Abstract
Background
Cardiovascular disease (CVD), particularly ischemic heart disease, remains the leading cause of death and morbidity in patients with type 1 diabetes. Detecting subclinical atherosclerosis could enhance cardiovascular risk stratification and enable individualised therapies. The aim of this study is to investigate the prevalence and predictors of subclinical atherosclerosis in patients with type 1 diabetes without overt cardiovascular disease (CVD) and to assess its impact on patient survival over a follow-up period of at least 5 years.
Methods
This observational study included 507 patients treated at the Diabetes Unit of the Hospital of Girona Doctor Josep Trueta between 2015 and 2023. The inclusion criteria for patients were as follows: those aged 18 and older with diabetes for a minimum of 10 years or those aged 40 and older with a diabetes for at least 5 years. Subclinical atherosclerosis was identified via ultrasound imaging of the carotid and femoral arteries. Clinical and biochemical evaluations were also conducted. Major cardiovascular events (MACE) and deaths from other causes were monitored, and survival analysis was performed using Kaplan‒Meier methods.
Results
Subclinical atherosclerosis was detected in 218 patients (43%). Multivariate analysis revealed that the male sex, diabetic nephropathy, tobacco exposure, higher HbA1c levels, older age, and longer diabetes duration were significant predictors. During a mean follow-up of 70.64 ± 27.08 months, 19 patients experienced MACE, and 13 died from any cause. The probability of MACE or death was greater in patients with subclinical atherosclerosis, with a hazard ratio (HR) of 25.1 (95% CI 5.81–108, p < 0.001) for MACE and an odds ratio (OR) of 7.57 (95% CI 1.97–53.9, p = 0.004) for death.
Conclusion
Subclinical atherosclerosis is independently associated with increased overall mortality and MACE in patients with type 1 diabetes. Identifying clinical predictors can improve risk stratification and personalised therapeutic strategies to prevent MACEs in this high-risk population.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02455-0.
Keywords: Subclinical atherosclerosis, Type 1 diabetes, Cardiovascular disease mortality
Background
Cardiovascular disease (CVD), particularly ischemic heart disease, remains the leading cause of death and morbidity in patients with type 1 diabetes [1]. However, the role of traditional cardiovascular risk factors (CVRFs), such as hypertension, dyslipidaemia and smoking, in this population is not well defined, and the pathophysiology of cardiovascular events is not fully understood. Even patients under 40 years old without traditional CVRFs, both men and women, are 5–10 times more likely to develop coronary disease [2], with the risk increasing with an earlier diagnosis of type 1 diabetes [2, 3].
The prevalence of CVD in people with type 1 diabetes is known to depend on diabetes duration, age, sex, and glycaemic control [4, 5], and microvascular complications further increase the risk of CVD in these patients [6–11]. Consequently, cardiovascular event prediction models used for the general population, which rely on traditional CVRFs, are not applicable to individuals with type 1 diabetes because their risk is underestimated [5, 12, 13]. Specific CVD prediction models for type 1 diabetes patients, such as the Steno Type 1 Risk Engine [14] (ST1RE), may be more accurate because they consider the key etiopathogenic factors for atherosclerosis in this population.
The detection of subclinical atherosclerosis could enhance cardiovascular risk stratification and enable individualised therapeutic strategies, potentially preventing it [15, 16]. However, routine screening for asymptomatic patients with type 1 diabetes is currently not recommended [17].
This study aimed to determine the prevalence of subclinical atherosclerosis and its clinical predictors in a patient cohort with type 1 diabetes without overt CVD and, secondarily, to study the impact of subclinical atherosclerosis on patient survival over a follow-up period of at least 5 years.
Methods
Participants and setting
Patients with type 1 diabetes treated in the Diabetes Unit of our centre were consecutively recruited between 2015 and 2023 during their annual medical evaluation for chronic complications and CVRF assessment. The inclusion criteria were patients with type 1 diabetes who were over 18 years of age with diabetes for at least 10 years or patients with type 1 diabetes who were over 40 years of age with diabetes for at least 5 years. Patients were excluded if they had a history of CVD confirmed by clinical history review or if they were diagnosed with a life-threatening disease with a shortened life expectancy at the time of recruitment.
Clinical evaluation included age, sex, weight, height, BMI and abdominal perimeter, which were measured by standardised methods and recorded in the electronic medical history. Blood pressure (BP) (average of 2 measurements separated by 5 min) was measured with a BP monitor (DINAMAP V100 Carescape) after 10 min of sitting. Hypertension (HTA) was defined as a BP above 140/90 mmHg or the use of antihypertensive treatment. The duration of type 1 diabetes, the presence of micro- or macrovascular complications, dyslipidaemia, and medical treatment (statins, antiplatelet agents, and antihypertensive agents) were systematically recorded. Microangiopathy was defined by the presence of either retinopathy (diagnosed by either screening retinography or ophthalmologist examination), nephropathy (diagnosed by urine albuminuria tests and estimated glomerular filtrate rate) or polyneuropathy (diagnosed by typical symptoms and clinical exploration). Severe hypoglycaemic events requiring third party assistance within the last 5 years were also recorded.
Exposure to tobacco was defined as smoking at any time in life, regardless of current smoking habit. Tobacco consumption was measured in pack-years, where one pack per day for one year was considered a pack-year. Patients were considered to be physically active if they engaged in exercise for 3.5 or more hours per week, outside of working hours, regardless of the intensity and type of exercise. Metabolic syndrome (MetS) was defined according to the criteria of National Cholesterol Education Programme Adult Treatment Panel III (ATP III) [18], excluding the diagnosis of diabetes.
Biochemical measures: Blood and urine samples were collected in a fasting state and analysed locally using standardised methods to measure glucose, glycosylated haemoglobin (HbA1c) and lipid profiles (including total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and triglyceride levels).
The cardiovascular risk (CVR) for all individuals was estimated using the Steno Type 1 Risk Engine calculator (ST1RE) [14].
