Abstract
Background
Borderline Personality Disorder (BPD) is a prevalent and debilitating psychiatric condition often accompanied by Post-Traumatic Stress Disorder (PTSD), with a substantial prevalence of trauma history among affected individuals. The clinical, cognitive, and cerebral parallels shared with PTSD suggest a trauma-related etiology for BPD. Studies consistently demonstrate a reduction in hippocampal volume in individuals with BPD, echoing findings in PTSD. However, the interpretation of this shared neurobiological profile remains contentious, with ongoing debates regarding the independence of these pathologies or the potential exacerbation of diminished hippocampal volume in BPD due to concurrent PTSD. Differential impacts on hippocampal subfields across both disorders may further complicate interpretation, suggesting the volume of hippocampal subfields as a potential discriminant biomarker. This study aims to characterize the multidimensional specific and shared profiles of BPD and PTSD-related alterations, with a particular emphasis on hippocampal subfields during adolescence, a crucial period in BPD development.
Methods
This study focuses on female adolescents, who are more prevalent in the BPD population. Participants are categorized into three groups: BPD, BPD with comorbid PTSD, and a control group of matched healthy individuals. Data collection encompasses clinical, cognitive, and neuroimaging domains commonly affected in both disorders, utilizing various imaging markers (including gray matter macrostructure, white matter microstructural integrity, and regional functional connectivity).
Discussion
This study examines adolescent BPD with and without comorbid PTSD on clinical, neuroimaging, and cognitive levels. It is the first to use a comprehensive multi-modal approach within the same sample. Additionally, it uniquely explores hippocampal subfield volume differences in adolescents. Analysis of the relationship between the investigated domains and the effects of PTSD comorbidity will elucidate specific and shared alteration profiles in both disorders.
Trial registration
IDRCB number 2019-A00366-51 / clinicaltrials.gov ID: NCT0485274. Registered on 21/04/2021.
Keywords: Adolescent, Female, Post-traumatic stress disorder, Neuroanatomy, Borderline personality disorder, Comorbidity, Psychopathology, Emotional regulation, Hippocampus
Introduction
Borderline personality disorder (BPD) is a debilitating mental disorder, characterized by significant instability in affective, identity, and interpersonal functioning [1, 2]. More specifically, the core clinical features of BPD include emotional hyperreactivity, identity diffusion, and self-injurious behaviors [1, 3]. The lifetime prevalence of BPD is approximately 5% in the general population, with a sex ratio of 3 females to 1 male [4]. Patients with BPD represent 10 to 25% of patients in psychiatric clinics or hospitals, and globally display severe morbidity, poor functioning, and high mortality, primarily due to suicide [5]. The substantial individual and societal impact of BPD highlights its status as a major public health concern [2, 5].
The etiopathogenesis of BPD is complex, involving both genetic vulnerability and environmental factors. Among these, exposure to potentially traumagenic adverse life events in childhood stands out as one of the most significant factors [6]. This is reflected in the high comorbidity between BPD and post-traumatic stress disorder (PTSD), a trauma- and stressor-related disorder characterized by long-lasting symptoms such as psychic intrusions, avoidant behavior, and hyperarousal following exposure to a traumagenic event [3]. Concomitant PTSD is observed in up to 80% of BPD patients [7] and is associated with increased severity of BPD symptoms and reduced treatment efficacy [7, 8]. Moreover, patients with both BPD and PTSD experience more severe PTSD symptoms compared to those with PTSD alone [7].
Traumagenic events that have been prospectively associated with BPD mainly consist of childhood abuse, including emotional, physical, and sexual trauma [6], the same association being shown for childhood maltreatment as a whole [9, 10]. These findings highlight the developmental aspects of BPD, which show emotional and behavioral precursors in childhood [11, 12] and typically manifest during adolescence [1, 3]. Adolescence is indeed a critical period for the development and stabilization of emotional regulation [13], identity [14], and social cognitive functioning [15]. These developmental processes may be linked to the progressive maturation of the prefrontal cortex during puberty and its interaction with limbic structures [16].
Limited knowledge exists regarding the brain underpinnings of BPD during adolescence. A few structural neuroimaging studies have indicated a reduction in gray matter volume (GMV) within the limbic cortical circuit [17–22] and the internal temporal lobe, including the hippocampus [23, 24]. In adults with BPD, structural studies have reported decreased GMV in fronto-limbic structures, such as the cingulate cortex, orbitofrontal cortex, prefrontal cortex, amygdala, and hippocampus [25]. Fewer studies have investigated the integrity of white matter (WM) tracts in BPD, with the most consistently observed findings showing decreased integrity of WM tracts in the corpus callosum and the hippocampal fornix [26].
These structural alterations closely resemble those observed in PTSD [27]. Notably, patients with PTSD exhibit decreased GMV within fronto-limbic structures [28, 29] and alterations in the integrity of WM tracts, including those in the corpus callosum and fronto-limbic fibers [28, 30]. Furthermore, resting-state fMRI studies in BPD have demonstrated disruptions in functional connectivity within the default mode network [25, 31], which aligns with patterns observed in PTSD patients [32].
Importantly, meta-analyses of brain structure in BPD underscores a reduction in hippocampal volume as a prominent finding [33–35]. This observation aligns with the critical role of the hippocampus within cortico-limbic circuits and some of the cognitive functions they’re involved in, such as emotional regulation [36], impulsivity [37, 38], and higher-order cognitive dysfunctions, especially in memory functioning [39, 40].
Hippocampal volume and function also appear to be involved in the development and maintenance of PTSD symptoms [41]. Notably, reduced hippocampal volume is associated with an increased susceptibility to PTSD [42]. Conversely, heightened hippocampal activity has been linked to fewer PTSD symptomatology following exposure to potentially traumagenic events [43]. Moreover, a negative correlation between hippocampal volume and the severity of reexperiencing symptoms has been reported in PTSD patients [44].
