Abstract
Histamine, a neurotransmitter, plays a predominant role in maintaining wakefulness. Furthermore, our previous studies showed that histamine N-methyltransferase (HNMT), a histamine-metabolizing enzyme, is important for regulating brain histamine concentration. However, the effects of pharmacological HNMT inhibition on mouse behavior, including the sleep–wake cycle and cataplexy, in a mouse model of narcolepsy have not yet been investigated. In the present study, we investigated the effects of metoprine, an HNMT inhibitor with high blood-brain barrier permeability, in wild-type (WT) and orexin-deficient (OxKO) narcoleptic mice. Metoprine increased brain histamine concentration in a time- and dose-dependent manner without affecting peripheral histamine concentrations. Behavioral tests showed that metoprine increased locomotor activity in both novel and familiar environments, but did not alter anxiety-like behavior. Sleep analysis showed that metoprine increased wakefulness and decreased non-rapid eye movement (NREM) sleep through the activation of the histamine H1 receptor (H1R) in WT mice. In contrast, the reduction of rapid eye movement (REM) sleep by metoprine occurred independent of H1R. In OxKO mice, metoprine was found to prolong wakefulness and robustly suppress cataplexy. In addition, metoprine has a greater therapeutic effect on cataplexy than pitolisant, which induces histamine release in the brain and has been approved for patients with narcolepsy. These data demonstrate that HNMT inhibition has a strong effect on wakefulness, demonstrating therapeutic potential against cataplexy in narcolepsy.
Keywords: histamine, histamine N-methyl transferase, metoprine, narcolepsy, wakefulness
Graphical Abstract
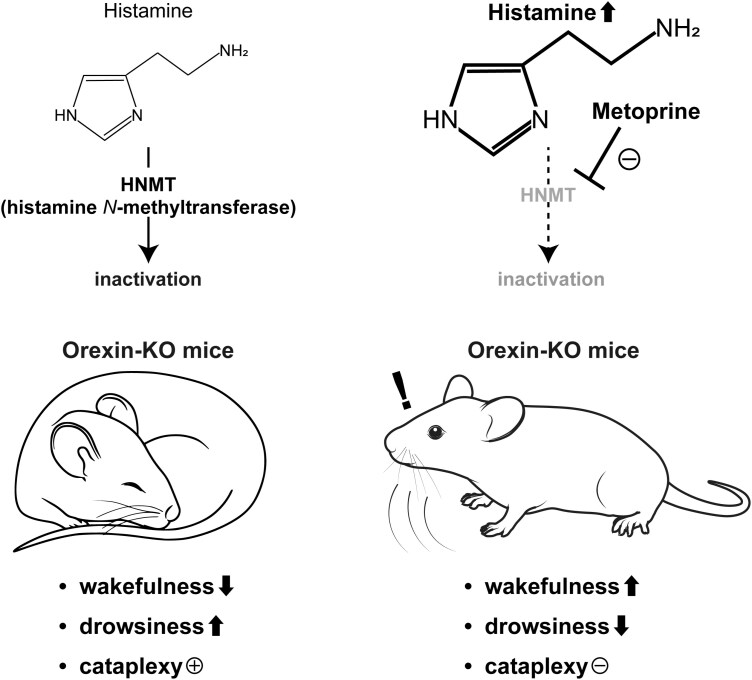
Graphical Abstract.
Statement of Significance.
Patients with narcolepsy experience severe daytime sleepiness and cataplexy. However, approved psychostimulants for narcolepsy have side effects including drug dependence. Histamine is a neurotransmitter that promotes wakefulness and inhibits drug dependence. In the present study, we found that metoprine, an inhibitor of the histamine-metabolizing enzyme (HNMT), increased histamine levels in the brain without affecting levels of other monoamines. Metoprine dramatically prolonged wakefulness and eliminated cataplexy in a mouse model of narcolepsy. In addition, the effects of metoprine were greater than those of an approved drug, pitolisant. Taken together, these data suggest that pharmacological inhibition of HNMT could be an effective therapeutic strategy for the treatment of narcolepsy.
Histamine is a neurotransmitter synthesized from histidine by histidine decarboxylase (HDC) and inactivated to 1-methylhistamine by histamine N-methyltransferase (HNMT) [1]. Histaminergic neurons exist exclusively in the tuberomammillary nucleus of the posterior hypothalamus, while their axons extend throughout the brain [2, 3]. Previous studies have provided consistent evidence that histamine plays an important role in promoting wakefulness. For example, chemogenetic activation of histaminergic neurons increases wakefulness and decreases non-rapid eye movement (NREM) sleep, but not rapid eye movement (REM) sleep [4, 5]. A recent study using genetically encoded sensors to measure histamine release demonstrated that extracellular concentration of histamine was high during wakefulness, and weak or silent during NREM and REM sleep [6]. Pharmacological assays using histamine receptor antagonists have shown that histaminergic signals via the histamine H1 receptor (H1R) expressed on the postsynaptic membrane strongly increase wakefulness and decrease NREM sleep [7]. Clinical studies have further shown the importance of the histamine H3 receptor (H3R) as a therapeutic target for hypersomnolence. Because H3R is an inhibitory auto-receptor on histaminergic neurons, which is mainly expressed on synaptic terminals and negatively regulates histamine release and synthesis, many pharmaceutical companies have developed H3R inverse agonists to enhance histamine release and maintain wakefulness in patients with excessive daytime sleepiness. Indeed, pitolisant, an H3R inverse agonist, has been approved in the European Union and United States as a medication for excessive daytime sleepiness and cataplexy in narcolepsy [8, 9] and for improving sleepiness in obstructive sleep apnea syndrome in the European Union [10]. These lines of evidence demonstrate the importance of the histaminergic system in sleep–wake regulation and drug development targeting sleep disorders.
Our previous studies have demonstrated that genetic HNMT disruption substantially increases brain histamine concentrations [11], indicating a beneficial impact of histamine elevation by HNMT inhibitors on hypersomnia. Several studies have examined the effects of HNMT inhibitors on increasing brain histamine concentrations [12, 13]. Therefore, in the present study, we examined the pharmacological effects of metoprine, an HNMT inhibitor with high blood-brain barrier (BBB) permeability [12], on histamine concentration, locomotor activity, and sleep–wake regulation in mice. We further investigated the therapeutic potential of HNMT inhibitors in a mouse model of narcolepsy by comparing the efficacies of metoprine and pitolisant on cataplexy.
Methods
Animals
We used a closed colony of male ICR mice (Japan SLC, Hamamatsu, Japan) for all experiments, except for the cataplexy analysis, for which we used male orexin knockout (OxKO) mice as the narcolepsy model [14]. There are sex differences in the sleep–wake architecture and female gonadal hormones influence sleep–wake behaviors [15]. Therefore, we used only male mice of both mouse lines in this study. OxKO mice present with symptoms similar to human narcolepsy, such as cataplexy during the active phase and excessive daytime sleepiness. All mice were maintained on a 12h–12h light–dark cycle under humid and temperature-controlled conditions. All animal experiments were approved by the Animal Committees of Tohoku University (2022MdA-051), Hokkaido University (23-0130), and Tohoku Medical and Pharmaceutical University (23043-cn). All gene modification experiments using OxKO mice were approved by the Centers for Gene Research of Hokkaido University (2023-095) and Tohoku Medical and Pharmaceutical University (2023-24).
Brain homogenization
Two hours after intraperitoneal (i.p.) injection of vehicle (10 % lactic acid with phosphate-buffered saline, 1:9 v/v) or metoprine (TargetMol, Boston, MA) in ICR mice, we harvested either the whole brain or the brain dissected into the cortex, diencephalon, brainstem, and cerebellum. Brain samples were then homogenized in a 10-time volume of 0.4 M perchloric acid (Wako, Osaka, Japan) containing 100 μM 3-methylhistamine as an internal control. After centrifugation 3 times at 4°C, supernatants were applied to a high-performance liquid chromatography (HPLC) system (EICOM, Kyoto, Japan) to measure histamine, 1-methylhistamine, and 3-methylhistamine concentrations. Whole-brain samples were analyzed using another HPLC system (EICOM) to measure monoamines and their metabolites.
