rutinoside) | C33H33O16 | 685.1763 | 685.1766 | −0.4 | 377 | 240.3 | |
| 83 | 25.08 | pyr-base-(cyanidin-glucoside) | C27H23O12 | 539.1184 | 539.1183 | 0.2 | 377 | 219.0 | |
| 84 | 25.52 | pyr-base-(cyanidin-arabinoside) | C26H21O11 | 509.1078 | 509.1071 | 1.5 | 377 | 214.8 | |
| 85 | 27.18 | pyr-ac-(cyanidin-glucoside) | C43H43O22 | 911.2240 | 911.2244 | −0.4 | 749, 423, 377 | 282.4 | |
| 86 | 27.50 | pyr-diac-(cyanidin-glucoside) | C45H45O23 | 953.2346 | 953.2345 | 0.1 | 791, 423, 377 | 283.4 | |
| 87 | 30.58 | pyr-ac-cou-(delphinidin-rutinoside) | 376–514 | C58H59O29 | 1219.3137 | 1219.3132 | 0.4 | 911, 765, 439, 393 | 325.4 |
| 88 | 30.84 | pyr-cou-(delphinidin-glucoside) | C50H47O24 | 1031.2452 | 1031.2450 | 0.2 | 869, 723, 439, 393 | 299.6 | |
| 89 | 30.98 | pyr-ac-cou-(cyanidin-rutinoside) | C58H59O28 | 1203.3187 | 1203.3186 | 0.1 | 895, 749, 423, 377 | 322.7 | |
| 90 | 31.17 | pyr-ac-cou-(delphinidin-glucoside) | 374–514 | C52H49O25 | 1073.2557 | 1073.2562 | −0.4 | 911, 765, 439, 393 | 307.4 |
| 91 | 31.28 | pyr-cou-(cyanidin-glucoside) | C50H47O23 | 1015.2503 | 1015.2500 | 0.2 | 853, 707, 423, 377 | 296.1 | |
| 92 | 31.52 | pyr-ac-cou-(cyanidin-glucoside) | 356–510 | C52H49O24 | 1057.2608 | 1057.2607 | 0.1 | 895, 749, 423, 377 | 303.9 |
| 93 | 31.84 | pyr-ac-cou-(cyanidin-arabinose) | C51H47O23 | 1027.2503 | 1027.2490 | 1.3 | 895, 749, 423, 377 | 301.8 | |
| 94 | 32.18 | pyr-ac-cou-(delphinidin-glucoside) | C52H49O25 | 1073.2557 | 1073.2546 | 1.0 | 911, 765, 439, 393 | 308.9 | |
| 95 | 32.70 | pyr-ac-cou-(cyanidin-glucoside) | 352–508 | C52H49O24 | 1057.2608 | 1057.2596 | 1.2 | 895, 749, 423, 377 | 306.9 |
| 96 | 33.29 | pyr-ac-cou-(pelargonidin-glucoside) | C52H49O23 | 1041.2659 | 1041.2651 | 0.8 | 879, 733, 407, 361 | 307.3 | |
| 97 | 33.47 | pyr-diac-cou-(delphinidin-glucoside) | C54H51O26 | 1115.2663 | 1115.2668 | −0.4 | 953, 807, 439, 393 | 311.4 | |
| 98 | 34.15 | pyr-diac-cou-(cyanidin-glucoside) | C54H51O25 | 1099.2714 | 1099.2720 | −0.5 | 937, 791, 423, 377 | 308.7 | |
| 99 | 34.29 | pyr-ac-cou-(delphinidin-glucoside) | C52H49O25 | 1073.2557 | 1073.2560 | −0.3 | 911, 765, 439, 393 | 304.8 | |
| 100 | 34.39 | pyr-ac-cou-(cyanidin-glucoside) | C52H49O24 | 1057.2608 | 1057.2608 | 0.5 | 895, 749, 423, 377 | 304.2 | |
2.1.1. Phenolic Acids
According to our data, the extract contained various species of quinic acid derivates like chlorogenic acid isomers, [M − H]− theoretical m/z 353.0878 (15, 23, 25), and feruloyl quinic acid isomers, [M − H]− theoretical m/z 367.1035 (24, 29). Most of the signals could be assigned to phenolic glycosides, which have been reported to occur in the plant family of Salicaceae [9,23]. The main phenolic glycosides observed were annotated as idesin or its isomer salirepin, [M − H]− theoretical m/z 301.0928 (8, 11, 19), which both have been isolated from the Salicaceae Idesia polycarpa Maxim., native to the eastern part of Asia [24]. Further, it is described that in the presence of 1-hydroxy-6-oxo-2-cyclohexene-1-carboxylic acid (HCC), salicin as well as idesin form the compounds salicortin, [M − H]− theoretical m/z 423.1657 (43, 44, 47), and idescarpin or its salirepin analog, [M − H]− theoretical m/z 439.1246 (33, 36, 48, 59) [25,26]. Salicortin as well as some of its structural relatives have been isolated from the bark of willow trees (Salix spp.) [26]. Both idescarpin and salicortin have shown promising pharmaceutical benefits in different studies [9]. Further coumaroyl derivates of those species have been annotated. The coumaroyl species of salicin are trichocarposide, [M − H]− theoretical m/z 431.1348 (37, 41, 46, 51, 53); coumaroyl idesin, [M − H]− theoretical m/z 447.1297 (50); and coumaroyl idescarpin, [M − H]− theoretical m/z 585.1614 (63). As described in the literature, the hexose moiety of salicinoids can be acetylated as well, resulting in more structural variety [26]. Like the coumaroylated species, three different isomers of an acetylated coumaroyl idescarpin or the salirepin analoga have been detected, [M − H]− theoretical m/z 627.1719 (64, 65). Figure 3 illustrates the major salicinoids present in the methanolic extract of the berries of Azara serrata Ruiz & Pav., as reflected in their detector counts (MS) and UV/Vis absorbance (cf. Figure 2).
Figure 3.
Chemical structures of some salicinoids in the berries of Azara serrata Ruiz & Pav.
2.1.2. Anthocyanins
Apart from the more commonly occurring cyanidin- and delphinidin-glycosides (67–73), which have also been reported by Bridle Stott et al. (1973) in Salix purpurea, most of the observed anthocyanins have not been, to the best of our knowledge, described yet and therefore represent potential novelties [16,27]. We postulate that these previously unreported types of pyranoanthocyanin are formed by a reaction of the respective anthocyanin with the double bond within the HCH unit of idescarpin or the salirepin isomer, as illustrated in Figure 4. The reaction may involve an unknown precursor of the HCH molecule, as well as a catalyst, which could be an enzyme or external stimulus.
Figure 4.
Proposed mechanism of the formation of the pyranoanthocyanin species in the berries of Azara serrata Ruiz & Pav.
The chromatographic pattern, as well as the mass difference of m/z 16 between delphinidin and cyanidin, persisted throughout the analysis, but the typical fragments of m/z 287 for cyanidin and m/z 303 for delphinidin could not be detected. Apparently, all of the novel anthocyanins (74–100) have a fragment of m/z 377 for the cyanidin species and m/z 393 for the delphinidin species. An important observation throughout the analysis was the difference in the CCS values between the cyanidin and delphinidin species, being ∆ 3.7 Å2 for the glucosides, [M]+ m/z 465.1023 and [M]+ m/z 449.1075 (67 & 69), and ∆ 3.3 Å2 for the rutinosides, [M]+ m/z 611.1608 and [M]+ m/z 595.1660 (68 & 70), which remained in the same order of magnitude. Therefore, we observed ∆ 3.5 Å2 for the glucosides, [M]+ m/z 885.2084 and [M]+ m/z 869.2135 (75 & 79), of the other species and ∆ 2.5 Å2 for the rutinosides, [M]+ m/z 1031.2664 and [M]+ m/z 1015.2173 (74 & 78). This observation carried through to the higher mass anthocyanins eluting at the end of the chromatographic program (85–100), which were the coumaroyl and acetylated counterparts of the idesin, respectively, salirepin and analogas, described above. Because of this very similar pattern regarding the factors—retention time, the difference in the mass-to-charge ratio of 16, and the almost identical difference in the CCS values between cyanidin and delphinidin species—we are confident in our observation that the here-found anthocyanin species originate from the cyanidin- and delphinidin-glycosides present in the plant. Additionally, we observed a shift in the UV/Vis maximum towards 500 nm for these substances, which has been described as characteristic of pyranoanthocyanins [28,29]. This subclass of the anthocyanins is often reported in red wines, resulting from a condensation reaction between malvidin-glycosides and other phenolic compounds found in red wines [27]. A wide range of pyranoanthocyanins in red wine has been described. The group known as vitisins is formed by a reaction between pyruvic acid, acetaldehyde, acetoacetic acid, or acetone with malvidin-glycosides. Another group referred to as pinotins is formed by the reaction of anthocyanins with a vinyl compound such as different vinylphenols or hydroxycinnamic acids [30]. Pyranoanthocyanin-flavanols are formed by the reaction of 8-vinylcatechin with the respective anthocyanin [27]. Lastly, the group of vinylpyranoanthocyanins, termed portisins, is described in red wines due to the reaction of the already-formed carboxypyranoanthocyanin with either vinylflavan-3-ol or hydroxycinnamic acid [31]. Apart from red wines, pyranoanthocyanins have been isolated from blood orange juice (Citrus sinensis L.) [32], black carrot juice (Daucus carota L.) [33], strawberry (Fragaria ananassa) [34], red onion (Allium cepa) [35], and, recently, wines made from bilberry (Vaccinium myrtillus L.) [36].
