Abstract
Background
Although anaplastic lymphoma kinase tyrosine kinase inhibitors (ALK-TKIs) have improved the survival rates of lung cancer patients with ALK fusion mutations, their effectiveness varies significantly across different subtypes. We report a case of small intestine metastasis in a lung adenocarcinoma patient with co-occurring echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion variant 3 (V3) and tumor protein 53 (TP53) mutations after distinct responses to ALK-TKIs.
Case presentation
A 45-year-old woman was diagnosed with stage IV lung adenocarcinoma with brain metastasis. Next-generation sequencing revealed EML4-ALK V3 and TP53 co-mutations. After the initial treatment with ensartinib, the patient experienced intracranial disease progression. Radiation therapy (RT) was then administered. Despite good response to RT for the intracranial disease, the primary tumor enlarged. Thus, the patient was treated with oral ensartinib concurrent with chemotherapy, with a partial response in both the primary tumor and intracranial metastases. However, after three cycles of treatment, the patient discontinued chemotherapy because of acute kidney injury. Subsequent thoracic RT resulted in a partial response of the primary tumor; however, new brain and bone metastases were detected, prompting a switch to lorlatinib. The patient developed symptoms of intestinal obstruction 14 months after the initial diagnosis. Surgical intervention revealed a poorly differentiated metastatic lung adenocarcinoma of the upper jejunum. Genetic testing confirmed EML4-ALK V3 and TP53 co-mutations and high expression of programmed cell death-ligand 1. Despite pembrolizumab treatment, the patient's condition deteriorated, and she passed away.
Conclusion
We reported a rare case of small intestinal metastasis in a lung adenocarcinoma patient with concurrent EML4-ALK V3/TP53 mutations after distinct responses to ALK-TKIs in different lesions. Our findings revealed heterogeneity in ALK mutations and responses to ALK-TKIs, necessitating the close monitoring of genetic subtypes and associated mutations for tailored treatment strategies. Maintaining a heightened awareness of potential intestinal metastasis and vigilance in monitoring intestinal symptoms and abdominal metastases are pivotal for managing advanced lung adenocarcinoma.
Keywords: Lung adenocarcinoma, Anaplastic lymphoma kinase, Lorlatinib, Metastasis, Small intestine
1. Introduction
Lung cancer is the leading cause of cancer-related death. It primarily comprises non-small cell lung cancer (NSCLC), which accounts for 85 % of all lung cancer cases [1,2]. The major histological subtypes of NSCLC are squamous cell carcinoma and lung adenocarcinoma. Most NSCLC patients are diagnosed at an advanced stage with distant metastases. Metastases to the liver, bone, and brain are frequently observed in lung cancer patients. The occurrence of gastrointestinal tract metastasis is relatively rare, ranging from approximately 0.3 %–1.7 % [3], and the involvement of the lower gastrointestinal tract is even rarer. Notably, postmortem investigations have revealed a 4.6 % incidence of small intestinal metastases in NSCLC patients [4]. The higher rate of intestinal metastasis observed in postmortem cases may be attributed to several factors. First, in many cases, metastasis of the tumor to the intestines often does not exhibit noticeable symptoms and typically raises concerns among physicians only when severe complications, such as intestinal obstruction or perforation, occur. Second, advancements in scientific technologies and medications have led to prolonged patient survival and an increased risk of distant organ metastasis. Lung cancer patients with intestinal metastases have been reported to have unfavorable prognoses [5].