Microdialysis
ICR mice were anesthetized with 0.3 mg/kg medetomidine (Nippon Zenyaku Kogyo, Tokyo, Japan), 4.0 mg/kg midazolam (Teva Takeda Pharma, Nagoya, Japan), and 5.0 mg/kg butorphanol (Meiji Seika Pharma, Tokyo, Japan), after which guide cannulas (AG-4, EICOM) and dummy probes were stereotactically implanted in the prefrontal cortex (AP = 1.8 mm, RL = 0.8 mm, DV = −2.2 mm from bregma) [7]. At least 1 week after surgery, the dummy probe was replaced with a microdialysis probe (AI-4-2, membrane length of 2 mm, EICOM). The probe was perfused with artificial cerebrospinal fluid (147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 0.85 mM MgCl2) at a flow rate of 2 μL/minute using a glass gas-tight syringe (1002, Hamilton, Reno, NV) and micro syringe pump (ESP-36, EICOM). The dialysate samples were automatically collected and mixed with equal volumes of 0.1 M phosphate buffer (pH3.5) every 20 minutes in the vial using the fraction collector (EFC-82, EICOM) at 4°C. Dialysate samples were collected from freely moving mice for 2 hours to determine baseline histamine concentrations. Two hours after fraction collection, metoprine (10 mg/kg) or vehicle was injected intraperitoneally, and further 4-hour dialysate samples were collected. The collected samples were analyzed using an HPLC system (EICOM) to measure the histamine concentration.
HPLC measurement of brain histamine and 1-methylhistamine, or extracellular histamine concentration
Histamine and 1-methylhistamine measurement in homogenate samples.
Samples were separated by SC-5ODS (3.0 mm, i.d. × 150 mm, EICOM) with 0.1 M sodium acetate buffer (pH 4.6)-methanol (91:9, v/v) containing 220 mg/L sodium 1-octanesulfonate (flow rate: 500 μL/min) at 40°C. After mixing with 80 mg/L o-phthalaldehyde solution-ethanol (499:1, v/v) containing 40 μL/L 2-mercaptoethanol (flow rate: 100 μL/min) and 0.5 M potassium carbonate solution (flow rate: 100 μL/min), fluorescence from histamine, 1-methylhistamine, and 3-methylhistamine was excited at 335 nm and measured at 450 nm using a fluorescence detector (L-2485, Hitachi, Tokyo, Japan).
Histamine measurement in microdialysis samples.
Samples were separated by SC-5ODS (3.0 mm, i.d. × 150 mm, EICOM) with 0.1 M sodium dihydrogen phosphate buffer-methanol (9:1, v/v) containing 170 mg/L sodium 1-octanesulfonate (flow rate: 500 μL/minutes). After mixing with 80 mg/L o-phthalaldehyde solution-methanol (40:1, v/v; flow rate: 100 μL/minutes) and 0.5 M potassium carbonate solution (flow rate: 100 μL/minutes), fluorescence from histamine was excited at 340 nm and measured at 450 nm by fluorescence detector (L-2485, Hitachi).
Behavioral analysis
Open field test.
The open-field test was performed in the light period (zeitgeber time 2-6) as previously described [16]. In brief, mice freely explored an open field arena (50 × 50 cm box, BTA-1®, Muromachi, Tokyo, Japan) for 30 minutes 2 hours after the i.p. injection of vehicle or metoprine (10 mg/kg). We measured the total movement distance, total movement time, average speed, and time spent in the central area using the photo beam sensor controller and software (BSC-001W®, Muromachi).
Elevated zero maze test.
The elevated zero maze test was performed in the light period (ZT2-6) as previously described [17]. In brief, 2 hours after i.p. injection of vehicle or metoprine (10 mg/kg), mice were allowed to move freely in an elevated zero maze for 10 minutes, and the time spent in the open arm was recorded using an overhead camera and tracking system (SMART®, Panlab, Barcelona, Spain).
Home-cage locomotor test.
Home-cage locomotor tests were performed as previously described [18]. In brief, mice were transferred to individual home cages and habituated for at least 5 days prior to recording. Vehicle or metoprine (10 mg/kg) was injected at the ZT0 or ZT12. The locomotor activity of each mouse was then measured for 24 hours using an activity monitoring system equipped with an infrared-beam apparatus (SUPERMEX, Muromachi). Data were collected and analyzed using CompACT AMS® software (Muromachi).
Sleep analysis
Sleep analysis was performed as previously described [16]. Male ICR mice (8 weeks old) were anesthetized with 0.3 mg/kg medetomidine, 4 mg/kg midazolam, and 5 mg/kg butorphanol, after which they were implanted with electrodes to allow electroencephalogram (EEG) and electromyogram (EMG) recording. At least 1 week after the surgery, the mice were connected to the recording cable and habituated for at least 5 days. Sleep–wake recordings were then initiated. We injected the vehicle, metoprine (10 mg/kg), metoprine + pyrilamine (an H1R antagonist; Sigma-Aldrich, St. Louis, MO, USA; 10 mg/kg each), or metoprine + zolantidine (an H2R antagonist; Tocris, Bristol, UK; 10 mg/kg each) by i.p. injection at ZT3 and collected the EEG and EMG data for 6 h. The order of injections was randomized and there were at least 5 days between two injections in the same mouse. The doses of histamine receptor antagonists were determined based on previous studies to sufficiently reduce the function of each receptor [7, 11]. The collected EEG and EMG signals were amplified and digitized using the SIRENIA® data acquisition system and software (Pinnacle Technology, Oregon, KS). The digitized EEG and EMG data was divided into 12 s epochs and scored manually for one of the three sleep–wake episodes (wake, NREM sleep, or REM sleep) using SleepSign® 3 Software (Kissei Comtec, Nagano, Japan). The obtained data were normalized as a percentage of the total power at 0-20 Hz, and the theta (6–9 Hz)/delta (1–4 Hz) ratio was calculated.
Cataplexy analysis
Cataplexy analysis was performed using the OxKO mice. As described above (see Sleep analysis), male OxKO mice (8 weeks old) were implanted with electrodes, and EEG and EMG recordings were initiated after recovery from surgery. OxKO mice were injected intraperitoneally with the vehicle, metoprine (10 mg/kg), metoprine + pyrilamine (10 mg/kg each), metoprine + zolantidine (10 mg/kg each), or pitolisant (an H3R inverse agonist; Sigma-Aldrich, 20 mg/kg) at ZT12. Additionally, cataplexy was induced in OxKO mice by chocolate intake [19]. A single Hershey’s Kiss chocolate sample was placed in the cage immediately after the drug injection. The order of injections was randomized and there were at least 5 days between two injections in the same mouse. Digitized EEG and EMG data were recorded 6 hours after the drug injection. Twelve-second epochs were scored manually for one of four sleep–wake episodes: wake, NREM sleep, REM sleep, or cataplexy. Power spectrum analysis during wakefulness was conducted by fast Fourier transform using SleepSign® 3 Software. The obtained data were normalized as a percentage of the total power at 0–20 Hz, and the theta (6–9 Hz)/delta (1–4 Hz) ratio was calculated.
Statistical analysis
All data were analyzed using Prism 10.0.3 software (GraphPad, La Jolla, CA). Significant differences were assessed using unpaired t-test, one-way ANOVA followed by Tukey’s or Dunnett’s multiple comparisons tests, or two-way repeated measurement ANOVA followed by Sidak’s or Tukey’s multiple comparisons. p-values < .05 indicated statistically significant. All data are presented as the mean ± SEM. The normality of data distribution was examined by using Shapiro–Wilk test.
