Abstract
Introduction
Since the introduction of insulin therapy, it has become apparent that type 1 diabetes (T1D) is accompanied by long-term microvascular and macrovascular complications. In the context of the many benefits of continuous glucose monitoring (CGM), there remain opportunities to study the large amount of data now available in order to maximise its potential in the endeavour to reduce the occurrence of diabetes tissue complications in the longer term.
Methods
Continuous glucose monitoring values were downloaded for 89 type 1 diabetes mellitus (T1D) individuals for up to 18 months from 2021 to 2023. Data for patient demographics was also taken from the patient record which included Sex, Date of Birth, and Date of Diagnosis. The recorded laboratory glycated haemoglobin (HbA1c) test results were also recorded. The glucose management index (GMI) was calculated from average glucose readings for 18 months using the formula GMI (%) = (0.82 − (Average glucose/100)). This was then adjusted to give GMI (mmol/mol) = 10.929 * (GMI (%) − 2.15). Average Glucose Fluctuation (AGF) was calculated by adding up the total absolute change value between all recorded results over 18 months and dividing by the number of results minus one. The % Above Critical Threshold (ACT) was calculated by summing the total number of occurrences for each result value. A cumulative 95% limit was then applied to identify the glucose value that only 5% of results exceeded in the overall population. Using this value, we estimated the percentage of total tests that were above the Critical Threshold (ACT).
Results
The mean age of the participants was 42.6 years, and the mean duration of T1D was 18.4 years. A total of 3.22 million readings were analysed, yielding an average blood glucose level of 10.3 mmol/l and a GMI of 57.2 mmol/mol. There was a strong correlation between GMI and measured HbA1c (r2 = 0.82). However, there were patients who had an above-critical threshold (ACT) of 4–10% at a GMI of 60 mmol/mol or less.
The percentage average value at the time of day (%AVTD) was applied to all blood glucose readings at each 15-min interval throughout the day, averaged over 18 months. The %AVTD of GMI (overall average 57.2 mmol/mol) increased after midday, dipped at 18:00, and peaked at 22:00. The %AVTD of AGF (overall average 0.60 mmol/l) showed higher change rates after 09:00 declining at the end of the day. The %AVTD of ACT peaked at 22:00, with those having the highest %ACT showing an additional peak at 15:00.
Conclusions
We have shown here that the percentage glucose results above 18 mmol/l (top 5% of distribution) increased exponentially above 54 mmol/mol HbA1c. The %AVTD is introduced as a useful measure. Our data indicate that over the 24-h period, improvement in metabolic control could be focussed on the afternoon and evening, when there are higher-than-average levels of GMI, a higher-than-average degree of glucose change, and higher-than-average risks of being above the critical threshold. In conclusion, a measure of glycaemic variation based on the amplitude of glucose change to a population mean could be used to provide valuable clinical insights into glucose change over a 24-h period.
Keywords: Glucose variability, Continuous glucose monitoring, Glycated haemoglobin (HbA1c), Type 1 diabetes (T1D)
Key Summary Points
| Why carry out this study? |
| In the context of the many benefits of continuous glucose monitoring, there remain opportunities to analyse the large amount of data now available in order to maximise its potential in the endeavour to reduce the occurrence of diabetes tissue complications in the longer term. |
| What was learned from this study? |
| We have shown that the percentage glucose results above 18mmol/l (top 5% of distribution) increased exponentially above 54 mmol/mol glycated haemoglobin (HbA1c). |
| Our data indicate that over the 24-h period, the improvement in metabolic control could be focussed on the afternoon and evening when there are higher-than-average levels of glucose management indicator (GMI), a higher-than-average degree of glucose change, and higher-than-average risks of being above the critical threshold. |
| A measure of glycaemic variation based on the amplitude of glucose change to a population mean could be used to provide valuable clinical insights into glucose change over a 24-h period. |
Introduction
Since the introduction of insulin therapy, it has become apparent that type 1 diabetes (T1D) is accompanied by long-term microvascular and macrovascular complications, leading to associated morbidity and shortened life expectancy. The Diabetes Control and Complications Trial (DCCT) [1, 2] demonstrated the importance of glycaemic control in preventing microvascular complications and the utility of glycated haemoglobin (HbA1c) monitoring for risk prediction. So far, HbA1c measurement continues to be a cornerstone of diabetes assessment [3], as it reflects the time-averaged glucose exposure. However, it is a crude indicator of glucose control, as it provides no information on glycaemic variability [4]. Furthermore, the DCCT trial [5] showed that a person’s diabetes complication risk is not fully explained by HbA1c [6]. Acute hyperglycaemic fluctuations may cause increased oxidative stress, inflammation, endothelial dysfunction, and altered gene expression (7). Glycaemic variability has therefore been implicated in the development of microvascular disease, although some studies report the association being lost after adjustment for HbA1c.