Genetic testing and targeted therapy have significantly improved the survival outcomes of lung cancer patients with driver gene mutations. Anaplastic lymphoma kinase (ALK) is a driver found in approximately 3%–7% of all lung adenocarcinoma cases [6]. Several anaplastic lymphoma kinase tyrosine kinase inhibitors (ALK-TKIs) have been globally approved for the treatment of this type of lung cancer. The first-generation ALK-TKI, crizotinib, has achieved breakthrough progress, leading to a significantly prolonged overall survival of over 4 years [7]. Second-generation ALK-TKIs include ceritinib, alectinib, brigatinib. Among them, alectinib and brigatinib have demonstrated superior efficacy compared to crizotinib, with a median progression-free survival (PFS) of >2 years [[8], [9], [10]]. Moreover, lorlatinib, a novel third-generation ALK-TKI, exhibits broader activity and effectiveness against certain ALK-resistant mutations [11]. However, it is controversial whether lorlatinib can be used as a first-line treatment owing to its specific adverse events that may affect patients’ quality of life. Ensartinib, a novel, potent, second-generation ALK-TKI, was developed to overcome crizotinib resistance. The global, randomized eXalt3 trial demonstrated the superior systemic (PFS 31.3 vs. 12.7 months) and intracranial (ORR: 63.6 vs. 21.1 %) efficacy of ensartinib versus crizotinib in treatment-naïve patients with advanced ALK-positive NSCLC [12]. Based on these data, ensartinib has been approved as a first-line treatment for ALK-positive NSCLC in China in 2022.
However, the treatment efficacy of ALK-TKIs may vary among the different subtypes of ALK-positive NSCLC. Patients with variant 3 (V3) exhibit poorer responses than those with variant 1 (V1) [13]. Patients with concurrent mutations in ALK, particularly those with ALK and tumor protein 53 (TP53) co-mutations, also demonstrate inferior outcomes [14], with poorer outcomes in patients with concurrent V3 and TP53 mutations [15].
Herein, we presented a case of lung adenocarcinoma with co-occurring ALK V3 and TP53 mutations. Despite initial ensartinib treatment, the patient developed intracranial disease progression. The subsequent administration of lorlatinib showed limited effectiveness. The patient developed small intestinal metastasis 14 months after diagnosis and ultimately passed away.
2. Case presentation
A 45-year-old non-smoking woman presented with persistent cough and chest discomfort on February 24, 2022. Positron emission tomography-computed tomography (PET-CT) (Fig. 1A) revealed a highly metabolic mass near the hilum of the right lower lobe with distal lung collapse, pleural effusion, and multiple hyper-metabolic lymph nodes in the mediastinal, right hilar, and supraclavicular regions. Cranial magnetic resonance imaging (MRI) revealed scattered abnormal signals and enhancement in the left frontal cortex, indicating potential metastatic lesions (Fig. 1 A). There was no evidence of visceral metastasis on CT. A pathological diagnosis of lung adenocarcinoma was established based on a biopsy of the right supraclavicular lymph node (cT2N3M1, stage IV). Hematoxylin and eosin (H&E) staining of the right supraclavicular lymph node was positive ( × 200) (Fig. 2A). Immunohistochemical analysis showed positive staining for transcription factor-1 (TTF-1) (Fig. 2B) and Napsin A (Fig. 2C) ( × 100). Next-generation sequencing (NGS) using a panel of 425 cancer-related genes (Nanjing Geneseeq Technology Inc., China) conducted on March 9, 2022, indicated echinoderm microtubule-associated protein-like 4 (EML4): exon 6-ALK: exon 20 fusion (V3) (mutation abundance: lymph node 1.4 %, plasma 0.8 %). Reciprocal ALK translocation was detected in the plasma sample, albeit with a weak signal (one read at the ALK end and two at the EML4 end), whereas no such signal was detected in the tissue sample. A TP53 mutation was detected in the plasma (0.7 %) but not in the tissue sample. The programmed cell death-ligand 1 (PD-L1) testing on tissue polypeptide specific-antigen revealed a tumor proportion score (TPS) of 60 %.
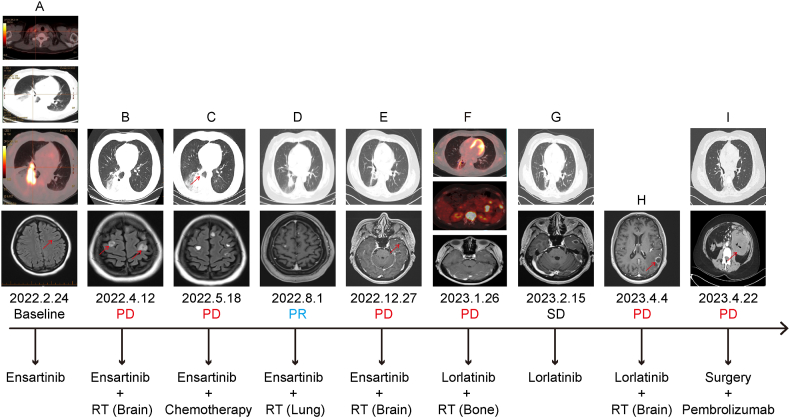
Fig. 1.