Results
HNMT inhibition by metoprine increased brain histamine levels, with no effect on levels of other monoamine
To investigate the impact of metoprine on brain histamine concentration, we intraperitoneally injected ICR mice with metoprine (5, 10, or 20 mg/kg) or vehicle. Two hours after injection, we harvested brain samples and measured histamine concentrations in homogenates of the cortex, diencephalon, brainstem, and cerebellum using an HPLC system. Metoprine significantly increased histamine concentrations (Figure 1A), and decreased 1-methylhistamine concentrations in all brain regions (Figure 1B). In contrast, metoprine did not change the levels of norepinephrine, dopamine, or serotonin in these brain regions (Figure S1). We also confirmed that metoprine did not alter the level of monoamine concentrations in more selective brain regions such as the prefrontal cortex, striatum, and midbrain (Figure S2). We further confirmed that metoprine did not change the histamine and 1-methylhistamine levels in the skin and stomach (Table S1). Next, we investigated the time-dependent effect of 10 mg/kg metoprine, finding that metoprine-induced histamine elevation was confirmed 1 h after injection and lasted for at least 8 h in whole brain homogenates (Figure 1C). Previous studies have demonstrated the cytosolic localization of HNMT [1, 20]. To clarify the effect of metoprine on extracellular histamine concentration, we investigated it using microdialysis methods. Microdialysis further revealed that the extracellular histamine concentration in the prefrontal cortex [7] was significantly increased by metoprine treatment (Figure 1D). These data demonstrate that HNMT inhibition by metoprine has a strong effect on the elevation of brain histamine concentration.
Figure 1.
Metoprine increased brain histamine concentration. (A) The i.p. injection of metoprine significantly increased brain histamine concentration in the cortex, diencephalon, brainstem, and cerebellum in a dose-dependent manner. *p < .05, **P < .01: One-way ANOVA followed by Tukey’s multiple comparisons test (n = 4; for cortex: F = 11.25, p = .0008, for diencephalon: F = 7.35, p = .005, for brainstem: F = 7.04, p = .006, for cerebellum: F = 12.40, p = .0005). (B) metoprine decreased 1-methylhistamine concentration. *p < .05, **p < .01: One-way ANOVA followed by Tukey’s multiple comparisons test (n = 4; for cortex: F = 5.31, p = .015, for diencephalon: F = 4.88, p = .019, for brainstem: F = 9.04, p = .0021, for cerebellum: F = 7.06, p = .0054). (C) Metoprine also increased and decreased extracellular histamine and 1-methylhistamine concentrations in a time-dependent manner, respectively. *p < .05, **p < .01: One-way ANOVA followed by Dunnett’s multiple comparisons test (0 h vs several time points; n = 4; for histamine: F = 5.31, p = .0036, for 1-methyl histamine: F = 5.14, p = .0042). (D) Metoprine continually increases extracellular histamine concentration in the prefrontal cortex. (analyzed 4 hours of data after vehicle or metoprine injection) †p < .05; Two-way RM ANOVA for “time” and ‘drug injection’ (n = 4; interaction F (11,66) = 1.5, p = .14, time F(11,66) = 1.28, p = .25, drug F (1,6) = 8.28, p = .028). The Shapiro–Wilk test confirmed the normal distribution of the data.
Metoprine increased locomotor activity in novel and familiar environments, without affecting anxiety-like behaviors
Next, we investigated whether the increase in brain histamine levels induced by metoprine affected locomotor activity. In the open field test, travel time, distance, and average speed were found to be significantly increased by metoprine (Figure 2A-C), although the time spent in the central area did not change (Figure 2D). In the elevated zero mase test, performed to confirm the effect of metoprine on the anxiety-like behavior, the time spent in the open arms was not changed by metoprine (Figure 2E), confirming that metoprine did not affect anxiety-like behavior. We subsequently investigated the effects of metoprine on locomotor activity in the home cage. Results of this analysis showed that metoprine injection during the light period (resting period in mice) significantly increased locomotor activity (Figure 2F); however, no significant difference in locomotor activity was observed following metoprine injection during the dark period (active period in mice; Figure 2G). These behavioral data demonstrate that metoprine increased locomotor activity in both novel and familiar environments, but did not affect anxiety-like behaviors.
Figure 2.
Metoprine increased locomotor activity without the effect on anxiety-like behaviors. In the open field test, metoprine significantly increased traveled time (A), traveled distance (B) and average speed (C) without an effect on the time spent in the central area (D). ***p < .005; Two-way RM ANOVA followed by Sidak’s multiple comparisons test. (n = 10, Two-way RM ANOVA for “time” and ‘drug injection’, for (A): interaction F (5,90) = 9.44, p < .0001, time F (3.35,60.4) = 9.76, p < .0001, drug F (1,18) = 87.83, p < .0001, for (B): interaction F (5,90) = 7.12, p < .0001, time F (5,90) = 9.33, p < .0001, drug F (1,18) = 112.3, p < .0001, for (C): interaction F (5,90) = 4.44, p = .0012, time F (5,90) = 7.64, p < .0001, drug F (1,18) = 66.48, p < .0001, for (D): interaction F (5,90) = 1.78, p = .12, time F (5,90) = 7.87, p < .0001, drug F (1,18) = 2.03, p = .17). (E) In the elevated zero maze, metoprine did not affect the time spent in the open area (n = 10, unpaired t test, p = .70). (F) Metoprine injection at ZT0 (starting time of light period) significantly increased locomotor activity in the home cage. (G) Metoprine injection at ZT12 (starting time of dark period) tended to increase the locomotor activity in the home cage. †p < .05, ††p < .01; Two-way RM ANOVA for “time” and ‘drug injection’ (n = 12; for (F), interaction F (7,154) = 2.05, p = .53, time F (3.31,72.85) = 22.87, p < .0001, drug F (1,22) = 6.07, p = .022, for (G), interaction F (7,154) = 2.41, p = .023, time F (2.97,65.32) = 26.17, p < .0001, drug F (1,22) = 2.13, p = .16). There were no significant differences using Sidak’s multiple comparisons test.
The increase in brain histamine by metoprine induced prolonged wakefulness and reduced NREM sleep through H1R activation
Numerous studies have shown that histamine acts as a wake-promoting amine, leading us to hypothesize that prolonged wakefulness caused by HNMT inhibition would result in increased locomotor activity (Figure 2). Therefore, we conducted sleep analysis using EEG and EMG recordings. Because metoprine significantly increased locomotor activity during the light period (Figure 2F), we injected mice with metoprine during the light period (ZT3) to evaluate its arousal effect. Overall, our results showed that HNMT inhibition by metoprine robustly increased the total amount of wakefulness due to the prolonged bout duration over 6h (Figure 3A-C and S4). Because of the powerful action of metoprine on wakefulness, NREM and REM sleep were absent in the first 3 hours and were strongly reduced in the 4-6 hours after injections (Figure 3D-I). Pyrilamine, an H1R antagonist, significantly decreased the prolonged wakefulness induced by metoprine (Figure 3A), as well as the mean duration of wakefulness (Figure 3C). Pyrilamine partially restored NREM sleep, which was reduced by metoprine (Figure 3D-F), while REM sleep reduction by metoprine was not reversed by pyrilamine (Figure 3G-I). Zolantidine, an H2R antagonist, did not influence the metoprine-dependent alteration of sleep–wake behavior (Figure 3A-I). In addition, metoprine markedly prolonged latency to first NREM sleep dependent on H1R and extended the latency to initial REM sleep independent of H1R and H2R (Figure 3J). These data indicate that metoprine-induced activation of H1R, but not H2R, resulted in prolonged wakefulness and shortened NREM sleep. However, the metoprine-induced reduction in REM sleep occurred independently of H1R and H2R. We further calculated the power spectral density (PSD) during wakefulness for 3 hours after drug administration. Power spectrum analysis revealed that metoprine significantly increased the theta (6–9 Hz)/delta (1–4 Hz) ratio, which correlates with high arousal [21], compared to the vehicle control, implying that metoprine increased the level of wakefulness (Figure S6).
Figure 3.