In 2014, the FreeStyle Libre Flash Glucose Monitoring System (Abbott Diabetes Care, Oxon, UK) for continuous glucose monitoring became available as a potential alternative to the self-monitoring of capillary glucose. Flash glucose sensors have since been found to significantly improve HbA1c over capillary monitoring [8], with subcutaneous glucose monitoring providing more data points and information than capillary glucose self-monitoring. Metrics provided with a continuous glucose monitor (CGM) trace include time in range (TIR), the glucose management indicator (GMI), and the coefficient of variation (CV).
Contrary to HbA1c measurement, CGM data also enable longer-term evaluation of daily glucose variability. How best to evaluate these data remains to be determined. Here, we have examined CGM data for the contribution of glycaemic variability to GMI.
Methods
This was an observational, retrospective, real-world study. A consecutive sample of patients with T1D, using the Freestyle Libre 2, was included. All participants were users of smartphones, allowing utilisation of the Freestyle Libre app. All were administering insulin in a basal-bolus regime. The data downloads were taken separately and do not overlap for any individual. We obtained consecutive data downloads for up to 18 months for each individual. There was no repeated sampling.
The slices were adjacent based on the date we took all the samples falling within consecutive 100-day periods for each patient.
The period from which data was taken was 1 January 2021 to 31 July 2023 (the exact 18-month time window varied between individuals). The 15-min glucose values were downloaded for 89 T1D individuals managed by the East Cheshire diabetes service in the UK. HbA1c test results were taken from the patient record, as were demographic details which included: sex, date of birth, and date of diagnosis of diabetes. Three measures were used to establish the various aspects of blood glucose control achieved during a given period:
Average blood glucose: The glucose management indicator (GMI) was calculated from the average interstitial glucose over 100 days using the formula GMI (%) = (0.82- [average glucose/100]). This was then adjusted to give GMI (mmol/mol) = 10.929 * (GMI [%] - 2.15) [9]. To examine the correlation between GMI value and laboratory-measured HbA1c, a GMI was calculated from the average blood glucose values for the 100 days before the laboratory sample date and was compared by Pearson’s correlation to the actual HbA1c test result.
Change in blood glucose: Average glucose fluctuation (AGF) between glucose readings was calculated by the sum of the total absolute change between recorded results over time, divided by the number of results minus 1. This gave the total average change over 24 h (this is different from the standard deviation, as we are considering a deviation from the previous glucose concentration, not from the mean)
Time above high blood glucose: Above critical threshold (ACT). The total for each result value was consolidated and a cumulative 95% limit was applied to establish the glucose value, at which only 5% of results were greater overall. Applying this value to determine the percent of the total number of tests above this value gave an estimated percentage (ACT) within a period.
The relative independence of these three variables was shown by calculating the Pearson correlation coefficient between these measures, for each patient’s results that were divided into 100-day periods.
The average value at the time of day (AVTD) was calculated across the 24-h clock by applying the above measures to all the blood glucose readings, at each given 15-min time of day, for 18 months. This was then standardized by dividing the overall average for that class.
Cluster Analysis
As these three measures are not entirely independent of each other, cluster analysis was applied between the GMI and AGF to determine the interaction size, as well as to evaluate how dependent they were on demographic factors (sex, age, duration). This was achieved by splitting the cohort into halves by the median value.