Ultrasound images were obtained interchangeably by two trained researchers using a high-resolution B-mode ultrasound system (Philips ClearVue 550) equipped with a 15 MHz linear array probe. The bilateral extracranial carotid trees (common carotid artery (CCA), carotid bifurcation (CB), internal carotid artery (ICA) and external carotid artery (ECA)) were examined to assess the presence of plaques on both the proximal and distal walls of all carotid segments. The 20 mm segment of the common femoral artery (CFA) proximal to the bifurcation of the deep femoral artery was examined to assess the presence of plaques on both the proximal and distal walls. The 20 mm segment of the superficial femoral artery (SFA) proximal to the bifurcation was also examined. Plaques were defined as a thickening from the intima-lumen to the half-adventitia of > 1.5 mm or focal thickening of the wall with an arterial luminal encroachment of at least 50% of the surrounding intima-media thickness [19]. The number and location of plaques were recorded.
Subclinical atherosclerosis was defined as the presence of at least one plaque in any of the carotid or femoral segments examined. The atherosclerotic burden was defined as the total number of plaques found in a patient.
Acute myocardial infarction (AMI), angina (confirmed by exercise stress testing or cardiac catheterisation), stroke (haemorrhagic or ischemic), peripheral arterial disease with or without amputation, and deaths of cardiovascular origin were considered major cardiovascular events (MACE). Silent AMI was defined by ECG changes suggestive of myocardial ischemia in patients with previously normal ECG, without chest pain but with elevated cardiac enzymes (Creatin Kinase and troponin T), and afterwards confirmed by echocardiogram with either segmental wall motion abnormalities, resting perfusion defects on nuclear scintigraphy or coronary angiography. Vital status data were obtained from the electronic medical records of patients who did not attend follow-up.
The study was approved by the local ethical review board.
Statistical analysis
Sample calculation and justification
The primary aim of our study was to determine the prevalence of subclinical atherosclerosis in patients with type 1 diabetes in our area. Given that the number of patients who met the inclusion criteria typically followed by our unit was 1000 and that previous studies had reported a 35% prevalence of subclinical atherosclerosis in patients with similar characteristics [20], to detect subclinical atherosclerosis in 35% of patients with 3% precision and a confidence level of 95%, a sample size of n = 493 patients was required. Although there are no generally accepted methods for estimating sample size requirements for risk prediction model studies [21], empirical investigations suggest a rule of thumb of having 10–15 events per variable (EPV) to produce stable estimates [22–24]. Therefore, assuming a 43% event rate and at least 10 potential predictors, 233 patients are needed to achieve stable estimates.
A descriptive analysis of participants’ characteristics was conducted. Categorical variables are presented as counts and percentages, while continuous variables are expressed as the means with standard deviations (SDs) or medians with interquartile ranges.
The results were stratified by the presence or absence of subclinical atherosclerosis and by sex. The normality of the distribution was assessed using the Shapiro‒Wilk test. The Student’s t test or the Mann‒Whitney U test was used for continuous variables to detect group differences. The chi-square test or Fisher's exact test was used for categorical comparisons.
Regression analyses included univariate and multivariate binary logistic regressions to identify factors significantly impacting subclinical atherosclerosis and atherosclerotic burden. Clinically important variables [25] and those having a significance level of p < 0.20 in univariate analysis were included in the multivariate analysis using a stepwise forward procedure. Sex, tobacco exposure, and Hb1Ac were included as potential confounders irrespective of significance. The results of the final model are reported as odds ratios (ORs) with 95% confidence intervals (CIs). Model adequacy was assessed using the Hosmer‒Lemeshow test, and multicollinearity was checked using variance inflation factors.
Survival analysis was performed using the Kaplan‒Meier method, with group comparisons conducted using the log-rank test. Statistical analyses were carried out using SPSS Statistics 26 (IBM Corp., Armonk, NY), Stata/IC 13.1 (StataCorp LP, College Station, TX), and RStudio v4.3.0. All tests were two-sided, and the results were considered statistically significant at p < 0.05.
Results
Cohort description
A total of 507 patients who met the inclusion criteria were enrolled at our centre, 53.6% of whom were male. Over a mean follow-up period of 70.64 ± 27.08 months, 6 patients were lost to follow-up, 13 patients died, and there were 19 fatal and nonfatal major adverse cardiovascular events (MACEs). (Fig. 1).
Fig. 1.
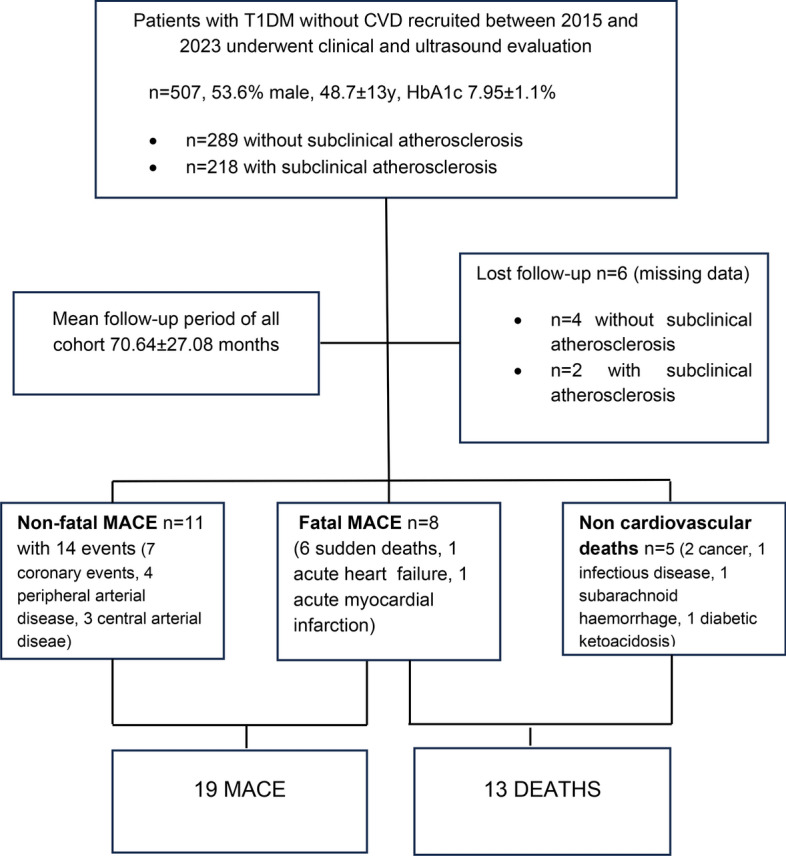
Study flowchart
Characteristics of the cohort
Table 1 presents the baseline clinical and analytical characteristics of the cohort. Subclinical atherosclerosis was identified in 218 patients (43%), with a greater prevalence among men (61.9% vs. 38.1%; p = 0.002) (Table 1). Patients with atherosclerotic plaques were older (56.9 ± 10.8 vs. 42.5 ± 11.1 years; p < 0.001) and had diabetes for a longer period of time (26.4 ± 12.5 vs. 20.9 ± 8.58 years; p < 0.001), higher rates of microangiopathy (62.8% vs. 44.07%; p < 0.001), and more episodes of severe hypoglycaemia (45% vs. 31.1%; p = 0.007) than those without plaques. Patients with subclinical atherosclerosis also exhibited higher creatinine levels (0.87 ± 0.3 mg/dl vs. 0.79 ± 0.2 mg/dl; p < 0.001) and triglyceride levels (107 ± 71.3 mg/dl vs. 90.7 ± 49.8 mg/dl; p = 0.004), while LDL-c levels were slightly lower (89.2 ± 26.9 mg/dl vs. 93.8 ± 24.6 mg/dl). Statin use was more prevalent in patients with subclinical atherosclerosis (67.9% vs. 27%).