Metoprine significantly increased wakefulness and decreased NREM sleep via H1R activation. The 3-hourly percentages, bout number, and mean duration of each sleep–wake episode after vehicle or drug(s) injection at ZT3. Pyrilamine and zolantidine are H1R antagonists and H2R antagonists, respectively. The percentage of wakefulness (A), NREM sleep (B) and REM sleep (C). The bout number of wakefulness (D), NREM sleep (E) and REM sleep (F). The mean duration of wakefulness (G). NREM sleep (H) and REM sleep (I). (J) The latency to first NREM sleep and REM sleep after drug administration. (A-I) *p < .05, **p < .01, ***p < .001; Two-way RM ANOVA followed by Tukey’s multiple comparisons test (n = 7, Two-way RM ANOVA for “time” and ‘drug injection’, for (A): interaction F (3,20) = 2.93, p = .059, time F (1,20) = 42.47, p < .0001, drug F (3,20) = 25.15, p < .0001, for (B): interaction F (3,20) = 3.24, p = .044, time F (1,20) = 43.63, p < .0001, drug F (3,20) = 21.27, p < .0001, for (C): interaction F (3,20) = 0.11, p = .96, time F (1,20) = 6.18, p = 0.017, drug F (3,20) = 36.42, p < .0001, for (D): interaction F (3,20) = 5.09, p = .0088, time F (1,20) = 31.84, p < .0001, drug F (3,20) = 14.44, p < .0001, for (E): interaction F (3,20) = 4.86, p = .011, time F (1,20) = 33.22, p < .0001, drug F (3,20) = 14.51, p < .0001, for (F): interaction F (3,20) = 1.13, p = .36, time F (1,20) = 4.70, p = .043, drug F (3,20) = 17.44, p < .0001, for (G): interaction F (3,20) = 18.50, p < .0001, time F (1,20) = 51.40, p < .0001, drug F (3,20) = 18.41, p < .0001, for (H): interaction F (3,20) = 2.10, p = .13, time F (1,20) = 15.54, p = .0008, drug F (3,20) = 2.97, p = .057, for (I): interaction F (3,20) = 1.11, p = .37, time F (1,20) = 7.46, p = .013, drug F (3,20) = 12.16, p < .0001). (J) ***p < .001; One-way ANOVA followed by Tukey’s multiple comparisons test (NREM) F (3, 20) = 32.92, p < .0001, (REM) F (3, 20) = 32.43, p < .0001.
Metoprine robustly reduced cataplexy in narcolepsy-model mice
As several clinical studies have indicated that cerebrospinal fluid (CSF) histamine levels are lower in patients with narcolepsy than in healthy controls [22, 23], we examined the effect of chronic orexin deficiency on brain histamine concentrations. We confirmed that brain histamine levels in Ox-KO mice were significantly lower than in littermate C57BL6 mice (Table S2). On the other hand, other monoamines were not significantly lower (Table S2). We also investigated the effect of metoprine on the brain histamine concentration of Ox-KO mice and confirmed that metoprine significantly increased brain histamine concentration in the absence of orexin (Figure S3). We further examined the significance of HNMT inhibition during cataplexy treatment in OxKO mice. These drugs were administered during the active period, as this is when cataplexy occurs. Overall, the results showed that metoprine induced sustained wakefulness with reduced NREM and REM sleep in OxKO mice (Figure 4A-I and S5), and completely eliminated the total duration of cataplexy episodes, which accounted for approximately 5 % of the total time in the control OxKO mice (Figure 4J-L). In addition, the latency to first NREM sleep and cataplexy is significantly prolonged by metoprine (Figure 4M). Pyrilamine also significantly reduced the impact of metoprine on wakefulness and NREM sleep, confirming the importance of H1R in wakefulness and NREM sleep in OxKO mice. Pyrilamine and zolantidine had no effect on metoprine-induced reductions in REM sleep and cataplexy (Figure 4G-L), suggesting that histaminergic signals via H1R and H2R did not contribute to the reduction of REM sleep and cataplexy-mediated by metoprine. Overall, these data show that brain histamine concentration was decreased in Ox-KO mice and the increase in histamine concentrations induced by HNMT inhibition might have therapeutic effects in narcolepsy.
Figure 4.
Metoprine significantly increased wakefulness and suppressed cataplexy in OxKO mice. The 3-hourly percentages, bout number, and mean duration of each sleep–wake episodes or cataplexy after vehicle or drug(s) injection at ZT12. The percentage of wakefulness (A), NREM sleep (B), REM sleep (C) and cataplexy (D). The bout number of wakefulness (E), NREM sleep (F), REM sleep (G) and cataplexy (H). The mean duration of wakefulness (I). NREM sleep (J), REM sleep (K) and cataplexy (L). (M) The latency to first NREM sleep and REM sleep after drug administration. (A-L) *p < .05, **p < .01, ***p < .001; Two-way RM ANOVA followed by Tukey’s multiple comparisons test (n = 7, Two-way RM ANOVA for “time” and ‘drug injection’, for (A): interaction F (3,24) = 2.34, p = .099, time F (1,24) = 17.86, p = .0003, drug F (3,20) = 7.78, p = .0008, for (B): interaction F (3,24) = 2.46, p = .087, time F (1,24) = 10.80, p = .0031, drug F (3,24) = 7.18, p = .0031, for (C): interaction F (3,24) = 4.84, p = .0090, time F (1,24) = 5.05, p = .034, drug F (3,24) = 5.12, p = .0070, for (D): interaction F (3,24) = 10.61, p = .0001, time 1,24) = 18.70, p = .0002, drug F (3,24) = 69.12, p < .0001, for (E): interaction F (3,24) = 1.85, p = .17, time F (1,24) = 17.26, p = .0004, drug F (3,24) = 8.87, p = .0004, for (F): interaction F (3,24) = 2.65, p = .072, time F (1,24) = 8.96, p = .0063, drug F (3,24) = 9.85, p = .0002, for (G): interaction F (3,24) = 5.00, p = .0078, time F (1,24) = 4.91, p = .037, drug F (3,24) = 4.31, p = .014, for (H): interaction F (3,24) = 3.76, p = .024, time F (1,24) = 10.98, p = .0029, drug F (3,24) = 29.63, p < .0001, for (I): interaction F (3,24) = 4.37, p = .014, time F (1,24) = 15.00, p = .0007, drug F (3,24) = 8.79, p = .0004, for (J): interaction F (3,24) = 2.19, p = .12, time F (1,24) = 4.28, p = .049, drug F (3,24) = 4.20, p = .016, for (K): interaction F (3,24) = 1.91, p = .16, time F (1,24) = 1.88, p = .18, drug F (3,24) = 7.14, p = .0014, for (L): interaction F (3,24) = 2.67, p = .070, time F (1,24) = 1.36, p = .25, drug F (3,24) = 92.86, p < .0001). (M) *p < .05, **p < .01, ***p < .001; One-way ANOVA followed by Tukey’s multiple comparisons test (NREM) F (3, 24) = 8.04, p = .0007, (REM) F (3, 20) = 2.88, p = .057, (cataplexy) F (3, 24) = 112.6, p < .0001.
Metoprine had a greater therapeutic effect on suppressing cataplexy than pitolisant
Finally, we compared the effect of metoprine on cataplexy in OxKO mice (same dataset as in Figure 4) with that of pitolisant, which has been shown to increase the release of histamine in the rat brain [24] and reduce cataplexy in narcolepsy patients [25]. Although pitolisant significantly increased wakefulness and reduced cataplexy with decreasing bout numbers (Figure 5A-I and S5), the therapeutic effect of metoprine was stronger than that of pitolisant (Figure 5J-M). Power spectrum analysis to calculate PSD during wakefulness for 3 hours after drug administration revealed that metoprine, but not pitolisant, increased the theta/delta ratio compared to the vehicle control (Figure 5N and 5O). Therefore, these data indicate that the therapeutic potential of HNMT inhibitors for cataplexy and daytime sleepiness in narcolepsy may be greater than that of H3R inverse agonists.