Daily Variation
The average value of each factor (GMI, AGF, and ACT) was taken from each 15-min interval over the 24-h period. Patients were then split into tertiles by their average of the above three measures. To improve comparisons between tertiles, the values at each period were standardized as the percentage of the average for that tertile. Unless stated otherwise, data are expressed as mean (standard deviation). This study was a service evaluation exercise. No patient-identifiable information was included in the analysis, so ethics permission was therefore not required.
Ethics
All participants were attending the clinic of one of the authors AHH. The FreeStyle Libre data are available in their entirety as part of their usual care for analysis by the clinical team. All patients were given the option to opt out of their data being analysed. Such data analysis in relation to clinical care is acceptable under UK research ethics guidance. We have kept the patients whose data were analysed fully informed in accordance with the UK guidance at https://digital.nhs.uk/services/national-data-opt-out/understanding-the-national-data-opt-out/protecting-patient-data. All data were full anonymised prior to analysis.
Statistics
Individual patients' blood glucose records at 15-min intervals were downloaded from the LibreView system as CSV files. These records were anonymised ID labelled and consolidated. The overall data set was imported into Excel Power Pivot and analysed, which included dividing each patient total period into 100-day (as HbA1c) sequential segments, establishing fluctuation value as the absolute change from the previous value, and if record was above critical threshold (18 mmol/l).
The patients’ other data including sex, date of birth, date of diagnosis, and HbA1c values were extracted from clinical records and labelled with anonymised ID to link with their BGM scores within Power Pivot. Excel was then used to calculate the Pearson correlation coefficient and plots for selected outputs for the various time interval and cohorts including average blood glucose, converting ABG to GMI, SGF fluctuation, and % results above critical threshold. The given formula was applied to convert the average blood glucose values to GMI.
The patient-recorded HbA1c mmol/mol measurement was compared with the CGM GMI calculated from the average blood glucose over the 100 days previous to the HbA1c sample date. The total number of records for each BG value was plotted covering all patients over all periods was plotted.
Pearson correlation linking GMI, AGF, and ACT were calculated over all the patient 100-day periods. Cluster plots for GMI versus AGF showed value for each patient relative the overall average split by sex and by median value for age and duration with T1D. The last analysis performed considered how the average value for GMI, AGF, and ACT varied across the 24-h day, and also split into level terciles.
Results
Results for 89 individuals (44 men and 45 women) were analysed from an 18-month data collection period. The mean age was 42.6 (standard deviation [SD] 12.7) years, and the mean duration of diabetes was 18.4 (11.8) years. There was a total of 3.22 million glucose values, giving a mean blood glucose of 10.3 mmol/l. This reflected a mean GMI of 56.9 mmol/mol. Table 1 presents data on patient demographics.
Table 1.
Descriptive data of the 89 individuals included in our study
| Class | Number of individuals | Average age (SD) | Average diabetes mellitus duration (SD) | Number of results | Average blood glucose, mmol/l (SD) | GMI | AGF | ACT % | |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 89 | 42.6 (12.7) | 18.4 (11.8) | 3,227,948 | 10.3 (4.4) | 56.9 | 0.596 | 9.5 | |
| Sex | Female | 45 | 44.8 (11.6) | 20.8 (11.9) | 1,597,920 | 10.4 (4.5) | 58.1 | 0.612 | 10.2 |
| Male | 44 | 40.2 (13.5) | 15.9 (11.2) | 1,630,028 | 10.1 (4.3) | 55.7 | 0.580 | 8.7 | |
| Age | < 45 | 44 | 32.1 (7.6) | 14.9 (9.6) | 1,601,357 | 10.2 (4.5) | 56.5 | 0.615 | 9.8 |
| > = 45 | 45 | 53 (6.7) | 22 (12.7) | 1,626,591 | 10.3 (4.3) | 57.3 | 0.577 | 9.1 | |
| Diabetes mellitus duration | < 17 | 43 | 39.2 (12.7) | 8.3 (4.8) | 1,517,162 | 10.3 (4.5) | 57.5 | 0.591 | 10.7 |
| > = 17 | 46 | 46.5 (10.9) | 27.7 (8.7) | 1,710,786 | 10.2 (4.3) | 56.3 | 0.600 | 8.4 | |
4/89 of the patients were people of colour. SD standard deviation, GMI glucose management indicator, AGF average glucose fluctuation, ACT % above critical threshold
There were 428 results of 100-day averages across the 89 patients. The Pearson correlation coefficient was 0.19 between GMI and ACF, 0.81 between GMI and ACT, and 0.21 between AGF and ACT.