The hippocampus has frequently been found to be involved in stress or -related psychopathology, mainly because of its sensitivity to biological stress [45, 46]. The glucocorticoid cascade hypothesis posits that persistent exposure to stress, such as childhood adversity, leads to a reduction in hippocampal volume through the cytotoxic impact of elevated cortisol levels [45–47]. This hypothesis has also been put forth as a neurobiological model for BPD [25].
The scientific literature exploring the role of PTSD on hippocampal alterations in patients with BPD is scarce and has yielded contrasting results. While some studies report that hippocampal GMV reduction in BPD patients is independent of PTSD comorbidity [34, 48–51], other ones suggest that decreased hippocampal volume in BPD may be amplified by, or even a consequence of, concurrent PTSD [52].
Specific profiles of GMV alterations in BPD and PTSD might be derived from the study of hippocampal subfields, allowing for more subtle analyses of volumetric differences. Interestingly, hippocampal GMV reduction in adolescents with PTSD has been shown to be driven by smaller volumes of some hippocampal substructures, the Cornu Ammonis (CA), specifically the CA2-CA3-Dentate Gyrus (DG) complex, which correlated negatively with the severity of reexperiencing symptoms [53]. Consequently, the volume of hippocampal subfields could be an interesting candidate as a predictive biomarker of PTSD outcome, as well as a biomarker of susceptibility to PTSD.
Hippocampal subfields have received very limited attention in BPD to date. A single study in BPD patients without comorbid PTSD has shown a reduction in volume in the stress-sensitive subfields DG-CA4 and CA2-3 compared to healthy controls. Interestingly, this volume loss exhibited no significant relationship with levels of childhood adversity [54]. Investigating the precise location of hippocampal volume loss might thus provide information on the differential role of the hippocampus in both BPD and PTSD psychopathology.
Methods
Aims and objectives
The BORDERSTRESS-ADO research project aims to comprehensively investigate the impact of chronic stress through the experience of adverse childhood events, with a specific focus on the influence of comorbid PTSD on the neurobiology and psychopathology of BPD. Notably, BPD typically emerges during adolescence, a critical period for its development, yet no such comparison has been conducted in adolescents to date. By identifying new markers associated with BPD, the outcomes of this study hold the potential to enhance the accuracy of BPD diagnosis in adolescents and will also provide evidence to inform treatment decisions for this particularly severe condition.
Primary objective
The primary objective of this study is to investigate morphological modifications specific to PTSD in BPD population by comparing hippocampal volume in adolescents with BPD and those with comorbid BPD and PTSD. Each clinical group will first be compared to a healthy developing age-matched group.
Secondary objective
Primary analyses will be completed by comparisons of hippocampal subfields volumes across both clinical groups.
Exploratory objectives
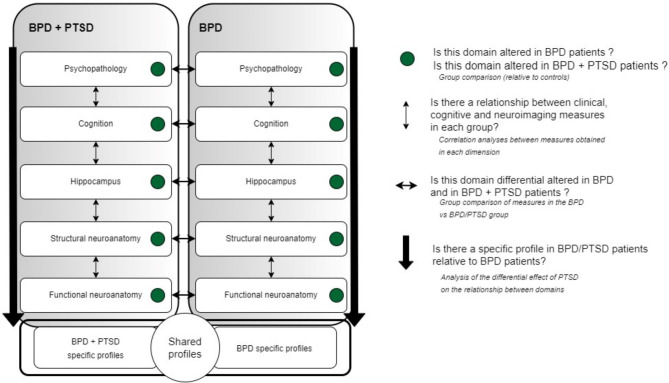
Other secondary analyses will aim identifying specific and shared profiles of alterations between BPD and BPD with comorbid PTSD. Comparative and correlation analyses will be conducted on several measures, including WM microstructural integrity, resting-state functional connectivity (rsFC), and a range of cognitive and clinical variables of interest. Additionally, we aim to investigate the relationship between cerebral alterations and behavioral impairments in domains commonly affected in both BPD and PTSD. This will allow us to examine the impact of comorbid PTSD on the relationship between these variables and establish specific and shared profiles of multidimensional alterations in BPD and PTSD (see Fig. 1). This study will also assess the association between hippocampal volumes and the evolution of symptom profiles, employing a cross-sectional and repeated-measure design at 18 months post-inclusion.
Fig. 1.
Objectives of the BORDERSTRESS-ADO study. BPD: Borderline Personality Disorder, PTSD: Posttraumatic Stress Disorder
Study design and overview
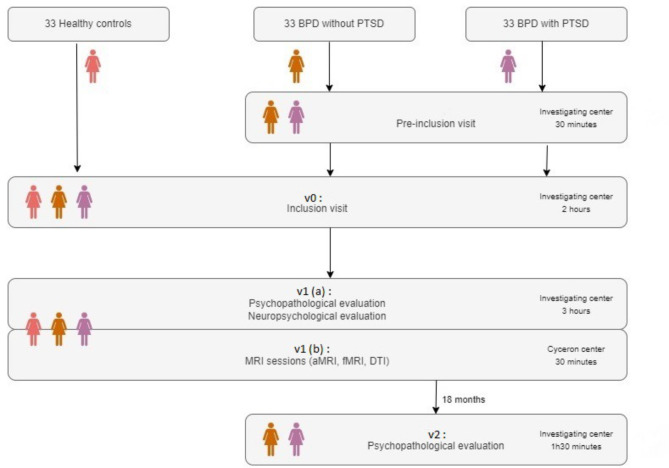
This study is a multi-center comparative study. We aim to recruit female adolescents from July 2022 to 2025 across 3 investigating centers (CHU Caen, CHU Rouen, GH Le Havre), 33 participants in each group (BPD, BPD/PTSD and healthy controls). Patients are pre-screened by a psychiatrist to check the inclusion criteria that are summarized in Table 1. Healthy controls interested in taking part in the study are pre-screened by a licensed psychologist during a telephonic interview. After providing an oral consent to participate, an interview is planned with the investigating psychiatrist, during which participants and their legal representatives are given the information notice, the consent forms and an answer to any question about the study. The inclusion visit (v0) is then scheduled after receiving informed written consent (see Fig. 2 for a depiction of participants’ flowchart). This visit takes place in the inclusion site for patients, and in the research center for controls, and is divided into two parts. First, a standardized psychopathological evaluation is conducted by a psychiatrist. Data obtained during this stage are used as objective arguments for group assignment during the team consensus best estimate diagnosis process (see measures and outcomes). The second part of the visit is conducted by a psychologist and aims to verify the cognitive inclusion criteria (Wechsler’s scales of intelligence). After this visit, two other ones are scheduled for neuropsychological assessments (v1(a)) and neuroimaging data collection (v1(b), Cyceron neuroimaging center, Caen). A last visit after a period of 18 months is also planned for patients to conduct a clinical assessment (v2). Please see measures and outcomes for more details.