Figure 5.
The efficacy of metoprine on cataplexy suppression was stronger than that of pitolisant. The 3-hourly percentages, bout number, and mean duration of each sleep–wake episode or cataplexy after vehicle or drug(s) injection at ZT12. The percentage of wakefulness (A), NREM sleep (B), REM sleep (C) and cataplexy (D). The bout number of wakefulness (E), NREM sleep (F), REM sleep (G) and cataplexy (H). The mean duration of wakefulness (I). NREM sleep (J), REM sleep (K) and cataplexy (L). (M) The latency to first NREM sleep and REM sleep after drug administration. (N) spectral distribution of EEG power densities during wakefulness. (O) The theta–delta ratio of EEG during wakefulness. (A-L) *p < .05, **p < .01, ***p < .001; Two-way RM ANOVA followed by Tukey’s multiple comparisons test (n = 7, Two-way RM ANOVA for “time” and ‘drug injection’, for (A): interaction F (2,18) = 5.27, p = .016, time F (1,18) = 9.11, p = .0074, drug F (2,18) = 11.41, p = .0006, for (B): interaction F (2,18) = 2.02, p = .16, time F (1,18) = 0.17, p = .68, drug F (2,18) = 3.21, p = .064, for (C): interaction F (2,18) = 2.85, p = .084, time F (1,18) = 7.27, p = .015, drug F (2,18) = 4.00, p = .037, for (D): interaction F (2,18) = 11.16, p = .0007, time F (1,18) = 47.52, p < .0001, drug F (2,18) = 27.03, p < .0001, for (E): interaction F (2,18) = 3.77, p = .043, time F (1,18) = 10.04, p = .0053, drug F (2,18) = 9.66, p = .0014, for (F): interaction F (2,18) = 1.03, p = .38, time F (1,18) = 0.39, p = .54, drug F (2,18) = 2.11, p = .15 for (G): interaction F (2,18) = 2.51, p = .11, time F (1,18) = 8.76, p = .0084, drug F (2,18) = 3.62, p = .045, for (H): interaction F (2,18) = 3.70, p = .045, time F (1,18) = 31.91, p < .0001, drug F (2,18) = 12.96, p = .0003, for (I): interaction F (2,18) = 1.51, p = .24, time F (1,18) = 6.16, p = .023, drug F (2,18) = 14.67, p = .0002, for (J): interaction F (2,18) = 1.10, p = .35, time F (1,18) = 0.0070, p = .93, drug F (2,18) = 4.65, p = .024, for (K): interaction F (2,18) = 1.14, p = .34, time F (1,18) = 5.39, p = .032, drug F (2,18) = 3.24, p = .063, for (L): interaction F (2,18) = 1.76, p = .20, time F (1,18) = 1.07, p = .31, drug F (2,18) = 22.13, p < .0001). (M and O) *p < .05, *** p < .001; one-way ANOVA followed by Tukey’s multiple comparisons test (M, n = 7, for NREM, F (2,18) = 4.35, p = .029, for REM, F (2,18) = 2.89, p = .081, for cataplexy, F (2,18) = 104.5, p < .0001) (O, n = 7, F (2,18) = 4.05, p = .035).
Discussion
The present study revealed that the HNMT inhibitor, metoprine, increased brain histamine levels without altering the levels of monoamine neurotransmitters in the brain and increased wakefulness and locomotor activity in normal mice. We also found that metoprine increased wakefulness and suppressed cataplexy in an animal model of narcolepsy and these effects were greater compared to the H3R inverse agonist, pitolisant, currently approved for the treatment of narcolepsy. Our data strongly suggest that HNMT inhibitors may serve as a novel treatment option for narcolepsy.
Several HNMT inhibitors have so far been developed as pharmacological tools to examine the importance of HNMT in the regulation of histamine concentrations. For example, metoprine, a derivative of 2,4-diaminopyrimidine, has frequently been used to examine the impact of HNMT on brain function, as it can cross the BBB [12]. However, it cannot be ruled out that the low specificity of metoprine affected the results because metorprine inhibits dihydrofolate reductase [26, 27]. Another HNMT inhibitor, SKF91488, specifically inhibits enzymatic activity without histamine receptor agonist activity. However, owing to the poor BBB permeability of SKF91488, its clinical application in brain disorders is unfortunately quite difficult [28, 29]. Therefore, it is important to identify specific HNMT inhibitors with sufficient BBB permeability to develop novel drugs for patients with hypersomnia.
HNMT is widely distributed across various brain regions [30]. This study confirmed that pharmacological HNMT inhibition was sufficient to increase the histamine concentration in each brain area. Our previous study revealed that HNMT deficiency did not affect the histamine content in the skin and stomach, or the monoamine concentration in the brain [11]. Overall, these data indicate that HNMT mainly contributes to histamine clearance in the brain and that systemic HNMT inhibition could dominantly affect the activity of the brain histaminergic system. Further studies are required to elucidate the brain regions responsible for the therapeutic effects of metoprine. Recent studies have revealed the crucial roles of specific brain regions, such as the basolateral amygdala or ventrolateral periaqueductal (vlPAG), in cataplexy [31, 32]. Our previous studies reported that histaminergic neurons highly project to the amygdala and vlPAG [4] and that HNMT is expressed in these brain areas [30], suggesting that these brain regions may be important for narcolepsy treatment by HNMT inhibition.
In the present study, metoprine was shown to increase locomotor activity and wakefulness but had no effect on anxiety-like behaviors (Figure 2). Our power spectrum analysis revealed that metoprine increased theta–delta ratio of EEG during the light period in ICR mice (Figure S6). The hippocampal local field potential exhibited a higher theta–delta ratio during the active wake state than during the quiet wake state [33]. The cortical EEG and hippocampal local field potentials have also been shown to be highly congruent during wakefulness [34] Thus, these data suggested that metoprine induced the high arousal state, which resulted in high locomotor activity in the open field test. Previous studies have also shown that acute histaminergic activation by chemoactivation or H3R inverse agonism also leads to increased locomotor activity without affecting anxiety-like behaviors [4, 35]. These data supported our results. On the other hand, some psychostimulants, such as methamphetamine and methylphenidate, have been approved for narcolepsy treatment because of their strong wake-promoting effects. However, previous reports have shown that these drugs also affect anxiety-like behaviors [36, 37]. As normal responses to anxious conditions are important for preventing dangerous situations, HNMT inhibitors could be used to treat narcolepsy more safely than psychostimulants. Additionally, metoprine did not change dopamine concentration, whereas psychostimulants induced dopamine release and promoted drug dependence. Moreover, metoprine inhibited methamphetamine-induced drug dependence in mice by increasing brain histamine levels [38, 39]. These results suggest that HNMT inhibitors could be used for narcolepsy treatment with a low risk of drug dependence, without changing anxiety-like behaviors.
Higher wakefulness with concomitant reductions in NREM sleep after metoprine was attenuated by pyrilamine but not zolantidine, suggesting that the H1R but not H2R activation mediated these effects [5, 40, 41]. While metoprine also decreased REM sleep, neither antagonist restored their levels, suggesting that REM-suppressing effects are independent of these receptors. Measurement of extracellular histamine using a G-protein coupled receptor activation-based strategy in a previous study that the histamine concentration during REM sleep is lower than that during wakefulness and NREM sleep [6]. The present study further showed that acute metoprine-induced histamine elevation robustly reduced the duration of REM sleep (Figure 3G). Several pharmacological assays have previously reported that H1R antagonists do not affect REM sleep in humans [42] or mice [43]. This evidence supports the suppressive role of histamine in REM sleep, which probably acts independently of H1R. Histamine also exerts its effects by activating H3R, an autoreceptor expressed on histaminergic neurons, and a presynaptic heteroreceptor expressed on non-histaminergic neuronal terminals. Previous studies have shown that histamine inhibits the action of melanin-concentrating hormone (MCH)-positive neurons [44], which are involved in promoting REM sleep [45, 46]. In addition to MCH neurons, several neural systems such as glutamatergic neurons in mPFC [47] and cholinergic neurons in the pedunculopontine tegmental nucleus [48] promote REM sleep. Previous reports demonstrated that H3R as a heteroreceptor inhibited the release of acetylcholine and glutamate [49]. Thus, metoprine may suppress REM sleep by inhibiting REM-promoting neurons such as MCH neurons, glutamatergic neurons, and/or cholinergic neurons via H3R activation. However, further studies are required to clarify the detailed mechanisms underlying REM sleep regulation by the histaminergic system.