Table 1.
Clinical and analytical description of the whole cohort and comparison between patients with and without subclinical atherosclerosis (SA)
| Variables | N = 507 | Without SA N = 289 | With SA N = 218 | P |
|---|---|---|---|---|
| Sex | ||||
| Female | 235 (46.4%) | 152 (52.6%) | 83 (38.1%) | 0.002 |
| Male | 272 (53.6%) | 137 (47.4%) | 135 (61.9%) | |
| Age (years) | 48.7 ± 13.0 | 42.5 ± 11.1 | 56.9 ± 10.8 | < 0.001 |
| Age at diabetes diagnosis (years) | 25.0 ± 14.2 | 21.1 ± 12.7 | 30.1 ± 14.5 | < 0.001 |
| Diabetes duration (years) | 23.2 ± 10.8 | 20.9 ± 8.58 | 26.4 ± 12.5 | < 0.001 |
| HbA1c (%) mmol/mol |
7.95 ± 1.1 63 ± 5 |
7.90 ± 1.14 63 ± 5 |
8.02 ± 1.03 64 ± 5 |
0.231 |
| Microangiopathy n (%) | 265(52.3%) | 128(44.3%) | 137(62.8%) | < 0.001 |
| Patients with any severe Hypoglycaemia episodes n(%) | 187(37.1%) | 89(31.1%) | 98(45%) | 0.007 |
| Other autoimmune disease n (%) | 195(38.5%) | 114(39.4%) | 81(37.2%) | 0.665 |
| Exercise n(%) | 236(48.5%) | 138(47.8%) | 98(45%) | 0.532 |
| Tobacco exposure n (%) | 221(43.6%) | 106(36.7%) | 115(52.8%) | < 0.001 |
| Tobacco consumption (pack/year) | 8.1 ± 14.2 | 4.89 ± 10.7 | 12.5 ± 17 | < 0.001 |
| Current Smoking | 111(21.9%) | 58 (19.9%) | 53(24.5%) | 0.215 |
| HTA n (%) | 244(48.1%) | 99(34.0%) | 145(67.1%) | < 0.001 |
| sBP (mmHg) | 132 ± 16.2 | 129 ± 14.3 | 135 ± 17.5 | < 0.001 |
| dBP (mmHg) | 74.6 ± 9.4 | 74.2 ± 9.44 | 75.2 ± 9.46 | 0.226 |
| Total Cholesterol mg/dl | 177 ± 30.7 | 178 ± 29.2 | 177 ± 32.6 | 0.622 |
| HDL-c mg/dl | 65.8 ± 18.6 | 65.8 ± 16.5 | 65.8 ± 21.2 | 0.982 |
| LDL-c mg/dl | 91.8 ± 25.7 | 93.8 ± 24.6 | 89.2 ± 26.9 | 0.046 |
| Tryglicerides mg/dl | 97.7 ± 60.4 | 90.7 ± 49.8 | 107 ± 71.3 | 0.004 |
| Creatinine mg/dl | 0.83 ± 0.29 | 0.79 ± 0.2 | 0.87 ± 0.3 | < 0.001 |
| BMI (kg/m2) | 26.3 ± 4.21 | 25.8 ± 4.31 | 27 ± 3.97 | 0.001 |
| BMI > or = 30 kg/m2 | 82 (16.2%) | 42 (14.5%) | 40 (18.3%) | 0.301 |
| Central Obesity (ATP III) | 219 (43.9%) | 107 (37.8%) | 112 (51.9%) | 0.002 |
| MetS (ATPIII) n (%) | 48 (9.62%) | 18 (6.3%) | 30 (14.0%) | 0.003 |
| Statins n (%) | 226 (44.6%) | 78 (27%) | 148 (67.9%) | < 0.001 |
| Antihypertensives n (%) | 186 (36.7%) | 61 (21.1%) | 125 (57.3%) | < 0.001 |
| Antiplatelets n (%) | 128 (25.2%) | 30 (10.4%) | 98 (45%) | < 0.001 |
| ST1RE | ||||
| Low n (%) | 169 (33.5%) | 151 (52.6%) | 18 (8.3%) | < 0.001 |
| Medium n (%) | 181 (35.9%) | 99 (34.5%) | 82 (37.8%) | |
| High n (%) | 154 (30.6%) | 37 (12.9%) | 117 (53.9%) | |
Data are shown as percentages for categorical variables and mean ± SD for continuous variables with normal distribution
SA subclinical atherosclerosis; HbA1c glycated haemoglobin; HTA hypertension; sBP systolic blood pressure; dBP diastolic blood pressure; HDL-c high-density lipoprotein cholesterol; LDL-c low-density lipoprotein cholesterol; BMI body mass index, ATPIII National Cholesterol Education Program Adult Treatment Panel III (ATP III) criteria [13]; MetS metabolic syndrome; ST1RE Steno T1 Risk Engine
Regarding CVRFs, patients with subclinical atherosclerosis were more likely to have smoked (52.8% vs. 36.7%; p < 0.001) and had greater cumulative tobacco exposure (12.5 ± 17 vs. 4.89 ± 10.7 pack-years; p < 0.001) than those without plaques. Additionally, patients with plaques were more frequently diagnosed with hypertension (67.1% vs. 34%; p < 0.001) and had a greater BMI (27 ± 3.97 vs. 25.8 ± 4.31 kg/m2; p < 0.001) than those without plaques. No significant differences were observed in HbA1c levels, active smoking status, exercise habits, or the presence of other autoimmune diseases between the groups. All patients included in the study were receiving intensive insulin therapy.