2.2. Quantitation of Anthocyanins
A chromatogram of the UHPLC-DAD analysis of the enriched anthocyanin extract is depicted in Figure 5. Preparation of the extract using analytical membrane chromatography (cf. Section 3.3.3) was carried out in triplicate; for comparison, a chromatogram of the purified extract at the wavelength of 280 nm is in the Supplementary Materials (Figure S2).
Figure 5.
Chromatogram of the UHPLC-DAD analysis of the anthocyanin-rich extract of the berries of Azara serrata Ruiz & Pav. at 520 nm.
For quantitation purposes, only anthocyanins with an S/N > 10 were considered. Therefore, the content of the major anthocyanins is summarized in Table 2. Quantitation was performed using a cyanidin-3-O-β-d-glucoside calibration curve ranging from 1 to 75 mg L−1; the curve including its key figures is in the Supplementary Materials Figure S3.
Table 2.
Results of the quantitative analysis of the enriched anthocyanin extract measured by UHPLC-DAD and reported as cyanidin-3-O-β-d-glucoside equivalents. Pyr = pyrano; ac = acetylated.
| Peak Number | Retention Time [min] | Compound Name | µg/g DW Berries |
|---|---|---|---|
| 67 | 5.32 | delphinidin-glucoside | 81.20 ± 8.00 |
| 68 | 6.22 | delphinidin-rutinoside | 20.45 ± 1.90 |
| 69 | 6.66 | cyanidin-glucoside | 115.8 ± 9.38 |
| 70 | 7.76 | cyanidin-rutinoside | 21.37 ± 1.58 |
| 71 | 8.13 | delphinidin-arabinoside | 7.59 ± 0.43 |
| 73 | 9.67 | cyanidin-arabinoside | 7.99 ± 0.35 |
| 74 | 11.07 | pyr-delphinidin-rutinoside | 14.65 ± 1.42 |
| 75 | 11.42 | pyr-delphinidin-glucoside | 37.73 ± 3.13 |
| 76 | 11.62 | pyr-delphinidin-arabinoside | 4.23 ± 0.35 |
| 78 | 12.81 | pyr-cyanidin-rutinoside | 17.37 ± 1.18 |
| 79 | 13.15 | pyr-cyanidin-glucoside | 75.55 ± 4.67 |
| 80 | 13.41 | pyr-cyanidin-arabinoside | 4.62 ± 0.20 |
| 87 | 20.66 | pyr-ac-coumaroyl-delphinidin-rutinoside | 3.21 ± 0.36 |
| 90 | 21.49 | pyr-ac-coumaroyl-delphinidin-glucoside | 9.61 ± 0.69 |
| 92 | 22.29 | pyr-ac-coumaoryl-cyanidin-glucoside | 18.66 ± 1.18 |
| 95 | 23.58 | pyr-ac-coumaroyl-cyanidin-glucoside | 1.47 ± 0.11 |
| sum | 441.49 ± 34.93 |
The most abundant anthocyanin was cyanidin-3-O-β-d-glucoside, with a content of 115.8 ± 9.38 µg g−1 (DW), followed by delphinidin-3-O-β-d-glucoside with 81.20 ± 8.00 µg g−1 (DW). For the pyranoanthocyanin species, a similar observation was made, with the pyranocyanidin-type glucoside having a content of 75.55 ± 4.67 µg g−1 (DW) and the pyranodelphinidin-type glucoside having a content of 37.73 ± 3.13 µg g−1 (DW). Since the contents are expressed as cyanidin-3-O-β-d-glucoside equivalents, we assume that the actual content of the pyranoanthocyanins might have been slightly higher due to the shift in the maximum light absorbance. The total anthocyanin content in the dry berries of Azara serrata Ruiz & Pav. was 441.49 ± 34.93 µg g−1 (DW). Given that the dry weight was approximately 1/10th of the fresh weight, the anthocyanin content was in the same order of magnitude as that of red currant (Ribes L.), whose berries have been reported to contain 4.95 ± 0.24 mg 100 g−1 of anthocyanins at fresh weight [37]. With 60.26% of the total anthocyanin content, the cyanidin species was more abundant in the plant compared to the 39.74% total content of the delphinidin species.
2.3. Phytochemical and Antioxidant Activity
Comparative Determination of Total Anthocyanins, Phenols, Flavonoids, and Antioxidant Capacities
Phenolic compounds, known for their strong antioxidant properties, are secondary metabolites found in various plant species. The total phenolic and flavonoid contents of the fruits are depicted in Table 3. The values for TAC were several times less than those reported for the Chilean maqui berries Aristotelia chilensis (between 22 and 50 mg/g DW) [11,38]; however, TPC was higher than that of blueberries Bluegold, Brigitta, and Draper (19.12, 10.54, and 15.69 mg GAE/g DW, respectively); murta berries (34.92 mg GAE/g DW); and maqui berries (31.16 mg GAE/g DW) [39]. These differences may be due to the different and strong phenolic compounds detected in this plant belonging to salicaceae. On the other hand, ORAC values were also close to those of blueberries Bluegold, Brigitta, and Draper (336, 213, and 333 μmol TE/g dry weight, respectively) and maqui berries (371 μmol TE/g dry weight) and higher than those of murta Ugni molinae berries (222.78 μmol TE/g dry weight) [39]. TFC was also higher than that reported for arrayan Luma apiculata and Ugni molinae fruits (29.44 and 5.54 mg QE/g dry weight, respectively) [1]. Bleaching of ABTS radicals was more sensitive than DPPH, while ferric-reducing antioxidant power (FRAP) showed the same trend as ORAC (Table 3).
Table 3.
a TAC expressed as μg cyanidin glucoside equivalent CGE/g dry weight; b TFC expressed as μg QE/g dry weight; c TPC expressed as μg GAE/g dry weight; d DPPH, ABTS, ORAC, and FRAP expressed as μmol TE/g dry weight.
| Sample | A. serrata Fruits |
|---|---|
| TAC a | 500.40 ± 9.67 |
| TFC b | 2400.64 ± 94.45 |
| TPC c | 5700.410 ± 47.24 |
| DPPH d | 350.301 ± 10.56 |
| ABTS d | 490.626 ± 7.87 |
| ORAC d | 387.21 ± 5.27 |
| FRAP d | 426.96 ± 15.23 |
2.4. Enzyme Inhibitory Properties of Azara serrata. Ruiz & Pav.
Several important enzymes related to NCD were assayed with the methanolic extract from the A. serrata endemic fruits. The results are shown in Table 4. Some of the enzymes involved in the development of metabolic syndromes are glucosidases, amylases, and lipases. Starches are the main foods in the human diet and several enzymes participate in the digestion of carbohydrates, such as amylases in the saliva and pancreas, which act on oligosaccharides and fragment them into monosaccharides for their consequent absorption. Indeed, type-2 diabetes mellitus is one of the most prevalent metabolic diseases in the world and is characterized by hyperglycemia. Alpha-glucosidase and alpha-amylase are hydrolases that release glucose by digesting glycogen and starch and are involved in diabetes and a variety of other diseases, such as infections and cancer [40]. The inhibition of α-amylase and α-glucosidase retards the digestion and absorption of carbohydrates and subsequently suppresses postprandial hyperglycemia. The inhibition can benefit patients with altered glucose metabolism because it can retard carbohydrate absorption, producing a lowering effect on postprandial insulin levels and glucosa in the blood [41,42]. The search for natural inhibitors such as flavonoids and phenolic acids [42] could lead to the discovery of a useful natural-origin drug for subsequent treatment. In this study, the most remarkable results were of amylase (IC50: 7.23 ± 0.0), which was close to that of standard acarbose (IC50: 6.56 ± 0.0), even though glucosidase inhibition was low compared to acarbose and lipase inhibition was IC50: 32.43 ± 0.0, 15 times lower than the standard drug orlistat (Table 4). On the other side, cholinesterase enzymes (ChE) play a major role in the development of Alzheimer’s disease because they catalyze the hydrolysis and inactivation of the acetylcholine neurotransmitter, yielding choline and acetate. Cholinesterase inhibitors such as some phenolic compounds improve the cholinergic function of AD, preserving the levels of acetylcholine, and are therefore a good approach in the symptomatic treatment of AD [43]. The inhibition of these enzymes could be helpful also for dementia and Parkinson’s disease [44], autism, and schizophrenia [45]. In this research, other important inhibition results were of cholinesterases (hBuChE IC50: 12.24 ± 0.03 and TcAChE IC50: 3.92 ± 0.23), which were not too distant from those of the standard inhibitor drug galantamine (hBuChE IC50: 3.81 ± 0.02 and TcAChE IC50: 0.57 ± 0.03). Finally, tyrosinase inhibition of the Azara methanolic extract was 12 times lower than that of standard kojic acid (Table 4).