In the present study, we demonstrated the therapeutic potential of HNMT inhibition in the treatment of cataplexy of OxKO mice. Orexinergic projections to TMN histaminergic neurons play a dominant role in orexin-dependent arousal effects [12, 50, 51]. Therefore, loss of orexin signaling in patients with narcolepsy may lead to lower histaminergic activity, resulting in exacerbation of their symptoms. Indeed, several clinical studies have reported that the histamine concentration in the CSF of narcolepsy patients is lower than that in healthy controls [22, 23]. A recent metabolome analysis of the CSF from patients with narcolepsy type-1 (NT1) also confirmed an increase in histidine and a decrease in histamine in the CSF of NT1 patients [52]. In this study, we revealed that brain histamine concentrations were significantly decreased in OxKO mice (Figure S1). These lines of evidence emphasize that reduced orexin signaling induces lower histamine concentrations in the brain. However, there are some previous reports showing that the number of HDC-immunoreactive cells increased in patients with narcolepsy [53] and narcolepsy model mice [54, 55]. The precise interaction between orexin and histamine systems should be determined in the future study.
Modafinil, a drug used for narcolepsy, increases histamine release [56] and induces wakefulness in orexin-null mice [57]. The therapeutic effect of pitolisant, which increases brain histamine level, on cataplexy with narcolepsy is widely recognized, although the exact mechanism of action of pitolisant on cataplexy is not well understood. This study clearly demonstrated that histamine elevation by HNMT inhibition reduced cataplexy. These lines of evidence support the importance of histamine elevation in the treatment of narcolepsy. However, further studies are necessary to determine the neural mechanisms underlying the cataplexy-suppressing effects of HNMT inhibition. Pyrilamine and zolantidine could not cancel the cataplexy suppression by metoprine (Figure 4J-L), confirming that histamine signal via H1R and H2R was not involved. As well as REM sleep suppression, the elevated histamine by HNMT inhibition could suppress cataplexy via activation of H3R signaling. Since several neurotransmitters such as GABA and MCH, which are negatively regulated by H3R [58], increased cataplexy in a narcolepsy model mice [19, 45], inhibition of non-histaminergic neurons through H3R activation might contribute to the therapeutic actions of metoprine.
Previous studies indicated that a lower theta–delta ratio was observed during the quiet wake state [33] and the PSD of theta waves in OxKO mice decreased during the dark period [59]. The present study revealed that metoprine decreased PSD in the delta frequency range, but increased PSD in the theta frequency range during wakefulness (Figure 5N). The theta/delta ratio was significantly higher in the metoprine-treated group than in the vehicle-treated group of Ox-KO mice (Figure 5N). Together, these results indicate that the increased theta/delta ratio by metoprine could induce a high arousal state in OxKO mice. Since patients with narcolepsy feel drowsy throughout the day, HNMT inhibitors may also relieve daytime somnolence symptoms.
Taken together, these data indicated that pharmacological inhibition of HNMT may be a safe and useful new therapeutic strategy for excessive daytime sleepiness and cataplexy in narcolepsy.
Supplementary material
Supplementary material is available at SLEEP online.
Acknowledgments
This research was supported by AMED (Japan Agency for Medical Research and Development) under Grant Number JP23ym0126802j0002 (to TY), and partly JP21zf0127005 (to MY). This work was also supported by a Grant-in-Aid for Scientific Research (B) (22H02808 to KY) and a Grant-in-Aid for Scientific Research (C) (22K07373 to FN), and partly the World Premier International Research Center Initiative (WPI, to MY) from the Japan Society for the Promotion of Science (JSPS) and a Research grant of The Akiyama Life Science Foundation (to TY) and Takeda Science Foundation 2023 (to FN) and National Institutes of Health grant R01NS119223 (to RV). We acknowledge the support of the Turkish Funding student support (to GB).
Contributor Information
Fumito Naganuma, Department of Neuropharmacology, Hokkaido University Graduate School of Medicine, Hokkaido, Japan; Department of Pharmacology, Tohoku University Graduate School of Medicine, Miyagi, Japan; Division of Pharmacology, Faculty of Medicine, Tohoku Medical and Pharmaceutical University, Miyagi, Japan.
Birkan Girgin, Department of Neuropharmacology, Hokkaido University Graduate School of Medicine, Hokkaido, Japan.
Anne Bernadette S Agu, Department of Neuropharmacology, Hokkaido University Graduate School of Medicine, Hokkaido, Japan.
Kyosuke Hirano, Department of Neuropharmacology, Hokkaido University Graduate School of Medicine, Hokkaido, Japan.
Tadaho Nakamura, Division of Pharmacology, Faculty of Medicine, Tohoku Medical and Pharmaceutical University, Miyagi, Japan; Division of Bioregulatory Pharmacology, Department of Pharmacology, Iwate Medical University, Iwate, Japan.
Kazuhiko Yanai, Cyclotron and Radioisotope Center, Tohoku University, Miyagi, Japan.
Ramalingam Vetrivelan, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Takatoshi Mochizuki, International Institute for Integrative Sleep Medicine (WPI-IIIS), University of Tsukuba, Ibaraki, Japan.
Masashi Yanagisawa, International Institute for Integrative Sleep Medicine (WPI-IIIS), University of Tsukuba, Ibaraki, Japan.
Takeo Yoshikawa, Department of Neuropharmacology, Hokkaido University Graduate School of Medicine, Hokkaido, Japan; Department of Pharmacology, Tohoku University Graduate School of Medicine, Miyagi, Japan.
Author Contributions
Fumito Naganuma, Birkan Girgin, and Takeo Yoshikawa designed the study. Fumito Naganuma, Birkan Girgin, Anne Bernadette S. Agu, Kyosuke Hirano, Tadaho Nakamura, Ramalingam Vetrivelan, Takatoshi Mochizuki, and Takeo Yoshikawa performed experiments. Fumito Naganuma, Birkan Girgin, Anne Bernadette S. Agu, Kyosuke Hirano, Takatoshi Mochizuki, and Takeo Yoshikawa analyzed data. Fumito Naganuma, Kazuhiko Yanai, Masashi Yanagisawa, and Takeo Yoshikawa accumulated fundings. Fumito Naganuma and Takeo Yoshikawa administrated project. Takatoshi Mochizuki, Masashi Yanagisawa, Tadaho Nakamura, and Takeo Yoshikawa provided resource. Kazuhiko Yanai, Ramalingam Vetrivelan, Takatoshi Mochizuki, Masashi Yanagisawa, and Takeo Yoshikawa supervised project. Fumito Naganuma and Birkan Girgin wrote draft. Tadaho Nakamura, Ramalingam Vetrivelan, Masashi Yanagisawa, and Takeo Yoshikawa reviewed and edited the draft.
Disclosure Statements
Financial disclosure: Ramalingam Vetrivelan has received research funding from Harmony Biosciences for a different study. Nonfinancial disclosure: none.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author (tyoshikawa@pop.med.hokudai.ac.jp).