Table 1.
Inclusion and non-inclusion criteria
| BPD | BPD/PTSD | HC | |
|---|---|---|---|
| Inclusion criteria |
• Female • 13–17 years old • Good understanding of written and spoken French • DSM-5 based diagnosis of BPD • Score > 20 CGA-S |
• Female • 13–17 years old • Good understanding of written and spoken French • DSM-5 based diagnosis of BPD • DSM-5 based diagnosis of PTSD • Score > 20 CGA-S |
• Female • 13–17 years old • Good understanding of written and spoken French • Absence of any mental disorders |
| Non-inclusion criteria |
• Medicated attention deficit • Illiteracy • Presence of a major sensory disorder • Severe comorbid psychiatric disorder • History or presence of severe neurological disorder and/or alertness deficit • History of anoxic coma • Contraindication to MRI • Severe ongoing physiological disease • Inclusion in another biomedical research protocol during the study period • IQ < 70 |
||
BPD: Borderline Personality Disorder; PTSD: Post-traumatic stress disorder; HC: Healthy Controls; CGA-S: Clinical Global Assessment Scale
Fig. 2.
Participants’ flowchart. There are three visits (v0, v1 and v2). The v1 visit is divided into two parts, v1 (a) at the Investigating center and v1 (b) at Cyceron center. BPD: Borderline Personality Disorder, PTSD: Posttraumatic Stress Disorder
Study population
This study aims to compare female adolescents (N = 99) with a diagnosis of BPD (N = 33), a dual diagnosis of BPD and PTSD (N = 33), and matched healthy controls (N = 33). We decided to include only females to limit the heterogeneity in the population due to the sex ratio of 3 females for 1 male in the general population.
This sample size (N = 99) was defined empirically, based on the recruitment capacity across the timespan of the study rather than based on a statistical hypothesis. Of note, the difference and standard deviation of hippocampal volume between groups, if any, are currently unknown. Nonetheless, the comparison of 33 subjects per group allows the detection of an anticipated effect size ES = 0.84 (large), with a power of 0.915 and a two-tailed alpha risk of 0.05. This computation was performed with a software for a-priori Sample Size Calculator for Student t-Tests [55].
Patients are recruited in child and adolescent psychiatry department in the 3 investigating sites. Controls are recruited to match patients’ age, sociodemographic status, and education level by diffusion of flyers in schools and on social media. Patients planning to move to another region in the next 18 months following their inclusion are not included. Finally, patients with ongoing or past pharmacological treatment are not excluded, except in the case of a medicated attention deficit. Treatment’s type and posology are registered and considered when analyzing neuroimaging data. The inclusion and exclusion criteria are further described in Table 1.
Measures and outcomes
Behavioral measures & analyses
Clinical evaluation: v0
The inclusion visit consists of a clinical evaluation to ensure the respect of inclusion and non-inclusion criteria, as well as to dispatch patients in the adequate group.
To determine group assignment (BPD vs. BPD/PTSD) for patients, the presence of PTSD is examined in a case conference using the team-consensus best estimate diagnoses method [56]. The decision is based on clinical data collected during the inclusion visit (T0), in the presence of the patient’s usual medical team. If the PTSD diagnosis is not confirmed in the case conference, the patient is assigned to the BPD group. Measures used for diagnosis and severity assessment are described in Table 2.
Table 2.
Clinical assessments
| Visit | Assessment | Test |
|---|---|---|
|
v0 Clinical evaluation |
BPD diagnosis | SIDP-IV : Structured Interview for DSM-IV Personality [57] |
| PTSD diagnosis | K-SADS-PL : Kiddie-Schedule for Affective Disorders and Schizophrenia [58] | |
| Severity of psychopathology | CGAS : Clinical Global Assessment Scale [59] | |
| Severity of PTSD symptoms | UCLA PTSD-RI C/A : Post-Traumatic Stress Disorder Reaction Index for Children and Adolescents [60] | |
| Presence of concomitant depression | ADRS : Adolescent Depression Rating Scale [61] | |
| Intensity of BPD symptoms | Echelle de Traits de Personnalité Limite chez les Enfants [62], BPFSC-11 : French version of the Borderline Feature Scale for Children [63] | |
| Intelligence | WISC-V : Weschler’s Intelligence Scale for Children V [64] | |
| WAIS-IV : Weschler’s Adult Intelligence Scale IV [65] | ||
| Adverse life events | CTQ : Childhood Trauma Questionnaire [66, 67] |
BPD: Borderline Personality Disorder; PTSD: Post-traumatic stress disorder
Cognitive functioning: v1(a)
All participants will undergo a set of neuropsychological evaluations, corresponding to affective and cognitive domains frequently altered in BPD and/or PTSD: emotional functioning, social cognition and more particularly theory of mind, identity diffusion and autobiographical memory and other associated abilities such as executive functions and quality of attachment (See Table 3 for details). All tests have shown good validity and reliability in adolescents.
Table 3.