Overall, the estimated CVRs of ST1RE in our cohort were 9.6 ± 9.31% at 5 years and 17.4 ± 14.3% at 10 years, with no differences between the sexes. Stratification of CVRs by ST1RE classified 30.6% of patients as high risk, 35.9% as moderate risk, and 33.5% as low risk. Subclinical atherosclerosis was diagnosed in 18 out of 169 (10.7%) patients classified as low risk, in 82 out of 181 (45.3%) patients classified as moderate risk, and in 117 out of 154 (76%) patients classified as high risk (p < 0.001).
The distribution of plaques among vascular territories in patients with subclinical atherosclerosis was as follows: 116 (53.2%) patients had both carotid and femoral plaques, 61 (27.9%) had only plaques in the femoral region, and 41 (18.8%) presented only plaques in the carotid region. Regarding atheromatous burden, 109 (50%) patients had plaques in 1–2 segments, while the remainder had plaques in 3 segments or more. Twenty-four (11%) patients had 7 or more segments with plaques.
Multivariate analysis revealed male sex (OR 3.23, p < 0.001), diabetic nephropathy (OR 2.33, p 0.006), tobacco exposure (OR 1.64, p 0.037), HbA1c (OR 1.25, p 0.035), age (OR 1.14, p < 0.001), and diabetes duration (OR 1.02, p 0.022) as predictors of the presence of subclinical atherosclerosis (Fig. 2).
Fig. 2.
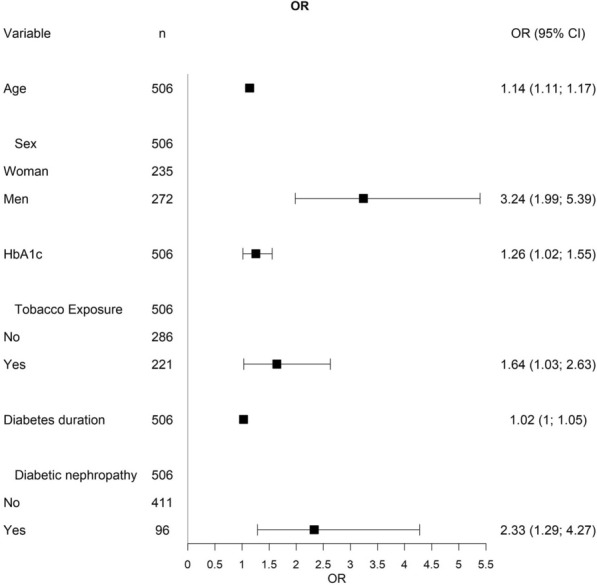
Clinical predictors of the presence of subclinical atherosclerosis
Diabetes nephropathy (OR 2.69, p 0.032), age (OR 1.08, p < 0.001), tobacco consumption (packs/year) (OR 1.05, p 0.003), and diabetes duration (OR 1.04, p 0.018) were identified as predictors of atherosclerotic burden (Fig. 3).
Fig. 3.
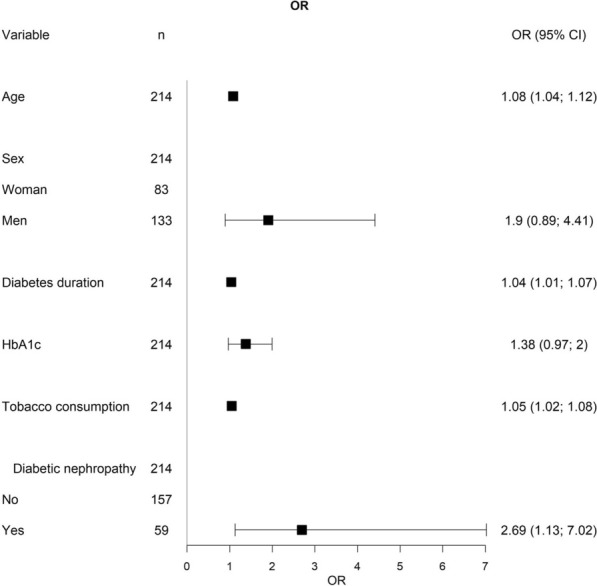
Clinical predictors of atherosclerotic burden (defined as the number of atherosclerotic plaques)
Follow-up data
During a mean follow-up of 70.64 ± 27.08 months, there were 13 deaths, comprising 8 fatal MACE and 5 non cardiovascular deaths (2 due to cancer (1 myeloma, 1 lung cancer), 1 due to infectious disease, 1 due to subarachnoid haemorrhage, and 1 due to diabetic ketoacidosis). The characteristics of the patients who either died or experienced MACE during follow-up are listed in Table 2.
Table 2.