References
- 1. Yoshikawa T, Naganuma F, Iida T, et al. Molecular mechanism of histamine clearance by primary human astrocytes. Glia. 2013;61(6):905–916. doi: https://doi.org/ 10.1002/glia.22484 [DOI] [PubMed] [Google Scholar]
- 2. Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4(2):121–130. doi: https://doi.org/ 10.1038/nrn1034 [DOI] [PubMed] [Google Scholar]
- 3. Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–1241. doi: https://doi.org/ 10.1152/physrev.00043.2007 [DOI] [PubMed] [Google Scholar]
- 4. Naganuma F, Nakamura T, Kuroyanagi H, et al. Chemogenetic modulation of histaminergic neurons in the tuberomamillary nucleus alters territorial aggression and wakefulness. Sci Rep. 2021;11(1):17935. doi: https://doi.org/ 10.1038/s41598-021-95497-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thakkar MM. Histamine in the regulation of wakefulness. Sleep Med Rev. 2011;15(1):65–74. doi: https://doi.org/ 10.1016/j.smrv.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong H, Li M, Yan Y, et al. Genetically encoded sensors for measuring histamine release both in vitro and in vivo. Neuron. 2023;111(10):1564–1576.e6. doi: https://doi.org/ 10.1016/j.neuron.2023.02.024 [DOI] [PubMed] [Google Scholar]
- 7. Huang ZL, Mochizuki T, Qu WM, et al. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc Natl Acad Sci U S A. 2006;103(12):4687–4692. doi: https://doi.org/ 10.1073/pnas.0600451103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kollb-Sielecka M, Demolis P, Emmerich J, Markey G, Salmonson T, Haas M. The European Medicines Agency review of pitolisant for treatment of narcolepsy: summary of the scientific assessment by the committee for medicinal products for human use. Sleep Med. 2017;33:125–129. doi: https://doi.org/ 10.1016/j.sleep.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 9. Syed YY. Pitolisant: first global approval. Drugs. 2016;76(13):1313–1318. doi: https://doi.org/ 10.1007/s40265-016-0620-1 [DOI] [PubMed] [Google Scholar]
- 10. Dauvilliers Y, Verbraecken J, Partinen M, et al. ; HAROSA II Study Group collaborators. Pitolisant for daytime sleepiness in patients with obstructive sleep apnea who refuse continuous positive airway pressure treatment. a randomized trial. Am J Respir Crit Care Med. 2020;201(9):1135–1145. doi: https://doi.org/ 10.1164/rccm.201907-1284OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naganuma F, Nakamura T, Yoshikawa T, et al. Histamine N-methyltransferase regulates aggression and the sleep-wake cycle. Sci Rep. 2017;7(1):15899. doi: https://doi.org/ 10.1038/s41598-017-16019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hough LB, Khandelwal JK, Green JP. Inhibition of brain histamine metabolism by metoprine. Biochem Pharmacol. 1986;35(2):307–310. doi: https://doi.org/ 10.1016/0006-2952(86)90530-7 [DOI] [PubMed] [Google Scholar]
- 13. Klein MC, Gertner SB. Evidence for a role of endogenous histamine in central cardiovascular regulation: inhibition of histamine-N-methyltransferase by SKF 91488. J Pharmacol Exp Ther. 1981;216(2):315–320. [PubMed] [Google Scholar]
- 14. Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: https://doi.org/ 10.1016/s0092-8674(00)81973-x [DOI] [PubMed] [Google Scholar]
- 15. Choi J, Kim SJ, Fujiyama T, et al. The role of reproductive hormones in sex differences in sleep homeostasis and arousal response in mice. Front Neurosci. 2021;15:739236. doi: https://doi.org/ 10.3389/fnins.2021.739236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamada Y, Yoshikawa T, Naganuma F, Kikkawa T, Osumi N, Yanai K. Chronic brain histamine depletion in adult mice induced depression-like behaviours and impaired sleep-wake cycle. Neuropharmacology. 2020;175:108179. doi: https://doi.org/ 10.1016/j.neuropharm.2020.108179 [DOI] [PubMed] [Google Scholar]
- 17. Mohsen A, Yoshikawa T, Miura Y, et al. Mechanism of the histamine H(3) receptor-mediated increase in exploratory locomotor activity and anxiety-like behaviours in mice. Neuropharmacology. 2014;81:188–194. doi: https://doi.org/ 10.1016/j.neuropharm.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 18. Yoshikawa T, Nakamura T, Shibakusa T, et al. Insufficient intake of L-histidine reduces brain histamine and causes anxiety-like behaviors in male mice. J Nutr. 2014;144(10):1637–1641. doi: https://doi.org/ 10.3945/jn.114.196105 [DOI] [PubMed] [Google Scholar]
- 19. Mahoney CE, Agostinelli LJ, Brooks JN, Lowell BB, Scammell TE. GABAergic neurons of the central amygdala promote cataplexy. J Neurosci. 2017;37(15):3995–4006. doi: https://doi.org/ 10.1523/JNEUROSCI.4065-15.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuhar MJ, Taylor KM, Snyder SH. The subcellular localization of histamine and histamine methyltransferase in rat brain. J Neurochem. 1971;18(8):1515–1527. doi: https://doi.org/ 10.1111/j.1471-4159.1971.tb00014.x [DOI] [PubMed] [Google Scholar]
- 21. Dean JG, Fields CW, Brito MA, et al. Inactivation of prefrontal cortex attenuates behavioral arousal induced by stimulation of basal forebrain during sevoflurane anesthesia. Anesth Analg. 2022;134(6):1140–1152. doi: https://doi.org/ 10.1213/ANE.0000000000006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bassetti CL, Baumann CR, Dauvilliers Y, Croyal M, Robert P, Schwartz JC. Cerebrospinal fluid histamine levels are decreased in patients with narcolepsy and excessive daytime sleepiness of other origin. J Sleep Res. 2010;19(4):620–623. doi: https://doi.org/ 10.1111/j.1365-2869.2010.00819.x [DOI] [PubMed] [Google Scholar]
- 23. Nishino S, Sakurai E, Nevsimalova S, et al. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep. 2009;32(2):175–180. doi: https://doi.org/ 10.1093/sleep/32.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ligneau X, Perrin D, Landais L, et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: preclinical pharmacology. J Pharmacol Exp Ther. 2007;320(1):365–375. doi: https://doi.org/ 10.1124/jpet.106.111039 [DOI] [PubMed] [Google Scholar]
- 25. Guevarra JT, Hiensch R, Varga AW, Rapoport DM. Pitolisant to treat excessive daytime sleepiness and cataplexy in adults with narcolepsy: rationale and clinical utility. Nat Sci Sleep. 2020;12:709–719. doi: https://doi.org/ 10.2147/NSS.S264140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamrell MR. Inhibition of dihydrofolate reductase and cell growth by antifolates in a methotrexate-resistant cell line. Oncology (Huntingt). 1984;41:343–348. doi: https://doi.org/ 10.1159/000225851 [DOI] [PubMed] [Google Scholar]
- 27. Cavallito JC, Nichol CA, Brenckman WD, et al. Lipid-soluble inhibitors of dihydrofolate reductase. I. Kinetics, tissue distribution, and extent of metabolism of pyrimethamine, metoprine, and etoprine in the rat, dog, and man. Drug Metab Dispos. 1978;6:329–337. [PubMed] [Google Scholar]
- 28. Beaven MA, Shaff RE. Inhibition of histamine methylation in vivo by the Dimaprit analog, SKF compound 91488. Agents Actions. 1979;9:455–460. doi: https://doi.org/ 10.1007/BF01968110 [DOI] [PubMed] [Google Scholar]
- 29. Beaven MA, Shaff RE. New inhibitors of histamine-N-methyltransferase. Biochem Pharmacol. 1979;28:183–188. doi: https://doi.org/ 10.1016/0006-2952(79)90500-8 [DOI] [PubMed] [Google Scholar]
- 30. Otsuka R, Naganuma F, Nakamura T, et al. Contribution of astrocytic histamine N-methyltransferase to histamine clearance and brain function in mice. Neuropharmacology. 2022;212:109065. doi: https://doi.org/ 10.1016/j.neuropharm.2022.109065 [DOI] [PubMed] [Google Scholar]