GMI estimation compared to laboratory-measured HbA1c is shown in Fig. 1, with Pearson’s correlation coefficient (r2) of 0.82.
Fig. 1.
Glucose management indicator (GMI) calculated from 100 days of data, taken before a laboratory measured HbA1c. Each dot refers to a patient’s HbA1c result
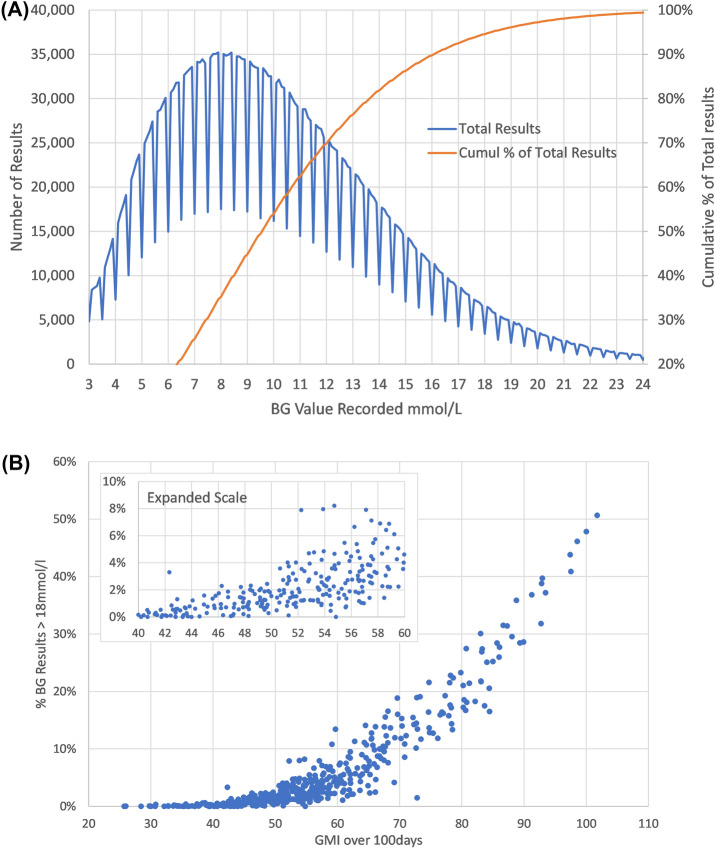
The distribution of glucose values is shown in Fig. 2A. This highlights that 5% of values fell above 18 mmol/l. An artefact of the Libre algorithm leads to fewer values ending in 0.0, or 0.5 mmol/l, leading to a saw-tooth effect. This effect is the same for all and so should not affect the analysis undertaken.
Fig. 2.
A Distribution of glucose values for all patients summated over 18 months. B The 18-month period for each patient was divided into 100-day slices and the % above critical threshold (ACT) and glucose management indicator (GMI) were calculated for each of these slices. Each dot refers to 90 days of glucose monitoring data for a given patient. BG blood glucose
The 18-month period for each patient was divided into 100-day slices, with the %ACT and GMI calculated for each of these slices (Fig. 2B). This shows the link between GMI and the percentage of results ACT for each of these (n = 428) 100-day patient periods analysed.
While the ACT percentage glucose results above 18 mmol/l (the threshold value of the top 5% of the glucose distribution) started to increase exponentially above 54 mmol/mol, there were still episodes with ACT 4–10% with a GMI of 60 mmol/mol or less.
For all of the 89 patients, the individual’s average daily glucose change was compared to that of the group’s mean daily change and plotted against the difference between the individual’s GMI and that of the group’s mean GMI (Fig. 3).
Fig. 3.