Table 4.
Enzymatic inhibitory activity (IC50, in µg/mL) of A. serrata methanolic extract against hBuChE, TcAChE, tyrosinase, lipase, glucosidase, and amylase.
| Assay | hBuChE | TcAChE | Tyrosinase | Lipase | Glucosidase | Amylase |
|---|---|---|---|---|---|---|
| A. serrata extract | 12.24 ± 0.03 | 3.92 ± 0.23 | 11.12 ± 0.10 | 32.43 ± 0.0 | 371.6 ± 0.0 | 7.23 ± 0.0 |
| Galantamine | 3.81 ± 0.02 | 0.57 ± 0.03 | - | - | - | - |
| Acarbose | - | - | - | - | 7.25 ± 0.0 | 6.56 ± 0.0 |
| Orlistat | - | - | - | 1.72 ± 0.0 | - | - |
| Kojic acid | - | - | 0.73 ± 0.04 | - | - | - |
All values are expressed as IC50, means ± SD (n = 3).
2.4.1. Docking Calculations
Identified idescarpin, coumaroyl idescarpin, and coumaroyl idesin (Figure 6) from A. serrata methanolic extract as well as the corresponding enzymatic inhibitors (galantamine, kojic acid, acarbose, and orlistat) were subjected to docking assays into each enzyme catalytic site to analyze the docking energy descriptor and the different molecular interactions between the amino acids of the catalytic site and the already-mentioned chosen molecules. The latter aimed to provide a rationale for the inhibition activities observed over the enzymes by the extract (see Table 4).
Figure 6.
Structures of a selection of salicinoids in the berries of A. serrata used for the docking calculations.
Acetylcholinesterase (TcAChE) Docking Results
Idescarpin, coumaroyl idescarpin, and coumaroyl idesin, listed in Table 5, mainly engaged in hydrogen bond interactions with the residues within the acetylcholinesterase catalytic site. Idescarpin formed several hydrogen bonds due to the nature of its structure, which possesses many oxygen atoms and polar hydrogen atoms. Therefore, idescarpin formed eight hydrogen bonds with Trp84, Gly117, Gly118, Gly119, Ser122, Tyr130, Glu199, and Ser200, as well as a π-π interaction with Phe330 (see Figure 7A). Coumaroyl idescarpin corresponds to the idescarpin molecule but esterifies with p-coumaric acid. Consequently, it shared the ability of the former to carry out multiple hydrogen bond interactions with the amino acids of the acetylcholinesterase catalytic site. The amino acids involved were Tyr70, Val71, Gln74, Tyr121, Glu199, Phe288, and His440 in hydrogen bond interactions, and there was a T-shaped interaction between Phe331 and the benzene ring of the idescarpide scaffold of coumaroyl idescarpide (Figure 7B). The latter indicated that both derivatives arranged in different manners into the catalytic site of the enzyme. On the other hand, coumaroyl idesin bears p-coumaric acid esterified with a sugar moiety and possesses a different hydroxymethylphenol ring compared to coumaroyl idescarpide. As can be seen in Figure 7C, this derivative performed seven hydrogen bond interactions with Tyr70, Tyr121, Gly118, Gly119, Ser200, Glu199, and His440. No π-π or T-shaped interactions were exhibited by the coumaroyl idesin.
Table 5.
Binding energies obtained from docking experiments of selected compounds from the A. serrata methanolic extract, as well as the known inhibitors galantamine, acarbose, orlistat, and kojic acid, over acetylcholinesterase (TcAChE), butyrylcholinesterase (hBuChE), tyrosinase, lipase, glucosidase, and amylase.
| Compound | Binding Energy (kcal/mol) Acetylcholinesterase |
Binding Energy (kcal/mol) Butyrylcholinesterase |
Binding Energy (kcal/mol) Tyrosinase |
Binding Energy (kcal/mol) Lipase |
Binding Energy (kcal/mol) Glucosidase |
Binding Energy (kcal/mol) Amylase |
|---|---|---|---|---|---|---|
| Idescarpin | −17.847 | −15.512 | −11.054 | −12.127 | −12.160 | −9.210 |
| Coumaroyl idescarpin | −16.071 | −15.115 | −10.050 | −12.831 | −11.933 | −10.150 |
| Coumaroyl idesin | −18.210 | −12.103 | −10.009 | −12.605 | −11.032 | −11.074 |
| Galantamine | −12.989 | −7.125 | ---- | ---- | ---- | ---- |
| Acarbose | ---- | ---- | ---- | ---- | −18.591 | −12.626 |
| Orlistat | ---- | ---- | ---- | −8.334 | ---- | |
| Kojic acid | ---- | ---- | −6.050 | ---- | ---- | ---- |
Figure 7.
Predicted intermolecular interactions of selected compounds in A. serrata methanolic extract and the residues of the Torpedo Californica acetylcholinesterase (TcAChE) catalytic site. Yellow dotted lines indicate hydrogen bond interactions, cyan dotted lines represent π-π interactions, and magenta dotted lines represent T-shaped interactions. (A). Idescarpin in the catalytic site. (B). Coumaroyl idescarpin in the catalytic site. (C). Coumaroyl idesin in the catalytic site.
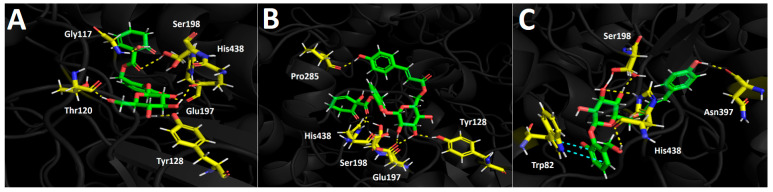
Butyrylcholinesterase (hBuChE) Docking Results
Compounds selected from the Azara serrata methanolic extract demonstrated favorable binding energies when subjected to docking experiments targeting the butyrylcholinesterase catalytic site. Once again, as seen in acetylcholinesterase docking descriptors, hydrogen bond interactions predominantly occurred with the residues of the catalytic pocket in all three derivatives tested. Idescarpin, which exhibited the best binding energy (−15.512 kcal/mol, see Table 5), formed six different hydrogen bond interactions with Gly117, Thr120, Tyr128, Glu197, Ser198, and His438 (Figure 8A). On the other hand, the binding energy exhibited by coumaroyl idescarpin was of the same order of magnitude as that of idescarpin (−15.115 kcal/mol). Although coumaroyl idescarpin formed one more hydrogen bond interaction than idescarpin (Figure 8B), the former also had greater contact with the water solvent due to the p-coumaric acid core in its structure. The solvent contact could explain the slightly unfavorable energy for this derivative, which formed hydrogen bonds with Tyr128, Glu197, Ser198, Pro285, and His438. Figure 8C shows the coumaroyl idesin arrangement in the butyrylcholinesterase catalytic site. This derivative formed four hydrogen bond interactions with Ser198, Asn 397, and His438, as well as one π-π interaction between Trp82 and the phenyl ring of the idesin moiety. Even though coumaroyl idesin formed less interactions compared with the other evaluated compounds, it also showed a good energy profile (−12.103 kcal/mol).
Figure 8.
Predicted intermolecular interactions of selected compounds in A. serrata methanolic extract and the residues of the human butyrylcholinesterase (hBuChE) catalytic site. Yellow dotted lines indicate hydrogen bond interactions and cyan dotted lines represent π-π interactions. (A). Idescarpin in the catalytic site. (B). Coumaroyl idescarpin in the catalytic site. (C). Coumaroyl idesin in the catalytic site.
Tyrosinase Docking Results
Inhibition assays of the A. Serrata methanolic extract over tyrosinase showed similar potency to the known inhibitor kojic acid (11.12 ± 0.10 µg/mL and 0.73 ± 0.04 µg/mL, respectively, see Table 4). In this sense, the binding energies of the selected compounds that underwent docking assays were consistent with the experimental evidence of enzyme inhibition employing the inhibitor (Table 5). Therefore, the energy profiles suggested that each tested derivative could exert a similar inhibition potency over the tyrosinase enzyme.
Concerning the intermolecular interactions of the selected compounds, the docking descriptors suggested that mainly hydrogen bond interactions with the residues of the catalytic site occurred, except for coumaroyl idesin, which presented fewer of these interactions and consequently had the least favorable energy profile. Although it presented a π-π interaction with His263, this interaction did not appear to contribute significantly to the binding energy (Figure 9A). Idescarpin formed hydrogen bonds with Asn260, Arg268, Met280, Gly281, Ser282, and Val283, while coumaroyl idescarpin showed hydrogen bond interactions with His85, His244, Glu322, and Asn260 and one π-π interaction with Phe264 through the phenyl aromatic ring of p-coumaric acid core ester (Figure 9B,C).