Neuropsychological assessments
| Visit | Assessment | Test |
|---|---|---|
|
v1(a) Neuropsychological evaluation |
Insecurity of attachment | Ca-Mir : Cartes des Modèles Individuels de Relations [68] |
| Emotional Dysregulation | Echelle Neuro-Affective de Personnalité (French version of the Affective Neuroscience Personality Scale; ANPS) [69] | |
| Sustained attention | CPT : Continuous Performance Test [70] | |
| Executive functions | WSCT : Wisconsin Card Sorting Test [71] | |
| Social cognition: Theory of Mind | MASC : Movie Assessment of Social Cognition [72] | |
| RFQ : Reflective Functioning Questionnaire [73] | ||
| Mental representations of self and others | AIDA : Assessment of Identity Development in Adolescence [74] | |
| Autobiographical memory | NaCCS : Narrative Coherence Coding Scheme [75] |
Neuroimaging measures and analyses
Neuroimaging data will be collected using an MRI 3T Signa Premier General Electric Healthcare with a 48-channels head-coil, located in the Cyceron Center (Caen, France).
Structural MRI
Data collection
Anatomical data will be collected using classical T1-weighted imaging on the whole brain. Volumetric T1-weighted images will be acquired at 1mm3 isotropic resolution to measure grey matter density and volumetry using a three-dimensional (3D) fast field echo sequence (sagittal acquisition; repetition time 2.2s; echo time 2.7 ms; flip angle 8⁰; 180 slices; slice thickness 1 mm; matrix size, 256 × 256).
To investigate volumetric alterations specifically within the hippocampal subfields, a high-resolution two-dimensional T2-weighted image of the hippocampus will also be acquired (repetition time 8s; echo time 47 ms; flip angle 122⁰; 35 slices, slice thickness 2 mm; matrix size 448 × 448; in-plane resolution 0.4 × 0.4 mm2).
Data analysis
Preprocessing of volumetric data will be done using SPM software (Statistical Parametric Mapping; Wellcome Department of Cognitive Neurology, London, UK), implemented in MATLAB (Mathworks Inc., Natick, MA). Obtained images will be normalized in a reference space based on the mean space from all the participants’ data, using SPM’s DARTEL.
Hippocampal subfields will be segmented with an automated method, developed in our research unit [53, 76]. This method is based on previous manual segmentation data and is comprised of a double random-blind measure regarding the participant’s identity and clinical status. Whole brain voxel-based morphometry (VBM) analyses will be performed on MRI data using the SPM software. VBM analysis allows for a voxel-by-voxel group comparison of anatomical neuroimaging data.
Segmented hippocampal subfields will be used for group comparison of volume using Generalized Linear Models (GLM).
Resting state functional MRI (rs-fMRI)
Data collection
Spontaneous cerebral activity will be measured through the temporal variations in BOLD’s signal amplitude. The resting state BOLD signal will be obtained through a specific acquisition sequence (500 EPI volumes acquired with repetition time 1.2s; echo time 30ms; flip angle 66⁰; 60 slices with slice thickness 2.4 mm; matrix size 90 × 90; in-plane resolution 2.4mm2). Participants will lay down in the MRI machine, with the lights off, equipped with noise cancelling headphones. Instructions will be to avoid movement and speech, while letting their thoughts roam freely. A debriefing will take place after the MRI scanning session to evaluate participants’ thoughts during the sequence. Additionally, physiological parameters known to affect the variations of the BOLD signal, such as respiratory and heart rate variations, will be collected throughout the acquisition. These parameters will be integrated in a model-based correction of physiological noise, which offers better denoising results than global signal removal [77].
Data analysis
Preprocessing of resting-state fMRI (rsfMRI) data will consist in a volume-by-volume realignment and temporal interpolation of slices to correct for the temporal latency. rsfMRI measures spontaneous cerebral connectivity, which provides information on the way cerebral structures interact with each other at rest. Whole-brain connectivity analyses will be performed and used for group comparison.
Diffusion Tensor Imaging (DTI)
Data collection
Diffusion data will be used to measure white matter microstructural integrity (A 2D-diffusion weighted sequence 64 directions; repetition time, 5.3s; echo time 73ms; flip angle 90°; slice thickness, 2 mm; matrix size, 108 × 108).
These data will be preprocessed to supply indirect measures of position, orientation, and anisotropy of white matter tracts. A reduction in anisotropy could reflect an alteration in density, diameter or thickness, or the thickness of the axonal myelin gains. Reconstructed DTI data will provide information on fractional anisotropy (FA) of white matter tracks and apparent diffusion coefficient (ADC). A reduced FA and/or ADC is thought to reflect a loss in white matter tracks integrity, possibly underlying impairments in cerebral connectivity.
Data analysis
Preprocessing of DTI data will be done using FSL software (FMRIB Software Library v5.0, Oxford, UK) in order to produce eigenvalue and eigenvector images, which will be used to create fractional anisotropy maps, allowing tractography analyses (structural connectivity between preselected regions of interest, ROIs). Analyses of DTI data will be run in FSL, using the tract-based spatial statistics (TBSS) procedure, the equivalent of VBM for white matter analysis. This will permit group comparisons of the integrity of white matter tracks.
Statistical analyses
General outline
Descriptive statistics of the participants will be computed through means (Standard deviation), medians (Interquartile range), or numbers (percentages), depending on whether the collected data are quantitative or qualitative.
All significance levels will be set at nominal p-value < 0.05, with two-sided hypothesis testing, due to the exploratory nature of this study, with no adjustment for multiple comparisons. Missing data are not expected but will be replaced using methods such as multiple imputations, assuming that the data will be missing at random [78].
Primary objective
Linear regression analyses will be performed on whole hippocampal volume with clinical status as a predictor to investigate the volume differences between BPD, BPD/PTSD and healthy participants. Regression analyses will be adjusted for the effect of relevant demographic and cerebral parameters.
Secondary objective
Segmented hippocampal subfields will be considered as ROIs and between-group volume differences will be assessed with linear regression with clinical status as a regressor. Regression analyses will be adjusted for the effect of relevant demographic and cerebral parameters.