Characteristics of patients with MACE (fatal and non-fatal) and non CV deaths
| Patient number | Sex | Age (years) | Diabetes duration (years) | ST1RE estimation 5/10 years (%) (risk stratification) | Localisation of subclinical atherosclerosis | Atherosclerotic burden (number of plaques) | Type of CV event |
|---|---|---|---|---|---|---|---|
| Non-Fatal MACE n = 11 | |||||||
| Patient 1 | Male | 62 | 41 | 18.2/33.2 (High) | Carotid and femoral | 10 | AMI no Q |
| Patient 2 | Male | 66 | 31 | 32/53.8 (High) | Carotid and femoral | 5 |
Silent AMI Minor amputation |
| Patient 3 | Male | 70 | 41 | 18.3/33.2 (High) | Carotid and femoral | 10 | Silent AMI |
| Patient 4 | Male | 73 | 18 | 22.9/40.5 (High) | Carotid and femoral | 4 | Angina |
| Patient 5 | Male | 73 | 42 | 24/42.3 (High) | Carotid and femoral | 9 | Silent AMI |
| Patient 6 | Male | 49 | 12 | 8.3/16 (Moderate) | Carotid and femoral | 5 | AMI |
| Patient 7 | Male | 68 | 60 | 37.4/60.9 (High) | Carotid and femoral | 6 |
Silent AMI Minor amputation |
| Patient 8 | Male | 50 | 34 | 11.6/21.8 (High) | Femoral | 1 |
Lacunar Ictus Limb revascularisation |
| Patient 9 | Male | 61 | 19 | 16.1/29.7 (High) | Carotid and femoral | 6 | Central retinal artery occlusion |
| Patient 10 | Male | 78 | 23 | 17.7/32.3 (High) | Carotid and femoral | 5 | Basal ganglia haematoma |
| Patient 11 | Female | 68 | 58 | 42.8/63.7 (High) | Femoral | 4 | Grade I Chronic Ischaemia |
| CV deaths n = 8 | |||||||
| Patient 12 | Female | 47 | 20 | 12.8/24 (High) | Carotid and femoral | 4 | Sudden death |
| Patient 13 | Female | 91 | 30 | 56.5/81.1 (High) | Carotid and femoral | 8 | Acute Heart Failure |
| Patient 14 | Male | 41 | 26 | 6.9/13.4 (Moderate) | Femoral | 2 | Sudden death |
| Patient 15 | Male | 59 | 45 | 70.6/94.4 (High) | Carotid | 2 | AMI |
| Patient 16 | Male | 77 | 37 | 71.2/91.7 (High) | Carotid and femoral | 7 | Sudden death |
| Patient 17 | Male | 81 | 48 | 73.1/ 92.7 (High) | Carotid and femoral | 6 | Sudden death |
| Patient 18 | Male | 54 | 40 | 11.7/22.1 (High) | Carotid and femoral | 8 | Sudden death |
| Patient 19 | Female | 78 | 52 | 41.8/66.1 (High) | Carotid and femoral | 8 | Sudden death |
| Non CV deaths n = 5 | |||||||
| Patient 20 | Female | 53 | 45 | 16.9/30.9 (High) | Carotid and femoral | 4 | Infection |
| Patient 21 | Female | 78 | 34 | 40.6/64.7 (High) | Carotid and femoral | 7 | Ketoacidosis |
| Patient 22 | Female | 81 | 19 | 22.2/39.5 (High) | None | 0 | Multiple Myeloma |
| Patient 23 | Male | 37 | 36 | 2.7/5.3 (Low) | Carotid and femoral | 2 | Subarachnoidal Haemorrhage |
| Patient 24 | Male | 48 | 32 | 2.8/5.5 (Low) | None | 0 | Lung Cancer |
MACE major cardiovascular event; CV cardiovascular; ST1RE Steno T1 Risk Engine; AMI acute myocardial infarction
Patients who experienced either fatal or nonfatal MACE (15 men vs. 4 women, p = 0.025) were older (65.2 ± 12.8 vs. 47.8 ± 12.5 years, p < 0.001), had a longer duration of diabetes (35.7 ± 13.3 vs. 22.6 ± 10.3 years, p < 0.001), had higher HbA1c levels (8.6 ± 8.4% vs. 7.9 ± 7.8%, p = 0.006), and had a greater frequency of microangiopathy (84.2% vs. 50.5%, p = 0.004). These patients also had a more frequent history of severe hypoglycaemia episodes (47.4% vs. 36.7%, p = 0.001), had higher creatinine levels (1.92 ± 0.0 vs. 0.8 ± 0.8 mg/dl, p < 0.001), and were diagnosed with hypertension (78.9% vs. 46.3%, p = 0.005), but a lower diastolic blood pressure was observed (69.2 ± 9 vs. 74.9 ± 9.4, p = 0.001). No differences were detected in systolic blood pressure control, tobacco exposure and consumption, exercise practices, or lipid profiles. All patients except two (classified as moderate risk) were categorised as high risk by the ST1RE. All patients who experienced MACEs had subclinical atherosclerosis.
When analysing mortality (fatal MACE and other causes) based on the presence or absence of subclinical atherosclerosis, 11 out of 218 patients with subclinical atherosclerosis died (6 sudden deaths, 1 fatal AMI, 1 heart failure, and 3 deaths due to other causes), compared to 2 deaths unrelated to cardiovascular causes among the 285 patients without subclinical atherosclerosis (OR 7.57; 95% CI 1.97–53.9).
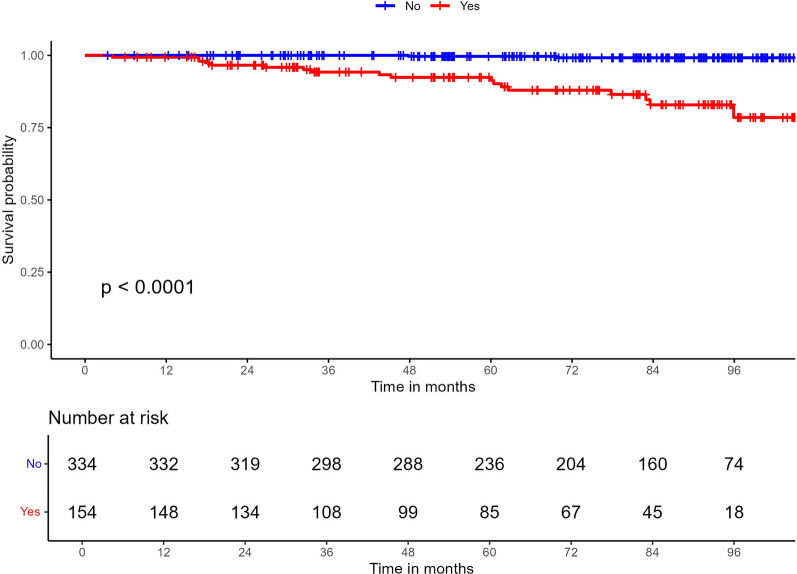
The probability of experiencing a MACE during follow-up of the whole cohort was 25 times greater in patients with carotid and/or femoral plaques than in those without plaques in these regions (HR 25.1; 95% CI 5.81–108; p < 0.001) (Fig. 4).
Fig. 4.
Development of MACE in the whole cohort depending on the presence of subclinical atherosclerosis
All patients in the cohort who experienced MACE had subclinical atherosclerosis, whereas according to ST1RE calculations, 17 out of 19 patients were classified as high risk, and 2 patients were classified as moderate risk.