Figure 9.
Predicted intermolecular interactions of selected compounds in the A. serrata methanolic extract and the residues of the human Agaricus bisporus mushroom tyrosinase catalytic site. Yellow dotted lines indicate hydrogen bond interactions and cyan dotted lines represent π-π interactions. (A). Idescarpin in the catalytic site. (B). Coumaroyl idescarpin in the catalytic site. (C). Coumaroyl idesin in the catalytic site.
Lipase Docking Results
The binding energies of the selected three compounds from the Azara serrata methanolic extract showed significant variations, reflecting their different inhibitory capacities. All compounds exhibited good binding energies in our docking assays compared to the known inhibitor orlistat (−8.334 kcal/mol). Nonetheless, the experimental inhibition assays indicated that the extract possessed lower inhibitory potency (Table 4). Probably, antagonistic interactions among the different compounds present in the extract interfered. Although each compound individually showed good binding energy with the catalytic site of the lipase, in the context of the extract, they could have interfered with each other in their ability to inhibit the enzyme.
Each compound separately showed the following interactions depicted in Figure 10A–C: Idescarpin exhibited four hydrogen bond interactions with Tyr125, Glu193, and Ser194. Coumaroyl idescarpin showed six different hydrogen bonds with Gln66, Gly107, Tyr125, and Glu193 and a π-cation interaction, illustrated by the red lines in figure LL-B. Finally, coumaroyl idesin formed only three hydrogen bond interactions with Gly107, Glu193, and Glu388 as well as a π-π interaction with the residue of Phe324 in the catalytic site.
Figure 10.
Predicted intermolecular interactions of selected compounds in the A. serrata methanolic extract and the residues of the human bile salt activated lipase catalytic site. Yellow dotted lines indicate hydrogen bond interactions, cyan dotted lines represent π-π interactions, and red lines represent the π-cation. (A). Idescarpin in the catalytic site. (B). Coumaroyl idescarpin in the catalytic site. (C). Coumaroyl idesin in the catalytic site.
Glucosidase Docking Results
Table 4 shows that the potency of glucosidase enzyme inhibition by the methanolic extract was lower compared to the known inhibitor acarbose (371.6 ± 0.0 μg/mL and 1.72 ± 0.0 μg/mL, respectively). These data are consistent with docking experiments conducted for the three selected compounds from the methanolic extract, which showed less favorable binding energies for idescarpin, coumaroyl idescarpin, and coumaroyl idesin (Table 5). This suggests that the compounds would act as glucosidase inhibitors but with slightly lower potency. Nonetheless, each compound exhibited a good interaction profile, and, therefore, each one could behave as an effective glucosidase inhibitor on its own. In this sense, idescarpin formed nine hydrogen bond interactions with Asp1157, Asp1276, Asp1420, Lys1460, Trp1355, Trp1523, Asp1526, and His1584 (Figure 11A). Furthermore, idescarpin formed two T-shaped interactions with Phe1559 and Tyr1251. Figure 11B shows that coumaroyl idescarpin executed six hydrogen bond interactions with Asp1157, Gln1158, Asp1279, Asp1420, and Asp1526. Finally, coumaroyl idesin to the glucosidase catalytic site formed hydrogen bonds with Asp1157, Asp1279, Lys1460, and Asp1420. Tyr1251 and Phe1559 formed two T-shaped interactions with the benzene ring of the p-coumaric acid framework of this derivative.
Figure 11.
Predicted intermolecular interactions of selected compounds in the A. serrata methanolic extract and the residues of the human glucosidase catalytic site. Yellow dotted lines indicate hydrogen bond interactions, cyan dotted lines represent π-π interactions, and magenta dotted lines represent T-shaped interactions. (A). Idescarpin in the catalytic site. (B). Coumaroyl idescarpin in the catalytic site. (C). Coumaroyl idesin in the catalytic site.
Amylase Docking Results
The methanolic extract showed a strong capacity to inhibit the amylase. Table 4 shows that the extract exhibited a potency comparable to that of the inhibitor acarbose, turning it into a good agent as a glucosidase inhibitor. Similarly, docking assays showed that the binding energy profiles for the selected tested compounds were very similar to each other and comparable to the energy obtained by acarbose (−12.626 kcal/mol, Table 5).
Just like in the other enzyme docking experiments, the main interactions for all derivatives were hydrogen bonds. Therefore, idescarpin formed six hydrogen bond interactions with Tyr151, Thr163, His201, Glu233, Asp300, and Gly306 (Figure 12A). Coumaroyl idescarpin executed seven hydrogen bonds with Trp59, Gln63, Thr163, Asp197, Glu233, and Asp300 (Figure 12B). Coumaroyl idesin formed seven hydrogen bond interactions with Arg195, Asp197, Glu233, Ile235, and His305, and a T-shaped interaction was also observed, which suggested a strong and stable binding configuration.
Figure 12.
Predicted intermolecular interactions of selected compounds in the A. serrata methanolic extract and the residues of the human pancreatic alpha-amylase catalytic site. Yellow dotted lines indicate hydrogen bond interactions and magenta dotted lines represent T-shaped interactions. (A). Idescarpin in the catalytic site. (B). Coumaroyl idescarpin in the catalytic site. (C). Coumaroyl idesin in the catalytic site.
3. Materials and Methods
3.1. Plant Material
The berries of Azara serrata Ruiz & Pav. were collected in Valdivia’s Botanical Garden (39°48′16″ S 73°15′01″ W/−39.8045, −73.2502), southern Chile, in May 2023 and were identified and deposited with the voucher specimen UAChAS-230412. The berries were freeze-dried (Labconco Freezone 4.5 l, Kansas, MO, USA), and part of them was sealed in a vacuum bag and brought to Germany to perform the chromatographic analyses and prepare an enriched anthocyanin extract.
3.2. Chemicals
For the preparation of the enriched anthocyanin extract, Amberlite™ XAD-7HP was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and Sartobind® S strong acidic cation exchanger MA 75 was obtained from Sartorius Stedim Biotech GmBH (Göttingen, Germany). Chemicals used for the extraction procedure were double-deionized water (Nanopure®, Werner GmbH, Leverkusen, Germany), hexane (HPLC-grade), methanol (HPLC grade), and dichloromethane (HPLC grade), purchased from Fisher Scientific (Loughborough, U.K.); formic acid (ACS Reagent 99–100% purity) and ethyl acetate (HPLC grade), purchased from VWR Chemicals (Radnow, PA, USA); and sodium chloride (≥99.5%. p.a., ACS, ISO), disodium hydrogen phosphate dihydrate (≥99.0%, p.a.), and citric acid (≥99.5%, p.a.); obtained from Carl Roth GmbH & Co. KG (Karlsruhe, Germany). For the UHPLC-DAD analyses, water and formic acid were obtained as mentioned above and acetonitrile (HPLC-grade) was purchased from Honeywell Specialty Chemicals (Seelze, Germany). The solvents used for the UHPLC-TIMS-TOF analyses were water (LC-MS grade) and acetonitrile (UHPLC-MS-grade), purchased from TH. Geyer GmbH & Co. KG (Renningen, Germany), and formic acid (LC-MS grade), purchased from Fisher Scientific (Loughborough, UK). For quantitation of the anthocyanins, cyanidin-3-O-β-d-glucoside chloride (purity ≥ 98.00%) was purchased from Phytolab (Vestenbergsgreuth, Germany). Commercial Folin–Ciocalteu reagent, ferric chloride hexahydrate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-s-triazine, Trolox, dimethyl sulfoxide (DMSO), tyrosinase (EC1.14.18.1), acetylcholinesterase (TcAChE, EC 3.1.1.7), butyrylcholinesterase (hBuChE, EC 3.1.1.8), porcine pancreatic lipase, 4-nitrophenyl-dodecanoate, phosphate buffer, dinitrosalicylic acid, L-DOPA, kojic acid, trichloroacetic acid (Merck, Darmstadt, Germany), fetal calf serum (FCS, Gibco, Grand Island, NY, USA), L-glutamine (Merck, Darmstadt, Germany), α-amylase, α-glucosidase, standard p-nitrophenyl-d-glucopyranoside, acarbose, orlistat, sodium persulfate sodium carbonate, ferrous sulfate, sodium acetate, sodium sulfate anhydrous, and absolute ethanol were obtained from Sigma Aldrich Chem. Co. (Sigma, St. Louis, MO, USA).
3.3. Sample Preparation
3.3.1. Extract for Elucidation of the Phenolic Profiles
The extract for elucidation of the phenolic profiles was prepared by macerating approximately 30 mg of dried pulverized berries in 20 mL of a mixture of methanol/water/formic acid (49.5/49.5/1; v/v/v) for 12 h while stirring at room temperature. After filtration, the methanol was removed using a rotary evaporator at 200 mbar and 40 °C. The aqueous solution then was freeze-dried and then diluted in 1 mL of water/acetonitrile/formic acid (95/4/1; v/v/v), filtered through a 0.2 µm PTFE syringe filter, and stored at 4 °C until measured using the UHPLC-DAD-TIMS-TOF method.