Exploratory analyses
Group comparison analyses for each domain will be performed first between each clinical group, i.e. BPD and BPD/PTSD, and the healthy control group using Student t-test or Wilcoxon sum-rank test in case of departure from the normal distribution. Second, comparisons will be performed between the two clinical groups. Then, within-group correlation using Spearman coefficient and least square linear regression analyses between measures obtained in each domain will be conducted to provide information on the relationship between clinical, cognitive and neuroimaging measures in each group. Finally, profile analyses will be conducted to determine the effect of an additional PTSD diagnosis on the relationship between clinical, cognitive and neuroimaging measures (see Fig. 1).
To investigate the hypothesis that different clinical parameters and symptomatology affect different hippocampal subfields, regression analyses will be performed in each pathological group with hippocampal subfields as dependent variables and behavioral and clinical measures as regressors.
The putative role of the hippocampal volume as a predictor of the evolution of symptoms will be analyzed using repeated measures of clinical symptoms (baseline and 18 months). Repeated measures data will be analyzed with mixed models (Proc MIXED, SAS v9.4 software). The group will be set as fixed effect, and the intercept will be randomly defined to account for repeated measures across time. To test the role of the hippocampus on the evolution, mediation analyses on repeated clinical measures will be conducted with hippocampal volume as a mediator.
Discussion
This study aims to characterize adolescent BPD with or without comorbid PTSD on the clinical, neuroimaging and cognitive levels. Given the higher prevalence of BPD in females, our sample comprises female adolescents only to limit sex-related heterogeneity. To our knowledge, no previous study has compared adolescents with BPD to those with both BPD and PTSD. Furthermore, our study is the first to conduct a comprehensive assessment of psychological symptoms, cognition, and brain functioning in the same sample of adolescents with BPD adolescents, both with and without PTSD. Using multiple modalities within the same sample provides a unique opportunity to paint a more representative picture of the altered domains in BPD and PTSD, which is particularly important given the heterogeneous presentations of BPD. Notably, the volume of hippocampal subfields may be an interesting marker candidate differentiating between adolescents with BPD and those with both BPD and PTSD. A previous study conducted in our team indeed showed that hippocampal hypotrophies observed in youths with PTSD are driven by smaller volumes in specific hippocampal subfields [53]. Studying the precise location of hippocampal volume alterations in BPD may enhance our understanding of the specific brain changes associated with BPD in adolescence, while controlling for comorbid PTSD. Identifying specific and shared alterations in these populations could indeed be a stepping stone towards improving of the accuracy of BPD diagnosis in adolescents.
Acknowledgements
Not applicable.
Abbreviations
- ADC
Apparent Diffusion Coefficient
- ADRS
Adolescent Depression Rating Scale
- AIDA
Assessment of Identity Development in Adolescence
- ANPS
Affective Neuroscience Personality Scale
- BOLD
Blood-oxygen level dependent
- BPFSC-11
Borderline Personality Features Scale for Children 11
- BPD
Borderline Personality Disorder
- CA
Cornu Ammonis
- Ca-MIR
Cartes des Modèles Individuels de Relations (Maps of individual models of relationship)
- CGA-S
Clinical Global Assessment Scale
- CPT
Continuous Performance Test
- CTQ
Childhood Trauma Questionnaire
- DG
Dentate Gyrus
- DTI
Diffusion Tensor Imaging
- FA
Fractional anisotropy
- fMRI
function Magnetic Resonance Imaging
- GLM
General Linear Model
- GMV
Grey Matter Volume
- IQ
Intellectual Quotient
- K-SADS-PL
Kiddie-Schedule for Affective Disorders and Schizophrenia
- MASC
Movie Assessment of Social Cognition
- MRI
Magnetic Resonance Imaging
- NaCCS
Narrative Coherence Coding Scheme
- PTSD
Posttraumatic Stress Disorder
- RFQ
Reflective Functioning Questionnaire
- ROIs
Regions of Interest
- rsFC
Resting state Functional Connectivity
- rs-fMRI
Resting state functional Magnetic Resonance Imaging
- SIDP-IV
Structured Interview for DSM-IV Personality
- SPM
Statistical and Parametrical Mapping
- TBSS
Tract-Based Spatial Statistics
- UCLA PTSD-RI C/A
University of California Los Angeles Posttraumatic Stress Disorder Reaction Index for Children and Adolescents
- VBM
Voxel Based Morphometry
- WAIS-IV
Weschler’s’ Adult Intelligence Scale IV
- WISC-V
Weschler’s’ Intelligence Scale for Children V
- WCST
Wisconsin Card Sorting Test
Author contributions
All authors were involved in developing the protocol and approved the final protocol manuscript. BGG, FG, and HD designed the study protocol. JJP substantially contributed to the statistical analyses plan. MR drafted the manuscript, and BGG, FG, JJP and SS revised it critically for important intellectual content. SS and AV substantially contributed to neuroimaging collection and analyses plan. ML contributed to neuropsychological data collection. FG, GA and PG coordinated patient inclusions. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by Groupement Inter-Régional de Recherche Clinique et d’Innovation (GIRCI) Nord-Ouest for the AAP – Programme Hospitalier de Recherche Clinique Inter-Régional (PHRC-IR) grant number API 18 − 15, awarded following peer review of the study protocol by two independent reviewers. This work is managed by the GSC G4 and labelled by AVIESAN.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study follows the guidelines established in the French methodology of reference for studies in medical health (MR-001). Automated monitoring of health-related data is in line with the European Guidelines regarding the processing of personal data for scientific research purposes as established by the European Data Protection Board (April 27th, 2016). Clinical, neuropsychological, and socio-demographic data will be anonymized, and each participant will be identified with a code. Imaging data will be anonymized according to the guidelines provided by the DICOM standard committee (Clinical Trials De-Identification Profiles) and transmitted to the investigating team.