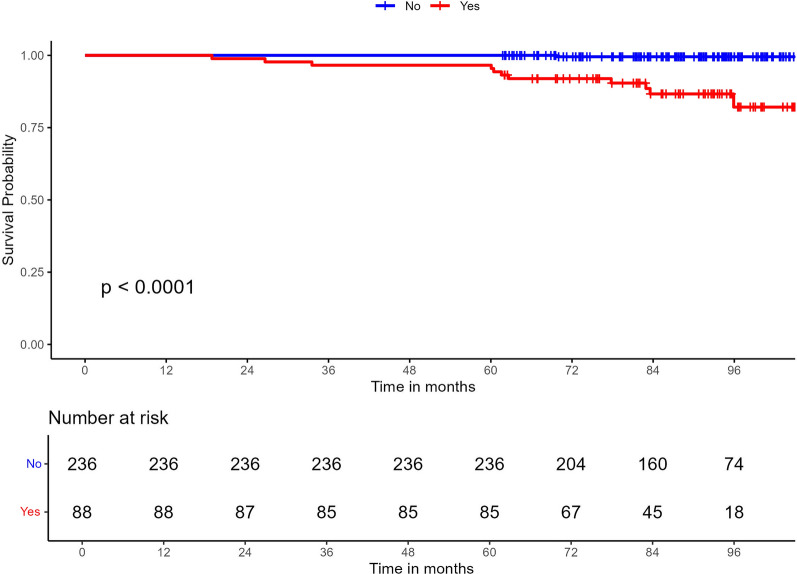
The probability of experiencing MACE in patients followed up for at least 5 years (n = 324) was 33 times greater in patients with carotid and/or femoral plaques than in those without plaques in these regions (HR 33.3; 95% CI 4.30–258; p < 0.001) (Fig. 5).
Fig. 5.
Development of MACE in patients followed up for at least 5 years depending on the presence of subclinical atherosclerosis
Discussion
To our knowledge, this is the first report describing the relationship between subclinical atherosclerosis and increased mortality in patients with type 1 diabetes without known CVD, as well as highlighting an elevated risk of developing MACE with a standardised real-world follow-up protocol for at least 5 years.
Several studies in the general population have demonstrated that subclinical atherosclerosis, identified through various methods, is independently associated with overall mortality and cardiovascular events [26–29]. Specifically, carotid plaque has been identified as an independent risk factor for all-cause mortality and cardiovascular events in both the general population [30–32] and specific populations [33, 34], although prospective studies in patients with type 1 diabetes are lacking [35].
In our cohort, the prevalence of subclinical atherosclerosis diagnosed via carotid and femoral ultrasound was 43%. In our geographic area, most studies in patients with type 1 diabetes have utilised only carotid ultrasound to assess subclinical atherosclerosis [20, 36, 37], reporting prevalences ranging from 11 to 57%, influenced by varying ages and durations of diabetes. However, our data advocate for the inclusion of femoral ultrasound alongside standard carotid ultrasound to enhance the detection of subclinical atherosclerosis. Notably, 61 out of 507 patients (12%) in our cohort exhibited femoral plaques without carotid plaques; thus, these individuals would not have been properly classified without the addition of femoral ultrasound. In the general population in Spain, the prevalence of subclinical atherosclerosis detected through combined carotid, iliofemoral, and aortic ultrasound among individuals aged 40–54 years can reach 63% [38]. Therefore, screening methodology is critical, and previous studies in patients with type 1 diabetes in our region may have underestimated the prevalence of subclinical atherosclerosis.
Consistent with prior research, our cohort showed a positive correlation between cardiovascular risk calculated by ST1RE [20, 39] and the prevalence of subclinical atherosclerosis. Subclinical atherosclerosis was identified in 10.7% (n = 18 out of 161) of patients classified as having a low ST1RE risk, 45.3% (n = 82 out of 181) of those classified as having a moderate ST1RE risk, and 76% (n = 117 out of 154) of those classified as having a high ST1RE risk. Thus, the diagnosis of subclinical atherosclerosis using carotid and femoral ultrasound in our cohort led to the reclassification of 100 patients (20.1%) initially classified as low or moderate CV risk by ST1RE into the high-risk category, necessitating more stringent therapeutic targets [40].
In our study, smoking was the only primary modifiable factor strongly associated with the presence and severity of subclinical atherosclerosis, while the male sex, HbA1c, age, diabetes duration and nephropathy were nonmodifiable factors linked to its presence. Given that routine screening for asymptomatic patients with type 1 diabetes mellitus is currently not recommended, these clinical predictors could aid in identifying patients who would benefit most from vascular ultrasound to improve risk stratification.
During follow-up, 19 out of 501 patients (3.79%) experienced fatal and nonfatal MACE. Patients with type 1 diabetes have an almost threefold higher mortality rate than the general population, largely attributed to the early onset of CVD, with men and women having relative risks of coronary events five and ten times greater, respectively, even at ages < 40 years [41].
In our cohort, all patients who experienced cardiovascular events had subclinical atherosclerosis. Although ST1RE risk assignment successfully categorised 17 out of 19 patients (79%) experiencing MACE as high risk, it failed to classify 2 patients who experienced events in the high-risk category; these patients were classified as moderate risk (n = 2). Based on the presence of subclinical atherosclerosis, however, these patients would have been categorised as high risk.
Limitations
Despite the extended follow-up period and patient selection based on age and diabetes duration, the low number of events detected limits the analysis of the relative impact of cardiovascular risk factors or other disease-specific factors on the development of CVD in this population. Our study evaluated both the presence and number of plaques; however, recent studies underscore the high predictive value of both plaque characterisation and size, areas beyond the scope of our study due to technical complexity and equipment feasibility [42–44].
Strengths
Our study is an observational study utilising real-world data from routine clinical practice. Patient adherence to clinic visits was high, with intervals ranging from 12 to 24 months. Although retrospectively recorded, the standardised follow-up protocol of the included patients allowed for robust data collection.
Conclusions
This study demonstrates that subclinical atherosclerosis is independently associated with overall mortality and cardiovascular events in patients with type 1 diabetes. Screening for subclinical atherosclerosis via carotid and femoral ultrasound could aid in re-stratifying CV risk and refining treatment strategies. Further prospective studies are necessary to assess the effectiveness of interventions aimed at delaying mortality and reducing cardiovascular events in this patient population.
Supplementary Information
Acknowledgements
The authors wish to thank the nurses and all the endocrinologists of the diabetes unit for their daily work in collecting clinical data from patients. We appreciate Dr. Ricart’s contributions and advice during the preparation of this manuscript.