3.3.2. Enriched Anthocyanin Extract for Quantitation
For the quantitation of the major anthocyanins, approximately 1 g of berries was milled using a laboratory mill (IKA-Werke, Staufen im Breisgau, Germany) and then degreased using 20 mL of n-hexane; this was repeated three times. The n-hexane was discarded and the degreased berries were then extracted using the method including analytical membrane chromatography, as described by Hopfstock et al. (2024) [46]. This procedure was carried out three times. After enrichment of the anthocyanins, the extracts were diluted in 1 mL of water/acetonitrile/formic acid (95/4/1; v/v/v), filtered through a 0.2 µm PTFE syringe filter, and stored at 4 °C until measured by UHPLC-DAD and UHPLC-DAD-TIMS-TOF. Calibration at seven levels was prepared using cyanidin-3-O-β-d-glucoside. The concentrations were 75, 50, 25, 10, 5, 2.5, and 1 mg L−1, with each level measured in duplicate. The concentration of the anthocyanins was reported as micrograms of cyanidin-3-O-β-d-glucoside equivalents per gram of dry weight (DW).
3.3.3. Purified Anthocyanin Extract
Approximately 10 g of dried berries was treated as described in the method above. The volume of the used solvents was upscaled to account for the higher amount of plant material. The extraction resulted in a yield of 8.9 mg of enriched anthocyanin extract, which was stored at 4 °C until being shipped to Chile for the enzyme tests.
3.4. Experimental Parameters
3.4.1. Ultra High Performance Liquid Chromatography (UHPLC) Diode Array Detector (DAD) Analysis for the Quantitation of the Anthocyanins
For the quantitation of the anthocyanins, an Agilent 1290 Infinity II System (Agilent Technologies, Waldbronn, Germany) equipped with a binary solvent manager, an autosampler, a column heater, and a diode array detector was used. The column was a C18 Kinetex core-shell column (2.1 mm i.d. × 100 mm, 1.7 µm). The mobile phases were (A) water/formic acid (95/5; v/v) and (B) acetonitrile/formic acid (95/5; v/v), with a column temperature of 40 °C, flow of 0.3 mL min−1, and an injection volume of 5 µL. The gradient was as follows: at initial conditions, 3% B; at 20 min, 20% B; at 30 min, 35% B; at 35 min, 95% B; at 37 min, 95% B; at 38 min, 3% B, until 40 min. The operating software for the system was OpenLAB Version 3.4 (Agilent Technologies, Waldbronn, Germany).
3.4.2. UHPLC Trapped Ion Mobility Spectrometry (TIMS) Time of Flight (TOF) Mass Spectrometry
The chromatographic analysis was performed on an Agilent 1290 Infinity system including the same parts as the system used for the qualification. The column used was an Agilent Eclipse Plus C18 RRHD (2.1 mm i.d. × 100 mm, 1.8 µm). The used mobile phases were (A) water containing 0.1% formic acid and (B) acetonitrile containing 0.1% formic acid, with a temperature of 30 °C, flow of 0.2 mL min−1, and injection volume of 2 µL. The gradient was as follows: at initial conditions, 2% B; at 25 min, 20% B; at 35 min, 35% B; at 40 min, 98% B; at 43 min, 98% B; at 45 min, 2% B, until 50 min. For the mass spectrometry, the system was a timsTOF equipped with an electrospray ionization source (Bruker Daltonik, Bremen, Germany). For ESI-negative measurements, the settings were m/z 20–1300; inversed ion mobility range 1/k0, 0.45–1.45 V s cm−2; ramp time, 100 ms; capillary voltage, 3600 V; nebulizing gas pressure, 2.20 bar (N2); dry gas flow rate, 10 L min−1 (N2); nebulizer temperature, 220 °C; collision energy, 10–50 eV (stepping). For ESI-positive measurements, the settings were scan range, m/z 100–1350; inversed ion mobility range 1/k0, 0.55–1.90 V s cm−2; ramp time, 100 ms; capillary voltage, 4500 V; nebulizing gas pressure, 2.20 bar (N2); dry gas flow rate, 10 L min−1 (N2); nebulizer temperature, 220 °C; collision energy, 10–30 eV (stepping). To calibrate the mass spectrometer and trapped ion mobility, the ESI-L Low Concentration Tuning Mix (Agilent Technologies, Waldbronn, Germany) was used; for the negative mode, the mixture was diluted by a factor of twenty. To operate the system, the software was Bruker Compass Hystar Version 6.2 and otofControl Version 6.2 (Bruker Daltonik, Bremen, Germany). For evaluating the analyses, Bruker Compass Data Analysis Version 5.3 (Bruker Daltonik, Bremen, Germany) was used.
3.5. Phytochemical Analysis
Determination of Total Phenol and Flavonoid Contents
The total phenol content (TPC) was determined using the Folin–Ciocalteau method. TPC quantification was performed based on the gallic acid standard curve and the results were expressed in μg gallic acid equivalent GAE/g (μg GAE)/g dry weight. The total flavonoid content (TFC) was determined by the aluminum chloride colorimetric method. TFC quantification was performed based on the quercetin standard curve and the results were expressed in mg quercetin equivalent (QE)/g dry weight. The total anthocyanins content (TAC) was estimated using the pH differential method [11]. The Azara methanolic extract was diluted with pH 1.0 and pH 4.5 buffers. The absorbance was measured at 510 nm and 700 nm for each of the buffers. TAC (expressed as cyanidin-3-glucoside) was calculated using the following equations: TAC = (A × MW × DF × V × 1000)/(ε × 1 × M) and A = (A510 − A700) pH 1.0 − (A510 − A700) pH 4.5, where DF is the dilution factor, MW is the molecular weight of cyanidin-3-glucoside (449 g/mol), V is the volume of extract, ε is the molar extinction coefficient of cyanidin-3-glucoside (26,900), and M is the mass of the Azara serrata extract.
3.6. Antioxidant Activity Assays
3.6.1. Ferric-Reducing Antioxidant Power (FRAP)
The ferric-reducing antioxidant power of the samples was determined according to previous procedures [47]. Briefly, 12 mM sodium acetate trihydrate buffer, pH 3.6; 10 mM TPTZ solution (2,4,6-tripyridyl-s-triazine), made up to volume with diluted HCl (40 mM) and prepared fresh the same day as used; and 20 mM FeCl3·6H2O solution were used in the assay. FRAP reagent was prepared by mixing 1020 µL pH 3.6 buffer solution, 100 µL 10 mM TPTZ, and 100 µL 20 mM FeCl3·6H2O. The microplate reaction was performed with 10 µL sample (or standard) plus 290 µL FRAP reagent, reacted for 60 min, and then read at 593 nm. Data were expressed as µmol the Trolox equivalent (TE)/g DW.
3.6.2. Free Radical Scavenging (DPPH)
Briefly, 50 µL standard or sample dissolved in EtOH plus 150 µL of DPPH solution (156 µM in EtOH) was incubated for 30 min in the dark at 35 °C. Then, the absorbance was measured at 517 nm [47]. The Trolox calibration curve was recorded between 20 and 200 µM. The results were expressed as micromoles of Trolox equivalents per g dry sample (TE/g DW).
3.6.3. ABTS Assay
The ABTS assay was performed by bleaching the cationic radical ABTS•+ as described previously [47]. Briefly, ABTS stock solution was prepared by mixing ABTS and sodium persulfate in distilled H2O, considering a final concentration of 7 mM ABTS and a final concentration of 3.6 mM for sodium persulfate (Na2S2O8). The results were expressed as micromoles of Trolox equivalents per g dry sample (TE/g DW). The Trolox curve ranged between 20 and 200 µM.
3.6.4. ORAC Assay
The ORAC assays were performed as previously described [47]. The reaction was prepared with 175 µL 108 nM fluorescein, 45 μL sample/Trolox/blank incubated at 37 °C for 30 min, and 50 μL AAPH (2,2′-Azobis(2-amidinopropane) dihydro-chloride). The mixture was then read for 2 h every 2 min at an excitation wave of 480 nm and an emission wave of 520 nm. The curve went from 1 to 20 µM. The results were expressed as micromoles of Trolox equivalents per g dry sample (TE/g DW).