Systemic treatment strategies: (A) Pre-treatment PET-CT revealing a hypermetabolic mass near the hilum of the right lower lung lobe, accompanied by enlarged right supraclavicular lymph nodes and distal lung atelectasis, measuring approximately 4.8 cm × 2.8 cm. Brain-enhanced MRI showing small metastases in the left frontal lobe cortex. (B) One-month follow-up after ensartinib treatment showed a reduction in the size of the soft tissue mass in the right lower lobe, measuring approximately 2.8 cm × 3.5 cm. Additionally, new brain metastases were observed, with the largest measuring about 1.3 cm. (C) Follow-up imaging on May 18, 2022, revealing an enlargement of the soft tissue mass in the right lower lobe, measuring approximately 5.6 cm × 5.6 cm, while a reduction in the intracranial tumor size is observed. (D) Follow-up imaging on August 1, 2022, indicating a reduction in the tumor in the right lower lobe of the lung, measuring approximately 3.8 cm × 3.6 cm. A significant reduction in brain metastatic tumor size is noted. (E) Follow-up imaging on December 27, 2022, revealing a continued reduction in the tumor in the right lower lobe of the lung and the development of a new metastatic tumor in the left temporal lobe, measuring approximately 1.8 cm × 1.2 cm. (F) PET-CT scan conducted on January 26, 2023, revealing a marked reduction in size and reduced metabolic activity of the malignant tumor in the right lung. Additionally, bone metastasis to the T12 vertebra and multiple small retroperitoneal lymph node metastases are observed. (G) Follow-up imaging on February 15, 2023, showing a reduction in the right lung lesion, measuring approximately 0.8 cm × 0.9 cm. Changes observed in the left temporal lobe tumor after radiation therapy. (H) Head MRI on April 4, 2023, indicating a new metastasis in the left parietal lobe, measuring approximately 2.7 cm × 1.5 cm. (I) Follow-up imaging on April 22, 2023, revealing stability in lung lesions, localized thickening of the small intestine wall in the upper left abdomen, and multiple enlarged lymph nodes in the retroperitoneum, suggestive of metastasis.
Fig. 2.
Biopsy pathologic images of the right supraclavicular lymph node. (A) Hematoxylin and eosin (H&E) staining is positive (H&E × 200). Immunohistochemical (IHC) analysis showing positive staining for TTF-1 (B) and Napsin A (C) (IHC × 100).
On March 10, 2022, the patient started ensartinib 225 mg orally once daily. One month later, efficacy evaluation showed intracranial disease progression, while the primary tumor showed a partial response (Fig. 1B). NGS revealed a plasma EML4-ALK fusion mutation abundance of 0.1 %; however, no mutations were detected in the cerebrospinal fluid. Additionally, no TP53 mutations were identified. Consequently, the patient underwent radiation therapy (RT) with a prescribed dose of 45.5 Gy in 10 fractions (45.5 Gy/10f) for the primary tumor and 30 Gy/10f for the whole brain. Imaging analyses performed on May 18, 2022 (Fig. 1C), indicated an increase in the size of the primary tumor and a decrease in the size of the intracranial tumors. Concurrent with oral ensartinib, the patient was administered a three-cycle chemotherapy regimen of pemetrexed and carboplatin. On August 1, 2022, follow-up imaging indicated shrinkage of the primary lung lesion and brain metastases with a partial response (Fig. 1D). However, after three cycles of treatment, the patient experienced acute kidney injury. Chemotherapy was discontinued, and the patient continued to receive oral ensartinib. On August 17, 2022, the patient underwent localized RT targeting the primary tumor and corresponding lymphatic drainage area, with a total dose of 59.92 Gy/28f.