A Overall relation between percentage difference from the mean AGF and percentage difference from mean glucose management indicator (GMI). A circle denotes 50% of the population centred on the median value. Data are A overall, B split by sex, C split by age, and D split by duration of type 1 diabetes (T1D). Blue lines represent terciles. The larger points reflect the median values for both classes. Overlaid numbers represent the number of patients per nine quantiles and the change associated with the class shown. AGF average glucose fluctuation
Figure 3A shows the difference in daily glucose levels (AGF) to the group average, plotted against the difference in HbA1c to that of the group mean. Some individuals have low AGF and high GMI with others having the opposite relation, thus indicating that these measures are capturing different aspects of glucose control. In a bivariate analysis, a slight difference by sex (Fig. 3B) was apparent. Importantly, younger patients (Fig. 3C) and patients with shorter duration of diabetes (Fig. 3D) both showed that for a given GMI value, per individual, the daily glucose change values can vary considerably.
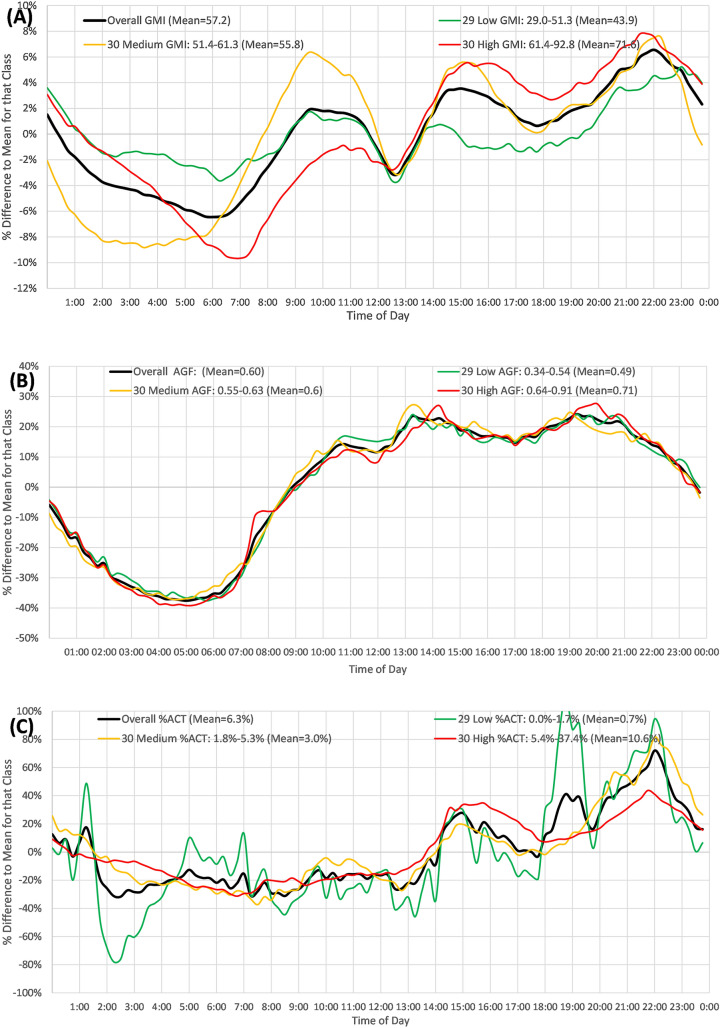
Figure 4A shows the GMI equivalent (mean 57 mmol/mol) in the overall population over a 24-h period. This declined by 10% from clock time 22:00–06:00 followed by an increment to 10:00, a decline to 12:30, and then a steady rise to a peak at 22:00. Patients in the lowest GMI tertile (29–51 mmol/mol) had a much flatter curve, with a total 6% difference between maximum and minimum. Those in the maximum tertile had a 16% difference between maximum and minimum in relation to the mean for the group.
Fig. 4.