3.7. Inhibition of Alpha-Amylase, Alpha-Glucosidase, and Lipase Enzymes
The ability of Azara serrata to inhibit the alpha-glucosidase was measured using the method described by Nampoothiri et al. [48] and then slightly modified [49]. Briefly, 50 μL 5 mM p-nitrophenyl-D-glucopyranoside and 50 μL 100 mM extract in sodium phosphate buffer (pH 6.9) were put together in a 96-well microplate and incubated at 37 °C for 5 min. Then, 0.1 U/mL glucosidase was added to each well at 37 °C and readings were performed at 405 nm for 30 min. The inhibitory effects of the extracts were expressed as IC50-values, which refer to the concentration that inhibits 50% of the enzyme activity. The inhibition of alpha-amylase was performed using the method described in a previous work of our group [49]. A solution of 1% starch and 100 μL extract in 20 mM sodium phosphate buffer was mixed (pH 6.9 with 6 mM sodium chloride) at 25 °C. Then, 100 μL porcine pancreatic alpha-amylase (0.5 mg/mL) was added and incubated at 25 °C for 10 min. The reaction was stopped by adding 200 μL dinitrosalicylic acid reagent at 100 °C for 5 min. The samples were cooled and 50 μL was taken from each tube and diluted by adding 200 μL water, and the absorbance was measured at 540 nm. The inhibitory effects of the extracts were expressed as IC50. Both activities were calculated using the following equation: Inhibition (%) = (1 − extract absorbance/control absorbance) × 100. Acarbose was included as a positive control.
The lipase assay was performed as reported [7,50]. Porcine pancreatic lipase type II was suspended in pure water at 20 μg/mL. Briefly, the assay mixture was Tris-HCl buffer (0.1 M, pH 8.5), lipase (10 mg/mL), and solutions of the extracts at different concentrations; the substrate solution (5 mM) was incubated for 15 min at 37 °C and the absorbance was measured at 410 nm on a microplate reader (BioTek Instrument, Inc., Winooski, VT, USA). The results were expressed as IC50-values. Orlistat was used as a positive control.
3.8. Inhibition of Acetylcholinesterase, Butyrylcholinesterase, and Tyrosinase Enzymes
The inhibitory activity of the ChE enzymes was evaluated as described by Ellman et al. [51]. Briefly, a solution was made with 5-dithio-bis(2-nitrobenzoic) acid (DTNB) in Tris-HCl buffer (pH 8.0) containing MgCl2 0.02 M and NaCl 0.1 M. Then, the Azara methanolic extract (50 mL, 2 mg/mL) was mixed in a 96-well microplate with 125 mL DTNB, acetylcholinesterase (TcAChE), or butyrylcholinesterase (hBuChE) solution (25 mL); dissolved in Tris-HCl buffer (pH 8.0); and incubated for 15 min at 25 °C. The reaction was started by the addition of acetylthiocholine iodide (ATCI) or butyrylthiocholine chloride (BTCl) (25 mL). After 10 min of reaction, the absorbance was measured at a wavelength of 405 nm and the IC50 (μg/mL) was calculated [49]. Tyrosinase inhibitory activity was measured using the modified dopachrome method with L-DOPA as a substrate [52]. The extract solution (25 μL, 2 mg/mL) was mixed with tyrosinase solution (40 μL) and phosphate buffer (100 μL, pH 6.8) in a 96-well microplate reader (BioTek Instrument, Inc., Winooski, VT, USA) and incubated for 15 min at 25 °C. The reaction was then initiated with the addition of L-DOPA (40 μL). The sample and blank absorbances were recorded at 492 nm after incubation for 10 min at 25 °C. The absorbance of the blank was subtracted from that of the sample and the tyrosinase inhibitory activity was expressed as the IC50 values, with kojic acid used as a positive control.
3.9. Docking Simulations
The partial charges plus the geometries of all compounds shown in Figure 6 were fully set using the DFT method, with set B3LYP-6-311G+ (d p) as the standard basis [53,54] in Gaussian 09W software (Gaussian, Inc.: Wallingford, CT, USA) [55]. Then, deprotonations and energetic minimizations were acquired using the LigPrep tool in Maestro Schrödinger suite v.11.8 (Schrödinger, LLC, New York, NY, USA) [56]. Torpedo Californica acetylcholinesterase structure (TcAChE; PDBID: 1DX6 code [57]) plus human butyrylcholinesterase (hBuChE; PDBID: 4BDS code [58]) and the tyrosinase obtained from Agaricus bisporus mushroom (tyrosinase; PDBID: 2Y9X code [59]) were obtained from the Protein Data Bank RCSB PDB [60]. Human bile salt-activated lipase (lipase; PDBID: 1F6W code [61]), human Maltase-Glucoamylase (Glucosidase; PDBID: 3TOP code [62]), human pancreatic alpha-amylase (Amylase; PDBID: 1B2Y code [63]), and lipase according to Terzyan et al. [61] and amylase and glucosidase according to Nahoum et al. [63] and Ren et al. [62] were obtained from the Protein Data Bank RCSB-PDB [64]. Enzyme optimizations were obtained using the Protein Preparation Wizard from Maestro software, where water molecules and ligands of the crystallographic protein active sites were removed. In the same way, all polar hydrogen atoms at pH 7.4 were added. Appropriate ionization states for acid and basic amino acid residues were considered. The OPLS3e force field was employed to minimize protein energy. The enclosing box size was set to a cube with sides of 26 Å in length. The presumed catalytic sites of each enzyme in the centroid of selected residues were chosen, considering their accepted catalytic amino acids: Ser200 for acetylcholinesterase (TcAChE) [65,66], Ser198 for butyrylcholinesterase (hBuChE) [67,68], and His263 for tyrosinase [59,69,70]. The Glide Induced Fit Docking protocol was employed for the final pairings [71]. Compounds were identified by the Glide scoring function in the extra-precision mode (Glide XP; Schrödinger, LLC, New York, NY, USA) [72] and selected by the best scores and best RMS values (cutting criterion: less than 1 unit) to obtain the potential intermolecular interactions between the enzymes and compounds plus the binding mode and docking descriptors. The different complexes were shown using a Visual Molecular Dynamics program (VMD 1.8.3 and Pymol 3.0 (Delano Scientific: San Carlos, CA, USA) [73].
3.10. Statistical Analyses
All the experiments were performed at least three times. The results were expressed as the mean plus standard deviation (SD) using the software GraphPad Prism 8. Results were compared using one-way analysis of variance (ANOVA), followed by Tukey’s HSD (honest significant difference) test (p < 0.05).
3.11. Metabolite Identification
Identification was performed (Bruker Compass DataAnalysis Version 5.3 and MetaboScape® 2022, both by Bruker Daltonik, Bremen, Germany) using the exact molecular formula and MS/MS spectra and confirmed by the available literature. The MS/MS databases used were mzCloud (https://www.mzcloud.org/, accessed on 26 July 2024, Thermo Fisher Scientific), Mass Bank of North America (MoNA, http://mona.fiehnlab.ucdavis.edu, accessed on 26 July 2024), Global Natural Product Social Molecular Networking (GNPS, http://gnps.ucsd.edu, accessed on 26 July 2024), and Human Metabolome Database (HMBD, http://www.hmdb.ca/, accessed on 26 July 2024).
4. Conclusions
In this study, the berries of the endemic species Azara serrata Ruiz & Pav. from the Chilean Valdivian Forest were studied regarding their chemical composition and bioactivities. The fruits proved to be a good source of interesting phytochemicals. Apart from containing the phenolic compounds typically associated with plants from the Salicaceae family, we were able to identify a potentially novel species of anthocyanins. Further studies are required to isolate and characterize the newly detected compounds in this species.
The phenolic and anthocyanin profiles were established using UHPLC coupled with UV/Vis detection and trapped ion mobility mass spectrometry (UHPLC-DAD-TIMS-TOF). Quantification of the anthocyanin derivatives was performed using external calibration as cyanidin-3-β-d-glucoside equivalents and various spectrophotometric assays also accounted for the other phenolics. Enzyme docking calculations were performed for a selection of compounds, showing promising results regarding their activity.
Therefore, we conclude that the berries of this ancient Mapuche medicine could be used as a source of health-promoting compounds for the preparation of nutritional supplements.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants13192756/s1: Figure S1: UHPLC-DAD-TIMS-TOF analysis of the purified anthocyanin extract of the berries from Azara serrata Ruiz & Pav.: base peak all MS positive I, DAD signal at 280 nm II, and DAD signal at 520 nm III.; Figure S2: Chromatogram of the UHPLC-DAD analysis of the purified anthocyanin extract of the berries of Azara serrata Ruiz & Pav. at 280 nm.; Figure S3: External calibration curve of cyanidin-3-O-β-d-glucoside ranging from 1 to 75 mg L−1.