Four months later, a follow-up assessment showed a reduction in the primary tumor size but also revealed intracranial disease progression and a new metastatic lesion identified in the left temporal lobe (Fig. 1E). Subsequently, the patient underwent stereotactic RT for newly diagnosed brain lesions at a total dose of 27Gy/3f. A PET-CT scan on January 26, 2023 (Fig. 1F), indicated notable reductions in the primary tumor and intracranial tumors. However, bone metastasis to T12 and multiple small retroperitoneal lymph node metastases were observed without abdominal abnormalities. Considering the intense pain in the twelfth thoracic vertebra, the patient underwent RT at a dose of 30 Gy/10f. The patient was also administered oral lorlatinib (100 mg, once daily). The treatment was well tolerated, and the patient did not experience any side effects. A subsequent follow-up examination (Fig. 1G) on February 15, 2023, suggested a stable condition of the intracranial lesions and further reduction in the size of the primary tumor.
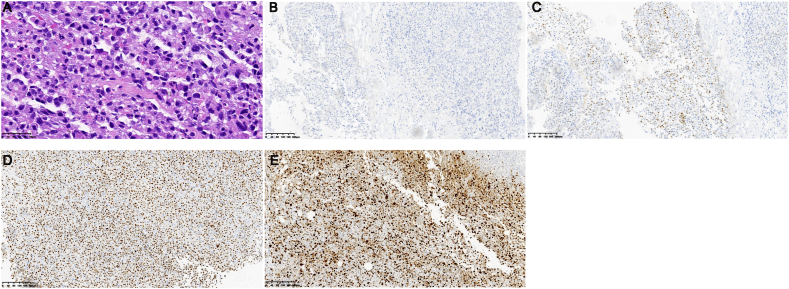
On April 4, 2023, the patient suddenly developed expressive aphasia. MRI revealed a new metastatic lesion in the left parietal lobe (Fig. 1H). The patient underwent stereotactic RT at a prescribed dose of 27Gy/3f. The aphasia symptoms gradually resolved. On April 22, 2023, the patient presented with abdominal distension and difficulty in passing gas and stools. A CT scan (Fig. 1I) showed thickening of the small intestinal wall in the left upper abdomen and multiple enlarged retroperitoneal lymph nodes, raising the suspicion of metastatic tumors. Laparoscopic surgery revealed tumor infiltration in the mesentery and lumen of the upper jejunum, with multiple nodules measuring approximately 1–3 cm in diameter located 30 cm–100 cm from the ligament of Treitz. Postoperative pathological examination indicated poorly differentiated metastatic lung adenocarcinoma (Fig. 3A). Immunohistochemical analysis showed positive expression of thyroid transcription factor-1(Fig. 3C), negative expression of caudal-related homeobox 2 (Fig. 3B), cytokeratin 7, and cytokeratin 20. Approximately 80 % of the cells stained positive for P53 (Fig. 3D) and 70 % stained positive for Ki-67 (Fig. 3E). Further assessment through NGS on May 17, 2023, confirmed the concurrent mutations of EML4-ALK V3 (plasma 17.15 %, tissue 54.51 %) and TP53 (plasma 10.88 %, tissue 55.56 %). The patient exhibited high PD-L1 expression, with a TPS of 70 %. Despite treatment with pembrolizumab (200 mg), the patient's condition deteriorated with notable abdominal distension, ascites, pulmonary infection, anemia, hypoalbuminemia, and coagulation dysfunction. The patient passed away on June 14, 2023.
Fig. 3.
(A) Hematoxylin and eosin (H&E) staining revealing adenocarcinoma (H&E × 400). Immunohistochemical (IHC) analysis showing negative staining for CDX-2 (B) and positive staining for TTF-1 (C), p53 (D), and Ki-67 (E) (IHC × 100).
3. Discussion
To our knowledge, this is the first documented case of EML4-ALK V3 and TP53 co-mutated lung adenocarcinoma with small intestine metastasis in a patient receiving ALK-TKIs. Despite being EML4-ALK fusion positive, the patient showed distinct responses to ALK-TKIs. Considering the rarity of this occurrence and the limited response to targeted therapy, we recommend that clinicians pay more attention to NSCLC patients with TP53 and EML4-ALK V3 co-mutations. Moreover, we should remain vigilant regarding the potential of primary NSCLC to metastasize to the intestinal tract. Early and effective treatment is imperative for diagnosis.