- 31. Hasegawa E, Miyasaka A, Sakurai K, Cherasse Y, Li Y, Sakurai T. Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science. 2022;375(6584):994–1000. doi: https://doi.org/ 10.1126/science.abl6618 [DOI] [PubMed] [Google Scholar]
- 32. Seifinejad A, Vassalli A, Tafti M. Neurobiology of cataplexy. Sleep Med Rev. 2021;60:101546. doi: https://doi.org/ 10.1016/j.smrv.2021.101546 [DOI] [PubMed] [Google Scholar]
- 33. Giri B, Miyawaki H, Mizuseki K, Cheng S, Diba K. Hippocampal reactivation extends for several hours following novel experience. J Neurosci. 2019;39(5):866–875. doi: https://doi.org/ 10.1523/JNEUROSCI.1950-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Durán E, Oyanedel CN, Niethard N, Inostroza M, Born J. Sleep stage dynamics in neocortex and hippocampus. Sleep. 2018;41(6). doi: https://doi.org/ 10.1093/sleep/zsy060 [DOI] [PubMed] [Google Scholar]
- 35. Sakai N, Onodera K, Maeyama K, Yanai K, Watanabe T. Effects of thioperamide, a histamine H3 receptor antagonist, on locomotor activity and brain histamine content in mast cell-deficient W/Wv mice. Life Sci. 1991;48(25):2397–2404. doi: https://doi.org/ 10.1016/0024-3205(91)90373-j [DOI] [PubMed] [Google Scholar]
- 36. Jager A, Kanters D, Geers F, Buitelaar JK, Kozicz T, Glennon JC. Methylphenidate dose-dependently affects aggression and improves fear extinction and anxiety in BALB/cJ mice. Front Psychiatry. 2019;10:768. doi: https://doi.org/ 10.3389/fpsyt.2019.00768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ortman HA, Newby ML, Acevedo J, Siegel JA. The acute effects of multiple doses of methamphetamine on locomotor activity and anxiety-like behavior in adolescent and adult mice. Behav Brain Res. 2021;405:113186. doi: https://doi.org/ 10.1016/j.bbr.2021.113186 [DOI] [PubMed] [Google Scholar]
- 38. Kitanaka J, Kitanaka N, Hall FS, Uhl GR, Takemura M. Brain histamine N-Methyltransferase as a possible target of treatment for methamphetamine overdose. Drug Target Insights. 2016;10:1–7. doi: https://doi.org/ 10.4137/DTI.S38342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kitanaka N, Hall FS, Kobori S, et al. Metoprine, a histamine N-methyltransferase inhibitor, attenuates methamphetamine-induced hyperlocomotion via activation of histaminergic neurotransmission in mice. Pharmacol Biochem Behav. 2021;209:173257. doi: https://doi.org/ 10.1016/j.pbb.2021.173257 [DOI] [PubMed] [Google Scholar]
- 40. Monti JM, Orellana C, Boussard M, Jantos H, Olivera S. Sleep variables are unaltered by zolantidine in rats: are histamine H2-receptors not involved in sleep regulation? Brain Res Bull. 1990;25(2):229–231. doi: https://doi.org/ 10.1016/0361-9230(90)90065-8 [DOI] [PubMed] [Google Scholar]
- 41. Monti JM, Pellejero T, Jantos H. Effects of H1- and H2-histamine receptor agonists and antagonists on sleep and wakefulness in the rat. J Neural Transm. 1986;66(1):1–11. doi: https://doi.org/ 10.1007/BF01262953 [DOI] [PubMed] [Google Scholar]
- 42. Katayose Y, Aritake S, Kitamura S, et al. Carryover effect on next-day sleepiness and psychomotor performance of nighttime administered antihistaminic drugs: a randomized controlled trial. Hum Psychopharmacol. 2012;27(4):428–436. doi: https://doi.org/ 10.1002/hup.2244 [DOI] [PubMed] [Google Scholar]
- 43. Wang YQ, Takata Y, Li R, et al. Doxepin and diphenhydramine increased non-rapid eye movement sleep through blockade of histamine H1 receptors. Pharmacol Biochem Behav. 2015;129:56–64. doi: https://doi.org/ 10.1016/j.pbb.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 44. Parks GS, Olivas ND, Ikrar T, et al. Histamine inhibits the melanin-concentrating hormone system: implications for sleep and arousal. J Physiol. 2014;592(10):2183–2196. doi: https://doi.org/ 10.1113/jphysiol.2013.268771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Naganuma F, Bandaru SS, Absi G, Mahoney CE, Scammell TE, Vetrivelan R. Melanin-concentrating hormone neurons contribute to dysregulation of rapid eye movement sleep in narcolepsy. Neurobiol Dis. 2018;120:12–20. doi: https://doi.org/ 10.1016/j.nbd.2018.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vetrivelan R, Kong D, Ferrari LL, et al. Melanin-concentrating hormone neurons specifically promote rapid eye movement sleep in mice. Neuroscience. 2016;336:102–113. doi: https://doi.org/ 10.1016/j.neuroscience.2016.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hong J, Lozano DE, Beier KT, Chung S, Weber F. Prefrontal cortical regulation of REM sleep. Nat Neurosci. 2023;26(10):1820–1832. doi: https://doi.org/ 10.1038/s41593-023-01398-1 [DOI] [PubMed] [Google Scholar]
- 48. Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747–765. doi: https://doi.org/ 10.1016/j.neuron.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abdulrazzaq YM, Bastaki SMA, Adeghate E. Histamine H3 receptor antagonists - Roles in neurological and endocrine diseases and diabetes mellitus. Biomed Pharmacother. 2022;150:112947. doi: https://doi.org/ 10.1016/j.biopha.2022.112947 [DOI] [PubMed] [Google Scholar]
- 50. Huang ZL, Qu WM, Li WD, et al. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001;98(17):9965–9970. doi: https://doi.org/ 10.1073/pnas.181330998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mochizuki T, Arrigoni E, Marcus JN, et al. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc Natl Acad Sci U S A. 2011;108(11):4471–4476. doi: https://doi.org/ 10.1073/pnas.1012456108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shimada M, Miyagawa T, Kodama T, Toyoda H, Tokunaga K, Honda M. Metabolome analysis using cerebrospinal fluid from narcolepsy type 1 patients. Sleep. 2020;43(11). doi: https://doi.org/ 10.1093/sleep/zsaa095 [DOI] [PubMed] [Google Scholar]
- 53. John J, Thannickal TC, McGregor R, et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann Neurol. 2013;74(6):786–793. doi: https://doi.org/ 10.1002/ana.23968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berteotti C, Lo Martire V, Alvente S, et al. Orexin/Hypocretin and histamine cross-talk on hypothalamic neuron counts in mice. Front Neurosci. 2021;15:660518. doi: https://doi.org/ 10.3389/fnins.2021.660518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Valko PO, Gavrilov YV, Yamamoto M, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013;74(6):794–804. doi: https://doi.org/ 10.1002/ana.24019 [DOI] [PubMed] [Google Scholar]
- 56. Ishizuka T, Sakamoto Y, Sakurai T, Yamatodani A. Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett. 2003;339(2):143–146. doi: https://doi.org/ 10.1016/s0304-3940(03)00006-5 [DOI] [PubMed] [Google Scholar]
- 57. Willie JT, Renthal W, Chemelli RM, et al. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience. 2005;130(4):983–995. doi: https://doi.org/ 10.1016/j.neuroscience.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 58. Takei H, Yamamoto K, Bae YC, Shirakawa T, Kobayashi M. Histamine H(3) heteroreceptors suppress glutamatergic and GABAergic synaptic transmission in the rat insular cortex. Front Neural Circuits. 2017;11:85. doi: https://doi.org/ 10.3389/fncir.2017.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vassalli A, Franken P. Hypocretin (orexin) is critical in sustaining theta/gamma-rich waking behaviors that drive sleep need. Proc Natl Acad Sci U S A. 2017;114(27):E5464–E5473. doi: https://doi.org/ 10.1073/pnas.1700983114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author (tyoshikawa@pop.med.hokudai.ac.jp).