Author Contributions
Conceptualization, P.H., R.G. and M.S.; methodology, P.H., J.R.-P., R.G. and M.S.; software, P.H. and J.R.-P.; validation, P.H., R.G. and M.S.; formal analysis, P.H. and J.R.-P.; investigation, P.H., R.G. and M.S.; resources, P.W. and M.S.; data curation, P.H. and M.S.; writing—original draft preparation, P.H., J.R.-P. and M.S., writing—review and editing, R.G. and P.W.; supervision, R.G. and M.S.; project administration, P.W. and M.S.; funding acquisition, P.W. and M.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by fondecyt regular 1220075.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Simirgiotis M.J., Bórquez J., Schmeda-Hirschmann G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequén. Food Chem. 2013;139:289–299. doi: 10.1016/j.foodchem.2013.01.089. [DOI] [PubMed] [Google Scholar]
- 2.Brito A., Areche C., Sepúlveda B., Kennelly E.J., Simirgiotis M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules. 2014;19:10936–10955. doi: 10.3390/molecules190810936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Don D., Johnst I.M., Fundación R.A. Philippi de Estudios Naturales Santiago de Chile. [(accessed on 26 July 2024)]. Available online: https://fundacionphilippi.cl/catalogo/azara-petiolaris/
- 4.Sagareishvili T.G., Alaniya M.D., Kemertelidze P. A new flavonol glycoside from Azara microphylla. Chem. Nat. Compd. 1983;19:275–278. doi: 10.1007/BF00579757. [DOI] [Google Scholar]
- 5.Giordano A., Retamal M., Leyton F., Martínez P., Bridi R., Velásquez P., Montenegro G. Bioactive polyphenols and antioxidant capacity of Azara petiolaris and Azara integrifolia Honeys. CyTA—J. Food. 2018;16:484–489. doi: 10.1080/19476337.2018.1426631. [DOI] [Google Scholar]
- 6.Paz C., González-Chavarría I., Freire E., Ortiz L., Karpiński T.M., Duprat F., Baggio R. Phytochemical and crystallographic studies of Azara dentata extracts and its cytotoxic effects on human breast cancer cell, MCF-7. J. Chil. Chem. Soc. 2020;65:4891–4894. doi: 10.4067/s0717-97072020000204891. [DOI] [Google Scholar]
- 7.Ramos L.C., Palacios J., Barrientos R.E., Gómez J., Castagnini J.M., Barba F.J., Tapia A., Paredes A., Cifuentes F., Simirgiotis M.J. UHPLC-MS Phenolic Fingerprinting, Aorta Endothelium Relaxation Effect, Antioxidant, and Enzyme Inhibition Activities of Azara dentata Ruiz & Pav Berries. Foods. 2023;12:643. doi: 10.3390/foods12030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeckler G.A., Gershenzon J., Unsicker S.B. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry. 2011;72:1497–1509. doi: 10.1016/j.phytochem.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Tawfeek N., Mahmoud M.F., Hamdan D.I., Sobeh M., Farrag N., Wink M., El-Shazly A.M. Phytochemistry, Pharmacology and Medicinal Uses of Plants of the Genus Salix: An Updated Review. Front. Pharmacol. 2021;12:593856. doi: 10.3389/fphar.2021.593856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqi K. Non-Communicable diseases. In: Walley J., Wright J., editors. Public Health. Oxford University Press; Oxford, UK: 2010. pp. 287–308. [Google Scholar]
- 11.Ramirez J.E., Zambrano R., Sepúlveda B., Kennelly E.J., Simirgiotis M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC-HR-ESI-ToF-MS. Food Chem. 2015;176:106–114. doi: 10.1016/j.foodchem.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Brito A., Ramirez J.E., Areche C., Sepúlveda B., Simirgiotis M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules. 2014;19:17400–17421. doi: 10.3390/molecules191117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez-Aspee F., Theoduloz C., Vieira M.N., Rodríguez-Werner M.A., Schmalfuss E., Winterhalter P., Schmeda-Hirschmann G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in human AGS cells. J. Funct. Foods. 2016;26:11–26. doi: 10.1016/j.jff.2016.06.036. [DOI] [Google Scholar]
- 14.Gironés-Vilaplana A., Baenas N., Villaño D., Speisky H., García-Viguera C., Moreno D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods. 2014;7:599–608. doi: 10.1016/j.jff.2013.12.025. [DOI] [Google Scholar]
- 15.Wu X., Beecher G.R., Holden J.M., Haytowitz D.B., Gebhardt S.E., Prior R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006;54:4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 16.Bridle P., Stott K.G., Timberlake C.F. Anthocyanins in Salix species: A new anthocyanin in salix purpurea bark. Phytochemistry. 1973;12:1103–1106. doi: 10.1016/0031-9422(73)85023-X. [DOI] [Google Scholar]
- 17.Alcalde-Eon C., García-Estévez I., Rivas-Gonzalo J.C., La Rodríguez de Cruz D., Escribano-Bailón M.T. Anthocyanins of the anthers as chemotaxonomic markers in the genus Populus L. Differentiation between Populus nigra, Populus alba and Populus tremula. Phytochemistry. 2016;128:35–49. doi: 10.1016/j.phytochem.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W., Zhao C., Karrar E., Du M., Jin Q., Wang X. Analysis of Chemical Composition and Antioxidant Activity of Idesia polycarpa Pulp Oil from Five Regions in China. Foods. 2023;12:1251. doi: 10.3390/foods12061251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bochi V.C., Godoy H.T., Giusti M.M. Anthocyanin and other phenolic compounds in Ceylon gooseberry (Dovyalis hebecarpa) fruits. Food Chem. 2015;176:234–243. doi: 10.1016/j.foodchem.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Ajay S., Panicker J.S., Krishnan R.R., Prema K.H. Greener Extraction of Anthocyanin Pigment from Syzygium samarangenese and Flacourtia jangomas: An Alternative to Synthetic pH Indicators. Waste Biomass Valorization. 2023;15:1175–1184. doi: 10.1007/s12649-023-02245-x. [DOI] [Google Scholar]
- 21.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng H., Fang T., Lin Q., Song H., Liu B., Chen L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017;66:153–165. doi: 10.1016/j.tifs.2017.05.015. [DOI] [Google Scholar]
- 23.Feistel F., Paetz C., Menezes R.C., Veit D., Schneider B. Acylated Quinic Acids Are the Main Salicortin Metabolites in the Lepidopteran Specialist Herbivore Cerura vinula. J. Chem. Ecol. 2018;44:497–509. doi: 10.1007/s10886-018-0945-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.H., Jang Y.P., Sung S.H., Kim Y.C. Inhibitory activity of phenolic glycosides from the fruits of Idesia polycarpa on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Planta Medica. 2007;73:167–169. doi: 10.1055/s-2006-951771. [DOI] [PubMed] [Google Scholar]
- 25.Kim T.B., Kim H.W., Lee M., Lee H.H., Kim S.H., Kang S.K., Sung S.H. Isolation and structure elucidation of (−)-Idescarparide, a new spiro compound from Idesia polycarpa. Tetrahedron Lett. 2014;55:5447–5449. doi: 10.1016/j.tetlet.2014.08.010. [DOI] [Google Scholar]
- 26.Ward J.L., Wu Y., Harflett C., Onafuye H., Corol D., Lomax C., Macalpine W.J., Cinatl J., Wass M.N., Michaelis M., et al. Miyabeacin: A new cyclodimer presents a potential role for willow in cancer therapy. Sci. Rep. 2020;10:6477. doi: 10.1038/s41598-020-63349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Freitas V., Mateus N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011;401:1463–1473. doi: 10.1007/s00216-010-4479-9. [DOI] [PubMed] [Google Scholar]
- 28.He J., Carvalho A.R.F., Mateus N., de Freitas V. Spectral features and stability of oligomeric pyranoanthocyanin-flavanol pigments isolated from red wines. J. Agric. Food Chem. 2010;58:9249–9258. doi: 10.1021/jf102085e. [DOI] [PubMed] [Google Scholar]
- 29.Cruz L., Petrov V., Teixeira N., Mateus N., Pina F., de Freitas V. Establishment of the chemical equilibria of different types of pyranoanthocyanins in aqueous solutions: Evidence for the formation of aggregation in pyranomalvidin-3-O-coumaroylglucoside-(+)-catechin. J. Phys. Chem. B. 2010;114:13232–13240. doi: 10.1021/jp1045673. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz M., Jerz G., Winterhalter P. Isolation and structure of Pinotin A, a new anthocyanin derivative from Pinotage wine. Vitis. 2003;42:105–106. [Google Scholar]
- 31.Mateus N., Silva A.M.S., Rivas-Gonzalo J.C., Santos-Buelga C., de Freitas V. A new class of blue anthocyanin-derived pigments isolated from red wines. J. Agric. Food Chem. 2003;51:1919–1923. doi: 10.1021/jf020943a. [DOI] [PubMed] [Google Scholar]
- 32.Hillebrand S., Schwarz M., Winterhalter P. Characterization of anthocyanins and pyranoanthocyanins from blood orange Citrus sinensis (L.) Osbeck juice. J. Agric. Food Chem. 2004;52:7331–7338. doi: 10.1021/jf0487957. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz M., Wray V., Winterhalter P. Isolation and identification of novel pyranoanthocyanins from black carrot (Daucus carota L.) juice. J. Agric. Food Chem. 2004;52:5095–5101. doi: 10.1021/jf0495791. [DOI] [PubMed] [Google Scholar]