Symptoms of lung cancer metastasizing to the intestine often manifest as bleeding, obstruction, or perforation, occasionally accompanied by nonspecific symptoms such as fatigue, abdominal pain, and weight loss [[16], [17], [18], [19]]. Consequently, such cases are frequently misattributed to adverse reactions to anti-tumor treatment or other ailments, resulting in a delayed or even missed diagnosis of intestinal metastasis until postmortem examination in some cases. In addition, gastrointestinal metastasis commonly arises during a patient's treatment journey, particularly in the advanced stages of the disease. This results in a limited number of reported cases at baseline, contributing to the relative scarcity of literature on this subject. A mean time of 13.5 months (range: 3–49) from the diagnosis of lung cancer to the detection of gastrointestinal metastasis was reported in a study of 2066 patients [5]. Compared to patients with other distant metastases, patients with gastrointestinal metastasis have a poorer prognosis, with a mean survival time of 100.6 days from the detection of gastrointestinal metastasis [5]. Our patient showed no symptoms of gastrointestinal metastasis at baseline. Multiple retroperitoneal lymph node metastases were noted 11 months later, followed by the detection of intestinal obstruction in the 14th month. Subsequent surgical pathology confirmed metastasis to the small intestine, and the patient survived for an additional 49 days. Consequently, when lung cancer patients exhibit gastrointestinal symptoms, the possibility of gastrointestinal metastasis should be carefully considered during a comprehensive evaluation. In cases of peritoneal metastasis, close monitoring of digestive symptoms is imperative.
Gastrointestinal metastases in lung cancer can be mistaken for primary gastrointestinal tumors, intestinal tuberculosis, or a diverticulum. Early stages often lack symptoms, necessitating reliance on imaging like CT and PET-CT scans, though they may have limited sensitivity. Endoscopic examination with histopathology is the gold standard; however, metastasis in the submucosal layer with intact mucosa can yield poor biopsy results, especially in deep sites of the small intestine. Immunohistochemical testing for TTF1, CDX2, cytokeratin 7, and cytokeratin 20 can be instrumental in enhancing the diagnostic accuracy of primary and metastatic small intestinal tumors, thus establishing a foundation for subsequent treatment [20]. In our case, abdominal CT revealed a thickening of the small intestinal wall in the left upper abdomen, suggesting possible metastatic tumors. The immunohistochemical results supported the diagnosis of lung adenocarcinoma metastasis. Currently, there is no standard treatment for gastrointestinal metastases of lung cancer. Considering the patient's condition and advice from a multidisciplinary team, we opted for palliative surgery. Postsurgical pathology confirmed a poorly differentiated metastatic lung adenocarcinoma with EML4-ALK mutation. Although there are no specific reports on the pathways of lung cancer metastasis to the gastrointestinal tract, blood and lymphatic dissemination are considered the most likely routes. Notably, all the studies reviewed here were retrospective, indicating the existence of data selection bias.