Explicit and informed consent from the participants and their legal representatives will be collected after they have been informed of the study objectives, its duration and procedure, as well as the potential risks and benefits of taking part in the research. Information notice will be given to both the participants and their legal representatives. Consent forms will be signed and dated by all involved parties, including a designated investigator. The medical procedures used in this study follow the guidelines of the Helsinki Declaration, as well as those of the law n°2012 − 300 (5th of march, 2012) and its application decree n° 2016 − 1537 (16th November 2016). Patients can retract their consent at any moment and can always exert their right to access and rectify their data. The promoter of the study will keep the participants informed of the results of the study at the end of the research.
An ID-RCB registration number has been requested from the Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM): IDRCB number 2019-A00366-51. This study was approved by the Comité de Protection des Personnes (Ethics Committee) on 05 January 2021 (RI-HPS), after being registered as a clinical trial on clinicaltrials.gov (trial number: NCT0485274, registered on 21/04/2021).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bohus M, Stoffers-Winterling J, Sharp C, Krause-Utz A, Schmahl C, Lieb K. Borderline personality disorder. Lancet Lond Engl. 2021;398(10310):1528–40. [DOI] [PubMed] [Google Scholar]
- 2.Leichsenring F, Heim N, Leweke F, Spitzer C, Steinert C, Kernberg OF. Borderline Personality Disorder: Rev JAMA. 2023;329(8):670–9. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th edition. Arlington, VA: American Psychiatric Association. 2013. 947 p.
- 4.Ellison WD, Rosenstein LK, Morgan TA, Zimmerman M. Community and clinical epidemiology of Borderline personality disorder. Psychiatr Clin North Am. 2018;41(4):561–73. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson JG, Herpertz SC, Skodol AE, Torgersen S, Zanarini MC. Borderline personality disorder. Nat Rev Dis Primer. 2018;4(1):1–20. [DOI] [PubMed] [Google Scholar]
- 6.Stepp SD, Lazarus SA, Byrd AL. A systematic review of risk factors prospectively associated with borderline personality disorder: taking stock and moving forward. Personal Disord Theory Res Treat. 2016;7(4):316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frías Á, Palma C. Comorbidity between post-traumatic stress disorder and Borderline personality disorder: a review. Psychopathology. 2015;48(1):1–10. [DOI] [PubMed] [Google Scholar]
- 8.Barnicot K, Crawford M. Posttraumatic stress disorder in patients with Borderline personality disorder: treatment outcomes and mediators. J Trauma Stress. 2018;31(6):899–908. [DOI] [PubMed] [Google Scholar]
- 9.Battle CL, Shea MT, Johnson DM, Yen S, Zlotnick C, Zanarini MC, et al. Childhood maltreatment Associated with Adult Personality disorders: findings from the collaborative Longitudinal Personality disorders Study. J Personal Disord. 2004;18(2):193–211. [DOI] [PubMed] [Google Scholar]
- 10.Porter C, Palmier-Claus J, Branitsky A, Mansell W, Warwick H, Varese F. Childhood adversity and borderline personality disorder: a meta-analysis. Acta Psychiatr Scand. 2020;141(1):6–20. [DOI] [PubMed] [Google Scholar]
- 11.Sharp C, Kim S. Recent advances in the Developmental aspects of Borderline personality disorder. Curr Psychiatry Rep. 2015;17(4):21. [DOI] [PubMed] [Google Scholar]
- 12.Fonagy P, Luyten P, Allison E, Campbell C. What we have changed our minds about: part 2. Borderline personality disorder, epistemic trust and the developmental significance of social communication. Borderline Personal Disord Emot Dysregulation. 2017;4(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweizer S, Gotlib IH, Blakemore SJ. The role of affective control in emotion regulation during adolescence. Emotion. 2020;20(1):80. [DOI] [PMC free article] [PubMed]
- 14.Branje S, de Moor EL, Spitzer J, Becht AI. Dynamics of Identity Development in Adolescence: a decade in review. J Res Adolesc. 2021;31(4):908–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilford EJ, Garrett E, Blakemore, Sarah-Jayne. The development of social cognition in adolescence: an integrated perspective. Neurosci Biobehav Rev. 2016;70:106–20. [DOI] [PubMed] [Google Scholar]
- 16.Constantinidis C, Luna B. Neural substrates of Inhibitory Control Maturation in Adolescence. Trends Neurosci. 2019;42(9):604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanen AM, Velakoulis D, Carison K, Gaunson K, Wood SJ, Yuen HP, et al. Orbitofrontal, amygdala and hippocampal volumes in teenagers with first-presentation borderline personality disorder. Psychiatry Res Neuroimaging. 2008;163(2):116–25. [DOI] [PubMed] [Google Scholar]
- 18.Whittle S, Chanen AM, Fornito A, McGorry PD, Pantelis C, Yücel M. Anterior cingulate volume in adolescents with first-presentation borderline personality disorder. Psychiatry Res. 2009;172(2):155–60. [DOI] [PubMed] [Google Scholar]
- 19.Brunner R, Henze R, Parzer P, Kramer J, Feigl N, Lutz K, et al. Reduced prefrontal and orbitofrontal gray matter in female adolescents with borderline personality disorder: is it disorder specific? NeuroImage. 2010;49(1):114–20. [DOI] [PubMed] [Google Scholar]
- 20.Goodman M, Hazlett EA, Avedon JB, Siever DR, Chu KW, New AS. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid major depression. J Psychiatr Res. 2011;45(6):803–7. [DOI] [PubMed] [Google Scholar]
- 21.Maier-Hein KH, Brunner R, Lutz K, Henze R, Parzer P, Feigl N, et al. Disorder-specific white matter alterations in adolescent Borderline personality disorder. Biol Psychiatry. 2014;75(1):81–8. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Q, Fu Y, Yi X, Ding J, Han Z, Zhang Z, et al. Altered cortical thickness and emotional dysregulation in adolescents with borderline personality disorder. Eur J Psychotraumatology. 2023;14(1):2163768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jovev M, Whittle S, Yücel M, Simmons JG, Allen NB, Chanen AM. The relationship between hippocampal asymmetry and temperament in adolescent borderline and antisocial personality pathology. Dev Psychopathol. 2014;26(1):275–85. [DOI] [PubMed] [Google Scholar]
- 24.Richter J, Brunner R, Parzer P, Resch F, Stieltjes B, Henze R. Reduced cortical and subcortical volumes in female adolescents with borderline personality disorder. Psychiatry Res. 2014;221(3):179–86. [DOI] [PubMed] [Google Scholar]
- 25.Ruocco AC, Carcone D. A neurobiological model of Borderline personality disorder: systematic and integrative review. Harv Rev Psychiatry. 2016;24(5):311–29. [DOI] [PubMed] [Google Scholar]