- 34.Andersen Ø.M., Fossen T., Torskangerpoll K., Fossen A., Hauge U. Anthocyanin from strawberry (Fragaria ananassa) with the novel aglycone, 5-carboxypyranopelargonidin. Phytochemistry. 2004;65:405–410. doi: 10.1016/j.phytochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Fossen T., Andersen Ø.M. Anthocyanins from red onion, Allium cepa, with novel aglycone. Phytochemistry. 2003;62:1217–1220. doi: 10.1016/S0031-9422(02)00746-X. [DOI] [PubMed] [Google Scholar]
- 36.Liu S., Laaksonen O., Yang W., Zhang B., Yang B. Pyranoanthocyanins in bilberry (Vaccinium myrtillus L.) wines fermented with Schizosaccharomyces pombe and their evolution during aging. Food Chem. 2020;305:125438. doi: 10.1016/j.foodchem.2019.125438. [DOI] [PubMed] [Google Scholar]
- 37.Ponder A., Hallmann E., Kwolek M., Średnicka-Tober D., Kazimierczak R. Genetic Differentiation in Anthocyanin Content among Berry Fruits. Curr. Issues Mol. Biol. 2021;43:36–51. doi: 10.3390/cimb43010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genskowsky E., Puente L.A., Pérez-Álvarez J.A., Fernández-López J., Muñoz L.A., Viuda-Martos M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui Aristotelia chilensis (Molina) Stuntz a Chilean blackberry. J. Sci. Food Agric. 2016;96:4235–4242. doi: 10.1002/jsfa.7628. [DOI] [PubMed] [Google Scholar]
- 39.INTA Portal Antioxidantes. 2024. [(accessed on 26 July 2024)]. Available online: https://portalantioxidantes.com/orac-base-de-datos-actividad-antioxidante-y-contenido-de-polifenoles-totales-en-frutas/
- 40.Assefa S.T., Yang E.-Y., Chae S.-Y., Song M., Lee J., Cho M.-C., Jang S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants. 2019;9:2. doi: 10.3390/plants9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Laar F.A., Lucassen P.L., Akkermans R.P., van de Lisdonk E.H., Rutten G.E., van Weel C. Alpha-Glucosidase inhibitors for patients with type 2 diabetes: Results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 42.Liu K., Luo M., Wei S. The Bioprotective Effects of Polyphenols on Metabolic Syndrome against Oxidative Stress: Evidences and Perspectives. Oxidative Med. Cell. Longev. 2019;2019:6713194. doi: 10.1155/2019/6713194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar A., Singh A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Rolinski M., Fox C., Maidment I., McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst. Rev. 2012;2012:CD006504. doi: 10.1002/14651858.CD006504.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frye R.E., Rossignol D.A. Treatments for biomedical abnormalities associated with autism spectrum disorder. Front. Pediatr. 2014;2:66. doi: 10.3389/fped.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hopfstock P., Punyasiri P.A.N., Kiene M., Kottawa-Arachchi J.D., Gök R., Winterhalter P. Characterization and Quantitation of Anthocyanins of the Pigmented Tea Cultivar TRI 2043 (Camellia sinensis L.) from Sri Lanka. Separations. 2024;11:157. doi: 10.3390/separations11050157. [DOI] [Google Scholar]
- 47.Larrazábal-Fuentes M.J., Fernández-Galleguillos C., Palma-Ramírez J., Romero-Parra J., Sepúlveda K., Galetovic A., González J., Paredes A., Bórquez J., Simirgiotis M.J., et al. Chemical Profiling, Antioxidant, Anticholinesterase, and Antiprotozoal Potentials of Artemisia copa Phil. (Asteraceae) Front. Pharmacol. 2020;11:594174. doi: 10.3389/fphar.2020.594174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nampoothiri S.V., Prathapan A., Cherian O.L., Raghu K.G., Venugopalan V.V., Sundaresan A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011;49:125–131. doi: 10.1016/j.fct.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Muñoz-Molina N., Parada J., Simirgiotis M., Montecinos-González R. The Potential of Using Cochayuyo (Durvillaea incurvata) Extract Obtained by Ultrasound-Assisted Extraction to Fight against Aging-Related Diseases. Foods. 2024;13:269. doi: 10.3390/foods13020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres-Benítez A., Ortega-Valencia J.E., Sánchez M., Hillmann-Eggers M., Gómez-Serranillos M.P., Vargas-Arana G., Simirgiotis M.J. UHPLC-MS Chemical Fingerprinting and Antioxidant, Enzyme Inhibition, Anti-Inflammatory In Silico and Cytoprotective Activities of Cladonia chlorophaea and C. gracilis (Cladoniaceae) from Antarctica. Antioxidants. 2022;12:10. doi: 10.3390/antiox12010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellman G.L., Courtney K.D., Andres V., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 52.Mocan A., Zengin G., Simirgiotis M., Schafberg M., Mollica A., Vodnar D.C., Crişan G., Rohn S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017;32:153–168. doi: 10.1080/14756366.2016.1243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adamo C., Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999;110:6158–6170. doi: 10.1063/1.478522. [DOI] [Google Scholar]
- 54.Petersson G.A., Bennett A., Tensfeldt T.G., Al-Laham M.A., Shirley W.A., Mantzaris J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988;89:2193–2218. doi: 10.1063/1.455064. [DOI] [Google Scholar]
- 55.Frisch M.J., Hiscocks J. Gaussian 09. Gaussian, Inc.; Wallingford, CT, USA: 2009. Revision B. 01. [Google Scholar]
- 56.Release S. 2: Maestro, Version 11.8. Schrödinger, LLC; New York, NY, USA: 2018. [Google Scholar]
- 57.Greenblatt H.M., Kryger G., Lewis T., Silman I., Sussman J.L. Structure of acetylcholinesterase complexed with (-)—Galanthamine at 2.3 A resolution. FEBS Lett. 1999;463:321–326. doi: 10.1016/S0014-5793(99)01637-3. [DOI] [PubMed] [Google Scholar]
- 58.Nachon F., Carletti E., Ronco C., Trovaslet M., Nicolet Y., Jean L., Renard P.-Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl-and butyryl-cholinesterase. Biochem. J. 2013;453:393–399. doi: 10.1042/BJ20130013. [DOI] [PubMed] [Google Scholar]
- 59.Ismaya W.T., Rozeboom H.J., Weijn A., Mes J.J., Fusetti F., Wichers H.J., Dijkstra B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry. 2011;50:5477–5486. doi: 10.1021/bi200395t. [DOI] [PubMed] [Google Scholar]
- 60.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., Shindyalov I.N., Bourne P.E. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terzyan S., Wang C.S., Downs D., Hunter B., Zhang X.C. Crystal structure of the catalytic domain of human bile salt activated lipase. Protein Sci. 2000;9:1789–1790. doi: 10.1110/ps.9.9.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren L., Qin X., Cao X., Wang L., Bai F., Bai G., Shen Y. Structural insight into substrate specificity of human intestinal maltase-glucoamylase. Protein Cell. 2011;2:827–836. doi: 10.1007/s13238-011-1105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nahoum V., Roux G., Anton V., Rougé P., Puigserver A., Bischoff H., Henrissat B., Payan F. Crystal structures of human pancreatic alpha-amylase in complex with carbohydrate and proteinaceous inhibitors. Biochem. J. 2000;346:201–208. doi: 10.1042/bj3460201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westbrook J. RCSB Protein Data Bank. Structural biology views for basic and applied research. Acta Crystallogr. 2017;A73:a308. doi: 10.1107/S0108767317096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sussman J.L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 66.Silman I., Harel M., Axelsen P., Raves M., Sussman J.L. Three-Dimensional structures of acetylcholinesterase and of its complexes with anticholinesterase agents. Biochem. Soc. Trans. 1994;22:745–749. doi: 10.1042/bst0220745. [DOI] [PubMed] [Google Scholar]
- 67.Nicolet Y., Lockridge O., Masson P., Fontecilla-Camps J.C., Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- 68.Tallini L.R., Bastida J., Cortes N., Osorio E.H., Theoduloz C., Schmeda-Hirschmann G. Cholinesterase Inhibition Activity, Alkaloid Profiling and Molecular Docking of Chilean Rhodophiala (Amaryllidaceae) Molecules. 2018;23:1532. doi: 10.3390/molecules23071532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Da Silva A.P., Silva N.d.F., Andrade E.H.A., Gratieri T., Setzer W.N., Maia J.G.S., Da Silva J.K.R. Tyrosinase inhibitory activity, molecular docking studies and antioxidant potential of chemotypes of Lippia origanoides (Verbenaceae) essential oils. PLoS ONE. 2017;12:e0175598. doi: 10.1371/journal.pone.0175598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J., Ye Y., Ran M., Li Q., Ruan Z., Jin N. Inhibition of Tyrosinase by Mercury Chloride: Spectroscopic and Docking Studies. Front. Pharmacol. 2020;11:81. doi: 10.3389/fphar.2020.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sherman W., Day T., Jacobson M.P., Friesner R.A., Farid R. Novel Procedure for Modeling Ligand/Receptor Induced Fit Effects. J. Med. Chem. 2006;49:534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- 72.Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R., Halgren T.A., Sanschagrin P.C., Mainz D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 73.DeLano W.L. The PyMOL Molecular Graphics System. Delano Scientific; San Carlos, CA, USA: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.