Several ALK fusion partners have been identified, with EML4-ALK fusion being the most common and predominantly found in young non-smoking patients with lung adenocarcinoma [21]. Based on the different exons involved in the EML4-ALK fusion, this fusion type can be further classified into different variants. Among these, V1 (involving exon 13 of EML4 and exon 20 of ALK) and V3a/3b (involving exon 6a/b of EML4 and exon 20 of ALK) are the most common, accounting for 43 % and 40 % of the ALK-positive NSCLC, respectively [22]. V3 shows poor efficacy, with 69 % of patients experiencing metastasis at initial diagnosis [23]. Among patients with EML4-ALK-positive NSCLC, approximately 20 % exhibit TP53 mutations. Notably, TP53 mutations, along with EML4-ALK V3, are associated with increased metastatic dissemination and poor prognosis [15]. The co-occurrence of TP53 mutations is an adverse prognostic factor in ALK-positive patients. Compared to those with wild-type TP53, patients with ALK/TP53 co-mutations have significantly reduced median PFS (3.9 months versus 10.3 months) and overall survival (15.0 months versus 50.0 months) [24]. NSCLC patients with consistently negative TP53 expression during treatment have the best prognosis, while those with initially positive TP53 expression have the poorest outcomes [25]. Lorlatinib reportedly leads to inferior PFS in NSCLC patients with concomitant ALK and TP53 mutations [26]. Subgroup analysis of the Asian cohort in the eXalt3 trial indicated that patients with baseline brain metastases had a median PFS of 11.8 months with ensartinib treatment, significantly surpassing the 7.5 months achieved with crizotinib [27]. Based on the genetic testing results of our patient, ensartinib was prescribed as the first-line treatment. The patient initially responded to ensartinib treatment with a partial reduction in lung lesions but developed intracranial disease progression. The ALK fusion was negative in the cerebrospinal fusion. After RT for brain metastases, improvements in the intracranial lesions were observed. The patient exhibited heterogeneity in lung and brain lesions. Therefore, although targeted drugs are effective against primary tumors in patients with brain metastases, close monitoring of intracranial progression remains necessary. The sensitivity of genetic testing in cerebrospinal fluid should not be overlooked. Subsequently, despite switching to the third-generation ALK-TKI lorlatinib, the brain metastases continued to progress, further confirming the heterogeneity of the tumors. After the diagnosis of intestinal metastases, dynamic NGS revealed persistent V3/TP53 co-mutations and increased TP53 abundance. It was speculated that although third-generation TKI was effective against ALK, the TP53 mutation became the dominant subclone, leading to continued disease progression. For this specific subtype, the patient exhibited some response to concurrent ensartinib and chemotherapy, but the treatment had to be discontinued because of impaired renal function. Additionally, during the relatively stable disease phase with only residual lesions in the lungs, localized RT was administered, leading to a partial response in the intrathoracic lesions until the patient's death. The brain metastases also responded well to RT, highlighting the advantages of a comprehensive treatment approach.
Although the patient showed high PD-L1 expression, we initially pursued targeted therapy in line with the principles of targeted treatment. Owing to disease progression, we intended to initiate immunotherapy; however, the patient tested positive for the novel coronavirus disease. After the patient tested negative, her condition rapidly deteriorated, and despite administering immunotherapy, the response was suboptimal. Therefore, prioritizing frontline targeted therapy or implementing frontline immunotherapy with or without chemotherapy remains a challenge.
A previous study by Zhang et al. indicated that patients with non-reciprocal/reciprocal ALK translocations accounted for 18.7 % of those with ALK-rearranged NSCLC [28]. These patients had a notably shorter median PFS with first-line crizotinib therapy compared to patients with solely 3′-ALK fusions (6.1 months versus 12.0 months, p = 0.001) or those with EML4-ALK fusions alone (6.1 m vs. 12.6 m, p = 0.001). The occurrence of non-reciprocal/reciprocal ALK translocations was an independent predictor of the therapeutic effectiveness of crizotinib (p = 0.0046). Additionally, patients with non-reciprocal/reciprocal ALK translocations exhibited a higher rate of brain metastases. A reciprocal ALK translocation was identified in the plasma sample of our case, albeit with a weak signal. Conversely, no translocation was detected in tissue samples. This discrepancy may contribute to the suboptimal response to ALK-TKIs. However, it is unclear whether other second-generation ALK-TKIs would yield similar results, warranting further investigation.
4. Conclusion
A middle-aged woman with advanced lung adenocarcinoma harboring EML4-ALK V3 and TP53 co-mutations developed small intestinal metastases after distinct responses to ALK-TKIs. Primary resistance to ALK-TKIs in lung carcinoma is rare and complex, likely owing to tumor heterogeneity, concurrent genetic mutations, reciprocal ALK translocations, and patient immunological profiles. The investigation of these mechanisms is crucial. Clinicians should consider tumor histology and genetic testing for tailored treatments. Timely diagnosis of intestinal metastases from lung cancer is vital for personalized treatment. Further refinement of the management of ALK fusion-positive patients is clinically significant.