Abbreviations
- CVD
Cardiovascular disease
- MACE
Major cardiovascular event
- CVRF
Cardiovascular risk factors
- CVR
Cardiovascular risk
- ST1RE
Steno type 1 risk engine
- BP
Blood pressure
- MetS
Metabolic syndrome
- HbA1c
Glycosylated haemoglobin
- HDLc
High-density lipoprotein cholesterol
- LDLc
Low-density lipoprotein cholesterol
- CCA
Common carotid artery
- CB
Carotid bifurcation
- ICA
Internal carotid artery
- ECA
External carotid artery
- CFA
Common femoral artery
- AMI
Acute myocardial infarction
- OR
Odds ratio
- HR
Hazard ratio
- CI
Confidence interval
Author contributions
L.S. researched the data, contributed to the discussion, wrote the first draft of the manuscript and reviewed the manuscript. M.F. researched the data, contributed to the discussion and reviewed and edited the manuscript. M.R., A.A., M.A., N.A., P.P., C.B., B.F., M.E., L.R., R.B., G.X., E.E., J.B., D.P., G.G., S.M., E.C. and M.W. researched the data and contributed to the discussion. J.M. and M.B. performed the statistical analysis and reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Funding
None.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the local Ethical committee of the University Hospital Dr. Josep Trueta of Girona (Project-ID: CEIM 2020.092). Informed consent was obtained from all participants before study-specific procedures begin.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Colom C, Rull A, Sanchez-Quesada JL, Pérez A. Cardiovascular disease in type 1 diabetes mellitus: epidemiology and management of cardiovascular risk. J Clin Med. 2021;10:1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, Eliasson B, Gudbjörnsdottir S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-Specific trends from 2000–2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care. 2016;39:1018–26. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Risk factors for cardiovascular disease in type 1 diabetes. Diabetes. 2016;65:1370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlqvist S, Clements M, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–82. [DOI] [PubMed] [Google Scholar]
- 6.Allen KV, Frier BM. Nocturnal hypoglycemia: clinical manifestations and therapeutic strategies toward prevention. Endocr Pract. 2003;9(6):530–43. [DOI] [PubMed] [Google Scholar]
- 7.Marcovecchio ML, Chiesa ST, Armitage J, Daneman D, Donaghue KC, Jones TW, Mahmud FH, Marshall SM, Neil HAW, Dalton RN, Deanfield J, Dunger DB. Renal and cardiovascular risk according to tertiles of urinary albumin-to-creatinine ratio: the adolescent type 1 diabetes cardio-renal intervention trial (AdDIT). Diabetes Care. 2018;41:1963–9. [DOI] [PubMed] [Google Scholar]
- 8.Xu XH, Sun B, Zhong S, Wei DD, Hong Z, Dong AQ. Diabetic retinopathy predicts cardiovascular mortality in diabetes: a meta-analysis. BMC Cardiovasc Disord. 2020;20:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury M, Nevitt S, Eleftheriadou A, Kanagala P, Esa H, Cuthbertson DJ, Tahrani A, Alam U. Cardiac autonomic neuropathy and risk of cardiovascular disease and mortality in type 1 and type 2 diabetes: a meta-analysis. BMJ Open Diabetes Res Care. 2021;9:e002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacchetta L, Chiriacò M, Nesti L, Leonetti S, Forotti G, Natali A, Solini A, Tricò D. Synergistic effect of chronic kidney disease, neuropathy, and retinopathy on all-cause mortality in type 1 and type 2 diabetes: a 21-year longitudinal study. Cardiovasc Diabetol. 2022;21:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah VN, Bailey R, Wu M, Foster NC, Pop-Busui R, Katz M, Crandall J, Bacha F, Nadeau K, Libman I, Hiers P, Mizokami-Stout K, DiMeglio LA, Sherr J, Pratley R, Agarwal S, Snell-Bergeon J, Cengiz E, Polsky S, Mehta SN. Risk factors for cardiovascular disease (CVD) in adults with type 1 diabetes: findings from prospective real-life T1D exchange registry. J Clin Endocrinol Metab. 2020;105:e2032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawshani A, Rawshani A, Sattar N, Franzén S, McGuire DK, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, Rosengren A, Gudbjörnsdottir S. Relative prognostic importance and optimal levels of risk factors for mortality and cardiovascular outcomes in type 1 diabetes mellitus. Circulation. 2019;139(16):1900–12. [DOI] [PubMed] [Google Scholar]
- 13.Zgibor JC, Piatt GA, Ruppert K, Orchard TJ, Roberts MS. Deficiencies of cardiovascular risk prediction models for type 1 diabetes. Diabetes Care. 2006;29:1860–5. [DOI] [PubMed] [Google Scholar]
- 14.Vistisen D, Andersen GS, Hansen CS, Hulman A, Henriksen JE, Bech Nielsen H, Jørgensen ME. Prediction of first cardiovascular disease event in type 1 diabetes mellitus: the steno type 1 risk engine. Circulation. 2016;133:1058–66. [DOI] [PubMed] [Google Scholar]
- 15.Ibañez B, Fernández-Ortiz A, Fernández-Friera L, García-Lunar I, Andrés V, Fuster V. Progression of Early Subclinical Atherosclerosis (PESA) study: JACC focus seminar 7/8. J Am Coll Cardiol. 2021;78:156–79. [DOI] [PubMed] [Google Scholar]
- 16.Wong ND, Budoff MJ, Ferdinand K, Graham IM, Michos ED, Reddy T, Shapiro MD, Toth PP. Atherosclerotic cardiovascular disease risk assessment: an American Society for Preventive Cardiology clinical practice statement. Am J Prev Cardiol. 2022;10:100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipsy RJ. The national cholesterol education program adult treatment panel III guidelines. J Manag Care Pharm. 2003;9(Suppl 1):2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis.2012;34:290-296 [DOI] [PMC free article] [PubMed]
- 20.Viñals C, Conget I, Pané A, Boswell L, Perea V, Blanco AJ, Ruiz S, Giménez M, Vinagre I, Esmatjes E, Ortega E, Amor AJ. Steno type 1 risk engine and preclinical atherosclerosis in Mediterranean individuals with type 1 diabetes. Diabetes Metab Res Rev. 2020;36:e3320. [DOI] [PubMed] [Google Scholar]
- 21.Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73. [DOI] [PubMed] [Google Scholar]
- 22.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. [DOI] [PubMed] [Google Scholar]