A–C Variation across 24 h of the average result from all 15-min samples over total time as a percentage of the difference to the overall mean for that category. A Glucose management indicator (GMI) (mmol/mol) readings. B Average daily glucose fluctuation, AGF (mmol/l/day). C Percentage above critical threshold (ACT%)
Figure 4B shows the AGF equivalent to Fig. 4A. The %AVDT of AGF (overall average 0.60 mmol/l) was negative from clock time 24:00 to 09:00 (i.e., slower change in blood glucose), which was subsequently positive, representing higher change rates later in the day. There was little difference in the pattern for those with high and low fluctuations.
Figure 4C shows the %ACT summated at each point in time over 18 months. This is below mean values for each group from clock time 01:00 to 14:00 (lower number of above threshold readings) and above mean thereafter, peaking at 22:00. Those in the lowest tertile of ACT (0–1.8%) showed the largest fluctuation in ACT. Those patients with the highest percentage of ACT readings showed an additional peak at 15:00.
Discussion
We found that the GMI compares favourably to measured HbA1c (Fig. 1) [10, 11]. Poor correlation can occur in routine clinical practice if significant glycaemic change occurs for the 2 weeks before GMI calculation, as the sensors base the calculation on that timeframe. In our study, we based the GMI calculation on 100 days of data, which might have better approximated the laboratory measurement of HbA1c.
For any given GMI value, the daily “glucose change” values can vary considerably (Fig. 3). Furthermore, greater daily glucose variation can be partly attributed to younger age and shorter duration of diabetes. In our sample, Libre monitoring showed that younger individuals and those with a shorter duration of T1D had greater glucose variability (Fig. 3), which is concerning. GMI fluctuations at a particular time as summated are less in the first half of the day vs. the second half.
Early optimisation of glucose control significantly reduces the risk of microvascular complications [12, 13]. Greater daily glucose fluctuation, occurring between peaks and troughs, is linked to an increased occurrence of hypoglycaemic episodes [14, 15]. Although greater glycaemic variation is known in adolescents [13, 16], our population focused on adults over 18 years of age, so hormonal or psychological aspects of development would not be the cause. Structured education can reduce glycaemic variability and it may be that younger people with shorter duration of diabetes have yet to take part in such a programme. Although it may be assumed that younger people are more active and that blood glucose responses to physical activity may be highly variable, recent studies suggest that overall, physical activity levels are not associated with measures of glucose variability [17–19].
Glycaemic variability metrics fall into two general categories: The first is metrics of amplitude and the second is time-dependency [20]. These are representative of the duration of events. The metric we employed is from the first category and does not quantify the absolute time spent out of normoglycaemia. Time in range will quantify duration but not give an estimation of amplitude [21]. For this, standard reports usually give a CV of < 36%, which is considered to identify those at lesser risk of hypoglycaemia [22]. However, a problem with using the CV is that when the mean is large, even a moderate amount of variability (measured by the standard deviation), may seem small relative to the magnitude of the mean [23]. In Fig. 4, we show that glycaemic variability (AGF) is greatest in the second half of the day and then declines toward midnight with the degree of GMI for summated glucose levels over 18 months being greater for those with higher GMI (by tertile of GMI).
Above a GMI of 54 mmol/mol, the percentage of values > 18 mmol/l rose exponentially (Fig. 2b), but even at values of 54 mmol/mol, some individuals showed high variation in glucose levels from one reading to the next. Furthermore, those in the highest tertile of % > 18 mmol/l had peaks in the % above 18 mmol/l at both clock times 15:00 and 22:00. This emphasizes the limitations of HbA1c (approximated by GMI) with regard to identifying glucose variability. In such individuals, a change in clinical approach might be needed, even though HbA1c is at, or close to, target.
We also show that the summated GMI over time was at its lowest for all GMI categories (tertiles of GMI) at just before midday (Fig. 4) and that there was much less variation in summated GMI over time for those in the lowest versus the highest tertile of GMI. Meal consumption is positively associated with glucose variability [24] and was likely having an effect. Newer, rapid-acting insulin analogues may result in reduced early postprandial hyperglycaemia and less hypoglycaemia several hours after the meal [25, 26]. However, the effectiveness is related to rates of insulin absorption. As well as determining glycaemic variability, the use of either CGM or flash glucose monitoring can also help to reduce hypoglycaemia and improve glycaemic variability [27–29].