Ethics statement
This study has been reviewed and approved by The Ethics Committee of the First Hospital of Hebei Medical University. Written informed consent was obtained from the patient for the publication of all her clinical data and images.
Date Availability
All datasets generated for this study are included in the manuscript.
CRediT authorship contribution statement
Lingling Zhu: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Yingchun Zhao: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Yongqian Zhang: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Zhai Liu: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Wenhua Ma: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Ying Guo: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Qian Wang: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Yan Guo: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Hengxu Lv: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Min Zhao: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Investigation, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the patient involved in this case study and all the research staff who participated in this study.
References
- 1.Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Thai A.A., Solomon B.J., Sequist L.V., Gainor J.F., Heist R.S. Lung cancer. Lancet. 2021;398(10299):535–554. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 3.Jevremovic V. Is gastrointestinal metastasis of primary lung malignancy as rare as reported in the literature? A comparison between clinical cases and post-mortem studies. Oncology & Hematology Review (US) 2016;12:51. [Google Scholar]
- 4.Stenbygaard L.E., Sørensen J.B. Small bowel metastases in non-small cell lung cancer. Lung Cancer. 1999;26(2):95–101. doi: 10.1016/s0169-5002(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 5.Taira N., Kawabata T., Gabe A., Furugen T., Ichi T., Kushi K., et al. Analysis of gastrointestinal metastasis of primary lung cancer: clinical characteristics and prognosis. Oncol. Lett. 2017;14(2):2399–2404. doi: 10.3892/ol.2017.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addeo A., Tabbò F., Robinson T., Buffoni L., Novello S. Precision medicine in ALK rearranged NSCLC: a rapidly evolving scenario. Crit. Rev. Oncol. Hematol. 2018;122:150–156. doi: 10.1016/j.critrevonc.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Mok T., Camidge D.R., Gadgeel S.M., Rosell R., Dziadziuszko R., Kim D.W., et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020;31(8):1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- 8.Cho B.C., Kim D.W., Batra U., Park K., Kim S.W., Yang C.T., et al. Efficacy and safety of ceritinib 450 mg/day with food and 750 mg/day in fasted state in treatment-naïve patients with ALK+ non-small cell lung cancer: results from the ASCEND-8 asian subgroup analysis. Cancer Res Treat. 2023;55(1):83–93. doi: 10.4143/crt.2021.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camidge D.R., Dziadziuszko R., Peters S., Mok T., Noe J., Nowicka M., et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J. Thorac. Oncol. 2019;14(7):1233–1243. doi: 10.1016/j.jtho.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Ahn M.J., Kim H.R., Yang J.C.H., Han J.Y., Li J.Y., Hochmair M.J., et al. Efficacy and safety of brigatinib compared with crizotinib in asian vs. Non-asian patients with locally advanced or metastatic ALK-inhibitor-naive ALK+ non-small cell lung cancer: final results from the phase III ALTA-1L study. Clin. Lung Cancer. 2022;23(8):720–730. doi: 10.1016/j.cllc.2022.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Lu S., Zhou Q., Liu X., Du Y., Fan Y., Cheng Y., et al. Lorlatinib for previously treated ALK-positive advanced NSCLC: primary efficacy and safety from a phase 2 study in people's Republic of China. J. Thorac. Oncol. 2022;17(6):816–826. doi: 10.1016/j.jtho.2022.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Selvaggi G., Wu Y., Wang Z., Wu G., Poddubskaya E., Reck M., et al. FP14.12 quality of life and subgroup analysis in a phase 3 randomized study of ensartinib vs crizotinib in ALK–positive NSCLC patients: eXalt3. J. Thorac. Oncol. 2021;16(3):S232. S3. [Google Scholar]