- 26.Kelleher-Unger I, Tajchman Z, Chittano G, Vilares I. Meta-analysis of white matter diffusion tensor imaging alterations in borderline personality disorder. Psychiatry Res Neuroimaging. 2021;307:111205. [DOI] [PubMed] [Google Scholar]
- 27.Lou J, Sun Y, Cui Z, Gong L. Common and distinct patterns of gray matter alterations in borderline personality disorder and posttraumatic stress disorder: a dual meta-analysis. Neurosci Lett. 2021;741:135376. [DOI] [PubMed] [Google Scholar]
- 28.Kunimatsu A, Yasaka K, Akai H, Kunimatsu N, Abe O. MRI findings in posttraumatic stress disorder. J Magn Reson Imaging. 2020;52(2):380–96. [DOI] [PubMed] [Google Scholar]
- 29.Del Casale A, Ferracuti S, Barbetti AS, Bargagna P, Zega P, Iannuccelli A, et al. Grey Matter volume reductions of the Left Hippocampus and Amygdala in PTSD: a coordinate-based Meta-analysis of magnetic resonance Imaging studies. Neuropsychobiology. 2022;81(4):257–64. [DOI] [PubMed] [Google Scholar]
- 30.Dennis EL, Disner SG, Fani N, Salminen LE, Logue M, Clarke EK, et al. Altered white matter microstructural organization in posttraumatic stress disorder across 3047 adults: results from the PGC-ENIGMA PTSD consortium. Mol Psychiatry. 2021;26(8):4315–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visintin E, De Panfilis C, Amore M, Balestrieri M, Wolf RC, Sambataro F. Mapping the brain correlates of borderline personality disorder: a functional neuroimaging meta-analysis of resting state studies. J Affect Disord. 2016;204:262–9. [DOI] [PubMed] [Google Scholar]
- 32.Amad A, Radua J, Vaiva G, Williams SC, Fovet T. Similarities between borderline personality disorder and post traumatic stress disorder: evidence from resting-state meta-analysis. Neurosci Biobehav Rev. 2019;105:52–9. [DOI] [PubMed] [Google Scholar]
- 33.Nunes PM, Wenzel A, Borges KT, Porto CR, Caminha RM, de Oliveira IR. Volumes of the Hippocampus and Amygdala in patients with Borderline personality disorder: a Meta-analysis. J Personal Disord. 2009;23(4):333–45. [DOI] [PubMed] [Google Scholar]
- 34.Ruocco AC, Amirthavasagam S, Zakzanis KK. Amygdala and hippocampal volume reductions as candidate endophenotypes for borderline personality disorder: a meta-analysis of magnetic resonance imaging studies. Psychiatry Res Neuroimaging. 2012;201(3):245–52. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Hu L, Zeng J, Tan Y, Cheng B. Default mode network and frontolimbic gray matter abnormalities in patients with borderline personality disorder: a voxel-based meta-analysis. Sci Rep. 2016;6(1):34247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson RJ, Fox A, Kalin NH. Neural bases of emotion regulation in Nonhuman Primates and humans. Handbook of emotion regulation. New York, NY, US: The Guilford Press; 2007. pp. 47–68. [Google Scholar]
- 37.Mitchell MR, Potenza MN. Recent insights into the Neurobiology of Impulsivity. Curr Addict Rep. 2014;1(4):309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pattij T, Vanderschuren LJMJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29(4):192–9. [DOI] [PubMed] [Google Scholar]
- 39.Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci. 2017;20(11):1434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montagrin A, Saiote C, Schiller D. The social hippocampus. Hippocampus. 2018;28(9):672–9. [DOI] [PubMed] [Google Scholar]
- 41.Chaposhloo M, Nicholson AA, Becker S, McKinnon MC, Lanius R, Shaw SB, et al. Altered resting-state functional connectivity in the anterior and posterior hippocampus in post-traumatic stress disorder: the central role of the anterior hippocampus. NeuroImage Clin. 2023;38:103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Rooij SJH, Stevens JS, Ely TD, Hinrichs RC, Michopoulos V, Winters SJ, et al. The role of the hippocampus in predicting future PTSD symptoms in recently traumatized civilians. Biol Psychiatry. 2018;84(2):106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keding TJ, Herringa RJ. Abnormal structure of fear circuitry in Pediatric post-traumatic stress disorder. Neuropsychopharmacology. 2015;40(3):537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malhi GS, Das P, Outhred T, Irwin L, Gessler D, Bwabi Z, et al. The effects of childhood trauma on adolescent hippocampal subfields. Aust N Z J Psychiatry. 2019;53(5):447–57. [DOI] [PubMed] [Google Scholar]
- 46.Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2013;52:24–37. [DOI] [PubMed] [Google Scholar]
- 47.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A. 2012;109(9):E563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niedtfelt I, Schulze L, Krause-Utz A, Demirakca T, Bohus M, Schmahl C. Voxel-based morphometry in women with Borderline personality disorder with and without Comorbid Posttraumatic stress disorder. PLoS ONE. 2013;8(6):e65824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res Neuroimaging. 2003;122(3):193–8. [DOI] [PubMed] [Google Scholar]
- 50.Weniger G, Lange C, Sachsse U, Irle E. Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci JPN. 2009;34(5):383–8. [PMC free article] [PubMed] [Google Scholar]
- 51.Zetzsche T, Preuss UW, Frodl T, Schmitt G, Seifert D, Münchhausen E, et al. Hippocampal volume reduction and history of aggressive behaviour in patients with borderline personality disorder. Psychiatry Res Neuroimaging. 2007;154(2):157–70. [DOI] [PubMed] [Google Scholar]
- 52.Rodrigues E, Wenzel A, Ribeiro MP, Quarantini LC, Miranda-Scippa A, de Sena EP, et al. Hippocampal volume in borderline personality disorder with and without comorbid posttraumatic stress disorder: a meta-analysis. Eur Psychiatry. 2011;26(7):452–6. [DOI] [PubMed] [Google Scholar]
- 53.Postel C, Viard A, André C, Guénolé F, de Flores R, Baleyte J, et al. Hippocampal subfields alterations in adolescents with post-traumatic stress disorder. Hum Brain Mapp. 2018;40(4):1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bøen E, Westlye LT, Elvsåshagen T, Hummelen B, Hol PK, Boye B, et al. Smaller stress-sensitive hippocampal subfields in women with borderline personality disorder without posttraumatic stress disorder. J Psychiatry Neurosci JPN. 2014;39(2):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soper DS. A-priori Sample Size for Student t-Tests. [cited 2024 Jul 22]. https://www.danielsoper.com/statcalc
- 56.Klein DN, Ouimette PC, Kelly HS, Ferro T, Riso LP. Test-retest reliability of team consensus best-estimate diagnoses of axis I and II disorders in a family study. Am J Psychiatry. 1994;151(7):1043–7. [DOI] [PubMed] [Google Scholar]
- 57.Pfohl B, Blum N, Zimmerman M, Personality. SIDP-IV. American Psychiatric Pub; 1997. 50.