As there are no established critical clinical levels to be applied for age and duration with the condition, splitting the cohort into halves by age and duration gave samples of equivalent size and allowed more confident comparison between the two groups. The use of terciles to highlight and quantify the impact of age and duration showed that younger individuals in the early years of living with the condition were at higher risk of increases in both GMI and AGF.
It is important to point out that the link between the three chosen metrics GMI, AGF, and % ACT was weak with R2 between them < 0.2, suggesting that these metrics are relatively independent of each other and may each be capturing different aspects of the physiology of glucose handling in people with T1D.
Limitations
This study utilized data from intermittent scanning CGM (flash glucose monitoring), which required participants to swipe a reader over the sensor. This might introduce bias, as there may be a greater propensity to swipe at extremes of glucose. However, the sensor records the glucose reading each minute and stores a glucose reading every 15 min for the previous 8 h [30]. These data are then captured after each swipe. Another limitation is that we have not been able to include systematic data on insulin dosing or carbohydrate intake. This will be the subject of a future study. It should also be pointed out that DEXCOM® and other continuous glucose monitoring systems are available. However, LIBRE was the technology most widely used by our patients at the time of this study.
Conclusions
Our data indicate that over the 24-h period, improvement in metabolic control could be focussed on the afternoon and evening when there are higher-than-average levels of GMI summated for this time of day, a higher-than-average degree of glucose change, and higher-than-average risks of being ACT. We have shown that the percentage of glucose results above 18 mmol/l (top 5% of the distribution) increased exponentially above 54 mmol/mol HbA1c.
A key aspect of this paper is that we have summated the glucose data over a long period of up to 18 months, rather than the shorter periods usually taken. The %AVTD is introduced as a useful measure. We are not suggesting this as an alternative to GMI estimated over all readings taken in a given period /HBA1c, rather as a helpful addition to existing measures.
Thus, a measure of glycaemic variation based on the amplitude of glucose change summated over time, for particular times of the day, could be used to provide valuable clinical insights to enable optimisation of insulin regime in people with T1D.
Acknowledgements
Vernova Healthcare provided the diabetes service that all the participants attend. We also thank the participants of the study.
Author Contribution
Adrian H Heald and Mike Stedman conceived the study. Mike Stedman led on data analysis. John Warner-Levy and Lleyton Belston supported data management and contributed to data analysis. Angela Paisley provided expert input in relation to T1D management. Aleksandra Jotic and Nebojsa Lalic provided essential technical insights. Martin Gibson provided invaluable insight in relation to the context of the study while Hellena H Habte-Asres, Martin Whyte, and Angus Forbes let on the interpretation of the glucose monitoring data and the implications of the findings for people with T1D.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Conflict of Interest
Adrian H Heald, Mike Stedman, John Warner-Levy, Lleyton Belston, Angela Paisley, Aleksandra Jotic, Nebojsa Lalic, Martin Gibson, Hellena H Habte-Asres, Martin Whyte, and Angus Forbes have nothing to disclose.
Ethical Approval
All participants were attending the clinic of one of the authors AHH. The FreeStyle Libre data are available in its entirety as part of their usual care for analysis by the clinical team. All patients were given the option to opt out of their data being analysed. Such data analysis in relation to clinical care is acceptable under UK research ethics guidance. We kept the patients whose data were fully analyzed informed in accordance with UK guidelines at https://digital.nhs.uk/services/national-data-opt-out/understanding-the-national-data-opt-out/protecting-patient-data. All data were full anonymised prior to analysis.