- 23.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–21. [DOI] [PubMed] [Google Scholar]
- 24.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–10. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S. Model-building strategies and methods for logistic regression. In: Hosmer DW, Lemeshow S, editors. Applied logistic regression. New York: John Wiley & Sons; 2000. p. 91–142. [Google Scholar]
- 26.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) study, 1987–1993. Am J Epidemiol. 1997;146:483–94. [DOI] [PubMed] [Google Scholar]
- 27.Wu C, Zhang K, Odden MC, Kucharska-Newton AM, Palta P, Matsushita K, Gottesman RF, Windham BG. Subclinical vascular disease burden and premature mortality among middle-aged adults: the Atherosclerosis Risk in Communities study. J Gen Intern Med. 2021;36:2048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaMonte MJ, FitzGerald SJ, Church TS, Barlow CE, Radford NB, Levine BD, Pippin JJ, Gibbons LW, Blair SN, Nichaman MZ. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162:421–9. [DOI] [PubMed] [Google Scholar]
- 29.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Ma F, Jiang YM, Li JJ, Song L, Chen SH, Liu XM, Li XQ, Wu SL. Association between carotid artery plaques and all-cause mortality and cardiovascular events. Zhonghua Xin Xue Guan Bing Za Zhi. 2017;24:1086–90. [DOI] [PubMed] [Google Scholar]
- 31.Amato M, Veglia F, de Faire U, Giral P, Rauramaa R, Smit AJ, Kurl S, Ravani A, Frigerio B, Sansaro D, Bonomi A, Tedesco CC, Castelnuovo S, Mannarino E, Humphries SE, Hamsten A, Tremoli E, Baldassarre D, IMPROVE Study Group. Carotid plaque-thickness and common carotid IMT show additive value in cardiovascular risk prediction and reclassification. Atherosclerosis. 2017;263:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CW, Guo YC, Li CI, Liu CS, Lin CH, Liu CH, Wang MC, Yang SY, Li TC, Lin CC. Subclinical atherosclerosis markers of carotid intima-media thickness, carotid plaques, carotid stenosis, and mortality in community-dwelling adults. Int J Environ Res Public Health. 2020;17:4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanna DB, Moon JY, Haberlen SA, French AL, Palella FJ Jr, Gange SJ, Witt MD, Kassaye S, Lazar JM, Tien PC, Feinstein MJ, Kingsley LA, Post WS, Kaplan RC, Hodis HN, Anastos K. Carotid artery atherosclerosis is associated with mortality in HIV-positive women and men. AIDS. 2018;23:2393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai T, Ohishi M, Takeya Y, Onishi M, Ito N, Oguro R, Yamamoto K, Kamide K, Rakugi H. Carotid plaque score and intima media thickness as predictors of stroke and mortality in hypertensive patients. Hypertens Res. 2013;36:902–9. [DOI] [PubMed] [Google Scholar]
- 35.Serés-Noriega T, Perea V, Amor AJ. Screening for subclinical atherosclerosis and the prediction of cardiovascular events in people with type 1 diabetes. J Clin Med. 2024;13(4):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernández M, López C, Real J, Valls J, Ortega-Martinez de Victoria E, Vázquez F, Rubinat E, Granado-Casas M, Alonso N, Molí T, Betriu A, Lecube A, Fernández E, Leslie RD, Mauricio D. Preclinical carotid atherosclerosis in patients with latent autoimmune diabetes in adults (LADA), type 2 diabetes and classical type 1 diabetes. Cardiovasc Diabetol. 2017;28:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguilera E, Serra-Planas E, Granada ML, Alonso N, Pellitero S, Pizarro E, Reverter JL, Salinas I, Soldevila B, Mauricio D, Puig-Domingo M. Low prevalence of subclinical atherosclerosis in asymptomatic patients with type 1 diabetes in a European Mediterranean population. Diabetes Care. 2014;37:814–20. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, Ibañez B, López-Melgar B, Laclaustra M, Oliva B, Mocoroa A, Mendiguren J, Martínez de Vega V, García L, Molina J, Sánchez-González J, Guzmán G, Alonso-Farto JC, Guallar E, Civeira F, Sillesen H, Pocock S, Ordovás JM, Sanz G, Jiménez Borreguero LJ, Fuster V. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: The PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation. 2015;131:2104–13. [DOI] [PubMed] [Google Scholar]
- 39.Serés-Noriega T, Giménez M, Perea V, Boswell L, Viñals C, Blanco J, Vinagre I, Pané A, Esmatjes E, Conget I, Amor AJ. Use of the steno T1 risk engine identifies preclinical atherosclerosis better than use of ESC/EASD-2019 in adult subjects with type 1 diabetes at high risk. Diabetes Care. 2022;45:2412. [DOI] [PubMed] [Google Scholar]
- 40.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Angelantonio ED, Franco OH, Halvorsen S, Richard Hobbs FD,Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B, ES Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies. With the special contribution of the European Association of Preventive Cardiology (EAPC). Rev Esp Cardiol.2022;75:429. [DOI] [PubMed]
- 41.Livingstone SJ, Looker HC, Hothersall EJ, Wild SH, Lindsay RS, Chalmers J, Cleland S, Leese GP, McKnight J, Morris AD, Pearson DW, Peden NR, Petrie JR, Philip S, Sattar N, Sullivan F, Colhoun HM. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;2012(9):e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zavodni AE, Wasserman BA, McClelland RL, Gomes AS, Folsom AR, Polak JF, Lima JA, Bluemke DA. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology. 2014;2271:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brunner G, Virani SS, Sun W, Liu L, Dodge RC, Nambi V, Coresh J, Mosley TH, Sharrett AR, Boerwinkle E, Ballantyne CM, Wasserman BA. Associations between carotid artery plaque burden, plaque characteristics, and cardiovascular events: the ARIC carotid magnetic resonance imaging study. JAMA Cardiol. 2021;1:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van der Toorn JE, Bos D, Ikram MK, Verwoert GC, van der Lugt A, Ikram MA, Vernooij MW, Kavousi M. Carotid plaque composition and prediction of incident atherosclerotic cardiovascular disease. Circ Cardiovasc Imaging. 2022;2022(15):e013602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.