- 58.Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N. K-SADS-PL DSM-5. Pittsburgh: Western Psychiatric Institute and Clinic; 2016. [Google Scholar]
- 59.Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228–31. [DOI] [PubMed] [Google Scholar]
- 60.Kaplow JB, Rolon-Arroyo B, Layne CM, Rooney E, Oosterhoff B, Hill R, et al. Validation of the UCLA PTSD reaction index for DSM-5: a developmentally informed Assessment Tool for Youth. J Am Acad Child Adolesc Psychiatry. 2020;59(1):186–94. [DOI] [PubMed] [Google Scholar]
- 61.Revah-Levy A, Birmaher B, Gasquet I, Falissard B. The adolescent Depression Rating Scale (ADRS): a validation study. BMC Psychiatry. 2007;7(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ensink K, Bégin M, Kotiuga J, Sharp C, Normandin L. Psychometric properties of the French Version of the Borderline personality features scale for children and adolescents. Adolesc Psychiatry. 2020;10(1):48–58. [Google Scholar]
- 63.Sharp C, Steinberg L, Temple J, Newlin E. An 11-item measure to assess borderline traits in adolescents: refinement of the BPFSC using IRT. Personal Disord Theory Res Treat. 2014;5(1):70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wechsler D. Échelle d’intelligence de Wechsler pour enfants et adolescents, 5ème édition (WISC-V)—Adaptation française. ECPA Par Pearson. 2016.
- 65.Wechsler D. WAIS-IV Nouvelle version de l’échelle d’intelligence de wechsler pour adultes. Paris Ed Cent Psychol Appliquée; 2011.
- 66.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent Psychiatric Population. J Am Acad Child Adolesc Psychiatry. 1997;36(3):340–8. [DOI] [PubMed] [Google Scholar]
- 67.Corcos M, Pham-Scottez A, Speranza M. Troubles de la personnalité borderline à l’adolescence. Paris (FR): Dunod. 2013.
- 68.Pierrehumbert B, Karmaniola A, Sieye A, Meister C, Miljkovitch R, Halfon O. Les modéles de relations: Développement d’un auto-questionnaire d’attachement pour adultes. Psychiatrie Enf. 1996;1:161–206.
- 69.Pahlavan F, Mouchiroud C, Zenasni F, Panksepp J. Validation De L’adaptation française de l’échelle neuro-affective de personnalité. Eur Rev Appl Psychol. 2008;58(3):155–63. [Google Scholar]
- 70.Conners CK. CPT-II, continuous performance test II. Toronto. Ont Can Multi-Health Syst; 2002.
- 71.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin card sorting test manual. Odessa, Fla. Psychol Assess Resour. 1981.
- 72.Martinez G, Alexandre C, Mam-Lam-Fook C, Bendjemaa N, Gaillard R, Garel P, et al. Phenotypic continuum between autism and schizophrenia: evidence from the movie for the Assessment of Social Cognition (MASC). Schizophr Res. 2017;185:161–6. [DOI] [PubMed] [Google Scholar]
- 73.Badoud D, Luyten P, Fonseca-Pedrero E, Eliez S, Fonagy P, Debbané M. The French Version of the reflective functioning questionnaire: Validity Data for adolescents and adults and its Association with Non-suicidal Self-Injury. PLoS ONE. 2015;10(12):e0145892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goth K, Foelsch P, Schlüter-Müller S, Birkhölzer M, Jung E, Pick O, et al. Assessment of identity development and identity diffusion in adolescence - theoretical basis and psychometric properties of the self-report questionnaire AIDA. Child Adolesc Psychiatry Ment Health. 2012;6(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reese E, Haden CA, Baker-Ward L, Bauer P, Fivush R, Ornstein PA. Coherence of personal narratives across the Lifespan: a Multidimensional Model and Coding Method. J Cogn Dev off J Cogn Dev Soc. 2011;12(4):424–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.La Joie R, Fouquet M, Mézenge F, Landeau B, Villain N, Mevel K, et al. Differential effect of age on hippocampal subfields assessed using a new high-resolution 3T MR sequence. NeuroImage. 2010;53(2):506–14. [DOI] [PubMed] [Google Scholar]
- 77.Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009;47(4):1448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: A Data Analyst’s perspective. Multivar Behav Res. 1998;33(4):545–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.