References
- 1.Deckert T, Poulsen JE, Larsen M. Prognosis of diabetics with diabetes onset before the age of thirty-one. survival, causes of death, and complications. Diabetologia. 1978;14(6):363–70. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group, Nathan DM. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- 3.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40:994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinemann L, Freckmann G, Müller-Wieland D, Kellerer M. Critical reappraisal of the time-in-range: alternative or useful addition to glycated hemoglobin? J Diabetes Sci Technol. 2020;14(5):922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968–83. [PubMed] [Google Scholar]
- 7.Kilpatrick ES, Rigby AS, Atkin SL. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care. 2009;32(10):1901–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leelarathna L, Evans ML, Neupane S, Rayman G, Lumley S, Cranston I, Narendran P, Barnard-Kelly K, Sutton CJ, Elliott RA, Taxiarchi VP, Gkountouras G, Burns M, Mubita W, Kanumilli N, Camm M, Thabit H, Wilmot EG. Intermittently scanned continuous glucose monitoring for type 1 diabetes. N Engl J Med. 2022;387(16):1477–87. [DOI] [PubMed] [Google Scholar]
- 9.Bergenstal RM, Beck RW, Close KL, et al. Glucose Management Indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Peralta F, Choudhary P, Cosson E, Irace C, Rami-Merhar B, Seibold A. Understanding the clinical implications of differences between glucose management indicator and glycated haemoglobin. Diabetes Obes Metab. 2022;24(4):599–608. [DOI] [PubMed] [Google Scholar]
- 11.Leelarathna L, Beck RW, Bergenstal RM, Thabit H, Hovorka R, APCam11, AP@home04 and APCam08 Investigators. Glucose management indicator (GMI): insights and validation using guardian 3 and navigator 2 sensor data. Diabetes Care. 2019;42(4):e60–1. [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008;31(11):2198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virk SA, Donaghue KC, Cho YH, et al. Association between HbA1c variability and risk of microvascular complications in adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2016;101(9):3257–63. [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50:2553–61. [DOI] [PubMed] [Google Scholar]
- 15.Toschi E, Slyne C, Sifre K, et al. The relationship between CGM-derived metrics, A1C, and risk of hypoglycemia in older adults with type 1 diabetes. Diabetes Care. 2020;43(10):2349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Volkening LK, Laffel LM. Distinct patterns of daily glucose variability by pubertal status in youth with type 1 diabetes. Diabetes Care. 2020;43(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biankin SA, Jenkins AB, Campbell LV, Choi KL, Forrest QG, Chisholm DJ. Target-seeking behavior of plasma glucose with exercise in type 1 diabetes. Diabetes Care. 2003;26(2):297–301. [DOI] [PubMed] [Google Scholar]
- 18.Montt-Blanchard D, Sánchez R, Dubois-Camacho K, Leppe J, Onetto MT. Hypoglycemia and glycemic variability of people with type 1 diabetes with lower and higher physical activity loads in free-living conditions using continuous subcutaneous insulin infusion with predictive low-glucose suspend system. BMJ Open Diabetes Res Care. 2023;11(2): e003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Kandari J, Al Ozairi E, Irshad M, Varghese A, Gray SR. Association of physical activity metrics with glucose variability in people with type 1 diabetes: A cross-sectional study. Eur J Sport Sci. 2024;24:210–6. [Google Scholar]
- 20.Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354–69. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19(3):178–81. [DOI] [PubMed] [Google Scholar]
- 22.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahola AJ, Mutter S, Forsblom C, et al. Meal timing, meal frequency, and breakfast skipping in adult individuals with type 1 diabetes – associations with glycaemic control. Sci Rep. 2019;9:20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutta D, Mohindra R, Mahajan K, Sharma M. Performance of fast-acting aspart insulin as compared to aspart insulin in insulin pump for managing type 1 diabetes mellitus: a meta-analysis. Diabetes Metab J. 2023;47(1):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell-Jones D, et al. Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial. Diabetes Care. 2017;40(7):943–50. [DOI] [PubMed] [Google Scholar]
- 27.Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391:1367–77. [DOI] [PubMed] [Google Scholar]
- 28.Breton MD, Patek SD, Lv D, et al. Continuous glucose monitoring and insulin informed advisory system with automated titration and dosing of insulin reduces glucose variability in type 1 diabetes mellitus. Diabetes Technol Ther. 2018;20:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2017;61(539–550):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.https://www.england.nhs.uk/london/wp-content/uploads/sites/8/2019/07/dia-FreeStyle-Libre-training-pack-for-HCP-and-patients-052018.pdf: accessed 30 March 2024
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.