- 13.Zhang S.S., Nagasaka M., Zhu V.W., Ou S.I. Going beneath the tip of the iceberg. Identifying and understanding EML4-ALK variants and TP53 mutations to optimize treatment of ALK fusion positive (ALK+) NSCLC. Lung Cancer. 2021;158:126–136. doi: 10.1016/j.lungcan.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Qin K., Hou H., Liang Y., Zhang X. Prognostic value of TP53 concurrent mutations for EGFR- TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: a meta-analysis. BMC Cancer. 2020;20(1):328. doi: 10.1186/s12885-020-06805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christopoulos P., Budczies J., Kirchner M., Dietz S., Sültmann H., Thomas M., et al. Defining molecular risk in ALK(+) NSCLC. Oncotarget. 2019;10(33):3093–3103. doi: 10.18632/oncotarget.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanriverdi O., Alkan A., Ozseker B., Solak-Ozseker H., Kilinc R.M. Synchronous duodenum and descending colon metastasis from primary lung neuroendocrine small-cell carcinoma: a case report and review of the literature. J. Oncol. Pharm. Pract. 2020;26(6):1524–1529. doi: 10.1177/1078155220904133. [DOI] [PubMed] [Google Scholar]
- 17.Chen J. Undiagnosed primary lung carcinoma with initial manifestation of intestinal obstruction: a case report and literature review. J. Cancer Res. Therapeut. 2015;11(Suppl 1):C134–C137. doi: 10.4103/0973-1482.163873. [DOI] [PubMed] [Google Scholar]
- 18.Lin M.W., Wu C.T., Chang Y.L. Intussusception caused by intestinal metastasis from lung pleomorphic carcinoma. Ann. Thorac. Cardiovasc. Surg. 2014;20(Suppl):635–638. doi: 10.5761/atcs.cr.13-00099. [DOI] [PubMed] [Google Scholar]
- 19.Chen H.F., Zhang Q.X., Zhu Y.C., Du K.Q., Li X.F., Wu L.X., et al. Intestinal metastasis from primary ROS1-positive lung adenocarcinoma cancer patients responding to crizotinib. OncoTargets Ther. 2018;11:7821–7825. doi: 10.2147/OTT.S178985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi G., Marchioni A., Romagnani E., Bertolini F., Longo L., Cavazza A., et al. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J. Thorac. Oncol. 2007;2(2):115–120. [PubMed] [Google Scholar]
- 21.Shaw A.T., Yeap B.Y., Mino-Kenudson M., Digumarthy S.R., Costa D.B., Heist R.S., et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 2009;27(26):4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J.J., Zhu V.W., Yoda S., Yeap B.Y., Schrock A.B., Dagogo-Jack I., et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J. Clin. Oncol. 2018;36(12):1199–1206. doi: 10.1200/JCO.2017.76.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noh K.W., Lee M.S., Lee S.E., Song J.Y., Shin H.T., Kim Y.J., et al. Molecular breakdown: a comprehensive view of anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer. J. Pathol. 2017;243(3):307–319. doi: 10.1002/path.4950. [DOI] [PubMed] [Google Scholar]
- 24.Kron A., Alidousty C., Scheffler M., Merkelbach-Bruse S., Seidel D., Riedel R., et al. Impact of TP53 mutation status on systemic treatment outcome in ALK-rearranged non-small-cell lung cancer. Ann. Oncol. 2018;29(10):2068–2075. doi: 10.1093/annonc/mdy333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christopoulos P., Dietz S., Kirchner M., Volckmar A.L., Endris V., Neumann O., et al. Detection of TP53 mutations in tissue or liquid rebiopsies at progression identifies ALK+ lung cancer patients with poor survival. Cancers. 2019;11(1) doi: 10.3390/cancers11010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost N., Christopoulos P., Kauffmann-Guerrero D., Stratmann J., Riedel R., Schaefer M., et al. Lorlatinib in pretreated ALK- or ROS1-positive lung cancer and impact of TP53 co-mutations: results from the German early access program. Ther Adv Med Oncol. 2021;13 doi: 10.1177/1758835920980558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn L., Wang Z., Wu G., Poddubskaya E., Mok T., Reck M., et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):1617–1625. doi: 10.1001/jamaoncol.2021.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Zeng L., Zhou C., Li Y., Wu L., Xia C., et al. Detection of nonreciprocal/reciprocal ALK translocation as poor predictive marker in patients with first-line crizotinib-treated ALK-rearranged NSCLC. J. Thorac. Oncol. 2020;15(6):1027–1036. doi: 10.1016/j.jtho.2020.02.007. [DOI] [PubMed] [Google Scholar]